Abstract
Background:
From a chemistry point of view, we hypothesized that superlative dual cytotoxicity-radical scavenging bioefficacies of series 4 FQs correlate to their acidic groups and C8-C7 ethylene diamine Chelation Bridge.
Methodology:
Newly synthesized 16 lipophilic-acid chelating FQs have been screened for in vitro duality of proliferation inhibition and radical scavenging capacities.
Results:
Substantially in LPS prompted RAW264.7 macrophages inflammation, IC50 values (µM) in the ascending order of new FQs’ NO scavenging/antiinflammation capacity were 4e<4b<3d<4f<5c<indomethacin (17.6<25.5<27.7<38.5<46.1< indomethacin’s 55.1, respectively). Exceptionally reduced FQ 4b exhibited comparable DPPH radical scavenging capacity to ascorbic acid (IC50 values (µM) 4.3 vs. 3.4, p>0.05). In comparison to classical and robust antineoplastic agent cisplatin and unlike triazoloFQs; nitroFQs (3a, 3b and 3f) and the reduced FQs (4a, 4c, 4d and 4e) exerted antiproliferation IC50 values <50 µM in leukaemia K562. Besides nitroFQ 3, the reduced FQs (4c and 4f) exhibited antineoplastic IC50 values <50 µM in lung A549 carcinoma. NitroFQ 3c and reduced FQs 4b, 4c, and 4f in breast MCF7 and reduced 4c in pancreatic PANC1 had reduction of viability IC50 values <50 µM. NitroFQ 3e, reduced FQs 4b and, 4c and triazoloFQ 5a exerted antiproliferation IC50 values <50 µM in breast T47D cells. Also nitroFQ 3e, reduced FQ 4c and triazoloFQ 5f exhibited antineoplastic IC50 values <50 µM in PC3 prostate cancer cells. Exceptionally triazoloFQ 5a, but neither nitro- nor reduced FQs, had cytotoxicity IC50 value <50 µM in resistant melanoma A375 cells. Unequivocally 4b antineoplastic effectiveness linked with its radical scavenging and antiinflammation effects while 3d and 5c lacked matching antiproliferation potentialities to their exquisite antiinflammation capacities. Explicitly reduced 4e and 4f exerted antiinflammation-selective cytotoxicity duality in vitro.
Conclusion:
Collectively, this work reveals lipophilic-acidic chelator FQs as authentic agents for the repurposing approach in anticancer chemotherapy/prevention.
Key Words: Fluoroquinolones and Triazoloquinolones, NO-radical scavenging, Sulphorodhamine B
Introduction
Incidence rates and prevalence of cancer are substantially high globally with difficulties involving cost, resistance and intolerable side effects of existing chemotherapy. Moderate increases of ROS contribute to several pathologic conditions, among which are tumor promotion and progression as well as inducing DNA mutation and programmed cell death. Tumor cells can principally develop a mechanism where they adjust to the high ROS by expressing elevated levels of antioxidant proteins to detoxify them while maintaining pro-tumorigenic signaling and resistance to apoptosis (Arfin et al., 2021). However ROS-based therapeutic strategies can act as Trojan horses to eliminate cancer cells (Perrillo et al., 2020). Moreover, several epidemiological studies have demonstrated that Inflammation is strictly associated with cancer and plays a key role in tumor development and progression. Targeting inflammation and the molecules involved in the inflammatory process could represent a good strategy for cancer prevention and therapy. Moreover, clinical studies have demonstrated that many anti-inflammatory agents, including non-steroidal anti-inflammatory drugs (NSAIDs) could intervene with the tumor microenvironment by reducing cell migration and increasing apoptosis and chemo-sensitivity. Among many, COX-2 inhibitors were emphasized as novel anti-inflammatory agents with significant anticancer activity (Rayburn et al., 2009; Zappavign et al., 2020). Moreover, Indomethacin was ascribed from anti-inflammatory agent to anticancer therapy (Kassab, 2018). Conventional market FQs such as Ciprofloxacin and levofloxacin were reported to attenuate microglia inflammatory response via TLR4/NF-kB pathway (Zusso et al., 2019). Recently antilipolytic-Antiproliferative Activities of Novel AntidiabesityTriazolo/Fluoroquinolones were substantially signified for anti-inflammatory responses (Arabiyat et al., 2020) exceeding the sensitive and robust indomethacin in against LPS-induced nitric oxide production in studied cells. Essentially more optimally Functionalized FQs as a Potentially Novel Class of Anti-inflammatory and Anti-glycation Compounds were subjected to Pharmacological Appraisal (Hamdan et al., 2019; AlShahrabi et al., 2020). This kind of activity can suggestively be associated with the chelating properties possessed by the synthesized FQs (Hamdan et al., 2019). Drug repurposing pharmacology of new safe therapeutic drugs has emerged as an alternative approach to overcome high toxicity and poor safety profile of clinical anticancer agents. Vosaroxin is a commercial fluoroquinolone (FQ) with antineoplastic propensities in acute myeloid leukemia comparable to anticancer agents doxorubicin and cisplatin (Bruno et al., 2020). Newly developed synthetic chelator Fluoroquinolones (FQs) had the scaffold for FQs but carried extra lipophilic and chelator groups as structural functionalities for optimal cytotoxicities similar to doxorubicin (Arabiyat, et al., 2016a,b; 2019; Khaleel et al., 2021; AlHallaq et al., 2022; Qashou et al., 2022). Our hypothesis was based to dual lipophilic- acidic chelating FQs with a genuine potential of antiproliferative propensities based to their dual DPPH- and NO- radicals scavenging biocapacities using cell based – and colorimetric assays vs. respective reference agents as their molecular action mechanism. In this work, 16 lipophilic-acid chelating FQs have been screened against seven different cancer cell lines (melanoma A375; breast T47D and MCF7, prostate PC3; Lung A549, Pancreatic Panc-1, and Leukaemia K562) using sulforhodamine B colorimetric bioassay vs. cisplatin. Parameters including potency, toxicity, and selectivity (potency/toxicity) have been reported along with DPPH- and NO- radicals’ scavenging propensities in comparison to ascorbic acid and indomethacin respectively. Their selectivity indices for safety examination were calculated using normal periodontal ligament fibroblasts (PDL) in comparison to cisplatin’s lack of differential cytotoxicity.
Materials and Methods
(Supplementary)
Results
DPPH radical- and RAW264.7 cell line NO radical- scavenging properties of tested compounds of new 3 FQs series (Table 1).
Table 1.
Selectivity Index (SI) and IC50 Values (μM; µg/mL) of in vitro DPPH-radical and RAW264.7 Cell Line NO- radical scavenging properties of the tested compounds of new 3 FQs series vs. respective reference agents
| Treatment | DPPH radical scavenging IC50 value µM (μg/mL)+ | NOS- IC50 value μM (µg/mL)++ |
IC50 PDL Fibro µM (µg/mL) | SI |
|---|---|---|---|---|
| Nitrous FQs series | ||||
| 3a | NI | 121.33±2.02**** | 368.83±23.08**** | 10.35 (K562) |
| (52.1± 0.87) | (158.37±9.91) | |||
| 3b | 4086.1±405.59**** | 351.39± 39.95*** | 153.10±17.13*** | 11.14 (K562) |
| (1754.73±174.18) | (150.9± 17.15) | (65.75±7.36) | ||
| 3c | 71.59±14.22** | 98.74± 9.96** | 407.61±11.38**** | 21.61 (MCF7) |
| (30.74±6.10) | (42.4± 4.28) | (175.03±4.89) | ||
| 3e | 640.82±28.50**** | 71.39± 5.28** | 81.96±9.84*** | 1.24 (A549) |
| (313.45±13.94) | (32.8± 2.4248711) | (37.66±4.52) | ||
| 3d | 440.16±39.85**** | 27.67± 3.37*** | 10.62±1.15*** | 1.83 (T47D) |
| (202.22±18.31) | (13.53± 1.65) | (5.19±0.56) | ||
| 3f | NI | 181.55± 18.05*** | NI | NI |
| (77.97± 7.75) | ||||
| Reduced FQ series | ||||
| 4a | 37.67±3.03*** | 84.21± 9.83** | 51.45±7.11*** | 8.68 (K562) |
| (15.05±1.21) | (33.63± 3.93) | (20.55±2.84) | ||
| 4b | 3.61±0.31NS | 25.54± 2.62*** | 38.05±2.97**** | 3.03 (MCF7) |
| (1.55±0.13) | (10.97± 1.12) | (16.34±1.27) | ||
| 4c | 276.81±28.69**** | 76.78± 5.38** | 95.31±2.55**** | 27.15 (K562) |
| (110.56±11.46) | (30.67± 2.15) | (38.07±1.02) | ||
| 4d | 251.32±28.81*** | 63.49± 6.08ns | 95.29±12.28*** | 10.19 (K562) |
| (107.93±12.37) | (27.27± 2.61) | (40.92±5.27) | ||
| 4e | 229.45±19.08**** | 17.63± 3.07**** | 7.52±0.14**** | 0.36 (K562) |
| (105.42±8.77) | (8.1± 1.41) | (52.49±3.45) | ||
| 4f | 80.17±15.92** | 38.51± 2.90** | 6.07±1.14** | 0.36 (MCF7) |
| (30.74±6.10) | (14.77± 1.12) | (2.33±0.44) | ||
| TriazoloFQ series | ||||
| 5a | 7891.68±886.16*** | 82.73± 9.94** | 9.31±1.45*** | 0.42 (T47D) |
| (3238.67±363.67) | (33.95± 4.08) | (3.82±0.59) | ||
| 5b | 1716.52±237.77*** | 130.09± 2.81**** | 240.05±11.87**** | 3.40 (K562) |
| (737.14±102.11) | (55.87± 1.21) | (103.09±5.10) | ||
| 5c | 193.48±21.20*** | 46.13± 3.79NS | 350.70±23.26**** | 3.65 (A375.s2) |
| (79.4±8.7) | (18.93± 1.55) | (143.92±9.54) | ||
| 5f | 1743.04±191.73**** | 103.42± 10.19** | 96.75±3.23**** | 8.14 (K562) |
| (609±66.99) | (36.13± 3.55) | (33.80±1.13) | ||
| Reference Drug | Ascorbic 3.37±0.29 | Indomethacin | Cisplatin | 1.01 (A375.s2) |
| (1.55±0.13) | 55.06± 2.25 | 0.71 ± 0.13 | ||
| (26.93± 1.10) | (0.21 ± 0.04) | |||
Results are mean ± SD (n = 3 independent replicates). + IC50 values (concentration at which 50% inhibition of DPPH in comparison to non-induced basal 30 minutes incubations or cell proliferation in comparison to non-induced basal incubations) were calculated within testing dose range. ++ The IC50 value is the concentration at which 50% inhibition of Nitric oxide synthase took place in comparison to non-induced basal 24h incubations. P-value is calculated by unpaired t-test between test compound IC50 values μM and ascorbic acid IC50 values μM (DPPH) or Indomethacin IC50 values μM (NOS) using GraphPad Prism software version 8.0.1 * When P<0.05 and ** when P<0.01 or 0.001, *** when P< 0.001or 0.0001 , **** when P<0.0001, NS: not significantly different from reference agent. Bolded numerals stand out as the least IC50 values (most active) among others enlisted in the same tested cell line. NI, Non-Inhibitory.
