Abstract
Appendiceal diverticulosis is a rare finding associated with appendiceal neoplasms. Both can masquerade as appendicitis in patients and are overlooked in differentials of right upper quadrant pain. A 37-year-old African American female presented with appendicitis-like symptoms to the emergency room with fever and leukocytosis. Appendectomy was performed with pathological evaluation revealing coexisting appendiceal diverticula and carcinoid of the appendix with lymphovascular invasion and mesoappendiceal involvement. In line with the National Comprehensive Cancer Network guidelines, right hemicolectomy with lymph node dissection was performed which was negative for neoplastic invasion but positive for colonic diverticulosis. While there have been many case reports of appendiceal diverticula with coexisting appendiceal carcinoid, a concurrent colonic diverticulum in the right hemicolectomy specimen during the oncologic resection of the appendiceal carcinoid has not been previously reported. We propose colonic diverticula as another possible feature that may be associated with appendiceal diverticula especially with an underlying appendiceal neoplasm.
Keywords: Appendiceal diverticula, appendiceal carcinoid, colonic diverticula, surgical oncology, colorectal surgery
Introduction
Appendicitis is one of the most encountered surgical emergencies. Oftentimes diverticulitis of the appendix can mimic appendicitis and present with similar symptoms. Appendiceal diverticulitis is the result of inflammation of the appendiceal diverticulum and is commonly misdiagnosed as acute appendicitis preoperatively. A resected appendix is sent for histopathological examination which in about 0.4%–1.7% of the cases yields acquired appendiceal diverticulum.1,2 Interestingly, often concurrent with the appendiceal diverticula, about 0.5% of the resected appendices have neoplastic transformation. 1 The association between diverticular appendix and appendiceal neoplasm is an underrated part of appendiceal pathology and can be critical for diagnosing appendiceal neoplasms, among which carcinoids are the most common. Classified into two types, congenital and acquired, appendiceal diverticula are a rare entity generally found incidentally. 2 Neuroendocrine tumors (NETs) are derived from enterochromaffin cells and represent the most common gastrointestinal NETs. NETs are frequently seen in the small intestine (45%), followed by the rectum (20%), appendix (17%), colon (11%), and stomach (7%).2–4 The diagnosis of a carcinoid tumor is generally confirmed on initial histology with positive staining for neuroendocrine markers, most commonly chromogranin A or synaptophysin. However, due to the slow-growing nature of the carcinoid tumors, the initial identification can be challenging. To aid in diagnosis, one must identify the classic symptoms of carcinoid syndrome including skin flushing, chronic diarrhea, bronchospasm, and hypotension. The symptoms are thought to be caused by the release of vasoactive substances, most reliably 5-hydroxytryptophan (5-HT) also known as serotonin. 5-HT is synthesized nearly exclusively by the enterochromaffin cells and aids in early diagnosis with the use of 24-h urine collection of its inactive metabolite, 5-hydroxyindoleacetic acid (5-HIAA), which is found to be elevated.3,4
Incidentally found appendiceal carcinoids appear to be indolent with very low rate of metastasis (0%–1%); however, for larger appendiceal carcinoids (>2 cm), the risk of metastasis increases to 25%–40% with the 5-year survival for localized tumor, regional metastasis, and distant metastasis postulated to be 92%, 81%, and 31%, respectively.5–7 While most of the appendiceal carcinoids are diagnosed and cured following appendectomy for appendicitis, we rarely encounter a more advanced form of the disease with distant metastasis, most commonly to the liver with accompanying carcinoid syndrome. In the more advanced stages of appendiceal carcinoids, cytoreductive partial hepatectomy has shown to improve the 5-year prognosis to 70% and complete resolution of carcinoid symptoms in 86% of patients. 8 Early diagnosis and treatment of carcinoid tumor is important to prevent the progression of gastrointestinal carcinoid metastasis.
