Summary
More than 20 million individuals worldwide suffer from congenital or acquired bone defects annually. The development of bone scaffold materials that simulate natural bone for bone defect repair remains challenging. Recently, ncRNA-based therapies for bone defects have attracted increasing interest because of the great potential of ncRNAs in disease treatment. Various types of ncRNAs regulate gene expression in osteogenesis-related cells via multiple mechanisms. The delivery of ncRNAs to the site of bone loss through gene vectors or scaffolds is a potential therapeutic option for bone defect repair. Therefore, this study discusses and summarizes the regulatory mechanisms of miRNAs, siRNAs, and piRNAs in osteogenic signaling and reviews the widely used current RNA delivery vectors and scaffolds for bone defect repair. Additionally, current challenges and potential solutions of delivery scaffolds for bone defect repair are proposed, with the aim of providing a theoretical basis for their future clinical applications.
Subject areas: Biomolecules, Nucleic acids, Tissue engineering
Graphical abstract
Biomolecules; Nucleic acids; Tissue engineering
Introduction
With the increasing aging of the global population, osteoarthritis, fractures, osteoporosis, and other bone-degenerative diseases have received widespread attention worldwide (Bhattacharjee et al., 2017). According to statistics, more than 20 million people worldwide suffer from congenital or acquired bone defects annually (Habibovic, 2017). Bone defects refer to bone loss owing to trauma, bone tumors, inflammation, or other bone diseases that may result in localized loss of function in patients (Wei et al., 2020). Most bone defects heal spontaneously because of the strong ability of bones to regenerate and repair themselves, particularly in young individuals (El-Rashidy et al., 2017; Walmsley et al., 2016). However, when the bone defect is larger than the critical size of the human support bone, external surgical intervention is required for healing (El-Rashidy et al., 2017; Wei et al., 2020).
Currently, autologous bone grafting is considered the “gold standard” for the treatment of bone defects; however, its widespread use is restricted by the limited availability of bone grafts and morbidity of the donor area (Baldwin et al., 2019; Habibovic, 2017; Sheikh et al., 2015; Wei et al., 2020). Allogeneic bone grafting is widely available and does not cause donor site morbidity, which makes it an elective choice for the treatment of bone defects. Nevertheless, it may lead to immune stress responses or the transmission of diseases (Baldwin et al., 2019; Brown and Carter, 2018; Fillingham and Jacobs, 2016; Giannoudis et al., 2005).
Bone scaffolds circumvent the limitations of autologous or allogeneic bone grafting to some extent (Fillingham and Jacobs, 2016). Traditional bone scaffolds mainly include inert metal materials such as stainless steel, titanium, and its alloys, which are widely used owing to their excellent mechanical properties and biocompatibility. However, they lack bioactivity, osteoconductivity, and osteointegration (Bekmurzayeva et al., 2018; Wei et al., 2020). In addition, as the traditional bone scaffolds are non-degradable, surgery is required for their removal, which increases the pain of the patient and the cost of treatment (Sheikh et al., 2015). Moreover, the difference in elastic modulus between metal implants and natural bone has a stress shielding effect on the bone, which interferes with bone metabolism and increases the risk of osteoporosis (Kamrani and Fleck, 2019). Second-generation bone scaffolds, based on bioactive and biodegradable materials, have been developed to promote bone tissue regeneration (Sheikh et al., 2015; Wei et al., 2020). These materials degrade at a rate commensurate with the rate of bone tissue healing and reduce the strength of second-generation bone scaffolds as much as possible while meeting load-bearing requirements (Kamrani and Fleck, 2019). Recently, with the improvement in tissue engineering technology, the regeneration of bone tissue has been facilitated through the modulation of seed cells, scaffolds, and growth factors at the intended site. Scaffolds are critical in bone tissue engineering, as they not only act as structural braces but also as templates to guide the formation of new bone tissue (Shadjou and Hasanzadeh, 2016; Shadjou et al., 2018). Therefore, ideal bone tissue engineering scaffold materials should have more than just good biocompatibility and biodegradability properties; they also need to be porous, osteoinductive, osteoconductive, osteogenic, and have mechanical properties similar to those of the bone at the graft site (El-Rashidy et al., 2017; Shadjou et al., 2018). For example, bioactive glass (BG) has been effectively utilized as a bone tissue engineering scaffold for bone defect repair. BG forms a layer of carbonated hydroxyapatite (HCA) on the glass surface after implantation, which firmly adheres to bone tissue and induces bone tissue regeneration (Kong et al., 2018; Yan et al., 2004). Furthermore, the by-products of BG degradation, containing soluble ions such as Si and Ca ions, can influence the proliferation and differentiation of osteoblasts as well as the expression of genes related to osteogenic differentiation (El-Rashidy et al., 2017; Kong et al., 2018). In addition, some growth factors, such as bone morphogenetic proteins (BMPs), transforming growth factor β (TGF-β), vascular endothelial growth factor (VEGF), and platelet-derived growth factor, are used in combination with BG scaffolds can improve the osteogenic performance of the scaffolds. Notably, the synthesis of highly ordered mesoporous bioactive glasses using nonionic block copolymers as structural guides was reported in 2004 by Yan et al. (2004). In addition to its stronger biological activity than that of traditional bioactive glass (BG) (Yan et al., 2006), the mesoporous bioactive glass also makes the delivery of biomolecules more effective and convenient through its controlled mesoporous structure (Kong et al., 2018).
Gene therapy has great potential in regenerative medicine (Wang et al., 2019). Plasmid DNA and RNA are the main components of gene therapy. Although plasmid DNA therapy has been applied to bone tissue engineering with favorable results, some limitations, such as security and efficiency, remain. In recent years, RNA has been considered an alternative option because of its high transfection efficiency. Specifically, in terms of delivery efficiency, plasmid DNA must be delivered to the nucleus before transcription; it may integrate into the host DNA during delivery, causing unwanted changes in genetic information. In contrast, RNA only needs to be delivered to the cytoplasm for transcription and can control disease progression by regulating gene expression (Nguyen et al., 2018). From the viewpoint of range of application, plasmid DNA induces the production of encoded proteins only in dividing cells, whereas RNA can induce it in both dividing and non-dividing cells (Youn and Chung, 2015). With persistent improvement in the high-throughput sequencing technology and the Encyclopedia of DNA Elements (ENCODE) project, once considered gene "transcriptional noise,” non-coding RNAs (ncRNAs) have been reported to play important roles in gene expression regulation, ontogeny, and disease development (Zhang et al., 2019).
As RNA therapeutic drugs are susceptible to degradation by nucleases in tissues and cells and are negatively charged, there exists an electrostatic repulsion between these drugs and the negatively charged cell membranes, which results in insufficient endocytosis and endosome escape of the drugs. The biggest obstacle to RNA therapy is achieving targeted and efficient delivery of RNA therapeutic drugs to the target tissues and cells (Mao et al., 2011). To overcome these obstacles, suitable vectors loaded with RNA therapeutic drugs need to be developed for efficient delivery to the target cells and effective escape from endosomes to achieve efficient drug expression. In addition, compared with sncRNAs, lncRNAs have a wide variety of functions; however, their biological functions have not yet been fully clarified. This study systematically reviews the action mechanisms of ncRNAs and the vectors used for ncRNA delivery and provides insights into their clinical applications.
Mechanisms of the ncRNA-mediated regulation of osteogenic signaling
RNA can be classified into two categories according to its function of encoding proteins — mRNA, which encodes proteins, and ncRNA, which is not capable of encoding proteins. Although ncRNAs cannot encode proteins, their content in the human gene transcriptome is more than 98% (Zhang et al., 2019), indicating that ncRNAs might have rich biological functions in living organisms (Nacher, 2013). Based on their molecular size, ncRNAs can be further divided into long ncRNAs (lncRNAs; length ≥200 nucleotides) and small ncRNAs (sncRNAs; length <200 nucleotides). Studies have shown that lncRNAs can directly interact with transcription factors, functional RNA molecules, and chromatin remodeling modifiers to direct the expression of target genes at numerous levels (Chen and Carmichael, 2010), and sncRNAs can participate in gene shearing and modification, mediate gene silencing, and regulate gene transcription and translation (Panwar et al., 2014).
As the complex regulatory mechanisms of lncRNAs and their biological functions are not fully understood, more studies have concentrated on using vectors to deliver small ncRNAs for bone defect repair. However, several recent studies have shown that lncRNAs can affect the osteogenic differentiation of bone marrow mesenchymal stem cells (BMSCs) through a mechanism known as competing endogenous RNAs (ceRNAs), which has received increasing attention (Hong et al., 2020; Lai et al., 2022; Wang et al., 2020b, 2021). The experimental results of Ouyang et al. (2020) showed that lncRNA ENST00000563492 can act as a ceRNA for miR-205-5p to upregulate the expression of CDH11 and VEGF, thereby promoting the osteogenic differentiation of BMSCs. Furthermore, lncRNA-PAGBC as a ceRNA combines with the osteogenic inhibitory microRNA miR-133b, which targets Runx2, to induce the osteogenesis of adipose-derived mesenchymal stem cells (AMSCs) (Ru et al., 2020). In conclusion, the above-mentioned studies confirmed that lncRNAs have the potential to treat bone defects in clinical settings.
Small ncRNAs that direct gene expression at the post-transcriptional level through direct excision of target mRNAs or inhibition of their translation are widely found in higher and lower organisms. Small ncRNAs are classified into two primary categories — microRNAs (miRNAs) and small interfering RNAs (siRNAs). miRNAs and siRNAs differ in their production and action mechanisms (Leng et al., 2020). P-element-induced wimpy testis (Piwi) interacting RNAs (piRNAs) are a less-studied class of small ncRNAs that may be involved in the regulation of osteogenic differentiation. No previous study has detailed the regulatory role of piRNAs in osteogenesis differentiation. These types of small ncRNAs are described separately later in discussion.
MicroRNAs
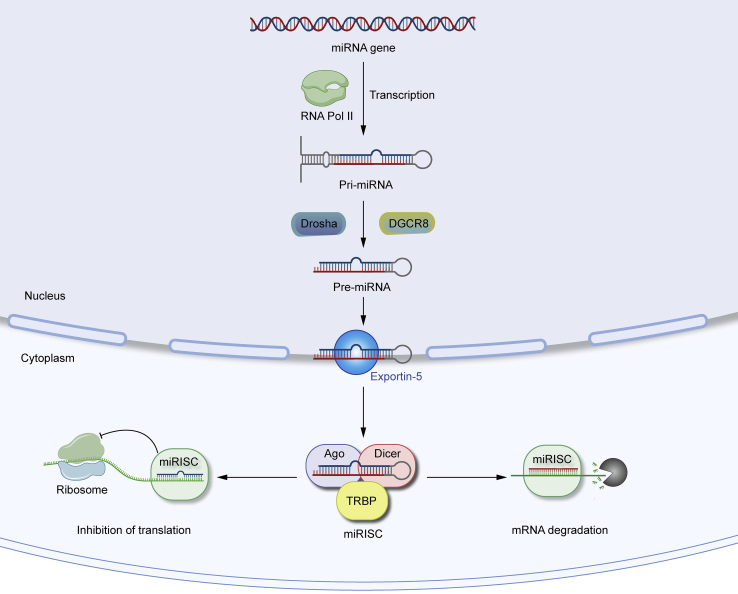
MicroRNAs (miRNAs) are a class of non-coding single-stranded RNA molecules that are 18–24 nucleotides in length, encoded by endogenous genes, and play vital regulatory roles in most cellular processes in eukaryotic cells (Yang et al., 2020). Most miRNAs are transcribed in the nucleus by RNA polymerase II to generate pri-miRNAs, which are further cleaved by Drosha and its cofactor DGCR8 to form hairpin-shaped precursor miRNAs (pre-miRNAs) of approximately 60–70 nucleotides and then exported to the cytoplasm by exportin-5 (XPO-5). Finally, the RNase Dicer1 binds to the ends of pre-miRNAs in the cytoplasm to generate mature double-stranded miRNAs. One strand combines with the RNA-induced silencing complex (RISC) assembled by Dicer1, trans-activated responsive RNA-binding protein (TRBP), and Argonaute proteins to create a new silencing complex called miRISC, which further performs post-transcriptional regulatory functions (Bellavia et al., 2019b; Pu et al., 2019). miRISC recognizes mRNA and binds to the 3′UTR or 5′UTR of the target mRNA (Leng et al., 2020). The effect of miRISC on mRNA expression depends mainly on its degree of complementarity with the target mRNA. miRISC cleaves the mRNA when it binds to the target mRNA in full complementarity. When miRISC binds to the target mRNA with incomplete complementarity, it inhibits the translation of the mRNA, which plays a critical role in directing gene expression. The formation and regulatory mechanisms of miRNA are shown as below (Figure 1).
Figure 1.
The formation and regulation mechanisms of miRNA
miRISC can cleave the mRNA or inhibit the translation of the mRNA depends on its degree of complementarity with the target mRNA.
