Abstract
Pain and wound after haemorrhoidectomy constantly bothered the patient's convenience. Recurrently, topical sucralfate is used to treat excoriations and burns. It is considered to enhance epidermal growth and tissue granulation, thus, alleviating patients' problems. This study evaluated topical sucralfate's feasibility, safety, and superiority after haemorrhoidectomy. We searched randomised controlled trial (RCT) studies in PubMed, Google Scholar, Europe PMC, and ClinicalTrials.gov until March 29th, 2022. We investigated the influence of topical sucralfate on pain score postoperatively (24 hours, 7 days, and 14 days), pethidine usage, diclofenac usage, and wound healing rate compared to placebo. This study was conducted following the PRISMA guidelines. This study sorted the final six studies with 439 patients underwent haemorrhoidectomy. Topical sucralfate demonstrated significant outcomes on VAS 24 hours post‐operative [Std. Mean Difference −1.00 (95% CI −1.70, −0.31), P = .005], VAS 7 days post‐operative [Std. Mean Difference −2.29 (95% CI −3.34, −1.25), P < .0001], VAS 14 days post‐operative [Std. Mean Difference −1.88 (95% CI −2.74, −1.01), P < .0001], pethidine usage within 24 hours post‐operative [Std. Mean Difference −0.62 (95% CI −0.96, −0.27), P = .0004], diclofenac usage 7 days post‐operative [Std. Mean Difference −1.76 (95% CI −2.61, −0.92), P < .0001], diclofenac usage 14 days post‐operative [Std. Mean Difference −1.64 (95% CI −2.38, −0.91), P < .0001], and wound healing rate at 28‐day post‐operative [RR 1.45 (95% CI 1.25–1.68), P < .00001]. Topical sucralfate alleviated pain, improved wound healing, and minimised the usage of pethidine and diclofenac compared to placebo.
Keywords: haemorrhoidectomy, pain, systematic review, topical sucralfate, wound healing
1. INTRODUCTION
Haemorrhoids are common benign anorectal conditions, defined as the symptomatic enlargement and distal displacement of the anal cushions. 1 This is remaining an issue since the ancient Egypt era in 1700 BC. 2 The prevalence of haemorrhoids in the general population was 11%, the highest prevalence found in middle‐aged people, while women were slightly over‐represented compared with men. The anorectal apparatus's supportive connective tissue framework weakens due to age or increased intra‐abdominal pressures. Pregnancy, obesity, constipation, straining during defecation, sitting for a long period on the toilet seat, repetitive Valsalva manoeuvre, and chronic cough can all increase intra‐abdominal pressures, leading to haemorrhoids. 3 , 4 , 5 , 6
Treatment of haemorrhoids ranges from dietary and lifestyle modification to open surgery, depending on the degree and severity of symptoms. Pain remains one of the most patient complaints after haemorrhoidectomy. 7 Uncontrolled pain is associated with delayed discharge from an outpatient facility, unplanned hospital admission, prolonged hospital stay, and delayed return to normal daily activities. Opioids and NSAIDs relieve post haemorrhoidectomy pain but have a short duration of action and well‐known side effects. Due to these factors, alternative treatments for post‐haemorrhoidectomy pain are needed. 8 , 9
Edward Campbell Milligan and Clifford Naughton Morgan established the Milligan‐Morgan technique, a classic operative technique for open excision haemorrhoidectomy. It seems obsolete yet valuable and has become the most common technique in developing countries and remote areas. 10 , 11 A V‐shape incision of the anal skin, continuing to ligate the hemorrhoidal pedicle, which contains the mucosa, submucosa, the terminal branch of the superior hemorrhoidal artery, and vein with non‐mucosal closure, might aggravate the pain. Moreover, wound healing is slower than other techniques. Thus, persisting the pain even months to years. 12 , 13 , 14 , 15
Pethidine is a synthetic opioid analgesic drug, approved for use since 1943. It has been used widely for moderate and severe acute pain such as labour and post‐operative patient and post‐open haemorrhoidectomy. Although it is commonly used and considered safe, several adverse events were noted, including dizziness, nausea, vomiting, and constipation. 16 , 17
Diclofenac is a non‐steroidal anti‐inflammatory drug (NSAID) widely used in mild and moderate pain, with common side effects such as nausea and vomiting and renal impairment in chronic usage. It is ubiquitous to be given in post‐open haemorrhoidectomy and has been used either orally, intravenously, or via rectal. 18 Its usage has also been a factor to be noted when comparing several haemorrhoidectomy techniques. 19
Sucralfate is a sucrose sulphate and aluminium hydroxide complex that has been widely used to protect and cure gastric mucosa in acid peptic disease. 20 , 21 Sucralfate serves as a mechanical barrier by binding epidermal growth more quickly, causing it to accumulate at wound sites and stimulating epithelial cell proliferation. 22 It also promotes angiogenesis, which aids wound healing by increasing tissue granulation. Compared to placebo, topical sucralfate can reduce pain and promote faster wound healing on days 7 and 14 after haemorrhoidectomy. Sucralfate has been found to be a dependable medicine in all research done so far using it topically. There were no harmful local or systemic effects. 23 , 24 , 25 However, there is no evidence of its efficacy for post‐haemorrhoidectomy pain reduction from a systematic review. This study aimed to conduct a systematic review, meta‐analysis, and meta‐regression to compare the effectiveness of topical sucralfate ointment over placebo for reducing post‐operative pain and improving wound healing following haemorrhoidectomy.
