Abstract
Dietary exposure to mycotoxins is a matter of great concern in terms of public health and regulatory bodies worldwide. Contamination of meat products with mycotoxigenic fungi and production of aflatoxins (AFs), ochratoxin A (OTA) and other mycotoxins can occur at different points of the manufacturing steps, from farm to fork. Among all microorganisms, moulds (mycobiota) are groups of microorganisms that can contaminate dry-cured meats, so they may carry the risk of mycotoxicosis. Samarella (tsamarella in Greek) is one of Cyprus’s traditional, sun-dried and salted meat products. Mycological studies on this product have not been reported, and the risk of AFs or OTA has not been studied. This point of view aimed to conduct a survey study in terms of mycotoxin risk in samarella. With this aim, samples (n = 30) were collected from all commercial brands from markets in Northern Cyprus and analysed by ELISA. According to the results of this study, 14 of 30 and 9 of 30 samples were above Quantitative Measurement Limits (LOQ) for Total AFs, and AFB1, respectively. On the other hand, no result was obtained above LOQ for OTA. It was obtained that among all detectable results for total AFs, even the min result (5.3 μg/kg) was above 4 μg/kg, defined as a critical limit for directly consumed foods. None of the AFB1 and OTA results was above the determined critical limit.
Keywords: Mycotoxin, Samarella, Tsamarella, Aflatoxin, Ochratoxin A
Introduction
Dietary exposure to mycotoxins is a matter of great concern in terms of public health and regulatory bodies worldwide. Aflatoxins (AFs) and Ochratoxin A (OTA) are among the most critical mycotoxins, which continue to be a worldwide problem causing millions of dollars of losses also severe problems in human health and animal health (Perroe et al. 2019). Despite stringent legislation, many food products that exceed maximum permissible limits (MPLs) of AFs and other mycotoxins reach the market. Thus, complete removal of these contaminants is somehow not feasible or rather complicated (Chiocchetti et al. 2019). The challenge is that many developing countries and transitional nations are unaware of the prevalence of mycotoxins in animal products, while most do not have strict monitoring and surveillance practices regarding the safety of animal products, especially livestock products (Adegbeye et al. 2020). The resulting diseases because of mycotoxins are referred to as mycotoxicosis and characterised as carcinogenic, genotoxic, teratogenic, nephrotoxic, hepatotoxic, immunotoxic, amongst other debilitating clinical conditions (Benkerroum 2016) and even possible death in times of high exposure (Sherif et al. 2009; Paterson and Lima 2010). Contamination of meat products with mycotoxigenic fungi and production of AFs, OTA and other mycotoxins can occur at different points of the manufacturing steps, from the farm (animal feed contamination) to fork (consumption of end product) (Kademi et al. 2017).
The drying method can be accepted as one of the oldest preservation techniques used to prolong the shelf-life of foods. From this point of view, dried-cured meat products are widespread and well-known worldwide with a long history of safe use. Under poor hygienic conditions, those stable safety products can be contaminated with various harmful agents such as chemicals and microorganisms. Among all microorganisms, moulds (mycobiota) are groups of microorganisms that can contaminate dry-cured meat products. The predominant mycobiota of dry-cured meat, especially sun-dried and salted meat products, comprises Aspergillus, Fusarium and Penicillium species (Adeyeye 2016). Although there are certain moulds desirable to be present in dry-cured meat products, as they actively participate in the improvement of organoleptic qualities of these products, some of the fungal genera can contaminate dry-cured meats by producing certain mycotoxins such as AFs and OTA, which can produce toxicological and deleterious effects in humans (Montanha et al. 2018).
When meat-producing animals are fed with contaminated feeds and/or meat production ingredients are heavily contaminated, the most toxic groups of AFs (B1, B2, G1 and G2) residues can be present in meat and meat products (Markov et al. 2013; Amirkhizi et al. 2015; Montanha et al. 2018). Once the product is contaminated, decontamination may be very difficult. The reason for that is the resistance of mycotoxins to extreme environmental conditions and physical, chemical and biological treatments designed specifically for their inactivation or decontamination (Kabak 2009). Therefore, mycotoxins in meat and meat products must be intensively controlled from farm to fork (Asefa et al. 2011).