Osteogenesis refers to the differentiation of mesenchymal stem cells (MSCs) into mature osteoblasts, and miRNAs maintain bone homeostasis by regulating the processes associated with bone formation and bone resorption (Fang et al., 2015). Upregulating or deregulating miRNAs based on the effect on bone of different miRNAs is an important treatment for bone defect repair (Bellavia et al., 2019a). Several miRNAs have been shown to promote osteoblast differentiation by regulating the elements associated with osteogenic differentiation. The WNT/β-catenin signaling pathway is one of the vital classical pathways involved in the regulation of osteogenic differentiation of BMSCs and bone metabolism through a series of mechanisms. Dickkopf-1 (DKK1) and secreted frizzled-related protein-1 (SFRP1) are negative regulators of the WNT signaling pathway and are often considered markers of osteoporosis. miR-433-3p promotes osteoblast differentiation by reducing DKK1 expression (Tang et al., 2017). miR-542-3p plays a critical role in bone formation by repressing the expression of SFRP1 to induce the osteogenic differentiation of MSCs (Zhang et al., 2018b). Sclerostin (SOST) could be a negative regulator of bone formation. miR-96 promotes osteoblast differentiation and bone formation by binding to SOST to activate the WNT signaling pathway (Ma et al., 2019). Intracellular adhesion molecule I (ICAM1) can direct bone remodeling by promoting osteoclast formation, which is vital in multiple inflammatory bone diseases. miR-187-5p promotes osteogenesis by targeting ICAM1 and thus is a potential therapeutic target for bone diseases (Sun et al., 2020). The phosphatidylinositol three-kinase (PI3K)/AKT signaling pathway is another significant pathway that regulates bone metabolism. miR-216a can direct the PI3K/AKT signaling pathway by targeting c-Cbl to promote the osteogenic differentiation of BMSCs (Li et al., 2015; Yang et al., 2020).
Studies have shown that miRNAs not only promote osteoblast differentiation but also inhibit bone formation. Runt-related transcription factor 2 (Runx2), as a key transcription factor, directs the differentiation of MSCs to osteoblasts for bone formation (Garcia and Delany, 2021; Wang et al., 2017a). Zhang et al. (2017) found that miR-221 represses osteoblast differentiation by directly targeting Runx2. Additionally, miR-467g negatively regulates osteogenesis by targeting Runx2 (Kureel et al., 2017). Apart from miR-221 and miR-467g, miR-133a-5p also inhibits the expression of Runx2 by targeting the 3′UTR of Runx2, which controls osteoblast differentiation (Zhang et al., 2018a). Although recent studies have delved into the mechanisms by which miRNAs regulate Runx2, more studies are needed to reveal the exact network among miRNAs, Runx2, and osteoblast differentiation (Fang et al., 2015; Kim and Lim, 2014). The TGF-β superfamily includes TGFβs, BMPs, growth differentiation factors (GDFs), and activins, which play key roles in the regulation of bone homeostasis (Chen et al., 2012; Garcia and Delany, 2021; Thielen et al., 2019; Wang et al., 2017a). Activin A receptor type I (ACVR1) belongs to the TGF-β superfamily, and miR-208a-3p inhibits osteoblast differentiation and bone formation by targeting ACVR1 (Arfat et al., 2018). Smad proteins are signal transduction molecules downstream of the TGF-β signaling pathway that translocate to the nucleus to direct the expression of target genes (Garcia and Delany, 2021; Macias et al., 2015; Thielen et al., 2019). Pais et al. (2010) developed a new approach based on computational analysis of promoter sequences and mRNA microarray experiments and found that miR-140 inhibits the TGF-β pathway by suppressing Smad3. In addition, both miR-16 and miR-21 inhibit osteoblast differentiation by targeting Smad5 (Mencía Castaño et al., 2019; Wei et al., 2017).
Small interfering RNAs
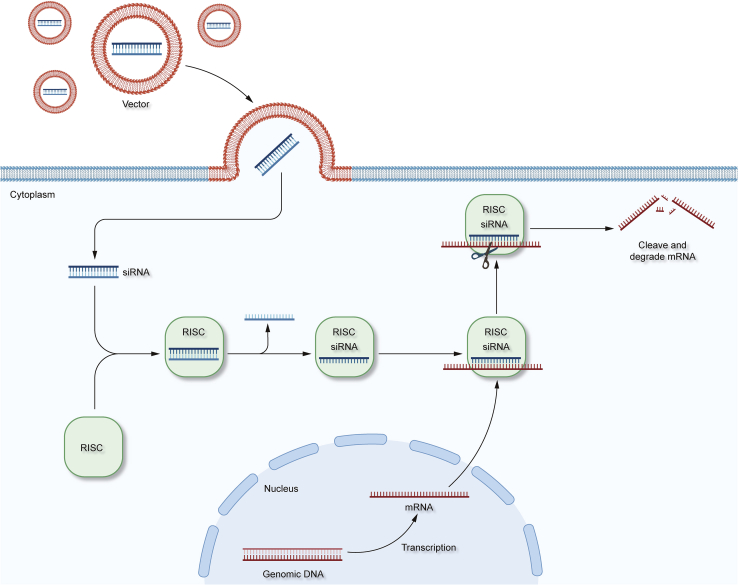
Small interfering RNAs (siRNAs) are double-stranded RNAs (dsRNAs) that are 19–30 nucleotides in length and have two prominent nucleotides at the 3′ UTR (Leng et al., 2020; Wang et al., 2010). Compared to miRNAs, siRNAs are produced primarily through the cleavage of dsRNAs by Dicer ribonucleases. siRNAs consist of a sense strand and an antisense strand, which are combined into the RNA-induced silencing complex (RISC), wherein the siRNA interacts with its Argouate two component, leading to the degradation of the sense strand (Alshaer et al., 2021; Fakhr et al., 2016; Xu et al., 2018a). The antisense strand then directs the RISC complex to the target mRNA and binds it in a fully complementary manner, followed by precise cleavage of the mRNA molecule at the binding site, ultimately leading to the degradation of the mRNA, which silences the gene encoding the mRNA; this is known as the RNAi (RNAi) phenomenon (Figure 2).
Figure 2.
The gene silencing mechanism of siRNA
Delivery of siRNA to cells via membrane fusion and endocytosis using specific vectors leads to gene silencing.
In addition to their molecular structures, production mechanisms, and action mechanisms, siRNAs differ from miRNAs in several other ways. miRNAs can be fully paired, incompletely paired, or even mismatched with target mRNAs, whereas siRNAs are fully paired with mRNAs. In addition, siRNAs are highly specific as a kind of siRNA targeting only complementary mRNA, whereas a type of miRNA can bind to multiple target mRNAs at the same time (Leng et al., 2020; Xu et al., 2018a).
RNAi (RNAi) is an effective and valuable tool in bone tissue engineering because of its ability to silence the expression of genes that negatively affect bone tissue regeneration. Receptor activator of nuclear factor kappa B ligand (RANKL)/RANK signaling promotes osteoclast activation and differentiation. Bilecen et al.(Sezlev Bilecen et al., 2019) used nano-poly (lactic-co-glycolic acid) (PLGA) capsules encapsulating poly (ethylene imine) (PEI) –RANK siRNA complexes to significantly reduce RANK mRNA levels and inhibit osteoclast activity. Additionally, S100A4 may inhibit the expression of osteogenesis-related genes in periodontal ligament (PDL) cells, thereby suppressing mineralization (Duarte et al., 1998, 1999; Strutz et al., 1995). It has been shown that S100A4 may be a part of the S100 calcium-binding protein family (Schäfer et al., 1995), which is synthesized and secreted by PDL cells. Kato et al. (2005) reported that siRNA targeting S100A4 increases the expression of osteogenic markers (such as osteocalcin and osteopontin) as well as the osteogenesis-specific transcription factors Runx2/Cbfa1 and Osterix, leading to the differentiation of PDL cells toward osteoblasts.
Noggin is a major extracellular antagonist of bone morphogenetic proteins (BMPs) that negatively regulates the BMP-induced osteoblast differentiation. Takayama et al. (2009) demonstrated that the siRNA-mediated silencing of noggin enhances BMP-induced osteoblast differentiation and eliminates the negative regulation of BMPs caused by noggin. Nguyen et al. (2018) found that the sustained release of siRNA targeting noggin (siNoggin) in polyethylene glycol (PEG) hydrogels implanted in situ in a rat cranial defect model promoted the osteogenic differentiation of human bone marrow mesenchymal stem cells (hMSCs) encapsulated in PEG hydrogels, which accelerated the repair of cranial defects. Plekho1, also known as casein kinase2 interaction protein 1 (Ckip-1), is a negative regulator of bone formation. Liang et al. (2015) screened aptamer CH6 with high specificity for osteoblasts using the cell-SELEX technique and used CH6 aptamer-functionalized lipid nanoparticles (LNPs) encapsulated with siRNA targeting Plekho1(siCkip-1), which led to the silencing of the Plekho1 gene in osteoblasts, further promoting bone formation. Silencing Ckip-1 using siCkip-1 promotes bone formation through the WNT3/β-catenin signaling pathway (Zhou et al., 2017). Notably, angiogenesis during bone formation is often overlooked. Jia et al. (2014) combined two siRNAs, siRNA targeting casein kinase2 interaction protein 1 (siCkip-1) and siRNA targeting soluble VEGF receptor 1 (siFlt-1), into a chitosan sponge. Results showed that the target genes were markedly repressed, and osteocalcin, alkaline phosphatase (ALP), and VEGF were significantly upregulated, which indicated strong osteogenesis and angiogenesis effects. Moreover, tumor necrosis factor alpha (TNF-α), which is also a negative regulator of bone formation, is considered a potential target for siRNA silencing in bone defect repair (Guo et al., 2013).
Piwi-interacting RNAs
P-element-induced wimpy testis (Piwi) interacting RNAs (piRNAs) are a novel class of less-studied small ncRNAs originally found in germ cells, which play critical roles in the differentiation and maintenance of germ cells (Yamashiro and Siomi, 2018). piRNAs are 24–31 nucleotides in length and exhibit 2′-O-methyl modifications at the 3′ terminus (Chen et al., 2021b). Unlike miRNAs and siRNAs, piRNAs are slightly longer and are generated from single-stranded precursors that do not involve Dicer or the formation of secondary structures (Lin et al., 2021). Statistically, there are more than 20,000 known piRNAs in the human genome, which is more than 10 times the number of miRNAs; this suggests that piRNAs may play an important role in gene expression (Chen et al., 2021b; Kozomara et al., 2019; Kozomara and Griffiths-Jones, 2014; Lin et al., 2021).
piRNAs can bind to the PIWI family subproteins to create piRNA/PIWI complexes (piRC); additionally, piRNAs are guided by PIWI proteins to recognize and silence target mRNAs to regulate the gene translation process (Liu et al., 2019b; Wu et al., 2021; Yang et al., 2019). Wang et al. (2020a) found that piRNAs are highly expressed in the exosomes of BMSCs; additionally, they revealed that piRNAs may be involved in processes such as the proliferation and osteogenic differentiation of BMSCs, using GO enrichment and KEGG pathway analyses. Furthermore, Wu et al. (2010) discovered the expression of PiwiL2 protein and related piRNAs in the BMSCs of mice and found that siRNA-mediated gene knockdown could regulate the cell cycle and enhance the proliferation of BMSCs.
Sun et al.(Li et al., 2011; Sun et al., 2010) showed that the Piwi2 gene Zili represses TGF-β signaling by binding its N-terminal structural domain to Smad4 and blocking the formation of Smad2/3/4 and Smad1/5/9/4 complexes. However, another study (Della Bella et al., 2020) demonstrated that the expression of piRNA-36741 is markedly upregulated during the osteogenic differentiation of BMSCs. Although studying the underlying mechanism, Liu et al. (2021a) demonstrated that the piR-36741-PIWI4 complex promotes the expression of BMP-2 by interacting with METTL3 and preventing the METTL3-mediated N6-methyladenosine (m6A) modification of BMP-2 mRNA transcripts, which, in turn, reverses the inhibitory effects of piR-36741 on the osteogenic differentiation and Smad signaling pathway in BMSCs. However, Chen et al. (2021a) demonstrated that piR-63049 represses bone formation via the WNT2b/β-catenin signaling pathway, which may be a promising target for the treatment of bone defects.
Notably, when designing and running piRNA-related experiments, it should be considered that the levels of several ncRNAs in trypsin-digested hMSCs change rapidly, leading to unexpected experimental results (Della Bella and Stoddart, 2019). In addition, the lack of standardized classification is a limiting factor in piRNA research (Della Bella et al., 2020). The same piRNA may have different names; therefore, establishing a uniform piRNA nomenclature is an urgent requirement and would be beneficial.
Circular RNAs
Circular RNAs (circRNAs) are a class of endogenous covalently closed circular RNA molecules formed mainly by reverse splicing, without 5' to 3′ polarity or poly-adenylated tails (Hong et al., 2019; Li et al., 2021b). CircRNAs are mainly derived from exons and are highly stable in tissues owing to their closed circular structures, being less susceptible to degradation by ribonucleases (Li et al., 2021b).