2. MATERIALS AND METHODS
2.1. Eligibility criteria
We conducted a systematic review and meta‐analysis study from randomised clinical trial studies. The inclusion criteria using PICOS formulation were as follows: (1) P – Population: patients with the hemorrhoidal disease who undergo haemorrhoidectomy procedure; (2) I – Intervention: receiving topical sucralfate in any dosage following the surgical procedure; (3) C – Control: did not receive topical sucralfate or only receiving placebo as a topical treatment after surgical procedure; (4) O – Outcome: reporting at least one of the following outcomes: VAS at 24 hours post‐operative, VAS at 7 days post‐operative, VAS at 14 days post‐operative, amount pethidine used (mg) within 24 hours post‐operative, amount of diclofenac used (mg) at 7 days post‐operative, amount of diclofenac used (mg) at 14 days post‐operative, and wound healing rate at 28 days post‐operative; (5) S – Study design: randomised clinical trials; (6) presentation as a full‐text article (which included preprints). Exclusion criteria were as follows: (1) articles reported in language other than English; (2) articles besides randomised clinical trials (non‐randomised clinical trials, cohort, case–control, case‐series, case‐report, cross‐sectional studies); (3) studies with no control group; (4) unpublished study or abstract; and (5) non‐primary research.
2.2. Search strategy and study selection
Database searching was done systemically for studies with English‐language constraints sourced from four databases (PubMed, Google Scholar, Europe PMC, and ClinicalTrials.gov). The search terms included “(topical OR ointment OR cream) AND (sucralfate) AND (haemorrhoid OR hemorrhoid OR hemorrhoidectomy OR pile surgery OR anal surgery)” were used to filter the intended studies through March 29th, 2022. The details of the literature search strategies are summarised in Table S1. The initial step was identifying eligible articles by screening titles and abstracts by two reviewers. Additional references from eligible studies were also evaluated to search for more potential articles. Duplicate articles were removed. Finally, two reviewers independently screened full‐text articles (RR, RVH), with discrepancies resolved through discussion with the senior author (FH). Our study is in accordance with Meta‐analysis of Observational Studies in Epidemiology (MOOSE) 26 and Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines. 27
2.3. Data extraction and quality assessment
Two authors (RR, RVH) conducted the data extraction. An extraction form was developed to list information about the study, such as author's names, year of study, sample size, study design, haemorrhoid grade, sucralfate dose, age, gender, number of patients in the intervention group, the control group in included studies, as well as the outcome of interest.
The methodological quality of the included studies was assessed independently by two authors (RR, RVH). The quality of the included clinical trial studies will be evaluated using Risk of Bias version 2 (RoB v2) from Cochrane Collaborations. 28 This tool comprises five domains for methodological evaluation: (a) randomization process; (b) deviations from intended interventions; (c) missing outcome data; (d) measurement of the outcome; and (e) selection of the reported result. The RCT was classified as low risk of bias (low risk of bias for all domains), high risk (high risk of bias for one or more domains), or unclear risk (unclear risk of bias for one or more key domains).
2.4. Statistical analysis
Meta‐analysis was done using Review Manager 5.4 (Cochrane Collaboration) software. The Mantel–Haenszel formula with random‐effect models, regardless of heterogeneity was employed to calculate the risk ratio (RR) and its 95% confidence interval (95% CI) for the dichotomous variable outcomes. Meanwhile, the Inverse‐Variance formula with random effect models was used to calculate the standardised mean difference (SMD) and its standard deviations (SD) for the continuous variable outcomes. This meta‐analysis assessed heterogeneity between studies by I‐squared (I 2; Inconsistency). The I 2 statistic with a value of <25% is considered a low degree of heterogeneity, 26%–50% moderate degree of heterogeneity, and >50% a high degree of heterogeneity. 29 I 2 of at least 50% is regarded as substantial heterogeneity, indicating at least half of the total variability among effect sizes is due to true heterogeneity between studies. Meta‐regression with a random‐effects model was performed using a restricted‐maximum likelihood for pre‐specified variables including age, gender, and haemorrhoid grade 3 prevalence to see the interaction effect between topical sucralfate and these variables in influencing the outcomes of interest. When data were reported as medians and interquartile ranges or as medians and minimum‐to‐maximum ranges, we converted them to means and standard deviations for meta‐analysis pooling using the formula by Wan X et al 30 Funnel plot analysis was utilised to assess the qualitative risk of publication bias.
3. RESULTS
3.1. Study selection and characteristics
The initial database search yielded 234 studies, of which 13 were eligible after screening titles and abstracts and removing duplicates. Seven articles were further excluded from these eligible studies after the full‐text screening. Four articles were not primary research, one article did not have a comparison/control group, one article was not a randomised clinical trial, and one article did not have data about the specified outcome of interest, thus resulting in the final number of six randomised clinical trial studies 31 , 32 , 33 , 34 , 35 , 36 which included a total of 439 haemorrhoid patients undergoing haemorrhoidectomy procedure for the analysis (Figure 1). Out of 6 studies, four were double‐blind RCT, one was single‐blind RCT, and the remaining one articles did not specify the blinding methods. Sample sizes ranged from 40 to 116. All the samples in the included studies have grade III or IV haemorrhoids. The dose for topical sucralfate used in the included studies varied from 7% to 10% after the surgery. Table 1 gives out the details of each included research.
FIGURE 1.
