Abstract
Coronavirus disease 2019 (COVID-19) has yet to be proven to alter male reproductive function, particularly in the majority of mild/asymptomatic patients. The purpose of this study was to explore whether mild/asymptomatic COVID-19 affects semen quality and sex-related hormone levels. To find suitable comparative studies, a systematic review and meta-analysis was done up to January 22, 2022, by using multiple databases (Web of Science, PubMed, and Embase). Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were used to identify and choose the studies. Meta-analysis was used to examine the semen parameters and sex-related hormones of mild/asymptomatic COVID-19 patients before and after infection. The effects of semen collection time, fever, and intensity of verification on semen following infection were also investigated. A total of 13 studies (n = 770) were included in the analysis, including three case-control studies, six pre-post studies, and four single-arm studies. A meta-analysis of five pre-post studies showed that after infection with COVID-19, sperm concentration (I2 = 0; P = 0.003), total sperm count (I2 = 46.3%; P = 0.043), progressive motility (I2 = 50.0%; P < 0.001), total sperm motility (I2 = 76.1%; P = 0.047), and normal sperm morphology (I2 = 0; P = 0.001) decreased. Simultaneously, a systematic review of 13 studies found a significant relationship between semen collection time after infection, inflammation severity, and semen parameter values, with fever having only bearing on semen concentration. Furthermore, there was no significant difference in sex-related hormone levels before and after infection in mild/asymptomatic patients. Mild/asymptomatic COVID-19 infection had a significant effect on semen quality in the short term. It is recommended to avoid initiating a pregnancy during this period of time.
Keywords: coronavirus disease 2019, male infertility, meta-analysis, semen quality, severe acute respiratory syndrome coronavirus 2
INTRODUCTION
According to the World Health Organization (WHO) figures, there were more than 360 million confirmed cases of coronavirus disease 2019 (COVID-19) and more than 5.6 million deaths as of January 24, 2022.1 Since the WHO announced it as a global pandemic in March 2020, it has affected hundreds of millions of people worldwide. Many studies have shown that COVID-19 can cause more harm and a higher mortality rate to men.2,3,4 However, there are no clear studies showing that severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) can cause serious and lasting damage to male reproductive function.
Although there is no definite evidence that male infertility can be a potential indicator of future health, some previous studies have suggested that impaired male fertility may be related to the occurrence and mortality of chronic diseases in old age.5,6,7 Studies have shown that infertile men have a higher risk of death than fertile men, and the risk of death increases with the degree of impaired semen quality.8,9 There is also a significant increase in the risk of future malignant tumors (prostate and testicular cancer) in men with reproductive impairment.10 Therefore, attention should be paid to whether SARS-CoV-2 will damage male fertility.
Owing to the blood–testis barrier, the testis has an immune privilege that prevents harmful substances from interfering with spermatogenesis and damaging spermatozoa,11 but some viruses can cross the blood–testis barrier. So far, 27 viruses, including RNA viruses, have been found in human semen, such as human immunodeficiency virus (HIV) and mumps virus (MuV).12 Orchitis caused by viruses or its effect on the testis is one of the causes of male infertility and decline in reproductive function.13 Although a recent meta-analysis found differences in semen quality between COVID-19 infected and uninfected men, the results were heterogeneous. In addition, it is not clear whether there is any relationship between the degree of COVID-19 inflammation and semen quality.14
Considering that testicular parenchyma may be a potential target for SARS-CoV-2 infection, sex hormone levels and some biomarkers related to reproductive function may also be affected.15 It is well known that testosterone plays an important physiological role in affecting spermatogenesis and maintaining normal sexual function. Therefore, the damage of Leydig cells by SARS-CoV-2 may seriously affect the level of testosterone in the body.16 In addition, in some studies, increased serum luteinizing hormone (LH) concentrations have been observed in men and women infected with COVID-19, which indicates the possibility of hypogonadism.17 Other serum markers related to reproductive function, such as type 2 cysteine, are rarely mentioned in the literature, but they may also be affected by COVID-19.18 Therefore, the purpose of this study was to explore the effects of mild/asymptomatic COVID-19 on semen parameters and sex-related hormone levels and to analyze the relationship between semen parameter values and semen collection time after infection, fever, and severity of COVID-19.
MATERIALS AND METHODS
This systematic review and meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.19 The study protocol has been registered with PROSPERO (CRD42022309634).
Search strategy and eligibility criteria
We searched the Web of Science, PubMed, and Embase databases as of January 22, 2022, for articles on semen and COVID-19 infection. The main search terms were “COVID-19”, “SARS-CoV-2”, “severe acute respiratory syndrome coronavirus 2”, “Novel Coronavirus (NCOV)”, “2019 NCOV”, “semen”, “seminal”, “sperm”, and “spermatozoa”. The detailed retrieval strategy is described in the appendix (Supplementary Tables 1–3).
Supplementary Table 1.
Search strategy for PubMed
| Search | Search terms |
|---|---|
| #1 | "covid 19"[All Fields] OR "covid 19"[MeSH Terms] OR "covid 19 vaccines"[All Fields] OR "covid 19 vaccines"[MeSH Terms] OR "covid 19 serotherapy"[All Fields] OR "covid 19 serotherapy"[Supplementary Concept] OR "covid 19 nucleic acid testing"[All Fields] OR "covid 19 nucleic acid testing"[MeSH Terms] OR "covid 19 serological testing"[All Fields] OR "covid 19 serological testing"[MeSH Terms] OR "covid 19 testing"[All Fields] OR "covid 19 testing"[MeSH Terms] OR "sars cov 2"[All Fields] OR "sars cov 2"[MeSH Terms] OR "severe acute respiratory syndrome coronavirus 2"[All Fields] OR "ncov"[All Fields] OR "2019 ncov"[All Fields] OR (("coronavirus"[MeSH Terms] OR "coronavirus"[All Fields] OR "cov"[All Fields]) AND 2019/11/01:2022/1/22[Date - Publication]) |
| #2 | "semen"[MeSH Terms] OR "semen"[All Fields] OR "semen s"[All Fields] OR "semens"[All Fields] |
| #3 | "sperm s"[All Fields] OR "spermatozoa"[MeSH Terms] OR "spermatozoa"[All Fields] OR "sperm"[All Fields] OR "sperms"[All Fields] |
| #4 | "seminal"[All Fields] |
| #5 | (#1) AND ((#2) OR (#3) OR (#4)) |
Date: January 22, 2022: 283 citations found
Supplementary Table 3.
