Abstract
Noninvasive blood pressure measurement devices have gained popularity in recent years as an alternative to radiotelemetry and other invasive blood pressure measurement techniques. While many factors must be considered when choosing a measurement method, specific variables should be evaluated when using a tail-cuff blood pressure technique. Rodents have complex and dynamic thermal biology processes that involve fluctuating vasomotor tone of the tail. This and other factors that affect vascular tone, such as the autonomic response to stress, significantly affect peripheral blood flow. Awareness and consideration of thermoregulatory states and vasomotor tone can increase success and decrease variability when measuring blood pressure measurements using a tail-cuff measurement technique.
Abbreviations: AHA, American Heart Association; BP, blood pressure; HR, heart rate; MAP, mean arterial blood pressure; TNZ, thermoneutral zone
Introduction
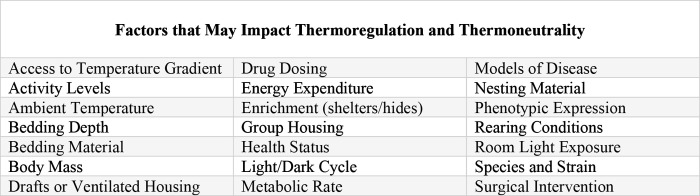
The complex and dynamic thermal biology of rodents and the effects of thermoregulation on their physiology have been well documented, especially in recent years.2,6,8,15,18,28,29,33,37 Thermoregulatory processes affecting animal behavior and physiology have been a subject of interest across a multitude of topics ranging from animal welfare to pharmacologic and toxicologic studies. The rodent thermoneutral zone (TNZ) is defined as the range of temperatures at which the animal’s metabolic heat production is at equilibrium with evaporative heat loss to the environment.3,18 TNZ is often incorrectly assumed to be a static range, with thermoregulatory responses similar across experimental conditions. In reality, the specific temperature range of the TNZ is affected by a wide range of variables, including experimental parameters. Many environmental factors influence an individual animal’s TNZ, such as rearing environment, caging conditions, bedding type and depth, environmental room parameters, air exchange rates, light/dark cycles, and others (Figure 1).6,14,15,18,29 Despite the ability of rodents to survive in a wide range of temperatures, their TNZs are surprisingly narrow due in part to a high metabolic rate and large surface to volume ratio.17 Unlike larger endotherms, rodents do not have stable in core temperatures even when they are in thermoneutral states. Rodents routinely exhibit rapid and continuous fluctuations in their core body temperatures.3,14,15,18 As a result, body temperature and thermoneutrality should be viewed as dynamic conditional states based on consideration of environmental conditions relative to the TNZ. Thermoregulatory responses can alter experimental results and affect study reproducibility,8,18 and therefore rodent thermal biology is an important study variable that should be understood and acknowledged.
Figure 1.
A multitude of factors related to housing status, the animal’s ability to perform natural thermoregulatory behaviors, and study protocols can affect an individual animal’s thermoregulatory processes and thermoneutral zone.14,15,18,29
Temperature regulation and thermoregulatory equilibrium are maintained by a combination of behavioral and autonomic responses. Thermoregulatory behaviors typically precede any long-lasting physiologic responses to increase body heat, and include core behaviors such as nest building, social huddling, increased activity, and moving toward a warmer environment.3,14,15,17 Physiologic thermoregulation includes autonomic responses that most significantly involve changes in peripheral vasomotor tone and metabolic heat production (thermogenesis).18,28 Heat loss primarily occurs in areas with a high surface area to volume ratio, namely the legs and tail.8,18,28 When exposed to cold, peripheral blood flow rapidly decreases via vasoconstriction in the extremities to reduce heat loss.18 Laser Doppler imaging and infrared thermography have been used to validate the relationship between peripheral blood flow and skin temperature. An increase in skin surface temperature indicates greater vasomotor tone, consistent with increased blood flow to the underlying tissue; the inverse also occurs.4,8,29,36 This relationship allows tail temperature to be used as a simple indicator of a rodent’s thermoregulatory status.
