Abstract
Atrial fibrillation has a multifactorial pathophysiology influenced by cardiac autonomic innervation. Both sympathetic and parasympathetic influences are pro-fibrillatory. Innovative therapies targeting the neurocardiac axis include catheter ablation or pharmacologic suppression of ganglionated plexi, renal sympathetic denervation, low-level vagal stimulation, and stellate ganglion blockade. To date, these therapies have variable efficacy. As our understanding of atrial fibrillation and the cardiac nervous system expands, our approach to therapeutic neuromodulation will continue evolving for the benefit of those with AF.
Keywords: Atrial fibrillation, Autonomics, Neurocardiac axis, Ganglionated plexi, Neuromodulation
INTRODUCTION
Atrial fibrillation (AF) is a common arrhythmia that will ultimately affect 1 in 5 individuals1. The frequency of AF is matched by its complexity. AF has a multifactorial pathophysiology influenced by cardiac autonomic innervation2, 3. There are even distinct autonomic-driven phenotypes of AF with distinct management strategies (Figure 1). For example, in patients with vagal AF, defined as occurring postprandially or nocturnally, beta-blocker therapy may aggravate or precipitate persistent or permanent AF4. Both AHA and ESC guidelines suggest that disopyramide may be useful for vagal AF due to its anticholinergic activity, although the evidence is limited.5, 6
Figure 1.
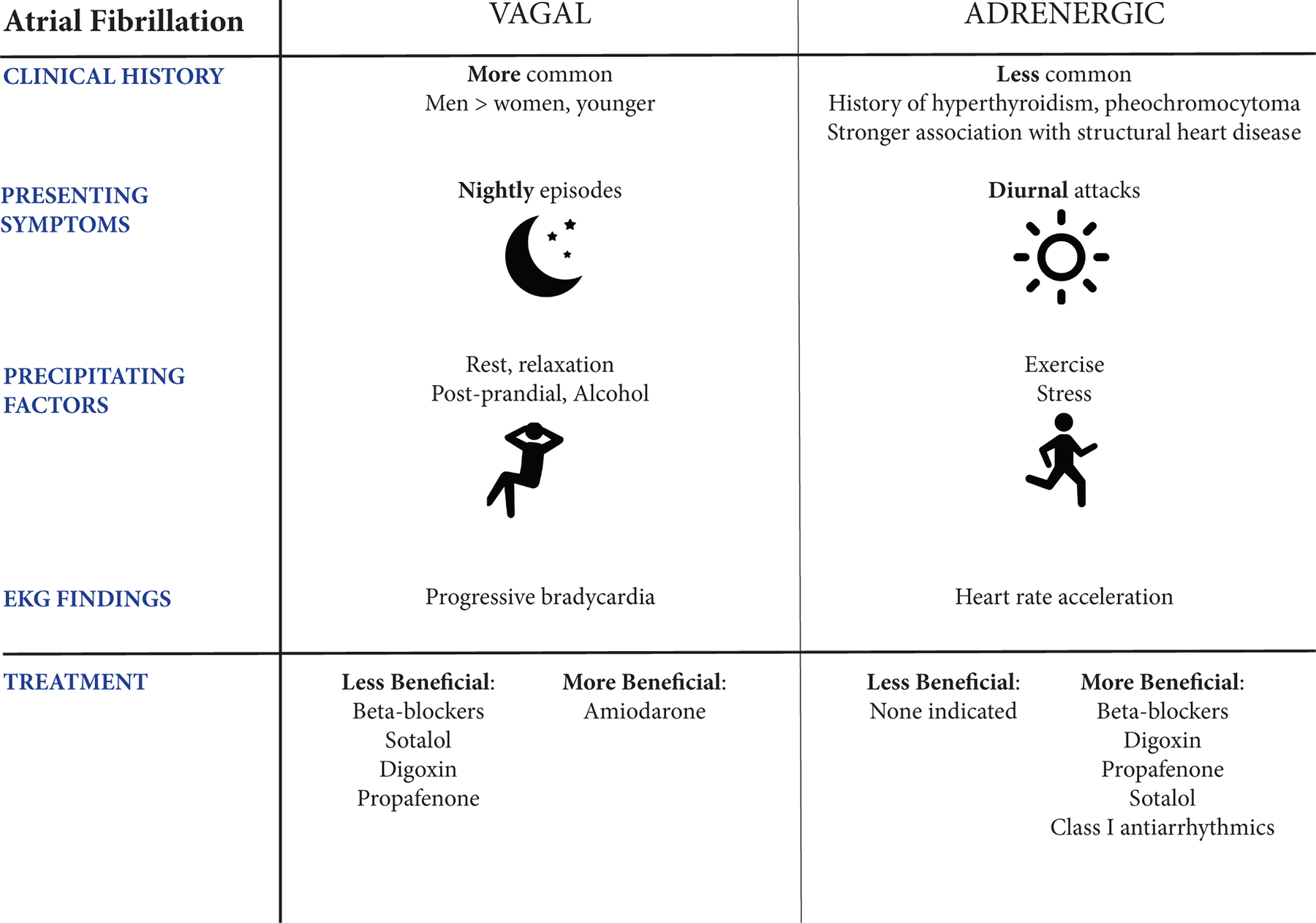
Vagal versus Adrenergic Atrial Fibrillation, adapted from Coumel, et al.50 and de Vos, et al. 4
A comprehensive understanding of the neurocardiac axis is essential in treating AF. The present review will elucidate the anatomy and physiology of the neurocardiac axis as well as the rationale and evidence for therapeutic neuromodulation in atrial fibrillation.
ANATOMY & PHYSIOLOGY OF CARDIONEURAL AXIS
The autonomic nervous system (ANS) influences heart rate (chronotropy), conduction speed (dromotropy), contractility (inotropy), and relaxation (lusitropy), with opposing parasympathetic (PNS) and sympathetic (SNS) effects7. ANS innervation is categorized as central, arising from the brain cortex, brainstem, and spinal cord; intrathoracic extra-cardiac, characterized by sympathetic chain ganglia; and intrinsic cardiac, comprised of neurons organized into ganglionated plexi with interconnecting neurons within epicardial fat pads8.
Sympathetic and Parasympathetic Pathways to the Heart
Central autonomic signaling arises from the cingulate cortex, amygdala, thalamus, and cerebellum, with parasympathetic and sympathetic anatomical variation9. SNS output descends to the cervical and thoracic spinal cord where preganglionic neurons in the intermediolateral columns project to superior cervical, middle cervical, cervicothoracic (stellate), and mediastinal ganglia10. Long, postganglionic axons project to epicardial ganglia and cardiac myocytes directly, stimulating the release of norepinephrine to beta-1 and beta-2 receptors of the sinoatrial (SA) and atrioventricular (AV) nodes, as well as atrial and ventricular myocytes11.
In contrast, long preganglionic parasympathetic neurons originate in the brainstem, at the nucleus accumbens and dorsal motor nucleus of the medulla oblongata. These project through the vagus nerve to short postganglionic neurons within intrinsic cardiac ganglia, releasing acetylcholine at muscarinic (M2) receptors of the SA and AV nodes as well as atrial myocytes.
Sympathetic innervation at adrenergic receptors classically stimulates G-protein coupled receptor (GPCR) Gs which activates adenylyl cyclase (AC), increasing intracellular cAMP12. cAMP plays a complex role in mediating chronotropy, predominantly through binding hyperpolarization-activated cyclic nucleotide gated (HCN) channels to enhance channel opening probability by positively shifting voltage thresholds13, 14. cAMP additionally activates protein kinase A (PKA), which phosphorylates molecules implicated in automaticity pathways regulated by calcium cycling15. Many nonclassical pathways are associated with beta-2 activation and influence chronotropy through cAMP-independent mechanisms12. Sympathetic innervation additionally strengthens contractility through cAMP/PKA-mediated augmentation of intracellular calcium11, 12 SNS-driven, inward calcium via L-type channels, in addition to promoting automaticity and contractility, has been implicated in early afterdepolarizations that trigger AF through promoting forward Na/Ca exchange16.
Parasympathetic release of acetylcholine at M2 receptors slows depolarization and heart rate by both negatively influencing GPCR activation as well as binding to muscarinic-gated potassium channels (IKAch) for an inward, hyperpolarizing current17, 18. Acetylcholine impacts heart rate greater than contractility due to higher M2 receptor density in nodal and atrial tissue11. Parasympathetic stimulation ultimately shortens the atrial effective refractory period (AERP)19, which synergistically with sympathetic input promotes AF initiation16. Local circuit neurons facilitate SNS/PNS communication in the cardiac conduction system11, 20.
Anatomy and Physiology of Epicardial Ganglionated Plexi
Extrinsic autonomic nerves enter the intrinsic cardiac nervous system (ICNS) through the cardiac hilum and synapse onto epicardial ganglionated plexi (GPs)10, 21, 22. One GP contains 200–1000 local, interconnecting neurons embedded in epicardial adipose tissue23. Some have colloquially referred to the ICNS as the heart’s “little brain”24.
Atrial and Ventricular Ganglionated Plexi
GP topography is variably described but consistent between hearts; 75% of epicardial ganglia lie in the cardiac dorsum, with the highest density at the left atrial (LA) - pulmonary vein (PV) junction25. An early study identified 5 atrial and 5 ventricular ganglia, in which 4 major atrial GP were localized near the PVs. These 4 atrial GP were further explored as ablation targets and renamed: (1) anterior right (ARGP), concentrated immediately anterior to the right PVs, (2) superior left (SLGP), residing in the LA roof medial to the left superior PV, (3) inferior right (IRGP) and (4) inferior left (ILGP), both located inferior to the right and left PVs on the LA posterior wall23, 25–27.
Atrial GP integrate signals from bilateral vagosympathetic trunks to the SA and AV nodes28, 29. Input to the SA node is mediated by the ARGP, which receives innervation either directly from the right vagosympathetic trunk (predominant pathway) or indirectly from bilateral vagosympathetic trunks after traversing the SLGP28. The SLGP and ARGP converge on the IRGP before modulating the AV node29. The ILGP also influences the AV node and contributes to ventricular slowing23, 28, 29. Pauza, et al. apply a different nomenclature to their findings in human hearts, which largely coincides with the aforementioned GPs but adds emphasis to ventral GPs residing along coronary arteries and atrial surfaces21, 30 (Figure 2).
Figure 2.