Search strategy for Web of Science
| Search | Search terms |
|---|---|
| #1 | ALL=(COVID-19 OR SARS-CoV-2 OR Severe Acute Respiratory Syndrome Coronavirus 2 OR NCOV OR 2019 NCOV OR coronavirus OR COV) |
| #2 | ALL=(semen OR semen’s OR semens) |
| #3 | AL=(sperm OR sperm’s OR spermatozoa OR sperms) |
| #4 | ALL=(seminal) |
| #5 | #4 OR #3 OR #2 |
| #6 | #1 AND #5 |
Date: January 22, 2022: 247 citations found
This study’s inclusion criteria are peer-reviewed studies in which almost or the vast majority of patients are mild/asymptomatic COVID-19, defined as those treated at home or do not need supplemental oxygen. Studies without raw data (i.e., case report, reviews, comments, letters, and conference summaries) were excluded, but the reference list was checked to determine whether other studies of interest were included. The pre-post studies were included in the meta-analysis, and the case-control studies, pre-post studies, and single-arm studies were included in the systematic review.
Two reviewers (PC and YY) screened abstracts of all studies identified by the initial search, excluding those that did not meet the inclusion criteria. Then, the full text of the qualified research is obtained. In the event of disagreement, a third reviewer (BWC) made the decision.
Data extraction and quality assessment
Data on sperm parameters (semen volume, sperm concentration, total sperm count, progressive motility, sperm motility, and normal sperm morphology) and sex-related hormone parameters (follicle-stimulating hormone [FSH], LH, and testosterone [TT]) were extracted using predefined data extraction forms. For the study of the report with median and range or interquartile range (IQR), a verified mathematical model was used to convert the median (range or IQR) to mean (standard deviation [s.d.]).20
To assess the quality of observational studies, two reviewers (PC and YY) used the Newcastle–Ottawa scale (NOS). The reviewer applied the scale to each study independently, and if no agreement could be reached, the difference was resolved through discussion or negotiation with a third-party reviewer (BWC). The highest score on the scale was 9. A total score of 5 or less was considered low quality, 6 and 7 were considered medium quality, and 8 and 9 were considered high quality.
Statistical analyses
For continuous results, the weighted mean difference (WMD) was used to measure the difference. The statistical heterogeneity between studies was evaluated by I2 and the Cochrane Q test. I2 represented low heterogeneity, moderate heterogeneity, and high heterogeneity in the range of I2 < 40%, I2 ≥ 40 and I2 ≤ 75%, and I2 >75%, respectively.21 If there was no significant heterogeneity between studies (I2 < 50%; P > 0.10), the fixed-effects model was used to calculate the combined estimate; otherwise, the random-effects model was used to calculate the combined estimate (I2 > 50%; P < 0.10). The significance level was set to P = 0.05, and 95% confidence interval (CI) was taken. Funnel charts, Egger’s test, and Begg’s test were used to evaluate publication bias. Sensitivity analysis was used to detect influential studies. Fisher’s method was used to combine the P values (P < 0.05) obtained from semen changes in various studies.22 The statistical software StataMP (version 14.2; StataCorp LP, College Station, TX, USA) was used for data merging.
RESULTS
Study selection
A total of 943 articles were retrieved. After eliminating repetition, 505 articles were selected, and 35 papers were considered to meet the requirements for full-text review. Of these studies, 22 were excluded because they did not provide relevant or extractable data. Finally, 13 articles met the inclusion criteria of the systematic review.23,24,25,26,27,28,29,30,31,32,33,34,35 Of the 13 studies, only 5 pre-post studies included comparable semen data from mild/asymptomatic COVID-19 patients, which could be used for meta-analysis26,27,28,29,31 (Figure 1).
Figure 1.
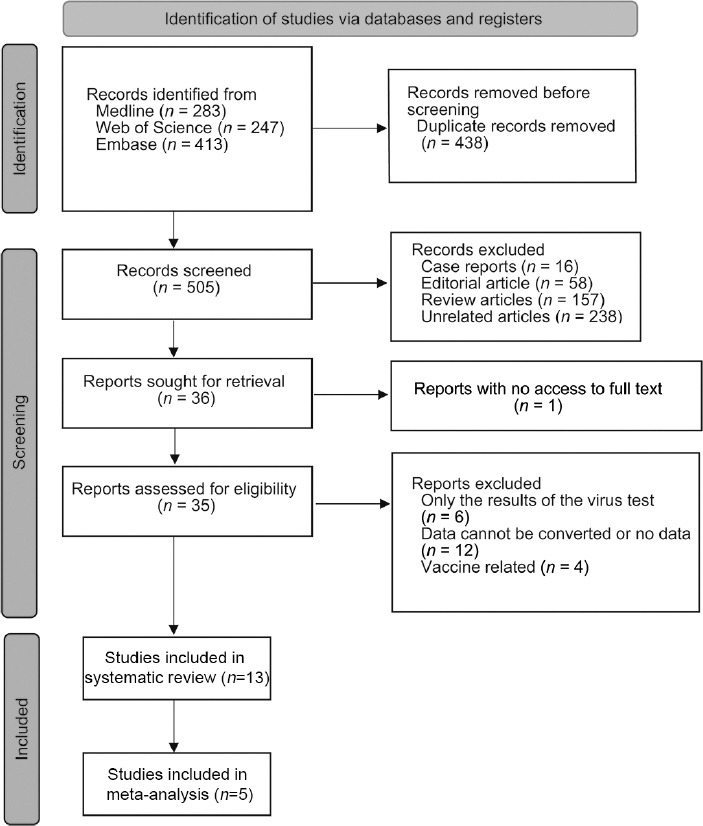
PRISMA flowchart of the study identification process. PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Study characteristics and quality assessment
Table 1 summarizes the characteristics of the studies included. The enrolled population included laboratory-confirmed COVID-19 patients aged ≥18 years. Of the 13 studies, three were case-control studies,23,24,25 six were pre-post studies,26,27,28,29,30,31 and four were single-arm studies.32,33,34,35 Five of the 13 studies obtained semen data of mild/asymptomatic patients before and after infection,26,27,28,29,31 four studies obtained semen data about 70 days before and after infection,23,30,31,33 two studies obtained data of patients with fever/nonfever after infection,27,29 and three studies obtained data of mild/nonmild patients.27,32,34 Only five studies (n = 325) tested for SARS-CoV-2 in semen and found no detection of the virus in semen.24,25,32,34,35 According to NOS, two studies27,28 (15.4%) were classified as high-quality studies, seven studies23,24,25,26,29,30,31 (53.8%) were medium-quality studies, and four single-arm studies32,33,34,35 (30.8%) could not be classified.
Table 1.
