Abstract
We present a case of eosinophil‐rich linear IgA bullous disease (LABD) following the administration of a messenger RNA COVID‐19 booster vaccine. A 66‐year‐old man presented to the emergency department with a 3‐week history of a pruritic blistering rash characterized by fluid‐filled bullae and multiple annular and polycyclic plaques. He was initially diagnosed with bullous pemphigoid based on a biopsy showing a subepidermal blister with numerous eosinophils. However, direct immunofluorescence studies showed linear IgA and IgM deposition along the basement membrane zone with no immunoreactivity for C3 or IgG. Additionally, indirect immunofluorescence was positive for IgA basement membrane zone antibody. The patient was subsequently diagnosed with LABD and initiated on dapsone therapy with resolution of his lesions at 3‐month follow‐up. This case illustrates the growing number of autoimmune blistering adverse cutaneous reactions from vaccination. Dermatopathologists should be aware that features of autoimmune blistering diseases can overlap and may not be distinguishable based on these histopathological findings alone. Confirmation with direct immunofluorescence and/or serological studies may be necessary for accurate diagnosis.
Keywords: COVID‐19, direct immunofluorescence, linear IgA bullous dermatosis, Moderna booster, vaccine
1. INTRODUCTION
Cutaneous adverse reactions in the setting of COVID‐19 vaccination have recently been the subject of great interest, with several studies and registries reporting various clinical presentations 1 , 2 , 3 , 4 , 5 and histopathological findings. 6 , 7 , 8 Among these, reports of autoimmune bullous dermatoses (AIBD) following COVID‐19 infections, 9 , 10 as well as vaccinations, 11 , 12 , 13 , 14 , 15 are some of the most serious reactions. While bullous pemphigoid (BP) has been the most commonly reported vaccine‐related AIBD, linear IgA bullous dermatosis (LABD) has also been reported in the setting of both the recombinant Oxford‐AstraZeneca and messenger RNA (mRNA) Pfizer‐BioNTech COVID‐19 vaccines. 16 , 17 Here, we present a case of LABD triggered by the mRNA Moderna COVID‐19 vaccine booster with histopathological features resembling BP that required confirmation with direct immunofluorescence (DIF) and indirect immunofluorescence (IIF) studies for accurate diagnosis.
2. CASE REPORT
A 66‐year‐old male with a past medical history of hyperlipidemia and nasal congestion presented to the emergency department with a 3‐week history of a pruritic blistering rash on both his lower extremities. The eruption began on the bilateral upper thighs 5 days after receiving the Moderna booster vaccine and progressed to involve his extremities and trunk with pruritic and tense bullae. He reported no other systemic symptoms or other new exposures. His medications included rosuvastatin, mometasone furoate nasal spray, and oral montelukast sodium, which had been unchanged.
Physical examination revealed multiple annular and polycyclic blanchable plaques with central areas of clearing spread over his lower extremities, upper extremities, and trunk. There were grouped tense fluid‐filled bullae on his upper thighs on an erythematous base (Figure 1). Mucosal membranes were uninvolved.
FIGURE 1.
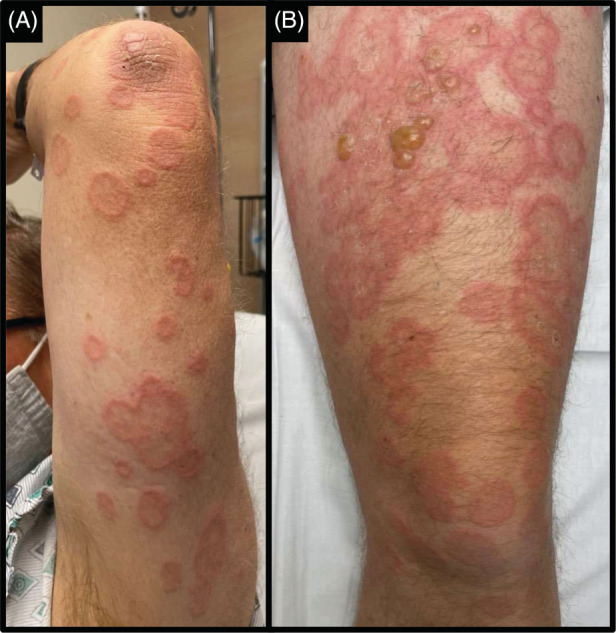
(A) Polycyclic erythematous plaques with central clearing were noticed on the left arm. (B) Yellow tense fluid‐filled bullae were present on a background of annular and polycyclic erythematous plaques on the left thigh.
