Abstract
Clinical trials frequently include multiple end points that mature at different times. The initial report, typically based on the primary end point, may be published when key planned coprimary or secondary analyses are not yet available. Clinical Trial Updates provide an opportunity to disseminate additional results from studies, published in JCO or elsewhere, for which the primary end point has already been reported.
The purpose of this update was to determine differences in patient-reported chronic toxicity and disease outcomes with intensity-modulated radiation therapy (IMRT) compared with conventional pelvic radiation. Patients with cervical and endometrial cancers who received postoperative pelvic radiation were randomly assigned to conventional radiation therapy (CRT) or IMRT. Toxicity and quality of life were assessed using Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events, Expanded Prostate Cancer Index Composite (EPIC) bowel and urinary domains, and Functional Assessment of Cancer Therapy–General. Between 2012 and 2015, 279 eligible patients were enrolled to the study with a median follow-up of 37.8 months. There were no differences in overall survival (P = .53), disease-free survival (P = .21), or locoregional failure (P = .81). One year after RT, patients in the CRT arm experienced more high-level diarrhea frequency (5.8% IMRT v 15.1% CRT, P = .042) and a greater number had to take antidiarrheal medication two or more times a day (1.2% IMRT v 8.6% CRT, P = .036). At 3 years, women in the CRT arm reported a decline in urinary function, whereas the IMRT arm continued to improve (mean change in EPIC urinary score = 0.5, standard deviation = 13.0, IMRT v –6.0, standard deviation = 14.3, CRT, P = .005). In conclusion, IMRT reduces patient-reported chronic GI and urinary toxicity with no difference in treatment efficacy at 3 years.
INTRODUCTION
Postoperative radiotherapy (RT) has been shown to reduce locoregional recurrences in both cervical1,2 and endometrial cancers.3,4 Unfortunately, RT in this setting leads to significant morbidity. We previously reported the initial results of the first large, multicenter randomized trial comparing the impact of pelvic intensity-modulated radiation therapy (IMRT) and conventional radiation therapy (CRT) on acute patient-reported toxicity, demonstrating that IMRT resulted in significantly reduced acute GI and urinary toxicity.5 Now, with a 3-year follow-up, we report results on chronic toxicity and treatment efficacy.
METHODS
Study Design
NRG Oncology's RTOG 1203 trial was a phase III multicenter randomized controlled trial. Patients with cervical or endometrial cancer with indications for postoperative pelvic RT were eligible for inclusion and randomly assigned 1:1 to either CRT or IMRT. Radiation dose (45 Gy or 50.4 Gy) and administration of concurrent once weekly cisplatin 40 mg/m2 were determined by the treating physician. The primary end point was acute GI toxicity at week 5 of RT measured with the bowel domain of the Expanded Prostate Cancer Index Composite (EPIC) patient-reported outcome (PRO) instrument. Secondary end points included disease outcomes and chronic toxicity. The details of inclusion criteria, radiation treatment planning, and PRO assessments have been discussed in previous reports.6,7
Assessments
Patients completed the EPIC,8 the Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE),9 the Functional Assessment of Cancer Therapy instrument with cervix subscale,10 and EuroQOL's EQ-5D at the following time points: baseline, week 3 of RT (only EPIC), week 5 of RT, 4-6 weeks after RT, 1 year after RT, and 3 years after RT. Further details on these instruments can be found in previous reports.5 Validation of EPIC in this patient population has been reported separately.11 Physicians reported toxicity using CTCAE, version 4.0.
Statistics
Between-group comparisons for categorical and continuous variables were performed using chi-square tests and two-sided t-tests, respectively. Mixed-effects models were used to assess longitudinal change while incorporating stratification factors, patient characteristics, treatment arm, and treatment by time interaction. Overall survival (OS), disease-free survival (DFS), and locoregional failure (LRF) were measured from the date of random assignment to the date of death because of any cause, date of progression or death because of any cause, and date of LRF, respectively. Patients without events were censored at their last known follow-up time. Death without experiencing LRF was treated as a competing risk. OS and DFS were estimated using the Kaplan-Meier method12 and compared between arms using the log-rank test. The cumulative incidence approach was used to estimate failure rates for LRF with Gray's test to compare between arms.13,14 Cox proportional hazards models were used to obtain hazard ratios.
RESULTS
Of the 289 randomly assigned patients, 10 were found to be ineligible, leaving 279 eligible patients (Appendix Fig A1, online only). The median follow-up for the 279 eligible patients was 37.8 months (range, 0.33-66.18 months). Patient characteristics were well balanced between arms (Table 1).
TABLE 1.
Patient Characteristics

Treatment Efficacy
There were no differences between arms in any measured treatment efficacy end point (Appendix Fig A2, online only). The 3-year OS rates were 92.4% (95% CI, 87.7 to 97.2) in the IMRT arm versus 97.0% (95% CI, 94.1 to 99.9) in the CRT arm. The 3-year DFS rates were 85.5% (79.2 to 91.9) in the IMRT arm versus 80.8% (74.2 to 87.3) in the CRT arm. The 3-year LRF rates were 3.5% (95% CI, 1.1 to 8.1) in the IMRT arm versus 2.2% (95% CI, 0.6 to 5.7) in the CRT arm.
