Abstract
Nrf1 is a member of the nuclear erythroid 2-like family of transcription factors that regulate stress-responsive gene expression in animals. Newly synthesized Nrf1 is targeted to the endoplasmic reticulum (ER) where it is N-glycosylated. N-glycosylated Nrf1 is trafficked to the cytosol by the ER-associated degradation (ERAD) machinery and is subject to rapid proteasomal degradation. When proteasome function is impaired, Nrf1 escapes degradation and undergoes proteolytic cleavage and deglycosylation. Deglycosylation results in deamidation of N-glycosylated asparagine residues to edit the protein sequence encoded by the genome. This truncated and “sequence-edited” form of Nrf1 enters the nucleus where it induces up-regulation of proteasome subunit genes. Thus, Nrf1 drives compensatory proteasome biogenesis in cells exposed to proteasome inhibitor drugs and other proteotoxic insults. In addition to its role in proteasome homeostasis, Nrf1 is implicated in responses to oxidative stress, and maintaining lipid and cholesterol homeostasis. Here, we describe the conserved and complex mechanism by which Nrf1 is regulated and highlight emerging evidence linking this unusual transcription factor to development, aging, and disease.
Following proteasome inhibition, cells recover proteasome function via transcriptional activation of proteasome biogenesis (Fleming et al. 2002; Meiners et al. 2003). In animal cells, this response depends on a conserved endoplasmic reticulum (ER)-associated transcription factor named SKN-1A (in Caenorhabditis elegans), CnC-C (in Drosophila), and Nrf1 (in mammals). Inactivation of SKN-1A/CnC-C/Nrf1 results in pronounced sensitivity to killing by proteasome inhibitors (Radhakrishnan et al. 2010; Lehrbach and Ruvkun 2016; Iyer et al. 2019). Depletion of skn-1 causes synthetic lethality with diverse proteasome subunit mutations in C. elegans, indicating that this compensatory response is not limited to drugs that target the proteolytic active site of the proteasome (Keith et al. 2016; Lehrbach and Ruvkun 2016). SKN-1A/CnC-C/Nrf1 is primarily thought to mediate proteasome inhibitor resistance by increasing proteasome levels (Radhakrishnan et al. 2010; Grimberg et al. 2011; Li et al. 2011; Sha and Goldberg 2014), but it is also possible that SKN-1A/Nrf1 may act to promote alternative protein turnover pathways such as autophagy, mitophagy, or activate expression of other genes that confer resilience to proteotoxic stressors (Yang et al. 2018; Cui et al. 2021). Of note, oxidative protein damage can result in proteasome impairment (Friguet and Szweda 1997; Reinheckel et al. 2000), and Nrf1 appears to redundantly mediate oxidative stress responses together with its close relative Nrf2 (Chen et al. 2003; Leung et al. 2003).
The role of SKN-1A/CnC-C/Nrf1 in animal cells is analogous to the regulation of proteasome subunit gene expression by the RPN4 transcription factor in yeast. But unlike SKN-1A/Nrf1, RPN4 is not ER-associated and is regulated by a cytosolic ubiquitin ligase and a direct ubiquitin-independent interaction with the proteasome (Xie and Varshavsky 2001; Ju and Xie 2004; Wang et al. 2004). The more complex pathway that exists in animals may be necessary to correctly regulate proteasome levels in different tissues and physiological states. In this article, we describe the evolutionarily conserved mechanism by which ER-associated degradation (ERAD), proteolytic cleavage and N-linked glycosylation regulate Nrf1 and highlight the functions of this transcriptional regulator in stress responses, development, and aging.
EVOLUTION OF THE NRF FAMILY OF TRANSCRIPTION FACTORS
Vertebrate genomes encode three nuclear factor erythroid 2-like (Nrf) transcription factors: Nrf1/NFE2L1, Nrf2/NFE2L2, and Nrf3/NFE2L3 (Chan et al. 1993; Moi et al. 1994; Kobayashi et al. 1999). Named for their similarity to nuclear factor erythroid 2 (NFE2), which regulates erythropoiesis and megakaryogenesis (Shivdasani et al. 1995), the Nrfs are widely expressed and control stress-responsive gene expression programs that are conserved throughout the animal kingdom (Sykiotis and Bohmann 2010; Blackwell et al. 2015). The Nrfs contain cap'n’collar (CNC) and basic leucine zipper (bZIP) domains near their carboxyl-termini through which they bind to DNA as heterodimers with small Maf proteins. The Nrf/Maf dimers recognize a DNA sequence motif termed the antioxidant response element (ARE) and function largely as transcriptional activators (Itoh et al. 1995; Johnsen et al. 1996, 1998; Kobayashi et al. 1999). Despite their similar mode of DNA binding, Nrf1, Nrf2, and Nrf3 perform distinct biological functions and regulate sets of target genes that only partially overlap (Ohtsuji et al. 2008; Liu et al. 2019; Ibrahim et al. 2020). Nrf1 is unique in its ability to activate proteasome subunit genes, Nrf2 activates antioxidant defenses under conditions of oxidative stress, whereas the function of Nrf3 is not well understood (Ma 2013; Kobayashi 2020).
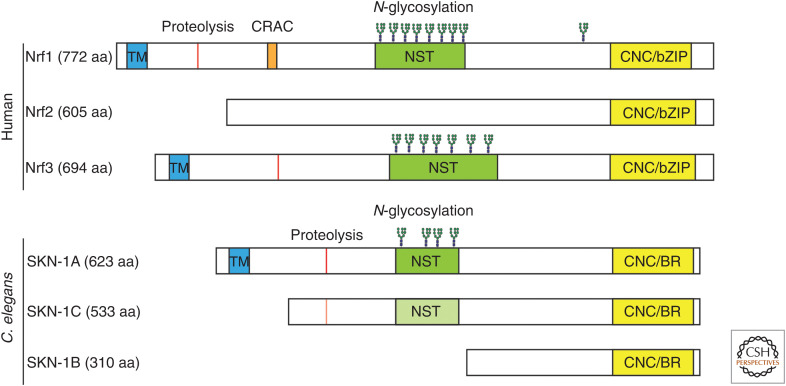
Nrfs are present throughout animals, but unlike vertebrates, most invertebrate genomes contain only one Nrf family gene. The Nrf genes of C. elegans and Drosophila melanogaster are called skn-1 and CNC, respectively, and have been studied in detail (Sykiotis and Bohmann 2010; Blackwell et al. 2015). The mode of DNA binding has diverged between human Nrfs and SKN-1, which binds to DNA as a monomer (Blackwell et al. 1994). Even though the stress-responsive functions of Nrf transcription factors are conserved, it was unclear how a single Nrf ortholog fulfills functions that are divided between different mammalian proteins. skn-1 and CNC each encode multiple protein isoforms through use of alternative transcriptional start sites (see Fig. 1 for a comparison between human Nrf1/2/3 and the protein isoforms of C. elegans SKN-1). Phylogenetic analysis suggests that the vertebrate Nrfs evolved via duplication of an ancestral Nrf locus that encoded functionally distinct protein isoforms (Pitoniak and Bohmann 2015; Fuse and Kobayashi 2017). Such gene duplications in vertebrates of an ancestral gene that has not undergone duplication in insects or nematodes are common. Functional and molecular evidence indicates that different protein isoforms of invertebrate SKN-1/CNC are equivalent to different mammalian Nrfs. Both Nrf2 and SKN-1C are regulated in the cytosol by redox-sensitive ubiquitin ligases and regulate oxidative stress responses (Itoh et al. 1999; Choe et al. 2009; Fukushige et al. 2017; Xu et al. 2018). Nrf1, SKN-1A, and Drosophila CNC-C all contain an amino-terminal transmembrane domain and mediate stress-responsive regulation of the ubiquitin-proteasome system (Zhang et al. 2006; Radhakrishnan et al. 2010; Grimberg et al. 2011; Glover-Cutter et al. 2013; Lehrbach and Ruvkun 2016). We will hereafter refer to SKN-1A/Nrf1 when describing conserved aspects of this pathway.
