Abstract
Virus-induced gene silencing (VIGS) is a powerful tool for high-throughput analysis of gene function. Here, we developed the VIGS vector pCF93, from which expression of the cucumber fruit mottle mosaic virus genome is driven by the cauliflower mosaic virus 35S promoter to produce viral transcripts in inoculated plants. To test the utility of the pCF93 vector, we identified candidate genes related to male sterility (MS) in watermelon (Citrullus lanatus), which is recalcitrant to genetic transformation. Specifically, we exploited previously reported reference-based and de novo transcriptome data to define 38 differentially expressed genes between a male-sterile line and its fertile near-isogenic line in the watermelon cultivar DAH. We amplified 200- to 300-bp fragments of these genes, cloned them into pCF93, and inoculated DAH with the resulting VIGS clones. The small watermelon cultivar DAH enabled high-throughput screening using a small cultivation area. We simultaneously characterized the phenotypes associated with each of the 38 candidate genes in plants grown in a greenhouse. Silencing of 8 of the 38 candidate genes produced male-sterile flowers with abnormal stamens and no pollen. We confirmed the extent of gene silencing in inoculated flowers using reverse transcription–qPCR. Histological analysis of stamens from male-fertile and male-sterile floral buds and mature flowers revealed developmental defects and shrunken pollen sacs. Based on these findings, we propose that the pCF93 vector and our VIGS system will facilitate high-throughput analysis for the study of gene function in watermelons.
A vector based on cucumber fruit mottle mosaic virus from Cucumis melo facilitates large-scale validation of male sterility-related gene functions in watermelon (Citrullus lanatus).
Introduction
Plant male sterility (MS) is characterized by flowers producing nonviable pollen or no pollen at all, a failure of anther dehiscence, or the production of defective stamens. MS is a useful tool for hybrid production, as it prevents self-pollination and bypasses the need to emasculate flowers manually, which can increase crop yield by 3.5%–200% in some crops (Chen and Liu, 2014; Kim and Zhang, 2018). Thus, the dissection of MS through functional genomic studies can offer major benefits for crop breeders and farmers. Watermelon (Citrullus lanatus) has two flowering patterns: monoecious and andromonoecious. Monoecious watermelon plants carry both male and female flowers on the same plant, while andromonoecious watermelon plants have both male and hermaphrodite flowers on the same plant (Rudich 1985). To date, six watermelon male-sterile mutants have been reported: glabrous male-sterile (Watt, 1962, 1967; Ray and Sherman, 1988), male-sterile dwarf (Huang Hexun, 1998), ms1 (Zhang and Wang, 1990, Rhee et al., 2015), ms2 (Dyutin, 1990), ms3 (Bang et al., 2005), and Se18 (Wang et al., 2020). We recently identified the causal genomic region of the genic MS (GMS) observed in DAH3615-MS (ms1-mutant breeding line), which harbors a 10-bp deletion in gene Cla97C06G117840 on chromosome 6, which encodes a basic helix–loop–helix transcription factor. We also developed single-nucleotide polymorphism markers linked to this trait to facilitate genotyping (Jang et al., 2021).
Several techniques have been developed to facilitate functional genomics analyses, including targeting induced local lesions in genomes, ethyl methanesulfonate mutagenesis, and the production of transgenic lines via T-DNA insertions and RNA interference (RNAi). Furthermore, the discovery and introduction of sequence-specific nucleases in plant genomes, such as zinc-finger nucleases (ZFNs) and transcription activator-like effector nucleases (TALENs), revolutionized gene editing (Gupta and Musunuru, 2014). However, TALENs and ZFNs targeting specific sequences are difficult to design and are relatively expensive. Notably, gene editing based on clustered regularly interspaced short palindromic repeats (CRISPR) and CRISPR-associated nucleases (Cas) has been successfully applied in humans, animals, and plants (Pickar-Oliver and Gersbach, 2019). Although CRISPR/Cas9-mediated editing has opened new avenues for functional genomics and precise gene editing, it does typically require the generation of transgenic plants, via for example, Agrobacterium tumefaciens-mediated or particle bombardment transformation to introduce and express the transgene encoding the Cas nuclease and various single-guide RNAs for target specificity (Fister et al., 2018; Shan et al., 2020). This bottleneck needs to be addressed before CRISPR/Cas9 can be deployed in plants that are recalcitrant to genetic modification. In general, cucurbit plants exhibit an extremely low transformation efficiency, although several studies have reported the generation of cucurbit knockout mutants by using CRISPR/Cas9 (Hu et al., 2017; Tian et al., 2017, 2018). However, there remains a need for the rapid testing of gene function to complement stable transformation techniques.
Virus-induced gene silencing (VIGS) represents one such alternative method, as it allows the rapid screening of genes in a high-throughput manner for functional genomics. Improved VIGS vectors are critical to reach high silencing efficiency. For instance, the maize dwarf mosaic virus vector targets multiple genes simultaneously in maize (Zea mays) (Xie et al., 2021) and a pseudorecombinant chimeric cucumber mosaic virus vector adopted ligation-independent cloning for high-throughput cloning (Li et al., 2021). A modified tobacco rattle virus vector was also developed for efficient validation of gene silencing by incorporating a fragment of the PHYTOENE DESATURASE (PDS) gene upstream of the multiple cloning site (MCS) in the vector (Yamamoto et al., 2021); silencing of PDS in photosynthetic tissues will result in photobleaching and white tissues.
VIGS exploits the posttranscriptional gene silencing (PTGS) that takes place when host cells are infected by virus (Robertson, 2004). Viral double-stranded RNAs (dsRNA) are formed during most RNA-based virus replication. These dsRNAs are degraded by the endoribonucleases DICER-LIKE 2 (DCL2) and DCL4, resulting in the generation of short interfering RNAs (siRNAs). The siRNAs are in turn incorporated into the RNA-induced silencing complex and targeted endogenous mRNAs and viral RNA harboring a complementary sequence and initiate RNA cleavage and degradation. To harness the potential of PTGS in functional genomics, plant virus vectors have been extensively developed in deploy VIGS in various plant species, such as tomato (Solanum lycopersicum) (Liu et al., 2002; Fu et al., 2005; Senthil‐Kumar and Mysore, 2011; Rhee et al., 2018), Chinese cabbage (Brassica rapa ssp. pekinensis) (Yu et al., 2019), rice (Oryza sativa) (Purkayastha et al., 2010; Kant et al., 2015), maize (Benavente et al., 2012; Mei et al., 2016), barley (Hordeum vulgare) (Liu et al., 2016), strawberry (Fragaria × ananassa) (Li et al., 2019), and, more recently, cucurbits (Igarashi et al., 2009; Bu et al., 2019; Liao et al., 2019) as well as banana (Musa acuminata) (Tzean et al., 2019).
We previously constructed a virus vector for functional genomic studies in cucurbits, particularly of their flowers and fruits, using cucumber fruit mottle mosaic virus (CFMMV). CFMMV is a member of the Tobamovirus genus and has a monopartite genome of 6.5 kb that comprised four open reading frames (ORFs), which encode 127- and 187-kDa proteins involved in virus replication as RNA-dependent RNA polymerases (RdRps), a 30-kDa movement protein (MP) required for cell-to-cell movement of the virus, and a 17-kDa coat protein (CP) that is essential for long-distance movement (Antignus et al., 2001). CFMMV infects a wide range of cucurbits encompassing major commercial crops such as cucumber (Cucumis sativus), melon (Cucumis melo), squash (Cucurbita pepo), and watermelon. Our groups previously isolated and sequenced CFMMV-Cm from melon to construct a full-length infectious clone (Rhee et al., 2014). We further confirmed that viral in vitro transcripts stably replicate in cucurbit fruits. Subsequently, we mapped the subgenomic promoter (SGP) controlling CP expression and constructed a CFMMV vector, which successfully expressed enhanced green fluorescent protein (EGFP) in cucumber, melon, and watermelon (Rhee et al., 2016).
Functional genomics has been challenging in watermelon because it is recalcitrant to the generation of transgenic plants. In addition, the study of watermelon fruit development demands a relatively long time commitment and large areas for cultivation. In this study, we report an efficient cucurbit-infecting virus vector system based on the CFMMV-VIGS vector that exhibited high and long-lasting gene silencing efficiency in cucurbit plants, with clear phenotypes. Our monopartite CFMMV vector is relatively easy to manipulate and allows the high-throughput screening of gene function in watermelon. This CFMMV-VIGS system will help advance the exploration of gene function in cucurbit reproductive organ development.
