Abstract
Background:
A systemic proinflammatory state plays a central role in the development of heart failure with preserved ejection fraction (HFpEF). Low-level transcutaneous vagus nerve stimulation (LLTS) suppresses inflammation in animals and humans, mediated by an alpha7-nicotinic acetylcholine receptor-dependent pathway. We examined the effects of LLTS on cardiac function, inflammation and fibrosis in the presence of alpha7-nicotinic acetylcholine receptor pharmacological blockade in a rat model of HFpEF.
Methods:
Dahl salt-sensitive (DS) rats at 7 weeks of age were treated with high salt diet for 6 weeks to induce HFpEF, followed by 4 weeks of a) LLTS, b) LLTS with the alpha7-nicotinic acetylcholine receptor blocker methyllycaconitine (MLA), c) sham and d) Olmesartan. Blood pressure, cardiac function by echocardiography, heart rate variability and serum cytokines were measured at 13 and 17 weeks of age. Cardiac fibrosis, inflammatory cell infiltration and gene expression were determined at 17 weeks.
Results:
LLTS attenuated the increase in blood pressure, improved cardiac function, decreased inflammatory cytokines, macrophage infiltration and fibrosis, and improved survival compared to other groups. MLA attenuated these effects, while Olmesartan did not improve cardiac function or fibrosis despite maintaining similar blood pressure as LLTS. Heart rate variability was similarly improved in the LLTS and LLTS plus MLA groups, but remained low in the other groups. LLTS reversed the dysregulated inflammatory signaling pathways in HFpEF hearts.
Conclusion:
Neuromodulation with LLTS improved cardiac function in a rat model of HFpEF through its anti-inflammatory and anti-fibrotic effects. These results provide the basis for further clinical trials in humans.
Keywords: heart failure with preserved ejection fraction, neuromodulation, inflammation, fibrosis
Introduction
Heart failure with preserved ejection fraction (HFpEF) remains one of the most vexing cardiovascular conditions 1, 2. Recent animal and human studies suggest that a systemic proinflammatory state, produced by comorbidities, including diabetes, obesity and aging, plays a central role in the development of HFpEF 3. Therefore, attenuating the proinflammatory state is a promising therapeutic target for HFpEF.
Vagus nerve stimulation exerts prominent anti-inflammatory effects in multiple experimental models of systemic inflammation and sepsis 4, 5, as well as in humans with rheumatoid arthritis 6 and atrial fibrillation undergoing cardiac surgery 7. Moreover, low-level transcutaneous vagus nerve stimulation (LLTS), delivered at the tragus of the ear, where the auricular branch of the vagus nerve is located 8, for just one hour, significantly suppressed atrial fibrillation and decreased systemic inflammatory cytokines in clinical trials 9, 10. Importantly, we have recently shown that LLTS ameliorated diastolic dysfunction in a well-established rat model of HFpEF 11, but the exact mechanism has not been yet elucidated. In this translational study, we hypothesized that the anti-inflammatory effects of LLTS, which are mediated through an alpha7 nicotinic acetylcholine receptor (α7nAchR)-dependent pathway, 5, 12 underlie the improvement in cardiac function and fibrosis. Therefore, we examined the effects of LLTS on cardiac function, inflammation and fibrosis in the presence of pharmacological blockade of the α7nAchR in Dahl salt-sensitive (DS) rats with HFpEF.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Experimental design
All animals were obtained from Charles River Laboratories (Wilmington, MA) and the protocol was approved by the University of Oklahoma Health Sciences Center Institutional Animal Care and Use Committee. DS rats of both sexes were used in this study. The experimental protocol is summarized in Figure 1A. Animals were fed a 0.3% NaCl [low-salt (LS)] diet until 7 weeks of age, followed by 8% NaCl [high-salt (HS)] diet for 6 weeks, to induce HFpEF 13. Water was provided at libitum. At 13 weeks of age, rats on HS diet were randomized into 4 groups: 1) Active LLTS (n=40; 50% female); 2) Active LLTS plus methyllaconitine (MLA) (5mg/kg i.p. daily), a selective blocker of the α7nAChR, which mediates the anti-inflammatory effects of LLTS 5, 12 (n=36, 50% female); 3) Sham (n=48, 50% female) and 4) Antihypertensive treatment with olmesartan (1mg/kg daily via oral gavage 14; n=18, 50% female). The latter group was included to decipher whether the LLTS effect on cardiac function and fibrosis was due to its blood pressure-lowering effect 11. Rats treated with LS diet for the total duration of the study represented the control group (n=24, 50% female). Electrical stimulation was delivered through a transcutaneous electrical nerve stimulation device (InTENSity™ Twin StimR, Current Solutions LLC, Austin, TX), for 30 minutes daily under 2% isoflurane anesthesia, using the same parameters as in our previous study (20 Hz frequency, 0.2 ms pulse duration, 3 mA amplitude) 11. In the active LLTS group, the electrodes were placed over the auricular concha region with cathode inside and anode outside. In the sham group, electrodes were placed over the tip of auricular margin, as previously described 11. During active, but not sham stimulation, LLTS resulted in a modest, yet statistically significant drop in heart rate, which was dose dependent (baseline: 393 ± 24 bpm vs. active LLTS 3mA: 382 ± 26 bpm; vs. active LLTS 6mA 373 ± 27 bpm p<0.001), suggesting that activation of vagal afferents induced reflex changes in the cardiac hierarchy that includes increased vagal efferent activity 15 (Figure 1B, C).
Figure 1.
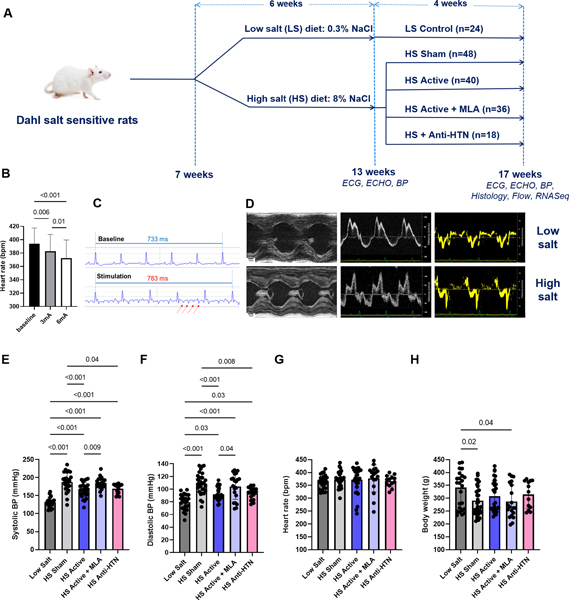
Study design, baseline data and effect of treatment on vital signs. A. Schematic of the study design, group assignment and endpoints. B. Changes in heart rate before and during LLTS at baseline (13 weeks), at different stimulation intensities (n=12). C. Representative ECG tracings from an animal at baseline and during active LLTS at 3mA, indicating a modest prolongation of the RR interval during active stimulation. Arrows indicate the stimulation artifact. D. Representative examples of echocardiographic images at 13 weeks from an animal treated with low salt diet compared to a high salt diet-treated animal, indicating development of left ventricular hypertrophy and diastolic dysfunction in the latter group. E. Systolic blood pressure (BP). F. Diastolic BP. G. Heart rate. H. Body weight. N=12–34 per group. LS = low salt. HS = high salt. Anti-HTN = antihypertensive (Olmesartan). MLA = methyllycaconitine. ECG = electrocardiogram. ECHO = echocardiogram. BP = blood pressure.