Figure 1.
Nrf family transcription factors of humans and Caenorhabditis elegans. The Human Nrfs are encoded by distinct genes, whereas the various SKN-1 proteins of C. elegans are generated by alternative transcription start sites and splicing at the skn-1 locus. The amino acid sequences of SKN-1A/C/B are identical aside from the amino-terminal 90 amino acids of SKN-1A, and the amino-terminal 77 amino acids of SKN-1B, which are each encoded by isoform-specific exons. Nrf1, Nrf3, and SKN-1A contain an amino-terminal transmembrane (TM) domain. The site of DDI-1/DDI2-dependent proteolysis of SKN-1A, Nrf1, and Nrf3 is marked in red. Nrf1 additionally contains a cholesterol recognition amino acid consensus (CRAC) domain. Nrf1 contains eight N-glycosylation sites within its N-X-S/T-rich (NST) domain, and one site further carboxy terminal. Nrf3 also contains a central NST domain and is N-glycosylated. SKN-1A contains four N-glycosylation sites within its NST domain. All three vertebrate Nrfs contain a highly homologous cap'n’collar (CNC) basic leucine zipper (bZIP) DNA-binding domain. All SKN-1 isoforms contain an identical DNA-binding domain that consists of a CNC domain followed by a carboxy-terminal basic region (BR). The DDI-1-dependent proteolysis sequence and NST domain are present in SKN-1C, but SKN-1C does not undergo N-glycosylation or proteolysis since endoplasmic reticulum (ER)-association is required for both these post-translational modifications. For ease of interpretation, the proteins and their domains are depicted to approximate scale.
BIOGENESIS AND REGULATION OF ER-ASSOCIATED SKN-1A/Nrf1
SKN-1A/Nrf1 is Synthesized in the ER and Degraded via ERAD
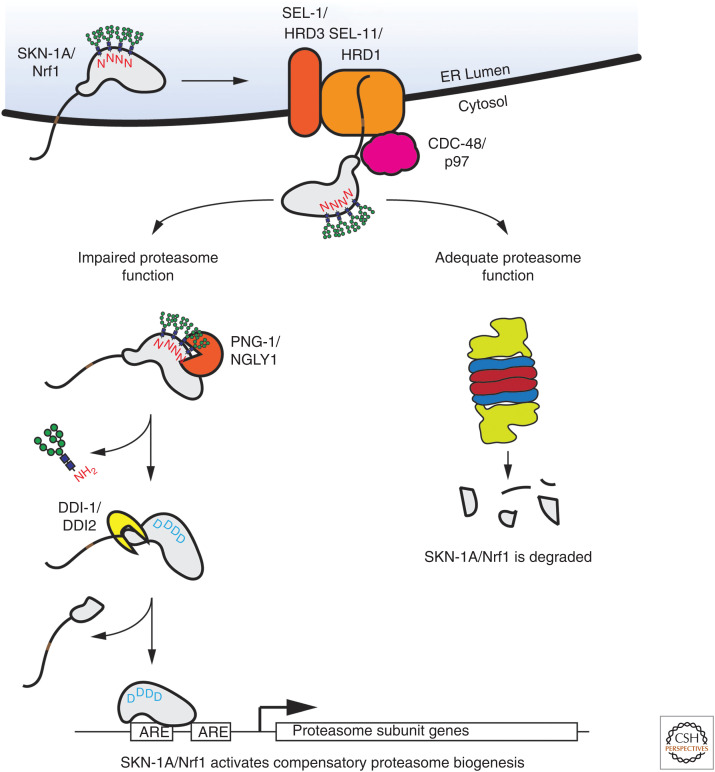
Both Nrf1 and SKN-1A possess an amino-terminal transmembrane domain that targets the protein to the ER (Zhang et al. 2006; Glover-Cutter et al. 2013). SKN-1A/Nrf1 is inserted into the ER such that most of the protein, including the carboxy-terminal DNA-binding domain, resides within the ER lumen (Wang and Chan 2006; Zhang et al. 2006). In the ER, SKN-1A/Nrf1 undergoes N-linked glycosylation, before being returned to the cytosol by the ERAD pathway, where it is degraded by proteasomes (Fig. 2). ERAD-dependent trafficking and degradation of Nrf1 requires the HRD1 ubiquitin ligase and p97 (Steffen et al. 2010; Radhakrishnan et al. 2014). In C. elegans, the HRD1 ortholog SEL-11, and the HRD3 ortholog SEL-1 are required for ERAD-dependent trafficking and degradation of SKN-1A (Lehrbach and Ruvkun 2016). Failure of ERAD results in the sequestration of SKN-1A/Nrf1 in the ER, preventing its nuclear localization and activation of target genes. The main function of the ERAD pathway is removal and degradation of misfolded proteins in the ER (Wu and Rapoport 2018; Sun and Brodsky 2019). It is not known whether SKN-1A/Nrf1 misfolds in the ER or is recognized by the ERAD machinery via some other mechanism. For some ERAD substrates, N-linked glycosylation mediates interactions with ER-resident protein quality control factors, but N-linked glycosylation is not essential for the ERAD-mediated degradation of SKN-1A/Nrf1 (Zhang et al. 2014b; Lehrbach et al. 2019; Yoshida et al. 2021).
Figure 2.
The mechanism of SKN-1A/Nrf1 activation under proteasome dysfunction. SKN-1A/Nrf1 is inserted into the endoplasmic reticulum (ER). The amino-terminal transmembrane domain is anchored within the ER membrane and the carboxyl terminus of the protein is extruded into the ER lumen. SKN-1A/Nrf1 is N-glycosylated in the ER. The SKN-1A/Nrf1 glycoprotein is extracted from the ER and ubiquitinated by the ER-resident ERAD factors SEL-11/HRD1 and SEL-1/HRD3. The extraction of SKN-1A/Nrf1 from the ER requires the p97 ATPase. In cells with sufficient proteasome capacity, cytosolic SKN-1A/Nrf1 is rapidly degraded. In cells with impaired proteasome function, SKN-1A/Nrf1 half-life may be increased to the point that it is cleaved by the protease DDI-1/DDI2 and deglycosylated by the peptide:N-glycanase PNG-1/NGLY1. In this model, deglycosylation precedes proteolysis, but the order of these events is not known. Proteolytic cleavage liberates the transmembrane domain-containing amino terminus of SKN-1A/Nrf1. Removal of N-linked glycans post-translationally converts N-glycosylated asparagine (N) residues into aspartates (D). Proteolytically processed and N-to-D edited SKN-1A/Nrf1 activates the expression of proteasome subunits and other target genes via binding to antioxidant response elements (AREs) within their promoters. Elevated proteasome subunit gene expression leads to increased proteasome biogenesis, thus restoring adequate proteasome function.
Proteolytic Activation of SKN-1A/Nrf1 by DDI-1/DDI2
In cells with deficient proteasome function, Nrf1 undergoes proteolytic cleavage that removes ∼100 amino acids from the amino terminus of the protein (Radhakrishnan et al. 2014). Similarly, SKN-1A undergoes a proteolytic cleavage that removes an ∼150 amino acid amino-terminal peptide (Lehrbach and Ruvkun 2016; Lehrbach et al. 2019). This cleavage generates a truncated form of SKN-1A/Nrf1 that lacks the transmembrane domain but is the activated transcription factor for up-regulation of proteasome gene expression. This cleavage requires a conserved aspartic protease called DDI-1 in C. elegans and DDI2 in humans (Koizumi et al. 2016; Lehrbach and Ruvkun 2016). In cells lacking DDI-1/DDI2, or expressing a protease active site mutant, SKN-1A/Nrf1 remains full-length and fails to up-regulate proteasome subunit genes. It is not fully understood why cleavage by DDI-1/DDI2 is required for activation of SKN-1A/Nrf1. In some cell types, DDI2 is necessary for release of Nrf1 from the ER (Koizumi et al. 2016). However, cleavage is not essential for this step in C. elegans and some human cell lines (Lehrbach and Ruvkun 2016; Northrop et al. 2020). In these cases, it appears that uncleaved SKN-1A/Nrf1 is released from the ER and localizes to the nucleus but is defective in activating target gene expression. In C. elegans, loss of DDI-1 causes accumulation of SKN-1A in nuclear puncta not seen in wild-type animals, suggesting that the retained transmembrane domain causes SKN-1A/Nrf1 to aggregate (Lehrbach and Ruvkun 2016).
