Abstract
Prostate cancer (PCa) is the most common noncutaneous malignancy in men and is the second leading cause of cancer mortality in men in the United States. Current practice requires histopathological confirmation of cancer achieved through biopsy for diagnosis. The transrectal approach for prostate biopsy has been the standard for several decades. However, the risks and limitations of transrectal biopsies have led to a recent resurgence of transperineal prostatic biopsies. Recent studies have demonstrated the transperineal approach for prostate biopsies to be effective, associated with minimal complications and superior in several aspects to traditional transrectal biopsies. While sextant and extended sextant templates are widely accepted templates for transrectal biopsy, there are a diverse set of transperineal biopsy templates available for use, without consensus on the optimal sampling strategy. We aim to critically appraise the salient features of established transperineal biopsy templates.
Keywords: 10-sector template, 12-core, Barzell technique, Ginsburg protocol, prostate cancer, prostate mapping, transperineal template biopsies
Introduction
Prostate cancer (PCa) is the most common noncutaneous malignancy in men and is the second leading cause of cancer mortality in men in the United States.1,2 Approximately, 1 million prostate biopsies are performed in the United States on an annual basis and have continued to increase over recent years. 3 Prostate biopsy techniques have varied over the past century, undergoing many refinements in technique with the goal of improving cancer detection and risk stratification. The earliest efforts to sample prostate tissue involved biopsy of areas with palpable abnormalities. Systematic sampling of prostate became popular in the 1980s, as it would identify tumors previously missed by targeted sampling. Notably, transperineal biopsy (TP-Bx) was the prevailing method of choice for prostate gland access, until the establishment and popularity of the systematic sextant 12-core whole gland sampling via the transrectal route by Hodge et al. in 1989.4,5 This proposed technique, coupled with the introduction of the transrectal ultrasound (TRUS), displayed superior diagnostic accuracy and remains the standard of care today. 6 However, subsequent studies assessing transrectal biopsy (TR-Bx) in recent years have drawn attention to the higher risks of infectious complications, including hospital admissions due to sepsis.7,8 In addition, a significant number of cancers can be missed by a transrectal approach, particularly in the antero-apical portions of the gland.9,10 With improved access to hard-to-reach portions of the prostate gland and minimal infection rates, TP-Bx has re-emerged positioning itself as the solution to these problems.11–14
While TP-Bx is increasingly being used, there is a considerable variation in transperineal template-guided biopsy schemes used among clinicians. The absence of consensus highlights the need for evidence-based recommendations to optimize cancer detection. Several schemes have been described with varying combinations of regions sampled and cores obtained (Table 1). Both the mapping approach (Barzell) and the sector approach (Ginsburg) are examples of techniques that have been utilized with excellent results. Other schemes, such as the 10-sector, 12-core, and MUSIC (Michigan Urological Surgery Improvement Collaborative) approaches have also been routinely used. To our knowledge, while numerous approaches exist, there is a scarcity in literature regarding a review of these commonly used TP-Bx template types. Thus, our goal is to provide a brief overview of target zones and the effectiveness of the five commonly used TP templates, namely, (1) Barzell, (2) Ginsburg, (3) 12-core, (4) 10-sector, and (5) MUSIC.
Table 1.
Summary table of templates.
| Transperineal biopsy template type | Brief description | Median # cores | Cancer detection rates | Complication rates | Article |
|---|---|---|---|---|---|
| Barzell technique | Eight-sector template divided by three planes. —Four to eight
cores are taken from each region for a total of 32–64
cores Eight-sector template, cores taken at every 5 mm; volume-dependent Twenty-sector template, modified Barzell created from two lateral sagittal sections per side (Modified Barzell) |
32–64 46 (SD ±19) 40 (IQR = 22–53) N/A |
Overall CDR: N/A, CsCDR: 40%
(n = 65) Overall CDR: 78%, CsCDR: N/A (n = 110) Overall CDR: N/A, CsCDR: 62% (n = 182) Overall CDR: 71%, CsCDR: 40% (n = 576) |
No major complications 8% catheter drainage, 2% had hematuria. 1% hospitalization No major complications |
Barzell and Whitmore
15
Onik and Barzell 16 Kasivisvanathan et al. 17 Ahmed et al. 18 |
| Ginsburg protocol | Twelve-sector template, two cores/sector with two to four cores from targeted lesions for a total of 26–28 cores. More number of cores for larger prostates | 28 28 |
Overall CDR: 96%, CsCDR: 97%
(n = 120) Overall CDR: 75%, CsCDR: 45% (n = 487) |
No major complications reported No serious complications or hospital readmissions |
Radtke et al.