Results
Constructing an efficient CFMMV vector for VIGS
We previously isolated and constructed a CFMMV-Cm-based full-length infectious clone (Rhee et al., 2014) and a virus vector for heterologous expression (Rhee et al., 2016). We had developed 14 CFMMV vectors, carrying various lengths of sequence upstream of the CP translation start site up to 100 bp into the SGP (Rhee et al., 2016).
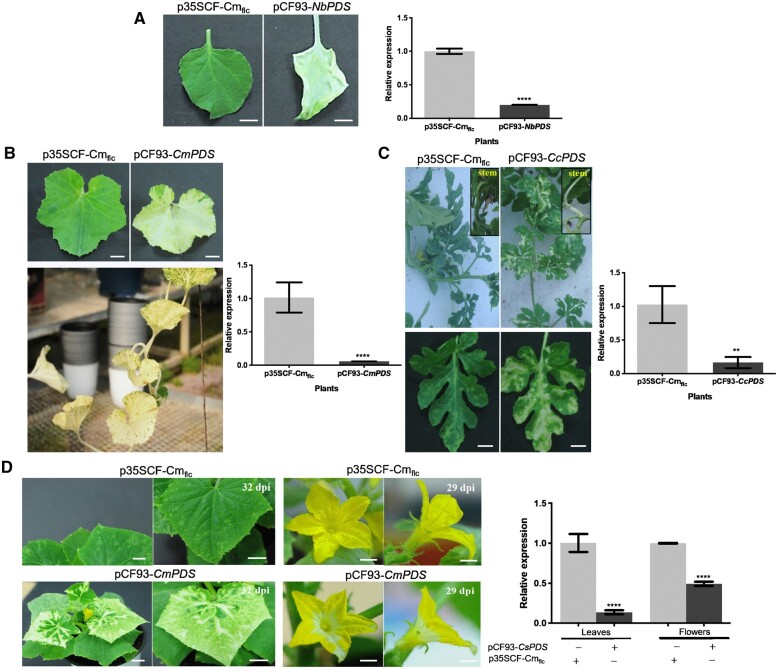
To select the most efficient VIGS vector, we assessed four of the 14 CFMMV-Cm-based vectors (pCF93 and pCF93K, with 93 bp of sequence upstream of CP; pCF157, and pCF157K, with 157 bp of sequence upstream of CP) from our previous report (Rhee et al., 2016). pCF93 and pCF157 each harbored one 3′-nontranslated region (3′-NTR), while pCF93K and pCF157K included an additional 3′-NTR between the MCS (XhoI/PmeI) and CP region (Figure 1A). To visualize the gene silencing efficiency of CFMMV-Cm-based vectors, we inserted a partial PDS fragment into each vector. We noticed infection symptoms at 5–6 days postinoculation (dpi) on the second (noninoculated) leaf above the leaf inoculated with pCF93-NbPDS or pCF157-NbPDS, with the development of a photobleaching phenotype at 12–15 dpi (Figure 1, B and C). The pCF93-NbPDS vector produced wider white-leaf sectors on the leaves of inoculated plants compared to those seen with the pCF157-NbPDS vector. We did not observe the typical photobleaching phenotype on Nicotiana benthamiana plants inoculated with pCF157K-NbPDS and observed only mild symptoms on plants inoculated with pCF93K-NbPDS, although both sets of plants displayed typical virus infection phenotypes (Figure 1C).
Figure 1.
VIGS of the PDS gene results in a photobleaching phenotype in N. benthamiana. A, Schematic diagrams of the vectors pCF93-NbPDS and pCF157-NbPDS and the nucleotide sequence around the MCS. XhoI and PmeI are the restriction sites to clone the insert of interest. 35S, cauliflower mosaic virus 35S promoter; MP, triple gene block. B and C, comparison of VIGS phenotypes in N. benthamiana plants infected by pCF93-NbPDS and pCF93K-NbPDS (B) or pCF157-NbPDS and pCF157K-NbPDS (C) at 12 dpi. Of the four constructs tested, pCF93 exhibited the strongest silencing. Scale bars, 2 cm.
To assess the stability of the inserted fragment in the virus vector in planta, we performed reverse transcription–PCR (RT–PCR) with a primer pair flanking the MCS. Specifically, after inoculating N. benthamiana plants with pCF93-NbPDS, pCF93K-NbPDS, and pCF157-NbPDS, we isolated total RNA from fourth upper leaves above the inoculated leaves. We obtained products of expected size after RT–PCR (Supplemental Figure S1). However, plants inoculated with pCF157K-NbPDS appeared to have lost the artificially inserted MCS and returned to the genome structure of wild-type CFMMV via homologous recombination (Supplemental Figure S1). Although pCF93K-NbPDS induced photobleaching phenotypes, gene silencing efficiency was low compared to pCF93-NbPDS and pCF157-NbPDS, indicating that vectors with a single 3′NTR are better suited for VIGS. We thus selected pCF93 as the most efficient VIGS vector for functional gene analysis.
Evaluation of pCF93 as a VIGS vector in cucurbits
We tested the efficiency of pCF93-PDS vectors carrying specific PDS fragments for each cucurbit plant species of interest by inoculating cotyledons of cucumber, melon, and watermelon. The pCF-PDS vectors successfully induced gene silencing not only in leaves but also in the reproductive organs of all cucurbits tested here. Relative PDS expression levels in N. benthamiana inoculated with pCF93-NbPDS were only ∼20% those measured in N. benthamiana plants inoculated with the control CFMMV-Cm vector (Figure 2A). Melon plants inoculated with pCF93-CmPDS showed photobleaching phenotypes including stems and leaves, and maintained these phenotypes in all newly developed leaves until the natural death of the plant (Figure 2B). Likewise, the leaves of watermelon plants exhibited photobleaching and a drop in relative PDS transcript level of up to 333-fold when compared to control plants inoculated with a full-length clone of CFMMV, p35SCF-Cmflc (Figure 2C). Similarly, cucumber plants also presented evidence of PDS silencing phenotypes in the leaves and flowers, the latter being consistent with the involvement of PDS in the β-carotene biosynthesis pathway that is implicated in the color of flowers. Relative PDS transcript levels in the leaves of cucumber inoculated with pCF93-CsPDS were ∼seven-fold lower than in p35SCF-Cmflc-inoculated cucumber plants. Flowers also exhibited gene silencing phenotypes and an ∼ 2.5-fold reduction in PDS transcript level upon inoculation with pCF93-CsPDS relative to p35SCF-Cmflc, as determined by RT–qPCR (Figure 2D). Of note, we transferred all plants inoculated with the tested virus vectors from the growth room to a greenhouse under the natural sunlight to enhance photobleaching (PDS silencing) efficiency.
Figure 2.
Photobleaching was observed not only in leaves but also in stems and flowers in cucurbit plants. A, Photobleaching phenotypes and relative PDS expression levels in N. benthamiana leaves infected with pCF93-NbPDS or wild-type CFMMV. Scale bars, 2 cm. B, Comparison of gene silencing efficiency in melon leaves infected with p35SCF-Cmflc (top, left) or pCF93-CmPDS (top, right). pCF93-CmPDS-induced VIGS phenotypes in melon grown in the greenhouse (bottom). Relative PDS expression levels are shown on the right. Scale bars, 3 cm. C, Photobleaching phenotypes on watermelon leaves and stems infected with pCF93-CcPDS (left) and relative PDS expression levels in leaves inoculated with pCF93-CcPDS or wild-type CFMMV. Scale bars, 3 cm. D, Photobleaching of leaves and flowers from cucumber plants inoculated with pCF93-CsPDS (left). Scale bars, 3 cm (leaf) and 1 cm (flower). Right: relative PDS expression levels in leaves and flowers from cucumber plants inoculated with p35SCF-Cmflc (CF-leaf and CF-flower) and pCF93-CsPDS (PDS-leaf and PDS-flower). Relative PDS expression levels were determined using PDS-specific primers and normalized to GAPDH (A) or 18S rRNA expression levels by RT–qPCR (B, C, and D). The relative expression levels in CFMMV-Cm samples were set to 1. Significant differences were determined using a Student’s t test (****P < 0.0001 and 0.001 <**P < 0.01).