DDI-1/DDI2 is highly conserved in eukaryotes and orthologous to yeast DNA damage–induced 1 (Ddi1). Ddi1 contains a highly conserved aspartic protease domain, which is structurally related to that of the retroviral aspartic proteases that are responsible for proteolytic processing of viral polyproteins (Sirkis et al. 2006). Ddi1 additionally contains three domains capable of binding ubiquitin: an atypical ubiquitin-like (UBL) domain, a ubiquitin-associated (UBA) domain, and a “helical domain of Ddi1” (HDD) (Kaplun et al. 2005; Nowicka et al. 2015; Yip et al. 2020). These domains are conserved in Ddi1 orthologs, although the UBA domain is absent in several species, including C. elegans. S. cerevisiae Ddi1 is a ubiquitin-dependent endopeptidase that binds to and preferentially cleaves proteins modified by long polyubiquitin chains (Yip et al. 2020). Human DDI2 similarly cleaves polyubiquitinated proteins and preferentially cleaves Nrf1 when it is modified by very long polyubiquitin chains (Dirac-Svejstrup et al. 2020). The length of polyubiquitin chain required to stimulate cleavage by Ddi1 or DDI2 is much longer than the length normally required to cause proteasomal degradation (Yip et al. 2020). As the only known ubiquitin-dependent endopeptidase aside from the proteasome itself, Ddi1/DDI-1/DDI2 may act to ensure degradation (by proteasomes or other proteases) of highly ubiquitinated proteins in cells with impaired proteasome function. The preference of DDI-1/DDI2 for long polyubiquitin chains may also help to ensure that proteolytic activation of SKN-1A/Nrf1 only occurs if the proteasome is impaired, and may also provide an opportunity for fine-tuned regulation of SKN-1A/Nrf1 activity by cytosolic ubiquitin ligases and/or deubiquitinating enzymes.
Deglycosylation-Dependent “Sequence Editing” of SKN-1A/Nrf1 by PNG-1/NGLY1
Nrf1 contains eight N-linked glycosylation motifs (N-X-S/T) in a central domain of the protein and a ninth more carboxy-terminal motif (Fig. 1). It is likely that some or all these sites are N-glycosylated sequentially as the nascent chain of Nrf1 translation at the ER membrane emerges from the Sec61 translocon into the ER (Zhang and Hayes 2013; Zhang et al. 2014b; Yoshida et al. 2021). C. elegans SKN-1A contains a cluster of four N-linked glycosylation motifs similarly positioned in a central domain of the protein (Lehrbach et al. 2019). N-linked glycosylation of these sites is not required for ER-associated SKN-1A/Nrf1 to undergo ERAD-dependent trafficking and degradation, but is important for activation of target genes after SKN-1A/Nrf1 is released from the ER (Zhang et al. 2014b; Lehrbach et al. 2019; Yoshida et al. 2021). Despite this, the active form of SKN-1A/Nrf1 that accumulates in the nuclei of cells with impaired proteasome function is not N-glycosylated.
Deglycosylation of SKN-1A/Nrf1 after release from the ER requires peptide:N-glycanase (termed NGLY1 in mammals and PNG-1 in C. elegans), a cytosolic enzyme responsible for deglycosylation of ERAD substrate glycoproteins prior to their proteasomal degradation (Suzuki et al. 2016). In cells lacking peptide:N-glycanase activity (through pharmacological inhibition or mutation), SKN-1A/Nrf1 is unable to activate proteasome subunit gene expression (Lehrbach and Ruvkun 2016; Tomlin et al. 2017). The deglycosylation reaction carried out by PNG-1/NGLY1 couples release of the N-linked glycan to conversion of the glycosylated Asn residue to Asp (Suzuki et al. 1994). SKN-1A/Nrf1 with Asn to Asp amino acid substitution mutations engineered at N-glycosylation motifs are functional and have the additional capacity to completely bypass the requirement for PNG-1/NGLY1 for regulation of proteasome subunit genes (Lehrbach et al. 2019; Yoshida et al. 2021). Thus, sequential N-glycosylation and deglycosylation serve to post-translationally alter the amino acid sequence of SKN-1A/Nrf1 and this “sequence editing” is essential for regulation of the proteasome, but can be bypassed by altering the sequence of the SKN-1A/Nrf1 gene. Interestingly, genomic mutations to bypass the requirement for protein sequence editing by NGLY1/PNG-1 is observed in phylogenetic comparisons of SKN-1A/Nrf1 protein sequences (Lehrbach et al. 2019; Ruvkun et al. 2021).
Some of the Nrf1 Asn to Asp edits carried out by NGLY1 were validated in a recent comprehensive proteomic analysis of protein modifications of HLA-presented peptides (Mei et al. 2020). Most of the 450 deamidations detected in this study were derived from Asn residues within consensus N-glycosylation motifs (N-X-S/T), strongly suggesting that the deamidation was dependent on previous N-glycosylation and thus likely to be mediated by NGLY1. The HLA display of Nrf1 peptides suggests that the immune system may use Nrf1 as a sign of aberrant protein homeostasis, for example, in viral infection (Ruvkun et al. 2021).
How does “sequence editing” of SKN-1A/Nrf1 affect its function? The N-glycosylated Asn residues lie within a putative transactivation domain. Acidic residues are important for the function of some transactivation domains, so their conversion to Asp may potentiate activation of SKN-1A/Nrf1 targets (Triezenberg 1995). Consistently, mutation of multiple Nrf1 glycosylation sites to Asp increases the activation of an ARE-containing reporter construct (Zhang et al. 2014b). “Sequence editing” of SKN-1A/Nrf1 differentially effects activation of specific target genes. For example, introduction of Asp residues is essential for regulation of proteasome subunit genes but is dispensable for activation of some other targets, notably those implicated in responding to oxidative stress (Lehrbach et al. 2019; Yoshida et al. 2021). Thus, deglycosylation-dependent conversion of specific N-glycosylated Asn residues to Asp serves to bias the transcriptional output of SKN-1A/Nrf1 activation toward distinct stress responses. In C. elegans, this allows the ER-associated and cytosolic isoforms of SKN-1 to perform distinct functions corresponding to those of mammalian Nrf1 and Nrf2 (see Fig. 1). The impact of sequence editing on target selection also raises the exciting possibility that N-glycosylation of SKN-1A/Nrf1 may be regulated to fine-tune stress-responsive gene expression programs. It will be of great interest to determine whether this is the case, and to understand how Asn to Asp sequence editing controls SKN-1A/Nrf1 target specificity.
Deglycosylation of Nrf1 also prevents its ubiquitination by a cytosolic E3 ubiquitin ligase complex containing the F-box protein Fbxo6, which specifically recognizes sugar chains of N-linked glycoproteins (Yoshida et al. 2003, 2021). Expression of Fbxo6 in NGLY1-deficient cells leads to severe defects in proteostasis and cell death. This effect is, at least in part, driven by hyperubiquitination of N-glycosylated Nrf1. Since N-glycosylated Nrf1 is already defective in regulating target gene transcription, it is unclear how its ubiquitination has such a profound effect on proteasome function and cell viability. Possibly, Nrf1 that is both N-glycosylated and hyperubiquitinated mediates a toxic effect through a transcription-independent effect on proteasomes or other components of the proteostasis machinery. This finding suggests that the interplay between glycosylation and ubiquitination of Nrf1 plays a role in regulation of proteasome function that deserves further attention.
