Abstract
Malignancies with unknown primaries contribute to a small yet significant percentage of overall tumors. Neuroendocrine carcinomas, a rare disease with a poor prognosis, have been known to present as an unknown primary. Treatment consists of cytotoxic chemotherapy but given the latter’s high toxicity profile new treatment options are being explored. In this case report, we describe a case of a patient with poorly differentiated neuroendocrine carcinoma of unknown primary treated with compassionate oral everolimus after his refusal of intravenous chemotherapy.
Keywords: Everolimus, hematology, oncology, poorly differentiated neuroendocrine carcinoma
Introduction
About 5% of invasive cancers are of unknown primary, 1 with less than 5% of those being neuroendocrine carcinomas, 2 rendering this entity a very rare disease, with poor prognosis.3,4
Treatment of poorly differentiated neuroendocrine carcinoma (PDNEC) is based on intravenous chemotherapy combining platinum compounds and etoposide, with high toxicity risk especially in the elderly, or enrollment into a clinical trial.3,4 In this case report, we describe a case of a patient with PDNEC of unknown primary treated with compassionate oral everolimus after his refusal of intravenous chemotherapy.
Case presentation
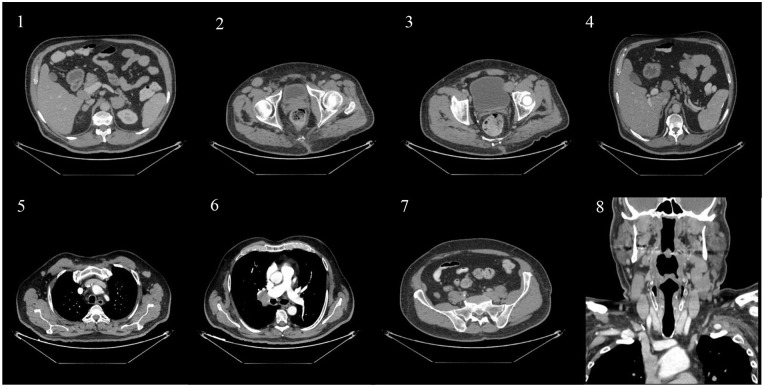
A 63-year-old male patient, non-smoker, with a history of type II diabetes and peripheral vascular disease presented for left flank pain. He was admitted for management of left obstructive kidney stone by JJ insertion. On physical examination, the patient was found to have multiple enlarged lymph nodes, predominantly in the cervical and right inguinal areas. Initial computed tomography (CT) scan that aided in the diagnosis of obstructive pyelonephritis was consistent with the physical examination findings of generalized lymphadenopathy. It revealed enlarged cervical, axillary, mediastinal, hilar, mesenteric, retroperitoneal, and pelvic adenopathies. Splenic hypodensities and adrenal nodules were also discovered (Figure 1).
Figure 1.
Coronal scans of the adrenals (1), inguinal region (2), pelvic area (3), portocaval (4), axillary (5), hilar (6), and peri-psoas muscle areas (7). Sagittal scan of the cervical area (8).
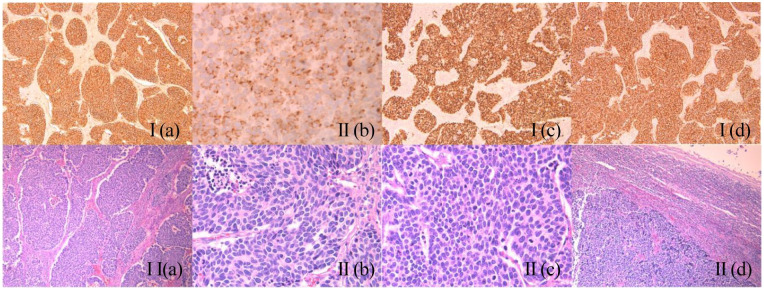
An excisional biopsy of the right inguinal lymph node was performed. Histopathology showed a lymph node entirely occupied by a malignant neoplasm with high mitotic index and patchy areas of necrosis. The neoplastic cells were CK7 (cytoplasmic paranuclear dots), CD56, TTF1, and synapthophysin positive, whereas they were CK20 and chromogranin negative. Ki-67 index was 80%. A diagnosis of PDNEC was made (Figure 2 and figure 3).
Figure 2.