Patient-Reported GI Toxicity
The EPIC questionnaire was completed by 97.1% of patients at baseline, 88.9% at week 3 of RT, 86.7% at week 5 of RT, 85.6% at 4-6 weeks after RT, 77.1% at 1 year, and 55.1% at 3 years. There was no difference in mean change in EPIC bowel summary score at 1 and 3 years between arms, and both arms showed a clear improvement from week 5 of RT to 1 and 3 years (Fig 1A and Appendix Fig A3A, online only). Longitudinal modeling showed that IMRT had a significant effect on EPIC bowel score over time compared with CRT (estimate = -3.14, SE = 1.38, P = .023; Appendix Table A1, online only).
FIG 1.
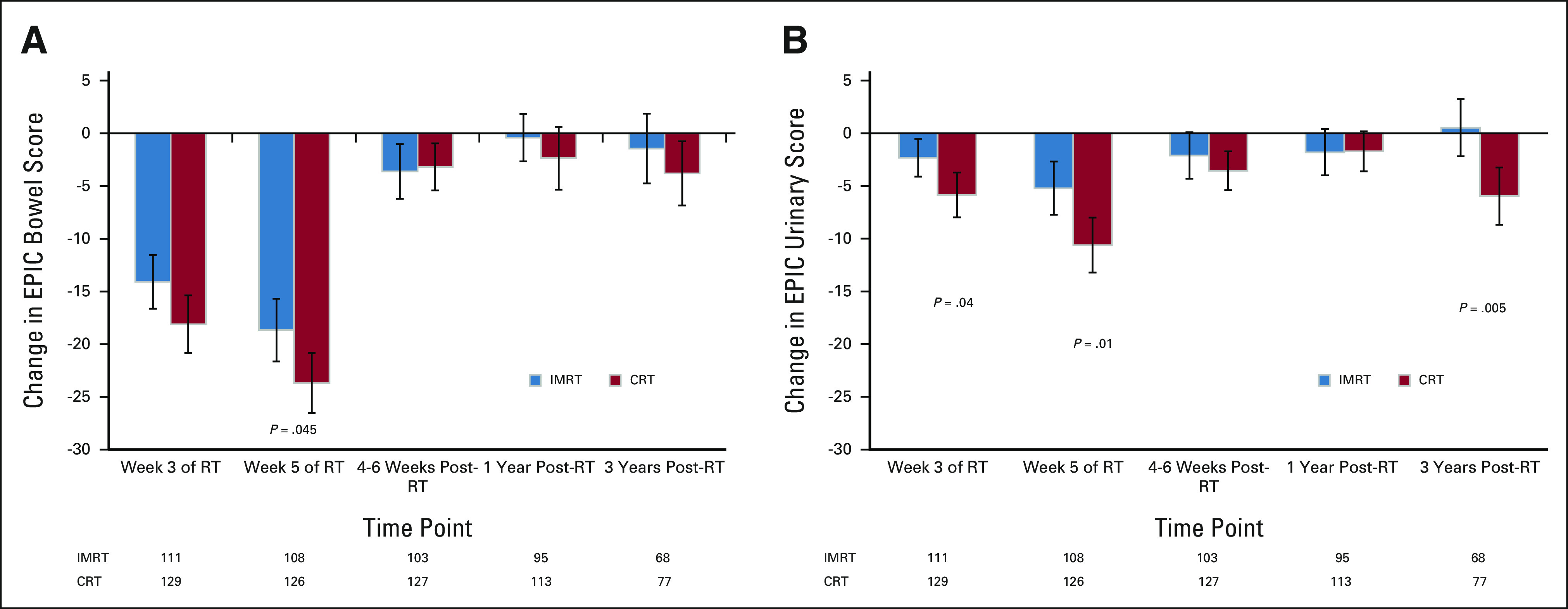
EPIC assessment of GI toxicity depicting changes in EPIC (A) bowel and (B) urinary summary scores between baseline and subsequent time points. Greater negative numbers reflect an increase in worsening of symptoms from baseline. Error bars represent 95% CIs. P values not listed are > .05. CRT, conventional radiation therapy; EPIC, Expanded Prostate Cancer Index Composite; IMRT, intensity-modulated radiation therapy; RT, radiation therapy.
The PRO-CTCAE questionnaire was completed within the timeframe by 95.8% of patients at baseline, 85.5% at week 5 of RT, 84.1% at 4-6 weeks after RT, 77.9% at 1 year, and 55.6% at 3 years. At 1 year after RT, fewer patients in the IMRT arm reported taking an antidiarrheal two or more times daily (1.2% IMRT v 8.6% CRT, P = .036) and diarrhea frequently or almost constantly (5.75% IMRT v 15.05% CRT, P = .042) compared with patients in the CRT arm (Fig 2A). This difference resolved at 3 years (Fig 2B). There were no significant differences between arms in regard to fecal incontinence or abdominal pain at 1 or 3 years (Appendix Table A2, online only).
FIG 2.