CELLULAR AND ORGANISMAL FUNCTIONS OF SKN-1A/Nrf1
Physiological Roles of Proteasome Regulation by SKN-1A/Nrf1
In the mouse, Nrf1 is essential for embryonic development. Nrf1 knockout embryos are anemic and rarely survive beyond mid-gestation (Chan et al. 1998). Tissue-specific disruption of Nrf1 in bone, adipocytes, liver, and brain all cause abnormalities, suggesting that Nrf1 is required for the normal development and/or function of many if not all mammalian tissues (Chen et al. 2003; Xu et al. 2005; Ohtsuji et al. 2008; Kim et al. 2010; Kobayashi et al. 2011; Lee et al. 2011; Widenmaier et al. 2017). At least some of these defects are likely to result from failure to maintain adequate proteasome function. Reduced basal proteasome expression, reduced proteasome activity, and increased accumulation of ubiquitinated proteins are observed following tissue-specific depletion of Nrf1 in liver or the nervous system (Kobayashi et al. 2011; Lee et al. 2011, 2013). Inactivation of SKN-1A/CnC-C similarly causes reduced basal proteasome expression and activity in C. elegans and Drosophila (Grimberg et al. 2011; Li et al. 2011; Lehrbach et al. 2019). Clearly, SKN-1A/Nrf1 optimizes proteasome levels as part of normal physiology and development, not solely as a response to acute exposure to exogenous stressors.
What are the endogenous physiological cues that influence the regulation of the proteasome by SKN-1A/Nrf1? Several lines of evidence suggest that SKN-1A/Nrf1 is a downstream target of nutrient sensing and growth factor signaling pathways. mTORC1, which promotes protein synthesis in response to growth factor signaling and nutrient sufficiency, also increases proteasome levels, through an effect on Nrf1 transcription (Zhang et al. 2014a). The extracellular signal-regulated kinases (ERK1 and ERK2) regulate diverse cellular processes, including cell growth, proliferation, and metabolism (Lavoie et al. 2020). Genetic screening in C. elegans identified the ERK1/2 ortholog MPK-1 as essential for activation of proteasome gene expression by SKN-1A (Zhang et al. 2021). This study also found that pharmacological inhibition of ERK1/2 in human cells caused reduced phosphorylation and nuclear localization of Nrf1 suggesting direct regulation of SKN-1A/Nrf1 by MPK-1/ERK. O-linked N-acetylglucosamine (O-GlcNac) modification of Nrf1 by the cytosolic O-linked N-acetylglucosamine transferase (OGT) enzyme also impacts proteasome regulation (Chen et al. 2015; Han et al. 2017; Sekine et al. 2018). OGT activity is dependent on N-acetylglucosamine availability, and so this modification may adjust Nrf1 activity according to cellular metabolic state (Jozwiak et al. 2014). Regulation of SKN-1A/Nrf1 by ERK1/2, mTORC1, and OGT may serve to coordinate protein degradation capacity to ensure protein quality control is maintained in cells that are metabolically active, growing, and/or proliferating. Interestingly, in C. elegans, SKN-1C/Nrf2 is phosphorylated by additional growth-factor and nutrient-responsive kinases and is also regulated by O-linked glycosylation (An et al. 2005; Inoue et al. 2005; Tullet et al. 2008; Li et al. 2017). The residues of SKN-1C modified by these enzymes are shared with SKN-1A, but the extent of SKN-1A regulation by these factors is not known.
In addition to responding to extrinsic cues, SKN-1A/Nrf1 also regulates the proteasome for cell-autonomous adaptation to perturbed proteostasis. Accumulation of proteins with a propensity to misfold and aggregate, including the 42 amino acid human amyloid β peptide (Aβ1–42), causes activation of proteasome subunit gene expression by SKN-1A in C. elegans (Lehrbach and Ruvkun 2019). Activation of the proteasome in this context requires the same factors as the response to acute proteasome inhibition, indicating that a common mechanism of SKN-1A/Nrf1 activation mediates both responses. However, this response appears to occur in the absence of overt proteasome dysfunction, so it is unclear what prompts SKN-1A to escape proteasomal degradation. In any case, the increased expression of proteasome subunit genes driven by SKN-1A may maintain homeostasis by ensuring the efficient degradation of misfolded protein(s). Indeed, SKN-1A activation protects against the age-dependent aggregation and toxicity of Aβ1–42. Inactivation of Nrf in the mouse brain leads to neurodegeneration and formation of ubiquitinated protein aggregates (Kobayashi et al. 2011; Lee et al. 2011). In humans and rodent models, NGLY1 deficiency is associated with neurodegenerative symptoms and accumulation of intracellular protein aggregates (Lam et al. 2017; Asahina et al. 2020; Mueller et al. 2020).
These findings are suggestive of a role for SKN-1A/Nrf1 in homeostatic fine-tuning of the proteasome to ensure protein quality control during development and in adult tissues. Failure of protein quality control is a hallmark of aging (López-Otín et al. 2013). Interventions that slow age-related decline in protein degradation may serve to promote healthy aging and increase overall life span. Consistently, increased expression of SKN-1A is sufficient to extend life span, whereas the average life span of mutants lacking SKN-1A is shorter than that of the wild-type (Lehrbach and Ruvkun 2019). A wealth of literature links C. elegans skn-1 to life span extension by several interventions known to promote longevity in both the worm and mammals. These include reduced insulin/IGF signaling, reduced mTOR signaling, dietary restriction, and germline ablation (Blackwell et al. 2015). Genetic analyses of the role of skn-1 in aging have largely relied on mutations or RNAi reagents that inactivate both SKN-1A/Nrf1 and SKN-1C/Nrf2. Thus, the extent to which SKN-1A-dependent regulation of the proteasome promotes longevity in each of these contexts remains to be determined.
SKN-1A/Nrf1 as an Anti-Cancer Target
Proteasome inhibitors are used to treat cancers that challenge proteasome capacity such as multiple myeloma, but their usefulness is limited by cellular resistance mechanisms (Wallington-Beddoe et al. 2018). The requirement for Nrf1 in cellular responses to proteasome dysfunction suggests that pharmacological approaches to inhibit Nrf1 activation may be a promising means by which to enhance these drugs’ effectiveness. Prior to the assignment of NGLY1/PNG-1 deglycosylation to the C. elegans proteasome homeostasis pathway, it was generally assumed that aberrantly folded glycoproteins were the major clients for N-deglycosylation as a step in their proteolysis. The genetic analysis of C. elegans proteasomal homeostasis showed that a single, unexpected and non-abundant N-glycosylated target of NGLY1/PNG-1, the transcription factor SKN-1A/NRF1, is the key target of NGLY1/PNG-1 deglycosylation (Lehrbach et al. 2019). Strikingly, mapping the gene dependencies for growth of diverse cancer cell lines suggests that Nrf1 is similarly the key substrate for NGLY1-dependent deglycosylation in cancer cells (Tsherniak et al. 2017; Wang et al. 2017). Diverse tumor cell lines, ranging from gliomas to leukemias to skin cancers, are sensitive to loss of NGLY1, and almost all those cell lines are similarly sensitive to loss of the Nrf1 transcription factor or the DDI2 aspartic protease. This correlation is highly statistically significant—aside from Nrf1 and DDI2, there are no other genes that similarly correlate in essentiality with NGLY1 in the Cancer Dependency Map. Thus, as suggested by studies in C. elegans, Nrf1 is the major NGLY1 client protein that impacts growth in human tumor cell lines. The cancer cells that depend on Nrf1/NGLY1/DDI2 for proliferation arise from a range of tissues and harbor diverse oncogenic mutations. Why does growth of this diverse subset of cancer cell lines depend on proteasome homeostasis? Perhaps those cancers require constitutive Nrf1 activity to counter the effects of mutations or chromosomal abnormalities that disrupt protein homeostasis. An attractive model, not yet tested, is that the cancer cell lines that are most sensitive to the loss of NGLY1/Nrf1/DDI2 are aneuploid in chromosomal regions that challenge proteasome capacity by mismatching gene dosage between components of abundant protein complexes (Oromendia and Amon 2014). Nrf1, NGLY1, and DDI2 might therefore be attractive targets for cancer treatment even without cotreatment with a proteasome inhibitor drug.
