Abstract
Intestinal T/NK-cell lymphomas include enteropathy-associated T-cell lymphoma (EATL), monomorphic epitheliotropic intestinal T-cell lymphoma (MEITL), indolent T-cell lymphoproliferative disorders of the GI tract (ITCLPD), extranodal NK/T-cell lymphoma, nasal type (ENKTL), and intestinal T-cell lymphoma NOS (ITCL-NOS). Here we describe a case of surface CD3-negative MEITL. A 63-year-old Japanese female had a tumor located in the conglomerated ileum, which formed multiple mass lesions. The resected tissue showed a diffuse infiltration of monomorphic medium-sized lymphocytes with epitheliotropism. Flowcytometry using a fresh specimen of the tumor revealed positivity for CD7, CD8, CD38, and CD56, but not surface CD3. On immunohistochemistry, the tumor showed positivity for cytoplasmic CD3, CD8, CD56, TIA-1, Granzyme B, and perforin. EBER with in situ hybridization was negative. Moreover, H3K36me3, which is negative in MEITL with SETD2-mutation, was positive. This is an important case of MEITL due to its oncogenesis.
Keywords: monomorphic epitheliotropic intestinal T-cell lymphoma, intestinal T/NK-cell lymphoma, surface CD3, flowcytometry
INTRODUCTION
The gastrointestinal (GI) tract is the most common site for extranodal lymphomas comprising 30-40% of all cases.1-3 Various types of lymphomas occurring in specific sites of the GI tract have been described.1 Although diffuse large B-cell lymphoma NOS (DLBCL) is the most frequent type in the world, different frequencies of other types of lymphoma according to region and race have been reported.1,3 T/NK-cell lymphomas of the GI tract include enteropathy-associated T-cell lymphoma (EATL), monomorphic epitheliotropic intestinal T-cell lymphoma (MEITL), indolent T-cell lymphoproliferative disorders of the GI tract (ITCLPD), extranodal NK/T-cell lymphoma, nasal type (ENKTL), and intestinal T-cell lymphoma NOS (ITCL-NOS). Both EATL related to Celiac disease (CD) and MEITL unrelated to CD display proliferation of neoplastic T-cells with epitheliotropism.4 MEITL is characterized by the immunohistochemical phenotype of cytoplasmic CD3 (cCD3), CD8, and CD56. T-cell receptor (TCR) γδphenotype is more frequent than αβ in Asia.5 ITLPD is known to have a quite indolent clinical course and specific endoscopic findings, and ITCL-NOS is defined as lacking the clinical and pathological features of other lymphomas.
NK-cell lymphomas are known to show a predilection for extranodal sites, mainly in nasal sites, and are rarely found outside of nasal sites such as the skin or GI tract. 6 The neoplastic cells in most cases harbor the EBV genome that was demonstrated by EBER with in situ hybridization. These cases were diagnosed as ENKTL. Other than ENKTL, intestinal NK-cell lymphomas are exceptionally rare.
We herein report a case of surface CD3 (sCD3)-negative MEITL diagnosed histopathologically using flowcytometry. Because sCD3 is a critical marker for distinguishing T-cells, which are sCD3-positive, and NK-cells, which are sCD3-negative, this is an important case of MEITL.
CASE REPORT
A 63-year-old Japanese female complained of constipation for 6 months with recent abdominal pain and appetite loss. She had lost 15 kilograms in weight. Frequent urination, intermittent abdominal pain, and abnormal sensation in the abdomen appeared. Her past history was not significant except for a Cesarean section and hysterectomy due to multiple leiomyomas. On admission, an abdominal mass was palpated around the umbilical region. Laboratory examination showed normocytic anemia (8.2 g/dl) with abnormal elevation of ferritin (132.4 μg), CRP (4.12 mg/dl), and serum IL-2 receptor (1,990 U/ml), and decreased albumin (2.5 g/dl). LDH was in the normal range (125 IU/l). Non-contrast enhanced computed tomography showed a mass in the ileum compressing the urinary bladder (Figure 1). Partial resection of the ileum to the ileocecal region was performed because of a suspicion of malignancy. Postoperative PET-CT showed the presence of an iso-intensity mass (SUV max=10.3) beside the apex of the urinary bladder. This was interpreted as a residual lymphoma. Chemotherapy of DeVIC regimen (etoposide, ifosfamide, and carboplatin) was successfully completed without prominent adverse reactions, and the patient has achieved complete remission so far.
