Abstract
Receptor for Advanced Glycated End‐products (RAGE) is highly expressed in diabetes and impairs wound healing. We proposed that administering an antibody that blocks RAGE will hasten the healing of dorsal wounds in diabetic pigs compared with a non‐immune IgG. Two purpose‐bred diabetic (D) Yucatan minipigs (Sinclair, Auxvasse MO) each underwent 12 2 × 2 cm full thickness dorsal wounds: four wounds received decellularized porcine skin patches (Xylyx Bio, Bklyn NY): four anti‐RAGE Ab (CR‐3) infused patches, four saline infused patches and four wounds were left open. One pig received anti‐RAGE Ab (CR‐3) 1 mg/kg IM q 10 days and other received non‐immune IgG. Wounds were measured at 2 and 4 weeks followed by euthanasia and wound harvesting. At 2 weeks few of the patches appeared to be incorporated into the wound. By 4 weeks all patches in pigs treated systemically with CR‐3 were detached and the wounds almost healed. For all 24 wounds for both pigs regardless of presence of patch or type of patch, the average IgG treated pig wound size at 4 weeks was 69.2 ± 14.6% of initial size and the average CR‐3 treated pig wound size was 40.9 ± 11.3% of initial size (P = 0.0002). Quantitative immunohistology showed greater staining for collagen in the CR‐3 treated wounds compared with IgG treated. Staining was positive for RAGE, Mac, and IL‐6 in the IgG treated wounds and negative in the CR‐3 treated wounds. From these pilot experiments, we conclude that a RAGE blocking antibody given parenterally improved wound healing in a diabetic pig while patches were not effective.
Keywords: diabetes, RAGE, wound healing
List of Abbreviations
- CR‐3
anti‐RAGE antibody
- D
diabetic
- DM
diabetes mellitus
- ECM
extracellular matrix
- IL
interleukin
- RAGE
receptor for advance glycated end‐product
1. INTRODUCTION
Diabetes mellitus (DM) is a chronic metabolic disorder that affects 170 million people worldwide, 29 million in the US. 1 , 2 A common major complication of diabetes is non‐healing leg and foot ulcers that arise from a combination of poor tissue perfusion, inhibition of reepithelialisation, and poor collagen formation. 3 , 4 Current standard of care for non‐healing wounds includes reperfusion therapy, nutritional supplementation, offloading, compression, and management of comorbidities. 5 These measures are largely supportive and fail to address the underlying molecular pathology.
Receptor for Advanced Glycation Endproducts (RAGE), a multiligand member of the immunoglobulin superfamily of cell surface molecules, plays a key role in the pathogenesis of impaired wound healing in diabetics. 6 , 7 , 8 , 9 Binding of RAGE to it ligands such as AGEs and S100 calgranulins are responsible for inducing pro‐coagulant initiator tissue factors that include cytokines, such as interleukin (IL)‐6, and cell adhesion molecules, such as VCAM‐1. 9 , 10 , 11 , 12 These cytokines affect the immune response causing prolonged wound exposure time and increasing susceptibility to bacterial infection. RAGE is responsible for failure of the normal angiogenic response to tissue hypoxia in diabetes and failure of angiogenesis is a major contributor to loss of tissue viability. 13 , 14
The blockade of RAGE promotes angiogenesis, improves blood flow to hypoxic sites of a wound, reduces the pro‐inflammatory response, and reduces pro‐apoptotic signalling to a chronic non‐healing wound. 6 , 10 , 11 , 12 In this pilot study, we aimed to investigate the effect of blocking RAGE on wound healing in diabetes via two approaches: (1) systemically administering our RAGE blocking antibody and (2) local delivery of the Ab via a bioengineered extracellular matrix (ECM) patch.