Exceptionally in LPS prompted RAW264.7 macrophages inflammation using Griess assay, NitroFQ 3d NO scavenging IC50 value was < 50µM (with IC50 value (µM) 27.67 vs. indomethacin’s 55.06) but lacking on DPPH scavenging activity in 96well based colorimetric assay (Table 1). Reduced FQ 4b was proven for equipotency to vitamin C and highly significantly > potent than NO-scavenging anti-inflammatory indomethacin (the same table). Besides reduced 4e was most potent anti-inflammatory FQ (with the least IC50 value (µM) 17.63 vs. indomethacin’s 55.06) but lacking on comparable DPPH scavenging propensities to ascorbic acid (Table 1). Reduced FQ 4f had not DPPH scavenging bioactivity but exhibited substantially > NO-scavenging/anti-inflammatory potency than indomethacin (with IC50 value (µM) 38.51 vs. indomethacin’s 55.06). 5c was the Fonly triazoloFQ that could demonstrate a promising euipotency to indomethacin (with IC50 value (µM) 46.13 vs. indomethacin’s 55.06; p>0.05) without similar DPPH scavenging effectiveness (the same Table 1). The rest of the 3 series of new FQS were significantly ineffective radical scavengers as compared to either ascorbic acid or indomethacin (p<0.01-0.001). Most notably, none of the tested compounds of the 3 new FQs series had cytotoxity over 72h incubations as checked by SRB bioassay.
Antiproliferative activity of the tested compounds in new 3 FQs series
In this work, 16 lipophilic-acid chelating FQs have been screened against seven different cell lines (Malignant Melanoma A375; Leukaemia K562; breast T47D and MCF7 cancer and prostate cancer cell PC3 lines; Lung cancer cell A549 and Pancreatic1 Panc-1cancer) in comparison to the cisplatin, using sulforhodamine B colorimetric bioassay (Table 2). Their spectrum of selectivity indices values for safety examination was calculated in normal periodontal ligament fibroblasts (PDL)-based 72h incubactions in comparison to cisplatin’s lack of differential cytotoxicity (Table 1).
Table 2.
Cytotoxicity (as of %Control) IC50 Values in µM (µg/mL) of the Tested Compounds of New 3 FQs Series vs. Cisplatin
| Treatment | A549 | A375.s2 | MCF7 | PANC-1 | PC-3 | K562 | T47D |
|---|---|---|---|---|---|---|---|
| Nitrous FQs series | |||||||
| 3a | 1003.52± 140.23*** | 173.69± 21.29*** | 70.91 2.14* | 1935.38± 211.18**** | 526.55 ±37.51* | 35.63 ±6.12 | 309.67 ±6.19**** |
| (430.9± 60.21) | (74.58± 9.14) | (30.45± 0.92) | (831.03 ±90.68) | (226.10 ±16.11) | (15.30 ±2.63) | (132.97 ±2.66) | |
| 3b | 79.96± 12.26*** | 405.76± 60.03*** | 122.88± 7.80* | 2541.43± 51.14**** | 303.12 ±30.58** | 13.74 ±1.32** | 302.47 ±10.75**** |
| (34.34± 5.26) | (174.25± 25.78) | (52.77± 3.35) | (1091.39± 21.96) | (130.17 ±13.13) | (5.90±0.57) | (129.89 ±4.61) | |
| 3c | 149.15± 22.13*** | 393.83± 10.13**** | 18.86± 2.31*** | NI | 1065.26±140.57** | 588.82 ±32.51**** | 710.82± 90.34** |
| (64.04± 9.50) | (169.11± 4.35) | (8.10 ±0.99) | (457.41± 60.36) | (252.83± 13.96) | (305.22 v3.79) | ||
| 1310.5± 194.14*** | 285.14±38.54** (131.00±17.71) | 182.48± 12.20**** | 148.74 ±8.84**** | 211.64 ±24.11*** | 606.45 ±25.20**** | ||
| 3d | 66.05±9.60*** | (602.07± 89.19) | (83.83 ±5.61) | (68.33 ±4.06) | (97.23 ±11.08) | (278.61 ±11.58) | |
| (30.34±4.41) | |||||||
| 3e | 45.14± 6.64** | 56.25± 6.90*** | 79.87± 10.43 | 99.28± 5.86**** | 49.61± 5.30**** | 54.90 ±1.39* | 5.80 ±0.22**** |
| (22.08± 3.24) | (27.52± 3.37) | (39.07± 5.10) | (48.56 ±2.87) | (24.27± 2.59) | (22.53 ±0.57) | (2.84 ±0.11) | |
| 3f | NI | 1323.73±120.05*** | 162.62± 3.378*** | NI | NI | 38.54 ±0.49* | NI |
| (553.51± 50.20) | (68.00± 1.41) | (16.55 ±0.21) | |||||
| Reduced FQs series | |||||||
| 4a | 83.60± 7.36**** | 151.55± 19.98*** | 62.77± 5.31* | 172.53± 15.0**** | 255.17 ±45.32*** | 8.68 ±1.67** | 327.96± 26.79**** |
| (33.39±2.94) | 60.53 ±7.98 | 25.07± 2.12 | (68.91± 5.99) | 101.92 ±18.10 | 3.47 ±0.67 | (130.99 ±10.70) | |
| 4b | 75.42± 8.78*** | 50.99± 6.26*** | 12.57 ±0.66*** | 308.62 ±38.38*** | 131.79± 17.67**** | 37.96 ±6.47 | 44.04 ±3.41 |
| (32.39± 3.77) | 21.9 ±2.69 | (5.40 ±0.28 ) | (132.54 ±21.05) | (56.60 ±7.59) | (16.30 ±2.78) | (18.91± 1.47) | |
| 4c | 12.29± 2.17 | 75.81± 10.89*** | 16.14± 2.26*** | 30.41± 4.77** | 13.14± 2.55**** | 3.51 ±0.66*** | 6.73 ±1.16*** |
| (4.908± 0.87) | (30.28± 4.35) | (6.45± 0.90) | (12.15± 1.90) | (5.25± 1.02) | (1.40± 0.26) | (2.69 ±0.46) | |
| 4d | 72.43±6.79*** | 373.87± 11.31**** | 348.59± 29.31*** | 2509.19± 44.84**** | 1192.24± 83.72*** | 9.35 ±1.80** | 533.6±40.00**** |
| (31.10±2.92) | (160.55± 4.85) | (149.70± 12.59) | (1077.55± 19.26) | (512.0 ±35.95) | (4.01 ±0.77) | (229.15±17.18) | |
| 4e | 137.40± 16.05** | 90.38 ±12.62*** | 158.67± 7.56** | 159.98 ±21.17*** | 179.30± 21.03**** | 21.13± 1.49*** | 102.07± 16.87*** |
| (63.13± 7.38) | (41.53± 5.79) | (72.90 ±3.47) | (73.51 ±9.73) | (82.38 ±9.66) | (8.67± 0.61) | (46.9 ±7.75) | |
| 4f | 26.37± 5.09* | 109.41± 15.17*** | 16.95 ±2.65*** | 93.42± 2.97**** | 256.13± 12.05**** | 36.95 ±4.54 | 213.74 ±22.46*** |
| (10.11± 1.95) | (41.95± 5.81) | (6.5± 1.01) | (35.82± 1.14) | (98.20 ±4.62) | (14.17 ±1.74) | (81.95 ±8.61) | |
| TriazoloFQs series | |||||||
| 5a | 132.54± 23.17*** | 30.57± 3.08**** | 77.57± 13.77ns | 840.61± 146.74*** | 1525.28±140.75*** | 277.05± 24.93**** | 22.32 ±1.41**** |
| (54.39± 9.51) | (12.54± 1.26) | (31.83 ±6.65) | (344.98± 60.22) | (625.96± 73.50) | (113.70 ±10.23) | (9.16 ±0.58) | |
| 5b | 164.06± 12.29**** | 923.39± 62.70**** | 656.20± 11.86**** | 788.13 ±49.02**** | 494.84 ±40.46 | 70.44 ±11.36** | 142.52± 14.45*** |
| (70.45± 5.28) | (396.54± 26.93) | (281.80 ±5.09) | (338.45 ±21.05) | (212.50 ±17.38) | (30.25 ±4.88 ) | (61.20 ±6.21) | |
| 5c | 125.17± 8.37**** | 96.07± 18.05*** | 431.30 ±58.58*** | 1745.17± 287.74*** | 548.37± 95.89 | 2017.35±27.22**** | 133.92± 4.05** |
| (51.37± 3.43) | (39.42± 7.40) | (177.0 ±24.04) | (716.2 ±118.09) | (225.05± 39.35) | (827.90 ±11.17) | (54.96 ±1.66) | |
| 5f | 138.88± 16.86*** | 86.66± 12.45*** | 131.9 ±19.52* | 519.06 5.94**** | 490.65 ±55.33 | 11.88 ±0.61** | 527.59 ±13.35*** |
| (48.53± 5.89) | (30.28± 4.35) | (46.08 ±6.82) | (181.35±2.08) | (171.43± 19.33) | (4.15 ±0.21) | (184.33 ±4.66) | |
| 12.27 ± 2.05 | 0.7 ± 0.1 | 92.58±12.54 | 7.01 ± 1.17 | 454.37±10.65 | 29.3 ± 5 | 45.15 ± 7.84 | |
| (3.68 ± 0.62) | (0.22 ± 0.03) | (42.54 5.76) | (2.10 ± 0.35) | 208.77±4.89 | (8.8 ± 1.5) | (13.55 ± 2.35) | |
Results are mean ± SD (n = 3-4 independent replicates). IC50 values (concentration at which 50% inhibition of cell proliferation took place in comparison to non-induced basal 72 h incubations) were calculated within 0.1-200 μg/mL range. NI is a lack of cytotoxicity within the tested 0.1-200 μg/mL concentration range. P-value calculated by unpaired t-test between test compound IC50 values and cisplatin's (μM) using Graph Pad Prism software version 8.0.1. * When P<0.05 and ** when P<0.01 or 0.001, *** when P< 0.001or 0.0001, **** when P<0.0001, NS: not significantly different from reference agent.
