Abstract
Most cholesterol in mammalian cells is stored in the plasma membrane (PM). Cholesterol transport from the PM to low-sterol regulatory regions of the endoplasmic reticulum (ER) controls cholesterol synthesis and uptake, and thereby influences the rates of cholesterol flux between tissues of complex organisms. Cholesterol transfer to the ER is also required for steroidogenesis, oxysterol and bile acid synthesis, and cholesterol esterification. The ER-resident Aster proteins (Aster-A, -B, and -C) form contacts with the PM to move cholesterol to the ER in mammals. Mice lacking Aster-B have low adrenal cholesteryl ester stores and impaired steroidogenesis because of a defect in cholesterol transport from high-density lipoprotein (HDL) to the ER. This work reviews the molecular characteristics of Asters, their role in HDL- and low-density lipoprotein (LDL)-cholesterol movement, and how cholesterol transferred to the ER is utilized by cells. The roles of other lipid transporters and of membrane lipid organization in maintaining aspects of cholesterol homeostasis are also highlighted.
Cholesterol is an integral component of mammalian cell membranes. Metabolites of cholesterol, which include oxysterols, bile acids, steroid hormones, and vitamin D, play diverse roles in physiology. Most cells obtain cholesterol from endogenous synthesis from acetyl CoA or by exogenous uptake in the form of low-density lipoproteins (LDLs) or high-density lipoproteins (HDLs), although unesterified cholesterol is also delivered directly to intestinal epithelial cells in food and bile. The ability of mammalian cells to survive and function depends on tight control of cellular free cholesterol levels. A delicate balance between synthesis, uptake, modification, and efflux allows cells to maintain the appropriate distribution of cholesterol among organelles. Certain cells such as adipocytes, hepatocytes, and enterocytes also store excess cholesterol as cholesteryl esters (CEs) in lipid droplets.
Impaired or excessive cholesterol flux through cellular membranes defines several genetic and chronic diseases. For example, lysosomal cholesterol accumulation is linked to neurodegeneration in people with mutations in Niemann–Pick C1 (NPC1) or NPC2 (Vanier 2010); impaired mitochondrial cholesterol import causes adrenal hyperplasia in people with defects in steroidogenic acute regulatory protein (STARD1) (Clark et al. 1994; Bose et al. 1996); and impaired plasma membrane (PM) cholesterol mobilization to extracellular acceptors causes multiorgan lipid accumulation in people with mutations in ATP-binding cassette subfamily A member 1 (Tangier disease) (Brooks-Wilson et al. 1999). Sustained LDL-cholesterol accumulation by macrophages in the artery wall leads to foam cell formation and the development of atherosclerosis (Brown and Goldstein 1986).
Up to 90% of cholesterol in mammalian cells resides in the PM where it accounts for ∼30–40 mol% of PM lipids (Lange et al. 1989; Slotte et al. 1989; Das et al. 2014). By contrast, the endoplasmic reticulum (ER), which contains the sterol response element-binding protein (SREBP) machinery that regulates cholesterol synthesis and uptake, contains just ∼1% of total cellular cholesterol (Radhakrishnan et al. 2008). Sterols move from the PM to the ER and vice versa with a half-time of ∼10 min (DeGrella and Simoni 1982; Baumann et al. 2005; Wüstner et al. 2005). This rapid and continuous sterol flow from the PM to the low-sterol regulatory regions of the ER allows relatively small changes in PM cholesterol to be detected with high sensitivity by SREBPs in the ER (Infante and Radhakrishnan 2017). When cholesterol is scarce and its movement to the ER is low, SREBP-2 is processed, and cholesterol is actively synthesized and internalized (for review, see Brown et al. 2018). When cholesterol availability increases, more cholesterol is transported to the ER, turning off synthesis and uptake. Production of liver X receptor (LXR) agonists in response to ER cholesterol delivery also coordinately activates genes in the cholesterol efflux pathway. Excess ER cholesterol is effluxed, esterified, oxidized, or reverted to other organelles to keep ER cholesterol levels low. In this way, the facilitated transport of cholesterol between the PM and ER controls the balance between endogenous cholesterol synthesis and exogenous uptake, and thereby influences the rates of cholesterol flux between tissues of complex organisms.
Recent studies have described Aster-A, -B and -C (encoded by the Gramd1a-c genes), a family of integral ER membrane proteins that mediate PM–ER cholesterol transfer in mammalian cells (Sandhu et al. 2018). The central importance of these proteins in cholesterol movement to the ER has been confirmed independently by several groups (Naito et al. 2019; Trinh et al. 2022). When PM cholesterol levels rise, Asters form contacts with the PM to move cholesterol down a concentration gradient to the ER (Sandhu et al. 2018; Naito et al. 2019). By delivering cholesterol to ER sterol sensors and modifiers, Asters play key roles in cholesterol homeostasis.
CHOLESTEROL ACCESSIBILITY
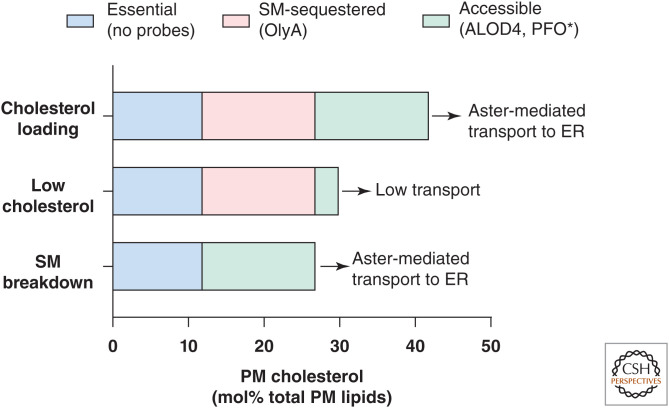
Cholesterol in excess of a membranes’ capacity to sequester it in phospholipids and sphingolipids becomes “accessible” on the membrane surface for transport, binding, or modification by nearby proteins (Radhakrishnan and McConnell 2000). Accessible cholesterol has traditionally been measured based on its availability for extraction from membranes by cyclodextrins (Haynes et al. 2000) or modification by cholesterol oxidase (Lange et al. 2004). The development of specific probes based on the cholesterol-binding toxins perfringolysin O (PFO*) and domain 4 of anthrolysin O (ALOD4) has greatly facilitated the study of accessible cholesterol (Flanagan et al. 2009; Das et al. 2013; Gay et al. 2015). These probes do not measure total PM cholesterol, rather the portion of cholesterol that is not sequestered by phospholipids. When the cholesterol content of a membrane is low, cholesterol accessibility is low and remains low until cholesterol levels rise above a threshold determined by their phospholipid content; after this point, cholesterol accessibility increases dramatically (Radhakrishnan and McConnell 2000). Because accessible cholesterol is readily transported or modified, the accessible cholesterol pool constitutes only a minor portion of total membrane cholesterol content under physiological conditions (Fig. 1; Das et al. 2014).
Figure 1.
Three pools of cholesterol in the plasma membrane. The accessible pool (green) is available for Aster-mediated transport to the endoplasmic reticulum (ER). The sphingomyelin-sequestered pool (pink) is not available for transport but can be made accessible by hydrolysis of plasma membrane sphingomyelin. The essential pool (blue) is sequestered by phospholipids and proteins and is not available for transport. Depletion of the essential pool results in impaired plasma membrane (PM) integrity. The probes ALOD4 and PFO* bind accessible cholesterol, OlyA binds sphingomyelin (SM)-sequestered cholesterol, and the essential pool currently lacks probes. (Figure based on Das et al. 2014.)
Phospholipid and sphingolipid species differ in their capacity to form complexes with cholesterol (Demel et al. 1977; Keller et al. 2000; Lönnfors et al. 2011). Therefore, the point at which cholesterol becomes accessible depends in part on the phospholipid composition of the membrane. Cholesterol has the highest affinity for sphingomyelin (SM) and other sphingolipids, followed by phospholipids with saturated fatty acyl chains, and forms lower affinity complexes with unsaturated phospholipids (Niu and Litman 2002; Lange et al. 2013). The outer leaflet of the PM is enriched in SM and saturated phosphatidylcholine species, resulting in high-capacity cholesterol retention in the PM (Verkleij et al. 1973). Cholesterol complexed with SM comprises ∼15 mol% total PM lipids and is not available for transport but can be biochemically probed with a modified version of the fungal protein Ostreolysin A (OlyA) (Fig. 1; Endapally et al. 2019). Release of the SM-sequestered pool by hydrolysis of SM expands the accessible cholesterol pool, making it available for transport to the ER, with little change to total membrane cholesterol (Fig. 1). Another pool of cholesterol (∼12 mol% total PM lipids), termed the “essential” pool, is sequestered by phospholipids and other membrane factors, and currently lacks probes (Fig. 1; Das et al. 2014). Cholesterol becomes accessible in the PM when it reaches ∼35 mol% total PM lipids. Although the sequestered pools of PM cholesterol tend to remain constant, the accessible pool fluctuates depending on whether the cell is cholesterol replete or deplete (Das et al. 2014).
In contrast to the PM, the ER has a high polyunsaturated phospholipid content and is almost completely devoid of complex sphingolipid, conferring it with lower capacity to sequester cholesterol (van Meer et al. 2008). Accordingly, cholesterol becomes available to interact with cholesterol homeostatic machinery such as SREBP cleavage-activating protein (SCAP) in the ER when it reaches just ∼5 mol% total ER lipids (Radhakrishnan et al. 2008; Sokolov and Radhakrishnan 2010). When ER cholesterol is low, SCAP exists in a conformation that allows it to associate with COPII-coated vesicles and escort SREBP-2 to the Golgi for processing to an active transcription factor that promotes cholesterol synthesis and uptake (Brown et al. 2002; Radhakrishnan et al. 2008). When ER cholesterol rises above ∼5 mol%, sterols bind SCAP to ER retention insulin-induced gene proteins (INSIGs) so that SREBP-2 processing is blocked (Yang et al. 2002; Radhakrishnan et al. 2008). Excess ER cholesterol is esterified by acyl-CoA:cholesterol acyltransferase (ACAT) to CE for storage in lipid droplets (or in the case of hepatocytes and enterocytes, export as lipoproteins). Surplus ER cholesterol is also converted to oxysterols, which further blunts SREBP-2 processing and activates LXRs to promote cholesterol efflux (for review, see Wang and Tontonoz 2018). Because of the continuous transport of PM accessible cholesterol to the ER for sensing, oxidation, esterification, or export, the steady-state levels of accessible cholesterol in the PM and ER likely do not differ substantially, allowing bidirectional cholesterol movement without the need to overcome large concentration gradients.
