Abstract
Juvenile idiopathic arthritis (JIA) is a heterogeneous group of chronic inflammatory arthritides that if inadequately treated, may be associated with chronic disability and deformity. Early diagnosis and treatment initiation is essential in the management of patients with JIA. Conventional means of evaluation of disease presence, disease activity and response to therapy including physical exam, labs and x-rays are at times limited and may be insufficient in making an accurate assessment. Musculoskeletal ultrasound (MSUS) is a well-established modality that is patient and family-friendly, non-invasive, does not require sedation and can be performed at the bedside in real-time. MSUS offers information that cannot be attained by standard outcome measures, and may help to advance both diagnosis and treatment of patients with JIA ultimately improving patient outcomes. This review explores the background of MSUS and the current evidence to support its potential role as a diagnostic, disease activity monitoring and interventional tool.
Keywords: Juvenile idiopathic arthritis, ultrasound, enthesitis
Introduction
Juvenile idiopathic arthritis (JIA) is a heterogeneous group of arthritides of unknown etiology persisting >6 weeks with an age of onset of <16 years. The estimated prevalence of JIA is 0.6–1.9 in 1000 children (1). In childhood inflammatory arthropathies, such as JIA, conventional methods of assessing disease presence and activity (e.g., X-ray or physical examination) cannot objectively evaluate the synovium, tendon, or enthesis of a growing skeleton in an accurate manner (2). The infiltration of inflammatory cells into the synovium augments proinflammatory cytokines and ultimately the formation of a pannus, in turn contributing to significant joint deformity and morbidity in JIA (3). Effusion and synovial thickening are often present even in clinically silent disease (4–6).
In adults, musculoskeletal ultrasound (MSUS) has become an essential tool for timely diagnosis, monitoring response to treatment, and providing guidance for interventional procedures (7). High-resolution ultrasonography is particularly well suited for the evaluation of musculoskeletal disorders in the immature skeleton in which there is an increased ratio of cartilage to bone. MSUS not only allows the examiner to readily distinguish cartilage, soft tissue, and bone but also demonstrates changes in structural relationships that occur with motion and more favorably permits the comparison of a region in different planes and to the normal contralateral side. Importantly, MSUS is a safe, non-invasive, cost-effective, and widely available method that allows bedside evaluation real-time. Patient and family involvement in the decision-making process has recently been recognized as one of the overarching principles when treating JIA. US has the potential to help inform and educate children and parents about the presence of disease, disease activity and response to medication, and guide therapeutic interventions (8). Despite the many advantages of MSUS, there are some disadvantages including operator dependence and machine variability. Obtaining quality images requires knowledge and training specific to age-related findings of the growing skeleton. The reliability of MSUS findings is adequate if standardized training, scanning protocols, and scoring systems are in place (9–11).
This review highlights the current state of MSUS in pediatric rheumatology and includes a general description of sonographic settings and findings in children, the use of MSUS in JIA including synovitis and enthesitis, and a review of US-guided procedures in pediatrics.
MSUS Principles
Technological improvements, including faster microprocessors, digital imaging systems, and high-frequency linear-array transducers in the early 1990s, greatly improved US image resolution and tissue contrast, allowing the assessment of inflammatory arthritis (12). Successful use of MSUS by the clinician requires a clear understanding of the principles of sonography, thereby optimizing the ability of the sonographer to adjust image settings and enhance the identification of image artifacts, which may mimic pathology.
Transducer design is important and is partly responsible for image quality. Higher frequency transducers (7.5–18 MHz) enhanced the visualization of more superficial structures, e.g., tendons and ligaments, as well as small joints. The choice of transducer is always a trade-off between depth of penetration and resolution. Higher frequencies provide greater resolution but compromise depth. In general, the use of a high-frequency linear transducer is preferred for MSUS in children.
The reduction of speckle-, noise-, and angle-related artifacts by the use of real-time spatial compound US imaging is of great benefit when scanning soft tissues. In this technique, the transducer angles the beam over several different angles of insonation with the image composed of the resulting echoes from each of these. The enhancement of contrast resolution and tissue differentiation provided by compound imaging improves image quality over conventional US (13).
Harmonic imaging utilizes the phenomenon of non-linear propagation of US waves through the body, leading to multiple primary echo frequency returning from reflective body interfaces (harmonics) around the primary transducer frequency (14). Using harmonic imaging improves image clarity due to the marked reduction in interference and a significant improvement in the signal-to-noise ratio. Harmonic frequencies are generated maximally at the focal zone.
Another important requirement is the presence of a detailed and accurate knowledge of anatomy. This knowledge paired with the background understanding of joint pathophysiology by the rheumatologist gives a new dimension, a more “hands on” practice to the clinician. Recognition of the sonographic appearance of the developing skeleton is crucial when applying this modality to children. Ultrasonographically, owing to its high water content, unossified cartilage appears hypo- to anechoic. Occasionally, internal hyperechoic echoes may be seen within the cartilaginous epiphysis, corresponding to vascular channels (15). The physis is also seen as a relatively linear, but undulating, hypoechoic structure (also being unossified cartilage); the metaphysis and diaphysis, being ossified, exhibit linear, strongly reflective echoes. With increasing age, the chondroepiphysis begins ossification, initially a central reflective irregular echo with posterior acoustic shadowing followed by progressive mineralization until ultimately the whole epiphysis is ossified and covered by articular cartilage, which can be seen as a hypoechoic rim overlying the bone (14). Avoidance of mistaking the hypoechoic articular cartilage for joint fluid can be done by applying pressure to the probe and by using dynamic examination as the hyaline cartilage is not compressible nor will it change its shape with movement.
Special attention should be given to the quality of the US equipment used for MSUS. High-resolution systems capable of generating an accurate and well-delineated image of the target tissue are desirable. B-mode, which is synonymous with grayscale (GS), provides a two-dimensional image in different intensities of gray, representing US echoes reflected by various structures. Color Doppler and/or Power Doppler (PD) settings are required for the assessment of inflammatory arthropathies. Additional factors to take into consideration are portability and whether MSUS will be used to guide diagnostic and/or therapeutic decisions. Different types of probes are necessary to cover the full frequency range required for routine MSUS examination depending upon a patients’ size, i.e., a different transducer frequency is required for assessing the hip joint of an adolescent than for examining the small joints of the hands. A linear-array transducer is usually used in most musculoskeletal applications (16). The transducer size is also important. A transducer with a smaller footprint (surface contact area) allows better angulation between the small joints, decreasing the chance of “blind spots” and reducing the risk of artifact. However, a bigger footprint can be useful particularly for larger, more complex joints, such as the shoulder, where orientation is sometimes challenging. In this case, a broader visualization of a region of interest that includes key landmarks can optimize the identification and assessment of a specific structure of interest.
MSUS in JIA
MSUS is highly sensitive, detecting as little as 1 mL of fluid, but it lacks specificity in identifying a cause (17). Therefore, definitions of the expected sonographic findings in developing children, as well as in juvenile arthritis, are crucial. The pediatric sub-task force of the Outcome Measures in Rheumatology (OMERACT) Ultrasound Working Group has methodically outlined MSUS definitions for normal pediatric joint, as well as features of arthritis in children. In addition to US findings in synovitis in children, these definitions outline the sonographic appearance of the growing skeleton including hyaline cartilage, ossification centers, and Doppler findings in healthy children (Tables 1 and 2) (15, 18, 19). Figures 1 and 2 clearly demonstrate synovitis on GS and PD, respectively. More recently, the reliability of US in the assessment of normal vascularization and the grading of skeletal maturation in healthy children have been described (20).
Table 1.
Definitions for the sonographic features of joints in healthy children (15) including DOPPLER technique (18)
| Terms | Definitions |
|---|---|
| Hyaline Cartilage | The hyaline cartilage will present as a well-defined anechoic structure (with/without bright echoes/dots) that is non-compressible. The cartilage surface can (but does not have to) be detected as a hyperechoic line. |
| Ossification Center | With advancing maturity, the epiphyseal secondary ossification center will appear as a hyperechoic stucture, with a smooth or irregular surface within the cartilage. |
| Joint Capsule | Normal joint capsule-A hyperechoic structure which can (but does not have to) be seen over bone, cartigale and other intraarticular tissue of the joint. |
| Synovial membrane | Normal synovial membrane - Under normal circumstance, the thin synovial membrance is undetectable. |
| Articular bone | The ossified portion of articular bone is detected as a hyperechoic line. Interruptions of this hyperechoic line may be detected at the growth plate and at the junction of two or more ossification centers. |
| Joint features in healthy children when using DOPPLER technique (18) | |
| Physiological vascularity | Physiological vascularity can be detected by PD as Doppler signal in the joint structures at any age during growth. |
| Physiological intraarticular vascularity can be detected in children within the fat pads and unossified joint structures (i.e., the physis, the cartilage of epiphysis and the short bones cartilage). | |
| Detection of physiological vascularity and its intraarticular anatomical position is joint and age (particularlly in the youngest children dependent). | |
| Physis | Physis can be detected in children as an anechoic unossified structure, intra- or extra-articular according to its anatomical location. |
| Fat pad | Fat pad can be detected as an intra-articular structure with heterogeneous echotexture (similar to the subcutaneous tissue), which might show vascularity. |
| Ossification | In different age groups of children, due to the skeletal development, ossification centers can be detected with different maturation state. |
| Ossification grade is age and joint dependent. | |
Table 2.