The anatomy of the cardiac autonomic ganglionated plexi, as discovered through anatomic dissection of canines (Armour et al., black 26), anatomic dissection of human hearts (Pauza et al., blue 21, 30), and electroanatomic mapping of human hearts (Po et al., green 27). (A) Posterior, (B) Cross-sectional, (C) Right, (D) Left. Abbreviations: Armour et al., PL-LAGP=posterolateral left atrial, OMGP=obtuse marginal, SLAGP=superior left atrial, PDGP=posterior descending, PM-LAGP=posteromedial left atrial, PRAGP=posterior right atrial, SRAGP=superior right atrial; Po et al., SLGP=superior left, ILGP=inferior left, ARGP=anterior right, IRGP=inferior right; Pauza et al., MDsGP=middle dorsal ganglionated subplexus, LDsGP=left dorsal ganglionated subplexus, VLAsGP=ventral left atrial ganglionated subplexus, DRAsGP=dorsal right atrial ganglionated subplexus, VRAsGP=ventral right atrial ganglionated subplexus, LCsGP=left coronary ganglionated subplexus, RCsGP=right coronary ganglionated subplexus. Anatomy: SVC=superior vena cava, PA=pulmonary artery, LAA=left atrial appendage, VOM=vein of Marshall, LSPV=left superior pulmonary vein, LIPV=left inferior pulmonary vein, RSPV=right superior pulmonary vein, RIPV=right inferior pulmonary vein, IVC=inferior vena cava, SA=sinoatrial, AV=atrioventricular.
Pulmonary Veins and Vein of Marshall
PV ectopy plays an established role in initiating and maintaining AF31, 32. The PVs become encased in atrial tissue as they return from the lungs and enter the LA, forming a myocardial sheath composed of circumferentially oriented cardiomyocytes resembling a “mesh-like” network23, 32, 33. The irregular orientation of these fibers may contribute to anisotropy and re-entry. Studies have also identified both cholinergic and adrenergic activity neighboring the PV–LA junctions23, 34. Strategies for pulmonary vein isolation (PVI) have evolved to circumferential ablation for increased maintenance of sinus rhythm32, 35, which further improves when neighboring autonomic innervation is eliminated36. PV innervation by both adrenergic and vagal fibers is highly co-localized34, engendering difficulty in selectively ablating either pathway.
The Vein of Marshall (VOM) runs within the vestigial ligament of Marshall (LOM) and is another landmark implicated in AF37. The intracardiac VOM extends from the coronary sinus to the left superior PV base and pulmonary artery, attaching superiorly to the pericardium23, 38. Not only is VOM ectopy seen in patients with sustained AF39, but also the VOM is richly innervated by cholinergic and adrenergic fibers implicated in atrial tachyarrhythmias40, 41. Recent clinical trial data has demonstrated that adjunctive alcohol ablation of the VOM improves maintenance of sinus rhythm in persons with persistent AF treated with PVI42.
Inaccessible Ganglionated Plexi
Several GP are difficult to access with an endocardial approach due to their association with the great vessels, including: (1) transverse sinus GP (TSGP), between the pulmonary artery and base of the aorta, and (2) superior vena caval-aortic GP (SVC-Ao GP), in the posteromedial wall of the SVC, the anterolateral aortic wall, and superior to the right pulmonary artery23, 26, 43. The SVC-Ao GP has been identified as the “head station” of parasympathetic input to the heart, receiving most vagal fibers that ultimately synapse on the atria, SA, and AV nodes23, 44. The SVC-Ao GP may also be implicated in apnea-related AF, with reduced AF inducibility following ablation in canines45.
Renal Influences on Sympathetic Nervous System Activity
The kidney contains mechanical and chemical receptors which ascend along afferent renal fibers and travel through renal nerves and dorsal roots (T9-L4) that ultimately project centrally to enact widespread sympathetic activation46. This pathway is implicated in hypertension and its denervation has been targeted for refractory hypertension with varying efficacy47, 48. Recent data suggests that renal denervation in conjunction with PVI reduces the risk of recurrent atrial arrhythmias (hazard ratio=0.57; 95% CI, 0.38 to 0.85; P=0.006)49.
RATIONALE FOR TARGETING THE AUTONOMIC NERVOUS SYSTEM
Role of the ANS in Atrial Fibrillation
While early clinical studies formed the basis of our understanding of “vagal AF” versus “adrenergic AF”4, 50, this distinction is not always clinically apparent. Biomarkers may be useful in delineating autonomic profiles that contribute to AF. Insulin-like growth factor 1 (IGF1) and N-terminal pro-B-type natriuretic peptide (NT-proBNP) are associated with the risk of incident AF51–53. Decreased IGF1 corresponds with increased risk of AF51, possibly due to IGF1’s inverse relationship with age54, as well as its deactivating role on the SNS that may be protective against arrhythmia. No molecular biomarkers have been identified that distinguish vagal versus adrenergic AF.
Heart rate variability (HRV) is a physiologic biomarker that measures autonomic imbalance with both time and frequency domain measurements55. High frequency (HF) represents vagal predominance while low frequency (LF), when expressed in normalized units, reflects sympathetic tone55, 56. In 782 patients with hypertension, higher HRV and HF were associated with AF occurrence57, while prior studies noted lower HRV and LF to confer AF risk58, 59. This inconsistency may be influenced by varied autonomic phenotypes. Interestingly, patients undergoing PVI without recurrent AF have lower HF and elevated LF/HF in the months after ablation compared to those with recurrence36.
No study has distinguished HRV patterns in patients with clinical vagal or adrenergic AF. Future studies should evaluate the utility of physiologic or molecular biomarkers in confirming phenotypic variants in those with symptoms, as well as distinguishing phenotypes when clinical indicators are lacking. This approach may clarify conflicting evidence on these classifications and the varying efficacy of AF treatments.
Ultimately, defining “vagal” versus “adrenergic” AF may oversimplify the interdependent relationship of sympathetic and parasympathetic activation. In the first large observational study of vagal versus adrenergic AF, an autonomic pattern was only identifiable in 33% of patients with paroxysmal AF (PAF)4. Another later study could not distinguish autonomic phenotypes by history alone; however, there was evidence of increased adrenergic tone in the 20 minutes preceding AF followed by a predominance of vagal tone in the last 5 minutes in both patients with lone AF and those with structural heart disease60, suggesting a synergistic role of both systems.
Sharifov, et al. demonstrated that isoproterenol and adrenaline infusions induced AF in 21% and 17% of canines, respectively, while acetylcholine induced AF in 100%. The effects of acetylcholine were potentiated with simultaneous isoproterenol infusion, decreasing the threshold acetylcholine concentration required for AF induction61. This study, and others, have demonstrated that the parasympathetic and sympathetic systems exert independent and synergistic effects on AF62, 63.
Electrophysiologic Effects of Parasympathetic and Sympathetic Stimulation
Proposed autonomic-driven mechanisms underlying AF include those that facilitate reentry or provoke calcium mishandling causing early or delayed afterdepolarizations64, 65.
Parasympathetic input shortens the atrial effective refractory period (AERP)19, 66, 67, which is a known trigger for AF and has been implicated in PV re-entry, as suggested by optical mapping68. Medications like verapamil and class III antiarrhythmic drugs attenuate AERP shortening69, 70. Patients with PAF exhibit greater baseline AERP dispersion compared to those without AF, and the degree of AERP dispersion increases during episodes of supraventricular arrhythmia71. PVs also have, on average, shorter AERPs when compared with the LA and when compared with PVs of patients without AF72.
Sympathetic stimulation, in contrast, predominantly influences AF by predisposing myocytes to afterdepolarizations. Delayed afterdepolarizations (DADs) occur after full repolarization and typically arise from abnormal diastolic increases in cytoplasmic calcium (usually from abnormal ryanodine receptors) causing inward depolarizing sodium through Na/Ca exchange73, a mechanism for AF common in heart failure74. In contrast, early afterdepolarizations (EADs) have been associated with sympathetic stimulation and are caused by excitation during phase 2 or 3 of myocyte repolarization65. EADs can arise from prolonged repolarization allowing L-type calcium channel recovery75, and more recently have been associated with brief refractory periods coupled with increased vulnerability to calcium cycling16, 72.
Sympathetic stimulation can provoke AF via the latter mechanism by transiently augmenting calcium flow through L-type calcium channels, often termed calcium transient currents16, 19, 76. In a canine model, Patterson, et al. demonstrated that within PV myocytes, EAD formation is favored by enhanced calcium transient currents and subsequent increased forward Na/Ca exchange16. In these same myocytes, norepinephrine and isoproterenol infusions significantly increased calcium transient currents and EAD formation, both independently and with the addition of pacing interventions.
Both early (INa, early) and late (INa,late) sodium currents are also implicated in arrhythmogenesis. INa, early drives the initial upstroke in the non-pacemaker action potential, while INa, late courses through recovered sodium channels that have reopened during the plateau phase77. Increased INa,late can prolong action potential duration77, 78 and provokes EADs through the former mechanism of increased calcium current through recovered L-type channels77. Sympathetic stimulation can enhance INa,late and contribute to AF through PKA mediated posttranslational modifications79, 80.
Autonomic modulation of the SA node may also promote reentry. In computer models, cholinergic stimulation caudally shifts the leading pacemaker site which, when coupled with an atrial premature beat and/or unidirectional block within the SA node, promotes reentry and atrial tachycardia81. This is attributed to an intrinsic heterogeneity in response to autonomic stimulation within the SA node, and is influenced by enhanced L-type calcium currents that increase the probability of atrial premature beats. Another study describes the onset of AF as “flickering” between sinus rhythm and AF, and that simultaneous sympathetic and parasympathetic modulation of the sinus node contribute to this “tug-of-war.”82
Finally, while sympathetic and parasympathetic stimulation have distinct electrophysiologic effects, their ability to provoke AF is synergistic. In the study by Patterson, et al.,EADs provoked by sympathetic stimulation only led to sustained arrhythmias when acetylcholine was administered simultaneously16. Burashnikov, et al. similarly found that EADs formed after both rapid pacing and after AF termination in the presence of acetylcholine, but not in its absence, and that acetylcholine was an additional prerequisite for AF initiation83. Interestingly, simultaneous PNS and SNS activation shortens AERPs as an algebraic sum of their individual effects62.
These findings unify theories of parasympathetic and sympathetic synergy; parasympathetic activation shortens effective refractory periods and action potential durations, which allows calcium mishandling induced by sympathetic stimulation to favor EADs that ultimately incite AF. Furthermore, this pattern of rapid atrial pacing followed by termination bears some similarity to patterns of HRV (increased adrenergic followed by vagal predominance) that occur prior to the onset of AF60, underscoring that surges in parasympathetic and sympathetic tone, taken together, play a collaborative role in precipitating and sustaining AF.
Overall Autonomic Tone and AF
The heart has physiologic, circadian variations in rhythm84 implicated in both atrial85 and ventricular86 arrhythmias, as well as sudden cardiac death87, 88. Patients with frequent tachyarrhythmias often have increased atrial tachycardia and AF at night89, possibly mediated by nocturnal increases in vagal activity90.
Cardiac autonomic neuropathy, often observed in diabetes, is associated with AF91. Diabetes can cause both sympathetic and parasympathetic cardiac denervation92. Changes in HRV have been associated with asymptomatic episodes of AF in patients with type 2 diabetes and no prior history of AF93. Additionally, increased epicardial adipose tissue (where most autonomic GP reside) is an independent predictor of AF recurrence, possibly due to underlying inflammatory responses in adipose tissue or electrical remodeling94.
