Abstract
Secretory proteins are transported from the endoplasmic reticulum (ER) to the Golgi complex in carriers that are formed by the concerted activities of cytoplasmic proteins in the coat protein complex II (COPII). COPII was first described in Saccharomyces cerevisiae and its basic functions are largely conserved throughout eukaryotes. The discovery of the TANGO1 (transport and Golgi organization 1) family of proteins is revealing insights into how cells can adapt COPII proteins to reorganize the ER exit site for the export of the most abundant and bulky molecules, collagens.
Eukaryotic cells are subdivided into functionally distinct membrane-bound organelles and the controlled sorting and trafficking of proteins between them is central to the regulation of cellular function. The endoplasmic reticulum (ER) is the largest organelle in the cell and is a major site of protein synthesis and transport, protein folding, and lipid synthesis.
In the ER, newly synthesized proteins undergo a series of modifications and encounter several molecular chaperones, which together assist proteins in their folding and release from the ER. Proteins receive posttranslational modifications (PTMs) including N-linked glycosylation, disulfide bond formation, proline isomerization, and oligomerization. The ER is enriched in chaperones that help proteins fold and achieve their quaternary structure. Defects in folding and assembly are controlled in the ER and misfolded proteins are degraded by ER-associated degradation (ERAD) by extraction of clients into the cytoplasm or by degradation in lysosomes.
COMPARTMENTS OF THE SECRETORY PATHWAY AND VESICLES AS TRANSPORT INTERMEDIATES
George Palade's microscopy-based analyses of highly secretory, pancreatic acinar cells revealed the pathway followed by secretory proteins from the ER to the Golgi complex and on to the cell exterior. This schema of vesicle-mediated traffic of secretory cargoes has laid the foundation for a molecular understanding of inter-organelle protein traffic (Fig. 1).
Figure 1.
The secretory pathway. A schema based on a foundation laid by George Palade's classical electron microscopic characterization of the cellular secretory pathway. Secretory proteins are synthesized and folded in the endoplasmic reticulum (ER). They then gather at specialized subdomains of the ER called ER exit sites, marked by coat protein complex II (COPII) proteins. In the conventional model, proteins are packaged into COPII-coated vesicles, which detach from the ER and fuse with the next compartment, the ERGIC (ER-Golgi intermediate compartment) and thence on through the Golgi apparatus to their final destination. Materials that need to be retrieved are packaged into a similar class of vesicles called COPI-coated vesicles for their retrograde transport to the ER.
Subsequent landmark genetic studies and in vitro reconstitutions have revealed that ER-to-Golgi bidirectional cargo transport is performed by concerted activities of coat protein complexes, coatomer proteins (COP), COPI, and COPII. These are cytoplasmic proteins, which are recruited to the site of cargo export, and assemble into lattices to sculpt membranes and produce vesicles of uniform size and shape. A framework for how cytoplasmic proteins called coatomers might assemble into a stereotypical lattice and mediate carrier formation was available from landmark studies on clathrin-dependent endocytosis (Pearse 1976; Goldstein et al. 1979) and COPI-mediated intra-Golgi transport (Orci et al. 1986; Malhotra et al. 1989; Serafini et al. 1991a,b; Waters et al. 1991). COPI coatomers function at the Golgi, controlling formation of COPI-coated vesicles for intra-Golgi transport, and for retrograde trafficking from the Golgi to the ER. Exiting the ER by COPII-coat-dependent carriers is the subject of this review.
The initial description of the COPII-coated vesicle as an ER-Golgi transport intermediate was based on studies in Saccharomyces cerevisiae and described the assembly of a 10-nm-thick electron dense coat on vesicles of ∼60 nm diameter. As secretory cargoes increase in size and complexity through evolution, COPII machinery has adapted concomitantly. Unlike S. cerevisiae, the activity of COPII is constrained at specialized subdomains of the ER called ER exit sites (ERES) (Orci et al. 1991; Bannykh et al. 1996).
Mammalian cells have ∼300 ERES, which are distributed throughout the ER with 60% closely apposed to the Golgi apparatus (Hammond and Glick 2000). ERES, also called transitional ER (tER) is collection of tubulovesicular membranes and COPII-coated buds emanating from the ER in metazoans, but their definition and organization remains unclear in yeast. Studies in plant, Pichia pastoris, and mammalian cells suggest that an ERES should be viewed as part of an integrated unit together with the associated post-ER compartment such as the ER Golgi intermediate compartment (ERGIC) in metazoans (Ito et al. 2012, 2017; Glick 2014; Robinson et al. 2015).
The ERGIC is composed of tubules and vesicles, is defined by the presence of the transmembrane protein ERGIC-53 (Schweizer et al. 1988). ERGIC is likely to be a sorting station that controls trafficking between the ER and the Golgi. Interestingly, it has also been shown recently to provide membranes for the biogenesis of other cellular organelles such as autophagosomes (Ge et al. 2013).
COPII AND VESICLE FORMATION AT THE ER
A density-enrichment screen identified 23 complementation groups required for protein secretion in yeast (Novick and Schekman 1979; Novick et al. 1980). Molecular analyses of these SEC genes allowed the Schekman group to delineate distinct stages of biosynthetic protein secretion from the ER to extracellular space. Genetic analyses and cell-free reconstitution assays from these and further studies placed Sar1, Sec12, Sec16, Sec23/24, Sec13/31 in steps leading to COPII vesicle biogenesis and cargo export from the ER.
COPII-Coated Vesicle Formation
The small GTPase (Sar1) is recruited to a site at the ER membrane by the transmembrane guanine nucleotide exchange factor (GEF) Sec12 (Nakano et al. 1988; Barlowe and Schekman 1993). GTP-bound Sar1 exposes its amphipathic amino terminus, which is inserted into the ER membrane, thereby generating curvature (Bielli et al. 2005; Lee et al. 2005). Membrane-bound Sar1 recruits an inner layer of the COPII coat, composed of Sec23/24 heterodimers (Hicke et al. 1992). Sec23 is a GTPase activating protein (GAP) for Sar1 and provides an arginine into the GTP binding site of Sar1 to facilitate GTP hydrolysis (Yoshihisa et al. 1993). The inner coat then recruits an outer COPII layer composed of heterotetramers of Sec13/31 that assembles into an intrinsically curved, polyhedral lattice. Sec31 provides a tryptophan residue to the catalytic site of Sar1 that further stimulates the GAP activity of Sec23 (Antonny et al. 2001). GTP hydrolysis is directly linked to the fission of the vesicle from the ER membrane and its uncoating (Antonny et al. 2001). Collectively, the concerted activities of these COPII components selects cargo and sculpts membranes into vesicles (Fig. 2).
Figure 2.
Coat protein complex II (COPII)-coated vesicle biogenesis. Cytosolic machinery assembles in a hierarchical manner at an endoplasmic reticulum (ER) exit site (ERES). The small GTPase Sar1 is recruited to the ER membrane. This in turn recruits an inner COPII coat of Sec23/Sec24. Secretory cargo in the ER binds to cargo receptors, which bind to Sec24. The Sar1-Sec23/24 “prebudding” complex recruits an outer COPII coat of Sec13/31 that sculpts the site into a vesicle. The vesicle buds off of the ER and forms a 60-nm coated vesicle. Vesicle uncoating exposes machinery required for vesicle targeting and fusion to a downstream compartment.
In addition to the COPII components described above, the gene SEC16 is essential for viability and encodes a protein that is necessary for cargo export from the ER. Sec16 interacts with various components at ERES to scaffold and organize exit site formation (Shaywitz et al. 1997; Connerly et al. 2005; Watson et al. 2006; Iinuma et al. 2007; Ivan et al. 2008; Hughes et al. 2009; Shindiapina and Barlowe 2010; Kung et al. 2012). Structural studies showed that Sec16 interactions with Sec13 provide a platform for the organized assembly of Sec23-24 and Sec13-31 at the ERES (Whittle and Schwartz 2010). Sec16 might also regulate the GTP cycle of the COPII coat and to stabilize the outer coat proteins after Sar1 GTP hydrolysis, thereby potentiating COPII vesicle biogenesis (Supek et al. 2002).
A Sec23-interacting protein (Sec23IP), also known as p125A, is present at ERESs in 1:1 stoichiometry with Sec13/31. Changing levels of p125A affects ERES morphology and cargo export (Tani et al. 1999; Shimoi et al. 2005; Klinkenberg et al. 2014).
TFG (Trk-fused gene) is a metazoan COPII-regulation factor with a proline-rich carboxy-terminal region that competes with Sec31 for binding to the inner coat. Binding of TFG to Sec23 is thought to promote release of Sec31 and uncoating to regulate the COPII cycle of assembly and disassembly (Hanna et al. 2017). Its function has been reported to mediate clustering of COPII membranes between the ER and ERGIC (Johnson et al. 2015; McCaughey et al. 2016; Hanna et al. 2017, 2018; Peotter et al. 2019).
Cargo Capture into COPII Vesicles by Receptors
For export from the ER, secretory cargo is captured and packaged into COPII-coated vesicles at ERES. These processes require export motifs in fully folded and export-competent client proteins. Export motifs are often short (2–15 residue) sequences nested within the cargo polypeptide chain. Export motifs are recognized by transmembrane sorting receptors that couple secretory cargo to COPII through interactions with both cargo and coat proteins. Cargo receptors are packaged into the COPII-vesicle and then transported to the next compartment of the secretory pathway. Receptors release bound cargo in pre-Golgi or Golgi compartments, and then they are recycled back to the ER for further cargo export. Different receptors recognize different signals in the cargo including carbohydrate and/or polypeptide signals (Appenzeller et al. 1999; Belden and Barlowe 2001; Barlowe and Helenius 2016). Receptor recycling is brought about by COPI-dependent vesicles via the ERGIC to the ER.
On model membranes such as liposomes, Sar1 and COPII coat proteins are sufficient to select transmembrane cargos, deform membranes into buds, and finally generate vesicles (Matsuoka et al. 1998). COPII components show a high degree of functional and structural conservation throughout evolution and yeast and human proteins can substitute for each other's functions.
A role for Sec23/Sec24 in cargo recruitment into nascent vesicles was proposed when a complex of Sar1, Sec23/24, was shown to interact with transmembrane cargoes and receptors for secretory proteins (Aridor et al. 1998; Kuehn et al. 1998). Genetic, biochemical, and structural data have provided a molecular basis for how Sec24 subunits recognize and bind specific ER exit motifs in cargoes for their selective uptake into vesicles (Miller et al. 2003; Mossessova et al. 2003; Mancias and Goldberg 2007). Sec24 has several cargo-binding sites, which allows for the recognition of several different cargoes by the COPII coat. Multiple isoforms of Sec24 provide additional diversity in interactions with cargo. Yeast expresses three Sec24 homologs (Sec24, Iss1/Sfb2, and Lst1/Sfb3), whereas mammals express four (Sec24A-D). Based on sequence identity, the four isoforms are separated into two subfamilies, Sec24A/B and Sec24C/D (Pagano et al. 1999; Tang et al. 1999).
The ERGIC53 and ER vesicle (Erv) families of proteins are the best characterized cargo receptors that function in capturing cargo into COPII vesicles for ER export (Dancourt and Barlowe 2010). ERGIC-53, so named because it is a 53 kDa protein that localizes to the ERGIC, is an oligomeric single-pass transmembrane protein with a bulky amino-terminal ER-lumenal domain and a short, cytoplasmic carboxy terminal. The cytoplasmic stretch has a diphenylalanine (FF) motif, which interacts with COPII proteins for incorporation into COPII vesicles (Kappeler et al. 1997). There is also a dilysine (KKXX) signal, which promotes capture into COPI-dependent retrograde carriers to the ER (Kappeler et al. 1997). The ERGIC53 lumenal domain is homologous to L-type lectins that show Ca2+-dependent binding to high-mannose oligosaccharides. The cargo, cathepsin Z-related protein, was shown to bind ERGIC-53 in the ER and dissociate in a post-ER compartment (Appenzeller et al. 1999).
Yeast Erv proteins show similar general properties as ERGIC53 cargo receptors. Deletion of individual ERV genes cause specific secretory proteins to be retained in the ER. Erv proteins cycle between the ER and Golgi using similar signals in their cytoplasmic sequences for recognition by COPII and COPI machinery (Belden and Barlowe 2001; Malkus et al. 2002).