Definition for synovitis on ultrasonography in children (19)
| Synovitis detection by MSUS in children includes the assessment of B-mode and Doppler mode (color or power Doppler) findings. | |
|---|---|
|
| |
| Terms | Definitions |
| Synovitis | Synovitis can be detected on the basis of B-mode findings alone. Synovitis cannot be detected based on color/power Doppler findings alone. |
| B-mode findings | B-mode findings include synovial effusion and synovial hypertrophy. |
| Synovial effusion | Synovial effusion is defined as an abnormal, intraarticular, anechoic, or hypoechoic material that is displaceable. |
| Synovial hypertrophy | Synovial hypertrophy is defined as an abnormal, intraarticular, hypoechoic material that is non-displaceable. |
| Doppler signal | Color/power Doppler signals must be detected within synovial hypertrophy to be considered as a sign of synovitis. |
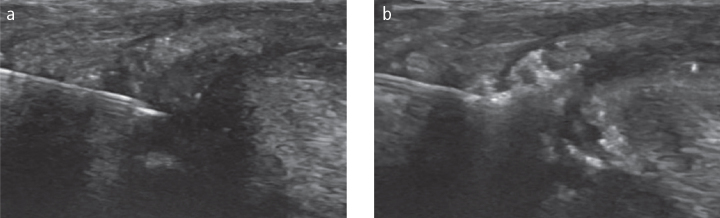
Figure 1.
A longitudinal view of the suprapatellar knee joint with evidence for a large effusion and synovial hypertrophy.
Figure 2.

Power Doppler (PD) image of a radiocarpal wrist joint with evidence for active synovitis demonstrating large effusion, synovial hypertrophy and PD signal.
Ultrasound vs. physical exam
Accurate assessment of disease activity in JIA can often be challenging. Multiple outcome measures have been established to evaluate the heterogeneity of disease activity in different JIA subtypes (21). MSUS may provide additional insight into disease status and offer information not captured by traditional measures. It has repeatedly demonstrated heightened sensitivity compared with physical examination in the identification of active disease (4, 7, 22–25). Enhanced identification directly impacts disease categorization, ultimately impacting treatment decisions (26).
Following the MSUS of 52 joints in 32 patients with JIA, 5 patients originally classified as oligoarthritis or monoarthritis were reclassified as polyarticular (22). A moderate to strong correlation between swelling and GS and PD abnormalities was observed. However, there was a poor correlation for tenderness, pain on movement, and reduced range of motion, highlighting the importance that pain in itself is not indicative of active disease. In the study by Haslam (23), subclinical synovitis was identified in 6 patients, with 1 out of 17 patients being reclassified as having polyarthritis following US. Janow et al. (24) demonstrated improved detection of subclinical disease with US, revealing active disease in 14 joints that were deemed inactive on physical examination.
A pilot study examining MSUS as a non-adherence intervention in 8 patients with JIA demonstrated a 63% discrepancy between US examination and physical examination, with US detecting greater pathology than physical examination. A therapeutic intervention was made in half of the patients (n=4), including initiation of three new biologics, with a clinically meaningful improvement in clinical Juvenile Arthritis Disease Activity Score (25).
MSUS not only enhances sensitivity in detecting the number of joints involved in JIA but also greatly improves the ability to correctly identify the exact location of an inflammatory process and differentiate between tenosynovitis and synovitis (5, 6). Rooney et al. (2), in an effort to clarify the lack of response of intra-articular steroid (IAS) injection and heightened risk of recurrence in ankle synovitis, clinically evaluated swollen ankles and ultrasonographically identified the specific anatomic locations contributing to the appearance of swelling (i.e., articular or synovial). In a cohort of 34 children with JIA (19 polyarticular, 13 oligoarticular, 1 systemic, and 1 psoriatic JIA), 49 clinically swollen ankles were identified and examined using MSUS. US revealed isolated tibiotalar effusion in 29% of swollen ankles, whereas 39% had tenosynovitis alone and 71% had both tenosynovitis and synovitis. Their study also highlighted the fact that medial ankle tenosynovitis was most commonly involved in oligoarticular JIA (81%), whereas tibiotalar involvement was only present in 43% and an isolated tibiotalar effusion was even less common (19%).
In a subsequent prospective study involving 42 children with JIA, clinical evaluation focusing on three regions (tibiotalar, medial tendons, and lateral tendons) was compared with MSUS assessment in 61 swollen ankles (27). MSUS demonstrated superior diagnostic ability compared with clinical examination. Tibiotalar involvement was suspected in 43 out of 61 ankles on clinical examination; however, 14 (32%) patients had no evidence of tibiotalar involvement on MSUS. In addition, of the 31 patients suspected to be free from tibialis posterior tendon (PTT) involvement, 13 (42%) patients had evidence of PTT tenosynovitis on MSUS. Clinical assessment supported 19 patients with peroneal tendon involvement, but this was only appreciated in 8 (42%) patients on MSUS. Similar to the previous study by Rooney et al., tibiotalar involvement was more common when accompanied by tendon involvement (37 ankles, 60.6%) than tibiotalar involvement alone (12 ankles, 19.7%). Medial tendon involvement was twice as frequent as lateral tendon involvement (32 vs. 17) (28).
MSUS as a “biomarker” in disease
MSUS has a growing role in the assessment of disease activity. In a study of 20 children with JIA and 20 age-matched healthy controls, MSUS demonstrated higher knee synovial thickness in children with JIA than in healthy patients (4.2±2.4 mm vs. 1.7±0.3 mm, p<0.001) (29). The study also showed significant positive correlations between mean knee synovial thickness and disease activity score, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), articular index score, and clinical knee scores. Similar significant positive correlations were seen between these measures and knee effusion volume and visual analog scores.
An association between joint vascularity as depicted by PD and inflammatory cytokines, most notably interleukin (IL)-6 (30), and more recently IL-37 (31), has been described. Serum amyloid A (SAA) was also found to be higher in patients with active JIA, and these patients with increased SAA levels had a significantly greater mean MSUS score than those with normal SAA levels (32). PD positivity has also been correlated with markers of angiogenesis including vascular endothelial growth factor (33) and has demonstrated higher sensitivity in the assessment of disease activity than ESR and CRP (34).
Predicting flare
Reliable assessment of disease activity requires well-established, valid definitions, with measures that are reproducible and feasible.
Psoriatic arthritis provides an excellent model for the detection of subclinical synovitis by MSUS. Patients with psoriatic arthritis often present with psoriasis, typically preceding the onset of joint disease by several years. MSUS has been used to detect subclinical joint disease in patients with psoriasis and in adult studies has identified a higher incidence of subclinical synovitis than in healthy controls (50.7% vs. 32.6%, respectively) (35). In a cross-sectional study of adult patients with psoriasis without clinical musculoskeletal involvement, subclinical active synovitis and enthesitis as evidenced by MSUS were seen in 27.5% and 20%, respectively (36). Miotto et al. (37) demonstrated subclinical synovitis in 15 (41.7%) out of 36 patients with JIA with clinically inactive disease (CID) compared with 4 (11.1%) of healthy controls. A total of 34 joints were examined per patient for the presence of synovitis, which they defined as synovial fluid/hypertrophy with or without any PD signal.
Subclinical synovitis has also been appreciated in peripheral small joints (38). A study focusing on US evaluation of metatarsophalangeals (MTPs) and metacarpophalangeals (MCPs) defined US synovitis as synovial hypertrophy with PD signal with or without effusion. Clinical swelling was identified in 75% of MCPs and approximately 25% of MTPs of the joints identified as having synovitis on MSUS.
Rebollo-Polo et al. (6) evaluated the presence of GS and PD abnormalities in 28 patients with JIA with a minimum of 3 months of CID. Previously involved joints and uninvolved contralateral joints were evaluated for GS abnormalities including synovial thickening and joint effusion, as well as abnormal PD signal. GS abnormalities were seen in the wrist of 8 (57.1%) out of 14 previously affected patients and 2 (50%) out of 4 previously unaffected patients. Ankle GS abnormalities were seen in 6 (40%) out of 15 previously affected patients and 1 (12.5%) out of 8 unaffected patients. Corresponding PD was 21.5% and 0% for the wrist and 6.7% and 0% for the ankle in previously affected and previously unaffected patients, respectively. No GS pathology or PD abnormalities were appreciated in either previously affected or unaffected knees. Although the study demonstrated ongoing pathology on US in some patients meeting the definition of CID, determining the exact clinical significance of these findings is ongoing.
Although there are well-established definitions of CID (39, 40), the advent of advanced imaging prompts reevaluation of current definitions. Indeed, subclinical arthritis was identified in 45% of children with CID on magnetic resonance imaging (MRI) (41) and is frequently detected on MSUS. Taking into account the superiority of MSUS to physical examination in the detection of synovitis (10, 22, 23, 38, 42, 43) and the good to excellent intra- and interobserver reliability in recognizing active inflammatory lesions, as well as evidence of damage (10), there is great interest in the role of MSUS in predicting disease flare. The ability to identify and accurately assess key variables may assist in a more accurate prediction of disease flares, as well as overall prognosis, and while preliminary, MSUS may be a helpful tool (44).
Magni-Manzoni et al. (45) sought to determine if the identification of pathologic US findings in CID could predict flare. The group evaluated 52 joints in each of 39 children with JIA and CID of at least 3 months duration. Baseline evaluation revealed synovial hyperplasia in nearly 77%, joint effusion in 66.7%, PD signal in 33.3%, and tenosynovitis in 15.4% of patients. During the 2-year follow-up, 24 (61.5%) patients continued in CID. Based on their findings, the identification of ongoing pathology in patients with CID was not predictive of disease flare. However, a subsequent study noted an increased rate of clinical flare in patients with JIA with CID found to have positive PD (46). In this prospective study of 35 patients with CID followed up for a 30-month period, 20 (57.1%) patients flared, with the risk of flare being five times greater in those with positive PD signal. Patients with CID on medication had 14 times higher risk of flare. Additionally, subclinical synovitis, defined as synovitis with or without positive PD signal, was associated with more erosions.