Evaluation of PDS gene silencing efficiency in three watermelon cultivars
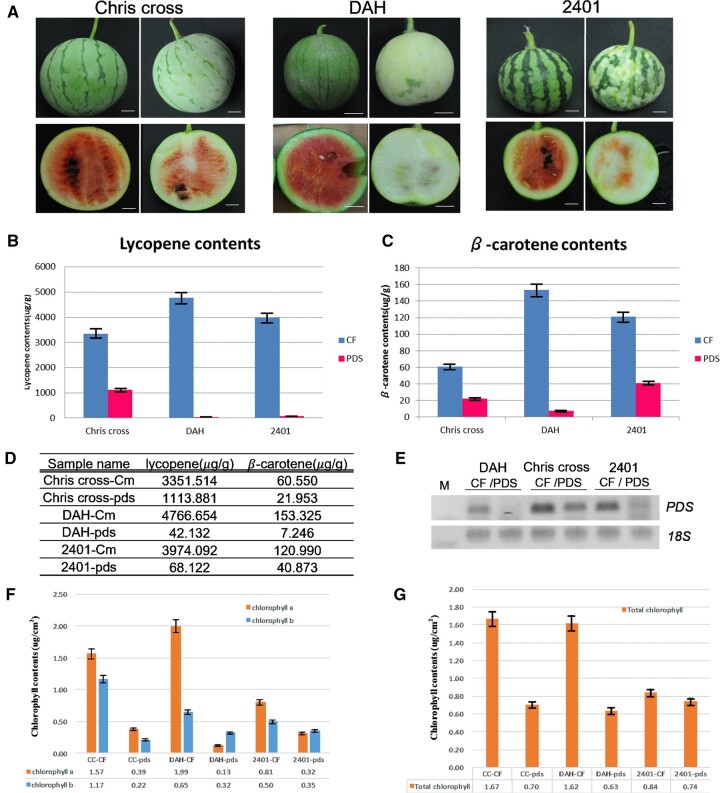
Flowers and fruits are the major reproductive organs producing the edible parts of plants, especially cucurbits. Cucurbit fruit and flower development is, therefore, intensely studied (Lin et al., 2007; Wang et al., 2010; Manzano et al., 2014; Switzenberg et al., 2015). We inoculated watermelon cotyledons with Agrobacteria harboring the pCF93-CcPDS vector and grew the plants in the greenhouse without any additional inoculations. We tested PDS VIGS efficiency in three watermelon cultivars, all with small fruits: “2401”, “Chris cross”, and “DAH”. Accordingly, we harvested watermelon fruits at 45-day after pollination (dap) and assessed the photobleaching phenotypes on fruit peels (Figure 3A). PDS downregulation can lead to the inhibition of lycopene and β-carotene biosynthesis, resulting in a color change of watermelon flesh from red to white. Indeed, the fruits produced by watermelon plants inoculated with pCF93-CcPDS were characterized by lighter green peels and white flesh. As the red flesh of watermelon is packed with lycopene and β-carotene (Tomes et al., 1963), we hypothesized that the white color is caused by photobleaching and the degradation of chlorophyll. To test this hypothesis, we thus measured the contents of lycopene and β-carotene by high-performance liquid chromatography (HPLC) and determined PDS expression levels via end-point RT–PCR. The lycopene and β-carotene contents of fruits (45 dap) decreased dramatically in all three watermelon cultivars inoculated with the pCF93-CcPDS vector. Specifically, lycopene contents were 110-fold lower and β-carotene contents 2.7- to 21-fold lower in fruits from plants inoculated with pCF93-CcPDS compared to those from p35SCF-Cmflc fruit (Figure 3, B–D). PDS transcripts also accumulated to lower levels in all watermelons experiencing PDS silencing (Figure 3E). In agreement with the photobleaching phenotype, chlorophyll a contents in the fruit peels of watermelon plants inoculated with pCF93-CcPDS dropped ∼2.5– to 15-fold compared to p35SCF-Cmflc plants, with chlorophyll b contents being 1.4– to 8-fold lower. Total chlorophyll contents decreased by ∼1.1– to 2.5-fold (Figure 3, F and G).
Figure 3.
Estimation of lycopene and β-carotene contents in watermelon fruits silenced for PDS. A, VIGS phenotypes in the fruits of three watermelon cultivars. Control fruits are on the left, and VIGS fruits are on the right. Scale bars, 2 cm. B and C, Lycopene (B) and β-carotene (C) contents in the flesh of watermelon fruits inoculated with p35SCF-Cmflc (CF) or pCF93-CcPDS (PDS). D, Lycopene and β-carotene contents, as determined by HPLC. E, Semi-quantitative RT–PCR analysis of PDS expression levels using a CcPDS-specific primer set. F, Contents of chlorophyll a and b in the peels of watermelon fruits inoculated with p35SCF-Cmflc (CF) or pCF93-PDS vectors. G, Total chlorophyll contents. Watermelon cultivars “Chris cross,” “DAH,” and “2401” infected by p35SCF-Cmflc are denoted as “CC-CF,” “DAH-CF,” and “2401-CF,” respectively. “Chris cross,” “DAH,” and “2401” infected by pCF93-ccpds are denoted as “CC-pds,” “DAH-pds,” and “2401-pds,” respectively.
Testing candidate genes related to MS in watermelon with the CFMMV-VIGS system
We previously conducted reference-based and de novo transcriptome deep sequencing (RNA-seq) analysis to identify differentially expressed genes (DEGs) between the male-sterile and male-fertile lines of DAH3615 (Rhee et al., 2015, 2017). We detected 38 DEGs and amplified fragments for each gene, which we then cloned into pCF93 vectors for gene function analysis (Supplemental Table S1). For each experiment, we inoculated about 550 watermelon plants with vectors to silence each DEG and grew the plants together in the greenhouse (Supplemental Figure S2).
We observed typical MS phenotypes for eight of the DEGs, included CALCIUM- DEPENDENT PROTEIN KINASE 2 (CDPK2), ELONGATION FACTOR 1-ALPHA (EF1a), LATE EMBRYOGENESIS ABUNDANT PROTEIN 1 (LEA1), LIN-11, Isl-1 and MEC-3 domain protein (LIM), MITOTIC ARREST DEFICIENT 2 (MAD2), POLYGALACTURONASE (PG), FASCICLIN-LIKE ARABINOGALACTAN PROTEIN 5 (FLA5), and EXPANSIN-A9 (EXPA9) (Table 1). All DAH plants infected with the eight constructs produced complete or partially abnormal MS flowers in the same plant (Figure 4; Supplemental Figure S3).
Table 1.
Eight candidate genes from reference-based and de novo transcriptome data, selected for VIGS-mediated silencing in watermelon
| Gene symbol | Research | Gene_ID | Annotation |
|---|---|---|---|
| CDPK2 | Reference based | Cla010883 | Calcium-dependent protein kinase 2 |
| EF1a | Reference based | Cla000553 | EF1a |
| LEA1 | Reference based | Cla007634 | LEA1 |
| LIM | Reference based | Cla001608 | LIM |
| MAD2 | Reference based | Cla009991 | Mitotic spindle assembly checkpoint protein MAD2 |
| PG | Reference based | Cla016924 | PG |
| FLA5 | de novo | c9925_g1 | FLA5 |
| EXPA9 | de novo | c29764_g1 | EXPA9 |
Uniprot ID: CDPK2, Q3YAT0_PETIN; EF1a, B9SPV9_RICCO; LEA1, LEA1_CICAR; LIM, Q306K1_BRANA; MAD2, D2V3A0_NAEGR; PG, E3VSV7_CUCPE; FLA5, FLA5_ARATH; EXPA9, EXPA9_ARATH.
Figure 4.
Male-sterile flowers develop on watermelon plants silenced for candidate genes via VIGS. Complete male sterility was observed in some silenced plants, while other plants exhibited partial male sterility, with flowers having various degrees of male sterility. Mock, representative flowers from watermelon plants inoculated with pCF93-PDS-int; MS phenotype, watermelon plants silenced for EXPA9. Partially abnormal male-sterile flowers developed on LIM-, MAD2-, FAP5-, and PG-silenced plants (from left to right). Arrows indicate aborted stamen. Scale bars, 0.5 cm.
Wild-type watermelon male flower have three individual stamens. In complete male-sterile flowers, the stamens were 3 times smaller than those of wild-type flowers and were green and immature with no pollen. Partial male-sterile flowers were characterized by one or two abnormal stamens and also had no pollen grains, although the remaining one or two normal stamens from these flowers developed normally and produced pollens. The silencing of LEA1 resulted in plants with only complete male-sterile flowers. The silencing via VIGS of any one of the remaining seven candidate genes produced both complete and partially abnormal male-sterile flowers. Notably, all virus-induced male-sterile flowers were phenotypically comparable and resembled those of the male-sterile line “DAH3615-MS”.