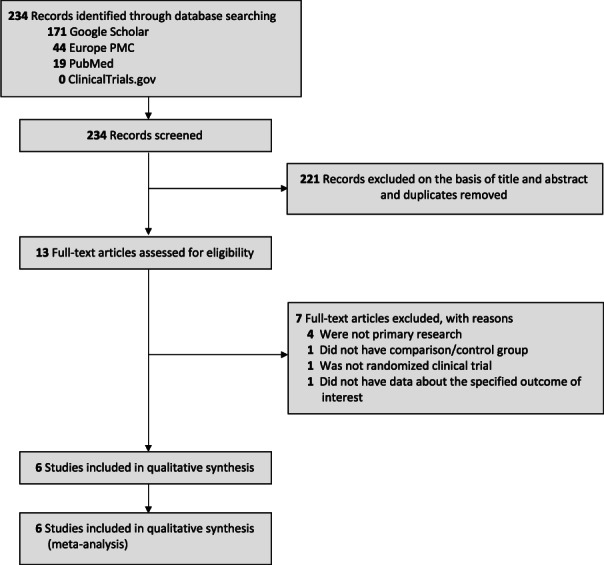
PRISMA diagram of the detailed process of selection of studies for inclusion in the systematic review and meta‐analysis
TABLE 1.
Characteristics of included studies
| Study | Sample size | Design | Age (years) | Male (%) | Hemorrhoid grade | Sucralfate dose | Control group | Outcomes a |
|---|---|---|---|---|---|---|---|---|
| Ala et al 31 | 48 | Double‐blind RCT | 41.5 ± 12.1 | 50% | Grade III and IV | Sucralfate 10% | Placebo | 1,2,3,4,5,6 |
| Albatanony et al 32 | 90 | Double‐blind RCT | 37.2 ± 10.7 | 45.5% |
Grade III: 80% Grade IV: 20% |
Sucralfate 10% | Placebo | 1,2,3,4,5,6,7 |
| Al Khateeb et al 33 | 50 | Prospective RCT | 41.2 ± 10.8 | 46% |
Grade III: 54% Grade IV: 46% |
Sucralfate 8% | Placebo | 1,2,3,7 |
| Alkhateep et al 34 | 95 | Double‐blind RCT | 42.4 ± 7.7 | 50.5% |
Grade III: 77.8% Grade IV: 22.2% |
Sucralfate 10% | Placebo | 1,2,3,7 |
| Gupta et al 35 | 116 | Double‐blind RCT | 44.9 ± 11 | 52.5% |
Grade III: 74.1% Grade IV: 25.9% |
Sucralfate 7% | Placebo | 2,3,7 |
| Vejdan et al 36 | 40 | Single‐blind RCT | N/A | N/A | Grade III and IV | Sucralfate 10% | Placebo | 1 |
Outcomes: 1 = VAS at 24 hours post‐operative; 2 = VAS at 7 days post‐operative; 3 = VAS at 14 days post‐operative; 4 = Pethidine used (mg) within 24 hours post‐operative; 5 = diclofenac used (mg) at 7 days post‐operative; 6 = diclofenac used (mg) at 14 days post‐operative; 7 = wound healing rate at 28 days post‐operative.
3.2. Quality of study assessment
Risk of Bias version 2 (RoB v2) from Cochrane was used to evaluate the quality of randomised clinical trial studies. Three of the included studies have a low risk of bias in all five assessed domains. Two out of six studies were judged to have some concerns risk of bias, one because the methods for the randomization process were not explained clearly, and the other one because the measurement of the outcome was not clearly explained whether it is blinded from the outcome's assessor or not. The remaining two studies were judged to have a high risk of bias because the outcome measurement was not blinded by the assessor/investigator, which may have affected the results. The summary risk of bias assessment is presented in Table 2.
TABLE 2.
Risk of bias version 2 (RoB v2) for assessment of clinical trial studies

|
3.3. Topical sucralfate versus placebo
3.3.1. VAS at 24 hours post‐operative
Five studies (n = 323) reported the VAS at 24 hours post‐operative outcome. Our pooled analysis showed that topical sucralfate after haemorrhoidectomy could reduce VAS at 24 hours post‐operative compared with placebo [Std. Mean Difference −1.00 (95% CI −1.70, −0.31), P = .005, I 2 = 88%, random‐effect modelling] (Figure 2A).
FIGURE 2.
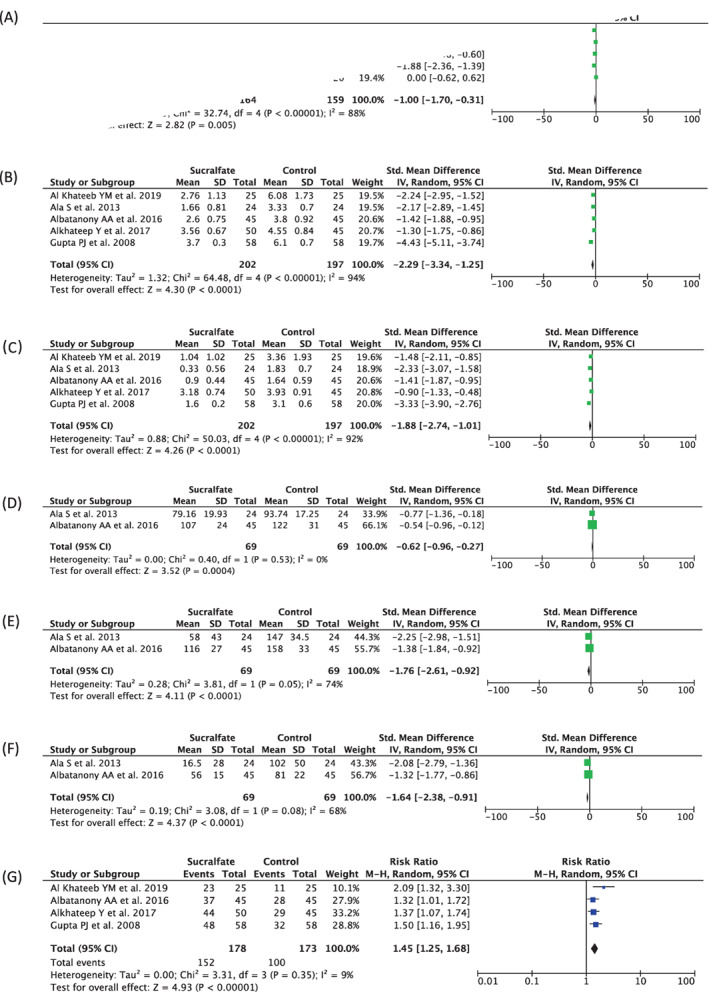
Forest plot that demonstrates the comparison between using topical sucralfate and placebo after haemorrhoidectomy in terms of VAS at 24 hours‐post‐operative (A), VAS at 7 days post‐operative (B), VAS at 14 days post‐operative (C), pethidine used (mg) within 24 hours post‐operative (D), diclofenac used (mg) at 7 days post‐operative (E), diclofenac used (mg) at 14 days post‐operative (F), and wound healing rate at 28 days post‐operative (G) outcomes
3.3.2. VAS at 7 days post‐operative
Five studies (n = 399) reported the VAS at 7 days post‐operative outcome. Our pooled analysis showed that topical sucralfate after haemorrhoidectomy can reduce VAS at 7 days post‐operative compared with placebo [Std. Mean Difference −2.29 (95% CI −3.34, −1.25), P < .0001, I 2 = 94%, random‐effect modelling] (Figure 2B).