Zhao et al. (47) recently evaluated the ability of MSUS to predict a flare in patients with JIA with CID, demonstrating a low positive predictive value (PPV) of 12%. In a separate commentary by Roth (48), a discrepancy is revealed in their description of what is considered abnormal on MSUS. They highlight that although 11 wrists were considered abnormal on the basis of PD positivity, only one of these 11 joints had abnormalities on GS. Such discrepancies were also present in the tibiotalar joint. Roth (28) emphasized that Doppler positivity outside of an area of synovial abnormality cannot be considered synovitis. This is a key factor in pediatric musculoskeletal ultrasonography given the frequent detection of normal blood flow within a joint but outside of the synovium.
De Lucia (49) demonstrated an increased risk of flare in patients with JIA with at least 3 months of CID followed up over a 4-year period (odds ratio (OR) 3.8, 95% confidence interval (CI) (1.2–11.5), with the combination of GS and PD abnormalities giving a PPV of relapse of 65% compared with 33% PPV for GS alone, in contrast to the study by Zhao et al. They determined a 94% remission probability with normal MSUS at 1 year and 55% remission probability in the event of abnormal MSUS. It is this combination of GS synovitis and PD positivity that appears to best predict flare of JIA. Interestingly, the joints that flared in De Lucia’s study were different from those joints identified as having subclinical synovitis on baseline US. The authors hypothesized that patients with greater systemic inflammation are more likely to develop synovitis in different joints from those at the time of presentation.
Nieto Gonzalez et al. (50) sought to determine the predictive value of subclinical synovitis in 56 patients with JIA in remission following tapering of tumor necrosis factor (TNF) inhibitors for 1 year. Although GS synovitis was frequent at baseline (83.9% of patients and 10.1% of joints), only 19 (1.3%) out of 1456 joints examined had GS synovitis grade 2 or 3. PD synovitis was seen at baseline in 5 (8.9%) patients, also demonstrating GS synovitis grade 2–3. During the 12-month period, 18 (32.1%) out of 56 experienced a flare, but no significant differences were appreciated in US findings in those patients who flared versus those remaining in remission and none of the 5 patients with concomitant GS synovitis and PD synovitis flared. Of note, although patients were weaning from TNFs, none of them stopped biologic therapy.
Ongoing studies are essential in determining the clinical significance of subclinical synovitis by MSUS and if perhaps the meaning of this finding is variable based upon the JIA subgroup.
Scoring
The growing use of MSUS in routine clinical practice heralds the need for scoring systems to more accurately assess synovitis. Ting et al. (11) established a scoring system utilizing B-mode and PD and determined its reliability in evaluating knee synovitis. Following a consensus-driven approach, a protocol for image acquisition was established, and a standardized scoring system was created. The final image acquisition protocol could be completed within 10 min and consisted of a midline suprapatellar longitudinal view, medial parapatellar transverse, and lateral parapatellar transverse view. A semiquantitative scoring system ranging from 0 (normal) to 3 (severe) was created for B-mode and PD, only including Doppler signals within areas of the synovial recess and synovial hypertrophy. A series of three exercises was performed, ultimately demonstrating suprapatellar view B-mode interclass correlation coefficient (ICC) of 0.89 (95% CI 0.86–0.98) and PD of 0.55 (95% CI 0.41–0.69), medial parapatellar view B-mode ICC of 0.76 (95% CI 0.68–0.83) and PD of 0.75 (95% CI 0.66–0.83), and lateral parapatellar view B-mode ICC of 0.82 (95% CI 0.75–0.88) and PD of 0.75 (95% CI 0.66–0.84).
Collado et al. (51) evaluated a semiquantitative scoring system for pediatric synovitis including 44 joints imaging the fingers, radiocarpal, elbow, tibiotalar, and midfoot joints. They defined synovitis as the presence of synovial hypertrophy and/or effusion. A consensual 4-point semiquantitative scale of GS synovitis and 3-point semiquantitative scale for PD was determined. Tenosynovitis and bursitis were scored dichotomously for GS and PD, with 0, absence and 1, presence. MSUS was more sensitive than physical examination in the detection of synovitis. Various models were used to then create a reduced PD ultrasonography assessment, which ultimately included 10 joints and encompassed the bilateral knee, ankle, wrist, elbow, and second MCP joints. All of the patients with GS synovitis and PD signal were detected using the reduced PD composite score. Correlations between the 44-joint and reduced 10-joint assessments were high at baseline, 3, and 6 months (r>0.8, all p<0.0005). The reduced score took an average of 17.3 min compared with 40.8 min for the 44-joint evaluation. This study highlights the feasibility of a standardized limited scoring system in the assessment of JIA. Nevertheless, it was performed prior to the establishment of preliminary definitions for synovitis (28). A scoring system demonstrated good reliability in four joints (wrist, 2nd MCP, knee, and ankle) following the OMERACT creation of preliminary definitions of synovitis (20). Good to excellent intra- and interobserver reliability for inflammatory and structural lesions of the wrist and MCP has also been reported using this scoring system (10).
The establishment of reliable scoring systems is essential not only in the grading of synovitis but also in the determination of response to treatment. The relationship between the US indices Color Fraction (CF), which measures local vascularity in regions of hyperemic synovium, and Resistive Index (RI), which measures the velocity of blood flow, and clinical and laboratory parameters were evaluated in JIA knees at baseline and 2 months following IAS injection. Patients with JIA were age and sex matched to healthy controls (52). Baseline evaluation demonstrated increased CF and RI in patients with JIA in comparison with matched healthy controls. There was a significant improvement in CF and RI in patients with JIA 2 months after IAS injection. CF positively correlated with swelling score, active joint count, and JADA score, and there was a negative correlation between RI and active joint count and morning stiffness duration. A very high intra- and interobserver correlation coefficient was also appreciated.
Damage
Chronic synovial inflammation leads to deterioration and irreversible structural changes of osteocartilaginous structures, resulting in functional limitation (53). The measurement of cartilage thickness by US has been validated in both healthy children (54, 55), as well as in children with JIA (56). In 2013, Pradsgaard et al. ultrasonographically measured the cartilage thickness in 95 patients with JIA and compared findings to age- and sex-matched healthy controls. Patients with JIA were found to have thinner cartilage than healthy controls in both previously affected arthritic joints, as well as previously healthy joints. Children with polyarticular and systemic JIA also demonstrated thinner cartilage than those with oligoarticular JIA. Mitra et al. (57) again highlighted significant cartilage thinning in children with JIA, most notably polyarticular disease, and in boys, independent of disease duration and markers of inflammation.
A subsequent validation study comparing US and MRI measurements of cartilage thickness determined the best site for reproducibility and reliability, ultimately establishing the intercondylar notch of the distal femur in a flexed knee as the ideal location (58).
Enthesitis
Enthesitis-related arthritis (ERA) makes up approximately 10%–37% of patients with JIA (59, 60) and is characterized predominantly by lower limb arthritis and enthesitis, affecting boys aged >6 years who are frequently HLA-B27 positive. Some children may also develop inflammatory back pain and acute anterior uveitis (61), and unfortunately, many have similarly poor outcomes as adults with spondyloarthropathies (60, 62). Treatment generally involves nonsteroidal anti-inflammatory drugs and conventional disease-modifying antirheumatic drugs, but often biologic therapy is needed to prevent the progression of disease (60). Therefore, making an early diagnosis of enthesitis is important with respect to the appropriate diagnosis and timely implementation of treatment interventions. Unfortunately, clinical assessment of enthesitis can be challenging in the growing pediatric population. In addition to the recent Juvenile Spondyloarthritis Disease Activity Score, another challenge is the lack of validated outcome measures for pediatric enthesitis (63). Thus, the potential for US to aid in both establishing a diagnosis and treatment monitoring is key.
US and whole-body MRI are emerging as helpful adjuncts to clinical examination in the diagnosis of enthesitis (64). MSUS is an effective, highly sensitive method of detecting enthesitis for adult spondylarthropathies (65–68). While diagnosis remains clinical (i.e.,., localized pain, tenderness, and swelling at the attachment site), US is increasingly being utilized to detect subclinical enthesitis (69–71) and for injection guidance for enthesitis in JIA (60). Recent studies indicate a prevalence of active enthesitis (by MSUS) in approximately 12.5%–13% of patients with JIA-ERA (69–72). Jousse-Joulin et al. (69) evaluated a cohort of 213 enthesis sites among patients with JIA, in which 27 (12.5%) were clinically abnormal, whereas 20 (9.4%) were abnormal by PD MSUS with the distal patellar ligament (30%) and Achilles tendons (20%) primarily affected. Interestingly, while MSUS assessed fewer abnormal entheses, 10 (50%) were deemed clinically normal, indicating discordance with clinical examination. Notably, tenderness and swelling on examination correlated well with abnormal PD MSUS (69). Weiss et al. reported on a cohort of 30 patients with JIA-ERA and 30 control patients assessed by both MSUS and dolorimetry. While dolorimetry was unreliable, abnormal MSUS findings most frequently involved the quadriceps tendon insertion (30%), common extensor tendon (12%), and Achilles tendon (10%) (70). Shenoy et al. found 47 abnormal entheses on MSUS in 25 out of 30 male patients with JIA-ERA, whereas only 27 (in 15 out of 30 patients) were clinically abnormal (primarily at the patellar tendon insertion at the tibial tuberosity). The concordance rate between clinical examination and MSUS was better than previous studies at 89.4% (out of 360 enthesis sites) with discordance occurring at the tibial tuberosity, superior patellar pole, and Achilles tendon sites (71). MSUS assessment of enthesitis is feasible and may be superior to clinical examination in JIA. Given it is quick and portable, the routine use of MSUS in clinical care of children with enthesitis warrants further optimization.