Case presentation
A 37-year-old African American female presented to the emergency department at the NYC Health and Hospitals-Metropolitan with a complaint of severe abdominal pain in right lower quadrant. The pain had initially started 2 years ago with associated symptoms of diarrhea, early satiety, shortness of breath, and skin flushing. The symptoms became progressively worse over the past week, and 2 days before presentation, patient had developed fever and mild nausea. Patient denied having hematochezia, melena, chest pain, and chills. She denied a previous history of appendicitis. Her vital signs were within normal limits. Abdominal examination revealed right lower quadrant tenderness with no guarding, rebound, or a palpable mass in the abdomen. Laboratory tests yielded a white blood cell count of 11.08 × 103 cells/μL (polymorphonuclears: 59.4%). Computed tomography (CT) scan revealed an inflamed appendix with no peri-appendiceal wall thickening or fat stranding (Figure 1). The patient was admitted with a diagnosis of appendicitis and underwent urgent laparoscopic appendectomy. The appendiceal specimen was sent for histopathologic evaluation which revealed no appendicitis, rather an acquired appendiceal diverticulum (Figure 2) and more notably a carcinoid tumor measuring 1.25 cm in size with invasion to the lamina propria, submucosa, muscularis propria, and mesoappendix, as well as lymphovascular and perineural involvement (Figures 3 and 4). Of note, the appendiceal diverticulum was not grossly visible intraoperatively. The carcinoid tumor was confirmed by positive immunostaining for neuroendocrine markers chromogranin A and synaptophysin. It was graded as low grade <2%, G1 well-differentiated NET, margins negative, and staged as pT1, pNx (Table 1). 9 Postoperatively, the case was discussed in multidisciplinary tumor board and the decision was made to return to the operating room, during elective readmission with bowel prep 1 week later, for a more extensive surgery than previously performed simple appendectomy by proceeding with a right hemicolectomy and ileocolic anastomosis. The ileocecal segment was found to have no consequent findings of carcinoid in the ileocolonic segment; however, a colonic diverticulum was noted to be microscopically visible in right hemicolectomy specimen. The 11 lymph nodes resected showed sinus lymphocytosis. In postoperative surveillance, patient underwent nuclear medicine octreotide scan (also known as somatostatin receptor scintigraphy) a year later to evaluate for previous lymphovascular invasion and possible locoregional or distant metastasis. The scan revealed mild radiotracer uptake, likely in a loop of small bowel in left upper quadrant, indicating low suspicion for recurrent carcinoid. Patient was recommended to undergo further evaluation with contrast-enhanced CT of abdomen; however she was lost to follow-up.
Figure 1.
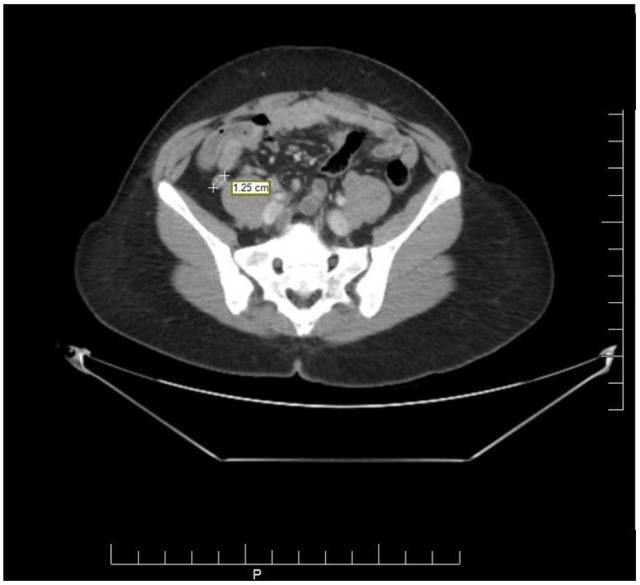
CT scan of the abdomen showing an inflamed appendix with no peri-appendiceal wall thickening or fat stranding. No appendiceal diverticulitis was visible.
Figure 2.
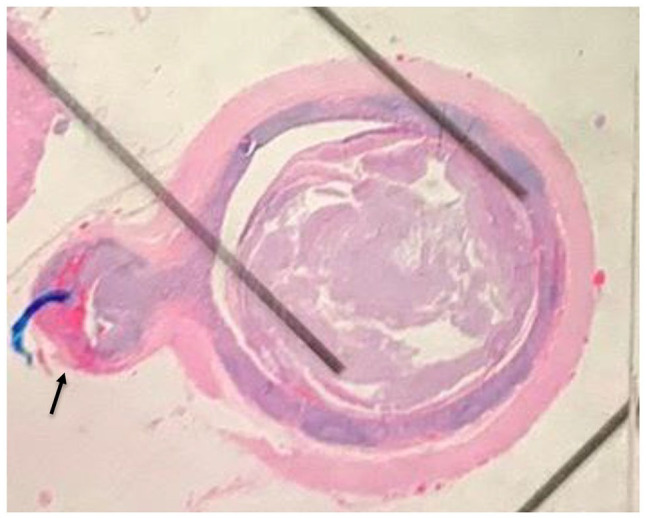
Appendiceal diverticulosis gross histopathology. Appendiceal diverticulosis (arrow). No appendicitis was seen.