ASTER PROTEINS
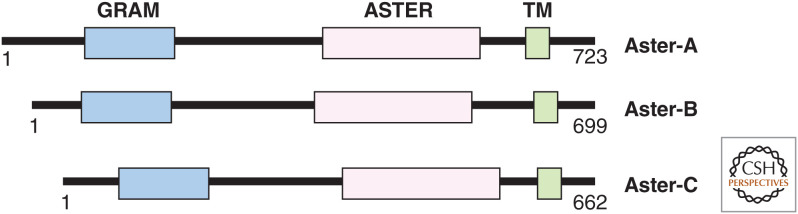
The Aster (Greek for “star”) proteins (encoded by gramd1a-c) have an amino-terminal GRAM domain that facilitates their phosphatidylserine (PS)-dependent recruitment to cholesterol-enriched PMs, a central cholesterol-carrying ASTER domain, and a single-pass transmembrane domain at the carboxyl terminus that anchors them to the ER (Fig. 2; Sandhu et al. 2018). Aster-A is ubiquitously expressed in tissues and cell lines, with particularly high copy numbers in the brain; Aster-B is highly expressed in macrophages, steroidogenic tissues, and the brain; Aster-C is abundant in the liver and testes, but poorly expressed in most immortalized cell lines. Asters are localized throughout ER membranes in cholesterol-deplete conditions but rapidly relocate to contact sites between the ER and PM in cells loaded with HDL or cholesterol-cyclodextrin complexes (Sandhu et al. 2018), or after the liberation of SM-sequestered cholesterol by exogenous addition of a sphingomyelinase enzyme (Naito et al. 2019). Aster-B transports HDL cholesterol in the adrenal gland, and mice lacking Aster-B have low adrenal CEs and impaired steroidogenesis (Sandhu et al. 2018). Aster-mediated cholesterol transport in other tissues remains to be characterized.
Figure 2.
The domain structure of Aster-A, -B and -C. The GRAM domain interacts with accessible cholesterol and phosphatidylserine (PS) at the plasma membrane. The ASTER domain binds and transports cholesterol. The transmembrane (TM) domain anchors the proteins to the endoplasmic reticulum (ER).
The Cholesterol-Carrying ASTER Domain
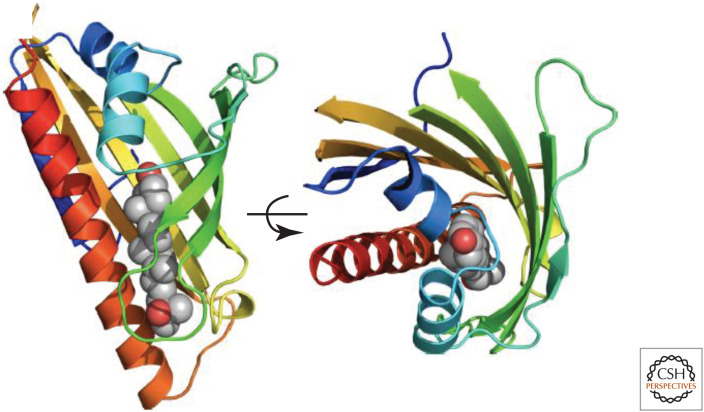
The central ASTER domain of Aster-A, -B, and -C binds cholesterol and select oxysterols and mediates their transfer between membranes in vitro (Sandhu et al. 2018; Naito et al. 2019). The three-dimensional crystal structure of the ASTER domain of Aster-A in complex with 25-hydroxycholesterol shows a seven-stranded β-sheet that curves into a basket to hold the oxysterol ligand (Fig. 3). The basket is closed by an extended carboxy-terminal helix and two shorter helices after the amino-terminal β-strand, with a small opening between β-strands 3 and 4. This opening is likely dynamic to allow sterols to gain access to the pocket. Therefore, the three-dimensional structure of the ASTER domain resembles the sterol-binding START domain (Tsujishita and Hurley 2000) and the START-like domain found in yeast lipid transfer proteins anchored at membrane contact sites (LAM proteins) (Elbaz-Alon et al. 2015; Gatta et al. 2015; Murley et al. 2015; Horenkamp et al. 2018; Jentsch et al. 2018; Tong et al. 2018), despite low sequence similarity. The closed basket conformation of the lipid-carrying domain requires Asters to carry one lipid molecule at a time and necessitates rotation of the pocket to dump cholesterol into the ER after receiving it at the PM (Sandhu et al. 2018). Molecular dynamics experiments suggest that water penetration into the sterol-binding cavity of the yeast START-like domain is involved in sterol uptake and release (Khelashvili et al. 2019). Further structural insights are required to fully understand the dynamics of ligand binding and release by Asters and other lipid transporters.
Figure 3.
Crystal structure of the ASTER domain of Aster-A (mouse). The ribbons are colored from blue to red moving from the amino to the carboxyl terminus. The 25-OHC is displayed as atomic spheres (gray = carbon, red = oxygen). The structure is rotated 90° about the indicated axis in the right-hand display. The sterol-binding pocket is located between a curved β-sheet and an extended carboxy-terminal helix. Structure determined with Dr. John Schwabe. (Figure reprinted from Sandhu et al. 2018 with permission from Elsevier © 2018.)
A screen to identify ASTER domain-binding compounds revealed that U18666A, best known for its ability to bind the sterol-sensing domain of NPC1 and block lysosomal cholesterol egress, inhibits cholesterol transport by each of the Asters (Xiao et al. 2021). This observation calls for caution when using U18666A to study intracellular cholesterol trafficking because, in addition to blocking lysosomal cholesterol export, it also blocks PM-to-ER cholesterol movement. The ability of U18666A to inhibit Aster function explains some previous observations of the effects of this compound on cellular metabolism. For example, U18666A has been reported to slow cholesterol esterification in cells treated with exogenous sphingomyelinase (Härmälä et al. 1994; Skiba et al. 1996), which induces PM–ER cholesterol movement by liberating SM-sequestered cholesterol. U18666A also blunts 27-hydroxycholesterol production and 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGCR) degradation in fibroblasts loaded with cyclodextrin-cholesterol (Lange et al. 2009). Xiao et al. (2021) identified the U18666A derivative AI-3d as a selective inhibitor of Asters. This compound blocks the ability of exogenously supplied cholesterol to suppress SREBP-2 processing and promote CE synthesis (Xiao et al. 2021). A small-molecule inhibitor of Aster-A has also been described (Laraia et al. 2019).
The Membrane-Binding GRAM Domain
The amino-terminal GRAM domain of Aster proteins is both necessary and sufficient for cholesterol-dependent recruitment to PMs (Sandhu et al. 2018). The GRAM domain is a phospholipid-interacting motif initially identified in glucosyltransferases, Rab-like GTPase activators, and myotubularins, and has since been described in other membrane-associated proteins (Doerks et al. 2000; Begley et al. 2003). The GRAM domain is structurally similar to the pleckstrin homology (PH) domain, another membrane recognition motif commonly present in lipid-transfer proteins (Harlan et al. 1994; Lemmon et al. 1995). An ectopically expressed GRAM43–189 domain of Aster-B (i.e., lacking all components of the protein except for the membrane-sensing motif) localizes to the cytoplasm of fibroblasts and is more rapidly recruited to the PM in response to cholesterol loading compared to the full-length protein. Conversely, a truncated Aster-B that lacks the GRAM fails to move to the PM (Sandhu et al. 2018). Recruitment of the GRAM domain to accessible cholesterol-containing membranes is enhanced by the presence of the anionic phospholipid PS (Naito et al. 2019; Ferrari et al. 2020), which is enriched on the inner leaflet of the PM and is known to mediate charge-dependent interactions between the PM and ER proteins (Yeung et al. 2008) (the importance of PS recognition by Asters for LDL–cholesterol transport is discussed in the subsection LDL–Cholesterol Transport). It was recently proposed that the Aster-B GRAM domain contains a basic patch that recognizes PS and a distinct site for sensing cholesterol (Ercan et al. 2021). Neutralizing the charge in this basic patch impaired the ability of PS to enhance GRAM domain binding to cholesterol-containing membranes. Furthermore, a point mutation in a separate region of the GRAM domain of Aster-B (R189W), which is associated with intellectual disability in humans (Reuter et al. 2017; Santos-Cortez et al. 2018), impaired its recruitment to membranes containing accessible cholesterol (Ercan et al. 2021).
Aster-A, -B and -C all contain disordered regions upstream of their GRAM domains, a common feature in lipid transfer proteins located at organelle contact sites (Jamecna and Antonny 2021). The close proximity of the disordered region to the membrane-binding domain might better facilitate recruitment to membranes that are characterized by large shifts in composition compared to domains with rigid, inflexible coils (Jamecna and Antonny 2021). Although binding of the GRAM domain to cholesterol in the presence of anionic lipids occurs in vitro without additional proteins (Sandhu et al. 2018; Naito et al. 2019; Ferrari et al. 2020), the GRAM and its upstream disordered region could interact with currently unidentified PM proteins in cells.
In summary, cholesterol becomes accessible in the PM when it reaches ∼35 mol% total PM lipids, the approximate concentration at which Asters are engaged to move cholesterol to the ER (Sandhu et al. 2018; Naito et al. 2019; Ferrari et al. 2020). Aster-mediated cholesterol transport regulates PM accessible cholesterol levels and SREBP-2 pathway activity in macrophages (Ferrari et al. 2020) and mouse, hamster, and human cell lines (Sandhu et al. 2018; Naito et al. 2019; Xiao et al. 2021; Trinh et al. 2022).
Transcriptional Regulation of Aster-B by Liver X Receptors
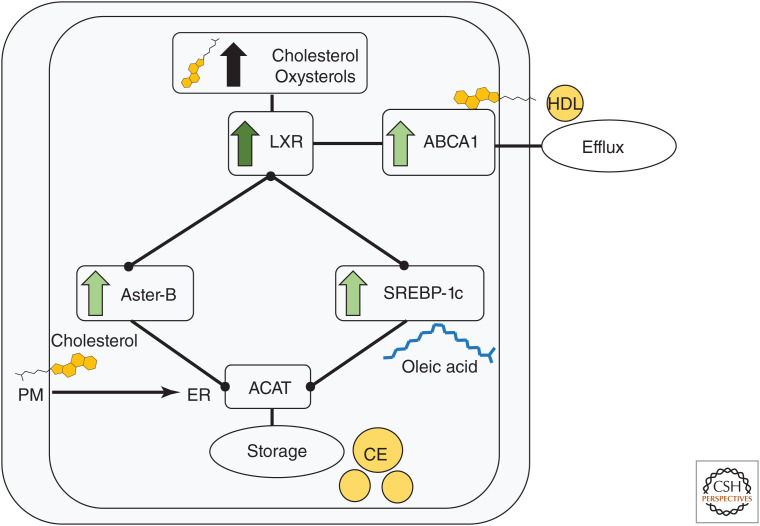
LXRs regulate a transcriptional program that maintains cellular unesterified cholesterol levels within a viable range upon its binding to cholesterol-derived oxysterol ligands (for review, see Wang and Tontonoz 2018). In addition to inducing ABC transporters that promote cholesterol efflux, LXRs control the expression of the transcription factor SREBP-1c, which promotes fatty acid synthesis and desaturation via fatty acid synthase, acetyl-CoA carboxylase, and stearoyl-CoA desaturase (Fig. 4; Repa et al. 2000). Stimulation of fatty acid synthesis in response to LXR activation generates fatty acyl chains for cholesterol esterification (Repa et al. 2000). The Gramd1b transcript (encodes Aster-B) is also strongly induced in macrophages treated with synthetic ligands for LXR and its heterodimer partner retinoid X receptor in an LXR-dependent manner (Fig. 4; Sandhu et al. 2018). The PM-to-ER movement of excess unesterified cholesterol by Asters promotes cholesterol esterification and suppresses SREBP-2 pathway activation (Sandhu et al. 2018; Ferrari et al. 2020). It is noteworthy that Aster-B does not stimulate exogenous cholesterol uptake but instead promotes the redistribution of excess PM cholesterol to the ER for storage. The factors that regulate Aster-A and Aster-C expression are currently undefined but uncovering these signals may shed light on the physiological roles for accessible cholesterol movement in various tissues.