2. METHODS
2.1. Animals
All animal experiments were performed with the approval of the Institutional Animal Care and Use Committee of Columbia University. Purpose bred diabetic Yucatan mini‐pigs (castrated male 27–30 kg) were obtained from Sinclair Laboratories (Auxvasse MO). The Sinclair diabetic Yucatan minipigs have type I diabetes induced with Alloxan and blood sugar stabilised in the range of 300–500 mg/dL on 7–8 units NPH Humulin N (range 2–12). Upon arrival at CU, each pig was continued on the dose of insulin, determined by Sinclair, given once daily SQ with blood sugar monitored twice daily with a handheld glucometer (Accu‐check Aviva, Roche). Additional doses of regular insulin were given as needed. The veterinary staff weighed pigs weekly and observed them daily for signs of hyperglycemia or hypoglycemia.
2.2. Patches
We used tissueSpec™ Matrix patches (Xylyx, Brooklyn NY). These patches are derived from porcine skin cells and contain free natural matrix biomaterial collagenous skin matrix (type I, III) and other skin‐specific matrix components. The raw material is natural healthy skin tissue (porcine, 6 months, 44 kg) from which the hair, subcutaneous fat are removed. The patch producing process utilises serine proteases, polysorbate‐type non‐ionic surfactants for membrane solubilisation, bile acid salts for cell lysis, intracellular component dissolution, and hyper‐ and hypotonic solutes for osmotic stress.
2.3. Antibody
The anti‐RAGE Ab binds a unique peptide sequence on the V domain of the receptor which cross‐reacts to human, porcine, murine tissue. We immunised mice to the unique peptide and developed hybridomas producing murine monoclonal antibodies of IgG2a isotype with kappa light chain. Subsequently, we found the antibody had blocking properties in cell culture experiments by Western blot. We humanised the anti‐RAGE Ab using the chimeric Ab intermediary process. The Ab cDNA was cloned and expressed on mammalian cells and then large‐scale antibody produced using plasmid technology (AvantGen San Diego CA). The humanised antibody is called CR‐3. The average apparent affinity of CR‐3 for the receptor and ligands is in the range of 42 nanomolar by ELISA. Eight patches (four for each pig) were immersed in 0.6 mg of antibody solution for 24 h and four immersed in normal saline.
2.4. Wound placement and patch placement
Day 1, pigs were subjected to full thickness wounds along the dorsal column of the spine. Using a deep anaesthetic by intraperitoneal injection of ketamine and xylazine, the backs of the pigs were shaved and sterilised using a povidone‐iodine solution and alcohol. Twelve 2 × 2 cm full thickness wounds were created by removing the skin and panniculus carnosus layers. Four patches pre‐treated with CR‐3 and four ‐untreated TissueSpec™ Matrix patches were placed in wounds and sutured with 8 sutures at corners and middle of each side to remain in place. Four wounds were left to heal by secondary intention. Wounds were marked for identification: TissueSpec™ Matrix patch with antibody (A1‐A4), TissueSpec™ Matrix patch without antibody (B1‐B4), and patch alone (C1‐C4). Wounds were then covered with a 4 cm × 10 cm piece of Tegaderm™ dressing (3 M, St. Paul, MN, USA) and mesh jackets placed on each pig. Benadryl was given to reduce itching and prevent animals from scratching their sides on cage.
2.5. Wound monitoring
Dressings were changed and wounds monitored every week. Wounds were considered closed when moist granulated tissue was no longer visible and new epithelial growth present. At days 14 and 28 post‐wounding, calliper measurements of each wound were made and area expressed as width × length = mm2. Digital photos of the wounds were taken. The wound areas were expressed as a percentage of their original area (200 mm2), using the following formula: wound area on day X/wound area on day 0 × 100.
2.6. Histology
At the end of experiment, pigs were euthanized, wounds were excised, formalin fixed, embedded, and sectioned. Tissue sections were stained with H&E for morphology, Movat Pentachrome for the presence of collagen, and immunohistochemistry for RAGE, macrophages, TNF‐α, and IL‐6. Briefly, serial sections were deparaffinised in xylene, treated with 0.3% hydrogen peroxide for 20 min, and incubated in protein‐free block (Dako Inc., Carpinteria, CA, USA) for 10 min to inhibit the non‐specific binding of primary antibody. For immunohistochemical staining, tissue sections were incubated overnight with humanised anti‐RAGE antibody (50 μg/mL) followed by incubating for 30 min with biotinylated secondary antibody (1:200). To stain for macrophages (1:50; Santa Cruz Biotechnology), IL‐6 (1:500; Abcam), and TNF‐α (1:100; Abcam) tissue sections were treated for 30 min with VECTASTAIN ABC reagent (Vector Laboratories), followed by 3′,3′‐diaminobenzidine (DAB substrate kit for peroxidase; Vector Laboratories, Burlingame, CA, USA), and counterstaining with Gill's haematoxylin solution. Morphometric and immunohistochemical analyses of the wound sections were performed using a Nikon microscope (Tokyo, Japan) and Image‐Pro Plus software (Media Cybernetics Inc., Silver Spring, MD, USA).