Finally, manipulation of autonomic outflow from GPs induces AF. ARGP stimulation can convert isolated premature depolarizations (PD) into AF-inducing PDs95. Stimulating the base of the right superior PV progressively slows the canine heart rate and initiates AF after fewer PDs96. This effect is attenuated by lidocaine injection at the site of GP stimulation96. Ablation of epicardial and coronary sinus atrial tissue attenuates vagally induced AERP shortening in canines and reduces AF inducibility from cervical vagal stimulation97. Moreover, high frequency stimulation of atrial GP, when synchronized with AERPs, can cause PV ectopy and subsequent AF in humans98. Finally, response to GP stimulation independently predicts the recurrence of AF following PVI in patients with PAF, further suggesting that GP ablation may reduce AF99.
THERAPEUTIC INTERVENTIONS
Clinical Trials Evaluating Therapeutic Modulation of the Neurocardiac Axis
Modulation of the neurocardiac axis has emerged as a promising therapy for treating AF. Techniques include GP ablation, pharmacological treatments, renal denervation, low-level stimulation of the vagus nerve, and blockade of the sympathetic ganglia, including the stellate ganglion (Figure 3).
Figure 3.
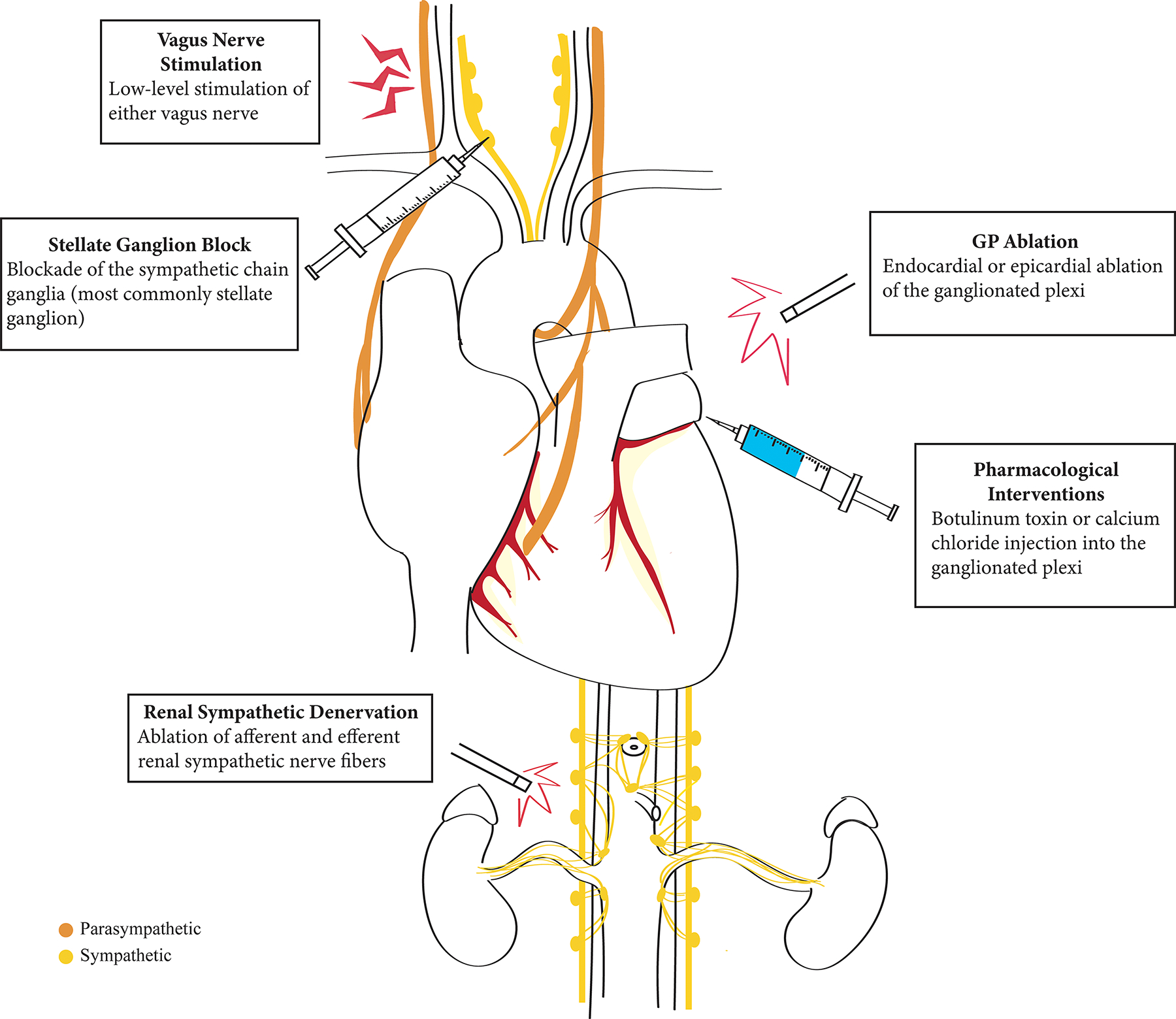
Therapeutic Modulation of the Neurocardiac Axis.
Therapeutic Interventions Targeting the Ganglionated Plexi
GP identification can be guided by (1) empiric/anatomical targeting and/or (2) high frequency stimulation (HFS). The latter method involves identifying GP regions that, when stimulated, induce sinus rate slowing (R-R lengthening) and/or blood pressure changes consistent with “vagal responses”100. Additional methods for GP identification include imaging-based approaches (SPECT/CT guidance)101 or ectopy-triggered approaches102. The best method for identifying GPs has not yet been established as each method has its own limitations.
Ganglionated Plexi Ablation
Approaches to GP ablation (GPA) include transcatheter, endocardial techniques and thoracoscopic or open epicardial ablation (Supplementary Table 1). The cornerstone of AF ablation is PVI103–105, and GPA has been investigated both alone and adjunct to PVI with varying results.
Endocardial Approaches to Ganglionated Plexi Ablation
Early studies established the feasibility and safety of endocardial GPA both when guided by HFS27, 36, 106 or anatomic mapping107. The preferred order of GPA is (1) SLGP, (2) ILGP, (3) ARGP, (4) IRGP, because the IRGP acts as a gateway for the other GP to access the AV node. Early IRGP ablation may prematurely eliminate parasympathetic responses to stimulation of remaining GPs, which can make the endpoint of ablation ill-defined27.
The efficacy of GPA when performed alone is variable, which is partly influenced by ablation methods. In small observational studies, Lemery, et al. found that HFS-guided GPA alone led to 50% freedom from AF at 8 months (N=14 patients)106, while Katritsis, et al. employed anatomic-guided GPA with less promising results (GPA alone: 26% freedom from AF, PVI: 63%)107. However, in a larger study of 80 patients randomized to HFS- or anatomic-guided GPA, Pokushalov, et al. demonstrated that anatomic-guidance was superior to HFS (77.5% freedom from AF vs 42.5%, p=0.02)108. They postulated that their “cloud-like” lesions were superior to the “linear” lesions employed by Katritsis, et al., conferring greater efficacy with a higher area of ablation. Furthermore, GP sites have both sympathetic and parasympathetic innervation; in those with significant sympathetic contribution, HFS may not induce adequate vagal responses, ultimately underestimating GP regions requiring ablation109. Interestingly, even in a study that only included patients with “vagal AF,” endocardial HFS-guided GPA alone was unsuccessful in eliminating AF inducibility; 17 of 18 patients required additional PVI to achieve freedom from AF at 15 months110.
Several studies suggest that the efficacy of endocardial GPA combined with PVI is greater than the efficacy of either technique alone. In an observational study of 297 patients with PAF, Pappone, et al. ablated GP targets with vagal reflexes (sinus bradycardia<40 bpm) in addition to PVI and found greater 1-year freedom from symptomatic AF compared to PVI alone (99% vs 85%, p=0.0002)36. Katritsis, et al. randomized 242 patients with symptomatic PAF to (1) PVI alone, (2) anatomic GPA alone, and (3) PVI plus anatomic GPA. Two-year freedom from recurrent arrhythmia was most optimal in the PVI + GP ablation group (PVI only: 56%, GPA only: 48%, PVI+GPA: 74%, p=0.004)109, with no differences in safety events, including post-procedural atrial flutter. In this trial, Katritsis et al. employed “cloud-like” lesions instead of the linear lesions they used previously107. In another randomized trial of 264 patients with persistent forms of AF, PVI + GPA was superior to PVI + LA linear ablation with increased maintenance of sinus rhythm at three years (49% vs 34%, p=0.035) and less atrial flutter (6% vs 18%, p=0.002)111. Taken together, these studies demonstrate that endocardial GPA improves maintenance of sinus rhythm when performed with PVI.
Epicardial Approaches to Ganglionated Plexi Ablation
Epicardial approaches to GPA have more variable efficacy. The Ganglion Plexus Ablation in Advanced Atrial Fibrillation Trial (AFACT) randomized 240 patients with paroxysmal or persistent AF undergoing surgical ablation (PVI+/−Dallas lesion set) to additional epicardial ablation of the 4 major GPs and LOM versus no additional ablation. At 1-year there was no added benefit of GP and LOM ablation112. The GPA group also required more frequent pacemaker implantation due to sinus node dysfunction and AV block. Similarly, an observational study found no added benefit of epicardial GPA added to LA cryoablation during cardiac surgery in maintaining sinus rhythm with or without antiarrhythmic medications113.
The lack of benefit observed in the AFACT trial may be influenced by inadvertent GPA during PVI in the control group. While HFS-vagal responses were present in at least 1 GP in 87% of control patients compared to 0% of the GPA group, the differences between patients who only had 1 active GP site after PVI alone and patients who received full GPA may be too small for a clinical impact. Moreover, if the IRGP was ablated first, absent vagal responses to HFS may have prematurely indicated successful ablation. Ultimately, the epicardial approach to GPA does not appear to offer significant safety or efficacy advantages.
Hybrid approaches that combine epicardial ablation with endocardial ablation may show promise. While not designed to test GPA, the Converge Trial is the largest and only randomized clinical trial comparing hybrid and endocardial ablation in patients with persistent forms of AF114. The hybrid ablation arm (epicardial PVI followed by endocardial ablation to close gaps) was superior to endocardial ablation alone (67.7% freedom from AF vs 50%, p=0.036). Further studies are needed to clarify the impact of hybrid approaches on GP elimination, autonomic modulation, and improved outcomes.
Right Sided versus Left Sided Left Atrial GP Ablation
Ablation of either right-sided or left-sided left atrial GP (LAGP) alone can reduce AF inducibility thresholds independently in preclinical studies115. AF inducibility is virtually eliminated after ablation of all 4 GP plus the LOM115, suggesting that global GPA is most optimal.