In recent years, normative data of entheses in healthy children have been evaluated. Normal vascular changes of the tendinous (10–13 year old) and peritendinous (4–9 year old) regions were noted among healthy children (73), indicating the need for caution while interpreting MSUS findings. Tendon thickness appears to increase with age or weight (74), whereas cartilage thickness declines over time (73, 75). These features are important to be aware of in growing children as the OMERACT definition of MSUS enthesitis in adults (76) includes the presence of tendon thickening and abnormal Doppler signal along with hypoechoic appearance, loss of fibrillar architecture, possible calcification, and bony erosion(s). At this time, there are no MSUS enthesitis definitions specific to children. Furthermore, although adult enthesitis scoring recommendations have been well-established (77), pediatric scoring systems are still lacking.
Overall, these limited studies indicate a potential bedside role of MSUS in the assessment of enthesitis; however, additional studies are necessary to develop imaging protocols and scoring systems and to ascertain the ability of MSUS to assess change.
Steroid injection procedures
The utility of IAS injections is well recognized in the treatment of inflammatory arthritis. Indeed, it is a common intervention and treatment modality in JIA (78). IAS injections have been shown to improve synovitis and functional outcomes within days and typically are associated with very few systemic side effects (79). However, the efficacy of the procedure generally relies on the accuracy of the steroid placement within the synovial capsule of the joint or tendon sheath. Currently, pediatric joint injections are performed blindly either with palpation by the clinician or with imaging guidance (fluoroscopy, computed tomography enhanced, MRI, and/or US) via interventional radiology in many pediatric rheumatology clinics. With advances in use and training of MSUS, US-guided injections have increased in frequency in the treatment of JIA.
Several studies in adults with arthritis indicate the benefits of utilizing MSUS for steroid injection guidance with improved efficacy and accuracy (80, 81), whereas the evidence in JIA remains limited. Current studies evaluating the use of MSUS for steroid injection procedures in JIA have primarily been retrospective and descriptive reports with radiologists performing the procedures. Two large cohorts from Peters (82) and Young (83) reported on a combined 2719 procedures with MSUS guidance. Of these, 1030 (37.8%) were for tendon sheaths with the majority of injections occurring in the ankles (75%–84.9%, respectively) (82, 83). Generally, all studies on MSUS-guided injections in JIA have been effective both clinically and via MSUS findings in the wrist (84), ankle (83, 85, 86), knee (52, 87), hip (87), sacroiliac joint (88), tendon sheath (82), and temporomandibular joint (TMJ) (89–91); however, long-term outcomes were difficult to assess given concurrent immunotherapy and limited follow-up. Indirect assessments of outcome were documented by duration to repeat injection, which was reported on average from 6.3 to 24.8 (range 0.5–130.7) months after the initial procedure (83–86).
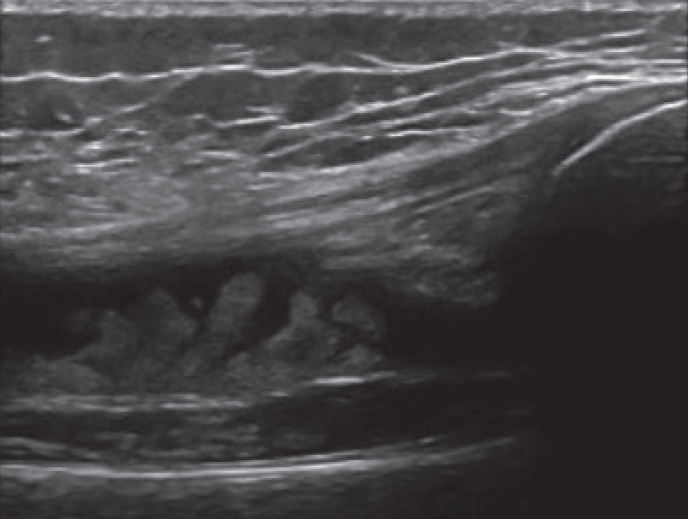
MSUS provides real-time assessment and confirmation of disease activity, as well as direct visualization of the needle tip in the affected region and immediate confirmation of the medication injected. MSUS has been shown to be superior to clinical examination, particularly with respect to ankle tenosynovitis (2, 85). Peters et al. (82), in their 10-year review of tendon sheath injections, examined the frequency of clinical assessment of tenosynovitis with US findings. For every 100 injections requested, 77 were completed, suggesting a 23% discordance between examination and imaging findings. Injections of an uninflamed tendon can have negative consequences, increasing the risk of tear or rupture and steroid atrophy side effects. Furthermore, ineffective steroid injections may be due to incorrect steroid placement. Rooney et al. (2) found that 39% of patients with JIA with clinically swollen ankles have only tenosynovitis on MSUS. Thus, if the tibiotalar joint was mistakenly injected with IASs, a considerable proportion of patients would have had incomplete or no response at all. Therefore, the use of MSUS prior to a joint/tendon injection can further confirm inflammatory findings and guide appropriateness of the procedure. Figure 3a and b depicts accurate needle placement and confirmatory injection of steroid into a tendon sheath, respectively.
Figure 3. a, b.
Transverse image of a tendon sheath injection (a); Visualization of steroid as it is injected into the tendon sheath (b).
MSUS-guided injection procedures have been associated with very low (2%–5%) complication rates most commonly related with steroid atrophy (82–86, 91). Subcutaneous atrophy is a known complication from steroid injections, noted more often in small or complex joints (ankle and wrist) in children aged <4 years (92) or with large injection volumes (93). MSUS guidance may minimize extravasation into the tissue given visible localization of and confirmation of medication into the injection site.
Although the majority of studies hint toward the utility of MSUS-guided steroid injections, Resnick et al. (90) found that the only difference between MSUS-guided TMJ injections versus the anatomic identification/palpation performed by an oral maxillofacial surgeon is a time difference of 49 min more for MSUS. The other authors indicate that the time of actual procedures averaged 5–15 min (84, 85) performed by a musculoskeletal trained radiologist. Overall, the role of MSUS-guided steroid injections appears promising with very good outcomes and few complications.
MRI vs. MSUS
MRI has generally been considered to be the most sensitive, non-invasive modality for imaging inflammatory disease given the ability to evaluate all layers of tissue including joints, tendons, entheses, cartilage, and bony erosions, as well as bone marrow edema (94, 95). Contrast-enhanced MRI further adds the ability to assess active synovitis and is recommended in most instances, but does not appear to be needed in the assessment of the sacroiliac joint (96, 97), cartilage, bone edema, or erosions (97). However, MRI use is limited by the long duration of study, potential need for sedation for young or anxious children, limited to single extremity (whole body offers less detail), need for intravenous (IV) contrast, access, and cost. Similar to MSUS, the use of MRI is further affected by a lack of normative data of MRI findings in healthy children, making scoring and interpretation more difficult.
Given the usability and lower cost of MSUS, several studies have compared the utility of the two modalities. Only MRI can assess bone marrow edema, which has been shown to be predictive of erosive disease in RA (98, 99) though it remains uncertain if this is similar in JIA. MRI has improved sensitivity compared with MSUS and radiography with regard to bony erosions (100, 101) and appears to have a slight advantage over both clinical examination and MSUS in the assessment of inflammatory arthritis (102) particularly with respect to the TMJ (103–105). MSUS has demonstrated a potential diagnostic role for TMJ disease, especially in the presence of condylar involvement (106), and may be a helpful screening tool (107). Nevertheless, a recent systematic review demonstrated low sensitivity of MSUS and advocated that it may play a more valuable role in established disease based on the initial diagnosis by MRI (108). In a recent study assessing 92 patients with JIA and TMJ involvement, contrast-enhanced MRI detected inflammation in 64.7% of joints, whereas PD MSUS did not detect inflammation in any of the joints (105). While MSUS may play a potential role in the evaluation of TMJ arthritis, at this point, MRI remains superior and cannot be replaced. Phatak et al. (109) reported that MRI has a 74% concordance with clinical examination and a 72% concordance with MSUS with respect to the midfoot. However, Eich et al. (87) performed a prospective study of 15 knees and hips among patients with JIA and evaluated findings pre- and post-steroid injection with both MSUS and MRI. Overall, MSUS was as sensitive as MRI in identifying synovial effusion and pannus. Studies comparing MSUS and MRI show a high level of agreement in the assessment of cartilage thickness in both healthy children (54) and among patients with JIA (58). In addition, a recent comparison study of MSUS and MRI in hemophilia arthropathy indicated a very high reliability (110).
With advances in technology and validated scoring systems, each imaging modality may become more accurate and cost-effective. For instance, diffusion-weighted MRI imaging may be a non-invasive alternative to IV contrast enhancement (111, 112). In MSUS, superb microvascular imaging (SMI) is an emerging Doppler modality using advanced algorithms to focus on a specific area of interest. It is capable of effectively suppressing background tissue motion without impacting slow flow signals that are characteristically seen in synovitis. In a study of knee synovitis in patients with JIA, SMI was superior to PD in depicting microvascular blood flow in both patients with clinically active disease (i.e.,., those with disease on physical examination and US), as well as CID (i.e.,., those with no disease on physical examination but positive US findings consisting of synovial hypertrophy and effusion) (113). Ultimately, future studies comparing the two modalities in JIA are warranted.