19
Hansen et al. 20 |
| 10-sector template | Ten-sector template, targeting peripheral zones, one to two cores/sector | 16 (IQR = 14–20) 20 for large prostate |
Overall CDR: 70.9%, CsCDR: 51.3% (n = 117) | No major complications | Ristau et al. 21 |
| 12-core template | Twelve-core peripheral template obtained in a fan-like pattern. Two cores from transition zone | 12 (+2 if patient on surveillance) | Overall CDR: 49%, CsCDR: 16% (n = 43) | 7% had CGII complications. 5% had AUR, 2% had hematuria | Meyer et al. 22 |
| MUSIC | Twelve core, omits midline cores of MB template, targets peripheral zone. Two of 12 sectors found in anterior zone | 12 | Overall CDR: 53%, CsCDR: 33.5%
(n = 215) Overall CDR: 47.2%, CsCDR: 25% (n = 144) |
0.17% had rates of infectious hospitalization No major complications were reported |
Maruf et al.
23
Dupati et al. 24 |
AUR, Acute urinary retention; CDR, cancer detection rate; CGII, Clavien–Dindo classification grade II; CsCDR, clinically significant cancer detection rate; IQR, interquartile range; MB, modified Barzell; MpMRI, multiparametric magnetic resonance imaging; MRI, magnetic resonance imaging; SD, standard deviation; TRUS, transrectal ultrasound.
Zonal anatomy of prostate
Transperineal templates are designed to systematically sample the prostate gland to maximize cancer detection rates (CDRs) and are based largely on the zonal anatomy of prostate. The prostate is divided into four zones: the central zone (CZ), transition zone (TZ), peripheral zone (PZ), and anterior fibromuscular stroma (Figure 1). 25 The CZ is derived from Wolffian duct, whereas the rest of the prostate is derived from urogenital sinus. In adult men, PZ comprises 70% of glandular tissue, whereas the CZ and the TZ comprise 25% and 5% of glandular tissues, respectively.26,27
Figure 1.

Prostate anatomy zones with cancer frequency.
As men age by the age of 60 years, the TZ and the PZ mainly contribute to prostate enlargement except that once the total volume of prostate exceeds 50 g, the growth is mainly accounted by TZ and may eventually lead to benign prostatic hyperplasia (BPH). 28 The PZ accounts for 75% of cancers; the TZ although enlarged in older males accounts for 20% of the cancer, while the CZ accounts for 5–8% of cancer. There is considerable evidence that cancers arising in the TZ are clinically and biologically different from PZ cancers. 29
Barzell technique and its modifications
Sextant template biopsies using transrectal approach have been shown to undergrade and understage PCa. 30 In an effort to overcome the random and uneven sampling of all areas of the prostate, a systematic transperineal prostate biopsy using brachytherapy grid was proposed that would later become known as the Barzell technique. Established in 2003 by Barzell and Whitmore, 15 the use of a grid in combination with TRUS was proposed to ensure reproducible and accurate systematic sampling of the prostate that would help minimize human error and provide precise localization of cancer foci. With this approach, a fixed set of reproducible coordinates would allow for accurate mapping of the lesions allowing for targeted therapy as a feasible option. Access to the antero-apical regions of the prostate was also a benefit of this approach given, it was to be performed through a transperineal approach.
In the Barzell template, the prostate is divided into eight sectors. A transverse plane separates prostate into proximal (base) and distal (apex) halves, a sagittal plane divides each half further into right and left lobes, and finally, a coronal plane divides into anterior and posterior regions (Figure 2). The resulting regions formed are as follows: left anterior apex (LAA), left anterior base (LAB), left posterior apex (LPA), left posterior base (LPB), right anterior apex (RAA), right anterior base (RAB), right posterior apex (RPA), and right posterior base (RPB). 31 The procedure is performed under general anesthesia or intravenous (IV) sedation anesthesia as it requires a significant number of cores. Local anesthesia has recently been utilized with success, allowing room for a speedier process and lower risk of complications that come with general anesthesia. 6 Using a bi-plane TRUS for guidance, four to eight cores are taken from each of the eight sectors in a craniocaudal fashion from apex to base, for a total of 32–64 biopsies depending on volume of prostate. Special care is taken in the anterior zone where biopsy gun can transverse through urethra and increase the risk of complications. In Barzell’s description, oral antibiotics for 3 days were prescribed to all to prevent infection and sepsis, while most patients were given alpha-blockers to aid with voiding post catheter removal. In 65 patients, Barzell and Whitmore 15 demonstrated a 40% clinically significant cancer detection without major complications.
Figure 2.
Eight-sector Barzell template.
