Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has numerous effects on different systemic organs other than the lungs. In this case report, we look at the presentation of a young female who was diagnosed with autoimmune hemolytic anemia (AIHA), kidney injury and thrombocytopenia during coronavirus disease 2019 (COVID-19) infection. She recovered well without the need for steroids. As demonstrated by this case, COVID-19 infection can be associated with the development of AIHA. The purpose of this report is to indicate that COVID-19 can present unusually with different clinical manifestations enough to require hospitalization.
Keywords: hemolytic anemia, covid 19, acute kidney injury, aiha, thrombocytopenia, infection
Introduction
Information from the medical literature on coronavirus disease 2019 (COVID-19) infection and its hematological consequences is rapidly growing. COVID-19 is mostly associated with respiratory illness and complications. However, the virus can result in multi-organ failure as a result of its pathology [1]. To our knowledge, this is one of the few cases of relatively healthy patients presenting with COVID-19 and autoimmune hemolytic anemia (AIHA). Here, we present the case of a lady who deteriorated dramatically during her admission as a result of COVID-19 infection. This case adds to the evidence for the association between AIHA and COVID-19 and will help in raising awareness of the unusual symptomatology of COVID-19.
Case presentation
A 32-year-old Middle Eastern female with a history of multiple sclerosis presented with fever for two days to the emergency department. There were no other associated symptoms. She was on teriflunomide and tizanidine for multiple sclerosis. She did not receive any COVID-19 vaccination. Upon initial assessment, her vital signs were within normal range with oxygen saturation of 98% on room air, and systemic examination was unremarkable. The polymerase chain reaction (PCR) swab for COVID-19 was positive. Here chest x-ray showed no abnormalities in lung fields. Laboratory findings were significant for leukocytosis with a white blood cell (WBC) count of 14.5 x 103/uL, while others including inflammatory markers and complete metabolic profile were unremarkable. Since she was not hypoxic, according to our local protocol, she did not qualify to receive any steroids or antiviral medications.
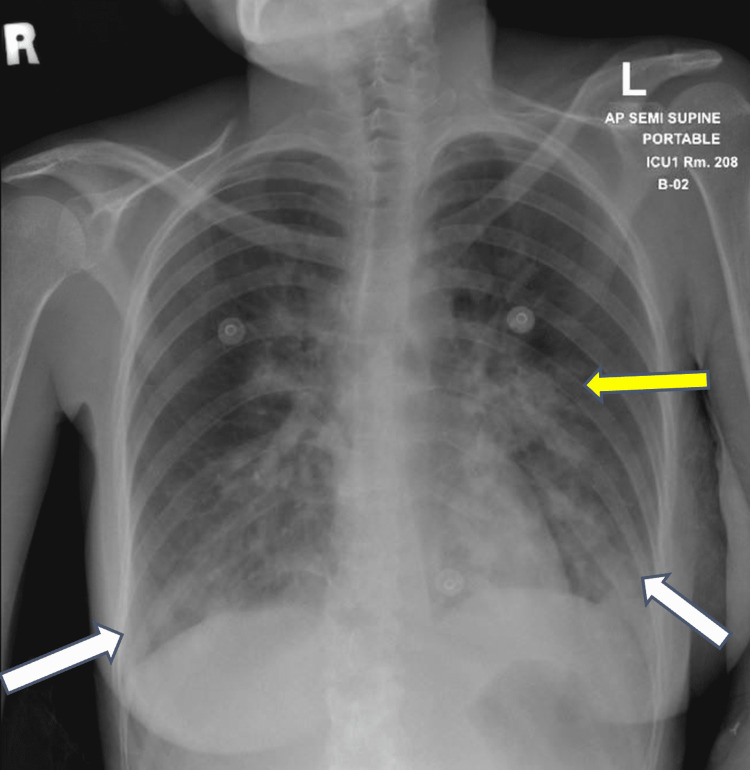
On the third day of hospital admission, she became dyspneic, tachycardic and hypoxic. The electrocardiogram (ECG) showed normal sinus rhythm and troponins were negative. Chest x-ray was repeated and showed bilateral lower lung zone opacities (Figure 1). She was transferred to the intensive care unit (ICU) as a case of moderate-severe COVID-19 infection and was managed with nasal cannula only and did not require invasive ventilation.
Figure 1. Chest x-ray showing findings of pneumonic consolidation in the bilateral lower lung zone (white arrows) and left-sided perihilar region (yellow arrow).
