Abstract
Scleromyxedema is a rare but important mucinosis disorder of the skin that is presented with dermatological manifestations such as waxy papules, diffuse induration, and nondermatologic involvements like neurological and renal disorders. We report a case series of the data regarding the characteristics and treatment of 14 patients diagnosed with scleromyxedema and their follow-up. Patients entered the study based on scleromyxedema diagnosis criteria. Comorbidities were also recorded to evaluate their effect on the treatment process. Clinicopathological and laboratory findings and responses to their treatment were evaluated separately. There was a significant improvement after administering intravenous immunoglobulin (IVIG). Despite the lack of a definite treatment for this condition, the present study shows that the application of IVIG can improve both cutaneous and systemic symptoms. Younger patients, in particular, responded significantly to the use of IVIG. More studies are required to investigate the potential efficacy of IVIG in the treatment of scleromyxedema.
Keywords: Scleromyxedema, Treatment, Intravenous immunoglobulin, Monoclonal gammopathy
Introduction
Scleromyxedema is a progressive mucinosis disease of the skin that can lead to various systemic involvements. With the exact pathology still unknown, treatment with intravenous immunoglobulin (IVIG) has been indicated to be efficient in controlling the progression of the disease. In this retrospective cross-sectional study, we studied the clinicopathological data, laboratory findings, and treatment of 14 patients with scleromyxedema [1, 2, 3].
Case Series
From January 2008 to July 2020, data of patients with a definite diagnosis of scleromyxedema referred to Razi Skin Hospital, Tehran University of Medical Sciences, Tehran, Iran, were evaluated. The study was also approved by the Ethics Committee of the Tehran University of Medical Sciences. The diagnostic criteria for scleromyxedema included generalized waxy papules, accompanied by mucin deposition, fibrosis, and fibroblast proliferation in histopathology [3]. Data concerning patients' demographics; clinical characteristics, such as the history of hypertension, diabetes mellitus, and thyroid problems; laboratory data, such as albumin and immunoglobulin levels; and treatments were extracted from their medical records. The treatment response was measured based on waxy papules' flattening, skin-thickness improvement, improved joint mobility, and reduced systemic symptoms.
A total of 14 patients (8 [57.1%] females, 6 [42.9%] males) with an average age of 73.10 ± 29.51 years were included. Six (42.9%) patients had monoclonal gammopathy at diagnosis, while two more were found during follow-up. Therefore, gammopathy was detected in 8 (57.2%) of the 14 patients. Diabetes (35.3%) and hypertension (29.4%) were the most common comorbidities, whereas 23.5% of patients had no comorbidity.
Concerning skin findings, symmetrical involvement of the face, trunk, and extremities was present in all patients; however, only one (9.1%) patient presented with skin lesions on the scalp. In 11 (78.6%) patients, lesions were predominantly red, whereas 3 (21.4%) patients had brown lesions. Reduced mouth and finger movements were detected in four (27.8%), and pruritus was noticed in five (35.8%) patients.
Regarding the extracutaneous involvements of scleromyxedema, physical weakness (50%), arthralgia (22%), renal disorders (11%), and rheumatological disorders (5%) were detected. The patients were followed for 62.4 ± 20.1 months. The most common treatment was high-dose IVIG (2 g/kg divided over 2–5 consecutive days) (71.4%), oral retinoids (14.3%), corticosteroids (7.1%), and melphalan (7.1%). IVIG was administered monthly to 40% of patients, while 40% and 20% were treated with IVIG every 2 or 3 months, respectively.