AS, anterior fibromuscular stroma; LAA, left anterior apex; LAB, left anterior base; LPA, left posterior apex; LPB, left posterior base; PZ, peripheral zone; RAA, right anterior apex; RAB, right anterior base; RPA, right posterior apex; RPB, right posterior base; TZ, transition zone.
In 2008, Onik and Barzell 16 demonstrated increased reliability using the Barzell technique in the detection of clinically significant cancer if the prostatic tissue was sampled at intervals of every 5 mm as compared with an arbitrary 32/64 cores. The theory behind the proposed utilization of the 5 mm technique comes from the fact that fewer clinically significant cancers would be missed while allowing adequate sampling of larger prostates. 32 This theory was first reported using a computer simulation carrying out TP-Bx on 86 autopsy and 20 radical prostatectomy specimens. It demonstrated that the 5 mm protocol detected 95% (38/40) of clinically significant cancers. 33 This approach, aptly named as template prostate mapping (TPM) biopsy, achieved the maximal cancer detection for clinically significant cancer as lesions smaller than 5 mm would likely be clinically insignificant cancers. Also, 5 mm grid proved to be better for cancer detection over 10 mm grid, detecting cancer in 75%, an increase of 25% over those with the 10 mm grid. Barzell and Onik carried out a study with similar parameters in 110 patients achieving a remarkably high positive biopsy rate of 78%. 16 The median number of cores taken in their study was 46 (SD ±19). Nine (8%) patients had urinary retention requiring short-term indwelling catheter drainage, two (1.8%) had hematuria, and one (0.9%) required hospital readmission for bladder irrigation. While a core-by-core coordinate yields most accurate information, there is a considerable excess cost and time associated with this approach. Some groups have proposed maintaining 5 mm sampling while grouping the cores geographically based on the Barzell zones. Such an approach would still facilitate a targeted prostate treatment while reducing the need for logistic and histopathological support. Researchers at the University College of London adopted the above approach by modifying the Barzell template into 20 separate sectors and renamed it as modified Barzell (MB) template (Figure 3). 31 In this modification, similar to original Barzell template, the prostate is divided into left and right halves, anterior and posterior prostate, and apex and base. The cores are obtained from medial to lateral sectors. On each side, the sectors are labeled as parasagittal anterior apex and base, parasagittal posterior apex and base, medial anterior apex and base, medial posterior apex and base, and lateral sectors. This modification also allowed for sampling of midline prostate using midline apex and midline base sectors. Kasivisvanathan et al., 17 using the MB template, revealed a clinically significant CDR of 62% in 182 men with a median number of 40 cores. No major complications were reported. In the PROstate MR Imaging Study (PROMIS) trial, utilization of similar approach led to an overall CDR of 71% in 576 men, with 40% having cancer that was clinically significant. 18 The median number of cores were estimated to be in the 40–60 range as cores were taken at every 5 mm. Eight (1%) patients were reported to have sepsis secondary to urinary tract infection and 58 (10%) had urinary retention. These infections were likely confounded and not likely due to TP-Bx as this study had TR-Bx performed right after TP-Bx.
Figure 3.
Twenty-sector modified Barzell.
AS, anterior fibromuscular stroma; LL, left lateral; LMAP, left medial anterior apex; LMAB, left medial anterior base; LMPA, left medial posterior apex; LMPB, left medial posterior base; LPAP, left parasagittal anterior apex; LPAB, left parasagittal anterior base; LPPA, left parasagittal posterior apex; LPPB, left parasagittal posterior base; MA, midline apex; MB, midline base; PZ, peripheral zone; RL, right lateral; RMAP, right medial anterior apex; RMAB, right medial anterior base; RMPA, right medial posterior apex; RMPB, right medial posterior base; RPAP, right parasagittal anterior apex; RPAB, right parasagittal anterior base; RPPA, right parasagittal posterior apex; RPPB, right parasagittal posterior base; TZ, transition zone.
Excellent spatial localization is a major benefit in the use of this approach, appropriate for those patients who met the criteria for focal therapy intervention. However, given the high number of samples obtained, the technique is not without its drawbacks of increased costs and time required for pathology processing, increased risks of procedure-related morbidity, and possible findings of clinically insignificant cancers leading to overdiagnosis and potential overtreatment.34–36 Thus, this template has mainly been utilized in those with prior-negative prostate biopsies and with persistent clinical suspicion. Complications associated with this technique have been higher rates of acute urinary retention and prostatic bleeding. While the exact mechanism behind the higher rates of urinary retention is not well known, it is thought that prostatic edema could be the cause. In attempt to find a solution to this problem, Bozlu et al. 37 demonstrated decreased incidence of acute urinary retention with the use of tamsulosin in the perioperative period. However, most of the associated complications of acute urinary retention have been transient, not requiring intervention as they often resolve on their own.