Figure 3.
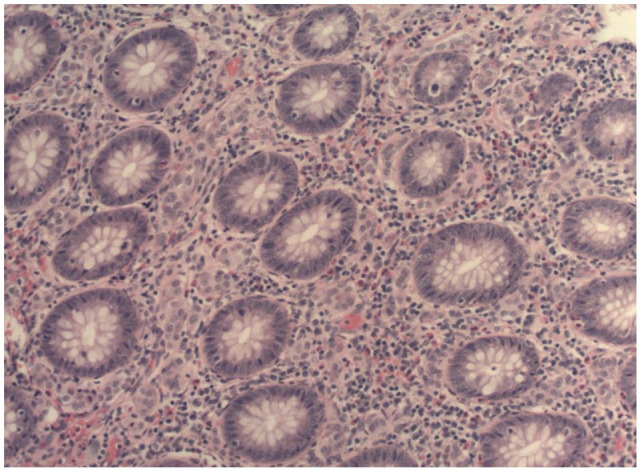
Neuroendocrine cells in mucosa of appendix (magnification: 100×).
Figure 4.
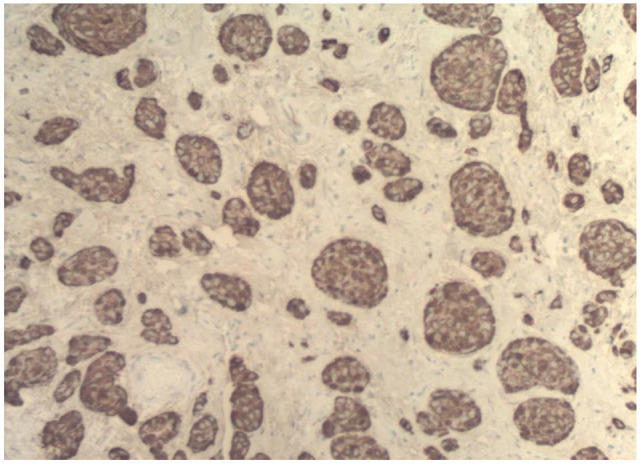
Chromogranin stain of appendiceal specimen (magnification: 100×).
Table 1.
Stages and grades of the carcinoid tumors of the appendix.
| AJCC stage | Stage grouping a |
|---|---|
| I | T1 N0 M0 |
| II | T2 N0 M0 |
| T3 N0 M0 | |
| III | T4 N0 M0 |
| Any T N1 M0 | |
| IV | Any T Any N M1 |
T1 (<2 cm); T2 (2–4 cm); T3 (>4 cm OR invades into the subserosa or the mesoappendix); T4 (invades into the peritoneum or into nearby organs); N0 (no spread to nodes), N1 (spread to nearby lymph nodes); M0 (no spread to distant parts of the body); M1 (spread to distant parts of the body).
Additional categories not listed above:
TX: Main tumor cannot be assessed due to lack of information.
T0: No evidence of a main tumor.
NX: Nearby lymph nodes cannot be assessed due to lack of information.
| Grade | Mitotic count per 10 hpf | Percent of cells Ki67+ |
|---|---|---|
| G1 | <2 | <2 |
| G2 | 2–20 | 3–20 |
| G3 | >20 | >20 |
Discussion
Although the coexistence of appendix diverticulum and appendiceal carcinoids has been published several times previously, a concurrent colonic diverticulum in the right hemicolectomy specimen during the oncologic resection of the appendiceal carcinoid has not been previously reported. While the causal relationship is unclear, the rarity of coexistence of the appendix diverticulum, appendiceal carcinoid, and colonic diverticula brings into question the behavior of carcinoid neoplasms as it relates to diverticular disease. Owing to the rarity of the appendiceal carcinoids (17%), and colonic carcinoids (11%),2,3 the relationship between carcinoid neoplasms and diverticular disease remains largely unknown. On the contrary, there have been several systematic reviews and meta-analyses that assessed the risk of colonic adenocarcinoma in patients with acute diverticulitis in the past. It is reported that carcinogenesis of adenocarcinoma is in part caused by the changes in the intestinal bacterial flora caused by repeated chronic inflammation, especially in the setting of complicated diverticulitis. 10 Whether appendiceal carcinoids or even adenocarcinoma of the colon if arising within the colonic diverticulum follows similar mechanism of carcinogenesis remains unknown. In fact, to date, there is only one report in English literature that describes a case of carcinoid tumor arising within a colonic (rectosigmoid) diverticulum. 11 While this is a single report that describes concurrent colonic (right-sided) diverticula with appendiceal carcinoid and appendiceal diverticula, it is a noteworthy finding that can play a significant role in understanding the pathogenesis of appendiceal diverticulum and possibly serve to redefine the current guidelines of NETs of the appendix, especially for carcinoid tumors sized 1–2 cm in diameter.
