Abstract
Background
Tortoises of the genus Testudo are widely distributed throughout the Mediterranean region and southwestern Asia. However, the evolutionary mechanisms of diversification in this genus are still poorly understood.
Methods
In this study, we assessed the evolutionary patterns in the climate niches of five species and 11 subspecies of the genus Testudo using ecological niche models and evaluated the niche overlap based on species phylogenetic distances.
Results
The ecological models indicated that most species differ in their climate niches, but show overlap, with gradual transitions at range boundaries. As expected, the ecological divergence among subspecies was lower than that among species. Evaluation of the phylogenetic signal indicated that climate niches have been weakly conserved, but sister species also show high evolutionary divergence.
Keywords: Ecology, Evolution, Reptiles, Testudo, Glacial cycles, Refugia
Introduction
Closely related species tend to respond similarly to environmental variation because they share similar life histories and physiological traits (Cruz et al., 2011; Dahlke et al., 2020). This lineage-specific evolutionary inertia in environmental response is known as phylogenetic niche conservatism (Crisp & Cook, 2012). Niche conservatism is common during the speciation process of a lineage, and it is produced by the fragmentation of ancestral subpopulations in environmentally analogous regions (Peterson & Nyari, 2008). Climatic oscillations, including glacial phases, promote niche diversification, as some subpopulations are subject to substantial contractions in their ranges during unfavourable periods, reducing or preventing gene flow (Leroy et al., 2017; Andersen et al., 2019).
A vicariant mode of speciation is evident in several lineages of Mediterranean reptiles (Kindler et al., 2017; Senczuk et al., 2017). Most reptiles are ectothermic, having entire reliance on external heating sources to control body temperature and complete embryonic development (Brattstrom, 1965; Booth, 2006). Therefore, the temperature is a major environmental factor influencing species richness among reptile assemblages in temperate regions (Rodríguez, Belmontes & Hawkins, 2005). The oscillatory phases throughout the Pleistocene, which involved a general drop in temperature and increased aridification (Magri & Parra, 2002), had profound impacts on the process of reptile diversification and distribution (Araújo et al., 2008; Fitze et al., 2011; Anadón et al., 2015). However, the influence of recent climatic history on the diversification process of Mediterranean tortoises is little known.
In this study, we tested for phylogenetic climatic niche conservatism and modelled the ranges of the tortoise species of the genus Testudo. This genus includes species that are morphologically similar (size ranges between 20 and 35 cm) and are generalist herbivores (Bonin, Devaux & Dupré, 2006). The genus Testudo comprises five species and 15 subspecies, distributed throughout Eurasia and North Africa in open and relatively warm environments (Calzolai & Chelazzi, 1991; Fritz et al., 2009; Escoriza et al., 2022). Most Testudo species are either parapatrically or allopatrically distributed, although some species are sympatric in a partial range of their distribution in the southern Balkans, such as T. graeca-T. hermanni and T. hermanni-T. marginata (Valakos et al., 2008). This pattern of occurrence suggests: (i) that the climatic niche of the species is conserved, being isolated by competitive exclusion or environmental barriers (Pasch, Bolker & Phelps, 2013; Jankowski et al., 2013; Li, Shao & Li, 2020); or (ii) that the species are ecologically divergent but contact in narrow transitional areas within the margins of environmental tolerance of the two parapatric species (Jones et al., 2020). We tested these hypotheses using ecological niche models (ENMs).
Materials & Methods
Study region and species
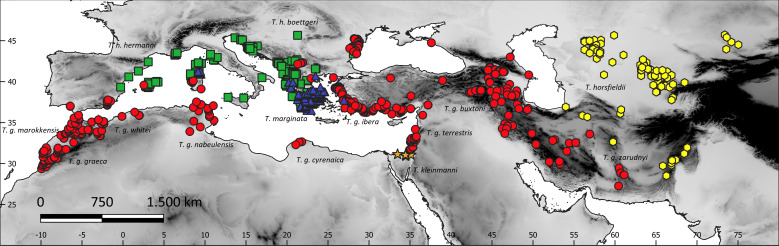
The study area included the western Palaearctic biogeographic realm (southern Europe and northern Africa) and southwestern Asia between Pakistan and Turkey (Fig. 1). Data on the occurrence of all the five species of the genus Testudo were obtained during several samplings in the region (Escoriza & Ben Hassine, 2017; Escoriza & Pascual, 2021), from bibliographic sources, the GBIF (Global Biodiversity Information Facility, https://www.gbif.org) and iNaturalist (only Testudo marginata; https://www.inaturalist.org/) databases (Appendix S1 and S2). These databases are to be reliable data sources for biodiversity research (García-Roselló et al., 2015; Hochmair et al., 2020). We selected from the GBIF those records added since 2000, with a fine spatial resolution (error < 1,000 m), far from cities and within the known ranges of the species, as defined by Bonin, Devaux & Dupré (2006). To reduce spatial autocorrelation bias (Boria et al., 2014), record points that are closer than 10 km from each other were removed from the analysis using spThin (Aiello-Lammens et al., 2015) in R (R Core Team, 2022). This process reduced the number of locations by 54% (from 964 to 519) (Appendix S1): T. graeca (274 records), T. hermanni (103), T. horsfieldii (77), T. kleinmanni (4), and T. marginata (61) (Fig. 1).
Figure 1. The study region and species sites, with superimposed terrain elevation (grayscale).
Red circles, Testudo graeca; green squares, Testudo hermanni; yellow hexagons, Testudo horsfieldii; orange stars, Testudo kleinmanni; blue triangles, Testudo marginata.