I(a): CD56 ×100; Positive membranous staining. I(b): CK7 ×400; Positive cytoplasmic staining of tumor cells with characteristic perinuclear dots. I(c): TTF1 ×100; Positive nuclear staining. I(d): Synaptophysin ×100; Positive cytoplasmic staining. II(a): HE ×100; The tumor cells are arranged in nests of variable size and shape, some of which exhibiting peripheral nuclear palisading, set within a desmoplastic stroma. II(b): HE ×400; Mitotic figures are also easily detected. II(c): HE ×400; Tumor cells have high nuclear to cytoplasmic ratio and exhibit an oval to round nucleus with finely granular chromatin. Occasional necrotic areas are present (left upper area). II(c): HE ×40; Sheets of metastatic small cell carcinoma cells (left lower half) in a lymph node (residual lymphocytes at the periphery).
Figure 3.
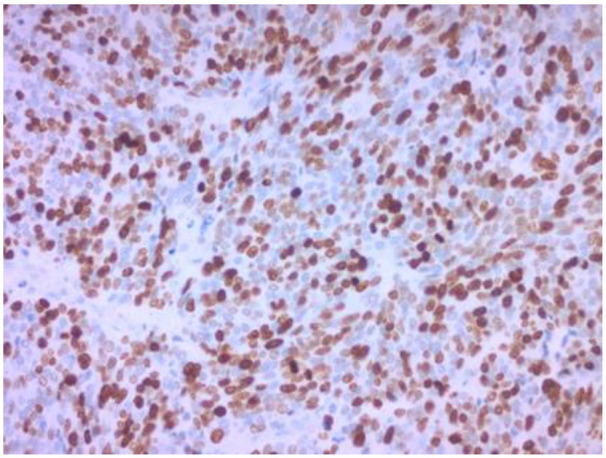
Ki-67 immunohistochemical stain revealing expression by approximately 80% of the neoplastic cells. Original magnification 200×.
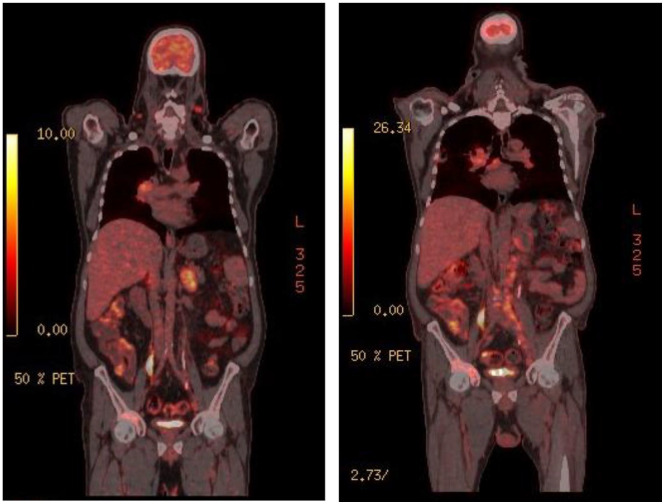
Whole-body fluoro deoxy glucose (FDG) PET was performed for initial staging (Figure 4) showing increased FDG uptake in bilateral cervical nodes with an SUV = 6.7, a solitary liver lesion with an SUV max of 5.5, bilateral adrenal foci and metastatic bone disease—in the right acetabulum and right iliac bone—with an SUV = 8.6. Scattered FDG avid retroperitoneal and pelvic adenopathies with an SUV max of 7.1 and 5.7, respectively. A satellite lesion in the right lower lung lobe was also noted with an SUV = 6.9 (Figure 4).
Figure 4.
Initial (left) FDG-PET showing increased uptake in several organs versus mild progress seen in a comparable image 6 months later (right).
The patient vigorously declined intravenous chemotherapy despite being in good general health status. He was started on compassionate everolimus 10 mg daily. Clinical follow-up at 2 and 4 months revealed stable cervical and inguinal lymph nodes. The patient maintained a good performance status (ECOG PS = 1) that allowed him to keep on with his usual physical activities. Follow-up FDG PET at 6 months showed disease progression with mainly peri-portal lymphadenopathies using RECIST 1.1 criteria. Everolimus was stopped as the patient was complaining of diarrhea along with numbness and tingling his feet bilaterally. The patient still refused intravenous chemotherapy and death occurred 8 months after diagnosis from a complicated myocardial infarction.