Figure 4.
Liver X receptor (LXR) signaling in macrophages. Excess unesterified cholesterol is converted to oxysterols. Oxysterols activate LXR to promote cholesterol efflux by inducing ABCA1. LXRs also induce Aster-B to deliver unesterified cholesterol from the plasma membrane (PM) to endoplasmic reticulum (ER), where acyl-CoA:cholesterol acyltransferase (ACAT) resides. Fatty acid substrates for ACAT-mediated esterification of excess cholesterol are provided by LXR-dependent induction of SREBP-1c (and its targets fatty acid synthase, acetyl-CoA carboxylase, and stearoyl-CoA desaturase).
MOVEMENT OF LIPOPROTEIN CHOLESTEROL TO THE ER
Cholesterol biosynthesis is energetically costly and therefore most cells preferentially internalize lipoprotein cholesterol. Indeed, seminal balance studies in the 1930s showed that although mice can make their own cholesterol, they make less when cholesterol is supplied in the diet (Schoenheimer and Breusch 1933). De novo cholesterol synthesis, however, becomes particularly important when lipoprotein availability is limited. For example, the mammalian brain relies on de novo cholesterol synthesis because it has limited access to lipoproteins in the systemic circulation (Andersson et al. 1990; Jurevics and Morell 1995). Furthermore, cholesterol synthesis is stimulated in cultured cells grown in lipoprotein-deficient media (Brown et al. 1973) and in the livers of humans taking ezetimibe, which blocks dietary cholesterol absorption and biliary cholesterol reabsorption in the gut (Sudhop et al. 2002).
LDL–Cholesterol Transport
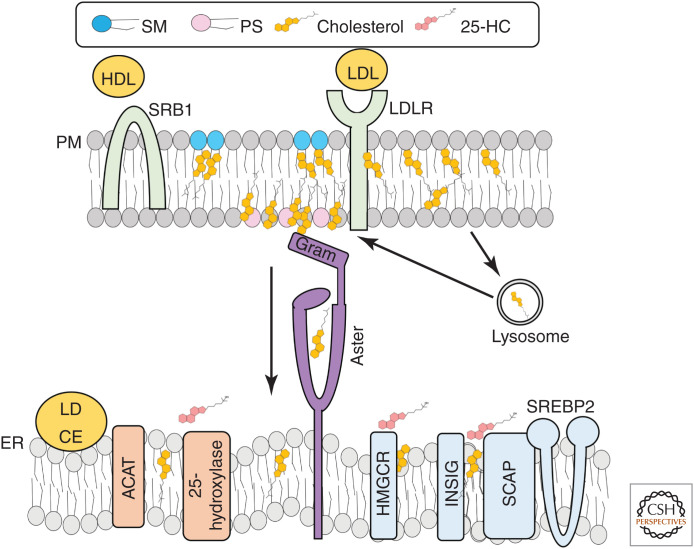
LDL binds LDL receptors (LDLRs) on the PM and the receptor-particle complex is endocytosed via clathrin-coated pits (Fig. 5; Goldstein and Brown 1974; Anderson et al. 1977). LDLRs also mediate the uptake of chylomicron remnants derived from intestinal lipids (Ellsworth et al. 1987). Following its endocytosis, LDL cholesterol is released in late endosomes/lysosomes by acid lipase–mediated hydrolysis of CE (Anderson and Sando 1991). Unesterified cholesterol is then exported from the late endosome/lysosome lumen by sequential steps involving soluble NPC2, which transfers cholesterol to the amino-terminal domain of the membrane-embedded NPC1 (Kwon et al. 2009) in an exchange that is low-pH-dependent (Qian et al. 2020). NPC1 funnels cholesterol through a cavity to the outer endosomal/lysosomal membrane (Gong et al. 2016; Li et al. 2016; Winkler et al. 2019) where it becomes available for distribution to other organelles.
Figure 5.
Lipoprotein–cholesterol transport to the endoplasmic reticulum (ER). High-density lipoprotein (HDL) cholesterol is funneled into the plasma membrane (PM) by SR-B1. Low-density lipoprotein (LDL) cholesterol is endocytosed by the LDL receptor (LDLR) and released in lysosomes before transport to the PM. When PM sphingomyelin (SM)/cholesterol complexes are saturated, cholesterol becomes accessible for Aster-mediated transport to the ER. The Aster GRAM domain recognizes cholesterol in the presence of PS. At the ER, cholesterol is esterified by acyl-CoA:cholesterol acyltransferase (ACAT) for storage in lipid droplets or converted to 25-hydroxycholesterol by 25-hydroxylase. Cholesterol can also be transported to mitochondria to undergo further modifications including conversion to 27-hydroxcholesterol, bile acids (liver), or steroid hormones (adrenal, gonads). Cholesterol and oxysterols in the ER enhance the proteolytic degradation of 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGCR) and the binding of insulin-induced gene proteins (INSIGs) to SREBP cleavage-activating protein (SCAP) to prevent SREBP-2 movement to the nucleus.
From the late endosomal/lysosomal membrane surface, most LDL-derived cholesterol is first transported to the PM before moving to the ER (Tabas et al. 1988; Brasaemle and Attie 1990; Neufeld et al. 1996; Lange et al. 1997; Das et al. 2014). The mechanisms of post-NPC1 cholesterol movement to the PM have not been fully elucidated. However, oxysterol-binding protein related-protein 2 (OSBPL2/ORP2) moves some cholesterol from late endosomes toward the PM by a mechanism involving exchange of cholesterol for phosphatidylinositol 4,5-bisphosphate (Wang et al. 2019; Takahashi et al. 2021) with at least a portion of this cholesterol moving through recycling endosomes on its way to the PM (Takahashi et al. 2021). A minor portion of LDL cholesterol bypasses the PM and moves directly to the ER (Neufeld et al. 1996; Underwood et al. 1998), peroxisomes (Chu et al. 2015), and possibly other internal membranes after the lysosome. The rate and extent of cholesterol flux though these alternative routes to the ER is likely cell-type-dependent. Both OSBPL5 (ORP5) and OSBP1L (ORP1L) move LDL-derived cholesterol from late endosomes to the ER (Du et al. 2011; Zhao and Ridgway 2017), whereas the specific transporters involved in lysosome-peroxisome cholesterol transport have yet to be identified. Aster-B interacts with NPC1 in HeLa cells, suggesting that Aster-B might move cholesterol directly from lysosomes to the ER under certain conditions (Höglinger et al. 2019). Nevertheless, the majority of LDL-derived cholesterol moves to the PM before the ER, ensuring that PM cholesterol requirements are met before termination of synthesis and uptake (Infante and Radhakrishnan 2017).
When LDL cholesterol reaches the PM, it fills the SM-sequestered cholesterol pool before becoming accessible for trafficking to the ER (Das et al. 2014; Johnson et al. 2019). Addition of the cholesterol-binding probe ALOD4 to the extracellular media of cells traps most LDL-derived cholesterol at the PM (preventing its movement to the ER) and thus blocks suppression of SREBP-2 and stimulation of ACAT activity (Infante and Radhakrishnan 2017). Exogenous addition of SM to the exofacial surface of cells also stimulates cholesterol synthesis (Gatt and Bierman 1980), suggesting that cholesterol is being trapped at the PM. The ability of extracellular ALOD4 to bind cholesterol arriving at the PM from endosomal compartments confirms that cholesterol readily equilibrates between the two PM leaflets, as reported previously (Steck et al. 2002). Trapping of as little as 1% total PM cholesterol by ALOD4 appears sufficient to stimulate cholesterol synthesis, underscoring that the ER is highly responsive to cholesterol movement from the PM (Infante and Radhakrishnan 2017).
The internalization and subsequent delivery of LDL cholesterol to the PM is normal in Aster-deficient cells (Trinh et al. 2022). However, PM–ER transport of LDL cholesterol downstream of NPC1 is impaired with Aster deficiency. Specifically, loss of Aster function causes accessible cholesterol accumulation on the PM while blunting the suppression of SREBP-2 processing and CE formation by LDL in Chinese hamster cells (Xiao et al. 2021; Trinh et al. 2022). Although physiological consequences of impaired Aster-mediated HDL–cholesterol movement in the adrenals are clear from the phenotype of global Aster-B-deficient mice (i.e., impaired steroidogenesis) (Sandhu et al. 2018), Asters have not yet been shown to transport LDL cholesterol in vivo.
Subcellular PS distribution largely overlaps with cholesterol distribution, with each being enriched in the PM and its endo-lysosomal derivatives and scarce in the ER (Maekawa and Fairn 2015). PS depletion in Chinse hamster cells by genetic deletion of PS synthase-1 expands the PM accessible cholesterol pool and impairs the movement of LDL-derived cholesterol to the ER (Trinh et al. 2020). Follow-up studies with Aster-A, -B, and -C knockout cells showed that loss of Aster function largely accounts for the cholesterol trafficking defect in PS-deficient cells (Trinh et al. 2022). Interestingly, PS is highly abundant in SM/cholesterol-rich PM caveolae (Fairn et al. 2011). The coupling of PS with SM/cholesterol complexes could help Aster recruitment to these regions when the cholesterol-sequestering capacity of SM is exceeded and where cholesterol is likely to first become accessible.
HDL–Cholesterol Transport
Cholesterol carried by apolipoprotein A1-containing HDL particles primarily enters cells via the scavenger receptor B1 (SR-B1) (Fig. 5; Acton et al. 1996). SR-B1 is particularly abundant in steroidogenic tissues and the liver where it supplies cholesterol for steroid hormone synthesis and bile acid production, respectively. In contrast to LDL, which is rapidly endocytosed after binding to its receptor, HDL cholesterol is funneled by SR-B1 into the PM through a hydrophobic channel (Neculai et al. 2013). Lipid-depleted apoprotein A1 loses affinity for SR-B1 and re-enters circulation to continue its role as an extracellular acceptor of cholesterol and phospholipids from peripheral tissues (Rodrigueza et al. 1999). SR-B1 avoids endocytosis by forming stable multimers on the PM, allowing it to mediate gradual lipid removal from HDL (estimated half-life of 12 h per SR-B1 molecule) (Marques et al. 2019). HDL loading recruits Asters to the PM in cultured cells (Sandhu et al. 2018). Multimers of SR-B1 on the PM could cooperate with Asters, which also form homo- and heteromeric complexes (Naito et al. 2019), to mediate bulk lipid transfer from HDL.
