Abstract
The endoplasmic reticulum (ER) is the largest organelle and has multiple roles in various cellular processes such as protein secretion, lipid synthesis, calcium storage, and organelle biogenesis. The quantity and quality of this organelle are controlled by the ubiquitin-proteasome system and autophagy (termed “ER-phagy”). ER-phagy is defined as the degradation of part of the ER by the vacuole or lysosomes, and there are at least two types of ER-phagy: macro-ER-phagy and micro-ER-phagy. In macro-ER-phagy, ER fragments are enclosed by autophagosomes, which is mediated by ER-phagy receptors. In micro-ER-phagy, a portion of the ER is engulfed directly by the vacuole or lysosomes. In these two pathways, some proteins in the ER lumen can be recognized selectively and subjected to ER-phagy. This review summarizes our current knowledge of ER-phagy, focusing on its membrane dynamics, molecular mechanisms, substrate specificity, and physiological significance.
The endoplasmic reticulum (ER) is a dynamic organelle that is involved in various cellular processes such as protein secretion, lipid synthesis, calcium storage, detoxification, redox regulation, and organelle biogenesis (Schwarz and Blower 2016; Joshi et al. 2017; Yoboue et al. 2018). To maintain its function and integrity, the ER undergoes continuous renovation, which is achieved by two major mechanisms: the ubiquitin-proteasome system and autophagy. Misfolded polypeptides are retrotranslocated from the ER to the cytosol where they are degraded by the ubiquitin-proteasome system, which is termed “ER-associated protein degradation” (ERAD) (Ruggiano et al. 2014; Wu and Rapoport 2018; Oikonomou and Hendershot 2019; Sun and Brodsky 2019). In contrast, autophagy targets ER fragments, including luminal proteins (even aggregates) and ER membranes, for lysosomal degradation. Autophagic degradation of the ER is termed “ER-phagy” (Chino and Mizushima 2020; Hübner and Dikic 2020; Wilkinson 2020; Ferro-Novick et al. 2021; Fregno et al. 2021). ER-phagy was first observed by electron microscopy ∼50 years ago in rat hepatocytes recovering from phenobarbital-induced smooth ER expansion (Bolender and Weibel 1973; Feldman et al. 1980). However, the mechanisms and roles of ER-phagy have not been investigated extensively until recently. This review summarizes the recent advances in our understanding of various types of ER-phagy, focusing on their membrane dynamics, mechanisms, and potential functions.
CLASSIFICATION OF ER-PHAGY
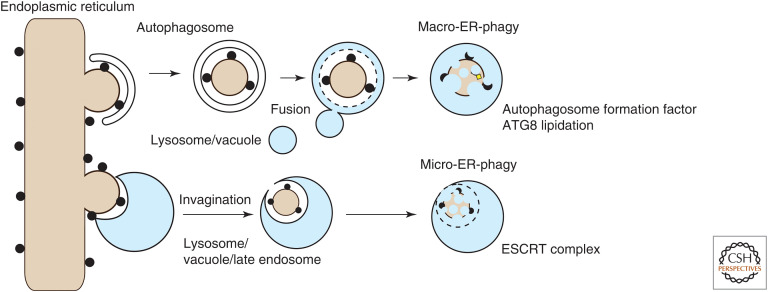
ER-phagy can be classified into two distinct categories: macro-ER-phagy and micro-ER-phagy (Fig. 1). Macro-ER-phagy uses autophagosomes to enclose fragments of the ER and deliver them to lysosomes (De Leonibus et al. 2019; Stolz and Grumati 2019). This pathway requires a set of autophagy-related (ATG) genes (Mizushima and Komatsu 2011). Micro-ER-phagy does not use autophagosomes and instead involves the inward invagination of lysosomal (or vacuolar) membranes to engulf parts of the ER (Schuck et al. 2014; Wilkinson 2019). Micro-ER-phagy uses the endosomal sorting complex required for transport (ESCRT) machinery for scission of the lysosomal membrane, but depends on only some, or even no, ATG genes (see below). Recently, another form of ER-phagy, called the vesicular transport pathway, was proposed. In that pathway, ER-derived vesicles fuse directly with lysosomes for degradation (Fregno et al. 2018; Fregno and Molinari 2019). This pathway is also dependent on some ATG genes (see below).
Figure 1.
Different types of endoplasmic reticulum (ER)-phagy. Macro-ER-phagy: Fragments of the ER are enclosed by autophagosomes. After fusion with lysosomes, the inner autophagosomal membrane and sequestered materials, including ER fragments, are degraded. This pathway requires the core ATG factors involved in autophagy initiation and ATG8 lipidation. Micro-ER-phagy: A small portion of the ER is engulfed directly by inward invagination of lysosomal/endosomal or vacuolar membranes and then degraded. This pathway depends on some or no ATG factors and requires the endosomal sorting complex required for transport (ESCRT) machinery for the closure step.
MECHANISM OF MACRO-ER-PHAGY
During macroautophagy, cytoplasmic components are engulfed by autophagosomes (Mizushima and Komatsu 2011). This sequestration is generally nonselective but can also be selective for specific substrates (Farre and Subramani 2016; Kirkin and Rogov 2019; Lamark and Johansen 2021). In the case of ER sequestration, it is difficult to tell whether the ER is selectively recognized by just using electron microscopy. Because autophagosomes are formed on the ER (Kovacs et al. 2007; Axe et al. 2008; Eskelinen 2008; Hayashi-Nishino et al. 2009), ER fragments may sometimes be engulfed by chance. However, the recent identification of ER-phagy receptors clearly indicates that ER fragments are specifically recognized by autophagosomal membranes.
Recognition of the ER by Autophagosomes
The molecules that link the ER and autophagosomes are called ER-phagy receptors (or ER-phagy adaptors). ER-phagy receptors can be either ER transmembrane proteins or soluble proteins. To date, nine transmembrane ER-phagy receptors (FAM134B, FAM134A, FAM134C, RTN3L [a long isoform of RTN3], CCPG1, SEC62, TEX264, and ATL3) and three soluble ER-phagy receptors (CALCOCO1, C53, and p62 [also known as SQSTM1]) have been identified in mammals (Fig. 2; Khaminets et al. 2015; Fumagalli et al. 2016; Grumati et al. 2017; Smith et al. 2018; An et al. 2019; Chen et al. 2019; Chino et al. 2019; Ji et al. 2019; Kohno et al. 2019; Liang et al. 2020; Nthiga et al. 2020; Smith 2020; Stefely et al. 2020; Stephani et al. 2020; Reggio et al. 2021). In addition, two membrane ER-phagy receptors (Atg39 and Atg40) in the yeast Saccharomyces cerevisiae and one soluble ER-phagy receptor (Epr1) in Schizosaccharomyces pombe were identified (Fig. 2; Mochida et al. 2015; Zhao et al. 2020). These ER-phagy receptors have several common and unique features.
Figure 2.
Structure and characteristics of endoplasmic reticulum (ER)-phagy receptors in mammals and yeast. Membrane topology and other characteristic features (e.g., the presence of the intrinsically disordered region [IDR] between the LC3-interacting region [LIR] and transmembrane domain) of mammalian and yeast ER-phagy receptors are shown. (a.a.) Amino acid, (AIM) Atg8-interacting motif, (ATZ) α1-antitrypsin Z variant, (NPC1) Niemann-Pick disease type C1, (PC) procollagen, (RHD) reticulon homology domain, (S.c.) Saccharomyces cerevisiae, (S.p.) Schizosaccharomyces pombe, (TMD) transmembrane domain, (UPR) unfolded protein response.
First, all ER-phagy receptors bind to autophagosomal ATG8 family proteins (the LC3 or GABARAP subfamilies in mammals and Atg8 in yeast) through a region termed the “LC3-interacting region” (LIR), GABARAP-interaction motif (GIM), or Atg8-interacting motif (AIM) (Johansen and Lamark 2020; Lamark and Johansen 2021). ER-phagy receptors must possess at least one of these regions for the recognition of ER fragments by autophagosomal membranes (Fig. 2; Chino and Mizushima 2020; Hübner and Dikic 2020; Wilkinson 2020; Molinari 2021). In addition, CALCOCO1 contains a ubiquitin-interacting motif (UIM)-like sequence, which interacts with the UIM-docking site of GABARAP (Nthiga et al. 2020). In general, the binding affinity of the LIR can be regulated by the phosphorylation of serine and threonine residues upstream of the core LIR sequence. In fact, phosphorylation of the upstream serine residues in the LIR of TEX264 by casein kinase 2 strengthens its affinity to ATG8 family proteins (Chino et al. 2022). However, even in the absence of membrane-bound LC3-II (in cells lacking ATG conjugation system components such as ATG5 and ATG3), the ER can still make contact with the outer and inner autophagosomal membranes, suggesting that ATG8 binding is not the only mechanism for the association of ER with autophagosomes (Sou et al. 2008; Kishi-Itakura et al. 2014). An alternate mechanism could involve interactions with ER-phagy receptors and autophagy-initiation complex components, such as FIP200, ULK1, and ATG13 in mammals and Atg11 in yeast. For example, CCPG1 has two FIP200-interacting regions (Smith et al. 2018), which are activated by phosphorylation (Zhou et al. 2021). TEX264 and C53 also interact with initiation complex components, including FIP200 (An et al. 2019; Stephani et al., 2020). ATL3 (and ATL2) interacts directly with ULK1 and ATG13 and facilitates the assembly of the ULK1 complex on the ER during autophagosome formation (Liu et al. 2021). Yeast Atg39 interacts with Atg11 through the Atg11-binding region (Mochida et al. 2015). The interactions of ER with the initiation complex could be important for the active induction of autophagy (i.e., cargo-driven autophagy), whereas the interactions of ER with ATG8 family proteins are important for recognition of a downstream step. This is also the case for the selective autophagy of other cargos such as mitochondria, intracellular bacteria, p62 condensates, and yeast Cvt substrates (Kamber et al. 2015; Torggler et al. 2016; Ravenhill et al. 2019; Turco et al. 2019; Vargas et al. 2019). Furthermore, there are some additional molecular interactions between the ER and autophagosomes. For example, the ER membrane proteins VAPA and VAPB, which are involved in membrane contact between various organelles, interact with FIP200, ULK1, and WIPI2 on autophagic membranes (Zhao et al. 2018).