A shave biopsy of the lateral edge of a bulla was performed, and the resulting specimen with an H&E stain showed a subepidermal bulla with numerous eosinophils within the cavity in addition to a perivascular predominantly eosinophilic infiltrate. At the periphery of the blister, both neutrophils and eosinophils were seen within dermal papillae and lining the dermal‐epidermal junction (Figure 2). Based on these histopathological findings, an initial diagnosis of BP was made.
FIGURE 2.
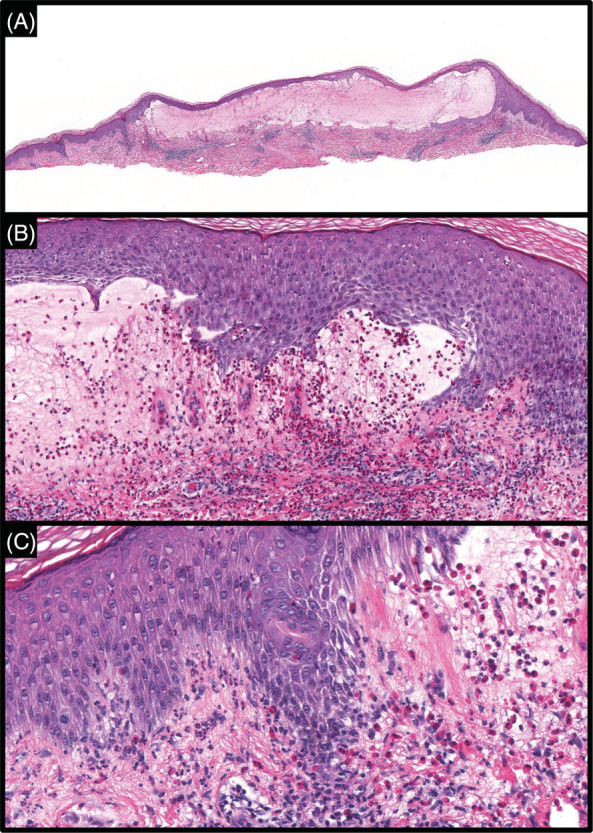
(A) Lesional skin specimen with H&E stain revealed broad subepidermal blister with a superficial perivascular infiltrate (×4). (B) Numerous eosinophils are seen within the blister cavity (×20). (C) Both eosinophils and neutrophils are seen lining the dermal‐epidermal junction and within dermal papillae (×40).
A punch biopsy specimen from perilesional skin was also submitted for DIF and showed linear deposition of IgA and IgM along the basement membrane zone (Figure 3) with no immunoreactivity for C3 or IgG. IIF was positive for IgA basement membrane zone antibody. Antibodies for BPAg1 and BPAg2 were not detected.
FIGURE 3.
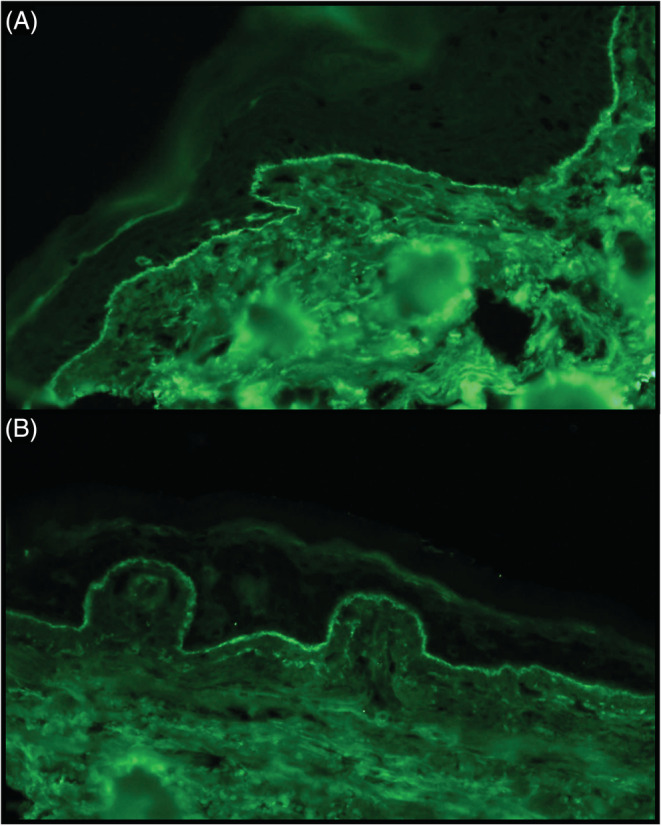
(A) Direct Immunofluorescence of clinically perilesional skin revealed a strong linear deposition of IgA (×40) and (B) weaker IgM co‐reactivity along the dermal‐epidermal junction (×40).