The dynamic thermal biology of mice and rats also affects experimental results.8,18 As a result, a more comprehensive understanding of rodent thermoregulation can improve study reproducibility across multiple disciplines, particularly those affected by metabolic conditions or blood flow. A common example is the use of noninvasive blood pressure measurement devices to indirectly measure systolic arterial blood pressure10 (referred to from this point forward as BP). Noninvasive, indirect monitoring has gained popularity in recent years as an alternative to radiotelemetry and other invasive measurement techniques. Due to its frequent use and the need for reproducibility, the American Heart Association (AHA) published recommendations to aid in the selection of optimal BP measurement methods for research animals.10,20 Direct BP measurements, such as radiotelemetry or fluid-filled catheters, have historically been the standard method of monitoring BP in rodents.10,20 The use of indirect methods, most commonly a variety of tail-cuff techniques, have been used more frequently over the last decade due to ease of use and overall cost-effectiveness as compared with direct BP methods, which often require surgery for implementation. While radiotelemetry remains the gold standard for systolic BP measurements in some situations, the AHA recommends tail-cuff techniques over direct BP measurements for their advantages in high-throughput experimental protocols and when quantifying changes in BP over time.10,20 However, the accuracy and use of different tail-cuff methods can vary significantly between tail-cuff and telemetry measurements, leading to discrepant findings with different protocols.7,9,11,17,38,39 This overview aims to increase awareness and consideration of thermoregulatory states when using tail-cuff BP methods in an effort to control these variables. Controlling the effects of vasomotor tone, restraint, and temperature on BP and peripheral blood flow can limit their unintended effects on tail-cuff measurements and improve reproducibility.
Thermoregulatory Impacts on Blood Pressure and Tail-Cuff Techniques
Thermoregulatory responses have been well studied over the last several decades primarily in rats, particularly with regard to the role of the tail in heat dissipation.8,18,23,28,40 In rats, the tail accounts for up to 7% of total body surface area and, due to this large surface area and arteriovenous anastomoses, it has the capacity to dissipate up to 25% of total heat production when fully vasodilated.17,39 Similar regulation occurs in mice due to structural parallels and comparable surface area ratios, though to a lesser extent.32
Several tail-cuff BP measurement systems are commercially available, and are most commonly based on volume-pressure recording, pulse-based, or flow-based technologies.10 While accuracy and study suitability vary among the 3 types of systems, they each can be significantly affected by the thermoregulatory status of the animal. Regardless of the tail-cuff method technology or tail-cuff system manufacturer, the cuff must be able to detect enough tail blood flow to measure BP accurately. Therefore, any physiologic state leading to a decrease in vasomotor tone should be avoided, as tail vasoconstriction will limit the ability of a tail-cuff ability to accurately measure BP. Maintaining the animal within the TNZ ensures that thermoregulation will not alter vasomotor tone and thereby interfere with the accuracy of the measurement.
Three factors should be established to determine whether a rodent is within the TNZ: 1) degree of fluctuation in tail skin temperature, 2) closeness of tail skin temperature to median of the full range of that fluctuation, and 3) a negative correlation between core temperature and tail skin temperature. In general, skin surface temperature is not a good measure of body temperature, although it is significantly more responsive to changes in thermal environment and physiologic status than is core body temperature.28,31 Monitoring tail skin temperature is a simple and inexpensive way of determining whether an animal remains in TNZ range under experimental conditions.8,29 Changes in tail vasomotor tone, which leads to a change in tail temperature, typically precedes an effect on body temperature and heat loss by several minutes.41 This relationship between temperature and vasomotor tone is often applied in protocols that require vasodilation. For example, protocols that require tail vein injections often use a warming method to promote vasodilation of the tail vein for visual vein identification and ease of needle positioning. Many methods of warming focus on maintaining specific core body temperatures and do not include thermoregulatory monitoring capabilities, leading to the development of specialized devices that aim to warm the skin to a target temperature of 32 to 35 °C prior to tail vein injections. This temperature range is associated with a consistent and adequate degree of vasodilation.40 For similar reasons, this is also the ideal target tail skin temperature range during tail-cuff BP monitoring.7 A tail temperature within the 32 to 35 °C range provides consistent vasomotor tone that can be replicated across multiple animals.
