ABSTRACT
Background
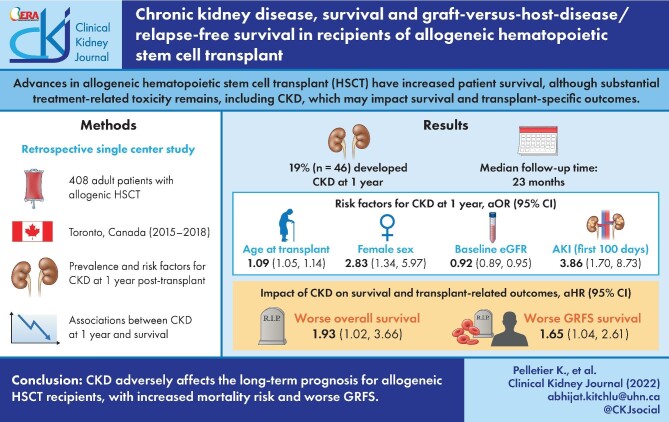
Advances in allogeneic hematopoietic stem cell transplant (HSCT) have increased patient survival, although substantial treatment-related toxicity remains, including chronic kidney disease (CKD). We assessed the association between CKD and survival and transplant-specific outcomes in HSCT recipients.
Methods
We conducted a retrospective study of all 408 adult patients with allogenic HSCT at Princess Margaret Cancer Centre (Toronto, Canada, 2015–18). We used logistic regression to identify risk factors for CKD at 1 year post-transplant. Associations between CKD at 1 year and overall survival, relapse-free survival, graft-versus-host-disease (GVHD)-free/relapse-free survival, relapse and transplant-related mortality were examined using extended time-varying Cox models. In a sensitivity analysis, we restricted the cohort to survivors at 1 year, using standard Cox proportional hazard models to examine associations between CKD and overall survival, relapse-free survival and GVHD-free/relapse-free survival, and Fine and Gray's competing risk models to determine associations between CKD and relapse/transplant-related mortality.
Results
The prevalence of CKD at 1 year was 19% (46 patients) with median follow-up of 23 months. Multivariable regression identified age at transplant [adjusted OR (aOR) 1.09, 95% confidence interval (95% CI) = 1.05–1.14; P < 0.0001), female gender (aOR 2.83, 95% CI = 1.34–5.97; P = 0.006) and acute kidney injury during the first 100 days (aOR 3.86, 95% CI = 1.70–8.73; P = 0.001) as risk factors for CKD at 1 year. Patients with CKD at 1 year had significantly poorer overall survival than those without CKD, when adjusted for relevant covariates [adjusted HR (aHR) 1.93, 95% CI = 1.02–3.66; P = 0.04 in the time-varying Cox model, and aHR 2.06, 95% CI = 1.04–4.07; P = 0.04 using the standard Cox model]. CKD at 1 year was also associated with worse GVHD-free/relapse-free survival (aHR 1.65, 95% CI = 1.04–2.61; P = 0.03).
Conclusions
CKD adversely affects the long-term prognosis for allogeneic HSCT recipients, with increased mortality risk and worse GVHD-free/relapse-free survival.
Keywords: allogeneic hematopoietic stem cell transplant, chronic kidney disease, GVHD-free/relapse-free survival, long-term survival
Graphical Abstract
Graphical Abstract.
INTRODUCTION
Hematopoietic stem cell transplantation (HSCT) is a potentially curative therapy for many life-threatening hematologic diseases [1]. Advances in the techniques used for HSCT have increased patient survival in the past decades, although substantial treatment-related toxicity remains [2]. Allogeneic transplantation usually involves a conditioning regimen of chemotherapy and radiation before the infusion of donor stem cells. Patients who undergo allogenic HSCT are at high risk of renal injury due to radiation, substantial incidence of sepsis and use of medications to prevent graft-versus-host disease (GVHD), such as calcineurin inhibitors [3]. Acute kidney injury (AKI) is a common complication following allogenic HSCT, with a reported incidence of 50–75% depending on the definition of AKI [3–5]. Chronic kidney disease (CKD) also frequently occurs in patients who undergo allogenic HSCT, with reported incidence rates between 7% and 66%, again depending on the definition used and the length of follow-up [4, 6].
CKD has important sequelae in patients post-HSCT. Apart from the increased cardiovascular risk, hypertension and metabolic sequelae, CKD may have specific implications in the post-HSCT population. This may include modification or reduction in immunosuppressive treatment (and associated GVHD-related risks), ineligibility for subsequent HSCT or further cancer therapies, and challenges with the use of antiviral agents and other post-transplant medications. CKD leads to end-stage kidney disease (ESKD) in about 4% of patients [7], with very high subsequent mortality [8]. Risk factors for CKD that have been identified in previous studies include age at transplant, history of AKI, GVHD and reduced pre-transplantation glomerular filtration rate [4, 7, 9–11]. However, published studies have yielded discrepant results regarding CKD effect on overall survival (OS) after allogenic HSCT. Moreover, many of the previous studies regarding CKD incidence, risk factors and outcome after HSCT were conducted with earlier HSCT protocols and kidney function estimation methods, and may not reflect current practice. Importantly, the association between CKD and the more recently defined outcome of GVHD-free/relapse-free survival (GRFS) [12], which represents the ideal recovery after allogeneic HSCT (i.e. reflecting survival in the absence of morbidity from both relapsed disease and GVHD), has not previously been examined.
We conducted a retrospective single-center study to determine prevalence and risk factors for CKD at 1 year after allogeneic HSCT in 408 adults with hematologic malignancies. We also assessed the impact of CKD at 1 year on OS, relapse-free survival (RFS), transplant-related mortality (TRM), relapse risk and GRFS.
MATERIALS AND METHODS
Study population and design
We conducted a retrospective cohort study of all adult patients who had allogeneic HSCT for any indication at our institution (Princess Margaret Cancer Center, Toronto, Canada) between 1 January 2015 and 1 January 2018. All patients who underwent an allogeneic HSCT during this period were included in our study. There was no exclusion on the basis of minimum follow-up period. Demographics, clinical characteristics and transplant-related variables were extracted from patient records and the institutional database. Our institutional database is a unique and centralized database that contains all clinical and laboratory data for all HSCT recipients for the entirety of the follow-up period. This study was approved by our institutional Ethics Board and the Cancer Registry Data Access Committee (CRDAC) and was conducted according to the guidelines of the Declaration of Helsinki.