Second, ER-phagy receptors can be membrane or soluble proteins. Membrane ER-phagy receptors have one or multiple domains that are embedded in a membrane (Fig. 2). Receptors with one transmembrane domain such as CCPG1 and TEX264 are thought to serve as simple receptors or sensors of the luminal milieu, whereas those with reticulon homology domains (RHDs), which consist of two short hairpin-like membrane domains (i.e., FAM134 family proteins, RTN3L, and Atg40), have ER-fragmentation activity. The RHDs generate membrane curvature by partially inserting into, but not fully penetrating, the membrane (Zurek et al. 2011). For example, overexpression of FAM134B and RTN3L or multimerization of RTN3L induces ER fragmentation (Grumati et al. 2017). FAM134B contains not only RHDs but also two amphipathic helices, which also induce membrane curvature (Brady et al. 2015; Bhaskara et al. 2019). The Atg8-mediated superassembly of Atg40 also generates highly curved ER regions depending on its RHD (Mochida et al. 2020). Thus, it would be purposeful that ER-phagy receptors mediate ER fragmentation and ER transport functions during ER-phagy. Soluble ER-phagy receptors can localize to the ER through an interaction with ER membrane proteins (Fig. 2). For example, p62 is recruited to ER membranes through its interaction with ubiquitinated TRIM13. Fission yeast Epr1 and its mammalian homolog CALCOCO1 localize to the ER through an interaction with Scs2/Scs22 and VAPA/VAPB, respectively (Nthiga et al. 2020, 2021; Stefely et al. 2020; Zhao et al. 2020). C53 is recruited to the ER by binding to a complex consisting of the UFMylation ligase UFL1 and its membrane adaptor DDRGK1 (Stephani et al. 2020). This C53-UFL1-DDRGK1 axis seems to be conserved in mammals.
Third, the majority of ER-phagy receptors have a long intrinsically disordered region (IDR) (Fig. 2). The ER and autophagosomal membranes are more than 20 nm apart because ER-associated ribosomes are located between them. The IDR, which is flexible and does not fold into a compact structure, is present between the transmembrane domain and LIR (or AIM) and thus can bridge the >20 nm gap between both membranes. This function simply depends on its length rather than a specific amino acid sequence (Chino et al. 2019). The IDR may have another function; that is, IDR-containing ER-phagy receptors might induce molecular crowding at the site facing autophagosomes, leading to steric hindrance, which would lead to the generation of curvature and eventual membrane scission (Busch et al. 2015; Snead et al. 2017).
Why do so many ER-phagy receptors exist, particularly in mammals? These receptors may at least partially function redundantly (Chino et al. 2019). For example, TEX264, FAM134B, and CCPG1 cooperatively contribute to a large fraction of basal and starvation-induced ER-phagy (Chino et al. 2019), yet these receptors also have different functions (Fig. 3). The ER consists of various domains such as the nuclear envelope, ER sheets, and ER tubules, which exert different functions (Voeltz et al. 2002). Different regions of the ER appear to be targeted by different receptors. For example, the two ER-phagy receptors in S. cerevisiae, Atg39 and Atg40, localize to and degrade the perinuclear and cortical ER, respectively (Mochida et al. 2015). Likewise, FAM134B, TEX264, CCPG1, and SEC62 primarily mediate the degradation of ER sheets, while RTN3L, ATL3, and CALCOCO1 mediate the degradation of ER tubules (Khaminets et al. 2015; Grumati et al. 2017; Chen et al. 2019; Nthiga et al. 2020). FAM134A and FAM134C are present throughout the ER network (Reggio et al. 2021).
Figure 3.
Various models of endoplasmic reticulum (ER)-phagy. Models of macro-ER-phagy and micro-ER-phagy in yeast and mammalian cells are depicted. Structures, ER-phagy receptors, and related factors and proteins to be degraded are shown. Please see the text for details of each process. (ERPHS) ER-phagy site, (ERES) ER exit sites.
Different ER-phagy receptors can be used depending on the type of ER-phagy-inducing conditions. Yeast Atg39 and Atg40 are up-regulated during nutrient starvation or rapamycin treatment (Mochida et al. 2015) and ER stress (Mizuno et al. 2020; Mizuno and Irie 2021), whereas Atg40 is also induced in mid- to late meiosis to degrade cortical ER for programmed changes in ER morphology (Otto et al. 2021). Atg39 and Atg40 are required for starvation-induced ER-phagy, but they are not needed for constitutive ER-phagy (Lipatova et al. 2020). In mammals, starvation-induced ER-phagy is mediated by FAM134B, RTN3L, ATL3, TEX264, and CALCOCO1 (Khaminets et al. 2015; Grumati et al. 2017; An et al. 2019; Chen et al. 2019; Chino et al. 2019; Nthiga et al. 2020), whereas CCPG1 and SEC62 mediate ER-phagy during ER stress and the recovery phase of ER stress, respectively (Fumagalli et al. 2016; Smith et al. 2018). p62 plays a role in the elimination of excess ER induced by xenobiotics in the liver (Yang et al. 2016). More recently, p62 was shown to be involved in ER-phagy under basal and ER stress conditions, which is mediated by binding to ubiquitinated TRIM13 (an ER membrane E3 ligase) as an N-degron substrate (Ji et al. 2019). Epr1, a fission yeast ER-phagy receptor, is also up-regulated and required for ER-phagy under ER stress conditions (Zhao et al. 2020). In addition, under ER-stress conditions, activated CAMK2B phosphorylates FAM134B, which enhances FAM134B oligomerization and ER-phagy (Jiang et al. 2020). STING, a DNA-sensing innate immune signaling molecule, contains three LIRs and may also function like an ER-phagy receptor (Moretti et al. 2017; Moretti and Blander 2018; Liu et al. 2019; Fischer et al. 2020; Smith 2020).
Finally, these ER-phagy receptors are differentially expressed among tissues. TEX264, SEC62, and ATL3 are expressed in nearly all tissues (Rismanchi et al. 2008; Chino et al. 2019; Molinari 2021). FAM134C is also ubiquitously expressed, with the exception of the pancreas where FAM134B is highly expressed, and in the brain and testis where FAM134A is highly expressed (GTEx Consortium 2015; Wang et al. 2019). FAM134B-2, an amino-terminal-truncated isoform of FAM134B, is abundant in the liver, kidney, spleen, and white adipose tissue (Kohno et al. 2019; Keles et al. 2020). CCPG1 is expressed predominantly in the pancreas, kidney, and liver (Smith et al. 2018; Chino et al. 2019), which is consistent with the finding that systemic CCPG1 hypomorphic mice accumulate abnormal protein aggregates in the pancreas (see below).
PHYSIOLOGICAL ROLES OF MACRO-ER-PHAGY
General Principle
There is some evidence indicating that ER-phagy controls ER levels. ER-phagy is induced after treatment with drugs such as phenobarbital and is hypothesized to play a role in the elimination of excess ER (Bolender and Weibel 1973; Feldman et al. 1980). Abnormally concentric ER membranes accumulate in autophagy-deficient hepatocytes (Komatsu et al. 2005). Plasma cell–specific and pancreatic acinar cell–specific autophagy-deficient mice show ER expansion (Pengo et al. 2013; Antonucci et al. 2015). In addition, ER expansion and the accumulation of ER proteins are observed in autophagy-deficient T lymphocytes (Jia et al. 2011). These data suggest that autophagy is important for the control of ER quantity.
The phenotypes of humans and mice lacking ER-phagy receptors have also provided insights into the role of ER-phagy in vivo. Patients with hereditary sensory and autonomic neuropathy (HSAN) type 2 have loss-of-function mutations in FAM134B (Kurth et al. 2009). Consistently, Fam134b-deficient mice demonstrate ER expansion and degeneration of sensory neurons (Khaminets et al. 2015). Mutations in ATL3 (Y192C [in the first LIR] and P338R) result in the loss of binding to GABARAP and cause HSAN type 1 (Chen et al. 2019; Xu et al. 2019). CCPG1 hypomorphic mice develop ER protein aggregation and hyperactivation of the unfolded protein response in pancreatic acinar cells (Smith et al. 2018). Furthermore, in yeast, epr1Δ cells show reduced viability compared to wild-type, indicating that Epr1-mediated ER-phagy is crucial for cell survival against ER stress (Zhao et al. 2020). These findings suggest that ER-phagy is also important for ER quality control.