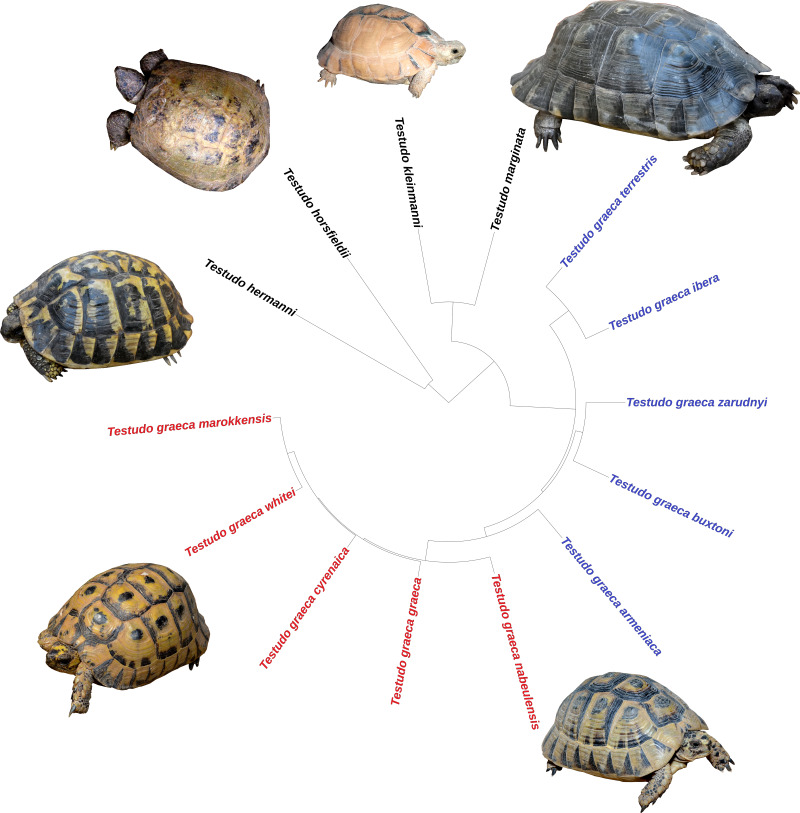
The taxonomic classification of Rhodin et al. (2021) was used i.e., only considering as valid those subspecies genetically and morphologically divergent. The niche analyses evaluated the ecological differentiation among species and subspecies, but also of two well-supported subclades within the genus (Fritz et al., 2007; Graciá et al., 2017): the eastern (Asian) group of subspecies of T. graeca (T. g. armeniaca, T. g. buxtoni, T. g. ibera, T. g. terrestris, T. g. zarudnyi) and the western (African) group (T. g. cyrenaica, T. g. graeca, T. g. marokkensis, T. g. nabeulensis, T. g. whitei) (Figs. 2 and 3).
Figure 2. Phylogenetic relationships among species and subspecies of the genus Testudo, based on estimated times of molecular divergence.
In T. graeca the names in red represent the western group of subspecies and in blue the eastern group. Clockwise: Testudo marginata (blackish specimen), T. graeca nabeulensis, T. graeca whitei, T. hermanni hermanni, T. horsfieldii, T. kleinmanni.
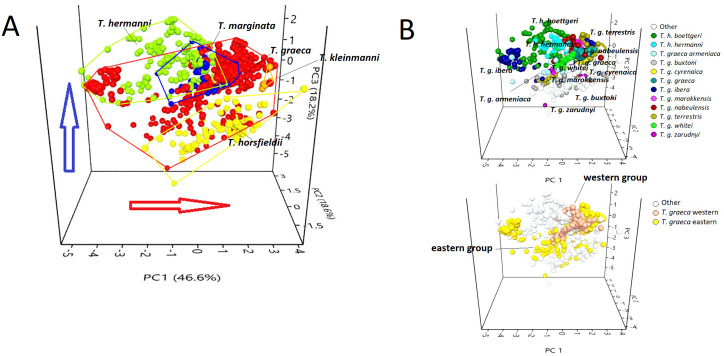
Figure 3. PCA triplot showing (A) the position of each species of the genus Testudo and (B) the position of the subspecies (above) and T. graeca western-eastern subclades (below), based on data obtained from bioclimatic variables.
Red circles, Testudo graeca; green circles, Testudo hermanni; yellow circles, Testudo horsfieldii; orange circles, Testudo kleinmanni; blue circles, Testudo marginata. The red arrow indicates an increase in temperature; the blue arrow indicates an increase in rainfall.
Environmental data
Data on 19 bioclimatic variables, available in World Clim2 (Fick & Hijmans, 2017), were obtained from the species occurrences. Model complexity was reduced by estimating the increase in the variance inflation factor (VIF) (Craney & Surles, 2002). To estimate the VIF, we defined buffer polygons of 200 km around the location of each species and generated 1000 random points within these polygons. The climate data obtained for the random points and the location of each species were included in a logistic regression and used to estimate the variations in VIF for each new variable added to the model. We started with a simple model including only bio 10 (mean temperature of the warmest quarter), bio 11 (mean temperature of the coldest quarter), and bio 12 (annual precipitation). These variables were initially chosen because of their relevance in the life cycles of tortoises, and they included summer and winter temperatures (specifically associated with embryonic development and hibernation) and moisture availability (Lambert, 1983; Anadón et al., 2012). Variables with VIF > 10 were excluded (Schroeder, Lander & Levine-Silverman, 1990).
Data analyses
We visualized the realised niche of each species using principal component analysis (PCA) of the normalised variables. The climate niche of each species was generated using Maxent, as this method is suitable for models based only on presence data (Elith et al., 2011). The best Maxent model was selected by comparing several candidates built using various features (L: linear; Q: quadratic; H: hinge; P: product; T: threshold) and regularisation multipliers (RMs). This iterative selection was conducted using the Akaike criterion corrected for finite samples (AICc), using the ENMeval functions (Muscarella et al., 2014) in R. The features and the RM for the optimal model were used to generate 30 replicates in Maxent 3.4.4 (Phillips, Anderson & Schapire, 2006), calibrated using 75% of the species occurrences. The reliability of these models was assessed using the AUC statistic and variable contributions based on permutations of the presence–background data (Phillips, Anderson & Schapire, 2006).
The best ENMs were used to infer the location and extent of potential palaeoclimate refugia. To do this, we projected the current relationships between climate and species to the paleoclimatic conditions, using the default clamping function in Maxent (Zhao et al., 2021). We used several palaeoclimatic layers covering the period from the early Pleistocene (ca. 787 ka), and a complete glacial cycle that included the last interglacial (ca. 130 ka), the last glacial maximum (ca. 21 ka), and the mid-Holocene (ca. 6 ka) (Otto-Bliesner et al., 2006; Brown et al., 2018). The logistic result of these models was binarised using the fuzzify function in Quantum GIS (mid point = 0.5). The four layers generated (along with one generated for present conditions) were summed, producing a new layer in which values close to five identified those areas that were climatically suitable as refugia throughout most of the Pleistocene–Holocene periods. The niche of T. kleinmanni was not modelled, because the number of localities was low.
We also evaluated the niche divergence between pairs of species and subspecies of the genus Testudo. These comparisons were conducted for all species and between parapatric conspecific subspecies. Those taxa having fewer than 19 occurrences were excluded from the niche tests (Jenkins & Quintana-Ascencio, 2020). Niche divergence was estimated using a suite of tests (overlap, linear and blob range-breaking, and identity and background tests) that estimated the D index, which varies between 0 (no overlap) and 1 (full overlap) (Schoener, 1970). The overlap test quantified the similarity between ENMs using Latin hypercube sampling (Warren et al., 2021). The range-breaking tests assess the occurrence of sharp changes in environmental conditions at the range boundaries of a species. The linear test assumes that this limit is linear, whereas the blob test assumes that the limit is geometrically irregular; the latter is more robust if the species occupy geographical areas differing in extent, and the sample sizes differ (Glor & Warren, 2011). Identity tests assessed whether the models generated for the occurrence of two species differed statistically from those generated from random subsamples of their occurrences (Warren, Glor & Turelli, 2008). Background tests evaluated the environmental conditions that surrounded a species range, enabling assessment of whether the niches of two species were more similar to each other than to the available conditions (Warren, Glor & Turelli, 2008). We defined background regions as buffers of 500 km for the species and 250 km for the subspecies and natural micro-endemic T. marginata. These background regions were defined according to the distribution of each species or subspecies, maximizing the inclusion of environmental variation around the occurrence areas while minimizing the effect of non-informative regions (such as temperate-boreal biomes or deserts, not occupied by any species of the genus) (Acevedo et al., 2012).