3.3.3. VAS at 14 days post‐operative
Five studies (n = 399) reported the VAS at 14 days post‐operative outcome. Our pooled analysis showed that topical sucralfate after haemorrhoidectomy can reduce VAS at 14 days post‐operative compared with placebo [Std. Mean Difference −1.88 (95% CI −2.74, −1.01), P < .0001, I 2 = 92%, random‐effect modelling] (Figure 2C).
3.3.4. Pethidine used (mg) within 24 hours post‐operative
Two studies (n = 138) reported on the pethidine used (mg) within 24 hours post‐operative outcome. Our pooled analysis showed that the amount of pethidine used (mg) within 24 hours post‐operative was lower in the topical sucralfate group compared with control group [Std. Mean Difference −0.62 (95% CI −0.96, −0.27), P = .0004, I 2 = 0%, random‐effect modelling] (Figure 2D).
3.3.5. Diclofenac used (mg) at 7 days post‐operative
Two studies (n = 138) reported on the diclofenac used (mg) at 7 days post‐operative outcome. Our pooled analysis showed that the amount of diclofenac used (mg) at 7 days post‐operative was lower in the topical sucralfate group compared with control group [Std. Mean Difference −1.76 (95% CI −2.61, −0.92), P < .0001, I 2 = 74%, random‐effect modelling] (Figure 2E).
3.3.6. Diclofenac used (mg) at 14 days post‐operative
Two studies (n = 138) reported on the diclofenac used (mg) at 14 days post‐operative outcome. Our pooled analysis showed that the amount of diclofenac used (mg) at 14 days post‐operative was lower in the topical sucralfate group compared with control group [Std. Mean Difference −1.64 (95% CI −2.38, −0.91), P < .0001, I 2 = 68%, random‐effect modelling] (Figure 2F).
3.3.7. Wound healing rate at 28 days post‐operative
Four studies (n = 351) reported the wound healing rate at 28 days post‐operative. Our pooled analysis showed that the use of topical sucralfate after haemorrhoidectomy was associated with a higher wound healing rate at 28 days post‐operative compared with placebo [RR 1.45 (95% CI 1.25–1.68), P < .00001, I 2 = 9%, random‐effect modelling] (Figure 2G).
3.4. Meta‐regression
Meta‐regression was performed to identify risk factors influencing the relationship between topical sucralfate treatment and all outcomes of interest. Our meta‐regression revealed that variability in those outcomes in post‐haemorrhoidectomy patients receiving sucralfate treatment could not be explained by known patient factors associated with predictors of treatment outcomes (Table S2). From our meta‐regression analysis, it was revealed that the association between topical sucralfate treatment and VAS at 24 hours post‐operative was not significantly influenced by age (P = .8058) (Figure S1A) and gender (P = .8639) (Figure S1B). In terms of VAS at 7 days post‐operative, the association was not influenced by age (P = .0787) (Figure S2A), gender (P = .2070) (Figure S2B), and haemorrhoid grade 3 prevalence (P = .8292) (Figure S2C). In terms of VAS at 14 days post‐operative, all variables such as age (p = .2297) (Figure S3A), gender (p = .2201) (Figure S3B), and haemorrhoid grade 3 prevalence (P = .9946) (Figure S3C) were not significantly influenced those relationships. Finally, the relationship between topical sucralfate and wound healing rate at 28 days post‐operative also was not influenced by age (P = .5832) (Figure S4A), gender (P = .9384) (Figure S4B), and haemorrhoid grade 3 (P = .0731) (Figure S4C). For other outcomes of interest, such as the amount of pethidine used (mg) within 24 hours post‐operative, amount of diclofenac used (mg) at 7 days post‐operative, and amount of diclofenac used (mg) at 14 days post‐operative, the number of included studies in each outcome was not sufficient to conduct regression analysis.
3.5. Publication bias
We used Funnel plot analysis for the publication bias assessment. This analysis showed a relatively symmetrical inverted plot for all outcomes of interest in this study, indicating no publication bias (Figure 3A–G).
FIGURE 3.