Considering the redundancy among ER-phagy receptors, the physiological significance of ER-phagy might have been underestimated in single ER-phagy receptor-knockout mice. It should also be noted that some ER-phagy receptors may have ER-phagy-independent functions. For example, TEX264 also localizes to the nuclear periphery and plays a crucial role in DNA repair (Fielden et al. 2020). To prove that the phenotypes observed in these knockout mice and human patients are indeed due to a defect in ER-phagy, it is essential to investigate animal models specifically lacking ER-phagy function.
Elimination of Misfolded Proteins
Misfolded proteins inside the ER are typically degraded by ERAD (Ruggiano et al. 2014). However, tightly aggregated proteins cannot be unfolded to be subjected to the ERAD pathway (Wu and Rapoport 2018). ER subdomains containing these abnormal proteins can instead be degraded by ER-phagy. One of the most well-studied substrates of ER-phagy is procollagen, a major component of the extracellular matrix. Procollagen is generated in the ER by the assembly of two Proα1 chains and one Proα2 chain (Gomez-Navarro and Miller 2016); however, due to the complicated folding process, about 15% of newly synthesized procollagen is degraded (Bienkowski and Gotkin 1995). During this process, mutant procollagen, which fails to form trimers, is degraded by ERAD, whereas misfolded procollagen that trimerizes and subsequently aggregates in the ER is degraded by ER-phagy (Ishida et al. 2009). Procollagen degradation is mediated by both macro- and micro-ER-phagy (see below). In macro-ER-phagy, the ER-resident lectin chaperone calnexin recognizes the N-glycans of luminal misfolded procollagen and also interacts with FAM134 to deliver misfolded procollagen to lysosomes (Fig. 3; Forrester et al. 2019; Fregno et al. 2021; Reggio et al. 2021). Notably, FAM134A mediates procollagen degradation in an LIR-independent manner, whereas FAM134B and FAM134C do so in an LIR-dependent manner. During the development of mice and fish, ER-phagy and FAM134B expression are induced in chondrocytes, and type II procollagen accumulates in chondrocytes if Fam134b is depleted in fish (Cinque et al. 2020). A disease-causing mutant of Niemann–Pick disease type C1 protein, (NPC1I106T), is also degraded by FAM134B-dependent ER-phagy (Schultz et al. 2018).
An aggregate-prone mutant (E342K) of α1-antitrypsin Z variant (ATZ) was reported to be degraded by autophagy. Two distinct pathways, macro-ER-phagy and a vesicular transport pathway, were reported. ATZ degradation by macro-ER-phagy involves p62 and polyubiquitinated TRIM13 (Fig. 4; Ji et al. 2019). In the second pathway, ER-derived single-membrane vesicles containing ATZ fuse with lysosomes for degradation (Fig. 4; Fregno et al. 2018; Fregno and Molinari 2019). Fusion with endolysosomes depends on the SNAREs syntaxin 17 and VAMP8. This pathway also depends on the formation of a calnexin-FAM134B-LC3 complex, but not FIP200, ULK1/2, ATG13, and ATG9. Monoglucosylated oligosaccharides on misfolded polypeptides generated by the removal of the two outermost glucose residues are required for calnexin engagement (Fregno et al. 2021). During this process, UGGT1, which catalyzes the reglucosylation of deglucosylated nonnative proteins, is required for the lysosomal delivery of ATZ, suggesting that a deglucosylation and reglucosylation cycle is required.
Figure 4.
Two distinct endoplasmic reticulum (ER)-phagy pathways for ATZ degradation. p62-dependent macro-ER-phagy and FAM134B-calnexin-mediated vesicular transport pathways for ATZ degradation are depicted.
ER-phagy degrades several insoluble aggregate-prone mutant prohormones, including the Akita mutant of proinsulin, which causes early-onset diabetes, the pro-opiomelanocortin (POMC) C28F mutant, which causes early-onset obesity, and the pro-arginine-vasopressin G57S mutant, which causes the autosomal-dominant neurogenerative disorder neurohypophyseal diabetes insipidus (Kim et al. 2018; Cunningham et al. 2019; Spiess et al. 2020). Although RTN3L does not possess an intraluminal domain, two mechanisms enable the RTN3L-dependent selective degradation of prohormones. One is mediated by the ER membrane protein PGRMC1, a binding partner of RTN3L (Chen et al. 2021). PGRMC1 has a long luminal domain that interacts with low molecular forms of mutant prohormones, such as proinsulin and POMC (Chen et al. 2021). Another mechanism was proposed for the RTN3L-mediated selective degradation of higher-molecular-weight misfolded cargos such as Akita mutant proinsulin. The Akita mutant forms large aggregates inside the ER in autophagy-deficient and RTN3-depleted cells (Cunningham et al. 2019). The formation of these large macromolecular aggregates may change the biophysical properties of the ER membrane and recruit RTN3 to the site of autophagosome formation (Chen et al. 2020b). SEC62, CCPG1, and FAM134B-2 are also involved in the degradation of unfolded protein response–induced chaperone-containing ER subdomains, insoluble secretory enzymes in pancreatic acinar cells, and secretory proteins in hepatocytes, respectively (Fumagalli et al. 2016; Smith et al. 2018; Kohno et al. 2019).
However, it remains largely unclear how ER-phagy receptors selectively recognize intraluminal proteins besides the aforementioned mechanisms. One possible mechanism is the formation of ER subdomains where misfolded proteins are enriched and subjected to ER-phagy en bloc. In fact, misfolded procollagen accumulates at sites where secretory cargo leave the ER. There, ER exit sites (ERES) are selectively degraded by micro-ER-phagy (Omari et al. 2018). It has also been reported that a mutant form of the arginine vasopressin precursor accumulates at a specific compartment in the ER that is surrounded by LC3-positive membranes (Hagiwara et al. 2014; Miyata et al. 2020). Therefore, these subdomains may serve as entry points for unwanted secretory cargos into autophagosomes. Moreover, the ERES may be suitable sites to drive ER-phagy because ERES are also considered to be important for autophagosome formation itself (Graef et al. 2013; Wang et al. 2014; Ge et al. 2017; Shima et al. 2019).
The coat protein complex II (COPII) cargo adaptor complex provides another ER domain termed ER-phagy site (ERPHS), which is the site where ER is packaged into autophagosomes during ER-phagy (Cui et al. 2019). In yeast, the Lst1 (also called Sfb3)–Sec23 complex binds to Atg40 and sorts their cargos into autophagosomes, preventing the accumulation of aggregate-prone proteins inside the ER (Cui et al. 2019). The formation of ERPHS requires the ER junction formation factor, Lnp1 (Chen et al. 2015, 2018), Atg40, and Vps13, a lipid transfer protein (Chen et al. 2020a). More recently, it was shown that contact between the cortical ER and endocytic pits is also required for Atg40-dependent ER-phagy (Liu et al. 2022). Contact is mediated by Scs2/22, the oxysterol-binding proteins Osh2/3, and the type I myosins Myo3/5.
The unconventional role of Lst1 in ER-phagy is conserved in mammals. The mammalian Lst1 homolog SEC24C interacts with RTN3L to form ERPHS (Grumati et al. 2017; Parashar et al. 2021). At the ERPHS, mutant prohormones such as proinsulin (Akita), G57S Pro-AVP, and C28F POMC form small liquid-like condensates, recruiting RTN3L and SEC24C to package ER fragments into autophagosomes (Cui et al. 2019; Parashar et al. 2021). These findings, together with the fact that Atg40 and RTN3 are present in ER tubules, suggests that RTN3L, rather than FAM134B, is the mammalian counterpart of yeast Atg40 (Parashar et al. 2021).
Where misfolded proteins accumulate in the ER depends on the type of cargo. Dominant-interfering prohormones such as proinsulin (Akita), G57S Pro-AVP, and C28F POMC accumulate and form liquid-like condensates in ER tubules, which are degraded in a RTN3L-SEC24C-dependent manner (Parashar et al. 2021). On the other hand, type I procollagen aggregates inside ER sheets, which are degraded in a manner dependent on FAM134B, but not RTN3L and SEC24C (Parashar et al. 2021). Because the internal space of ER tubules is narrow compared with sheets, sequestering fluid-like cargos in ER tubules could prevent the expansion of the condensates and keep their size small, so they can be engulfed by autophagosomes (Parashar and Ferro-Novick 2022).