There must be sufficient diversity in cargo capture to permit specific selection of a large number of cargoes (Barlowe 2003). ER export motifs have been identified as hydrophobic and aromatic carboxy-terminal amino acids, such as the two phenylalanine residues (FF) present at the carboxyl terminus of cargo receptors like ERGIC53 and the p24 family, or a single valine residue, as present on the carboxyl terminus of CD8 (Iodice et al. 2001; Nufer et al. 2002). Another export motif comprises di-acidic Glu-X-Asp (ExD) or DxE sequences (Nishimura and Balch 1997; Ma et al. 2001). There is some diversity in the how these motifs (either the FF or DXE) interact with COPII machinery that might both provide greater stringency in cargo selection and drive cargo packaging out of the ER. Both kinds of motifs bind Sec23/24. Other binding sites have been identified in Sec24 for the YNNSNPF signal of the yeast SNARE Sed5, and for LxxLE and DxE class signals present on certain SNARE and cargo proteins.
Several cargo-receptor interactions and motifs are yet to be characterized, which will reveal the diversity and cargo specificity of protein trafficking out of the ER. Export motifs facilitate the export of membrane proteins from the ER, but they are not always sufficient to promote export, suggesting that combinatorial interactions between coat proteins, the lipid membrane, accessory proteins, and other motifs on the cargo protein may build avidity for cargo selection. For some cargoes, the transport signal is a conformational epitope formed only when the cargo is in an export-competent conformation (Mancias and Goldberg 2007). More recent evidence has identified that some lysosomal proteins need to assemble into complexes in the ER before they are exported together as a single functional unit (Bajaj et al. 2020; Devireddy and Ferguson 2022). Export motif recognition by ERES machinery can therefore provide a regulation point that may control ER export.
ER exit is highly selective, while secretory cargo is being packaged for export, resident chaperones, and partially folded proteins must be retained (Barlowe and Helenius 2016; Gomez-Navarro and Miller 2016). COPII recognition of the export motif can mediate a five- to 20-fold concentration of secretory cargo into COPII-dependent vesicles. ERES machinery, receptors, and cargo exclude resident and misfolded proteins, both by active concentration of p24 receptors that drive out nonsecretory cargoes or by crowding out of other proteins (Ma et al. 2017; Gomez-Navarro et al. 2020).
Additional components of the budded COPII-dependent container include proteins such as the small GTPase Rab1 (Segev et al. 1988) and v-SNAREs that allow for fusion to the target membrane.
Some proteins that escape the ER and need to be retrieved from the ERGIC and Golgi apparatus to the ER. This retrograde cargo recruitment into COPI-dependent vesicles is mediated by the KDEL receptor for soluble cargoes that contain a carboxy-terminal sequence of amino acids K/RDEL (Semenza et al. 1990; Tang et al. 1993). Cargo receptors, KDEL receptors, and other transmembrane proteins contain the motif KKXX for capture into COPI-coated vesicle for retrograde transport to the ER.
Is All Biosynthetic Transport Mediated by COPII-Coated Vesicles?
Unlike yeast, metazoans secrete collagens and other similarly bulky proteins that compose the extracellular matrix (ECM) or chylomicrons and mucins. Although the export of these molecules is COPII-dependent, they are too big to be encased in a standard COPII-coated vesicle of the kind described above. This is not a small problem because collagens compose 25% of our dry protein weight and they are in fact the most abundant metazoan proteins. This led to a search for a cellular mechanism to increase the size of COPII-coated vesicles to form megacarriers.
ADAPTING COPII FOR BULKY CARGOES
A conceptually straightforward solution to the problem of big cargo export would be if cells assemble a bigger COPII coat lattice to extract a larger volume of ER, creating a COPII megacarrier. Changing the dimensions and geometry of COPII lattice assembly could be brought about by using specific COPII protein isoforms or different adaptors. One isoform of Sec24 called Lst1 in yeast was identified that allowed for COPII-coated carriers to increase from 75 nm up to 87 nm in diameter (Shimoni et al. 2000). But the resulting increase in size of a COPII vesicle is still too small for metazoan collagens.
Using correlative light and electron microscopy (CLEM) and superresolution imaging, it was shown subsequently that monoubiquitination of Sec31A, mediated by the klhl12 adaptor subunit of the Cullin 3 (CRL3) E3 ligase complex, might increase the dimension of the outer COPII coat. Overexpression of klhl12 formed enlarged COPII structures and enhanced the rate of collagen secretion (Jin et al. 2012). KLHL12- and COPII-containing structures were visualized, isolated, and were shown to contain collagen-1 (Gorur et al. 2017; Yuan et al. 2018). CUL3 knockdown has a more profound effect than klhl12 knockdown, suggesting redundancy in CUL3 adaptors for this process. Collagen-containing COPII vesicles contain hsp47, a chaperone that stabilizes the triple helical form of procollagen, which is either recycled back to the ER (Nakai et al. 1992; van Dijk et al. 2020) or as also shown, does not exit the ER (Omari et al. 2020). Furthermore, these vesicles were observed in cells and in a cell-free reaction. A similar cell-free COPII budding reaction revealed megacarriers containing another bulky cargo, ApoB-containing very light density lipoprotein (VLDL) (Gusarova et al. 2003; Melville et al. 2019).
More recently, it has been shown that CRL3KLHL12 influences collagen synthesis in human skin fibroblasts and that transport of collagen-1 is independent of CRL3KLHL12 (Kim et al. 2018). Subsequent reports have described structures with ERES markers that contain overexpressed tagged collagen, which are targeted for lysosomal degradation (Omari et al. 2018; Gorrell et al. 2021). A model procollagen-2 with characterized mutations that result in misfolded triple helices were further enriched in these degradative structures (Omari et al. 2018). These collagen-1-containing degradative structures were shown to contain klhl12, Cul3, and LC3 and the investigators suggested that KLHL12 and Cul3-mediated ubiquitination is involved in collagen degradation (Omari et al. 2018). Luini and colleagues have argued that collagen export is performed by uncoated, pleomorphic carriers (Mironov et al. 2003). On the other hand, live confocal video microscopy from Stephens and colleagues showed collagen transport from ER to the Golgi without the involvement of long-range trafficking of large vesicular structures (McCaughey et al. 2018).
Other reported ways to control COPII-mediated collagen export from the ER include the activity of Sedlin, which binds and regulates efficient cycling of Sar1 to allow nascent carriers to grow (Venditti et al. 2012). Additionally, TFG has been reported to cluster ERES-ERGIC machinery to affect the dynamics and size of COPII-dependent carriers, although the details are unresolved (Johnson et al. 2015; McCaughey et al. 2016).
It is clear that collagen export is COPII-dependent, but export can be for either secretion, or degradation, from an ERES. The morphology of the proposed containers for these two distinct routes, their composition, the conditions for their formation, and the presence or absence coatomers (partial or total coating) needs to be clarified. A handle to understand the mechanism of collagen export and how luminal collagen and cytoplasmic COPII coats are linked, stems from the discovery of the TANGO1 (transport and Golgi organization 1) family of proteins.
TANGO1 IS REQUIRED FOR BULKY AND HIGH-VOLUME CARGOES
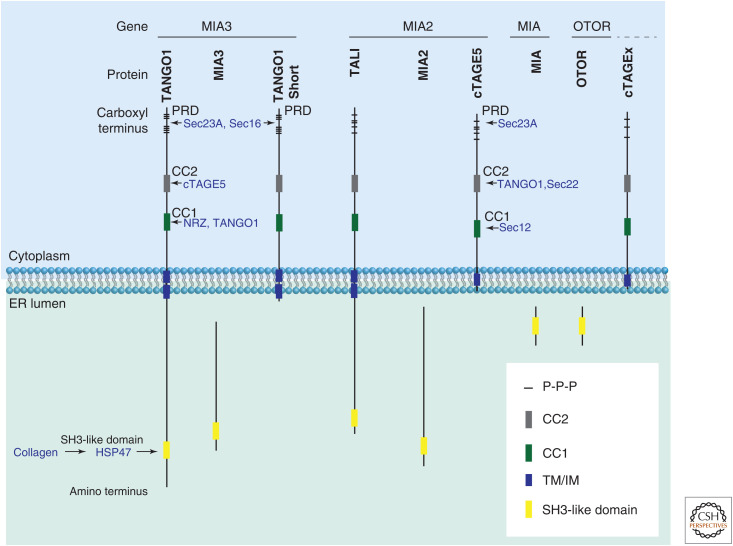
In a genome-wide RNAi screen for genes whose depletion led to reduced secretion of an engineered horseradish peroxidase (Bard et al. 2006), the protein TANGO1 was identified. In invertebrates, there is only one TANGO1 gene producing a single protein. In vertebrates, the whole gene and some individual portions have duplicated to give rise to the MIA (melanoma inhibitory activity) family of proteins. In mammals, the family consists of four genes, OTOR (otoraplin), MIA, MIA2, and MIA3 (TANGO1) (Fig. 3). At least two translated isoforms each of the paralogs MIA2 and MIA3 have been characterized thus far; the gene for MIA3 produces TANGO1 and TANGO1-Short and the paralog MIA2 produces TALI and cTAGE5 (cutaneous T-cell lymphoma-associated antigen 5).
Figure 3.
The transport and Golgi organization 1 (TANGO1) family of proteins. Mammalian TANGO1 family of proteins. Full-length TANGO1 or TANGO1-like (TALI) consists of an endoplasmic reticulum (ER)-lumenal part, with extensive regions that are predicted to be intrinsically disordered and an SH3-like domain. The cytoplasmic face of the ER membrane contains coiled coils (CC1 and CC2) and a carboxy-terminal proline-rich domain (PRD). Secreted isoforms MIA and otoraplin are almost exclusively an SH3-like domain. Other paralogs and their isoforms include TANGO1-Short, cTAGE5, and several predicted cTAGEx proteins (where x is 2, 4, 6, 8, 9, and 15). Each domain interacts with a specific set of proteins as indicated.
TANGO1 and cTAGE5 localize exclusively to ERES, doing so via interactions of their proline-rich domains (PRDs) to Sec23A and Sec16. Once at an ERES, TANGO1, cTAGE5, Sec12, and Sec23 mutually recruit and restrict each other along with their interactors. Data from Drosophila, zebrafish, medaka fish, and mammals suggest that the TANGO1 family is required for the secretion of several different collagens including I, II, III, IV, VII, and IX from chondrocytes, fibroblasts, endothelial cells, and mural cells (Saito et al. 2009; Wilson et al. 2011; Lerner et al. 2013; Tiwari et al. 2015; Ishikawa et al. 2017; Lekszas et al. 2020; Christen et al. 2021; Clark and Link 2021; Guillemyn et al. 2021). TANGO1 is also required for cargoes that are secreted in huge volumes including mucin (Zhang et al. 2014). TANGO1, TALI, and cTAGE5 show a similar effect on the ER export of another bulky cargo, apolipoproteins (Pitman et al. 2011; Santos et al. 2016). Collagen-1 hypersecretion in pathological fibrogenesis is mediated by TANGO1 in mice and its genetic depletion is protective against liver fibrosis (Maiers et al. 2017).
Altogether, these data suggest that TANGO1 has arisen in metazoa as an adaptation for the ER export of bulky cargoes like collagens. Although TANGO1 is an ERES-resident, protein-linking, export-competent cargo to COPII machinery, it is not a conventional cargo receptor. Unusually, it does not leave the ER along with the departing cargo. A collagen-1-containing intermediate that also contained klhl12, Sec31, TANGO1, cTAGE5, and Sec12 has been reported as the megacarrier for collagen export from the ER (Yuan et al. 2018). It is also important to note that unlike cargo receptors, TANGO1 binds Sec23A, not Sec24 (Saito et al. 2009; Ma and Goldberg 2016).
Mechanism of TANGO1 Function
Current data indicate that TANGO1 has at least two distinct functions: (1) to capture and export collagen, and (2) to organize the ERES.
In the ER lumen, an SH3-like domain is required for TANGO1 to interact with collagen (Saito et al. 2009). In vertebrates, the SH3-like domain binds to the collagen-specific chaperone HSP47 (SerpinH1) (Ishikawa et al. 2016). HSP47 binds to triple-helical procollagen and therefore it could serve as a link between TANGO1 and most procollagens. Perhaps not all collagens interact via Hsp47; they might bind directly to TANGO1 or use other linking mechanisms. It remains unclear how the SH3-like domain functions in invertebrates, which lack HSP47, but one possibility is that it may interact directly with procollagen (Ishikawa et al. 2016; Arnolds and Stoll 2022).