Conclusion
The diagnostic challenges of synovitis, tenosynovitis, and enthesitis in a pediatric patient with JIA along with the evidence of poor prognosis related to (subclinical) disease suggest the need for a quick, readily available, non-invasive imaging tool(s), such as MSUS. MSUS plays a valuable role in enhancing diagnosis, assessing response to therapy, and improving interventional modalities to overall advance the care and improve outcomes of children with JIA.
Main Points.
Musculoskeletal ultrasound (MSUS) is a highly sensitive, non-invasive bedside instrument of obtaining clinical information that supplements physical exam without the need for sedation and may enhance both the diagnosis and treatment.
Additional studies are necessary to establish the routine use of MSUS in pediatric rheumatology. Among others, there is a need to determine what are the best MSUS findings to predict active JIA and the clinical significance of sub-clinical synovitis.
MSUS-guided steroid injections are promising with good outcomes and few complications.
Footnotes
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - E.B., T.T., P.V.F.; Design - E.B., T.T., P.V.F.; Supervision - E.B., T.T., P.V.F.; Data Collection and/or Processing - E.B., T.T., P.V.F.; Analysis and/or Interpretation - E.B., T.T., P.V.F.; Literature Search - E.B., T.T., P.V.F.; Writing Manuscript - E.B., T.T., P.V.F.; Critical Review - E.B., T.T., P.V.F.;
Conflict of Interest: The authors have no conflict of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Thierry S, Fautrel B, Lemelle I, Guillemin F. Prevalence and incidence of juvenile idiopathic arthritis: a systematic review. Joint Bone Spine. 2014;81:112–7. doi: 10.1016/j.jbspin.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 2.Rooney ME, McAllister C, Burns JF. Ankle disease in juvenile idiopathic arthritis: ultrasound findings in clinically swollen ankles. J Rheumatol. 2009;36:1725–9. doi: 10.3899/jrheum.080508. [DOI] [PubMed] [Google Scholar]
- 3.Brown AK, Conaghan PG, Karim Z, Quinn MA, Ikeda K, Peterfy CG, et al. An explanation for the apparent dissociation between clinical remission and continued structural deterioration in rheumatoid arthritis. Arthritis Rheum. 2008;58:2958–67. doi: 10.1002/art.23945. [DOI] [PubMed] [Google Scholar]
- 4.Filippou G, Cantarini L, Bertoldi I, Picerno V, Frediani B, Galeazzi M. Ultrasonography vs. clinical examination in children with suspected arthritis. Does it make sense to use poliarticular ultrasonographic screening? Clin Exp Rheumatol. 2011;29:345–50. [PubMed] [Google Scholar]
- 5.Hendry GJ, Gardner-Medwin J, Steultjens MP, Woodburn J, Sturrock RD, Turner DE. Frequent discordance between clinical and musculoskeletal ultrasound examinations of foot disease in juvenile idiopathic arthritis. Arthritis Care Res (Hoboken) 2012;64:441–7. doi: 10.1002/acr.20655. [DOI] [PubMed] [Google Scholar]
- 6.Rebollo-Polo M, Koujok K, Weisser C, Jurencak R, Bruns A, Roth J. Ultrasound findings on patients with juvenile idiopathic arthritis in clinical remission. Arthritis Care Res (Hoboken) 2011;63:1013–9. doi: 10.1002/acr.20478. [DOI] [PubMed] [Google Scholar]
- 7.Sparchez M, Fodor D. What’s new in musculoskeletal ultrasound in pediatric rheumatology? Med Ultrason. 2018;20:371–8. doi: 10.11152/mu-1604. [DOI] [PubMed] [Google Scholar]
- 8.Ravelli A, Consolaro A, Horneff G, Laxer RM, Lovell DJ, Wulffraat NM, et al. Treating juvenile idiopathic arthritis to target: recommendations of an international task force. Ann Rheum Dis. 2018;77:819–28. doi: 10.1136/annrheumdis-2018-213030. [DOI] [PubMed] [Google Scholar]
- 9.Naredo E, Moller I, Moragues C, de Agustin JJ, Scheel AK, Grassi W, et al. Interobserver reliability in musculoskeletal ultrasonography: results from a “Teach the Teachers” rheumatologist course. Ann Rheum Dis. 2006;65:14–9. doi: 10.1136/ard.2005.037382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ventura-Rios L, Faugier E, Barzola L, De la Cruz-Becerra LB, Sanchez-Bringas G, Garcia AR, et al. Reliability of ultrasonography to detect inflammatory lesions and structural damage in juvenile idiopathic arthritis. Pediatr Rheumatol Online J. 2018;16:58. doi: 10.1186/s12969-018-0275-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ting TV, Vega-Fernandez P, Oberle EJ, De Ranieri D, Bukulmez H, Lin C, et al. Novel Ultrasound Image Acquisition Protocol and Scoring System for the Pediatric Knee. Arthritis Care Res (Hoboken) 2019;71:977–85. doi: 10.1002/acr.23746. [DOI] [PubMed] [Google Scholar]
- 12.Wakefield RJ, Balint PV, Szkudlarek M, Filippucci E, Backhaus M, D’Agostino MA, et al. Musculoskeletal ultrasound including definitions for ultrasonographic pathology. J Rheumatol. 2005;32:2485–7. [PubMed] [Google Scholar]
- 13.Entrekin RR, Porter BA, Sillesen HH, Wong AD, Cooperberg PL, Fix CH. Real-time spatial compound imaging: application to breast, vascular, and musculoskeletal ultrasound. Semin Ultrasound CT MR. 2001;22:50–64. doi: 10.1016/S0887-2171(01)90018-6. [DOI] [PubMed] [Google Scholar]
- 14.Basra HAS, Humphries PD. Juvenile idiopathic arthritis: what is the utility of ultrasound? Br J Radiol. 2017;90:20160920. doi: 10.1259/bjr.20160920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roth J, Jousse-Joulin S, Magni-Manzoni S, Rodriguez A, Tzaribachev N, Iagnocco A, et al. Definitions for the sonographic features of joints in healthy children. Arthritis Care Res (Hoboken) 2015;67:136–42. doi: 10.1002/acr.22410. [DOI] [PubMed] [Google Scholar]
- 16.Wakefield RJ, Gibbon WW, Emery P. The current status of ultrasonography in rheumatology. Rheumatology (Oxford) 1999;38:195–8. doi: 10.1093/rheumatology/38.3.195. [DOI] [PubMed] [Google Scholar]
- 17.Bellah R. Ultrasound in pediatric musculoskeletal disease: techniques and applications. Radiol Clin North Am. 2001;39:597–618. ix. doi: 10.1016/S0033-8389(05)70302-X. [DOI] [PubMed] [Google Scholar]
- 18.Collado P, Windschall D, Vojinovic J, Magni-Manzoni S, Balint P, Bruyn GAW, et al. Amendment of the OMERACT ultrasound definitions of joints’ features in healthy children when using the DOPPLER technique. Pediatr Rheumatol Online J. 2018;16:23. doi: 10.1186/s12969-018-0240-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roth J, Ravagnani V, Backhaus M, Balint P, Bruns A, Bruyn GA, et al. Preliminary Definitions for the Sonographic Features of Synovitis in Children. Arthritis Care Res (Hoboken) 2017;69:1217–23. doi: 10.1002/acr.23130. [DOI] [PubMed] [Google Scholar]
- 20.Vojinovic J, Magni-Manzoni S, Collado P, Windschall D, Ravagnani V, Hernandez-Diaz C, et al. SAT0636 Ultrasonography definitions for synovitis grading in children: the omeract pediatric ultrasound task force. Ann Rheum Dis. 2017;76:1015. doi: 10.1136/annrheumdis-2017-eular.6199. [DOI] [Google Scholar]
- 21.Consolaro A, Giancane G, Schiappapietra B, Davi S, Calandra S, Lanni S, et al. Clinical outcome measures in juvenile idiopathic arthritis. Pediatr Rheumatol Online J. 2016;14:23. doi: 10.1186/s12969-016-0085-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Magni-Manzoni S, Epis O, Ravelli A, Klersy C, Veisconti C, Lanni S, et al. Comparison of clinical versus ultrasound-determined synovitis in juvenile idiopathic arthritis. Arthritis Rheum. 2009;61:1497–504. doi: 10.1002/art.24823. [DOI] [PubMed] [Google Scholar]
- 23.Haslam KE, McCann LJ, Wyatt S, Wakefield RJ. The detection of subclinical synovitis by ultrasound in oligoarticular juvenile idiopathic arthritis: a pilot study. Rheumatology (Oxford) 2010;49:123–7. doi: 10.1093/rheumatology/kep339. [DOI] [PubMed] [Google Scholar]
- 24.Janow GL, Panghaal V, Trinh A, Badger D, Levin TL, Ilowite NT. Detection of active disease in juvenile idiopathic arthritis: sensitivity and specificity of the physical examination vs ultrasound. J Rheumatol. 2011;38:2671–4. doi: 10.3899/jrheum.110360. [DOI] [PubMed] [Google Scholar]