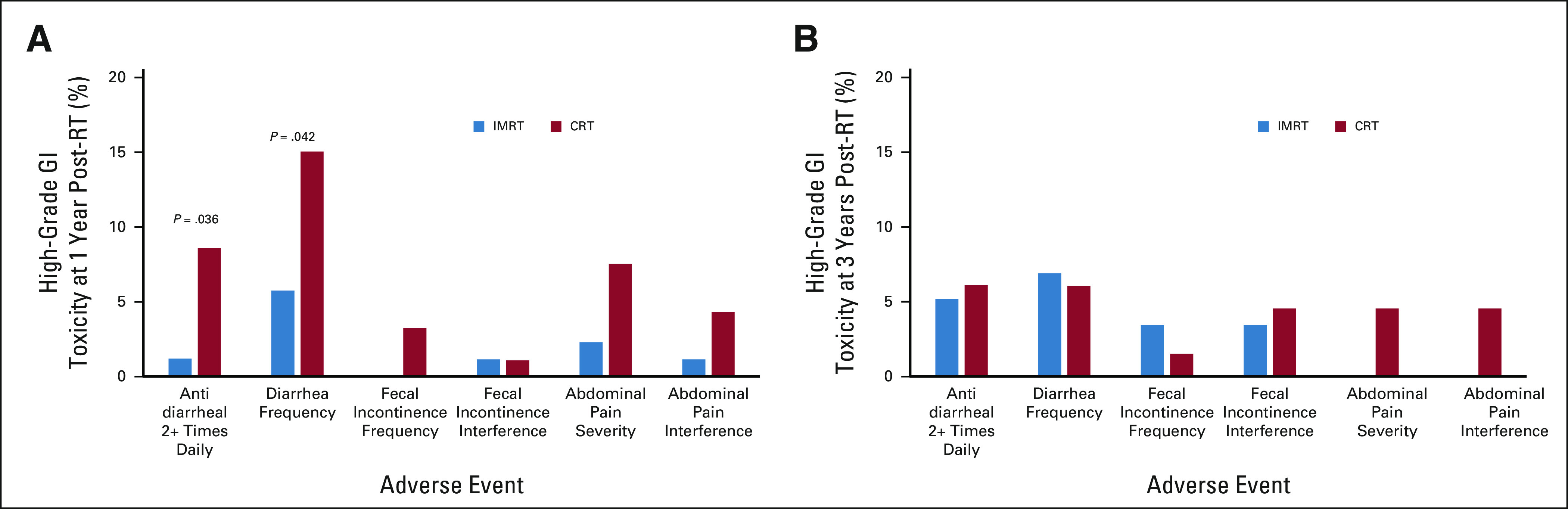
PRO-CTCAE assessment of high-grade (score 3+) GI toxicity at (A) 1 year and (B) 3 years after RT. A PRO-CTCAE score of 3 or 4 represents an adverse event frequency of frequently or almost constantly, severity of severe or very severe, or interference with usual or daily activities of quite a bit or very much. P values not listed are > .05. CRT, conventional radiation therapy; IMRT, intensity-modulated radiation therapy; PRO-CTCAE, Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events; RT, radiation therapy.
Patient-Reported Urinary Toxicity
Both arms showed a clear improvement in the mean change in EPIC urinary summary score from week 5 of RT to 1 year (Fig 1B). At 1 year after RT, there was no difference in the mean change in EPIC urinary score between arms (P = .96), but the improvement in the IMRT arm at 3 years was significant (P = .005). Longitudinal modeling showed a significant interaction between treatment and time (Appendix Table A3, online only). At 3 years, the CRT arm showed increased worsening compared with 1 year, signifying a decline in urinary function with further follow-up, whereas the IMRT arm continued to show improvement from 1 year to 3 years (Fig 1B and Appendix Fig A3B, online only).
Physician-Reported Toxicity
There was no late grade 2+ urinary toxicity reported in either arm. There was no difference in late grade 2+ GI toxicity between arms: 11.2% in the IMRT arm versus 11.8% in the CRT arm. There were no grade 5 toxicities reported, only one grade 4 toxicity, and other reproductive system and breast disorders, related to treatment reported in the CRT arm.
Quality of Life
At 1 and 3 years, there was no significant difference in change from baseline in Functional Assessment of Cancer Therapy–General total score (Appendix Fig A3C, online only). Longitudinal modeling showed a significant interaction between treatment and time (Appendix Table A4, online only).
DISCUSSION
It is well known that pelvic RT results in lasting GI and urinary toxicity.15 One way to mitigate the late toxicity of pelvic EBRT is to use IMRT. Multiple retrospective reviews have shown a reduction of physician-reported late toxicity with IMRT.16-19 However, few prospective, randomized trials have been performed directly comparing pelvic IMRT with CRT.20-22 Most recently, the PARCER trial, an Indian phase III trial comparing IMRT versus CRT in the adjuvant treatment of cervical cancer, demonstrated a reduction in 3-year grade 2+ chronic GI toxicity in the IMRT arm (21.1% v 42.2%, P < .001) and no difference in DFS.23 In comparison, to our knowledge, the current study is the first large, multicenter phase III trial comparing pelvic IMRT and CRT using PROs to evaluate toxicity from the patients' perspective. Although the PARCER trial demonstrated a reduction in physician-reported toxicity, the current study did not, but did, reveal a reduction in patient-reported toxicity. We believe that this is due to increased overall toxicity in the PARCER trial given that about 75% of patients received concurrent chemotherapy, compared with only 25% in the current study.