Outcome measures
Blood pressure at 13 and 17 weeks was measured non-invasively using the tail-cuff method (PowerLab Data Acquisition System; ADInstruments, Bella Vista, NSW, Australia). Needle electrodes were inserted under the skin of the four limbs of the animals to obtain six-lead ECG recordings (iWorx Systems, Dover, NH). Heart rate was calculated before and during LLTS by averaging 20 cycle lengths on the ECG recording. Echocardiography was performed using a Vevo 3100 system (Visual Sonics, Toronto, ON) equipped with a 25MHz linear array transducer, under 2% isoflurane anesthesia at 13 and 17 weeks to assess cardiac function. Briefly, 2-dimensional parasternal long and short axis, as well as apical 4-chamber left ventricular (LV) images were obtained. Systolic function was determined by measuring LV ejection fraction from the parasternal short axis view as described previously 11, 16. Pulse-wave Doppler spectra of mitral inflow and mitral annulus tissue Doppler spectra were recorded from the apical 4-chamber view. The early diastolic mitral annulus velocity (e’), a sensitive marker for diastolic dysfunction 17, the ratio of early to late mitral inflow Doppler velocity (E/A ratio), a marker of LV diastolic relaxation and the ratio of the early mitral inflow Doppler velocity to the early diastolic mitral annulus velocity (E/e’ ratio), which correlates with LV filling pressures 17, were measured. LV circumferential strain, a sensitive marker of LV systolic function 18, was obtained off line using a speckle-tracking algorithm (Vevo strain). We only analyzed circumferential strain due to the poor reproducibility of apical long axis views in rodents, which would render longitudinal strain inaccurate 19. The echocardiographic analysis was performed in a blinded fashion to ensure rigor and avoid bias.
To assess heart rate variability (HRV), a surrogate marker of autonomic tone 20, 3-minute ECG recordings (sampling rate 1000 Hz) were performed at 13 and 17 weeks, using the iWorx data recorder (iWorx Systems Inc., Dover, NH), under isoflurane anesthesia. To minimize the effect of circadian rhythms on HRV, all ECG recordings were performed between 9 and 11 am. Frequency domain HRV parameters using the FFT method [low frequency (LF; 0.2–0.8 Hz), high frequency (HF; 0.8–2.5 Hz), and LF/HF ratio], and nonlinear measures (SD1, SD2 and SD2/SD1 ratio from Poincare plots) 16, 21 were calculated in a blinded fashion, using the Kubios software (Kubios HRV Premium 3.3.1, Kuopio, Finland). In addition, we used the symbolic dynamics method 22 to assess HRV. This method converts the RR intervals into a sequence of symbols, by dividing the full range of values into six equal levels, and evaluates the dynamics of each three consecutive symbols, which are classified according to their variation pattern: zero variation (0V – all symbols are equal), one variation (1V – sequences with two consecutive equal symbols and one different), two like variations (2LV – three different symbols but with the same variations direction, i.e. in ascending or descending order) or two unlike variations (2UV – sequences that form a peak or a valley, i.e. with two different variations, in opposite directions). The percentage of patterns classified in each family is then used for analysis. Importantly, the incidence of 1V and 2UV patterns is related to sympathetic and vagal modulation, respectively 22.
To assess serum cytokines, approximately 0.5 ml of blood was collected from the tail vein of each animal at 13 and 17 weeks. Serum was saved frozen at −80° C and processed in batches of 18–20. Tumor necrosis factor (TNF)-α, monocyte chemoattractant protein (MCP)-1, osteopontin and transforming growth factor (TGF)-β were measured using a commercially available immunoassay (Ella Automated Immunoassay system; R&D Systems, Minneapolis, MN). All immunoassays were run in triplicate and read according to manufacturer’s instructions, in a blinded fashion.
Animals were monitored twice daily for signs and symptoms of heart failure, including distress, decreased mobility, labored breathing, body edema and cachexia. A scoring system, which incorporates these parameters was used to quantify the development of heart failure, as previously described 16, 21. In brief, each of the 5 parameters (appearance, breathing, mobility, edema and weight) was assigned a score of 0 (normal), 1 (mildly abnormal) or 2 (severely abnormal) and the composite score was calculated by adding the individual scores in each category 16, 21.
At the end of the experiment (week 17), LV tissue was harvested, immediately fixed in 4% formalin, and embedded in paraffin. Paraffin blocks were cut into 5 μm sections and stained with hematoxylin/eosin to assess myocardial inflammation and Masson’s trichrome stain to determine cardiac fibrosis. Collagen (fibrosis) stains blue, whereas cytoplasm and cardiac muscle stain red. The stained slides were imaged at 20x magnification and the percentage of fibrosis was quantified as the collagen-positive tissue of the total stained LV tissue using the Bioquant software (BIOQUANT Image Analysis, Nashville, TN), as previously described 11. A semi-quantitative score was used to assess myocardial inflammation (0: no infiltrate; 1+, infiltrates involving <25% myocardium; 2+, infiltrates involving 25%–50%; 3+, infiltrates involving 50%–75% of the myocardium; and 4+, infiltrates involving 75%–100% of the myocardium), as previously described 23. The investigators performing all the above measurements and data analysis were blinded to group assignment to avoid bias.
Isolation of single cells and flow cytometry
Left ventricular tissue was first minced using a sterile razor blade, then digested for 1.5 hours at 37˚C with the following combination of enzymes: Collagenase XI (2500U/g of tissue), DNase I (2000U/g of tissue), Hyaluronidase (2600U/g of tissue) (Millipore Sigma, St. Louis, MO) and Collagenase I (1200U/g of tissue) (Worthington Biochemical Corporation, Lakewood, NJ). The suspension was passed through a 100 μm filter and any remaining tissue was pressed through the filter and washed with Phosphate-buffered saline. Cells were first stained with Zombie Red fixable viability dye (Biolegend, San Diego, CA) according to the manufacturer protocol, followed by surface marker staining for 20 minutes at room temperature. At least 2 million cells were stained per sample. Cells were fixed and permeabilized using the Leucoperm reagent kit (Bio-Rad, Hercules, CA) according to the manufacturer protocol and stained for intracellular markers. T cells were stained with anti-rat CD45 (clone Ox-1), CD3 (clone IF4), CD4 (clone W3/25), and CD8 (clone G28) (Biolegend, San Diego, CA). Macrophages were stained with anti-rat CD45 (clone Ox-1), CD86 (clone 24F) (Biolegend, San Diego, CA), CD163 (clone ED2), and CD68 (clone ED1) (Bio-Rad, Hercules, CA). Stained cells were analyzed using the Stratedigm S1200Ex flow cytometer (Stratedigm, Inc., San Jose, CA), and analyzed using FlowJo Version 10.4.1 (FlowJo, LLC, Ashland, OR).
Transcriptomic analysis
We performed transcriptomic profiling of hearts from LS rats, as well as HS sham, HS active and HS active + MLA rats (n=5–6/group). Briefly, RNA was isolated from homogenized LV samples using RNeasy Mini Kit and QIAzol Lysis Reagent (Qiagen Sciences Inc., Germantown, MD), according to manufacturer’s instructions. RNA-seq analysis was performed using an Illumina NovSeq 6000 system at the Laboratory for Molecular Biology and Cytometry Research at the University of Oklahoma Health Sciences Center. RNA-seq libraries were constructed using NEBNext Ultra II Library Prep Kit (New England Biolabs, Inc., Ipswich, MA) according to the established protocols. The RNA-Seq data were aligned to the reference genome (rattus norvegicus rn6) using HISAT2 software. The transcripts were filtered (at least 0.5 cpm present in each sample per group), yielding a total of 12,979 transcripts, and normalized for library depth 24. Differential expression gene lists were created with a 1.5-fold change cutoff and false discovery rate (FDR) <0.05 to identify genes that were upregulated or downregulated under each condition using the limma-voom procedure 25 in Partek Flow (Partek, Inc., Saint Louis, MO). Principal component analysis and unsupervised hierarchical clustering (Euclidean distance, average linkage) was performed using Partek Flow (Partek, Inc., Saint Louis, MO). KEGG pathway analysis was performed on genes with greater than 1.5-fold change in expression level between experimental groups at FDR <0.05 (Benjamini-Hochberg correction procedure).