The treatment for appendiceal diverticula depends on the underlying pathology. In many cases, appendiceal carcinoid tumors present with appendicitis-like symptoms and undergo an appendectomy. After the appendectomy, specimens are sent to pathology in which carcinoid tumor can either be identified or confirmed. Subsequent steps are determined by the appendiceal carcinoid specimen. Current guidelines by the National Comprehensive Cancer Network (NCCN) suggest that if the tumor size is >2 cm, a right hemicolectomy is warranted. 12 For small carcinoid tumors (<1 cm), simple appendectomy is often adequate for cure. For moderate-sized carcinoid tumors (1–2 cm), however, the optimal management continues to be controversial. Both simple appendectomy and surveillance versus right hemicolectomy have been proposed in this circumstance. 13 In this case, given the lymphovascular invasion and the spread to the mesoappendix, our patient with carcinoid tumor size of 1.25 cm underwent elective right hemicolectomy. Other features to further characterize the tumor include tumor-positive resection margins, cellular pleomorphism with a high mitotic index, and presence of proliferation markers. 3 Given that approximately one-third of patients with large carcinoid tumors (>2 cm) either present with or develop nodal and distant metastases, careful attention should be given to assess the risk for carcinoid syndrome in patients presenting with symptoms of appendicitis through precise radiological and clinical correlation to differentiate appendiceal diverticulitis from acute appendicitis. In a retrospective study of 451 post-appendectomy patients in which 44 (9.7%) were diagnosed with appendiceal diverticulitis postoperatively, Ito et al. 14 demonstrate the common features of appendiceal diverticulitis including the age of patients, a longer duration of illness, and a higher rate of perforation, as well as the common CT findings including the absence of a fluid collection in the appendix, absence of an appendicolith, and abscess formation. Compared to the typical imaging findings of acute appendicitis such as enlarged appendices, appendiceal wall thickening, and peri-appendiceal fat stranding, those of appendiceal diverticulitis showed appendices that could not be easily visualized, however, often with a localizing abscess formation. This was consistent with the CT findings of our patient which revealed an inflamed appendix with no peri-appendiceal wall thickening or fat stranding (Figure 1). Given the similar presentation of the two diseases and the frequency of misdiagnosis, a surgeon must be vigilant to rule out the possibility of appendiceal diverticulitis and underlying appendiceal neoplasm in all cases of appendectomy. The appendectomy specimen should be carefully examined intraoperatively to evaluate for diverticula, especially in between the leaves of the mesoappendix and at the distal tip where the acquired diverticula are most frequently found. 15 Similarly, if a surgeon were to recognize appendiceal diverticula during intraabdominal operation for other reasons, prophylactic appendectomy should be considered to rule out appendiceal carcinoid.