Selective Removal of Stalled Nascent Polypeptides
Secretary proteins are cotranslationally or post-translationally translocated across the ER membrane through the SEC61 translocon (Rapoport et al. 2017). During this process, some nascent chains fail to translocate and remain stuck in the translocon machinery (Fregno and Molinari 2019). Post-translationally translocating proteins are more frequently stuck because they are prematurely folded before translocation is completed (Fregno and Molinari 2019). In this situation, the ER-associated zinc metalloprotease Ste24/ZMPSTE24 removes the polypeptide chain from the translocon (Ast et al. 2016). However, the translocon can be blocked by stalled ribosomes translating faulty mRNAs during cotranslational translocation (Walczak et al. 2019; Liang et al. 2020; Wang et al. 2020). As mentioned above, DDRGK1-UFL1-mediated UFMylation of the ribosomal protein RPL26 removes stalled ribosomes from translocons (Stephani et al. 2020). This modification recruits the ER-phagy receptor C53, which is also a UFMylation adaptor, to translocons and facilitates the lysosomal degradation of arrested products (Walczak et al. 2019; Liang et al. 2020; Stephani et al. 2020; Wang et al. 2020).
Degradation of the Nuclear Membrane
Because the nuclear membrane is connected to the ER, the proteasome-dependent and lysosome-dependent pathways can eliminate undesirable nuclear components similar to ER-phagy. Indeed, the ER-phagy machinery is involved in the autophagic degradation of part of the nucleus (Mijaljica and Devenish 2013). The degradation of nuclear components in yeast was first reported as piecemeal microautophagy (see below for more details) under nutrient-rich and short-term nitrogen starvation conditions (Fig. 3; Papandreou and Tavernarakis 2019). In contrast, after prolonged nitrogen starvation (18–24 h), macroautophagy of the nuclear membranes (termed “macronucleophagy”) is induced (Fig. 3; Mochida et al. 2015). Atg39 has a long carboxy-terminal domain that associates with the inner nuclear membrane using its amphipathic helices to drive budding of nuclear membrane–derived double-membrane vesicles (Chandra et al. 2021; Mochida et al. 2022). These mechanisms enable the degradation of the outer and inner nuclear membranes and nucleolar proteins (Mochida et al. 2015). Nucleophagy is important for nuclear shaping and viability in yeast.
The nuclear pore complex is huge machinery consisting of hundreds of proteins and can be turned over by autophagy (Lee et al. 2020; Tomioka et al. 2020). In this case, nuclear envelope–embedded nuclear pore complexes are engulfed by autophagosomes through binding between Atg8 and Nup159. Nup159 has an AIM and serves as a receptor (Lee et al. 2020). Alternatively, only Nup159 proteins or Nup159-containing subcomplex could be enclosed by autophagosomes (termed nucleoporinophagy) (Tomioka et al. 2020). These pathways are dependent on Atg11, but not or only partially on Atg39.
In mammals, macronucleophagy has been reported in relation to cancers and neurodegenerative disorders (Park et al. 2009). RAS activation triggers nuclear membrane blebbing and autophagic degradation of lamin B1 and heterochromatin (Dou et al. 2015). The interaction between lamin B1 and ATG8 family proteins mediates the degradation of nuclear lamin B1 (Dou et al. 2015). Inhibition of the autophagic degradation of lamin B1 delays oncogene-induced senescence. Macronucleophagy is also observed in laminopathies causing muscular dystrophy and premature aging syndrome (Park et al. 2009). Damaged portions of the nucleus and extruded nuclear components are degraded. Nucleophagy is thought to be important for the turnover of part of the nucleus and rapid repair of the nuclear membrane.
MICRO-ER-PHAGY
In some cases, the ER can be degraded by the microautophagy pathway. Although macroautophagy depends on evolutionarily conserved core ATG factors (ATG1, 2, 3, 4, 5, 6, 7, 8, 9, 10, (11), 12, 13, 14, 16, and 18), the ATG-dependency of microautophagy is variable and complicated (Oku and Sakai 2018; Schuck 2020). The first observation of microautophagy was piecemeal micronucleophagy (Fig. 3; Roberts et al. 2003; Kvam and Goldfarb 2007). Carbon and nitrogen starvation increase nucleus–vacuole junctions through interactions between Vac8 on the vacuolar membrane and Nvj1 in the nuclear envelope and promotes sequestration of part of the nucleus into the vacuole by membrane invagination (Roberts et al. 2003; Luo et al. 2016). A nonessential portion of the nucleus is degraded via this process. During piecemeal micronucleophagy, the core ATG genes are not required for the formation of invaginated blebs but are required for complete enclosure by the vacuolar membrane (Krick et al. 2008). This may be similar to micropexophagy, in which ATG genes are required for the formation of a sealing membrane termed the “micropexophagy-specific membrane apparatus (MIPA)” (Mukaiyama et al. 2004; Oku and Sakai 2018).
In addition to the nucleus, the ER itself is also degraded by microautophagy in yeast. Yeast cells under ER stress conditions form large ER whorls (Bernales et al. 2006, 2007). These stress-induced ER whorls are subjected to microautophagy rather than macroautophagy (Fig. 3; Schuck et al. 2014). This process does not require core autophagy factors such as Atg1, Atg6, Atg7, Atg8, Atg14, and Atg16 nor does it require the macro-ER-phagy receptors Atg39 and Atg40, but it does require the Nem1-Spo7 phosphatase complex and the ESCRT machinery for scission of the vacuolar membrane (Schuck et al. 2014; Schäfer et al. 2020). Because no cytoplasm is included, the engulfment of ER whorls is highly selective. However, the mechanisms behind the selective degradation of ER whorls remain unknown.
In mammals, piecemeal micro-ER-phagy is observed during recovery from ER stress induced by cyclopiazonic acid, a reversible inhibitor of Ca2+-ATPase (Fumagalli et al. 2016; Loi et al. 2019). In this case, ER fragments are engulfed directly by endolysosomes (Fig. 3; Loi et al. 2019). Micro-ER-phagy depends on SEC62, ATG8-conjugation-system components such as ATG4B, ATG7, and ATG16L1, as well as ESCRT-III components but not autophagy initiation factors such as ULK1, ULK2, ATG13, and ATG14 or SNARE proteins such as STX17 and VAMP8, which are required for autophagosome–lysosome fusion (Loi et al. 2019). ER subdomains containing specific misfolded ERAD-resistant proteins can also be degraded by micro-ER-phagy (Omari et al. 2018). Misfolded procollagen is degraded by macro-ER-phagy, as mentioned above, but it is also degraded by micro-ER-phagy. Procollagen-containing ERESs recruit some of the core ATG proteins (e.g., LC3, ATG14, and ATG9), p62, and ubiquitin and induce micro-ER-phagy (Fig. 3; Omari et al. 2018). The roles of these ATG proteins in this process remain elusive.
CONCLUDING REMARKS
Organelle degradation is a hot topic in autophagy research. Because the ER is a multifunctional organelle, much attention has been paid to understanding the mechanisms and function of ER-phagy. Although there has been significant progress in the last decade, many important issues remain. First, how the ER-phagy machinery recognizes intraluminal proteins is largely unknown, except for a few examples introduced in this review. A more general mechanism may exist. Second, the physiological significance of ER-phagy is also almost unknown at the organismal level. As discussed in this review, simple deletion of ER-phagy receptors is not an ideal approach to reveal their ER-phagy-dependent function. It is important to know how ER-phagy turnover of the ER impacts cell and tissue homeostasis in physiological and pathological conditions. Third, monitoring ER-phagy activity is crucial, but the available methods are still limited (Chino and Mizushima 2020). The development of more sensitive and quantitative methods is essential. Finally, there may be additional mechanisms that contribute to ER degradation other than ER-phagy. One apparent example occurs in the lens. All organelles, including the ER, are degraded during the differentiation of lens fiber cells to make the lens transparent. This is executed by the cytosolic phospholipase PLAAT (phospholipase A and acyltransferase) (Morishita et al. 2021). It is currently unknown whether this system works in non-lens cells. There may still be unknown pathways for organelle degradation.
ACKNOWLEDGMENTS
This work was supported by Exploratory Research for Advanced Technology (ERATO) (No. JPMJER1702) from the Japan Science and Technology Agency.