Fig. 1.
Computed tomographic scan without contrast enhancement on admission. An ileal mass with wall thickening is identified (arrow) but intestinal obstruction is not seen (A). Peritonitis is suspected due to thickening of the peritoneum and mild increased intensity of the intrapelvic adipose tissue (ellipse) (B).
PATHOLOGICAL FINDINGS
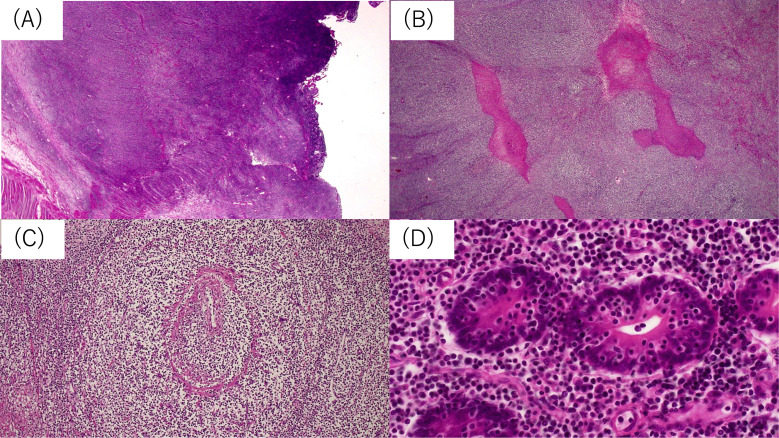
A whitish tumor mass measuring 120 x 80 x 60 mm located on the ileum infiltrating the mesentery was resected (Figure 2). The mucosa covering the tumor was ulcerated. Histological sections showed a diffuse proliferation of monomorphic medium-sized lymphocytes from the lamina propria to the subserosa with geographical coagulative necrosis (Figure 3A, B). Nuclear irregularity was not prominent. An angio-destructive pattern (Figure 3C) and epitheliotropism (Figure 3D) were also seen.
Fig. 2.
Gross features of the cut surface of the ileal mass. A whitish protruding tumor with surface erosion and scatted focal necrosis is seen. The ileal wall shows diffuse thickening and tumor infiltrated the mesentery.
Fig. 3.
Lymphoma cells diffusely infiltrated the entire ileal wall (A). Focal coagulated necrosis (B) and angioinvasion (C) are seen. Lymphoma cells are monomorphic and show infiltration into the crypt epithelium (D, epitheliotropism).
Immunohistochemically, the medium-sized atypical lymphocytes were positive for CD3 (Figure 4A), CD8 (Figure 4B), CD56 (Figure 4C), TIA-1 (Figure 4D), Granzyme B (Figure 4E), perforin (Figure 4F), BCL2, and antibody recognizing demethylated lysine 36 of histone H3 (marked as H3K36me3) (Figure 4G), but negative for CD4, CD5, CD10, CD20, CD30, BCL6, MUM1, and Cyclin D1. Follicular dendritic cell meshwork was not identified by CD21 immunostaining. The MIB1 labeling index was approximately 40% (Figure 4H). EBV-encoded small RNA (EBER) with in situ hybridization was totally negative.
Fig. 4.
Immunophenotypes of lymphoma cells. Lymphoma cells are positive for CD3 (A), CD8 (B), CD56 (C), TIA-1 (D), Granzyme B (E), perforin (F), and H3K36me3 (G). The MIB1 index is approximately 40% (H).
Flowcytometry using a fresh specimen of tumor revealed positivity for CD7, CD8, CD38, and CD56, and negativity for CD2, surface CD3, CD4, CD5, CD10, CD19, CD20, and CD23 (Figure 5). In addition, T-cell receptor (TCR)-αβ and -γδ were both negative. Karyotyping analysis was unavailable.
Fig. 5.
Flowcytometric analysis of CD45 gated lymphoma cells of the ileal wall. The lymphoma cells are positive for cytoplasmic CD3, CD7, CD8, CD38, and CD56 and negative for CD2, surface CD3, CD4, CD5, CD10, CD19, CD20, and CD23.
PCR-amplification of the TCR-β and γ genes using extracted DNA from the formalin-fixed paraffin embedded tissue (FFPE) sections showed rearrangement of TCR-β, but that of the TCR-γ gene failed due to DNA degradation.