Statistical Analysis
Categorical data are presented as percentages and continuous data as mean ± standard deviation or median (range), as applicable. Categorical data were compared between groups using Fisher’s exact test. Continuous data were compared between groups using repeated measures analysis of variance (ANOVA) or one-way ANOVA, as applicable. All pair-wise testing was adjusted for multiple comparisons by Tukey’s method. Logarithmic transformation was used as appropriate to satisfy modeling assumptions. The effect of sex as a biological variable was assessed by introducing a sex by group interaction in all the ANOVA models. The interaction was dropped from the model if it did not reach statistical significance. Statistical significance was set at p<0.05. Survival was calculated using the Kaplan-Meier method and groups were compared using the log-rank test. Statistical significance was declared at p<0.05. All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
Results
After 6 weeks of HS diet, animals developed hypertension, LV hypertrophy and diastolic dysfunction (Figure 1D), as documented by decreased e’ velocity, reversal of E/A ratio and elevated E/e’ ratio (Table 1). No animal showed a reduced LV ejection fraction at any time point during the study. The HS animals developed signs of heart failure, including mild to moderate distress, edema, decreased mobility and tachypnea. The heart failure composite score, which incorporates all these parameters 16, 21, was significantly higher in the HS group compared to the LS group (3.0 ± 1.0 vs. 0; p <0.001), indicating the development of compensated HFpEF.
Table 1.
Baseline (13 week) characteristics of rats treated with high salt and low salt diets for 6 weeks
| Variable | Low salt (n=24) | High salt (n=142) | P value |
|---|---|---|---|
| Systolic blood pressure (mmHg) | 127.6±23.4 | 174.5±24.9 | <0.0001 |
| Diastolic blood pressure (mmHg) | 77.8±14.8 | 109.0±18.5 | <0.0001 |
| Heart rate (bpm) | 323.6±87.6 | 329.2±73.2 | 0.67 |
| Body weight (g) | 325.0±70.3 | 307.4±67.8 | 0.14 |
| LV ejection fraction (%) | 76.8±5.1 | 76.9±4.4 | 0.99 |
| Interventricular septum diameter (mm) | 1.8±0.3 | 2.2±0.4 | <0.0001 |
| E/A | 1.6±0.3 | 1.1±0.5 | 0.01 |
| Mitral annular velocity (e’, cm/s) | 5.4±1.1 | 4.6±0.9 | 0.004 |
| E/e’ | 17.5±4.6 | 20.6±5.1 | 0.009 |
E/A = ratio of early to late mitral inflow Doppler velocity
E/e’ = ratio of the early mitral inflow Doppler velocity to the early diastolic mitral annulus velocity
After 4 weeks of treatment, LLTS significantly attenuated the blood pressure elevation in the HS active group compared to the HS sham group (169.3±12.9 vs. 188.3±12.8mmHg, respectively; p<0.001), while this effect was blocked by MLA (184.3±13.6mmHg; Figure 2A, B). Within each group, blood pressure was lower in female rats compared to male rats, but there was no sex by group interaction. Olmesartan decreased blood pressure to a similar degree as LLTS (171.1±15.0 vs. 169.3±12.9, respectively; p=0.98). There was no effect of treatment on heart rate (373.6±27.4 bpm vs. 372.1±27.4 bpm vs. 377.9±29.5 bpm vs. 366.5±27.6 bpm in HS sham, HS active HS active+MLA and HS anti-HTN groups, respectively; p=0.92) or body weight (290.5±25.4 bpm vs. 307.6±29.0 bpm vs. 287.1±32.2 bpm vs. 314.4±31.5 bpm in HS sham, HS active HS active+MLA and HS anti-HTN groups, respectively; p=0.42) (Figure 1E-H).
Figure 2.
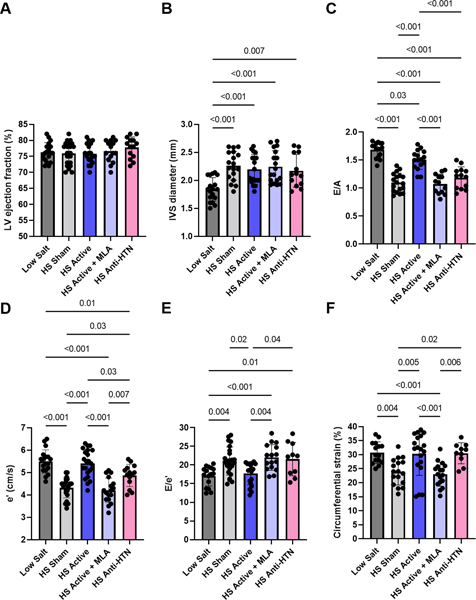
Effect of treatment on echocardiographic parameters. A. Left ventricular (LV) ejection fraction. B. Interventricular septum (IVS) diameter. C. Ratio of the early to late mitral inflow Doppler velocity (E/A). D. Early diastolic mitral annulus Doppler velocity (e’). E. Ratio of the early mitral inflow Doppler velocity to the early diastolic mitral annulus velocity (E/e’). F. Circumferential strain (absolute values are plotted). N=10–24 per group. LS = low salt. HS = high salt. Anti-HTN = antihypertensive (Olmesartan). MLA = methyllycaconitine.
While there was no effect of LLTS on LV ejection fraction or LV hypertrophy (Figure 2A, B), echocardiographic markers of diastolic dysfunction, including E/A ratio(1.5±0.2 vs. 1.2±0.2, respectively; p<0.001), e’ velocity(5.4±0.2 vs. 4.5±0.2 cm/s, respectively; p<0.001), and E/e’ ratio (17.8±3.0 vs. 20.3±3.0, respectively; p<0.001) were significantly improved in the active group compared to the sham group (Figure 2C, D, E). This effect was reversed in the presence of MLA(1.1±0.3, 4.2±0.2 cm/s and 21.9±3.3, respectively; p<0.001 vs. LLTS for each of E/A, e’ and E/e’), while Olmesartan failed to induce similar changes despite similar reductions in blood pressure (1.2±0.3, 4.9±0.3 cm/s and 20.5±3.0, respectively; p<0.001 vs. LLTS for each of E/A, e’ and E/e’). There was no significant sex by group interaction in any other above parameters; therefore, we report the combined results for both sexes. Circumferential strain, a subtle marker of LV systolic dysfunction 18, was improved by both LLTS and Olmesartan, compared to the other two HS groups (Figure 2F), consistent with the well-characterized beneficial effects of angiotensin receptor blockers on systolic dysfunction 26. Similarly, inflammatory cytokines, including TNF-α, MCP-1, osteopontin and TGF-β were decreased in the active group, but not in the sham group, while MLA attenuated the effect of LLTS (Figure 3A-D). Olmesartan decreased osteopontin and TGF-β, but not TNF-α or MCP-1, consistent with previous reports 27.
Figure 3.
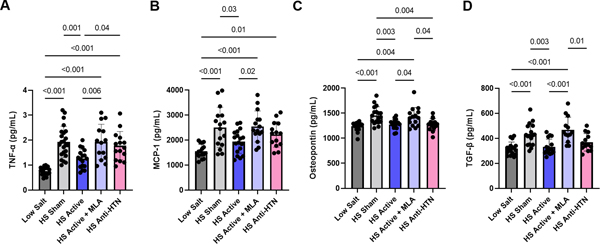
Effect of treatment on serum inflammatory cytokines. A. Tumor necrosis factor (TNF)-α. B. Monocyte chemoattractant protein (MCP)-1. C. Osteopontin. D. Transforming growth factor (TGF)-β. N=12–24 per group. LS = low salt. HS = high salt. Anti-HTN = antihypertensive (Olmesartan). MLA = methyllycaconitine.
Autonomic tone, as assessed by HRV, was restored to the control levels in animals treated with LLTS, even in the presence of MLA (Figure 4). Both LF and LF/HF ratio, as well as SD1 and SD1/SD2 ratio were reduced with LLTS, suggesting an anti-adrenergic effect 20. On the contrary, animals treated with Olmesartan showed altered HRV parameters, similar to HS sham animals (Figure 4A, B). Symbolic dynamics analysis demonstrated that LLTS reduced the occurrence of 0V and 1V patterns, compared to sham, and increased the incidence of 2UV, suggesting sympathetic inhibition and parasympathetic enhancement, respectively 22 (Figure 4C). MLA did not block this effect, while Olmesartan resulted in similar parameters to the sham group.