It is interesting that our patient even had a consequent colonic diverticulum removed during the right hemicolectomy. This raises the question if prophylactic diagnostic colonic imaging or intraoperative attempt to rule out colonic diverticula via gross imaging should be conducted in addition to the established guidelines regarding NETs of the appendix. For carcinoid tumors that are 1–2 cm in diameter, there are currently no clear guidelines to indicate right hemicolectomies. Investigating the significance of this rare triad and understanding the role of appendiceal carcinoids in the formation of diverticulum extending beyond the walls of the appendix can shed new insights into the complex pathophysiology of diverticular disease as well as the characteristics of carcinoid neoplasms. Several factors are thought to contribute to the pathogenesis of colonic diverticula among which include age, diet, genetic predispositions, inflammatory factors, increased luminal pressure secondary to changes in gut motility, and microflora leading to fecal stasis and subsequent exposure to intraluminal toxins and antigens. 16 While the causal relationship between carcinoids and diverticula remains unclear, based on a review of literature (Table 2), we know that there is an association between the two disease processes at least in the appendix. The question is whether that association is the inflammatory effect of carcinoid tumor giving rise to the formation of appendix diverticulum or the mechanical effect of the mass causing increased luminal pressure of the appendix and the subsequent outpouching or a combination of both. The mechanical theory is consistent with the current theories of appendiceal diverticulum pathogenesis. It states that the mucosa and submucosa herniate near the weak point of penetrating vessels through the muscularis when exposed to prolonged pressure caused by fecalith, proximal tumors, excessive luminal mucus. 2 Nonetheless, it is also possible that the excess endogenous serotonin produced by the carcinoid tumor changes the appendiceal bacterial flora and gut motility leading to increased luminal pressures and eventual development of diverticulum. This chemical theory, however, is refuted by several previous studies. In 2008, in an experimental study that analyzed serotonin signaling including content, release, and serotonin transporter expression in relation to diverticulosis, Costedio et al. 17 proposed that alterations in the serotonin signaling do not appear to be responsible for the development of diverticula. Despite the less likely causal relationship of serotonin in the development of diverticular disease, serotonin has proven to play an integral role in the motility disorders of diverticular disease. In the same study, Costedio et al. 17 proposed that the decreased expression of serotonin transporter in patients with previous attacks of diverticulitis explained the persistent altered intestinal motility seen in the recovery phase of acute diverticulitis. In addition, in 2019, Puzikov et al. 18 described that exogenous serotonin-induced stimulation of diverticulosis enhanced the contractile activity in experimental colon diverticulosis and reduced the inflammatory factors, suggesting the protective role of exogenous serotonin in diverticulosis. Given that this proposed protective mechanism of exogenous serotonin was based on the principle of feedback mechanism that prevents the release of endogenous serotonin, it is plausible to think that the endogenous serotonin produced by carcinoids may have a role in the disease process of appendiceal diverticulitis and possibly the formation of appendiceal diverticulum.
Table 2.
Incidence of appendiceal carcinoid and diverticula reported in the literature.
| Author and title | Method and aim | Findings |
|---|---|---|
| Collins
15
Diverticula of the vermiform appendix: a study based on 30 cases |
A review of literature and deriving both surgical and postmortem data from multiple studies over 32 years (1902–1934) to determine the average incidence, most common location, and accompanying pathology of appendiceal diverticula |