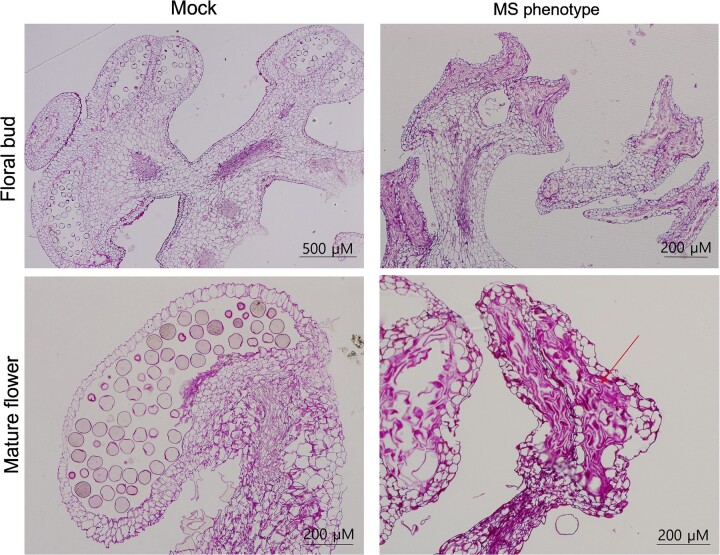
To investigate the anatomical differences between male-sterile and male-fertile stamens, we examined histological cross-sections of anthers. To this end, we collected male-fertile floral buds and flowers from plants mock inoculated with pCF93-PDS-int. We also collected flowers from plants silenced for EXPA9, which produced fully sterile male flowers. Male-fertile floral buds and flowers produced normal pollen sacs and pollen, whereas male-sterile flowers showed developmental disruption of tapetum cells and microspore mother cells. The mature male-sterile flowers also had atrophied pollen sacs that consisted of abnormal shrunken cells (Figure 5).
Figure 5.
Histological analysis of stamens from floral buds and mature flowers of mock-inoculated and silenced plants. The floral buds of plants inoculated with pCF93-PDS-int (mock) formed normal pollen sacs containing pollen grains. In contrast, floral buds of plants silenced for a candidate gene (EXPA9 here) had no pollen. Pollen sacs of mature mock flowers displayed a normal morphology, but those of silenced plants were atrophied (arrow). Scale bars, 200 and 500 µm.
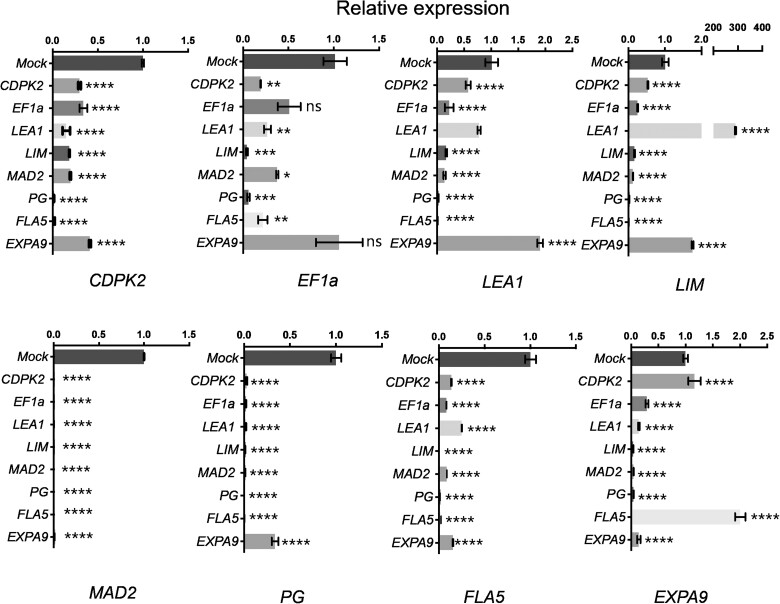
We also performed RT–qPCR to determine the expression levels of the eight DEGs and explore their interaction in watermelon plants silenced for each individual gene. Accordingly, we collected three mature flowers showing a complete male-sterile phenotype and pooled them for each replicate. Most of the candidate genes tended to be less expressed in VIGS-silenced male-sterile flowers and relative to mock-inoculated controls (Figure 6). Surprisingly, flowers silenced for LEA1 also exhibited a dramatic 290-fold induction of the LIM gene compared to that of mock-inoculated plants. Conversely, flowers silenced for LIM showed lower LEA1 expression levels. EXPA9 expression was induced when FLA5 was downregulated, while CDPK2, MAD2, PG, and FLA5 transcript levels were all decreased in all male-sterile flowers. In particular, all male-sterile flowers had extremely low levels of MAD2 transcripts.
Figure 6.
Relative expression levels of male sterility-related candidate genes in VIGS plants. Relative transcript levels were determined by RT–qPCR and normalized to 18S rRNA levels. The relative transcript levels of the eight candidate genes were measured in inoculated plants and mock controls. Relative expression levels in the mock control inoculated with pCF93-PDS-int were set to 1. Significant differences were determined using one-way ANOVA, followed by Tukey’s post-hoc test for multiple comparisons.
Discussion
Genetic transformation of cucurbits is difficult, thus hindering the progress of functional genomic research in these plant species compared to other plants. To address this issue, we previously isolated and constructed a CFMMV-Cm-based full-length infectious clone (Rhee et al., 2014) and a virus vector for heterologous expression (Rhee et al., 2016). In previous studies on tobacco mosaic virus (TMV), a gene insertion site closer to the 3′-NTR gave rise to an increase in expression of the foreign gene (Culver et al., 1993). When the MP gene, which is originally far from the 3′-NTR, was cloned in front of the 3′-NTR, expression levels increased up to 20 times relative to that from the wild-type virus (Culver et al., 1993). In addition, insertion of additional NTRs between the two ORFs resulted a similar increase in expression levels for both genes (Lindbo, 2007). Proximity to the 3′-NTR was thus suggested to be crucial for enhancing expression. TMV vectors have also been designed with a duplication of the TMV 3′-NTR between the foreign ORF and the CP gene (Shivprasad et al., 1999). Based on these previous findings, we developed two types of virus vectors herein: one with one copy of the 3′-NTR and one with tandemly duplicated 3′-NTRs, named CFMMV and CFMMV-K, respectively.
Of our previously developed vectors, pCF93 and pCF157 resulted in the highest expression levels for EGFP. We therefore modified these two vectors to harbor one or two copies of the 3′-NTR to evaluate VIGS efficiency in the current work. Nicotiana benthamiana inoculated with pCF93-NbPDS, pCF93K-NbPDS, or pCF157-NbPDS developed photobleaching phenotypes to various degree, whereas the pCF157K-NbPDS vector failed to induce photobleaching in the inoculated plants (Figure 1). In addition, we determined that the inserted fragments in pCF93K-NbPDS and pCF157K-NbPDS are easily lost due to homologous recombination. pCF93 vector caused a five-fold drop in NbPDS transcript levels in the leaves of inoculated N. benthamiana plants relative to plants inoculated with the pCF157-NbPDS (Figure 2A). Taken together, pCF93-NbPDS was the best VIGS vector for N. benthamiana.
When tested on cucurbits, pCF93-PDS also effectively silenced the respective PDS gene in melon, cucumber, and watermelon, resulting in photobleaching in leaves and fruit peels, as well as lower lycopene and β-carotene contents in fruit flesh, which changed to white in watermelons.
VIGS is sensitive to the genotypes of the plant species being inoculated (Bekele et al., 2019), prompting us to test three watermelon cultivars. Importantly, all cultivars were silenced for CcPDS, with fruits developing white peels due to chlorophyll degradation and lower lycopene content. Among the evaluated cultivars, DAH showed the most pronounced silencing effects and was selected for subsequent experiments. All three cultivars are small and can be grown in 16-cm pots with stable fruit development and ripening, thus, requiring a relatively small cultivation area relative to common cultivated watermelon. Taken together, the VIGS approach described herein allows for convenient and relatively effortless watermelon research.
We wished to functionally explore the 38 DEGs identified between a male-sterile and a male-fertile watermelon line. To this end, we cloned fragments specific to each gene individually into the pCF93 vector before inoculating DAH male-fertile seedlings via Agrobacterium-mediated infiltration. Eight of the 38 candidate genes appeared to induce MS when silenced, yielding male-sterile flowers of varying severity within a single plant. To identify crucial genes implicated in MS, we evaluated the expression of these eight genes in male-sterile flower. Most of the candidate genes were downregulated in male-sterile flowers, suggesting a complex regulatory network underlying flower development and gene expression.