- 25.Favier LA, Ting TV, Modi AC. Feasibility of a musculoskeletal ultrasound intervention to improve adherence in juvenile idiopathic arthritis: a proof-of concept trial. Pediatr Rheumatol Online J. 2018;16:75. doi: 10.1186/s12969-018-0292-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ringold S. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Treatment of Juvenile Idiopathic Arthritis: Therapeutic Approaches forNon-Systemic Polyarthritis, Sacroiliitis, and Enthesitis. Arthritis Care Res (Hoboken) 2019;71:717–34. doi: 10.1002/acr.23870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pascoli L, Wright S, McAllister C, Rooney M. Prospective evaluation of clinical and ultrasound findings in ankle disease in juvenile idiopathic arthritis: importance of ankle ultrasound. J Rheumatol. 2010;37:2409–14. doi: 10.3899/jrheum.091262. [DOI] [PubMed] [Google Scholar]
- 28.Roth J, Ravagnani V, Backhaus M, Balint P, Bruns A, Bruyn GA, et al. Preliminary Definitions for the Sonographic Features of Synovitis in Children. Arthritis Care Res (Hoboken) 2017;69:1217–23. doi: 10.1002/acr.23130. [DOI] [PubMed] [Google Scholar]
- 29.Algergawy S, Haliem T, Al-Shaer O. Clinical, laboratory, and ultrasound assessment of the knee in juvenile rheumatoid arthritis. Clinical medicine insights Arthritis and musculoskeletal disorders. 2011;4:21–7. doi: 10.4137/CMAMD.S4371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shahin AA, Shaker OG, Kamal N, Hafez HA, Gaber W, Shahin HA. Circulating interleukin-6, soluble interleukin-2 receptors, tumor necrosis factor alpha, and interleukin-10 levels in juvenile chronic arthritis: correlations with soft tissue vascularity assessed by power Doppler sonography. Rheumatol Int. 2002;22:84–8. doi: 10.1007/s00296-002-0191-1. [DOI] [PubMed] [Google Scholar]
- 31.El-Barbary AM, Hussein MS, Almedany SH, Rageh EM, Alsalawy AM, Aboelhawa MA, et al. Role of Interleukin 37 as a Novel Proangiogenic Factor in Juvenile Idiopathic Arthritis. J Clin Rheumatol. 2019;25:85–90. doi: 10.1097/RHU.0000000000000779. [DOI] [PubMed] [Google Scholar]
- 32.Dev S, Singh A. Study of role of serum amyloid A (SAA) as a marker of disease activity in juvenile idiopathic arthritis. J Family Med Prim Care. 2019;8:2129–33. doi: 10.4103/jfmpc.jfmpc_339_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Swidrowska-Jaros J, Smolewska E. A fresh look at angiogenesis in juvenile idiopathic arthritis. Cent Eur J Immunol. 2018;43:325–30. doi: 10.5114/ceji.2018.80052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sparchez M, Fodor D, Miu N. The role of Power Doppler ultrasonography in comparison with biological markers in the evaluation of disease activity in Juvenile Idiopathic Arthritis. Med Ultrason. 2010;12:97–103. [PubMed] [Google Scholar]
- 35.Naredo E, Moller I, de Miguel E, Batlle-Gualda E, Acebes C, Brito E, et al. High prevalence of ultrasonographic synovitis and enthesopathy in patients with psoriasis without psoriatic arthritis: a prospective case-control study. Rheumatology (Oxford) 2011;50:1838–48. doi: 10.1093/rheumatology/ker078. [DOI] [PubMed] [Google Scholar]
- 36.Zuliani F, Zabotti A, Errichetti E, Tinazzi I, Zanetti A, Carrara G, et al. Ultrasonographic detection of subclinical enthesitis and synovitis: a possible stratification of psoriatic patients without clinical musculoskeletal involvement. Clin Exp Rheumatol. 2019;37:593–9. [PubMed] [Google Scholar]
- 37.Bugni Miotto e Silva V, de Freitas Tavares da Silva C, de Aguiar Vilela Mitraud S, Nely Vilar Furtado R, Esteves Hilario MO, Natour J, et al. Do patients with juvenile idiopathic arthritis in remission exhibit active synovitis on joint ultrasound? Rheumatol Int. 2014;34:937–45. doi: 10.1007/s00296-013-2909-7. [DOI] [PubMed] [Google Scholar]
- 38.Breton S, Jousse-Joulin S, Cangemi C, de Parscau L, Colin D, Bressolette L, et al. Comparison of clinical and ultrasonographic evaluations for peripheral synovitis in juvenile idiopathic arthritis. Semin Arthritis Rheum. 2011;41:272–8. doi: 10.1016/j.semarthrit.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 39.Wallace CA, Giannini EH, Huang B, Itert L, Ruperto N. American College of Rheumatology provisional criteria for defining clinical inactive disease in select categories of juvenile idiopathic arthritis. Arthritis Care Res (Hoboken) 2011;63:929–36. doi: 10.1002/acr.20497. [DOI] [PubMed] [Google Scholar]
- 40.Wallace CA, Ruperto N, Giannini E. Preliminary criteria for clinical remission for select categories of juvenile idiopathic arthritis. J Rheumatol. 2004;31:2290–4. [PubMed] [Google Scholar]
- 41.Brown A, Hirsch R, Laor T, Hannon MJ, Levesque MC, Starz T, et al. Do patients with juvenile idiopathic arthritis in clinical remission have evidence of persistent inflammation on 3T magnetic resonance imaging? Arthritis Care Res (Hoboken) 2012;64:1846–54. doi: 10.1002/acr.21774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Collado P, Jousse-Joulin S, Alcalde M, Naredo E, D’Agostino MA. Is ultrasound a validated imaging tool for the diagnosis and management of synovitis in juvenile idiopathic arthritis? A systematic literature review. Arthritis Care Res (Hoboken) 2012;64:1011–9. doi: 10.1002/acr.21644. [DOI] [PubMed] [Google Scholar]
- 43.Wakefield RJ, Green MJ, Marzo-Ortega H, Conaghan PG, Gibbon WW, McGonagle D, et al. Should oligoarthritis be reclassified? Ultrasound reveals a high prevalence of subclinical disease. Ann Rheum Dis. 2004;63:382–5. doi: 10.1136/ard.2003.007062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guzman J, Oen K, Loughin T. Predicting disease severity and remission in juvenile idiopathic arthritis: are we getting closer? Curr Opin Rheumatol. 2019 doi: 10.1097/BOR.0000000000000620. [DOI] [PubMed] [Google Scholar]
- 45.Magni-Manzoni S, Scire CA, Ravelli A, Klersy C, Rossi S, Muratore V, et al. Ultrasound-detected synovial abnormalities are frequent in clinically inactive juvenile idiopathic arthritis, but do not predict a flare of synovitis. Ann Rheum Dis. 2013;72:223–8. doi: 10.1136/annrheumdis-2011-201264. [DOI] [PubMed] [Google Scholar]
- 46.Miotto ESVB, Mitraud SAV, Furtado RNV, Natour J, Len CA, Terreri M. Patients with juvenile idiopathic arthritis in clinical remission with positive power Doppler signal in joint ultrasonography have an increased rate of clinical flare: a prospective study. Pediatr Rheumatol Online J. 2017;15:80. doi: 10.1186/s12969-017-0208-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao Y, Rascoff NE, Iyer RS, Thapa M, Reichley L, Oron AP, et al. Flares of Disease in Children with Clinically Inactive Juvenile Idiopathic Arthritis Were Not Correlated with Ultrasound Findings. J Rheumatol. 2018;45:851–7. doi: 10.3899/jrheum.170681. [DOI] [PubMed] [Google Scholar]
- 48.Roth J. Predictive Value of Musculoskeletal Ultrasound for Flares in Juvenile Idiopathic Arthritis. J Rheumatol. 2019;46:113. doi: 10.3899/jrheum.180735. [DOI] [PubMed] [Google Scholar]
- 49.De Lucia O, Ravagnani V, Pregnolato F, Hila A, Pontikaki I, Gattinara M, et al. Baseline ultrasound examination as possible predictor of relapse in patients affected by juvenile idiopathic arthritis (JIA) Ann Rheum Dis. 2018;77:1426–31. doi: 10.1136/annrheumdis-2017-211696. [DOI] [PubMed] [Google Scholar]
- 50.Nieto-Gonzalez JC, Rodriguez A, Gamir-Gamir ML, Boteanu A, Lopez-Robledillo JC, Garulo DC, et al. Can ultrasound-detected subclinical synovitis be an indicator of flare recurrence in juvenile idiopathic arthritis remission patients on tapered TNFi? Clin Exp Rheumatol. 2019;37:705–12. [PubMed] [Google Scholar]
- 51.Collado P, Naredo E, Calvo C, Gamir ML, Calvo I, Garcia ML, et al. Reduced joint assessment vs comprehensive assessment for ultrasound detection of synovitis in juvenile idiopathic arthritis. Rheumatology (Oxford) 2013;52:1477–84. doi: 10.1093/rheumatology/ket148. [DOI] [PubMed] [Google Scholar]
- 52.Baikar T, Chhabra A, Yadav TP, Sachdev N, Dewan V. Power Color Doppler and Spectral Doppler Ultrasonography to Evaluate Response to Intra-articular Steroid Injection in Knee Joints in Juvenile Idiopathic Arthritis. Indian J Pediatr. 2017;84:826–32. doi: 10.1007/s12098-017-2418-x. [DOI] [PubMed] [Google Scholar]
- 53.Prakken BAS, Martini A. Juvenile idiopathic arthritis. Lancet. 2011;18:2138–49. doi: 10.1016/S0140-6736(11)60244-4. [DOI] [PubMed] [Google Scholar]
- 54.Spannow AH, Stenboeg E, Pfeiffer-Jensen M, Fiirgaard B, Haislund M, Ostergaard M, et al. Ultrasound and MRI measurements of joint cartilage in healthy children: a validation study. Ultraschall in der Medizin (Stuttgart, Germany : 1980) 2011;32(Suppl 1):S110–6. doi: 10.1055/s-0029-1245374. [DOI] [PubMed] [Google Scholar]
