Abstract
Objective
This study assessed the influence of chitosan nanoparticles on the fluoride-releasing ability of 4 glass ionomer cement (GIC) through an in vitro analysis.
Methods
Four types of GIC (type II light cure universal restorative, type II universal restorative, GC Fuji VII, and type IX) were modified with nanochitosan particles; 10% chitosan was added to the glass ionomer liquid. Six specimens for each of the 4 groups were created, using expendable Teflon moulds. Discs of each type of GIC (n = 6) were immersed in deionised water at various time intervals. Electrodes selective for fluoride ions were employed to analyse the amount of released fluoride at 1, 7, 14, 21, and 28 days.
Results
Chitosan-modified GICs showed greater fluoride release than conventional GICs at all time points. All samples showed an initial high release of fluoride that tapered off with time. The total amount of fluoride released increased from the 1st day to the 28th day on adding chitosan to all the 4 types of GIC. Amongst those, type IX high-strength posterior extra with chitosan released a considerably higher quantity of fluoride at all time intervals.
Conclusions
In all the experimental groups, adding chitosan to the glass ionomer liquid had an accelerating effect on its fluoride-releasing property.
Key words: Chitosan, Nanoparticles, Fluoride release, Glass ionomer cement, Modified, Restoration, Cement
Introduction
Dental caries is the most prevalent noncommunicable disease worldwide.1 According to the latest available data from the Global Burden of Disease Study 2019, dental caries affects more than 2.5 billion people, affecting health and quality of life.2 Fluoride is an effective anticariogenic agent that can reduce the incidence of dental caries.3,4 Fluorides exert an anticariogenic effect by attenuating the formation of the pellicle, microbial growth, and metabolism whilst augmenting remineralisation.5,6
Presently, we have numerous fluoride-releasing materials, including fluoridated composites, polyacid modified composite resins, and glass ionomers, both conventional and resin-modified.7 They release different levels of fluoride, which affects their cariostatic and antibacterial properties.8
Glass ionomer cement (GIC) is a widely used restorative cement and has a sustained release of fluoride ions. GIC cumulatively releases 5 times more fluoride than a compomer and 21 times more fluoride than a fluoride-containing composite resin.9 This property of the cement is responsible for its anticariogenic potential, protecting the tooth surface from microorganisms.10 Manufacturers have modified GICs over the years by subtly altering their composition to improve their properties.11 The latest in this long line of modified GICs are chitosan-modified GICs.12
Chitosan is a natural biocompatible and biodegradable polysaccharide recognised as being safe by the US Food and Drug Administration. Chitosan is derived from chitin, the most abundant organic compound in nature, after cellulose.13 Chitin is a biopolymer making up the exoskeletons of arthropods such as shrimps, lobsters, and cell walls of fungi.14,15 Chitosan is an organic polymer with a multitude of applications in dentistry ranging from drug carriers for the treatment of periodontitis to antimicrobial agents in endodontic therapy and pulp regeneration.[16], [17], [18], [19] Its unique cationic nature allows it to form multilayer structures or electrostatic complexes with other natural or synthetic polymers, making it suitable as a drug delivery agent. It possesses antibiotic, antimycotic, and anticarcinogenic properties, and its formulations have been widely employed in the pharmacologic field.20 Cationic chitosan oligomers attack negatively charged cell membranes of microbes causing leakage of cellular contents.21 Chitosan nanoparticles have the properties of chitosan and the characteristics of nanoparticles, showing controlled release, mucoadhesivity, antibacterial activity, and quantum size effects.22,23
Bioadhesive chitosan-fluoride microparticles help in increased fluoride availability and uptake, providing protection against caries.24 GIC modified with chitosan solutions has enhanced antibacterial properties against Streptococcus mutans.25 The addition of 10% chitosan in the liquid phase improved the mechanical properties of GIC.26 Chitosan interferes with tooth demineralisation by inhibiting phosphorus release and mineral loss.27 The addition of chitosan to GIC increases flexural resistance and increases the amount of fluoride ions released.28
Whilst the addition of chitosan polymers to improve the mechanical properties is a growing field, it is unclear to what extent the addition of chitosan to GIC can influence its fluoride release. Few studies have analysed the effects of chitosan addition to the anticarcinogenic properties of GIC. There is a notable paucity of studies examining chitosan-modified GICs focusing on their anticariogenic fluoride release. GICs are classified based on their clinical indication. Type II GIC is indicated for restorations, type VII is a high fluoride-releasing, and type IX is paediatric GIC that is condensable and less moisture-sensitive.29 The present study aimed to compare the influence of chitosan nanoparticles on the fluoride-releasing ability of 4 commonly used types of GIC.