Many recent studies have shown that circRNAs can indirectly regulate the expression of miRNA downstream target genes by acting as miRNA sponges. miRNA sponges were first proposed in 2007 for long-acting inhibition of miRNAs (Ebert et al., 2007). The so-called miRNA sponge refers to that cicrRNAs can compete with the RISC to bind free miRNAs with specific RNA-binding domains in the cytoplasm as circRNAs have a large number of miRNAs binding sites, functioning like a sponge to regulate the expression of miRNA downstream target genes. Liu et al. and Hong et al.(Hong et al., 2019; Liu et al., 2019a) found a novel circRNA called cpwwp2a, which is a miR-579 sponge and upregulates the expression of miR-579 target genes including angiopoietin one and phosphoinositide-dependent protein kinase 1 (PDK1) by inhibiting the activity of miR-579. Dexamethasone (DEX) is highly cytotoxic to human osteoblasts, and its mechanism of action is related to circRNAs. Zhu et al. (2019) found that the expression of circRNA HIPK3 (circHIPK3) was downregulated in human osteoblasts treated with DEX, resulting in the accumulation of miR-124, a key miRNA sponged by circHIPK3, which leads to cytotoxicity. Additionally, Li et al. (2018) showed that circRNA CDR1as acts as an inhibitor of miR-7, provoking the upregulation of growth differentiation factor 5 (GDF5) and subsequent phosphorylation of Smad1/5/8 and p38 mitogen-activated protein kinases (p38 MAPK), promoting the osteogenic differentiation of periodontal ligation stem cells (PDLSCs). Overall, making use of the miRNA sponge effect of circRNAs to regulate the expression of miRNA target genes for bone tissue engineering has a positive clinical prospect, which needs continued exploration by more and more related scholars.
Several ncRNAs with wide application in bone tissue engineering have been well summarized above. Additionally, bone tissue engineering vectors incorporating recombinant proteins or mRNA agonists/antagonists also have potential for application in bone regenerative medicine. Many traumatologists consider certain BMPs as the gold standard for bone regeneration (Paulini et al., 2021). Recombinant human BMP-2 has been approved by the Food and Drug Administration (FDA) for clinical use; however, its release in vivo may lead to ectopic bone formation, inflammation, and the risk of cancer (Mikos et al., 2006). In addition, mRNA agonists/antagonists have some applications in bone tissue engineering, but how to specifically activate or inhibit the expression of target mRNAs to minimize off-target effects is a matter that requires a solution in the future (Pal et al., 2019). Compared with the conventional pharmacological approaches for bone defect repair, ncRNA-based therapies as a novel strategy for bone tissue engineering can impact the fate decisions of BMSCs by regulating various signal molecules downstream (Winkle et al., 2021). However, owing to the instability and negative charge of oligonucleotides, designing efficient vectors for ncRNAs delivery is an extremely attractive strategy.
NcRNA delivery scaffolds for bone defect therapy
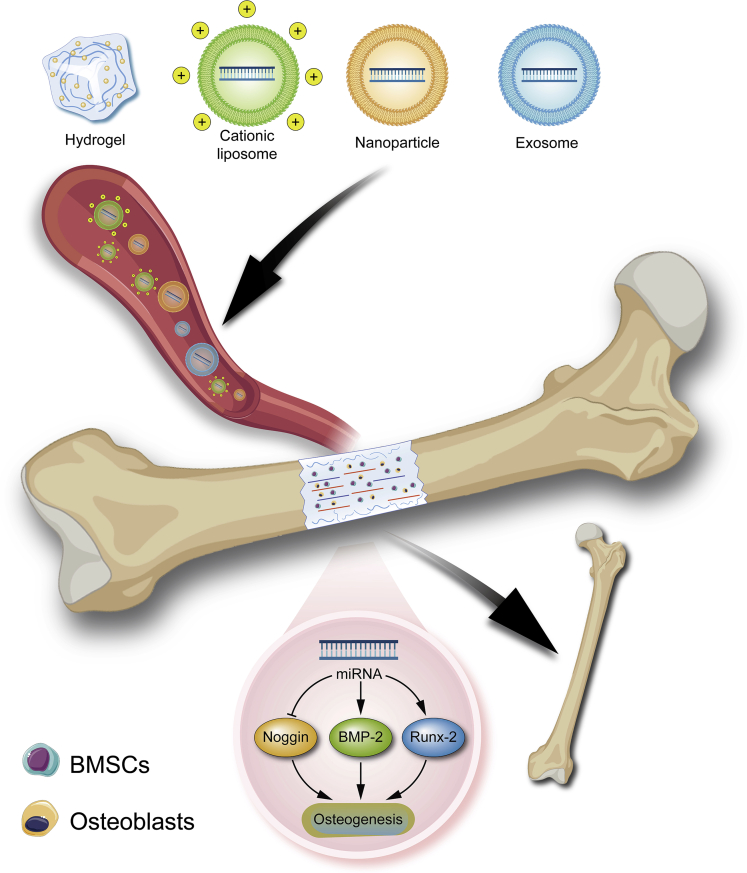
There are no RNAi therapeutic drugs available for bone tissue engineering regeneration in clinical practice (Malcolm et al., 2020). The main reason for the failure of RNAi therapy is that RNA therapeutic drugs are vulnerable to degradation by nucleases in tissues and cells, and it is difficult for them to enter the cell membrane owing to electrostatic repulsion. However, with increasing awareness of gene regulatory networks and the continuous development of technologies in the materials field, multifunctional vectors loaded with RNAi drugs have been developed to achieve high targeting and efficiency of drugs. RNAi therapies are expected to be further developed. Currently, vectors can be divided into viral and non-viral vectors. Viral vectors are less commonly used in gene therapy because of their immunogenicity and limited drug-loading capacity. Non-viral vectors are particularly desirable as gene delivery systems owing to their accessibility for mass production and chemical characterization (Picanço-Castro et al., 2020). In addition, they are capable of high reproducibility during mass production, which is of great significance for both research and clinical applications. A few common non-viral vectors are used to deliver ncRNAs to treat bone defects. The strategies of using non-viral vectors to deliver ncRNA via blood circulation for the treatment of bone defect are displayed below (Figure 3).
Figure 3.
The strategies of ncRNA delivery for bone defect
Vectors encapsulating gene therapy drugs are delivered to the bone defect site via blood circulation and undergo a series of regulatory mechanisms to promote osteogenesis, thereby repairing the bone defect.
Liposome-based scaffolds
Liposomes are closed vesicles with a lipid bilayer structure, ranging in size from 20 to 1000 nm, and are usually prepared by adding appropriate amounts of cholesterol to the ingredients, which can be integrated into the lipid membrane to fluidize the membrane (Ickenstein and Garidel, 2019). Size is one of the key parameters determining the drug encapsulation rate and cycle half-life of liposomes. As smaller liposomes are more likely to escape uptake by phagocytes (Tenchov et al., 2021), LNPs with sizes less than 100 nm represent an effective delivery platform for RNA therapy (Eygeris et al., 2022). Liposomes are considered the earliest generation of LNPs because they are composed of lipids and, in most cases, are nanosized (Tenchov et al., 2021). Because of the negative charge of RNA drugs, cationic LNPs complexed with RNA can stabilize RNA and enhance its resistance to nucleases. Therefore, they are the most widely used non-viral delivery systems for RNA drugs. In addition, because the cell membrane is negatively charged, the electrostatic attraction promotes the adsorption of LNPs onto the cell membrane and endocytosis of the membrane. After entering the cell, the intracellular anionic lipids neutralize the positive charge carried by the cationic lipids to disrupt the electrostatic interaction between RNA and cationic lipids, which leads to the release of nucleic acids (Tenchov et al., 2021). It has been shown that cationic liposome delivery systems have the advantages of high efficiency, low toxicity, and low immunogenicity as a delivery platform for RNA therapeutics (Pedroso de Lima et al., 2003). However, delivery systems consisting of permanently positively charged cationic lipids may damage cell membranes owing to electrostatic effects, thereby causing cytotoxicity (Eygeris et al., 2022). Therefore, ionizable lipids that are only positively charged intracellularly and uncharged in the blood owing to pH changes are the best choice (Hajj et al., 2019; Tenchov et al., 2021). Zhang et al. (2012) used a cationic liposome based on dioleoyl trimethylammonium propane (DOTAP), which was connected to six repetitive sequences of aspartate, serine, and serine ((AspSerSer)6), to specifically deliver siRNA targeting the Plekho1 gene to the bone-forming surface. This approach facilitates the effective enrichment of osteogenic siRNA in osteoblasts and silencing of expression of the Plekho1 gene, ultimately achieving the goal of promoting bone formation. miRNA-132-3p is a mechanosensitive miRNA. When patients are mechanically unloaded for reasons such as prolonged bed rest, the expression of miRNA-132-3p increases, leading to the inhibition of the osteogenic differentiation of BMSCs. Hu et al. (2020) delivered an antagonist of miRNA-132-3p to the site of bone formation via a bone-targeted (AspSerSer)6-cationic liposome system, which effectively inhibited the expression of miRNA-132-3p and reduced the negative impact of mechanical unloading on osteogenic differentiation. In addition, Herman et al. (2015) used a cationic liposome-based delivery system to deliver siRNA targeting heterogeneous nuclear RNP A2/B1 (hnRNP A2/B1), which efficiently silenced the expression of target genes, thereby inhibiting inflammation and bone erosion.
PEG modification is frequently used to enhance the pliability and hydrophilicity of LNPs. PEG provides a polymer layer for LNPs, which reduces the interaction of LNPs with plasma proteins and prolongs circulation time in vivo (Eygeris et al., 2022). Binding antibodies or ligands to the PEG terminus maintain both a long circulating time and recognition of the target site. Gao et al. (2022) modified PEG into an organically synthesized (DSS)6-liposome nanoparticle (NP) system to target the surface of bone formation sites, which significantly enhanced the delivery of siRNA targeting the Ckip-1 gene to osteoblasts, without apparent cytotoxicity. In vivo osteogenesis-related analysis showed that bone mass was significantly increased, with improved bone microarchitecture and enhanced mechanical properties. Several other functionalized modifications have also been adopted for the preparation of liposomes to enhance their targeting. Liang et al. (2015) used the cell SELEX technology to screen for CH6, a specific aptamer for osteoblasts, to develop CH6 aptamer-functionalized LNPs encapsulated with an siRNA targeting the Plekho1 gene, which eventually led to specific silencing of the Plekho1 gene in vivo and promoted the formation of bone tissue. Using such functionalized LNPs, the specificity of osteogenesis-related siRNAs for targeted delivery increases from the tissue to the cellular level. Additionally, because aspartate 8 peptide (D-asp8 peptide) binds strongly to the highly crystalline hydroxyapatite, Liu et al. (2015) decorated D-asp8 peptide onto liposomes to purposefully target antagomiR-148a, a regulator of miR-148a, to the bone resorption surface. The results showed that this liposome delivery system promoted the enrichment of antagomiR-148a in osteoblasts and reduced miR-148a expression, which further reduced bone resorption and the deterioration of bone trabecular structures.
With the continuous development of liposome technology, a new type of liposomes, sterosomes, comprising single-chain amphiphilic molecules and a high sterol ratio, has been developed. Compared with those of conventional liposomes, both the positive charge on the surface and stability of the sterosomes are high. Cui et al. (2015) delivered siRNA using sterosomes composed of stearylamine (SA) and cholesterol (Chol), which significantly enhanced the cellular uptake and gene knockdown efficiency compared to the commercially available Lipofectamine 2000. Moreover, cytotoxicity is greatly reduced, which has a high potential for application. Nevertheless, there are some drawbacks to liposome delivery. Owing to the unstable physical properties of liposomes, it is difficult to control the mode and timing of drug release. Future studies should focus on identifying appropriate target sites for therapy to achieve efficient drug delivery and minimize cytotoxicity.
Hydrogel scaffolds
Hydrogels are a class of extremely hydrophilic three-dimensional network-structured gels composed of natural or synthetic polymers. Owing to their biocompatibility, biodegradability, and ability to sustainably release RNA therapeutic drugs, hydrogels are a promising platform for RNA therapeutic drug delivery (Wang and Burdick, 2017; Yu et al., 2020b). Kim et al. (2021) prepared a sulfonate hydrogel using sulfoacetic acid to modify chitosan, and siRNA targeting noggin was covalently bound to the sulfonate hydrogel via visible blue light cross-linking. The sulfonate hydrogel-siRNA conjugate significantly prolonged the release of siRNA to sustainably inhibit Noggin, which enhanced osteogenic differentiation. McMillan et al. (2021) developed photocrosslinked dextran hydrogel microspheres encapsulating siRNA micelles, which were bound to hMSC aggregates, to continuously deliver siRNA for targeted gene silencing. The delivery system used in this study may drive the differentiation of hMSCs into the desired specific lineage. Carthew et al. (2020) combined MSCs and miR-100-5p/miR-143-3p into a light-cross-linkable gelatin-PEG hydrogel. The data revealed superior mineralization properties and high expression of osteogenesis-related genes in the MSCs. The osteogenic potential of MSCs can be significantly enhanced by using this system. Few studies have aimed to promote bone formation by enhancing innervation. Lei et al. (2019) developed an injectable mesoporous silica NP (MSN)-embedded core-shell structured poly (ethylene glycol)-b-poly (lactic-co-glycolic acid)-b-poly (N-isopropylacrylamide) (PEG-PLGA-PNIPAM) hydrogel, which was used to realize long-term co-delivery of microRNA-222 and aspirin (ASP) in the form of a miR222/MSN/ASP hydrogel. miR-222 promotes the differentiation of hMSCs into neurogenic cells via WNT/β-catenin/Nemo-like kinase signaling. Disulfide bonds and amino-modified MSNs are used to bind miR222 and stabilize miR222 extracellularly. When the miR222/MSN complex escapes from the hydrogel and is endocytosed by cells, the disulfide bond breaks, as it is susceptible to redox reactions with endogenous GSH, and miR222 is released. In a rat mandibular bone defect model, injection of the miR222/MSN/ASP hydrogel resulted in enhanced bone formation.