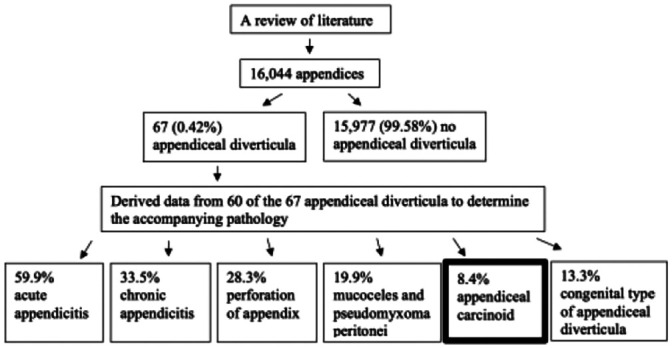
|
| Chong
20
Diverticula of the vermiform appendix: a report of nine cases |
Retrospective analysis of all appendectomy specimens over 8 years (1967–1975) to determine epidemiology and pathogenesis of appendiceal diverticula |
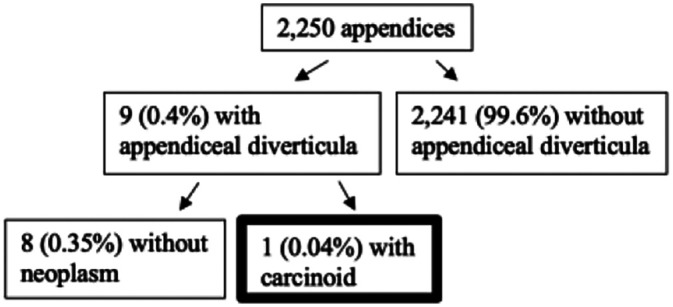
|
| Dupre
2
Diverticular disease of the vermiform appendix: a diagnostic clue to underlying appendiceal neoplasm |
Retrospective analysis of all appendectomy specimens over 4 years (2002–2006) to investigate the frequency of appendiceal neoplasms with acquired diverticulosis |
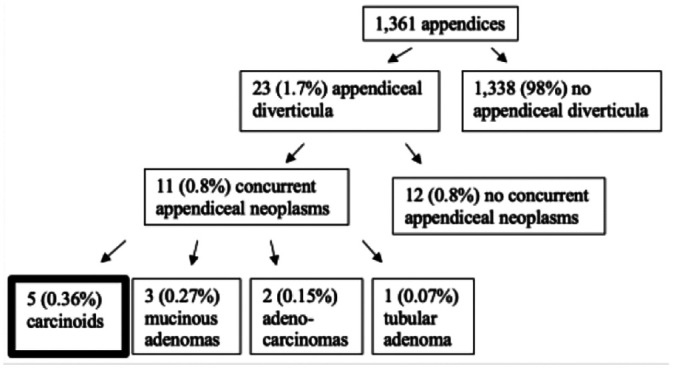
|
| Käser
21
Prevalence and clinical implications of diverticulosis of the vermiform appendix |
Retrospective analysis of all appendectomy specimens over 4 years (2003–2008) to determine epidemiology and etiology of inflammatory diseases of vermiform appendix |

|
| Al-Brahim
22
Clinicopathological study of 25 cases of diverticular disease of the appendix: experience from Farwaniya Hospital |
Case series of 25 appendices with diverticula over 8 years (2003–2011) to characterize clinicopathological features of appendiceal diverticula and its association with appendiceal neoplasms |
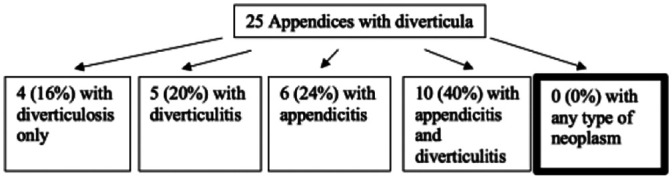
|
Appendiceal diverticula was first described in 1819 when Villar et al. 19 reported an instance of perforation of an appendiceal diverticulum with the subsequent formation of a generalized pseudomyxoma peritonei. It is interesting that 200 years later, so little is known about this disease. In the United States, nearly 300,000 appendectomies are performed each year, and based on a review of literature,2,15,20- 22 there may be over 1200–5100 appendiceal diverticula in any given year that we fail to thoroughly investigate without proper pathological examination. Moreover, carcinoid neoplasm which comprises 0.49%–1% of all malignancies appears to have increased in incidence in the past 30 years; 2 however, its etiology also remains largely unknown. Diverticulosis, on the other hand, has been extensively studied in the past and is very common with the left-sided diverticulosis affecting nearly 50% of adults above 60 years of age in the United States. 10 While diverticulosis of the cecum and the ascending colon are much less common in Western countries (1%–2%), it continues to be a huge health burden in Asian countries (43%–50%). 23 To our knowledge, there has been no report of this rare triad of appendix diverticulum, appendiceal carcinoid, and colonic diverticula. Increased awareness of this rare triad and investigation of the pathological relevance between carcinoid neoplasms and appendiceal and colonic diverticula may play a significant role in the unfolding of mysteries of diverticular disease.
Conclusion
The high frequency of association between appendiceal diverticula and appendiceal neoplasms is of pathological relevance. Carefully examining the gross and histological appendectomy specimen for appendiceal diverticula can guide the risk assessment of appendiceal carcinoid tumors and the optimal treatment before cancer progression. We propose colonic diverticula as another possible feature that may be associated with appendiceal diverticula especially with an underlying appendiceal neoplasm begging the question if prophylactic diagnostic colonic imaging or intraoperative attempt to rule out colonic diverticula via gross imaging should be conducted in addition to the established guidelines regarding NETs of the appendix, especially those not managed with right hemicolectomies.
Acknowledgments
I wish to extend my special thanks to the patient of this case report.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: Our institution does not require ethical approval for reporting individual cases or case series.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed consent: Written informed consent was obtained from the patient(s) for their anonymized information to be published in this article.