Although unesterified cholesterol taken up by SR-B1 can be directly transported to the ER, CEs must first be hydrolyzed at the PM by neutral cholesteryl esterases that have yet to be identified. ACAT-1 deficiency results in dramatic loss of adrenal CE stores, a phenotype that would not develop if intact CEs were delivered directly to the ER (Meiner et al. 1996). Aster-B mediates PM–ER cholesterol movement after uptake by SR-B1 (Sandhu et al. 2018). Thus, mice with genetic deficiency of any of the key steps in HDL cholesterol movement and storage, including ApoA1, SR-B1, Aster-B, or ACAT-1, have low adrenal CE levels (Meiner et al. 1996; Plump et al. 1996; Rigotti et al. 1997; Sandhu et al. 2018). It is noteworthy that although mice and rats transport most of their circulating cholesterol in HDL and use this as the major cholesterol source for steroidogenesis, most cholesterol in human circulation is in LDL (Gwynne et al. 1976; Kovanen et al. 1979; Andersen and Dietschy 1981). Because Aster proteins also mediate PM-to-ER movement of LDL cholesterol, Asters also likely supply sterol substrates for human adrenal steroidogenesis.
FATE OF PM CHOLESTEROL DELIVERED TO THE ER
Changes to ER cholesterol content alter membrane organization in a way that promotes the activity of some proteins and processes and blocks the activity of others (Fig. 5). A well-defined example is how ER membranes with relatively low cholesterol content allow SCAP to exist in a conformation that promotes its association with COPII vesicles for transport to the Golgi with SREBP-2 (Brown et al. 2002). At the same time, low ER cholesterol content prevents the loading of COPII vesicles with certain cargo that is unrelated to sterol homeostasis (Runz et al. 2006; Saher et al. 2009). Consistent with the idea that selective mechanisms exist to promote SREBP-2 processing during sterol depletion, cell death–inducing DFFA-like effector-B (CIDEB) was found to tether SREBP-2/SCAP and Sec12 to COPII complexes at ER exit sites in the liver, thereby enhancing their delivery to the Golgi (Su et al. 2019).
A rise in cholesterol delivery to the ER leads to the proteolytic degradation of the cholesterol biosynthetic enzymes HMGCR (Roitelman and Simoni 1992; McGee et al. 1996; Ravid et al. 2000) and squalene monooxygenase (Gill et al. 2011; Sharpe et al. 2019). Conversely, sterols stabilize INSIG1 by binding it to SCAP as a mechanism to inhibit its escort of SREBP-2 to the Golgi. INSIG1 undergoes ubiquitin–mediated proteasomal degradation in low sterol conditions (Gong et al. 2006; Lee et al. 2006). Additionally, excess cholesterol in hepatocytes is associated with the induction of the pro-inflammatory transcription factor Wwtr1 (encoding TAZ) during the steatosis-to-fibrosis transition (Wang et al. 2016). TAZ induction is blunted by antisense oligonucleotide-mediated knockdown of Aster-B and -C in mice fed a profibrotic diet (Wang et al. 2020), suggesting that PM–ER cholesterol movement is involved in TAZ induction during fibrosis development.
Cholesterol Esterification
ACATs are integral ER membrane proteins that place a long-chain fatty acyl-CoA on carbon 3 of cholesterol to produce CEs (Chang et al. 1993; Cases et al. 1998). ACAT-1 is ubiquitously expressed, with particularly high levels in macrophages and steroidogenic tissues (Sakashita et al. 2000), whereas ACAT-2 is selectively expressed in the intestine and liver (Chang et al. 2000; Parini et al. 2004). ACAT-1-deficient mice have low CEs in their adrenals and peritoneal macrophages but have normal intestinal cholesterol absorption and substantial liver ACAT activity (Meiner et al. 1996). ACAT-2-deficient mice have almost undetectable CE synthesis in the intestine and liver (Buhman et al. 2000), impaired intestinal cholesterol absorption (Repa et al. 2004), and blunted very low-density lipoprotein (VLDL)-CE secretion (Lee et al. 2005). Both ACAT-1 and -2 are allosterically activated by cholesterol (Liu et al. 2005). Therefore, although CE synthesis is low when cholesterol is scare, ACAT activity increases dramatically when PM cholesterol becomes accessible for transport to the ER (Slotte and Bierman 1988; Xu and Tabas 1991; Lange and Steck 1997). Aster proteins deliver cholesterol to ACATs for normal rates of CE formation following HDL loading (Sandhu et al. 2018) or LDL loading (Xiao et al. 2021; Trinh et al. 2022) of cultured cells. Although a role for Asters in delivery of HDL cholesterol to adrenal ACAT-1 for CE synthesis has been described (Sandhu et al. 2018), it remains to be determined whether Asters deliver cholesterol to ACAT-2 for chylomicron or VLDL secretion in vivo.
Oxysterol Production
Oxysterols are formed by the addition of an oxygenated group to cholesterol, commonly a hydroxyl or keto moiety to position-24, -25, or -27 on the side chain, or position-7 of the B-ring (reviewed in Brown et al. 2021). The hydroxylases that produce oxysterols are ER- and mitochondria-localized (Andersson et al. 1989; Lund et al. 1998). The hepatic bile acid synthetic pathway produces oxysterols as intermediates though the actions of ER-localized cytochrome P450 family 7 subfamily A member 1 (CYP7A1) and CYP7B1, and mitochondria-localized CYP27A1 (Brown et al. 2021). Oxysterols are typically present at low levels in cells (103- to 106-fold lower concentrations than cholesterol) but their synthesis is stimulated ∼30-fold when cellular cholesterol content is increased by 60% after a cholesterol depletion period (Lange et al. 2009). Oxysterols signal sterol excess and synergize with cholesterol to block cholesterol synthesis. Specifically, side-chain oxysterols bind INSIGs to SCAP, preventing SREBP-2/SCAP movement to the cis-Golgi membrane (Radhakrishnan et al. 2007). Additionally, 25-hydroxycholesterol and 27-hydroxycholesterol accelerate the proteolytic degradation of HMGCR in an INSIG-dependent manner (Faust et al. 1982; Ravid et al. 2000; Sever et al. 2003). Furthermore, oxysterols stimulate CE formation by promoting PM–ER cholesterol transport (Brown et al. 1975; Lange and Steck 1997) by a mechanism that might include their expansion of the accessible cholesterol pool due to a disordering effect on PM phospholipids (Olsen et al. 2011). The three purified Aster domains have different binding affinities to various oxysterols (Xiao et al. 2021). For example, 20α-hydroxycholesterol binds with high affinity to Aster-B and -C, but has lower affinity for Aster-A. However, although Asters can bind oxysterols in vitro, it is currently unknown whether they mediate oxysterol transfer in cells.
Macrophages produce 25-hydroxycholesterol after infection or interferon stimulation (Blanc et al. 2013), resulting in rapid internalization of PM-accessible cholesterol (Abrams et al. 2020; Zhou et al. 2020). 25-hydroxycholesterol depletion of PM cholesterol blocks cytolysin binding to macrophage PMs (Zhou et al. 2020) and also acts as a paracrine signal to deplete PM cholesterol of neighboring epithelial cells, blocking bacterial entry (Abrams et al. 2020). The protective autocrine and paracrine effects of PM accessible cholesterol depletion are lost in macrophages lacking the ER-localized 25-hydrolase enzyme that produces 25-hydroxycholesterol (Abrams et al. 2020; Zhou et al. 2020). Notably, activation of ACAT is required for full accessible cholesterol depletion and pathogen protection in response to 25-hydroxycholesterol (Abrams et al. 2020; Zhou et al. 2020). ACAT inhibition also increases PM cholesterol in CD8+ T cells (Yang et al. 2016). It is conceivable that cholesterol transported to the ER in the setting of ACAT inactivation is rerouted back to the PM to prevent unesterified cholesterol accumulation in the ER. The specific transporters that deliver PM accessible cholesterol to the ER in response to 25-hydroxycholesterol remain to be defined.
CHOLESTEROL TRANSPORT FROM THE ER TOWARD THE PM
Most ER-to-PM cholesterol transport is nonvesicular (Urbani and Simoni 1990; Heino et al. 2000). Although specific proteins that mediate direct ER–PM trafficking of newly synthesized cholesterol have yet to be identified, these mechanisms must be efficient to prevent termination of synthesis before PM cholesterol stores are replete. A few sterol transfer proteins have been implicated in nonvesicular cholesterol trafficking away from the ER to the endo-lysosomal system and Golgi in the direction of the PM. For example, STARD3 (which has an amino-terminal transmembrane domain that anchors it to endosomes and a di-phenylalanine in an acidic tract [FFAT] motif that contacts ER-localized vesicle-associated membrane protein-associated protein [VAP]) shuttles cholesterol from the ER to endosomes with its carboxy-terminal START domain (Wilhelm et al. 2017). OSBP (composed of an amino-terminal PH domain that recognizes phosphatidylinositol-4-phosphate, a FFAT domain that binds VAP, and a carboxy-terminal OSBP-related domain that carries lipids) counterexchanges sterol for phosphatidylinositol-4-phosphate at ER–Golgi contacts (Mesmin et al. 2013). OSBP also transfers cholesterol from the ER to the lysosomal surface to activate mTORC1 (Lim et al. 2019). Similarly, OSBP1L (which contains PH, FFAT, and OSBP-related domains like OSBP but additionally contains amino-terminal ankyrin repeats that link to late endosomal RAB7) transfers cholesterol across ER-late endosome contacts to generate intraluminal vesicles (Eden et al. 2016). Uncovering the transporters that mediate direct ER–PM cholesterol movement will shed light on how cells and organisms maintain PM cholesterol levels during sterol depletion.
CONCLUDING REMARKS
Additional research is needed to characterize Aster function in tissues with high-cholesterol flux including the liver, intestines, and brain. Identification of Aster-independent pathways of PM–ER cholesterol movement will also be important. The spatial organization of ACAT, SCAP/SREBP2, and 25-hydroxylase relative to cholesterol transporters, and whether cholesterol is preferentially delivered to different ER regions depending on cell type or cellular cholesterol status, also requires further study.
Variations in GRAMD1B encoding Aster-B are linked to intellectual disability (Reuter et al. 2017; Santos-Cortez et al. 2018) and leukemia/lymphoma (Di Bernardo et al. 2008; Conde et al. 2010). Understanding the role of cholesterol transport in these disorders (and identifying more humans with loss-of-function mutations in Asters) may shed further light on the biology of these transporters and direct therapeutic development. Uncovering transcriptional regulators (in addition to LXRs for Aster-B) and homeostatic cues (e.g., metabolic, inflammatory) that influence Aster function will also provide insight into the roles of accessible cholesterol movement in physiology and disease.
ACKNOWLEDGMENTS
We thank Xu Xiao, Yajing Gao, Alessandra Ferrari, Emily Whang, and Jaspreet Sandhu for helpful discussions. This work was supported by National Institutes of Health (NIH) grants HL146358 and DK126779 and a Fondation Leducq Transatlantic Network of Excellence (19CVD04). J.P.K. is supported by a postdoctoral fellowship from the American Heart Association (903306).