To protect RNA molecules from hydrolysis by nucleases and to improve the delivery efficiency of RNA in hydrogels, several strategies, including the use of hydrogels as vectors for the delivery of RNA in the form of RNA complexes or encapsulated in NPs, have been developed (Yu et al., 2020b). Nguyen et al. (2018) synthesized a PEG hydrogel in which siRN–PEI complexes targeting Noggin and hMSCs were encapsulated. In vitro experiments showed enhanced osteogenic differentiation of hMSCs owing to the continuous release of siNoggin from the PEG hydrogel into the encapsulated hMSCs. When applied to the rat cranial defect model, the hydrogel led to a greater extent of new bone formation than the siRNA-free hydrogel. Wang et al. (2017b) developed a hybrid NP/hydrogel system, wherein the hydrogel enabled sustained drug delivery by controlling the release of the encapsulated siRNA/NP complex, while the NPs provided protection to siRNA with efficient accumulation. In a specific application, Wang et al. encapsulated siRNA targeting WW domain-containing E3 ubiquitin-protein ligase 1 (Wwp1) in a PEG-based hydrogel after complexing it with NPs to observe its effect after implantation into the femoral fracture site in mice. Compared to that in the control group, this system was able to substantially silence the Wwp1 gene and accelerate bone formation, which is highly promising in promoting fracture healing. Wu et al. (2018) incorporated chitosan/tripolyphosphate/hyaluronic acid/antimiRNA-138 NPs (CTH/antimiR-138 NPs) into a chitosan/β-sodium glycerol phosphate (CS/GP) hydrogel to construct an NP/hydrogel composite system for the treatment of critical-size cranial defects in rats. Sustained release of antimiR-138 over 21 days improved the osteogenic differentiation of MSCs and enhanced the expression of osteopontin (OPN), collagen type-1 (COL-1), and osteocalcin (OCN) proteins. DNA nanostructures have also been used to bind RNA and form complexes. Li et al. (2022) used tetrahedral DNA nanostructures (TDNs) to deliver miR355-5P, targeting the DKK1 gene, and heparin lithium hydrogel (Li-hep-gel) for the delivery of MiR@TDNs. The MiR@TDNs/Li-hep-gel composite promoted the expression of alkaline phosphatase and calcium nodule deposition, along with the generation of vascular-like structures, after implantation at the site of bone defects. The advantage of the MiR@TDNs/Li-hep-gel composite is that it utilizes the dual delivery of lithium and MiR@TDNs to promote bone regeneration through the upregulation of the WNT signaling pathway. To demonstrate the effect of delivering RNA therapeutic drugs as complexes, Lolli et al. (2019) bound or unbound inhibitors targeting miR-221 (antimiR-221) to lipofectamine using fibrin/hyaluronan (Fb/HA) hydrogels as vectors. hMSCs were then inoculated onto the hydrogel surface. The results showed that antimiR-221, combined with Lipofectamine, significantly enhanced intracellular miR-221 silencing and improved the efficiency of gene transfection. Moreover, plasma small extracellular vesicles (sEVs) containing osteogenic siRNAs targeting osteoprogenitor cells (Xia et al., 2021) and exosomes rich in miR-375 (Exo(miR-375)) (Chen et al., 2019) were delivered using hydrogels to repair bone defects.
Achieving a further controlled release of RNA drugs in hydrogels is a persistent problem. Huynh et al. (2017) developed a photodegradable PEG-hydrogel system. In the absence of UV light, RNA was released from the hydrogel in a sustained manner, whereas UV irradiation significantly accelerated the release of siRNA from the hydrogel. siRNA targeting noggin induced the osteogenic differentiation of hMSCs with or without UV light. In 2016, Huynh et al. (2016) similarly induced osteogenesis in hMSCs using photodegradable hydrogels. Cholesterol-modified miR-26a (Chol-miR-26a) was used to improve the osteogenic differentiation of hMSCs. Gan et al. (2021) linked Chol-miR-26a to injectable PEG hydrogels via UV light-cleavable ester bonds. Under UV light, Gel-chol-miR-26a selectively released chol-miR-26a to repair severe bone defects in a rat cranial defect model. This study suggests that injectable hydrogels that control the release of RNA for osteogenesis hold great potential for bone defect repair.
In bone tissue engineering, a series of studies have demonstrated the potential of hydrogels to convey RNA therapeutic molecules to bone defect sites for gene therapy (Yu et al., 2020b). Nevertheless, the sustained release of RNA therapeutic agents in hydrogels requires further modulation to facilitate their further development as RNA delivery platforms for bone defect repair.
Exosomes
All cells in an organism secrete extracellular vesicles (EVs) (Barile and Vassalli, 2017). Exosomes are nanoscale EVs that originate from the endosome, fuse with molecules present in the ER, and are processed in the Golgi apparatus to form multivesicular endosomes. When multivesicular endosomes mature and integrate with the plasma membrane, their contents are discharged as exosomes into the extracellular compartment (Barile and Vassalli, 2017; Wang and Thomsen, 2021). Exosomes carry nucleic acids, proteins, lipids, and metabolites that can target receptor cells to be taken up through membrane fusion or endocytosis. In addition, exosomes can cross barriers, such as the blood-brain barrier, which makes them natural vectors in vivo (Ha et al., 2016; Lu et al., 2021). Recently, exosomes have been utilized as non-viral vectors for RNA delivery, which indicates their high biocompatibility, low clearance, and suitability for targeting cells (Duan et al., 2021). Lee et al.(Won Lee et al., 2020) isolated and purified exosomes from the plasma of rabbits and loaded miR-140 into the exosomes, which acted as an RNA delivery system. BMSCs were cultured in vitro, and the results showed a significant increase in collagen type II and glycoprotein synthesis as well as enhanced gene expression of SOX9 and aggrecan, which demonstrated the differentiation of BMSCs toward chondrocytes. In a study by Liu et al. (2021b), exosomes were used as nanocarriers for the protection and delivery of miR-181b. exo-181b suppressed inflammatory responses by inhibiting PRKCD and activating p-AKT to enhance M2 macrophage polarization, which promoted osteogenesis in vitro and osseointegration in vivo. Chen et al. (2019) isolated miR-375-rich exosomes from human adipose mesenchymal stem cells overexpressing miR-375 after lentiviral transfection. The experimental results showed that exosomes could deliver miR-375 to hMSCs, thereby inhibiting the expression of IGFBP3 (a negative regulator of osteogenic differentiation) and playing an enhanced osteogenic role. Furthermore, selective targeting of specific sites can be achieved by engineering the surface proteins of exosomes to avoid accumulation at non-targeted sites, which reduces the toxic side effects of the drug (Duan et al., 2021).
However, currently available research results related to exosomes should be treated with caution from the perspective of clinical application. The main reason for this is that there is no universal method for exosome isolation and purification, and current isolation techniques may lead to the inevitable mixing of some other components with exosomes. Moreover, there is no uniform standard for the isolation of exosomes and clinical safety is difficult to guarantee. Therefore, the standardization of exosome-related experiments is required to facilitate subsequent research (Lu et al., 2021).
Synthetic NPs
NPs, which are solid particles with a size of 10–1000 nm, have also been used as delivery vectors for RNA therapeutics (Xin et al., 2017). Common NPs for RNA delivery usually contain cationic components for complexing with RNA and adsorbing to the cell membrane to promote the uptake of cells via electrostatic interactions (Malcolm et al., 2020). Currently, NPs for RNA delivery can be classified as organic and inorganic NPs (Xin et al., 2017).
Organic NPs mainly include liposome-based and cationic polymer-based NPs. Liposome-based NPs have been described Liposome-based scaffolds. Cationic polymer-based NPs can be further classified into synthetic cationic polymer-based and natural cationic polymer-based NPs (Malcolm et al., 2020; Xin et al., 2017). Among the cationic polymers, PEI is the most widely used, mainly because PEI with a high molecular weight and high charge density can effectively compress and protect DNA, has a high transfection efficiency, and can escape from the lysosome owing to the proton sponge impact (Chen et al., 2020; Li et al., 2021a). Branched PEI (25 kDa) and 22 kDa linear PEI are currently considered the "gold standard" in the field of polymeric gene vectors (Chen et al., 2020). However, the cytotoxicity caused by the high surface charge density and non-biodegradability of these two PEIs limits their clinical application. Therefore, polymer molecules, such as PEG, are usually utilized to modify PEI to reduce its cytotoxicity (Xin et al., 2017). Li et al. (2021a) constructed selenomethionine (SEMET)-modified PEG-PEI NP (SeNP) gene vectors based on PEG-PEI, loaded with miR-132-3p (BMSCs osteogenic differentiation inhibitor by targeting BMP-2) inhibitor, and then uniformly dispersed them into a gel solution for titanium implants as a novel coating to promote osseointegration. Compared to PEG-PEI, SeNPs have better biocompatibility, based on equivalent transfection efficiency. SeNP-modified titanium implants significantly promote the osteogenic differentiation of BMSCs and bone regeneration. Although the cationic polymer PEI itself can serve as a delivery vector for gene therapy, the modification of the NP surface by PEI can facilitate the complexation of the gene with the NP. Mora-Raimundo et al. (2021) functionally modified the surface of MSNs with alendronate-modified PEG to increase their stability and bone targeting. Moreover, the cationic polymer PEI was coated on the NP surface for the co-delivery of osteostatin and siRNA targeting SOST. The increased expression of important osteogenic markers and vascularization-related genes leads to enhanced bone formation. Similarly, in 2019, Mora-Raimundo et al. (2019) enhanced bone formation using PEI-modified MSNs for the combined delivery of siRNA targeting SOST and osteostatin. In addition, biodegradable polymeric NPs based on PEG and poly (disulfide amide) (PDSA), developed by Xu and Wu et al.(Xu et al., 2018b), are likely to be used to improve the delivery efficiency of RNA drugs in RNAi therapy, because these NPs can effectively encapsulate siRNA extracellularly and react with glutathione (GSH) in the cytoplasm to induce rapid intracellular release of siRNA.
Delivery systems based on natural cationic polymers are also viable options for gene delivery. Chitosan (a cationic polysaccharide)-based delivery systems have been used for gene delivery without cytotoxicity and at low costs (Jia et al., 2014; Jiang et al., 2020; Wu et al., 2016). Jiang et al. (2020) prepared chitosan-tripolyphosphate-hyaluronic acid (CTH) NPs using ionic gel technology for the delivery of antigomiR-133a/b to inhibit the expression of miR-133a and miR-133b (inhibitors of osteogenesis) in BMSCs. In vitro experiments showed that this delivery system was not significantly cytotoxic and could enhance calcium deposition and the expression of osteogenesis-specific genes. In a mouse cranial defect model, the use of CTH-antigomiR-133a/b NPs resulted in sustained release of antigomiR-133a/b and promoted the repair of bone defects. PLGA is a class of degradable functional polymers with good biocompatibility, non-immunogenicity, and sustained drug release and is widely used as a gene vector. Cationic modifications are usually applied to increase transfection efficiency. Sezlev Bilecen et al.(Sezlev Bilecen et al., 2019) developed a PLGA-based siRNA delivery system. siRNA targeting RANK was complexed with PEI and encapsulated in PLGA nanocapsules. The encapsulation rate of the PEI–siRNA complex in PLGA nanocapsules was 48%. Once the complex entered the cell, the PEI–siRNA complex was continuously released into the cytoplasm, leading to a significant reduction in RANK mRNA levels, resulting in the inhibition of osteoclast activity.
Inorganic NPs, such as MSNs and metal NPs, have also been used as vectors for RNA therapy. MSNs with large specific surface areas, high porosity, and biodegradability are relatively versatile and promising inorganic NPs for gene-drug delivery (Kesse et al., 2019; Rimpei et al., 2018; Yan et al., 2020). Yan et al. (2020) loaded miR-26a, which promotes osteogenic differentiation, into the pores of MSNs and coated their surface with PEI to protect miR-26a from degradation. This delivery system entered the lysosomes of BMSCs through endocytosis and escaped through the proton sponge effect of PEI. With the degradation of MSNs, miR-26 was discharged into the cytoplasm and induced the osteogenic differentiation of BMSCs. Modification with functionalized polymers on the surface of MSN_miR-26a@PEI could enhance targeting and further improve biocompatibility. Furthermore, Liu et al. (2017) combined a miR-204 (BMSCs osteogenic differentiation inhibitor) inhibitor with gold NPs to form AuNPs-antigomiR204 and dispersed them in a PLGA solution for the surface coating of titanium implants, which promoted osteointegration.
In vivo adsorption of nonspecific proteins is a limitation of NPs for clinical gene-drug delivery, which can result in high mononuclear phagocytic system (MPS)-mediated clearance and often requires an increased dose to achieve therapeutic effects (Malcolm et al., 2020; Stefan et al., 2016). Therefore, optimizing the structure of NPs to avoid the nonspecific adsorption of proteins as much as possible is one of the significant issues that needs to be addressed in future studies. Table 1 compares and summarizes the above four carriers to give a clear and intuitive understanding.
Table 1.