Figure 4.
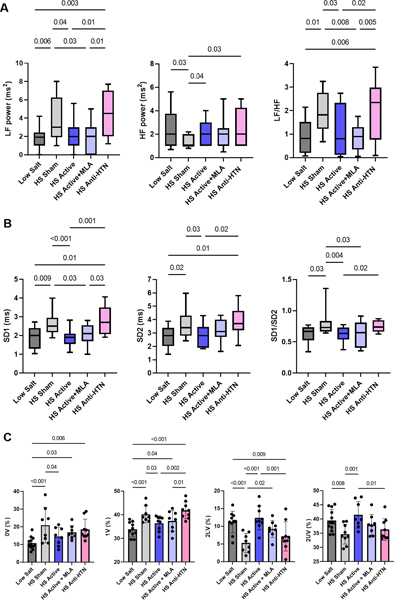
Effect of treatment on heart rate variability. A. Frequency domain analysis using the FFT method (LF: low frequency. HF: high frequency. LF/HF: low frequency to high frequency ratio). B. Nonlinear analysis from Poincare plots [SD1: the standard deviation of the instantaneous beat-to-beat RR interval variability (minor axis of the ellipse), SD2: the standard deviation of the continuous long-term RR interval variability (major axis of the ellipse), SD1/SD2: the ratio of SD1 to SD2]. Logarithmic transformation was performed to satisfy the modeling assumptions. Box represents median values with interquartile intervals and whiskers represent 10th and 90th percentiles. N=10–24 per group. C. Symbolic dynamics analysis (0V: zero variation family; 1V: one variation family; 2LV: two like variations family; 2UV: two unlike variations family). N=8–12 per group. LS = low salt. HS = high salt. Anti-HTN = antihypertensive (Olmesartan). MLA = methyllycaconitine.
At 17 weeks of age, heart weight (normalized to tibia length) was lower in the active and Olmesartan groups compared to sham and active plus MLA groups (Figure 5A). However, despite similar reductions in heart weight, Olmesartan failed to decrease fibrosis (perivascular and interstitial combined) to a similar extent as LLTS (2.0±0.3% vs. 0.9±0.2%, respectively; p=0.02), suggesting that LLTS-induced decrease in inflammation accounted for the amelioration of cardiac fibrosis, independent of the blood pressure lowering effect (Figure 5A, D, E). MLA attenuated the effect of LLTS (2.3±0.4% vs. 0.9±0.2%, respectively; p=0.008), resulting in similar fibrosis as sham stimulation (2.3±0.4% vs. 2.4±0.4%, respectively; p=0.99), suggesting that the anti-fibrotic effect of LLTS was mediated by an α7nAchR-dependent pathway.
Figure 5.
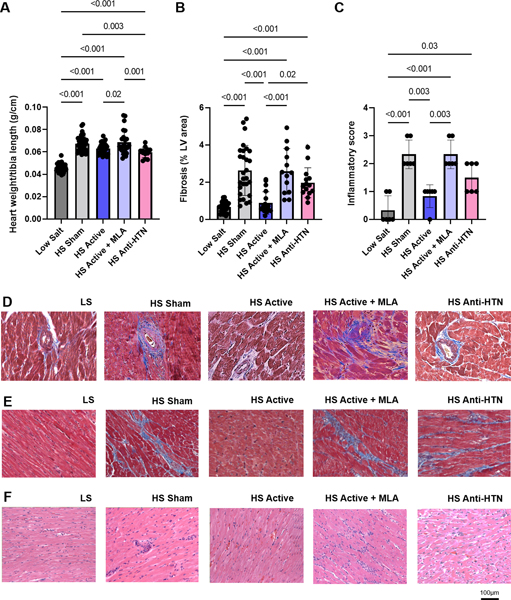
Effect of treatment on heart weight, fibrosis and inflammatory infiltration. A. Heart weight normalized to tibia length (N=13–34 per group). B. Fibrosis area (as a percent of the left ventricular area) (N=12–24 per group). C. Inflammatory score (N=6 per group). D. Representative examples of histological images from animals from each group stained with Masson’s trichrome showing perivascular fibrosis. E. Representative examples of histological images from animals from each group stained with Masson’s trichrome showing interstitial fibrosis. F. Representative examples of histological images from animals from each group stained with hematoxylin and eosin. LS = low salt. HS = high salt. Anti-HTN = antihypertensive (Olmesartan). MLA = methyllycaconitine.
Inflammatory infiltration of the myocardium was significantly higher (p=0.001) in sham hearts compared with LLTS hearts. Similarly, the inflammatory score 23 was significantly (p=0.01) higher in MLA hearts when compared with LLTS hearts, while the effect of Olmesartan was intermediate between that of LLTS and sham (Figure 5B, F). Flow cytometry indicated that the number of macrophages was increased in LV tissue in the sham group compared to the LS group, consistent with previous reports 28. Treatment with LLTS restored the number of CD68+ macrophages to that of LS, while MLA attenuated this effect (Figure 6), without affecting the number of T cells or granulocytes.
Figure 6.
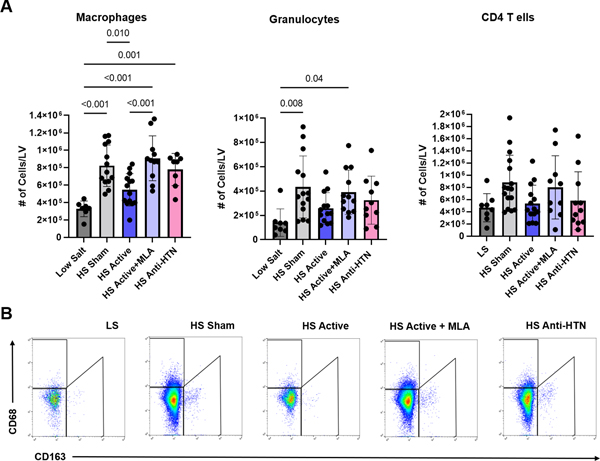
Flow cytometry from left ventricular (LV) tissue isolated at endpoint. A. Effect of treatment on macrophages, granulocytes and CD4 T cells (N=8–12 per group). B. Representative examples of flow cytometry analysis of macrophage subsets in the hearts of animals from each group. LS = low salt. HS = high salt. Anti-HTN = antihypertensive (Olmesartan). MLA = methyllycaconitine.
During the course of the study, 13 sham (27%), 6 active (15%), 12 active plus MLA (33%) and 5 Olmesartan (28%) animals died (Figure 7). Thus, survival was significantly better in the active group compared to the rest of the HS-induced HFpEF groups (p=0.02 vs. all other HFpEF groups combined; Figure 7). To assess whether the addition of MLA had any untoward effects on mortality, we treated 5 LS rats (3 males and 2 females) with MLA alone starting at week 13. None of the animals died, suggesting that MLA (and/or α7nAChR blockade) and did not have any untoward effects on mortality and its effect was attributed predominantly to reversal of the effect of LLTS. As in our previous study, female rats had a significantly lower mortality rate compared to male rats, across all treatment groups (11% vs. 39%, p=0.0002), with no significant sex by group interaction.
Figure 7.
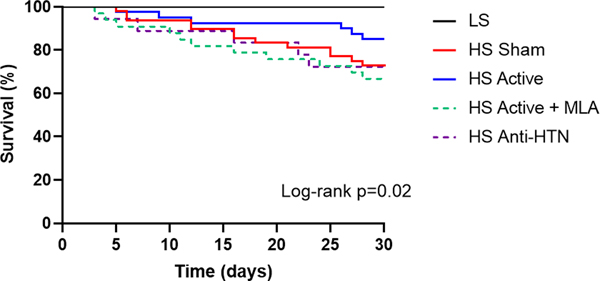
Effect of treatment on survival. Kaplan-Meier curve of survival according to treatment group. For this analysis, time 0 was considered when the mice were 13 weeks old. Survival was significantly better in the HS active group compared to all other HS groups. LS = low salt. HS = high salt. Anti-HTN = antihypertensive (Olmesartan). MLA = methyllycaconitine.