EF1a is essential for translation, particularly elongation; the encoding gene is ubiquitously expressed, including reproductive organs (Cosgrove et al., 1997; Wang et al., 2004). Thus, the downregulation of EF1a might affect MS-related gene expression. LIM, LEA, and Expansin play important roles in pollen formation and pollen tube elongation (Ye and Xu, 2012). Thus, we suspected that the four genes listed above might affect male fertility but might not be directly involved in the underlying mechanism. Of note, CDPK2, FLA5, PG, and MAD2 were downregulated in all male-sterile flowers regardless of the gene being silenced. Several reports have suggested that CDPKs contributed to the regulation of pollen tube growth (Myers et al., 2009; Li et al., 2018). Thus, we suspect that the downregulation of CDPK2 might cause the lack of pollen grain. PG is a representative hydrolytic enzyme involved in various functions, such as plant cell wall deposition, fruit ripening, abscission, pollen intine, and exine formation, as well as pollen tube growth via pectin rearrangement (Kulikauskas and McCormick, 1997; Huang et al., 2008; Jiang et al., 2014; Lyu et al., 2015). As male-sterile flowers in inoculated plants did not produce pollen grain and exhibited an immature stamen, the loss of PG activity may have compromised stamen formation and pollen production. FLAs are a subclass of arabinogalactan proteins, which hold pleiotropic functions in plant reproduction and pollen development (Pennell and Roberts, 1990; Coimbra et al., 2007). Knockdown lines of Arabidopsis (Arabidopsis thaliana) FLA3 produced abnormal pollen, while FLA3 overexpression resulted in defective stamen filaments (Li et al., 2010). FLA3 may therefore participate in the development of microspores and reproductive organs.
We noticed an unusual increase in transcript levels in some VIGS lines. In flowers silenced for LEA, LIM transcript levels rose 290-fold compared to mock-inoculated flowers. LEA and LIM have an important role in pollen formation and pollen tube elongation (Wang et al., 2008; Ye and Xu, 2012). Apart from their role in development, LIM domain proteins are key regulators of plant stress responses and candidates for stress management (Srivastava and Verma, 2017). LEA proteins also protect cells against various stresses. An RNAi construct targeting LEA5 in rice resulted in greater plant sensitivity to drought and salt stress (Huang et al., 2018). As plants silenced for LEA1 are particularly vulnerable to abiotic stress, the strong induction of LIM expression might mitigate sensitivity to stress. Expansin is also involved in biotic and abiotic stress responses, as indicated by the greater sensitivity of Expansin RNAi lines to salt and drought stress (Jadamba et al., 2020). The upregulation of LEA1, LIM, and EF1a in EXPA9-silenced plants might reflect an attempt by the plants to mount a response to stress.
FLA12 regulates cellulose biosynthesis or deposition (Ma et al., 2022). A double mutant in Arabidopsis lacking FLA11 and FLA12 function exhibited reduced stem tensile strength and tensile modulus elasticity, as well as altered cell wall architecture and composition (MacMillan et al., 2010). Expansin is a representative structural protein that was reported to loosen plant cell walls in a pH-dependent manner (Marowa et al., 2016). We thus hypothesize that the silencing of FLA may stimulate EXPA9 expression to loosen cell walls.
Remarkably, we failed to detect any MAD2 transcripts in all virus-induced male-sterile flowers, even when MAD2 was not the primary target (Figure 6). MAD2 is essential for chromosome segregation during mitosis, as it controls the metaphase-to-anaphase transition (Shah and Cleveland, 2000). The spindle assembly checkpoint is a crucial regulator for genome stability in humans, animals, and yeast (Saccharomyces cerevisiae) (Lara-Gonzalez et al., 2012). While the functional importance of plant MAD2 is still unclear, equal chromosome segregation is clearly essential for normal development. Arabidopsis and maize MAD2 homologs localize to the kinetochore, and maize MAD2 was reported to be sensitive to microtubule attachment during meiosis and mitosis (Yu et al., 1999). While a loss-of-function mutant in Arabidopsis MAD2 did not affect reproductive organ development, it did compromise root growth. The loss of Budding Uninhibited by Benzimidazole function, one of the components of the spindle assembly checkpoint along with MAD2, induced MS in rice (Burgos-Rivera and Dawe, 2012). Taken together, the suppression of mitosis-related genes may give rise to MS in plants.
In this study, we established a high-throughput screening system for the identification of putative MS-related genes deduced from massive RNA-seq data. This approach will facilitate functional gene studies in watermelon, which normally requires large cultivation areas (Supplemental Figure S1). The use of watermelon cultivars with small fruits allowed their planting in fairly small (16 cm in diameter) pots, an essential prerequisite for reducing the needed growing area, which may also lower the risk of soil contamination.
The current work adds to the body of evidence regarding the major advantage of the VIGS system in some plant species. The pCF93 vector tested here established silencing in the various organs throughout the lifetime of the plant. We propose that pCF93 is a very useful tool for high-throughput screening and is particularly advantageous in fruit development. The CFMMV-VIGS system establishes a theoretical and practical basis for future studies focused on functional genomics analysis in cucurbits.
Materials and methods
Plant materials
VIGS was conducted on N. benthamiana, cucumber (C. sativus L.) “Parisian pickling,” melon (C. melo L.) “Early hanover,” and watermelon (C. lanatus L.) “Chris cross,” “2401,” and “DAH” plants. Seeds for the watermelon cultivars were obtained from the Gene Bank of the National Agrobiodiversity Center, Rural Development Administration (RDA), Jeonju, South Korea. The plants were grown under a constant temperature of 28°C with a 16-h-light/8-h-dark photoperiod. Cucumber, melon, and watermelon plants were transferred to greenhouse 2–3 weeks after VIGS inoculation. Each shelf in the greenhouse is 150 cm × 270 cm and could hold 28 pots. For one VIGS experimental set, each candidate gene was tested with 14 replicate plants. Twenty shelves were used for each set of MS VIGS experiments within the same time frame. Individual plants were planted in 16-cm pots on the shelves, with a distance of 15 cm between neighbor pots (Supplemental Figure S1).
RNA extraction and cDNA synthesis
Total RNA was extracted using TRIZOL reagent (GIBCOBRL-Life technologies, MD, USA) and subsequently treated with 1 µL DNase (10 U µL−1) at 37°C for 30 min. Total RNA (2 µg) was reverse transcribed to first-strand cDNA with Superscript III Reverse transcriptase (Invitrogen, CA, USA), in a reaction consisting of 2 µg total RNA, 0.5 mM of dNTP, and 100 ng of oligo d(T) or random hexamer. The reaction mixture was incubated at 65°C for 5 min and then placed on ice for 1 min, followed by the addition of 5 × first-strand buffer, 5-mM DTT, 1-µL RNase inhibitor, 1-µL Superscript III Reverse transcriptase, and incubation at 50°C for 50 min and at 70°C for 15 min to terminate the reverse transcription.
Cloning of target insert genes for VIGS
GenBank was searched for PDS genes. Insert fragments were amplified from N. benthamiana (NbPDS) and C. melo L. (CmPDS) PDS transcripts via RT–qPCR (Accession No. DQ469932 and KC507802, respectively). NbPDS was amplified with the primer pair Nbpds-F and Nbpds-R. Cucumber (CsPDS) and melon (CmPDS) PDS fragments were amplified with the primer pair cucurbit pds-F and cucurbit pds-R; watermelon PDS was amplified from Chris cross cultivar (CcPDS) with the primer pair ccpds-F and ccpds-R (Supplemental Table S2). The resulting PDS fragments were cloned into the vectors pCF93, pCF157, pCF93K, and pCF157K (Figure 1) (Rhee et al., 2016). A list of candidate MS-related genes was obtained from a comparison of reference-based and de novo RNA-seq data between male-sterile and male-fertile watermelon lines previously reported by our group (Rhee et al., 2015, 2017). pCF93-PDS-int, constructed by inserting a partial fragment of a PDS intron into the MCS of pCF93, was used as mock control. To amplify the intron of watermelon PDS, genomic DNA was extracted from the watermelon cultivar Chris cross with a DNeasy plant mini kit (Qiagen, CA, USA) following the manufacturer’s instructions. The target genes and primer sequences are presented in Supplemental Tables S1 and S2, respectively. The primer sets were designed based on the watermelon 97,103 genome v1 accessed at CuGenDB (http://cucurbitgenomics.org/).
Total RNA was reverse-transcribed to first-strand cDNA with Superscript III Reverse transcriptase (Invitrogen, Carlsbad, CA, USA) and oligo d(T) primers. The cDNA was then amplified using Phusion DNA polymerase (Thermo, Waltham, MA, USA). The PCR conditions were as follows: 98°C for 30 s, 35 cycles of 98°C for 10 s, 60°C for 30 s, 72°C for 30 s, and 72°C for 2 min. Each XhoI–PmeI-digested PCR fragment was ligated into the pCF93 vector at the same sites within the MCS.