Basic study characteristics
| Study | Country | Case size (n) | Mild/asymptomatic samples, n (%) | Age (year), mean±s.d. | SARS-CoV-2 RNA in semen, n (%) | Outcomes reported | NOS |
|---|---|---|---|---|---|---|---|
| Case-control study | |||||||
| Guo et al.23 2021 | China | 41 | 29 (70.7) | 26.4±2.7 | - | Semen volume, sperm concentration, total sperm count, progressive motility, sperm motility; FSH, LH, TT | 6 |
| Best et al.24 2021 | USA | 30 | - | - | 0 (0) | Semen volume, sperm concentration, total sperm count | 7 |
| Ma et al.25 2021 | China | 119 | - | 38.3±5.5 | 0 (0) | Progressive motility, sperm morphology; FSH, LH, TT | 6 |
| Pre-post study | |||||||
| Koç and Keseroğlu26 2021 | Turkey | 21 | 21 (100.0) | 32.0±6.3 | - | Semen volume, sperm concentration, total sperm count, progressive motility, total motility, sperm morphology; FSH, LH, TT | 7 |
| Erbay et al.27 2021 | Turkey | 69 | 26 (37.7) | 30.1±4.4 | - | Semen volume, sperm concentration, total sperm count, progressive motility, total motility | 8 |
| Gul et al.28 2021 | Turkey | 29 | 26 (89.7) | 31.2±5.5 | - | Semen volume, sperm concentration, total sperm count, progressive motility, total motility; FSH, LH, TT | 8 |
| Pazir et al.29 2021 | Turkey | 24 | 24 (100.0) | 34.7±6.4 | - | Semen volume, sperm concentration, progressive motility, total motility | 6 |
| Rafiee and Bagher30 2021 | Iran | 200 | - | 36.1±4.1 | - | Semen volume, sperm concentration, total motility, sperm morphology | 7 |
| Hamarat et al.31 2021 | Turkey | 41 | 39 (95.1) | 31.3±6.0 | - | Semen volume, sperm concentration, total sperm count, progressive motility, total motility, sperm morphology | 7 |
| Single-arm study | |||||||
| Scroppo et al.32 2021 | Italy | 15 | 10 (66.7) | 30.6±8.7 | 0 (0) | Semen volume, sperm concentration, total sperm count, progressive motility, sperm morphology; FSH, LH, TT | - |
| Falahieh et al.33 2021 | Iran | 20 | 0 (0) | - | - | Semen volume, sperm concentration, progressive motility, sperm morphology | - |
| Gacci et al.34 2021 | Italy | 43 | 19 (44.2) | - | 0 (0) | Semen volume, sperm concentration, total sperm count, progressive motility, sperm morphology | - |
| Donders et al.35 2021 | Belgium | 118 | 110 (93.2) | 34.7±9.1 | 0 (0) | Sperm concentration, total sperm count, progressive motility, sperm morphology | - |
s.d.: standard deviation; FSH: follicle-stimulating hormone; LH: luteinizing hormone; TT: testosterone; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2; NOS: the Newcastle-Ottawa Scale; -: no relevant data
Effects of mild/asymptomatic COVID-19 on semen parameters and sex-related hormone levels in men
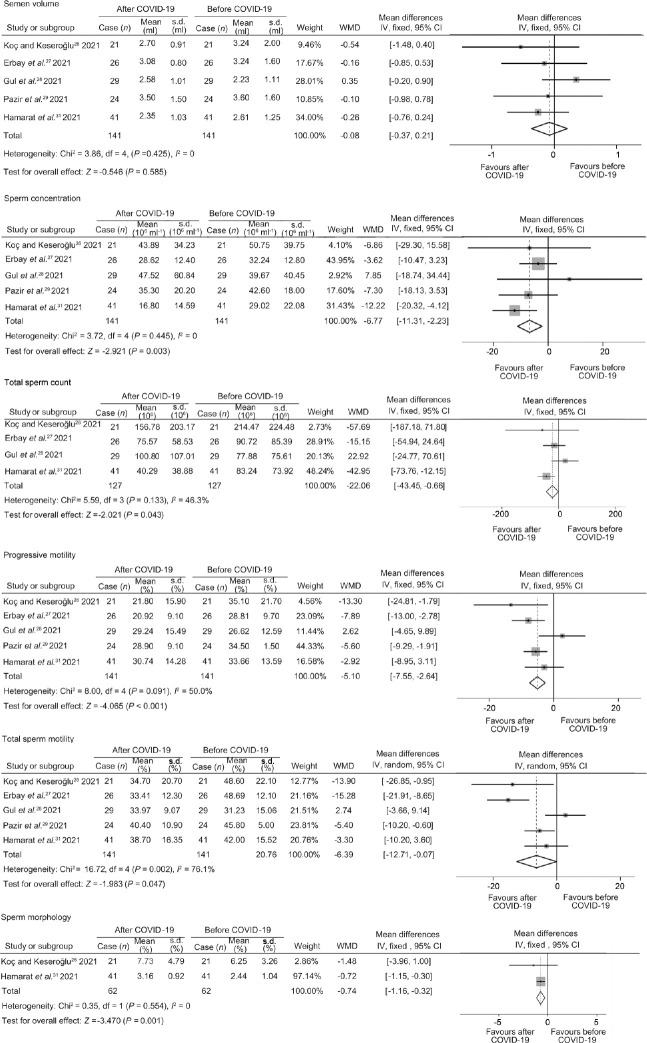
Except for semen volume (I2 = 0; P = 0.585), there were significant differences in sperm concentration (I2 = 0; P = 0.003), total sperm count (I2 = 46.3%; P = 0.043), progressive motility (I2 = 50.0%; P < 0.001), sperm motility (I2 = 76.1%; P = 0.047), and normal sperm morphology (I2 = 0; P = 0.001) before and after infection in mild/asymptomatic COVID-19 patients (Figure 2). Except for the sperm motility, the funnel patterns of other semen parameters were symmetrical (Supplementary Figure 1 (148.6KB, tif) ). There was no publication bias in any semen parameters by Egger’s and Begg’s test (Table 2). The results of the sperm motility were highly heterogeneous, and the funnel diagram was asymmetric. However, sensitivity analysis showed that the combined effect did not change significantly after excluding any studies which indicated that the result was relatively stable (Supplementary Figure 2 (171.8KB, tif) ).
Figure 2.
Forest plots of semen parameters before and after COVID-19 infection. COVID-19: coronavirus disease 2019; WMD: weighted mean difference; df: degree of freedom; IV: inverse-variance; CI: confidence interval; s.d.: standard deviation.
Table 2.