Discussion
The biological and clinical features of PDNEC resemble small cell lung carcinoma with respect to their rapid growth, morphological and biological heterogeneity as well as the relatively high sensitivity to platinum-based chemotherapy. 5
Following the 2019 WHO classification and grading of neuroendocrine neoplasms of the gastrointestinal tract and hepato-pancreato-biliary organs, NET G1, G2, and G3 are well-differentiated tumors with a low, intermediate, and high grade, respectively, have a mitotic rate of <2 mitoses/2 mm², 2–20 mitoses/2 mm², and >20 mitoses/2 mm², respectively, and a Ki67 index of <3%, 3–20%, and >20%, respectively. Small-cell type and large-cell type neuroendocrine carcinomas are poorly differentiated and considered high grade with a mitotic rate of >20 mitoses/2 mm² and a ki-67 index of >20%. 6
PDNECs’ response to platinum agents significantly fluctuates. A hallmark of PDNEC is the key loss of function mutations in tumor suppressor genes, PTEN and/or TSC and, as a result, the PI3K/AKT/mTOR pathway becomes highly active. 7 This presents an opportunity for tyrosine kinase inhibitors (TKIs) and mammalian target of rapamycin (mTOR) inhibitors, which target the PI3K/AKT/mTOR pathway.
The recent WHO classification recognized two distinct morphological subtypes of PDNEC: the small cell type and the large-cell type. Despite the current treatments, based on the combination etoposide-cisplatin, the prognosis of both subtypes is poor, with median survival durations in patients with localized, regional, and distant disease of 34, 14, and 5 months, respectively.8,9 Lindholm et al. 10 revealed the efficacy of temozolomide, an alkylating agent, along with bevacizumab, a monoclonal antibody targeting tumor angiogenesis, after etoposide-cisplatin failed. There is a huge potential for future targeted therapies to be evaluated in PDNECs.
mTOR signaling pathway has a crucial role in controlling cancer cell-cycle and growth. 11 The activation of the mTOR pathway plays a major role in the pathogenesis of NETs. 12 Shida et al. 13 assessed the expression of phospho-mTOR between well-differentiated and poorly differentiated neuroendocrine neoplasms. More than 65% of PDNECs had a high expression of phosphor-mTOR as compared to only 27% well-differentiated NETs and carcinomas. Furthermore, Catena et al. 14 were able to reveal than 80% of tumors in 36 patients diagnosed with PDNECs had a high expression of phosphor-mTOR.
The RADIANT trials were clinical studies that studied the efficacy and safety of everolimus, an oral inhibitor of the mTOR pathway in patients with NETs of different origins. 15 – 18 They showed significant clinical benefit of everolimus in patients with well and moderately differentiated advanced pancreatic, lung, and gastrointestinal NET’s. Duran et al. evaluated the efficacy of temsirolimus, another mTOR inhibitor, in advanced neuroendocrine carcinoma. In this phase II study, having a higher baseline level of phospho-mTOR was predictive of tumor response and a decrease in phospho-mTOR after temsirolimus treatment was associated with increased time to progression. 19 Bollard et al. 20 also demonstrated a strong expression of phospho-mTOR in several cases of PDNECs. After utilizing xenografted mice with everolimus, a decrease in the number of nodules as well as the mean surface and total tumor surface was observed. This as explained by a decrease in proliferation rather than an increase in apoptosis as a decrease in Ki67 index between control and treated animals was observed, but there was difference in the percentage of cells expressing active caspase 3.9,20
Gilabert et al. 21 report a case series of six patients newly diagnosed patients (4 males and 2 females, median age 55 years (range 40–70)) with a metastatic PDNEC that refused chemotherapy or deteriorated at the time of the diagnosis and underwent targeted therapies, either sunitinib or everolimus.
Panzuto et al. 22 investigated the use of everolimus in well-moderately differentiated pancreatic neuroendocrine carcinoma in 15 patients with a median Ki67 of 30% and Eastern Cooperative Oncology Group performance status 0 to 1. Of these, 11 had been pretreated with chemotherapy or peptide receptor radionuclide therapy and only four received everolimus as first-line therapy. Median progression-free survival was 6 months, and median overall survival was 28 months. At 3 months follow-up, 11 patients achieved disease stabilization. Six patients (40%) maintained the latter for at least 12 months. Three of four patients who received everolimus as first-line therapy had sustained disease stabilization, progression-free survival, 12, 17, and 22 months.