Agrobacterium-mediated inoculations
All constructed vectors were introduced into Agrobacterium (A. tumefaciens) strain GV3101. A single resistant colony was picked up and grown overnight in 5 mL of LB media at 28°C. A 100-µL inoculum from the overnight culture was transferred to 30 mL of fresh LB medium containing 0.01-M MES, pH 5.6, and 20 µM of acetosyringone. The cells were grown to an optical density (OD)600 of 1.0 and then harvested via centrifugation. The harvested cells were resuspended in 4.4g L-1 Murashige and Skoog salt, 0.01 M MES, 20g L-1 sucrose, 200 µM acetosyringone (MMA) medium (4.4 g L−1 Murashige and Skoog salt, 0.01-M MES (pH 5.6), 20-g L−1 sucrose) to a final OD600 of 0.9, before adding 200-µM acetosyringone. The resuspended cells were incubated at room temperature for 4 h with gentle agitation. The cells were then inoculated onto three true leaves per plant for N. benthamiana and two cotyledons of cucurbits. The Agrobacterium suspension was injected into the abaxial side of leaves with a 1-mL needleless syringe.
RT–qPCR
Leaves were collected and pooled from individual N. benthamiana and cucurbit plants. Total RNA was isolated from three individual plants in one replicate to determine PDS silencing efficiency via RT–qPCR. Relative expression levels were calculated by setting the expression levels measured in mock-inoculated plants with the vector pCF93-PDS-int to 1. Significant differences were determined using one-way ANOVA, followed by Tukey’s post-hoc test for multiple comparisons.
To determine MS-related gene expression levels in flowers, three individual flowers were used for total RNA isolation and cDNA synthesis. One microliter of cDNA was subjected to qPCR. The 18S rDNA and GLYCERALDEHYDE 3-PHOSPHATE DEHYDROGENASE (GAPDH) gene were used as internal controls for N. benthamiana and cucurbits, respectively. The coding sequences were obtained from the watermelon genome at the Cucurbit Genomics Database (http://cucurbitgenomics.org/), and primers were designed for candidate genes accordingly (Supplemental Table S2). qPCR was conducted using an Eco Real-Time PCR (Illumina, San Diego, CA, USA). Each reaction included 10 µL 2 × Realtime PCR mix (Biofact, Daejeon, Korea), 1-µL Evagreen dye, 1 µL of each 10-µM primers, 1-µL first-strand cDNA, and ddH2O up to 20 µL. PCR conditions were as follows: 95°C for 12 min, 40 cycles of 95°C for 10 s, 60°C for 15 s, and 68°C for 15 s. Relative gene expression levels were determined with the ΔΔCT method. Significant differences were determined by one-way ANOVA. Dunnett’s post-hoc test was applied using PRISM version 6 software.
Quantification of chlorophyll contents
Samples consisting of 0.5 g of watermelon peels (approximately 10 discs, 10 mm in diameter) were collected and placed in 30 mL of absolute acetone overnight in the dark. Chlorophyll a and b contents were measured using a spectrophotometer and 1-cm quartz cuvettes with absorbance values at 651 and 664 nm, respectively. The formulas to calculate chlorophyll contents were as follows (Mackinney, 1941): chlorophyll a = (16.5 × OD664 − 8.3 × OD651) × A; chlorophyll b = (33.5 × OD651 – 12.5 × OD664) × A; total chlorophyll = (25.5 × OD651 − 4 × OD664) × A (A = Methanol volume (L) × 100 πr2 (cm) × number of leaf disc). A was calculated as 0.385 (A = (30/1000) × 100 × 3.14 × 0.52 × 10).
Quantification of lycopene and β-carotene contents by using HPLC
For sample preparation, 10 g of fresh watermelon flesh was freeze-dried for 1 week. The freeze-dried samples (∼0.1 g) were ground and mixed with silica beads (3–5 µm) (1:1, w/w). One milliliter of ethanol containing 0.5-mM butylated hydroxytoluene was added to the samples and struck for 30 s. The bead-containing samples were then transferred to 15-mL tubes, to each of which, 3 mL of petroleum ether and 8 mL of 20% (w/v) NaCl were gradually added with vortexing. The samples were centrifuged at 1,700g for 10 min, and the supernatant was collected. Finally, Na2SO4 was added to each supernatant, which was then filtrated on a polytetrafluoroethylene (PTFE) membrane filter (13 mm, 0.2 µm; Advantec, Irvine, CA, USA). The pretreated samples were analyzed by HPLC by using a Waters chromatography system equipped with a reverse-phase column (Kinetex 2.6 µm, C18 100a, 100 × 460 mm; Phenomenex, USA).
Histological analysis and microscopy
To investigate differences in anther development between the male-sterile and male-fertile lines, floral buds and mature flowers were collected from both lines. The samples were soaked in 2.5% (w/v) glutaraldehyde for 90 min and washed in 0.1-M phosphate buffer, pH 7.2. The samples were then soaked in 1% (w/v) osmic acid for 90 min and washed in 0.1-M phosphate buffer, pH 7.2, at 4°C. Subsequently, the fixed samples were dehydrated using a graded ethanol series (40% [v/v], 60%, 80%, 90%, 95%, and 100%) and then embedded in Epon-812 resin. Ultrathin cross sections of 1.5 µm in thickness were prepared using an ultramicrotome (PT-X, RMC, USA) and stained with Periodic acid–Schiff stain.
Accession numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers: N. benthamiana GLYCERALDEHYDE 3-PHOSPHATE DEHYDROGENASE (GAPDH, Accession No. AB937979) and PHYTOENE DESATURASE (Nbpds, Accession No. DQ469932) and C. melo PHYTOENE DESATURASE (Cmpds, Accession No. KC507802), and 18S rDNA (Accession No. AY030241).
Supplementary Material
Acknowledgments
We would like to show our gratitude to the gene bank of the National Agrobiodiversity Center (NAC) of Rural Development Administration (Gimje, Korea) for providing seeds.
Funding
This work was carried out with the support of the “Cooperative Research Program for Agriculture Science & Technology Development (Project No. PJ01421302)’’ Rural Development Administration of the Republic of Korea. This research was supported by the Chung-Ang University Graduate Research Scholarship in 2020.
Conflict of interest statement. The authors declare no competing interests.
Contributor Information
Sun-Ju Rhee, Department of Plant Science and Technology, Chung-Ang University, Anseong, 17546, Republic of Korea.
Yoon Jeong Jang, Department of Plant Science and Technology, Chung-Ang University, Anseong, 17546, Republic of Korea.
Jun-Young Park, Department of Plant Science and Technology, Chung-Ang University, Anseong, 17546, Republic of Korea.
Jisu Ryu, Department of Plant Science and Technology, Chung-Ang University, Anseong, 17546, Republic of Korea.
Gung Pyo Lee, Department of Plant Science and Technology, Chung-Ang University, Anseong, 17546, Republic of Korea.
Data availability statement
All relevant data can be found within this manuscript and its supporting information files.
Supplemental data
The following materials are available in the online version of this article.
Supplemental Figure S1. RT–PCR and sequence analysis of progeny virus from pCF157K-NbPDS to confirm homologous recombination in N. benthamiana.
Supplemental Figure S2. Plot layout in the greenhouse.
Supplemental Figure S3. Male-sterile flowers in watermelon plants silenced for individual candidate genes.
Supplemental Table S1. List of 38 DEGs functionally validated via VIGS.
Supplemental Table S2. Primers used for cloning in this study.
Supplemental Table S3. Primers for RT–qPCR.
G.L. and S.R. designed the study. S.R. performed the experiments, analyzed the data, and drafted the manuscript. Y.J. performed the cloning and histological analysis. J.P. and J.R. performed the agroinoculation, phenotyping, and revising manuscript. G.L. supervised the data analysis, revised the manuscript, and funded the project. All authors reviewed and approved the submitted version of the manuscript.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (https://academic.oup.com/plphys/pages/general-instructions) is Gung Pyo Lee (gplee@cau.ac.kr).