Technique of allogenic HSCT
Patients received a conditioning regimen followed by infusion of donor hematopoietic cells on day zero, by convention. Myeloablative conditioning regimens (MACs) consisted of fludarabine 50 mg/m2 and busulfan 3.2 mg/kg × 4 days with total body irradiation (TBI) 400 cGy in a majority of patients who received this regimen. Reduced intensity conditioning regimen (RIC) was used in a majority of patients in this cohort and usually consisted of fludarabine 30 mg/m2 × 4 days and busulfan 3.2 mg/kg × 2 days with TBI 200 cGy. According to our radiation oncology protocol, the kidneys were not shielded during TBI because radiation dose received was significantly below threshold dose associated with radiation nephropathy [13].
For GVHD prophylaxis, the majority of patients received anti-thymocyte globulin (ATG) 0.5–4 mg, IV cyclophosphamide (PTCy) 50 mg/kg on days 3 and 4 post-transplant and calcineurin-inhibitor cyclosporine (CsA) 2.5 mg/kg twice a day. Standard prophylaxis for bacterial and viral infections included ciprofloxacin from day +6 to engraftment, acyclovir from day +6 to 1 year following transplant and trimethoprim-sulfamethoxazole starting at the time of engraftment and for a minimum of 1 year post-transplant. Standard fungal prophylaxis was IV micafungin followed by oral posaconazole upon engraftment. Cytomegalovirus (CMV) prophylaxis was not given as standard of care.
Definition of CKD and other covariates
The following initial parameters were collected for all patients: age, sex, initial hematologic disease, donor type, conditioning regimen, GVHD prophylaxis regimen, recipient CMV status, baseline serum albumin, baseline serum creatinine, length of initial hospitalization as well as prior diagnosis of hypertension and diabetes. Post-transplant complications, and their dates of first occurrence, were collected as follows: acute GVHD (aGVHD), chronic GVHD (cGVHD), veno-occlusive disease (VOD), thrombotic microangiopathy (TMA), CMV viremia, BK viremia, AKI during the first 100 days post-transplant, relapse, death and cause of death. Diagnosis and grading of aGVHD (grade 3–4 versus grade 1–2 or none) and cGVHD (moderate/severe versus mild or none) were done according to established criteria [14, 15] (see Supplementary data, Tables S1 and S2). AKI was defined and graded using the Kidney Disease: Improving Global Outcomes (KDIGO) criteria, based on peak creatinine value during first 100 days post-transplant [16]. Estimated glomerular filtration rate (eGFR) was calculated based on serum creatinine, using the validated CKD-EPI equation [17], at baseline (day 0), 100 days post-transplant, 1 year post-transplant and last follow-up. Definition of CKD was eGFR <60 mL/min/1.73 m2, based on at least two serum creatinine measurements obtained 30–90 days apart, and was assessed by two nephrologists. CKD stage was defined using the KDIGO definition [18].
OS was defined by patients who were still alive at the time of last follow-up. GRFS is a novel composite endpoint defined as the absence of grade 3–4 aGVHD, systemic therapy—requiring cGVHD, relapse or death [12]. TRM was defined as death due to any cause in the absence of relapse or progression of primary disease.
Statistical analyses
Descriptive data are reported as median (range), mean ± standard deviation or frequency of positive occurrences with their corresponding percentages. Characteristics of patients with or without CKD at 1 year were compared using Fisher's exact tests or chi-squared tests for categorical variables and two-sample independent t-tests or Wilcoxon tests for continuous variables. Repeated measures ANOVAs were used to compare mean eGFR of the cohort at different time points during follow-up. Univariable and multivariable logistic regression models were used to analyze the association between CKD at 1 year and the following covariates: age at transplant, sex, initial hematologic disease, type of donor, conditioning regimen, GVHD prophylaxis, length of initial hospitalization, hypertension, diabetes, aGVHD, cGVHD, VOD, TMA, CMV viremia, BK viremia and AKI during the first 100 days post-transplant. Variables with statistical relevance (P < 0.10) and/or considered predictors of interest were retained for the final multivariable model.
Associations between CKD and OS, RFS, GRFS, TRM and relapse were examined in two different ways using multivariate analyses. In the primary model, we used extended Cox proportional hazard models and extended Fine and Gray's competing risk models, where CKD was treated as a time-varying covariate that could potentially change status at the 1 year time point. This permitted us to examine the associations between CKD and survival and transplant outcomes for the full cohort. The date of allogeneic HSCT served as the time-origin for survival analysis (i.e. the index date). In a subsequent sensitivity analysis, we restricted the cohort to patients who survived to 1 year post-transplant and examined associations between CKD status at 1 year (treated as a fixed, baseline covariate) and long-term survival and transplant outcomes. For this (multivariate) analysis, standard Cox proportional hazard models were used to examine associations between CKD and OS, RFS and GRFS, whereas Fine and Gray's competing risk models were used to determine associations between CKD and relapse and TRM. The date 1 year post-allogeneic HSCT served as the index date in these models. Again, to build each of the multivariable models, variables with P < 0.10 from the univariable analyses were chosen, on which a backward selection method was applied to retain variables with statistical relevance (P < 0.05). Variables with clinical importance were also included, regardless of their statistical significance. Kaplan–Meier plots were generated to depict the association between CKD at 1 year and OS. In a sensitivity analyses, we also reported results of a model with variable selection based only on the lowest P-values in univariate testing.
Statistical significance threshold was considered for a two-tailed P-value < 0.05. All analyses were conducted using the SAS 9.4 software (Cary, NC, USA).