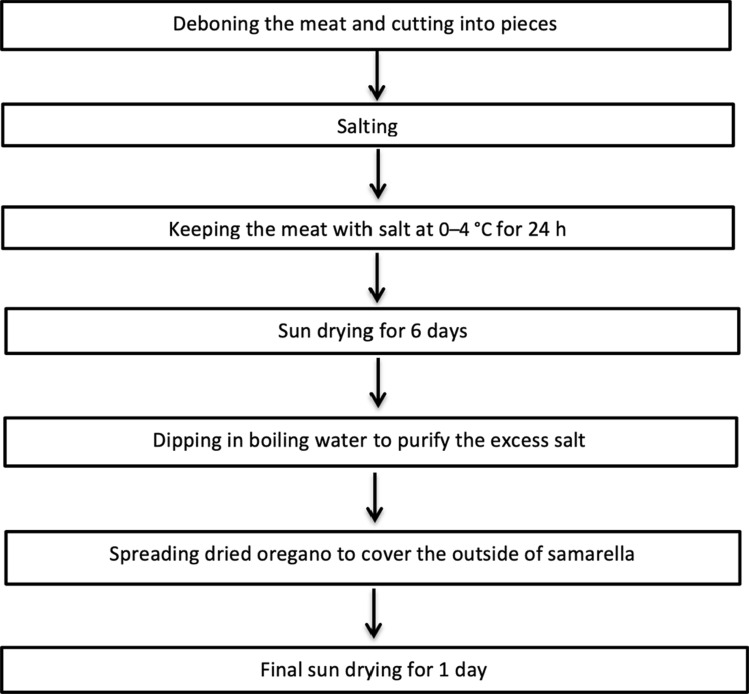
Traditionally known as “Cypriot pastrami”, samarella (tsamarella-τσαμαρελλα in Greek) is one of the kinds of sun-dried and salted meat made by deboned sheep and mainly goats’ meat (Ulusoy et al. 2018; Hakeri 2003; Kabataş 2007). It is trendy in both South and North parts of the Island and has been produced in the foothills of the Troodos Mountain in the past, especially in the areas of Paphos and Dillirga, to preserve meat, generally from the meat of mouflon (Ovis orientalis orientalis, is a subspecies group of the wild sheep in Cyprus) (Yorgancıoğlu 2000). Today, it is produced from the meat of goats or sheep of mature age and is still dried in the sun by traditional methods (Fig. 1). After the drying process, it is washed and sprinkled with dry oregano, giving the product a unique flavour. The traditional production flow chart of samarella (Tsamarella) is given in Fig. 2. Samarella is also a significant Cypriot meat product under the protection of The Slow Food Presidia Project (Anon 2020).
Fig. 1.
Traditional sun drying methods of samarella (Tsamarella)
Fig. 2.
Production flow chart of samarella (Tsamarella)
To the best of our knowledge, the information contained in the scientific sources about samarella is quite limited. Additionally, microbiological and mycological studies on this product have not been reported, and the risk of aflatoxin has not been studied. Therefore, it was aimed to conduct a survey study in terms of mycotoxin risk in samarella. With this aim, samples (n = 30) were collected from all commercial brands in markets of North Cyprus.
Materials and methods
Collection of samples
A total of 30 samarella samples (from all the manufacturers) were collected from supermarkets in different regions (Famagusta, Nicosia, Kyrenia and Morphous) of Northern Cyprus and transferred to the laboratory under the cold chain. Samples were kept in their original packages at 0–4 °C until analysed.
Extraction procedures
In order to determine the Total Aflatoxins, AFB1 and OTA levels in samarella samples, extraction procedures before ELISA were performed according to the manufacturers’ instructions (R-Biopharm Ag, Darmstadt, Germany).