References
- Antignus Y, Wang Y, Pearlsman M, Lachman O, Lavi N, Gal-On A (2001) Biological and molecular characterization of a new cucurbit-infecting tobamovirus. Phytopathology 91: 565–571 [DOI] [PubMed] [Google Scholar]
- Bang H, King SR, Liu W (2005) A new male sterile mutant identified in watermelon with multiple unique morphological features. Cucurbit Genet Coop Rep 28: 46–48 [Google Scholar]
- Bekele D, Tesfaye K, Fikre A (2019) Applications of virus induced gene silencing (VIGS) in plant functional genomics studies. J Plant Biochem Physiol 7: 1–7 [Google Scholar]
- Benavente LM, Ding XS, Redinbaugh MG, Nelson R, Balint-Kurti P (2012) Virus-induced gene silencing in diverse maize lines using the Brome mosaic virus-based silencing vector. Maydica 57: 206–214 [Google Scholar]
- Bu R, Wang R, Wei Q, Hu H, Sun H, Song P, Yu Y, Liu Q, Zheng Z, Li T (2019) Silencing of glycerol-3-phosphate acyltransferase 6 (GPAT6) gene using a newly established virus induced gene silencing (VIGS) system in cucumber alleviates autotoxicity mimicked by cinnamic acid (CA). Plant Soil 438: 329–346 [Google Scholar]
- Burgos-Rivera B, Dawe RK (2012) An Arabidopsis tissue-specific RNAi method for studying genes essential to mitosis. PLoS One 7: e51388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Liu YG (2014) Male sterility and fertility restoration in crops. Annu Rev Plant Biol 65: 579–606 [DOI] [PubMed] [Google Scholar]
- Coimbra S, Almeida J, Junqueira V, Costa ML, Pereira LG (2007) Arabinogalactan proteins as molecular markers in Arabidopsis thaliana sexual reproduction. J Exp Bot 58: 4027–4035 [DOI] [PubMed] [Google Scholar]
- Cosgrove DJ, Bedinger P, Durachko DM (1997) Group I allergens of grass pollen as cell wall-loosening agents. Proc Natl Acad Sci USA 94: 6559–6564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culver JN, Lehto K, Close SM, Hilf ME, Dawson WO (1993) Genomic position affects the expression of tobacco mosaic virus movement and coat protein genes. Proc Natl Acad Sci USA 90: 2055–2059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyutin KSS (1990) Spontaneous mutant of watermelon with male sterility. Tsitol Genetika 24: 56–57 [Google Scholar]
- Fister AS, Landherr L, Maximova SN, Guiltinan MJ (2018) Transient expression of CRISPR/Cas9 machinery targeting TcNPR3 enhances defense response in Theobroma cacao. Front Plant Sci 9: 268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu DQ, Zhu BZ, Zhu HL, Jiang WB, Luo YB (2005) Virus-induced gene silencing in tomato fruit. Plant J 43: 299–308 [DOI] [PubMed] [Google Scholar]
- Gupta RM, Musunuru K (2014) Expanding the genetic editing tool kit: ZFNs, TALENs, and CRISPR-Cas9. J Clin Invest 124: 4154–4161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B, Li D, Liu X, Qi J, Gao D, Zhao S, Huang S, Sun J, Yang L (2017) Engineering non-transgenic gynoecious cucumber using an improved transformation protocol and optimized CRISPR/Cas9 system. Mol Plant 10: 1575–1578 [DOI] [PubMed] [Google Scholar]
- Huang Hexun ZX, Zhencheng Wei, Qinghuai Li, Xi Li (1998) Inheritance of male-sterility and dwarfism in watermelon [Citrullus lanatus (Thunb.) Matsum. and Nakai]. Sci Hortic 74: 175–181 [Google Scholar]
- Huang L, Cao J, Ye W, Liu T, Jiang L, Ye Y (2008) Transcriptional differences between the male-sterile mutant bcms and wild-type Brassica campestris ssp. chinensis reveal genes related to pollen development. Plant Biol (Stuttg) 10: 342–355 [DOI] [PubMed] [Google Scholar]
- Huang L, Zhang M, Jia J, Zhao X, Huang X, Ji E, Ni L, Jiang M (2018) An atypical late embryogenesis abundant protein OsLEA5 plays a positive role in ABA-induced antioxidant defense in Oryza sativa L. Plant Cell Physiol 59: 916–929 [DOI] [PubMed] [Google Scholar]
- Igarashi A, Yamagata K, Sugai T, Takahashi Y, Sugawara E, Tamura A, Yaegashi H, Yamagishi N, Takahashi T, Isogai M, et al. (2009) Apple latent spherical virus vectors for reliable and effective virus-induced gene silencing among a broad range of plants including tobacco, tomato, Arabidopsis thaliana, cucurbits, and legumes. Virology 386: 407–416 [DOI] [PubMed] [Google Scholar]
- Jadamba C, Kang K, Paek NC, Lee SI, Yoo SC (2020) Overexpression of rice expansin7 (Osexpa7) confers enhanced tolerance to salt stress in rice. Int J Mol Sci 21: 454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang YJ, Sim TY, Ryu J, Rhee SJ, Kim Y, Lee GP (2021) Identification of a candidate locus and development of a molecular marker for male sterility in watermelon. Hortic Sci Technol 39: 673–683 [Google Scholar]
- Jiang J, Yao L, Yu Y, Lv M, Miao Y, Cao J (2014) PECTATE LYASE-LIKE10 is associated with pollen wall development in Brassica campestris. J Integr Plant Biol 56: 1095–1105 [DOI] [PubMed] [Google Scholar]
- Kant R, Sharma S, Dasgupta I (2015) Virus-induced gene silencing (VIGS) for functional genomics in rice using rice tungro bacilliform virus (RTBV) as a vector. Plant Gene Silencing. Springer, Berlin, Germany, pp 201–217 [DOI] [PubMed] [Google Scholar]
- Kim YJ, Zhang D (2018) Molecular control of male fertility for crop hybrid breeding. Trends Plant Sci 23: 53–65 [DOI] [PubMed] [Google Scholar]
- Kulikauskas R, McCormick S (1997) Identification of the tobacco and Arabidopsis homologues of the pollen-expressed LAT59 gene of tomato. Plant Mol Biol 34: 809–814 [DOI] [PubMed] [Google Scholar]
- Lara-Gonzalez P, Westhorpe FG, Taylor SS (2012) The spindle assembly checkpoint. Curr Biol 22: R966–980 [DOI] [PubMed] [Google Scholar]
- Li C, Yamagishi N, Kasajima I, Yoshikawa N (2019) Virus-induced gene silencing and virus-induced flowering in strawberry (Fragaria x ananassa) using apple latent spherical virus vectors. Hortic Res 6: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Zhang D, Xie K, Wang Y, Liao Q, Hong Y, Liu Y (2021) Efficient and high-throughput pseudorecombinant-chimeric Cucumber mosaic virus-based VIGS in maize. Plant Physiol 187: 2865–2876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Li Y, Deng Y, Chen P, Feng F, Chen W, Zhou X, Wang Y (2018) A calcium-dependent protein kinase, ZmCPK32, specifically expressed in maize pollen to regulate pollen tube growth. PLoS One 13: e0195787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Yu M, Geng LL, Zhao J (2010) The fasciclin-like arabinogalactan protein gene, FLA3, is involved in microspore development of Arabidopsis. Plant J 64: 482–497 [DOI] [PubMed] [Google Scholar]
- Liao JJ, Wang CH, Xing QJ, Li YP, Liu XF, Qi HY (2019) Overexpression and VIGS system for functional gene validation in oriental melon (Cucumis melo var. makuwa Makino). Plant Cell Tissue Organ Cult 137: 275–284 [Google Scholar]
- Lin MK, Belanger H, Lee YJ, Varkonyi-Gasic E, Taoka K, Miura E, Xoconostle-Cazares B, Gendler K, Jorgensen RA, Phinney B, et al (2007) FLOWERING LOCUS T protein may act as the long-distance florigenic signal in the cucurbits. Plant Cell 19: 1488–1506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindbo JA (2007) TRBO: a high-efficiency tobacco mosaic virus RNA-based overexpression vector. Plant Physiol 145: 1232–1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N, Xie K, Jia Q, Zhao J, Chen T, Li H, Wei X, Diao X, Hong Y, Liu Y (2016) Foxtail mosaic virus-induced gene silencing in monocot plants. Plant Physiol 171: 1801–1807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Schiff M, Dinesh-Kumar SP (2002) Virus-induced gene silencing in tomato. Plant J 31: 777–786 [DOI] [PubMed] [Google Scholar]
- Lyu M, Liang Y, Yu Y, Ma Z, Song L, Yue X, Cao J (2015) Identification and expression analysis of BoMF25, a novel polygalacturonase gene involved in pollen development of Brassica oleracea. Plant Reprod 28: 121–132 [DOI] [PubMed] [Google Scholar]
- Ma Y, MacMillan CP, de Vries L, Mansfield SD, Hao P, Ratcliffe J, Bacic A, Johnson KL (2022) FLA11 and FLA12 glycoproteins fine-tune stem secondary wall properties in response to mechanical stresses. New Phytol 233: 1750–1767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackinney G (1941) Absorption of light by chlorophyll solutions. J Biol Chem 140: 315–322 [Google Scholar]