RESULTS
Study cohort
The study included 408 adult patients, with a median follow-up time of 22.5 (range 1.0–70.0) months. Baseline demographic and clinical data for the entire cohort are presented in Table 1. Median age at transplantation was 57 (range 18–74) years and 40% of our cohort was 60 years old or above. The most frequent indication for allogenic HSCT was acute myelogenous leukemia (49%). Hypertension was present in 37% of patients, and diabetes mellitus in 13% of patients. Seventeen (4%) patients had CKD at baseline. Median serum creatinine value at baseline was 0.8 (range 0.5–1.5) mg/dL and mean eGFR at baseline was 94 ± 18 mL/min/1.73 m2. Overall incidence of AKI at 100 days was 64% (262 patients; stage 1: 62.6%, stage 2: 22.5% and stage 3: 14.8%). Dialysis was required in five (2%) patients; four of them recovered renal function after AKI, but three died within 1 year post-transplant. Only 13 (3%) patients were referred to nephrology at any point during follow-up. CMV viremia and aGVHD were both frequent post-transplant complications, occurring, respectively, in 52% and 49% of the patients.
Table 1.
Patient demographic data and clinical characteristics at baseline (n = 408)
| Patient characteristic | |
|---|---|
| Age at transplantation (years) ≥60 years old |
57 (18–74) 163 (40) |
| Female | 171 (42) |
| Diagnosis Acute myelogenous leukemia Myelodysplastic syndrome Chronic myelogenous leukemia Lymphoma Acute lymphocytic leukemia Chronic lymphocytic leukemia Chronic myelomonocytic leukemia Myelofibrosis Other |
199 (49) 66 (16) 13 (3) 23 (6) 28 (7) 18 (4) 9 (2) 31 (8) 21 (5) |
| Conditioning regimen Reduced intensity Myeloablative conditioning |
318 (78) 90 (22) |
| Stem cell source Bone marrow Peripheral blood |
8 (2) 400 (98) |
| GHVD prophylaxis ATG-PTCy-CSA Other |
253 (62) 155 (38) |
| Baseline kidney function | |
| Baseline serum creatinine (mg/dL) | 0.8 (0.5–1.5) |
| Baseline eGFR (mL/min/1.73 m2)a | 96 (42–143) |
| CKD at baselineb | 17 (4) |
| Comorbidities Hypertension Diabetes |
152 (37) 55 (13) |
| Positive CMV serostatus | 309 (76) |
| Complications during first 100 days AKI (any stage) AKI stage ≥ 2 RRT CMV viremia BK viremia VOD TMA aGVHD |
262 (64) 98 (37) 5 (2) 212 (52) 39 (10) 56 (14) 11 (3) 200 (49) |
Values are expressed as frequency (%) or median (range). GVHD = graft-versus-host disease; ATG-PTCy-CSA = anti-thymocyte globulin with post-transplant cyclophosphamide and cyclosporine; eGFR = estimated glomerular filtration rate; CKD = chronic kidney disease; CMV = cytomegalovirus; AKI = acute kidney injury; RRT = renal replacement therapy; VOD = veno-occlusive disease; TMA = thrombotic microangiopathy; aGVHD = acute graft-versus-host disease.
aBaseline eGFR is calculated according to the CKD-EPI equation.
bCKD at baseline is defined as eGFR <60 mL/min/1.73 m2.
Changes in eGFR following transplant and prevalence of CKD
Over time change in median eGFR for the entire cohort is presented in Figure 1. Mean ± SD eGFR was 87 ± 20 mL/min/1.73 m2 at 100 days post-transplant, 82 ± 23 mL/min/1.73 m2 at 1 year and 79 ± 22 mL/min/1.73 m2 at last follow-up (all P < 0.0005). In total, 32 patients (9%) had CKD 100 days post-transplant, and 46 patients (19%) had CKD 1 year post-transplant. Of those, 33 patients (72%) had stage 3a CKD, 11 patients (24%) had stage 3b and 2 patients (4%) had stage 4. At last follow-up, the prevalence of CKD among survivors remained stable at 19% (35/189 patients). The mean decline in kidney function (eGFR) within the first-year post-transplantation was 13.5 ± 16.4 mL/min/1.73 m2. Between 1 year and last follow-up, the mean decline in eGFR was 6.6 ± 6.9 mL/min/1.73 m2 per year.
FIGURE 1:
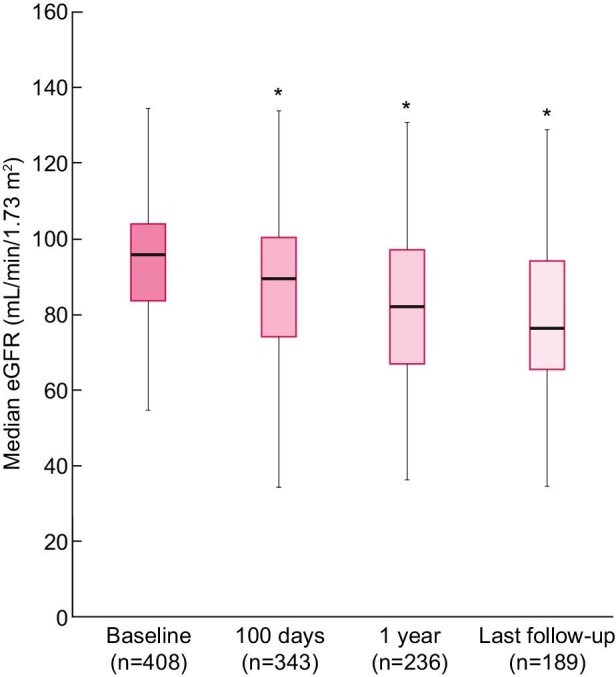
Median eGFR of the cohort among alive patients at baseline (time of transplant), 100 days post-transplant, 1 year post-transplant and last follow-up. Median total follow-up time was 22.5 (range 1.0–70.0) months. Box plots show 25th and 75th percentiles.
Clinical characteristics of patients with CKD
Descriptive analyses comparing patients with and without CKD at 1 year are given in Table 2. Compared with patients with normal kidney function, patients with CKD at 1 year were older [median age at transplant 62 (range 43–74) versus 54 (range 18–74)]; P < 0.001) and more frequently female (57% versus 39%; P = 0.04). Thirty-five patients (76%) with CKD at 1 year experienced AKI during the first 100 days after transplant, which is significantly more than patients with no CKD (52%; P = 0.002). Past medical history of hypertension and diabetes was more frequent in patients with CKD at 1 year (50% versus 33%; P = 0.03 and 17% versus 7%; P = 0.05, respectively). There was no difference between the groups regarding conditioning regimen, GVHD prophylaxis regimen, aGVHD, cGVHD, TMA, VOD, BK viremia and CMV viremia.