DISCUSSION
A differential diagnosis of GI tract NK-cell and T-cell lymphoma is sometimes challenging.6 Differentiation between NK-cells and T-cells by immunohistochemistry of FFPE seems to be difficult because both NK-cells and T-cells express cCD3. However, it can be detected by sCD3-negativity in NK-cells and sCD3-positivity in T-cells on flowcytometry. In our case, the immunohistochemical phenotype of cCD3, CD8, CD56, and cytotoxic markers, such as TIA-1, Granzyme B, and perforin, on immunohistochemistry, and EBER-negativity made us consider the cytotoxic T-cell phenotype. In addition, the presence of epitheliotropism suggested a diagnosis of MEITL. However, the flowcytometric phenotype of sCD3(-), CD5(-), CD7(+), CD8(+), and CD56(+) indicated that this case may be derived from NK-cells. CD16, which is a marker of NK-cells, was negative. Histopathological comparisons including the flowcytometry of MEITL, ENKTL, and the current case are summarized in Table 1.
Table 1. Comparison of extranodal NK/T cell lymphoma, nasal type (NK/T), monomorphic epitheliotropic intestinal T- cell lymphoma (MEITL) and the current case.
| NK/T | MEITL | Current case | |
|---|---|---|---|
| Morphological features | |||
| Vascular damage and destruction | + | − | + |
| Necrosis | + | − | + |
| Epitheliotropism | − | + | + |
| Nuclear features | various | monomorphic | monomorphic |
| Immunohistochemical features | |||
| Surface CD3 | − | + | − |
| Cytoplasmic CD3 | + | + | + |
| CD8 | −/+ | + | + |
| CD38 | − | − | + |
| CD56 | + | − | + |
| EBER in situ hybridization | + | − | − |
| Flowcytometry | |||
| Surface CD3 | − | + | − |
| TCR rearrangement | silent | γδ>αβ | silent |
In the English-language literature, almost all of the cases reported as intestinal NK-cell lymphoma may be included as ENKTL under the current WHO classification.7,8 Chuang and Jung reported a 37-year-old male with intestinal multicentric lymphoma with epitheliotropism expressing CD3, CD8, cytotoxic granules, and EBV and germline mutation for TCR.9 Fujiwara et al. reported a 63-year-old male with ulcerated tumor in the jejunum showing medium to large-sized lymphoma cells with angiocentric patterns, but not epitheliotropism.10 The lymphoma cells were positive for CD2, CD7, CD8, and CD56, but negative for sCD3. EBER was positive. This case was suggested as ENKTL. On the other hand, Aoyama et al. described an 83-year-old male with MEITL that showed sCD3-negativity on flowcytometry.11 However, PCR examination of the TCR gene revealed monoclonal gene rearrangements of TCR-β and TCR-γ. This case was suggested to be MEITL without sCD3 expression. Therefore, our case seems to be similar to Aoyama’s case. CD8 expression is found in the subset of cytotoxic T-cell lymphoma and in T-cell lineage of ENKTL, but not usually in the NK-cell lineage of ENKTL.12 Abnormal expression of CD8 is occasionally found in some cases of aggressive NK-cell leukemia.13 It has been reported that a normal subset of CD8-positive NK-cells existed.14
Flowcytometry is one of the most powerful modalities for diagnosing malignant lymphomas. Altered expression of the pan T-cell antigens CD2, CD3, CD5, and CD7 are frequently identified in T-cell lymphoproliferative neoplasms.15 Only 4 cases of MEITL with flowcytometry data were reported in the English-language literature. Among them, sCD3 was positive in two cases,16,17 positive (dim) in one case,18 and negative in one case as described by Aoyama et al.11 The sCD3-negative case was reported to be double negative for CD4 and CD8 and negative for TIA-1, suggesting a strange immunophenotype for MEITL. Therefore, the current case seems to be the second case of MEITL with a typical immunophenotype except sCD3 loss by flowcytometry.
MEITL showed alterations of the tumor suppressor gene SETD2 by mutations and/or loss and mutations in one or more genes of the JAK/STAT pathway, suggesting that the combination of epigenetic deregulation and cell signaling activation represent major oncogenic events in the pathogenesis of MEITL.19,20 SETD2 encodes a methyltransferase that in humans is non-redundantly responsible for specifically trimethylating lysine 36 of histone H3 using H3K36me2 as a substrate. Therefore, the H3K36me3 is an epigenetic modification to the DNA packaging protein Histone H3. Tomita et al. reported that most cases of MEITL with SETD2-mutation were negative for H3K36me3.21 This case was positive for H3K36me3, suggesting a different oncogenesis from MEITL.