Warming mice to a tail temperature of 35 °C does not appear to cause any additional stress factors that affect BP.30,38 Furthermore, raising the tail skin temperature by as much as 5 °C does not appear to significantly increase core body temperature.30,38 This is due in part to the dynamic effects of thermoregulation on vasomotor tone. The relationship between temperature, blood flow, BP, heart rate (HR), and other cardiovascular effects is complex. Thermographic recordings show fluctuations in tail temperature indicating that when rats are in the TNZ, the vasomotor tone of the tail frequently transitions between vasoconstriction and vasodilation.8,29 This fluctuation is also correlated with changes in mean arterial pressure (MAP) and HR, suggesting a linear relationship based on ambient temperature.4,8,33-35 During a state of vasoconstriction, MAP and HR show an associated increase. Similarly, states of vasodilation are associated with a decrease in MAP and HR.4,8 These changes in MAP and HR are likely to be trivial with respect to BP measurements, provided that the animal remains within the TNZ despite the fluctuations in vasomotor tone, which occur at a rate of 2 to 7 per hour.8 However, BP values may be affected if the animal remains outside of the TNZ and in a state of significant vasoconstriction or vasodilation. Temperature monitoring and control is important for both mouse and rat studies, but due to their greater surface-area-to-volume ratio and rapid metabolic rate, mice are more likely to exhibit temperature-related changes in MAP as compared with rats.33 Controlling temperature by providing thermal support and monitoring tail skin temperature to maintain it in the range of 32 to 35 °C can improve consistency and limit unintended thermoregulatory effects on MAP or HR.
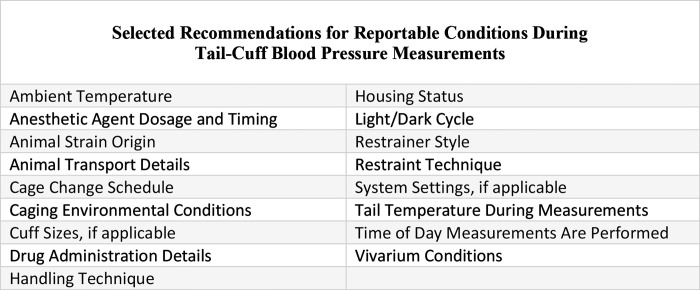
Given the narrow range of the rodent TNZ and the significance of thermoregulatory responses, vasomotor activity can be expected to contribute to variation in tail-cuff BP measurements. Ensuring that the animal is maintained within TNZ limits with stable and sufficient vasomotor tone, indicated by a tail temperature of 32 to 35 °C, will improve repeatability. However, temperature and thermal maintenance during tail-cuff BP measurements is not consistently reported in the literature,7,9,11,17,38,39 which likely contributes to reported differences in accuracy of tail-cuff methods as compared with telemetry.11,17,38,39 The use of reporting guidelines, such as the ARRIVE Guidelines (Animals in Research: Reporting In Vivo Experiments),19 could improve both consistency and reproducibility among similar scientific studies. Comprehensive reporting provides a more complete picture of study conditions and methodologies.1,19 Describing the details of the methodology and reporting thermoregulatory variables, particularly those involving the tail, are important, yet often overlooked, components of accurate and reproducible tail-cuff BP measurement (Figure 2). Without this information, accurate comparisons of the results of individual tail-cuff BP studies are likely not feasible. Monitoring and reporting of both environmental and physiologic conditions are equally important and will increase data accuracy and reproducibility.
Figure 2.
Several conditions should be documented based on ARRIVE and ACLAM reporting guidelines.1,19 Identifying and reporting these potential variables, among others, can help to increase reproducibility among tail-cuff blood pressure studies.