On the cytoplasmic face of the ER, TANGO1 has two extended stretches predicted to adopt a coiled-coil fold. The membrane-proximal coiled coil (CC1) is required for self-association of TANGO1 in both human and Drosophila TANGO1 (Raote et al. 2018; Reynolds et al. 2019). Interestingly, the same 40 amino acids required for the self-association are also the site of interaction with a retrograde multisubunit tether complex called the NRZ tether (Raote et al. 2018). Drosophila TANGO1 interacts with the golgin GM130, which is also associated with membrane tethering (Liu et al. 2017). Tethers are extended proteins or multisubunit complexes present on target membranes, sampling the environment for incoming membrane-bound carriers. These carriers dock to the tethers and finally fuse to the target membrane in a SNARE-dependent process.
The TANGO1 paralog cTAGE5 recruits Sec12 to ERES via a membrane proximal coiled coil. Carboxy-terminal, proline-rich domains of TANGO1 family members bind Sec23 and Sec16 (Saito et al. 2009; Ma and Goldberg 2016; Maeda et al. 2017).
TANGO1 depletion in Drosophila tissues reduces ERES size and number and increases the spatial segregation of ERES from the Golgi apparatus, suggesting that TANGO1 contributes to COPII clustering, ERES architecture, and ER-Golgi tethering. Superresolution microscopy in human cell lines, in Drosophila fat bodies, and larval salivary glands showed linear, filamentous arrays of TANGO1 surrounding exit sites (Liu et al. 2017; Raote et al. 2017; Reynolds et al. 2019), suggesting that TANGO1 assembles into a ring-like filament in the plane of the ER membrane, encircling and corralling COPII components (Raote et al. 2020).
Structural data suggest that the proline tripeptide (PPP) motifs in the proline-rich domains of TANGO1 and cTAGE5 engage in multivalent interactions with multiple copies of the pre-budding complex of Sar1A-Sec23-Sec24. This binding allows for flexibility in COPII lattices, which can now adopt a different shape to stabilize an alternate membrane curvature. The proposed model was that TANGO1 and cTAGE5 act as master scaffolds to switch COPII inner coat lattice between a cylindrical form and its usual spherical structure (Ma and Goldberg 2016; Hutchings et al. 2018).
TANGO1-Dependent ER Exit
Combining all these aspects of COPII function and how the TANGO1 family of proteins can remodel ERES machinery, we can arrive at a possible model of TANGO1-dependent export from the ER. TANGO1 and cTAGE5 along with the scaffolding protein Sec16 define the location and size of an ERES. At the neck of this export route, cTAGE5 recruits Sec12, which would ensure a stable supply of Sar1A at the site (Sato and Nakano 2005; Saito et al. 2014; Sasaki et al. 2018). COPII proteins including coatomers and Sar1, and Sec16 remain as a collar (Kurokawa et al. 2016; Iwasaki et al. 2017; Shomron et al. 2021) forming a treadmilling sheet of COPII that polymerizes at the neck in which cTAGE5 and TANGO1 activate Sar1, and depolymerizes further away from the ER (Fig. 4).
Figure 4.
TANGO1 (transport and Golgi organization 1) stabilizes a transient tunnel between the endoplasmic reticulum (ER) and the ER-Golgi intermediate compartment (ERGIC). TANGO1 at the base of nascent bud, binds to multiple Sec23/24 and promotes a cylindrical coat protein complex II (COPII) assembly. Via the NRZ tether, TANGO1 holds the ERGIC (pink membranes) in place at the ER exit sites (ERES). This could result in a transient continuity generated between the ER (blue) and the ERGIC (pink).
TANGO1 recruits tethering proteins, the NRZ complex in mammals or GM130 in Drosophila. Via the tether, TANGO1 recruits post-ER membranes to the ERES. Once tethered at the site, these retrograde ERGIC membranes need to fuse back to the ER for anterograde movement of collagen (Santos et al. 2015).
Vesicles, Tubules, or Tunnels for Cargo Export from the ER
Free COPII vesicles have been reported in yeast, in thin sections of cultured mammalian cells (Zeuschner et al. 2006), and in cell-free reactions. One could argue that the prevalence of free COPII vesicles in the cell-free reactions is caused by the relaxed organization and decreased proximity of the ERGIC and the ERES compared with intact cells. Nonetheless, isolated COPII vesicles, at least those isolated from yeast cell-free reactions, are functional in cargo delivery to the Golgi.
Extended tubular structures emanating from an ERES have also been reported in electron micrographs over the past four decades, along with more recent video microscopy, suggesting that there could be continuities between the ER and the ERGIC or even the Golgi (Claude 1970; Morré et al. 1971; Lindsey and Ellisman 1985; Sesso et al. 1994; Stinchcombe et al. 1995; Bannykh et al. 1996). Whether these are physiologically relevant is questioned from observations of similar tubules under conditions of defective transport—Fromme et al. observed these tubules in cranio-lenticulo-sutural dysplasia (CLSD) skin cells that had a heterozygous missense mutation in SEC23A. Bacia et al. observed constricted tubules projecting from GUVs in incubations conducted with nonhydrolyzable GTP (Fromme et al. 2007; Bacia et al. 2011).
Schekman and colleagues speculated that the coat may depolymerize from the tip of a bud, thus leaving a collar of COPII that persists until vesicle constriction and fission. Recent studies have indeed shown that instead of forming only a spherical vesicle, COPII can be visualized as a collar at the ER–ERES boundary, with an anastomosis of membranes at the ERES in mammalian cells as well as in Drosophila (Shomron et al. 2021; Weigel et al. 2021; Yang et al. 2021).
An uncoated tip of a nascent carrier will have exposed fusion machinery that could allow it to fuse with a target membrane, even before the carrier has been cut off from the ER. Such a fusion would result in a short-lived tunnel between the ER and the next compartment (Raote and Malhotra 2019). Transient continuities between the ER and the cis-Golgi have been suggested to transfer cargo (termed hug-and-kiss) in S. cerevisiae (Kurokawa et al. 2014). A similar association of ERES and cis-Golgi membranes is seen in P. pastoris, which is driven by the Dsl1 multisubunit retrograde tether complex, ortholog of the metazoan NRZ tether (Roy Chowdhury et al. 2020). It is not clear how these associations between ERES and retrograde tethers are maintained in non-metazoan eukaryotes because these species do not express TANGO1, but it is possible that they are formed and stabilized by stochastic processes resulting from transient higher local concentrations of machinery for anterograde and retrograde machinery. Metazoan activities like TANGO1 may have evolved to reinforce this functionally integrated unit of the ERES-Golgi interface, by controlling the timing and shape COPII assembly and physically recruiting tethers to the ERES.
Under these circumstances, COPII components remain at the ERES although anterograde cargo departs in anterograde transport intermediates marked by components of the downstream compartment that were tethered at the ERES.
Altogether, TANGO1 organizes the early secretory pathway, functionally linking cargo, coatomers, Sar1 activity, and a downstream compartment (Raote and Malhotra 2021). Other secretory modules have also been incorporated into the metazoan repertoire to control some of these aspects of ER export and the ER-Golgi interface, with a common feature being that they link activities at the ERES to a downstream compartment. Collagen folding in the ER lumen is brought together with cytoplasmic ER-ERGIC tethering activities by TMEM131 (Zhang et al. 2020). COPII-coated transport carriers may be tethered at the ER-ERGIC interface and their coat assembly and disassembly may be regulated in time and space by TFG (Hanna et al. 2017, 2018). The coat component p125A (Sec23ip) is a major Sec31A-interacting protein and, like TANGO1, controls the interactions between the Sec31A and Sec23A via tri-proline repeats (Shimoi et al. 2005; Ong et al. 2010). In addition, p125A also recognizes and binds to phospholipid (PI4P) enriched at the ERGIC, again serving to couple COPII budding with the presence of ERGIC membranes (Klinkenberg et al. 2014). The combination of all these proteins and activities ensure that COPII budding domains at the ER are always closely juxtaposed to ERGIC membranes.
REGULATING ER EXPORT
A challenge that awaits the field is to develop an integrated understanding of how ERES machinery is regulated to allow for rapid, dynamic changes in secretory requirements. Traffic out of the ER generates large fluxes of proteins and lipids, which must be compensated for dynamically by new synthesis or by retrograde transport. The ERES-ERGIC-Golgi interface also provides proteins and membranes for several processes, including the biogenesis of autophagosomes and lipid droplets (Ishihara et al. 2001; Ge et al. 2013, 2017; Graef et al. 2013; Wang et al. 2014). Failure of these homoeostatic mechanisms can lead to a disruption of cellular functioning.
One form of regulation comes from posttranslational modifications of COPII subunits and primer proteins that might modulate their activity (Table 1, derived from Bisnett et al. 2021); however, the mechanistic bases of these controls remain unclear. ER-resident leukocyte tyrosine kinase (LTK)-mediated Sec12 phosphorylation modulates the number of ERESs and amount of ER-Golgi traffic (Centonze et al. 2019). When large amounts of cargo arrive at ERESs, AREX (autoregulation of secretory flux) is activated by a cargo–Sec24 complex, which triggers kinases to enhance cargo export from the ER (Subramanian et al. 2019).
Table 1.
Post-translational modifications (PTMs) of COPII proteins
| Protein | PTM | Regulator | Effect | References |
|---|---|---|---|---|
| Sar1A | Ubiquitination | Unknown | Unknown | Hornbeck et al. 2015 |
| Sar1B | Phosphorylation Ubiquitination |
PKCz Unknown |
Unknown COPII response to large cargoes |
Siddiqi and Mansbach 2012; Hornbeck et al. 2015 |
| Sec12 |
N-glycosylation O-mannosylation Phosphorylation |
Unknown Unknown Hrr25/CK1δ LTKE |
Unknown Unknown Negative regulation of vesicle budding Positive regulator of ERES abundance and export |
Nakano et al. 1988; d'Enfert et al. 1991; Sato et al. 1996 Murakami et al. 1999 Centonze et al. 2019 |
| Sec16 | MARylation Phosphorylation Phosphorylation Phosphorylation Phosphorylation |
dPARP16 ERK2 ERK7/MAPK15 ULK1/2 |
Sec body biogenesis ERES biogenesis Localization to ERES Control ER export rates Sec23 interaction |
Aguilera-Gomez et al. 2016 Farhan et al. 2010; Tillmann et al. 2014; Zacharogianni et al. 2011 Joo et al. 2016 Yorimitsu and Sato 2020 |
| Sec23 | Phosphorylation Ubiquitination |
Hrr25/CK1δ Uba1, Ubc4, Rsp5, Ubp3, Bre5, Cdc48 |
Vesicle uncoating and membrane fusion Sec23 turnover |
Lord et al. 2011 Cohen et al. 2003 |
| Sec23A |
O-GlcNAcylation Phosphorylation Ubiquitination |
OGT ULK1 Unknown |
Collagen export from the ER Controls ER export Protein localization and turnover |
Cox et al. 2018 Gan et al. 2017 Amodio et al. 2009 |
| Sec23B | Phosphorylation | ULK1 | Autophagosome biogenesis | Jeong et al. 2018 |
| Sec24 | Phosphorylation | Hrr25/CK1δ | Unknown | Lord et al. 2011 |
| Sec24C | Phosphorylation Phosphorylation O-GlcNAcylation |
Akt Unknown OGT |
Control Sec23A affinity Unknown Mitotic shutdown of ERES export |
Sharpe et al. 2011 Dudognon et al. 2004; Kettenbach et al. 2011 Dudognon et al. 2004; Cox et al. 2018 |
| Sec24D | Phosphorylation | Akt | Control Sec23A binding | Sharpe et al. 2011 |
| Sec13 | Phosphorylation | Unknown | Unknown | Hornbeck et al. 2015 |
| Sec31 | Phosphorylation Phosphorylation Phosphorylation Phosphorylation O-GlcNAcylation O-GlcNAcylation Ubiquitination Ubiquitination |
Unknown Unknown Unknown Unknown OGT OGT ARIH1 KLHL12 |
Unknown Unknown Unknown Localization to ERES Localization to ERES Formation of enlarged COPII-containing structures |
Shugrue et al. 1999 Koreishi et al. 2013 Cho and Mook-Jung 2018 Cox et al. 2016 Scott et al. 2016 Yamasaki et al. 2006; Shibata et al. 2007; Jin et al. 2012 |
| TANGO1 | Phosphorylation O-GlcNAcylation |
CK1 PGANT4 Unknown |
Mitotic ERES breakdown Secretory granule apical secretion Insulin secretion |
Maeda et al. 2020 Zhang et al. 2014 |
ER-to-Golgi transport can be modulated by ALG-2 binding to ERES and altering the recruitment of coat proteins to the ERES. PEF1 and ALG2 acts as a co-adaptor to link cytosolic Ca2+ to Cul3KLHL12 recognition and sustained ubiquitination of the substrate Sec31 (McGourty et al. 2016). Short bursts of agonist-driven Ca2+ signaling increases ALG-2-dependent outer coat targeting to ERES to stimulate transport, whereas extended Ca2+ has the opposite effect (Sargeant et al. 2021). TFG also functions in a Ca2+-dependent manner through the association with the ALG-2 (Kanadome et al. 2017). Altogether, there are multiple indications for a regulatory role for Ca2+ levels in COPII budding.