Table 2 reveals that:
TriazoloFQs
-Unlike Nitro-FQs and Reduced FQs; TraizoFQs exhibited the least antineoplastic activity; where only 5a in skin melanoma A375 (with IC50 value (µM) 30.8 vs. cisplatin’s 0.7), and in breast cancer T47D (with IC50 values (µM) 22.3 < cisplatin’s 45.2) and 5f in leukaemia K562 (with IC50 values (µM) 11.9 < cisplatin’s 29.3) had appreciably antiproliferatice IC50 values < 50 µM like cisplatin. The rest of the TriazoloFQs series were significantly ineffective in lung cancer A549, breast cancer MCF7, pancreatic cancer Panc-1, and prostate cancer PC-3 cell lines as compared to cisplatin.
Reduced FQs
-Exceptionally skin melanoma A375 cell line was resistant to any reasonably moderate antiproliferative capacity of nitro- and reduced FQs (IC50 values ≥ 100 µM, unlike cisplatin (IC50 value of 0.7µM).
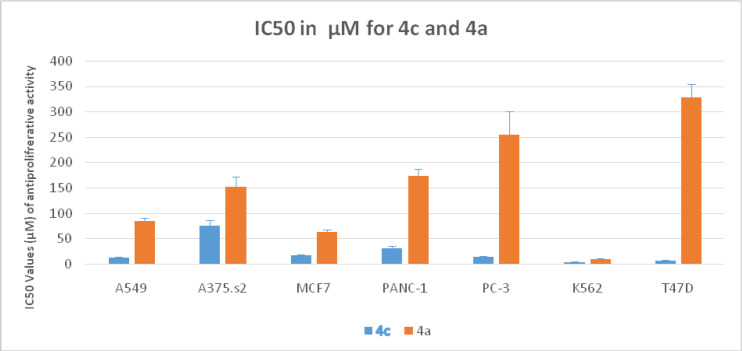
-In lung cancer A549 cell line; 4c and 4f (with respective IC50 value (µM) of 12.3 and 26.4 vs. cisplatin’s 12.3) displayed cytotoxicity IC50 values< 50 µM like cisplatin
-In breast carcinoma MCF7 and T47D cell lines; evidently both 4b (with IC50 values (µM) of 12.6 vs. cisplatin’s 92.6 in MCF7, and 44 vs. cisplatin’s 45 in T47D) and 4c ((with IC50 values (µM) of 16.16 vs. cisplatin’s 92.6 in MCF7, and 6.7 vs. cisplatin’s 45 in T47D) had IC50 values< 50 µM as cisplatin’s. Equally 4f exerted antineoplastic IC50 value (µM) < 50 µM in MCF7 only (17 vs. cisplatin’s 92.6).
-In pancreatic Panc-1 and prostate PC3 carcinoma cell lines; explicitly 4c displayed cytotoxicity IC50 values< 50 µM (with respective IC50 values (µM) of 30.4 vs. cisplatin’s 7 and 13.1 vs. cisplatin’s 454). Implicitly the rest of reduced FQs had cytotoxicity IC50 values>100 µM in both cell lines.
-In leukaemia K562; marked reduction of viability IC50 values (µM) < 50 µM by reduced FQs in ascending order were 4c<4a<4d<4e<cisplan’s (3.5<8.9<9.4<21<cisplatin’s 29.3, p<0.01-.001; respectively. Reduced 4b and 4f displayed cytotoxicity IC50 values (µM) > 100 µM unlike cisplatin (respective 38 and 37 vs. reference’s 29.3)
Nitro-FQs:
-In lung cancer A549 cell line; 3e of antiproliferation IC50 value of 45.1 µM and cisplatin’s of 12.3 µM were both < 50 µM, alike.
-In breast carcinoma MCF7 and T47D cell lines; obviously both 3c and 3e (with IC50 values (µM) of 18.9 vs. cisplatin’s 92.6 in MCF7, and 5.8 vs. cisplatin’s 45 in T47D, respectively) had IC50 values< 50 µM as cisplatins’.
-As in melanoma 72h incubations; pancreatic PANC-1 carcinoma cells were similarly resistant to nitroFQs (IC50 values ≥ 100 µM, unlike cisplatin (IC50 value of 7µM).
-In PC-3 prostate cancer cell line; explicitly 3e displayed cytotoxicity IC50 value ≤ 50 µM (with respective IC50 values (µM) of 49.6 vs. cisplatin’s 454.4)
-In leukaemia K562; pronounced reductions of viability by nitro-FQs in ascending order of IC50 values (µM) were 3b’s (13.7) <cisplatin’s (29.3) <3a’s (35.6) <3f’s (38.4) <50 µM.
Discussion
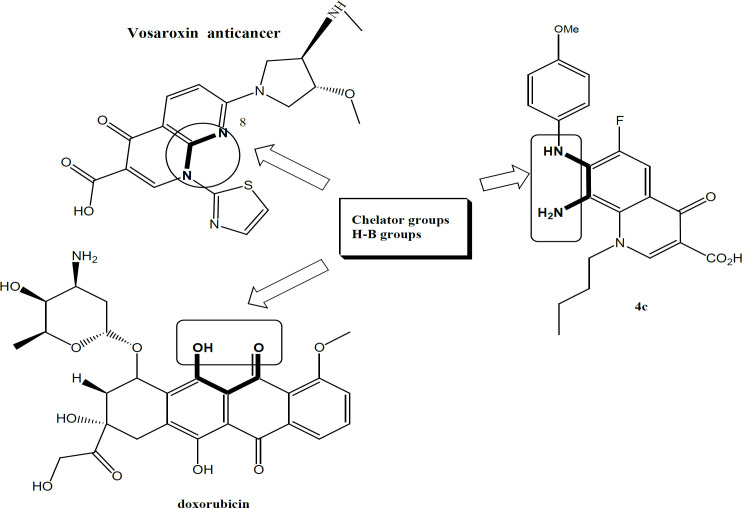
This research aims at investigating new FQs already prepared in our laboratory against serious cancer cell lines. They include two breast cancer cell lines MCF7 and T47D, Leukemia K562 cell line, Melanoma A375.S2 cancer cell line, Lung A549 cancer cell line, Prostate PC-3 and Pancreatic cancer Panc1 cell line. Recently, quinolones represented by the drug Vosaroxin; a topoisomerase II inhibitor causing site-selective DNA damage; have been introduced in the fight against cancer. Vosaroxin is under phase III clinical trial investigation for acute myelogenous leukemia (AML) sponsored by Sunesis. Vosaroxin is an inhibitor of Topoisomerase II enzymes- which are essential for the survival of eukaryotic cells. Vosaroxin hinders the reunion of topoisomerase II-induced double-strand breaks at selective sites in DNA, resulting in G2 arrest and cell death by apoptosis. The advantage of quinolone drug (vosaroxin) over other anticancer drugs is the fact that it is one of few drugs that target liquid tumors such as acute myeloid leukemia. Although it is used against ovarian cancer solid tumor, such use is minimal in clinical trials.