Methods
Preparation of chitosan solution
A flask of 100 mL capacity was filled with glacial acetic acid (1.8 mL) and distilled water (100 mL); 0.3 N acetic acid was used to dissolve 20 mg of chitosan nanoparticles (Sigma-Aldrich), which contributed to 100 mL in the flask of 100 mL capacity, in turn resulting in a solution of chitosan of 0.2 mg/mL.30
Formulation of glass ionomer liquid modified with chitosan
Then, 0.1 mL of prepared solution of chitosan (0.2 mg/mL) was added to 0.9 mL of the glass ionomer liquid to achieve a strength of 10 v/v% of glass ionomer liquid incorporated with chitosan.
Sample grouping
Four different kinds of GICs (type II light cure universal restorative, type II universal restorative, GC Fuji VII [pink], and GC HS posterior) were examined to evaluate the quantity of fluoride released from the specimens immersed in deionised or deionized (DI) water at various time intervals. Figure 1 depicts the various study groups.
Fig. 1.
Sample grouping of the glass ionomer cements.
Sample preparation
Six disc-shaped specimens were prepared for every group with the help of expendable Teflon moulds of 10 mm in diameter and 2 mm in depth. All samples were prepared by manipulating the GIC based on guidelines provided by the manufacturer. To guard against performance bias, all samples were prepared by a single operator. The proportioned powder and liquid were mixed by hand using an agate spatula and mixing pad. For samples belonging to groups IA, IIA, IIIA, and IVA, the liquid provided by the manufacturer was used. For samples belonging to groups IB, IIB, IIIB, and IVB, glass ionomer liquid incorporated with chitosan was used. Hand-mixing of the proportioned powder and liquid was done with the help of an agate spatula and mixing pad. This GIC was then packed into expendable Teflon moulds. The excess cement in all the prepared samples was discarded by pressing microscopic slides against these moulds on either side. A short length of waxed dental floss was incorporated into the cement specimen before it was set to allow the set sample to be suspended in the deionised water storage medium. The setting was carried out at room temperature for 10 minutes for the groups IA, IB, IIIA, IIIB, IVA, and IVB. The samples in groups IIA and IIB were exposed to a curing light source (OrtholuxTM Luminous LED, 3M Unitek) for 20 seconds on both sides. Discs extracted from the expendable Teflon moulds were suspended in deionised water (4 mL) in plastic vials.
The samples were then incubated at 37 °C. The DI water in each vial was replaced after the 1st, 7th, 14th, 21st, and 28th days. Equal volumes of total ionic strength acid buffer were added to maintain the solution of pH 5.0 to provide an ionic background and decomplex the fluoride. A fluoride ion-selective electrode was employed to measure the release of fluoride. Analysis was carried out with a precalibrated (calibrated to 0.20, 1.00, 2.00, 10.00, 20.00,100 ppm F containing a standard solution of fluoride ion) ion analyser. Time-dependent variations in the fluoride release concentration were assessed. At each interval, data on the total amount of fluoride released were recorded.
Statistical analysis
SPSS Software v.22 (IBM Corp.) was used for tabulating and analysing the data. Analysis of variance (ANOVA) followed by Tukey's honestly significant difference (post hoc) tests were applied for statistical analysis of the registered data. t test was used for comparative analysis. Significance levels were set at P < .05.
Results
The fluoride ion release profiles on deionised or DI water from 4 different GICs with and without chitosan were recorded for 28 days at 5 specific intervals. The quantity of fluoride released was documented in parts per million (ppm).
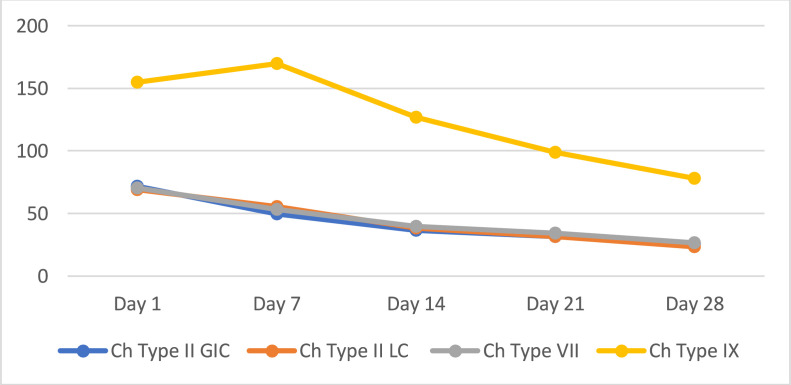
Table 1, Table 2, and Figure 2 show the comparative evaluation of fluoride release amongst the 4 types of cement with and without chitosan. GICs containing chitosan released a statistically greater quantity of fluoride than those without chitosan. The 2 groups reported the highest fluoride released after the 1st day, followed by a progressive decline until the 28th day. The results of this in vitro study showed that there was a significant elevation in the amount of fluoride released from the 1st to the 28th day when chitosan was added to all 4 groups of GIC. Amongst the varieties of GIC examined, type IX high-strength posterior extra, modified by chitosan, released a considerably greater quantity of fluoride at all time intervals tested.