The identity and background tests were conducted based on ENMs (Warren, Glor & Turelli, 2008) and an ordination method (ecospat) (Broennimann et al., 2012). The ecospat method has the advantage that it reduces the chances of model overfitting (Broennimann et al., 2012). We also estimated the Spearman rank correlation between environmental variation and predicted suitability (Warren et al., 2021). This correlation varies from −1 to 1, with 0 representing random correlation (Warren et al., 2021). These tests were implemented using generalized linear models and their statistical significance was assessed after 500 replicates (Warren et al., 2021). These analyses were conducted using the ENMTools package (Warren, Glor & Turelli, 2010) in R.
The evolutionary relationships among several species and subspecies of the genus Testudo were taken from a published molecular phylogeny (Graciá et al., 2017) (Fig. 2). The phylogenetic signal was determined only for a set of taxa in which the extent of divergence was quantified for the same genes (Fig. 2). The test of phylogenetic influence over environmental niche occupancy (defined by the same subset of low-correlated variables) was determined using Blomberg’s K (Blomberg, Garland Jr & Ives, 2003). Blomberg’s K values vary between 0 and ∞, where values of K < 1 represent less phylogenetic signal than that expected under Brownian motion (Blomberg, Garland Jr & Ives, 2003). Blomberg’s K provides an acceptable estimate of the phylogenetic signal even when sample sizes are limited, although is susceptible to false positives (Blomberg, Garland Jr & Ives, 2003; Münkemüller et al., 2012). The value of the phylogenetic metric was calculated after 10,000 resamplings of the climate matrix, thus avoiding the error associated with estimations based only on the mean value per species (Harmon & Losos, 2005). These calculations were performed using the phytools package (Revell, 2012) in R.
Results
The scatter plot (Fig. 3) showed that the species occupy well-differentiated positions along the environmental gradient, from a mesophilic species (with high scores in the variables that describe precipitations), such as T. hermanni, other species that occupied an intermediate position (T. graeca, T. marginata), to xerophilic species such as T. horsfieldii and T. kleinmanni, that occupy one of the climate extremes (Fig. 3A). The subspecies show a tendency to overlap or occupy contiguous positions in the niche space (Fig. 3B), although the western group of T. graeca occupies a more limited niche than the eastern group of subspecies (Fig. 3B).
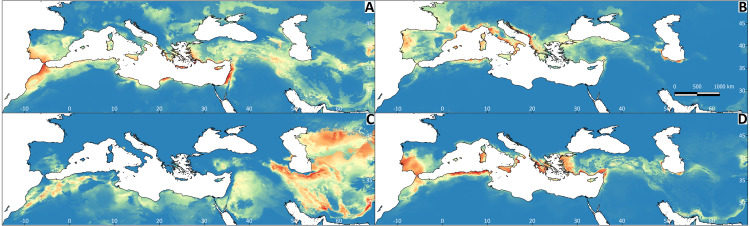
All Maxent models showed good performance (Appendix S2). The explanatory capacity of the climatic variables determining the species distributions differed significantly (Appendix S2). For T. graeca the variable having the greatest importance was annual precipitation (39.1%), for T. hermanni it was annual precipitation (77.9%), for T. horsfieldii it was the temperature of the wettest quarter and precipitation in the warmest quarter (29.1%), and for T. marginata it was precipitation in the warmest quarter (32.3%). The mapped results of the ENMs are shown in Fig. 4. The ENM projections identified several palaeoclimate refugia, which differed in most of the species (Fig. 5).
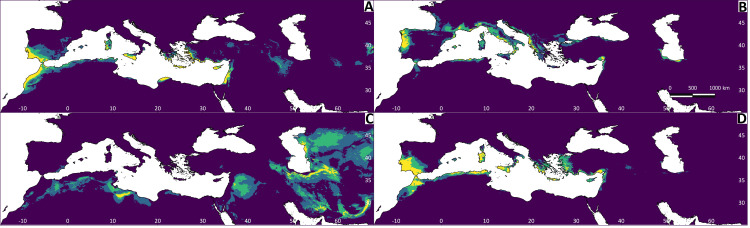
Figure 4. Results of the Maxent models representing the niche of Testudo species, obtained from climatic variables.
The red colour indicates greater environmental suitability, and the blue colour reduced suitability. (A) Testudo graeca; (B) Testudo hermanni; (C) Testudo horsfieldii; (D) Testudo marginata.
Figure 5. Regions most likely to represent stable climate refugia throughout the Pleistocene-Holocene periods (787 ka to 0 ya).
The yellow colour indicates a higher probability (high suitability for at least five time periods), and the blue colour indicates high suitability for less than two time periods. (A) Testudo graeca; (B) Testudo hermanni; (C) Testudo horsfieldii; (D) Testudo marginata.
The results of the niche tests did not indicate that these species are niche conservative, both in the group of parapatric species and between sister species (T. hermanni-T. horsfieldii). The lack of statistical significance for most of the comparisons of the range breaking tests allows us to reject the hypothesis of sharp environmental variations at the species’ range boundaries (Table 1). In general, climatic niche overlap between species was lower than between subspecies, and species tend to occur in distinct climate niches (Tables 1 and 2). Similarly, species showed negative values or close to 0 in their correlations between climate variation and predicted suitability, while in some pairs of subspecies (e.g., T. g. graeca and T. g. marokkensis) this correlation was positive, indicating similar environmental responses (Tables 1 and 2). The phylogenetic signal estimation indicated lability during the evolutionary processes for the genus Testudo, with absent or weak phylogenetic signal for the entire subset of bioclimatic variables included in the ENMs (i.e., BIO8–BIO12, BIO15, and BIO18) (K ≤ 0.475; Table 3).