Table 2.
Descriptive analysis for survivors with and without CKDa at 1 year (n = 236)
| Variable | No CKD (n = 190) | CKD (n = 46) | P-value |
|---|---|---|---|
| Age at transplant (years) | 54 (18–74) | 62 (43–74) | <0.001 |
| Female gender | 75 (39) | 26 (57) | 0.04 |
| Hypertension | 63 (33) | 23 (50) | 0.03 |
| Diabetes | 14 (7) | 8 (17) | 0.05 |
| Baseline albumin (g/L) | 39 (30–49) | 39 (32–44) | 0.18 |
| Length of initial hospitalization (days) | 28 (17–219) | 32 (22–75) | 0.04 |
| Conditioning regimen MAC RIC |
45 (24) 145 (76) |
5 (11) 41 (89) |
0.06 |
| AKI within 100 days post-transplant |
28 (15) | 16 (35) | 0.002 |
| AKI stage 1 2 3 |
72 (38) 20 (11) 7 (4) |
11 (24) 22 (48) 7 (15) |
0.002 |
| Recipient CMV positive | 142 (75) | 33 (72) | 0.68 |
| CMV viremia | 88 (46) | 25 (54) | 0.33 |
| BK viremia | 9 (5) | 4 (9) | 0.29 |
| TMA | 5 (3) | 1 (2) | 0.99 |
| VOD | 17 (9) | 8 (17) | 0.11 |
| GVHD prophylaxis regimen ATG-PTCy-CSA Other |
126 (66) 64 (34) |
29 (63) 17 (37) |
0.67 |
| aGVHD Grade 1–2 Grade 3–4 |
74 (76) 23 (24) |
23 (88) 3 (12) |
0.24 |
| cGVHD Mild Moderate to severe |
18 (9) 53 (28) |
5 (11) 15 (33) |
0.47 |
| Baseline creatinine (mg/dL) | 0.8 (0.6–1.2) | 0.9 (0.6–1.4) | <0.001 |
| Baseline eGFR (mL/min/1.73 m2) | 97 (61–143) | 77 (46–110) | <0.001 |
| eGFR at 100 days (mL/min/1.73 m2) | 94 (51–134) | 62 (34–99) | <0.001 |
| CKD at baselinea | 0 (0) | 6 (13) | <0.001 |
| CKD at 100 daysa | 4 (2) | 20 (43) | <0.001 |
| eGFR at 1 year (mL/min/1.73 m2) | 88 (61–131) | 50 (15–60) | <0.001 |
| 1 year eGFR slope (mL/min/1.73 m2) | –8.0 (–56.8 to 34.2) | –24.6 (–82.4 to 2.9) | <0.001 |
Values are expressed as frequency (%) or median (range). CKD = chronic kidney disease; MAC = myeloablative conditioning; RIC = reduced intensity regimen; AKI = acute kidney injury; CMV = cytomegalovirus; VOD = veno-occlusive disease; TMA = thrombotic microangiopathy; GVHD = graft-versus-host disease; ATG-PTCy-CSA = anti-thymocyte globulin with post-transplant cyclophosphamide and cyclosporine; aGVHD = acute graft-versus-host disease; cGVHD = chronic graft-versus-host disease; eGFR = estimated glomerular filtration rate; eGFR is calculated according to CKD-EPI equation.
aCKD is defined as eGFR < 60 mL/min/1.73 m2.
Risk factors for CKD at 1 year
Univariable analysis identified age at transplant (P < 0.0001), baseline eGFR (P < 0.0001), female gender (P = 0.04), hypertension (P = 0.04), diabetes (P = 0.05), AKI within 100 days (P = 0.004) and MAC regimen (P = 0.05) to be significantly associated with CKD at 1 year (Table 3). In the multivariable model, age at transplant [adjusted OR (aOR) 1.09, 95% confidence interval (95% CI) = 1.05–1.14; P < 0.0001], female sex (aOR 2.83, 95% CI = 1.34–5.97; P = 0.006) and AKI during the first 100 days (aOR 3.86, 95% CI = 1.70–8.73; P = 0.001) remained significant risk factors for developing CKD at 1 year (Table 3, see also Supplementary data, Table S3).
Table 3.
Univariable and multivariable analysis for risk factors for CKD at 1 year
| Variable | Univariable analysisOR (95% CI) | P-value | Multivariable analysisOR (95% CI) | P-value |
|---|---|---|---|---|
| Age at transplant | 1.09 (1.05–1.13) | <0.0001 | 1.09 (1.05–1.14) | <0.0001 |
| Female gender | 2.01 (1.05–3.88) | 0.04 | 2.83 (1.34–5.97) | 0.006 |
| Hypertension | 2.02 (1.05–3.89) | 0.04 | ||
| Diabetes | 2.51 (0.98–6.41) | 0.05 | 1.63 (0.58–4.56) | 0.35 |
| Baseline eGFR | 0.91 (0.89–0.94) | <0.0001 | ||
| AKI within 100 days post-transplant | 2.95 (1.41–6.16) | 0.004 | 3.86 (1.70–8.73) | 0.001 |
| Transplant regimen (MAC versus RIC) | 0.37 (0.14–0.99) | 0.05 | ||
| GVHD prophylaxis (ATG-PTCy-CSA versus others) | 0.91 (0.47–1.78) | 0.79 | ||
| aGVHD (grade 3–4) | 0.53 (0.15–1.87) | 0.32 | ||
| cGVHD (moderate/severe) | 1.17 (0.58–2.34) | 0.66 | 1.31 (0.60–2.84) | 0.50 |
Univariable and multivariable logistic regression results are presented. Patients who died within 1 year post-transplant were removed from analyses. Due to lack of power, only five variables were included in the multivariable model to avoid over-fit. For this reason, hypertension and baseline eGFR were excluded from the model. Diabetes was included in the model as it is a recognized major risk factor for CKD. We also retained cGVHD in the model (although nonsignificant in univariate analysis), as cGVHD has been reported as a significant risk factor for kidney disease after transplantation in multiple recent studies [19–21]. Alternative multivariate model incorporating baseline eGFR and using only variables with the lowest P-value in univariate analysis is provided as Supplementary material.