Extraction procedure for Total Aflatoxin and AFB1 25 g sample was mixed with 80% methanol and was blended using a mixer at high speed for 2 min and centrifuged at 4000 rpm for 10 min. 12 ml of the supernatant obtained was diluted with 60 ml of 0.1% Tween 20 phosphate buffer solution (PBS). Diluted supernatant was filtered through filter paper, and the filtrate was passed through AflaPREP column (AFLAPREP®, Art. No.: RBRP07, R-Biopharm Ag, Darmstadt, Germany) at a flow rate of 2 ml/min. It was then passed through the column with 20 ml of PBS at a 5 ml/min flow rate. In the last step, mycotoxins retained in the column were eluted using 1 ml of 100% methanol; following elution passed 1 ml of water to a 2 ml total volume. The collected eluate was used in the total aflatoxin and AFB1 ELISA test.
Extraction procedure for OTA 50 g sample was homogenised, and 1 g of homogenised sample was mixed with 0.5 mL of 1 M phosphoric acid for 1 min in a vortex. Then 3 ml of ethyl acetate was added and mixed for 30 s in vortex again. This mixture was centrifuged at 2000 g for 1 min at room temperature to obtain the supernatant (this process was repeated twice). Then 3 ml 0.65 M sodium bicarbonate was added and mixed for 15 min. After centrifugation again at 2000 g for 5 min, the aqueous phase at the bottom of the tube was transferred to another glass tube and heated for 3 min at 100 °C. Then, cooled and 4 ml distilled water was added. Then, an aliquot was diluted with 0.13 M sodium bicarbonate at a ratio of 1:1 and was used in the ELISA test.
Immunoassay analysis of mycotoxins
Total Aflatoxin, AFB1 and OTA were quantified in accordance with the manufacturer’s instructions and guidelines using commercially available enzyme-linked immunosorbent assay (ELISA) kits (RidaScreen® Aflatoxin Total, Art. No.: R4701; RidaScreen® Ochratoxin A30/15, Art. No.: R1211; RidaScreen® Aflatoxin B1 30/15, Art. No.: R1311, R-Biopharm AG, Darmstadt, Germany). The analysis results (as optical density) of the samples were obtained using a microtiter plate reader (MR-96A Microplate reader, Mindray, Shenzhen, China). Test results were calculated according to the manufacturer’s instructions using RIDA SOFT soft software (version 1.96, R-Biopharm AG, Darmstadt, Germany).
Results and discussion
The American, Asian, African, and other European continents do not have legislation that indicates the maximum permitted of OTA or AFs in meat products as the other researchers also mentioned (Perrano et al. 2019; Montanha et al. 2018; Junker 2015; EU Regulation 1881/2006; EU Regulation 165/2010). According to the critical limits given in Annex of Commission Regulation (EC) No 1881/2006 (Setting Maximum Levels for Certain Contaminants in Foodstuffs) and also European Commission Regulation (EU) No. 165/2010 (for only aflatoxins), products (such as groundnuts, dried fruits) thereof, intended for direct human consumption or use as an ingredient in foodstuffs, the maximum level of total AFs should be 4 μg/kg, and AFB1 should be 2 μg/kg. Also, no limit is defined for OTA in meat products. Only in Italy, the Ministry of Health has recommended, since 1999, the maximum value of 1 μg/kg of OTA in meat or meat products (Montanha et al. 2018). Also, according to Commission Regulation (EC) No 1881/2006 regarding OTA; all products derived from unprocessed cereals, including processed cereal products and cereals intended for direct human consumption, should carry a maximum of 3 μg/kg, and wine varieties should carry a maximum of 2 μg/kg. Samarella is not similar to those mentioned foodstuffs, but the common point is to be a ready-to-consume product. All results for all samples are given in Table 1. As presented in Table 2, 14 of 30 samples (47%) was above quantitative measurement limits (LOQ) for Total AFs, 9 of 30 samples (30%) was above LOQ for AFB1and no result was obtained above LOQ for OTA. It was obtained that among all detectable results for total AFs, even the min result (5.3 μg/kg) was above 4 μg/kg, defined as a critical limit for directly consumed foods. None of the AFB1 and OTA results was above the determined critical limit.
Table 1.