Table 1. Evaluation of ecological niche overlap between pairs of species of the genus Testudo.
| Overlap | Lin | Blob | Id | BG | IdB | BGB | ||
|---|---|---|---|---|---|---|---|---|
| T. graeca vs | D | 0.656 | 0.368 | 0.368 | 0.370 | 0.343 | 0.386 | 0.327 |
| T. hermanni | P | 0.079 | 0.396 | 0.009 | 0.009 | 0.008 | 0.099 | |
| Rho | −0.036 | −0.036 | −0.035 | −0.051 | ||||
| T. graeca vs | D | 0.372 | 0.392 | 0.387 | 0.387 | 0.359 | 0.148 | 0.148 |
| T. horsfieldii | P | 0.416 | 0.396 | 0.009 | 0.009 | 0.001 | 0.174 | |
| Rho | −0.430 | −0.448 | −0.448 | −0.476 | ||||
| T. graeca vs | D | 0.226 | 0.251 | 0.251 | 0.245 | 0.243 | 0.361 | 0.361 |
| T. marginata | P | 0.475 | 0.347 | 0.009 | 0.009 | 0.596 | 0.073 | |
| Rho | −0.034 | −0.034 | −0.052 | −0.056 | ||||
| T. hermanni vs | D | 0.231 | No | No | 0.252 | 0.247 | 0.009 | 0.009 |
| T. horsfieldii | P | 0.004 | 0.004 | 0.001 | 0.430 | |||
| Rho | −0.277 | −0.205 | ||||||
| T. hermanni vs | D | 0.341 | 0.440 | 0.440 | 0.438 | 0.442 | 0.197 | 0.197 |
| T. marginata | P | 0.030 | 0.003 | 0.010 | 0.044 | 0.001 | 0.366 | |
| Rho | 0.548 | 0.548 | 0.547 | 0.692 | ||||
| T. horsfieldii vs | D | 0.130 | No | No | 0.241 | 0.235 | 0.145 | 0.155 |
| T. marginata | P | 0.005 | 0.015 | 0.019 | 0.145 | |||
| Rho | 0.005 | 0.005 |
Notes.
- Lin
- linear range–breaking test
- Blob
- blob rangebreaking test
- Id
- identity test
- BG
- background test
- IdB
- identity test (ecospat)
- BGB
- background test (ecospat)
- D
- Schoener’s D
- Rho
- Spearman rank correlation
- No
- no geographic contact
Table 2. Evaluation of ecological niche overlap between pairs of species of the genus Testudo.
| Overlap | Lin | Blob | Id | BG | IdB | BGB | ||
|---|---|---|---|---|---|---|---|---|
| T. g. graeca vs | D | 0.193 | 0.285 | 0.277 | 0.277 | 0.151 | 0.096 | 0.096 |
| T. g. marokkensis | P | 0.109 | 0.406 | 0.010 | 0.010 | 0.001 | 0.439 | |
| Rho | 0.338 | 0.330 | 0.330 | 0.129 | ||||
| T. g. marokkensis vs | D | 0.117 | 0.194 | 0.191 | 0.191 | 0.155 | 0.277 | 0.277 |
| T. g. whitei | P | 0.168 | 0.228 | 0.010 | 0.158 | 0.061 | 0.397 | |
| Rho | −0.357 | −0.354 | −0.354 | 0.018 | ||||
| T. g. whitei vs | D | 0.623 | 0.313 | 0.316 | 0.321 | 0.298 | 0.452 | 0.452 |
| T. g. nabeulensis | P | 0.277 | 0.495 | 0.010 | 0.375 | 0.657 | 0.057 | |
| Rho | 0.286 | 0.273 | 0.284 | 0.375 | ||||
| T. graeca western vs | D | 0.309 | No | No | 0.158 | 0.146 | 0.230 | 0.230 |
| T. graeca eastern | P | 0.010 | 0.010 | 0.001 | 0.132 | |||
| Rho | −0.645 | −0.668 | ||||||
| T. g. ibera vs | D | 0.260 | 0.282 | 0.282 | 0.286 | 0.239 | 0.333 | 0.333 |
| T. g. terrestris | P | 0.149 | 0.069 | 0.010 | 0.040 | 0.838 | 0.021 | |
| Rho | −0.089 | −0.089 | −0.081 | −0.228 | ||||
| T. g. ibera vs | D | 0.250 | 0.260 | 0.263 | 0.264 | 0.336 | 0.116 | 0.116 |
| T. g. buxtoni | P | 0.416 | 0.227 | 0.010 | 0.099 | 0.085 | 0.350 | |
| Rho | −0.618 | −0.612 | −0.614 | −0.351 | ||||
| T. g. buxtoni vs | D | 0.174 | 0.221 | 0.218 | 0.219 | 0.235 | 0.057 | 0.057 |
| T. g. terrestris | P | 0.238 | 0.248 | 0.009 | 0.012 | 0.001 | 0.490 | |
| Rho | −0.141 | −0.141 | −0.127 | 0.412 | ||||
| T. h. hermanni vs | D | 0.366 | 0.195 | 0.198 | 0.197 | 0.084 | 0.206 | 0.206 |
| T. h. boettgeri | P | 0.208 | 0.378 | 0.005 | 0.010 | 0.013 | 0.058 | |
| Rho | −0.121 | −0.133 | −0.123 | −0.504 |
Notes.
- Lin
- linear range-breaking test
- Blob
- blob rangebreaking test
- Id
- identity test
- BG
- background test
- IdB
- identity test (ecospat)
- BGB
- background test (ecospat)
- D
- Schoener’s D
- Rho
- Spearman rank correlation
- No
- no geographic contact
Table 3. Estimation of the phylogenetic signal (Blomberg’s K) in the diversification of the ecological niche in the genus Testudo.
| Blomberg’s K | |
|---|---|
| Temperature warmest quarter | 0.365 ± 0.001 |
| Temperature coldest quarter | 0.475 ± 0.002 |
| Temperature wettest quarter | 0.352 ± 0.001 |
| Temperature driest quarter | 0.360 ± 0.002 |
| Annual precipitation | 0.437 ± 0.002 |
| Precipitation seasonality | 0.263 ± 0.001 |
| Precipitation warmest quarter | 0.467 ± 0.002 |
Notes.
The mean value and the standard error after 10,000 resamplings of the possible values of the niche for each species are shown.
Discussion
The combined results indicate niche partitioning among the species of the genus Testudo. The temperate and humid region is largely occupied by T. hermanni and as the conditions become more arid, the species progressively replace one another, appearing two species entirely adapted to extreme climates, cold steppes (T. horsfieldii) or specialized in sand semi-deserts (T. kleinmanni) (Bringsøe & Buskirk, 1998; Valakos et al., 2008; Bondarenko & Peregontsev, 2017; Arakelyan et al., 2018). Consistently, we found that climate niches are not phylogenetically conserved, as the values for Blomberg’s K in our analysis range from 0.263 to 0.475. Mapped projections of the ENMs showed marked differences in the climate niches of these species of tortoise, and niche overlap was generally low. The analyses also suggest that climate (and particularly annual precipitation, and precipitation and temperature of the warmest quarter) plays a key role in determining tortoise distribution in the study area, given the high predictive performance of the ENMs, as already indicated by previous studies involving the eastern clades of T. graeca (Turkozan, Karacaoğlu & Parham, 2021).