Effects of Restraint and Handling Stress on Thermoregulation
When measuring BP in conscious animals, possible stress effects caused by tail-cuff techniques are a topic of concern.38 Much like the response to cold, peripheral vasoconstriction is an autonomic response to a stressor.4 Restraint stress-induced hypothermia can also affect tail-cuff BP measurements.13,21,23,24 Restraint and stress can both affect the accuracy of tail-cuff BP measurements by causing a reduction in tail blood flow.
Handling stress is a major concern for noninvasive BP measurements, namely the possibility of elevated BP values related to restraint stress.12,21,24,38 Animal handling (for example, the act of lifting the animal from the cage) causes a greater effect on BP than does physical restraint (for example, time spent in an animal holder). The act of moving a mouse cage causes stress responses similar to those of handling individual animals, including an increase in BP and HR.38 Careful attention to animal handling practices before making BP measurements may affect data collected during a session. Differences in animal handling has been cited as a possible variable during tail-cuff BP measurements.10 The most common method of picking up mice and rats involves lifting from the tail base prior to supporting the body; this procedure causes anxiety in both mice and rats.16 Moving mice by using a container to lift them causes less anxiety and stress than does tail handling.16 Many tail-cuff BP measurement devices include animal holders that can be used as handling tunnels to reduce handling stress prior to a measurement session. Regardless of the handling method used, consistency is key to avoid the introduction of unintended variables and reporting the handling method is important for study reproducibility.
The effects of restraint stress are also model and species specific. When first restrained, most commonly used rat strains (Sprague–Dawley, Long-Evans, etc.) exhibit an initial rapid increase in body temperature and a reduction in tail temperature, followed by an overall decrease in body temperature.2 The range of limits of normothermia is significantly lower in restrained rats as compared with unrestrained rats, indicating that restraint can reduce the rat’s ability to thermoregulate in certain ambient conditions.2 Restraint-induced hypothermia has also been well documented in both mice and rats and has been attributed to anxiety, increased peripheral vasodilation, changes in ambient temperature and a combination of other factors.13,21,23,24 Infrared thermography has been used to monitor temperature changes in animals after stress.27 In rodents, stressors such as handling or restraint increase the surface temperature of the head and back and decrease in the temperature of the tail. This indicates that restraint induces peripheral vasoconstriction.9,13,37 Because tail blood flow is a critical component of noninvasive BP measurements, these effects should be mitigated to obtain accurate readings. Monitoring and reporting tail temperatures is important because significant differences in recorded tail temperatures can explain differences in BP.
The AHA recommends tail-cuff techniques for high-throughput experimental protocols,20 and many studies use tail-cuff methods to measure BP in multiple animals simultaneously. BP measured in the presence of an animal’s littermate or cage-mate reduces the necessary time for stabilization (acclimation) by up to several minutes.5 A reduction in animal stress can promote accurate and reproducible results by reducing animal movement artifacts and increasing the number of usable measurements.
Restraint-related hypothermia can cause significant variation in tail-cuff BP measurements, especially because the effects vary significantly between strains and individual animals, based on the degree of immobilization, time of day, animal age, and animal sex. A much greater hypothermic response occurs in female mice and rats compared with male animals of the same strain.9,23 While the exact mechanism of restraint-related hypothermia is not fully understood, a hypothesis is that heat loss may be accelerated through dissipation caused by contact with restrainer walls.23 Prewarming animal holders reduces the temperature decrease associated with restraint-related hypothermia,38 and supplemental heat throughout the duration of restraint can mitigate effects on thermoregulatory status caused by restraint and handling. Prewarming of the animal holder and providing supplemental heat can reduce the likelihood of hypothermic effects that may impact tail-cuff BP measurements. In addition, differences in temperature related to time of day indicates a relationship to circadian rhythms. Although phase-temperature relationships can be affected by several factors, dark phases are generally associated with higher basal temperatures than are light phases,15,18,29 which may offset the heat loss related to heat dissipation through contact with the restrainer walls. Taking tail-cuff BP measurements at the same time each day will control for the potential effects of phase. Furthermore, reporting the time of day at which measurements are made as well as other environmental factors known to affect thermoregulation will improve reproducibility between studies.