Table 3.
Overall comparison of glass ionomer cements with chitosan.
| Groups | Days (Mean ± SD) ppm |
||||
|---|---|---|---|---|---|
| Day 1 | Day 7 | Day 14 | Day 21 | Day 28 | |
| IB | 71.67 ± 7.89a | 49.50 ± 3.51a | 36.50 ± 3.51a | 31.33 ± 2.73a | 25.50 ± 1.97a |
| IIB | 69.17 ± 4.67a | 55.50 ± 3.45a | 38.17 ± 2.71a | 31.38 ± 0.98a | 23.33 ± 0.82a |
| IIIB | 70.17 ± 6.94 a | 53.00 ± 4.15a | 39.83 ± 3.37a | 34.33 ± 0.82 a | 26.33 ± 2.66a |
| VB | 155.00 ± 23.45b | 170.00 ± 10.95b | 126.67 ± 13.66b | 98.83 ± 8.82 b | 78.00 ± 12.20b |
| P value amongst groups | <.001*** | <.001*** | <.001*** | <.001*** | <.001*** |
Denotes significance at 0.001 level. Different alphabets between groups denote significance at 1% level using tukey HSD test.
Table 1.
Comparison of fluoride release amongst groups.
| Groups | Days (Mean ± SD) ppm |
P value between days | ||||
|---|---|---|---|---|---|---|
| Day 1 | Day 7 | Day 14 | Day 21 | Day 28 | ||
| IA | 30.83 ± 1.17e | 20.33 ± 1.03d | 17.67 ± 0.82c | 14.67 ± 1.37b | 10.67 ± 0.52a | <.001*** |
| IB | 71.67 ± 7.89d | 49.50 ± 3.51c | 36.50 ± 3.51 b | 31.33 ± 2.73ab | 25.50 ± 1.97a | <.001*** |
| IIA | 17.00 ± 2.37d | 12.33 ± 0.52c | 11.33 ± 0.52bc | 10.17 ± 0.99ab | 8.60 ± 0.46a | <.001*** |
| IIB | 69.17 ± 4.67e | 55.50 ± 3.45d | 38.17 ± 2.71c | 31.38 ± 0.98b | 23.33 ± 0.82a | <.001*** |
| IIIA | 55.83 ± 4.75e | 34.33 ± 2.88d | 22.50 ± 1.64c | 22.67 ± 1.37bc | 16.50 ± 1.22a | <.001*** |
| IIIB | 70.17 ± 6.94e | 53.00 ± 4.15d | 39.83 ± 3.37c | 34.33 ± 0.82b | 26.33 ± 2.66a | <.001*** |
| IVA | 117.67 ± 35.22e | 49.17 ± 4.92d | 42.33 ± 2.25cd | 30.33 ± 4.23ab | 20.00 ± 0.89a | <.001*** |
| IVB | 155.00 ± 23.45e | 170.00 ± 10.95d | 126.67 ± 13.66 c | 98.83 ± 8.82b | 78.00 ± 12.20a | <.001*** |
Denotes significance at 0.001 level. Different alphabets between groups denote significance at 1% level using tukey HSD test.
Table 2.
Overall comparisons of glass ionomer cements.
| Groups | Days (Mean ± SD) ppm |
||||
|---|---|---|---|---|---|
| Day 1 | Day 7 | Day 14 | Day 21 | Day 28 | |
| IA | 30.83 ± 1.17ab | 20.33 ± 1.03b | 17.67 ± 0.82b | 14.67 ± 1.37b | 10.67 ± 0.52b |
| IIA | 17.00 ± 2.37a | 12.33 ± 0.52a | 11.33 ± 0.52a | 10.17 ± 0.99a | 8.60 ± 0.46a |
| IIIA | 55.83 ± 4.75b | 34.33 ± 2.88c | 22.50 ± 1.64c | 22.67 ± 1.37 c | 16.50 ± 1.22c |
| IVA | 117.67 ± 35.22c | 49.17 ± 4.92d | 42.33 ± 2.25d | 30.33 ± 4.23d | 20.00 ± 0.89d |
| P value amongst groups | <.001*** | <.001*** | <.001*** | <.001*** | <.001*** |
Denotes significance at 0.001 level. Different alphabets between groups denote significance at 1% level using tukey HSD test.