The analyses revealed that, in most cases, the geographical boundaries between these tortoise species and subspecies were not determined by rapid climate transitions. This implies that the species can partially differ in their niches, but also share zones of environmental tolerance, as shown by the ENM results. These regions (e.g., the southern Balkans or Sardinia) are environmentally suitable for several species, and natural populations (or populations resulting from historical introductions; Vamberger et al., 2011) of two or three species coexist. In transitional regions, the species turnover may be determined by negative interspecific interactions or habitat properties, but they can also occur sympatrically (Wright, Steer & Hailey, 1988; Valakos et al., 2008).
The ENMs and ecospat identity tests did not show complete consistency. The results of the ecospat method were more conservative (i.e., they rejected the null hypothesis of niche equivalency less frequently). We considered that the species are ecologically divergent if the results of the two identity tests were consistent. These tests showed a pattern of niche divergence for most of the species pairs, except for T. graeca–T. marginata. However, in this case, the environmental response was not convergent (i.e., the values of the rank correlation coefficient were close to 0), and they were no more conservative than expected given their available conditions (i.e., the background tests were not statistically significant).
The subspecies showed greater similarities in their climate niches, with pairs occurring in equivalent climate niches and with convergent environmental responses (for example, the T. g. whitei–T. g. nabeulensis pair). Lower niche divergence was expected between subspecies since their genetic separation spans smaller timescales (Peterson, 2011; Meynard et al., 2017).
Niche divergence was especially marked for the eastern and western groups of T. graeca, as indicated by significant differences in the identity tests and negative values for the rank correlation coefficient. Indeed, western populations of T. graeca are confined to warm and dry environments in southern Europe, whereas the species extends to colder regions in eastern Europe (Anadón et al., 2006; Buică, Iosif & Cogălniceanu, 2013). This high niche divergence observed between both clades would initially support their separation as species, as proposed by Van der Kuyl, Ballasina & Zorgdrager (2005). The North African subclade has evolved isolated in the southern Mediterranean, in a hot and arid region on the fringes of the Sahara, which could have reduced its cold tolerance, compared to Asian subspecies. However, it should be evaluated whether these differences in the realized niches between the western and eastern subclades of T. graeca also extend to the fundamental niches, through experimental mechanistic studies (Jiménez et al., 2019; Bujan et al., 2022).
Complementary to the niche tests, evaluation of the phylogenetic signal suggested a poorly conserved climate niche. This result contrasts with data for other reptile groups from the region, including lacertid lizards, which show a strong phylogenetic signal in species climate niches (Escoriza, Pascual & Mestre, 2021). However the power of Blomberg’s K tests is limited when using small phylogenies (Münkemüller et al., 2012), and future studies have to evaluate these evolutionary patterns with a larger number of tortoise species.
Conclusions
The speciation process of tortoises from the genus Testudo is closely linked to regional climate history. The distribution of subspecies can be explained by the presence of stable climate refugia throughout the Pleistocene. Most species occupy well-defined and separate climate niches, reducing the chances of contact and interspecific interactions. In the case of occupation of equivalent niches, the species may occur in separate geographic regions (e.g., the T. graeca–T. marginata).
Supplemental Information
The thinned records represent the spatially independent coordinates +10 km.
Acknowledgments
The authors wish to thank their support during the fieldwork and data gathering of Dr Soumia Fahd (University of Tetouan, Morocco), John B. Iverson (Earlham College), Julia Ferrer (Fundació Emys), and Ridha Ouni (University of Tunis el Manar, Tunisia), and Guillem Pascual and Juan Gabriel Ureña (Barcelona Zoological Garden) for providing pictures of T. horsfieldii (GP) and T. kleinmanni (JGU).
Funding Statement
The authors received no funding for this work.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Daniel Escoriza conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the article, and approved the final draft.
Jihene Ben Hassine conceived and designed the experiments, performed the experiments, authored or reviewed drafts of the article, and approved the final draft.
Data Availability
The following information was supplied regarding data availability:
The raw measurements are available in the Supplementary File.
References
- Acevedo et al. (2012).Acevedo P, Jiménez-Valverde A, Lobo JM, Real R. Delimiting the geographical background in species distribution modelling. Journal of Biogeography. 2012;39:1383–1390. doi: 10.1111/j.1365-2699.2012.02713.x. [DOI] [Google Scholar]
- Aiello-Lammens et al. (2015).Aiello-Lammens ME, Boria RA, Radosavljevic A, Vilela B, Anderson RP. spThin: an R package for spatial thinning of species occurrence records for use in ecological niche models. Ecography. 2015;38:541–545. doi: 10.1111/ecog.01132. [DOI] [Google Scholar]
- Anadón et al. (2012).Anadón JD, Giménez A, Graciá E, Pérez I, Ferrández M, Fahd S, El Mouden H, Kalboussi M, Jdeidi T, Larbes S, Rouag R. Distribution of Testudo graeca in the western Mediterranean according to climatic factors. Amphibia-Reptilia. 2012;33:285–296. doi: 10.1163/156853812X643710. [DOI] [Google Scholar]
- Anadón et al. (2006).Anadón JD, Giménez A, Martínez M, Martínez J, Pérez I, Esteve MA. Factors determining the distribution of the spur-thighed tortoise Testudo graeca in south-east Spain: a hierarchical approach. Ecography. 2006;29:339–346. doi: 10.1111/j.2006.0906-7590.04486.x. [DOI] [Google Scholar]