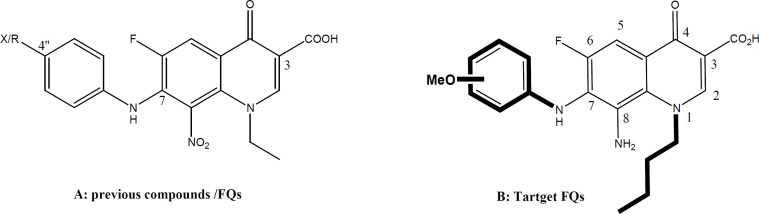
Similar to vosaroxin, we have developed new FQs with anticancer properties. This research has been established by our group since 2013. Our research has revealed many new lipophilic FQs against different cell lines, especially solid cancer. Our group has developed FQs with a lipophilic alkane chain at N1 and substituted anilines at C7. In this work, we aimed to test and investigate methoxy substitution at the aniline group pointed out at C7 of the basic FQ nucleus. Both the position and number of methoxy derivatives (anisidines) will be tested (Chart 1). To investigate similarity to vosaroxin, it is planned to test against liquid cancer cell lines represented by K562 (target in acute/chronic myelogenous leukemia /AML) and available solid cancer cell lines. It is worth mentioning that the targeted FQs have more lipophilic characters to ease and facilitate accessibility into the lipophilic cell membrane of cancer cell lines, unlike the hydrophilic drug vosaroxin. This work is an attempt among the fight against cancer indestructible challenges. Cancer statistics are devastating with increased cases each year. Resistance and serious side effects in addition to the cost of existing therapy add to the problem. Our derivatives offer cheaper compounds with a possible new mechanism and lower resistance since they are introduced recently. We hope that these FQs will be a new addition in this area, especially to overcome resistance issues.
Chart 1.
Target FQs with more Lipophilic
Chemical properties of the new FQs tested
The use of FQs as antiproliferative compounds is highly justified. They are clinically used antibacterial drugs with an equitable safety profile. They have a negligible side effect without any significant cardiotoxicity like anthracyclines (Owens and Ambrose, 2005). Moreover, quinolones have superior physicochemical properties with outstanding bioavailability and pharmacokinetics (Bisacchi and Hale, 2016). Quinolones are easily formulated as both oral and parenteral preparations and their stability is well established. In particular, the quinolone drug vosaroxin’s scaffold confers chemical and pharmacologic characteristics distinct from classic topoisomerase II poisons used in acute myeloid leukemia (AML) treatment. Compared with currently approved topoisomerase II inhibitors, vosaroxin is minimally metabolized because of its stable quinolone core. Their structure has never been linked to reactive oxygen species ROS (Jamieson et al., 2016).
In fact, vosaroxin is not associated with the significant formation of free radicals or ROS. It is worth mentioning that the targeted FQs tested in this work have the same pharmacophoric scaffold of vasoroxin with well-established stability as pure compounds. They have lipophilic characters that further contributor to their chemical stability and add to their improved kinetic profile. Lipophilicity was assumed to ease and facilitate crossing of lipophilic cell membranes of cancer cell lines, unlike the hydrophilic drug vosaroxin. The idea of this work was based on previous work by our group involving new lipophilic FQ derivatives with noticeably promising antiproliferative activity against human colon and breast cancer cell lines (Arabiyat, et al 2016 a,b; Al-Nuaimi, et al., 2021). It was revealed that the FQ scaffold (6-fluoro-8-nitro-4-oxo-1, 4-dihydroquinoline-3-carboxylic acid) exhibited reasonable antiproliferative activity. It was also spotted that lipophilicity and size were the most important requirements for such activity in our FQs. Therefore, this work aims at testing new lipophilic FQs prepared previously against cancer cell lines for the first time (Elsheikhi, 2018). The new FQs hold methoxy functionality at the C-7 aniline group in addition to the N-Butyl group at N1 (chart 1: B). Both aniline and Butyl groups were introduced to increase the lipophilicity and investigate its effect on activity. Both liquid and solid cancer cell lines were used including K562 and MCF7.
Antiproliferative activity of methoxy FQs 3-5 (a-f)
Table 2 and Scheme 1 (Supplementary) disclose the antiproliferative results of 16 new FQs compounds while Table 1 shows selectivity index data; Antioxidant and antiinflamation activity are detailed in Table 1. Starting with antiproliferative activity, 16 FQs and cisplatin were tested against 7 cancer cell lines (Table 2). The results show that our FQs have good antiproliferative activity against most cell lines with superior activity against Leukaemia cell line K562. Another marked activity was also shown against MCF-7, against A549, and the T47D breast cell lines. Due to the similarity of the chemical structural scaffold to vasoroxin, it was assumed that our FQs will mainly work on the same cell line (Leukaemia cell line K562). This hypothesis is validated in the results of this work since the pattern of topoisomerase II (TOP II) inhibitors involves higher potency against liquid tumours compared to any solid one. The tested FQs go smoothly with this trend since they have marked activity against Leukaemia cell lines (such K562) compared to other solid cancer cell lines tested. Such findings indicate clearly that these FQs possibly have a similar target to that of vasoroxin which is TOP II. Weaker but the sole noticeable activity of our FQs was against solid breast cell lines, especially the reduced series 4. Obviously this point out that the tested FQs have additional mechanism or target when compared to vasoroxin. Another support for this assumption comes from testing another TOP II inhibitor; doxorubicin. In fact, previous work by our group has revealed a similar pattern of activity and mechanism between FQs and doxorubicin, meaning they share the same target and mechanism which is TOP II inhibitor (Khaleel, et al., 2021). It is highly tolerable to suggest that our FQs mechanism is similar to that of both vasoroxin and doxorubicin as they are both TOP II inhibitors.
Antiproliferative activity of strong FQs
This research has produced a huge amount of data that could not be easily interpreted. As a result, it was decided to categorize our data to three assemblies to make sense of these records (Table 2):
A. Strong to the reasonable antiproliferative effect of tested drugs’ with IC50 values below 50 μM.
B. Intermediate antiproliferative effect of tested drugs’ with IC50 values from 50 μM to 100 μM.
C. Weak effect antiproliferative effect of tested drugs’ with IC50 values over 100 μM.
This classification is totally acceptable due to the fact that there were no clear cut-off numerals for sorting on the basis of IC50 values in the general HTS approach for potential anticancer hits and druggable candidate leads. Actually, this subject is totally controversial in the literature. Some scientists debate that 500 to 1000 µM can be a reasonable range for further lead/hit/candidate optimization, whereas others went lower values of 10 µM to initiate a mechanistic investigation. However, the majority abide by equally marked tendencies as in solubility, kinetics and bioavailability and safety profiles. The antiproliferative activities of strong compounds with IC50 values (µM) below 50 μM are displayed in Table 2 . Obviously 10 FQs out of 15 exhibited IC50 value below 50 µM against leukemia cell lines K562. Indeed 6 compounds showed stronger IC50 values compared to the reference cisplatin, whereas the rest were of comparable effectiveness. Compound (4c) has shown the strongest IC50 value impending 3.51±0.66 µM IC50 against K562.Similar wise, compounds 4a and 4d showed superior IC50 values compared to cisplatin against K562 with values below 10 µM.
On the other hand, series 4 revealed superlative activity to cisplatin against MCF-7 breast cell line with IC50 value below 20 µM. Again most of them were from the reduced series 4, with the outstanding activity of 4c and 4b. Although weaker than the reference cisplatin, 3 compounds have displayed strong activity against lung cell line A549. Only 4c was equally potent to the reference with an IC50 value of 12.29± 2.17 µM. 4c and 3e, in particular, have revealed superior activity to reference against the T47D breast cell line. The non-matched activity of 4c and reduced series 4 in general attained against both leukemia K562 and breast cancer cells propose this group as good hits for further investigation. The calculation of the selectivity index was carried out as a symbolic feature to define the safety of tested FQs. SI was calculated by dividing IC50 of the tested compound on a normal cell line by IC50 of the same compound on a specific pathological cell line (Hoffmann, et al., 2011). The cell which showed the strongest value (lowest IC50) was selected for such calculation.
Unpredictably all active FQs, particularly 4a-d, have shown selectivity index upper than cisplatin demonstrating high selectivity and safety profile for these compounds. Table 1 displays the selectivity index for all compounds in comparison against cisplatin in addition to the IC50 (µM) values of PDL fibroblasts. The IC50 values of PDL fibroblast of all active compounds exceeded again the IC50 value of cisplatin indicating again high safety profile of these hits. In fact, the most active FQ 4c showed an IC50 value of 95.31 µM which was much higher than marginal cisplatin’s value of 0.71 µM (IC50 value, Table 1). From a pharmaceutical point of view, these results in Table 1 reveal excellent safety profile and it is highly acceptable to consider these FQs as druggable candidate leads. LD50 and ED50 for the active hits along with an assessment of IC50 and doses may be planned for the future work.
Structure-activity relationship of antiproliferative activity (SARS, Figure 1 )
Figure 1.