Fig. 2.
Fluoride release of chitosan-modified glass ionomer cements over time.
Discussion
GICs have several advantages recommending them as a restoration material in primary and permanent teeth. They have minimum shrinkage and a capacity to chemically bond with tooth structures including dentin and enamel.31 They have high pulpal and periodontal biocompatibility as well as the ability to release fluoride. GIC and its modified dental cements are associated with lower incidence of secondary caries.32
Chitosan nanoparticles are drug-delivery agents that have been extensively employed in medicine and carry key advantages including higher solubility of the drug, low toxicity, and the ability for an optimal stable drug release property.33 However, there remains a lack of clarity about the dynamics of fluoride release from chitosan-modified GICs. This study evaluated the impact chitosan nanoparticles have on the release of fluoride ions from 4 different types of GIC (type II light cure universal restorative, type II universal restorative, GC Fuji VII [pink], and GC HS posterior extra).
Chitosan-modified GICs showed a greater fluoride release than commercial GICs in all groups. This could translate into greater availability of fluoride for uptake into the tooth structure.34 Our results corroborate the findings of Patel et al, who reported that chitosan-modified resin-modified GIC (RMGIC) showed greater fluoride release than conventional RMGIC at 15 and 30 days.35 Our findings match those observed by Kumar et al, who found that nanochitosan-modified type II showed higher fluoride release at 1-hour, 24-hour, and 7-day time points.36 GIC chitosan nanoparticles may have a catalytic effect on fluoride release, eliciting rapid sustained diffusion, benefitting remineralisation, and preventing secondary caries.
We observed that all groups had the greatest amount of fluoride release on day 1. This result is likely to be related to the “burst phenomenon” observed in GICs, where high amounts of fluoride are released in the initial periods.[37], [38], [39], [40] The burst phenomenon of high fluoride release is attributed to the setting reaction of GIC.41 Our findings are consistent with previous observational studies by Neelakantan et al, who reported a similar high initial fluoride release. The burst phenomenon of fluoride release may have beneficial biological and bactericidal effects immediately after restoration.8
We observed that after an initial rapid release, fluoride release declines during the first week, going on to stabilise after 3 weeks. Comparison of the findings with those of other studies by Hasan et al, Pellizari et al, and Nagi et al confirms this pattern of fluoride release, with GIC showing the highest amount of fluoride release in the first day and tapering off to stabilise over weeks.[42], [43], [44]
There is a statistically significant difference in the amount of fluoride released amongst the various groups. Amongst the restorative materials, the chitosan-modified Fuji IX showed the highest fluoride release. This is in agreement with previous research by Mazzaoui et al, who found that Fuji IX showed the highest fluoride release compared to Fuji II light cure and a composite resin.45 The higher fluoride release by Fuji IX is presumably due to its high fluoride content and increased water uptake. Fluoride release values show a wide range of variation in the literature due to differences in protocols followed during experiments.46 The highest release of fluoride occurred in the first week, with the most rapid release occurring in the first 24 hours. This release profile is similar to the results obtained in the present study.
Fluoride release in deionised water ranges from 10 ppm to 100 ppm at 28 days for conventional GICs.47 RMGICs show a relatively decreased amount of fluoride release, ranging from 7 to 50 ppm over the same period. This is in agreement with the results of the present study, with the chitosan-modified Fuji IX releasing more fluoride than the chitosan-modified GIC—Fuji II light cure. However, Xu and Burgess have claimed that RMGICs have a longer fluoride release time than conventional GICs.8 This was not evident in the results of the present study.
The release of fluoride is a complex process. It depends on various intrinsic and environmental factors: organic matrix and filler composition, manipulation method, solubility and porosity of the mass, surface area, and pH.48 For all the samples investigated, the quantity of fluoride released was the highest after the 1st day, followed by a progressive decline in release up to the 28th day. Gradual and short-term release are considered the 2 major aqueous-based fluoride-releasing mechanisms from GIC. Fluoride rapidly dissolves from the surface in the short-term release.