Publication bias
| Outcomes | P of Begg’s test | Egger’ s test | |
|---|---|---|---|
|
| |||
| P | 95% CI | ||
| Semen volume | 0.81 | 0.54 | −7.55–4.87 |
| Sperm concentration | 0.81 | 0.63 | −2.91–4.10 |
| Total sperm number | 0.73 | 0.82 | −8.66–9.74 |
| Progressive sperm motility | 1.00 | 0.97 | −6.81–6.99 |
| Sperm motility | 0.81 | 0.64 | −13.73–9.87 |
| Normal sperm morphology | 1.00 | NA | NA |
| FSH | 1.00 | NA | NA |
| LH | 1.00 | NA | NA |
| TT | 1.00 | NA | NA |
FSH: follicle-stimulating hormone; LH: luteinizing hormone; TT: testosterone; 95% CI: 95% confidence interval; NA: not applicable
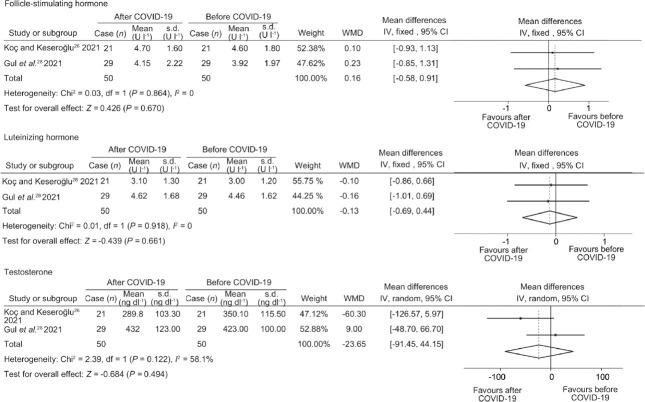
In addition, meta-analysis of two studies26,28 showed that there was no significant difference in FSH (I2 = 0; P = 0.670), LH (I2 = 0; P = 0.661) and TT (I2 = 58.1%; P = 0.494) in mild/asymptomatic COVID-19 patients before and after infection (Figure 3, Supplementary Figure 3 (89.1KB, tif) , and Table 2).
Figure 3.
Forest plots of sex-related hormone before and after COVID-19 infection. COVID-19: coronavirus disease 2019; WMD: weighted mean difference; df: degree of freedom; IV: inverse-variance; CI: confidence interval; s.d.: standard deviation.
Supplementary Table 2.
Search strategy for Embase
| Search | Search terms |
|---|---|
| #1 | COVID-19 OR SARS-CoV-2 OR Severe Acute Respiratory Syndrome Coronavirus 2 OR NCOV OR 2019 NCOV OR coronavirus OR COV |
| #2 | Semen OR semens OR sperm OR spermatozoa OR sperms OR seminal |
| #3 | #1 AND #2 |
Date: January 22, 2022: 413 citations found
Effect of semen collection time on semen quality after COVID-19 infection
Four studies23,30,31,33 compared semen quality before and after 70 days of COVID-19 infection. Although the results of each study were different, the P values of semen parameters combined with each study concluded that significant decreases in sperm concentration (P < 0.001), total sperm count (P = 0.001), progressive motility (P = 0.006), sperm motility (P = 0.004), and normal sperm morphology (P < 0.001) were observed after infection with COVID-19 (Supplementary Table 4).
Supplementary Table 4.
Effect of semen collection time (≤70 day or >70 day) on semen quality after infection with coronavirus disease 2019
| Characteristic | Guo et al.23 2021 | Rafiee and Bagher30 2021 | Hamarat et al.31 2021 | Falahieh et al.33 2021 | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||
| Number of patients | Value | Number of patients | Value | Number of patients | Value | Number of patients | Value | |
| Semen volume | ||||||||
| ≤70 days (ml), mean±s.d. | 22 | 2.97±0.38 | 100 | 2.50±0.30 | 15 | 2.51±0.81 | 20 | 3.80±1.20 |
| >70 days (ml), mean±s.d. | 22 | 3.27±0.30 | 100 | 4.44±1.32 | 26 | 2.26±1.16 | 20 | 4.10±1.30 |
| P | 0.065 | 0.040 | 0.470 | 0.439 | ||||
| Combine P | 0.058 | |||||||
| Sperm concentration | ||||||||
| ≤70 days (×106 ml−1), mean±s.d. | 22 | 43.39±13.54 | 100 | 58.8±25.10 | 15 | 9.14±4.41 | 20 | 47.60±21.90 |
| >70 days (×106 ml−1), mean±s.d. | 22 | 69.29±21.43 | 100 | 63.54±54.21 | 26 | 30.20±14.25 | 20 | 52.10±24.30 |
| P | 0.007 | 0.071 | <0.001 | 0.542 | ||||
| Combine P | <0.001 | |||||||
| Total sperm count | ||||||||
| ≤70 days (×106), mean±s.d. | 22 | 113.51±47.36 | 100 | - | 15 | 26.09±21.46 | 20 | - |
| >70 days (×106), mean±s.d. | 22 | 207.51±60.79 | 100 | - | 26 | 50.58±41.65 | 20 | - |
| P | 0.003 | - | 0.041 | - | ||||
| Combine P | 0.001 | |||||||
| Progressive motility | ||||||||
| ≤70 days (%), mean±s.d. | 22 | 32.50±6.39 | 100 | - | 15 | 27.35±12.21 | 20 | 30.60±8.20 |
| >70 days (%), mean±s.d. | 22 | 38.37±6.24 | 100 | - | 26 | 32.70±13.78 | 20 | 44.10±9.90 |
| P | 0.566 | - | 0.220 | <0.001 | ||||
| Combine P | 0.006 | |||||||
| Total motility | ||||||||
| ≤70 days (%), mean±s.d. | 22 | 39.62±6.97 | 100 | 37.50±25.10 | 15 | 35.63±16.70 | 20 | 32.80±8.90 |
| >70 days (%), mean±s.d. | 22 | 43.31±5.81 | 100 | 43.30±21.43 | 26 | 40.47±16.39 | 20 | 47.50±9.80 |
| P | 0.624 | 0.060 | 0.371 | <0.001 | ||||
| Combine P | 0.004 | |||||||
| Normal sperm morphology | ||||||||
| ≤70 days (%), mean±s.d. | 22 | - | 100 | 2.00±2.10 | 15 | 1.85±1.06 | 20 | 1.30±1.10 |
| >70 days (%), mean±s.d. | 22 | - | 100 | 3.00±0.90 | 26 | 2.81±0.87 | 20 | 3.20±1.70 |
| P | - | 0.060 | 0.003 | <0.001 | ||||
| Combine P | <0.001 | |||||||
s.d.: standard deviation
Effect of fever/nonfever on semen quality after COVID-19 infection
In two studies,27,29 the semen quality in patients with fever/nonfever after COVID-19 infection was compared. Except for semen volume, total sperm count, and normal sperm morphology, the P values of semen parameters were combined, and only sperm concentration was decreased significantly (P = 0.006). There was no significant difference in the presence or absence of fever on progressive motility and total motility (both P > 0.05; Supplementary Table 5).
Supplementary Table 5.