Order of Activity Based on the Structural Scaffold in a Strong Group against K562
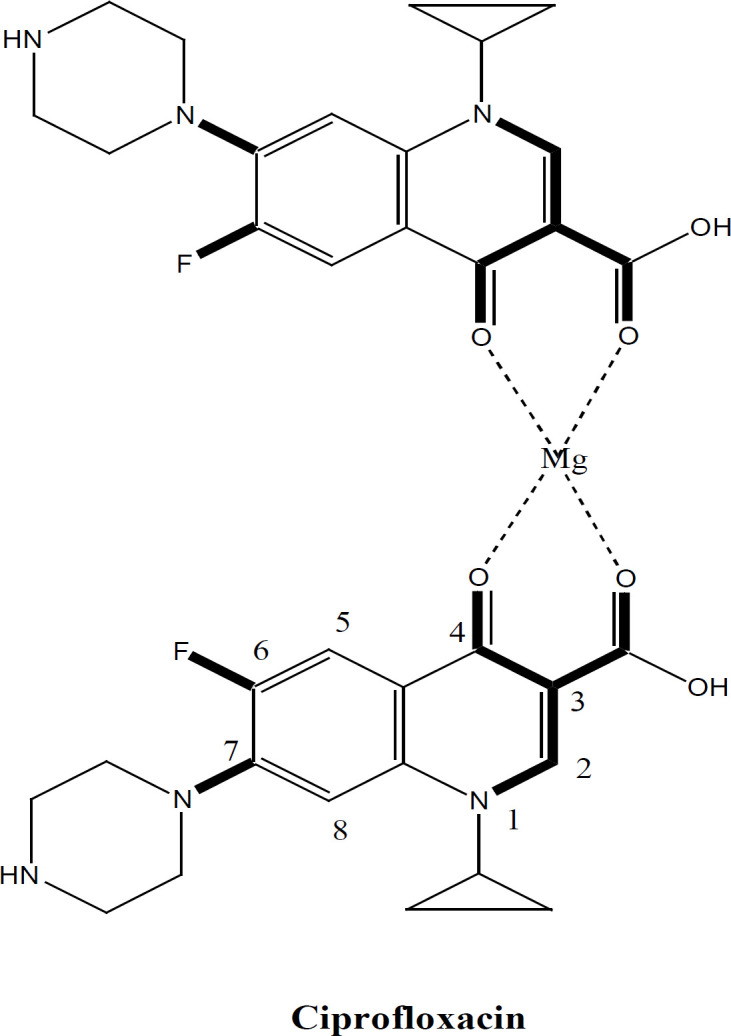
This work proposes that lipophilic acidic FQs would have potential antiproliferative activity against solid and liquid tumor cell lines. All active FQs are derived from free acidic 3-COOH -4-oxo quinolone basic structure. This acidic chelator group is essential to provide a point of H-B attachment and ionic interaction with any targeted receptor. On the other hand, all FQs exhibit lipophilic properties impeded by the C-7 phenyl and N-butyl group. These lipophilic characters do help in cell membrane penetration since cancer cell lines have phospholipid bilayer membrane allowing only lipophilic compounds diffusion. It is postulated that lipophilicity has overcome the active process needed for penetration of clinically used polar FQs. In fact, it is reported that they cross the cell membrane as dimers in the form of a tetradentate co-ordinated complex with Mg (Charts 1 and 2).
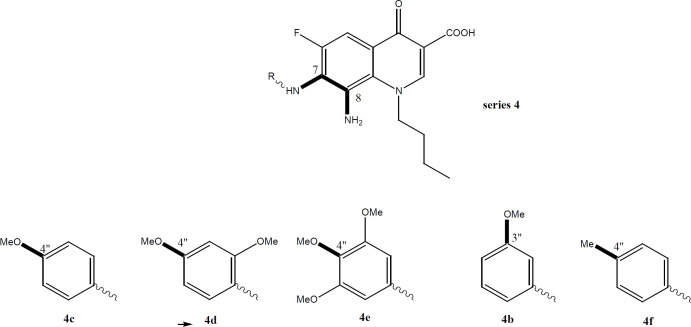
Such 4-oxo-3-COOH – Mg complex enforces final lipophilic properties allowing the drug to cross the cell membrane. The data of this work (Tables 2 and 4) validate our assumptions regarding structural requirements and confirm that many compounds have a superior activity to cisplatin. In fact, superlative activity was revealed against the Leukemia K562 cell line mainly similar to the quinolone drug Vosaroxin. A satisfactory activity was also noticed against breast MCF, T47D, and A549 lung cancer cell line. The very Table 2 illustrates the unmatched activity of the reduced series 4 against K562 detailing that 4a, 4c, 4d, and 4e have IC50 values significantly lower than cisplatin. 4f and 4b have equipotent values to cisplatin. Less number of compounds from the nitro series 3 has displayed activity (3b, 3a, 3f). From the triazole series 5; 5f has exhibited a reasonably appreciable IC50 value. This emphasizes clearly that the active hits against liquid tumours (K562) belong to the reduced series 4, followed by nitro (3) and triazole (5) series respectively (Tables 2 and 4). Similar findings and order were exposed against solid tumor cell line MCF7. Although fewer compounds were active, the 3 compounds of the reduced series 4b, 4c, and 4f have superior and significant activity compared to the reference. Only one compound of series 3 was active whereas no triazole derivative has shown any activity. Again, series 4 had the exquisitely superb compounds with activity against T47D and A549. This incremental increase in scaffold activity order indicates that the H-B acceptor and donor properties might have contributed a significant role besides it seems that increasing the number of H-B (A/D) percentage does enhance activity substantially. Series 4 has shown strong to intermediate activity against all cell lines with particular properties of 4c (Table 2). Compound 4c exhibited strong and remarkable IC50 values against all 6/7 cell lines tested with IC50 values < 50 µM. This confirms the impact of methoxy substitution on C-4’’ of aniline (4-anisdine).
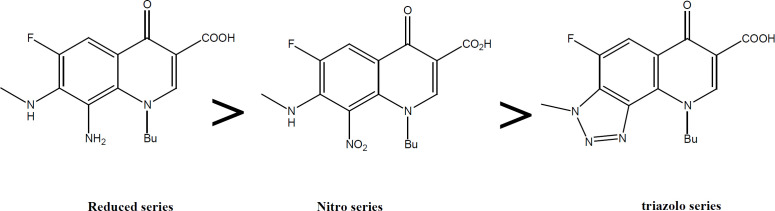
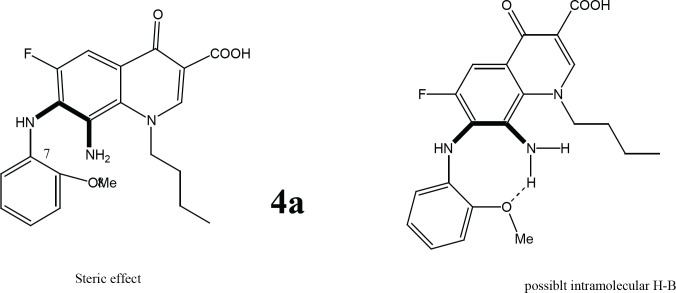
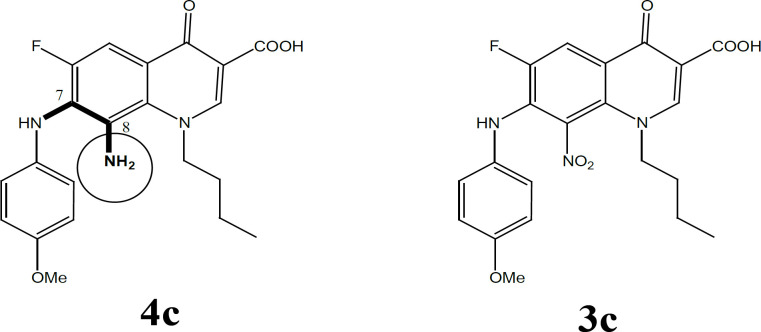
Further validation for the importance of position 4-methoxy substituent arises from the finding that the strongest reduced series exhibit methoxy group on C4’’ in addition to other substituents (Figure 2). Compounds 4c, 4d, and 4e (with at least one methoxy group at C-4’’) revealed stronger IC50 values than the reference. Even active compound 4b had the methoxy at a Meta position (3’’) which is far from the vicinity of the diamine bridge at C-7 and C-8. This points out clearly that Para (4’’) or Meta (3’’) methoxy group is essential for cytotoxicity. Although the number of methoxy substituents did not affect activity, the position seems important and should be far from the space or vicinity of the diamine bridge. The methoxy group has possibly added H-B bond capability to the molecule and provided better interaction with the target inside the cancer cells. Lower activity imposed by 2’’ methoxy substituents might be explained by steric/size repulsion effect or possibly intramolecular H-B that makes it in close proximity to space of the diamine bridge (Figures 3 and 4). This points out clearly that the main contributor in the antiproliferative activity is the diamine bridge between C-7 and C-8 of the 4-oxo-quinoline-3-COOH structural core, due to C8-amino group.
Figure 2.
4''-methoxy Substitution Effect on Activity K562
Figure 3.
Possible Effects that Explain the Weak Activity of 4a
The role of C-8 amino group in cytotoxicity
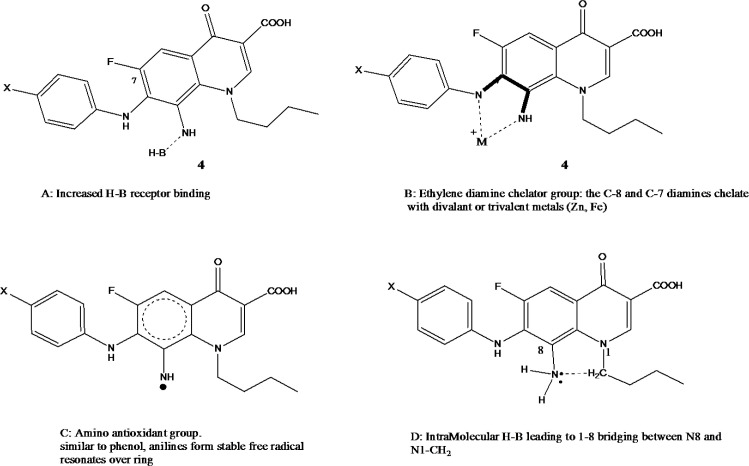
The role of the C8 amino group (series 4) in antiproliferative activity was noticed with all active FQs hits. In fact, the only difference between the most active compound 4c and its precursor 3c is the conversion of the C8 nitro into C8 amine (Figure 5). This means only that the add-on effectiveness correlated to this particular group. It is possible that this group has increased the total number of H-B capabilities that further provides extra linkage with the receptor. The C-8 amino has contributed to both H-bond donor/acceptor incremental addition to growth inhibition which spots the light on the importance of such position and such group. In fact, the C8-amino substituent provides 2 H bond donor atoms and 1 H bond acceptor. This implements that 8-amino is essential for acting as the case in all reduced FQs and provides good vicinity for enzyme interaction with FQ through the freely accessible interfacing H-B. One further justification for the super activity of the reduced 4 series comes also from the role of the 8-amino group as a chelator group and as an antioxidant group (Figure 6a,b). The ethylene diamine bridge created with reduced series offers an excellent nitrogenous based chelator group between C-7 and C-8. Moreover, the aromatic amine of C-8 arranges for the stability of free ionizable electron radical through resonance which makes such a group as an antioxidant group, (Figure 6a,b). Those 2 phenomena can further contribute to the stunning activity of the reduced FQs series 4. It is also reported in vosaroxin that N8 and S Bridge is highly impactful for activity. This might be the case with our compound 4 due to the 1-8 H-B bridging effect.