Pathophysiological Conditions
Most mammalian COPII subunits have one or more paralogs with some divergence and partial redundancy in function, as loss-of-function of individual copies often results in genetic pathophysiologies. COPII genes are associated with several diseases (Table 2), several of which have no clear links to cargo packaging. Notably, congenital dyserythropoietic anemia type II (CDAII) is caused by mutations in Sec23B (Bianchi et al. 2009; Schwarz et al. 2009) but there is no known cargo packaging defect. Analysis of the mechanistic link between the gene and the phenotype will reveal new aspects of how these proteins work and are regulated under physiological conditions.
Table 2.
Pathological conditions associated with ERES machinery
| Protein | Disease/phenotype | References |
|---|---|---|
| Sar1A | Chylomicron retention | Jones et al. 2003 |
| Sar1B | Chylomicron retention | Jones et al. 2003; Charcosset et al. 2008 |
| Abetalipoproteinemia | Magnolo et al. 2013 | |
| Hypocholesterolemia | Levic et al. 2015 | |
| Sec12 | Diabetes mellitus (non-insulin-dependent) | Park et al. 2018 |
| Prolactinoma | Zhang et al. 2010 | |
| Sec16 | Obesity | Hotta et al. 2009 |
| Sec23A | Craniolenticulosutural dysplasia | Boyadjiev et al. 2006 |
| Sec23B | Congenital dyserythropoietic anemia | Bianchi et al. 2009; Schwarz et al. 2009 |
| Sec24D | Osteogenesis imperfecta (Cole Carpenter syndrome) | Garbes et al. 2015; Takeyari et al. 2018 |
| Sec24B | Neural tube defects | Yang et al. 2013 |
| Sec13 | Von Hippel–Lindau syndrome | Swaroop et al. 1994 |
| Sec31 | Neurodevelopmental disorder with spastic quadriplegia, optic atrophy, seizures, and structural brain anomalies | Halperin et al. 2019 |
| p125A (Sec23ip) | Cranial, skeletal, and neurodevelopmental effects | Arimitsu et al. 2011; Reuter et al. 2017 |
| TANGO1 (MIA3) | Coronary artery disease | Samani et al. 2007 |
| Clinodactyly, brachodactyly, diabetes, scoliosis, pruritic skin lesions | Lekszas et al. 2020; Guillemyn et al. 2021 | |
| MIA2 | Lymphoma, T cell, cutaneous | Koch et al. 2003 |
| Fahr's syndrome | Lemos et al. 2011 |
Multiple bacteria and viruses remodel secretory organelle membranes to generate specialized sites for their RNA replication or assembly and often use host COPII machinery in the remodeling (den Boon and Ahlquist 2010; Hsu et al. 2010; Inoue and Tsai 2013). ERES machinery repurposing by intracellular pathogens is also revealing novel aspects of how the machinery can work and which specific steps and interactions are useful for therapeutic interventions.
CONCLUSIONS AND OPEN QUESTIONS
Decades of studies have cemented our understanding of how COPII proteins mold and pinch a portion of ER into a small-coated vesicle for cargo export. The discovery of the TANGO1 family of proteins is helping in addressing long-standing questions of how bulky cargoes are exported by a COPII-dependent pathway. The challenge now is to address how metazoan proteins like TANGO1 influence COPII proteins to restrict their assembly as a collar at the neck of a carrier emanating from the ER, how bulky cargo is packed into the ensuing container, and what the biochemical properties are of the cargo-filled container.
ACKNOWLEDGMENTS
We acknowledge the support of the Spanish Ministry of Science and Innovation to the EMBL partnership, the Centro de Excelencia Severo Ochoa, and the CERCA Programme/Generalitat de Catalunya. We acknowledge financial support from the following sources: Ministerio de Economía y Competitividad (IJCI-2017-34751 to I.R., SEV-2012-0208, BFU2013-44188-P, CSD2009-00016 to V.M.). This project has received funding from the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation programme (Grant Agreement No. 951146). This publication is part of the Project TARTAFI (Ref. PDC2021-121870-I00), funded by MCIN/AEI/10.13039/501100011033 and the European Union “NextGenerationEU”/PRTR.
Footnotes
Editors: Susan Ferro-Novick, Tom A. Rapoport, and Randy Schekman
Additional Perspectives on The Endoplasmic Reticulum available at www.cshperspectives.org
REFERENCES
- Aguilera-Gomez A, van Oorschot MM, Veenendaal T, Rabouille C. 2016. In vivo vizualisation of mono-ADP-ribosylation by dPARP16 upon amino-acid starvation. eLife 5: e21475. 10.7554/eLife.21475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amodio G, Renna M, Paladino S, Venturi C, Tacchetti C, Moltedo O, Franceschelli S, Mallardo M, Bonatti S, Remondelli P. 2009. Endoplasmic reticulum stress reduces the export from the ER and alters the architecture of post-ER compartments. Int J Biochem Cell Biol 41: 2511–2521. 10.1016/j.biocel.2009.08.006 [DOI] [PubMed] [Google Scholar]
- Antonny B, Madden D, Hamamoto S, Orci L, Schekman R. 2001. Dynamics of the COPII coat with GTP and stable analogues. Nat Cell Biol 3: 531–537. 10.1038/35078500 [DOI] [PubMed] [Google Scholar]
- Appenzeller C, Andersson H, Kappeler F, Hauri HP. 1999. The lectin ERGIC-53 is a cargo transport receptor for glycoproteins. Nat Cell Biol 1: 330–334. 10.1038/14020 [DOI] [PubMed] [Google Scholar]
- Aridor M, Weissman J, Bannykh S, Nuoffer C, Balch WE. 1998. Cargo selection by the COPII budding machinery during export from the ER. J Cell Biol 141: 61–70. 10.1083/jcb.141.1.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arimitsu N, Kogure T, Baba T, Nakao K, Hamamoto H, Sekimizu K, Yamamoto A, Nakanishi H, Taguchi R, Tagaya M, et al. 2011. P125/Sec23-interacting protein (Sec23ip) is required for spermiogenesis. FEBS Lett 585: 2171–2176. 10.1016/j.febslet.2011.05.050 [DOI] [PubMed] [Google Scholar]
- Arnolds O, Stoll R. 2022. A new fold in TANGO1 evolved from SH3 domains for the export of bulky cargos. bioRxiv 10.1101/2022.02.02.478833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacia K, Futai E, Prinz S, Meister A, Daum S, Glatte D, Briggs JAG, Schekman R. 2011. Multibudded tubules formed by COPII on artificial liposomes. Sci Rep 1: 17. 10.1038/srep00017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajaj L, Sharma J, di Ronza A, Zhang P, Eblimit A, Pal R, Roman D, Collette JR, Booth C, Chang KT, et al. 2020. A CLN6-CLN8 complex recruits lysosomal enzymes at the ER for Golgi transfer. J Clin Invest 130: 4118–4132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannykh SI, Rowe T, Balch WE. 1996. The organization of endoplasmic reticulum export complexes. J Cell Biol 135: 19–35. 10.1083/jcb.135.1.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bard F, Casano L, Mallabiabarrena A, Wallace E, Saito K, Kitayama H, Guizzunti G, Hu Y, Wendler F, Dasgupta R, et al. 2006. Functional genomics reveals genes involved in protein secretion and Golgi organization. Nature 439: 604–607. 10.1038/nature04377 [DOI] [PubMed] [Google Scholar]
- Barlowe C. 2003. Signals for COPII-dependent export from the ER: what's the ticket out? Trends Cell Biol 13: 295–300. 10.1016/S0962-8924(03)00082-5 [DOI] [PubMed] [Google Scholar]
- Barlowe C, Helenius A. 2016. Cargo capture and bulk flow in the early secretory pathway. Annu Rev Cell Dev Biol 32: 197–222. 10.1146/annurev-cellbio-111315-125016 [DOI] [PubMed] [Google Scholar]
- Barlowe C, Schekman R. 1993. SEC12 encodes a guanine-nucleotide-exchange factor essential for transport vesicle budding from the ER. Nature 365: 347–349. 10.1038/365347a0 [DOI] [PubMed] [Google Scholar]
- Belden WJ, Barlowe C. 2001. Role of Erv29p in collecting soluble secretory proteins into ER-derived transport vesicles. Science 294: 1528–1531. 10.1126/science.1065224 [DOI] [PubMed] [Google Scholar]
- Bianchi P, Fermo E, Vercellati C, Boschetti C, Barcellini W, Iurlo A, Marcello AP, Righetti PG, Zanella A. 2009. Congenital dyserythropoietic anemia type II (CDAII) is caused by mutations in the SEC23B gene. Hum Mutat 30: 1292–1298. 10.1002/humu.21077 [DOI] [PubMed] [Google Scholar]
- Bielli A, Haney CJ, Gabreski G, Watkins SC, Bannykh SI, Aridor M. 2005. Regulation of Sar1 NH2 terminus by GTP binding and hydrolysis promotes membrane deformation to control COPII vesicle fission. J Cell Biol 171: 919–924. 10.1083/jcb.200509095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisnett BJ, Condon BM, Lamb CH, Georgiou GR, Boyce M. 2021. Export control: post-transcriptional regulation of the COPII trafficking pathway. Front Cell Dev Biol 8: 618652. 10.3389/fcell.2020.618652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyadjiev SA, Fromme JC, Ben J, Chong SS, Nauta C, Hur DJ, Zhang G, Hamamoto S, Schekman R, Ravazzola M, et al. 2006. Cranio-lenticulo-sutural dysplasia is caused by a SEC23A mutation leading to abnormal endoplasmic-reticulum-to-Golgi trafficking. Nat Genet 38: 1192–1197. 10.1038/ng1876 [DOI] [PubMed] [Google Scholar]
- Centonze FG, Reiterer V, Nalbach K, Saito K, Pawlowski K, Behrends C, Farhan H. 2019. LTK is an ER-resident receptor tyrosine kinase that regulates secretion. J Cell Biol 218: 2470–2480. 10.1083/jcb.201903068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charcosset M, Sassolas A, Peretti N, Roy CC, Deslandres C, Sinnett D, Levy E, Lachaux A. 2008. Anderson or chylomicron retention disease: molecular impact of five mutations in the SAR1B gene on the structure and the functionality of Sar1b protein. Mol Genet Metab 93: 74–84. 10.1016/j.ymgme.2007.08.120 [DOI] [PubMed] [Google Scholar]
- Cho HJ, Mook-Jung I. 2018. O-GlcNAcylation regulates endoplasmic reticulum exit sites through Sec31A modification in conventional secretory pathway. FASEB J 32: 4641–4657. 10.1096/fj.201701523R [DOI] [PubMed] [Google Scholar]
- Christen M, Booij-Vrieling H, Oksa-Minalto J, de Vries C, Kehl A, Jagannathan V, Leeb T. 2021. MIA3 splice defect in Cane Corso dogs with dental-skeletal-retinal anomaly (DSRA). Genes (Basel) 12: 1497. 10.3390/genes12101497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark EM, Link BA. 2021. Complementary and divergent functions of zebrafish Tango1 and Ctage5 in tissue development and homeostasis. Mol Biol Cell 32: 391–401. 10.1091/mbc.E20-11-0745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claude A. 1970. Growth and differentiation of cytoplasmic membranes in the course of lipoprotein granule synthesis in the hepatic cell. I: Elaboration of elements of the Golgi complex. J Cell Biol 47: 745–766. 10.1083/jcb.47.3.745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M, Stutz F, Belgareh N, Haguenauer-Tsapis R, Dargemont C. 2003. Ubp3 requires a cofactor, Bre5, to specifically de-ubiquitinate the COPII protein, Sec23. Nat Cell Biol 5: 661–667. 10.1038/ncb1003 [DOI] [PubMed] [Google Scholar]
- Connerly PL, Esaki M, Montegna EA, Strongin DE, Levi S, Soderholm J, Glick BS. 2005. Sec16 is a determinant of transitional ER organization. Curr Biol 15: 1439–1447. 10.1016/j.cub.2005.06.065 [DOI] [PubMed] [Google Scholar]