To explore potential mechanisms for the differences in phenotype between groups, we performed transcriptional profiling at 17 weeks. As shown in Figure 8A, 1058 and 931 transcripts were differentially expressed in HS sham compared to LS hearts, and HS active compared to HS sham hearts, respectively, such that LLTS normalized the expression of 656 transcripts. Comparing HS active plus MLA heart with HS active hearts (Figure 8B), revealed 597 transcripts were differentially expressed, whereas MLA reversed the expression of 357 transcripts. The hierarchical cluster analysis plot (Figure 8C) and the principal component analysis plot (Figure 8D) indicate that LLTS nearly normalized the transcriptional profile of these rats, whereas the transcriptional profile of the HS active plus MLA hearts was intermediate between HS sham hearts and HS active hearts, suggesting that MLA treatment resulted in partial reversal of the effects of LLTS. KEGG pathways analysis (Figure 8E) on the 656 genes that were normalized (relative fold change ≥1.5, FDR < 0.05) in the LLTS-treated hearts compared to sham, indicated that the majority of these were members of inflammatory and profibrotic signaling pathways.
Figure 8.
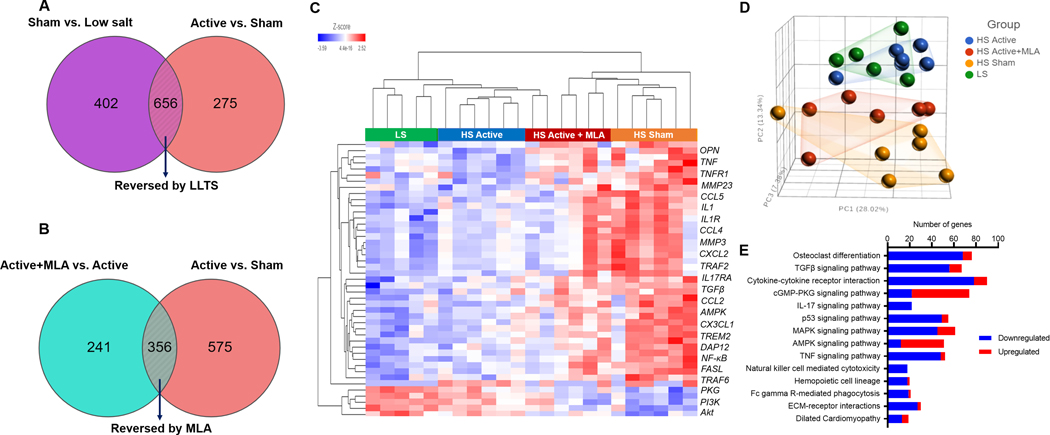
Effect of treatment of left ventricular transcriptional profile. A. Venn diagram showing the number of transcripts that were differentially expressed in high salt sham vs. low salt hearts and were reversed by treatment with low level transcutaneous vagus nerve stimulation (LLTS). In the lower panel, Venn diagram of the number of transcripts that were differentially expressed in high salt sham vs. high salt active hearts and were reversed by treatment with methyllycaconitine (MLA). B. Heat map showing differentially expressed genes in each treatment group. C. Principal component analysis of changes in gene expression in hearts from different treatment groups. D. KEGG pathway analysis of the genes (n=656) that were normalized in the LLTS-treated hearts compared to sham-treated hearts. N=5–6 per group.
Discussion
In this translational study, we showed that LLTS ameliorated diastolic dysfunction, decreased inflammatory cytokines, LV inflammatory infiltration and LV fibrosis, and improved survival in a well-established rat model of HFpEF through its anti-inflammatory properties, as evidenced by attenuation of these effects with pharmacological blockade of the α7nAchR. Moreover, transcriptional profiling using RNAseq revealed that treatment with LLTS attenuated the dysregulated proinflammatory and profibrotic signaling pathways in HFpEF hearts. Notably, this effect did not merely result from a decrease in blood pressure, since treatment with Olmesartan failed to reverse the cardiac phenotype or fibrosis despite maintaining similar blood pressure as LLTS. In contrast to previous studies showing that treatment with angiotensin receptor blockers improved cardiac function in DS rats, when started at 7 weeks, before cardiac remodeling occurred 29, 30, we started Olmesartan at 13 weeks, which may explain why we did not observe a significant effect on diastolic parameters or cardiac fibrosis, despite similar decrease in blood pressure compared to LLTS. Importantly, these results support the inflammatory paradigm for the pathogenesis of HFpEF 3 and suggest that inflammation and fibrosis are not only causative in HFpEF, but also potentially reversible. Our results provide mechanistic insights into the effects of LLTS in HFpEF and form the basis for the design of clinical trials to evaluate the effect of LLTS on clinical outcomes in patients with HFpEF. Notably, in a recent pilot randomized clinical trial, LLTS over 3 months improved global longitudinal strain, inflammatory cytokines, and quality of life in a selected group of patients with HFpEF 31.
The effect of LLTS on blood pressure is consistent with recent evidence suggesting that an α7nAChR-dependent cholinergic pathway is recruited by hypertensive stimuli to contribute to blood pressure regulation 32. Attenuation of the LLTS effect on blood pressure with the α7nAchR blocker MLA in our study is consistent with these results, and highlights the important role of hemodynamic load-induced proinflammatory signaling in mediating myocardial inflammation and fibrosis, eventually leading to HFpEF 3. Notably, hypertension carries the highest population attributable risk for heart failure in the general population and a more than two-fold increase in the risk for HFpEF vs. heart failure with reduced ejection fraction 33. This finding should be contrasted with recent human data, where no effect on blood pressure was observed with chronic LLTS in a cohort of patients with atrial fibrillation (who are at risk for HFpEF) 10, likely because these patients had established, long-standing myocardial and renal changes, beyond the point of no return. Consistent of this notion, the effectiveness of LLTS on blood pressure and cardiac hypertrophy was not as prominent as in our previous study, which examined the effects of LLTS in a milder phenotype of HFpEF 11. Collectively, these data suggest that targeting inflammation and cardiac fibrosis at an earliest stage of the disease is more likely to reverse hemodynamic load-induced adverse cardiac remodeling and improve the phenotype of HFpEF.
Our results corroborate recent animal and human studies showing that systemic and myocardial inflammation play a central role in the development of diastolic dysfunction and HFpEF 3, 28. Importantly, we showed that treatment with LLTS significantly altered the immune cell profile of the myocardium. This finding likely involves direct or indirect effects on tissue-resident and infiltrating macrophages, leading to a reduction in LV inflammation and fibrosis, as well as triggering tissue repair mechanisms 28, 34. Moreover, these data support the hypothesis that inhibiting the anti-inflammatory effect of LLTS attenuates the anti-fibrotic effect. The importance of these findings is highlighted by studies showing that elevated inflammatory cytokines and endothelial adhesion molecules predicted, respectively, the development of incident HFpEF 35 and incipient HFpEF-related LV dysfunction, as evidenced by depressed LV strain 36. Notably, secretion of osteopontin and TGF-β (both of which were decreased by LLTS) from cardiac macrophages activates fibroblasts to produce collagen resulting in cardiac fibrosis and increased cardiac stiffness 28. Nonetheless, it should be noted that there are multiple mechanisms that control myocardial stiffness, the substrate of diastolic dysfunction, including titin hypophosphorylation and deficient unfolding protein response, which in turn is caused by systemic inflammation triggering expression of inducible nitric oxide synthase in cardiomyocytes 3. The degree to which each of these mechanisms contributes to diastolic dysfunction in the individual patient remains unknown. Moreover, fibrosis is a highly dynamic process and intervention at the early rather than later stages of the disease may be beneficial 3. As such, relatively early intervention during the course of the disease was effective in ameliorating diastolic dysfunction in our study. Future studies may focus on identifying biomarkers that correlate with irreversible myocardial changes, beyond which interventions, including LLTS, are unlikely to prove beneficial.