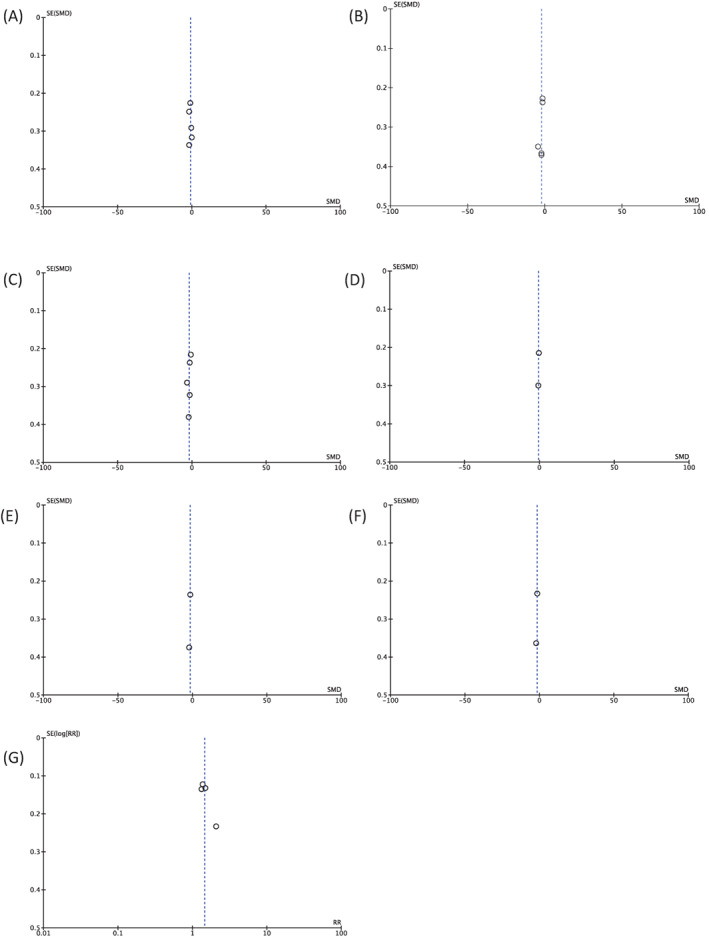
Funnel plot analysis for each outcome of interest in this study: VAS at 24 hours post‐operative (A), VAS at 7 days post‐operative (B), VAS at 14 days post‐operative (C), pethidine used (mg) within 24 hours post‐operative (D), diclofenac used (mg) at 7 days post‐operative (E), diclofenac used (mg) at 14 days post‐operative (F), and wound healing rate at 28 days post‐operative (G)
4. DISCUSSION
Based on our knowledge, this study is the first systematic review, meta‐analysis, and meta‐regression for investigating sucralfate usage and comparing several outcomes mentioned earlier. Moreover, other systematic reviews have been conducted to answer the interminable question: how can post‐operative pain be reduced significantly? 37 , 38 , 39 , 40
Post‐operative pain is the major issue after haemorrhoidectomy and improving patient satisfaction is also the precursor to surgical success. 41 Surgical wounds in the sensitive anoderm and perianal skin and oedema from tissue inflammation surrounding the wound cause the internal anal sphincter to spasm, worsening post‐operative pain. 42 , 43 Wound healing is another significant factor in the outcome of a haemorrhoidectomy. Large wound areas can delay healing and increase pain. 44
Principally, sucralfate is an essential aluminium salt of sucrose octa sulphate and acts as an anti‐ulcer drug. 45 Sucralfate has been shown to have analgesic properties in various conditions. The primary action of sucralfate is idiopathic; meanwhile, several studies showed the effect of sucralfate. 46 , 47 Aside from the anti‐peptic effect, sucralfate increases angiogenesis and the bioavailability of growth factors, including fibroblast growth factor (FGF), epidermal growth factor (EGF), and tissue growth factor (TGF); thus, it enhances epithelialization and mucosal protection resulting in the reduction of pain and improving wound healing. 36 , 48 , 49 In conjunction with the pharmacodynamics of sucralfate, this study demonstrated linear results in which sucralfate is beneficial and significantly improves pain and wound healing. Nonetheless, constipation after the haemorrhoidectomy remains an issue due to general anaesthesia, which initiates constipation. Opioid consumption also aggravates this condition. A strict diet, in conjunction with the limitation of opioid usage, help alleviates constipation and reduction in postoperative pain. 36
In concurrent with these facts, our study showed the superiority of sucralfate at all indicators (Figure 2), including VAS 24 hours post‐operative [Std. Mean Difference −1.00 (95% CI −1.70, −0.31), P = .005, I 2 = 88%, random‐effect modelling], VAS 7 days post‐operative [Std. Mean Difference −2.29 (95% CI −3.34, −1.25), P < .0001, I 2 = 94%, random‐effect modelling], VAS 14 days post‐operative [Std. Mean Difference −1.88 (95% CI −2.74, −1.01), P < .0001, I 2 = 92%, random‐effect modelling], pethidine usage within 24 hours post‐operative [Std. Mean Difference −0.62 (95% CI −0.96, −0.27), P = .0004, I 2 = 0%, random‐effect modelling], diclofenac usage 7 days post‐operative [Std. Mean Difference −1.76 (95% CI −2.61, −0.92), P < .0001, I 2 = 74%, random‐effect modelling], diclofenac usage 14 days post‐operative [Std. Mean Difference −1.64 (95% CI −2.38, −0.91), P < .0001, I 2 = 68%, random‐effect modelling], and wound healing rate at 28‐day post‐operative [RR 1.45 (95% CI 1.25–1.68), P < .00001, I 2 = 9%, random‐effect modelling]. Our meta‐regression demonstrated an insignificant influenced of the risk factors regarding topical sucralfate treatment and all outcomes measured.