OR = odds ratio; CKD = chronic kidney disease; AKI = acute kidney injury; MAC = myeloablative conditioning; RIC = reduced intensity regimen; GVHD = graft-versus-host disease; ATG-PTCy-CSA = anti-thymocyte globulin with post-transplant cyclophosphamide and cyclosporine; aGVHD = acute graft-versus-host disease; cGVHD = chronic graft-versus-host disease.
Impact of CKD on survival and transplant outcomes
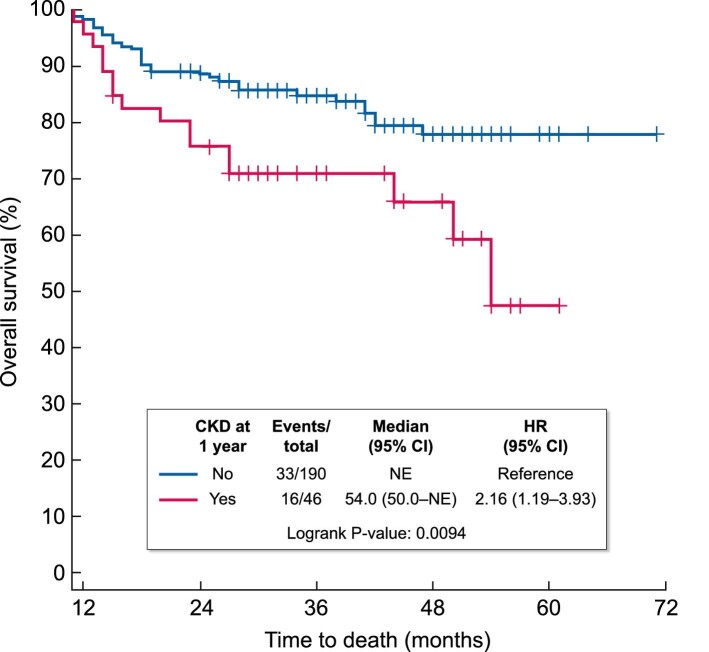
The overall mortality rate in the cohort was 54% (219/408 patients), and 47 of these patients died after 1 year post-transplant. Amongst the 46 patients who developed CKD 1 year after transplant, 6 died from relapse of primary disease, 6 died from infectious complications and 3 died from other causes. Patients with CKD at 1 year showed significantly poorer OS than those without CKD [hazard ratio (HR) for mortality 2.16, 95% CI = 1.19–3.93; P = 0.009), as shown in Figure 2. When adjusted for age, conditioning regimen, GVHD prophylaxis, diabetes, aGVHD (extended Cox model only) and cGVHD, CKD remained significantly associated with mortality (aHR 1.93, 95% CI = 1.02–3.66; P = 0.04) using a time-varying extended Cox model among the entire cohort (Table 4). Similarly, in the sensitivity analysis (using the standard Cox model restricted to survivors at 1 year), CKD was again associated with worse OS (aHR 2.06, 95% CI = 1.04–4.07; P = 0.04) (Table 5). Acute GVHD was excluded from the standard Cox model as it is an early complication of HSCT (within 100 days post-transplant), and this model looked only at survivors at 1 year post-transplant. In both models, CKD at 1 year had no significant impact on RFS, TRM and relapse risk. However, it was associated with worse GRFS (HR 1.65, 95% CI = 1.04–2.61; P = 0.03) among survivors at 1 year (Table 5).
FIGURE 2:
Overall survival at last follow-up based on CKD at 1 year status. Time origin is 1 year post-transplant, n = 236 (survivors at 1 year).
Table 4.
Impact of CKD at 1 year on survival and transplant outcomes using time-varying approach
| Mortality | RFS | GRFS | TRM | Relapse | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value |
| CKD at 1 year | 1.93 (1.02–3.66) | 0.04 | 1.73 (0.84–3.53) | 0.13 | 1.43 (0.53–3.89) | 0.48 | 1.47 (0.61–3.56) | 0.39 | 1.28 (0.42–3.89) | 0.66 |
| Age at transplant | 1.01 (0.99–1.02) | 0.35 | 1.01 (1.00–1.02) | 0.06 | 1.01 (0.99–1.02) | 0.16 | 1.04 (1.02–1.06) | 0.0007 | 0.99 (0.97–1.00) | 0.12 |
| Diabetes | 2.26 (1.52–3.38) | <0.0001 | 1.95 (1.25–3.04) | 0.003 | ||||||
| Conditioning regimen (MAC versus RIC) |
0.89 (0.57–1.40) | 0.61 | 0.91 (0.58–1.41) | 0.66 | 1.10 (0.80–1.51) | 0.58 | 1.04 (0.58–1.85) | 0.90 | 0.74 (0.40–1.40) | 0.35 |
| GVHD prophylaxis (ATG-PTCy-CSA versus others) |
0.49 (0.34–0.71) | 0.0001 | 0.69 (0.47–1.01) | 0.05 | 0.52 (0.40–0.68) | <0.0001 | 0.73 (0.48–1.11) | 0.14 | 0.89 (0.53–1.49) | 0.65 |
| aGVHD (grade 3–4) |
1.88 (1.25–2.83) | 0.0002 | 1.98 (1.29–3.06) | 0.002 | ||||||
| cGVHD (moderate/severe) |
0.35 (0.22–0.54) | <0.0001 | 0.36 (0.24–0.55) | <0.0001 | 0.15 (0.06–0.38) | <0.0001 | ||||
In this model, associations between CKD and OS, RFS, GRFS, TRM and relapse were examined using extended Cox proportional hazard models and extended Fine and Gray's competing risk models, where CKD was treated as a time-varying covariate that could potentially change status at 1 year time point. This permitted us to examine the associations between CKD and survival and transplant outcomes for the full cohort. The date of allogeneic HSCT served as the time-origin for survival analysis.