Autonomic dysfunction, with sympathetic predominance is present in HFpEF 37. However, it remains unclear whether increased sympathetic activity results in HFpEF, or is secondary to HFpEF 37. In our study, blockade of the α7nAchR with MLA attenuated the beneficial effect of LLTS on diastolic dysfunction, but not the effect on autonomic dysfunction, assessed by HRV, consistent with consistent with previous studies suggesting that α7nAchR is not required for parasympathetic control of the heart 38. On the other hand, Olmesartan had no effect on HRV. It is possible that the timing of initiation of Olmesartan may have attenuated its beneficial effects on HRV. Notably, in previous studies showing a sympathoinhibitory effect of angiotensin receptor blockers, these were administered either concurrently with 39 or even before the injury 40, whereas in our study, we initiated Olmesartan well after the establishment of HFpEF. Nonetheless, ameliorating sympathetic hyperactivity with other means, such as renal artery denervation, may improve the clinical phenotype in hypertensive patients with HFpEF, through different mechanisms, including reduced aortic stiffness and improved ventriculoarterial coupling 41. These findings support the notion that HFpEF is a heterogenous disease with distinct phenotypes, with differential response to therapy 42, 43. Further studies are required to elucidate the downstream signaling pathways of the pleiotropic effects of LLTS, including sympathoinhibition, which in turn may lead to identification of novel therapeutic targets.
Our results are consistent with the well-characterized anti-inflammatory effects of vagus nerve stimulation, which are mediated through the cholinergic anti-inflammatory pathway 4, 5. The finding that pharmacological inhibition of the α7nAchR was sufficient to reverse the cardiac phenotype induced by HS diet, lends credence to this notion. Notably, the anti-inflammatory effects of LLTS are characterized by a prominent memory effect, whereby short periods of stimulation lead to a long-lasting effect 6, 10. The findings of our studies clearly illustrate this property and support the premise of providing short periods of stimulation to ameliorate cardiac function and clinical outcomes. It should be noted, however, that the minimum duration of LLTS needed to achieve an improvement in relevant clinical outcomes remains unknown. Nonetheless, we cannot exclude an immunomodulatory role of LLTS on the non-neuronal cholinergic myocardial signaling pathway, which has been shown to decrease proinflammatory monocyte recruitment in the heart following tissue injury 23.
Anti-inflammatory interventions, including interleukin (IL)-1β and IL-6 blockade have been tested in patients with HFpEF, with promising results. The DHART pilot trial (Diastolic Heart Failure Anakinra Response Trial) demonstrated that IL-1β blockade with anakinra increased peak aerobic oxygen consumption 44, although this result was not replicated in the subsequent DHART2 trial, which observed a significant fall in C-reactive protein and N-terminal pro-B type natriuretic peptide, but did not meet its primary clinical endpoint 45. In the recent CANTOS trial (Canakinumab Anti-Inflammatory Thrombosis Outcomes Study), IL-1β blockade with canakinumab decreased hospitalizations for heart failure 46. In addition, IL-6 antagonism with tocilizumab decreased LV mass among patients with rheumatoid arthritis 47, consistent with animal data showing reduction in myocardial fibrosis with IL-6 deletion in rat model of pressure overload 48. These data support the notion that anti-inflammatory interventions may improve clinical outcomes in HFpEF. Nonetheless, it should be noted that LLTS has pleiotropic effects, beyond its anti-inflammatory effects, including increased nitric oxide availability 49 and sympathoinhibition 50, which might also contribute to the beneficial effect in HFpEF.
Limitations
This study has several limitations. The DS rat model, as other rodent models of HFpEF, does not recapitulate all the features of HFpEF, including the integrative complexity of the related comorbidities, such as aging and diabetes 13. Nonetheless, although the pathophysiology of the DS rat model results mainly from salt and water retention 13, it is also associated with a significant inflammatory response, with elevated serum cytokines, increased myocardial gene expression of cytokines and inflammatory infiltration of the myocardium 11, 51. We did not examine the effects of LLTS on intrinsic cardiac nervous system. Therefore, we cannot exclude that the beneficial effects observed might be due to, at least in part, targeting intrinsic cardiac neurons to mitigate sympathoexcitation and adverse cardiac remodeling 50. It remains unclear whether MLA blocked the α7nAChR on macrophages or other immune and non-immune cell subtypes. We focused our analysis on the LV and did not examine any effects on the right ventricle. In light of the detrimental role of α7nAChR in pulmonary hypertension and right ventricular dysfunction52, further studies are required to examine the effect of LLTS on right ventricular pathology. HRV was assessed under isoflurane anesthesia. Although it is possible that anesthesia affects the assessed parameters, the effect would be the same in all groups, therefore the results would still hold regardless of the use of anesthesia. Our intent was not to provide a detailed characterization of the cardiac immune cell profile. However, given the distinct origin and function of individual macrophage subsets in the heart 34, future studies should focus on further characterizing the effects of LLTS on resident and non-resident macrophages.
Clinical implications
Improving morbidity and mortality with pharmacological treatments in humans with HFpEF has been challenging 1. Our study provides the basis for examining the use of LLTS to treat HFpEF in clinical trials. However, in light of the notion that a “one-size-fits-all” approach may not be effective in HFpEF 1, 2, selection of patients who are likely to respond to this novel, noninvasive therapy, by identifying key biomarkers of response, becomes a key issue to maximize its efficacy. Moreover, our data suggest that α7nAchR may represent a novel therapeutic target for HFpEF. Notably, enhancing non-neuronal cholinergic signaling with pyridostigmine exerted a beneficial immunomodulatory effect and improved survival in mice following cardiac injury 23. Whether enhancing cholinergic signaling or α7nAchR pharmacologically may be beneficial in patients with HFpEF remains to be determined.
Conclusions
Neuromodulation with LLTS improved cardiac function and fibrosis in a well-established rat model of HFpEF through its anti-inflammatory effects. Our results support the emerging paradigm of noninvasive neuromodulation to treat selected patients with HFpEF and provide the basis for further randomized trials.
Summary.
What is new?
In Dahl salt-sensitive rats with heart failure with preserved ejection fraction, low-level transcutaneous vagus nerve stimulation attenuated the increase in blood pressure, improved diastolic dysfunction, decreased left ventricular fibrosis, and improved survival compared to other groups.
Pharmacological blockade of the alpha7-nicotinic acetylcholine receptor attenuated these effects, while antihypertensive treatment with Olmesartan did not improve cardiac function or fibrosis despite maintaining similar blood pressure as low-level transcutaneous vagus nerve stimulation.
We concluded that autonomic modulation with low-level transcutaneous vagus nerve stimulation improved cardiac function in this well-established rat model of heart failure with preserved ejection fraction through its anti-inflammatory effects.
What are the clinical implications?
These results provide the basis for examining the use of low-level transcutaneous vagus nerve stimulation to treat selected patient with heart failure with preserved ejection fraction in clinical trials.
These data also suggest that the alpha7-nicotinic acetylcholine receptor may represent a novel therapeutic target for heart failure with preserved ejection fraction.
These results support the inflammatory paradigm for the pathogenesis of heart failure with preserved ejection fraction and suggest that inflammation and fibrosis are not only causative in heart failure with preserved ejection fraction, but also potentially reversible.