Comparison and summary of the four carriers mentioned in this study
| Vector | Delivery mode | Advantages | Drawbacks | Examples | Mechanism | Effects |
|---|---|---|---|---|---|---|
| Liposome-based scaffolds | Complex or conjugate | High drug encapsulation rate; protection from the degradation of RNA by nucleases; low toxicity and immunogenicity; sustained drug release | Unstable physical properties; difficulty in controlling the timing of drug release | (AspSerSer)6-cationic liposome based on DOTAP/siRNA (Zhang et al., 2012) | Silence the expression of the Plekho1 gene | Promote bone formation |
| PEG-modified (DSS)6-liposome nanoparticle/siRNA (Gao et al., 2022) | Silence the expression of the Ckip-1 gene | Improve bone microarchitecture and enhance mechanical properties | ||||
| D-asp8 peptide-modified liposome/antagomiR-148a (Liu et al., 2015) | Downregulate the expression of miR-148a | Reduce bone resorption and the deterioration of bone trabecular structures. | ||||
| Hydrogel scaffolds | Directly conjugate; encapsulate RNA complexes or nanoparticles | Excellent biocompatibility and biodegradability; sustained release of the RNA molecules; ease of cell adhesion and migration; adjustable properties | In vivo applications need to be further studied | Sulfonate hydrogel-siRNA conjugates (Kim et al., 2021) | Silence the expression of Noggin | Enhance the osteogenic differentiation |
| PEG-PLGA-PNIPAM hydrogel/miR-222 (Lei et al., 2019) | Promote the differentiation of hMSCs into neurogenic-like cells via WNT/β-catenin/Nemo-like kinase signaling | Promote neurogenesis and bone formation | ||||
| PEG hydrogel/siRNA-PEI complexes (Nguyen et al., 2018) | Silence the expression of Noggin | Promote the osteogenic differentiation of hMSCs | ||||
| PEG hydrogel/nanoparticle encapsulating siRNA (Wang et al., 2017b) | Silence the expression of Wwp1 | Accelerate bone formation | ||||
| CS/GP hydrogel combining with CTH/antimiR-138 NPs (Wu et al., 2018) | Upregulate the expression of OCN, OPN, and COL-1 | Enhance the osteogenic differentiation of MSCs | ||||
| miR355-5p@TDNs/Li-hep-gel composite (Li et al., 2022) | Upregulate the WNT signaling pathway by targeting the DKK1 gene | Promote bone regeneration | ||||
| Exosomes | Encapsulate RNA directly | Ability to cross the natural barriers in the body; high biocompatibility; low clearance; suitability for targeting cells; ease of engineering modifications | Lack of cost-effective techniques for exosome isolation; limited understanding of exosomes | Exosomes/miR-140 (Won Lee et al., 2020) | Enhance the expression of SOX9 and aggrecan | Promote the differentiation of BMSCs toward chondrocytes and increase the synthesis of collagen type II and glycoproteins |
| Exosomes/miR-181b (Liu et al., 2021b) | Inhibit the PRKCD and activate the p-AKT signaling pathway | Promote osteogenesis in vitro and osseointegration in vivo | ||||
| Exosomes/miR-375 (Chen et al., 2019) | Inhibit the expression of IGFBP3 | Promote osteogenesis | ||||
| Synthetic nanoparticles | Chemical bonding or physical encapsulation | Large specific surface area; good biocompatibility and biodegradability; high efficiency of gene transfection; low cytotoxicity; mass production; protection of RNA from degradation by nucleases | Adsorption of nonspecific proteins | selenomethionine (SEMET)-modified PEG-PEI nanoparticles (SeNPs)/miR-132-3p inhibitors (Li et al., 2021a) | Enhance the expression of BMP-2 | Improve the osteogenic differentiation of BMSCs and bone regeneration |
| Alendronate-modified PEG-mesoporous silica nanoparticles (MSNs) (Mora-Raimundo et al., 2021) | Silence the expression of SOST | Promote osteogenesis | ||||
| Chitosan-tripolyphosphate- hyaluronic acid (CTH) nanoparticles/antigomiR-133a/b (Jiang et al., 2020) | Enhance the expression of Runx2 | Promote the repair of bone defects | ||||
| MSN_miR-26a@PEI (Yan et al., 2020) | Improve the expression of Runx2, Opn, Osx, and BMP-2 | Promote the osteogenic differentiation of RBMSCs | ||||
| AuNPs/antigomiR-204 (Liu et al., 2017) | Improve the expression of Runx2 | Promote osteointegration |
Other types of scaffolds
Nanofibers
Nanofibers are broadly utilized in bone tissue engineering because they contribute to the osteogenic differentiation, adhesion, and growth of BMSCs (Kharaghani et al., 2021; Rim et al., 2009; Yu et al., 2020a). Moreover, because of their excellent capacity to trap drugs, together with their high specific surface area and porosity, nanofibers are used for the delivery of gene drugs (Kharaghani et al., 2019, 2021). Nanofibers with different diameters, morphologies, and orientations can be prepared using the recently developed electrostatic spinning technique (Anamarija, 2014; Hoik et al., 2018; Izadpanahi et al., 2018). James et al. (2014) prepared a gelatin nanofiber loaded with a miR-29 inhibitor using the electrostatic spinning technique, which could release the inhibitor continuously within 72 h miR-29 is a negative regulator of extracellular matrix synthesis. Cells growing on the miR-29-loaded nanofibers showed an increase in the synthesis of osteonectin and collagen. Pinese et al. (2018) synthesized polycaprolactone (PCL) nanofibers using electrostatic spinning. Prolonged persistent drug discharge and effective silencing of target genes were achieved by surface adsorption or nanofiber encapsulation for continuous delivery of siRNA/MSN-PEI complexes. Using MSN-PEI, dual delivery of more small-molecule drugs can be achieved, which has certain application prospects.
Microspheres
The use of microspheres in drug delivery is well known (Sulaiman et al., 2020; Tharmalingam et al., 2011). The goal of drug delivery is to achieve sustained release of drugs by encapsulating the drug molecules inside the microspheres or adsorbing them on the microsphere surface. When microspheres are gradually dissolved in the physiological environment, the drug is released depending on the degradation of the microspheres and exerts its effect. Using microspheres to deliver drugs offers long-term controlled drug release and greatly improves patient compliance by reducing dosing frequency. McMillan et al. (2021) prepared photo-cross-linkable dextran microspheres encapsulating siRNA micelles using an aqueous emulsion method and added them to hMSC aggregates to achieve persistent in situ release of siRNA. Microspheres encapsulating siRNA targeting green fluorescent protein (GFP) were consolidated into GFP-hMSC aggregates, which achieved GFP silencing for 15 days. To achieve cell-free scaffold-based miRNA therapy for bone tissue engineering, Zhang et al. (2016) designed a hyperbranched polymer (HP) vector for miRNA delivery and attached short PEG chains and low-molecular-weight cationic PEI. The polyplex formed a nanosized spherical shell via self-assembly after the addition of miR-26a. To avoid the uncontrolled release of miR-26a, the polyplex was encapsulated with degradable polymer microspheres. Such a delivery strategy enables the dual delivery of miR-26a, which not only maintains sustained delivery but also offers high transfection efficiency through the polyplex. Additionally, microspheres were attached to the nanofiber scaffold to spatially control the discharge of miR-26a. This innovation was used to repair the critical size of bone defects by focusing on Gsk-3β. NFs and microspheres are summarized in Table 2.
Table 2.
Summary of nanofibers (NFs) and microspheres
| Vectors | Delivery mode | Advantages | Drawbacks | Examples | Mechanism | Effects |
|---|---|---|---|---|---|---|
| Nanofibers (NFs) | Surface adsorption or nanofiber encapsulation | Excellent ability to capture drugs; high specific surface area and porosity; ability to promote osteogenic differentiation and adhesion of BMSCs together with drug delivery | Lack of tissue-specific delivery, resulting in off-target effects and cytotoxicity; weak intracellular uptake | Gelatin nanofibers/miR-29 inhibitors (James et al., 2014) | Upregulate the mRNA levels of both IGF-1 and TGF-β1 | Increase the synthesis of osteonectin and type I collagen |
| Polycaprolactone (PCL) nanofibers/siRNA-MSN@PEI (Pinese et al., 2018) | Silence the expression of COLL1A1 | Inhibit fibrous capsule formation to promote host-implant integration | ||||
| Microspheres | Surface adsorption or encapsulation inside the microsphere | Sustained drug release; targeted drug delivery; biocompatibility and biodegradability; improved drug efficacy and patient compliance; prevention of nuclease degradation | Sudden release phenomenon in clinical applications; difficulty in large-scale preparation; biocompatibility issues of modified microspheres | Degradable polymer microspheres/miR-26a (Zhang et al., 2016) | Inhibit the expression of Gsk-3β | Promote bone regeneration |
Conclusions and perspectives
Signaling pathways such as WNT/β-catenin, PI3K/AKT, and TGF-β are important regulators of bone formation and osteogenic differentiation. Multiple small ncRNAs utilize these pathways to transduce signals to downstream molecules to regulate the osteogenic differentiation of BMSCs, which affects bone regeneration. The safe delivery of ncRNA to the target site without degradation by nucleases has always been a challenge. Cationic liposomes and synthetic NPs with cationic components are widely used for RNA delivery, as they can stabilize ncRNA via electrostatic forces and benefit cellular uptake. Exosomes are commonly used as vectors for RNA delivery because of their high biocompatibility and excellent cellular targeting. The application of microspheres to deliver ncRNA can help in achieving long-term controlled release of RNA agents and improving patient compliance. In addition, nanofibers and hydrogels can not only serve as vectors for RNA drugs but can also combine with RNA complexes, such as RNA/NPs and RNA/exosomes, to form composite materials for the dual release of RNA drugs, which has high clinical application value.
However, the delivery of exogenous ncRNAs to bone defect sites by vectors such as cationic liposomes may cause severe cellular toxicity and off-target effects which cannot be ignored in clinical treatment (Ganju et al., 2017). In situ the controlled release of ncRNAs at the site of bone defects is one way to improve the therapeutic effect of ncRNAs and reduce cytotoxicity. In addition, modifying bone-targeting molecules, such as (AspSerSer)6 peptide and CH6 aptamer, to the vector surface can significantly enhance the targeting ability of delivered ncRNAs to bone. In the future, the combination of vectors modified by bone-targeted molecules and gene therapy will contribute to the further development of bone tissue engineering.
In conclusion, a deeper understanding of ncRNAs is the basis for achieving breakthrough results in the field of bone defect repair. Further studies should focus on exploring the biological functions of ncRNAs and their regulatory mechanisms in osteogenic signaling. In addition, the instability of ncRNAs, off-target effects, and poor prognosis restrict the design of ncRNA delivery vectors and their further clinical applications. Designing novel low-toxicity and stable in vivo bone-targeting molecules is a potential strategy to reduce the off-target effects of ncRNAs. With the continuous development of biology, medicine, and engineering, "precision" custom-engineered ncRNA delivery scaffolds may become one of the hotspots for the development of novel bone repair materials.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (no. 51973243 and 52173150), the Shenzhen Basic Research Project (JCYJ20190807155801657), the INTERNATIONAL COOPERATION and Exchange of the National Natural Science Foundation of China (no. 51820105004).
Author contributions
Conceptualization: J.W. and Z. Z.; Methodology: S.Y.G. and Z. Z.; Investigation: S.Y.G. and Z. Z.; Visualization: S.Y.G.; Supervision: J.W.; Funding Acquisition: J.W.; Writing—original draft: S.Y.G. and Z. Z.; Writing—review & editing: J.W. and Z. Z.;
Declaration of interests
The authors declare no competing interests.