2.7. Statistical analysis
Statistics for data of each group are expressed as mean ± standard deviation (SD) followed by the range. Comparisons of means between two groups for all variables were made using two sample t tests with a P value <0.05 considered statistically significant.
3. RESULTS
3.1. Antibody retention in patches
We performed an in‐vitro experiment to show uptake and retention of the anti‐RAGE antibody in the patch material. Humanised anti‐RAGE antibody labelled with fluorescent dye (100 μg in 100 μL) was incubated overnight at 4 °C in wells. We washed the Skin matrix patch twice with 200 μL phosphate‐buffered saline. The wash fluids (first, second) and skin matrix patch were imaged in multi‐well plate on the Biospace Lab Photon Imager at 4 and 24 h. There was >95% antibody retention at 24 h and 80% retention at 4 days.
3.2. Wound observations
For the IgG treated diabetic pig at 2 weeks post wounding, group A and B TissueSpec™ Matrix patches were still in place and several appeared to be incorporating into the wounds while one appeared swollen and by 4 weeks was detached. The wounds without patches appeared unchanged at 2 weeks and shrank in size by 4 weeks but were not completely healed (Figure 1).
FIGURE 1.

Digital photographs of the dorsum of each pig focused on the area of wounding and patch placement. Row (A) patch plus CR‐3, row (B) patch alone, (C) wound without patch. Left images from pig receiving q 10 days IM injections of 1 mg/kg IgG and right images from pig receiving same dosing of CR‐3. The top panels show baseline photos and bottom panels photos taken at 4 weeks
For the CR‐3 treated pig, at 2 weeks the group A patches had fallen off and the wounds underneath appeared to be healing. The B patches appeared adherent to the wound. The open wounds were smaller and appeared to be healing. At 4 weeks all patches were detached and the wound beds appeared to be almost completely healed (Figure 1).
3.3. Calliper measurements
Figure 2 shows the graph plots and the tables of data used to plot the graphs for the 3 groups of patches in the two pigs. For the IgG treated pig, TissueSpec™ Matrix patches with anti‐RAGE Ab (group A) had a surface area of 74.9 ± 10.8% initial wound 2 weeks post wounding, and 73.1 ± 17.5% at week 4 post wounding. The TissueSpec™ Matrix patches alone (without antibody) (group B) for the IgG treated pig had values of 88.1 ± 11.1% surface area 2 weeks post wounding, and 73.8 ± 17.9% at week 4. The wound alone sites had the smallest % of initial surface area at 2 weeks at 57.3 ± 7.2% initial wound size and 60.8 ± 3.8% at week 4.
FIGURE 2.

Top graphs shows plot of serial values for average % wound size from baseline, 2 and 4 weeks for IgG treated pig on left and CR‐3 treated pig on right. Legend identifies the three groups: patch + CR‐3 (blue line), patch alone (orange line), and no patch (grey line). The tables on the bottom show the numerical values (average ± SD) for the data points plotted in the graphs for 2 and 4 weeks for the IgG treated pig on the left and the CR‐3 treated pig on the right
For the RAGE Ab treated diabetic pig, wound sizes as % initial size in Group A were 57.4 ± 13.6% at week 2 and a 32.9 ± 5.1% at week 4 post wounding. For the TissueSpec™ Matrix patches alone, the wound size was a 67.3 ± 10.5% initial wound size at week 2, with a 36.6 ± 4.4 at week 4 post wounding. Group C's values were 69.2 ± 8.1% at week 2, and 53.3 ± 10.7% at week 4.