Several potential limitations to this study exist. Recurrences may still occur after 2-3 years, which might not have been captured in these data with a median follow-up of about 3 years.24,25 Compliance in completing PRO forms decreased as the time since treatment increased, making long-term comparisons between arms less robust. Multiple testing was performed without multiplicity adjustment as these were secondary end points. Some results, such as those regarding antidiarrheals and frequency of diarrhea, might not have reached significance under type I error adjustment.
In conclusion, IMRT results in reduced patient-reported chronic diarrhea and urinary toxicity compared with CRT, with no difference in disease outcomes at 3 years. Clinical practice has shifted such that IMRT is now commonly used to treat women with cervical or endometrial cancer receiving postoperative pelvic RT. The updated results of this trial fully support its continuous use in this setting and suggest that IMRT should now be considered the standard of care.
APPENDIX
TABLE A1.
EPIC Bowel Score Mixed-Effects Model

TABLE A2.
PRO-CTCAE Assessment of High-Grade (score 3+) GI Toxicity at 1 Year and 3 Years After RT
TABLE A3.
EPIC Urinary Score Mixed-Effects Model

TABLE A4.
FACT-G Total Score Mixed-Effects Model

FIG A1.
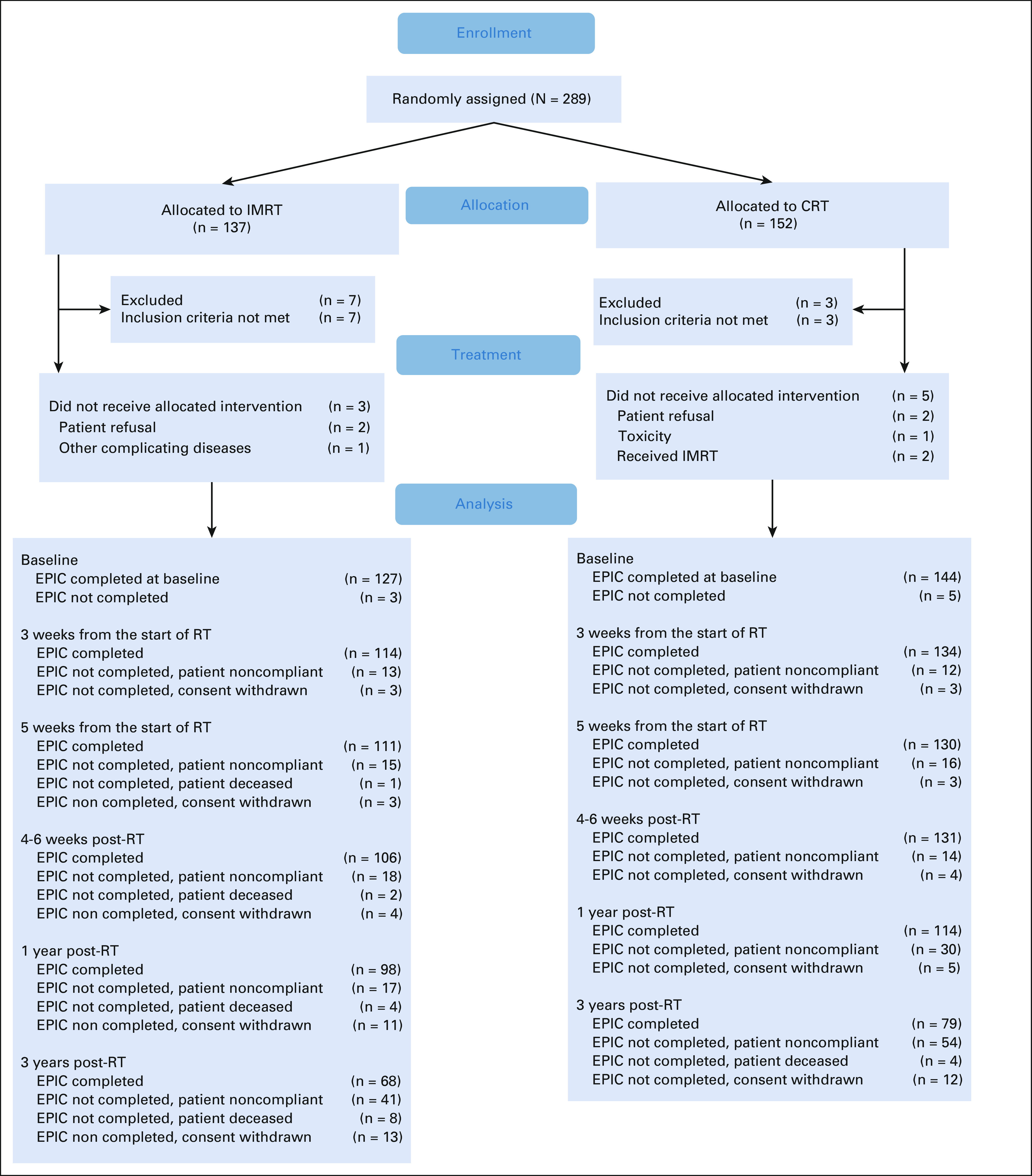
CONSORT diagram. CRT, conventional radiation therapy; EPIC, Expanded Prostate cancer Index Composite; IMRT, intensity-modulated radiation therapy; RT, radiation therapy.