References
- Alshaer W., Zureigat H., Al Karaki A., Al-Kadash A., Gharaibeh L., Hatmal M.M., Aljabali A.A.A., Awidi A. siRNA: mechanism of action, challenges, and therapeutic approaches. Eur. J. Pharmacol. 2021;905 doi: 10.1016/j.ejphar.2021.174178. [DOI] [PubMed] [Google Scholar]
- Rogina A. Electrospinning process: versatile preparation method for biodegradable and natural polymers and biocomposite systems applied in tissue engineering and drug delivery. Appl. Surf. Sci. 2014;296:221–230. [Google Scholar]
- Arfat Y., Basra M.A.R., Shahzad M., Majeed K., Mahmood N., Munir H. miR-208a-3p suppresses osteoblast differentiation and inhibits bone formation by targeting ACVR1. Mol. Ther. Nucleic Acids. 2018;11:323–336. doi: 10.1016/j.omtn.2017.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin P., Li D.J., Auston D.A., Mir H.S., Yoon R.S., Koval K.J. Autograft, allograft, and bone graft substitutes: clinical evidence and indications for use in the setting of orthopaedic trauma surgery. J. Orthop. Trauma. 2019;33:203–213. doi: 10.1097/BOT.0000000000001420. [DOI] [PubMed] [Google Scholar]
- Barile L., Vassalli G. Exosomes: therapy delivery tools and biomarkers of diseases. Pharmacol. Ther. 2017;174:63–78. doi: 10.1016/j.pharmthera.2017.02.020. [DOI] [PubMed] [Google Scholar]
- Bekmurzayeva A., Duncanson W.J., Azevedo H.S., Kanayeva D. Surface modification of stainless steel for biomedical applications: revisiting a century-old material. Mater. Sci. Eng. C Mater. Biol. Appl. 2018;93:1073–1089. doi: 10.1016/j.msec.2018.08.049. [DOI] [PubMed] [Google Scholar]
- Bellavia D., De Luca A., Carina V., Costa V., Raimondi L., Salamanna F., Alessandro R., Fini M., Giavaresi G. Deregulated miRNAs in bone health: epigenetic roles in osteoporosis. Bone. 2019;122:52–75. doi: 10.1016/j.bone.2019.02.013. [DOI] [PubMed] [Google Scholar]
- Bellavia D., Salamanna F., Raimondi L., De Luca A., Carina V., Costa V., Alessandro R., Fini M., Giavaresi G. Deregulated miRNAs in osteoporosis: effects in bone metastasis. Cell. Mol. Life. Sci. 2019;76:3723–3744. doi: 10.1007/s00018-019-03162-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharjee P., Kundu B., Naskar D., Kim H.W., Maiti T.K., Bhattacharya D., Kundu S.C. Silk scaffolds in bone tissue engineering: an overview. Acta Biomater. 2017;63:1–17. doi: 10.1016/j.actbio.2017.09.027. [DOI] [PubMed] [Google Scholar]
- Brown M.J., Carter T. ACL allograft: advantages and when to use. Sports Med. Arthrosc. Rev. 2018;26:75–78. doi: 10.1097/JSA.0000000000000194. [DOI] [PubMed] [Google Scholar]
- Carthew J., Donderwinkel I., Shrestha S., Truong V.X., Forsythe J.S., Frith J.E. In situ miRNA delivery from a hydrogel promotes osteogenesis of encapsulated mesenchymal stromal cells. Acta Biomater. 2020;101:249–261. doi: 10.1016/j.actbio.2019.11.016. [DOI] [PubMed] [Google Scholar]
- Chen G., Deng C., Li Y.P. TGF-β and BMP signaling in osteoblast differentiation and bone formation. Int. J. Biol. Sci. 2012;8:272–288. doi: 10.7150/ijbs.2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G., Wang S., Long C., Wang Z., Chen X., Tang W., He X., Bao Z., Tan B., Lu W.W., et al. PiRNA-63049 inhibits bone formation through Wnt/β-catenin signaling pathway. Int. J. Biol. Sci. 2021;17:4409–4425. doi: 10.7150/ijbs.64533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L.L., Carmichael G.G. Decoding the function of nuclear long non-coding RNAs. Curr. Opin. Cell. Biol. 2010;22:357–364. doi: 10.1016/j.ceb.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Ben S., Xin J., Li S., Zheng R., Wang H., Fan L., Du M., Zhang Z., Wang M. The biogenesis and biological function of PIWI-interacting RNA in cancer. J. Hematol. Oncol. 2021;14:93. doi: 10.1186/s13045-021-01104-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Tang Y., Liu Y., Zhang P., Lv L., Zhang X., Jia L., Zhou Y. Exosomes derived from miR-375-overexpressing human adipose mesenchymal stem cells promote bone regeneration. Cell. Prolif. 2019;52 doi: 10.1111/cpr.12669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Lv Z., Sun Y., Chi Z., Qing G. Recent advancements in polyethyleneimine-based materials and their biomedical, biotechnology, and biomaterial applications. J. Mater. Chem. B. 2020;8:2951–2973. doi: 10.1039/c9tb02271f. [DOI] [PubMed] [Google Scholar]
- Cui Z.K., Fan J., Kim S., Bezouglaia O., Fartash A., Wu B.M., Aghaloo T., Lee M. Delivery of siRNA via cationic Sterosomes to enhance osteogenic differentiation of mesenchymal stem cells. J. Contr. Release. 2015;217:42–52. doi: 10.1016/j.jconrel.2015.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della Bella E., Menzel U., Basoli V., Tourbier C., Alini M., Stoddart M.J. Differential regulation of circRNA, miRNA, and piRNA during early osteogenic and chondrogenic differentiation of human mesenchymal stromal cells. Cells. 2020;9:398. doi: 10.3390/cells9020398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della Bella E., Stoddart M.J. Cell detachment rapidly induces changes in noncoding RNA expression in human mesenchymal stromal cells. Biotechniques. 2019;67:286–293. doi: 10.2144/btn-2019-0038. [DOI] [PubMed] [Google Scholar]
- Duan L., Xu L., Xu X., Qin Z., Zhou X., Xiao Y., Liang Y., Xia J. Exosome-mediated delivery of gene vectors for gene therapy. Nanoscale. 2021;13:1387–1397. doi: 10.1039/d0nr07622h. [DOI] [PubMed] [Google Scholar]
- Duarte W.R., Iimura T., Takenaga K., Ohya K., Ishikawa I., Kasugai S. Extracellular role of S100A4 calcium-binding protein in the periodontal ligament. Biochem. Biophys. Res. Commun. 1999;255:416–420. doi: 10.1006/bbrc.1999.0214. [DOI] [PubMed] [Google Scholar]
- Duarte W.R., Kasugai S., Iimura T., Oida S., Takenaga K., Ohya K., Ishikawa I. cDNA cloning of S100 calcium-binding proteins from bovine periodontal ligament and their expression in oral tissues. J. Dent. Res. 1998;77:1694–1699. doi: 10.1177/00220345980770090501. [DOI] [PubMed] [Google Scholar]
- Ebert M.S., Neilson J.R., Sharp P.A. MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells. Nat. Methods. 2007;4:721–726. doi: 10.1038/nmeth1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Rashidy A.A., Roether J.A., Harhaus L., Kneser U., Boccaccini A.R. Regenerating bone with bioactive glass scaffolds: a review of in vivo studies in bone defect models. Acta Biomater. 2017;62:1–28. doi: 10.1016/j.actbio.2017.08.030. [DOI] [PubMed] [Google Scholar]
- Eygeris Y., Gupta M., Kim J., Sahay G. Chemistry of lipid nanoparticles for RNA delivery. Acc. Chem. Res. 2022;55:2–12. doi: 10.1021/acs.accounts.1c00544. [DOI] [PubMed] [Google Scholar]
- Fakhr E., Zare F., Teimoori-Toolabi L. Precise and efficient siRNA design: a key point in competent gene silencing. Cancer. Gene. Ther. 2016;23:73–82. doi: 10.1038/cgt.2016.4. [DOI] [PubMed] [Google Scholar]
- Fang S., Deng Y., Gu P., Fan X. MicroRNAs regulate bone development and regeneration. Int. J. Mol. Sci. 2015;16:8227–8253. doi: 10.3390/ijms16048227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillingham Y., Jacobs J. Bone grafts and their substitutes. Bone Joint Lett. J. 2016;98-b:6–9. doi: 10.1302/0301-620X.98B.36350. [DOI] [PubMed] [Google Scholar]
- Gan M., Zhou Q., Ge J., Zhao J., Wang Y., Yan Q., Wu C., Yu H., Xiao Q., Wang W., et al. Precise in-situ release of microRNA from an injectable hydrogel induces bone regeneration. Acta Biomater. 2021;135:289–303. doi: 10.1016/j.actbio.2021.08.041. [DOI] [PubMed] [Google Scholar]
- Ganju A., Khan S., Hafeez B.B., Behrman S.W., Yallapu M.M., Chauhan S.C., Jaggi M. miRNA nanotherapeutics for cancer. Drug. Discov. Today. 2017;22:424–432. doi: 10.1016/j.drudis.2016.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y., Xin H., Cai B., Wang L., Lv Q., Hou Y., Liu F., Dai T., Kong L. RNA interference-based osteoanabolic therapy for osteoporosis by a bone-formation surface targeting delivery system. Biochem. Biophys. Res. Commun. 2022;601:86–92. doi: 10.1016/j.bbrc.2022.02.080. [DOI] [PubMed] [Google Scholar]
- Garcia J., Delany A.M. MicroRNAs regulating TGFβ and BMP signaling in the osteoblast lineage. Bone. 2021;143 doi: 10.1016/j.bone.2020.115791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannoudis P.V., Dinopoulos H., Tsiridis E. Bone substitutes: an update. Injury. 2005;36:S20–S27. doi: 10.1016/j.injury.2005.07.029. [DOI] [PubMed] [Google Scholar]
- Guo H.H., Yu C.C., Sun S.X., Ma X.J., Yang X.C., Sun K.N., Jin Q.H. Adenovirus-mediated siRNA targeting TNF-α and overexpression of bone morphogenetic protein-2 promotes early osteoblast differentiation on a cell model of Ti particle-induced inflammatory response in vitro. Braz. J. Med. Biol. Res. 2013;46:831–838. doi: 10.1590/1414-431X20133092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha D., Yang N., Nadithe V. Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: current perspectives and future challenges. Acta Pharm. Sin. B. 2016;6:287–296. doi: 10.1016/j.apsb.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habibovic P. (∗) strategic directions in osteoinduction and biomimetics. Tissue Eng. Part A. 2017;23:1295–1296. doi: 10.1089/ten.TEA.2017.0430. [DOI] [PubMed] [Google Scholar]
- Hajj K.A., Ball R.L., Deluty S.B., Singh S.R., Strelkova D., Knapp C.M., Whitehead K.A. Branched-tail lipid nanoparticles potently deliver mRNA in vivo due to enhanced ionization at endosomal pH. Small. 2019;15 doi: 10.1002/smll.201805097. [DOI] [PubMed] [Google Scholar]
- Herman S., Fischer A., Presumey J., Hoffmann M., Koenders M.I., Escriou V., Apparailly F., Steiner G. Inhibition of inflammation and bone erosion by RNA interference-mediated silencing of heterogeneous nuclear RNP A2/B1 in two experimental models of rheumatoid arthritis. Arthritis. Rheum. 2015;67:2536–2546. doi: 10.1002/art.39223. [DOI] [PubMed] [Google Scholar]
- Hoik L., Masayoshi N., Daewon S., Jung Soon L., Ick Soo K. Control of the morphology of cellulose acetate nanofibers via electrospinning. Cellulose. 2018;25:2829–2837. [Google Scholar]
- Hong H., Sun Y., Deng H., Yuan K., Chen J., Liu W., Cui Z. Dysregulation of cPWWP2A-miR-579 axis mediates dexamethasone-induced cytotoxicity in human osteoblasts. Biochem. Biophys. Res. Commun. 2019;517:491–498. doi: 10.1016/j.bbrc.2019.07.095. [DOI] [PubMed] [Google Scholar]
- Hong S., Hu S., Kang Z., Liu Z., Yang W., Zhang Y., Yang D., Ruan W., Yu G., Sun L., Chen L. Identification of functional lncRNAs based on competing endogenous RNA network in osteoblast differentiation. J. Cell. Physiol. 2020;235:2232–2244. doi: 10.1002/jcp.29132. [DOI] [PubMed] [Google Scholar]
- Hu Z., Zhang L., Wang H., Wang Y., Tan Y., Dang L., Wang K., Sun Z., Li G., Cao X., et al. Targeted silencing of miRNA-132-3p expression rescues disuse osteopenia by promoting mesenchymal stem cell osteogenic differentiation and osteogenesis in mice. Stem Cell Res. Ther. 2020;11:58. doi: 10.1186/s13287-020-1581-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh C.T., Nguyen M.K., Naris M., Tonga G.Y., Rotello V.M., Alsberg E. Light-triggered RNA release and induction of hMSC osteogenesis via photodegradable, dual-crosslinked hydrogels. Nanomedicine (Lond) 2016;11:1535–1550. doi: 10.2217/nnm-2016-0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh C.T., Zheng Z., Nguyen M.K., McMillan A., Yesilbag Tonga G., Rotello V.M., Alsberg E. Cytocompatible catalyst-free photodegradable hydrogels for light-mediated RNA release to induce hMSC osteogenesis. ACS Biomater. Sci. Eng. 2017;3:2011–2023. doi: 10.1021/acsbiomaterials.6b00796. [DOI] [PubMed] [Google Scholar]
- Ickenstein L.M., Garidel P. Lipid-based nanoparticle formulations for small molecules and RNA drugs. Expert Opin. Drug Deliv. 2019;16:1205–1226. doi: 10.1080/17425247.2019.1669558. [DOI] [PubMed] [Google Scholar]
- Izadpanahi M., Seyedjafari E., Arefian E., Hamta A., Hosseinzadeh S., Kehtari M., Soleimani M. Nanotopographical cues of electrospun PLLA efficiently modulate non-coding RNA network to osteogenic differentiation of mesenchymal stem cells during BMP signaling pathway. Mater. Sci. Eng. C Mater. Biol. Appl. 2018;93:686–703. doi: 10.1016/j.msec.2018.08.023. [DOI] [PubMed] [Google Scholar]