Total Aflatoxin, Aflatoxin B1 and Ochratoxin A content in samerella samples (n = 30)
| Sample IDs | Total aflatoxin (ng/kg)* | Aflatoxin B1 (µg/kg)* | Ochratoxin A (ng/kg)* |
|---|---|---|---|
| 1 | < 50.00 | < 0.100 | < 1500 |
| 2 | < 50.00 | < 0.100 | < 1500 |
| 3 | < 50.00 | < 0.100 | < 1500 |
| 4 | 11,529 | 0.115 | < 1500 |
| 5 | 10,939 | 0.105 | < 1500 |
| 6 | 5278 | < 0.100 | < 1500 |
| 7 | < 50.00 | < 0.100 | < 1500 |
| 8 | < 50.00 | < 0.100 | < 1500 |
| 9 | < 50.00 | < 0.100 | < 1500 |
| 10 | < 50.00 | < 0.100 | < 1500 |
| 11 | 10,666 | < 0.100 | < 1500 |
| 12 | 11,906 | 0.101 | < 1500 |
| 13 | 12,452 | 0.109 | < 1500 |
| 14 | < 50.00 | < 0.100 | < 1500 |
| 15 | < 50.00 | < 0.100 | < 1500 |
| 16 | < 50.00 | < 0.100 | < 1500 |
| 17 | < 50.00 | < 0.100 | < 1500 |
| 18 | < 50.00 | < 0.100 | < 1500 |
| 19 | 9870 | < 0.100 | < 1500 |
| 20 | 11,073 | 0.110 | < 1500 |
| 21 | 12,152 | 0.110 | < 1500 |
| 22 | 5278 | < 0.100 | < 1500 |
| 23 | < 50.00 | < 0.100 | < 1500 |
| 24 | < 50.00 | < 0.100 | < 1500 |
| 25 | < 50.00 | < 0.100 | < 1500 |
| 26 | < 50.00 | < 0.100 | < 1500 |
| 27 | 10,241 | < 0.100 | < 1500 |
| 28 | 10,241 | 0.100 | < 1500 |
| 29 | 10,939 | 0.111 | < 1500 |
| 30 | 12,333 | 0.101 | < 1500 |
*Quantitative measurement limits (LOQ) for Total Aflatoxin, Aflatoxin B1 and Ochratoxin A are 50 ng/kg, 0.1 µg/kg and 1500 ng/kg, respectively
Table 2.
Mean, min–max values of results that above LOQ
| n = 30 | Total aflatoxin | Aflatoxin B1 | Ochratoxin A |
|---|---|---|---|
| # and % of samples above LOQ | 14/47 | 9/30 | 0/0 |
| Mean of the results above LOQ (µg/kg) | 10.3** | 0.11 | – |
| Max and min results (µg/kg) | 5.3–12.5** | 0.10–0.12 | – |
| # and % of samples above max levels* | 14/100 | 0/0 | 0 |
*4 μg/kg for total AFs, 2 μg/kg for AFB1, 2 μg/kg for Ochratoxin A (EC Com.)
**ng/kg values for total AFs in Table 1 were converted to μg/kg
In general, dry-cured and salted meats are recommended as shelf-stable and safe food products in terms of spoilage bacteria because of high salt content and low water activity (Aw). On the other hand, the high lipid content and salt concentration and the low Aw, supports the growth of xerotolerant and xerophilic fungi (Asefa et al. 2009); mainly belonging to the Penicillium and Aspergillus genera (Perrone et al. 2019). As reported in recent studies, there are beneficial species that contribute to the unique properties of dry-cured meats. Some of those are Penicillium nalgiovense, Penicillium chrysogenum, Penicillium solitum, and the recently identified one Penicillium salamii (Perrone et al. 2015). On the other hand, the others such as Penicillium nordicum, Penicillium verrucosum, Aspergillus westerdijkiae and Aspergillus ochraceus are mainly responsible for undesired mycotoxin production (Perrone et al. 2019; Asefa et al. 2009, 2011). Also, an increase of Aw in dry-cured meats may influence the metabolism of fungi may facilitate mycotoxins biosynthesis (Rodríguez et al. 2012). That can be because of the improper drying process. Nevertheless, unfortunately, as also Perrano et al. (2019) mentioned before, there are still few studies that monitor the presence of OTA and AFB1 in cured meats. As a result of the study on samarella conducted by Ulusoy et al (2018), the number of moulds was high (3.69 log10 CFU/g). Regarding the manufacturing process of samarella, it is expected that a high number of moulds may contaminate the product. Thus, it can explain the high level of total AFs (in 47% of samples) in the results we obtained. In general, the presence of mycotoxins in cured dry meat products could result from direct contamination as the secondary metabolites of moulds that are contaminated to the product during processing, handling and storage or from contaminated ingredients. Another possible pathway of contamination of meat products with mycotoxin is the carry-over effect which the mycotoxins are present in the meat itself, carried from the feed that animals consumed (Asefa et al. 2009, 2011; Comi and Iacumin 2013; Delgado et al. 2015; Perrone et al. 2019). However, low levels of mycotoxin contamination may not affect an animal’s growth or performance but may be carried to animals’ tissue products (Fowler et al. 2015).