Effects of fever (fever or nonfever) on semen quality after infection with coronavirus disease 2019
| Characteristic | Erbay et al.27 2021 | Pazir et al.29 2021 | ||
|---|---|---|---|---|
|
|
|
|||
| Number of patients | Value | Number of patients | Value | |
| Semen volume | ||||
| Fever (ml), mean±s.d. | 18 | - | 12 | 3.40±1.60 |
| Nonfever (ml), mean±s.d. | 8 | - | 12 | 3.50±1.60 |
| P | - | 0.880 | ||
| Combine P | - | |||
| Sperm concentration | ||||
| Fever (×106 ml−1), mean±s.d. | 18 | 29.52±10.70 | 12 | 3.50±1.60 |
| Nonfever (×106 ml−1), mean±s.d. | 8 | 28.48±15.80 | 12 | 33.90±22.10 |
| P | 0.695 | <0.001 | ||
| Combine P | 0.006 | |||
| Total sperm count | ||||
| Fever (×106), mean±s.d. | 18 | 78.37±48.67 | 12 | - |
| Nonfever (×106), mean±s.d. | 8 | 81.42±62.39 | 12 | - |
| P | 0.672 | - | ||
| Combine P | - | |||
| Progressive motility | ||||
| Fever (%), mean±s.d. | 18 | 21.08±6.70 | 12 | 33.9±22.10 |
| Nonfever (%), mean±s.d. | 8 | 22.38±9.20 | 12 | 27.50±6.90 |
| P | 0.671 | 0.349 | ||
| Combine P | 0.574 | |||
| Total motility | ||||
| Fever (%), mean±s.d. | 18 | 33.49±10.10 | 12 | 41.60±12.10 |
| Nonfever (%), mean±s.d. | 8 | 30.82±12.70 | 12 | 45.00±5.20 |
| P | 0.652 | 0.381 | ||
| Combine P | 0.594 | |||
| Normal sperm morphology | ||||
| Fever (%), mean±s.d. | 18 | - | 12 | - |
| Nonfever (%), mean±s.d. | 8 | - | 12 | - |
| P | - | - | ||
| Combine P | - | |||
s.d.: standard deviation
The influence of COVID-19 severity on semen quality
There were three studies27,32,34 in the grouping analysis of the inflammatory degree of the COVID-19. Only one of the three studies provided data on total motility and normal sperm morphology, so this part only combines the P values of other semen parameters (semen volume, sperm concentration, total sperm count, and progressive motility). After the P values were combined, it was found that the severity of COVID-19 infection was inversely proportional to semen volume (P = 0.01), sperm concentration (P = 0.002), and total sperm count (P = 0.009). The more serious COVID-19 was, the more obvious the decline in semen quality. In addition, the P value of progressive motility in the studies of Erbay et al.27 and Scroppo et al.32 suggested that it might not be related to the severity of the COVID-19 infection (P > 0.05; Supplementary Table 6).
Supplementary Table 6.
Effect of coronavirus disease 2019 degree (mild or nonmild) on semen quality
| Characteristic | Erbay et al.27 2021 | Scroppo et al.32 2021 | Gacci et al.34 2021 | |||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Number of patients | Value | Number of patients | Value | Number of patients | Value | |
| Semen volume | ||||||
| Mild (ml), mean±s.d. | 26 | 3.08±0.80 | 10 | 2.18±1.20 | 12 | 2.49±0.63 |
| Nonmild (ml), mean±s.d. | 43 | 2.74±0.90 | 5 | 2.39±0.88 | 26 | 1.95±0.34 |
| P | 0.159 | 0.700 | 0.002 | |||
| Combine P | 0.010 | |||||
| Sperm concentration | ||||||
| Mild (×106 ml−1), mean±s.d. | 26 | 28.62±12.40 | 10 | 30.20±20.80 | 12 | 61.20±10.19 |
| Nonmild (×106 ml−1), mean±s.d. | 43 | 30.63±17.20 | 5 | 38.20±21.63 | 26 | 22.51±16.79 |
| P | 0.065 | 0.500 | <0.001 | |||
| Combine P | 0.002 | |||||
| Total sperm count | ||||||
| Mild (×106), mean±s.d. | 26 | 75.57±58.53 | 10 | 67.50±65.81 | 12 | 137.80±60.76 |
| Nonmild (×106), mean±s.d. | 43 | 90.38±83.66 | 5 | 89.29±79.28 | 26 | 44.86±31.86 |
| P | 0.320 | 0.600 | <0.001 | |||
| Combine P | 0.009 | |||||
| Progressive motility | ||||||
| Mild (%), mean±s.d. | 26 | 20.92±9.10 | 10 | 45.20±14.34 | 12 | 38.14±9.82 |
| Nonmild (%), mean±s.d. | 43 | 21.40±10.10 | 5 | 40.60±14.72 | 26 | 25.42±7.86 |
| P | 0.847 | 0.570 | 0.004 | |||
| Combine P | 0.052 | |||||
| Total motility | ||||||
| Mild (%), mean±s.d. | 26 | 3.41±12.30 | 10 | - | 12 | - |
| Nonmild (%), mean±s.d. | 43 | 33.41±12.30 | 5 | - | 26 | - |
| P | 0.234 | - | - | |||
| Combine P | - | |||||
| Normal sperm morphology | ||||||
| Mild (%), mean±s.d. | 26 | - | 10 | 4.00±2.30 | 12 | - |
| Nonmild (%), mean±s.d. | 43 | - | 5 | 4.10±1.44 | 26 | - |
| P | - | 0.920 | - | |||
| Combine P | - | |||||
s.d.: standard deviation
DISCUSSION
This is the first systematic review and meta-analysis of mild/asymptomatic COVID-19 on male semen and sex-related hormones, and it reports the effects of semen collection time, fever, and inflammation severity on this result, in order to comprehensively evaluate the effect of mild/asymptomatic COVID-19 on male semen and fertility. By comparing the semen quality and sex-related hormone changes of mild/asymptomatic COVID-19 patients before and after infection, the potential deviation between different individuals can be minimized, which will more effectively indicate the possible impact of this epidemic on male reproductive function.
Our results show that COVID-19 has a certain effect on male reproductive function in the short term. Although this result is consistent with the results found by Tiwari and his colleagues, the difference is that their results are generally highly heterogeneous.14 The main reason may be that there are individual differences between the control group and the case group, as well as different types of literature included. In this study, the types of pre-post studies were included in the meta-analysis as a condition to reduce the impact of individual differences, thus successfully reducing heterogeneity. In addition, we also found that this result was affected by the time of semen collection after infection and the severity of inflammation. For sex-related hormone levels, there was no significant difference in COVID-19 before and after infection.