Regulation of Lipid and Cholesterol Metabolism by SKN-1A/Nrf1
Excess cholesterol causes full-length Nrf1 (in both glycosylated and nonglycosylated forms) to accumulate in the ER. Nrf1 contains a putative cholesterol binding (CRAC) domain and deletion of either the CRAC domain, or the ER-targeting amino terminus of Nrf1, renders its levels insensitive to cholesterol. This suggests that Nrf1 binds to excess cholesterol in the ER, and cholesterol-bound Nrf1 undergoes reduced ERAD-dependent retrotranslocation to the cytosol. Liver-specific knockout of Nrf1 in the mouse causes hypersensitivity to cholesterol-induced damage indicating a role for this ER-retained Nrf1 in maintaining cholesterol homeostasis in vivo (Widenmaier et al. 2017). Additional studies of Nrf1-deficient livers have identified increased oxidative stress, ER stress, fatty liver pathology, inflammation, and development of hepatic tumors (Xu et al. 2005; Lee et al. 2013). Adipocyte-specific depletion of Nrf1 causes adipocyte hypertrophy further cementing the connection to organismal lipid homeostasis (Hou et al. 2018). Intriguingly, mice heterozygous for an Nrf1 knockout allele are hypersensitive to hepatic steatosis induced by the proteasome inhibitor bortezomib suggesting that defects in lipid homeostasis caused by loss of Nrf1 derive from, or are aggravated by, proteasome dysfunction (Lee et al. 2013). Fatty liver has also been observed in NGLY1 deficiency models, implicating deglycosylated nuclear Nrf1 in regulating both proteasome levels and lipid metabolism (Fujihira et al. 2020). The links between lipid metabolism and Nrf1 signaling may be conserved in C. elegans. skn-1 drives both elevated proteasome activity and adaptive regulation of lipid metabolism to increase longevity in germline-ablated animals (Steinbaugh et al. 2015), and gain-of-function mutations in skn-1 prevent increased fat accumulation in animals fed a high-carbohydrate diet (Pang et al. 2014).
CONCLUDING REMARKS
SKN-1A/Nrf1 is a master regulator of proteasome biogenesis in animals that adjusts cellular protein degradation to meet changing cellular needs during development, tissue homeostasis, and in response to stress. But SKN-1A/Nrf1 is not just a homeostat for protein degradation. It is regulated by growth factors, nutrient sensing pathways, and responds to protein folding defects even in the absence of proteasome dysfunction. SKN-1A/Nrf1 is also required for normal lipid, cholesterol, and mitochondrial metabolism. Our growing understanding of how this elaborately regulated transcription factor coordinates metabolism and proteostasis promises to unlock new therapeutic approaches to diseases including cancer, neurodegeneration, and age-associated metabolic dysfunction.
ACKNOWLEDGMENTS
This work is supported by National Institutes of Health Grant R35 GM142728 to N.L. and R01AG16636 to G.R., as well as Grace Foundation support to N.L. and G.R.
Footnotes
Editors: Susan Ferro-Novick, Tom A. Rapoport, and Randy Schekman
Additional Perspectives on The Endoplasmic Reticulum available at www.cshperspectives.org
REFERENCES
- An JH, Vranas K, Lucke M, Inoue H, Hisamoto N, Matsumoto K, Blackwell TK. 2005. Regulation of the Caenorhabditis elegans oxidative stress defense protein SKN-1 by glycogen synthase kinase-3. Proc Natl Acad Sci 102: 16275–16280. 10.1073/pnas.0508105102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asahina M, Fujinawa R, Nakamura S, Yokoyama K, Tozawa R, Suzuki T. 2020. Ngly1−/− rats develop neurodegenerative phenotypes and pathological abnormalities in their peripheral and central nervous systems. Hum Mol Genet 29: 1635–1647. 10.1093/hmg/ddaa059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell TK, Bowerman B, Priess JR, Weintraub H. 1994. Formation of a monomeric DNA binding domain by Skn-1 bZIP and homeodomain elements. Science 266: 621–628. 10.1126/science.7939715 [DOI] [PubMed] [Google Scholar]
- Blackwell TK, Steinbaugh MJ, Hourihan JM, Ewald CY, Isik M. 2015. SKN-1/Nrf, stress responses, and aging in Caenorhabditis elegans. Free Radic Biol Med 88: 290–301. 10.1016/j.freeradbiomed.2015.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan JY, Han XL, Kan YW. 1993. Cloning of Nrf1, an NF-E2-related transcription factor, by genetic selection in yeast. Proc Natl Acad Sci 90: 11371–11375. 10.1073/pnas.90.23.11371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan JY, Kwong M, Lu R, Chang J, Wang B, Yen TS, Kan YW. 1998. Targeted disruption of the ubiquitous CNC-bZIP transcription factor, Nrf-1, results in anemia and embryonic lethality in mice. EMBO J 17: 1779–1787. 10.1093/emboj/17.6.1779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Kwong M, Lu R, Ginzinger D, Lee C, Leung L, Chan JY. 2003. Nrf1 is critical for redox balance and survival of liver cells during development. Mol Cell Biol 23: 4673–4686. 10.1128/MCB.23.13.4673-4686.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Liu X, Lü F, Liu X, Ru Y, Ren Y, Yao L, Zhang Y. 2015. Transcription factor Nrf1 is negatively regulated by its O-GlcNAcylation status. FEBS Lett 589: 2347–2358. 10.1016/j.febslet.2015.07.030 [DOI] [PubMed] [Google Scholar]
- Choe KP, Przybysz AJ, Strange K. 2009. The WD40 repeat protein WDR-23 functions with the CUL4/DDB1 ubiquitin ligase to regulate nuclear abundance and activity of SKN-1 in Caenorhabditis elegans. Mol Cell Biol 29: 2704–2715. 10.1128/MCB.01811-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui M, Atmanli A, Morales MG, Tan W, Chen K, Xiao X, Xu L, Liu N, Bassel-Duby R, Olson EN. 2021. Nrf1 promotes heart regeneration and repair by regulating proteostasis and redox balance. Nat Commun 12: 5270. 10.1038/s41467-021-25653-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirac-Svejstrup AB, Walker J, Faull P, Encheva V, Akimov V, Puglia M, Perkins D, Kümper S, Hunjan SS, Blagoev B, et al. 2020. DDI2 is a ubiquitin-directed endoprotease responsible for cleavage of transcription factor NRF1. Mol Cell 79: 332–341.e7. 10.1016/j.molcel.2020.05.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming JA, Lightcap ES, Sadis S, Thoroddsen V, Bulawa CE, Blackman RK. 2002. Complementary whole-genome technologies reveal the cellular response to proteasome inhibition by PS-341. Proc Natl Acad Sci 99: 1461–1466. 10.1073/pnas.032516399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friguet B, Szweda LI. 1997. Inhibition of the multicatalytic proteinase (proteasome) by 4-hydroxy-2-nonenal cross-linked protein. FEBS Lett 405: 21–25. 10.1016/S0014-5793(97)00148-8 [DOI] [PubMed] [Google Scholar]
- Fujihira H, Masahara-Negishi Y, Akimoto Y, Hirayama H, Lee HC, Story BA, Mueller WF, Jakob P, Clauder-Münster S, Steinmetz LM, et al. 2020. Liver-specific deletion of Ngly1 causes abnormal nuclear morphology and lipid metabolism under food stress. Biochim Biophys Acta Mol Basis Dis 1866: 165588. 10.1016/j.bbadis.2019.165588 [DOI] [PubMed] [Google Scholar]