Ginsburg protocol
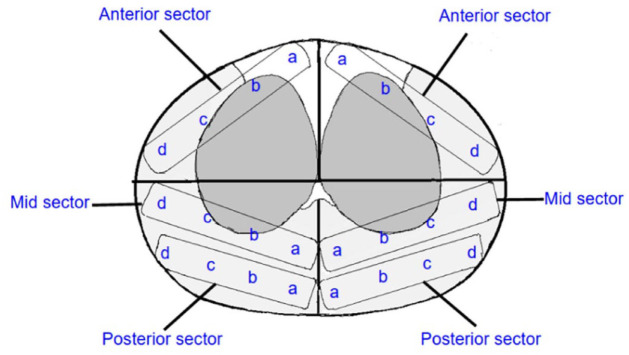
While the Barzell technique has been well received for its reliability and accuracy, the high tissue sampling is thought to limit its utility and wider adoption. To standardize TP systematic biopsies and to encourage prospective studies and multicenter collaborative data analysis, the Ginsburg consensus discussed the definitions to be incorporated and minimal optimal requirements regarding data points to be included in a prospective TP-Bx database (Figure 4). 38 The panelists had a concern that while the Barzell technique showed significant diagnostic quality, it had substantial limitations. A consensus was reached that the increased side effects and the added burden of pathology having to process increased sample numbers was not justified leading to the proposed technique that would go on to become the Ginsburg Biopsy Scheme (GBS). It was recommended that this template should ‘become the state of practice to be used by clinicians moving forward’.
Figure 4.
Ginsburg protocol template.
The Ginsburg protocol is usually used in combination with magnetic resonance imaging/ultrasound (MRI/US) fusion to obtain tissue samples. This technique divides the prostate into 12 anatomical sectors, with two biopsies obtained from each sector in a craniocaudal fashion leading to a 24-core systematic sampling with two additional MRI-targeted biopsies (TBs) (lesions found on imaging) thought to deliver maximal PCa detection rates while minimizing post-procedural complications. The consensus of the group was that the peripheral and anterior zone should be preferentially targeted, as these are the areas most likely to harbor disease, thus, the prostate was divided into the anterior zone, apical (mid sector) PZ, and posterior PZ. A total of four to six cores are to be obtained from four equally spaced areas from medial to lateral in these zones for each side of the gland. For those with longer prostates >4 cm or volumes >50 ml, an additional basal PZ and posterior TZs are added. Overall, 24 total cores should be obtained in smaller prostates up to 30 ml, whereas the bigger prostates as previously mentioned would require up to 38 ml (Table 2). The TB is to be completed prior to systematic sampling to prevent movement of lesions localized on imaging and was added to the protocol. In a study by Radtke et al., 19 the use of this template resulted in successfully detecting 96% of overall cancer with 97% of clinically significant PCa lesions. A median of 28 biopsies was taken per patient that was prostate volume adjusted and no major complications were reported. In a different study, Hansen et al. 20 showed an overall detection rate of 75%, with 45% being clinically significant cancer in 487 men. No significant complications were reported.
Table 2.
Ginsburg protocol cores obtained according to prostate volume and length.
| Prostate volume (ml) | Number of cores taken per sector (right + left) | Total number of cores | |||
|---|---|---|---|---|---|
| Anterior | Mid | Posterior | Basal | ||
| <30 ml | 4 + 4 | 4 + 4 | 4 + 4 | 0 | 24 |
| 30–50 ml and >4 cm length | 4 + 4 | 4 + 4 | 4 + 4 | 4 + 4 | 32 |
| >50 ml and >4 cm length | 5 + 5 | 5 + 5 | 5 + 5 | 4 + 4 | 38 |
While displaying satisfactory CDRs in the setting of reduced number of cores sampled, this protocol still has its shortcomings. GBS preferentially targets the PZ and systematically omits most of the transition and periurethral zones and thus risks missing cancers in these areas. 38 While the idea behind this approach is that most PCas occur around the PZ with minimal rates occurring elsewhere and the high risk of complications with sampling the omitted regions, a recent study published by Sigle et al., 9 found significant lesions were missed in 3.6% of 1084 patients. However, there have been no increased risks of urinary retention and hematuria across most studies using these templates. 7
12-core transperineal biopsy template
Another template, which has been popular in the outpatient office-based free-hand (FH) TP-Bx studies, is a 12-core biopsy template similar to the 12-core extended sextant template used in transrectal biopsies. Meyer et al.22,39 performed an in-office 12-core US-guided prostate biopsy under local anesthesia using transperineal access system (such as PrecisionPoint). In this template, the PZ of prostate is divided into two anterior zones, namely, right and left PZ anterior (PZa), and four posterior zones, namely, right and left PZ posterior medial (PZpm), right and left PZ posterior lateral (PZpl) on either side (Figure 5). Using a fan-like pattern, 12 cores are sampled (two cores from each PZ of the prostate) (Figure 6). Patients who are under active surveillance underwent two additional cores from TZ either transitional zone anterior (TZa) or transitional zone posterior (TZp). The procedure can be completed in one to two access punctures on either side. The authors reported no periprocedural antibiotic use for this approach.