SARS-CoV-2 has 79% sequence homology with SARS-CoV. It has been known that the SARS-CoV can damage multiple organs, including the human testis, and cause testicular inflammation leading to testicular damage and spermatogenic defects in patients. The external subdomain of the receptor binding domain of SARS-CoV-2 is similar to that of SARS-CoV, indicating that SARS-CoV-2 may also use angiotensin converting enzyme 2 (ACE2) as a cell receptor.36 Some studies have shown that ACE2 is mainly expressed in spermatogonia, interstitial cells, and Sertoli cells in the human testis.37,38 It is reported that the expression of ACE2 in the testis is the highest in men around 30 years old.39 The first step of SARS-CoV-2 infection is the initiation of protease-mediated protamine and host cell receptor, mainly through transmembrane protease serine 2 (TMPRSS2).40 TMPRSS2 is thought to cleave ACE2 receptors and help the virus enter host cells.25 The activation of the androgen receptor is necessary for the initiation of TMPRSS2 gene transcription. These results suggest that the testis may also be the target organ of SARS-CoV-2, especially in men of childbearing age. This can lead to testicular hormone secretion or sperm damage. Our research confirms this. After the mild/asymptomatic patients were infected with COVID-19, the semen quality of the patients was significantly lower than that before infection, and there was no significant change in sex-related hormones; this is mainly reflected in sperm concentration, total sperm count, progressive motility, sperm motility, and normal sperm morphology.
However, the duration of negative effects on male semen is not clear. Considering that the human spermatogenic cycle is estimated to take about 70 days, it is generally believed that the effect of COVID-19 may last 70 days.41 In a recent study, the semen parameters of patients with COVID-19 infection were compared at three different time points (less than 31 days, 32–62 days, and more than 63 days).35 It was found that the semen parameters decreased most significantly within 1 month after infection. After infection for 2 months or more, the semen quality parameters gradually returned to normal. This is consistent with our results that there are significant differences in semen parameters between more than 70 days after infection with COVID-19 and within 70 days, mainly on sperm concentration, total sperm count, progressive motility, sperm motility, and normal sperm morphology. This result is contrary to the changes in semen parameters of mild/asymptomatic patients before and after infection with COVID-19. This shows that the semen quality of the patient gradually recovers at the beginning of the next spermatogenic cycle after recovery. In addition, our results showed that the severity of COVID-19 inflammation was significantly related to semen volume, sperm concentration, and total sperm count. Therefore, for COVID-19 patients with different degrees of inflammation, the time for semen quality recovery remains to be considered.
Systemic or local symptoms caused by inflammation may also have a direct or indirect effect on spermatogenesis, such as fever.42 This is also a symptom observed in more than 80% of people infected with COVID-19. In particular, high fever may lead to changes in testicular temperature and damage to germ cells.43 In most infectious diseases, the inflammatory process caused by fever can destroy germ cells through leukocytic infiltration and reduce TT levels by affecting interstitial cells.44,45 Surprisingly, fever had no significant effect on semen parameters other than sperm concentration in this study.
With the continuation of the SARS-CoV-2 pandemic, new variants of the virus have emerged through continuous mutations, of which the most famous variants are B.1.617.2 (Delta) and B.1.1.529 (Omicron).46,47 In order to be able to spread in the face of rising population immunity when maintaining or increasing their replication suitability, most of the mutations involved in these variants are concentrated in the spike gene.48 Therefore, some variants, including these two variants, seem to be more easily transmitted or more lethal than wild-type SARS-CoV-2. More importantly, different variants seem to have different effects on semen parameters and sex-related hormones. A recent animal experiment confirmed that Delta and Omicron variants have different effects on semen parameters and TT levels in hamsters.16 This is mainly due to the difference in the degree of injury caused by the different viral loads in the testis. Unfortunately, none of the research included in this paper indicated the viral strain that was detected, making it hard to draw any conclusions.
Although almost all the existing literature shows that SARS-CoV-2 is not detected in semen, many reproductive medicine entities and regulators are still concerned about it.49,50,51 On the one hand, the virus is at risk of infiltrating semen, especially in patients with viremia or inflammation that can alter the blood–testis barrier. On the other hand, there is also a risk of exposure to aerosols containing viruses during semen analysis or semen treatment.52 As a result, many countries have decided to suspend fertility treatment to reduce the risk of spreading infection, but this will permanently deprive some special populations of their ability to seek fertility.50 At present, the effect of SARS-CoV-2 on sperm cryopreservation is only a theoretical risk, but with the continuous emergence of mutants, it may be a potential real threat in the future.53
Our research has some limitations. First of all, there is an inherent bias in the meta-analysis of the pre-post studies, which may cause a certain deviation in the results of COVID-19 effect on semen. Second, the technical differences and diagnostic criteria among different time periods and different research centers, as well as the heterogeneity among studies, are inevitable. Third, since COVID-19 has just lasted for about two years, there was no longer follow-up data. Finally, although we have searched three major databases, we have found only 13 studies that meet the inclusion criteria, and only five pre-post studies have been meta-analyzed. Therefore, more and longer follow-up cohort studies are needed to verify it in the future.
CONCLUSION
In summary, mild/asymptomatic COVID-19 has a certain impact on semen in men in the short term, especially within about 70 days after infection. Fever after infection only had a significant effect on sperm concentration. The difference in the effect of inflammation severity on semen quality may be mainly concentrated on the overall semen quality rather than the motility of individual spermatozoa. In addition, no SARS-CoV-2 was found in semen in the 13 studies included.
AUTHOR CONTRIBUTIONS
KFT conceptualized and initiated the study. BWC, PC, and YY screened the text. BWC and PC analyzed the data and created the forest plots. BWC and PC wrote the manuscript. YY, WL, TH, WJZ, SHX, JH, and ML participated in the discussion. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication. All authors contributed to the design of the study and read and approved the final manuscript.
COMPETING INTERESTS
All authors declare no competing interests.
Funnel diagram of semen parameters before and after COVID-19 infection. (a) Semen volume, (b) sperm concentration, (c) total sperm count, (d) progressive motility, (e) sperm motility, and (f) sperm morphology.
Funnel diagram of sex-related hormone before and after COVID-19 infection. (a) Semen volume, (b) sperm concentration, (c) total sperm count, (d) progressive motility, (e) sperm motility, and (f) sperm morphology.
Sensitivity analysis of semen parameters of COVID-19 before and after infection. (a) FSH, (b) LH, and (c) TT. FSH: follicle-stimulating hormone; LH: luteinizing hormone; TT: testosterone.
ACKNOWLEDGMENTS
This study was supported by the Science and Technology Fund Project of Guizhou Health Commission (gzwkj2021-211).
Supplementary Information is linked to the online version of the paper on the Asian Journal of Andrology website.
REFERENCES
- 1.WHO. WHO Coronavirus (COVID-19) Dashboard. 2022. [[Last accessed on 2022 Jan 24]]. Available from: https://covid19.who.int .