Footnotes
Editors: Susan Ferro-Novick, Tom A. Rapoport, and Randy Schekman
Additional Perspectives on The Endoplasmic Reticulum available at www.cshperspectives.org
REFERENCES
- An H, Ordureau A, Paulo JA, Shoemaker CJ, Denic V, Harper JW. 2019. TEX264 is an endoplasmic reticulum-resident ATG8-interacting protein critical for ER remodeling during nutrient stress. Mol Cell 74: 891–908.e810. 10.1016/j.molcel.2019.03.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonucci L, Fagman JB, Kim JY, Todoric J, Gukovsky I, Mackey M, Ellisman MH, Karin M. 2015. Basal autophagy maintains pancreatic acinar cell homeostasis and protein synthesis and prevents ER stress. Proc Natl Acad Sci 112: E6166–E6174. 10.1073/pnas.1519384112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ast T, Michaelis S, Schuldiner M. 2016. The protease Ste24 clears clogged translocons. Cell 164: 103–114. 10.1016/j.cell.2015.11.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axe EL, Walker SA, Manifava M, Chandra P, Roderick HL, Habermann A, Griffiths G, Ktistakis NT. 2008. Autophagosome formation from membrane compartments enriched in phosphatidylinositol 3-phosphate and dynamically connected to the endoplasmic reticulum. J Cell Biol 182: 685–701. 10.1083/jcb.200803137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernales S, McDonald KL, Walter P. 2006. Autophagy counterbalances endoplasmic reticulum expansion during the unfolded protein response. PLoS Biol 4: e423. 10.1371/journal.pbio.0040423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernales S, Schuck S, Walter P. 2007. ER-phagy: selective autophagy of the endoplasmic reticulum. Autophagy 3: 285–287. 10.4161/auto.3930 [DOI] [PubMed] [Google Scholar]
- Bhaskara RM, Grumati P, Garcia-Pardo J, Kalayil S, Covarrubias-Pinto A, Chen W, Kudryashev M, Dikic I, Hummer G. 2019. Curvature induction and membrane remodeling by FAM134B reticulon homology domain assist selective ER-phagy. Nat Commun 10: 2370. 10.1038/s41467-019-10345-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienkowski RS, Gotkin MG. 1995. Control of collagen deposition in mammalian lung. Proc Soc Exp Biol Med 209: 118–140. 10.3181/00379727-209-43886a [DOI] [PubMed] [Google Scholar]
- Bolender RP, Weibel ER. 1973. A morphometric study of the removal of phenobarbital-induced membranes from hepatocytes after cessation of treatment. J Cell Biol 56: 746–761. 10.1083/jcb.56.3.746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady JP, Claridge JK, Smith PG, Schnell JR. 2015. A conserved amphipathic helix is required for membrane tubule formation by Yop1p. Proc Natl Acad Sci 112: E639–E648. 10.1073/pnas.1415882112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch DJ, Houser JR, Hayden CC, Sherman MB, Lafer EM, Stachowiak JC. 2015. Intrinsically disordered proteins drive membrane curvature. Nat Commun 6: 7875. 10.1038/ncomms8875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra S, Mannino PJ, Thaller DJ, Ader NR, King MC, Melia TJ, Lusk CP. 2021. Atg39 selectively captures inner nuclear membrane into lumenal vesicles for delivery to the autophagosome. J Cell Biol 220: e202103030. 10.1083/jcb.202103030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Desai T, McNew JA, Gerard P, Novick PJ, Ferro-Novick S. 2015. Lunapark stabilizes nascent three-way junctions in the endoplasmic reticulum. Proc Natl Acad Sci 112: 418–423. 10.1073/pnas.1423026112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Cui Y, Parashar S, Novick PJ, Ferro-Novick S. 2018. ER-phagy requires Lnp1, a protein that stabilizes rearrangements of the ER network. Proc Natl Acad Sci 115: e6237–e6244. 10.1073/pnas.1707984115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Xiao Y, Chai P, Zheng P, Teng J, Chen J. 2019. ATL3 is a tubular ER-phagy receptor for GABARAP-mediated selective autophagy. Curr Biol 29: 846–855.e846. 10.1016/j.cub.2019.01.041 [DOI] [PubMed] [Google Scholar]
- Chen S, Mari M, Parashar S, Liu D, Cui Y, Reggiori F, Novick PJ, Ferro-Novick S. 2020a. Vps13 is required for the packaging of the ER into autophagosomes during ER-phagy. Proc Natl Acad Sci 117: 18530–18539. 10.1073/pnas.2008923117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YJ, Williams JM, Arvan P, Tsai B. 2020b. Reticulon protects the integrity of the ER membrane during ER escape of large macromolecular protein complexes. J Cell Biol 219: e201908182. 10.1083/jcb.201908182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YJ, Knupp J, Arunagiri A, Haataja L, Arvan P, Tsai B. 2021. PGRMC1 acts as a size-selective cargo receptor to drive ER-phagic clearance of mutant prohormones. Nat Commun 12: 5991. 10.1038/s41467-021-26225-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chino H, Mizushima N. 2020. ER-phagy: quality control and turnover of endoplasmic reticulum. Trends Cell Biol 30: 384–398. 10.1016/j.tcb.2020.02.001 [DOI] [PubMed] [Google Scholar]
- Chino H, Hatta T, Natsume T, Mizushima N. 2019. Intrinsically disordered protein TEX264 mediates ER-phagy. Mol Cell 74: 909–921.e906. 10.1016/j.molcel.2019.03.033 [DOI] [PubMed] [Google Scholar]
- Chino H, Yamasaki A, Ode KL, Ueda HR, Noda NN, Mizushima N. 2022. Phosphorylation by casein kinase 2 enhances the interaction between ER-phagy receptor TEX264 and ATG8 proteins. EMBO Rep 23: e54801. 10.15252/embr.202254801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinque L, De Leonibus C, Iavazzo M, Krahmer N, Intartaglia D, Salierno FG, De Cegli R, Di Malta C, Svelto M, Lanzara C, et al. 2020. Mit/TFE factors control ER-phagy via transcriptional regulation of FAM134B. EMBO J 39: e105696. 10.15252/embj.2020105696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y, Parashar S, Zahoor M, Needham PG, Mari M, Zhu M, Chen S, Ho HC, Reggiori F, Farhan H, et al. 2019. A COPII subunit acts with an autophagy receptor to target endoplasmic reticulum for degradation. Science 365: 53–60. 10.1126/science.aau9263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham CN, Williams JM, Knupp J, Arunagiri A, Arvan P, Tsai B. 2019. Cells deploy a two-pronged strategy to rectify misfolded proinsulin aggregates. Mol Cell 75: 442–456.e444. 10.1016/j.molcel.2019.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Leonibus C, Cinque L, Settembre C. 2019. Emerging lysosomal pathways for quality control at the endoplasmic reticulum. FEBS Lett 593: 2319–2329. 10.1002/1873-3468.13571 [DOI] [PubMed] [Google Scholar]
- Dou Z, Xu C, Donahue G, Shimi T, Pan JA, Zhu J, Ivanov A, Capell BC, Drake AM, Shah PP, et al. 2015. Autophagy mediates degradation of nuclear lamina. Nature 527: 105–109. 10.1038/nature15548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskelinen EL. 2008. Fine structure of the autophagosome. Methods Mol Biol 445: 11–28. 10.1007/978-1-59745-157-4_2 [DOI] [PubMed] [Google Scholar]
- Farre JC, Subramani S. 2016. Mechanistic insights into selective autophagy pathways: lessons from yeast. Nat Rev Mol Cell Biol 17: 537–552. 10.1038/nrm.2016.74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman D, Swarm RL, Becker J. 1980. Elimination of excess smooth endoplasmic reticulum after phenobarbital administration. J Histochem Cytochem 28: 997–1006. 10.1177/28.9.7410819 [DOI] [PubMed] [Google Scholar]
- Ferro-Novick S, Reggiori F, Brodsky JL. 2021. ER-phagy, ER homeostasis, and ER quality control: implications for disease. Trends Biochem 46: 630–639. 10.1016/j.tibs.2020.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fielden J, Wiseman K, Torrecilla I, Li S, Hume S, Chiang SC, Ruggiano A, Narayan Singh A, Freire R, Hassanieh S, et al. 2020. TEX264 coordinates p97- and SPRTN-mediated resolution of topoisomerase 1-DNA adducts. Nat Commun 11: 1274. 10.1038/s41467-020-15000-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer TD, Wang C, Padman BS, Lazarou M, Youle RJ. 2020. STING induces LC3B lipidation onto single-membrane vesicles via the V-ATPase and ATG16L1-WD40 domain. J Cell Biol 219: e202009128. 10.1083/jcb.202009128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester A, De Leonibus C, Grumati P, Fasana E, Piemontese M, Staiano L, Fregno I, Raimondi A, Marazza A, Bruno G, et al. 2019. A selective ER-phagy exerts procollagen quality control via a calnexin-FAM134B complex. EMBO J 38: e99847. 10.15252/embj.201899847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fregno I, Molinari M. 2019. Proteasomal and lysosomal clearance of faulty secretory proteins: ER-associated degradation (ERAD) and ER-to-lysosome-associated degradation (ERLAD) pathways. Crit Rev Biochem Mol Biol 54: 153–163. 10.1080/10409238.2019.1610351 [DOI] [PubMed] [Google Scholar]