- Cox NJ, Unlu G, Bisnett BJ, Meister TR, Condon BM, Luo PM, Smith TJ, Hanna M, Chhetri A, Soderblom EJ, et al. 2018. Dynamic glycosylation governs the vertebrate COPII protein trafficking pathway. Biochemistry 57: 91–107. 10.1021/acs.biochem.7b00870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dancourt J, Barlowe C. 2010. Protein sorting receptors in the early secretory pathway. Annu Rev Biochem 79: 777–802. 10.1146/annurev-biochem-061608-091319 [DOI] [PubMed] [Google Scholar]
- den Boon JA, Ahlquist P. 2010. Organelle-like membrane compartmentalization of positive-strand RNA virus replication factories. Annu Rev Microbiol 64: 241–256. 10.1146/annurev.micro.112408.134012 [DOI] [PubMed] [Google Scholar]
- d'Enfert C, Barlowe C, Nishikawa S, Nakano A, Schekman R. 1991. Structural and functional dissection of a membrane glycoprotein required for vesicle budding from the endoplasmic reticulum. Mol Cell Biol 11: 5727–5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devireddy S, Ferguson SM. 2022. Efficient progranulin exit from the ER requires its interaction with prosaposin, a Surf4 cargo. J Cell Biol 221: e202104044. 10.1083/jcb.202104044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudognon P, Maeder-Garavaglia C, Carpentier JL, Paccaud JP. 2004. Regulation of a COPII component by cytosolic O-glycosylation during mitosis. FEBS Lett 561: 44–50. 10.1016/S0014-5793(04)00109-7 [DOI] [PubMed] [Google Scholar]
- Farhan H, Wendeler MW, Mitrovic S, Fava E, Silberberg Y, Sharan R, Zerial M, Hauri HP. 2010. MAPK signaling to the early secretory pathway revealed by kinase/phosphatase functional screening. J Cell Biol 189: 997–1011. 10.1083/jcb.200912082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromme JC, Ravazzola M, Hamamoto S, Al-Balwi M, Eyaid W, Boyadjiev SA, Cosson P, Schekman R, Orci L. 2007. The genetic basis of a craniofacial disease provides insight into COPII coat assembly. Dev Cell 13: 623–634. 10.1016/j.devcel.2007.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan W, Zhang C, Siu KY, Satoh A, Tanner JA, Yu S. 2017. ULK1 phosphorylates Sec23A and mediates autophagy-induced inhibition of ER-to-Golgi traffic. BMC Cell Biol 18: 22. 10.1186/s12860-017-0138-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbes L, Kim K, Rieß A, Hoyer-Kuhn H, Beleggia F, Bevot A, Kim MJ, Huh YH, Kweon HS, Savarirayan R, et al. 2015. Mutations in SEC24D, encoding a component of the COPII machinery, cause a syndromic form of osteogenesis imperfecta. Am J Hum Genet 96: 432–439. 10.1016/j.ajhg.2015.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge L, Melville D, Zhang M, Schekman R. 2013. The ER–Golgi intermediate compartment is a key membrane source for the LC3 lipidation step of autophagosome biogenesis. eLife 2: e00947. 10.7554/eLife.00947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge L, Zhang M, Kenny SJ, Liu D, Maeda M, Saito K, Mathur A, Xu K, Schekman R. 2017. Remodeling of ER-exit sites initiates a membrane supply pathway for autophagosome biogenesis. EMBO Rep 18: 1586–1603. 10.15252/embr.201744559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick BS. 2014. Integrated self-organization of transitional ER and early Golgi compartments: ideas & speculations. Bioessays 36: 129–133. 10.1002/bies.201300131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JL, Anderson RGW, Brown MS. 1979. Coated pits, coated vesicles, and receptor-mediated endocytosis. Nature 279: 679–685. 10.1038/279679a0 [DOI] [PubMed] [Google Scholar]
- Gomez-Navarro N, Miller E. 2016. Protein sorting at the ER–Golgi interface. J Cell Biol 215: 769–778. 10.1083/jcb.201610031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Navarro N, Melero A, Li XH, Boulanger J, Kukulski W, Miller EA. 2020. Cargo crowding contributes to sorting stringency in COPII vesicles. J Cell Biol 219: e201806038. 10.1083/jcb.201806038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorrell L, Omari S, Makareeva E, Leikin S. 2021. Noncanonical ER–Golgi trafficking and autophagy of endogenous procollagen in osteoblasts. Cell Mol Life Sci 78: 8283–8300. 10.1007/s00018-021-04017-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorur A, Yuan L, Kenny SJ, Baba S, Xu K, Schekman R. 2017. COPII-coated membranes function as transport carriers of intracellular procollagen I. J Cell Biol 216: 1745–1759. 10.1083/jcb.201702135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graef M, Friedman JR, Graham C, Babu M, Nunnari J. 2013. ER exit sites are physical and functional core autophagosome biogenesis components. Mol Biol Cell 24: 2918–2931. 10.1091/mbc.e13-07-0381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillemyn B, Nampoothiri S, Syx D, Malfait F, Symoens S. 2021. Loss of TANGO1 leads to absence of bone mineralization. JBMR Plus 5: e10451. 10.1002/jbm4.10451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusarova V, Brodsky JL, Fisher EA. 2003. Apolipoprotein B100 exit from the endoplasmic reticulum (ER) is COPII-dependent, and its lipidation to very low density lipoprotein occurs post-ER. J Biol Chem 278: 48051–48058. 10.1074/jbc.M306898200 [DOI] [PubMed] [Google Scholar]
- Halperin D, Kadir R, Perez Y, Drabkin M, Yogev Y, Wormser O, Berman EM, Eremenko E, Rotblat B, Shorer Z, et al. 2019. SEC31A mutation affects ER homeostasis, causing a neurological syndrome. J Med Genet 56: 139–148. 10.1136/jmedgenet-2018-105503 [DOI] [PubMed] [Google Scholar]
- Hammond AT, Glick BS. 2000. Dynamics of transitional endoplasmic reticulum sites in vertebrate cells. Mol Biol Cell 11: 3013–3030. 10.1091/mbc.11.9.3013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna MG, Block S, Frankel EB, Hou F, Johnson A, Yuan L, Knight G, Moresco JJ, Yates JR, Ashton R, et al. 2017. TFG facilitates outer coat disassembly on COPII transport carriers to promote tethering and fusion with ER–Golgi intermediate compartments. Proc Natl Acad Sci 114: E7707–E7716. 10.1073/pnas.1709120114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna MG, Peotter JL, Frankel EB, Audhya A. 2018. Membrane transport at an organelle interface in the early secretory pathway: take your coat off and stay a while: evolution of the metazoan early secretory pathway. Bioessays 40: 1800004. 10.1002/bies.201800004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicke L, Yoshihisa T, Schekman R. 1992. Sec23p and a novel 105-kDa protein function as a multimeric complex to promote vesicle budding and protein transport from the endoplasmic reticulum. Mol Biol Cell 3: 667–676. 10.1091/mbc.3.6.667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornbeck PV, Zhang B, Murray B, Kornhauser JM, Latham V, Skrzypek E. 2015. PhosphoSitePlus, 2014: mutations, PTMs and recalibrations. Nucleic Acids Res 43: D512–D520. 10.1093/nar/gku1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotta K, Nakamura M, Nakamura T, Matsuo T, Nakata Y, Kamohara S, Miyatake N, Kotani K, Komatsu R, Itoh N, et al. 2009. Association between obesity and polymorphisms in SEC16B, TMEM18, GNPDA2, BDNF, FAIM2 and MC4R in a Japanese population. J Hum Genet 54: 727–731. 10.1038/jhg.2009.106 [DOI] [PubMed] [Google Scholar]
- Hsu N-Y, Ilnytska O, Belov G, Santiana M, Chen YH, Takvorian PM, Pau C, van der Schaar H, Kaushik-Basu N, Balla T, et al. 2010. Viral reorganization of the secretory pathway generates distinct organelles for RNA replication. Cell 141: 799–811. 10.1016/j.cell.2010.03.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes H, Budnik A, Schmidt K, Palmer KJ, Mantell J, Noakes C, Johnson A, Carter DA, Verkade P, Watson P, et al. 2009. Organisation of human ER-exit sites: requirements for the localisation of Sec16 to transitional ER. J Cell Sci 122: 2924–2934. 10.1242/jcs.044032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchings J, Stancheva V, Miller EA, Zanetti G. 2018. Subtomogram averaging of COPII assemblies reveals how coat organization dictates membrane shape. Nat Commun 9: 4154. 10.1038/s41467-018-06577-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iinuma T, Shiga A, Nakamoto K, O'Brien MB, Aridor M, Arimitsu N, Tagaya M, Tani K. 2007. Mammalian Sec16/p250 plays a role in membrane traffic from the endoplasmic reticulum. J Biol Chem 282: 17632–17639. 10.1074/jbc.M611237200 [DOI] [PubMed] [Google Scholar]
- Inoue T, Tsai B. 2013. How viruses use the endoplasmic reticulum for entry, replication, and assembly. Cold Spring Harb Perspect Biol 5: a013250–a013250. 10.1101/cshperspect.a013250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iodice L, Sarnataro S, Bonatti S. 2001. The carboxyl-terminal valine is required for transport of glycoprotein CD8α from the endoplasmic reticulum to the intermediate compartment. J Biol Chem 276: 28920–28926. 10.1074/jbc.M103558200 [DOI] [PubMed] [Google Scholar]
- Ishihara N, Hamasaki M, Yokota S, Suzuki K, Kamada Y, Kihara A, Yoshimori T, Noda T, Ohsumi Y. 2001. Autophagosome requires specific early Sec proteins for its formation and NSF/SNARE for vacuolar fusion. Mol Biol Cell 12: 3690–3702. 10.1091/mbc.12.11.3690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa Y, Ito S, Nagata K, Sakai LY, Bächinger HP. 2016. Intracellular mechanisms of molecular recognition and sorting for transport of large extracellular matrix molecules. Proc Natl Acad Sci 113: E6036–E6044. 10.1073/pnas.1609571113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa T, Toyama T, Nakamura Y, Tamada K, Shimizu H, Ninagawa S, Okada T, Kamei Y, Ishikawa-Fujiwara T, Todo T, et al. 2017. UPR transducer BBF2H7 allows export of type II collagen in a cargo- and developmental stage-specific manner. J Cell Biol 216: 1761–1774. 10.1083/jcb.201609100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y, Uemura T, Shoda K, Fujimoto M, Ueda T, Nakano A. 2012. cis-Golgi proteins accumulate near the ER exit sites and act as the scaffold for Golgi regeneration after brefeldin A treatment in tobacco BY-2 cells. Mol Biol Cell 23: 3203–3214. 10.1091/mbc.e12-01-0034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y, Uemura T, Nakano A. 2017. Golgi entry core compartment functions as the COPII-independent scaffold for ER-Golgi transport in plant cells. J Cell Sci 131: jcs.203893. 10.1242/jcs.203893 [DOI] [PubMed] [Google Scholar]
- Ivan V, de Voer G, Xanthakis D, Spoorendonk KM, Kondylis V, Rabouille C. 2008. Drosophila Sec16 mediates the biogenesis of tER sites upstream of Sar1 through an arginine-rich motif. Mol Biol Cell 19: 4352–4365. 10.1091/mbc.e08-03-0246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki H, Yorimitsu T, Sato K. 2017. Microscopic analysis of reconstituted COPII coat polymerization and Sec16 dynamics. J Cell Sci 130: 2893–2902. 10.1242/jcs.203844 [DOI] [PubMed] [Google Scholar]