A final diagnosis of drug‐induced LABD was made. He was initially treated with oral prednisone and a high potency topical steroid for rapid disease control. He then transitioned to dapsone, with resolution of his bullous eruption at approximately 3‐month follow‐up.
3. DISCUSSION
We present a challenging case of LABD induced by COVID‐19 vaccination. Although the patient presented with polycyclic and annular vesiculobullae that were characteristic of LABD, histopathological examination from a biopsy specimen showed features that more closely resembled BP. Accurate diagnosis and treatment required the detection of IgA deposition along the basement membrane zone on DIF and circulating IgA anti‐basement membrane zone antibodies in the patient's sera.
LABD is a relatively uncommon autoimmune disorder in which IgA autoantibodies develop against heterogeneous antigens of the basement membrane zone. LABD can present in a pediatric form (also known as chronic bullous disease of childhood), an idiopathic adult form, and a drug‐induced form. Clinical presentation is polymorphic and variable and can range from polycyclic or annular urticarial papules, to plaques, erosions, or papulovesicles that can be focal or generalized. A characteristic physical exam finding is grouped blisters in an annular configuration around the edges of the plaques (“string of pearls” sign). 18 , 19 , 20
In drug‐induced LABD, lesions typically present 2–21 days after administration of the offending medication, with vancomycin as the most commonly associated exposure. 21 , 22 Skin involvement tends to be more severe and extensive with large erosions compared to the idiopathic form. 23 , 24 The most common sites involved in drug‐induced LABD involve the upper and lower limbs. 19 , 25 Interestingly, mucosal and conjunctival lesions are less likely in drug‐induced LABD. 26 , 27 Drug‐induced LABD more commonly occurs in men and older patients with a mean age of 66.5 years. 22 , 28 These features are consistent with those of our patient, a 65‐year‐old male who developed polymorphic papulovesicles in an annular arrangement on the upper and lower extremities that started 5 days after his COVID‐19 mRNA booster vaccine.
On histopathology, LABD is typically characterized by a subepidermal separation with a neutrophil‐rich infiltrate that can line the dermal‐epidermal junction or concentrate within dermal papillae. In drug‐induced cases, however, eosinophils may predominate and mimic BP. 29 , 30 , 31 , 32 , 33 The histopathological differential diagnosis for classical LABD includes dermatitis herpetiformis (DH), epidermolysis bullosa acquisita (EBA), neutrophil‐rich BP, mucous membrane pemphigoid (MMP), and bullous systemic lupus erythematosus (SLE). In cases of LABD in which eosinophils predominate, it may be indistinguishable from BP.
DIF studies are, therefore, a critical component of appropriate workup and accurate diagnosis. LABD can be confirmed through DIF studies showing deposits of IgA with or without C3 in a linear fashion along the basement membrane zone. While IgA is often the sole immunoreactant, 33 weaker co‐reactivity with IgG or IgM have been reported. 33 , 34 , 35 , 36 DIF findings in DH are characterized by microgranular or fibrillar IgA deposition along the basement membrane zone and in the dermal papillae. 37 In BP, there is linear C3 with or without weaker IgG along the basement membrane zone. Linear C3 and IgG along the basement membrane zone can also be seen in EBA, MMP, and bullous SLE. Not uncommonly, IgA deposition can be seen in BP, EBA, MMP, and bullous SLE. 38 , 39 , 40 , 41 Distinction between pemphigoid and non‐pemphigoid group of disorders can be made through serration patterns, where immunoreactants exhibit an n‐serration pattern in pemphigoid and a u‐serration pattern in non‐pemphigoid groups, and through salt‐split studies to determine the localization of immunoreactant deposition to the roof, which favors pemphigoid, or to the floor, which points to EBA or bullous SLE.