- Fukushige T, Smith HE, Miwa J, Krause MW, Hanover JA. 2017. A genetic analysis of the Caenorhabditis elegans detoxification response. Genetics 206: 939–952. 10.1534/genetics.117.202515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuse Y, Kobayashi M. 2017. Conservation of the Keap1-Nrf2 system: an evolutionary journey through stressful space and time. Molecules 22: 436. 10.3390/molecules22030436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover-Cutter KM, Lin S, Blackwell TK. 2013. Integration of the unfolded protein and oxidative stress responses through SKN-1/Nrf. PLoS Genet 9: e1003701. 10.1371/journal.pgen.1003701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimberg KB, Beskow A, Lundin D, Davis MM, Young P. 2011. Basic leucine zipper protein Cnc-C is a substrate and transcriptional regulator of the Drosophila 26S proteasome. Mol Cell Biol 31: 897–909. 10.1128/MCB.00799-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han JW, Valdez JL, Ho DV, Lee CS, Kim HM, Wang X, Huang L, Chan JY. 2017. Nuclear factor-erythroid-2 related transcription factor-1 (Nrf1) is regulated by O-GlcNAc transferase. Free Radic Biol Med 110: 196–205. 10.1016/j.freeradbiomed.2017.06.008 [DOI] [PubMed] [Google Scholar]
- Hou Y, Liu Z, Zuo Z, Gao T, Fu J, Wang H, Xu Y, Liu D, Yamamoto M, Zhu B, et al. 2018. Adipocyte-specific deficiency of Nfe2l1 disrupts plasticity of white adipose tissues and metabolic homeostasis in mice. Biochem Biophys Res Commun 503: 264–270. 10.1016/j.bbrc.2018.06.013 [DOI] [PubMed] [Google Scholar]
- Ibrahim L, Mesgarzadeh J, Xu I, Powers ET, Wiseman RL, Bollong MJ. 2020. Defining the functional targets of cap'n’collar transcription factors NRF1, NRF2, and NRF3. Antioxidants (Basel) 9: 1025. 10.3390/antiox9101025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue H, Hisamoto N, An JH, Oliveira RP, Nishida E, Blackwell TK, Matsumoto K. 2005. The C. elegans p38 MAPK pathway regulates nuclear localization of the transcription factor SKN-1 in oxidative stress response. Genes Dev 19: 2278–2283. 10.1101/gad.1324805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh K, Igarashi K, Hayashi N, Nishizawa M, Yamamoto M. 1995. Cloning and characterization of a novel erythroid cell-derived CNC family transcription factor heterodimerizing with the small Maf family proteins. Mol Cell Biol 15: 4184–4193. 10.1128/MCB.15.8.4184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD, Yamamoto M. 1999. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev 13: 76–86. 10.1101/gad.13.1.76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer S, Mast JD, Tsang H, Rodriguez TP, DiPrimio N, Prangley M, Sam FS, Parton Z, Perlstein EO. 2019. Drug screens of NGLY1 deficiency in worm and fly models reveal catecholamine, NRF2 and anti-inflammatory-pathway activation as potential clinical approaches. Dis Model Mech 12: dmm040576. 10.1242/dmm.040576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnsen O, Skammelsrud N, Luna L, Nishizawa M, Prydz H, Kolsto AB. 1996. Small Maf proteins interact with the human transcription factor TCF11/Nrf1/LCR-F1. Nucleic Acids Res 24: 4289–4297. 10.1093/nar/24.21.4289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnsen O, Murphy P, Prydz H, Kolsto AB. 1998. Interaction of the CNC-bZIP factor TCF11/LCR-F1/Nrf1 with MafG: binding-site selection and regulation of transcription. Nucleic Acids Res 26: 512–520. 10.1093/nar/26.2.512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jozwiak P, Forma E, Brys M, Krzeslak A. 2014. O-GlcNAcylation and metabolic reprograming in cancer. Front Endocrinol (Lausanne) 5: 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju D, Xie Y. 2004. Proteasomal degradation of RPN4 via two distinct mechanisms, ubiquitin-dependent and -independent. J Biol Chem 279: 23851–23854. 10.1074/jbc.C400111200 [DOI] [PubMed] [Google Scholar]
- Kaplun L, Tzirkin R, Bakhrat A, Shabek N, Ivantsiv Y, Raveh D. 2005. The DNA damage-inducible UbL-UbA protein Ddi1 participates in Mec1-mediated degradation of Ho endonuclease. Mol Cell Biol 25: 5355–5362. 10.1128/MCB.25.13.5355-5362.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith SA, Maddux SK, Zhong Y, Chinchankar MN, Ferguson AA, Ghazi A, Fisher AL. 2016. Graded proteasome dysfunction in Caenorhabditis elegans activates an adaptive response involving the conserved SKN-1 and ELT-2 transcription factors and the autophagy-lysosome pathway. PLoS Genet 12: e1005823. 10.1371/journal.pgen.1005823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Xing W, Wergedal J, Chan JY, Mohan S. 2010. Targeted disruption of nuclear factor erythroid-derived 2-like 1 in osteoblasts reduces bone size and bone formation in mice. Physiol Genomics 40: 100–110. 10.1152/physiolgenomics.00105.2009 [DOI] [PubMed] [Google Scholar]
- Kobayashi A. 2020. Roles of NRF3 in the hallmarks of cancer: proteasomal inactivation of tumor suppressors. Cancers (Basel) 12: 2681. 10.3390/cancers12092681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi A, Ito E, Toki T, Kogame K, Takahashi S, Igarashi K, Hayashi N, Yamamoto M. 1999. Molecular cloning and functional characterization of a new cap'n’collar family transcription factor Nrf3. J Biol Chem 274: 6443–6452. 10.1074/jbc.274.10.6443 [DOI] [PubMed] [Google Scholar]
- Kobayashi A, Tsukide T, Miyasaka T, Morita T, Mizoroki T, Saito Y, Ihara Y, Takashima A, Noguchi N, Fukamizu A, et al. 2011. Central nervous system-specific deletion of transcription factor Nrf1 causes progressive motor neuronal dysfunction. Genes Cells 16: 692–703. 10.1111/j.1365-2443.2011.01522.x [DOI] [PubMed] [Google Scholar]
- Koizumi S, Irie T, Hirayama S, Sakurai Y, Yashiroda H, Naguro I, Ichijo H, Hamazaki J, Murata S. 2016. The aspartyl protease DDI2 activates Nrf1 to compensate for proteasome dysfunction. eLife 5: e18357. 10.7554/eLife.18357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam C, Ferreira C, Krasnewich D, Toro C, Latham L, Zein WM, Lehky T, Brewer C, Baker EH, Thurm A, et al. 2017. Prospective phenotyping of NGLY1-CDDG, the first congenital disorder of deglycosylation. Genet Med 19: 160–168. 10.1038/gim.2016.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoie H, Gagnon J, Therrien M. 2020. ERK signalling: A master regulator of cell behaviour, life and fate. Nat Rev Mol Cell Biol 21: 607–632. 10.1038/s41580-020-0255-7 [DOI] [PubMed] [Google Scholar]