As discussed previously, the recommended order of GPA ends with ARGP and IRGP to avoid falsely indicating inactivity in the SLGP and ILGP (and thereby successful ablation). In a retrospective analysis of 67 patients undergoing mini-maze surgery, patients were divided into: (1) right sided LAGP ablation first, and (2) left sided LAGP ablation first116. In those who underwent anatomic ablation, the order did not affect rates of AF recurrence. However, in those who underwent HFS-guided GPA, patients who received right sided ablation first had significantly higher AF recurrence than those with left sided ablation first (p=0.016), suggesting that HFS-guided ablation may cause incomplete ablation when approached from the right.
Ultimately, the efficacy of neuromodulation treatments is influenced by many factors. Which cells are responsible for ablation outcomes is not known. Not only are both sympathetic and parasympathetic cells highly co-localized34, but also ablation damages both neurons and glial cells117. In an immunohistochemical study of S100B-expressing cells in the ICNS, S100B+ glial cells were found in high density surrounding neuronal cell bodies, which in turn expressed the S100B receptor118. In the same study, of 112 patients with AF receiving PVI, reduced AF recurrence was associated with increased S100B following ablation118, suggesting that the extent of glial cell injury may influence ablation efficacy.
Furthermore, in their study of inputs from porcine right atrial GP on the SA node, Hanna, et al. identified a diversity of gene and protein receptor expression, giving rise to highly heterogeneous neuronal subpopulations by transcriptome analysis117. The authors postulated that the heterogeneity in neuronal subtypes within GP may contribute to the heterogeneity in GPA results. Interestingly, selectively ablating cardiac neuronal subpopulations has become an avenue of research for improving ablation outcomes, and has been the focus of some preclinical studies in attenuating apnea-induced AF119 or ventricular tachycardia/fibrillation subsequent to cardiac remodeling120, but has not yet been explored in humans.
Pharmacological Interventions
Studies have investigated botulinum toxin or calcium chloride injections into epicardial GP for preventing post-operative AF (POAF) (Supplementary Table 2). Botulinum toxin (BTX), the neurotoxin produced by Clostridium botulinum, interferes with parasympathetic signaling by blocking the release of acetylcholine from presynaptic vesicles121, while calcium chloride injections cause calcium induced neurotoxicity122.
Botulinum Toxin
In canines, BTX injection at sinus and AV nodal epicardial fat pads can temporarily suppress vagal AF for 1 week123. Similarly, BTX injection into the ARGP, IRGP, SLGP and ILGP temporarily prolongs AERPs and eliminates bradycardic responses, while producing a more prolonged effect on diminishing AF burden at 3 months in canines124.
In a pilot study, Pokushalov, et al. randomized 60 patients with PAF undergoing coronary artery bypass grafting (CABG) to either LA epicardial BTX injection or placebo. The BTX group not only had significantly less POAF (p=0.024), but also had 0% recurrent AF at 1 year compared to 27% of the placebo group (p=0.02)125, 126. A subsequent randomized clinical trial (N=130) investigated peri-operative BTX injection in both LA and anterior epicardial fat pads during cardiac surgery127. There was an 11% lower absolute risk of POAF in the BTX group compared with placebo that did not meet statistical significance, potentially due to lower than anticipated power. The patient population in this study was higher-risk (compared to the Pokushalov study), included patients undergoing valve surgery, had higher median age, and high prevalence of COPD; consequently, included patients may have had more arrhythmogenic substrate. Additionally, few patients had prior PAF, and those with a history of persistent AF were excluded. Because patients with AF are known to have GP hyperactivity when compared to healthy controls128, it is possible that in patients without prior AF, the pathophysiology of POAF is less related to autonomic remodeling and GP hyperactivity and rather due to inflammation or sympathetic hyperactivity129. A multicenter, international Phase 2 randomized clinical trial evaluating BTX for POAF after cardiac surgery is currently under way (NCT03779841)130.
Calcium Chloride Injection
Calcium chloride injection induces apoptosis within cardiac GP and prevents POAF in animal studies131. In a randomized clinical trial, 200 patients without AF were randomized to calcium chloride injection of the major GP or placebo during CABG132. At 7 days, only 15% of patients receiving calcium chloride injection developed POAF compared to 36% of placebo (p=0.001); those who developed AF had significantly reduced AF burden. Studies of HRV demonstrated a balanced reduction in parasympathetic and sympathetic tone.
Renal Denervation
Renal denervation (RDN) involves ablating renal afferent and efferent sympathetic nerves with the purpose of reducing sympathetic tone133. In animal models, RDN reduces blood pressure, progression of kidney injury, cardiac fibrosis and diastolic dysfunction134. While RDN was less promising in treating resistant hypertension in the SIMPLICITY-HTN-3 trial48, some suggest these results may have been influenced by incomplete denervation135. Nevertheless, RDN may abate AF through reducing overall sympathetic tone.
In the multicenter, single-blind ERADICATE-AF trial, 302 patients with PAF were randomized to either (1) PVI alone (N=148) or (2) PVI+RDN (N=154)49. After 12 months, the PVI+RDN group had significantly higher freedom from AF, atrial flutter, or tachycardia (72.1% vs 56.5%, p=0.006), with no significant difference in procedural complications. The benefit of RDN in patients with persistent AF has been demonstrated in smaller studies136, and will be further evaluated in an ongoing clinical trial (NCT04055285).
In a meta-analysis that included 6 trials (N=725 patients), adjunctive RDN significantly reduced recurrent AF compared to PVI alone (Risk Ratio 0.68, 95% CI 0.55–0.83, p=0.0002)137. Another trial in patients with paroxysmal or persistent AF and refractory hypertension trended towards increased efficacy in the ablation plus RDN group without reaching statistical significance at one year (p=0.34)138, but is still undergoing extended follow-up and patient recruitment. Additional randomized clinical trials are ongoing (NCT01990911, NCT02115100), including a trial in ultrasound-based RDN (NCT04182620).
Low-Level Vagal Nerve Stimulation
In a small study of patients with PAF, 1 hour of low-level tragus stimulation during AF ablation significantly suppressed pacing-induced AF139. In a sham-controlled, double-blind, randomized clinical trial in the ambulatory setting, patients with PAF received 1 hour of low-level tragus stimulation per day over 6 months via an ear clip (N=26) or sham control (N=27)140. Median AF burden in those receiving stimulation was 85% lower than in the control group, with no device related side effects. In the stimulation arm, device compliance decreased over time (from 86% at 1 month to 74% at 6 months), and AF burden was higher at baseline in the treatment group, possibly amplifying the intervention’s effects141. In another randomized study, low-level vagal stimulation delivered via a bipolar wire sutured to vagal pre-ganglionic fibers alongside the superior vena cava decreased the incidence of POAF142.
Stimulation parameters and patient selection optimization are crucial in adequately understanding the efficacy of neuromodulation treatments. The heterogeneity of prior studies in nerve stimulation for heart failure has been attributed to varying site, strength, and cycles of stimulation143, 144. Currently, there are few studies investigating nerve stimulation for arrhythmia suppression. Future studies should determine the comparative safety and efficacy of implantable or wearable devices, as well as define optimal stimulation parameters. Finally, while not directly related to vagus nerve stimulation, a pilot randomized study recently found that temporary spinal cord stimulation decreased POAF incidence from 31% to 4%145.
Stellate Ganglion Block
In a placebo-controlled study, 36 patients with PAF were randomized to temporary, transcutaneous stellate ganglion block (SGB) unilaterally with lidocaine, or placebo146. Prior to SGB, AF was inducible in all patients by atrial pacing; after SGB, AF was inducible in only 54% of patients (p < 0.01) and had significantly shorter duration (p < 0.01). The same results were achieved regardless of right-sided versus left-sided SGB. A pilot study of 25 patients undergoing cardiac surgery found that left-sided SGB had a lower POAF rate than the institution’s average (18.2% versus 27%)147. A larger, randomized clinical trial of 200 patients undergoing lung lobectomy similarly found that patients randomized to right-sided SGB had significantly lower POAF (3% versus 10%, p=0.045). Interestingly, the SGB group also had lower WBC counts, suggesting a potential interaction between autonomics and inflammation. The long-term effects of SGB on AF have yet to be studied.
Conclusions
Atrial fibrillation has a multifaceted, complex pathophysiology, of which cardiac autonomic innervation plays a central role. Modulation of the neurocardiac axis has emerged as an innovative therapy with varying efficacy. As our understanding of atrial fibrillation and the ICNS expands, our approach to therapeutic neuromodulation will continue to evolve for the benefit of those with AF.
Supplementary Material
Footnotes
Disclosures
MK reports no relevant disclosures. MF is supported by the National Heart, Lung, and Blood Institute (NHLBI) (K23HL151744), the American Heart Association (20IPA35310955), Mario Family Award, Duke Chair’s Award, Translating Duke Health Award, Bayer, Bodyport and BTG Specialty Pharmaceuticals. He receives consulting fees from Abbott, Audicor, AxonTherapies, Bodyguide, Bodyport, Boston Scientific, CVRx, Daxor, Edwards LifeSciences, Feldschuh Foundation, Fire1, Gradient, Intershunt, NXT Biomedical, Pharmacosmos, PreHealth, Shifamed, Splendo, Vironix, Viscardia, Zoll. JPP is supported by R01AG074185 from the National Institutes of Aging. He also receives grants for clinical research from Abbott, American Heart Association, Association for the Advancement of Medical Instrumentation, Bayer, Boston Scientific, and Philips and serves as a consultant to Abbott, Abbvie, Ablacon, Altathera, Biotronik, Boston Scientific, Bristol Myers Squibb, LivaNova, Medtronic, Milestone, ElectroPhysiology Frontiers, Pfizer, Sanofi, Philips, and Up-to-Date.