Salt‐split studies are less useful in LABD as immunoreactants can localize to either the roof or floor depending on the location of the autoantigen in the basement membrane zone. 42 This is because of the development of IgA autoantibodies against a heterogenous group of antigenic targets. These include the NC16a domain of BP180, 97‐ and 120‐kDa neoepitopes of BP180, 43 laminin‐332, laminin‐γ1, integrin α6β4, 44 and type VII collagen. 20 , 44 , 45 , 46
While histopathological examination with DIF is sufficient to diagnose many autoimmune blistering disorders, IIF or serological studies may be necessary to ultimately confirm the diagnosis, as in our case. As LABD generally responds well to dapsone, accurate diagnosis is of the utmost importance for selecting treatment.
With the recent COVID‐19 pandemic, AIBD, such as BP, has been found to be induced not only by COVID‐19 infections, 9 , 10 but also by newly developed vaccines to COVID‐19. 11 , 12 , 13 , 14 , 15 The exact mechanism of action is unknown for vaccine‐induced AIBDs, including LABD. One hypothesis is that vaccinations, in general, can lead to elevations of pre‐existing autoimmunity in patients with an immunological predisposition to blistering disorders. 47 For instance, there is evidence that some patients have circulating anti‐basement membrane antibodies but subclinical disease activity. 48 , 49 Another explanation for the development of AIBD after administration of a vaccine proposes that antigenic components of a vaccine share structural similarity to a host's antigen at the basement membrane zone. 16 In addition to molecular mimicry, it has been proposed that vaccines can activate interleukin and transforming growth factor‐beta production, leading to an increase in IgA synthesis. 50
While no cases of LABD have been reported with COVID‐19 infection, there are two reports of LABD being triggered by the recombinant Oxford‐AstraZeneca and mRNA Pfizer‐BioNTech COVID‐19 vaccines. In both these cases, eosinophils were seen in the infiltrate, although not as dense as seen in our patient 16 , 17 In these reports, a 61‐year‐old male developed LABD 3 days after the second dose of the Oxford‐AstraZeneca COVID‐19 vaccine and was treated with oral prednisolone with improvement, 16 while a 71‐year‐old male developed LABD 3 days after the second dose of the Pfizer‐BioNTech COVID‐19 vaccine and was treated with topical corticosteroids with improvement. 17
The Oxford‐AstraZeneca vaccine, available in other parts of the world but not in the United States, is a recombinant vaccine containing an altered adenovirus with the gene of the coronavirus spike protein. 51 The Pfizer‐BioNTech vaccine and Moderna vaccine utilize mRNA technology to prevent symptomatic COVID‐19 disease. 52 , 53 It has been proposed that cross‐reactions between SARS‐CoV‐2 spike protein antibody and interprotein crosslinks of the epidermis such as transglutaminase (TGase)2, TGase3, S100B, and collagen may play a role in the immune‐mediated response with vaccine‐induced LABD. 17 Additionally, COVID‐19 mRNA vaccines can induce spike‐antigen‐specific IgG and IgA levels in the serum of patients. 54 Thus, skin reactions could potentially occur months after vaccine administration. 3 With the recent recommendation of a second booster COVID‐19 vaccine, an increased number of immune‐mediated cutaneous reactions may potentially be observed. 55 , 56 , 57 , 58
Blistering disorders are being increasingly recognized as a cutaneous side effect of COVID‐19 vaccination. Dermatopathologists should continue to be aware of the various clinical and histopathological presentations of vaccine‐related reactions. In particular, appropriate workup for bullous diseases should be accompanied by DIF studies or other serological studies as histopathological features alone may not be adequately specific.