Footnotes
Editors: Susan Ferro-Novick, Tom A. Rapoport, and Randy Schekman
Additional Perspectives on The Endoplasmic Reticulum available at www.cshperspectives.org
REFERENCES
- Abrams ME, Johnson KA, Perelman SS, Zhang LS, Endapally S, Mar KB, Thompson BM, McDonald JG, Schoggins JW, Radhakrishnan A, et al. 2020. Oxysterols provide innate immunity to bacterial infection by mobilizing cell surface accessible cholesterol. Nat Microbiol 5: 929–942. 10.1038/s41564-020-0701-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acton S, Rigotti A, Landschulz KT, Xu S, Hobbs HH, Krieger M. 1996. Identification of scavenger receptor SR-BI as a high density lipoprotein receptor. Science 271: 518–520. 10.1126/science.271.5248.518 [DOI] [PubMed] [Google Scholar]
- Andersen JM, Dietschy JM. 1981. Kinetic parameters of the lipoprotein transport systems in the adrenal gland of the rat determined in vivo. Comparison of low and high density lipoproteins of human and rat origin. J Biol Chem 256: 7362–7370. 10.1016/S0021-9258(19)68971-4 [DOI] [PubMed] [Google Scholar]
- Anderson RA, Sando GN. 1991. Cloning and expression of cDNA encoding human lysosomal acid lipase/cholesteryl ester hydrolase. Similarities to gastric and lingual lipases. J Biol Chem 266: 22479–22484. 10.1016/S0021-9258(18)54597-X [DOI] [PubMed] [Google Scholar]
- Anderson RGW, Brown MS, Goldstein JL. 1977. Role of the coated endocytic vesicle in the uptake of receptor-bound low density lipoprotein in human fibroblasts. Cell 10: 351–364. 10.1016/0092-8674(77)90022-8 [DOI] [PubMed] [Google Scholar]
- Andersson S, Davis DL, Dahlbäck H, Jörnvall H, Russell DW. 1989. Cloning, structure, and expression of the mitochondrial cytochrome P-450 sterol 26-hydroxylase, a bile acid biosynthetic enzyme. J Biol Chem 264: 8222–8229. 10.1016/S0021-9258(18)83172-6 [DOI] [PubMed] [Google Scholar]
- Andersson M, Elmberger PG, Edlund C, Kristensson K, Dallner G. 1990. Rates of cholesterol, ubiquinone, dolichol and dolichyl-P biosynthesis in rat brain slices. FEBS Lett 269: 15–18. 10.1016/0014-5793(90)81107-Y [DOI] [PubMed] [Google Scholar]
- Baumann NA, Sullivan DP, Ohvo-Rekilä H, Simonot C, Pottekat A, Klaassen Z, Beh CT, Menon AK. 2005. Transport of newly synthesized sterol to the sterol-enriched plasma membrane occurs via nonvesicular equilibration. Biochemistry 44: 5816–5826. 10.1021/bi048296z [DOI] [PubMed] [Google Scholar]
- Begley MJ, Taylor GS, Kim SA, Veine DM, Dixon JE, Stuckey JA. 2003. Crystal structure of a phosphoinositide phosphatase, MTMR2: insights into myotubular myopathy and Charcot–Marie–Tooth syndrome. Mol Cell 12: 1391–1402. 10.1016/S1097-2765(03)00486-6 [DOI] [Google Scholar]
- Blanc M, Hsieh WY, Robertson KA, Kropp KA, Forster T, Shui G, Lacaze P, Watterson S, Griffiths SJ, Spann NJ, et al. 2013. The transcription factor STAT-1 couples macrophage synthesis of 25-hydroxycholesterol to the interferon antiviral response. Immunity 38: 106–118. 10.1016/j.immuni.2012.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose HS, Sugawara T, Strauss JF, Miller WL. 1996. The pathophysiology and genetics of congenital lipoid adrenal hyperplasia. N Engl J Med 335: 1870–1879. 10.1056/NEJM199612193352503 [DOI] [PubMed] [Google Scholar]
- Brasaemle D, Attie A. 1990. Rapid intracellular transport of LDL-derived cholesterol to the plasma membrane in cultured fibroblasts. J Lipid Res 31: 103–112. 10.1016/S0022-2275(20)42764-6 [DOI] [PubMed] [Google Scholar]
- Brooks-Wilson A, Marcil M, Clee SM, Zhang LH, Roomp K, van Dam M, Yu L, Brewer C, Collins JA, Molhuizen HOF, et al. 1999. Mutations in ABC1 in Tangier disease and familial high-density lipoprotein deficiency. Nat Genet 22: 336–345. 10.1038/11905 [DOI] [PubMed] [Google Scholar]
- Brown MS, Goldstein JL. 1986. A receptor-mediated pathway for cholesterol homeostasis. Science 232: 34–47. 10.1126/science.3513311 [DOI] [PubMed] [Google Scholar]
- Brown MS, Dana SE, Goldstein JL. 1973. Regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity in human fibroblasts by lipoproteins. Proc Natl Acad Sci 70: 2162. 10.1073/pnas.70.7.2162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MS, Dana SE, Goldstein JL. 1975. Cholesterol ester formation in cultured human fibroblasts. Stimulation by oxygenated sterols. J Biol Chem 250: 4025–4027. 10.1016/S0021-9258(19)41498-1 [DOI] [PubMed] [Google Scholar]
- Brown AJ, Sun L, Feramisco JD, Brown MS, Goldstein JL. 2002. Cholesterol addition to ER membranes alters conformation of SCAP, the SREBP escort protein that regulates cholesterol metabolism. Mol Cell 10: 237–245. 10.1016/S1097-2765(02)00591-9 [DOI] [PubMed] [Google Scholar]
- Brown MS, Radhakrishnan A, Goldstein JL. 2018. Retrospective on cholesterol homeostasis: the central role of SCAP. Annu Rev Biochem 87: 783–807. 10.1146/annurev-biochem-062917-011852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AJ, Sharpe LJ, Rogers MJ. 2021. Oxysterols: from physiological tuners to pharmacological opportunities. Br J Pharmacol 178: 3089–3103. 10.1111/bph.15073 [DOI] [PubMed] [Google Scholar]
- Buhman KK, Accad M, Novak S, Choi RS, Wong JS, Hamilton RL, Turley S, Farese RV. 2000. Resistance to diet-induced hypercholesterolemia and gallstone formation in ACAT2-deficient mice. Nat Med 6: 1341–1347. 10.1038/82153 [DOI] [PubMed] [Google Scholar]
- Cases S, Novak S, Zheng YW, Myers HM, Lear SR, Sande E, Welch CB, Lusis AJ, Spencer TA, Krause BR, et al. 1998. ACAT-2, a second mammalian acyl-CoA:Cholesterol acyltransferase: its cloning, expression, and characterization. J Biol Chem 273: 26755–26764. 10.1074/jbc.273.41.26755 [DOI] [PubMed] [Google Scholar]
- Chang CC, Huh HY, Cadigan KM, Chang TY. 1993. Molecular cloning and functional expression of human acyl-coenzyme A:cholesterol acyltransferase cDNA in mutant Chinese hamster ovary cells. J Biol Chem 268: 20747–20755. 10.1016/S0021-9258(19)36846-2 [DOI] [PubMed] [Google Scholar]
- Chang CC, Sakashita N, Ornvold K, Lee O, Chang ET, Dong R, Lin S, Lee CY, Strom SC, Kashyap R, et al. 2000. Immunological quantitation and localization of ACAT-1 and ACAT-2 in human liver and small intestine. J Biol Chem 275: 28083–28092. 10.1074/jbc.M003927200 [DOI] [PubMed] [Google Scholar]
- Chu BB, Liao YC, Qi W, Xie C, Du X, Wang J, Yang H, Miao HH, Li BL, Song BL. 2015. Cholesterol transport through lysosome-peroxisome membrane contacts. Cell 161: 291–306. 10.1016/j.cell.2015.02.019 [DOI] [PubMed] [Google Scholar]
- Clark BJ, Wells J, King SR, Stocco DM. 1994. The purification, cloning, and expression of a novel luteinizing hormone-induced mitochondrial protein in MA-10 mouse Leydig tumor cells. Characterization of the steroidogenic acute regulatory protein (StAR). J Biol Chem 269: 28314–28322. 10.1016/S0021-9258(18)46930-X [DOI] [PubMed] [Google Scholar]
- Conde L, Halperin E, Akers NK, Brown KM, Smedby KE, Rothman N, Nieters A, Slager SL, Brooks-Wilson A, Agana L, et al. 2010. Genome-wide association study of follicular lymphoma identifies a risk locus at 6p21.32. Nat Genet 42: 661–664. 10.1038/ng.626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A, Goldstein JL, Anderson DD, Brown MS, Radhakrishnan A. 2013. Use of mutant 125I-perfringolysin O to probe transport and organization of cholesterol in membranes of animal cells. Proc Natl Acad Sci 110: 10580–10585. 10.1073/pnas.1309273110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A, Brown MS, Anderson DD, Goldstein JL, Radhakrishnan A. 2014. Three pools of plasma membrane cholesterol and their relation to cholesterol homeostasis. eLife 3: e02882. 10.7554/eLife.02882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGrella RF, Simoni RD. 1982. Intracellular transport of cholesterol to the plasma membrane. J Biol Chem 257: 14256–14262. 10.1016/S0021-9258(19)45374-X [DOI] [PubMed] [Google Scholar]
- Demel RA, Jansen JW, van Dijck PW, van Deenen LL. 1977. The preferential interaction of cholesterol with different classes of phospholipids. Biochim Biophys Acta 465: 1–10. 10.1016/0005-2736(77)90350-9 [DOI] [PubMed] [Google Scholar]
- Di Bernardo MC, Crowther-Swanepoel D, Broderick P, Webb E, Sellick G, Wild R, Sullivan K, Vijayakrishnan J, Wang Y, Pittman AM, et al. 2008. A genome-wide association study identifies six susceptibility loci for chronic lymphocytic leukemia. Nat Genet 40: 1204–1210. 10.1038/ng.219 [DOI] [PubMed] [Google Scholar]
- Doerks T, Strauss M, Brendel M, Bork P. 2000. GRAM, a novel domain in glucosyltransferases, myotubularins and other putative membrane-associated proteins. Trends Biochem Sci 25: 483–485. 10.1016/S0968-0004(00)01664-9 [DOI] [PubMed] [Google Scholar]
- Du X, Kumar J, Ferguson C, Schulz TA, Ong YS, Hong W, Prinz WA, Parton RG, Brown AJ, Yang H. 2011. A role for oxysterol-binding protein-related protein 5 in endosomal cholesterol trafficking. J Cell Biol 192: 121–135. 10.1083/jcb.201004142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eden ER, Sanchez-Heras E, Tsapara A, Sobota A, Levine TP, Futter CE. 2016. Annexin A1 tethers membrane contact sites that mediate ER to endosome cholesterol transport. Dev Cell 37: 473–483. 10.1016/j.devcel.2016.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbaz-Alon Y, Eisenberg-Bord M, Shinder V, Stiller Sebastian B, Shimoni E, Wiedemann N, Geiger T, Schuldiner M. 2015. Lam6 regulates the extent of contacts between organelles. Cell Rep 12: 7–14. 10.1016/j.celrep.2015.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellsworth JL, Kraemer FB, Cooper AD. 1987. Transport of β-very low density lipoproteins and chylomicron remnants by macrophages is mediated by the low density lipoprotein receptor pathway. J Biol Chem 262: 2316–2325. 10.1016/S0021-9258(18)61656-4 [DOI] [PubMed] [Google Scholar]