Chemotherapeutic agents or other targeted therapies should be used in combination with mTOR inhibitors. In addition, a stronger efficacy is expected if the mTOR pathway is targeted at several levels, such as PI3kinase and mTOR levels, by using new inhibitors targeting both complexes mTORC1 and mTORC2, or by targeting several pathways simultaneously (mTOR and MAPkinase pathways).23,11 A completed phase II study, NCT02248012, evaluated the use of everolimus or temozolomide as therapies in the first-line setting in gastroenteropancreatic neuroendocrine carcinomas, and we are awaiting its result. 24
Table 1 summarizes the use of everolimus in neuroendocrine carcinoma.
Table 1.
RADIANT trials findings and case reports of everolimus use in neuroendocrine carcinoma.
| Authors | Study type | Summary |
|---|---|---|
| Yao et al. 17 | Radiant 1 trial | Daily everolimus, with or without concomitant octreotide LAR, demonstrates antitumor activity as measured by objective response rate and PFS and is well tolerated in patients with advanced pancreatic NETs after failure of prior systemic chemotherapy. |
| Pavel et al. 16 | Radiant 2 trial | Everolimus plus octreotide LAR, compared with placebo plus octreotide LAR, improved progression-free survival in patients with advanced neuroendocrine tumors associated with carcinoid syndrome. |
| Yao et al. 8 | Radiant 3 trial | Everolimus, as compared with placebo, improves progression-free survival in patients with advanced pancreatic neuroendocrine tumors. Grade 1 and 2 adverse events with everolimus. |
| Yao et al. 17 | Radiant 4 trial | Treatment with everolimus was associated with significant
improvement in progression-free survival in patients with
progressive lung or gastrointestinal neuroendocrine
tumors. Acceptable tolerability of everolimus. |
| Bollard et al. 20 | Case report | Anti-proliferative effect of everolimus in PDNEC tumor in a
patient with PDNEC of unknown primary origin. He maintained a good performance status and a stable disease for 6 months before progression |
| Gilabert et al. 21 | Case Report | Poorly differentiated pancreatic neuroendocrine carcinoma treated successfully with everolimus for 15 months with a survival of more than 2 years following diagnosis |
| Panzuto et al. 22 | Retrospective study | Everolimus is active in well-differentiated neuroendocrine carcinoma G3 with Ki67 less than 55%. Seventy-five percent of the patients receiving everolimus as a first line had a progression-free survival of 12, 17, and 22 months. |
PDNEC: poorly differentiated neuroendocrine carcinoma.
LAR: long-acting repeatable.
PFS: progression free survival.
Conclusion
The standard treatment for PDNEC is chemotherapy with platinum-based regimens. PDNEC was shown to exhibit high expression and activation of mTOR in immunohistochemical analyses. Everolimus was shown to have an anti-proliferative effect in these tumors in a preclinical study. In this case report, the patient had a PDNEC of unknown primary origin with a very poor prognosis. He maintained a good performance status and a stable disease for 6 months before progression. Another case report described a patient with a poorly differentiated pancreatic neuroendocrine carcinoma treated successfully with everolimus for 15 months with a survival of more than 2 years following diagnosis.
Everolimus could be an interesting palliative treatment option for patients with PDNEC of unknown primary. Unfortunately, given the rarity of the disease, confirming this hypothesis with multicentered phase III studies can be very challenging, and data from individual case reports and case series may remain the only objective evidence to rely on for the time being.