- 55.Spannow AH, Stenboeg E, Pfeiffer-Jensen M, Herlin T. Ultrasound measurement of joint cartilage thickness in large and small joints in healthy children: a clinical pilot study assessing observer variability. Pediatr Rheumatol Online J. 2007;5:3. doi: 10.1186/1546-0096-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pradsgaard DO, Spannow AH, Heuck C, Herlin T. Decreased cartilage thickness in juvenile idiopathic arthritis assessed by ultrasonography. J Rheumatol. 2013;40:1596–603. doi: 10.3899/jrheum.121077. [DOI] [PubMed] [Google Scholar]
- 57.Mitra S, Samui PP, Samanta M, Mondal RK, Hazra A, Mandal K, et al. Ultrasound detected changes in joint cartilage thickness in juvenile idiopathic arthritis. Int J Rheum Dis. 2019;22:1263–70. doi: 10.1111/1756-185X.13584. [DOI] [PubMed] [Google Scholar]
- 58.Pradsgaard DO, Fiirgaard B, Spannow AH, Heuck C, Herlin T. Cartilage thickness of the knee joint in juvenile idiopathic arthritis: comparative assessment by ultrasonography and magnetic resonance imaging. J Rheumatol. 2015;42:534–40. doi: 10.3899/jrheum.140162. [DOI] [PubMed] [Google Scholar]
- 59.Weiss PF. Update on enthesitis-related arthritis. Curr Opin Rheumatol. 2016;28:530–6. doi: 10.1097/BOR.0000000000000313. [DOI] [PubMed] [Google Scholar]
- 60.Mistry RR, Patro P, Agarwal V, Misra DP. Enthesitis-related arthritis: current perspectives. Open Access Rheumatol. 2019;11:19–31. doi: 10.2147/OARRR.S163677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Petty RE, Southwood TR, Manners P, Baum J, Glass DN, Goldenberg J, et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol. 2004;31:390–2. [PubMed] [Google Scholar]
- 62.Flato B, Hoffmann-Vold AM, Reiff A, Forre O, Lien G, Vinje O. Long-term outcome and prognostic factors in enthesitis-related arthritis: a case-control study. Arthritis Rheum. 2006;54:3573–82. doi: 10.1002/art.22181. [DOI] [PubMed] [Google Scholar]
- 63.Weiss PF, Colbert RA, Xiao R, Feudtner C, Beukelman T, DeWitt EM, et al. Development and retrospective validation of the juvenile spondyloarthritis disease activity index. Arthritis Care Res (Hoboken) 2014;66:1775–82. doi: 10.1002/acr.22411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sudol-Szopinska I, Znajdek M, Gietka P, Vasilevska-Nikodinovska V, Patrovic L, Salapura V. Imaging of juvenile spondyloarthritis. Part II: Ultrasonography and magnetic resonance imaging. J Ultrason. 2017;17:176–81. doi: 10.15557/JoU.2017.0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.D’Agostino MA. Ultrasound imaging in spondyloarthropathies. Best Pract Res Clin Rheumatol. 2010;24:693–700. doi: 10.1016/j.berh.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 66.D’Agostino MA, Said-Nahal R, Hacquard-Bouder C, Brasseur JL, Dougados M, Breban M. Assessment of peripheral enthesitis in the spondylarthropathies by ultrasonography combined with power Doppler: a cross-sectional study. Arthritis Rheum. 2003;48:523–33. doi: 10.1002/art.10812. [DOI] [PubMed] [Google Scholar]
- 67.Eder L, Barzilai M, Peled N, Gladman DD, Zisman D. The use of ultrasound for the assessment of enthesitis in patients with spondyloarthritis. Clinical radiology. 2013;68:219–23. doi: 10.1016/j.crad.2012.07.018. [DOI] [PubMed] [Google Scholar]
- 68.Zytoon AA, Eid H, Sakr A, El Abbass HA, Kamel M. Ultrasound assessment of elbow enthesitis in patients with seronegative arthropathies. J Ultrasound. 2014;17:33–40. doi: 10.1007/s40477-013-0057-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jousse-Joulin S, Breton S, Cangemi C, Fenoll B, Bressolette L, de Parscau L, et al. Ultrasonography for detecting enthesitis in juvenile idiopathic arthritis. Arthritis Care Res (Hoboken) 2011;63:849–55. doi: 10.1002/acr.20444. [DOI] [PubMed] [Google Scholar]
- 70.Weiss PF, Chauvin NA, Klink AJ, Localio R, Feudtner C, Jaramillo D, et al. Detection of enthesitis in children with enthesitis-related arthritis: dolorimetry compared to ultrasonography. Arthritis Rheumatol. 2014;66:218–27. doi: 10.1002/art.38197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shenoy S, Aggarwal A. Sonologic enthesitis in children with enthesitis-related arthritis. Clin Exp Rheumatol. 2016;34:143–7. doi: 10.1007/s10067-015-3029-4. [DOI] [PubMed] [Google Scholar]
- 72.Laurell L, Court-Payen M, Nielsen S, Zak M, Thomsen C, Miguel-Perez M, et al. Ultrasonography and color Doppler of proximal gluteal enthesitis in juvenile idiopathic arthritis: a descriptive study. Pediatr Rheumatol Online J. 2011;9:22. doi: 10.1186/1546-0096-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chauvin NA, Ho-Fung V, Jaramillo D, Edgar JC, Weiss PF. Ultrasound of the joints and entheses in healthy children. Pediatr Radiol. 2015;45:1344–54. doi: 10.1007/s00247-015-3313-0. [DOI] [PubMed] [Google Scholar]
- 74.Lin C, Diab M, Milojevic D. Grey-scale ultrasound findings of lower extremity entheses in healthy children. Pediatr Rheumatol Online J. 2015;13:14. doi: 10.1186/s12969-015-0012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jousse-Joulin S, Cangemi C, Gerard S, Gestin S, Bressollette L, de Parscau L, et al. Normal sonoanatomy of the paediatric entheses including echostructure and vascularisation changes during growth. Eur Radiol. 2015;25:2143–52. doi: 10.1007/s00330-014-3586-y. [DOI] [PubMed] [Google Scholar]
- 76.Terslev L, Naredo E, Iagnocco A, Balint PV, Wakefield RJ, Aegerter P, et al. Defining enthesitis in spondyloarthritis by ultrasound: results of a Delphi process and of a reliability reading exercise. Arthritis Care Res (Hoboken) 2014;66:741–8. doi: 10.1002/acr.22191. [DOI] [PubMed] [Google Scholar]
- 77.Balint PV, Terslev L, Aegerter P, Bruyn GAW, Chary-Valckenaere I, Gandjbakhch F, et al. Reliability of a consensus-based ultrasound definition and scoring for enthesitis in spondyloarthritis and psoriatic arthritis: an OMERACT US initiative. Ann Rheum Dis. 2018;77:1730–5. doi: 10.1136/annrheumdis-2018-213609. [DOI] [PubMed] [Google Scholar]
- 78.Bloom BJ, Alario AJ, Miller LC. Intra-articular corticosteroid therapy for juvenile idiopathic arthritis: report of an experiential cohort and literature review. Rheumatol Int. 2011;31:749–56. doi: 10.1007/s00296-010-1365-x. [DOI] [PubMed] [Google Scholar]
- 79.Cleary AG, Murphy HD, Davidson JE. Intra-articular corticosteroid injections in juvenile idiopathic arthritis. Arch Dis Child. 2003;88:192–6. doi: 10.1136/adc.88.3.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cunnington J, Marshall N, Hide G, Bracewell C, Isaacs J, Platt P, et al. A randomized, double-blind, controlled study of ultrasound-guided corticosteroid injection into the joint of patients with inflammatory arthritis. Arthritis Rheum. 2010;62:1862–9. doi: 10.1002/art.27448. [DOI] [PubMed] [Google Scholar]
- 81.Balint PV, Kane D, Hunter J, McInnes IB, Field M, Sturrock RD. Ultrasound guided versus conventional joint and soft tissue fluid aspiration in rheumatology practice: a pilot study. J Rheumatol. 2002;29:2209–13. [PubMed] [Google Scholar]
- 82.Peters SE, Laxer RM, Connolly BL, Parra DA. Ultrasound-guided steroid tendon sheath injections in juvenile idiopathic arthritis: a 10-year single-center retrospective study. Pediatr Rheumatol Online J. 2017;15:22. doi: 10.1186/s12969-017-0155-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Young CM, Shiels WE, 2nd, Coley BD, Hogan MJ, Murakami JW, Jones K, et al. Ultrasound-guided corticosteroid injection therapy for juvenile idiopathic arthritis: 12-year care experience. Pediatr Radiol. 2012;42:1481–9. doi: 10.1007/s00247-012-2487-y. [DOI] [PubMed] [Google Scholar]
- 84.Laurell L, Court-Payen M, Nielsen S, Zak M, Fasth A. Ultrasonography and color Doppler in juvenile idiopathic arthritis: diagnosis and follow-up of ultrasound-guided steroid injection in the wrist region. A descriptive interventional study. Pediatr Rheumatol Online J. 2012;10:11. doi: 10.1186/1546-0096-10-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Laurell L, Court-Payen M, Nielsen S, Zak M, Boesen M, Fasth A. Ultrasonography and color Doppler in juvenile idiopathic arthritis: diagnosis and follow-up of ultrasound-guided steroid injection in the ankle region. A descriptive interventional study. Pediatr Rheumatol Online J. 2011;9:4. doi: 10.1186/1546-0096-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Young CM, Horst DM, Murakami JW, Shiels WE., 2nd Ultrasound-guided corticosteroid injection of the subtalar joint for treatment of juvenile idiopathic arthritis. Pediatr Radiol. 2015;45:1212–7. doi: 10.1007/s00247-015-3291-2. [DOI] [PubMed] [Google Scholar]