- Anadón et al. (2015).Anadón JD, Graciá E, Botella F, Giménez A, Fahd S, Fritz U. Individualistic response to past climate changes: niche differentiation promotes diverging Quaternary range dynamics in the subspecies of Testudo graeca. Ecography. 2015;38:956–966. doi: 10.1111/ecog.01163. [DOI] [Google Scholar]
- Andersen et al. (2019).Andersen JC, Havill NP, Mannai Y, Ezzine O, Dhahri S, Jamâa MLBen, Caccone A, Elkinton JS. Identification of winter moth (Operophtera brumata) refugia in North Africa and the Italian Peninsula during the last glacial maximum. Ecology and Evolution. 2019;9:13931–13941. doi: 10.1002/ece3.5830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arakelyan et al. (2018).Arakelyan M, Türkozan O, Hezaveh N, Parham JF. Ecomorphology of tortoises (Testudo graeca complex) from the Araks river valley. Russian Journal of Herpetology. 2018;25:245–252. doi: 10.30906/1026-2296-2018-25-4-245-252. [DOI] [Google Scholar]
- Araújo et al. (2008).Araújo MB, Nogués-Bravo D, Diniz-Filho JAF, Haywood AM, Valdes PJ, Rahbek C. Quaternary climate changes explain diversity among reptiles and amphibians. Ecography. 2008;31:8–15. doi: 10.1111/j.2007.0906-7590.05318.x. [DOI] [Google Scholar]
- Blomberg, Garland Jr & Ives (2003).Blomberg SP, Garland Jr T, Ives AR. Testing for phylogenetic signal in comparative data: behavioral traits are more labile. Evolution. 2003;57:717–745. doi: 10.1111/j.0014-3820.2003.tb00285.x. [DOI] [PubMed] [Google Scholar]
- Bondarenko & Peregontsev (2017).Bondarenko DA, Peregontsev EA. Distribution of the Central Asian Tortoise Agrionemys horsfieldii (Gray, 1844) in Uzbekistan (range, regional and landscape distribution, populations density) Current Studies in Herpetology. 2017;17:124–146. doi: 10.18500/1814-6090-2017-17-3-4-124-146. [DOI] [Google Scholar]
- Bonin, Devaux & Dupré (2006).Bonin F, Devaux B, Dupré A. Turtles of the world. John Hopkins University Press; Baltimore: 2006. [Google Scholar]
- Booth (2006).Booth DT. Influence of incubation temperature on hatchling phenotype in reptiles. Physiological and Biochemical Zoology. 2006;79:274–281. doi: 10.1086/499988. [DOI] [PubMed] [Google Scholar]
- Boria et al. (2014).Boria RA, Olson LE, Goodman SM, Anderson RP. Spatial filtering to reduce sampling bias can improve the performance of ecological niche models. Ecological Modelling. 2014;275:73–77. doi: 10.1016/j.ecolmodel.2013.12.012. [DOI] [Google Scholar]
- Brattstrom (1965).Brattstrom BH. Body temperatures of reptiles. American Midland Naturalist. 1965;73:376–422. doi: 10.2307/2423461. [DOI] [Google Scholar]
- Bringsøe & Buskirk (1998).Bringsøe H, Buskirk JR. Distribution of Testudo kleinmanni Lortet, 1883 and Testudo graeca Linnaeus, 1758 in the Negev desert, southern Israel (Reptilia: Testudines: Testudinidae) Faunistische Abhandlungen Staatliches Museum fur Tierkunde Dresden. 1998;21:23–30. [Google Scholar]
- Broennimann et al. (2012).Broennimann O, Fitzpatrick MC, Pearman PB, Petitpierre B, Pellissier L, Yoccoz NG, Thuiller W, Fortin MJ, Randin C, Zimmermann NE, Graham CH. Measuring ecological niche overlap from occurrence and spatial environmental data. Global Ecology and Biogeography. 2012;21:481–497. doi: 10.1111/j.1466-8238.2011.00698.x. [DOI] [Google Scholar]
- Brown et al. (2018).Brown JL, Hill DJ, Dolan AM, Carnaval AC, Haywood AM. PaleoClim, high spatial resolution paleoclimate surfaces for global land areas. Scientific Data. 2018;5:1–9. doi: 10.1038/sdata.2018.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buică, Iosif & Cogălniceanu (2013).Buică G, Iosif R, Cogălniceanu D. Demography and conservation of an isolated Spur-thighed tortoise Testudo graeca population in Dobrogea (Romania) Ecologia Balkanica. 2013;5:97–106. [Google Scholar]
- Bujan et al. (2022).Bujan J, Ollier S, Villalta I, Devers S, Cerdá X, Amor F, Dahbi A, Bertelsmeier C, Boulay R. Can thermoregulatory traits and evolutionary history predict climatic niches of thermal specialists? Diversity and Distributions. 2022;28:1081–1092. doi: 10.1111/ddi.13511. [DOI] [Google Scholar]
- Calzolai & Chelazzi (1991).Calzolai R, Chelazzi G. Habitat use in a central Italy population of Testudo hermanni Gmelin (ReptiliaTestudinidae) Ethology, Ecology & Evolution. 1991;3:153–166. doi: 10.1080/08927014.1991.9525381. [DOI] [Google Scholar]
- Craney & Surles (2002).Craney TA, Surles JG. Model-dependent variance inflation factor cutoff values. Quality Engineering. 2002;14:391–403. doi: 10.1081/QEN-120001878. [DOI] [Google Scholar]
- Crisp & Cook (2012).Crisp MD, Cook LG. Phylogenetic niche conservatism: what are the underlying evolutionary and ecological causes? New Phytologist. 2012;196:681–694. doi: 10.1111/j.1469-8137.2012.04298.x. [DOI] [PubMed] [Google Scholar]
- Cruz et al. (2011).Cruz FB, Antenucci D, Luna F, Abdala CS, Vega LE. Energetics in Liolaemini lizards: implications of a small body size and ecological conservatism. Journal of Comparative Physiology B. 2011;181:373–382. doi: 10.1007/s00360-010-0524-4. [DOI] [PubMed] [Google Scholar]
- Dahlke et al. (2020).Dahlke FT, Wohlrab S, Butzin M, Pörtner HO. Thermal bottlenecks in the life cycle define climate vulnerability of fish. Science. 2020;369:65–70. doi: 10.1126/science.aaz3658. [DOI] [PubMed] [Google Scholar]
- Elith et al. (2011).Elith J, Phillips SJ, Hastie T, Dudík M, Chee YE, Yates CJ. A statistical explanation of MaxEnt for ecologists. Diversity and Distributions. 2011;17:43–57. doi: 10.1111/j.1472-4642.2010.00725.x. [DOI] [Google Scholar]
- Escoriza & Ben Hassine (2017).Escoriza D, Ben Hassine J. Niche separation among north-west African semi-aquatic reptiles. Hydrobiologia. 2017;797:47–56. doi: 10.1007/s10750-017-3157-8. [DOI] [Google Scholar]
- Escoriza et al. (2022).Escoriza D, Díaz-Paniagua C, Andreu A, Ben Hassine J. Testudo graeca Linnaeus 1758 (Western Subspecies Clade: Testudo g. graeca, T. g. cyrenaica, T. g. marokkensis, T. g. nabeulensis, T. g. whitei)—Mediterranean Spur-thighed Tortoise, Moorish Tortoise, Libyan Tortoise, Moroccan Tortoise, Tunisian Tortoise, Souss Valley Tortoise. In: Rhodin AGJ, Iverson JB, Van Dijk PP, Stanford CB, Goode EV, Buhlmann KA, Mittermeier RA, editors. Conservation biology of freshwater turtles and tortoises: a compilation project of the IUCN/SSC tortoise and freshwater turtle specialist group. Vol. 117. Arlington: Chelonian Research Foundation (CRF) & Turtle Conservancy (TC); 2022. pp. 1–18. (Chelonian Research Monographs). [Google Scholar]