In conclusion, mTOR inhibitors are known as effective treatment modality for well and moderately differentiated NEC. Studies should be conducted to assess efficacy in PDNEC especially of unknown primary.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: Our institution does not require ethical approval for reporting individual cases or case series.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Maroun Bou Zerdan  https://orcid.org/0000-0002-9229-3079
https://orcid.org/0000-0002-9229-3079
References
- 1. Greco FA, Hainsworth JD. Cancer of unknown primary site. Oncology 2006; 379: 1119–1132. [Google Scholar]
- 2. Spigel DR, Hainsworth JD, Greco FA, et al. Neuroendocrine carcinoma of unknown primary site. Semin Oncol 2009; 36: 52–59. [DOI] [PubMed] [Google Scholar]
- 3. Moertel CG, Lefkopoulo M, Lipsitz S, et al. Streptozocin–doxorubicin, streptozocin–fluorouracil, or chlorozotocin in the treatment of advanced islet-cell carcinoma. N Engl J Med 1992; 326(8): 519–523. [DOI] [PubMed] [Google Scholar]
- 4. Mitry E, Baudin E, Ducreux M, et al. Treatment of poorly differentiated neuroendocrine tumours with etoposide and cisplatin. Br J Cancer 1999; 81(8): 1351–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Basturk O, Yang Z, Tang LH, et al. The high grade (WHO G3) pancreatic neuroendocrine tumor category is morphologically and biologically heterogeneous and includes both well differentiated and poorly differentiated neoplasms. Am J Surg Pathol 2015; 39(5): 683–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nagtegaal ID, Odze RD, Klimstra D, et al. The 2019 WHO classification of tumours of the digestive system. Histopathology 2020; 76(2): 182–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Missiaglia E, Dalai I, Barbi S, et al. Pancreatic endocrine tumors: expression profiling evidences a role for AKT-mTOR pathway. J Clin Oncol 2010; 28(2): 245–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yao JC, Hassan M, Phan A, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol 2008; 26(18): 3063–3072. [DOI] [PubMed] [Google Scholar]
- 9. Sethi A, Islam M, Moses R, et al. Epidemiology and retrospective analysis in extrapulmonary neuroendocrine carcinoma. Cureus 2021; 13(1): e12748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lindholm DP, Eriksson B, Granberg D. Response to temozolomide and bevacizumab in a patient with poorly differentiated neuroendocrine carcinoma. Med Oncol 2012; 29(1): 301–303. [DOI] [PubMed] [Google Scholar]
- 11. Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell 2012; 149(2)): 274–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wolin EM. PI3K/Akt/mTOR pathway inhibitors in the therapy of pancreatic neuroendocrine tumors. Cancer Lett 2013; 335(1): 1–8. [DOI] [PubMed] [Google Scholar]
- 13. Shida T, Kishimoto T, Furuya M, et al. Expression of an activated mammalian target of rapamycin (mTOR) in gastroenteropancreatic neuroendocrine tumors. Cancer Chemother Pharmacol 2010; 65(5): 889–893. [DOI] [PubMed] [Google Scholar]
- 14. Catena L, Bajetta E, Milione M, et al. Mammalian target of rapamycin expression in poorly differentiated endocrine carcinoma: clinical and therapeutic future challenges. Target Oncol 2011; 6(2): 65–68. [DOI] [PubMed] [Google Scholar]
- 15. Yao JC, Phan AT, Chang DZ, et al. Efficacy of RAD001 (everolimus) and octreotide LAR in advanced low-to intermediate-grade neuroendocrine tumors: results of a phase II study. J Clin Oncol 2008; 26(26): 4311–4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pavel ME, Hainsworth JD, Baudin E, et al. Everolimus plus octreotide long-acting repeatable for the treatment of advanced neuroendocrine tumours associated with carcinoid syndrome (RADIANT-2): a randomised, placebo-controlled, phase 3 study. Lancet 2011; 378(9808): 2005–2012. [DOI] [PubMed] [Google Scholar]
- 17. Yao JC, Shah MH, Ito T, et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med 2011; 364(6): 514–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yao JC, Fazio N, Singh S, et al. Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): a randomised, placebo-controlled, phase 3 study. Lancet 2016; 387(10022): 968–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Duran I, Kortmansky J, Singh D, et al. A phase II clinical and pharmacodynamic study of temsirolimus in advanced neuroendocrine carcinomas. Br J Cancer 2006; 95(9): 1148–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bollard J, Couderc C, Blanc M, et al. Antitumor effect of everolimus in preclinical models of high-grade gastroenteropancreatic neuroendocrine carcinomas. Neuroendocrinology 2013; 97(4): 331–340. [DOI] [PubMed] [Google Scholar]
- 21. Gilabert M, Rho YS, Kavan P. Targeted therapies provide treatment options for poorly differentiated pancreatic neuroendocrine carcinomas. Oncology 2017; 92(3): 170–172. [DOI] [PubMed] [Google Scholar]
- 22. Panzuto F, Rinzivillo M, Spada F, et al. Everolimus in pancreatic neuroendocrine carcinomas G3. Pancreas 2017; 46(3): 302–305. [DOI] [PubMed] [Google Scholar]
- 23. Albert S, Serova M, Dreyer C, et al. New inhibitors of the mammalian target of rapamycin signaling pathway for cancer. Expert Opin Investig Drugs 2010; 19(8): 919–930. [DOI] [PubMed] [Google Scholar]
- 24. Herrera-Martínez AD, Hofland J, Hofland LJ, et al. Targeted systemic treatment of neuroendocrine tumors: current options and future perspectives. Drugs 2019; 79(1): 21–42. [DOI] [PMC free article] [PubMed] [Google Scholar]