FIG A2.
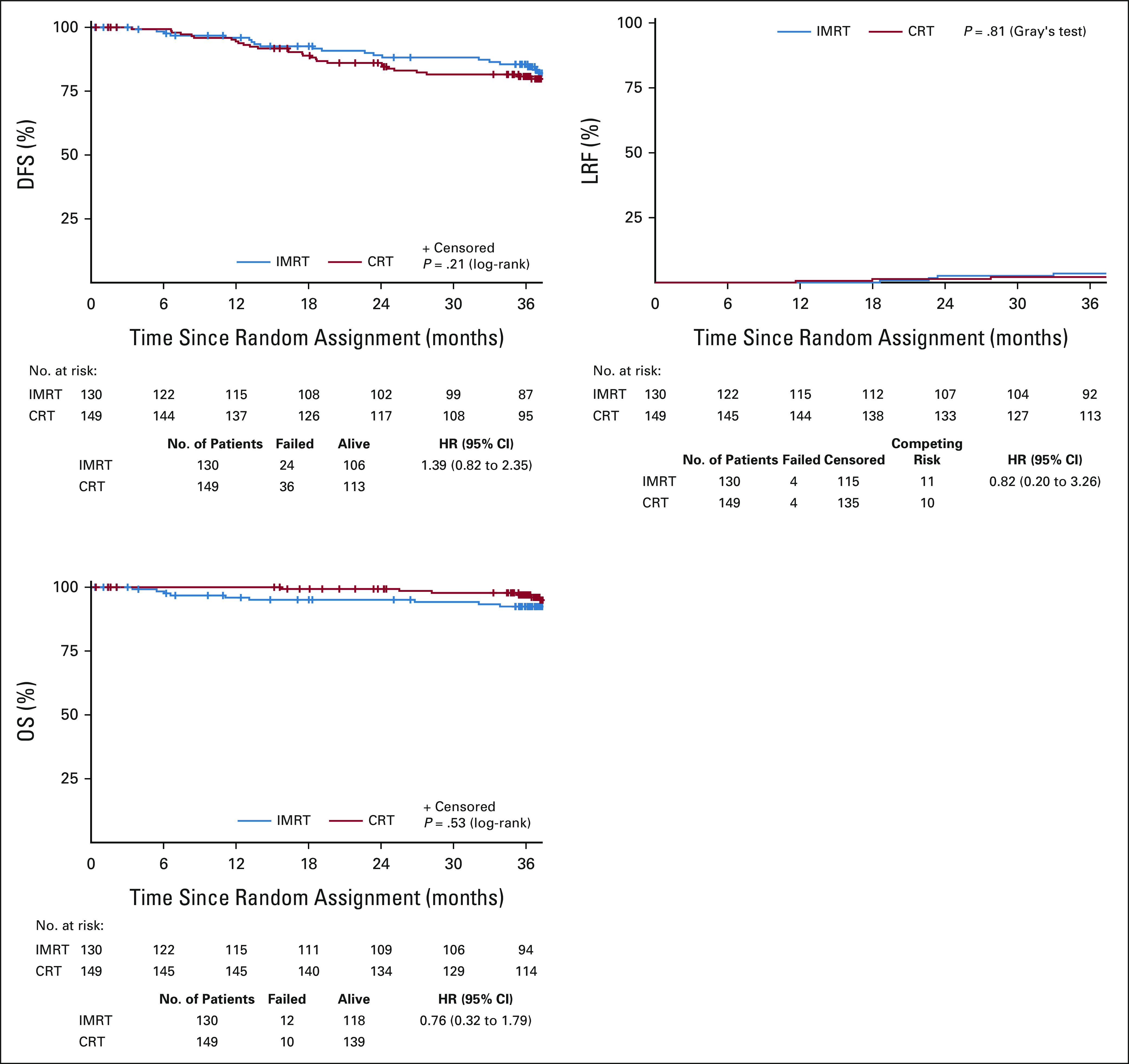
Kaplan-Meier survival curves for OS and DFS and cumulative incidence curve for LRF. CRT, conventional radiation therapy; DFS, disease-free survival; HR, hazard ratio; IMRT, intensity-modulated radiation therapy; LRF, locoregional failure; OS, overall survival.
FIG A3.