Effects of Anesthetic Agents on Thermoregulation
General anesthetics have significant effects on animal physiology, including body temperature. Most common anesthetic agents affect thermoregulation via a dose-dependent suppression of hypothalamic function, lowering the temperature at which the hypothalamus responds to hypothermia by several degrees.22,25,30 This reduction has 3 main effects: heat loss is increased, detection of cold is decreased, and thermogenic corrective actions are suppressed.18,30 Immediately after the onset of anesthesia, an initial period of increased vasodilation redistributes heat from the core to the periphery.22,25,30 This redistribution rapidly increases the rate of heat loss and is soon followed by an overall fall in core body temperature until hypothalamic function prompts vasoconstriction in the periphery.22,25,30 The resulting decrease in core body temperature can be as much as 8 °C in as little as 30 min after the onset of anesthesia in mice anesthetized with isoflurane at room temperature.12 Thus, standard practice includes the use of heating pads or other external heat sources to mitigate anesthetic-induced hypothermia.
Traditional warming methods measure rectal temperatures as a proxy for core body temperature. While this substitute is typically sufficient for monitoring for the occurrence of hypothermia during surgical procedures, it does not provide sufficient information about the animal’s thermoregulatory status. With regard to noninvasive BP measurements, core body temperatures are less informative than skin temperatures. In addition, any surgical or pharmacological actions that affect sympathetic responses will reduce peripheral blood flow, resulting in a further decrease in tail skin temperature.4 Because of these effects, a normal core body temperature does not necessarily correspond with the tail temperature range expected in a conscious animal maintained within the TNZ. Skin temperature can vary significantly from core body temperature and is often several degrees lower, especially when during anesthesia. Therefore, due to the inhibition of thermoregulation during anesthesia, particular attention should be afforded to thermal support and the resulting tail temperature during tail-cuff BP measurements. Although both mice and rats require thermal support during anesthetic administration, mice may require more warming to counter the effects of anesthetic-related hypothermia than do rats, in part because the small body mass to surface area ratio of mice accelerates the rate of heat loss during anesthesia.18
In addition to thermoregulatory effects, general anesthesia has well documented cardiovascular effects.22,25,26,30,33 Injected and inhalant anesthetics can affect both thermoregulation and cardiovascular status, but the route, significance, and implications of the effects vary by agent and dosage.8,12 As an example, injectable agents such as a ketamine-xylazine combination tend to cause a rapid and significant decrease in systolic and diastolic BP after the onset of anesthesia, and the effects persist well after anesthetic recovery.26 The AHA has recommended avoiding anesthesia during BP measurement due to the cardiovascular differences between conscious and anesthetized animals.20
While the effects of general anesthesia require consideration for all BP measurement techniques, this is particularly true for tail-cuff methods. If anesthetics must be used for BP measurement, additional steps can be taken to promote accurate measurement and reproducible results. Achieving and maintaining thermoneutrality by applying targeted heat and increasing the tail skin temperature will assist in vasodilation and counter the thermoregulatory effects of the anesthetic.8 Prewarming animals in a heated chamber prior to the onset of anesthesia can also raise overall skin temperature and delay the onset of hypothermia during general anesthesia.31 Using targeted warming to raise skin temperature by 4 to 5 °C reduces the gradient between core and peripheral temperatures, resulting in less overall heat loss.30 A prewarming step can be incorporated into tail-cuff BP protocols using anesthetized animals in order to lessen the effects of the anesthetic agent on blood flow. As in conscious animals, a tail temperature of 32 to 35 °C indicates tail vein vasodilation in anesthetized animals. Stabilizing the vasomotor tone from induction through anesthetic maintenance will help maintain consistent blood flow and accurate data during the tail-cuff measurement session.