- Lee CS, Lee C, Hu T, Nguyen JM, Zhang J, Martin MV, Vawter MP, Huang EJ, Chan JY. 2011. Loss of nuclear factor E2-related factor 1 in the brain leads to dysregulation of proteasome gene expression and neurodegeneration. Proc Natl Acad Sci 108: 8408–8413. 10.1073/pnas.1019209108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CS, Ho DV, Chan JY. 2013. Nuclear factor-erythroid 2-related factor 1 regulates expression of proteasome genes in hepatocytes and protects against endoplasmic reticulum stress and steatosis in mice. FEBS J 280: 3609–3620. 10.1111/febs.12350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrbach NJ, Ruvkun G. 2016. Proteasome dysfunction triggers activation of SKN-1A/Nrf1 by the aspartic protease DDI-1. eLife 5: e17721. 10.7554/eLife.17721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrbach NJ, Ruvkun G. 2019. Endoplasmic reticulum-associated SKN-1A/Nrf1 mediates a cytoplasmic unfolded protein response and promotes longevity. eLife 8: e44425. 10.7554/eLife.44425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrbach NJ, Breen PC, Ruvkun G. 2019. Protein sequence editing of SKN-1A/Nrf1 by peptide:N-glycanase controls proteasome gene expression. Cell 177: 737–750.e15. 10.1016/j.cell.2019.03.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung L, Kwong M, Hou S, Lee C, Chan JY. 2003. Deficiency of the Nrf1 and Nrf2 transcription factors results in early embryonic lethality and severe oxidative stress. J Biol Chem 278: 48021–48029. 10.1074/jbc.M308439200 [DOI] [PubMed] [Google Scholar]
- Li X, Matilainen O, Jin C, Glover-Cutter KM, Holmberg CI, Blackwell TK. 2011. Specific SKN-1/Nrf stress responses to perturbations in translation elongation and proteasome activity. PLoS Genet 7: e1002119. 10.1371/journal.pgen.1002119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Liu X, Wang D, Su L, Zhao T, Li Z, Lin C, Zhang Y, Huang B, Lu J, et al. 2017. O-GlcNAcylation of SKN-1 modulates the lifespan and oxidative stress resistance in Caenorhabditis elegans. Sci Rep 7: 43601. 10.1038/srep43601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Kerins MJ, Tian W, Neupane D, Zhang DD, Ooi A. 2019. Differential and overlapping targets of the transcriptional regulators NRF1, NRF2, and NRF3 in human cells. J Biol Chem 294: 18131–18149. 10.1074/jbc.RA119.009591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. 2013. The hallmarks of aging. Cell 153: 1194–1217. 10.1016/j.cell.2013.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q. 2013. Role of nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol 53: 401–426. 10.1146/annurev-pharmtox-011112-140320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei S, Ayala R, Ramarathinam SH, Illing PT, Faridi P, Song J, Purcell AW, Croft NP. 2020. Immunopeptidomic analysis reveals that deamidated HLA-bound peptides arise predominantly from deglycosylated precursors. Mol Cell Proteomics 19: 1236–1247. 10.1074/mcp.RA119.001846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meiners S, Heyken D, Weller A, Ludwig A, Stangl K, Kloetzel PM, Krüger E. 2003. Inhibition of proteasome activity induces concerted expression of proteasome genes and de novo formation of mammalian proteasomes. J Biol Chem 278: 21517–21525. 10.1074/jbc.M301032200 [DOI] [PubMed] [Google Scholar]
- Moi P, Chan K, Asunis I, Cao A, Kan YW. 1994. Isolation of NF-E2-related factor 2 (Nrf2), a NF-E2-like basic leucine zipper transcriptional activator that binds to the tandem NF-E2/AP1 repeat of the β-globin locus control region. Proc Natl Acad Sci 91: 9926–9930. 10.1073/pnas.91.21.9926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller WF, Jakob P, Sun H, Clauder-Münster S, Ghidelli-Disse S, Ordonez D, Boesche M, Bantscheff M, Collier P, Haase B, et al. 2020. Loss of N-glycanase 1 alters transcriptional and translational regulation in K562 cell lines. G3 (Bethesda) 10: 1585–1597. 10.1534/g3.119.401031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northrop A, Vangala JR, Feygin A, Radhakrishnan SK. 2020. Disabling the protease DDI2 attenuates the transcriptional activity of NRF1 and potentiates proteasome inhibitor cytotoxicity. Int J Mol Sci 21: 327. 10.3390/ijms21010327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowicka U, Zhang D, Walker O, Krutauz D, Castañeda CA, Chaturvedi A, Chen TY, Reis N, Glickman MH, Fushman D. 2015. DNA-damage-inducible 1 protein (Ddi1) contains an uncharacteristic ubiquitin-like domain that binds ubiquitin. Structure 23: 542–557. 10.1016/j.str.2015.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsuji M, Katsuoka F, Kobayashi A, Aburatani H, Hayes JD, Yamamoto M. 2008. Nrf1 and Nrf2 play distinct roles in activation of antioxidant response element-dependent genes. J Biol Chem 283: 33554–33562. 10.1074/jbc.M804597200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oromendia AB, Amon A. 2014. Aneuploidy: implications for protein homeostasis and disease. Dis Model Mech 7: 15–20. 10.1242/dmm.013391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang S, Lynn DA, Lo JY, Paek J, Curran SP. 2014. SKN-1 and Nrf2 couples proline catabolism with lipid metabolism during nutrient deprivation. Nat Commun 5: 5048. 10.1038/ncomms6048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitoniak A, Bohmann D. 2015. Mechanisms and functions of Nrf2 signaling in Drosophila. Free Radic Biol Med 88: 302–313. 10.1016/j.freeradbiomed.2015.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhakrishnan SK, Lee CS, Young P, Beskow A, Chan JY, Deshaies RJ. 2010. Transcription factor Nrf1 mediates the proteasome recovery pathway after proteasome inhibition in mammalian cells. Mol Cell 38: 17–28. 10.1016/j.molcel.2010.02.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhakrishnan SK, den Besten W, Deshaies RJ. 2014. p97-dependent retrotranslocation and proteolytic processing govern formation of active Nrf1 upon proteasome inhibition. eLife 3: e01856. 10.7554/eLife.01856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinheckel T, Ullrich O, Sitte N, Grune T. 2000. Differential impairment of 20S and 26S proteasome activities in human hematopoietic K562 cells during oxidative stress. Arch Biochem Biophys 377: 65–68. 10.1006/abbi.2000.1717 [DOI] [PubMed] [Google Scholar]
- Ruvkun G, Ji F, Sadreyev RI. 2021. Phylogenetic evidence for asparagine to aspartic acid protein editing of N-glycosylated SARS-CoV-2 viral proteins by NGLY1 deglycosylation/deamidation suggests an unusual vaccination strategy. bioRxiv 10.1101/2021.09.11.459891 [DOI] [Google Scholar]
- Sekine H, Okazaki K, Kato K, Alam MM, Shima H, Katsuoka F, Tsujita T, Suzuki N, Kobayashi A, Igarashi K, et al. 2018. O-GlcNAcylation signal mediates proteasome inhibitor resistance in cancer cells by stabilizing NRF1. Mol Cell Biol 38: e00252-18. 10.1128/MCB.00252-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha Z, Goldberg AL. 2014. Proteasome-mediated processing of Nrf1 is essential for coordinate induction of all proteasome subunits and p97. Curr Biol 24: 1573–1583. 10.1016/j.cub.2014.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivdasani RA, Rosenblatt MF, Zucker-Franklin D, Jackson CW, Hunt P, Saris CJ, Orkin SH. 1995. Transcription factor NF-E2 is required for platelet formation independent of the actions of thrombopoeitin/MGDF in megakaryocyte development. Cell 81: 695–704. 10.1016/0092-8674(95)90531-6 [DOI] [PubMed] [Google Scholar]
- Sirkis R, Gerst JE, Fass D. 2006. Ddi1, a eukaryotic protein with the retroviral protease fold. J Mol Biol 364: 376–387. 10.1016/j.jmb.2006.08.086 [DOI] [PubMed] [Google Scholar]
- Steffen J, Seeger M, Koch A, Krüger E. 2010. Proteasomal degradation is transcriptionally controlled by TCF11 via an ERAD-dependent feedback loop. Mol Cell 40: 147–158. 10.1016/j.molcel.2010.09.012 [DOI] [PubMed] [Google Scholar]