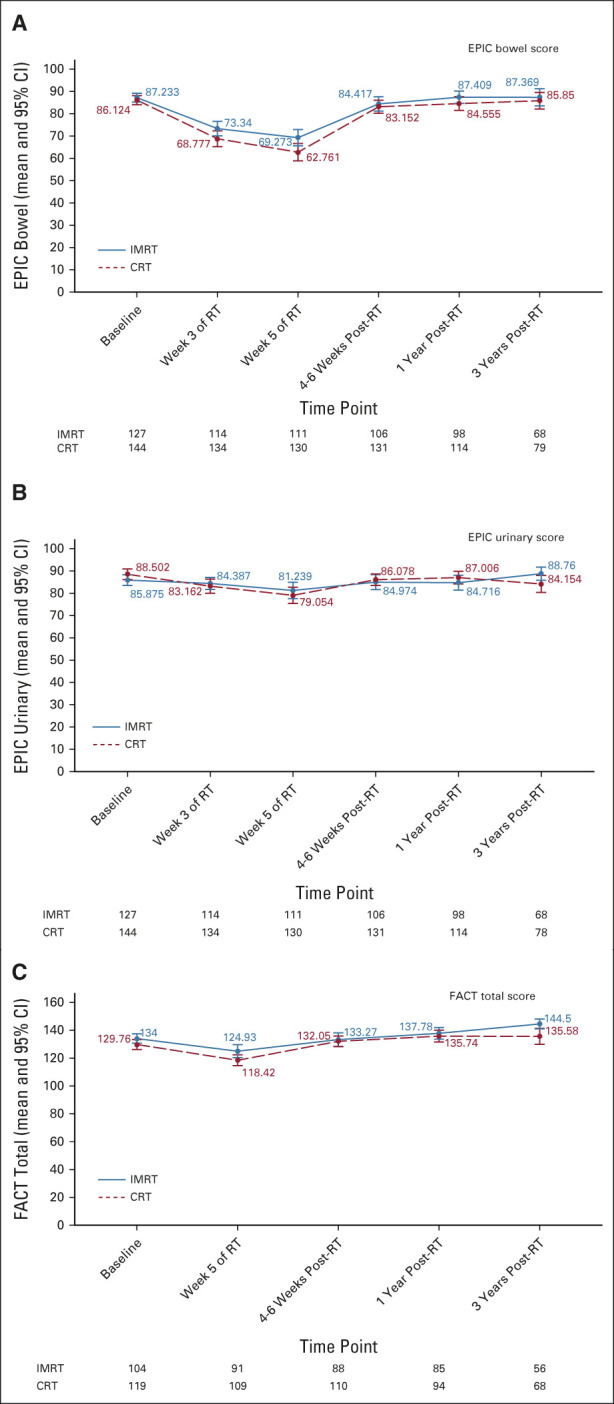
EPIC assessment of GI toxicity depicting changes in EPIC (A) bowel and (B) urinary summary scores across time. (C) Functional Assessment of Cancer Therapy (FACT) assessment of quality-of-life total scores over time. Error bars represent 95% CIs. Only significant P values are provided (P < .05). CRT, conventional radiation therapy; EPIC, Expanded Prostate Cancer Index Composite; IMRT, intensity-modulated radiation therapy.
Lari Wenzel
Consulting or Advisory Role: Array BioPharma
Shannon N. Westin
Consulting or Advisory Role: Roche, AstraZeneca, Genentech, Medscape, Clovis Oncology, Gerson Lehrman Group, Vaniam Group, Merck, BioAscent, Curio Science, OncLive, Targeted Oncology, GlaxoSmithKline, Eisai, Zentalis, Agenus, EQRX, Lilly, Vincerx Pharma, Mereo BioPharma, Immunogen, Mersana
Research Funding: AstraZeneca (Inst), Novartis (Inst), Bayer (Inst), Cotinga Pharmaceuticals (Inst), Clovis Oncology (Inst), Roche/Genentech (Inst), GOG Foundation (Inst), Mereo BioPharma (Inst), Bio-Path Holdings, Inc (Inst), GlaxoSmithKline (Inst), OncXerna Therapeutics (Inst), Zentalis (Inst)
Andre A. Konski
Employment: University of Pennsylvania
Stock and Other Ownership Interests: ImmunoCellular Therapeutics, Trevena
David K. Gaffney
Consulting or Advisory Role: Merck, AstraZeneca/MedImmune
Research Funding: Elekta
William Small Jr
Honoraria: Carl Zeiss Meditec, Varian Medical Systems
Consulting or Advisory Role: Novocure
Desiree E. Doncals
Research Funding: NRG Site Principle Investigator for Summa Health, DCISionRT Registry Site PI
David P. D'Souza
Honoraria: Ferring
Vijayananda Kundapur
Patents, Royalties, Other Intellectual Property: I hold a US patent for “mini beam collimator for medical linear accelerators”; Patent No.: US 10,702,711 B2; Date of Patent: July 7, 2020
Dasarahally S. Mohan
Employment: The Permanente Medical Group
Stephanie L. Pugh
Research Funding: Pfizer (Inst), Millennium (Inst)
Lisa A. Kachnic
Consulting or Advisory Role: New B Innovation
Research Funding: Varian Medical Systems
Patents, Royalties, Other Intellectual Property: UpToDate
Deborah W. Bruner
Employment: Emory University
Stock and Other Ownership Interests: AbbVie, Altria, Bristol Myers Squibb, GlaxoSmithKline, Johnson & Johnson, Pfizer, Procter & Gamble, Stryker, Viatris, Walgreens Boots Alliance
Honoraria: American Society of Radiation Oncology (ASTRO), Oncology Nursing Society, Memorial Sloan-Kettering Cancer Center, Alliance, Wilmot Cancer Center
Consulting or Advisory Role: Flatiron Health, Alliance for Clinical Trials in Oncology, University of Rochester
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented at the 61st Annual Meeting of the American Society of Radiation Oncology (ASTRO). September 15-19, 2019, Chicago, IL.