Conclusion
A basic understanding of rodent thermoregulation is beneficial for any groups using noninvasive tail-cuff BP measurement. Complex thermoregulatory processes in rodents can introduce unintended variables in tail-cuff BP measurements. Increased awareness and understanding of these processes and their possible effects can improve the accuracy and reproducibility of BP measurements. Thermoregulatory state, vasomotor tone, and peripheral blood flow should be monitored, reported, and controlled when feasible. Reporting and assessing factors that affect body temperature and thermoregulation are critical to forming conclusions and collecting accurate and reproducible data from tail-cuff BP measurement.
References
- 1.ACLAM Position statement on reproducibility. 2016. J Am Assoc Lab Anim Sci 55:824–825. [PMC free article] [PubMed] [Google Scholar]
- 2.Aydin C, Grace CE, Gordon CJ. 2011. Effect of physical restraint on the limits of thermoregulation in telemetered rats. Exp Physiol 96:1218–1227. 10.1113/expphysiol.2011.060301. [DOI] [PubMed] [Google Scholar]
- 3.Bligh J, Johnson KG. 1973. Glossary of terms for thermal physiology. J Appl Physiol 35:941–961. 10.1152/jappl.1973.35.6.941. [DOI] [PubMed] [Google Scholar]
- 4.Carrive P, Churyukanov M, Le Bars D. 2011. A reassessment of stress-induced “analgesia” in the rat using an unbiased method. Pain 152:676–686. 10.1016/j.pain.2010.12.019. [DOI] [PubMed] [Google Scholar]
- 5.Caszo B, Awang A, Gnanou J, Singh H. 2015. The presence of a littermate during measurement of blood pressure reduces the acclimatization time in conscious naive rats. Asian J Pharm Clin Res 8:151–153. [Google Scholar]
- 6.Chappell MA, Holsclaw DS. 1984. Effects of wind on thermoregulation and energy balance in deer mice (Peromyscus maniculatus). J Comp Physiol B 154:619–625. 10.1007/BF00684416. [DOI] [Google Scholar]
- 7.Daugherty A, Rateri D, Hong L, Balakrishnan A. 2009. Measuring blood pressure in mice using volume pressure recording, a tail-cuff method. J Vis Exp 27:e1291. 10.3791/1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.El Bitar N, Pollin B, Karroum E, Pincedé I, Mouraux A, Le Bars D. 2014. Thermoregulatory vasomotor tone of the rat tail and paws in thermoneutral conditions and its impact on a behavioral model of acute pain. J Neurophysiol 112:2185–2198. 10.1152/jn.00721.2013. [DOI] [PubMed] [Google Scholar]
- 9.Faraji J, Metz GAS. 2020. Infrared Thermography Reveals Sex-Specific Responses to Stress in Mice. Front Behav Neurosci 14:79. 10.3389/fnbeh.2020.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feng M, Whitesall S, Zhang Y, Beibel M, D’Alecy L, DiPetrillo K. 2008. Validation of volume-pressure recording tail-cuff blood pressure measurements. Am J Hypertens 21:1288–1291. 10.1038/ajh.2008.301. [DOI] [PubMed] [Google Scholar]
- 11.Fraser TB, Turner SW, Mangos GJ, Ludbrook J, Whitworth JA. 2001. Comparison of telemetric and tail-cuff blood pressure monitoring in adrenocorticotrophic hormone-treated rats. Clin Exp Pharmacol Physiol 28:831–835. 10.1046/j.1440-1681.2001.03531.x. [DOI] [PubMed] [Google Scholar]
- 12.Fueger BJ, Czernin J, Hildebrandt I, Tran C, Halpern BS, Stout D, Phelps ME, Weber WA. 2006. Impact of animal handling on the results of 18F-FDG PET studies in mice. J Nucl Med 47:999–1006. [PubMed] [Google Scholar]
- 13.Gjendal K, Franco NH, Ottesen JL, Sørensen DB, Olsson IAS. 2018. Eye, body or tail? Thermography as a measure of stress in mice. Physiol Behav 196:135–143. 10.1016/j.physbeh.2018.08.022. [DOI] [PubMed] [Google Scholar]