To build each of the multivariable models, variables with P < 0.10 from the univariable analyses (not shown) were chosen, on which backward selection method was applied to retain variables with statistical relevance (P < 0.05). Variables with clinical importance (i.e. variables associated with worse outcomes in previous published studies) were also included, regardless of their statistical significance.
CKD = chronic kidney disease; RFS = relapse-free survival; GRFS = graft-versus-host disease-free/relapse-free survival; TRM = transplant-related mortality; HR = hazard ratio; MAC = myeloablative conditioning; RIC = reduced intensity conditioning regimen; GVHD = graft-versus-host-disease; aGVHD = acute graft-versus-host disease; cGVHD = chronic graft-versus-host disease; ATG-PTCy-CSA = anti-thymocyte globulin with post-transplant cyclophosphamide and cyclosporine.
Table 5.
Impact of CKD at 1 year on survival and transplant outcomes among survivors at 1 year
| Mortality | RFS | GRFS | TRM* | Relapse | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value |
| CKD at 1 year | 2.06 (1.04–4.07) | 0.04 | 1.41 (0.75–2.64) | 0.29 | 1.65 (1.04–2.61) | 0.03 | 2.05 (0.82–5.09) | 0.12 | 1.27 (0.55–2.93) | .57 |
| Age at transplant | 1.00 (0.98–1.02) | 0.97 | 1.00 (0.98–1.02) | 0.68 | 0.99 (0.97–1.00) | 0.04 | 0.98 (0.96–1.01) | 0.20 | ||
| Diabetes | 2.99 (1.41–6.35) | 0.004 | 2.53 (1.24–5.15) | 0.01 | 1.30 (0.75–2.28) | 0.35 | 3.70 (1.30–10.58) | 0.01 | 1.67 (0.65–4.31) | 0.29 |
| Conditioning regimen (MAC versus RIC) |
0.73 (0.33–1.61) | 0.43 | 0.87 (0.30–2.53) | 0.78 | ||||||
| GVHD prophylaxis (ATG-PTCy-CSA versus others) |
0.56 (0.31–1.06) | 0.08 | 0.61 (0.32–1.15) | 0.13 | 0.76 (0.52–1.11) | 0.16 | 0.23 (0.09–0.57) | 0.002 | 1.47 (0.60–3.61) | 0.40 |
| cGVHD (moderate/severe) |
0.45 (0.22–0.93) | 0.03 | 0.41 (0.21–0.81) | 0.009 | 0.27 (0.09–0.77) | 0.01 | ||||
In this model, patients who died within 1 year post-transplant were excluded from the analyses. Impact of CKD at 1 year and other variables of interest on long-term (>1 year) survival and transplant outcomes were analyzed using Cox proportional hazard models (OS, RFS and GRFS) and Fine and Gray's completing risk model (TRM and relapse). The date 1 year post-allogeneic HSCT served as the index date in this model.
*There were only 21 events for TRM, thus limiting the amount of variables it was possible to include in the analyses.
CKD = chronic kidney disease; RFS = relapse-free survival; GRFS = graft-versus-host disease-free/relapse-free survival; TRM = transplant-related mortality; HR = hazard ratio; MAC = myeloablative conditioning; RIC = reduced intensity conditioning regimen; GVHD = graft-versus-host-disease; cGVHD = chronic graft-versus-host disease; ATG-PTCy-CSA = anti-thymocyte globulin with post-transplant cyclophosphamide and cyclosporine.
DISCUSSION
Our study explored the development of CKD after allogeneic HSCT and its impact on survival outcomes in a large, contemporary cohort of adult recipients. We found a significant decline in kidney function within the first year post-transplantation for the entire cohort, with a mean loss in eGFR of 13.5 ± 16.4 mL/min/1.73 m2. Prevalence of CKD, defined as eGFR <60 mL/min/1.73 m2, was 19% at 1 year post-transplant and at last follow-up. We identified age at transplant, female gender and history of AKI within first 100 days post-transplant as significant risk factors for CKD at 1 year. Furthermore, we demonstrated that CKD at 1 year was significantly associated with higher mortality as well as worse GRFS, when adjusting for relevant covariates.
Early data regarding the prevalence of CKD after HSCT were highly variable, mainly due to the heterogenicity of the included patients and type of transplant [22]. In the largest cohort study to date, with 1635 HSCT patients, the incidence rate of CKD was 23% in survivors 100 days after transplant [19]. In retrospective studies among long-term survivors after allogeneic HSCT, cumulative incidence of CKD was 25–34% at 10 years [10, 11]. Hingorani et al. [20] assessed a prospective cohort of 434 adults followed for a median of 5.3 years post-transplant. In keeping with our results, ∼20% of the cohort developed CKD by 1 year post-HSCT, and the largest decrease in kidney function occurred within the first year after transplantation, with eGFR decreasing from a median of 98 mL/min/1.73 m2 at baseline to 78 mL/min/1.73 m2 by 1 year. Moreover, as eGFR declined, risk of mortality progressively increased.
Previously, studies assessing the association with CKD and mortality have led to mixed results [11, 21, 23]. Furthermore, these results are difficult to compare as they include children and adult recipients, have relatively small sample sizes and are from earlier eras of transplant. Indeed, recent studies reported substantial improvement in the outcomes of patients having HSCT in the last decade, even if older patients were treated in recent years [24, 25]. The reasons for this improvement were mainly less organ damage from conditioning regimen, fewer infections during immune suppression, and lower incidence of severe aGVHD. For this reason, contemporary data assessing CKD and mortality risk were needed. We demonstrated an increased risk of overall mortality associated with CKD 1 year after transplant. Moreover, we demonstrated that CKD was associated with worse GRFS among survivors at 1 year, whereas it was not associated with RFS and TRM. Taken together, these results reinforce the association between development of CKD post-transplant and poorer survival outcomes in long-term survivors of HSCT, as relapse and TRM usually present within the first year post-transplant. GRFS is a clinically meaningful composite endpoint that acknowledges both survival and rates of other critical complications from transplant and its treatment, therefore representing an ‘ideal’ recovery from HSCT. To our knowledge, this is the first time the association between CKD post-transplant and GRFS has been studied. Our results highlight the importance of preserving kidney function post-transplant, as it affects long-term survival of patients.