REFERENCES
- 1.Staerk L, Wang B, Preis SR, et al. Lifetime risk of atrial fibrillation according to optimal, borderline, or elevated levels of risk factors. BMJ Apr 26 2018;361:k1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kornej J, Börschel CS, Benjamin EJ, Schnabel RB. Epidemiology of Atrial Fibrillation in the 21st Century. Circulation Research 2020;127:4–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhatt HV, Fischer GW. Atrial Fibrillation: Pathophysiology and Therapeutic Options. J Cardiothorac Vasc Anesth 2015;29:1333–1340. [DOI] [PubMed] [Google Scholar]
- 4.de Vos CB, Nieuwlaat R, Crijns HJGM, et al. Autonomic trigger patterns and anti-arrhythmic treatment of paroxysmal atrial fibrillation. EHJ 2008;29:632–639. [DOI] [PubMed] [Google Scholar]
- 5.January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation. JACC 2014;64:e1–e76. [DOI] [PubMed] [Google Scholar]
- 6.Hindricks G, Potpara T, Dagres N, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). EHJ 2020;42:373–498. [Google Scholar]
- 7.Kennedy A, Finlay DD, Guldenring D, Bond R, Moran K, McLaughlin J. The Cardiac Conduction System: Generation and Conduction of the Cardiac Impulse. Crit Care Nurs Clin North Am 2016;28:269–279. [DOI] [PubMed] [Google Scholar]
- 8.Hanna P, Rajendran PS, Ajijola OA, et al. Cardiac neuroanatomy - Imaging nerves to define functional control. Autonomic Neuroscience 2017;207:48–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beissner F, Meissner K, Bar KJ, Napadow V. The Autonomic Brain: An Activation Likelihood Estimation Meta-Analysis for Central Processing of Autonomic Function. Journal of Neuroscience 2013;33:10503–10511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kawashima T The autonomic nervous system of the human heart with special reference to its origin, course, and peripheral distribution. Anatomy and Embryology 2005;209:425–438. [DOI] [PubMed] [Google Scholar]
- 11.Gordan R, Gwathmey JK, Xie LH. Autonomic and endocrine control of cardiovascular function. World J Cardiol 2015;7:204–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lohse MJ, Engelhardt S, Eschenhagen T. What Is the Role of B2-Adrenergic Signaling in Heart Failure? Circulation Research 2003;93:896–906. [DOI] [PubMed] [Google Scholar]
- 13.DiFrancesco D, Tortora P. Direct activation of cardiac pacemaker channels by intracellular cyclic AMP. Nature 1991;351:145–147. [DOI] [PubMed] [Google Scholar]
- 14.Porro A, Thiel G, Moroni A, Saponaro A. cyclic AMP Regulation and Its Command in the Pacemaker Channel HCN4. Frontiers in Physiology 2020;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Behar J, Ganesan A, Zhang J, Yaniv Y. The Autonomic Nervous System Regulates the Heart Rate through cAMP-PKA Dependent and Independent Coupled-Clock Pacemaker Cell Mechanisms. Frontiers in Physiology 2016;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patterson E, Lazzara R, Szabo B, et al. Sodium-Calcium Exchange Initiated by the Ca2+Transient: An Arrhythmia Trigger Within Pulmonary Veins. JACC 2006;47:1196–1206. [DOI] [PubMed] [Google Scholar]
- 17.Krapivinsky G, Gordon EA, Wickman K, Velimirović B, Krapivinsky L, Clapham DE. The G-protein-gated atrial K+ channel IKAch is a heteromultimer of two inwardly rectifying K+-channel proteins. Nature 1995;374:135–141. [DOI] [PubMed] [Google Scholar]
- 18.Wickman K, Krapivinsky G, Corey S, et al. Structure, G Protein Activation, and Functional Relevance of the Cardiac G Protein-Gated K+ Channel, IKACh. Annals of the New York Academy of Sciences 1999;868:386–398. [DOI] [PubMed] [Google Scholar]
- 19.Chen P-S, Tan AY. Autonomic nerve activity and atrial fibrillation. Heart Rhythm 2007;4:S61–S64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hasan W Autonomic cardiac innervation. Organogenesis 2013;9:176–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pauza DH, Skripka V, Pauziene N, Stropus R. Morphology, distribution, and variability of the epicardiac neural ganglionated subplexuses in the human heart. Anat Rec 2000;259:353–382. [DOI] [PubMed] [Google Scholar]
- 22.Pauza DH, Pauziene N, Tamasauskas KA, Stropus R. Hilum of the heart. Anat Rec 1997;248:322–324. [DOI] [PubMed] [Google Scholar]
- 23.Avazzadeh S, McBride S, O’Brien B, et al. Ganglionated Plexi Ablation for the Treatment of Atrial Fibrillation. J Clin Med 2020;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herring N, Paterson DJ. The Heart’s Little Brain: Shedding New Light and CLARITY on the “Black Box”. Circ Res 2021;128:1297–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stavrakis S, Po S. Ganglionated Plexi Ablation: Physiology and Clinical Applications. Arrhythm Electrophysiol Rev 2017;6:186–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Armour JA, Murphy DA, Yuan BX, Macdonald S, Hopkins DA. Gross and microscopic anatomy of the human intrinsic cardiac nervous system. Anat Rec Feb 1997;247:289–298. [DOI] [PubMed] [Google Scholar]
- 27.Po SS, Nakagawa H, Jackman WM. Localization of left atrial ganglionated plexi in patients with atrial fibrillation. J Cardiovasc Electrophysiol Oct 2009;20:1186–1189. [DOI] [PubMed] [Google Scholar]
- 28.Hou Y, Scherlag BJ, Lin J, Zhang Y, Lu Z, Truong K, Patterson E, Lazzara R, Jackman WM, Po SS. Ganglionated Plexi Modulate Extrinsic Cardiac Autonomic Nerve Input: Effects on Sinus Rate, Atrioventricular Conduction, Refractoriness, and Inducibility of Atrial Fibrillation. Journal of the American College of Cardiology 2007/July/03/ 2007;50:61–68. [DOI] [PubMed] [Google Scholar]
- 29.Hou Y, Scherlag BJ, Lin J, Zhou J, Song J, Zhang Y, Patterson E, Lazzara R, Jackman WM, Po SS. Interactive atrial neural network: Determining the connections between ganglionated plexi. Heart Rhythm Jan 2007;4:56–63. [DOI] [PubMed] [Google Scholar]
- 30.Aksu T, Gupta D, Pauza DH. Anatomy and Physiology of Intrinsic Cardiac Autonomic Nervous System. JACC: Case Reports 2021;3:625–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haïssaguerre M, Jaïs P, Shah DC, Takahashi A, Hocini M, Quiniou G, Garrigue S, Le Mouroux A, Le Métayer P, Clémenty J. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med Sep 3 1998;339:659–666. [DOI] [PubMed] [Google Scholar]
- 32.Kircher S, Sommer P. Electrophysiological Evaluation of Pulmonary Vein Isolation. J Atr Fibrillation Oct-Nov 2013;6:934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nathan H, Eliakim M. The Junction Between the Left Atrium and the Pulmonary Veins. Circulation 1966;34:412–422. [DOI] [PubMed] [Google Scholar]
- 34.Tan AY, Li H, Wachsmann-Hogiu S, Chen LS, Chen PS, Fishbein MC. Autonomic innervation and segmental muscular disconnections at the human pulmonary vein-atrial junction: implications for catheter ablation of atrial-pulmonary vein junction. J Am Coll Cardiol Jul 4 2006;48:132–143. [DOI] [PubMed] [Google Scholar]
- 35.Arentz T, Weber R, Bürkle G, Herrera C, Blum T, Stockinger J, Minners J, Neumann FJ, Kalusche D. Small or large isolation areas around the pulmonary veins for the treatment of atrial fibrillation? Results from a prospective randomized study. Circulation Jun 19 2007;115:3057–3063. [DOI] [PubMed] [Google Scholar]
- 36.Pappone C, Santinelli V, Manguso F, et al. Pulmonary Vein Denervation Enhances Long-Term Benefit After Circumferential Ablation for Paroxysmal Atrial Fibrillation. Circulation 2004;109:327–334. [DOI] [PubMed] [Google Scholar]
- 37.Hwang C, Chen P-S. Ligament of Marshall: Why it is important for atrial fibrillation ablation. Heart Rhythm 2009;6:S35–S40. [DOI] [PubMed] [Google Scholar]
- 38.Rodríguez-Mañero M, Schurmann P, Valderrábano M. Ligament and vein of Marshall: A therapeutic opportunity in atrial fibrillation. Heart Rhythm Feb 2016;13:593–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kamanu S, Tan AY, Peter CT, Hwang C, Chen PS. Vein of Marshall activity during sustained atrial fibrillation. J Cardiovasc Electrophysiol Aug 2006;17:839–846. [DOI] [PubMed] [Google Scholar]
- 40.Doshi RN, Wu TJ, Yashima M, Kim YH, Ong JJ, Cao JM, Hwang C, Yashar P, Fishbein MC, Karagueuzian HS, Chen PS. Relation between ligament of Marshall and adrenergic atrial tachyarrhythmia. Circulation Aug 24 1999;100:876–883. [DOI] [PubMed] [Google Scholar]
- 41.Ulphani JS, Arora R, Cain JH, Villuendas R, Shen S, Gordon D, Inderyas F, Harvey LA, Morris A, Goldberger JJ, Kadish AH. The ligament of Marshall as a parasympathetic conduit. Am J Physiol Heart Circ Physiol Sep 2007;293:H1629–1635. [DOI] [PubMed] [Google Scholar]
- 42.Valderrábano M, Peterson LE, Swarup V, et al. Effect of Catheter Ablation With Vein of Marshall Ethanol Infusion vs Catheter Ablation Alone on Persistent Atrial Fibrillation: The VENUS Randomized Clinical Trial. Jama Oct 27 2020;324:1620–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hu TY, Kapa S, Cha YM, Asirvatham SJ, Madhavan M. Swallow-induced syncope: A case report of atrial tachycardia originating from the SVC. HeartRhythm Case Rep Jan 2016;2:83–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chiou CW, Eble JN, Zipes DP. Efferent vagal innervation of the canine atria and sinus and atrioventricular nodes. The third fat pad. Circulation Jun 3 1997;95:2573–2584. [DOI] [PubMed] [Google Scholar]
- 45.Ghias M, Scherlag BJ, Lu Z, Niu G, Moers A, Jackman WM, Lazzara R, Po SS. The role of ganglionated plexi in apnea-related atrial fibrillation. J Am Coll Cardiol Nov 24 2009;54:2075–2083. [DOI] [PubMed] [Google Scholar]
- 46.Stella A The kidney as a sensor: functional evidence. J Hypertens Suppl Dec 1992;10:S113–119. [PubMed] [Google Scholar]