Figure 5.
Transperineal 12-core biopsy template.
AS, anterior fibromuscular stroma; PZa, peripheral zone anterior; PZpl, peripheral zone posterior lateral; PZpm, peripheral zone posterior medial; TZa, transition zone anterior; TZp, transition zone posterior.
Figure 6.
Fan technique method as described by Emiliozzi et al. 40
In their experience on 43 patients, 49% men were found to have PCa of which 16% were found to have clinically significant cancer with only using 12 cores. Seven percent of patients developed complication after biopsy, of which 4.7% required urethral catheterization for urinary retention and 2.3% patients developed gross hematuria that also required catheterization. None of the patients developed post biopsy infection.
Ten-sector template
In a retrospective single-institution study by Ristau et al., 21 the authors suggested an alternative approach, proposing an outpatient FH transperineal prostate biopsy (fTP-Bx) using a 10-sector template. The 10-sector template involves dividing prostate into anterior and posterior halves (Figure 7). The anterior half is further divided into the right anterior lateral (RAL), right anterior medial (RAM), left anterior lateral (LAL), left anterior medial (LAM). The posterior half was divided into the right posterior lateral (RPL), right posterior medial (RPM), left posterior lateral (LPL) and left posterior medial (LPM). If prostate is large, then two additional sectors, namely, the right lateral base (RLB) and left lateral base (LLB), are biopsied at the base of the gland.
Figure 7.
Ten-sector template.
LAL, left anterior lateral; LAM, left anterior medial; LLB, left lateral base; LPL, left posterior lateral; LPM, left posterior medial; RAL, right anterior lateral; RAM, right anterior medial; RLB, right lateral base; RPL, right posterior lateral; RPM, right posterior medial.
An advantage to utilizing this technique is that it can easily be used with MRI prostate sector maps utilized in the PRECISE recommendation. 41 Biopsy is performed in an FH manner using a transperineal access system (such as PrecisionPoint) which allows the biopsy needle to always be aligned to the sagittal plane of the probe. This obviates the need for brachytherapy steppers and grid as well as general anesthesia. Of the 1000 fTP-Bx, 883 were performed using a 14-gauge hypodermic needle access system in a ‘fan-like’ method as described by Emiliozzi et al. 40 (Figure 6). In the other cohorts of 117 men, transperineal access system is used to take biopsies using the 10-sector template. The median core per prostate biopsy was 16 (IQR = 14–20) in the study. Total CDR with 10-sector template transperineal access system was more than ‘fan like’ pattern cohort (70.9% versus 59.3%) with similar findings for CDR for clinically significant GG ⩾ 2 (51.3% versus 38.8%). No major complications associated with this type of template biopsy. The potential shortcomings of both the 10- and 12-sector templates are lack of sampling of TZ and periurethral areas of prostate. However, by limiting the total number of cores to 12–16, these templates facilitate adoption of TP-Bx in the office setting and shorten the procedure times associated with other TP templates that plan for more extensive sampling.
Michigan Urological Surgery Improvement Collaborative
The MUSIC TP template was developed using core location-specific CDR data based on initial results from the adoption of MB template. It was developed to limit the overall number of cores obtained during TP sampling, while maintaining PZ sampling, where most of the cancers are commonly found (Figure 8). The template consists of obtaining biopsy from the six sectors of each prostate lobe, paramedian apex, paramedian base, posterior apex, posterior base, lateral, and anterior prostates. Each sector is biopsied once within each lobe, making it a 12-core biopsy and allowing a valid comparison with the 12-core transrectal biopsies.
Figure 8.
MUSIC template.
LA, left anterior; LL, left lateral; LPA, left posterior apex; LPB, left posterior base; LPMA, left paramedian apex; LPMB, left paramedian base; RA, right anterior; RL, right lateral; RPA, right posterior apex; RPB, right posterior base; RPMA, right paramedian apex; RPMB, right paramedian base.