The aflatoxins, namely B1, B2, G1 and G2, in relation to toxigenic fungi, are considered some of the most important mycotoxins in dry-cured meats (Montanha et al. 2018). AFs were detected in both fresh and processed muscles such as minced meat, burgers, luncheon (a type of dried meat), sausages and cured and aged meat products and offal meats like gizzard, liver, and kidney (Aziz and Youssef 1991; Refai et al. 2003; El-Desouky et al. 2014; Amirkhizi et al. 2015). In Croatia (Cvetnić and Pepeljnjak 1995), the prevalence of aflatoxigenic moulds in various smoke‐dried meat products was investigated. Four hundred twenty samples were collected from individual households in different regions of Croatia and analysed for aflatoxigenic moulds. A. flavus and A. parasiticus were present in 17.8% of the samples. The ability of AF‐producing moulds was tested in 75 isolates. A. flavus isolates produced mainly aflatoxin B1, at various concentrations from 1.4 to 3.12 mg/kg. Some isolates of A. parasiticus produced all four aflatoxins B1 B2 G1 G2, while the others produced AF B1 + G1 only, with AFs from 0.1 to 450 mg/kg. The prevalence of AFs in luncheon meat (which is a type of dried cured meat) was studied in Egypt by Ismail and Zaky (1999). Fifty (50) samples (25 each) were collected from two different companies and analysed, out of which seven (7) samples were positive for AFs. AFB1 was detected in 4 and 3 samples from the two different companies at concentrations between 0.5 and 11.1 μg/kg. Refai et al. (2003) investigated the incidence of AF residues in Basterma, a traditionally cured Egyptian meat. The samples were contaminated with total AFs in range of 2.8–47 μg/kg during the summer and 7.2–29 μg/kg in winter. We collected our samples at the period of the year between May to September, which are the months that the sun is sufficient for drying and samarella is commonly being produced. The results obtained by Refai et al. (2003) and the other reports mentioned above are similar to the ones we obtained.
The prevalence of surface moulds in some Croatian traditional dry-cured meats and correlation with AFB1 accumulation was studied by Zadravec et al. (2020). The authors reported that the contaminating moulds were Penicillium (79%), Aspergillus (11%), Eurotium (7%) and Mucor (4%). The maximal value of AFB1 was 1.92 μg/kg detected in 8% of the samples. We obtained the max value of AFB1 as 0.11 μg/kg, and 30% of the samples were detected to carry AFB1 amount above LOQ (0.10 μg/kg). Our study’s result was lower than the results reported by Zadravec et al. (2020). The occurrence of AFs in meat products marketed in Mansoura, Egypt, was evaluated by Abd-Elghany and Sallam (2015). A total of 50 samples (25 each of beef luncheon and beef burger) were purchased and analysed by VICAM AflaTest method. All the samples were contaminated with AFs. The mean values of AFs beef luncheon were 1.1 μg/kg, which are lower than we detected.