Acknowledgments
Funding: Funded by NIH R21AG057879 to Stavros Stavrakis
Non-standard Abbreviations and Acronyms
- HFpEF
Heart failure with preserved ejection fraction
- LLTS
Low-level transcutaneous vagus nerve stimulation
- α7nAchR
alpha7 nicotinic acetylcholine receptor
- DS
Dahl salt-sensitive
- LS
low salt
- HS
high salt
- MLA
methyllaconitine
- LV
Left ventricular
- e’
early diastolic mitral annulus velocity
- E/A
ratio of early to late mitral inflow Doppler velocity the ratio of the early mitral inflow
- E/e’
ratio of the early mitral inflow Doppler velocity to the early diastolic mitral annulus velocity
- HRV
heart rate variability
- TNF
Tumor necrosis factor
- MCP
Μonocyte chemoattractant protein
- TGF
Τransforming growth factor
- FFT
Fast Fourier Transform
- LF
low frequency
- HF
high frequency
- ANOVA
Analysis of variance
- FDR
false discovery rate
- IL
interleukin
Footnotes
Disclosures: None
References:
- 1.Shah SJ, Borlaug BA, Kitzman DW, McCulloch AD, Blaxall BC, Agarwal R, Chirinos JA, Collins S, Deo RC, Gladwin MT, et al. Research Priorities for Heart Failure With Preserved Ejection Fraction: National Heart, Lung, and Blood Institute Working Group Summary. Circulation. 2020;141:1001–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Woolley RJ, Ceelen D, Ouwerkerk W, Tromp J, Figarska SM, Anker SD, Dickstein K, Filippatos G, Zannad F, Metra M, et al. Machine learning based on biomarker profiles identifies distinct subgroups of heart failure with preserved ejection fraction. Eur J Heart Fail. 2021;23:983–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paulus WJ and Zile MR. From Systemic Inflammation to Myocardial Fibrosis: The Heart Failure With Preserved Ejection Fraction Paradigm Revisited. Circ Res. 2021;128:1451–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pavlov VA and Tracey KJ. Neural circuitry and immunity. Immunol Res. 2015;63:38–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, Li JH, Yang H, Ulloa L, Al-Abed Y, et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003;421:384–8. [DOI] [PubMed] [Google Scholar]
- 6.Koopman FA, Chavan SS, Miljko S, Grazio S, Sokolovic S, Schuurman PR, Mehta AD, Levine YA, Faltys M, Zitnik R, et al. Vagus nerve stimulation inhibits cytokine production and attenuates disease severity in rheumatoid arthritis. Proc Natl Acad Sci U S A. 2016;113:8284–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stavrakis S, Humphrey MB, Scherlag B, Iftikhar O, Parwani P, Abbas M, Filiberti A, Fleming C, Hu Y, Garabelli P, et al. Low-Level Vagus Nerve Stimulation Suppresses Post-Operative Atrial Fibrillation and Inflammation: A Randomized Study. JACC Clin Electrophysiol. 2017;3:929–938. [DOI] [PubMed] [Google Scholar]
- 8.Peuker ET and Filler TJ. The nerve supply of the human auricle. Clin Anat. 2002;15:35–7. [DOI] [PubMed] [Google Scholar]
- 9.Stavrakis S, Humphrey MB, Scherlag BJ, Hu Y, Jackman WM, Nakagawa H, Lockwood D, Lazzara R and Po SS. Low-level transcutaneous electrical vagus nerve stimulation suppresses atrial fibrillation. J Am Coll Cardiol. 2015;65:867–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stavrakis S, Stoner JA, Humphrey MB, Morris L, Filiberti A, Reynolds JC, Elkholey K, Javed I, Twidale N, Riha P, et al. TREAT AF (Transcutaneous Electrical Vagus Nerve Stimulation to Suppress Atrial Fibrillation): A Randomized Clinical Trial. JACC Clin Electrophysiol. 2020;6:282–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou L, Filiberti A, Humphrey MB, Fleming CD, Scherlag BJ, Po SS and Stavrakis S. Low-level transcutaneous vagus nerve stimulation attenuates cardiac remodelling in a rat model of heart failure with preserved ejection fraction. Exp Physiol. 2019;104:28–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cai L, Lu K, Chen X, Huang JY, Zhang BP and Zhang H. Auricular vagus nerve stimulation protects against postoperative cognitive dysfunction by attenuating neuroinflammation and neurodegeneration in aged rats. Neurosci Lett. 2019;703:104–110. [DOI] [PubMed] [Google Scholar]
- 13.Valero-Munoz M, Backman W and Sam F. Murine Models of Heart Failure with Preserved Ejection Fraction: a “Fishing Expedition”. JACC Basic Transl Sci. 2017;2:770–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pelisch N, Hosomi N, Ueno M, Nakano D, Hitomi H, Mogi M, Shimada K, Kobori H, Horiuchi M, Sakamoto H, et al. Blockade of AT1 receptors protects the blood-brain barrier and improves cognition in Dahl salt-sensitive hypertensive rats. Am J Hypertens. 2011;24:362–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ardell JL, Rajendran PS, Nier HA, KenKnight BH and Armour JA. Central-peripheral neural network interactions evoked by vagus nerve stimulation: functional consequences on control of cardiac function. Am J Physiol Heart Circ Physiol. 2015;309:H1740–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cho JH, Zhang R, Aynaszyan S, Holm K, Goldhaber JI, Marban E and Cingolani E. Ventricular Arrhythmias Underlie Sudden Death in Rats With Heart Failure and Preserved Ejection Fraction. Circ Arrhythm Electrophysiol. 2018;11:e006452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horgan S, Watson C, Glezeva N and Baugh J. Murine models of diastolic dysfunction and heart failure with preserved ejection fraction. J Card Fail. 2014;20:984–95. [DOI] [PubMed] [Google Scholar]
- 18.Matyas C, Kovacs A, Nemeth BT, Olah A, Braun S, Tokodi M, Barta BA, Benke K, Ruppert M, Lakatos BK, et al. Comparison of speckle-tracking echocardiography with invasive hemodynamics for the detection of characteristic cardiac dysfunction in type-1 and type-2 diabetic rat models. Cardiovasc Diabetol. 2018;17:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bonios M, Chang CY, Pinheiro A, Dimaano VL, Higuchi T, Melexopoulou C, Bengel F, Terrovitis J, Abraham TP and Abraham MR. Cardiac resynchronization by cardiosphere-derived stem cell transplantation in an experimental model of myocardial infarction. J Am Soc Echocardiogr. 2011;24:808–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stavrakis S, Kulkarni K, Singh JP, Katritsis DG and Armoundas AA. Autonomic Modulation of Cardiac Arrhythmias: Methods to Assess Treatment and Outcomes. JACC Clin Electrophysiol. 2020;6:467–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elkholey K, Morris L, Niewiadomska M, Houser J, Ramirez M, Tang M, Humphrey MB and Stavrakis S. Sex differences in the incidence and mode of death in rats with heart failure with preserved ejection fraction. Exp Physiol. 2021;106:673–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Silva LEV, Geraldini VR, de Oliveira BP, Silva CAA, Porta A and Fazan R. Comparison between spectral analysis and symbolic dynamics for heart rate variability analysis in the rat. Sci Rep. 2017;7:8428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rocha-Resende C, Weinheimer C, Bajpai G, Adamo L, Matkovich SJ, Schilling J, Barger PM, Lavine KJ and Mann DL. Immunomodulatory role of non-neuronal cholinergic signaling in myocardial injury. JCI Insight. 2019;5:e128961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bullard JH, Purdom E, Hansen KD and Dudoit S. Evaluation of statistical methods for normalization and differential expression in mRNA-Seq experiments. BMC Bioinformatics. 2010;11:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou X, Lindsay H and Robinson MD. Robustly detecting differential expression in RNA sequencing data using observation weights. Nucleic Acids Res. 2014;42:e91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maslov MY, Foianini S, Mayer D, Orlov MV and Lovich MA. Synergy between sacubitril and valsartan leads to hemodynamic, antifibrotic, and exercise tolerance benefits in rats with preexisting heart failure. Am J Physiol Heart Circ Physiol. 2019;316:H289–H297. [DOI] [PubMed] [Google Scholar]