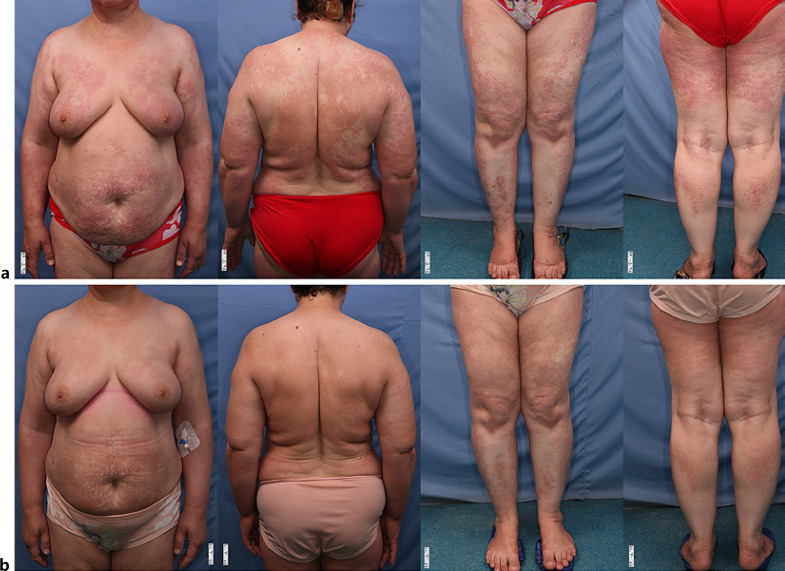
Regarding the treatment outcome, two of the patients showed a complete response (Fig. 1), seven experienced a partial response, two did not respond, as well as three patients died. The cause of death was ischemia (for one patient), lung involvement (for one patient), and encephalopathy (for another patient). Detailed characteristics of patients and treatment responses can be seen in Table 1.
Fig. 1.
The clinical picture of a 63-year-old woman with monoclonal gammopathy and scleromyxedema; at the diagnosis (a) 3 months after monthly administration of IVIG 2 g/kg (b).
Table 1.
Patients' characteristics and treatment response in the population study
| Improvement |
Total | p value | |||||
|---|---|---|---|---|---|---|---|
| complete | partial | without response | death | ||||
| Gender | |||||||
| Female | n (%) | 1 (12.5) | 6 (75) | 0 | 1 (12.5) | 8 (57.2) | 0.1 |
| Male | n (%) | 1 (16.7) | 1 (16.7) | 2 (33.3) | 2 (33.3) | 6 (42.8) | |
| Age | |||||||
| n (%) | 2 (11.8) | 7 (50) | 2 (11.8) | 3 (21.4) | 14 (100) | 0.03 | |
| Average age | 40.5 | 47.29 | 63.00 | 60.00 | 51.29 | ||
| Comorbidity | |||||||
| Hypertension | |||||||
| Yes | n (%) | 0 | 3 (60) | 1 (20) | 1 (20) | 5 (35.8) | 0.7 |
| No | n (%) | 2 (22.2) | 4 (44.4) | 1 (11.1) | 2 (22.2) | 9 (64.2) | |
| Diabetes | |||||||
| Yes | n (%) | 1 (16.7) | 2 (33.3) | 1 (16.7) | 2 (33.3) | 6 (42.8) | 0.7 |
| No | n (%) | 1 (12.5) | 5 (62.5) | 1 (12.5) | 1 (12.5) | 8 (57.2) | |
| Thyroid problem | |||||||
| Yes | n (%) | 0 | 0 | 1 (100) | 0 | 1 (7.2) | 0.09 |
| No | n (%) | 2 (15.4) | 7 (53.8%) | 1 (7.7) | 3 (23) | 13 (92.8) | |
| Monoclonal gammopathy | |||||||
| Yes | n (%) | 0 | 5 (62.5) | 1 (12.5) | 2 (25) | 8 (57.2) | 0.1 |
| No | n (%) | 2 (33.3) | 2 (33.3) | 1 (16.7) | 1 (16.7) | 6 (42.8) | |
| Treatment | |||||||
| IVIG | n (%) | 2 (20) | 4 (40) | 1 (10) | 3 (30) | 10 (71.4) | 0.8 |
| Corticosteroids | n (%) | 0 | 1 (100) | 0 | 0 | 1 (7.1) | |
| Retinoids | n (%) | 0 | 1 (50) | 1 (50) | 0 | 2 (14.3) | |
| Melphalan | n (%) | 0 | 1 (100) | 0 | 0 | 1 (7.1) | |
Scleromyxedema is a rare skin condition, and most of the previous studies include single case reports and series [4]. Rongioletti et al. [5, 6], who gathered the most significant number of patients from two manuscripts, showed that, similar to our findings, the onset of the disease took place in the fifth and sixth decades of life. Gender had no significant influence on the disease, and the most common extracutaneous manifestations were neurological (30%), rheumatological (25%), and cardiac (22%) [6]. Other authors, however, reported that the most common extracutaneous symptoms were related to the gastrointestinal tract and the pulmonary system [7, 8]. Previous reports about musculoskeletal and cardiac symptoms in patients with scleromyxedema are consistent with our findings in this study [8].