A study done by Maruf et al. 23 comparing MUSIC with traditional TR-Bx demonstrated that the overall CDR is 53.0% for MUSIC template versus 55.3% for TR-Bx and the rate of ⩾ GG2 cancer was 33.5% for MUSIC template versus 38% for TR-Bx. Multivariate analysis showed no significant difference in the odds of detecting any cancer ⩾ GG2 cancer for MUSIC compared with TR-Bx. The rates of hospitalization due to infection were lower in MUSIC template than in TR-Bx, though it was not statistically significant, likely due to state-wide quality improvement efforts achieving rates approaching 0.6%. Another study by Dupati et al., 24 found overall CDR of 47.2% with MUSIC template. CDR for clinically significant cancer was 25%. The study did not find any difference in CDR between MB and MUSIC templates, and noted that additional one to two midline core biopsies that were performed at apex and base did not improve in cancer diagnosis. Midline cores can potentially result in urethral injury and increased risk of hematuria, thus omitting those cores can potentially further reduce the risks associated with TP-Bxs.
Discussion
In this article, we describe some of the common TP-Bx templates. The clinical studies on these templates are heterogeneous and variable in the quality making direct comparison among these templates difficult. There is ample evidence that systematic saturation sampling of prostate, such as with Barzell or MB template, is reliable and accurate in detecting clinically significant PCa, however, a higher sampling leads to greater risk of complications as well as detection of clinically insignificant PCa due to higher biopsy density. Authors have significant experience with Ginsburg protocol that allows sampling from 12 sectors and with fixed number of cores range depending on prostate size. This template satisfactorily detects clinically significant cancer with a reduced number of cores by preferentially targeting peripheral and anterior zone where majority of cancer are present while misses cancer by omitting most of the transitional and periurethral zones. Currently, the authors utilize MUSIC template, most often as it has several advantages. First, it is a 12-core biopsy template, thus allowing valid comparison with 12-core transrectal sextant biopsies and easier adoption in office settings. It preferentially collects samples from PZ where majority of cancers are located as well as from anterior zone missed by traditional TRUS biopsy with a less thorough sampling of transitional zone. By avoiding sampling of the midline PZ like those done by MB, it can reduce the risk of hematuria and urinary retention. In the past, increasing the number of prostate cores with larger prostate size made sense as seen with Barzell templates; however, the benefit of such an approach is limited in the age of prostate MRI, which can identify clinically significant PCa outside the traditional biopsy templates. Therefore, MUSIC template might serve as a good compromise between CDRs and burden on patients.
Conclusion
The mainstay of PCa diagnosis has been systematic sampling of the prostate gland via a transrectal approach. The transperineal approach for systematic sampling has demonstrated similar if not superior CDRs and infectious complications approaching zero. With innovations in imaging, such as multiparametric MRI showing promising results, the landscape of PCa diagnosis is rapidly changing; however, systematic sampling remains an accepted and essential component of biopsy. The Barzell technique, the Ginsburg protocol, 12-core, the 10-sector, and MUSIC templates all represent excellent templates with data supporting their continued application. Studies done using these templates were conducted in different settings with nonhomogeneous patient populations, which make comparison between these templates very difficult. However, each template seems to have their relative advantages and shortcomings. Larger multi-institutional comparative studies will be needed to determine the relative efficacy, cancer detection, and complication rates of these templates. Until those data are available, physician discretion will likely dictate template selection during TP-Bx.
Footnotes
Ethics approval and consent to participate: Not applicable
Consent for publication: Not applicable
Author contributions: Abhinav Sidana: Conceptualization; Supervision; Writing – review & editing.
Fernando Blank: Resources; Writing – original draft; Writing – review & editing.
Hannah Wang: Writing – review & editing.
Nilesh Patil: Visualization; Writing – review & editing.
Arvin K George: Resources; Writing – review & editing.
Hasan Abbas: Resources; Supervision; Writing – original draft; Writing – review & editing.
ORCID iD: Abhinav Sidana  https://orcid.org/0000-0002-8290-936X
https://orcid.org/0000-0002-8290-936X
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Availability of data and materials: Not applicable
Contributor Information
Abhinav Sidana, Associate Professor of Surgery, Director of Urologic Oncology, Division of Urology, Department of Surgery, University of Cincinnati College of Medicine, 231 Albert Sabin Way, Cincinnati, OH 45267, USA.
Fernando Blank, Division of Urology, Department of Surgery, University of Cincinnati College of Medicine, Cincinnati, OH, USA.
Hannah Wang, Division of Urology, Department of Surgery, University of Cincinnati College of Medicine, Cincinnati, OH, USA.
Nilesh Patil, Division of Urology, Department of Surgery, University of Cincinnati College of Medicine, Cincinnati, OH, USA.