- 27.Yoshida J, Yamamoto K, Mano T, Sakata Y, Nishikawa N, Nishio M, Ohtani T, Miwa T, Hori M and Masuyama T. AT1 receptor blocker added to ACE inhibitor provides benefits at advanced stage of hypertensive diastolic heart failure. Hypertension. 2004;43:686–91. [DOI] [PubMed] [Google Scholar]
- 28.Hulsmans M, Sager HB, Roh JD, Valero-Munoz M, Houstis NE, Iwamoto Y, Sun Y, Wilson RM, Wojtkiewicz G, Tricot B, et al. Cardiac macrophages promote diastolic dysfunction. J Exp Med. 2018;215:423–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim S, Yoshiyama M, Izumi Y, Kawano H, Kimoto M, Zhan Y and Iwao H. Effects of combination of ACE inhibitor and angiotensin receptor blocker on cardiac remodeling, cardiac function, and survival in rat heart failure. Circulation. 2001;103:148–54. [DOI] [PubMed] [Google Scholar]
- 30.Sakata Y, Masuyama T, Yamamoto K, Doi R, Mano T, Kuzuya T, Miwa T, Takeda H and Hori M. Renin angiotensin system-dependent hypertrophy as a contributor to heart failure in hypertensive rats: different characteristics from renin angiotensin system-independent hypertrophy. J Am Coll Cardiol. 2001;37:293–9. [DOI] [PubMed] [Google Scholar]
- 31.Stavrakis S, Elkholey K, Morris L, Niewiadomska M, Asad ZUA and Humphrey MB. Neuromodulation of Inflammation to Treat Heart Failure With Preserved Ejection Fraction: A Pilot Randomized Clinical Trial. J Am Heart Assoc. 2022:e023582. [DOI] [PMC free article] [PubMed]
- 32.Carnevale D, Perrotta M, Pallante F, Fardella V, Iacobucci R, Fardella S, Carnevale L, Carnevale R, De Lucia M, Cifelli G, et al. A cholinergic-sympathetic pathway primes immunity in hypertension and mediates brain-to-spleen communication. Nat Commun. 2016;7:13035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee DS, Gona P, Vasan RS, Larson MG, Benjamin EJ, Wang TJ, Tu JV and Levy D. Relation of disease pathogenesis and risk factors to heart failure with preserved or reduced ejection fraction: insights from the framingham heart study of the national heart, lung, and blood institute. Circulation. 2009;119:3070–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wong NR, Mohan J, Kopecky BJ, Guo S, Du L, Leid J, Feng G, Lokshina I, Dmytrenko O, Luehmann H, et al. Resident cardiac macrophages mediate adaptive myocardial remodeling. Immunity. 2021;54:2072–2088 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kalogeropoulos A, Georgiopoulou V, Psaty BM, Rodondi N, Smith AL, Harrison DG, Liu Y, Hoffmann U, Bauer DC, Newman AB, et al. Inflammatory markers and incident heart failure risk in older adults: the Health ABC (Health, Aging, and Body Composition) study. J Am Coll Cardiol. 2010;55:2129–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patel RB, Colangelo LA, Reiner AP, Gross MD, Jacobs DR Jr., Launer LJ, Lima JAC, Lloyd-Jones DM and Shah SJ. Cellular Adhesion Molecules in Young Adulthood and Cardiac Function in Later Life. J Am Coll Cardiol. 2020;75:2156–2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Verloop WL, Beeftink MM, Santema BT, Bots ML, Blankestijn PJ, Cramer MJ, Doevendans PA and Voskuil M. A systematic review concerning the relation between the sympathetic nervous system and heart failure with preserved left ventricular ejection fraction. PLoS One. 2015;10:e0117332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deck J, Bibevski S, Gnecchi-Ruscone T, Bellina V, Montano N and Dunlap ME. Alpha7-nicotinic acetylcholine receptor subunit is not required for parasympathetic control of the heart in the mouse. Physiol Genomics. 2005;22:86–92. [DOI] [PubMed] [Google Scholar]
- 39.Rafiq K, Noma T, Fujisawa Y, Ishihara Y, Arai Y, Nabi AH, Suzuki F, Nagai Y, Nakano D, Hitomi H, et al. Renal sympathetic denervation suppresses de novo podocyte injury and albuminuria in rats with aortic regurgitation. Circulation. 2012;125:1402–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ye S, Zhong H, Duong VN and Campese VM. Losartan reduces central and peripheral sympathetic nerve activity in a rat model of neurogenic hypertension. Hypertension. 2002;39:1101–6. [DOI] [PubMed] [Google Scholar]
- 41.Kresoja KP, Rommel KP, Fengler K, von Roeder M, Besler C, Lucke C, Gutberlet M, Desch S, Thiele H, Bohm M, et al. Renal Sympathetic Denervation in Patients With Heart Failure With Preserved Ejection Fraction. Circ Heart Fail. 2021;14:e007421. [DOI] [PubMed] [Google Scholar]
- 42.Cohen JB, Schrauben SJ, Zhao L, Basso MD, Cvijic ME, Li Z, Yarde M, Wang Z, Bhattacharya PT, Chirinos DA, et al. Clinical Phenogroups in Heart Failure With Preserved Ejection Fraction: Detailed Phenotypes, Prognosis, and Response to Spironolactone. JACC Heart Fail. 2020;8:172–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shah SJ, Katz DH, Selvaraj S, Burke MA, Yancy CW, Gheorghiade M, Bonow RO, Huang CC and Deo RC. Phenomapping for novel classification of heart failure with preserved ejection fraction. Circulation. 2015;131:269–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Van Tassell BW, Arena R, Biondi-Zoccai G, Canada JM, Oddi C, Abouzaki NA, Jahangiri A, Falcao RA, Kontos MC, Shah KB, et al. Effects of interleukin-1 blockade with anakinra on aerobic exercise capacity in patients with heart failure and preserved ejection fraction (from the D-HART pilot study). Am J Cardiol. 2014;113:321–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Van Tassell BW, Trankle CR, Canada JM, Carbone S, Buckley L, Kadariya D, Del Buono MG, Billingsley H, Wohlford G, Viscusi M, et al. IL-1 Blockade in Patients With Heart Failure With Preserved Ejection Fraction. Circ Heart Fail. 2018;11:e005036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Everett BM, Cornel JH, Lainscak M, Anker SD, Abbate A, Thuren T, Libby P, Glynn RJ and Ridker PM. Anti-Inflammatory Therapy With Canakinumab for the Prevention of Hospitalization for Heart Failure. Circulation. 2019;139:1289–1299. [DOI] [PubMed] [Google Scholar]
- 47.Kobayashi H, Kobayashi Y, Giles JT, Yoneyama K, Nakajima Y and Takei M. Tocilizumab treatment increases left ventricular ejection fraction and decreases left ventricular mass index in patients with rheumatoid arthritis without cardiac symptoms: assessed using 3.0 tesla cardiac magnetic resonance imaging. J Rheumatol. 2014;41:1916–21. [DOI] [PubMed] [Google Scholar]
- 48.Zhao L, Cheng G, Jin R, Afzal MR, Samanta A, Xuan YT, Girgis M, Elias HK, Zhu Y, Davani A, et al. Deletion of Interleukin-6 Attenuates Pressure Overload-Induced Left Ventricular Hypertrophy and Dysfunction. Circ Res. 2016;118:1918–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jiang Y, Li L, Ma J, Zhang L, Niu F, Feng T and Li C. Auricular vagus nerve stimulation promotes functional recovery and enhances the post-ischemic angiogenic response in an ischemia/reperfusion rat model. Neurochem Int. 2016;97:73–82. [DOI] [PubMed] [Google Scholar]
- 50.Beaumont E, Wright GL, Southerland EM, Li Y, Chui R, KenKnight BH, Armour JA and Ardell JL. Vagus nerve stimulation mitigates intrinsic cardiac neuronal remodeling and cardiac hypertrophy induced by chronic pressure overload in guinea pig. Am J Physiol Heart Circ Physiol. 2016;310:H1349–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gallet R, de Couto G, Simsolo E, Valle J, Sun B, Liu W, Tseliou E, Zile MR and Marban E. Cardiosphere-derived cells reverse heart failure with preserved ejection fraction (HFpEF) in rats by decreasing fibrosis and inflammation. JACC Basic Transl Sci. 2016;1:14–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vang A, da Silva Goncalves Bos D, Fernandez-Nicolas A, Zhang P, Morrison AR, Mancini TJ, Clements RT, Polina I, Cypress MW, Jhun BS, et al. alpha7 Nicotinic acetylcholine receptor mediates right ventricular fibrosis and diastolic dysfunction in pulmonary hypertension. JCI Insight. 2021;6:e142945. [DOI] [PMC free article] [PubMed] [Google Scholar]