In addition to AFs, OTA is another important mycotoxin found in dry-cured meats (Volkel et al. 2011). It has been rated as a Group 2B carcinogen by the International Agency for Research on Cancer (Perrone et al. 2019). Considering the species Aspergillus carbonarius, A. westerdijkiae, A. steynii, A. Niger, A. ochraceus and the species of Penicillium verrucosum and P. nordicum are the primarily responsible fungi for the occurrence of OTA (Schmidt-Heydt et al. 2011; Montanha et al. 2018). OTA occurrence in meat products marketed in Mansoura, Egypt, was evaluated by Abd-Elghany and Sallam (2015). A total of 50 samples (25 each of beef luncheon and beef burger) were purchased and analysed by the OchraTest immunoaffinity fluorometric method. All the samples were contaminated with OTA. The mean values of OTA for beef luncheon were 5.23 μg/kg. Zadravec et al. (2020) obtained that OTA contamination was seen in 14% of samples, with concentration rising to the maximal 6.86 μg/kg in dry-cured traditional meat products of Croatia. None of the results we obtained for OTA analyses was above the critical limits.
Conclusion
There is a general need to develop specific standards and legislation regarding the maximum allowed levels of mycotoxins in dry-cured meat products; however, the presence of risk of those chemicals was presented in scientific researches. On the other hand, analyses of mycotoxins are focused on cereal, dairy and vegetable products but not on meat products. Primarily, dry-cured meats and samarella should be studied more. As far as we know, the presence of mycotoxins in cured meats is a real risk for consumers’ health so a hygienic management system based on HACCP (Hazard Analysis and Critical Control Points) should be conducted in samarella manufacturing. Much more fungi identification and mycotoxin survey studies should be performed for samarella. In our study, the results of total AFs analyses were high, but AFB1 and OTA analyses results were below the critical limits. Further fungi identification studies on the end product should be performed. Also, toxin and fungi studies should be conducted to understand if the risk of mycotoxin originated from the carry-over situation or not.
Authors' contributions
BHU Planned the experiment, evaluated the results, contribute writing manuscript. CH Planned the experiment, checked at the end. SS Planned the experiment, evaluated the results, contribute writing manuscript, performed the analyses. FKY Contribute writing manuscript, performed the analyses.
Funding
This research study was funded by Near East University Scientific Research Projects Unit with the project date 21/11/2017 and ID SAG-2017-1-066.
Data availability
Data and materials can be purchased from corresponding author is needed.
Declarations
Conflict of interest
Authors certify that there are no conflicts of interest with any organization, financial, or other regarding the material discussed in the manuscript.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abd-Elghany SM, Sallam KI. Rapid determination of total aflatoxins and ochratoxins A in meat products by immuno-affinity fluorimetry. Food Chem. 2015;179:253–256. doi: 10.1016/j.foodchem.2015.01.140. [DOI] [PubMed] [Google Scholar]
- Adegbeye MJ, Reddy PRK, Chilaka CA, Balogun OB, Elghandour MM, Rivas-Caceres RR, Salem AZ. Mycotoxin toxicity and residue in animal products: prevalence, consumer exposure and reduction strategies–A review. Toxicon. 2020;177:96–108. doi: 10.1016/j.toxicon.2020.01.007. [DOI] [PubMed] [Google Scholar]
- Adeyeye SAO. Fungal mycotoxins in foods: A review. Cogent Food Agric. 2016;2(1):1213127. doi: 10.1080/23311932.2016.1213127. [DOI] [Google Scholar]