- 47.Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE, Böhm M. Renal sympathetic denervation in patients with treatment-resistant hypertension (The Symplicity HTN-2 Trial): a randomised controlled trial. Lancet Dec 4 2010;376:1903–1909. [DOI] [PubMed] [Google Scholar]
- 48.Bhatt DL, Kandzari DE, O’Neill WW, et al. A controlled trial of renal denervation for resistant hypertension. N Engl J Med Apr 10 2014;370:1393–1401. [DOI] [PubMed] [Google Scholar]
- 49.Steinberg JS, Shabanov V, Ponomarev D, Losik D, Ivanickiy E, Kropotkin E, Polyakov K, Ptaszynski P, Keweloh B, Yao CJ, Pokushalov EA, Romanov AB. Effect of Renal Denervation and Catheter Ablation vs Catheter Ablation Alone on Atrial Fibrillation Recurrence Among Patients With Paroxysmal Atrial Fibrillation and Hypertension: The ERADICATE-AF Randomized Clinical Trial. Jama Jan 21 2020;323:248–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Coumel P Autonomic influences in atrial tachyarrhythmias. J Cardiovasc Electrophysiol Oct 1996;7:999–1007. [DOI] [PubMed] [Google Scholar]
- 51.Staerk L, Preis SR, Lin H, Lubitz SA, Ellinor PT, Levy D, Benjamin EJ, Trinquart L. Protein Biomarkers and Risk of Atrial Fibrillation. Circulation: Arrhythmia and Electrophysiology 2020;13:e007607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schnabel RB, Larson MG, Yamamoto JF, et al. Relations of Biomarkers of Distinct Pathophysiological Pathways and Atrial Fibrillation Incidence in the Community. Circulation 2010;121:200–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sinner MF, Stepas KA, Moser CB, et al. B-type natriuretic peptide and C-reactive protein in the prediction of atrial fibrillation risk: the CHARGE-AF Consortium of community-based cohort studies. EP Europace 2014;16:1426–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lam CSP, Chen M-H, Lacey SM, Yang Q, Sullivan LM, Xanthakis V, Safa R, Smith HM, Peng X, Sawyer DB, Vasan RS. Circulating Insulin-Like Growth Factor-1 and Its Binding Protein-3. Arteriosclerosis, Thrombosis, and Vascular Biology 2010;30:1479–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Malik M, Bigger JT, Camm AJ, Kleiger RE, Malliani A, Moss AJ, Schwartz PJ. Heart rate variability: Standards of measurement, physiological interpretation, and clinical use. European Heart Journal 1996;17:354–381. [PubMed] [Google Scholar]
- 56.Malliani A, Pagani M, Lombardi F, Cerutti S. Cardiovascular neural regulation explored in the frequency domain. Circulation Aug 1991;84:482–492. [DOI] [PubMed] [Google Scholar]
- 57.Kim SH, Lim KR, Seo J-H, Ryu DR, Lee B-K, Cho B-R, Chun KJ. Higher heart rate variability as a predictor of atrial fibrillation in patients with hypertension. Scientific Reports 2022/March/08 2022;12:3702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Agarwal SK, Norby FL, Whitsel EA, Soliman EZ, Chen LY, Loehr LR, Fuster V, Heiss G, Coresh J, Alonso A. Cardiac Autonomic Dysfunction and Incidence of Atrial Fibrillation: Results From 20 Years Follow-Up. Journal of the American College of Cardiology 2017/January/24/ 2017;69:291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Perkiömäki J, Ukkola O, Kiviniemi A, Tulppo M, Ylitalo A, Kesäniemi YA, Huikuri H. Heart Rate Variability Findings as a Predictor of Atrial Fibrillation in Middle-Aged Population. Journal of Cardiovascular Electrophysiology 2014;25:719–724. [DOI] [PubMed] [Google Scholar]
- 60.Bettoni M, Zimmermann M. Autonomic tone variations before the onset of paroxysmal atrial fibrillation. Circulation Jun 11 2002;105:2753–2759. [DOI] [PubMed] [Google Scholar]
- 61.Sharifov Oleg F, Fedorov Vadim V, Beloshapko Galina G, Glukhov Alexey V, Yushmanova Anna V, Rosenshtraukh Leonid V. Roles of adrenergic and cholinergic stimulation in spontaneous atrial fibrillation in dogs. Journal of the American College of Cardiology 2004/February/04 2004;43:483–490. [DOI] [PubMed] [Google Scholar]
- 62.Takei M, Furukawa Y, Narita M, Ren LM, Karasawa Y, Murakami M, Chiba S. Synergistic nonuniform shortening of atrial refractory period induced by autonomic stimulation. American Journal of Physiology-Heart and Circulatory Physiology 1991;261:H1988–H1993. [DOI] [PubMed] [Google Scholar]
- 63.Waldron NH, Fudim M, Mathew JP, Piccini JP. Neuromodulation for the Treatment of Heart Rhythm Disorders. JACC Basic Transl Sci Aug 2019;4:546–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pellman J, Sheikh F. Atrial fibrillation: mechanisms, therapeutics, and future directions. Compr Physiol Apr 2015;5:649–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Landstrom AP, Dobrev D, Wehrens XHT. Calcium Signaling and Cardiac Arrhythmias. Circulation Research 2017;120:1969–1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.ZIPES DP MIHALICK, ROBBINS GT. MJ Effects of selective vagal and stellate ganglion stimulation on atrial refractoriness1. Cardiovascular Research 1974;8:647–655. [DOI] [PubMed] [Google Scholar]
- 67.HIROSE M LEATMANORATN, LAURITA KR Z, CARLSON MD. Partial Vagal Denervation Increases Vulnerability to Vagally Induced Atrial Fibrillation. Journal of Cardiovascular Electrophysiology 2002;13:1272–1279. [DOI] [PubMed] [Google Scholar]
- 68.Po SS, Li Y, Tang D, Liu H, Geng N, Jackman WM, Scherlag B, Lazzara R, Patterson E. Rapid and Stable Re-Entry Within the Pulmonary Vein as a Mechanism Initiating Paroxysmal Atrial Fibrillation. Journal of the American College of Cardiology 2005;45:1871–1877. [DOI] [PubMed] [Google Scholar]
- 69.Wijffels MCEF Kirchhof CJHJ, Dorland R Allessie MA. Atrial Fibrillation Begets Atrial Fibrillation. Circulation 1995;92:1954–1968. [DOI] [PubMed] [Google Scholar]
- 70.Yu W-C, Chen S-A, Lee S-H, Tai C-T, Feng A-N, Kuo BI-T, Ding Y-A, Chang M-S. Tachycardia-Induced Change of Atrial Refractory Period in Humans. Circulation 1998;97:2331–2337. [DOI] [PubMed] [Google Scholar]
- 71.Chen Y-J, Chen S-A, Tai C-T, Wen Z-C, Feng A-N, Ding Y-A, Chang M-S. Role of atrial electrophysiology and autonomic nervous system in patients with supraventricular tachycardia and paroxysmal atrial fibrillation. Journal of the American College of Cardiology 1998;32:732–738. [DOI] [PubMed] [Google Scholar]
- 72.Jaïs P, Hocini M, Macle L, et al. Distinctive Electrophysiological Properties of Pulmonary Veins in Patients With Atrial Fibrillation. Circulation 2002;106:2479–2485. [DOI] [PubMed] [Google Scholar]
- 73.Nattel S, Dobrev D. The multidimensional role of calcium in atrial fibrillation pathophysiology: mechanistic insights and therapeutic opportunities. European Heart Journal 2012;33:1870–1877. [DOI] [PubMed] [Google Scholar]
- 74.Yeh Y-H, Wakili R, Qi X-Y, Chartier D, Boknik P, Kääb S, Ravens U, Coutu P, Dobrev D, Nattel S. Calcium-Handling Abnormalities Underlying Atrial Arrhythmogenesis and Contractile Dysfunction in Dogs With Congestive Heart Failure. Circulation: Arrhythmia and Electrophysiology 2008;1:93–102. [DOI] [PubMed] [Google Scholar]
- 75.January CT, Riddle JM. Early afterdepolarizations: mechanism of induction and block. A role for L-type Ca2+ current. Circulation Research 1989;64:977–990. [DOI] [PubMed] [Google Scholar]
- 76.Berlin JR, Cannell MB, Lederer WJ. Cellular origins of the transient inward current in cardiac myocytes. Role of fluctuations and waves of elevated intracellular calcium. Circulation Research 1989;65:115–126. [DOI] [PubMed] [Google Scholar]
- 77.Horváth B, Hézső T, Kiss D, Kistamás K, Magyar J, Nánási PP, Bányász T. Late Sodium Current Inhibitors as Potential Antiarrhythmic Agents. Frontiers in Pharmacology 2020-April-20 2020;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Coraboeuf E, Deroubaix E, Coulombe A. Effect of tetrodotoxin on action potentials of the conducting system in the dog heart. American Journal of Physiology-Heart and Circulatory Physiology 1979;236:H561–H567. [DOI] [PubMed] [Google Scholar]
- 79.Marionneau C, Abriel H. Cardiac Sodium Current Under Sympathetic Control. Circulation Research 2019;124:674–676. [DOI] [PubMed] [Google Scholar]
- 80.Hegyi B, Bányász T, Izu LT, Belardinelli L, Bers DM, Chen-Izu Y. β-adrenergic regulation of late Na+ current during cardiac action potential is mediated by both PKA and CaMKII. Journal of Molecular and Cellular Cardiology 2018/October/01/ 2018;123:168–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Muñoz MA, Kaur J, Vigmond EJ. Onset of atrial arrhythmias elicited by autonomic modulation of rabbit sinoatrial node activity: a modeling study. Am J Physiol Heart Circ Physiol Nov 2011;301:H1974–1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lan BL, Liew YW, Toda M, Kamsani SH. Flickering of cardiac state before the onset and termination of atrial fibrillation. Chaos May 2020;30:053137. [DOI] [PubMed] [Google Scholar]
- 83.Burashnikov A, Antzelevitch C. Reinduction of Atrial Fibrillation Immediately After Termination of the Arrhythmia Is Mediated by Late Phase 3 Early Afterdepolarization–Induced Triggered Activity. Circulation 2003;107:2355–2360. [DOI] [PubMed] [Google Scholar]
- 84.Degaute JP, Borne Pvd, Linkowski P, Cauter EV. Quantitative analysis of the 24-hour blood pressure and heart rate patterns in young men. Hypertension 1991;18:199–210. [DOI] [PubMed] [Google Scholar]
- 85.Irwin JM, McCarthy EA, Wilkinson WE, Pritchett EL. Circadian occurrence of symptomatic paroxysmal supraventricular tachycardia in untreated patients. Circulation 1988;77:298–300. [DOI] [PubMed] [Google Scholar]
- 86.Behrens S, Galecka M, Brüggemann T, Ehlers C, Willich SN, Ziss W, Dissmann R, Andresen D. Circadian variation of sustained ventricular tachyarrhythmias terminated by appropriate shocks in patients with an implantable cardioverter defibrillator. American Heart Journal 1995/July/01/ 1995;130:79–84. [DOI] [PubMed] [Google Scholar]