In various prior studies, CKD has been mainly associated with older age at transplant, female gender, AKI after transplant, hypertension, use of calcineurin inhibitors and TBI [7, 9–11, 26–28]. Importantly, the use of RIC as opposed to MAC was not associated with risk of CKD in our study. Few studies have examined the association between conditioning regimen and subsequent CKD, although specific entities such as radiation nephritis are well described and potentially associated with increased dose of TBI [6, 22]. Our data suggest that patients who may be otherwise considered for MAC should not necessarily be precluded from this on the basis of concerns regarding long-term kidney function alone. Acute and chronic GVHD have recently emerged as another risk factor for kidney injury after transplantation [19–21]. In our study population, however, neither acute (grade 3–4) nor chronic (moderate–severe) GVHD was significantly associated with CKD at 1 year. On the other hand, the high incidence of AKI in our cohort and its strong association with further development of CKD, as well as a significant decline in eGFR within the first year post-transplant, suggest that early intervention may be key to improving kidney outcome. In our study population, although AKI occurred in 64% of patients and CKD developed in 19%, only 13 (3%) patients were referred to nephrology. This indicates that better collaborative care between hematologists and nephrologists is needed, with the aim of improving kidney protection. Moreover, future studies should be designed to explore potential early therapeutic intervention, such as the use of renin–angiotensin system (RAS) blockade medication or sodium–glucose transport protein 2 (SGLT2) inhibitors.
Strengths of our study include a large cohort, composed of contemporary HSCT recipients with a wide number of clinical indications. We were able to include several clinically relevant covariates, including patient comorbidities, transplant characteristics (including conditioning regimen and GVHD prophylaxis regimen) and initial post-transplant complications. We were also able to assess clinically relevant outcomes, including longitudinal kidney function via CKD-EPI, and GRFS and TRM (in addition to OS). The association between CKD status at 1 year and OS was consistent in both the primary and sensitivity analyses.
Our study has some limitations. First is the number of patients who died over the time period of the study, limiting our power to perform broader multivariable analyses for risk factors of CKD and survival outcomes. Second is the duration of follow-up, which was relatively short considering that the incidence of CKD may accumulate over time; however, it is important to note that the majority of eGFR loss in this population appears to occur within the first year post-transplant. In the same vein, one could argue that we looked late, and ask why we did not look at the incidence of CKD at 6 months and its effect on survival and transplant outcomes. However, because CKD diagnosis necessitates at least two creatinine measurements obtained at a stable state 30–90 days apart, we felt more confident to identify ‘true’ CKD patients at 1 year post-transplant. Having looked at CKD at 6 months would have posed the risk of including patients with AKI in the early post-transplant period. Lastly, we did not have urinary data such as urinary protein excretion. As such, milder stages of CKD with hyperfiltration were not included in this study. Patients post-HSCT in our center do not routinely have monitoring of proteinuria via urine albumin-to-creatinine ratio or urinalyses, and given the low rate of referral to nephrology, we lack such data to assist with CKD prognostication. Given the association we observed between CKD and mortality, incorporation of this routine screening for proteinuria in patients post-HSCT may be a consideration, particularly in those with significant eGFR decline post-HSCT.
In conclusion, CKD is a common comorbidity among adult survivors of allogeneic HSCT, and AKI early after transplant appears as the main risk factor for the development of CKD. Presence of CKD adversely affects the long-term prognosis of patients after allogeneic HSCT, with increased risk of mortality. The association between CKD and worse GRFS outcome was also demonstrated for the first time with this study. The increasing number of transplants performed, and in older patients, will lead to an increase in the burden of kidney disease for the health care system. A collaborative, interdisciplinary approach, with early recognition of kidney injury and preventive measures for renal protection after transplantation, may be beneficial for the improvement of outcomes in long-term survivors of allogeneic HSCT.
DATA AVAILABILITY STATEMENT
The data underlying this article cannot be shared publicly to protect the privacy of individuals who participated in the study. The data will be shared on reasonable request to the corresponding author.
Supplementary Material
ACKNOWLEDGEMENTS
This study was approved by Princess Margaret Cancer Center (Toronto, Canada) Ethics Board and the Cancer Registry Data Access Committee (CRDAC).
Contributor Information
Karyne Pelletier, Department of Medicine, Division of Nephrology, University Health Network, Toronto, Canada.
Gabrielle Côté, Department of Medicine, Division of Nephrology, University Health Network, Toronto, Canada.
Kayla Madsen, Hans Messner Allogeneic Blood and Marrow Transplantation Program, Division of Medical Oncology and Hematology, Princess Margaret Cancer Centre, University Health Network, Toronto, Canada.
Shiyi Chen, Hans Messner Allogeneic Blood and Marrow Transplantation Program, Division of Medical Oncology and Hematology, Princess Margaret Cancer Centre, University Health Network, Toronto, Canada.
S Joseph Kim, Department of Medicine, Division of Nephrology, University Health Network, Toronto, Canada.
Christopher T Chan, Department of Medicine, Division of Nephrology, University Health Network, Toronto, Canada.
Jonas Mattsson, Hans Messner Allogeneic Blood and Marrow Transplantation Program, Division of Medical Oncology and Hematology, Princess Margaret Cancer Centre, University Health Network, Toronto, Canada.
Ivan Pasic, Hans Messner Allogeneic Blood and Marrow Transplantation Program, Division of Medical Oncology and Hematology, Princess Margaret Cancer Centre, University Health Network, Toronto, Canada.
Abhijat Kitchlu, Department of Medicine, Division of Nephrology, University Health Network, Toronto, Canada.