- Endapally S, Frias D, Grzemska M, Gay A, Tomchick DR, Radhakrishnan A. 2019. Molecular discrimination between two conformations of sphingomyelin in plasma membranes. Cell 176: 1040–1053.e1017. 10.1016/j.cell.2018.12.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ercan B, Naito T, Koh DHZ, Dharmawan D, Saheki Y. 2021. Molecular basis of accessible plasma membrane cholesterol recognition by the GRAM domain of GRAMD1b. EMBO J 40: e106524. 10.15252/embj.2020106524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairn GD, Schieber NL, Ariotti N, Murphy S, Kuerschner L, Webb RI, Grinstein S, Parton RG. 2011. High-resolution mapping reveals topologically distinct cellular pools of phosphatidylserine. J Cell Biol 194: 257–275. 10.1083/jcb.201012028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faust JR, Luskey KL, Chin DJ, Goldstein JL, Brown MS. 1982. Regulation of synthesis and degradation of 3-hydroxy-3-methylglutaryl-coenzyme A reductase by low density lipoprotein and 25-hydroxycholesterol in UT-1 cells. Proc Natl Acad Sci 79: 5205–5209. 10.1073/pnas.79.17.5205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari A, He C, Kennelly JP, Sandhu J, Xiao X, Chi X, Jiang H, Young SG, Tontonoz P. 2020. Aster proteins regulate the accessible cholesterol pool in the plasma membrane. Mol Cell Biol 40: e00255-20. 10.1128/MCB.00255-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan JJ, Tweten RK, Johnson AE, Heuck AP. 2009. Cholesterol exposure at the membrane surface is necessary and sufficient to trigger perfringolysin O binding. Biochemistry 48: 3977–3987. 10.1021/bi9002309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatt S, Bierman EL. 1980. Sphingomyelin suppresses the binding and utilization of low density lipoproteins by skin fibroblasts. J Biol Chem 255: 3371–3376. 10.1016/S0021-9258(19)85709-5 [DOI] [PubMed] [Google Scholar]
- Gatta AT, Wong LH, Sere YY, Calderón-Noreña DM, Cockcroft S, Menon AK, Levine TP. 2015. A new family of StART domain proteins at membrane contact sites has a role in ER–PM sterol transport. eLife 4: e07253. 10.7554/eLife.07253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay A, Rye D, Radhakrishnan A. 2015. Switch-like responses of two cholesterol sensors do not require protein oligomerization in membranes. Biophys J 108: 1459–1469. 10.1016/j.bpj.2015.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill S, Stevenson J, Kristiana I, Brown AJ. 2011. Cholesterol-dependent degradation of squalene monooxygenase, a control point in cholesterol synthesis beyond HMG-CoA reductase. Cell Metab 13: 260–273. 10.1016/j.cmet.2011.01.015 [DOI] [PubMed] [Google Scholar]
- Goldstein JL, Brown MS. 1974. Binding and degradation of low density lipoproteins by cultured human fibroblasts. comparison of cells from a normal subject and from a patient with homozygous familial hypercholesterolemia. J Biol Chem 249: 5153–5162. 10.1016/S0021-9258(19)42341-7 [DOI] [PubMed] [Google Scholar]
- Gong Y, Lee JN, Lee PC, Goldstein JL, Brown MS, Ye J. 2006. Sterol-regulated ubiquitination and degradation of insig-1 creates a convergent mechanism for feedback control of cholesterol synthesis and uptake. Cell Metab 3: 15–24. 10.1016/j.cmet.2005.11.014 [DOI] [PubMed] [Google Scholar]
- Gong X, Qian H, Zhou X, Wu J, Wan T, Cao P, Huang W, Zhao X, Wang X, Wang P, et al. 2016. Structural insights into the Niemann–Pick C1 (NPC1)-mediated cholesterol transfer and Ebola infection. Cell 165: 1467–1478. 10.1016/j.cell.2016.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwynne JT, Mahaffee D, Brewer HB, Ney RL. 1976. Adrenal cholesterol uptake from plasma lipoproteins: regulation by corticotropin. Proc Natl Acad Sci 73: 4329. 10.1073/pnas.73.12.4329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlan JE, Hajduk PJ, Yoon HS, Fesik SW. 1994. Pleckstrin homology domains bind to phosphatidylinositol-4,5-bisphosphate. Nature 371: 168–170. 10.1038/371168a0 [DOI] [PubMed] [Google Scholar]
- Härmälä A-S, Pörn MI, Mattjus P, Slotte JP. 1994. Cholesterol transport from plasma membranes to intracellular membranes is inhibited by 3β-[2-(diethylamino) ethoxy]androst-5-en-17-one. Biochim Biophys Acta 1211: 317–325. 10.1016/0005-2760(94)90156-2 [DOI] [PubMed] [Google Scholar]
- Haynes MP, Phillips MC, Rothblat GH. 2000. Efflux of cholesterol from different cellular pools. Biochemistry 39: 4508–4517. 10.1021/bi992125q [DOI] [PubMed] [Google Scholar]
- Heino S, Lusa S, Somerharju P, Ehnholm C, Olkkonen VM, Ikonen E. 2000. Dissecting the role of the Golgi complex and lipid rafts in biosynthetic transport of cholesterol to the cell surface. Proc Natl Acad Sci 97: 8375–8380. 10.1073/pnas.140218797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höglinger D, Burgoyne T, Sanchez-Heras E, Hartwig P, Colaco A, Newton J, Futter CE, Spiegel S, Platt FM, Eden ER. 2019. NPC1 regulates ER contacts with endocytic organelles to mediate cholesterol egress. Nat Commun 10: 4276. 10.1038/s41467-019-12152-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horenkamp FA, Valverde DP, Nunnari J, Reinisch KM. 2018. Molecular basis for sterol transport by StART-like lipid transfer domains. EMBO J 37: e98002. 10.15252/embj.201798002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Infante RE, Radhakrishnan A. 2017. Continuous transport of a small fraction of plasma membrane cholesterol to endoplasmic reticulum regulates total cellular cholesterol. eLife 6: e25466. 10.7554/eLife.25466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamecna D, Antonny B. 2021. Intrinsically disordered protein regions at membrane contact sites. Biochim Biophys Acta 1866: 159020. 10.1016/j.bbalip.2021.159020 [DOI] [PubMed] [Google Scholar]
- Jentsch JA, Kiburu I, Pandey K, Timme M, Ramlall T, Levkau B, Wu J, Eliezer D, Boudker O, Menon AK. 2018. Structural basis of sterol binding and transport by a yeast StARkin domain. J Biol Chem 293: 5522–5531. 10.1074/jbc.RA118.001881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KA, Endapally S, Vazquez DC, Infante RE, Radhakrishnan A. 2019. Ostreolysin A and anthrolysin O use different mechanisms to control movement of cholesterol from the plasma membrane to the endoplasmic reticulum. J Biol Chem 294: 17289–17300. 10.1074/jbc.RA119.010393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurevics H, Morell P. 1995. Cholesterol for synthesis of myelin is made locally, not imported into brain. J Neurochem 64: 895–901. 10.1046/j.1471-4159.1995.64020895.x [DOI] [PubMed] [Google Scholar]
- Keller SL, Radhakrishnan A, McConnell HM. 2000. Saturated phospholipids with high melting temperatures form complexes with cholesterol in monolayers. J Phys Chem B 104: 7522–7527. 10.1021/jp000958g [DOI] [Google Scholar]
- Khelashvili G, Chauhan N, Pandey K, Eliezer D, Menon AK. 2019. Exchange of water for sterol underlies sterol egress from a StARkin domain. eLife 8: e53444. 10.7554/eLife.53444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovanen PT, Schneider WJ, Hillman GM, Goldstein JL, Brown MS. 1979. Separate mechanisms for the uptake of high and low density lipoproteins by mouse adrenal gland in vivo. J Biol Chem 254: 5498–5505. 10.1016/S0021-9258(18)50623-2 [DOI] [PubMed] [Google Scholar]
- Kwon HJ, Abi-Mosleh L, Wang ML, Deisenhofer J, Goldstein JL, Brown MS, Infante RE. 2009. Structure of N-terminal domain of NPC1 reveals distinct subdomains for binding and transfer of cholesterol. Cell 137: 1213–1224. 10.1016/j.cell.2009.03.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange Y, Steck TL. 1997. Quantitation of the pool of cholesterol associated with acyl-CoA:cholesterol acyltransferase in human fibroblasts. J Biol Chem 272: 13103–13108. 10.1074/jbc.272.20.13103 [DOI] [PubMed] [Google Scholar]
- Lange Y, Swaisgood MH, Ramos BV, Steck TL. 1989. Plasma membranes contain half the phospholipid and 90% of the cholesterol and sphingomyelin in cultured human fibroblasts. J Biol Chem 264: 3786–3793. [PubMed] [Google Scholar]
- Lange Y, Ye J, Chin J. 1997. The fate of cholesterol exiting lysosomes. J Biol Chem 272: 17018–17022. 10.1074/jbc.272.27.17018 [DOI] [PubMed] [Google Scholar]
- Lange Y, Ye J, Steck TL. 2004. How cholesterol homeostasis is regulated by plasma membrane cholesterol in excess of phospholipids. Proc Natl Acad Sci 101: 11664–11667. 10.1073/pnas.0404766101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange Y, Steck TL, Ye J, Lanier MH, Molugu V, Ory D. 2009. Regulation of fibroblast mitochondrial 27-hydroxycholesterol production by active plasma membrane cholesterol. J Lipid Res 50: 1881–1888. 10.1194/jlr.M900116-JLR200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange Y, Tabei SMA, Ye J, Steck TL. 2013. Stability and stoichiometry of bilayer phospholipid–cholesterol complexes: relationship to cellular sterol distribution and homeostasis. Biochemistry 52: 6950–6959. 10.1021/bi400862q [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laraia L, Friese A, Corkery DP, Konstantinidis G, Erwin N, Hofer W, Karatas H, Klewer L, Brockmeyer A, Metz M, et al. 2019. The cholesterol transfer protein GRAMD1A regulates autophagosome biogenesis. Nat Chem Biol 15: 710–720. 10.1038/s41589-019-0307-5 [DOI] [PubMed] [Google Scholar]
- Lee RG, Shah R, Sawyer JK, Hamilton RL, Parks JS, Rudel LL. 2005. ACAT2 contributes cholesteryl esters to newly secreted VLDL, whereas LCAT adds cholesteryl ester to LDL in mice. J Lipid Res 46: 1205–1212. 10.1194/jlr.M500018-JLR200 [DOI] [PubMed] [Google Scholar]