- 2.Danielsen AC, Lee KM, Boulicault M, Rushovich T, Gompers A, et al. Sex disparities in COVID-19 outcomes in the United States: quantifying and contextualizing variation. Soc Sci Med. 2022;294:114716. doi: 10.1016/j.socscimed.2022.114716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lo SP, Hsieh TC, Pastuszak AW, Hotaling JM, Patel DP. Effects of SARS CoV-2, COVID-19, and its vaccines on male sexual health and reproduction: where do we stand? Int J Impot Res. 2022;34:138–44. doi: 10.1038/s41443-021-00483-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fortunato F, Martinelli D, Lo Caputo S, Santantonio T, Dattoli V, et al. Sex and gender differences in COVID-19: an Italian local register-based study. BMJ Open. 2021;11:e051506. doi: 10.1136/bmjopen-2021-051506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giudice FD, Kasman AM, Ferro M, Sciarra A, Berardinis ED, et al. Clinical correlation among male infertility and overall male health: a systematic review of the literature. Investig Clin Urol. 2020;61:355–71. doi: 10.4111/icu.2020.61.4.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen T, Belladelli F, Del GF, Eisenberg ML. Male fertility as a marker for health. Reprod Biomed Online. 2022;44:131–44. doi: 10.1016/j.rbmo.2021.09.023. [DOI] [PubMed] [Google Scholar]
- 7.Kasman AM, Del GF, Eisenberg ML. New insights to guide patient care:the bidirectional relationship between male infertility and male health. Fertil Steril. 2020;113:469–77. doi: 10.1016/j.fertnstert.2020.01.002. [DOI] [PubMed] [Google Scholar]
- 8.Giudice FD, Kasman AM, Chen T, Berardinis ED, Busetto GM, et al. The association between mortality and male infertility: systematic review and meta-analysis. Urology. 2021;154:148–57. doi: 10.1016/j.urology.2021.02.041. [DOI] [PubMed] [Google Scholar]
- 9.Marinelli L, Beccuti G, Zavattaro M, Cagnina S, Gesmundo L, et al. Testosterone as a biomarker of adverse clinical outcomes in SARS-CoV-2 pneumonia. Biomedicines. 2022;10:820. doi: 10.3390/biomedicines10040820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giudice FD, Kasman AM, Berardinis ED, Busetto GM, Belladelli F, et al. Association between male infertility and male-specific malignancies: systematic review and meta-analysis of population-based retrospective cohort studies. Fertil Steril. 2020;114:984–96. doi: 10.1016/j.fertnstert.2020.04.042. [DOI] [PubMed] [Google Scholar]
- 11.Mruk DD, Cheng CY. The mammalian blood-testis barrier: its biology and regulation. Endocr Rev. 2015;36:564–91. doi: 10.1210/er.2014-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salam AP, Horby PW. The breadth of viruses in human semen. Emerg Infect Dis. 2017;23:1922–4. doi: 10.3201/eid2311.171049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zafar MI, Yu J, Li H. Implications of RNA viruses in the male reproductive tract: an outlook on SARS-CoV-2. Front Microbiol. 2021;12:783963. doi: 10.3389/fmicb.2021.783963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tiwari S, Kc N, Thapa S, Ghimire A, Bijukchhe S, et al. Semen parameters in men recovered from COVID-19: a systematic review and meta-analysis. Middle East Fertil Soc J. 2021;26:44. doi: 10.1186/s43043-021-00089-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salciccia S, Del GF, Gentile V, Mastroianni CM, Pasculli P, et al. Interplay between male testosterone levels and the risk for subsequent invasive respiratory assistance among COVID-19 patients at hospital admission. Endocrine. 2020;70:206–10. doi: 10.1007/s12020-020-02515-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li C, Ye Z, Zhang AJ, Chan JF, Song W, et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections by intranasal or testicular inoculation induces testicular damage preventable by vaccination in golden Syrian hamsters. Clin Infect Dis. 2022 doi: 10.1093/cid/ciac142. Doi: 10.1093/cid/ciac142. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carneiro Gomes PR, Rodrigues da Rocha MD, da Rocha Coelho FA, Sousa Pinho de Lira JA, de Sousa Carmo RR, et al. Alterations of the male and female reproductive systems induced by COVID-19. Wien Klin Wochenschr. 2021;133:966–72. doi: 10.1007/s00508-021-01875-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giovannone R, Busetto GM, Antonini G, Cobelli DE, Ferro M, et al. Hyperhomocysteinemia as an early predictor of erectile dysfunction:International Index of Erectile Function (IIEF) and penile Doppler ultrasound correlation with plasma levels of homocysteine. Medicine (Baltimore) 2015;94:e1556. doi: 10.1097/MD.0000000000001556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, et al. The PRISMA 2020 statement:an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McGrath S, Zhao X, Steele R, Thombs BD, Benedetti A, et al. Estimating the sample mean and standard deviation from commonly reported quantiles in meta-analysis. Stat Methods Med Res. 2020;29:2520–37. doi: 10.1177/0962280219889080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 22.Fisher R. Statistical Methods for Research Workers. London: Oliver & Boyd; 1950. pp. 99–101. [Google Scholar]
- 23.Guo TH, Sang MY, Bai S, Ma H, Wan YY, et al. Semen parameters in men recovered from COVID-19. Asian J Androl. 2021;23:479–83. doi: 10.4103/aja.aja_31_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Best JC, Kuchakulla M, Khodamoradi K, Negris Lima TF, Frech FS, et al. Evaluation of SARS-CoV-2 in human semen and effect on total sperm number:a prospective observational study. World J Mens Health. 2021;39:489–95. doi: 10.5534/wjmh.200192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma L, Xie W, Li DY, Shi L, Ye GM, et al. Evaluation of sex-related hormones and semen characteristics in reproductive-aged male COVID-19 patients. J Med Virol. 2021;93:456–62. doi: 10.1002/jmv.26259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koç E, Keseroğlu BB. Does COVID-19 worsen the semen parameters?Early results of a tertiary healthcare center. Urol Int. 2021;105:743–8. doi: 10.1159/000517276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Erbay G, Sanli A, Turel H, Yavuz U, Erdogan A, et al. Short-term effects of COVID-19 on semen parameters:a multicenter study of 69 cases. Andrology. 2021;9:1060–5. doi: 10.1111/andr.13019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gul A, Zengin S, Dundar G, Ozturk M. Do SARS-CoV-2 infection (COVID-19) and the medications administered for its treatment impair testicular functions?Urol Int. 2021;105:944–8. doi: 10.1159/000517925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pazir Y, Eroglu T, Kose A, Bulut TB, Genc C, et al. Impaired semen parameters in patients with confirmed SARS-CoV-2 infection:a prospective cohort study. Andrologia. 2021;53:e14157. doi: 10.1111/and.14157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rafiee B, Bagher TS. The effect of N-acetyl cysteine consumption on men with abnormal sperm parameters due to positive history of COVID-19 in the last three months. Arch Ital Urol Androl. 2021;93:465–7. doi: 10.4081/aiua.2021.4.465. [DOI] [PubMed] [Google Scholar]