The current case showed CD38 by flowcytometry among 94% of CD45 gated cells. CD38, multifunctional transmembrane type II glycoprotein, is expressed on plasma cells and immature lymphocytes. Because of the development of the anti-CD38 antibody Daratumumab for plasma cell myeloma, the utility of Daratumumab is now expanding to other hematolymphoid malignancies.22 Expression of CD38 is a well-known biological marker in aggressive cases of CLL.23 Recently, Zaja et al. described that approximately 80% of angioimmnoblastic T-cell lymphomas (AITL) and 60% of peripheral T-cell lymphomas-NOS (PTCL-NOS) express CD38.24 In addition, CD38 is reported to be expressed by the majority of ENKTL and a rare and aggressive subtype of mature T- and natural killer cell lymphomas commonly associated with Epstein Barr Virus infection.24,25,26 In this context, anti CD38 Daratumumab can be a candidate for the treatment of AITL, PTCL-NOS, and ENKTL; however, CD38 expression has not been well investigated in MEITL. Although the current case was treated by the DeVIC regimen and has continued to be in complete remission so far, high CD38 positivity on flowcytometry may be interesting if the current regimen fails.
In conclusion, we report a case of MEITL with sCD3-negativity and H3K36me3-positivity. This is a unique case of MEITL from the perspectives of its oncogenesis. Integrated analyses of morphology, immunohistochemistry, flowcytometry, and TCR-gene are important for the diagnosis of intestinal NK/T-cell lymphoma.
Footnotes
FUNDING
The authors did not receive any financial support for the research, authorship, and/or publication of this article.
CONFLICT OF INTEREST
The authors have no potential conflicts of interest to declare with respect to the research, authorship, and/or publication of this article.
REFERENCES
- 1.Chan JKC, Fukayama M. Haematolymphoid tumours of digestive system: Introduction. In : The WHO Classification of Tumours Editorial Board (eds) : Digestive System Tumours. Lyon (France), International Agency for Research on Cancer. 2019; pp. 376-377. [Google Scholar]
- 2.Howell JM, Auer-Grzesiak I, Zhang J, et al. Increasing incidence rates, distribution and histological characteristics of primary gastrointestinal non-Hodgkin lymphoma in a North American population. Can J Gastroenterol. 2012; 26: 452-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olszewska-Szopa M, Wróbel T. Gastrointestinal non-Hodgkin lymphomas. Adv Clin Exp Med. 2019; 28: 1119-1124. [DOI] [PubMed] [Google Scholar]
- 4.Jaffe ES, Bhagat G, Chott A, et al. Intestinal T-cell lymphoma. In : The WHO Classification of Tumours Editorial Board (eds) : Digestive System Tumours. Lyon (France), International Agency for Research on Cancer. 2019; pp. 372-380. [Google Scholar]
- 5.Chan JKC, Chan ACL, Cheuk W, et al. Type II enteropathy-associated T-cell lymphoma: a distinct aggressive lymphoma with frequent γδ T-cell receptor expression. Am J Surg Pathol. 2011; 35: 1557-1569. [DOI] [PubMed] [Google Scholar]
- 6.Susan SSH, Ng SB, Wang S, Tan SY. Diagnostic approach to T- and NK-cell lymphoproliferative disorders in the gastrointestinal tract. Semin Diagn Pathol. 2021; 38: 21-30. [DOI] [PubMed] [Google Scholar]
- 7.Tang XF, Yang L, Duan S, Guo H, Guo QN. Intestinal T-cell and NK/T-cell lymphomas: A clinicopathological study of 27 Chinese patients. Ann Diagn Pathol. 2018; 37: 107-117. [DOI] [PubMed] [Google Scholar]
- 8.Jhuang JY, Chang ST, Weng SF, et al. Extranodal natural killer/T-cell lymphoma, nasal type in Taiwan: a relatively higher frequency of T-cell lineage and poor survival for extranasal tumors. Hum Pathol. 2015; 46: 313-321. [DOI] [PubMed] [Google Scholar]
- 9.Chuang SS, Jung YC. Natural killer cell lymphoma of small intestine with features of enteropathy but lack of association with celiac disease. Hum Pathol. 2004; 35: 639-642. [DOI] [PubMed] [Google Scholar]