SUPPORT
Supported by grants UG1CA189867 (NCORP) from the Division of Cancer Prevention (DCP) of the National Cancer Institute (NCI).
CLINICAL TRIAL INFORMATION
AUTHOR CONTRIBUTIONS
Conception and design: Anamaria R. Yeung, Ann H. Klopp, Karen M. Gil, Lari Wenzel, Andre A. Konski, David K. Gaffney, William Small Jr, Stephanie L. Pugh, Lisa A. Kachnic, Deborah W. Bruner
Administrative support: Lisa A. Kachnic
Provision of study materials or patients: J. Spencer Thompson, Amy Chang, Vijayananda Kundapur, Dasarahally S. Mohan, Catherine L. Ferguson
Collection and assembly of data: Anamaria R. Yeung, Snehal Deshmukh, Shannon N. Westin, William Small Jr, J. Spencer Thompson, Desiree E. Doncals, Amy Chang, Vijayananda Kundapur, Dasarahally S. Mohan, Yong Bae Kim, Catherine L. Ferguson, Stephanie L. Pugh
Data analysis and interpretation: Snehal Deshmukh, Karen M. Gil, Lari Wenzel, Shannon N. Westin, Andre A. Konski, William Small Jr, J. Spencer Thompson, Guilherme H.C. Cantuaria, Dasarahally S. Mohan, Michael L. Haas, Stephanie L. Pugh
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Intensity-Modulated Radiation Therapy Reduces Patient-Reported Chronic Toxicity Compared With Conventional Pelvic Radiation Therapy: Updated Results of a Phase III Trial
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Lari Wenzel
Consulting or Advisory Role: Array BioPharma
Shannon N. Westin
Consulting or Advisory Role: Roche, AstraZeneca, Genentech, Medscape, Clovis Oncology, Gerson Lehrman Group, Vaniam Group, Merck, BioAscent, Curio Science, OncLive, Targeted Oncology, GlaxoSmithKline, Eisai, Zentalis, Agenus, EQRX, Lilly, Vincerx Pharma, Mereo BioPharma, Immunogen, Mersana
Research Funding: AstraZeneca (Inst), Novartis (Inst), Bayer (Inst), Cotinga Pharmaceuticals (Inst), Clovis Oncology (Inst), Roche/Genentech (Inst), GOG Foundation (Inst), Mereo BioPharma (Inst), Bio-Path Holdings, Inc (Inst), GlaxoSmithKline (Inst), OncXerna Therapeutics (Inst), Zentalis (Inst)
Andre A. Konski
Employment: University of Pennsylvania
Stock and Other Ownership Interests: ImmunoCellular Therapeutics, Trevena
David K. Gaffney
Consulting or Advisory Role: Merck, AstraZeneca/MedImmune
Research Funding: Elekta
William Small Jr
Honoraria: Carl Zeiss Meditec, Varian Medical Systems
Consulting or Advisory Role: Novocure
Desiree E. Doncals
Research Funding: NRG Site Principle Investigator for Summa Health, DCISionRT Registry Site PI
David P. D'Souza
Honoraria: Ferring
Vijayananda Kundapur
Patents, Royalties, Other Intellectual Property: I hold a US patent for “mini beam collimator for medical linear accelerators”; Patent No.: US 10,702,711 B2; Date of Patent: July 7, 2020
Dasarahally S. Mohan
Employment: The Permanente Medical Group
Stephanie L. Pugh
Research Funding: Pfizer (Inst), Millennium (Inst)
Lisa A. Kachnic
Consulting or Advisory Role: New B Innovation
Research Funding: Varian Medical Systems
Patents, Royalties, Other Intellectual Property: UpToDate
Deborah W. Bruner
Employment: Emory University
Stock and Other Ownership Interests: AbbVie, Altria, Bristol Myers Squibb, GlaxoSmithKline, Johnson & Johnson, Pfizer, Procter & Gamble, Stryker, Viatris, Walgreens Boots Alliance
Honoraria: American Society of Radiation Oncology (ASTRO), Oncology Nursing Society, Memorial Sloan-Kettering Cancer Center, Alliance, Wilmot Cancer Center
Consulting or Advisory Role: Flatiron Health, Alliance for Clinical Trials in Oncology, University of Rochester
No other potential conflicts of interest were reported.