- Amirkhizi B, Arefhosseini SR, Ansarin M, Nemati M. Aflatoxin B1 in eggs and chicken livers by dispersive liquid–liquid microextraction and HPLC. Food Addit Contam B. 2015;8(4):245–249. doi: 10.1080/19393210.2015.1067649. [DOI] [PubMed] [Google Scholar]
- Anonymous (2020). Slow food foundation for biodiversity. http://www.fondazioneslowfood.com/en/slow-food-presidia/tsamarella/ (Accessed on 1 May 2020)
- Asefa DT, Gjerde RO, Sidhu MS, Langsrud S, Kure CF, Nesbakken T, Skaar I. Moulds contaminants on Norwegian dry-cured meat products. Int J Food Microbiol. 2009;128:435–439. doi: 10.1016/j.ijfoodmicro.2008.09.024. [DOI] [PubMed] [Google Scholar]
- Asefa DT, Kure CF, Gjerde RO, Langsrud S, Omer MK, Nesbakken T, Skaar I. A HACCP plan for mycotoxigenic hazards associated with dry-cured meat production processes. Food Control. 2011;22(6):831–837. doi: 10.1016/j.foodcont.2010.09.014. [DOI] [Google Scholar]
- Aziz NH, Youssef YA. Occurrence of aflatoxins and aflatoxin-producing moulds in fresh and processed meat in Egypt. Food Addit Contam. 1991;8(3):321–331. doi: 10.1080/02652039109373981. [DOI] [PubMed] [Google Scholar]
- Benkerroum N. Mycotoxins in dairy products: a review. Int Dairy J. 2016;62:63–75. doi: 10.1016/j.idairyj.2016.07.002. [DOI] [Google Scholar]
- Chiocchetti GM, Jadán-Piedra C, Monedero V, Zúñiga M, Vélez D, Devesa V. Use of lactic acid bacteria and yeasts to reduce exposure to chemical food contaminants and toxicity. Crit Rev Food Sci Nutr. 2019;59(10):1534–1545. doi: 10.1080/10408398.2017.1421521. [DOI] [PubMed] [Google Scholar]
- Comi G, Iacumin L. Ecology of moulds during the pre-ripening and ripening of San Daniele dry cured ham. Food Res Int. 2013;54:1113–1119. doi: 10.1016/j.foodres.2013.01.031. [DOI] [Google Scholar]
- Commission European. Commission regulation (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off J Eur Union. 2006;364:365–324. [Google Scholar]
- Cvetnić Z, Pepeljnjak S. Aflatoxin-producing potential of Aspergillus flavus and Aspergillus parasiticus isolated from samples of smoked-dried meat. Food Nahrung. 1995;39(4):302–307. doi: 10.1002/food.19950390409. [DOI] [PubMed] [Google Scholar]
- Delgado J, Acosta R, Rodríguez-Martín A, Bernúdez E, Núñez F, Asensio MA. Growth inhibition and stability of PgAFP from Penicillium chrysogenum against fungi common on dry-ripened meat products. Int J Food Microbiol. 2015;205:23–29. doi: 10.1016/j.ijfoodmicro.2015.03.029. [DOI] [PubMed] [Google Scholar]
- El-Desouky TA, Mohamed SR, Abou-Arab AA, Salim AB. Occurrence of aflatoxin B1 and M1 in some Egyptian chicken organs and their affected by ozonated water. Open Sci J Mod Phys. 2014;1:24–30. [Google Scholar]
- European Commission Commission regulation (EU) No. 165/2010 of 26 February 2010, amending regulation (EC) No. 1881/2006 setting maximum levels for certain contaminants in foodstuffs as regards aflatoxin. Off J Eur Union L. 2010;50:8–12. [Google Scholar]
- Fowler J, Li W, Bailey C. Effects of a calcium bentonite clay in diets containing aflatoxin when measuring liver residues of aflatoxin B1 in starter broiler chicks. Toxins. 2015;7:3455–3464. doi: 10.3390/toxins7093455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakeri BH (2003). Kıbrıs Türkçesi Sözlüğu; SAMTAY Vakfı Yayınları; KKTC: Gazimağusa, Northern Cyprus
- Ismail MA, Zaky ZM. Evaluation of the mycological status of luncheon meat with special reference to aflatoxigenic moulds and aflatoxin residues. Mycopathologia. 1999;146(3):147–154. doi: 10.1023/A:1007086930216. [DOI] [PubMed] [Google Scholar]
- Juncker JC (2015) Commission regulation (EU) 2015/1137 of 13 July 2015. Official J Eur Union 14 of July 2015. https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32015R1137&from=FR