All wound sizes as % initial size at week 4 for each pig‐ IgG treated and CR‐3 treated pig‐ were compared using two sample t‐test. Average for the IgG treated diabetic pig was 69 2 ± 14.6% and for the CR‐3 pig 40.9 ± 11.3%. The CR‐3 values were significantly lower than the IgG values, P = 0.0002 (Figure 3).
FIGURE 3.

Bars represent average ± SD for all wounds on the IgG treated pig (n = 12) (light grey) and for the CR‐3 treated pig (n = 12) (dark grey)
3.4. Immunohistology
H & E staining of the wounds at day 28 showed new epithelial layer growth for all groups. Patches that were incorporated into the wound site in IgG treated pig showed no epithelial layer at junction but reepithelialisation where patch was no longer attached. Collagen deposition identified as yellow/orange stain on Movat's pentachrome stain was greater in the anti‐RAGE Ab treated group then in the IgG treated groups indicating more advanced healing (Figure 4). Areas of wounds from the IgG treated pig with low collagen showed staining for RAGE localised to arterioles and macrophages while CR‐3 treated pig wounds with high collagen showed no staining for RAGE (Figure 5). Staining for inflammatory markers are shown in Figure 6. ICH showed greater staining qualitatively for macrophages, TNF‐α, and IL‐6 in the IgG treated pig compared with the CR‐3 treated pig.
FIGURE 4.

Pentachrome IHC staining on wound sections taken at necropsy on the two pigs. The % collagen (yellow stain) for each section is shown on the image. The yellow staining was higher in the CR‐3 treated pig for wounds receiving no patch, patch alone, and patch plus Cr‐3. The bottom images show “stitched” together sections to give an overall view of wound size and collagen content from IgG treated (left) and CR‐3 treated pig (right)
FIGURE 5.
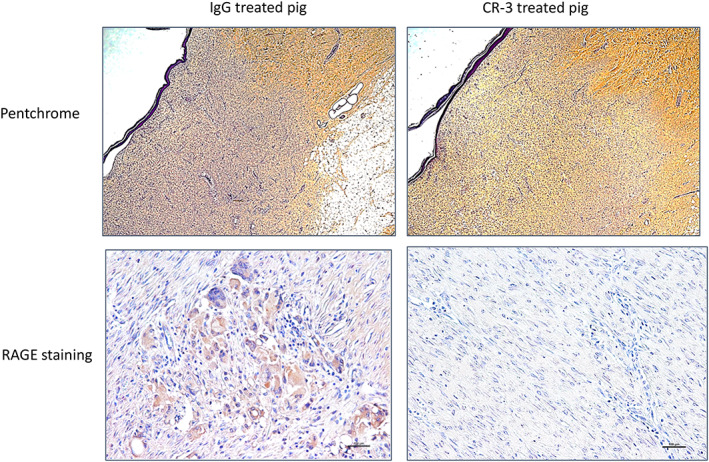
Pentachrome ICH from IgG treated pig wound on left and CR‐3 treated pig on right with pentachrome on top and RAGE staining on bottom
FIGURE 6.

IHC stained tissue sections for macrophages (Mac), TNF‐α in middle, and IL‐6 on right for diabetic pig treated with IgG top, and diabetic pig treated with CR‐3 on bottom row
4. DISCUSSION
Observations from clinical and experimental models of diabetes underscores its association to impaired wound healing and ulcer formation. 6 , 15 , 16 , 17 , 18 , 19 The current standard of care is managing the comorbidities, addressing the metabolic abnormalities, and supportive care through frequent dressing changes and offloading techniques, and revascularisation via endovascular or surgical approaches to restore blood flow but with limited success because of poor target vessels, poor wound healing, and chronic renal failure limiting the contrast load. 20 Treatment aimed at blocking pathw33ays contributing to poor wound healing in diabetics could be therapeutically very beneficial. Receptor for Advanced Glycation Endproducts (RAGE) expression in diabetes underlies several of the molecular pathways contributing to poor wound healing.