REFERENCES
- 1.Peters WA III, Liu PY, Barrett RJ II, et al. : Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J Clin Oncol 18:1606-1613, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Sedlis A, Bundy BN, Rotman MZ, et al. : A randomized trial of pelvic radiation therapy versus no further therapy in selected patients with stage IB carcinoma of the cervix after radical hysterectomy and pelvic lymphadenectomy: A Gynecologic Oncology Group study. Gynecol Oncol 73:177-183, 1999 [DOI] [PubMed] [Google Scholar]
- 3.Creutzberg CL, Nout RA, Lybeert ML, et al. : Fifteen-year radiotherapy outcomes of the randomized PORTEC-1 trial for endometrial carcinoma. Int J Radiat Oncol Biol Phys 81:e631-8, 2011 [DOI] [PubMed] [Google Scholar]
- 4.Keys HM, Roberts JA, Brunetto VL, et al. : A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: A Gynecologic Oncology Group study. Gynecol Oncol 92:744-751, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Klopp AH, Yeung AR, Deshmukh S, et al. : Patient-reported toxicity during pelvic intensity-modulated radiation therapy: NRG Oncology-RTOG 1203. J Clin Oncol 36:2538-2544, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roeske JC, Lujan A, Rotmensch J, et al. : Intensity-modulated whole pelvic radiation therapy in patients with gynecologic malignancies. Int J Radiat Oncol Biol Phys 48:1613-1621, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Ahamad A, D'Souza W, Salehpour M, et al. : Intensity-modulated radiation therapy after hysterectomy: Comparison with conventional treatment and sensitivity of the normal-tissue-sparing effect to margin size. Int J Radiat Oncol Biol Phys 62:1117-1124, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Wei JT, Dunn RL, Litwin MS, et al. : Development and validation of the expanded prostate cancer index composite (EPIC) for comprehensive assessment of health-related quality of life in men with prostate cancer. Urology 56:899-905, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Basch E, Dueck AC, Rogak LJ, et al. : Feasibility assessment of patient reporting of symptomatic adverse events in multicenter cancer clinical trials. JAMA Oncol 3:1043-1050, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cella DF, Tulsky DS, Gray G, et al. : The functional assessment of cancer therapy scale: Development and validation of the general measure. J Clin Oncol 11:570-579, 1993 [DOI] [PubMed] [Google Scholar]
- 11.Gil KM, Pugh SL, Klopp AH, et al. : Expanded validation of the EPIC bowel and urinary domains for use in women with gynecologic cancer undergoing postoperative radiotherapy. Gynecol Oncol 154:183-188, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaplan EL, Meier P: Nonparametric estimation from incomplete observations. J Am Stat Assoc 53:457-481, 1958 [Google Scholar]
- 13.Kalbfleish JD, Prentice RL: The Statistical Analysis of Failure Time Data. New York, NY, John Wiley & Sons, 1980, pp 167-169 [Google Scholar]
- 14.Gray RJ: A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat 16:1141-1154, 1988 [Google Scholar]
- 15.de Boer J, Wolf AL, Szeto YZ, et al. : Dynamic collimator angle adjustments during volumetric modulated arc therapy to account for prostate rotations. Int J Radiat Oncol Biol Phys 91:1009-1016, 2015 [DOI] [PubMed] [Google Scholar]
- 16.He S, Gill BS, Heron DE, et al. : Long-term outcomes using adjuvant pelvic intensity modulated radiation therapy (IMRT) for endometrial carcinoma. Pract Radiat Oncol 7:19-25, 2017 [DOI] [PubMed] [Google Scholar]
- 17.Viani GA, Viana BS, Martin JE, et al. : Intensity-modulated radiotherapy reduces toxicity with similar biochemical control compared with 3-dimensional conformal radiotherapy for prostate cancer: A randomized clinical trial. Cancer 122:2004-2011, 2016 [DOI] [PubMed] [Google Scholar]
- 18.Mundt AJ, Mell LK, Roeske JC: Preliminary analysis of chronic gastrointestinal toxicity in gynecology patients treated with intensity-modulated whole pelvic radiation therapy. Int J Radiat Oncol Biol Phys 56:1354-1360, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Chen MF, Tseng CJ, Tseng CC, et al. : Clinical outcome in posthysterectomy cervical cancer patients treated with concurrent cisplatin and intensity-modulated pelvic radiotherapy: Comparison with conventional radiotherapy. Int J Radiat Oncol Biol Phys 67:1438-1444, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Gandhi AK, Sharma DN, Rath GK, et al. : Early clinical outcomes and toxicity of intensity modulated versus conventional pelvic radiation therapy for locally advanced cervix carcinoma: A prospective randomized study. Int J Radiat Oncol Biol Phys 87:542-548, 2013 [DOI] [PubMed] [Google Scholar]
- 21.Lin Y, Chen K, Lu Z, et al. : Intensity-modulated radiation therapy for definitive treatment of cervical cancer: A meta-analysis. Radiat Oncol 13:177, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bruner DW: Outcomes research in cancer symptom management trials: The Radiation Therapy Oncology Group (RTOG) conceptual model. J Natl Cancer Inst Monogr 37:12-15, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Chopra S, Dora T, Gupta S, et al. : Phase III randomized trial of postoperative adjuvant conventional radiation (3DCRT) versus image guided intensity modulated radiotherapy (IG-IMRT) in cervical cancer (PARCER): Final analysis. Int J Radiat Oncol Biol Phys 108:S1-S2, 2020. (suppl 3) [Google Scholar]
- 24.de Foucher T, Bendifallah S, Ouldamer L, et al. : Patterns of recurrence and prognosis in locally advanced FIGO stage IB2 to IIB cervical cancer: Retrospective multicentre study from the FRANCOGYN group. Eur J Surg Oncol 45:659-665, 2019 [DOI] [PubMed] [Google Scholar]
- 25.Nomden CN, Pötter R, de Leeuw AAC, et al. : Nodal failure after chemo-radiation and MRI guided brachytherapy in cervical cancer: Patterns of failure in the EMBRACE study cohort. Radiother Oncol 134:185-190, 2019 [DOI] [PubMed] [Google Scholar]