Arvin K. George, Department of Urology, University of Michigan, Ann Arbor, MI, USA
Hasan Abbas, Division of Urology, Department of Surgery, University of Cincinnati College of Medicine, Cincinnati, OH, USA.
References
- 1. American Cancer Society. Cancer facts and figures 2021, https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2021.html
- 2. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021; 71: 209–249. [DOI] [PubMed] [Google Scholar]
- 3. Bhanji Y, Allaway MJ, Gorin MA. Recent advances and current role of transperineal prostate biopsy. Urol Clin North Am 2021; 48: 25–33. [DOI] [PubMed] [Google Scholar]
- 4. Chang DT, Challacombe B, Lawrentschuk N. Transperineal biopsy of the prostate – is this the future? Nat Rev Urol 2013; 10: 690–702. [DOI] [PubMed] [Google Scholar]
- 5. Hodge KK, McNeal JE, Terris MK, et al. Random systematic versus directed ultrasound guided transrectal core biopsies of the prostate. J Urol 1989; 142: 71–4; discussion 74–5. [DOI] [PubMed] [Google Scholar]
- 6. Ortner G, Tzanaki E, Rai BP, et al. Transperineal prostate biopsy: the modern gold standard to prostate cancer diagnosis. Turk J Urol 2021; 47(Supp. 1): S19–S26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Walker JT, Singla N, Roehrborn CG. Reducing infectious complications following transrectal ultrasound-guided prostate biopsy: a systematic review. Rev Urol 2016; 18: 73–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Forsvall A, Jönsson H, Wagenius M, et al. Rate and characteristics of infection after transrectal prostate biopsy: a retrospective observational study. Scand J Urol 2021; 55: 317–323. [DOI] [PubMed] [Google Scholar]
- 9. Sigle A, Jilg CA, Kuru TH, et al. Evaluation of Ginsburg scheme: where is significant prostate cancer missed. Cancers 2021; 13: 2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pepe P, Aragona F. Prostate biopsy: results and advantages of the transperineal approach – twenty-year experience of a single center. World J Urol 2014; 32: 373–377. [DOI] [PubMed] [Google Scholar]
- 11. Devetzis K, Kum F, Popert R. Recent advances in systematic and targeted prostate biopsies. Res Rep Urol 2021; 13: 799–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Xiang J, Yan H, Li J, et al. Transperineal versus transrectal prostate biopsy in the diagnosis of prostate cancer: a systematic review and meta-analysis. World J Surg Oncol 2019; 17: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Symons JL, Huo A, Yuen CL, et al. Outcomes of transperineal template-guided prostate biopsy in 409 patients. BJU Int 2013; 112: 585–593. [DOI] [PubMed] [Google Scholar]
- 14. Thomson A, Li M, Grummet J, et al. Transperineal prostate biopsy: a review of technique. Transl Androl Urol 2020; 9: 3009–3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Barzell WE, Whitmore WF. Transperineal template guided saturation biopsy of the prostate: rationale, indications, and technique. Urol Times 2003; 31: 41–42. [Google Scholar]
- 16. Onik G, Barzell W. Transperineal 3D mapping biopsy of the prostate: an essential tool in selecting patients for focal prostate cancer therapy. Urol Oncol 2008; 26: 506–510. [DOI] [PubMed] [Google Scholar]
- 17. Kasivisvanathan V, Dufour R, Moore CM, et al. Transperineal magnetic resonance image targeted prostate biopsy versus transperineal template prostate biopsy in the detection of clinically significant prostate cancer. J Urol 2013; 189: 860–866. [DOI] [PubMed] [Google Scholar]
- 18. Ahmed HU, El- Shater Bosaily A, Brown LC, et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet 2017; 389: 815–822. [DOI] [PubMed] [Google Scholar]
- 19. Radtke JP, Schwab C, Wolf MB, et al. Multiparametric magnetic resonance imaging (MRI) and MRI-transrectal ultrasound fusion biopsy for index tumor detection: correlation with radical prostatectomy specimen. Eur Urol 2016; 70: 846–853. [DOI] [PubMed] [Google Scholar]
- 20. Hansen NL, Barrett T, Lloyd T, et al. Optimising the number of cores for magnetic resonance imaging-guided targeted and systematic transperineal prostate biopsy. BJU Int 2020; 125: 260–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ristau BT, Allaway M, Cendo D, et al. Free-hand transperineal prostate biopsy provides acceptable cancer detection and minimizes risk of infection: evolving experience with a 10-sector template. Urol Oncol 2018; 36: 528.e15–528.e20. [DOI] [PubMed] [Google Scholar]