- 31.Hamarat MB, Ozkent MS, Yilmaz B, Aksanyar SY, Karabacak K. Effect of SARS-CoV-2 infection on semen parameters. Can Urol Assoc J. 2021;16:E173–7. doi: 10.5489/cuaj.7292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scroppo FI, Costantini E, Zucchi A, Illiano E, Trama F, et al. COVID-19 disease in clinical setting:impact on gonadal function, transmission risk, and sperm quality in young males. J Basic Clin Physiol Pharmacol. 2021;33:97–102. doi: 10.1515/jbcpp-2021-0227. [DOI] [PubMed] [Google Scholar]
- 33.Falahieh FM, Zarabadipour M, Mirani M, Abdiyan M, Dinparvar M, et al. Effects of moderate COVID-19 infection on semen oxidative status and parameters 14 and 120 days after diagnosis. Reprod Fertil Dev. 2021;33:683–90. doi: 10.1071/RD21153. [DOI] [PubMed] [Google Scholar]
- 34.Gacci M, Coppi M, Baldi E, Sebastianelli A, Zaccaro C, et al. Semen impairment and occurrence of SARS-CoV-2 virus in semen after recovery from COVID-19. Hum Reprod. 2021;36:1520–9. doi: 10.1093/humrep/deab026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Donders G, Bosmans E, Reumers J, Donders F, Jonckheere J, et al. Sperm quality and absence of SARS-CoV-2 RNA in semen after COVID-19 infection: a prospective, observational study and validation of the SpermCOVID test. Fertil Steril. 2021;117:287–96. doi: 10.1016/j.fertnstert.2021.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu RJ, Zhao X, Li J, Niu PH, Yang B, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–74. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Z, Xu X. scRNA-seq profiling of human testes reveals the presence of the ACE2 receptor, a target for SARS-CoV-2 infection in spermatogonia, leydig and sertoli cells. Cells. 2020;9:920. doi: 10.3390/cells9040920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fu J, Zhou B, Zhang L, Balaji KS, Wei CL, et al. Expressions and significances of the angiotensin-converting enzyme 2 gene, the receptor of SARS-CoV-2 for COVID-19. Mol Biol Rep. 2020;47:4383–92. doi: 10.1007/s11033-020-05478-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shen QY, Xiao X, Aierken A, Yue W, Wu XJ, et al. The ACE2 expression in Sertoli cells and germ cells may cause male reproductive disorder after SARS-CoV-2 infection. J Cell Mol Med. 2020;24:9472–7. doi: 10.1111/jcmm.15541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–80. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heller CG, Clermont Y. Spermatogenesis in man:an estimate of its duration. Science. 1963;140:184–6. doi: 10.1126/science.140.3563.184. [DOI] [PubMed] [Google Scholar]
- 42.Sergerie M, Mieusset R, Croute F, Daudin M, Bujan L. High risk of temporary alteration of semen parameters after recent acute febrile illness. Fertil Steril. 2007;88:970–1. doi: 10.1016/j.fertnstert.2006.12.045. [DOI] [PubMed] [Google Scholar]
- 43.Vahedian-Azimi A, Karimi L, Makvandi S, Jamialahmadi T, Sahebkar A. Does SARS-CoV-2 threaten male fertility? Adv Exp Med Biol. 2021;1321:139–46. doi: 10.1007/978-3-030-59261-5_12. [DOI] [PubMed] [Google Scholar]
- 44.Yang M, Chen S, Huang B, Zhong JM, Su H, et al. Pathological findings in the testes of COVID-19 patients: clinical implications. Eur Urol Focus. 2020;6:1124–9. doi: 10.1016/j.euf.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haghpanah A, Masjedi F, Alborzi S, Hosseinpour A, Dehghani A, et al. Potential mechanisms of SARS-CoV-2 action on male gonadal function and fertility: current status and future prospects. Andrologia. 2021;53:e13883. doi: 10.1111/and.13883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Araf Y, Akter F, Tang YD, Fatemi R, Alam Parvez MS, et al. Omicron variant of SARS-CoV-2:genomics, transmissibility, and responses to current COVID-19 vaccines. J Med Virol. 2022;94:1825–32. doi: 10.1002/jmv.27588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Raman R, Patel KJ, Ranjan K. COVID-19: unmasking emerging SARS-CoV-2 variants, vaccines and therapeutic strategies. Biomolecules. 2021;11:993. doi: 10.3390/biom11070993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tao K, Tzou PL, Nouhin J, Gupta RK, Oliveira TD, et al. The biological and clinical significance of emerging SARS-CoV-2 variants. Nat Rev Genet. 2021;22:757–73. doi: 10.1038/s41576-021-00408-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Corona G, Baldi E, Isidori AM, Paoli D, Pallotti F, et al. SARS-CoV-2 infection, male fertility and sperm cryopreservation: a position statement of the Italian Society of Andrology and Sexual Medicine (SIAMS) (SocietàItaliana di Andrologia e Medicina della Sessualità) J Endocrinol Invest. 2020;43:1153–7. doi: 10.1007/s40618-020-01290-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Esteves SC, Lombardo F, Garrido N, Alvarez J, Zini A, et al. SARS-CoV-2 pandemic and repercussions for male infertility patients: a proposal for the individualized provision of andrological services. Andrology. 2021;9:10–8. doi: 10.1111/andr.12809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Anifandis G, Tempest HG, Oliva R, Swanson GM, Simopoulou M, et al. COVID-19 and human reproduction: a pandemic that packs a serious punch. Syst Biol Reprod Med. 2021;67:3–23. doi: 10.1080/19396368.2020.1855271. [DOI] [PubMed] [Google Scholar]
- 52.Yakass MB, Quaye O, Woodward BJ. Risks of SARS-CoV-2 on male reproductive health and the practice of semen analysis and cryopreservation. Future Microbiol. 2020;15:1415–8. doi: 10.2217/fmb-2020-0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Anifandis G, Taylor TH, Messini CI, Chatzimeletiou K, Daponte A, et al. The impact of SARS-CoV-2 on sperm cryostorage, theoretical or real risk? Medicina (Kaunas) 2021;57:946. doi: 10.3390/medicina57090946. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Funnel diagram of semen parameters before and after COVID-19 infection. (a) Semen volume, (b) sperm concentration, (c) total sperm count, (d) progressive motility, (e) sperm motility, and (f) sperm morphology.
Funnel diagram of sex-related hormone before and after COVID-19 infection. (a) Semen volume, (b) sperm concentration, (c) total sperm count, (d) progressive motility, (e) sperm motility, and (f) sperm morphology.
Sensitivity analysis of semen parameters of COVID-19 before and after infection. (a) FSH, (b) LH, and (c) TT. FSH: follicle-stimulating hormone; LH: luteinizing hormone; TT: testosterone.