From the cement, there is a sustained ionic diffusion in the gradual release. After the preliminary burst, the release of fluoride slows down, followed by long-term sustained release. The overall quantity of released fluoride from the GIC in a short period is controlled by diffusion and accompanied by an attenuating gradient.49
Even though fluoride and its anticaries effect have been examined, a search of the literature did not find data regarding the minimum fluoride release necessary for inhibition of secondary caries.39 Hicks et al reported that a low concentration less than 1 ppm of fluoride is sufficient to reduce demineralisation and increase remineralisation.50
The chitosan and polyacrylic acid network surrounding the inorganic GIC particle strengthened the interfacial tension between the restorative glass ionomer constituents, increasing the mechanical efficiency at this strength.51 Previous studies report that the amount of fluoride released was less in artificial saliva than in deionised water, which in turn is because, in the latter, the fluoride-releasing property is well reflected in the absence of any effect from the solutions organic or mineral components.51 The amount of fluoride released by GIC was analysed for 28 days according to the previous study, as it almost remained unchanged after the 28th day.52
In the current research, ion-selective electrode-based potentiometer devices were chosen to ensure that the methods used for fluoride analysis are up to the universal standard. The method also has an easily accessible and lower detection threshold.53
Overall, the addition of chitosan to all the 4 types of GICs accelerated the quantity of fluoride released from baseline to the 28th day. Amongst the various groups tested, modified type IX HS posterior extra released a considerably higher quantity of fluoride at all time intervals. The addition of 0.0044 wt% of chitosan in GIC has a lytic influence on the release of the fluoride, which allows a faster fluoride diffusion. The effect causing the rapid diffusion is attributed to the polymeric network formation. This attaches firmly with inorganic filler. The fluoride ion release from the inorganic matrix was favoured when reinforced complexes (polyacrylic acid adsorbing onto chitosan, attached to the surface of the GIC particle) were sculpted.
Our study is limited by its in vitro design. Chatzistavrou et al reported that in vitro assessments of concentrations of fluoride ions released are often higher than in vivo assessments.48,54 Though there are considerable in vitro studies for chitosan-modified GIC, sufficient clinical trials and follow-ups should be considered.
Conclusions
The findings of this study clearly indicate that the addition of chitosan nanoparticles improves the fluoride release properties of glass-ionomer cements. It is possible that chitosan nanoparticles alter the structure of the cement matrix and allow the fluoride ions to diffuse more readily. Chitosan-modified type IX high demonstrated the highest quantity of fluoride ions released, and type II light cure GIC exhibited the least. The increased fluoride release from chitosan-modified GICs may serve to prevent secondary caries and enhance remineralisation. Further in vivo studies are needed to confirm and validate these findings. Future research should focus on incorporating antibacterial molecules such as chlorhexidine in improving the therapeutic efficacy of GICs.
Author contributions
Conceptualization: Cruz Nishanthine, Revathi Miglani, Abdullah Almalki, ThillaSekar Vinothkumar.
Design of the Study: Saravanan Poorni, Manali Ramakrishnan Srinivasan, Harisha Dewan.
Interpretation: Ali Robaian, Nassreen Hassan Mohammad Albar, Waseem Radwan.
Writing–original draft Preparation: Susen Faisal Rajeh Alhaidary, Sultan Binalrimal.
Writing–review and Editing: Indira R, Shankargouda Patil, Mubashir Baig Mirza, Shilpa Bhandi.
All authors have read and agreed to the published version of the manuscript.
Conflict of Interest
None disclosed.
Acknowledgements
This paper was published as a preprint in http://repository-tnmgrmu.ac.in/id/eprint/3922.
REFERENCES
- 1.Pitts NB, Twetman S, Fisher J, Marsh PD. Understanding dental caries as a non-communicable disease. Br Dent J. 2021;231(12):749–753. doi: 10.1038/s41415-021-3775-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Global Burden of Disease Study 2019 (GBD 2019). Reference Life Table | GHDx. Available from:https://ghdx.healthdata.org/record/ihme-data/global-burden-disease-study-2019-gbd-2019-reference-life-table Accessed 10 April 2022.