Discussion
Scleromyxedema is a rare progressive subtype of mucinosis, usually associated with systemic involvement and paraproteinemia [1]. The exact etiology of this disease remains unclear; however, it is speculated that unknown hematogenous factors stimulate the fibroblasts to increase mucin production and deposition in the skin [2, 9]. From a dermatological viewpoint, scleromyxedema manifests as waxy papules and diffuse induration from a dermatological perspective [3].
Different studies have reported variable levels of monoclonal gammopathy in patients with scleromyxedema; according to the Rongioletti et al. study, 90% of patients had gammopathy [6]. Meanwhile, in a recent systematic review, it was discovered that 72% of patients had the condition, while in the present study, it was detected in 57.2% of patients [10].
In a recently published systematic review, 85 patients with a mean age of 52 years and a male to female ratio of 1.16:1 were evaluated. Seventy-two percent of patients had a concomitant monoclonal gammopathy, whereas 45% had extracutaneous symptoms [10]. In terms of the treatments, high-dose IVIG (2 g/kg divided over 2–5 consecutive days) was the most frequent treatment with the highest efficacy on the skin (69%), systemic (63%), and paraproteinemia (75%) [8, 11, 12, 13]. The proposed mechanism of IVIG in scleromyxedema relates to its ability to eliminate immune complexes, inactivate antibodies, block Fc receptors, as well as immunoregulatory effects [14]. The most frequently administered treatments were thalidomide, autologous stem cell transplantation, alkylating agents, and systemic glucocorticoids [9, 15]. According to the prognosis of the disease, in the study of Rongioletti et al. [6], similar to the present study, 5 out of 21 patients died (23.8%), and 12 patients did not improve after different treatments. This finding is consistent with the poor prognosis of patients with scleromyxedema.
Our findings highlight that scleromyxedema is a chronic disorder, of unpredictable course, with many systemic effects, including neurological, renal, hematological, and rheumatological, rendering this condition of poor prognosis. Despite the lack of definitive treatment for this condition, the present study shows that IVIG can improve both the cutaneous and systemic symptoms. The study's retrospective nature and the study's low sample size are two of the main limitations of the present study due to the low prevalence of the disease. Further studies are necessary, preferably with a prospective design and a larger sample size, to better evaluate the best treatment option for scleromyxedema.
Conclusion
The present study highlights that scleromyxedema is a chronic and unpredictable disease with systemic effects, including neurologic, cardiovascular, and hematological, suggesting a guarded outlook. Despite the lack of a definitive treatment, our data indicate that IVIG can be used as a first-line treatment for scleromyxedema, given its relative effectiveness and safety.
Statement of Ethics
This study protocol was reviewed and approved by the Ethics Committee of Tehran University of Medical Sciences. Approval number: IR.TUMS.MEDICINE.REC.1399.100. Written informed consent was obtained from participants for publication of the details of their medical case and any accompanying images. Written informed consent was obtained from the next of kin of the patients who died for publication of the details of their medical case and any accompanying images.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
No funding received.
Author Contributions
The authors' contributions are in this list order: conceptualization: Hamidreza Mahmoudi; data creation: Abtin Ansari; formal analysis: Abtin Ansari; funding acquisition: none; investigation: Abtin Ansari, Maryam Daneshpazhooh, and Leila Mahmoudi; methodology: Kambiz Kamyab; project administration: Hamidreza Mahmoudi and Zahra Saffarian; resources: Zohre Erfani and Abtin Ansari; software: Zohre Erfani; supervision: Hamidreza Mahmoudi; validation: Kambiz Kamyab; visualization: Zohre Erfani; writing: Zohre Erfani.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.
Acknowledgments
We acknowledge that all the authors have only academic positions and these positions are nongovernmental.