Our findings contradicted with the previous systematic review study by Akkakraisee et al 50 They found that topical sucralfate ointment had a similar effect on pain score reduction to that of placebo on days 7 and 14 after haemorrhoidectomy; conversely, the result cannot be concluded due to a small number of studies and participants, as well as high heterogeneity.
There are several limitations of this study that might influence the overall judgements. Al‐Khateeb et al and Vejdan et al had a high risk of bias, and a high degree of heterogeneity due to the various dosage (7%‐10%) and applications (twice to a third a day) of sucralfate in each RCT, some studies did not mention a re‐application following defecation, and they did not state types of anaesthetic methods used intraoperatively (spinal, general anaesthesia, pudendal nerve block, et cetera) which could interfere the post‐operative pain scores and analgesic consumption. Adjunct medications might manipulate the overall outcomes, including metronidazole tablets and laxatives (lactulose and magnesium hydroxide). Other limitations are the small number of RCTs, the small size of samples, and short‐term observation. We suggest large‐scale, well‐designed, long‐term RCTs and equivalent dosage comparisons to abate the heterogeneity for further study.
5. CONCLUSIONS
Topical sucralfate improved pain scores, accelerated wound healing rate, and reduced analgesic usage. Consequently, topical sucralfate is a safe, feasible, and favourable treatment after haemorrhoidectomy.
AUTHORS' CONTRIBUTIONS
Conception and design: Reno Rudiman. Administrative support: Reno Rudiman, Ricarhdo Valentino Hanafi, Cecilia Evan. Provision of study materials or patients: Freda Halim. Collection and assembly of data: Reno Rudiman, Ricarhdo Valentino Hanafi. Data analysis and interpretation: Reno Rudiman, Ricarhdo Valentino Hanafi, Cecilia Evan. Manuscript writing: All authors. Final approval of manuscript: All authors.
FUNDING INFORMATION
The authors declare no grant was received for this study.
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
Supporting information
Figure S1 Bubble‐plot for meta‐regression. Meta‐regression analysis showed that the association between topical sucralfate treatment and VAS at 24 hours post‐operative was not significantly affected by age (A) and gender (B).
Figure S2 Bubble‐plot for meta‐regression. Meta‐regression analysis showed that the association between topical sucralfate treatment and VAS at 7 days post‐operative was not significantly affected by age (A), gender (B) and hemorrhoid grade 3 prevalence (C).
Figure S3 Bubble‐plot for meta‐regression. Meta‐regression analysis showed that the association between topical sucralfate treatment and VAS at 14 days post‐operative was not significantly affected by age (A), gender (B) and hemorrhoid grade 3 prevalence (C).
Figure S4 Bubble‐plot for meta‐regression. Meta‐regression analysis showed that the association between topical sucralfate treatment and wound healing rate at 28 days post‐operative was not significantly affected by age (A), gender (B) and hemorrhoid grade 3 prevalence (C).
Table S1 Literature search strategy.
Table S2 Results for the meta‐regression models for each outcomes of interest.
ACKNOWLEDGEMENTS
We are keen to make our data, analytic methods, and study materials available to other researchers. The data are accessible in the Data Availability section.
Rudiman R, Hanafi RV, Evan C, Halim F. The efficacy of topical sucralfate in improving pain and wound healing after haemorrhoidectomy procedure: A systematic review, meta‐analysis, and meta‐regression of randomised clinical trials. Int Wound J. 2023;20(2):543‐553. doi: 10.1111/iwj.13901
DATA AVAILABILITY STATEMENT
Zenodo: Underlying data for ‘The Efficacy of Topical Sucralfate in Improving Pain and Wound Healing After Hemorrhoidectomy Procedure: A Systematic Review, Meta‐Analysis, and Meta‐Regression of Randomized Clinical Trials. https://doi.org/10.5281/zenodo.6507581 Data are available under the terms of the Creative Commons Zero "No rights reserved" data waiver (CC0 4.0 Public domain dedication).