The wound healing process occurs in four phases: (1) coagulation and haemostasis, beginning immediately after injury; (2) inflammation, 24–26 h after injury; (3) proliferation, 3–14 days after injury; and (4) wound remoulding with the formation of scar tissue, 1–2 years after injury. 21 , 22 , 23 , 24 , 25 The molecular pathology of this process involves the ECM, actions of soluble mediators like growth factors and cytokines, and a variety of cell populations. Interactions between the ECM and its soluble mediators transduce signalling cascades that is pivotal to the wound healing process. 15 , 16 , 17 , 19 , 26 Abnormalities in the ECM have been shown to delay the healing process. 27 , 28 , 29
RAGE signalling plays a major role in poor wound healing in diabetes by blocking the hypoxic stimulus to angiogenesis thereby reducing blood flow to the wound, reducing the pro‐inflammatory response to infection, and reducing pro‐apoptotic signalling to a chronic non‐healing wound. 6 , 10 , 11 , 12 We developed an anti‐RAGE antibody that binds to a unique peptide sequence on the extracellular domain of RAGE and has blocking properties. In mouse model of hindlimb ischemia we showed the extent of RAGE expression and showed the effect of RAGE to inhibit the angiogenic response to hypoxia. 27 We treated diabetic mice with the murine anti‐RAGE Ab, and found that in mice treated with antibody, angiogenesis was enhanced compared with mice treated with vehicle. We extended this research to porcine model of diabetes with endovascular occlusion of the anterior femoral artery to show similar results of systemic administration of anti‐RAGE Ab to improve angiogenesis to the ischemic muscle in the limb occluded. 30 Other investigators demonstrated that soluble RAGE given as a decoy to diabetic db/db mice with full thickness dorsal wounds, showed accelerated wound healing in treated compared with untreated mice. 6
Local approaches to treat diabetic wounds have included sustained topical administration of drugs in patches. Bioengineered ECM patches can be fashioned from decelluarlized porcine ECM scaffolds to reproduce the tissue site. These scaffolds then act as a vehicle for administering drugs directly to a wound. Demonstration of a method for delivery of exosomes with similar molecular weights to whole IgG antibodies, from a scaffold applied to the myocardium has been established. 31 The molecular weights and sizes of exosomes are similar to antibodies and therefore the development for this type of delivery for antibodies directly to the wound should apply to patches applied directly to wounds carrying our anti‐RAGE antibody.
As a result of its passive physical support for cells and its role in transducing signals pivotal for tissue repair, the ECM provides the scaffolding needed during each stage of the healing process. 29 , 30 , 32 , 33 The ECM patch acts as a physical placeholder for the missing tissue and if not immediately incorporated into the wound would act more like a dressing. In this study, we observed that the patches on the IgG treated pig tended to stay attached to the wound and showed partial incorporation into the wounds. In the CR‐3 treated pigs, the patches were not incorporated into the wounds and fell off mid‐way through treatment associated with healing of the underlying tissue. In these wounds, the patches acted more as a covering or dressing for the wound. It is possible that there was some local delivery of antibody to the wound because the antibody plus patch in the CR‐3 treated pig healed faster than the patch without antibody but the numbers are very small. Improved collagen deposition in wounds of the CR‐3 treated pig strengthened the integrity of the wound.
We chose to perform our study in porcine model because of similarities of the dermal repair process to human wound healing. 32 , 34 Similarities in relative thickness of the dermis and epidermis, presence of similar density of dermal appendages, and the formation of keloid, hypertrophic and exuberant scar formation are a few examples that are not seen in other animal models. 32 The results of these experiments showing that our anti‐RAGE Ab improves wound healing must be interpreted as preliminary because of the sample size and need to be repeated in larger number of pigs but when combined with our other work in diabetic pigs, suggests that this antibody has potential to improve patients with chronic limb threatening ischemia.