- 3.Pizzo G, Piscopo MR, Pizzo I, Giuliana G. Community water fluoridation and caries prevention: a critical review. Clin Oral Investig. 2007;11(3):189–193. doi: 10.1007/s00784-007-0111-6. [DOI] [PubMed] [Google Scholar]
- 4.Petersson GH, Bratthall D. The caries decline: a review of reviews. Eur J Oral Sci. 1996;104:436–443. doi: 10.1111/j.1600-0722.1996.tb00110.x. (4, pt 2) [DOI] [PubMed] [Google Scholar]
- 5.Wiegand A, Buchalla W, Attin T. Review on fluoride-releasing restorative materials—fluoride release and uptake characteristics, antibacterial activity and influence on caries formation. Dent Mater. 2007;23(3):343–362. doi: 10.1016/j.dental.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 6.Buzalaf MAR, Pessan JP, Honório HM, Ten Cate JM. Mechanisms of action of fluoride for caries control. Monogr Oral Sci. 2011;22:97–114. doi: 10.1159/000325151. [DOI] [PubMed] [Google Scholar]
- 7.Hicks J, Garcia-Godoy F, Donly K, Flaitz C. Fluoride-releasing restorative materials and secondary caries. J Calif Dent Assoc. 2003;31(3):229–245. [PubMed] [Google Scholar]
- 8.Xu X, Burgess JO. Compressive strength, fluoride release and recharge of fluoride-releasing materials. Biomaterials. 2003;24(14):2451–2461. doi: 10.1016/S0142-9612(02)00638-5. [DOI] [PubMed] [Google Scholar]
- 9.Aboush YE, Torabzadeh H. Fluoride release from tooth-colored restorative materials: a 12-month report. J Can Dent Assoc. 1998;64(8) 561–4, 568. [PubMed] [Google Scholar]
- 10.Ngo H. Glass-ionomer cements as restorative and preventive materials. Dent Clin North Am. 2010;54(3):551–563. doi: 10.1016/j.cden.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 11.Culbertson BM. Glass-ionomer dental restoratives. Prog Polym Sci. 2001;26(4):577–604. doi: 10.1016/S0079-6700(01)00006-5. [DOI] [Google Scholar]
- 12.Petri DFS, Donegá J, Benassi AM, Bocangel JAJS. Preliminary study on chitosan modified glass ionomer restoratives. Dent Mater. 2007;23(8):1004–1010. doi: 10.1016/j.dental.2006.06.038. [DOI] [PubMed] [Google Scholar]
- 13.Martínez JP, Falomir MP, Gozalbo D. Chitin: a structural biopolysaccharide with multiple applications. eLS. 2014 doi: 10.1002/9780470015902.a0000694.pub3. [DOI] [Google Scholar]
- 14.Rinaudo M. Chitin and chitosan: properties and applications. Prog Polym Sci. 2006;31(7):603–632. [Google Scholar]
- 15.Crini G. Historical review on chitin and chitosan biopolymers. Environ Chem Lett. 2019;17(4):1623–1643. [Google Scholar]
- 16.Elshinawy MI, Al-Madboly LA, Ghoneim WM, El-Deeb NM. Synergistic effect of newly introduced root canal medicaments; ozonated olive oil and chitosan nanoparticles, against persistent endodontic pathogens. Front Microbiol. 2018;9:1371. doi: 10.3389/fmicb.2018.01371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khajuria DK, Patil ON, Karasik D, Razdan R. Development and evaluation of novel biodegradable chitosan based metformin intrapocket dental film for the management of periodontitis and alveolar bone loss in a rat model. Arch Oral Biol. 2018;85:120–129. doi: 10.1016/j.archoralbio.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 18.Aminu N, Chan S-Y, Yam M-F, Toh S-M. A dual-action chitosan-based nanogel system of triclosan and flurbiprofen for localised treatment of periodontitis. Int J Pharm. 2019;570 doi: 10.1016/j.ijpharm.2019.118659. [DOI] [PubMed] [Google Scholar]
- 19.Budai-szűcs M, Ruggeri M, Faccendini A, et al. Electrospun scaffolds in periodontal wound healing. Polymers (Basel) 2021;13(2):1–16. doi: 10.3390/polym13020307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mohire NC, Yadav AV. Chitosan-based polyherbal toothpaste: As novel oral hygiene product. Indian J Dent Res. 2010;21(3):380. doi: 10.4103/0970-9290.70808. [DOI] [PubMed] [Google Scholar]
- 21.Yilmaz Atay H. Functional chitosan. Springer;; Singapore: 2019. Antibacterial activity of chitosan-based systems; pp. 457–489. [DOI] [Google Scholar]