- Lee JN, Song B, DeBose-Boyd RA, Ye J. 2006. Sterol-regulated degradation of Insig-1 mediated by the membrane-bound ubiquitin ligase gp78. J Biol Chem 281: 39308–39315. 10.1074/jbc.M608999200 [DOI] [PubMed] [Google Scholar]
- Lemmon MA, Ferguson KM, O'Brien R, Sigler PB, Schlessinger J. 1995. Specific and high-affinity binding of inositol phosphates to an isolated pleckstrin homology domain. Proc Natl Acad Sci 92: 10472–10476. 10.1073/pnas.92.23.10472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Wang J, Coutavas E, Shi H, Hao Q, Blobel G. 2016. Structure of human Niemann-Pick C1 protein. Proc Natl Acad Sci 113: 8212–8217. 10.1073/pnas.1607795113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim CY, Davis OB, Shin HR, Zhang J, Berdan CA, Jiang X, Counihan JL, Ory DS, Nomura DK, Zoncu R. 2019. ER-lysosome contacts enable cholesterol sensing by mTORC1 and drive aberrant growth signalling in Niemann–Pick type C. Nat Cell Biol 21: 1206–1218. 10.1038/s41556-019-0391-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Chang CC, Westover EJ, Covey DF, Chang TY. 2005. Investigating the allosterism of acyl-CoA:cholesterol acyltransferase (ACAT) by using various sterols: in vitro and intact cell studies. Biochem J 391: 389–397. 10.1042/BJ20050428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lönnfors M, Doux JPF, Killian JA, Nyholm TKM, Slotte JP. 2011. Sterols have higher affinity for sphingomyelin than for phosphatidylcholine bilayers even at equal acyl-chain order. Biophys J 100: 2633–2641. 10.1016/j.bpj.2011.03.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund EG, Kerr TA, Sakai J, Li WP, Russell DW. 1998. cDNA cloning of mouse and human cholesterol 25-hydroxylases, polytopic membrane proteins that synthesize a potent oxysterol regulator of lipid metabolism. J Biol Chem 273: 34316–34327. 10.1074/jbc.273.51.34316 [DOI] [PubMed] [Google Scholar]
- Maekawa M, Fairn GD. 2015. Complementary probes reveal that phosphatidylserine is required for the proper transbilayer distribution of cholesterol. J Cell Sci 128: 1422–1433. 10.1242/jcs.164715 [DOI] [PubMed] [Google Scholar]
- Marques PE, Nyegaard S, Collins RF, Troise F, Freeman SA, Trimble WS, Grinstein S. 2019. Multimerization and retention of the scavenger receptor SR-B1 in the plasma membrane. Dev Cell 50: 283–295.e285. 10.1016/j.devcel.2019.05.026 [DOI] [PubMed] [Google Scholar]
- McGee TP, Cheng HH, Kumagai H, Omura S, Simoni RD. 1996. Degradation of 3-hydroxy-3-methylglutaryl-CoA reductase in endoplasmic reticulum membranes is accelerated as a result of increased susceptibility to proteolysis. J Biol Chem 271: 25630–25638. 10.1074/jbc.271.41.25630 [DOI] [PubMed] [Google Scholar]
- Meiner VL, Cases S, Myers HM, Sande ER, Bellosta S, Schambelan M, Pitas RE, McGuire J, Herz J, Farese RV. 1996. Disruption of the acyl-CoA:cholesterol acyltransferase gene in mice: evidence suggesting multiple cholesterol esterification enzymes in mammals. Proc Natl Acad Sci 93: 14041–14046. 10.1073/pnas.93.24.14041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesmin B, Bigay J, Moser von Filseck J, Lacas-Gervais S, Drin G, Antonny B. 2013. A four-step cycle driven by PI(4)P hydrolysis directs sterol/PI(4)P exchange by the ER–Golgi tether OSBP. Cell 155: 830–843. 10.1016/j.cell.2013.09.056 [DOI] [PubMed] [Google Scholar]
- Murley A, Sarsam RD, Toulmay A, Yamada J, Prinz WA, Nunnari J. 2015. Ltc1 is an ER-localized sterol transporter and a component of ER–mitochondria and ER–vacuole contacts. J Cell Biol 209: 539–548. 10.1083/jcb.201502033 [DOI] [Google Scholar]
- Naito T, Ercan B, Krshnan L, Triebl A, Koh DHZ, Wei F-Y, Tomizawa K, Torta FT, Wenk MR, Saheki Y. 2019. Movement of accessible plasma membrane cholesterol by the GRAMD1 lipid transfer protein complex. eLife 8: e51401. 10.7554/eLife.51401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neculai D, Schwake M, Ravichandran M, Zunke F, Collins RF, Peters J, Neculai M, Plumb J, Loppnau P, Pizarro JC, et al. 2013. Structure of LIMP-2 provides functional insights with implications for SR-BI and CD36. Nature 504: 172–176. 10.1038/nature12684 [DOI] [PubMed] [Google Scholar]
- Neufeld EB, Cooney AM, Pitha J, Dawidowicz EA, Dwyer NK, Pentchev PG, Blanchette-Mackie EJ. 1996. Intracellular trafficking of cholesterol monitored with a cyclodextrin. J Biol Chem 271: 21604–21613. 10.1074/jbc.271.35.21604 [DOI] [PubMed] [Google Scholar]
- Niu S-L, Litman BJ. 2002. Determination of membrane cholesterol partition coefficient using a lipid vesicle–cyclodextrin binary system: effect of phospholipid acyl chain unsaturation and headgroup composition. Biophys J 83: 3408–3415. 10.1016/S0006-3495(02)75340-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen BN, Schlesinger PH, Ory DS, Baker NA. 2011. 25-Hydroxycholesterol increases the availability of cholesterol in phospholipid membranes. Biophys J 100: 948–956. 10.1016/j.bpj.2010.12.3728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parini P, Davis M, Lada AT, Erickson SK, Wright TL, Gustafsson U, Sahlin S, Einarsson C, Eriksson M, Angelin B, et al. 2004. ACAT2 is localized to hepatocytes and is the major cholesterol-esterifying enzyme in human liver. Circulation 110: 2017–2023. 10.1161/01.CIR.0000143163.76212.0B [DOI] [PubMed] [Google Scholar]
- Plump AS, Erickson SK, Weng W, Partin JS, Breslow JL, Williams DL. 1996. Apolipoprotein A-I is required for cholesteryl ester accumulation in steroidogenic cells and for normal adrenal steroid production. J Clin Invest 97: 2660–2671. 10.1172/JCI118716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian H, Wu X, Du X, Yao X, Zhao X, Lee J, Yang H, Yan N. 2020. Structural basis of low-pH-dependent lysosomal cholesterol egress by NPC1 and NPC2. Cell 182: 98–111.e118. 10.1016/j.cell.2020.05.020 [DOI] [PubMed] [Google Scholar]
- Radhakrishnan A, McConnell HM. 2000. Chemical activity of cholesterol in membranes. Biochemistry 39: 8119–8124. 10.1021/bi0005097 [DOI] [PubMed] [Google Scholar]
- Radhakrishnan A, Ikeda Y, Kwon HJ, Brown MS, Goldstein JL. 2007. Sterol-regulated transport of SREBPs from endoplasmic reticulum to Golgi: Oxysterols block transport by binding to Insig. Proc Natl Acad Sci 104: 6511. 10.1073/pnas.0700899104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhakrishnan A, Goldstein JL, McDonald JG, Brown MS. 2008. Switch-like control of SREBP-2 transport triggered by small changes in ER cholesterol: a delicate balance. Cell Metab 8: 512–521. 10.1016/j.cmet.2008.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravid T, Doolman R, Avner R, Harats D, Roitelman J. 2000. The ubiquitin-proteasome pathway mediates the regulated degradation of mammalian 3-hydroxy-3-methylglutaryl-coenzyme A reductase. J Biol Chem 275: 35840–35847. 10.1074/jbc.M004793200 [DOI] [PubMed] [Google Scholar]
- Repa JJ, Liang G, Ou J, Bashmakov Y, Lobaccaro JM, Shimomura I, Shan B, Brown MS, Goldstein JL, Mangelsdorf DJ. 2000. Regulation of mouse sterol regulatory element-binding protein-1c gene (SREBP-1c) by oxysterol receptors, LXRα and LXRβ. Genes Dev 14: 2819–2830. 10.1101/gad.844900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repa JJ, Buhman KK, Farese RV Jr, Dietschy JM, Turley SD. 2004. ACAT2 deficiency limits cholesterol absorption in the cholesterol-fed mouse: impact on hepatic cholesterol homeostasis. Hepatology 40: 1088–1097. 10.1002/hep.20439 [DOI] [PubMed] [Google Scholar]
- Reuter MS, Tawamie H, Buchert R, Hosny Gebril O, Froukh T, Thiel C, Uebe S, Ekici AB, Krumbiegel M, Zweier C, et al. 2017. Diagnostic yield and novel candidate genes by exome sequencing in 152 consanguineous families with neurodevelopmental disorders. JAMA Psychiatry 74: 293. 10.1001/jamapsychiatry.2016.3798 [DOI] [PubMed] [Google Scholar]
- Rigotti A, Trigatti BL, Penman M, Rayburn H, Herz J, Krieger M. 1997. A targeted mutation in the murine gene encoding the high density lipoprotein (HDL) receptor scavenger receptor class B type I reveals its key role in HDL metabolism. Proc Natl Acad Sci 94: 12610–12615. 10.1073/pnas.94.23.12610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigueza WV, Thuahnai ST, Temel RE, Lund-Katz S, Phillips MC, Williams DL. 1999. Mechanism of scavenger receptor class B type I-mediated selective uptake of cholesteryl esters from high density lipoprotein to adrenal cells. J Biol Chem 274: 20344–20350. 10.1074/jbc.274.29.20344 [DOI] [PubMed] [Google Scholar]
- Roitelman J, Simoni RD. 1992. Distinct sterol and nonsterol signals for the regulated degradation of 3-hydroxy-3-methylglutaryl-CoA reductase. J Biol Chem 267: 25264–25273. 10.1016/S0021-9258(19)74035-6 [DOI] [PubMed] [Google Scholar]
- Runz H, Miura K, Weiss M, Pepperkok R. 2006. Sterols regulate ER-export dynamics of secretory cargo protein ts-O45-G. EMBO J 25: 2953–2965. 10.1038/sj.emboj.7601205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saher G, Quintes S, Möbius W, Wehr MC, Krämer-Albers EM, Brügger B, Nave KA. 2009. Cholesterol regulates the endoplasmic reticulum exit of the major membrane protein P0 required for peripheral myelin compaction. J Neurosci 29: 6094–6104. 10.1523/JNEUROSCI.0686-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakashita N, Miyazaki A, Takeya M, Horiuchi S, Chang CC, Chang TY, Takahashi K. 2000. Localization of human acyl-coenzyme A: cholesterol acyltransferase-1 (ACAT-1) in macrophages and in various tissues. Am J Pathol 156: 227–236. 10.1016/S0002-9440(10)64723-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandhu J, Li S, Fairall L, Pfisterer SG, Gurnett JE, Xiao X, Weston TA, Vashi D, Ferrari A, Orozco JL, et al. 2018. Aster proteins facilitate nonvesicular plasma membrane to ER cholesterol transport in mammalian cells. Cell 175: 514–529.e520. 10.1016/j.cell.2018.08.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Cortez RLP, Khan V, Khan FS, Mughal ZUN, Chakchouk I, Lee K, Rasheed M, Hamza R, Acharya A, Ullah E, et al. 2018. Novel candidate genes and variants underlying autosomal recessive neurodevelopmental disorders with intellectual disability. Hum Genet 137: 735–752. 10.1007/s00439-018-1928-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenheimer R, Breusch F. 1933. Synthesis and destruction of cholesterol in the organism. J Biol Chem 103: 439–448. 10.1016/S0021-9258(18)75823-7 [DOI] [Google Scholar]