REFERENCES
- 1. Lohsiriwat V. Hemorrhoids: from basic pathophysiology to clinical management. World J Gastroenterol. 2012;18(17):2009‐2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Agbo S. Surgical management of hemorrhoids. J Surg Tech Case Rep. 2011;3(2):68‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sheikh P, Régnier C, Goron F, Salmat G. The prevalence, characteristics and treatment of hemorrhoidal disease: results of an international web‐based survey. J Compar Effective Res. 2020;9(17):1219‐1232. [DOI] [PubMed] [Google Scholar]
- 4. Agbo S. Surgical management of hemorrhoids. J Surg Tech Case Rep. 2011;3(2):68‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Peery AF, Sandler RS, Galanko JA, et al. Risk factors for hemorrhoids on screening colonoscopy. PLoS One. 2015;10(9):e0139100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sun Z, Migaly J. Review of hemorrhoid disease: presentation and management. Clin Colon Rectal Surg. 2016;29(1):22‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Medina‐Gallardo A, Curbelo‐Peña Y, de Castro X , Roura‐Poch P, Roca‐Closa J, de Caralt‐Mestres E. Is the severe pain after Milligan‐Morgan hemorrhoidectomy still currently remaining a major postoperative problem despite being one of the oldest surgical techniques described? A case series of 117 consecutive patients. Int J Surg Case Rep. 2017;30:73‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Joshi GP, Rawal N, Kehlet H. Evidence‐based management of postoperative pain in adults undergoing open inguinal hernia surgery. Br J Surg. 2012;99(2):168‐185. [DOI] [PubMed] [Google Scholar]
- 9. Baratta JL, Schwenk ES, Viscusi ER. Clinical consequences of inadequate pain relief. Plast Reconstr Surg. 2014;134:15S‐21S. [DOI] [PubMed] [Google Scholar]
- 10. Lu M. Milligan‐Morgan hemorrhoidectomy with anal cushion suspension and partial internal sphincter resection for circumferential mixed hemorrhoids. World J Gastroenterol. 2013;19(30):5011‐5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gravié JF, Lehur PA, Huten N, et al. Stapled Hemorrhoidopexy versus Milligan‐Morgan Hemorrhoidectomy. Ann Surg. 2005;242(1):29‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Razaque SA, Ghafoor DA, Nasurullah S. An evaluation of Milligan‐Morgan and Ferguson procedures for haemorrhoidectomy at Liaquat University Hospital Jamshoro, Hyderabad, Pakistan. Pak J Med Sci. 2012;29(1):122‐127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Santos G d A, Coutinho CP, Meyer MMMMDE, Sampaio DV, Cruz GMG d. Surgical complications in 2,840 cases of hemorrhoidectomy by Milligan‐Morgan, Ferguson and combined techniques. J Coloproctol. 2012;32(3):271‐290. [Google Scholar]
- 14. Pata F, Gallo G, Pellino G, et al. Evolution of surgical Management of Hemorrhoidal Disease: an historical overview. Front Surg. 2021;8:1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kunitake H, Poylin V. Complications following anorectal surgery. Clin Colon Rectal Surg. 2016;29(1):14‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Moghaddam MRG, Ganjifard M, Ghasemi S. Effects of ketorolac versus pethidine on the management of postoperative acute pain and complications after hemorrhoidectomy. J Surg Trauma. 2019;7(2):48‐54. [Google Scholar]
- 17. Mather LE, Meffin PJ. Clinical pharmacokinetics pethidine. Clin Pharmacokinet. 1978;3(5):352‐368. [DOI] [PubMed] [Google Scholar]
- 18. Linares‐Gil MJ, Valls J, Hereu‐Boher P, et al. Topical analgesia with lidocaine plus diclofenac decreases pain in benign anorectal surgery: randomized, Doubleblind, and controlled clinical trial. Clin Transl Gastroenterol. 2018;9(11):e210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ozer MT, Yigit T, Uzar AI, et al. A comparison of different hemorrhoidectomy procedures. Saudi Med J. 2008;29(9):1264‐1269. [PubMed] [Google Scholar]
- 20. Hollander D, Tarnawski A. The protective and therapeutic mechanisms of Sucralfate. Scand J Gastroenterol. 1990;25(sup173):1‐5. [DOI] [PubMed] [Google Scholar]
- 21. Koshariya M, Shitole A, Agarwal V, Dave S. Role of topical Sucralfate in healing of burn wounds. Int Surg J. 2018;5(9):2995. [Google Scholar]
- 22. Masuelli L, Tumino G, Turriziani M, Modesti A, Bei R. Topical use of Sucralfate in epithelial wound healing: clinical evidence and molecular mechanisms of action. Recent Pat Inflamm Allergy Drug Discov. 2010;4(1):25‐36. [DOI] [PubMed] [Google Scholar]
- 23. Rahimi R, Abdollahi M. A systematic review of the topical drugs for post Hemorrhoidectomy pain. Int J Pharmacol. 2012;8(7):628‐637. [Google Scholar]
- 24. Cengiz TB, Gorgun E. Hemorrhoids: A range of treatments. Cleve Clin J Med. 2019;86(9):612‐620. [DOI] [PubMed] [Google Scholar]
- 25. Fisher RS. Sucralfate: a review of drug tolerance and safety. J Clin Gastroenterol. 1981;3(Suppl 2):181‐184. [PubMed] [Google Scholar]
- 26. Stroup DF, Berlin JA, Morton SC. Meta‐analysis of observational studies in epidemiology: A proposal for reporting. JAMA. 2000;283(15):2008‐2012. [DOI] [PubMed] [Google Scholar]
- 27. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. BMJ. 2009;339:1‐8. [PMC free article] [PubMed] [Google Scholar]
- 28. Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomized trials. BMJ. 2019;366:l4898. 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 29. Higgins JPT. Measuring inconsistency in meta‐analyses. BMJ. 2003;327(7414):557‐560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14(1):135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ala S, Saeedi M, Eshghi F, Rafati M, Hejazi V, Hadianamrei R. Efficacy of 10% Sucralfate ointment in the reduction of acute postoperative pain after open Hemorrhoidectomy: A prospective, double‐blind, randomized, placebo‐controlled trial. World J Surg. 2013;37(1):233‐238. [DOI] [PubMed] [Google Scholar]