- 22.Tiyaboonchai W. Chitosan nanoparticles: a promising system for drug delivery. Naresuan Univ J. 2003;11:51–66. [Google Scholar]
- 23.Qi L, Xu Z, Jiang X, Hu C, Zou X. Preparation and antibacterial activity of chitosan nanoparticles. Carbohydr Res. 2004;339(16):2693–2700. doi: 10.1016/j.carres.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 24.Keegan GM, Smart JD, Ingram MJ, Barnes L-M, Burnett GR, Rees GD. Chitosan microparticles for the controlled delivery of fluoride. J Dent. 2012;40(3):229–240. doi: 10.1016/j.jdent.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 25.Neelima B, Reddy JS, Singh PT, Suhasini K, Hemachandrika I, Hasanuddin S. Comparative evaluation of antimicrobial efficacy of glass ionomer cement added with propolis, chitosan, and chlorhexidine against Streptococcus mutans and Lactobacillus acidophilus: an in vitro study. J Indian Soc Pedod Prev Dent. 2020;38(4):367–373. doi: 10.4103/JISPPD.JISPPD_322_20. [DOI] [PubMed] [Google Scholar]
- 26.Ibrahim MA, Neo J, Esguerra RJ, Fawzy AS. Characterization of antibacterial and adhesion properties of chitosan-modified glass ionomer cement. J Biomater Appl. 2015;30(4):409–419. doi: 10.1177/0885328215589672. [DOI] [PubMed] [Google Scholar]
- 27.Arnaud TMS, de Barros Neto B, Diniz FB. Chitosan effect on dental enamel de-remineralization: an in vitro evaluation. J Dent. 2010;38(11):848–852. doi: 10.1016/j.jdent.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 28.Petri DFS, Donegá J, Benassi AM, Bocangel JAJS. Preliminary study on chitosan modified glass ionomer restoratives. Dent Mater. 2007;23(8):1004–1010. doi: 10.1016/j.dental.2006.06.038. [DOI] [PubMed] [Google Scholar]
- 29.Somani R, Jaidka S, Singh DJ, Sibal GK. Comparative evaluation of shear bond strength of various glass ionomer cements to dentin of primary teeth: an in vitro study. Int J Clin Pediatr Dent. 2016;9(3):192. doi: 10.5005/jp-journals-10005-1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karthick A, Kavitha M, Loganathan SC, Malarvizhi D. Evaluation of microshear bond strength of chitosan modified Gic. World J Med Sci. 2014;10(2):169–173. doi: 10.5829/idosi.wjms.2014.10.2.82184. [DOI] [Google Scholar]
- 31.Aboush YE, Torabzadeh H. Clinical performance of class II restorations in which resin composite is laminated over resin-modified glass-ionomer. Oper Dent. 2000;25(5):367–373. [PubMed] [Google Scholar]
- 32.Sidhu SK. Springer; London, UK: 2015. Glass-ionomers in dentistry. [Google Scholar]
- 33.Zeng Z. Recent advances of chitosan nanoparticles as drug carriers. Int J Nanomedicine. 2011;765 doi: 10.2147/IJN.S17296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Benelli EM, Serra MC, Rodrigues ALJ, Cury JA. In situ anticariogenic potential of glass ionomer cement. Caries Res. 1993;27(4):280–284. doi: 10.1159/000261551. [DOI] [PubMed] [Google Scholar]
- 35.Patel A, Dhupar JKMS, Jajoo SS, Shah P, Chaudhary S. Evaluation of adhesive bond strength, and the sustained release of fluoride by chitosan-infused resin-modified glass ionomer cement: an in vitro study. Int J Clin Pediatr Dent. 2021;14(2):254–257. doi: 10.5005/jp-journals-10005-1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Senthil Kumar R, Ravikumar N, Kavitha S, et al. Nanochitosan modified glass ionomer cement with enhanced mechanical properties and fluoride release. Int J Biol Macromol. 2017;104:1860–1865. doi: 10.1016/j.ijbiomac.2017.05.120. [DOI] [PubMed] [Google Scholar]
- 37.Delbem ACB, Pedrini D, França JGM, Machado TM. Fluoride release/recharge from restorative materials–effect of fluoride gels and time. Oper Dent. 2005;30(6):690–695. [PubMed] [Google Scholar]