- Escoriza & Pascual (2021).Escoriza D, Pascual G. Habitat occupancy by semi-aquatic reptiles on an aridity gradient in the western Mediterranean. River Research and Applications. 2021;37:1233–1242. doi: 10.1002/rra.3838. [DOI] [Google Scholar]
- Escoriza, Pascual & Mestre (2021).Escoriza D, Pascual G, Mestre L. Climate and habitat niche diversification in a southwest European squamate assemblage. Evolutionary Ecology. 2021;35:761–777. doi: 10.1007/s10682-021-10139-10144. [DOI] [Google Scholar]
- Fick & Hijmans (2017).Fick SE, Hijmans RJ. WorldClim 2: new 1-km spatial resolution climate surfaces for global land areas. International Journal of Climatology. 2017;37:4302–4315. doi: 10.1002/joc.5086. [DOI] [Google Scholar]
- Fitze et al. (2011).Fitze PS, Gonzalez-Jimena V, San-Jose LM, San-Mauro D, Aragón P, Suarez T, Zardoya R. Integrative analyses of speciation and divergence in Psammodromus hispanicus (Squamata: Lacertidae) BMC Evolutionary Biology. 2011;11:1–22. doi: 10.1186/1471-2148-11-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz et al. (2009).Fritz U, Auer M, Chirikova M, Duysebayeva TN, Eremchenko V, Kami HG, Kashkarov R, Masroor R, Moodley Y, Pindrani A, Široký P. Mitochondrial diversity of the widespread Central Asian steppe tortoise (Testudo horsfieldii Gray, 1844): implications for taxonomy and relocation of confiscated tortoises. Amphibia-Reptilia. 2009;30:245–257. doi: 10.1163/156853809788201135. [DOI] [Google Scholar]
- Fritz et al. (2007).Fritz U, Hundsdörfer A, Široký P, Auer M, Kami H, Lehmann J, Mazanaeva L, Türkozan O, Wink M. Phenotypic plasticity leads to incongruence between morphology-based taxonomy and genetic differentiation in western Palaearctic tortoises (Testudo graeca complex; Testudines, Testudinidae) Amphibia-Reptilia. 2007;28:97–121. doi: 10.1163/156853807779799135. [DOI] [Google Scholar]
- García-Roselló et al. (2015).García-Roselló E, Guisande C, Manjarrés-Hernández A, González-Dacosta J, Heine J, Pelayo-Villamil P, González-Vilas L, Vari RP, Vaamonde A, Granado-Lorencio C, Lobo JM. Can we derive macroecological patterns from primary Global Biodiversity Information Facility data? Global Ecology and Biogeography. 2015;24:335–347. doi: 10.1111/geb.12260. [DOI] [Google Scholar]
- Glor & Warren (2011).Glor RE, Warren D. Testing ecological explanations for biogeographic boundaries. Evolution. 2011;65:673–683. doi: 10.1111/j.1558-5646.2010.01177.x. [DOI] [PubMed] [Google Scholar]
- Graciá et al. (2017).Graciá EV, Vargas-Ramírez M, Delfino M, Anadón JD, Giménez A, Fahd S, Corti C, Jdeidi TB, Fritz U. Expansion after expansion: dissecting the phylogeography of the widely distributed spur-thighed tortoise, Testudo graeca (Testudines: Testudinidae) Biological Journal of the Linnean Society. 2017;121:641–654. doi: 10.1093/biolinnean/blx007. [DOI] [Google Scholar]
- Harmon & Losos (2005).Harmon LJ, Losos JB. The effect of intraspecific sample size on type I and type II error rates in comparative studies. Evolution. 2005;59:2705–2710. doi: 10.1111/j.0014-3820.2005.tb00981.x. [DOI] [PubMed] [Google Scholar]
- Hochmair et al. (2020).Hochmair HH, Scheffrahn RH, Basille M, Boone M. Evaluating the data quality of iNaturalist termite records. PLOS ONE. 2020;15:e0226534. doi: 10.1371/journal.pone.0226534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowski et al. (2013).Jankowski JE, Londoño GA, Robinson SK, Chappell MA. Exploring the role of physiology and biotic interactions in determining elevational ranges of tropical animals. Ecography. 2013;36:1–12. doi: 10.1111/j.1600-0587.2012.07785.x. [DOI] [Google Scholar]
- Jenkins & Quintana-Ascencio (2020).Jenkins DG, Quintana-Ascencio PF. A solution to minimum sample size for regressions. PLOS ONE. 2020;15:e0229345. doi: 10.1371/journal.pone.0229345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez et al. (2019).Jiménez L, Soberón J, Christen JA, Soto D. On the problem of modeling a fundamental niche from occurrence data. Ecological Modelling. 2019;397:74–83. doi: 10.1016/j.ecolmodel.2019.01.020. [DOI] [Google Scholar]
- Jones et al. (2020).Jones SE, Tobias JA, Freeman R, Portugal SJ. Weak asymmetric interspecific aggression and divergent habitat preferences at an elevational contact zone between tropical songbirds. Ibis. 2020;162:814–826. doi: 10.1111/ibi.12793. [DOI] [Google Scholar]
- Kindler et al. (2017).Kindler C, Chèvre M, Ursenbacher S, Böhme W, Hille A, Jablonski D, Vamberger M, Fritz U. Hybridization patterns in two contact zones of grass snakes reveal a new Central European snake species. Scientific Reports. 2017;7:7378. doi: 10.1038/s41598-017-07847-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert (1983).Lambert MR. Some factors influencing the Moroccan distribution of the western Mediterranean spur-thighed tortoise, Testudo graeca graeca L. and those precluding its survival in NW Europe. Zoological Journal of the Linnean Society. 1983;79:149–178. doi: 10.1111/j.1096-3642.1983.tb01164.x. [DOI] [Google Scholar]
- Leroy et al. (2017).Leroy T, Roux C, Villate L, Bodénès C, Romiguier J, Paiva JA, Dossat C, Aury JM, Plomion C, Kremer A. Extensive recent secondary contacts between four European white oak species. New Phytologist. 2017;214:865–878. doi: 10.1111/nph.14413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Shao & Li (2020).Li F, Shao L, Li S. Tropical niche conservatism explains the Eocene migration from India to Southeast Asia in ochyroceratid spiders. Systematic Biology. 2020;69:987–998. doi: 10.1093/sysbio/syaa006. [DOI] [PubMed] [Google Scholar]
- Magri & Parra (2002).Magri D, Parra I. Late Quaternary western Mediterranean pollen records and African winds. Earth and Planetary Science Letters. 2002;200:401–408. doi: 10.1016/S0012-821X(02)00619-2. [DOI] [Google Scholar]
- Meynard et al. (2017).Meynard CN, Gay PE, Lecoq M, Foucart A, Piou C, Chapuis MP. Climate-driven geographic distribution of the desert locust during recession periods: Subspecies’ niche differentiation and relative risks under scenarios of climate change. Global Change Biology. 2017;23:4739–4749. doi: 10.1111/gcb.13739. [DOI] [PubMed] [Google Scholar]