During her ICU stay, her hemoglobin and platelet levels dropped to 8.0 gm/dL and 105 x 103/uL, respectively (both within normal range initially) and subsequent blood tests were suggestive of hemolysis with a lactate dehydrogenase (LDH) level of of 583 U/L (reference, 135-214 U/L), haptoglobin level of <10 mg/dL (reference, 30-200 mg/dL) and reticulocyte count of 4.2% (reference, 0.5%-2.5%). A blood smear showed polychromasia with spherocytes suggestive of hemolytic anemia. The direct antiglobulin test (DAT) revealed significant levels of immunoglobulin G (IgG) anti-RBC autoantibodies. The Coombs profile confirmed warm-type autoimmune hemolytic anemia. In her other blood tests, ferritin was increased from 458 to 13,090 ng/mL (reference, 12-300 ng/mL). Additionally, she had an acute kidney injury (AKI) with creatinine 157 umol/L (reference, 44-80 umol/L). Ultrasound showed no renal stones or hydronephrosis, and spleen and liver were normal. A tentative diagnosis of thrombotic thrombocytopenic purpura (TTP) was ruled out on the basis of a low reticulocyte count and the positive direct Coombs test.
The patient was negative for infectious diseases (human immunodeficiency virus, hepatitis A/B/C, cytomegalovirus, Epstein-Barr virus, syphilis, and Mycoplasma pneumoniae). Her autoimmune workup was negative, and her vitamin B12 and glucose-6-phosphate dehydrogenase levels were normal. A summary of the investigation results during hospital stay is shown in Table 1.
Table 1. Blood investigations during hospitalization.
WBC, white blood cell; BUN, blood urea nitrogen; CRP, C-reactive protein; LDH, lactate dehydrogenase; AST, aspartate aminotransferase; ALT, alanine aminotransferase
| Reference range | Admission | Day 3 | Day 7 | Discharge |
| Hemoglobin (13-15 g/dL) | 12 | 8 | 9.4 | 10 |
| WBC (4-10 K/uL) | 14 | 15 | 5 | |
| Platelets (150-450 K/uL) | 220 | 105 | 76 | 140 |
| BUN (2.5-7.8 mmol/L) | 3 | 8.2 | 9 | 6.5 |
| Creatinine (44-80 umol/L) | 62 | 157 | 340 | 76 |
| CRP (0-5 mg/L) | 11 | 25 | 6 | 2 |
| Haptoglobin (30-200 mg/dL) | <10 | <10 | ||
| Procalcitonin (<0.5 ng/mL) | 0.3 | 0.5 | 0.5 | 0.1 |
| Ferritin (12-160 ug/L) | 458 | 13,090 | 238 | 140 |
| LDH (145-214 u/L) | 170 | 583 | 853 | 130 |
| Bilirubin (total) (0-21 umol/L) | 54 | 60 | 32 | |
| AST (0-32 u/L) | 44 | 42 | 40 | 30 |
| ALT (0-33 u/L) | 30 | 33 | 32 | 35 |
| Reticulocyte count (0.5%-2.5%) | 4.2% | 2.9% |
For next few days, her hemoglobin level and platelets continued to drop, and creatinine level continued to rise. She was given blood transfusions for anemia and required multiple sessions of hemodialysis for renal failure. We held a multidisciplinary meeting with the nephrologist and hematologist, and it was decided not to give her any steroids at that stage. After another few days, her hemoglobin, platelets, LDH, bilirubin, reticulocyte count and creatinine started to improve without any specific treatment. She was safely discharged home on folic acid after few more days of observations.
Discussion
AIHA is a unique immune condition caused by anti-RBC autoantibodies destroying red blood cells with or without the activation of the complement system [2]. With respect to its etiology, it can occur in association with several autoimmune disorders, neoplasia, infectious diseases or medications [3].
According to the literature, AIHA and COVID-19 infection have previously been linked to either warm or cold autoantibodies [4]. Lazarian et al. found that nine days was the median period between the development of AIHA and the first COVID-19 clinical features [5]. In our case report, AIHA occurred three days after the beginning of COVID-19 symptoms. According to Quinn and Murakhovskaya, a great proportion of the cases were the warm type of AIHA with the remainder being cold [6]. Similarly, our case also exhibited a positive IgG antiglobulin test. In another report, Hindilerden et al. described a case in which there was an association between the two diseases [7]. In contrast, in other cases, the patients suffered from malignant or lymphoproliferative disorders [5]. Our patient did not have any underlying neoplastic or hematological concomitant disorders.
The underlying pathophysiologic molecular mechanism in virus-induced cell death is not well known; however, it is thought to be an autoimmune reaction through antigenic mimicry, in which autoantibodies destroy self-antigens [8]. After ruling out all other possibilities, we can proclaim virus-induced AIHA as the culprit.