References
- 1.Mehta V, Balachandran C, Rao R. Arndt Gottron scleromyxedema: successful response to treatment with steroid minipulse and methotrexate. Indian J Dermatol. 2009;54((2)):193. doi: 10.4103/0019-5154.53183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mohammadi S, Khalili M, Mohebbi A, Aflatoonian M, Badakhsh H. Cutis verticis gyrata and leonine face in a patient with Darier disease: a case report and review of the literature. J Pakistan Assoc Dermatol. 2018;28((1)):114–6. [Google Scholar]
- 3.Rongioletti F, Rebora A. Updated classification of papular mucinosis, lichen myxedematosus, and scleromyxedema. J Am Acad Dermatol. 2001;44((2)):273–81. doi: 10.1067/mjd.2001.111630. [DOI] [PubMed] [Google Scholar]
- 4.Javinani A, Gharibdoost F, Jamshidi A, Kavosi H. Scleromyxedema associated with ankylosing spondylitis treated successfully with cyclosporine and high dose corticosteroids. Rheum Res. 2017;2((4)):145–9. [Google Scholar]
- 5.Rongioletti F, Merlo G, Carli C, Cribier B, Metze D, Calonje E, et al. Histopathologic characteristics of scleromyxedema: a study of a series of 34 cases. J Am Acad Dermatol. 2016;74((6)):1194–200. doi: 10.1016/j.jaad.2015.12.021. [DOI] [PubMed] [Google Scholar]
- 6.Rongioletti F, Merlo G, Cinotti E, Fausti V, Cozzani E, Cribier B, et al. Scleromyxedema: a multicenter study of characteristics, comorbidities, course, and therapy in 30 patients. J Am Acad Dermatol. 2013;69((1)):66–72. doi: 10.1016/j.jaad.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 7.Dinneen AM, Dicken CH. Scleromyxedema. J Am Acad Dermatol. 1995;33((1)):37–43. doi: 10.1016/0190-9622(95)90007-1. [DOI] [PubMed] [Google Scholar]
- 8.Blum M, Wigley FM, Hummers LK. Scleromyxedema: a case series highlighting long-term outcomes of treatment with intravenous immunoglobulin (IVIG) Medicine. 2008;87((1)):10–20. doi: 10.1097/MD.0b013e3181630835. [DOI] [PubMed] [Google Scholar]
- 9.Boin F, Hummers LK. Scleroderma-like fibrosing disorders. Rheum Dis Clin North Am. 2008;34((1)):199–220. doi: 10.1016/j.rdc.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haber R, Bachour J, El Gemayel M. Scleromyxedema treatment: a systematic review and update. Int J Dermatol. 2020;59((10)):1191–201. doi: 10.1111/ijd.14888. [DOI] [PubMed] [Google Scholar]
- 11.Fleming KE, Virmani D, Sutton E, Langley R, Corbin J, Pasternak S, et al. Scleromyxedema and the dermato‐neuro syndrome: case report and review of the literature. J Cutan Pathol. 2012;39((5)):508–17. doi: 10.1111/j.1600-0560.2012.01882.x. [DOI] [PubMed] [Google Scholar]
- 12.Caudill L, Howell E. Scleromyxedema: a case clinically and histologically responsive to intravenous immunoglobulin. J Clin Aesthet Dermatol. 2014;7((5)):45. [PMC free article] [PubMed] [Google Scholar]
- 13.Gholam P, Hartmann M, Enk A. Arndt-Gottron scleromyxoedema: successful therapy with intravenous immunoglobulins. Br J Dermatol. 2007;157((5)):1058–60. doi: 10.1111/j.1365-2133.2007.08169.x. [DOI] [PubMed] [Google Scholar]
- 14.Samuelsson A, Towers TL, Ravetch JV. Anti-inflammatory activity of IVIG mediated through the inhibitory Fc receptor. Science. 2001;291((5503)):484–6. doi: 10.1126/science.291.5503.484. [DOI] [PubMed] [Google Scholar]
- 15.Mecoli C, Talbot C, Jr, Fava A, Cheadle C, Boin F, Wigley F, et al. Clinical and molecular phenotyping in scleromyxedema pre-and post-treatment with intravenous immunoglobulin. Arthritis Care Res. 2020 Jun;72((6)):761–7. doi: 10.1002/acr.23908. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.