- Jeong YT, Simoneschi D, Keegan S, Melville D, Adler NS, Saraf A, Florens L, Washburn MP, Cavasotto CN, Fenyö D, et al. 2018. The ULK1-FBXW5-SEC23B nexus controls autophagy. eLife 7: e42253. 10.7554/eLife.42253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin L, Pahuja KB, Wickliffe KE, Gorur A, Baumgärtel C, Schekman R, Rape M. 2012. Ubiquitin-dependent regulation of COPII coat size and function. Nature 482: 495–500. 10.1038/nature10822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A, Bhattacharya N, Hanna M, Pennington JG, Schuh AL, Wang L, Otegui MS, Stagg SM, Audhya A. 2015. TFG clusters COPII-coated transport carriers and promotes early secretory pathway organization. EMBO J 34: 811–827. 10.15252/embj.201489032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B, Jones EL, Bonney SA, Patel HN, Mensenkamp AR, Eichenbaum-Voline S, Rudling M, Myrdal U, Annesi G, Naik S, et al. 2003. Mutations in a Sar1 GTPase of COPII vesicles are associated with lipid absorption disorders. Nat Genet 34: 29–31. 10.1038/ng1145 [DOI] [PubMed] [Google Scholar]
- Joo JH, Wang B, Frankel E, Ge L, Xu L, Iyengar R, Li-Harms X, Wright C, Shaw TI, Lindsten T, et al. 2016. The noncanonical role of ULK/ATG1 in ER-to-Golgi trafficking is essential for cellular homeostasis. Mol Cell 62: 491–506. 10.1016/j.molcel.2016.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanadome T, Shibata H, Kuwata K, Takahara T, Maki M. 2017. The calcium-binding protein ALG-2 promotes endoplasmic reticulum exit site localization and polymerization of Trk-fused gene (TFG) protein. FEBS J 284: 56–76. 10.1111/febs.13949 [DOI] [PubMed] [Google Scholar]
- Kappeler F, Klopfenstein D, Foguet M, Paccaud JP, Hauri HP. 1997. The recycling of ERGIC-53 in the early secretory pathway. J Biol Chem 272: 31801–31808. 10.1074/jbc.272.50.31801 [DOI] [PubMed] [Google Scholar]
- Kettenbach AN, Schweppe DK, Faherty BK, Pechenick D, Pletnev AA, Gerber SA. 2011. Quantitative phosphoproteomics identifies substrates and functional modules of aurora and polo-like kinase activities in mitotic cells. Sci Signal 4: rs5. 10.1126/scisignal.2001497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Moretti T, Park S, Kim J. 2018. Cullin3-RING ubiquitin ligases are intimately linked to the unfolded protein response of the endoplasmic reticulum. bioRxiv 10.1101/428136 [DOI] [Google Scholar]
- Klinkenberg D, Long KR, Shome K, Watkins SC, Aridor M. 2014. A cascade of ER exit site assembly that is regulated by p125A and lipid signals. J Cell Sci 127: 1765–1778. 10.1242/jcs.138784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch J, Dübel S, Usener D, Schadendorf D, Eichmüller S. 2003. cTAGE: a cutaneous T cell lymphoma associated antigen family with tumor-specific splicing. J Invest Dermatol 121: 198–206. 10.1046/j.1523-1747.2003.12318.x [DOI] [PubMed] [Google Scholar]
- Koreishi M, Yu S, Oda M, Honjo Y, Satoh A. 2013. CK2 phosphorylates Sec31 and regulates ER-to-Golgi trafficking. PLoS ONE 8: e54382. 10.1371/journal.pone.0054382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehn MJ, Herrmann JM, Schekman R. 1998. COPII–cargo interactions direct protein sorting into ER-derived transport vesicles. Nature 391: 187–190. 10.1038/34438 [DOI] [PubMed] [Google Scholar]
- Kung LF, Pagant S, Futai E, D'Arcangelo JG, Buchanan R, Dittmar JC, Reid RJD, Rothstein R, Hamamoto S, Snapp EL, et al. 2012. Sec24p and Sec16p cooperate to regulate the GTP cycle of the COPII coat. EMBO J 31: 1014–1027. 10.1038/emboj.2011.444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurokawa K, Okamoto M, Nakano A. 2014. Contact of cis-Golgi with ER exit sites executes cargo capture and delivery from the ER. Nat Commun 5: 3653. 10.1038/ncomms4653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurokawa K, Suda Y, Nakano A. 2016. Sar1 localizes at the rims of COPII-coated membranes in vivo. J Cell Sci 129: 3231–3237. doi 10.1242/jcs.189423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MCS, Orci L, Hamamoto S, Futai E, Ravazzola M, Schekman R. 2005. Sar1p N-terminal helix initiates membrane curvature and completes the fission of a COPII vesicle. Cell 122: 605–617. 10.1016/j.cell.2005.07.025 [DOI] [PubMed] [Google Scholar]
- Lekszas C, Foresti O, Raote I, Lietdke D, König E-M, Nanda I, Vona B, De Coster P, Cauwels R, Malhotra V, et al. 2020. Biallelic TANGO1 mutations cause a novel syndromal disease due to hampered cellular collagen secretion. eLife 9: e51319. 10.7554/eLife.51319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemos RR, Oliveira DF, Zatz M, Oliveira JRM. 2011. Population and computational analysis of the MGEA6 P521A variation as a risk factor for familial idiopathic basal ganglia calcification (Fahr's disease). J Mol Neurosci 43: 333–336. 10.1007/s12031-010-9445-7 [DOI] [PubMed] [Google Scholar]
- Lerner DW, McCoy D, Isabella AJ, Mahowald AP, Gerlach GF, Chaudhry TA, Horne-Badovinac S. 2013. A Rab10-dependent mechanism for polarized basement membrane secretion during organ morphogenesis. Dev Cell 24: 159–168. 10.1016/j.devcel.2012.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levic DS, Minkel J, Wang W-D, Rybski WM, Melville DB, Knapik EW. 2015. Animal model of Sar1b deficiency presents lipid absorption deficits similar to Anderson disease. J Mol Med 93: 165–176. 10.1007/s00109-014-1247-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey J, Ellisman M. 1985. The neuronal endomembrane system. I. Direct links between rough endoplasmic reticulum and the cis element of the Golgi apparatus. J Neurosci 5: 3111–3123. 10.1523/JNEUROSCI.05-12-03111.1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Feng Z, Ke H, Liu Y, Sun T, Dai J, Cui W, Pastor-Pareja JC. 2017. Tango1 spatially organizes ER exit sites to control ER export. J Cell Biol 216: 1035–1049. 10.1083/jcb.201611088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Bhandari D, Menon S, Ghassemian M, Nycz D, Hay J, Ghosh P, Ferro-Novick S. 2011. Sequential interactions with Sec23 control the direction of vesicle traffic. Nature 473: 181–186. 10.1038/nature09969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma W, Goldberg J. 2016. TANGO1/cTAGE5 receptor as a polyvalent template for assembly of large COPII coats. Proc Natl Acad Sci 113: 10061–10066. 10.1073/pnas.1605916113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma D, Zerangue N, Lin YF, Collins A, Yu M, Jan YN, Jan LY. 2001. Role of ER export signals in controlling surface potassium channel numbers. Science 291: 316–319. 10.1126/science.291.5502.316 [DOI] [PubMed] [Google Scholar]
- Ma W, Goldberg E, Goldberg J. 2017. ER retention is imposed by COPII protein sorting and attenuated by 4-phenylbutyrate. eLife 6: e26624. 10.7554/eLife.26624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda M, Katada T, Saito K. 2017. TANGO1 recruits Sec16 to coordinately organize ER exit sites for efficient secretion. J Cell Biol 216: 1731–1743. 10.1083/jcb.201703084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda M, Komatsu Y, Saito K. 2020. Mitotic ER exit site disassembly and reassembly are regulated by the phosphorylation status of TANGO1. Dev Cell 55: 237–250.e5. 10.1016/j.devcel.2020.07.017 [DOI] [PubMed] [Google Scholar]
- Magnolo L, Najah M, Fancello T, Di Leo E, Pinotti E, Brini I, Gueddiche NM, Calandra S, Slimene NM, Tarugi P. 2013. Novel mutations in SAR1B and MTTP genes in Tunisian children with chylomicron retention disease and abetalipoproteinemia. Gene 512: 28–34. 10.1016/j.gene.2012.09.117 [DOI] [PubMed] [Google Scholar]
- Maiers JL, Kostallari E, Mushref M, deAssuncao TM, Li H, Jalan-Sakrikar N, Huebert RC, Cao S, Malhi H, Shah VH. 2017. The unfolded protein response mediates fibrogenesis and collagen I secretion through regulating TANGO1 in mice. Hepatology 65: 983–998. 10.1002/hep.28921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra V, Serafini T, Orci L, Shepherd JC, Rothman JE. 1989. Purification of a novel class of coated vesicles mediating biosynthetic protein transport through the Golgi stack. Cell 58: 329–336. 10.1016/0092-8674(89)90847-7 [DOI] [PubMed] [Google Scholar]
- Malkus P, Jiang F, Schekman R. 2002. Concentrative sorting of secretory cargo proteins into COPII-coated vesicles. J Cell Biol 159: 915–921. 10.1083/jcb.200208074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancias JD, Goldberg J. 2007. The transport signal on Sec22 for packaging into COPII-coated vesicles is a conformational epitope. Mol Cell 26: 403–414. 10.1016/j.molcel.2007.03.017 [DOI] [PubMed] [Google Scholar]
- Matsuoka K, Orci L, Amherdt M, Bednarek SY, Hamamoto S, Schekman R, Yeung T. 1998. COPII-coated vesicle formation reconstituted with purified coat proteins and chemically defined liposomes. Cell 93: 263–275. 10.1016/S0092-8674(00)81577-9 [DOI] [PubMed] [Google Scholar]
- McCaughey J, Miller VJ, Stevenson NL, Brown AK, Budnik A, Heesom KJ, Alibhai D, Stephens DJ. 2016. TFG promotes organization of transitional ER and efficient collagen secretion. Cell Rep 15: 1648–1659. 10.1016/j.celrep.2016.04.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaughey J, Stevenson NL, Cross S, Stephens DJ. 2018. ER-to-Golgi trafficking of procollagen in the absence of large carriers. J Cell Biol 218: 929–948. 10.1083/jcb.201806035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGourty CA, Akopian D, Walsh C, Gorur A, Werner A, Schekman R, Bautista D, Rape M. 2016. Regulation of the CUL3 ubiquitin ligase by a calcium-dependent co-adaptor. Cell 167: 525–538.e14. 10.1016/j.cell.2016.09.026 [DOI] [PubMed] [Google Scholar]
- Melville D, Gorur A, Schekman R. 2019. Fatty-acid binding protein 5 modulates the SAR1 GTPase cycle and enhances budding of large COPII cargoes. Mol Biol Cell 30: 387–399. 10.1091/mbc.E18-09-0548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EA, Beilharz TH, Malkus PN, Lee MCS, Hamamoto S, Orci L, Schekman R. 2003. Multiple cargo binding sites on the COPII subunit Sec24p ensure capture of diverse membrane proteins into transport vesicles. Cell 114: 497–509. 10.1016/S0092-8674(03)00609-3 [DOI] [PubMed] [Google Scholar]
- Mironov AA, Mironov AA, Beznoussenko GV, Trucco A, Lupetti P, Smith JD, Geerts WJC, Koster AJ, Burger KNJ, Martone ME, et al. 2003. ER-to-Golgi carriers arise through direct en bloc protrusion and multistage maturation of specialized ER exit domains. Dev Cell 5: 583–594. 10.1016/S1534-5807(03)00294-6 [DOI] [PubMed] [Google Scholar]
- Morré DJ, Keenan TW, Mollenhauer HH. 1971. Golgi apparatus function in membrane transformations and product compartmentalization: studies with cell fractions isolated from rat liver. Adv Cytopharmacol 1: 159–182. [PubMed] [Google Scholar]
- Mossessova E, Bickford LC, Goldberg J. 2003. SNARE selectivity of the COPII coat. Cell 114: 483–495. 10.1016/S0092-8674(03)00608-1 [DOI] [PubMed] [Google Scholar]