- 87.Aronow WS, Ahn C. Circadian variation of primary cardiac arrest or sudden cardiac death in patients aged 62 to 100 years (mean 82). The American Journal of Cardiology 1993/June/15/ 1993;71:1455–1456. [DOI] [PubMed] [Google Scholar]
- 88.Portaluppi F, Hermida RC. Circadian rhythms in cardiac arrhythmias and opportunities for their chronotherapy. Adv Drug Deliv Rev Aug 31 2007;59:940–951. [DOI] [PubMed] [Google Scholar]
- 89.SHUSTERMAN V WARMAN, LONDON B E, SCHWARTZMAN D Nocturnal Peak in Atrial Tachyarrhythmia Occurrence as a Function of Arrhythmia Burden. Journal of Cardiovascular Electrophysiology 2012;23:604–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Black N, D’Souza A, Wang Y, Piggins H, Dobrzynski H, Morris G, Boyett MR. Circadian rhythm of cardiac electrophysiology, arrhythmogenesis, and the underlying mechanisms. Heart Rhythm Feb 2019;16:298–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang A, Green JB, Halperin JL, Piccini JP Atrial Fibrillation and Diabetes Mellitus: JACC Review Topic of the Week. J Am Coll Cardiol Aug 27 2019;74:1107–1115. [DOI] [PubMed] [Google Scholar]
- 92.Kuehl M, Stevens MJ. Cardiovascular autonomic neuropathies as complications of diabetes mellitus. Nature Reviews Endocrinology 2012/July/01 2012;8:405–416. [DOI] [PubMed] [Google Scholar]
- 93.Rizzo MR, Sasso FC, Marfella R, et al. Autonomic dysfunction is associated with brief episodes of atrial fibrillation in type 2 diabetes. Journal of Diabetes and its Complications 2015/January/01/ 2015;29:88–92. [DOI] [PubMed] [Google Scholar]
- 94.Zhou M, Wang H, Chen J, Zhao L. Epicardial adipose tissue and atrial fibrillation: Possible mechanisms, potential therapies, and future directions. Pacing Clin Electrophysiol Jan 2020;43:133–145. [DOI] [PubMed] [Google Scholar]
- 95.Zhou J, Scherlag BJ, Edwards J, Jackman WM, Lazzara R, Po SS. Gradients of atrial refractoriness and inducibility of atrial fibrillation due to stimulation of ganglionated plexi. J Cardiovasc Electrophysiol Jan 2007;18:83–90. [DOI] [PubMed] [Google Scholar]
- 96.Scherlag BJ, Yamanashi W, Patel U, Lazzara R, Jackman WM. Autonomically induced conversion of pulmonary vein focal firing into atrial fibrillation. J Am Coll Cardiol Jun 7 2005;45:1878–1886. [DOI] [PubMed] [Google Scholar]
- 97.Elvan A, Pride HP, Eble JN, Zipes DP. Radiofrequency catheter ablation of the atria reduces inducibility and duration of atrial fibrillation in dogs. Circulation Apr 15 1995;91:2235–2244. [DOI] [PubMed] [Google Scholar]
- 98.LIM PB MALCOLME-LAWESLC, STUBER T, WRIGHT I, FRANCIS DP, DAVIES DW, PETERS NS, KANAGARATNAM P Intrinsic Cardiac Autonomic Stimulation Induces Pulmonary Vein Ectopy and Triggers Atrial Fibrillation in Humans. Journal of Cardiovascular Electrophysiology 2011;22:638–646. [DOI] [PubMed] [Google Scholar]
- 99.Kurotobi T, Shimada Y, Kino N, Ito K, Tonomura D, Yano K, Tanaka C, Yoshida M, Tsuchida T, Fukumoto H. Features of intrinsic ganglionated plexi in both atria after extensive pulmonary isolation and their clinical significance after catheter ablation in patients with atrial fibrillation. Heart rhythm 2015/March// 2015;12:470–476. [DOI] [PubMed] [Google Scholar]
- 100.Schauerte P, Scherlag BJ, Pitha J, Scherlag MA, Reynolds D, Lazzara R, Jackman WM. Catheter Ablation of Cardiac Autonomic Nerves for Prevention of Vagal Atrial Fibrillation. Circulation 2000;102:2774–2780. [DOI] [PubMed] [Google Scholar]
- 101.Stirrup J, Gregg S, Baavour R, Roth N, Breault C, Agostini D, Ernst S, Underwood SR. Hybrid solid-state SPECT/CT left atrial innervation imaging for identification of left atrial ganglionated plexi: Technique and validation in patients with atrial fibrillation. J Nucl Cardiol Dec 2020;27:1939–1950. [DOI] [PubMed] [Google Scholar]
- 102.Kim M-Y, Coyle C, Tomlinson DR, et al. Ectopy-triggering ganglionated plexuses ablation to prevent atrial fibrillation: GANGLIA-AF study. Heart Rhythm 2022/April/01/ 2022;19:516–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Biase LD, Mohanty P, Mohanty S, et al. Ablation Versus Amiodarone for Treatment of Persistent Atrial Fibrillation in Patients With Congestive Heart Failure and an Implanted Device. Circulation 2016;133:1637–1644. [DOI] [PubMed] [Google Scholar]
- 104.January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration With the Society of Thoracic Surgeons. Circulation 2019;140:e125–e151. [DOI] [PubMed] [Google Scholar]
- 105.Oral H, Pappone C, Chugh A, et al. Circumferential Pulmonary-Vein Ablation for Chronic Atrial Fibrillation. New England Journal of Medicine 2006;354:934–941. [DOI] [PubMed] [Google Scholar]
- 106.Lemery R, Birnie D, Tang AS, Green M, Gollob M. Feasibility study of endocardial mapping of ganglionated plexuses during catheter ablation of atrial fibrillation. Heart Rhythm Apr 2006;3:387–396. [DOI] [PubMed] [Google Scholar]
- 107.Katritsis D, Giazitzoglou E, Sougiannis D, Goumas N, Paxinos G, Camm AJ. Anatomic approach for ganglionic plexi ablation in patients with paroxysmal atrial fibrillation. Am J Cardiol Aug 1 2008;102:330–334. [DOI] [PubMed] [Google Scholar]
- 108.Pokushalov E, Romanov A, Shugayev P, Artyomenko S, Shirokova N, Turov A, Katritsis DG. Selective ganglionated plexi ablation for paroxysmal atrial fibrillation. Heart Rhythm Sep 2009;6:1257–1264. [DOI] [PubMed] [Google Scholar]
- 109.Katritsis DG, Pokushalov E, Romanov A, Giazitzoglou E, Siontis GCM, Po SS, Camm AJ, Ioannidis JPA. Autonomic Denervation Added to Pulmonary Vein Isolation for Paroxysmal Atrial Fibrillation. Journal of the American College of Cardiology 2013;62:2318–2325. [DOI] [PubMed] [Google Scholar]
- 110.Danik S, Neuzil P, d’Avila A, Malchano ZJ, Kralovec S, Ruskin JN, Reddy VY. Evaluation of catheter ablation of periatrial ganglionic plexi in patients with atrial fibrillation. Am J Cardiol Sep 1 2008;102:578–583. [DOI] [PubMed] [Google Scholar]
- 111.Pokushalov E, Romanov A, Katritsis DG, Artyomenko S, Shirokova N, Karaskov A, Mittal S, Steinberg JS. Ganglionated plexus ablation vs linear ablation in patients undergoing pulmonary vein isolation for persistent/long-standing persistent atrial fibrillation: a randomized comparison. Heart Rhythm Sep 2013;10:1280–1286. [DOI] [PubMed] [Google Scholar]
- 112.Driessen AHG, Berger WR, Krul SPJ, van den Berg NWE, Neefs J, Piersma FR, Chan Pin Yin D, de Jong J, van Boven WP, de Groot JR. Ganglion Plexus Ablation in Advanced Atrial Fibrillation: The AFACT Study. J Am Coll Cardiol Sep 13 2016;68:1155–1165. [DOI] [PubMed] [Google Scholar]
- 113.Bárta J, Brát R. Assessment of the effect of left atrial cryoablation enhanced by ganglionated plexi ablation in the treatment of atrial fibrillation in patients undergoing open heart surgery. Journal of Cardiothoracic Surgery 2017/August/17 2017;12:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.DeLurgio DB, Crossen KJ, Gill J, et al. Hybrid Convergent Procedure for the Treatment of Persistent and Long-Standing Persistent Atrial Fibrillation. Circulation: Arrhythmia and Electrophysiology 2020;13:e009288. [DOI] [PubMed] [Google Scholar]
- 115.Lu Z, Scherlag BJ, Lin J, Yu L, Guo JH, Niu G, Jackman WM, Lazzara R, Jiang H, Po SS. Autonomic mechanism for initiation of rapid firing from atria and pulmonary veins: evidence by ablation of ganglionated plexi. Cardiovasc Res Nov 1 2009;84:245–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sharma PS, Kasirajan V, Ellenbogen KA, Koneru JN. Interconnections between Left Atrial Ganglionic Plexi: Insights from Minimally Invasive Maze Procedures and Their Outcomes. Pacing Clin Electrophysiol May 2016;39:427–433. [DOI] [PubMed] [Google Scholar]
- 117.Hanna P, Dacey MJ, Brennan J, et al. Innervation and Neuronal Control of the Mammalian Sinoatrial Node a Comprehensive Atlas. Circulation Research 2021;128:1279–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Scherschel K, Hedenus K, Jungen C, et al. Cardiac glial cells release neurotrophic S100B upon catheter-based treatment of atrial fibrillation. Science Translational Medicine 2019;11:eaav7770. [DOI] [PubMed] [Google Scholar]
- 119.Tavares L, Rodríguez-Mañero M, Kreidieh B, Ibarra-Cortez SH, Chen J, Wang S, Markovits J, Barrios R, Valderrábano M. Cardiac Afferent Denervation Abolishes Ganglionated Plexi and Sympathetic Responses to Apnea. Circulation: Arrhythmia and Electrophysiology 2019;12:e006942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yoshie K, Rajendran PS, Massoud L, Mistry J, Swid MA, Wu X, Sallam T, Zhang R, Goldhaber JI, Salavatian S, Ajijola OA. Cardiac TRPV1 afferent signaling promotes arrhythmogenic ventricular remodeling after myocardial infarction. JCI Insight Feb 13 2020;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pellizzari R, Rossetto O, Schiavo G, Montecucco C. Tetanus and botulinum neurotoxins: mechanism of action and therapeutic uses. Philos Trans R Soc Lond B Biol Sci Feb 28 1999;354:259–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Arundine M, Tymianski M. Molecular mechanisms of calcium-dependent neurodegeneration in excitotoxicity. Cell Calcium 2003/October/01/ 2003;34:325–337. [DOI] [PubMed] [Google Scholar]
- 123.Oh S, Choi EK, Zhang Y, Mazgalev TN. Botulinum toxin injection in epicardial autonomic ganglia temporarily suppresses vagally mediated atrial fibrillation. Circ Arrhythm Electrophysiol Aug 2011;4:560–565. [DOI] [PubMed] [Google Scholar]