- James E.N., Delany A.M., Nair L.S. Post-transcriptional regulation in osteoblasts using localized delivery of miR-29a inhibitor from nanofibers to enhance extracellular matrix deposition. Acta Biomater. 2014;10:3571–3580. doi: 10.1016/j.actbio.2014.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia S., Yang X., Song W., Wang L., Fang K., Hu Z., Yang Z., Shan C., Lei D., Lu B. Incorporation of osteogenic and angiogenic small interfering RNAs into chitosan sponge for bone tissue engineering. Int. J. Nanomed. 2014;9:5307–5316. doi: 10.2147/IJN.S70457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang F., Yin F., Lin Y., Xia W., Zhou L., Pan C., Wang N., Shan H., Zhou Z., Yu X. The promotion of bone regeneration through CS/GP-CTH/antagomir-133a/b sustained release system. Nanomedicine. 2020;24 doi: 10.1016/j.nano.2019.102116. [DOI] [PubMed] [Google Scholar]
- Kamrani S., Fleck C. Biodegradable magnesium alloys as temporary orthopaedic implants: a review. Biometals. 2019;32:185–193. doi: 10.1007/s10534-019-00170-y. [DOI] [PubMed] [Google Scholar]
- Kato C., Kojima T., Komaki M., Mimori K., Duarte W.R., Takenaga K., Ishikawa I. S100A4 inhibition by RNAi up-regulates osteoblast related genes in periodontal ligament cells. Biochem. Biophys. Res. Commun. 2005;326:147–153. doi: 10.1016/j.bbrc.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Kesse S., Boakye-Yiadom K.O., Ochete B.O., Opoku-Damoah Y., Akhtar F., Filli M.S., Asim Farooq M., Aquib M., Maviah Mily B.J., Murtaza G., Wang B. Mesoporous silica nanomaterials: versatile nanocarriers for cancer theranostics and drug and gene delivery. Pharmaceutics. 2019;11:E77. doi: 10.3390/pharmaceutics11020077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharaghani D., Gitigard P., Ohtani H., Kim K.O., Ullah S., Saito Y., Khan M.Q., Kim I.S. Design and characterization of dual drug delivery based on in-situ assembled PVA/PAN core-shell nanofibers for wound dressing application. Sci. Rep. 2019;9 doi: 10.1038/s41598-019-49132-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharaghani D., Kurniwan E.B., Khan M.Q., Yoshiko Y. MiRNA-nanofiber, the next generation of bioactive scaffolds for bone regeneration: a review. Micromachines. 2021;12:1472. doi: 10.3390/mi12121472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K.M., Lim S.K. Role of miRNAs in bone and their potential as therapeutic targets. Curr. Opin. Pharmacol. 2014;16:133–141. doi: 10.1016/j.coph.2014.05.001. [DOI] [PubMed] [Google Scholar]
- Kim S., Fan J., Lee C.S., Chen C., Lee M. Sulfonate hydrogel-siRNA conjugate facilitates osteogenic differentiation of mesenchymal stem cells by controlled gene silencing and activation of BMP signaling. ACS Appl. Bio Mater. 2021;4:5189–5200. doi: 10.1021/acsabm.1c00369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong C.H., Steffi C., Shi Z., Wang W. Development of mesoporous bioactive glass nanoparticles and its use in bone tissue engineering. J. Biomed. Mater. Res. B Appl. Biomater. 2018;106:2878–2887. doi: 10.1002/jbm.b.34143. [DOI] [PubMed] [Google Scholar]
- Kozomara A., Birgaoanu M., Griffiths-Jones S. miRBase: from microRNA sequences to function. Nucleic. Acids. Res. 2019;47:155–162. doi: 10.1093/nar/gky1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozomara A., Griffiths-Jones S. miRBase: annotating high confidence microRNAs using deep sequencing data. Nucleic. Acids. Res. 2014;42:D68–D73. doi: 10.1093/nar/gkt1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kureel J., John A.A., Dixit M., Singh D. MicroRNA-467g inhibits new bone regeneration by targeting Ihh/Runx-2 signaling. Int. J. Biochem. Cell. Biol. 2017;85:35–43. doi: 10.1016/j.biocel.2017.01.018. [DOI] [PubMed] [Google Scholar]
- Lai L., Wang Z., Ge Y., Qiu W., Wu B., Fang F., Xu H., Chen Z. Comprehensive analysis of the long noncoding RNA-associated competitive endogenous RNA network in the osteogenic differentiation of periodontal ligament stem cells. BMC. Genomics. 2022;23:1. doi: 10.1186/s12864-021-08243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei L., Liu Z., Yuan P., Jin R., Wang X., Jiang T., Chen X. Injectable colloidal hydrogel with mesoporous silica nanoparticles for sustained co-release of microRNA-222 and aspirin to achieve innervated bone regeneration in rat mandibular defects. J. Mater. Chem. B. 2019;7:2722–2735. doi: 10.1039/c9tb00025a. [DOI] [PubMed] [Google Scholar]
- Leng Q., Chen L., Lv Y. RNA-based scaffolds for bone regeneration: application and mechanisms of mRNA, miRNA and siRNA. Theranostics. 2020;10:3190–3205. doi: 10.7150/thno.42640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D., Sun H., Deng W., Tao D., Liu Y., Ma Y. Zili antagonizes Bmp signaling to regulate dorsal-ventral patterning during zebrafish early embryogenesis. Zool. Sci. 2011;28:397–402. doi: 10.2108/zsj.28.397. [DOI] [PubMed] [Google Scholar]
- Li D., Yang Z., Luo Y., Zhao X., Tian M., Kang P. Delivery of MiR335-5p-pendant tetrahedron DNA nanostructures using an injectable heparin lithium hydrogel for challenging bone defects in steroid-associated osteonecrosis. Adv. Healthc. Mater. 2022;11 doi: 10.1002/adhm.202101412. [DOI] [PubMed] [Google Scholar]
- Li H., Li T., Fan J., Li T., Fan L., Wang S., Weng X., Han Q., Zhao R.C. miR-216a rescues dexamethasone suppression of osteogenesis, promotes osteoblast differentiation and enhances bone formation, by regulating c-Cbl-mediated PI3K/AKT pathway. Cell. Death. Differ. 2015;22:1935–1945. doi: 10.1038/cdd.2015.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Zheng Y., Zheng Y., Huang Y., Zhang Y., Jia L., Li W. Circular RNA CDR1as regulates osteoblastic differentiation of periodontal ligament stem cells via the miR-7/GDF5/SMAD and p38 MAPK signaling pathway. Stem Cell Res. Ther. 2018;9:232. doi: 10.1186/s13287-018-0976-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Chen G., He Y., Yi C., Zhang X., Zeng B., Huang Z., Deng F., Yu D. Selenomethionine-modified polyethylenimine-based nanoparticles loaded with miR-132-3p inhibitor-biofunctionalized titanium implants for improved osteointegration. ACS Biomater. Sci. Eng. 2021;7:4933–4945. doi: 10.1021/acsbiomaterials.1c00880. [DOI] [PubMed] [Google Scholar]
- Li Z., Xue H., Tan G., Xu Z. Effects of miRNAs, lncRNAs and circRNAs on osteoporosis as regulatory factors of bone homeostasis (Review) Mol. Med. Rep. 2021;24:788. doi: 10.3892/mmr.2021.12428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang C., Guo B., Wu H., Shao N., Li D., Liu J., Dang L., Wang C., Li H., Li S., et al. Aptamer-functionalized lipid nanoparticles targeting osteoblasts as a novel RNA interference-based bone anabolic strategy. Nat. Med. 2015;21:288–294. doi: 10.1038/nm.3791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y., Zheng J., Lin D. PIWI-interacting RNAs in human cancer. Semin. Cancer. Biol. 2021;75:15–28. doi: 10.1016/j.semcancer.2020.08.012. [DOI] [PubMed] [Google Scholar]
- Liu C., Ge H.M., Liu B.H., Dong R., Shan K., Chen X., Yao M.D., Li X.M., Yao J., Zhou R.M., et al. Targeting pericyte-endothelial cell crosstalk by circular RNA-cPWWP2A inhibition aggravates diabetes-induced microvascular dysfunction. Proc. Natl. Acad. Sci. USA. 2019;116:7455–7464. doi: 10.1073/pnas.1814874116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Chen M., Ma L., Dang X., Du G. piRNA-36741 regulates BMP2-mediated osteoblast differentiation via METTL3 controlled m6A modification. Aging (Albany NY) 2021;13:23361–23375. doi: 10.18632/aging.203630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Dang L., Li D., Liang C., He X., Wu H., Qian A., Yang Z., Au D.W.T., Chiang M.W.L., et al. A delivery system specifically approaching bone resorption surfaces to facilitate therapeutic modulation of microRNAs in osteoclasts. Biomaterials. 2015;52:148–160. doi: 10.1016/j.biomaterials.2015.02.007. [DOI] [PubMed] [Google Scholar]
- Liu W., Yu M., Chen F., Wang L., Ye C., Chen Q., Zhu Q., Xie D., Shao M., Yang L. A novel delivery nanobiotechnology: engineered miR-181b exosomes improved osteointegration by regulating macrophage polarization. J. Nanobiotechnol. 2021;19:269. doi: 10.1186/s12951-021-01015-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Tan N., Zhou Y., Wei H., Ren S., Yu F., Chen H., Jia C., Yang G., Song Y. Delivery of antagomiR204-conjugated gold nanoparticles from PLGA sheets and its implication in promoting osseointegration of titanium implant in type 2 diabetes mellitus. Int. J. Nanomed. 2017;12:7089–7101. doi: 10.2147/IJN.S124584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Dou M., Song X., Dong Y., Liu S., Liu H., Tao J., Li W., Yin X., Xu W. The emerging role of the piRNA/piwi complex in cancer. Mol. Cancer. 2019;18:123. doi: 10.1186/s12943-019-1052-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lolli A., Sivasubramaniyan K., Vainieri M.L., Oieni J., Kops N., Yayon A., van Osch G.J.V.M. Hydrogel-based delivery of antimiR-221 enhances cartilage regeneration by endogenous cells. J. Contr. Release. 2019;309:220–230. doi: 10.1016/j.jconrel.2019.07.040. [DOI] [PubMed] [Google Scholar]
- Lu J., Zhang Y., Liang J., Diao J., Liu P., Zhao H. Role of exosomal MicroRNAs and their crosstalk with oxidative stress in the pathogenesis of osteoporosis. Oxid. Med. Cell. Longev. 2021;2021 doi: 10.1155/2021/6301433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S., Wang D.D., Ma C.Y., Zhang Y.D. microRNA-96 promotes osteoblast differentiation and bone formation in ankylosing spondylitis mice through activating the Wnt signaling pathway by binding to SOST. J. Cell. Biochem. 2019;120:15429–15442. doi: 10.1002/jcb.28810. [DOI] [PubMed] [Google Scholar]
- Macias M.J., Martin-Malpartida P., Massagué J. Structural determinants of Smad function in TGF-β signaling. Trends. Biochem. Sci. 2015;40:296–308. doi: 10.1016/j.tibs.2015.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malcolm D.W., Wang Y., Overby C., Newman M., Benoit D.S.W. Delivery of RNAi-based therapeutics for bone regeneration. Curr. Osteoporos. Rep. 2020;18:312–324. doi: 10.1007/s11914-020-00587-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao C.Q., Du J.Z., Sun T.M., Yao Y.D., Zhang P.Z., Song E.W., Wang J. A biodegradable amphiphilic and cationic triblock copolymer for the delivery of siRNA targeting the acid ceramidase gene for cancer therapy. Biomaterials. 2011;32:3124–3133. doi: 10.1016/j.biomaterials.2011.01.006. [DOI] [PubMed] [Google Scholar]
- McMillan A., Nguyen M.K., Huynh C.T., Sarett S.M., Ge P., Chetverikova M., Nguyen K., Grosh D., Duvall C.L., Alsberg E. Hydrogel microspheres for spatiotemporally controlled delivery of RNA and silencing gene expression within scaffold-free tissue engineered constructs. Acta Biomater. 2021;124:315–326. doi: 10.1016/j.actbio.2021.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mencía Castaño I., Curtin C.M., Duffy G.P., O'Brien F.J. Harnessing an inhibitory role of miR-16 in osteogenesis by human mesenchymal stem cells for advanced scaffold-based bone tissue engineering. Tissue Eng. Part A. 2019;25:24–33. doi: 10.1089/ten.TEA.2017.0460. [DOI] [PubMed] [Google Scholar]
- Mikos A.G., Herring S.W., Ochareon P., Elisseeff J., Lu H.H., Kandel R., Schoen F.J., Toner M., Mooney D., Atala A., et al. Engineering complex tissues. Tissue. Eng. 2006;12:3307–3339. doi: 10.1089/ten.2006.12.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora-Raimundo P., Lozano D., Benito M., Mulero F., Manzano M., Vallet-Regí M. Osteoporosis remission and new bone formation with mesoporous silica nanoparticles. Adv. Sci. 2021;8 doi: 10.1002/advs.202101107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora-Raimundo P., Lozano D., Manzano M., Vallet-Regí M. Nanoparticles to knockdown osteoporosis-related gene and promote osteogenic marker expression for osteoporosis treatment. ACS Nano. 2019;13:5451–5464. doi: 10.1021/acsnano.9b00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nacher J.C. Community structure of non-coding RNA interaction network. J. Integr. Bioinform. 2013;10:217. doi: 10.2390/biecoll-jib-2013-217. [DOI] [PubMed] [Google Scholar]