- Fregno I, Fasana E, Bergmann TJ, Raimondi A, Loi M, Solda T, Galli C, D'Antuono R, Morone D, Danieli A, et al. 2018. ER-to-lysosome-associated degradation of proteasome-resistant ATZ polymers occurs via receptor-mediated vesicular transport. EMBO J 37: e99259. 10.15252/embj.201899259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fregno I, Fasana E, Soldà T, Galli C, Molinari M. 2021. N-glycan processing selects ERAD-resistant misfolded proteins for ER-to-lysosome-associated degradation. EMBO J 40: e107240. 10.15252/embj.2020107240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fumagalli F, Noack J, Bergmann TJ, Cebollero E, Pisoni GB, Fasana E, Fregno I, Galli C, Loi M, Solda T, et al. 2016. Translocon component Sec62 acts in endoplasmic reticulum turnover during stress recovery. Nat Cell Biol 18: 1173–1184. 10.1038/ncb3423 [DOI] [PubMed] [Google Scholar]
- Ge L, Zhang M, Kenny SJ, Liu D, Maeda M, Saito K, Mathur A, Xu K, Schekman R. 2017. Remodeling of ER-exit sites initiates a membrane supply pathway for autophagosome biogenesis. EMBO Rep 18: 1586–1603. 10.15252/embr.201744559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Navarro N, Miller E. 2016. Protein sorting at the ER–Golgi interface. J Cell Biol 215: 769–778. 10.1083/jcb.201610031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graef M, Friedman JR, Graham C, Babu M, Nunnari J. 2013. ER exit sites are physical and functional core autophagosome biogenesis components. Mol Biol Cell 24: 2918–2931. 10.1091/mbc.e13-07-0381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grumati P, Morozzi G, Holper S, Mari M, Harwardt MI, Yan R, Muller S, Reggiori F, Heilemann M, Dikic I. 2017. Full length RTN3 regulates turnover of tubular endoplasmic reticulum via selective autophagy. eLife 6: e25555. 10.7554/eLife.25555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- GTEx Consortium. 2015. Human genomics. The genotype-tissue expression (GTEx) pilot analysis: multitissue gene regulation in humans. Science 348: 648–660. 10.1126/science.1262110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara D, Arima H, Morishita Y, Wenjun L, Azuma Y, Ito Y, Suga H, Goto M, Banno R, Sugimura Y, et al. 2014. Arginine vasopressin neuronal loss results from autophagy-associated cell death in a mouse model for familial neurohypophysial diabetes insipidus. Cell Death Dis 5: e1148. 10.1038/cddis.2014.124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi-Nishino M, Fujita N, Noda T, Yamaguchi A, Yoshimori T, Yamamoto A. 2009. A subdomain of the endoplasmic reticulum forms a cradle for autophagosome formation. Nat Cell Biol 11: 1433–1437. 10.1038/ncb1991 [DOI] [PubMed] [Google Scholar]
- Hübner CA, Dikic I. 2020. ER-phagy and human diseases. Cell Death Differ 27: 833–842. 10.1038/s41418-019-0444-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida Y, Yamamoto A, Kitamura A, Lamandé SR, Yoshimori T, Bateman JF, Kubota H, Nagata K. 2009. Autophagic elimination of misfolded procollagen aggregates in the endoplasmic reticulum as a means of cell protection. Mol Biol Cell 20: 2744–2754. 10.1091/mbc.e08-11-1092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji CH, Kim HY, Heo AJ, Lee SH, Lee MJ, Kim SB, Srinivasrao G, Mun SR, Cha-Molstad H, Ciechanover A, et al. 2019. The N-degron pathway mediates ER-phagy. Mol Cell 75: 1058–1072.e1059. 10.1016/j.molcel.2019.06.028 [DOI] [PubMed] [Google Scholar]
- Jia W, Pua HH, Li QJ, He YW. 2011. Autophagy regulates endoplasmic reticulum homeostasis and calcium mobilization in T lymphocytes. J Immunol 186: 1564–1574. 10.4049/jimmunol.1001822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Wang X, Ding X, Du M, Li B, Weng X, Zhang J, Li L, Tian R, Zhu Q, et al. 2020. FAM134B oligomerization drives endoplasmic reticulum membrane scission for ER-phagy. EMBO J 39: e102608. 10.15252/embj.2019102608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen T, Lamark T. 2020. Selective autophagy: ATG8 family proteins, LIR motifs and cargo receptors. J Mol Biol 432: 80–103. 10.1016/j.jmb.2019.07.016 [DOI] [PubMed] [Google Scholar]
- Joshi AS, Zhang H, Prinz WA. 2017. Organelle biogenesis in the endoplasmic reticulum. Nat Cell Biol 19: 876–882. 10.1038/ncb3579 [DOI] [PubMed] [Google Scholar]
- Kamber RA, Shoemaker CJ, Denic V. 2015. Receptor-bound targets of selective autophagy use a scaffold protein to activate the Atg1 kinase. Mol Cell 59: 372–381. 10.1016/j.molcel.2015.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keles U, Iscan E, Yilmaz HE, Karakülah G, Suner A, Bal E, Tasdemir N, Cavga AD, Ekin U, Mutlu Z, et al. 2020. Differential expression of full-length and NH(2) terminally truncated FAM134B isoforms in normal physiology and cancer. Am J Physiol Gastrointest Liver Physiol 319: g733–g747. 10.1152/ajpgi.00094.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaminets A, Heinrich T, Mari M, Grumati P, Huebner AK, Akutsu M, Liebmann L, Stolz A, Nietzsche S, Koch N, et al. 2015. Regulation of endoplasmic reticulum turnover by selective autophagy. Nature 522: 354–358. 10.1038/nature14498 [DOI] [PubMed] [Google Scholar]
- Kim GH, Shi G, Somlo DR, Haataja L, Song S, Long Q, Nillni EA, Low MJ, Arvan P, Jr MM, et al. 2018. Hypothalamic ER-associated degradation regulates POMC maturation, feeding, and age-associated obesity. J Clin Invest 128: 1125–1140. 10.1172/JCI96420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkin V, Rogov VV. 2019. A diversity of selective autophagy receptors determines the specificity of the autophagy pathway. Mol Cell 76: 268–285. 10.1016/j.molcel.2019.09.005 [DOI] [PubMed] [Google Scholar]
- Kishi-Itakura C, Koyama-Honda I, Itakura E, Mizushima N. 2014. Ultrastructural analysis of autophagosome organization using mammalian autophagy-deficient cells. J Cell Sci 127: 4089–4102. 10.1242/jcs.164293 [DOI] [PubMed] [Google Scholar]
- Kohno S, Shiozaki Y, Keenan AL, Miyazaki-Anzai S, Miyazaki M. 2019. An N-terminal-truncated isoform of FAM134B (FAM134B-2) regulates starvation-induced hepatic selective ER-phagy. Life Sci Alliance 2: e201900340. 10.26508/lsa.201900340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu M, Waguri S, Ueno T, Iwata J, Murata S, Tanida I, Ezaki J, Mizushima N, Ohsumi Y, Uchiyama Y, et al. 2005. Impairment of starvation-induced and constitutive autophagy in Atg7-deficient mice. J Cell Biol 169: 425–434. 10.1083/jcb.200412022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs AL, Palfia Z, Rez G, Vellai T, Kovacs J. 2007. Sequestration revisited: integrating traditional electron microscopy, de novo assembly and new results. Autophagy 3: 655–662. 10.4161/auto.4590 [DOI] [PubMed] [Google Scholar]
- Krick R, Muehe Y, Prick T, Bremer S, Schlotterhose P, Eskelinen EL, Millen J, Goldfarb DS, Thumm M. 2008. Piecemeal microautophagy of the nucleus requires the core macroautophagy genes. Mol Biol Cell 19: 4492–4505. 10.1091/mbc.e08-04-0363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurth I, Pamminger T, Hennings JC, Soehendra D, Huebner AK, Rotthier A, Baets J, Senderek J, Topaloglu H, Farrell SA, et al. 2009. Mutations in FAM134B, encoding a newly identified Golgi protein, cause severe sensory and autonomic neuropathy. Nat Genet 41: 1179–1181. 10.1038/ng.464 [DOI] [PubMed] [Google Scholar]
- Kvam E, Goldfarb DS. 2007. Nucleus–vacuole junctions and piecemeal microautophagy of the nucleus in S. cerevisiae. Autophagy 3: 85–92. 10.4161/auto.3586 [DOI] [PubMed] [Google Scholar]
- Lamark T, Johansen T. 2021. Mechanisms of selective autophagy. Annu Rev Cell Dev Biol 37: 143–169. 10.1146/annurev-cellbio-120219-035530 [DOI] [PubMed] [Google Scholar]
- Lee CW, Wilfling F, Ronchi P, Allegretti M, Mosalaganti S, Jentsch S, Beck M, Pfander B. 2020. Selective autophagy degrades nuclear pore complexes. Nat Cell Biol 22: 159–166. 10.1038/s41556-019-0459-2 [DOI] [PubMed] [Google Scholar]