- 22. Meyer AR, Joice GA, Schwen ZR, et al. Initial experience performing in-office ultrasound-guided transperineal prostate biopsy under local anesthesia using the precision point transperineal access system. Urology 2018; 115: 8–13. [DOI] [PubMed] [Google Scholar]
- 23. Maruf M, George A, Wei J, et al. PD38-08 multi-institutional prospective validation of the novel Michigan Urological Surgery improvement collaborative transperineal biopsy template. J Urol 2020; 203: e804. [Google Scholar]
- 24. Dupati A, Qi J, Dibianco J, et al. MP43-05 freehand transperineal biopsy sampling strategies: what is the optimal template? J Urol 2021; 206: e783–e783. [Google Scholar]
- 25. Kundra V, Matin SF, Kuban DA. Prostate cancer. Radiologic Key, 2016, https://radiologykey.com/prostate-cancer/
- 26. Bhavsar A, Verma S. Anatomic imaging of the prostate. Biomed Res Int 2014; 2014: 728539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rodriguez R, Ross A. Development, molecular biology, and physiology of the prostate. In: Wein A, Kavoussi L, Partin A, et al. (eds) Campbell Walsh Wein Urology, vol. 3. 11th ed. Philadelphia, PA: Elsevier, 2016, p. 2396. [Google Scholar]
- 28. Meikle AW, Stephenson RA, Lewis CM, et al. Effects of age and sex hormones on transition and peripheral zone volumes of prostate and benign prostatic hyperplasia in twins. J Clin Endocrinol Metab 1997; 82: 571–575. [DOI] [PubMed] [Google Scholar]
- 29. Ittmann M. Anatomy and histology of the human and murine prostate. Cold Spring Harb Perspect Med 2018; 8: a030346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Johnstone PA, Rossi PJ, Jani AB, et al. ‘Insignificant’ prostate cancer on biopsy: pathologic results from subsequent radical prostatectomy. Prostate Cancer Prostatic Dis 2007; 10: 237–241. [DOI] [PubMed] [Google Scholar]
- 31. Template mapping biopsies: an overview of technique and results. Radiologic Key, 2016, https://radiologykey.com/template-mapping-biopsies-an-overview-of-technique-and-results/
- 32. Sivaraman A, Sanchez-Salas R, Barret E, et al. Transperineal template-guided mapping biopsy of the prostate. Int J Urol 2015; 22: 146–151. [DOI] [PubMed] [Google Scholar]
- 33. Crawford ED, Wilson SS, Torkko KC, et al. Clinical staging of prostate cancer: a computer-simulated study of transperineal prostate biopsy. BJU Int 2005; 96: 999–1004. [DOI] [PubMed] [Google Scholar]
- 34. Pepe P, Garufi A, Priolo GD, et al. Multiparametric MRI/TRUS fusion prostate biopsy: advantages of a transperineal approach. Anticancer Res 2017; 37: 3291–3294. [DOI] [PubMed] [Google Scholar]
- 35. Tewes S, Peters I, Tiemeyer A, et al. Evaluation of MRI/Ultrasound fusion-guided prostate biopsy using transrectal and transperineal approaches. Biomed Res Int 2017; 2017: 2176471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Warlick C, Futterer J, Maruf M, et al. Beyond transrectal ultrasound-guided prostate biopsies: available techniques and approaches. World J Urol 37: 419–427. [DOI] [PubMed] [Google Scholar]
- 37. Bozlu M, Ulusoy E, Doruk E, et al. Voiding impairment after prostate biopsy: does tamsulosin treatment before biopsy decrease this morbidity? Urology 2003; 62: 1050–1053 [DOI] [PubMed] [Google Scholar]
- 38. Kuru TH, Wadhwa K, Chang RTM, et al. Definitions of terms, processess and a minimum dataset for transperineal prostate biopsies: a standardization approach of the Ginsburg study group for enhanced prostate diagnostics. BJU Int 2013; 112: 568–577. [DOI] [PubMed] [Google Scholar]
- 39. Zimmerman ME, Meyer AR, Carter HB, et al. In-office transperineal prostate biopsy using biplanar ultrasound guidance: a step-by-step guide. Urology 2019; 133: 247. [DOI] [PubMed] [Google Scholar]
- 40. Emiliozzi P, Longhi S, Scarpone P, et al. The value of a single biopsy with 12 transperineal cores for detecting prostate cancer in patients with elevated prostate specific antigen. J Urol 2001; 166: 845–850. [PubMed] [Google Scholar]
- 41. Giganti F, Kirkham A, Allen C, et al. Update on multiparametric prostate MRI during active surveillance: current and future trends and role of the PRECISE recommendations. AJR Am J Roentgenol 2021; 216: 943–951. [DOI] [PubMed] [Google Scholar]