AUTHOR CONTRIBUTIONS
William J. Nahm: writing—original draft. Michelle Juarez: supervision, writing—review and editing. Julie Wu: acquisition of data, writing—review and editing, supervision. Randie H. Kim—conceptualization, data analysis, writing—review and editing, supervision.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Nahm WJ, Juarez M, Wu J, Kim RH. Eosinophil‐rich linear IgA bullous dermatosis induced by mRNA COVID‐19 booster vaccine. J Cutan Pathol. 2022;1‐5. doi: 10.1111/cup.14305
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
REFERENCES
- 1. McMahon DE, Amerson E, Rosenbach M, et al. Cutaneous reactions reported after Moderna and Pfizer COVID‐19 vaccination: a registry‐based study of 414 cases. J Am Acad Dermatol. 2021;85(1):46‐55. doi: 10.1016/j.jaad.2021.03.092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Catala A, Munoz‐Santos C, Galvan‐Casas C, et al. Cutaneous reactions after SARS‐CoV‐2 vaccination: a cross‐sectional Spanish nationwide study of 405 cases. Br J Dermatol. 2022;186(1):142‐152. doi: 10.1111/bjd.20639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bataille V, Puig S. COVID‐19 vaccines and skin manifestations. Br J Dermatol. 2022;186(1):15. doi: 10.1111/bjd.20807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Robinson LB, Fu X, Hashimoto D, et al. Incidence of cutaneous reactions after messenger RNA COVID‐19 vaccines. JAMA Dermatol. 2021;157(8):1000‐1002. doi: 10.1001/jamadermatol.2021.2114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Maronese CA, Caproni M, Moltrasio C, et al. Bullous pemphigoid associated with COVID‐19 vaccines: an Italian multicentre study. Front Med. 2022;9:841506. doi: 10.3389/fmed.2022.841506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Niebel D, Wenzel J, Wilsmann‐Theis D, Ziob J, Wilhelmi J, Braegelmann C. Single‐center clinico‐pathological case study of 19 patients with cutaneous adverse reactions following COVID‐19 vaccines. Dermatopathology. 2021;8(4):463‐476. doi: 10.3390/dermatopathology8040049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fernandez‐Nieto D, Hammerle J, Fernandez‐Escribano M, et al. Skin manifestations of the BNT162b2 mRNA COVID‐19 vaccine in healthcare workers. ‘COVID‐arm’: a clinical and histological characterization. J Eur Acad Dermatol Venereol. 2021;35(7):e425‐e427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Larson V, Seidenberg R, Caplan A, Brinster NK, Meehan SA, Kim RH. Clinical and histopathological spectrum of delayed adverse cutaneous reactions following COVID‐19 vaccination. J Cutan Pathol. 2022;49(1):34‐41. doi: 10.1111/cup.14104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kridin K, Schonmann Y, Weinstein O, Schmidt E, Ludwig RJ, Cohen AD. The risk of COVID‐19 in patients with bullous pemphigoid and pemphigus: a population‐based cohort study. J Am Acad Dermatol. 2021;85(1):79‐87. doi: 10.1016/j.jaad.2021.02.087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Olson N, Eckhardt D, Delano A. New‐onset bullous pemphigoid in a COVID‐19 patient. Case Rep Dermatol Med. 2021;2021:5575111. doi: 10.1155/2021/5575111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Perez‐Lopez I, Moyano‐Bueno D, Ruiz‐Villaverde R. Bullous pemphigoid and COVID‐19 vaccine. Med Clin. 2021;157(10):e333‐e334. doi: 10.1016/j.medcle.2021.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tomayko MM, Damsky W, Fathy R, et al. Subepidermal blistering eruptions, including bullous pemphigoid, following COVID‐19 vaccination. J Allergy Clin Immunol. 2021;148(3):750‐751. doi: 10.1016/j.jaci.2021.06.