Figure 5.
C-8 Amino Group in 4c
Figure 6a.
Contribution of C-8 Amino Group (4 series) in Anti-Proliferative Activity; A- H-B; B-Chelating agents, C-Antioxidant and D- 1,8-bridging (rigidity)
Figure 6b.
Chelator Groups Shared by All Anti Proliferating FQs and Doxorubicin
In conclusion, we can propose that initial SAR/QSAR for optimal antiproliferation FQ requires (Table 3):
Table 3.
Molecular Descriptors of the Lipophilic FQs
| Name | Chemical Formula | MWt | Henry’s law | tPSA (Ų) | C log P | CMR | HB/D | HB/A |
|---|---|---|---|---|---|---|---|---|
| 3a | C21H20FN3O6 | 429.13 | 1.56 | 130.68 | 4.93 | 11.01 | 2 | 7 |
| 3b | C21H20FN3O6 | 429.13 | 1.56 | 130.68 | 4.93 | 11.01 | 2 | 7 |
| 3c | C21H20FN3O6 | 429.13 | 1.56 | 130.68 | 4.93 | 11.94 | 2 | 7 |
| 3d | C22H22FN3O7 | 459.14 | 1.56 | 139.91 | 4.90 | 11.63 | 2 | 8 |
| 3e | C23H24FN3O8 | 489.45 | 1.56 | 149.14 | 4.14 | 12.24 | 2 | 9 |
| 3f | C21H20FN3O5 | 413.4 | 1.56 | 121.45 | 5.51 | 10.86 | 2 | 6 |
| 4a | C21H22FN3O4 | 399.42 | 4.64 | 104.89 | 3.62 | 10.77 | 4 | 8 |
| 4b | C21H22FN3O4 | 399.42 | 4.64 | 104.89 | 3.62 | 10.77 | 4 | 8 |
| 4c | C21H22FN3O4 | 399.42 | 4.64 | 104.89 | 3.62 | 10.77 | 4 | 8 |
| 4d | C22H24FN3O5 | 429.44 | 4.64 | 114.12 | 3.59 | 11.38 | 4 | 9 |
| 4e | C23H26FN3O6 | 459.47 | 4.64 | 123.35 | 2.83 | 12.00 | 4 | 10 |
| 4d | C21H22FN3O3 | 383.42 | 4.64 | 95.66 | 4.19 | 10.61 | 4 | 7 |
| 5a | C21H19FN4O4 | 410.4 | 5.53 | 94.80 | 4.39 | 10.73 | 1 | 9 |
| 5b | C21H19FN4O4 | 410.4 | 5.53 | 94.80 | 4.39 | 10.73 | 1 | 9 |
| 5c | C21H19FN4O4 | 410.4 | 5.53 | 94.80 | 4.39 | 10.73 | 1 | 9 |
| 5f | C21H19FN4O3 | 394.4 | 5.53 | 85.57 | 4.76 | 10.57 | 1 | 8 |
| Doxorubicin | C27H29NO11 | 543.52 | 34.30 | 206.07 | 0.06 | 13.33 | 7 | 12 |
| Vosaroxin | C18H19N5O4S | 401.44 | 0.00 | 106.83 | -1.67 | 10.43 | 2 | 9 |
Chemical descriptors were calculated by using CS ChemDrawUltra V.11 software. Where MR: the Molar refractivity, CMR, calculated molar refractivity; Henry's Law: the inverse of the logarithm of Henry's law constant [-log (H)] (no units), MWt, molecular weight; and tPSA, topological polar surface area based on fragment contribution; Ų, Square angstroms; H-B, hydrogen bond; cLogP, the partition coefficient for n-octanol/water; All structures were drawn by using CS ChemDraw Ultra V.6.
1. Free acidic and chelator 4-Oxo quinolone-3-COOH is essential.
2. All FQs tested are acids with pKa range below 6 (COOH).
3. Lipophilic balance with high C log P values between 2.8 to 4.1.
4. Lipophilic properties by the C-7 phenyl and N-butyl group
5. Small size around 400 with tPSA of around 100-110 also important.
6. The high number of H-B donor and acceptor is a must-have functionality feature although the high number of donors is also a surplus criterion (ratio in reduced series 4 was ranging 4:9 A/D).
7. C-8 Amino H-B group or its isoster like N or OH or is the most important requirement for anti-proliferative FQ. (Henry’s law of reduced series is 4.64 while CMR is around 11)
8. C7-C8 Diamine Bridge with C8 amine related universal chelation is hugely important.
9. Methoxy group far from the vicinity of the Diamne Bridge is important.
10. The result of this research confirms the similarity pattern between the activity of 4 series and vosaroxin since both works on leukemia cell lines mainly. Similar to doxorubicin, vosaroxin is a Topo II inhibiting drug; therefore, we can assume that our 4 series also worked by the same mechanism. Table 3 shows clearly the most common property which gathers the three compounds in the H-B A/D ratio. As this ratio increases, the potency of the compound increases. The superb activity of doxorubicin can be related to its high A/D ratio. From a chemistry point of view, this ratio is a direct function of increased H-B groups in the molecule that allows the chelation. In other words, increasing the number of H-B group’s increase the number of chelation groups as it is the case in doxorubicin. This mechanism is a universal cytotoxicity mechanism allowing any drug to inhibit the proliferation of many cancers due to chelation with many metalloenzymes involved in cancer. This hypothesis has a precedent in literature since vosaroxin and doxorubicin have chelation groups and the C7-8 diamine nitrogen-based chelator is similar to chelator groups in both drugs.
Such idea SAR proposition can also explain the broad spectrum of anticancer activity against 4 cell lines. The stronger chelator group in 4c may also explain its universal activity against all cell lines. The C7-8 diamine bridge is similar to EDTA and has a strong chelation power that can chelate with di- and trivalent metals including Zn, Ag, Cu, Fe, Al. Zn and Fe metalo-enzymes are recently linked to cancer. It is also assumed that such chelators agents might exhibit a marked antioxidant activity due to chelation with enzymes involved in chelation. In fact, many anticancer agents do exhibit their actions through their antioxidive potency. Similarly, antiinflamation is also linked to anticancer activity in many antiinflamatory agents, possibly due to chelation in our case. To validate this hypothesis proposing linked radical scavenging-antioxidant and anti-inflammatory activities to the anticancer compounds, both anti oxidant (DPPH assay) and anti-inflammatory properties were validated.
Antiinflammatory activity
Infections and inflammation had been broadly linked to cancer, and the correlations among attendance of inflammation and the expansion of preneosplastic lesions at many different anatomic sites have been recognized. There is about a 14% increase in prostate cancer risk due to prostatitis, and a 25% in colorectal cancer risk due to ulcerative colitis. For this reason, the attendance of inflammation seems to induce or ease carcinogenesis. So inflammation causes cancer is sensible as the chronic inflammation is categorized by infiltration of mononuclear immune cells (including macrophages, lymphocytes, and plasma cells), tissue destruction, fibrosis, and increased angiogenesis (Rayburn et al., 2009). Also, there is another association with chronic inflammation like an increase in genomic damage, an increase in DNA synthesis, cellular proliferation, disruption of DNA repair pathways, inhibition of apoptosis, and the promotion of angiogenesis and invasion. These developmental processions have been concerned with the initiation and progression of cancers. Pro-inflammatory molecules, like cytokines, inducible nitric oxide synthase (iNOS), reactive oxygen species (ROS), and NF-kB (nuclear factor kappaB) are upregulated due to chronic inflammation. These mechanisms encourage microenvironment for the exponential progress of cancer cells. Thus, the inflammation afforded both the key mutations and a good environment to substitute tumor growth (Rayburn et al., 2009). As FQs were proved to be outstanding antineoplastic compounds, the request for antiinflammation potentiality can be very justified. Unpredictably, it was noticed that some of these NSAIDs have distinguished activity against leukemia cell line K562 similar to our FQs and vosaroxin. In the study that was primed with indomethacin (IND) on chronic myeloid leukemia (CML) cells demonstrate that IND can induce apoptosis and prevent the spread of K562 cells or newly isolated bone marrow cells from CML patients. IND has an inhibitory effect on cell proliferation and that effect is attributed to the detention of cell cycle progression and initiation of apoptosis. Examination of cell cycle phase spreading shows that the ratio of cells in the S phase increases while that in G0/G1 and G2/M phase significantly decreases, which reflects the inhibition of IND on cell progression from S phase to G2/M phase (sen Zhang, et al., 2000).