Johnson JM, Takebe Y, Zhang G, et al. Blocking RAGE improves wound healing in diabetic pigs. Int Wound J. 2023;20(3):678‐686. doi: 10.1111/iwj.13909
Funding information Dr. Lynne Johnson, Grant/Award Number: NHLBI R01 HL130056; TRx Resource: Pilot Award from Irving Insitute for Clinical and Translational Research 2017
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Fowkes FG, Aboyans V, Fowkes FJI, McDermott MM, Sampson UKA, Criqui MH. Peripheral artery disease: epidemiology and global perspectives. Nat Rev Cardiol. 2017;14(3):156‐170. [DOI] [PubMed] [Google Scholar]
- 2. Eldrup N, Sillesen H, Prescott E, Nordestgaard BG. Ankle brachial index, C‐reactive protein, and central augmentation index to identify individuals with severe atherosclerosis. Eur Heart J. 2006;27(3):316‐322. [DOI] [PubMed] [Google Scholar]
- 3. Anand SS, Caron F, Eikelboom JW, et al. Major adverse limb events and mortality in patients with peripheral artery disease: the COMPASS trial. J Am Coll Cardiol. 2018;71(20):2306‐2315. [DOI] [PubMed] [Google Scholar]
- 4. Low Wang CC, Blomster JI, Heizer G, et al. Cardiovascular and limb outcomes in patients with diabetes and peripheral artery disease: the EUCLID trial. J Am Coll Cardiol. 2018;72(25):3274‐3284. [DOI] [PubMed] [Google Scholar]
- 5. Boulton AJ, Vileikyte L, Ragnarson‐Tennvall G, Apelqvist J. The global burden of diabetic foot disease. Lancet. 2005;366(9498):1719‐1724. [DOI] [PubMed] [Google Scholar]
- 6. Schmidt AM, Yan SD, Wautier JL, Stern D. Activation of receptor for advanced glycation end products: a mechanism for chronic vascular dysfunction in diabetic vasculopathy and atherosclerosis. Circ Res. 1999;84(5):489‐497. [DOI] [PubMed] [Google Scholar]
- 7. Kalea AZ, Schmidt AM, Hudson BI. RAGE: a novel biological and genetic marker for vascular disease. Clin Sci (Lond). 2009;116(8):621‐637. [DOI] [PubMed] [Google Scholar]
- 8. Wendt T, Bucciarelli L, Qu W, et al. Receptor for advanced glycation endproducts (RAGE) and vascular inflammation: insights into the pathogenesis of macrovascular complications in diabetes. Curr Atheroscler Rep. 2002;4(3):228‐237. [DOI] [PubMed] [Google Scholar]
- 9. Shoji T, Koyama H, Morioka T, et al. Receptor for advanced glycation end products is involved in impaired angiogenic response in diabetes. Diabetes. 2006;55(8):2245‐2255. [DOI] [PubMed] [Google Scholar]
- 10. Tchaikovski V, Olieslagers Ś, Böhmer FD, Waltenberger J. Diabetes mellitus activates signal transduction pathways resulting in vascular endothelial growth factor resistance of human monocytes. Circulation. 2009;120(2):150‐159. [DOI] [PubMed] [Google Scholar]
- 11. Goova MT, Li J, Kislinger T, et al. Blockade of receptor for advanced glycation end‐products restores effective wound healing in diabetic mice. Am J Pathol. 2001;159(2):513‐525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hodde JP, Johnson CE. Extracellular matrix as a strategy for treating chronic wounds. Am J Clin Dermatol. 2007;8(2):61‐66. [DOI] [PubMed] [Google Scholar]
- 13. Hunt TK, Hopf H, Hussain Z. Physiology of wound healing. Adv Skin Wound Care. 2000;13(2 Suppl):6‐11. [PubMed] [Google Scholar]
- 14. Brem H, Jacobs T, Vileikyte L, et al. Wound‐healing protocols for diabetic foot and pressure ulcers. Surg Technol Int. 2003;11:85‐92. [PubMed] [Google Scholar]
- 15. Saluja S, Anderson SG, Hambleton I, et al. Foot ulceration and its association with mortality in diabetes mellitus: a meta‐analysis. Diabet Med. 2020;37(2):211‐218. [DOI] [PubMed] [Google Scholar]
- 16. Schultz GS, Davidson JM, Kirsner RS, Bornstein P, Herman IM. Dynamic reciprocity in the wound microenvironment. Wound Repair Regen. 2011;19(2):134‐148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schultz GS, Wysocki A. Interactions between extracellular matrix and growth factors in wound healing. Wound Repair Regen. 2009;17(2):153‐162. [DOI] [PubMed] [Google Scholar]