- Murakami A, Kimura K, Nakano A. 1999. The inactive form of a yeast casein kinase I suppresses the secretory defect of the sec12 mutant. J Biol Chem 274: 3804–3810. 10.1074/jbc.274.6.3804 [DOI] [PubMed] [Google Scholar]
- Nakai A, Satoh M, Hirayoshi K, Nagata K. 1992. Involvement of the stress protein HSP47 in procollagen processing in the endoplasmic reticulum. J Cell Biol 117: 903–914. 10.1083/jcb.117.4.903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano A, Brada D, Schekman R. 1988. A membrane glycoprotein, Sec12p, required for protein transport from the endoplasmic reticulum to the Golgi apparatus in yeast. J Cell Biol 107: 851–863. 10.1083/jcb.107.3.851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura N, Balch WE. 1997. A di-acidic signal required for selective export from the endoplasmic reticulum. Science 277: 556–558. 10.1126/science.277.5325.556 [DOI] [PubMed] [Google Scholar]
- Novick P, Schekman R. 1979. Secretion and cell-surface growth are blocked in a temperature-sensitive mutant of Saccharomyces cerevisiae. Proc Natl Acad Sci 76: 1858–1862. 10.1073/pnas.76.4.1858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick P, Field C, Schekman R. 1980. Identification of 23 complementation groups required for post-translational events in the yeast secretory pathway. Cell 21: 205–215. 10.1016/0092-8674(80)90128-2 [DOI] [PubMed] [Google Scholar]
- Nufer O, Guldbrandsen S, Degen M, Kappeler F, Paccaud J-P, Tani K, Hauri HP. 2002. Role of cytoplasmic C-terminal amino acids of membrane proteins in ER export. J Cell Sci 115: 619–628. 10.1242/jcs.115.3.619 [DOI] [PubMed] [Google Scholar]
- Omari S, Makareeva E, Roberts-Pilgrim A, Mirigian L, Jarnik M, Ott C, Lippincott-Schwartz J, Leikin S. 2018. Noncanonical autophagy at ER exit sites regulates procollagen turnover. Proc Natl Acad Sci 115: E10099–E10108. 10.1073/pnas.1814552115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omari S, Makareeva E, Gorrell L, Jarnik M, Lippincott-Schwartz J, Leikin S. 2020. Mechanisms of procollagen and HSP47 sorting during ER-to-Golgi trafficking. Matrix Biol 93: 79–94. 10.1016/j.matbio.2020.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong YS, Tang BL, Loo LS, Hong W. 2010. P125a exists as part of the mammalian Sec13/Sec31 COPII subcomplex to facilitate ER-Golgi transport. J Cell Biol 190: 331–345. 10.1083/jcb.201003005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orci L, Glick BS, Rothman JE. 1986. A new type of coated vesicular carrier that appears not to contain clathrin: its possible role in protein transport within the Golgi stack. Cell 46: 171–184. 10.1016/0092-8674(86)90734-8 [DOI] [PubMed] [Google Scholar]
- Orci L, Ravazzola M, Meda P, Holcomb C, Moore HP, Hicke L, Schekman R. 1991. Mammalian Sec23p homologue is restricted to the endoplasmic reticulum transitional cytoplasm. Proc Natl Acad Sci 88: 8611–8615. 10.1073/pnas.88.19.8611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano A, Letourneur F, Garcia-Estefania D, Carpentier JL, Orci L, Paccaud JP. 1999. Sec24 proteins and sorting at the endoplasmic reticulum. J Biol Chem 274: 7833–7840. 10.1074/jbc.274.12.7833 [DOI] [PubMed] [Google Scholar]
- Park JM, Kim MY, Kim TH, Min DK, Yang GE, Ahn YH. 2018. Prolactin regulatory element-binding (PREB) protein regulates hepatic glucose homeostasis. Biochim Biophys Acta Mol Basis Dis 1864: 2097–2107. 10.1016/j.bbadis.2018.03.024 [DOI] [PubMed] [Google Scholar]
- Pearse BM. 1976. Clathrin: a unique protein associated with intracellular transfer of membrane by coated vesicles. Proc Natl Acad Sci 73: 1255–1259. 10.1073/pnas.73.4.1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peotter J, Kasberg W, Pustova I, Audhya A. 2019. COPII-mediated trafficking at the ER/ERGIC interface. Traffic 20: 491–503. 10.1111/tra.12654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitman JL, Bonnet DJ, Curtiss LK, Gekakis N. 2011. Reduced cholesterol and triglycerides in mice with a mutation in Mia2, a liver protein that localizes to ER exit sites. J Lipid Res 52: 1775–1786. 10.1194/jlr.M017277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raote I, Malhotra V. 2019. Protein transport by vesicles and tunnels. J Cell Biol 218: 737–739. 10.1083/jcb.201811073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raote I, Malhotra V. 2021. Tunnels for protein export from the endoplasmic reticulum. Annu Rev Biochem 90: 605–630. 10.1146/annurev-biochem-080120-022017 [DOI] [PubMed] [Google Scholar]
- Raote I, Ortega Bellido M, Pirozzi M, Zhang C, Melville D, Parashuraman S, Zimmermann T, Malhotra V. 2017. TANGO1 assembles into rings around COPII coats at ER exit sites. J Cell Biol 216: 901–909. 10.1083/jcb.201608080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raote I, Ortega-Bellido M, Santos AJ, Foresti O, Zhang C, Garcia-Parajo MF, Campelo F, Malhotra V. 2018. TANGO1 builds a machine for collagen export by recruiting and spatially organizing COPII, tethers and membranes. eLife 7: e32723. 10.7554/eLife.32723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raote I, Ernst AM, Campelo F, Rothman JE, Pincet F, Malhotra V. 2020. TANGO1 membrane helices create a lipid diffusion barrier at curved membranes. eLife 9: e57822. 10.7554/eLife.57822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter MS, Tawamie H, Buchert R, Hosny Gebril O, Froukh T, Thiel C, Uebe S, Ekici AB, Krumbiegel M, Zweier C, et al. 2017. Diagnostic yield and novel candidate genes by exome sequencing in 152 consanguineous families with neurodevelopmental disorders. JAMA Psychiatry 74: 293. 10.1001/jamapsychiatry.2016.3798 [DOI] [PubMed] [Google Scholar]
- Reynolds HM, Zhang L, Tran DT, Ten Hagen KG. 2019. Tango1 coordinates the formation of endoplasmic reticulum/Golgi docking sites to mediate secretory granule formation. J Biol Chem 294: 19498–19510. 10.1074/jbc.RA119.011063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DG, Brandizzi F, Hawes C, Nakano A. 2015. Vesicles versus tubes: is endoplasmic reticulum-Golgi transport in plants fundamentally different from other eukaryotes? Plant Physiol 168: 393–406. 10.1104/pp.15.00124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy Chowdhury S, Bhattacharjee C, Casler JC, Jain BK, Glick BS, Bhattacharyya D. 2020. ER arrival sites associate with ER exit sites to create bidirectional transport portals. J Cell Biol 219: e201902114. 10.1083/jcb.201902114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito K, Chen M, Bard F, Chen S, Zhou H, Woodley D, Polischuk R, Schekman R, Malhotra V. 2009. TANGO1 facilitates cargo loading at endoplasmic reticulum exit sites. Cell 136: 891–902. 10.1016/j.cell.2008.12.025 [DOI] [PubMed] [Google Scholar]
- Saito K, Yamashiro K, Shimazu N, Tanabe T, Kontani K, Katada T. 2014. Concentration of Sec12 at ER exit sites via interaction with cTAGE5 is required for collagen export. J Cell Biol 206: 751–762. 10.1083/jcb.201312062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samani NJ, Erdmann J, Hall AS, Hengstenberg C, Mangino M, Mayer B, Dixon RJ, Meitinger T, Braund P, Wichmann HE, et al. 2007. Genomewide association analysis of coronary artery disease. N Engl J Med 357: 443–453. 10.1056/NEJMoa072366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos AJ, Raote I, Scarpa M, Brouwers N, Malhotra V. 2015. TANGO1 recruits ERGIC membranes to the endoplasmic reticulum for procollagen export. eLife 4: e10982. 10.7554/eLife.10982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos AJM, Nogueira C, Ortega-Bellido M, Malhotra V. 2016. TANGO1 and Mia2/cTAGE5 (TALI) cooperate to export bulky pre-chylomicrons/VLDLs from the endoplasmic reticulum. J Cell Biol 213: 343–354. 10.1083/jcb.201603072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargeant J, Seiler DK, Costain T, Madreiter-Sokolowski CT, Gordon DE, Peden AA, Malli R, Graier WF, Hay JC. 2021. ALG-2 and peflin regulate COPII targeting and secretion in response to calcium signaling. J Biol Chem 297: 101393. 10.1016/j.jbc.2021.101393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki N, Shiraiwa M, Maeda M, Yorimitsu T, Sato K, Katada T, Saito K. 2018. cTAGE5 acts as a Sar1 GTPase regulator for collagen export. bioRxiv 10.1101/452904 [DOI] [Google Scholar]
- Sato K, Nakano A. 2005. Dissection of COPII subunit-cargo assembly and disassembly kinetics during Sar1p-GTP hydrolysis. Nat Struct Mol Biol 12: 167–174. 10.1038/nsmb893 [DOI] [PubMed] [Google Scholar]
- Sato M, Sato K, Nakano A. 1996. Endoplasmic reticulum localization of Sec12p is achieved by two mechanisms: Rer1p-dependent retrieval that requires the transmembrane domain and Rer1p-independent retention that involves the cytoplasmic domain. J Cell Biol 134: 279–293. 10.1083/jcb.134.2.279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz K, Iolascon A, Verissimo F, Trede NS, Horsley W, Chen W, Paw BH, Hopfner K-P, Holzmann K, Russo R, et al. 2009. Mutations affecting the secretory COPII coat component SEC23B cause congenital dyserythropoietic anemia type II. Nat Genet 41: 936–940. 10.1038/ng.405 [DOI] [PubMed] [Google Scholar]
- Schweizer A, Fransen JA, Bächi T, Ginsel L, Hauri HP. 1988. Identification, by a monoclonal antibody, of a 53-kD protein associated with a tubulo-vesicular compartment at the cis-side of the Golgi apparatus. J Cell Biol 107: 1643–1653. 10.1083/jcb.107.5.1643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott DC, Rhee DY, Duda DM, Kelsall IR, Olszewski JL, Paulo JA, de Jong A, Ovaa H, Alpi AF, Harper JW, et al. 2016. Two distinct types of E3 ligases work in unison to regulate substrate ubiquitylation. Cell 166: 1198–1214.e24. 10.1016/j.cell.2016.07.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segev N, Mulholland J, Botstein D. 1988. The yeast GTP-binding YPT1 protein and a mammalian counterpart are associated with the secretion machinery. Cell 52: 915–924. 10.1016/0092-8674(88)90433-3 [DOI] [PubMed] [Google Scholar]
- Semenza JC, Hardwick KG, Dean N, Pelham HRB. 1990. ERD2, a yeast gene required for the receptor-mediated retrieval of luminal ER proteins from the secretory pathway. Cell 61: 1349–1357. 10.1016/0092-8674(90)90698-E [DOI] [PubMed] [Google Scholar]
- Serafini T, Orci L, Amherdt M, Brunner M, Kahn RA, Rothmant JE. 1991a. ADP-ribosylation factor is a subunit of the coat of Golgi-derived COP-coated vesicles: a novel role for a GTP-binding protein. Cell 67: 239–253. 10.1016/0092-8674(91)90176-Y [DOI] [PubMed] [Google Scholar]
- Serafini T, Stenbeck G, Brecht A, Lottspeich F, Orel L, Rothman JE, Wieland FT. 1991b. A coat subunit of Golgi-derived non-clathrin-coated vesicles with homology to the clathrin-coated vesicle coat protein β-adaptin. Nature 349: 215–220. 10.1038/349215a0 [DOI] [PubMed] [Google Scholar]