- Liang JR, Lingeman E, Luong T, Ahmed S, Muhar M, Nguyen T, Olzmann JA, Corn JE. 2020. A genome-wide ER-phagy screen highlights key roles of mitochondrial metabolism and ER-resident UFMylation. Cell 180: 1160–1177.e1120. 10.1016/j.cell.2020.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipatova Z, Gyurkovska V, Zhao SF, Segev N. 2020. Characterization of constitutive ER-phagy of excess membrane proteins. PLoS Genet 16: e1009255. 10.1371/journal.pgen.1009255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Wu H, Wang C, Li Y, Tian H, Siraj S, Sehgal SA, Wang X, Wang J, Shang Y, et al. 2019. STING directly activates autophagy to tune the innate immune response. Cell Death Differ 26: 1735–1749. 10.1038/s41418-018-0251-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N, Zhao H, Zhao YG, Hu J, Zhang H. 2021. Atlastin 2/3 regulate ER targeting of the ULK1 complex to initiate autophagy. J Cell Biol 220. 10.1083/jcb.202012091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Mari M, Li X, Reggiori F, Ferro-Novick S, Novick P. 2022. ER-phagy requires the assembly of actin at sites of contact between the cortical ER and endocytic pits. Proc Natl Acad Sci 119: e2117554119. 10.1073/pnas.2117554119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loi M, Raimondi A, Morone D, Molinari M. 2019. ESCRT-III-driven piecemeal micro-ER-phagy remodels the ER during recovery from ER stress. Nat Commun 10: 5058. 10.1038/s41467-019-12991-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo M, Zhao X, Song Y, Cheng H, Zhou R. 2016. Nuclear autophagy: an evolutionarily conserved mechanism of nuclear degradation in the cytoplasm. Autophagy 12: 1973–1983. 10.1080/15548627.2016.1217381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mijaljica D, Devenish RJ. 2013. Nucleophagy at a glance. J Cell Sci 126: 4325–4330. 10.1242/jcs.133090 [DOI] [PubMed] [Google Scholar]
- Miyata T, Hagiwara D, Hodai Y, Miwata T, Kawaguchi Y, Kurimoto J, Ozaki H, Mitsumoto K, Takagi H, Suga H, et al. 2020. Degradation of mutant protein aggregates within the endoplasmic reticulum of vasopressin neurons. iScience 23: 101648. 10.1016/j.isci.2020.101648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno T, Irie K. 2021. Msn2/4 transcription factors positively regulate expression of Atg39 ER-phagy receptor. Sci Rep 11: 11919. 10.1038/s41598-021-91480-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno T, Muroi K, Irie K. 2020. Snf1 AMPK positively regulates ER-phagy via expression control of Atg39 autophagy receptor in yeast ER stress response. PLoS Genet 16: e1009053. 10.1371/journal.pgen.1009053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima N, Komatsu M. 2011. Autophagy: renovation of cells and tissues. Cell 147: 728–741. 10.1016/j.cell.2011.10.026 [DOI] [PubMed] [Google Scholar]
- Mochida K, Oikawa Y, Kimura Y, Kirisako H, Hirano H, Ohsumi Y, Nakatogawa H. 2015. Receptor-mediated selective autophagy degrades the endoplasmic reticulum and the nucleus. Nature 522: 359–362. 10.1038/nature14506 [DOI] [PubMed] [Google Scholar]
- Mochida K, Yamasaki A, Matoba K, Kirisako H, Noda NN, Nakatogawa H. 2020. Super-assembly of ER-phagy receptor Atg40 induces local ER remodeling at contacts with forming autophagosomal membranes. Nat Commun 11: 3306. 10.1038/s41467-020-17163-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochida K, Otani T, Katsumata Y, Kirisako H, Kakuta C, Kotani T, Nakatogawa H. 2022. Atg39 links and deforms the outer and inner nuclear membranes in selective autophagy of the nucleus. J Cell Biol 221: e202103178. 10.1083/jcb.202103178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinari M. 2021. ER-phagy responses in yeast, plants, and mammalian cells and their crosstalk with UPR and ERAD. Dev Cell 56: 949–966. 10.1016/j.devcel.2021.03.005 [DOI] [PubMed] [Google Scholar]
- Moretti J, Blander JM. 2018. Detection of a vita-PAMP STINGs cells into reticulophagy. Autophagy 14: 1102–1104. 10.1080/15548627.2018.1441471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretti J, Roy S, Bozec D, Martinez J, Chapman JR, Ueberheide B, Lamming DW, Chen ZJ, Horng T, Yeretssian G, et al. 2017. STING senses microbial viability to orchestrate stress-mediated autophagy of the endoplasmic reticulum. Cell 171: 809–823.e813. 10.1016/j.cell.2017.09.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishita H, Eguchi T, Tsukamoto S, Sakamaki Y, Takahashi S, Saito C, Koyama-Honda I, Mizushima N. 2021. Organelle degradation in the lens by PLAAT phospholipases. Nature 592: 634–638. 10.1038/s41586-021-03439-w [DOI] [PubMed] [Google Scholar]
- Mukaiyama H, Baba M, Osumi M, Aoyagi S, Kato N, Ohsumi Y, Sakai Y. 2004. Modification of a ubiquitin-like protein Paz2 conducted micropexophagy through formation of a novel membrane structure. Mol Biol Cell 15: 58–70. 10.1091/mbc.e03-05-0340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nthiga TM, Kumar Shrestha B, Sjøttem E, Bruun JA, Bowitz Larsen K, Bhujabal Z, Lamark T, Johansen T. 2020. CALCOCO1 acts with VAMP-associated proteins to mediate ER-phagy. EMBO J 39: e103649. 10.15252/embj.2019103649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nthiga TM, Shrestha BK, Bruun JA, Larsen KB, Lamark T, Johansen T. 2021. Regulation of Golgi turnover by CALCOCO1-mediated selective autophagy. J Cell Biol 220: e202006128. 10.1083/jcb.202006128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oikonomou C, Hendershot LM. 2019. Disposing of misfolded ER proteins: a troubled substrate's way out of the ER. Mol Cell Endocrinol 500: 110630. 10.1016/j.mce.2019.110630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oku M, Sakai Y. 2018. Three distinct types of microautophagy based on membrane dynamics and molecular machineries. Bioessays 40: e1800008. 10.1002/bies.201800008 [DOI] [PubMed] [Google Scholar]
- Omari S, Makareeva E, Roberts-Pilgrim A, Mirigian L, Jarnik M, Ott C, Lippincott-Schwartz J, Leikin S. 2018. Noncanonical autophagy at ER exit sites regulates procollagen turnover. Proc Natl Acad Sci 115: e10099–e10108. 10.1073/pnas.1814552115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto GM, Cheunkarndee T, Leslie JM, Brar GA. 2021. Programmed cortical ER collapse drives selective ER degradation and inheritance in yeast meiosis. J Cell Biol 220: e202108105. 10.1083/jcb.202108105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papandreou ME, Tavernarakis N. 2019. Nucleophagy: from homeostasis to disease. Cell Death Differ 26: 630–639. 10.1038/s41418-018-0266-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parashar S, Ferro-Novick S. 2022. Architecture of the endoplasmic reticulum plays a role in proteostasis. Autophagy 18: 937–938. 10.1080/15548627.2022.2030175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parashar S, Chidambaram R, Chen S, Liem CR, Griffis E, Lambert GG, Shaner NC, Wortham M, Hay JC, Ferro-Novick S. 2021. Endoplasmic reticulum tubules limit the size of misfolded protein condensates. eLife 10: e71642. 10.7554/eLife.71642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park YE, Hayashi YK, Bonne G, Arimura T, Noguchi S, Nonaka I, Nishino I. 2009. Autophagic degradation of nuclear components in mammalian cells. Autophagy 5: 795–804. 10.4161/auto.8901 [DOI] [PubMed] [Google Scholar]
- Pengo N, Scolari M, Oliva L, Milan E, Mainoldi F, Raimondi A, Fagioli C, Merlini A, Mariani E, Pasqualetto E, et al. 2013. Plasma cells require autophagy for sustainable immunoglobulin production. Nat Immunol 14: 298–305. 10.1038/ni.2524 [DOI] [PubMed] [Google Scholar]
- Rapoport TA, Li L, Park E. 2017. Structural and mechanistic insights into protein translocation. Annu Rev Cell Dev Biol 33: 369–390. 10.1146/annurev-cellbio-100616-060439 [DOI] [PubMed] [Google Scholar]
- Ravenhill BJ, Boyle KB, von Muhlinen N, Ellison CJ, Masson GR, Otten EG, Foeglein A, Williams R, Randow F. 2019. The cargo receptor NDP52 initiates selective autophagy by recruiting the ULK complex to cytosol-invading bacteria. Mol Cell 74: 320–329.e326. 10.1016/j.molcel.2019.01.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reggio A, Buonomo V, Berkane R, Bhaskara RM, Tellechea M, Peluso I, Polishchuk E, Di Lorenzo G, Cirillo C, Esposito M, et al. 2021. Role of FAM134 paralogues in endoplasmic reticulum remodeling, ER-phagy, and collagen quality control. EMBO Rep 22: e52289. 10.15252/embr.202052289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rismanchi N, Soderblom C, Stadler J, Zhu PP, Blackstone C. 2008. Atlastin GTPases are required for Golgi apparatus and ER morphogenesis. Hum Mol Genet 17: 1591–1604. 10.1093/hmg/ddn046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts P, Moshitch-Moshkovitz S, Kvam E, O'Toole E, Winey M, Goldfarb DS. 2003. Piecemeal microautophagy of nucleus in Saccharomyces cerevisiae. Mol Biol Cell 14: 129–141. 10.1091/mbc.e02-08-0483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggiano A, Foresti O, Carvalho P. 2014. Quality control: ER-associated degradation: protein quality control and beyond. J Cell Biol 204: 869–879. 10.1083/jcb.201312042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer JA, Schessner JP, Bircham PW, Tsuji T, Funaya C, Pajonk O, Schaeff K, Ruffini G, Papagiannidis D, Knop M, et al. 2020. ESCRT machinery mediates selective microautophagy of endoplasmic reticulum in yeast. EMBO J 39: e102586. 10.15252/embj.2019102586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuck S. 2020. Microautophagy—distinct molecular mechanisms handle cargoes of many sizes. J Cell Sci 133: jcs246322. 10.1242/jcs.246322 [DOI] [PubMed] [Google Scholar]