- 14.Gordon CJ. 2004. Effect of cage bedding on temperature regulation and metabolism of group-housed female mice. Comp Med 54:63–68. [PubMed] [Google Scholar]
- 15.Gordon CJ. 2017. The mouse thermoregulatory system: Its impact on translating biomedical data to humans. Physiol Behav 179:55–66. 10.1016/j.physbeh.2017.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gouveia K, Hurst JL. 2013. Reducing mouse anxiety during handling: effect of experience with handling tunnels. PLoS One 8:e66401. 10.1371/journal.pone.0066401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haggerty CM, Mattingly AC, Gong MC, Su W, Daugherty A, Fornwalt BK. 2015. Telemetric blood pressure assessment in angiotensin II-infused ApoE−/−mice: 28 day natural history and comparison to tail-cuff measurements. PLoS One 10:e0130723. 10.1371/journal.pone.0130723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hankenson FC, Marx JO, Gordon CJ, David JM. 2018. Effects of rodent thermoregulation on animal models in the research environment. Comp Med 68:425–438. 10.30802/AALAS-CM-18-000049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. 2010. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol 8:e1000412. 10.1371/journal.pbio.1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kurtz TW, Griffin KA, Bidani AK, Davisson RL, Hall JE. 2005. Recommendations for blood pressure measurement in animals: summary of an AHA scientific statement from the Council on High Blood Pressure Research, Professional and Public Education Subcommittee. Arterioscler Thromb Vasc Biol 25:478–479. 10.1161/01.ATV.0000153088.15433.8f. [DOI] [PubMed] [Google Scholar]
- 21.Johnson EA, Sharp DS, Miller DB. 2000. Restraint as a stressor in mice: Against the dopaminergic neurotoxicity of D-MDMA, low body weight mitigates restraint-induced hypothermia and consequent neuroprotection. Brain Res 875:107–118. 10.1016/S0006-8993(00)02601-9. [DOI] [PubMed] [Google Scholar]
- 22.Matsukawa T, Sessler DI, Sessler AM, Schroeder M, Ozaki M, Kurz A, Cheng C. 1995. Heat flow and distribution during induction of general anesthesia. Anesthesiology 82:662–673. 10.1097/00000542-199503000-00008. [DOI] [PubMed] [Google Scholar]
- 23.McGivern RF, Zuloaga DG, Handa RJ. 2009. Sex differences in stress-induced hyperthermia in rats: restraint versus confinement. Physiol Behav 98:416–420. 10.1016/j.physbeh.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meijer MK, Spruijt BM, Van Zutphen LFM, Baumans V. 2006. Effect of restraint and injection methods on heart rate and body temperature in mice. Lab Anim 40:382–391. 10.1258/002367706778476370. [DOI] [PubMed] [Google Scholar]
- 25.Napolitano CA, Raatikainen MJ, Martens JR, Dennis DM. 1996. Effects of intravenous anesthetics on atrial wavelength and atrioventricular nodal conduction in guinea pig heart: potential antidysrhythmic properties and clinical implications. Anesthesiology 85:393–402. 10.1097/00000542-199608000-00022. [DOI] [PubMed] [Google Scholar]
- 26.Picollo C, Serra AJ, Levy RF, Antonio EL, dos Santos L, Tucci PJF. 2012. Hemodynamic and thermoregulatory effects of xylazine-ketamine mixture persist even after the anesthetic stage in rats. Arq Bras Med Vet Zootec 64:860–864. 10.1590/S0102-09352012000400011. [DOI] [Google Scholar]