- 124.Lo LW, Chang HY, Scherlag BJ, Lin YJ, Chou YH, Lin WL, Chen SA, Po SS. Temporary Suppression of Cardiac Ganglionated Plexi Leads to Long-Term Suppression of Atrial Fibrillation: Evidence of Early Autonomic Intervention to Break the Vicious Cycle of “AF Begets AF”. J Am Heart Assoc Jul 5 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Pokushalov E, Kozlov B, Romanov A, et al. Long-Term Suppression of Atrial Fibrillation by Botulinum Toxin Injection Into Epicardial Fat Pads in Patients Undergoing Cardiac Surgery. Circulation: Arrhythmia and Electrophysiology 2015;8:1334–1341. [DOI] [PubMed] [Google Scholar]
- 126.Pokushalov E, Kozlov B, Romanov A, et al. Botulinum Toxin Injection in Epicardial Fat Pads Can Prevent Recurrences of Atrial Fibrillation After Cardiac Surgery: Results of a Randomized Pilot Study. Journal of the American College of Cardiology 2014/August/12/ 2014;64:628–629. [DOI] [PubMed] [Google Scholar]
- 127.Waldron NH, Cooter M, Haney JC, et al. Temporary autonomic modulation with botulinum toxin type A to reduce atrial fibrillation after cardiac surgery. Heart Rhythm Feb 2019;16:178–184. [DOI] [PubMed] [Google Scholar]
- 128.Iso K, Okumura Y, Watanabe I, et al. Is Vagal Response During Left Atrial Ganglionated Plexi Stimulation a Normal Phenomenon? Circulation: Arrhythmia and Electrophysiology 2019;12:e007281. [DOI] [PubMed] [Google Scholar]
- 129.Dobrev D, Aguilar M, Heijman J, Guichard J-B, Nattel S. Postoperative atrial fibrillation: mechanisms, manifestations and management. Nature Reviews Cardiology 2019/July/01 2019;16:417–436. [DOI] [PubMed] [Google Scholar]
- 130.Piccini JP, Ahlsson A, Dorian P, et al. Design and Rationale of a Phase 2 Study of NeurOtoxin (Botulinum Toxin Type A) for the PreVention of Post-Operative Atrial Fibrillation - The NOVA Study. Am Heart J Oct 20 2021;245:51–59. [DOI] [PubMed] [Google Scholar]
- 131.O’Quinn MP, Dormer KJ, Huizar JF, Nguyen KT, Kaszala K, Sima A, Ellenbogen KA, Tan AY. Epicardial injection of nanoformulated calcium into cardiac ganglionic plexi suppresses autonomic nerve activity and postoperative atrial fibrillation. Heart Rhythm Apr 2019;16:597–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wang H, Zhang Y, Xin F, Jiang H, Tao D, Jin Y, He Y, Wang Q, Po SS. Calcium-Induced Autonomic Denervation in Patients With Post-Operative Atrial Fibrillation. J Am Coll Cardiol Jan 5 2021;77:57–67. [DOI] [PubMed] [Google Scholar]
- 133.Hering D, Lambert EA, Marusic P, Walton AS, Krum H, Lambert GW, Esler MD, Schlaich MP. Substantial Reduction in Single Sympathetic Nerve Firing After Renal Denervation in Patients With Resistant Hypertension. Hypertension 2013;61:457–464. [DOI] [PubMed] [Google Scholar]
- 134.Linz D, Hohl M, Schütze J, Mahfoud F, Speer T, Linz B, Hübschle T, Juretschke HP, Dechend R, Geisel J, Rütten H, Böhm M. Progression of kidney injury and cardiac remodeling in obese spontaneously hypertensive rats: the role of renal sympathetic innervation. Am J Hypertens Feb 2015;28:256–265. [DOI] [PubMed] [Google Scholar]
- 135.Esler M Illusions of truths in the Symplicity HTN-3 trial: generic design strengths but neuroscience failings. J Am Soc Hypertens Aug 2014;8:593–598. [DOI] [PubMed] [Google Scholar]
- 136.Qiu M, Shan Q, Chen C, Geng J, Guo J, Zhou X, Qian W, Tang L, Yin Y. Renal sympathetic denervation improves rate control in patients with symptomatic persistent atrial fibrillation and hypertension. Acta Cardiol Feb 2016;71:67–73. [DOI] [PubMed] [Google Scholar]
- 137.Turagam MK, Whang W, Miller MA, et al. Renal Sympathetic Denervation as Upstream Therapy During Atrial Fibrillation Ablation: Pilot HFIB Studies and Meta-Analysis. JACC Clin Electrophysiol Jan 2021;7:109–123. [DOI] [PubMed] [Google Scholar]
- 138.Kirstein B, Huo Y, Gaspar T, Salmas J, Tomala J, Mayer J, Sitzy J, Ulbrich S, Richter U, Piorkowski C. P309Preliminary results of a feasibility study to evaluate the effect of concomitant renal denervation and cardiac ablation on atrial fibrillation recurrence. EP Europace 2017;19:iii49–iii49. [Google Scholar]
- 139.Stavrakis S, Humphrey MB, Scherlag BJ, Hu Y, Jackman WM, Nakagawa H, Lockwood D, Lazzara R, Po SS. Low-level transcutaneous electrical vagus nerve stimulation suppresses atrial fibrillation. J Am Coll Cardiol Mar 10 2015;65:867–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Stavrakis S, Stoner JA, Humphrey MB, et al. TREAT AF (Transcutaneous Electrical Vagus Nerve Stimulation to Suppress Atrial Fibrillation): A Randomized Clinical Trial. JACC Clin Electrophysiol Mar 2020;6:282–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Qin D, Singh JP. Low-Level Tragus Stimulation for Atrial Fibrillation. JACC: Clinical Electrophysiology 2020;6:292–294. [DOI] [PubMed] [Google Scholar]
- 142.Stavrakis S, Humphrey Mary B, Scherlag B, et al. Low-Level Vagus Nerve Stimulation Suppresses Post-Operative Atrial Fibrillation and Inflammation. JACC: Clinical Electrophysiology 2017/September/01 2017;3:929–938. [DOI] [PubMed] [Google Scholar]
- 143.Byku M, Mann DL. NEUROMODULATION OF THE FAILING HEART: LOST IN TRANSLATION? JACC Basic Transl Sci Apr 2016;1:95–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ardell JL, Nier H, Hammer M, Southerland EM, Ardell CL, Beaumont E, KenKnight BH, Armour JA. Defining the neural fulcrum for chronic vagus nerve stimulation: implications for integrated cardiac control. J Physiol Nov 15 2017;595:6887–6903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Romanov A, Lomivorotov V, Chernyavskiy A, Murtazin V, Kliver E, Ponomarev D, Mikheenko I, Yakovlev A, Yakovleva M, Steinberg JS. Temporary Spinal Cord Stimulation to Prevent Postcardiac Surgery Atrial Fibrillation. Journal of the American College of Cardiology 2022;79:754–756. [DOI] [PubMed] [Google Scholar]
- 146.Leftheriotis D, Flevari P, Kossyvakis C, Katsaras D, Batistaki C, Arvaniti C, Giannopoulos G, Deftereos S, Kostopanagiotou G, Lekakis J. Acute effects of unilateral temporary stellate ganglion block on human atrial electrophysiological properties and atrial fibrillation inducibility. Heart Rhythm Nov 2016;13:2111–2117. [DOI] [PubMed] [Google Scholar]
- 147.Connors CW, Craig WY, Buchanan SA, Poltak JM, Gagnon JB, Curry CS. Efficacy and Efficiency of Perioperative Stellate Ganglion Blocks in Cardiac Surgery: A Pilot Study. J Cardiothorac Vasc Anesth Feb 2018;32:e28–e30. [DOI] [PubMed] [Google Scholar]
- 148.McClelland JH, Duke D, Reddy R. Preliminary results of a limited thoracotomy: new approach to treat atrial fibrillation. J Cardiovasc Electrophysiol Dec 2007;18:1289–1295. [DOI] [PubMed] [Google Scholar]
- 149.Mehall JR, Kohut RM, Schneeberger EW, Taketani T, Merrill WH, Wolf RK Intraoperative epicardial electrophysiologic mapping and isolation of autonomic ganglionic plexi. Ann Thorac Surg Feb 2007;83:538–541. [DOI] [PubMed] [Google Scholar]
- 150.Doll N, Pritzwald-Stegmann P, Czesla M, Kempfert J, Stenzel MA, Borger MA, Mohr F-W. Ablation of Ganglionic Plexi During Combined Surgery for Atrial Fibrillation. The Annals of Thoracic Surgery 2008/November/01/ 2008;86:1659–1663. [DOI] [PubMed] [Google Scholar]
- 151.Calò L, Rebecchi M, Sciarra L, Luca LD, Fagagnini A, Zuccaro LM, Pitrone P, Dottori S, Porfirio M, Ruvo Ed, Lioy E. Catheter Ablation of Right Atrial Ganglionated Plexi in Patients With Vagal Paroxysmal Atrial Fibrillation. Circulation: Arrhythmia and Electrophysiology 2012;5:22–31. [DOI] [PubMed] [Google Scholar]
- 152.Katritsis DG, Giazitzoglou E, Zografos T, Pokushalov E, Po SS, Camm AJ. Rapid pulmonary vein isolation combined with autonomic ganglia modification: a randomized study. Heart Rhythm May 2011;8:672–678. [DOI] [PubMed] [Google Scholar]
- 153.Al-Atassi T, Toeg H, Malas T, Lam B-K. Mapping and Ablation of Autonomic Ganglia in Prevention of Postoperative Atrial Fibrillation in Coronary Surgery: MAAPPAFS Atrial Fibrillation Randomized Controlled Pilot Study. Canadian Journal of Cardiology 2014;30:1202–1207. [DOI] [PubMed] [Google Scholar]
- 154.Budera P, Osmancik P, Talavera D, Kraupnerova A, Fojt R, Zdarska J, Vanek T, Straka Z. Two-staged hybrid ablation of non-paroxysmal atrial fibrillation: clinical outcomes and functional improvements after 1 year. Interactive CardioVascular and Thoracic Surgery 2017;26:77–83. [DOI] [PubMed] [Google Scholar]
- 155.Bagge L, Blomström P, Jidéus L, Lönnerholm S, Blomström-Lundqvist C. Left atrial function after epicardial pulmonary vein isolation in patients with atrial fibrillation. Journal of Interventional Cardiac Electrophysiology 2017/November/01 2017;50:195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zdarska J, Osmancik P, Budera P, Herman D, Prochazkova R, Talavera D, Straka Z. The absence of effect of ganglionated plexi ablation on heart rate variability parameters in patients after thoracoscopic ablation for atrial fibrillation. J Thorac Dis Dec 2017;9:4997–5007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Suwalski G, Marczewska MM, Kaczejko K, Mróz J, Gryszko L, Cwetsch A, Skrobowski A Left Atrial Ganglionated Plexi Detection is Related to Heart Rate and Early Recurrence of Atrial Fibrillation after Surgical Ablation. Brazilian Journal of Cardiovascular Surgery 2017; 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Garabelli P, Stavrakis S, Kenney JFA, Po SS. Effect of 28-mm Cryoballoon Ablation on Major Atrial Ganglionated Plexi. JACC Clin Electrophysiol Jun 2018;4:831–838. [DOI] [PubMed] [Google Scholar]
- 159.Haldar S, Khan HR, Boyalla V, et al. Catheter ablation vs. thoracoscopic surgical ablation in long-standing persistent atrial fibrillation: CASA-AF randomized controlled trial. European Heart Journal 2020;41:4471–4480. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