- 87.Eich GF, Halle F, Hodler J, Seger R, Willi UV. Juvenile chronic arthritis: imaging of the knees and hips before and after intraarticular steroid injection. Pediatr Radiol. 1994;24:558–63. doi: 10.1007/BF02012732. [DOI] [PubMed] [Google Scholar]
- 88.Klauser AS, Sailer-Hoeck M, Abdellah MM, Taljanovic MS, Siedentopf C, Auer T, et al. Feasibility of Ultrasound-Guided Sacroiliac Joint Injections in Children Presenting with Sacroiliitis. Ultraschall in der Medizin (Stuttgart, Germany : 1980) 2016;37:393–8. doi: 10.1055/s-0034-1399145. [DOI] [PubMed] [Google Scholar]
- 89.Habibi S, Ellis J, Strike H, Ramanan AV. Safety and efficacy of US-guided CS injection into temporomandibular joints in children with active JIA. Rheumatology (Oxford) 2012;51:874–7. doi: 10.1093/rheumatology/ker441. [DOI] [PubMed] [Google Scholar]
- 90.Resnick CM, Vakilian PM, Kaban LB, Peacock ZS. Is Intra-Articular Steroid Injection to the Temporomandibular Joint for Juvenile Idiopathic Arthritis More Effective and Efficient When Performed With Image Guidance? J Oral Maxillofac Surg. 2017;75:694–700. doi: 10.1016/j.joms.2016.09.045. [DOI] [PubMed] [Google Scholar]
- 91.Parra DA, Chan M, Krishnamurthy G, Spiegel L, Amaral JG, Temple MJ, et al. Use and accuracy of US guidance for image-guided injections of the temporomandibular joints in children with arthritis. Pediatr Radiol. 2010;40:1498–504. doi: 10.1007/s00247-010-1581-2. [DOI] [PubMed] [Google Scholar]
- 92.Job-Deslandre C, Menkes CJ. Complications of intra-articular injections of triamcinolone hexacetonide in chronic arthritis in children. Clin Exp Rheumatol. 1990;8:413–6. [PubMed] [Google Scholar]
- 93.Beukelman T, Arabshahi B, Cahill AM, Kaye RD, Cron RQ. Benefit of intraarticular corticosteroid injection under fluoroscopic guidance for subtalar arthritis in juvenile idiopathic arthritis. J Rheumatol. 2006;33:2330–6. [PubMed] [Google Scholar]
- 94.Sudol-Szopinska I, Grochowska E, Gietka P, Plaza M, Pracon G, Saied F, et al. Imaging of juvenile idiopathic arthritis. Part II: Ultrasonography and MRI. J Ultrason. 2016;16:237–51. doi: 10.15557/JoU.2016.0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sudol-Szopinska I, Jurik AG, Eshed I, Lennart J, Grainger A, Ostergaard M, et al. Recommendations of the ESSR Arthritis Subcommittee for the Use of Magnetic Resonance Imaging in Musculoskeletal Rheumatic Diseases. Semin Musculoskelet Radiol. 2015;19:396–411. doi: 10.1055/s-0035-1564696. [DOI] [PubMed] [Google Scholar]
- 96.Weiss PF, Xiao R, Biko DM, Johnson AM, Chauvin NA. Detection of inflammatory sacroiliitis in children with magnetic resonance imaging: is gadolinium contrast enhancement necessary? Arthritis Rheumatol. 2015;67:2250–6. doi: 10.1002/art.39159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hemke R, Kuijpers TW, van den Berg JM, van Veenendaal M, Dolman KM, van Rossum MA, et al. The diagnostic accuracy of unenhanced MRI in the assessment of joint abnormalities in juvenile idiopathic arthritis. Eur Radiol. 2013;23:1998–2004. doi: 10.1007/s00330-013-2770-9. [DOI] [PubMed] [Google Scholar]
- 98.Haavardsholm EA, Boyesen P, Ostergaard M, Schildvold A, Kvien TK. Magnetic resonance imaging findings in 84 patients with early rheumatoid arthritis: bone marrow oedema predicts erosive progression. Ann Rheum Dis. 2008;67:794–800. doi: 10.1136/ard.2007.071977. [DOI] [PubMed] [Google Scholar]
- 99.McQueen FM, Benton N, Perry D, Crabbe J, Robinson E, Yeoman S, et al. Bone edema scored on magnetic resonance imaging scans of the dominant carpus at presentation predicts radiographic joint damage of the hands and feet six years later in patients with rheumatoid arthritis. Arthritis Rheum. 2003;48:1814–27. doi: 10.1002/art.11162. [DOI] [PubMed] [Google Scholar]
- 100.Laurell L, Court-Payen M, Nielsen S, Zak M, Boesen M, Fasth A. Comparison of ultrasonography with Doppler and MRI for assessment of disease activity in juvenile idiopathic arthritis: a pilot study. Pediatr Rheumatol Online J. 2012;10:23. doi: 10.1186/1546-0096-10-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Malattia C, Damasio MB, Magnaguagno F, Pistorio A, Valle M, Martinoli C, et al. Magnetic resonance imaging, ultrasonography, and conventional radiography in the assessment of bone erosions in juvenile idiopathic arthritis. Arthritis Rheum. 2008;59:1764–72. doi: 10.1002/art.24313. [DOI] [PubMed] [Google Scholar]
- 102.El-Miedany YM, Housny IH, Mansour HM, Mourad HG, Mehanna AM, Megeed MA. Ultrasound versus MRI in the evaluation of juvenile idiopathic arthritis of the knee. Joint Bone Spine. 2001;68:222–30. doi: 10.1016/S1297-319X(01)00269-X. [DOI] [PubMed] [Google Scholar]
- 103.Weiss PF, Arabshahi B, Johnson A, Bilaniuk LT, Zarnow D, Cahill AM, et al. High prevalence of temporomandibular joint arthritis at disease onset in children with juvenile idiopathic arthritis, as detected by magnetic resonance imaging but not by ultrasound. Arthritis Rheum. 2008;58:1189–96. doi: 10.1002/art.23401. [DOI] [PubMed] [Google Scholar]
- 104.Muller L, Kellenberger CJ, Cannizzaro E, Ettlin D, Schraner T, Bolt IB, et al. Early diagnosis of temporomandibular joint involvement in juvenile idiopathic arthritis: a pilot study comparing clinical examination and ultrasound to magnetic resonance imaging. Rheumatology (Oxford) 2009;48:680–5. doi: 10.1093/rheumatology/kep068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zwir LF, Terreri MT, do Amaral ECA, Rodrigues WDR, Fernandes ARC. Is power Doppler ultrasound useful to evaluate temporomandibular joint inflammatory activity in juvenile idiopathic arthritis? Clin Rheumatol. 2019 doi: 10.1007/s10067-019-04731-x. [DOI] [PubMed] [Google Scholar]
- 106.Assaf AT, Kahl-Nieke B, Feddersen J, Habermann CR. Is high-resolution ultrasonography suitable for the detection of temporomandibular joint involvement in children with juvenile idiopathic arthritis? Dentomaxillofac Radiol. 2013;42:20110379. doi: 10.1259/dmfr.20110379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kirkhus E, Gunderson RB, Smith HJ, Flato B, Hetlevik SO, Larheim TA, et al. Temporomandibular joint involvement in childhood arthritis: comparison of ultrasonography-assessed capsular width and MRI-assessed synovitis. Dentomaxillofac Radiol. 2016;45:20160195. doi: 10.1259/dmfr.20160195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hechler BL, Phero JA, Van Mater H, Matthews NS. Ultrasound versus magnetic resonance imaging of the temporomandibular joint in juvenile idiopathic arthritis: a systematic review. Int J Oral Maxillofac Surg. 2018;47:83–9. doi: 10.1016/j.ijom.2017.07.014. [DOI] [PubMed] [Google Scholar]
- 109.Phatak S, Mohindra N, Zanwar A, Aggarwal A. Prominent midfoot involvement in children with enthesitis-related arthritis category of juvenile idiopathic arthritis. Clin Rheumatol. 2017;36:1737–45. doi: 10.1007/s10067-017-3733-3. [DOI] [PubMed] [Google Scholar]
- 110.Plut D, Kotnik BF, Zupan IP, Kljucevsek D, Vidmar G, Snoj Z, et al. Diagnostic accuracy of haemophilia early arthropathy detection with ultrasound (HEAD-US): a comparative magnetic resonance imaging (MRI) study. Radiol Oncol. 2019;53:178–86. doi: 10.2478/raon-2019-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Li M, Sauer A, Holl-Wieden A, Pabst T, Neubauer H. Diagnostic value of diffusion-weighted MRI for imaging synovitis in pediatric patients with inflammatory conditions of the knee joint. World J Pediatr. 2019 doi: 10.1007/s12519-019-00232-8. [DOI] [PubMed] [Google Scholar]
- 112.Sauer A, Li M, Holl-Wieden A, Pabst T, Neubauer H. Readout-segmented multi-shot diffusion-weighted MRI of the knee joint in patients with juvenile idiopathic arthritis. Pediatr Rheumatol Online J. 2017;15:73. doi: 10.1186/s12969-017-0203-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Alis D, Erol BC, Akbas S, Barut K, Kasapcopur O, Adaletli I. Superb Microvascular Imaging Compared With Power Doppler Ultrasound in Assessing Synovitis of the Knee in Juvenile Idiopathic Arthritis: A Preliminary Study. J Ultrasound Med. 2019 doi: 10.1002/jum.15079. [DOI] [PubMed] [Google Scholar]