- 32. Albatanony A. Sucralfate ointment reduces pain and improves healing following haemorrhoidectomy: a prospective, randomized, controlled and double‐blinded study. Egypt J Surg. 2016;35(2):102. [Google Scholar]
- 33. A1‐Khateeb Y, Al‐Abdel Sattar A, Batanony A. Evaluation of the role of sucralfate cream in decreasing pain intensity and improving healing following open hemorrhoidectomy: a randomized controlled study. Menoufia Med J. 2019;32(2):506. [Google Scholar]
- 34. Alkhateep Y, Fareed A. Double blinded randomized placebo‐controlled comparative study between sucralfate ointment and lidocaine ointment after Milligan Morgan hemorrhoidectomy. Int Surg J. 2017;4(12):3822. [Google Scholar]
- 35. Gupta PJ, Heda PS, Kalaskar S, Tamaskar VP. Topical sucralfate decreases pain after hemorrhoidectomy and improves healing: A randomized, blinded, controlled study. Dis Colon Rectum. 2008;51(2):231‐234. [DOI] [PubMed] [Google Scholar]
- 36. Vejdan AK, Khosravi M, Amirian Z, et al. Evaluation of the efficacy of topical sucralfate on healing haemorrhoidectomy incision wounds and reducing pain severity: A randomized clinical trial. Int Wound J. 2020;17(4):1047‐1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chen M, Tang TC, He TH, Du YJ, Qin D, Zheng H. Management of haemorrhoids: protocol of an umbrella review of systematic reviews and meta‐analyses. BMJ Open. 2020;10(3):e035287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sammour T, Barazanchi AWH, Hill AG. Evidence‐based management of pain after excisional haemorrhoidectomy surgery: A PROSPECT review update. World J Surg. 2017;41(2):603‐614. [DOI] [PubMed] [Google Scholar]
- 39. Lyons NJR, Cornille JB, Pathak S, Charters P, Daniels IR, Smart NJ. Systematic review and meta‐analysis of the role of metronidazole in post‐haemorrhoidectomy pain relief. Colorectal Dis. 2017;19(9):803‐811. [DOI] [PubMed] [Google Scholar]
- 40. Simillis C, Thoukididou SN, Slesser AAP, Rasheed S, Tan E, Tekkis PP. Systematic review and network meta‐analysis comparing clinical outcomes and effectiveness of surgical treatments for haemorrhoids. Br J Surg. 2015;102(13):1603‐1618. [DOI] [PubMed] [Google Scholar]
- 41. Chierici A, Frontali A. Post‐Hemorrhoidectomy pain management: the latest news. Rev Recent Clin Trials. 2021;16(1):32‐38. [DOI] [PubMed] [Google Scholar]
- 42. Rodríguez‐Wong U, Rodríguez‐Medina U, Medina‐Murillo GR. Randomized clinical trial with topical diltiazem for post‐hemorrhoidectomy wound healing. Rev Gastroenterol Mex. 2019;84(1):119‐122. [DOI] [PubMed] [Google Scholar]
- 43. Selvarajan R. Efficacy of haemorrhoidectomy versus haemorrhoidectomy with internal sphincterotomy in treatment of haemorrhoids: a retrospective randomized controlled trial study. Int Surg J. 2021;8(3):839. [Google Scholar]
- 44. Uzzaman MM, Siddiqui MRS. A brief literature review on the management of post‐Haemorrhoidectomy pain. Surg Techniq Dev. 2011;1(2):e32. [Google Scholar]
- 45. Zodpe P, Cho JG, Kang HJ, Hwang SJ, Lee HM. Efficacy of Sucralfate in the postoperative Management of Uvulopalatopharyngoplasty. Archiv Otolaryngol Head Neck Surg. 2006;132(10):1082. [DOI] [PubMed] [Google Scholar]
- 46. Taskin U, Yigit O, Sezim Sisman A, Acioglu E, Gor AP. Efficacy of Sucralfate in the early postoperative improvement of pediatric thermal welding Adenotonsillectomy morbidity. Turk Otolarengoloji Arsivi/Turk Arch Otolaryngol. 2013;51(1):15‐19. [Google Scholar]
- 47. Alexander A. Efficacy of Sucralfate in alleviating pain and morbidity in post‐tonsillectomy patients‐ A randomized control trial. Glob J Otolaryngol. 2018;17(2):31‐36. [Google Scholar]
- 48. Alvandipour M, Ala S, Tavakoli H, Yazdani Charati J, Shiva A. Efficacy of 10% sucralfate ointment after anal fistulotomy: A prospective, double‐blind, randomized, placebo‐controlled trial. Int J Surg. 2016;36:13‐17. [DOI] [PubMed] [Google Scholar]
- 49. Nagashima R. Mechanisms of action of sucralfate. J Clin Gastroenterol. 1981;3(Suppl 2):117‐127. [PubMed] [Google Scholar]
- 50. Akkakraisee S, Mitrapanont R, Srinathom A. Topical sucralfate ointment for postoperative pain reduction after hemorrhoidectomy: systematic review. Clinic Acad. 2019;43(3):81‐90. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Bubble‐plot for meta‐regression. Meta‐regression analysis showed that the association between topical sucralfate treatment and VAS at 24 hours post‐operative was not significantly affected by age (A) and gender (B).
Figure S2 Bubble‐plot for meta‐regression. Meta‐regression analysis showed that the association between topical sucralfate treatment and VAS at 7 days post‐operative was not significantly affected by age (A), gender (B) and hemorrhoid grade 3 prevalence (C).
Figure S3 Bubble‐plot for meta‐regression. Meta‐regression analysis showed that the association between topical sucralfate treatment and VAS at 14 days post‐operative was not significantly affected by age (A), gender (B) and hemorrhoid grade 3 prevalence (C).
Figure S4 Bubble‐plot for meta‐regression. Meta‐regression analysis showed that the association between topical sucralfate treatment and wound healing rate at 28 days post‐operative was not significantly affected by age (A), gender (B) and hemorrhoid grade 3 prevalence (C).
Table S1 Literature search strategy.
Table S2 Results for the meta‐regression models for each outcomes of interest.
Data Availability Statement
Zenodo: Underlying data for ‘The Efficacy of Topical Sucralfate in Improving Pain and Wound Healing After Hemorrhoidectomy Procedure: A Systematic Review, Meta‐Analysis, and Meta‐Regression of Randomized Clinical Trials. https://doi.org/10.5281/zenodo.6507581 Data are available under the terms of the Creative Commons Zero "No rights reserved" data waiver (CC0 4.0 Public domain dedication).