Acute renal impairment has been linked to an elevated death rate in COVID-19 hospitalized patients, with an 8.9% incidence reported in a recent meta-analysis [9,10]. The mechanism of this issue is not well known. Studies have shown that the pathogenesis is multifactorial, and both direct and indirect mechanisms are responsible for COVID-19-related AKI [11].
Thrombocytopenia was recently recognized as a common feature of the COVID-19 pandemic and was found in up to 36% of people [9]. It is also associated with high mortality and severe disease. One of the published studies in May 2020 reported a patient with COVID-19 who had no pulmonary abnormalities but significant thrombocytopenia. It illustrates the virus's abnormal presentation, which might range from asymptomatic cases to those with unexpected laboratory results [12].
From the management point of view, in the case of inability to treat the underlying cause, steroids remain the first-line treatment of choice for secondary AIHA patients [13]. Rituximab has been reported to be given following inadequate response to corticosteroids in only one case [5]. We did not use steroids for our patient as she was recovering without the need for them and her deterioration was reversible, and she had stable hemoglobin levels on her subsequent follow-ups.
Conclusions
There have been few reports of patients with concurrent COVID-19 and AIHA and the association remains to be elucidated. This serves as a reminder for clinicians that when encountering unexplained anemia in patients with a history of COVID-19, AIHA should be considered. It also highlights that treating the cause of secondary AIHA can improve patient condition without the need for steroids. More studies need to be done to establish the relationship between COVID-19 and AIHA.
Acknowledgments
We want to thank the patient and her family for allowing us to share her valuable details and making this article possible.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained or waived by all participants in this study
References
- 1.COVID-19 and multiorgan failure: a narrative review on potential mechanisms. Mokhtari T, Hassani F, Ghaffari N, Ebrahimi B, Yarahmadi A, Hassanzadeh G. J Mol Histol. 2020;51:613–628. doi: 10.1007/s10735-020-09915-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Autoimmune hemolytic anemia. Liebman HA, Weitz IC. Med Clin North Am. 2017;101:351–359. doi: 10.1016/j.mcna.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 3.New insights in the pathogenesis of autoimmune hemolytic anemia. Barcellini W. Transfus Med Hemother. 2015;42:287–293. doi: 10.1159/000439002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.COVID-19 associated with severe autoimmune hemolytic anemia. Jacobs J, Eichbaum Q. Transfusion. 2021;61:635–640. doi: 10.1111/trf.16226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Autoimmune haemolytic anaemia associated with COVID-19 infection. Lazarian G, Quinquenel A, Bellal M, et al. Br J Haematol. 2020;190:29–31. doi: 10.1111/bjh.16794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.SARS-CoV-2 and autoimmune cytopenia. Quinn R, Murakhovskaya I. Hemato. 2021;2:463–476. [Google Scholar]
- 7.Severe autoimmune hemolytic anemia in Covid-19 infection: autoimmune hemolytic anemia in Covid-19. Hindilerden F, Yonal-Hindilerden I, Akar E, Yesilbag Z, Kart-Yasar K. Mediterr J Hematol Infect Dis. 2020;12:0. doi: 10.4084/MJHID.2020.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Virus-induced autoimmune disease. von Herrath MG, Oldstone MB. Curr Opin Immunol. 1996;8:878–885. doi: 10.1016/S0952-7915(96)80019-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clinical characteristics of coronavirus disease 2019 in China. Guan WJ, Ni ZY, Hu Y, et al. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Incidence of acute kidney injury in COVID-19 infection: a systematic review and meta-analysis. Chen YT, Shao SC, Hsu CK, Wu IW, Hung MJ, Chen YC. Crit Care. 2020;24:346. doi: 10.1186/s13054-020-03009-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.COVID-19-associated acute kidney injury: consensus report of the 25th Acute Disease Quality Initiative (ADQI) Workgroup. Nadim MK, Forni LG, Mehta RL, et al. Nat Rev Nephrol. 2020;16:747–764. doi: 10.1038/s41581-020-00356-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Isolated severe thrombocytopenia in a patient with COVID-19: a case report. Sadr S, SeyedAlinaghi S, Ghiasvand F, Hassan Nezhad M, Javadian N, Hossienzade R, Jafari F. IDCases. 2020;21:0. doi: 10.1016/j.idcr.2020.e00820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Despotovic JM. New York City: Springer Publishing; 2018. Immune Hematology. Diagnosis and Management of Autoimmune Cytopenias. [Google Scholar]