- MacMillan CP, Mansfield SD, Stachurski ZH, Evans R, Southerton SG (2010) Fasciclin-like arabinogalactan proteins: specialization for stem biomechanics and cell wall architecture in Arabidopsis and Eucalyptus. Plant J 62: 689–703 [DOI] [PubMed] [Google Scholar]
- Manzano S, Martinez C, Garcia JM, Megias Z, Jamilena M (2014) Involvement of ethylene in sex expression and female flower development in watermelon (Citrullus lanatus). Plant Physiol Biochem 85: 96–104 [DOI] [PubMed] [Google Scholar]
- Marowa P, Ding A, Kong Y (2016) Expansins: roles in plant growth and potential applications in crop improvement. Plant Cell Rep 35: 949–965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei Y, Zhang C, Kernodle BM, Hill JH, Whitham SA (2016) A foxtail mosaic virus vector for virus-induced gene silencing in maize. Plant Physiol 171: 760–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers C, Romanowsky SM, Barron YD, Garg S, Azuse CL, Curran A, Davis RM, Hatton J, Harmon AC, Harper JF (2009) Calcium-dependent protein kinases regulate polarized tip growth in pollen tubes. Plant J 59: 528–539 [DOI] [PubMed] [Google Scholar]
- Pennell RI, Roberts K (1990) Sexual development in the pea is presaged by altered expression of arabinogalactan protein. Nature 344: 547–549 [Google Scholar]
- Pickar-Oliver A, Gersbach CA (2019) The next generation of CRISPR-Cas technologies and applications. Nat Rev Mol Cell Biol 20: 490–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purkayastha A, Mathur S, Verma V, Sharma S, Dasgupta I (2010) Virus-induced gene silencing in rice using a vector derived from a DNA virus. Planta 232: 1531–1540 [DOI] [PubMed] [Google Scholar]
- Ray DT, Sherman JD (1988) Desynaptic chromosome behavior of the GMS mutant in watermelon. J Heredity 79: 397–399 [Google Scholar]
- Rhee SJ, Jang YJ, Ko YJ, Lee GP (2018) Identification of the pleiotropic function of TOUSLED kinase in tomato (Solanum lycopersicum L.) using a Cucumber mosaic virus-based vector. Hortic Environ Biotechnol 59: 105–114 [Google Scholar]
- Rhee SJ, Hong JS, Lee GP (2014) Infectivity and complete nucleotide sequence of cucumber fruit mottle mosaic virus isolate Cm cDNA. Arch Virol 159: 1807–1811 [DOI] [PubMed] [Google Scholar]
- Rhee SJ, Jang YJ, Lee GP (2016) Identification of the subgenomic promoter of the coat protein gene of cucumber fruit mottle mosaic virus and development of a heterologous expression vector. Arch Virol 161: 1527–1538 [DOI] [PubMed] [Google Scholar]
- Rhee SJ, Kwon T, Seo M, Jang YJ, Sim TY, Cho S, Han SW, Lee GP (2017) De novo-based transcriptome profiling of male-sterile and fertile watermelon lines. PLoS One 12: e0187147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee SJ, Seo M, Jang YJ, Cho S, Lee GP (2015) Transcriptome profiling of differentially expressed genes in floral buds and flowers of male sterile and fertile lines in watermelon. BMC Genomics 16: 914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson D (2004) VIGS vectors for gene silencing: many targets, many tools. Annu Rev Plant Biol 55: 495–519 [DOI] [PubMed] [Google Scholar]
- Rudich J, Zamski E (1985) Citrullus lanatus. Handbook of Flowering, Vol. 2. CRC Press, Boca Raton, FL, pp 272–274 [Google Scholar]
- Senthil-Kumar M, Mysore KS (2011) Virus‐induced gene silencing can persist for more than 2 years and also be transmitted to progeny seedlings in Nicotiana benthamiana and tomato. Plant Biotechnol J 9: 797–806 [DOI] [PubMed] [Google Scholar]
- Shah JV, Cleveland DW (2000) Waiting for anaphase: Mad2 and the spindle assembly checkpoint. Cell 103: 997–1000 [DOI] [PubMed] [Google Scholar]
- Shan S, Soltis PS, Soltis DE, Yang B (2020) Considerations in adapting CRISPR/Cas9 in nongenetic model plant systems. Appl Plant Sci 8: e11314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivprasad S, Pogue GP, Lewandowski DJ, Hidalgo J, Donson J, Grill LK, Dawson WO (1999) Heterologous sequences greatly affect foreign gene expression in tobacco mosaic virus-based vectors. Virology 255: 312–323 [DOI] [PubMed] [Google Scholar]
- Srivastava V, Verma PK (2017) The plant LIM proteins: unlocking the hidden attractions. Planta 246: 365–375 [DOI] [PubMed] [Google Scholar]
- Switzenberg JA, Beaudry RM, Grumet R (2015) Effect of CRC:etr1-1 transgene expression on ethylene production, sex expression, fruit set and fruit ripening in transgenic melon (Cucumis melo L.). Transgenic Res 24: 497–507 [DOI] [PubMed] [Google Scholar]
- Tian S, Jiang L, Cui X, Zhang J, Guo S, Li M, Zhang H, Ren Y, Gong G, Zong M, Liu F, Chen Q, Xu Y (2018) Engineering herbicide-resistant watermelon variety through CRISPR/Cas9-mediated base-editing. Plant Cell Rep 37: 1353–1356 [DOI] [PubMed] [Google Scholar]
- Tian S, Jiang L, Gao Q, Zhang J, Zong M, Zhang H, Ren Y, Guo S, Gong G, Liu F, et al (2017) Efficient CRISPR/Cas9-based gene knockout in watermelon. Plant Cell Rep 36: 399–406 [DOI] [PubMed] [Google Scholar]
- Tomes M, Johnson K, Hess M (1963) The carotene pigment content of certain red fleshed watermelons. Proc Am Soc Hortic Sci 82: 460–464 [Google Scholar]
- Tzean Y, Lee MC, Jan HH, Chiu YS, Tu TC, Hou BH, Chen HM, Chou CN, Yeh HH (2019) Cucumber mosaic virus-induced gene silencing in banana. Sci Rep 9: 11553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang DH, Li F, Duan QH, Han T, Xu ZH, Bai SN (2010) Ethylene perception is involved in female cucumber flower development. Plant J 61: 862–872 [DOI] [PubMed] [Google Scholar]
- Wang W, Scali M, Vignani R, Milanesi C, Petersen A, Sari-Gorla M, Cresti M (2004) Male-sterile mutation alters Zea m 1 (β-expansin 1) accumulation in a maize mutant. Sex Plant Reprod 17: 41–47 [Google Scholar]
- Wang Y, Yang X, Yadav V, Mo Y, Yang Y, Zhang R, Wang Z, Chang J, Li H, Zhang Y, et al (2020) Analysis of differentially expressed genes and pathways associated with male sterility lines in watermelon via bulked segregant RNA-seq. 3 Biotech 10: 222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zhang WZ, Song LF, Zou JJ, Su Z, Wu WH (2008) Transcriptome analyses show changes in gene expression to accompany pollen germination and tube growth in Arabidopsis. Plant Physiol 148: 1201–1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt V (1962) A marked male-sterile mutant in watermelon. Proc Am Soc Hort Sci 81: 498–505 [Google Scholar]
- Watt V (1967) Development of disease resistance and seed production in watermelon stocks carrying msg gene. Proc Am Soc Hort Sci 91: 579–583 [Google Scholar]
- Xie W, Marty DM, Xu J, Khatri N, Willie K, Moraes WB, Stewart LR (2021) Simultaneous gene expression and multi-gene silencing in Zea mays using maize dwarf mosaic virus. BMC Plant Biol 21: 208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K, Grzech D, Koudounas K, Stander EA, Caputi L, Mimura T, Courdavault V, O'Connor SE (2021) Improved virus-induced gene silencing allows discovery of a serpentine synthase gene in Catharanthus roseus. Plant Physiol 187: 846–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J, Xu M (2012) Actin bundler PLIM2s are involved in the regulation of pollen development and tube growth in Arabidopsis. J Plant Physiol 169: 516–522 [DOI] [PubMed] [Google Scholar]
- Yu HG, Muszynski MG, Kelly Dawe R (1999) The maize homologue of the cell cycle checkpoint protein MAD2 reveals kinetochore substructure and contrasting mitotic and meiotic localization patterns. J Cell Biol 145: 425–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Gao L, Liu W, Song L, Xiao D, Liu T, Hou X, Zhang C (2019) Transcription coactivator ANGUSTIFOLIA3 (AN3) regulates leafy head formation in Chinese cabbage. Front Plant Sci 10: 520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Wang M (1990) A Genetic Male-sterile (ms) Watermelon from China. Cucurbit Genet Coop Rep 13: 45–46 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data can be found within this manuscript and its supporting information files.