- 18. Cantalupo A, Sasset L, Gargiulo A, et al. Endothelial sphingolipid De novo synthesis controls blood pressure by regulating signal transduction and NO via ceramide. Hypertension. 2020;75(5):1279‐1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sen CK, Gordillo GM, Roy S, et al. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen. 2009;17(6):763‐771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mustapha JA, Anose BM, Martinsen BJ, et al. Lower extremity revascularization via endovascular and surgical approaches: a systematic review with emphasis on combined inflow and outflow revascularization. SAGE Open Med. 2020;8:2050312120929239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kane CD, Greenhalgh DG. Expression and localization of p53 and bcl‐2 in healing wounds in diabetic and nondiabetic mice. Wound Repair Regen. 2000;8(1):45‐58. [DOI] [PubMed] [Google Scholar]
- 22. Meyer W, Schwarz R, Neurand K. The skin of domestic mammals as a model for the human skin, with special reference to the domestic pig. Curr Probl Dermatol. 1978;7:39‐52. [DOI] [PubMed] [Google Scholar]
- 23. Spampinato SF, Caruso GI, de Pasquale R, Sortino MA, Merlo S. The treatment of impaired wound healing in diabetes: looking among old drugs. Pharmaceuticals (Basel). 2020;13(4):1‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Olczyk P, Mencner L, Komosinska‐Vassev K. The role of the extracellular matrix components in cutaneous wound healing. Biomed Res Int. 2014;2014:747584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pleis JR, Lethbridge‐Cejku M. Summary health statistics for U.S. adults: National Health Interview Survey. Vital Health Stat 10. 2006;2007(235):1‐153. [PubMed] [Google Scholar]
- 26. Tekabe Y, Anthony T, Li Q, et al. Treatment effect with anti‐RAGE F(ab')2 antibody improves hind limb angiogenesis and blood flow in type 1 diabetic mice with left femoral artery ligation. Vasc Med. 2015;20(3):212‐218. [DOI] [PubMed] [Google Scholar]
- 27. Tekabe Y, Kollaros M, Li C, Zhang G, Schmidt AM, Johnson L. Imaging receptor for advanced glycation end product expression in mouse model of hind limb ischemia. EJNMMI Res. 2013;3(1):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Van Putte L, De Schrijver S, Moortgat P. The effects of advanced glycation end products (AGEs) on dermal wound healing and scar formation: a systematic review. Scars Burn Heal. 2016;2:2059513116676828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Velnar T, Bailey T, Smrkolj V. The wound healing process: an overview of the cellular and molecular mechanisms. J Int Med Res. 2009;37(5):1528‐1542. [DOI] [PubMed] [Google Scholar]
- 30. Johnson LL, Johnson J, Ober R, et al. Novel receptor for advanced glycation end products‐blocking antibody to treat diabetic peripheral artery disease. J Am Heart Assoc. 2021;10(1):e016696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Deuse T, Tediashvili G, Hu X, et al. Hypoimmune induced pluripotent stem cell‐derived cell therapeutics treat cardiovascular and pulmonary diseases in immunocompetent allogeneic mice. Proc Natl Acad Sci U S A. 2021;118(28):1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang JF, Olson ME, Reno CR, Wright JB, Hart DA. The pig as a model for excisional skin wound healing: characterization of the molecular and cellular biology, and bacteriology of the healing process. Comp Med. 2001;51(4):341‐348. [PubMed] [Google Scholar]
- 33. Wang Q, Zhu G, Cao X, Dong J, Song F, Niu Y. Blocking AGE‐RAGE signaling improved functional disorders of macrophages in diabetic wound. J Diabetes Res. 2017;2017:1428537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Middelkoop E, van den Bogaerdt AJ, Lamme EN, Hoekstra MJ, Brandsma K, Ulrich MMW. Porcine wound models for skin substitution and burn treatment. Biomaterials. 2004;25(9):1559‐1567. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