ORCID iD: Sunyoung Kim  https://orcid.org/0000-0001-7974-2418
https://orcid.org/0000-0001-7974-2418
References
- 1. Deans GT, Spence RA. Neoplastic lesions of the appendix. Br J Surg 1995; 82(3): 299–306. [DOI] [PubMed] [Google Scholar]
- 2. Dupre MP, Jadavji I, Matshes E, et al. Diverticular disease of the vermiform appendix: a diagnostic clue to underlying appendiceal neoplasm. Hum Pathol 2008; 39(12): 1823–1826. [DOI] [PubMed] [Google Scholar]
- 3. Pinchot SN, Holen K, Sippel RS, et al. Carcinoid tumors. Oncologist 2008; 13(12): 1255–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yamana I, Kawamoto S, Inada K, et al. Clinical characteristics of 12 cases of appendiceal diverticulitis: a comparison with 378 cases of acute appendicitis. Surg Today 2012; 42(4): 363–367. [DOI] [PubMed] [Google Scholar]
- 5. Chai QD, Pillai S, Mcclure R, et al. Carcinoid tumours of the appendix: an analysis of emergency appendicectomies over a 24-year period and outcomes of laparoscopic versus open resection. ANZ J Surg 2020; 90(10): 1975–1978. [DOI] [PubMed] [Google Scholar]
- 6. Kelly K. Management of appendix cancer. Clin Colon Rectal Surg 2015; 28(4): 247–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer 2003; 97(4): 934–959. [DOI] [PubMed] [Google Scholar]
- 8. Que FG, Sarmiento JM, Nagorney DM, et al. Hepatic surgery for metastatic gastrointestinal carcinoid tumors. Cancer Control 2002; 9: 67–79. [DOI] [PubMed] [Google Scholar]
- 9. American Joint Committee on Cancer. Neuroendocrine tumors of the appendix. In: Amin MB, Edge SB, Greene FL, et al. AJCC cancer staging manual. 8th ed. New York: Springer, 2017, pp. 389–394. [Google Scholar]
- 10. Strate LL, Modi R, Cohen E, et al. Diverticular disease as a chronic illness: evolving epidemiologic and clinical insights. Am J Gastroenterol 2012; 107(10): 1486–1493. [DOI] [PubMed] [Google Scholar]
- 11. Hernandez FJ, Fernandez BB. Mucus-secreting carcinoid tumor in a colonic diverticulum: report of a case. Dis Colon Rectum 1976; 19: 63–67. [DOI] [PubMed] [Google Scholar]
- 12. NCCN clinical practice guidelines in oncology: neuroendocrine tumors. Ver. 1. Fort Washington, PA: NCCN, 2019. [DOI] [PubMed] [Google Scholar]
- 13. Heller DR, Jean RA, Khan SA. Appendiceal carcinoid tumors: in reply to Sugarbaker. J Am Coll Surg 2019; 229(5): 519. [DOI] [PubMed] [Google Scholar]
- 14. Ito D, Miki K, Seiichiro S, et al. Clinical and computed tomography findings of appendiceal diverticulitis vs acute appendicitis. World J Gastroenterol 2015; 21(13): 3921–3927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Collins DC. Diverticula of the vermiform appendix: a study based on thirty cases. Ann Surg 1936; 104(6): 1001–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bugiantella W, Rondelli F, Longaroni M, et al. Left colon acute diverticulitis: an update on diagnosis, treatment and prevention. Int J Surg 2015; 13: 157–164. [DOI] [PubMed] [Google Scholar]
- 17. Costedio M, Coates M, Buttolph T, et al. Serotonin signaling in diverticular disease. J Am Coll Surg 2007; 205(3): S19–S20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Puzikov AM, Mikhailyants GS, Lychkova AE. The role of serotonin in the development of experimental diverticulosis. Biomed J Sci Tech Res 2019; 19(3): 14367–14371. [Google Scholar]
- 19. Villar Sur un cas d’appendicite chronique avec pseudo-myxoma diverticulaire. Bull. de l’Acad. de med., 79, 175, 181. [Google Scholar]
- 20. Chong KC. Diverticula of the vermiform appendix: a report of nine cases. Postgrad Med J 1976; 52(610): 504–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Käser SA, Willi N, Maurer CA. Prevalence and clinical implications of diverticulosis of the vermiform appendix. J Int Med Res 2013; 41: 1350–1356. [DOI] [PubMed] [Google Scholar]
- 22. Al-Brahim N, Al-Kandari I, Munahai M, et al. Clinicopathological study of 25 cases of diverticular disease of the appendix: experience from Farwaniya Hospital. Patholog Res Int 2013; 2013: 404308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Radhi JM, Ramsay JA, Boutross-Tadross O, et al. Diverticular disease of the right colon. BMC Res Notes 2011; 4: 383. [DOI] [PMC free article] [PubMed] [Google Scholar]