- Schuck S, Gallagher CM, Walter P. 2014. ER-phagy mediates selective degradation of endoplasmic reticulum independently of the core autophagy machinery. J Cell Sci 127: 4078–4088. 10.1242/jcs.154716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz ML, Krus KL, Kaushik S, Dang D, Chopra R, Qi L, Shakkottai VG, Cuervo AM, Lieberman AP. 2018. Coordinate regulation of mutant NPC1 degradation by selective ER autophagy and MARCH6-dependent ERAD. Nat Commun 9: 3671. 10.1038/s41467-018-06115-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz DS, Blower MD. 2016. The endoplasmic reticulum: structure, function and response to cellular signaling. Cell Mol Life Sci 73: 79–94. 10.1007/s00018-015-2052-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shima T, Kirisako H, Nakatogawa H. 2019. COPII vesicles contribute to autophagosomal membranes. J Cell Biol 218: 1503–1510. 10.1083/jcb.201809032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JA. 2020. STING, the endoplasmic reticulum, and mitochondria: is three a crowd or a conversation? Front Immunol 11: 611347. 10.3389/fimmu.2020.611347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MD, Harley ME, Kemp AJ, Wills J, Lee M, Arends M, von Kriegsheim A, Behrends C, Wilkinson S. 2018. CCPG1 is a non-canonical autophagy cargo receptor essential for ER-phagy and pancreatic ER proteostasis. Dev Cell 44: 217–232.e211. 10.1016/j.devcel.2017.11.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snead WT, Hayden CC, Gadok AK, Zhao C, Lafer EM, Rangamani P, Stachowiak JC. 2017. Membrane fission by protein crowding. Proc Natl Acad Sci 114: e3258–e3267. 10.1073/pnas.1616199114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sou YS, Waguri S, Iwata J, Ueno T, Fujimura T, Hara T, Sawada N, Yamada A, Mizushima N, Uchiyama Y, et al. 2008. The Atg8 conjugation system is indispensable for proper development of autophagic isolation membranes in mice. Mol Biol Cell 19: 4762–4775. 10.1091/mbc.e08-03-0309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiess M, Friberg M, Beuret N, Prescianotto-Baschong C, Rutishauser J. 2020. Role of protein aggregation and degradation in autosomal dominant neurohypophyseal diabetes insipidus. Mol Cell Endocrinol 501: 110653. 10.1016/j.mce.2019.110653 [DOI] [PubMed] [Google Scholar]
- Stefely JA, Zhang Y, Freiberger EC, Kwiecien NW, Thomas HE, Davis AM, Lowry ND, Vincent CE, Shishkova E, Clark NA, et al. 2020. Mass spectrometry proteomics reveals a function for mammalian CALCOCO1 in MTOR-regulated selective autophagy. Autophagy 16: 2219–2237. 10.1080/15548627.2020.1719746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephani M, Picchianti L, Gajic A, Beveridge R, Skarwan E, Sanchez de Medina Hernandez V, Mohseni A, Clavel M, Zeng Y, Naumann C, et al. 2020. A cross-kingdom conserved ER-phagy receptor maintains endoplasmic reticulum homeostasis during stress. eLife 9: e58396. 10.7554/eLife.58396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolz A, Grumati P. 2019. The various shades of ER-phagy. FEBS J 286: 4642–4649. 10.1111/febs.15031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z, Brodsky JL. 2019. Protein quality control in the secretory pathway. J Cell Biol 218: 3171–3187. 10.1083/jcb.201906047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomioka Y, Kotani T, Kirisako H, Oikawa Y, Kimura Y, Hirano H, Ohsumi Y, Nakatogawa H. 2020. TORC1 inactivation stimulates autophagy of nucleoporin and nuclear pore complexes. J Cell Biol 219: e201910063. 10.1083/jcb.201910063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torggler R, Papinski D, Brach T, Bas L, Schuschnig M, Pfaffenwimmer T, Rohringer S, Matzhold T, Schweida D, Brezovich A, et al. 2016. Two independent pathways within selective autophagy converge to activate Atg1 kinase at the vacuole. Mol Cell 64: 221–235. 10.1016/j.molcel.2016.09.008 [DOI] [PubMed] [Google Scholar]
- Turco E, Witt M, Abert C, Bock-Bierbaum T, Su MY, Trapannone R, Sztacho M, Danieli A, Shi X, Zaffagnini G, et al. 2019. FIP200 claw domain binding to p62 promotes autophagosome formation at ubiquitin condensates. Mol Cell 74: 330–346.e311. 10.1016/j.molcel.2019.01.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas JNS, Wang C, Bunker E, Hao L, Maric D, Schiavo G, Randow F, Youle RJ. 2019. Spatiotemporal control of ULK1 activation by NDP52 and TBK1 during selective autophagy. Mol Cell 74: 347–362.e346. 10.1016/j.molcel.2019.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voeltz GK, Rolls MM, Rapoport TA. 2002. Structural organization of the endoplasmic reticulum. EMBO Rep 3: 944–950. 10.1093/embo-reports/kvf202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walczak CP, Leto DE, Zhang L, Riepe C, Muller RY, DaRosa PA, Ingolia NT, Elias JE, Kopito RR. 2019. Ribosomal protein RPL26 is the principal target of UFMylation. Proc Natl Acad Sci 116: 1299–1308. 10.1073/pnas.1816202116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Tan D, Cai Y, Reinisch KM, Walz T, Ferro-Novick S. 2014. A requirement for ER-derived COPII vesicles in phagophore initiation. Autophagy 10: 708–709. 10.4161/auto.28103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Eraslan B, Wieland T, Hallström B, Hopf T, Zolg DP, Zecha J, Asplund A, Li LH, Meng C, et al. 2019. A deep proteome and transcriptome abundance atlas of 29 healthy human tissues. Mol Syst Biol 15: e8503. 10.15252/msb.20188503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Xu Y, Rogers H, Saidi L, Noguchi CT, Li H, Yewdell JW, Guydosh NR, Ye Y. 2020. UFMylation of RPL26 links translocation-associated quality control to endoplasmic reticulum protein homeostasis. Cell Res 30: 5–20. 10.1038/s41422-019-0236-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson S. 2019. ER-phagy: shaping up and destressing the endoplasmic reticulum. FEBS J 286: 2645–2663. 10.1111/febs.14932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson S. 2020. Emerging principles of selective ER autophagy. J Mol Biol 432: 185–205. 10.1016/j.jmb.2019.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Rapoport TA. 2018. Mechanistic insights into ER-associated protein degradation. Curr Opin Cell Biol 53: 22–28. 10.1016/j.ceb.2018.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Zhang C, Cao L, Song J, Xu X, Zhang B, Chen B, Zhao G. 2019. ATL3 gene mutation in a Chinese family with hereditary sensory neuropathy type 1F. J Peripher Nerv Syst 24: 150–155. 10.1111/jns.12309 [DOI] [PubMed] [Google Scholar]
- Yang H, Ni HM, Guo F, Ding Y, Shi YH, Lahiri P, Fröhlich LF, Rülicke T, Smole C, Schmidt VC, et al. 2016. Sequestosome 1/p62 protein is associated with autophagic removal of excess hepatic endoplasmic reticulum in mice. J Biol Chem 291: 18663–18674. 10.1074/jbc.M116.739821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoboue ED, Sitia R, Simmen T. 2018. Redox crosstalk at endoplasmic reticulum (ER) membrane contact sites (MCS) uses toxic waste to deliver messages. Cell Death Dis 9: 331. 10.1038/s41419-017-0033-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao YG, Liu N, Miao G, Chen Y, Zhao H, Zhang H. 2018. The ER contact proteins VAPA/B interact with multiple autophagy proteins to modulate autophagosome biogenesis. Curr Biol 28: 1234–1245.e1234. 10.1016/j.cub.2018.03.002 [DOI] [PubMed] [Google Scholar]
- Zhao D, Zou CX, Liu XM, Jiang ZD, Yu ZQ, Suo F, Du TY, Dong MQ, He W, Du LL. 2020. A UPR-induced soluble ER-phagy receptor acts with VAPs to confer ER stress resistance. Mol Cell 79: 963–977.e963. 10.1016/j.molcel.2020.07.019 [DOI] [PubMed] [Google Scholar]
- Zhou Z, Liu J, Fu T, Wu P, Peng C, Gong X, Wang Y, Zhang M, Li Y, Wang Y, et al. 2021. Phosphorylation regulates the binding of autophagy receptors to FIP200 claw domain for selective autophagy initiation. Nat Commun 12: 1570. 10.1038/s41467-021-21874-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurek N, Sparks L, Voeltz G. 2011. Reticulon short hairpin transmembrane domains are used to shape ER tubules. Traffic 12: 28–41. 10.1111/j.1600-0854.2010.01134.x [DOI] [PMC free article] [PubMed] [Google Scholar]