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dell'Antonia M, Anedda S, Usai F, Atzori L, Ferreli C. Bullous pemphigoid triggered by COVID‐19 vaccine: rapid resolution with corticosteroid therapy. Dermatol Ther. 2022;35(1):e15208. doi: 10.1111/dth.15208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rosinska‐Wieckowicz A, Jalowska M, Bowszyc‐Dmochowska M, Dmochowski M. Case report: infantile bullous pemphigoid: triggering by COVID‐19 is speculative. Front Med. 2021;8:760823. doi: 10.3389/fmed.2021.760823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Transient bullous pemphigoid with concurrent chronic urticaria followed by a transient T‐cell mediated dermatitis after mRNA COVID vaccination in a patient with underlying autoimmune pericarditis. Accessed December 8, 2022. https://www.aaaai.org/allergist-resources/ask-the-expert/answers/2021/bullouss
- 16. Hali F, Kerouach A, Alatawna H, Chiheb S, Lakhdar H. Linear IgA bullous dermatosis following Oxford AstraZeneca COVID‐19 vaccine. Clin Exp Dermatol. 2022;47(3):611‐613. doi: 10.1111/ced.15007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Coto‐Segura P, Fernandez‐Prada M, Mir‐Bonafe M, et al. Vesiculobullous skin reactions induced by COVID‐19 mRNA vaccine: report of four cases and review of the literature. Clin Exp Dermatol. 2022;47(1):141‐143. doi: 10.1111/ced.14835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jones DH, Todd M, Craig TJ. Early diagnosis is key in vancomycin‐induced linear IgA bullous dermatosis and Stevens‐Johnson syndrome. J Osteopath Med. 2004;104(4):157‐163. [PubMed] [Google Scholar]
- 19. Palmer RA, Ogg G, Allen J, et al. Vancomycin‐induced linear IgA disease with autoantibodies to BP180 and LAD285. Br J Dermatol. 2001;145(5):816‐820. doi: 10.1046/j.1365-2133.2001.04492.x [DOI] [PubMed] [Google Scholar]
- 20. Lammer J, Hein R, Roenneberg S, Biedermann T, Volz T. Drug‐induced linear IgA bullous dermatosis: a case report and review of the literature. Acta Derm Venereol. 2019;99(6):508‐515. doi: 10.2340/00015555-3154 [DOI] [PubMed] [Google Scholar]
- 21. Yamagami J, Nakamura Y, Nagao K, et al. Vancomycin mediates IgA autoreactivity in drug‐induced linear IgA bullous dermatosis. J Invest Dermatol. 2018;138(7):1473‐1480. doi: 10.1016/j.jid.2017.12.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fortuna G, Salas‐Alanis JC, Guidetti E, Marinkovich MP. A critical reappraisal of the current data on drug‐induced linear immunoglobulin A bullous dermatosis: a real and separate nosological entity? J Am Acad Dermatol. 2012;66(6):988‐994. doi: 10.1016/j.jaad.2011.09.018 [DOI] [PubMed] [Google Scholar]
- 23. Tran D, Kossard S, Shumack S. Phenytoin‐induced linear IgA dermatosis mimicking toxic epidermal necrolysis. Australas J Dermatol. 2003;44(4):284‐286. [DOI] [PubMed] [Google Scholar]
- 24. Waldman M, Black D, Callen J. Vancomycin‐induced linear IgA bullous disease presenting as toxic epidermal necrolysis. Clin Exp Dermatol. 2004;29(6):633‐636. [DOI] [PubMed] [Google Scholar]
- 25. Zone JJ, Pazderka Smith E, Powell D, Taylor TB, Smith JB, Meyer LJ. Antigenic specificity of antibodies from patients with linear basement membrane deposition of IgA. Dermatology. 1994;189(suppl 1):64‐66. doi: 10.1159/000246933 [DOI] [PubMed] [Google Scholar]
- 26. Kuechle MK, Stegemeir E, Maynard B, Gibson LE, Leiferman KM, Peters MS. Drug‐induced linear IgA bullous dermatosis: report of six cases and review of the literature. J Am Acad Dermatol. 1994;30(2 Pt 1):187‐192. doi: 10.1016/s0190-9622(94)70015-x [DOI] [PubMed] [Google Scholar]
- 27. Nousari HC, Kimyai‐Asadi A, Caeiro JP, Anhalt GJ. Clinical, demographic, and immunohistologic features of vancomycin‐induced linear IgA bullous disease of the skin. Report of 2 cases and review of the literature. Medicine. 1999;78(1):1‐8. doi: 10.1097/00005792-199901000-00001 [DOI] [PubMed] [Google Scholar]