- Sever N, Yang T, Brown MS, Goldstein JL, DeBose-Boyd RA. 2003. Accelerated degradation of HMG CoA reductase mediated by binding of insig-1 to its sterol-sensing domain. Mol Cell 11: 25–33. 10.1016/S1097-2765(02)00822-5 [DOI] [PubMed] [Google Scholar]
- Sharpe LJ, Howe V, Scott NA, Luu W, Phan L, Berk JM, Hochstrasser M, Brown AJ. 2019. Cholesterol increases protein levels of the E3 ligase MARCH6 and thereby stimulates protein degradation. J Biol Chem 294: 2436–2448. 10.1074/jbc.RA118.005069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skiba PJ, Zha X, Maxfield FR, Schissel SL, Tabas I. 1996. The distal pathway of lipoprotein-induced cholesterol esterification, but not sphingomyelinase-induced cholesterol esterification, is energy-dependent. J Biol Chem 271: 13392–13400. 10.1074/jbc.271.23.13392 [DOI] [PubMed] [Google Scholar]
- Slotte JP, Bierman EL. 1988. Depletion of plasma-membrane sphingomyelin rapidly alters the distribution of cholesterol between plasma membranes and intracellular cholesterol pools in cultured fibroblasts. Biochem J 250: 653–658. 10.1042/bj2500653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotte JP, Hedström G, Rannström S, Ekman S. 1989. Effects of sphingomyelin degradation on cell cholesterol oxidizability and steady-state distribution between the cell surface and the cell interior. Biochim Biophys Acta 985: 90–96. [DOI] [PubMed] [Google Scholar]
- Sokolov A, Radhakrishnan A. 2010. Accessibility of cholesterol in endoplasmic reticulum membranes and activation of SREBP-2 switch abruptly at a common cholesterol threshold. J Biol Chem 285: 29480–29490. 10.1074/jbc.M110.148254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steck TL, Ye J, Lange Y. 2002. Probing red cell membrane cholesterol movement with cyclodextrin. Biophys J 83: 2118–2125. 10.1016/S0006-3495(02)73972-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su L, Zhou L, Chen FJ, Wang H, Qian H, Sheng Y, Zhu Y, Yu H, Gong X, Le C, et al. 2019. Cideb controls sterol-regulated ER export of SREBP/SCAP by promoting cargo loading at ER exit sites. EMBO J 38: e100156. 10.15252/embj.2018100156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudhop T, Lütjohann D, Kodal A, Igel M, Tribble DL, Shah S, Perevozskaya I, von Bergmann K. 2002. Inhibition of intestinal cholesterol absorption by ezetimibe in humans. Circulation 106: 1943–1948. 10.1161/01.CIR.0000034044.95911.DC [DOI] [PubMed] [Google Scholar]
- Tabas I, Rosoff WJ, Boykow GC. 1988. Acyl coenzyme A:cholesterol acyl transferase in macrophages utilizes a cellular pool of cholesterol oxidase-accessible cholesterol as substrate. J Biol Chem 263: 1266–1272. 10.1016/S0021-9258(19)57295-7 [DOI] [PubMed] [Google Scholar]
- Takahashi K, Kanerva K, Vanharanta L, Almeida-Souza L, Lietha D, Olkkonen VM, Ikonen E. 2021. ORP2 couples LDL-cholesterol transport to FAK activation by endosomal cholesterol/PI(4,5)P2 exchange. EMBO J 40: e106871. 10.15252/embj.2020106871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong J, Manik MK, Im YJ. 2018. Structural basis of sterol recognition and nonvesicular transport by lipid transfer proteins anchored at membrane contact sites. Proc Natl Acad Sci 115: E856–E865. 10.1073/pnas.1719709115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinh MN, Brown MS, Goldstein JL, Han J, Vale G, McDonald JG, Seemann J, Mendell JT, Lu F. 2020. Last step in the path of LDL cholesterol from lysosome to plasma membrane to ER is governed by phosphatidylserine. Proc Natl Acad Sci 117: 18521. 10.1073/pnas.2010682117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinh MN, Brown MS, Seemann J, Vale G, McDonald JG, Goldstein JL, Lu F. 2022. Interplay between Asters/GRAMD1s and phosphatidylserine in intermembrane transport of LDL cholesterol. Proc Natl Acad Sci 119: e2120411119. 10.1073/pnas.2120411119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujishita Y, Hurley JH. 2000. Structure and lipid transport mechanism of a StAR-related domain. Nat Struct Biol 7: 408–414. 10.1038/75192 [DOI] [PubMed] [Google Scholar]
- Underwood KW, Jacobs NL, Howley A, Liscum L. 1998. Evidence for a cholesterol transport pathway from lysosomes to endoplasmic reticulum that is independent of the plasma membrane. J Biol Chem 273: 4266–4274. 10.1074/jbc.273.7.4266 [DOI] [PubMed] [Google Scholar]
- Urbani L, Simoni RD. 1990. Cholesterol and vesicular stomatitis virus G protein take separate routes from the endoplasmic reticulum to the plasma membrane. J Biol Chem 265: 1919–1923. 10.1016/S0021-9258(19)39918-1 [DOI] [PubMed] [Google Scholar]
- Vanier MT. 2010. Niemann-Pick disease type C. Orphanet J Rare Dis 5: 16. 10.1186/1750-1172-5-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Meer G, Voelker DR, Feigenson GW. 2008. Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Biol 9: 112–124. 10.1038/nrm2330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkleij AJ, Zwaal RF, Roelofsen B, Comfurius P, Kastelijn D, van Deenen LL. 1973. The asymmetric distribution of phospholipids in the human red cell membrane. A combined study using phospholipases and freeze-etch electron microscopy. Biochim Biophys Acta 323: 178–193. 10.1016/0005-2736(73)90143-0 [DOI] [PubMed] [Google Scholar]
- Wang B, Tontonoz P. 2018. Liver X receptors in lipid signalling and membrane homeostasis. Nat Rev Endocrinol 14: 452–463. 10.1038/s41574-018-0037-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Zheng Z, Caviglia JM, Corey KE, Herfel TM, Cai B, Masia R, Chung RT, Lefkowitch JH, Schwabe RF, et al. 2016. Hepatocyte TAZ/WWTR1 promotes inflammation and fibrosis in nonalcoholic steatohepatitis. Cell Metab 24: 848–862. 10.1016/j.cmet.2016.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Ma Q, Qi Y, Dong J, Du X, Rae J, Wang J, Wu WF, Brown AJ, Parton RG, et al. 2019. ORP2 delivers cholesterol to the plasma membrane in exchange for phosphatidylinositol 4, 5-bisphosphate (PI(4,5)P(2)). Mol Cell 73: 458–473.e457. 10.1016/j.molcel.2018.11.014 [DOI] [PubMed] [Google Scholar]
- Wang X, Cai B, Yang X, Sonubi OO, Zheng Z, Ramakrishnan R, Shi H, Valenti L, Pajvani UB, Sandhu J, et al. 2020. Cholesterol stabilizes TAZ in hepatocytes to promote experimental non-alcoholic steatohepatitis. Cell Metab 31: 969–986.e967. 10.1016/j.cmet.2020.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm LP, Wendling C, Védie B, Kobayashi T, Chenard MP, Tomasetto C, Drin G, Alpy F. 2017. STARD 3 mediates endoplasmic reticulum-to-endosome cholesterol transport at membrane contact sites. EMBO J 36: 1412–1433. 10.15252/embj.201695917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler MBL, Kidmose RT, Szomek M, Thaysen K, Rawson S, Muench SP, Wüstner D, Pedersen BP. 2019. Structural insight into eukaryotic sterol transport through Niemann–Pick Type C Proteins. Cell 179: 485–497.e418. 10.1016/j.cell.2019.08.038 [DOI] [PubMed] [Google Scholar]
- Wüstner D, Mondal M, Tabas I, Maxfield FR. 2005. Direct observation of rapid internalization and intracellular transport of sterol by macrophage foam cells. Traffic 6: 396–412. 10.1111/j.1600-0854.2005.00285.x [DOI] [PubMed] [Google Scholar]
- Xiao X, Kim Y, Romartinez-Alonso B, Sirvydis K, Ory DS, Schwabe JWR, Jung ME, Tontonoz P. 2021. Selective Aster inhibitors distinguish vesicular and nonvesicular sterol transport mechanisms. Proc Natl Acad Sci 118: e2024149118. 10.1073/pnas.2024149118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu XX, Tabas I. 1991. Lipoproteins activate acyl-coenzyme A:cholesterol acyltransferase in macrophages only after cellular cholesterol pools are expanded to a critical threshold level. J Biol Chem 266: 17040–17048. 10.1016/S0021-9258(19)47337-7 [DOI] [PubMed] [Google Scholar]
- Yang T, Espenshade PJ, Wright ME, Yabe D, Gong Y, Aebersold R, Goldstein JL, Brown MS. 2002. Crucial step in cholesterol homeostasis: sterols promote binding of SCAP to INSIG-1, a membrane protein that facilitates retention of SREBPs in ER. Cell 110: 489–500. 10.1016/S0092-8674(02)00872-3 [DOI] [PubMed] [Google Scholar]
- Yang W, Bai Y, Xiong Y, Zhang J, Chen S, Zheng X, Meng X, Li L, Wang J, Xu C, et al. 2016. Potentiating the antitumour response of CD8+ T cells by modulating cholesterol metabolism. Nature 531: 651–655. 10.1038/nature17412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung T, Gilbert GE, Shi J, Silvius J, Kapus A, Grinstein S. 2008. Membrane phosphatidylserine regulates surface charge and protein localization. Science 319: 210–213. 10.1126/science.1152066 [DOI] [PubMed] [Google Scholar]
- Zhao K, Ridgway ND. 2017. Oxysterol-binding protein-related protein 1L regulates cholesterol egress from the endo-lysosomal system. Cell Rep 19: 1807–1818. 10.1016/j.celrep.2017.05.028 [DOI] [PubMed] [Google Scholar]
- Zhou QD, Chi X, Lee MS, Hsieh WY, Mkrtchyan JJ, Feng AC, He C, York AG, Bui VL, Kronenberger EB, et al. 2020. Interferon-mediated reprogramming of membrane cholesterol to evade bacterial toxins. Nat Immunol 21: 746–755. 10.1038/s41590-020-0695-4 [DOI] [PMC free article] [PubMed] [Google Scholar]