- 38.Bansal R, Bansal T. A comparative evaluation of the amount of fluoride release and re-release after recharging from aesthetic restorative materials: an in vitro study. J Clin Diagn Res. 2015;9(8):ZC11–ZC14. doi: 10.7860/JCDR/2015/11926.6278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Forsten L. Fluoride release and uptake by glass-ionomers and related materials and its clinical effect. Biomaterials. 1998;19(6):503–508. doi: 10.1016/s0142-9612(97)00130-0. [DOI] [PubMed] [Google Scholar]
- 40.Neelakantan P, John S, Anand S, Sureshbabu N, Subbarao C. Fluoride release from a new glass-ionomer cement. Oper Dent. 2011;36(1):80–85. doi: 10.2341/10-219-LR. [DOI] [PubMed] [Google Scholar]
- 41.Crisp S, Lewis BG, Wilson AD. Glass ionomer cements: chemistry of erosion. J Dent Res. 1976;55(6):1032–1041. doi: 10.1177/00220345760550060501. [DOI] [PubMed] [Google Scholar]
- 42.Hasan AMHR, Sidhu SK, Nicholson JW. Fluoride release and uptake in enhanced bioactivity glass ionomer cement (“glass carbomerTM”) compared with conventional and resin-modified glass ionomer cements. J Appl Oral Sci. 2019;27 doi: 10.1590/1678-7757-2018-0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pellizzari VA, Michels AC, Luiz ST, de Souza EM, Tabchoury C, Rached RN. Fluoride ion release of self-adhesive resin cements and their potential to inhibit in situ enamel and dentin demineralization. Oper Dent. 2017;42(5):548–558. doi: 10.2341/16-115-L. [DOI] [PubMed] [Google Scholar]
- 44.Nagi SM, Moharam LM, El Hoshy AZ. Fluoride release and recharge of enhanced resin modified glass ionomer at different time intervals. Futur Dent J. 2018;4(2):221–224. doi: 10.1016/j.fdj.2018.06.005. [DOI] [Google Scholar]
- 45.Mazzaoui SA, Burrow MF, Tyas MJ. Fluoride release from glass ionomer cements and resin composites coated with a dentin adhesive. Dent Mater. 2000;16(3):166–171. doi: 10.1016/s0109-5641(00)00003-8. [DOI] [PubMed] [Google Scholar]
- 46.Rix D, Foley TF, Banting D, Mamandras A. A comparison of fluoride release by resin-modified GIC and polyacid-modified composite resin. Am J Orthod Dentofac Orthop Off Publ Am Assoc Orthod its Const Soc Am Board Orthod. 2001;120(4):398–405. doi: 10.1067/mod.2001.116083. [DOI] [PubMed] [Google Scholar]
- 47.de Araujo FB, García-Godoy F, Cury JA, Conceição EN. Fluoride release from fluoride-containing materials. Oper Dent. 1996;21(5):185–190. [PubMed] [Google Scholar]
- 48.Al Ibrahim NS, Tahmassebi JF, Toumba KJ. In vitro and in vivo assessment of newly developed slow-release fluoride glass device. Eur Arch Paediatr Dent Off J Eur Acad Paediatr Dent. 2010;11(3):131–135. doi: 10.1007/BF03262728. [DOI] [PubMed] [Google Scholar]
- 49.Yip HK, S RJ. Fluoride release from a polyacid-modified resin composite and 3 resin-modified glass-ionomer materials. Quintessence Int. 2000;31(4):261–266. [PubMed] [Google Scholar]
- 50.Hicks J, Garcia-Godoy F, Flaitz C. Biological factors in dental caries: role of remineralization and fluoride in the dynamic process of demineralization and remineralization (part 3) J Clin Pediatr Dent. 2004;28(3):203–214. doi: 10.17796/jcpd.28.3.w0610427l746j34n. [DOI] [PubMed] [Google Scholar]
- 51.Miranda LA, Weidlich P, Samuel SMW, Maltz M. Fluoride release from restorative materials coated with an adhesive. Braz Dent J. 2002;13(1):39–43. [PubMed] [Google Scholar]
- 52.Elsaka SE. Antibacterial activity and adhesive properties of a chitosan-containing dental adhesive. Quintessence Int. 2012;43(7):603–613. [PubMed] [Google Scholar]
- 53.Martínez-Mier EA, Cury JA, Heilman JR, et al. Development of gold standard ion-selective electrode-based methods for fluoride analysis. Caries Res. 2011;45(1):3–12. doi: 10.1159/000321657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chatzistavrou E, Eliades T, Zinelis S, Athanasiou AE, Eliades G. Fluoride release from an orthodontic glass ionomer adhesive in vitro and enamel fluoride uptake in vivo. Am J Orthod Dentofac Orthop Off Publ Am Assoc Orthod its Const Soc Am Board Orthod. 2010;137(4) doi: 10.1016/j.ajodo.2009.10.030. 458.e1–8; discussion 458–9. [DOI] [PubMed] [Google Scholar]