- Münkemüller et al. (2012).Münkemüller T, Lavergne S, Bzeznik B, Dray S, Jombart T, Schiffers K, Thuiller W. How to measure and test phylogenetic signal. Methods in Ecology and Evolution. 2012;3:743–756. doi: 10.1111/j.2041-210X.2012.00196.x. [DOI] [Google Scholar]
- Muscarella et al. (2014).Muscarella R, Galante PJ, Soley-Guardia M, Boria RA, Kass JM, Uriarte M, Anderson RP. ENM eval: an R package for conducting spatially independent evaluations and estimating optimal model complexity for Maxent ecological niche models. Methods in Ecology and Evolution. 2014;5:1198–1205. doi: 10.1111/2041-210X.12261. [DOI] [Google Scholar]
- Otto-Bliesner et al. (2006).Otto-Bliesner BL, Brady EC, Clauzet G, Tomas R, Levis S, Kothavala Z. Last glacial maximum and Holocene climate in CCSM3. Journal of Climate. 2006;19:2526–2544. doi: 10.1175/JCLI3748.1. [DOI] [Google Scholar]
- Pasch, Bolker & Phelps (2013).Pasch B, Bolker BM, Phelps SM. Interspecific dominance via vocal interactions mediates altitudinal zonation in neotropical singing mice. The American Naturalist. 2013;182:E161–E173. doi: 10.1086/673263. [DOI] [PubMed] [Google Scholar]
- Peterson (2011).Peterson AT. Ecological niche conservatism: a time-structured review of evidence. Journal of Biogeography. 2011;38:817–827. doi: 10.1111/j.1365-2699.2010.02456.x. [DOI] [Google Scholar]
- Peterson & Nyari (2008).Peterson AT, Nyari AS. Ecological niche conservatism and Pleistocene refugia in the thrush-like mourner, Schiffornis sp. in the neotropics. Evolution. 2008;62:173–183. doi: 10.1111/j.1558-5646.2007.00258.x. [DOI] [PubMed] [Google Scholar]
- Phillips, Anderson & Schapire (2006).Phillips SJ, Anderson RP, Schapire RE. Maximum entropy modeling of species geographic distributions. Ecological Modelling. 2006;190:231–259. doi: 10.1016/j.ecolmodel.2005.03.026. [DOI] [Google Scholar]
- R Core Team (2022).R Core Team . R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna: 2022. (accessed 20 January 2022) [Google Scholar]
- Revell (2012).Revell LJ. phytools: an R package for phylogenetic comparative biology (and other things) Methods in Ecology and Evolution. 2012;3:217–223. doi: 10.1111/j.2041-210X.2011.00169.x. [DOI] [Google Scholar]
- Rhodin et al. (2021).Rhodin AGJ, Iverson JB, Bour R, Fritz U, Georges A, Shaffer HB, Van Dijk PP. Turtles of the world: annotated checklist and atlas of taxonomy, synonymy, distribution, and conservation status (9th Ed.) Chelonian Research Monographs. 2021;8:1–472. doi: 10.3854/crm.8.checklist.atlas.v9.2021. [DOI] [Google Scholar]
- Rodríguez, Belmontes & Hawkins (2005).Rodríguez MÁ, Belmontes JA, Hawkins BA. Energy, water and large-scale patterns of reptile and amphibian species richness in Europe. Acta Oecologica. 2005;28:65–70. doi: 10.1016/j.actao.2005.02.006. [DOI] [Google Scholar]
- Schoener (1970).Schoener TW. Nonsynchronous spatial overlap of lizards in patchy habitats. Ecology. 1970;51:408–418. doi: 10.2307/1935376. [DOI] [Google Scholar]
- Schroeder, Lander & Levine-Silverman (1990).Schroeder MA, Lander J, Levine-Silverman S. Diagnosing and dealing with multicollinearity. Western Journal of Nursing Research. 1990;12:175–187. doi: 10.1177/019394599001200204. [DOI] [PubMed] [Google Scholar]
- Senczuk et al. (2017).Senczuk G, Colangelo P, De Simone E, Aloise G, Castiglia R. A combination of long term fragmentation and glacial persistence drove the evolutionary history of the Italian wall lizard Podarcis siculus. BMC evolutionary Biology. 2017;17:1–15. doi: 10.1186/s12862-016-0847-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkozan, Karacaoğlu & Parham (2021).Turkozan O, Karacaoğlu Ç, Parham JF. Reconstructions of the past distribution of Testudo graeca mitochondrial lineages in the Middle East and Transcaucasia support multiple refugia since the Last Glacial Maximum. Herpetological Journal. 2021;31:10–17. [Google Scholar]
- Van der Kuyl, Ballasina & Zorgdrager (2005).Van der Kuyl AC, Ballasina DL, Zorgdrager F. Mitochondrial haplotype diversity in the tortoise species Testudo graeca from North Africa and the Middle East. BMC Evolutionary Biology. 2005;5:29. doi: 10.1186/1471-2148-5-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valakos et al. (2008).Valakos ED, Pafilis P, Lymberakis P, Maragou P, Sotiropoulos K, Foufopoulos J. The amphibians and reptiles of Greece. Chimaira; Frankfurt: 2008. [Google Scholar]
- Vamberger et al. (2011).Vamberger M, Corti C, Stuckas H, Fritz U. Is the imperilled spur-thighed tortoise (Testudo graeca) native in Sardinia? Implications from population genetics and for conservation. Amphibia-Reptilia. 2011;32:9–25. doi: 10.1163/017353710X541869. [DOI] [Google Scholar]
- Warren, Glor & Turelli (2008).Warren DL, Glor RE, Turelli M. Environmental niche equivalency versus conservatism: quantitative approaches to niche evolution. Evolution. 2008;62:2868–2883. doi: 10.1111/j.1558-5646.2008.00482.x. [DOI] [PubMed] [Google Scholar]
- Warren, Glor & Turelli (2010).Warren DL, Glor RE, Turelli M. ENMTools: a toolbox for comparative studies of environmental niche models. Ecography. 2010;33:607–611. doi: 10.1111/j.1600-0587.2009.06142.x. [DOI] [Google Scholar]
- Warren et al. (2021).Warren DL, Matzke NJ, Cardillo M, Baumgartner JB, Beaumont LJ, Turelli M, Glor RE, Huron NA, Simões M, Iglesias TL, Piquet JC. ENMTools 1.0: an R package for comparative ecological biogeography. Ecography. 2021;44:504–511. doi: 10.1111/ecog.05485. [DOI] [Google Scholar]
- Wright, Steer & Hailey (1988).Wright J, Steer E, Hailey A. Habitat separation in tortoises and the consequences for activity and thermoregulation. Canadian Journal of Zoology. 1988;66:1537–1544. doi: 10.1139/z88-225. [DOI] [Google Scholar]
- Zhao et al. (2021).Zhao G, Cui X, Sun J, Li T, Wang Q, Ye X, Fan B. Analysis of the distribution pattern of Chinese Ziziphus jujuba under climate change based on optimized biomod2 and MaxEnt models. Ecological Indicators. 2021;132:108256. doi: 10.1016/j.ecolind.2021.108256. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The thinned records represent the spatially independent coordinates +10 km.
Data Availability Statement
The following information was supplied regarding data availability:
The raw measurements are available in the Supplementary File.