- 10.Fujiwara H, Akiyama T, Nishimura H, et al. Primary intestinal NK-cell lymphoma with a CD8+ CD56+ immunophenotype: a case report. Pathol Int. 2013; 63: 138-140. [DOI] [PubMed] [Google Scholar]
- 11.Aoyama Y, Tsunemine H, Zushi Y, et al. Colonal monomorphic epitheliotropic intestinal T-cell lymphoma with novel phenotype of cytoplasmic CD3 expression. J Clin Exp Hematop. 2018; 58: 102-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tan SY, Chuang SS, Tang T, et al. Type II EATL (epitheliotropic intestinal T-cell lymphoma): a neoplasm of intra-epithelial T-cells with predominant CD8αα phenotype. Leukemia. 2013; 27: 1688-1696. [DOI] [PubMed] [Google Scholar]
- 13.Li Y, Wei J, Mao X, et al. Flow Cytometric immunophenotyping is sensitive for the early diagnosis of de novo aggressive natural killer cell leukemia (ANKL): a multicenter retrospective analysis. PLoS One. 2016; 11: e0158827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McKinney EF, Cuthbertson I, Harris KM, et al. A CD8+ NK cell transcriptomic signature associated with clinical outcome in relapsing remitting multiple sclerosis. Nat Commun. 2021; 12: 635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Craig JW, Dorfman DM. Flow Cytometry of T cells and T-cell neoplasms. Clin Lab Med. 2017; 37: 725-751. [DOI] [PubMed] [Google Scholar]
- 16.Kato A, Takiuchi Y, Aoki K, et al. Enteropathy-associated T-cell lymphoma type II complicated by autoimmune hemolytic anemia. J Clin Exp Hematop. 2011; 51: 119-123. [DOI] [PubMed] [Google Scholar]
- 17.Tanaka H, Ambiru S, Nakamura S, et al. Successful diagnosis of type II enteropathy-associated T-cell lymphoma using flow cytometry and the cell block technique of celomic fluid manifesting as massive pyoid ascites that could not be diagnosed via emergency laparotomy. Intern Med. 2014; 53: 129-133. [DOI] [PubMed] [Google Scholar]
- 18.Antoniadou F, Dimitrakopoulou A, Voutsinas PM, et al. Monomorphic epitheliotropic intestinal T-cell lymphoma in pleural effusion: A case report. Diagn Cytopathol. 2017; 45: 1050-1054. [DOI] [PubMed] [Google Scholar]
- 19.Roberti A, Dobay MP, Bisig B, et al. Type II enteropathy-associated T-cell lymphoma features a unique genomic profile with highly recurrent SETD2 alterations. Nat Commun. 2016; 7: 12602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nairismägi ML, Tan J, Lim JQ, et al. JAK-STAT and G-protein-coupled receptor signaling pathways are frequently altered in epitheliotropic intestinal T-cell lymphoma. Leukemia. 2016; 30: 1311-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tomita S, Kikuti YY, Carreras J, et al. Monomorphic epitheliotropic intestinal T-cell lymphoma in Asia frequently shows SETD2 alterations. Cancers (Basel). 2020; 12: 3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Calabretta E, Carlo-Stella C. The Many facets of CD38 in Lymphoma: from tumor–microenvironment cell interactions to acquired resistance to immunotherapy. Cells. 2020; 9: 802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Damle RN, Wasil T, Fais F, et al. Ig V gene mutation status and CD38 expression as novel prognostic indicators in chronic lymphocytic leukemia. Blood. 1999; 94: 1840-1847. [PubMed] [Google Scholar]
- 24.Zaja F, Tabanelli V, Agostinelli C, et al. CD38, BCL-2, PD-1, and PD-1L expression in nodal peripheral T-cell lymphoma: possible biomarkers for novel targeted therapies? Am J Hematol. 2017; 92: E1-E2. [DOI] [PubMed] [Google Scholar]
- 25.Wang L, Wang H, Li PF, et al. CD38 expression predicts poor prognosis and might be a potential therapy target in extranodal NK/T cell lymphoma, nasal type. Ann Hematol. 2015; 94: 1381-1388. [DOI] [PubMed] [Google Scholar]
- 26.Hari P, Raj RV, Olteanu H. Targeting CD38 in refractory extranodal natural killer cell- T-cell lymphoma. N Engl J Med. 2016; 375: 1501-1502. [DOI] [PubMed] [Google Scholar]