- Steinbaugh MJ, Narasimhan SD, Robida-Stubbs S, Moronetti Mazzeo LE, Dreyfuss JM, Hourihan JM, Raghavan P, Operaña TN, Esmaillie R, Blackwell TK. 2015. Lipid-mediated regulation of SKN-1/Nrf in response to germ cell absence. eLife 4: e07836. 10.7554/eLife.07836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z, Brodsky JL. 2019. Protein quality control in the secretory pathway. J Cell Biol 218: 3171–3187. 10.1083/jcb.201906047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Seko A, Kitajima K, Inoue Y, Inoue S. 1994. Purification and enzymatic properties of peptide:N-glycanase from C3H mouse-derived L-929 fibroblast cells. Possible widespread occurrence of post-translational remodification of proteins by N-deglycosylation. J Biol Chem 269: 17611–17618. 10.1016/S0021-9258(17)32485-7 [DOI] [PubMed] [Google Scholar]
- Suzuki T, Huang C, Fujihira H. 2016. The cytoplasmic peptide:N-glycanase (NGLY1)—structure, expression and cellular functions. Gene 577: 1–7. 10.1016/j.gene.2015.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykiotis GP, Bohmann D. 2010. Stress-activated cap'n’collar transcription factors in aging and human disease. Sci Signal 3: re3. 10.1126/scisignal.3112re3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlin FM, Gerling-Driessen UIM, Liu YC, Flynn RA, Vangala JR, Lentz CS, Clauder-Muenster S, Jakob P, Mueller WF, Ordoñez-Rueda D, et al. 2017. Inhibition of NGLY1 inactivates the transcription factor Nrf1 and potentiates proteasome inhibitor cytotoxicity. ACS Cent Sci 3: 1143–1155. 10.1021/acscentsci.7b00224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triezenberg SJ. 1995. Structure and function of transcriptional activation domains. Curr Opin Genet Dev 5: 190–196. 10.1016/0959-437X(95)80007-7 [DOI] [PubMed] [Google Scholar]
- Tsherniak A, Vazquez F, Montgomery PG, Weir BA, Kryukov G, Cowley GS, Gill S, Harrington WF, Pantel S, Krill-Burger JM, et al. 2017. Defining a cancer dependency map. Cell 170: 564–576.e16. 10.1016/j.cell.2017.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tullet JM, Hertweck M, An JH, Baker J, Hwang JY, Liu S, Oliveira RP, Baumeister R, Blackwell TK. 2008. Direct inhibition of the longevity-promoting factor SKN-1 by insulin-like signaling in C. elegans. Cell 132: 1025–1038. 10.1016/j.cell.2008.01.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallington-Beddoe CT, Sobieraj-Teague M, Kuss BJ, Pitson SM. 2018. Resistance to proteasome inhibitors and other targeted therapies in myeloma. Br J Haematol 182: 11–28. 10.1111/bjh.15210 [DOI] [PubMed] [Google Scholar]
- Wang W, Chan JY. 2006. Nrf1 is targeted to the endoplasmic reticulum membrane by an N-terminal transmembrane domain. Inhibition of nuclear translocation and transacting function. J Biol Chem 281: 19676–19687. 10.1074/jbc.M602802200 [DOI] [PubMed] [Google Scholar]
- Wang L, Mao X, Ju D, Xie Y. 2004. Rpn4 is a physiological substrate of the Ubr2 ubiquitin ligase. J Biol Chem 279: 55218–55223. 10.1074/jbc.M410085200 [DOI] [PubMed] [Google Scholar]
- Wang T, Yu H, Hughes NW, Liu B, Kendirli A, Klein K, Chen WW, Lander ES, Sabatini DM. 2017. Gene essentiality profiling reveals gene networks and synthetic lethal interactions with oncogenic Ras. Cell 168: 890–903.e15. 10.1016/j.cell.2017.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widenmaier SB, Snyder NA, Nguyen TB, Arduini A, Lee GY, Arruda AP, Saksi J, Bartelt A, Hotamisligil GS. 2017. NRF1 is an ER membrane sensor that is central to cholesterol homeostasis. Cell 171: 1094–1109.e15. 10.1016/j.cell.2017.10.003 [DOI] [PubMed] [Google Scholar]
- Wu X, Rapoport TA. 2018. Mechanistic insights into ER-associated protein degradation. Curr Opin Cell Biol 53: 22–28. 10.1016/j.ceb.2018.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, Varshavsky A. 2001. RPN4 is a ligand, substrate, and transcriptional regulator of the 26S proteasome: A negative feedback circuit. Proc Natl Acad Sci 98: 3056–3061. 10.1073/pnas.071022298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Chen L, Leung L, Yen TS, Lee C, Chan JY. 2005. Liver-specific inactivation of the Nrf1 gene in adult mouse leads to nonalcoholic steatohepatitis and hepatic neoplasia. Proc Natl Acad Sci 102: 4120–4125. 10.1073/pnas.0500660102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Hu Y, Deng Y, Chen Y, Hua H, Huang S, Nie Q, Pan Q, Ma DK, Ma L. 2018. WDR-23 and SKN-1/Nrf2 coordinate with the BLI-3 dual oxidase in response to iodide-triggered oxidative stress. G3 (Bethesda) 8: 3515–3527. 10.1534/g3.118.200586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K, Huang R, Fujihira H, Suzuki T, Yan N. 2018. N-glycanase NGLY1 regulates mitochondrial homeostasis and inflammation through NRF1. J Exp Med 215: 2600–2616. 10.1084/jem.20180783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip MCJ, Bodnar NO, Rapoport TA. 2020. Ddi1 is a ubiquitin-dependent protease. Proc Natl Acad Sci 117: 7776–7781. 10.1073/pnas.1902298117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida Y, Tokunaga F, Chiba T, Iwai K, Tanaka K, Tai T. 2003. Fbs2 is a new member of the E3 ubiquitin ligase family that recognizes sugar chains. J Biol Chem 278: 43877–43884. 10.1074/jbc.M304157200 [DOI] [PubMed] [Google Scholar]
- Yoshida Y, Asahina M, Murakami A, Kawawaki J, Yoshida M, Fujinawa R, Iwai K, Tozawa R, Matsuda N, Tanaka K, et al. 2021. Loss of peptide:N-glycanase causes proteasome dysfunction mediated by a sugar-recognizing ubiquitin ligase. Proc Natl Acad Sci 118: e2102902118. 10.1073/pnas.2102902118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Hayes JD. 2013. The membrane-topogenic vectorial behaviour of Nrf1 controls its post-translational modification and transactivation activity. Sci Rep 3: 2006. 10.1038/srep02006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Crouch DH, Yamamoto M, Hayes JD. 2006. Negative regulation of the Nrf1 transcription factor by its N-terminal domain is independent of Keap1: Nrf1, but not Nrf2, is targeted to the endoplasmic reticulum. Biochem J 399: 373–385. 10.1042/BJ20060725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Nicholatos J, Dreier JR, Ricoult SJ, Widenmaier SB, Hotamisligil GS, Kwiatkowski DJ, Manning BD. 2014a. Coordinated regulation of protein synthesis and degradation by mTORC1. Nature 513: 440–443. 10.1038/nature13492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Ren Y, Li S, Hayes JD. 2014b. Transcription factor Nrf1 is topologically repartitioned across membranes to enable target gene transactivation through its acidic glucose-responsive domains. PLoS ONE 9: e93458. 10.1371/journal.pone.0093458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Qu HY, Wu Z, Na H, Hourihan J, Zhang F, Zhu F, Isik M, Walhout AJM, Feng YX, et al. 2021. ERK signaling licenses SKN-1A/NRF1 for proteasome production and proteasomal stress resistance. bioRxiv 10.1101/2021.01.04.425272 [DOI] [Google Scholar]