- 27.Rekant SI, Lyons MA, Pacheco JA, Arzt J, Rodriguez LL. 2016. Veterinary applications of infrared thermography. Am J Vet Res 77:98–107. 10.2460/ajvr.77.1.98. [DOI] [PubMed] [Google Scholar]
- 28.Romanovsky AA. 2014. Skin temperature: its role in thermoregulation. Acta Physiol (Oxf) 210:498–507. 10.1111/apha.12231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Romanovsky AA, Ivanov AI, Shimansky YP. 2002. Selected contribution: ambient temperature for experiments in rats: a new method for determining the zone of thermal neutrality. J Appl Physiol 92:2667–2679. 10.1152/japplphysiol.01173.2001. [DOI] [PubMed] [Google Scholar]
- 30.Rufiange M, Leung VSY, Simpson K, Pang DSJ. 2020. Pre-warming before general anesthesia with isoflurane delays the onset of hypothermia in rats. PLoS One 15:e0219722. 10.1371/journal.pone.0219722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schuster CJ, Pang DSJ. 2018. Forced-air pre-warming prevents peri-anaesthetic hypothermia and shortens recovery in adult rats. Lab Anim 52:142–151. 10.1177/0023677217712539. [DOI] [PubMed] [Google Scholar]
- 32.Škop V, Liu N, Guo J, Gavrilova O, Reitman ML. 2020. The contribution of the mouse tail to thermoregulation is modest. Am J Physiol Endocrinol Metab 319:E438–E446. 10.1152/ajpendo.00133.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Swoap SJ, Overton JM, Garber G. 2004. Effect of ambient temperature on cardiovascular parameters in rats and mice: a comparative approach. Am J Physiol Regul Integr Comp Physiol 287:R391–R396. 10.1152/ajpregu.00731.2003. [DOI] [PubMed] [Google Scholar]
- 34.Swoap SJ, Li C, Wess J, Parsons AD, Williams TD, Overton JM. 2008. Vagal tone dominates autonomic control of mouse heart rate at thermoneutrality. Am J Physiol Heart Circ Physiol 294:H1581–H1588. 10.1152/ajpheart.01000.2007. [DOI] [PubMed] [Google Scholar]
- 35.Vainer BG, Baranov VI, Vergunov EG. 2014. Infrared thermography as applied to the studies of cardiovascular system in rats. In: Proceedings of QIRT-2014 Conference, pp. 7-11. 10.21611/qirt.2014.157 [DOI]
- 36.Vanhoutte G, Verhoye M, Raman E, Roberts M, Van der Linden A. 2002. In-vivo non-invasive study of the thermoregulatory function of the blood vessels in the rat tail using magnetic resonance angiography. NMR Biomed 15:263–269. 10.1002/nbm.768. [DOI] [PubMed] [Google Scholar]
- 37.Weitkamp J. 2020. “Effect of tickling and gentling on eye and tail temperature of laboratory rats during manual restraint, using infrared thermography.” Master’s thesis.
- 38.Wilde E, Aubdool AA, Thakore P, Baldissera L, Jr, Alawi KM, Keeble J, Nandi M, Brain SD. 2017. Tail-cuff technique and its influence on central blood pressure in the mouse. J Am Heart Assoc 6:e005204. 10.1161/JAHA.116.005204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Whitesall SE, Hoff JB, Vollmer AP, D’Alecy LG. 2004. Comparison of simultaneous measurement of mouse systolic arterial blood pressure by radiotelemetry and tail-cuff methods. Am J Physiol Heart Circ Physiol 286:H2408–H2415. 10.1152/ajpheart.01089.2003. [DOI] [PubMed] [Google Scholar]
- 40.Yano J, Lilly EA, Noverr MC, Fidel PL. 2020. A Contemporary Warming/Restraining Device for Efficient Tail Vein Injections in a Murine Fungal Sepsis Model. J Vis Exp 165:e61961. 10.3791/61961. [DOI] [PubMed] [Google Scholar]
- 41.Young AA, Dawson NJ. 1982. Evidence for on–off control of heat dissipation from the tail of the rat. Can J Physiol Pharmacol 60:392–398. 10.1139/y82-057. [DOI] [PubMed] [Google Scholar]