- Sesso A, de Faria FP, Iwamura ES, Corrêa H. 1994. A three-dimensional reconstruction study of the rough ER-Golgi interface in serial thin sections of the pancreatic acinar cell of the rat. J Cell Sci 107: 517–528. 10.1242/jcs.107.3.517 [DOI] [PubMed] [Google Scholar]
- Sharpe LJ, Luu W, Brown AJ. 2011. Akt phosphorylates Sec24: new clues into the regulation of ER-to-Golgi trafficking. Traffic 12: 19–27. 10.1111/j.1600-0854.2010.01133.x [DOI] [PubMed] [Google Scholar]
- Shaywitz DA, Espenshade PJ, Gimeno RE, Kaiser CA. 1997. COPII subunit interactions in the assembly of the vesicle coat. J Biol Chem 272: 25413–25416. 10.1074/jbc.272.41.25413 [DOI] [PubMed] [Google Scholar]
- Shibata H, Suzuki H, Yoshida H, Maki M. 2007. ALG-2 directly binds Sec31A and localizes at endoplasmic reticulum exit sites in a Ca2+-dependent manner. Biochem Biophys Res Commun 353: 756–763. 10.1016/j.bbrc.2006.12.101 [DOI] [PubMed] [Google Scholar]
- Shimoi W, Ezawa I, Nakamoto K, Uesaki S, Gabreski G, Aridor M, Yamamoto A, Nagahama M, Tagaya M, Tani K. 2005. P125 is localized in endoplasmic reticulum exit sites and involved in their organization. J Biol Chem 280: 10141–10148. 10.1074/jbc.M409673200 [DOI] [PubMed] [Google Scholar]
- Shimoni Y, Kurihara T, Ravazzola M, Amherdt M, Orci L, Schekman R. 2000. Lst1p and Sec24p cooperate in sorting of the plasma membrane ATPase into COPII vesicles in Saccharomyces cerevisiae. J Cell Biol 151: 973–984. 10.1083/jcb.151.5.973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shindiapina P, Barlowe C. 2010. Requirements for transitional endoplasmic reticulum site structure and function in Saccharomyces cerevisiae. Mol Biol Cell 21: 1530–1545. 10.1091/mbc.e09-07-0605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shomron O, Nevo-Yassaf I, Aviad T, Yaffe Y, Zahavi EE, Dukhovny A, Perlson E, Brodsky I, Yeheskel A, Pasmanik-Chor M, et al. 2021. COPII collar defines the boundary between ER and ER exit site and does not coat cargo containers. J Cell Biol 220: e201907224. 10.1083/jcb.201907224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shugrue CA, Kolen ER, Peters H, Czernik A, Kaiser C, Matovcik L, Hubbard AL, Gorelick F. 1999. Identification of the putative mammalian orthologue of Sec31P, a component of the COPII coat. J Cell Sci 112: 4547–4556. 10.1242/jcs.112.24.4547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqi S, Mansbach CM. 2012. Phosphorylation of Sar1b protein releases liver fatty acid-binding protein from multiprotein complex in intestinal cytosol enabling it to bind to endoplasmic reticulum (ER) and bud the pre-chylomicron transport vesicle. J Biol Chem 287: 10178–10188. 10.1074/jbc.M111.327247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinchcombe JC, Nomoto H, Cutler DF, Hopkins CR. 1995. Anterograde and retrograde traffic between the rough endoplasmic reticulum and the Golgi complex. J Cell Biol 131: 1387–1401. 10.1083/jcb.131.6.1387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian A, Capalbo A, Iyengar NR, Rizzo R, di Campli A, Di Martino R, Lo Monte M, Beccari AR, Yerudkar A, del Vecchio C, et al. 2019. Auto-regulation of secretory flux by sensing and responding to the folded cargo protein load in the endoplasmic reticulum. Cell 176: 1461–1476.e23. 10.1016/j.cell.2019.01.035 [DOI] [PubMed] [Google Scholar]
- Supek F, Madden DT, Hamamoto S, Orci L, Schekman R. 2002. Sec16p potentiates the action of COPII proteins to bud transport vesicles. J Cell Biol 158: 1029–1038. 10.1083/jcb.200207053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaroop A, Yang-Feng TL, Liu W, Gieser L, Barrow LL, Chen KC, Agarwal N, Meisler MH, Smith DI. 1994. Molecular characterization of a novel human gene, SEC13R, related to the yeast secretory pathway gene SEC13, and mapping to a conserved linkage group on human chromosome 3p24-p25 and mouse chromosome 6. Hum Mol Genet 3: 1281–1286. 10.1093/hmg/3.8.1281 [DOI] [PubMed] [Google Scholar]
- Takeyari S, Kubota T, Miyata K, Yamamoto K, Nakayama H, Yamamoto K, Ohata Y, Kitaoka T, Yanagi K, Kaname T, et al. 2018. Japanese patient with Cole–Carpenter syndrome with compound heterozygous variants of SEC24D. Am J Med Genet 176: 2882–2886. 10.1002/ajmg.a.40643 [DOI] [PubMed] [Google Scholar]
- Tang BL, Wong SH, Qi XL, Low SH, Hong W. 1993. Molecular cloning, characterization, subcellular localization and dynamics of p23, the mammalian KDEL receptor. J Cell Biol 120: 325–338. 10.1083/jcb.120.2.325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang BL, Kausalya J, Low DYH, Lock ML, Hong W. 1999. A family of mammalian proteins homologous to yeast Sec24p. Biochem Biophys Res Commun 258: 679–684. 10.1006/bbrc.1999.0574 [DOI] [PubMed] [Google Scholar]
- Tani K, Mizoguchi T, Iwamatsu A, Hatsuzawa K, Tagaya M. 1999. P125 is a novel mammalian Sec23p-interacting protein with structural similarity to phospholipid-modifying proteins. J Biol Chem 274: 20505–20512. 10.1074/jbc.274.29.20505 [DOI] [PubMed] [Google Scholar]
- Tillmann KD, Reiterer V, Baschieri F, Hoffmann J, Millarte V, Hauser MA, Mazza A, Atias N, Legler DF, Sharan R, et al. 2014. Regulation of Sec16 levels and dynamics links proliferation and secretion. J Cell Sci 128: 670–682. 10.1242/jcs.157115 [DOI] [PubMed] [Google Scholar]
- Tiwari P, Kumar A, Das RN, Malhotra V, VijayRaghavan K. 2015. A tendon cell specific RNAi screen reveals novel candidates essential for muscle tendon interaction. PLoS ONE 10: e0140976. 10.1371/journal.pone.0140976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijk FS, Semler O, Etich J, Köhler A, Jimenez-Estrada JA, Bravenboer N, Claeys L, Riesebos E, Gegic S, Piersma SR, et al. 2020. Interaction between KDELR2 and HSP47 as a key determinant in osteogenesis imperfecta caused by bi-allelic variants in KDELR2. Am J Hum Genet 107: 989–999. 10.1016/j.ajhg.2020.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venditti R, Scanu T, Santoro M, Di Tullio G, Spaar A, Gaibisso R, Beznoussenko GV, Mironov AA, Mironov A Jr, Zelante L, et al. 2012. Sedlin controls the ER export of procollagen by regulating the Sar1 cycle. Science 337: 1668–1672. 10.1126/science.1224947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Tan D, Cai Y, Reinisch KM, Walz T, Ferro-Novick S. 2014. A requirement for ER-derived COPII vesicles in phagophore initiation. Autophagy 10: 708–709. 10.4161/auto.28103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters MG, Serafini T, Rothman JE. 1991. “Coatomer”: a cytosolic protein complex containing subunits of non-clathrin-coated Golgi transport vesicles. Nature 349: 248–251. 10.1038/349248a0 [DOI] [PubMed] [Google Scholar]
- Watson P, Townley AK, Koka P, Palmer KJ, Stephens DJ. 2006. Sec16 defines endoplasmic reticulum exit sites and is required for secretory cargo export in mammalian cells. Traffic 7: 1678–1687. 10.1111/j.1600-0854.2006.00493.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigel AV, Chang CL, Shtengel G, Xu CS, Hoffman DP, Freeman M, Iyer N, Aaron J, Khuon S, Bogovic J, et al. 2021. ER-to-Golgi protein delivery through an interwoven, tubular network extending from ER. Cell 184: 2412–2429.e16. 10.1016/j.cell.2021.03.035 [DOI] [PubMed] [Google Scholar]
- Whittle JRR, Schwartz TU. 2010. Structure of the Sec13–Sec16 edge element, a template for assembly of the COPII vesicle coat. J Cell Biol 190: 347–361. 10.1083/jcb.201003092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DG, Phamluong K, Li L, Sun M, Cao TC, Liu PS, Modrusan Z, Sandoval WN, Rangell L, Carano RA, et al. 2011. Global defects in collagen secretion in a Mia3/TANGO1 knockout mouse. J Cell Biol 193: 935–951. 10.1083/jcb.201007162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki A, Tani K, Yamamoto A, Kitamura N, Komada M. 2006. The Ca2+-binding protein ALG-2 is recruited to endoplasmic reticulum exit sites by Sec31A and stabilizes the localization of Sec31A. Mol Biol Cell 17: 4876–4887. 10.1091/mbc.e06-05-0444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XY, Zhou XY, Wang QQ, Li H, Chen Y, Lei YP, Ma XH, Kong P, Shi Y, Jin L, et al. 2013. Mutations in the COPII vesicle component gene SEC24B are associated with human neural tube defects. Human Mutat 34: 1094–1101. 10.1002/humu.22338 [DOI] [PubMed] [Google Scholar]
- Yang K, Liu M, Feng Z, Rojas M, Zhou L, Ke H, Pastor-Pareja JC. 2021. ER exit sites in Drosophila display abundant ER-Golgi vesicles and pearled tubes but no megacarriers. Cell Rep 36: 109707. 10.1016/j.celrep.2021.109707 [DOI] [PubMed] [Google Scholar]
- Yorimitsu T, Sato K. 2020. Sec16 function in ER export and autophagy is independent of its phosphorylation in Saccharomyces cerevisiae. Mol Biol Cell 31: 149–156. 10.1091/mbc.E19-08-0477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshihisa T, Barlowe C, Schekman R. 1993. Requirement for a GTPase-activating protein in vesicle budding from the endoplasmic reticulum. Science 259: 1466–1468. 10.1126/science.8451644 [DOI] [PubMed] [Google Scholar]
- Yuan L, Kenny SJ, Hemmati J, Xu K, Schekman R. 2018. TANGO1 and SEC12 are co-packaged with procollagen I to facilitate the generation of large COPII carriers. Proc Natl Acad Sci 115: E12255–E12264. 10.1073/pnas.1814810115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacharogianni M, Kondylis V, Tang Y, Farhan H, Xanthakis D, Fuchs F, Boutros M, Rabouille C. 2011. ERK7 is a negative regulator of protein secretion in response to amino-acid starvation by modulating Sec16 membrane association. EMBO J 30: 3684–3700. 10.1038/emboj.2011.253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeuschner D, Geerts WJC, van Donselaar E, Humbel BM, Slot JW, Koster AJ, Klumperman J. 2006. Immuno-electron tomography of ER exit sites reveals the existence of free COPII-coated transport carriers. Nat Cell Biol 8: 377–383. 10.1038/ncb1371 [DOI] [PubMed] [Google Scholar]
- Zhang W, Murao K, Imachi H, Iwama H, Chen K, Fei Z, Zhang X, Ishida T, Tamiya T. 2010. Suppression of prolactin expression by cabergoline requires prolactin regulatory element-binding protein (PREB) in GH3 cells. Horm Metab Res 42: 557–561. 10.1055/s-0030-1252064 [DOI] [PubMed] [Google Scholar]
- Zhang L, Syed ZA, van Dijk Härd I, Lim JM, Wells L, Ten Hagen KG. 2014. O-glycosylation regulates polarized secretion by modulating Tango1 stability. Proc Natl Acad Sci 111: 7296–7301. 10.1073/pnas.1322264111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Bai M, Barbosa GO, Chen A, Wei Y, Luo S, Wang X, Wang B, Tsukui T, Li H, et al. 2020. Broadly conserved roles of TMEM131 family proteins in intracellular collagen assembly and secretory cargo trafficking. Sci Adv 6: eaay7667. 10.1126/sciadv.aay7667 [DOI] [PMC free article] [PubMed] [Google Scholar]