- Kabak B (2009) Prevention and management of mycotoxins in food and feed. In: Mycotoxins in food, feed and bioweapons. Springer, Berlin, Heidelberg. Pp 201–227
- Kabatas O. Kıbrıs Türkçesinin Etimilojik Sözlüğü. Ankara: Öncü Basımevi; 2007. [Google Scholar]
- Kademi HI, Baba IA, Saad FT. Modelling the dynamics of toxicity associated with aflatoxins in foods and feeds. Toxicol Rep. 2017;4:358–363. doi: 10.1016/j.toxrep.2017.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markov K, Pleadin J, Bevardi M, Vahčić N, Sokolić-Mihalak D, Frece J. Natural occurrence of aflatoxin B1, ochratoxin A and citrinin in Croatian fermented meat products. Food Control. 2013;34(2):312–317. doi: 10.1016/j.foodcont.2013.05.002. [DOI] [Google Scholar]
- Montanha FP, Anater A, Burchard JF, Luciano FB, Meca G, Manyes L, Pimpao CT. Mycotoxins in dry-cured meats: a review. Food Chem Toxicol. 2018;111:494–502. doi: 10.1016/j.fct.2017.12.008. [DOI] [PubMed] [Google Scholar]
- Paterson RRM, Lima N. How will climate change affect mycotoxins in food? Food Res Int. 2010;43(7):1902–1914. doi: 10.1016/j.foodres.2009.07.010. [DOI] [Google Scholar]
- Perrone G, Samson RA, Frisvad JC, Susca A, Gunde-Cimerman N, Epifani F, Houbraken J. Penicillium salamii, a new species occurring during seasoning of dry-cured meat. Int J Food Microbiol. 2015;193:91–98. doi: 10.1016/j.ijfoodmicro.2014.10.023. [DOI] [PubMed] [Google Scholar]
- Perrone G, Rodriguez A, Magistà D, Magan N. Insights into existing and future fungal and mycotoxin contamination of cured meats. Curr Opin Food Sci. 2019;29:20–27. doi: 10.1016/j.cofs.2019.06.012. [DOI] [Google Scholar]
- Refai MK, Niazi ZM, Aziz NH, Khafaga NEM. Incidence of aflatoxin B1 in the Egyptain cured meat basterma and control by γ-irradiation. Food Nahrung. 2003;47(6):377–382. doi: 10.1002/food.200390085. [DOI] [PubMed] [Google Scholar]
- Rodríguez A, Rodríguez M, Martín A, Nuñez F, Córdoba JJ. Evaluation of hazard of aflatoxin B1, ochratoxin A and patulin production in dry-cured ham and early detection of producing moulds by qPCR. Food Control. 2012;27:118–126. doi: 10.1016/j.foodcont.2012.03.009. [DOI] [Google Scholar]
- Schmidt-Heydt M, Graf E, Batzler J, Geisen R. The application of tran- scriptomics to understand the ecological reasons of ochratoxin a biosynthesis by Penicillium nordicum on sodium chloriderich dry cured foods. Trends Food Sci Tech. 2011;22:39–48. doi: 10.1016/j.tifs.2011.02.010. [DOI] [Google Scholar]
- Sherif SO, Salama EE, Abdel-Wahhab MA. Mycotoxins and child health: the need for health risk assessment. Int J Hyg Envir Heal. 2009;212(4):347–368. doi: 10.1016/j.ijheh.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Ulusoy B, Hecer C, Kaynarca D, Berkan Ş. Effect of oregano essential oil and aqueous oregano infusion application on microbiological properties of samarella (tsamarella), a traditional meat product of Cyprus. Foods. 2018;7(4):43. doi: 10.3390/foods7040043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkel I, Schroer-Merker E, Czerny CP. The carry-over of mycotoxins in pro-ducts of animal origin with special regard to its implications for the European food safety legislation. Food Nutr Sci. 2011;2:852–867. doi: 10.4236/fns.2011.28117. [DOI] [Google Scholar]
- Yorgancıoğlu OM. Kıbrıs Türk Folkloru “Duydum, Gördüm, Yazdım”. Lefkosa: Cambulat Basımevi; 2000. [Google Scholar]
- Zadravec M, Vahčić N, Brnić D, Markov K, Frece J, Beck R, Pleadin J. A study of surface moulds and mycotoxins in Croatian traditional dry-cured meat products. Int J Food Microbiol. 2020;317:108459. doi: 10.1016/j.ijfoodmicro.2019.108459. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data and materials can be purchased from corresponding author is needed.