This had led to a search if other NSAIDs have a similar mechanism. Surprisingly, it was also found that other NSAIDs exhibit their antiproliferative activity through interfering cell cycle phases in a similar way that FQs do. In specific, numerous researches have established that aspirin and other NSAID treatments associate with a reduction in cell replication and with an improved proportion of cells arrested at the G0/G1 phase of the cell cycle. Aspirin behavior may affect the proliferation, variation, and apoptosis of the human colon adenocarcinoma Caco-2 cells; in specific, it depends on the doses. Aspirin behavior may boost apoptosis and a main DNA synthesis inhibition related to an adjustment in the level of the insulin-like growth factor II (IGF-II), an autocrine growth factor for this cell line. NS-398 has been demonstrated as a Cox-2 selective inhibitor, also affects Caco-2 DNA synthesis and apoptosis. Accumulating evidence proposes that intervention with cell cycle and/or with the intracellular growth factor (receptor)-activated signal transduction pathways are key modulators of cellular response to chemotherapeutic agent treatment unwell affects Caco-2 anticancer sensitivity, while aspirin treatment, counteracting anticancer drug-induced cell cycle modification and inducing the expression of bcl-2, is able to substantially alter Caco-2 anticancer drug-induced apoptosis and overall viability (Ricchi, et al., 2002). NSAIDs like salicylates (aspirin and sodium salicylate), a phenylacetic acid derivative (diclofenac), a propionic acid derivative (ibuprofen), and indoleacetic acid derivative (indomethacin) and a pyrroleacetic acid derivative (sulindac), are inhibited vascular smooth muscle cell (VSMC) proliferation in a concentration-dependent manner. They apply their effects at diverse phases of the VSM cell cycle. Thus, the non-salicylate NSAIDs, diclofenac, ibuprofen, indomethacin and sulindac, all block VSMCs at the G1 phase of the cell cycle, whereas salicylates induce a post G1/S block and arrest cells in the G2 and/or M phases. This work reveals clearly that any drug interferes with cell cycle phase G2 is topoisomerase II inhibitor, which the same target FQs inhibit. It is well detailed in the literature that NSAIDs exert Topo II inhibition effect possibly through chelator acidic groups (Brooks, et al., 2003).
Through our search for TOP II inhibitors, resveratrol, a polyphenol found in berries, nuts, traditional Asian medicines, and red wine, was spotted. It is widely used due to it is a benefit and likely side effect. Research has revealed that resveratrol to have pleiotropic properties, including potential anti-cancer, anti-inflammatory, cardioprotective, and neuroprotective activity. These privileges have led to the quick development of resveratrol supplementation; however, there is less clinical data on the efficacy of resveratrol and there is no logical agreement on the underlying metabolic and molecular mechanisms of resveratrol activity. So far, resveratrol has been reported to act on many different molecular targets including p53, PI3K/Akt, and NFκB. Eukaryotic DNA TOP II, in the last decade, has been added to resveratrol targets as a likely explanation for the anticancer effects of the agent. Topo II is a confirmed target of anticancer drugs, as it is obligatory for actively separating cells and interruption of enzyme function can induce the development of cytotoxic DNA damage. This anti-cancer effect was correlated to its resorcinol phenolic hydroxyl groups according to literature. Similarly, its antioxidant effect was explained with its phenolic groups (Lee, et al., 2017). Another natural anti-inflammatory and anti-topoisomerase ii is Flavonoids. Flavonoids are a set of natural plant metabolites found in a variety of fruits and vegetables. Although the effects of flavonoids have been examined for many years, their antioxidant, anticarcinogenic, and anti-inflammatory properties have only lately gained credit. The protective and therapeutic effects of flavonoids such as kaempferol, quercetin, luteolin, and apigenin have been reported (Kalındemirtaş, et al., 2019)
Antioxidative capacities
Antioxidants inhibit cellular damage throw out responding and eradicating oxidizing free radicals. Though, in cancer treatment, a method of action of some chemotherapeutic agents implicates the generation of free radicals to affect a cellular damage and necrosis of cancer cells. This reason is concern logically established as exogenous antioxidant compounds occupied alongside chemotherapy could decrease the helpful effect of chemotherapy on malignant cells. The standing of this fear is emphasized by a recent study which estimates 23 percent of cancer patients take antioxidants (Lamson et al., 1999). Table 2 displays that the supreme active antiproliferative compound is 4b, were the only group that expresses antioxidant activity in more than one compound is reduced series. In compound 4b the result was not significantly different from ascorbic acid. These results settle that radical scavenging activity maybe also has a potential mechanism of antiproliferation, surely due to the free radical scavenging amino group (NH2).
Antioxidant activity vs. cytotoxicity activity
Numerous classes of chemotherapy work by making a reactive oxygen compound or free radical.
Alkylating agents work to add alkyl groups to the negatively-charged group. They are identified to stop over tumor growing through cross-linking guanine nucleobases in strands of DNA, which straight damages to the DNA by making it unable to uncoil and separate. The cell, when argued in this way, is incapable of cardioprotective. While it may not die, it also cannot grow. Flavonoids such as quercetin and genistein have been approved in vitro and animal studies to have a benefit to patients receiving chemotherapy (Nepomuceno, 2011; Reyes-Farias, and Carrasco-Pozo, 2019; Tulli et al., 2019).
In conclusions, this research has introduced new FQs with anti-proliferative, antiinflammatory and antioxidant activities. Such activity is due to the ethylene diamine chelator group substituted on C7-8 carbons with a potential role for the 4-oxo-3-COOH group. In fact, such groups were responsible for superior anti-proliferative activity than the reference drug cisplatin represented by compound 4c. Their anti-inflammatory activity was also unique and more than the reference indomethacin with 4 compounds of series 4. The antioxidant activity was due to an aniline c-8 amino group that can form stable free radicals.
Chart 2.
Representative FQs Drugs
Figure 4.
Effect of Methoxy Substitution in 4c and 4a against All Cell Lines Tested
Author Contribution Statement
All authors contributed equally towards rationale conceptualization, experimental design, data collection and analyses, manuscript write up and proofreading. No animal or human studies were involved and as such no ethical permits were obtained. Data are available from corresponding author based to requests made directly to her.
Acknowledgments
We are grateful for the Deanship of Scientific Research at the University of Jordan for research funds (project 171/2018-2019, dated 28/8/2019, No. 2280). We also wish to thank the University of Jordan and Al-Zaytoonah Private University for providing research facilities and funds. This research was conducted during the sabbatical year granted to Professor Yusuf Al-Hiari by the University of Jordan in the academic year 2021/2022 at Al-Zaytoonah Private University. Authors declare no conflict of interest and equal contributions towards rationale conceptualization, experimental design, data collection and analyses, manuscript write up and proofreading. Authors declare no conflict of interest. No animal or human studies were involved and as such no ethical permits were obtained. Data are available based to requests made directly to corresponding author.
Future work
- Investigating the mechanism of action of these FQs and explore their LD50.
- Establishing a full toxicity profile of most active hits.
- Testing the active FQs against other cell lines or new activities.
- In vivo investigating of their proposed in vitro activities.
- Validating the chelation group theory in practice or directly on selected enzymes such as TOP II alpha and beta.