AUTHORS’ CONTRIBUTIONS
Study design was done by A.K., K.P. and G.C.; data acquisition was carried out by K.M., K.P. and G.C.; data analysis/interpretation was performed by A.K., K.P., G.C., S.J.K., K.M., I.P., C.T.C. and J.M.; statistical analysis was done by S.C., K.P. and A.K. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Majhail NS, Farnia SH, Carpenter PAet al. Indications for autologous and allogeneic hematopoietic cell transplantation: guidelines from the American Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant 2015; 21: 1863–1869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gooley TA, Chien JW, Pergam SAet al. Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med 2010; 363: 2091–2101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ganguli A, Sawinski D, Berns JS. Kidney diseases associated with haematological cancers. Nat Rev Nephrol 2015; 11: 478–490 [DOI] [PubMed] [Google Scholar]
- 4. Hingorani S. Renal complications of hematopoietic-cell transplantation. N Engl J Med 2016; 374: 2256–2267 [DOI] [PubMed] [Google Scholar]
- 5. Renaghan AD, Jaimes EA, Malyszko Jet al. Acute kidney injury and CKD associated with hematopoietic stem cell transplantation. Clin J Am Soc Nephrol 2020; 15: 289–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hingorani S. Chronic kidney disease in long-term survivors of hematopoietic cell transplantation: epidemiology, pathogenesis, and treatment. J Am Soc Nephrol 2006; 17: 1995–2005 [DOI] [PubMed] [Google Scholar]
- 7. Ando M, Ohashi K, Akiyama Het al. Chronic kidney disease in long-term survivors of myeloablative allogeneic haematopoietic cell transplantation: prevalence and risk factors. Nephrol Dial Transplant 2010; 25: 278–282 [DOI] [PubMed] [Google Scholar]
- 8. Cohen EP, Piering WF, Kabler-Babbitt C, Moulder JE. End-stage renal disease (ESRD) after bone marrow transplantation: poor survival compared to other causes of ESRD. Nephron 1998; 79: 408–412 [DOI] [PubMed] [Google Scholar]
- 9. Touzot M, Elie C, van Massenhove Jet al. Long-term renal function after allogenic haematopoietic stem cell transplantation in adult patients: a single-centre study. Nephrol Dial Transplant 2010; 25: 624–627 [DOI] [PubMed] [Google Scholar]
- 10. Shimoi T, Ando M, Munakata Wet al. The significant impact of acute kidney injury on CKD in patients who survived over 10 years after myeloablative allogeneic SCT. Bone Marrow Transplant 2013; 48: 80–84 [DOI] [PubMed] [Google Scholar]
- 11. Jo T, Arai Y, Kondo Tet al. Chronic kidney disease in long-term survivors after allogeneic hematopoietic stem cell transplantation: retrospective analysis at a single institute. Biol Blood Marrow Transplant 2017; 23: 2159–2165 [DOI] [PubMed] [Google Scholar]
- 12. Pasquini MC, Logan B, Jones RJet al. Blood and marrow transplant clinical trials network report on the development of novel endpoints and selection of promising approaches for graft-versus-host disease prevention trials. Biol Blood Marrow Transplant 2018; 24: 1274–1280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cohen EP, Robbins ME. Radiation nephropathy. Semin Nephrol 2003; 23: 486–499 [DOI] [PubMed] [Google Scholar]
- 14. Przepiorka D, Weisdorf D, Martin Pet al. 1994 Consensus conference on acute GVHD grading. Bone Marrow Transplant 1995; 15: 825–828 [PubMed] [Google Scholar]
- 15. Jagasia MH, Greinix HT, Arora Met al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant 2015; 21: 389–401.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. KDIGO Group . KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Inter Suppl 2012; 2: 1–138 [Google Scholar]
- 17. Levey AS, Stevens LA, Schmid CHet al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. KDIGO Group . KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int Suppl 2013; 3: 1–150 [DOI] [PubMed] [Google Scholar]
- 19. Hingorani S, Guthrie KA, Schoch Get al. Chronic kidney disease in long-term survivors of hematopoietic cell transplant. Bone Marrow Transplant 2007; 39: 223–229 [DOI] [PubMed] [Google Scholar]
- 20. Hingorani S, Pao E, Stevenson Pet al. Changes in glomerular filtration rate and impact on long-term survival among adults after hematopoietic cell transplantation: a prospective cohort study. Clin J Am Soc Nephrol 2018; 13: 866–873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sakellari I, Barbouti A, Bamichas Get al. GVHD-associated chronic kidney disease after allogeneic haematopoietic cell transplantation. Bone Marrow Transplant 2013; 48: 1329–1334 [DOI] [PubMed] [Google Scholar]
- 22. Ellis MJ, Parikh CR, Inrig JKet al. Chronic kidney disease after hematopoietic cell transplantation: a systematic review. Am J Transplant 2008; 8: 2378–2390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sakaguchi M, Nakayama K, Yamaguchi Het al. Risk factors for acute kidney injury and chronic kidney disease following allogeneic hematopoietic stem cell transplantation for hematopoietic malignancies. Acta Haematol 2020; 143: 452–464 [DOI] [PubMed] [Google Scholar]
- 24. McDonald GB, Sandmaier BM, Mielcarek Met al. Survival, nonrelapse mortality, and relapse-related mortality after allogeneic hematopoietic cell transplantation: comparing 2003–2007 versus 2013–2017 cohorts. Ann Intern Med 2020; 172: 229–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Penack O, Peczynski C, Mohty Met al. How much has allogeneic stem cell transplant-related mortality improved since the 1980s? A retrospective analysis from the EBMT. Blood Adv 2020; 4: 6283–6290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Glezerman IG, Devlin S, Maloy Met al. Long term renal survival in patients undergoing T-cell depleted versus conventional hematopoietic stem cell transplants. Bone Marrow Transplant 2017; 52: 733–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Abboud I, Porcher R, Robin Met al. Chronic kidney dysfunction in patients alive without relapse 2 years after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2009; 15: 1251–1257 [DOI] [PubMed] [Google Scholar]
- 28. Kal HB, van Kempen-Harteveld ML. Renal dysfunction after total body irradiation: dose–effect relationship. Int J Radiat Oncol Biol Phys 2006; 65: 1228–1232 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article cannot be shared publicly to protect the privacy of individuals who participated in the study. The data will be shared on reasonable request to the corresponding author.