- 28. Lings K, Bygum A. Linear IgA bullous dermatosis: a retrospective study of 23 patients in Denmark. Acta Derm Venereol. 2015;95(4):466‐471. doi: 10.2340/00015555-1990 [DOI] [PubMed] [Google Scholar]
- 29. Fulton E, Jan F, Zimarowski MJ. Flame figures in linear IgA bullous dermatosis: a novel histopathologic finding. Dermatol Online J. 2017;23(11):13030/qt1qs7m39f. [PubMed] [Google Scholar]
- 30. Kanda N, Nakadaira N, Otsuka Y, Ishii N, Hoashi T, Saeki H. Linear IgA bullous dermatosis associated with ulcerative colitis: a case report and literature review. Australas J Dermatol. 2020;61(1):e82‐e86. doi: 10.1111/ajd.13121 [DOI] [PubMed] [Google Scholar]
- 31. Jakhar D, Singal A, Sharma S, Kotru M. Norfloxacin‐induced linear IgA dermatosis. Skinmed. 2020;18(6):374‐377. [PubMed] [Google Scholar]
- 32. Billet SE, Kortuem KR, Gibson LE, El‐Azhary R. A morbilliform variant of vancomycin‐induced linear IgA bullous dermatosis. Arch Dermatol. 2008;144(6):774‐778. doi: 10.1001/archderm.144.6.774 [DOI] [PubMed] [Google Scholar]
- 33. Garel B, Ingen‐Housz‐Oro S, Afriat D, et al. Drug‐induced linear immunoglobulin A bullous dermatosis: a French retrospective pharmacovigilance study of 69 cases. Br J Clin Pharmacol. 2019;85(3):570‐579. doi: 10.1111/bcp.13827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ohata C, Ishii N, Koga H, Nakama T. A clinical and serological study of linear IgA bullous dermatosis without linear immunoglobulin deposition other than IgA at the basement membrane zone using direct immunofluorescence. Br J Dermatol. 2017;177(1):152‐157. doi: 10.1111/bjd.15232 [DOI] [PubMed] [Google Scholar]
- 35. Wojnarowska F, Marsden RA, Bhogal B, Black MM. Chronic bullous disease of childhood, childhood cicatricial pemphigoid, and linear IgA disease of adults. A comparative study demonstrating clinical and immunopathologic overlap. J Am Acad Dermatol. 1988;19(5 Pt 1):792‐805. doi: 10.1016/s0190-9622(88)70236-4 [DOI] [PubMed] [Google Scholar]
- 36. Guide SV, Marinkovich MP. Linear IgA bullous dermatosis. Clin Dermatol. 2001;19(6):719‐727. doi: 10.1016/s0738-081x(00)00185-1 [DOI] [PubMed] [Google Scholar]
- 37. Dmochowski M, Gornowicz‐Porowska J, Bowszyc‐Dmochowska M. An update on direct immunofluorescence for diagnosing dermatitis herpetiformis. Postepy Dermatol Alergol. 2019;36(6):655‐658. doi: 10.5114/ada.2019.91415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Schmidt E, Zillikens D. Pemphigoid diseases. Lancet. 2013;381(9863):320‐332. doi: 10.1016/S0140-6736(12)61140-4 [DOI] [PubMed] [Google Scholar]
- 39. Chan LS, Ahmed AR, Anhalt GJ, et al. The first international consensus on mucous membrane pemphigoid: definition, diagnostic criteria, pathogenic factors, medical treatment, and prognostic indicators. Arch Dermatol. 2002;138(3):370‐379. doi: 10.1001/archderm.138.3.370 [DOI] [PubMed] [Google Scholar]
- 40. Benoit S, Scheurlen M, Goebeler M, Stoevesandt J. Structured diagnostic approach and risk assessment in mucous membrane pemphigoid with oesophageal involvement. Acta Derm Venereol. 2018;98(7):660‐666. doi: 10.2340/00015555-2938 [DOI] [PubMed] [Google Scholar]
- 41. Gammon WR, Briggaman RA. Bullous SLE: a phenotypically distinctive but immunologically heterogeneous bullous disorder. J Invest Dermatol. 1993;100(1):28S‐34S. doi: 10.1111/1523-1747.ep12355210 [DOI] [PubMed] [Google Scholar]
- 42. Kim RH, Brinster NK. Practical direct immunofluorescence. Am J Dermatopathol. 2020;42(2):75‐85. doi: 10.1097/DAD.0000000000001516 [DOI] [PubMed] [Google Scholar]