Conflicts of interests
Authors declare no conflict of interest
References
- AbdulFattah T, Saeed A, Al-Hiari YM, et al. Functionalized Furo[3,2-c] coumarins as Anti-proliferative, Anti-lipolytic, and Antiinflammatory Compounds: Synthesis and Molecular Docking Studies. J Mol Struc. 2019;1179:390–400. [Google Scholar]
- Alabsi Y, Al-Hiari Y, Kasabri V, et al. In vitro modulation of pancreatic lipase and proliferation of obesity related-colorectal cancer cell line panel by novel synthetic FQs. Rev Roum Chim. 2018;63:1123–34. [Google Scholar]
- AlHallaq T, Al-Hiari Y, Kasabri V, et al. In vitro Antiproliferative Properties of Lipophililic -Acid Chelating Fluoroquinolones and triazoloFluoroquinolones with 7-dihaloanilinosubstitution. AntiCancer Agents Med Chem. 2022 doi: 10.2174/1871520622666220513154744. Accepted. [DOI] [PubMed] [Google Scholar]
- AlKhalil M, Al-Hiari Y, Kasabri V, et al. Selected pharmacotherapy agents as antiproliferative and antiinflammatory compounds. Drug Dev Res. 2020;81:470–90. doi: 10.1002/ddr.21640. [DOI] [PubMed] [Google Scholar]
- Al-Nuaimi A, Al-Hiari Y, Haddadin R, et al. A Novel class of Functionalized Synthetic Fluoroquinolones with Dual Antiproliferative - Antimicrobial Capacities. Asian Pac J Cancer Prev. 2021;22:1075–86. doi: 10.31557/APJCP.2021.22.4.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Shahrabi R, Kasabri V, Al-Hiari Y, et al. Functionalized Fluoroquinolones as a Potentially Novel Class of Antiinflammatory and Antiglycation Compounds; Synthesis and Pharmacological Appraisal. Ana Chem Lett. 2020;10:280–97. [Google Scholar]
- Arabiyat S, Al-Hiari Y, Bustanji YK, Zalloum H, Kasabri , V In Vitro modulation of pancreatic lipase andproliferation of obesity related colorectal cancer cell line panel by novel synthetic triazoloquinolones. Rev Roum Chim. 2016a;61:871–9. [Google Scholar]
- Arabiyat S. Synthesis docking studies and evaluation of noval fluoroquinolones and triazoloquinolonnes as pancreatic lipase inhibitors and anti-proliferative compounds. PhD thesis. The University of Jordan; 2016b. [Google Scholar]
- Arabiyat S, Kasabri V, Al-Hiari Y, et al. Dual glycation-inflammation modulation, DPP IV and pancraetic lipase inhibitory potentials and antiproliferative activity of novel FQs. Asian Pac J Cancer Prev. 2019;20:2503–14. doi: 10.31557/APJCP.2019.20.8.2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arabiyat S, Kasabri V, Al-Hiari Y. Antilipolytic-Antiproliferative Activity of Novel AntidiabesityTriazolo/Fluoroquinolones. Jordan J Pharm Sci. 2020;13:85–100. [Google Scholar]
- Arfin S, Jha NK, Jha SK, et al. Oxidative Stress in Cancer Cell Metabolism. Antioxidants. 2021;10:642. doi: 10.3390/antiox10050642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assanga SBI, Lujan LML, Gil-Salido AA, et al. Antiinflammatory activity and modulate oxidative stress of Bucidabuceras in lipopolysaccharide-stimulated RAW 264. 2017 macrophages;11:239. [Google Scholar]
- Bacchi S, Palumbo P, Sponta A, Coppolino MF. Clinical pharmacology of non-steroidal Antiinflammatory drugs: a review. Antiinflamm Antiallergy Agents Med Chem. 2012;11:52–64. doi: 10.2174/187152312803476255. [DOI] [PubMed] [Google Scholar]
- Bisacchi GS, Hale MR. A Double-Edged Scaffold: Antitumor Power within the Antibacterial Quinolone. Curr Med Chem. 2016;23:520–77. doi: 10.2174/0929867323666151223095839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks G, Yu XM, Wang Y, et al. Non-steroidal antiinflammatory drugs (NSAIDs) inhibit vascular smooth muscle cell proliferation via differential effects on the cell cycle. J Pharm Pharmacol. 2003;55:519–26. doi: 10.1211/002235702775. [DOI] [PubMed] [Google Scholar]
- Bruno PM, Lu M, Dennis KA, et al. The primary mechanism of cytotoxicity of the chemotherapeutic agent CX-5461 is topoisomerase II poisoning. PNAS. 2020;117:405–6. doi: 10.1073/pnas.1921649117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Hamoly T, El-Sharawy MS, Abd El-Rahman SS. L-thyroxine modifies nephrotoxicity by regulating the apoptotic pathway: The possible role of CD38/ADP-ribosyl cyclase-mediated calcium mobilization. PLoS One. 2017;12:e0184157. doi: 10.1371/journal.pone.0184157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsheikhi S. Antimicrobial effect of new fluoroquinolones on clinically important microorganisms. University of Jordan; 2018. [Google Scholar]
- Ghimeray AK, Lee HY, Kim YH, Ryu EK, Chang MS. Evaluation of antioxidant and antiinflammatory Effect of Rhododendron brachycarpum extract used in skin care product by in vitro and in vivo test. Tech Inv. 2015;6:105–11. [Google Scholar]
- Hamdan A, Kasabri V, Al-Hiari Y, et al. Dual anti-inflammatory and antiglycation propensities of a potentially novel class of functionalized fluoroquinolones. J Heterocyclic Chem. 2019;57:663. [Google Scholar]
- Hidayat MA, Fitri A, Kuswandi B. Scanometry as microplate reader for high throughput method based on DPPH dry reagent for antioxidant assay. Acta Pharm Sinica B. 2017;7:395–400. doi: 10.1016/j.apsb.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann H, Kunz A, Simona V, Palesea P, Shaw M. Broad-spectrum antiviral that interferes with de novo pyrimidine biosynthesis. Proc Nat Acad Sci U S A. 2011;108: 5777–82. doi: 10.1073/pnas.1101143108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Wang Y, Li C, Wang X, He X. Antiinflammatory Oleanolic Triterpenes from Chinese Acorns. Molecules. 2016;21:669–77. doi: 10.3390/molecules21050669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson GC, Fox JA, Poi M, Strickland SA. Molecular and pharmacologic properties of the anticancer quinolone derivative vosaroxin: A New Therapeutic Agent For Acute Myeloid Leukemia. Drugs. 2016;76:1245–55. doi: 10.1007/s40265-016-0614-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalındemirtaş FD, Birman H, Candöken E, et al. Cytotoxic Effects of Some Flavonoids and Imatinib on the K562 Chronic Myeloid Leukemia Cell Line: Data Analysis Using the Combination Index Method. Balkan Med J. 2019;36:96. doi: 10.4274/balkanmedj.galenos.2018.2017.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur G, Dufour JM. Cell lines, Valuable tools or useless artifacts. Spermatogenesis. 2012;2:1–5. doi: 10.4161/spmg.19885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaleel S, Al-Hiari Y, Kasabri V, et al. Antiproliferative properties of 7,8-Ethylene Diamine Chelator-Lipophilic Fluoroquinolone Derivatives Against colorectal cancer Cell Lines. Anticancer Agents Med Chem. 2021;21:1–17. doi: 10.2174/1871520621666210623111744. [DOI] [PubMed] [Google Scholar]
- Lamson DW, Brignall MS. Antioxidants in cancer therapy; their actions and interactions with oncologic therapies. Altern Med Rev. 1999;4:304–29. [PubMed] [Google Scholar]
- Litwinienko G, Ingold KU. Abnormal Solvent Effects on Hydrogen Atom Abstraction Resolution of the Curcumin Antioxidant Controversy. The Role of Sequential Proton Loss Electron Transfer. J Org Chem. 2004;69:5888–96. doi: 10.1021/jo049254j. [DOI] [PubMed] [Google Scholar]
- Mamdooh N, Kasabri V, Al-Hiari Y, et al. Evaluation of selected commercial pharmacotherapeutic drugs as potential pancreatic lipase inhibitors and antiproliferative compounds. Drug Dev Res. 2019;80:310–24. doi: 10.1002/ddr.21499. [DOI] [PubMed] [Google Scholar]
- Marinova G, Batchvarov V. Evaluation of the methods for determination of the free radical scavenging activity by DPPH. Bulg J Agric Sci. 2011;17:11–24. [Google Scholar]
- Owens RC, Ambrose PG. Animicrobial safety: focus on fluoroquinolones. Clin Inf Dis. 2005;41:57–144. doi: 10.1086/428055. [DOI] [PubMed] [Google Scholar]
- Perillo B, Di Donato M, Pezone A, et al. ROS in cancer therapy: the bright side of the moon. Exp Mol Med. 2020;52:192–203. doi: 10.1038/s12276-020-0384-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qashou E, Alhiari Y, Kasabri V, et al. Antiproliferative Activities Of Lipophililic Fluoroquinolones-Based Scaffold Against A Panel Of Solid And Liquid Cancer Cell Lines. Asian Pac J Cancer Prev. 2022;23:1529–37. doi: 10.31557/APJCP.2022.23.5.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayburn ER, Ezell SJ, Zhang R. Anti-inflammatory agents for cancer therapy. Mol Cell Pharm. 2009;1:29. doi: 10.4255/mcpharmacol.09.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes-Farias M, Carrasco-Pozo C. The anti-cancer effect of quercetin: Molecular implications in cancer metabolism. Int J Mol Sci. 2019;20:3177. doi: 10.3390/ijms20133177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricchi P, Di Matola T, Ruggiero G, et al. Effect of non-steroidal anti-inflammatory drugs on colon carcinoma Caco-2 cell responsiveness to topoisomerase inhibitor drugs. Brit J Cancer. 2002;86:1501–9. doi: 10.1038/sj.bjc.6600289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen Zhang G, Qing Tu, C , Ying Zhang G, et al. Indomethacin induces apoptosis and inhibits proliferation in chronic myeloid leukemia cells. Leuk Res. 2000;24:385–92. doi: 10.1016/s0145-2126(99)00198-8. [DOI] [PubMed] [Google Scholar]
- Shalaby EA, Shanab SMM. Antioxidant compounds, assays of determination and mode of action. Afri J Pharm Pharmacol. 2013;7:528–39. [Google Scholar]
- Sharma OP, Bhat TK. DPPH antioxidant assay revisited. Food Chem. 2009;113:1202–5. [Google Scholar]
- Thun MJ, Henley SJ, Patrono C. Nonsteroidal anti-inflammatory drugs as anticancer agents: mechanistic, pharmacologic, and clinical issues. J Nat Cancer Instit. 2002;94:252–66. doi: 10.1093/jnci/94.4.252. [DOI] [PubMed] [Google Scholar]
- Tuli HS, Tuorkey MJ, Thakral F, et al. A Molecular Mechanisms of Action of Genistein in Cancer: Recent Advances. Front Pharmacol. 2019;10 doi: 10.3389/fphar.2019.01336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vichai V, Kirtikara K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat Protoc. 2006;1:1112–6. doi: 10.1038/nprot.2006.179. [DOI] [PubMed] [Google Scholar]
- Zappavign S, Cossu AM, Grimaldi A, et al. Anti-Inflammatory Drugs as Anticancer Agents. Int J Mol Sci. 2020;21:2605. doi: 10.3390/ijms21072605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zusso M, Lunardi V, Franceschini D, et al. Ciprofloxacin and levofloxacin attenuate microglia inflammatory response via TLR4/NF-kB pathway. J Neuroinflamm. 2019;16:148. doi: 10.1186/s12974-019-1538-9. [DOI] [PMC free article] [PubMed] [Google Scholar]