- 43. Ishii N, Ohyama B, Yamaguchi Z, Hashimoto T. IgA autoantibodies against the NC16a domain of BP180 but not 120‐kDa LAD‐1 detected in a patient with linear IgA disease. Brit J Dermatol. 2008;158(5):1151‐1153. [DOI] [PubMed] [Google Scholar]
- 44. Li X, Tsuchisaka A, Qian H, et al. Linear IgA/IgG bullous dermatosis reacts with multiple laminins and integrins. Eur J Dermatol. 2015;25(5):418‐423. [DOI] [PubMed] [Google Scholar]
- 45. Hashimoto T, Ishiko A, Shimizu H, et al. A case of linear IgA bullous dermatosis with IgA anti‐type VII collagen autoantibodies. Brit J Dermatol. 1996;134(2):336‐339. [PubMed] [Google Scholar]
- 46. Zambruno G, Manca V, Kanitakis J, Cozzani E, Nicolas J‐F, Giannetti A. Linear IgA bullous dermatosis with autoantibodies to a 290 kd antigen of anchoring fibrils. J Am Acad Dermatol. 1994;31(5):884‐888. [DOI] [PubMed] [Google Scholar]
- 47. Corra A, Bonciolini V, Quintarelli L, Verdelli A, Caproni M. Linear IGA bullous dermatosis potentially triggered by vaccination. Int J Immunopathol Pharmacol. 2022;36:20587384211021218. doi: 10.1177/20587384211021218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Downs A. Does influenza vaccination induce bullous pemphigoid? A report of four cases. Br J Dermatol. 1998;13:363. [DOI] [PubMed] [Google Scholar]
- 49. Lear JT, Tan BB, English JS. Bullous pemphigoid following influenza vaccination. Clin Exp Dermatol. 1996;21(5):392. doi: 10.1111/j.1365-2230.1996.tb00136.x [DOI] [PubMed] [Google Scholar]
- 50. Alberta‐Wszolek L, Mousette AM, Mahalingam M, Levin NA. Linear IgA bullous dermatosis following influenza vaccination. Dermatol Online J. 2009;15(11):3. [PubMed] [Google Scholar]
- 51. Yadav T, Srivastava N, Mishra G, et al. Recombinant vaccines for COVID‐19. Hum Vaccin Immunother. 2020;16(12):2905‐2912. doi: 10.1080/21645515.2020.1820808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Fiolet T, Kherabi Y, MacDonald CJ, Ghosn J, Peiffer‐Smadja N. Comparing COVID‐19 vaccines for their characteristics, efficacy and effectiveness against SARS‐CoV‐2 and variants of concern: a narrative review. Clin Microbiol Infect. 2022;28(2):202‐221. doi: 10.1016/j.cmi.2021.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tenforde MW. Effectiveness of Pfizer‐BioNTech and Moderna vaccines against COVID‐19 among hospitalized adults aged ≥65 years—United States, January–March 2021. MMWR Morb Mortal Wkly Rep. 2021;70:674‐679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wisnewski AV, Campillo Luna J, Redlich CA. Human IgG and IgA responses to COVID‐19 mRNA vaccines. PLoS One. 2021;16(6):e0249499. doi: 10.1371/journal.pone.0249499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Dicks AB, Gray BH. Images in vascular medicine: leukocytoclastic vasculitis after COVID‐19 vaccine booster. Vasc Med. 2022;27(1):100‐101. doi: 10.1177/1358863X211055507 [DOI] [PubMed] [Google Scholar]
- 56. Drago F, Broccolo F, Ciccarese G. Pityriasis rosea, pityriasis rosea‐like eruptions, and herpes zoster in the setting of COVID‐19 and COVID‐19 vaccination. Clin Dermatol. 2022:S0738‐081X(22)00002‐5. doi: 10.1016/j.clindermatol.2022.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hause AM, Baggs J, Gee J, et al. Safety monitoring of an additional dose of COVID‐19 vaccine ‐ United States, August 12–September 19, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(39):1379‐1384. doi: 10.15585/mmwr.mm7039e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Phuan CZY, Choi EC, Oon HH. Temporary exacerbation of pre‐existing psoriasis and eczema in the context of COVID‐19 messenger RNA booster vaccination: a case report and review of the literature. JAAD Int. 2022;6:94‐96. doi: 10.1016/j.jdin.2021.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.