- Nguyen M.K., Jeon O., Dang P.N., Huynh C.T., Varghai D., Riazi H., McMillan A., Herberg S., Alsberg E. RNA interfering molecule delivery from in situ forming biodegradable hydrogels for enhancement of bone formation in rat calvarial bone defects. Acta Biomater. 2018;75:105–114. doi: 10.1016/j.actbio.2018.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang Z., Tan T., Zhang X., Wan J., Zhou Y., Jiang G., Yang D., Liu T. LncRNA ENST00000563492 promoting the osteogenesis-angiogenesis coupling process in bone mesenchymal stem cells (BMSCs) by functions as a ceRNA for miR-205-5p. Cell Death Dis. 2020;11:486. doi: 10.1038/s41419-020-2689-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pais H., Nicolas F.E., Soond S.M., Swingler T.E., Clark I.M., Chantry A., Moulton V., Dalmay T. Analyzing mRNA expression identifies Smad3 as a microRNA-140 target regulated only at protein level. Rna. 2010;16:489–494. doi: 10.1261/rna.1701210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal I., Safari M., Jovanovic M., Bates S.E., Deng C. Targeting translation of mRNA as a therapeutic strategy in cancer. Curr. Hematol. Malig. Rep. 2019;14:219–227. doi: 10.1007/s11899-019-00530-y. [DOI] [PubMed] [Google Scholar]
- Panwar B., Arora A., Raghava G.P.S. Prediction and classification of ncRNAs using structural information. BMC. Genomics. 2014;15:127. doi: 10.1186/1471-2164-15-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulini M., Camal Ruggieri I.N., Ramallo M., Alonso M., Rodriguez-Cabello J.C., Esbrit P., Mardegan Issa J.P., Feldman S. Recombinant proteins-based strategies in bone tissue engineering. Biomolecules. 2021;12:3. doi: 10.3390/biom12010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedroso de Lima M.C., Neves S., Filipe A., Düzgüneş N., Simões S. Cationic liposomes for gene delivery: from biophysics to biological applications. Curr. Med. Chem. 2003;10:1221–1231. doi: 10.2174/0929867033457430. [DOI] [PubMed] [Google Scholar]
- Picanço-Castro V., Pereira C.G., Covas D.T., Porto G.S., Athanassiadou A., Figueiredo M.L. Emerging patent landscape for non-viral vectors used for gene therapy. Nat. Biotechnol. 2020;38:151–157. doi: 10.1038/s41587-019-0402-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinese C., Lin J., Milbreta U., Li M., Wang Y., Leong K.W., Chew S.Y. Sustained delivery of siRNA/mesoporous silica nanoparticle complexes from nanofiber scaffolds for long-term gene silencing. Acta Biomater. 2018;76:164–177. doi: 10.1016/j.actbio.2018.05.054. [DOI] [PubMed] [Google Scholar]
- Pu M., Chen J., Tao Z., Miao L., Qi X., Wang Y., Ren J. Regulatory network of miRNA on its target: coordination between transcriptional and post-transcriptional regulation of gene expression. Cell. Mol. Life. Sci. 2019;76:441–451. doi: 10.1007/s00018-018-2940-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rim N.G., Lee J.H., Jeong S.I., Lee B.K., Kim C.H., Shin H. Modulation of osteogenic differentiation of human mesenchymal stem cells by poly[(L-lactide)-co-(epsilon-caprolactone)]/gelatin nanofibers. Macromol. Biosci. 2009;9:795–804. doi: 10.1002/mabi.200800358. [DOI] [PubMed] [Google Scholar]
- Kamegawa R., Naito M., Miyata K. Functionalization of silica nanoparticles for nucleic acid delivery. Nano Res. 2018;11:5219–5239. [Google Scholar]
- Ru J., Guo L., Ji Y., Niu Y. Hydrostatic pressure induces osteogenic differentiation of adipose-derived mesenchymal stem cells through increasing lncRNA-PAGBC. Aging (Albany NY) 2020;12:13477–13487. doi: 10.18632/aging.103448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer B.W., Wicki R., Engelkamp D., Mattei M.G., Heizmann C.W. Isolation of a YAC clone covering a cluster of nine S100 genes on human chromosome 1q21: rationale for a new nomenclature of the S100 calcium-binding protein family. Genomics. 1995;25:638–643. doi: 10.1016/0888-7543(95)80005-7. [DOI] [PubMed] [Google Scholar]
- Sezlev Bilecen D., Uludag H., Hasirci V. Development of PEI-RANK siRNA complex loaded PLGA nanocapsules for the treatment of osteoporosis. Tissue Eng. Part A. 2019;25:34–43. doi: 10.1089/ten.TEA.2017.0476. [DOI] [PubMed] [Google Scholar]
- Shadjou N., Hasanzadeh M. Graphene and its nanostructure derivatives for use in bone tissue engineering: recent advances. J. Biomed. Mater. Res. 2016;104:1250–1275. doi: 10.1002/jbm.a.35645. [DOI] [PubMed] [Google Scholar]
- Shadjou N., Hasanzadeh M., Khalilzadeh B. Graphene based scaffolds on bone tissue engineering. Bioengineered. 2018;9:38–47. doi: 10.1080/21655979.2017.1373539. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Sheikh Z., Najeeb S., Khurshid Z., Verma V., Rashid H., Glogauer M. Biodegradable materials for bone repair and tissue engineering applications. Materials. 2015;8:5744–5794. doi: 10.3390/ma8095273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm S., Tavares A.J., Dai Q., Ohta S., Audet J., Dvorak H.F., Chan W.C.W. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 2016;1:16014. [Google Scholar]
- Strutz F., Okada H., Lo C.W., Danoff T., Carone R.L., Tomaszewski J.E., Neilson E.G. Identification and characterization of a fibroblast marker: FSP1. J. Cell. Biol. 1995;130:393–405. doi: 10.1083/jcb.130.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulaiman S., Chowdhury S.R., Fauzi M.B., Rani R.A., Yahaya N.H.M., Tabata Y., Hiraoka Y., Binti Haji Idrus R., Min Hwei N. 3D culture of MSCs on a gelatin microsphere in a dynamic culture system enhances chondrogenesis. Int. J. Mol. Sci. 2020;21:E2688. doi: 10.3390/ijms21082688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H., Li D., Chen S., Liu Y., Liao X., Deng W., Li N., Zeng M., Tao D., Ma Y. Zili inhibits transforming growth factor-beta signaling by interacting with Smad4. J. Biol. Chem. 2010;285:4243–4250. doi: 10.1074/jbc.M109.079533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Wang X., Chen G., Song C., Ma X., Fu Y., Feng C., Yan J. miRNA-187-5p regulates osteoblastic differentiation of bone marrow mesenchymal stem cells in mice by targeting ICAM1. BioMed Res. Int. 2020;2020 doi: 10.1155/2020/6139469. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Takayama K., Suzuki A., Manaka T., Taguchi S., Hashimoto Y., Imai Y., Wakitani S., Takaoka K. RNA interference for noggin enhances the biological activity of bone morphogenetic proteins in vivo and in vitro. J. Bone. Miner. Metab. 2009;27:402–411. doi: 10.1007/s00774-009-0054-x. [DOI] [PubMed] [Google Scholar]
- Tang X., Lin J., Wang G., Lu J. MicroRNA-433-3p promotes osteoblast differentiation through targeting DKK1 expression. PLoS One. 2017;12 doi: 10.1371/journal.pone.0179860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenchov R., Bird R., Curtze A.E., Zhou Q. Lipid nanoparticles-from liposomes to mRNA vaccine delivery, a landscape of research diversity and advancement. ACS Nano. 2021;15:16982–17015. doi: 10.1021/acsnano.1c04996. [DOI] [PubMed] [Google Scholar]
- Tharmalingam T., Sunley K., Spearman M., Butler M. Enhanced production of human recombinant proteins from CHO cells grown to high densities in macroporous microcarriers. Mol. Biotechnol. 2011;49:263–276. doi: 10.1007/s12033-011-9401-y. [DOI] [PubMed] [Google Scholar]
- Thielen N.G.M., van der Kraan P.M., van Caam A.P.M. TGFβ/BMP signaling pathway in cartilage homeostasis. Cells. 2019;8 doi: 10.3390/cells8090969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walmsley G.G., Ransom R.C., Zielins E.R., Leavitt T., Flacco J.S., Hu M.S., Lee A.S., Longaker M.T., Wan D.C. Stem cells in bone regeneration. Stem Cell Rev. Rep. 2016;12:524–529. doi: 10.1007/s12015-016-9665-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A., Liu J., Zhuang X., Yu S., Zhu S., Liu Y., Chen X. Identification and Comparison of piRNA expression profiles of exosomes derived from human stem cells from the apical papilla and bone marrow mesenchymal stem cells. Stem Cell. Dev. 2020;29:511–520. doi: 10.1089/scd.2019.0277. [DOI] [PubMed] [Google Scholar]
- Wang H., Wei P., Zhang Y., Li Y., Yin L. LncRNA TCONS_00023297 regulates the balance of osteogenic and adipogenic differentiation in bone marrow mesenchymal stem cells and the coupling process of osteogenesis and angiogenesis. Front. Cell Dev. Biol. 2021;9 doi: 10.3389/fcell.2021.697858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Liu S., Shi J., Liu H., Li J., Zhao S., Yi Z. The role of lncRNAs in osteogenic differentiation of bone marrow mesenchymal stem cells. Curr. Stem Cell Res. Ther. 2020;15:243–249. doi: 10.2174/1574888X15666191227113742. [DOI] [PubMed] [Google Scholar]
- Wang J.G., Lu Z., Wientjes M.G., Au J.L. Delivery of siRNA therapeutics: barriers and carriers. Interact. Cardiovasc. Thorac. Surg. 2010;11:492–503. doi: 10.1208/s12248-010-9210-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L.L., Burdick J.A. Engineered hydrogels for local and sustained delivery of RNA-interference therapies. Adv. Healthc. Mater. 2017;6:1601041. doi: 10.1002/adhm.201601041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P., Perche F., Logeart-Avramoglou D., Pichon C. RNA-based therapy for osteogenesis. Int. J. Pharm. 2019;569 doi: 10.1016/j.ijpharm.2019.118594. [DOI] [PubMed] [Google Scholar]
- Wang T., Zhang X., Bikle D.D. Osteogenic differentiation of periosteal cells during fracture healing. J. Cell. Physiol. 2017;232:913–921. doi: 10.1002/jcp.25641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Thomsen P. Mesenchymal stem cell-derived small extracellular vesicles and bone regeneration. Basic Clin. Pharmacol. Toxicol. 2021;128:18–36. doi: 10.1111/bcpt.13478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Malcolm D.W., Benoit D.S.W. Controlled and sustained delivery of siRNA/NPs from hydrogels expedites bone fracture healing. Biomaterials. 2017;139:127–138. doi: 10.1016/j.biomaterials.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei F., Yang S., Guo Q., Zhang X., Ren D., Lv T., Xu X. MicroRNA-21 regulates osteogenic differentiation of periodontal ligament stem cells by targeting Smad5. Sci. Rep. 2017;7 doi: 10.1038/s41598-017-16720-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei S., Ma J.X., Xu L., Gu X.S., Ma X.L. Biodegradable materials for bone defect repair. Mil. Med. Res. 2020;7:54. doi: 10.1186/s40779-020-00280-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkle M., El-Daly S.M., Fabbri M., Calin G.A. Noncoding RNA therapeutics - challenges and potential solutions. Nat. Rev. Drug. Discov. 2021;20:629–651. doi: 10.1038/s41573-021-00219-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won Lee G., Thangavelu M., Joung Choi M., Yeong Shin E., Sol Kim H., Seon Baek J., Woon Jeong Y., Eun Song J., Carlomagno C., Miguel Oliveira J., et al. Exosome mediated transfer of miRNA-140 promotes enhanced chondrogenic differentiation of bone marrow stem cells for enhanced cartilage repair and regeneration. J. Cell. Biochem. 2020;121:3642–3652. doi: 10.1002/jcb.29657. [DOI] [PubMed] [Google Scholar]
- Wu G., Feng C., Hui G., Wang Z., Tan J., Luo L., Xue P., Wang Q., Chen X. Improving the osteogenesis of rat mesenchymal stem cells by chitosan-based-microRNA nanoparticles. Carbohydr. Polym. 2016;138:49–58. doi: 10.1016/j.carbpol.2015.11.044. [DOI] [PubMed] [Google Scholar]
- Wu G., Feng C., Quan J., Wang Z., Wei W., Zang S., Kang S., Hui G., Chen X., Wang Q. In situ controlled release of stromal cell-derived factor-1α and antimiR-138 for on-demand cranial bone regeneration. Carbohydr. Polym. 2018;182:215–224. doi: 10.1016/j.carbpol.2017.10.090. [DOI] [PubMed] [Google Scholar]
- Wu Q., Ma Q., Shehadeh L.A., Wilson A., Xia L., Yu H., Webster K.A. Expression of the Argonaute protein PiwiL2 and piRNAs in adult mouse mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2010;396:915–920. doi: 10.1016/j.bbrc.2010.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W., Lu B.F., Jiang R.Q., Chen S. The function and regulation mechanism of piRNAs in human cancers. Histol. Histopathol. 2021;36:807–816. doi: 10.14670/HH-18-323. [DOI] [PubMed] [Google Scholar]
- Xia W., Xie J., Cai Z., Liu X., Wen J., Cui Z.K., Zhao R., Zhou X., Chen J., Mao X., et al. Damaged brain accelerates bone healing by releasing small extracellular vesicles that target osteoprogenitors. Nat. Commun. 2021;12:6043. doi: 10.1038/s41467-021-26302-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin Y., Huang M., Guo W.W., Huang Q., Zhang L.Z., Jiang G. Nano-based delivery of RNAi in cancer therapy. Mol. Cancer. 2017;16:134. doi: 10.1186/s12943-017-0683-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J.Z., Zhang J.L., Zhang W.G. Antisense RNA: the new favorite in genetic research. J. Zhejiang Univ. - Sci. B. 2018;19:739–749. doi: 10.1631/jzus.B1700594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Wu J., Liu S., Saw P.E., Tao W., Li Y., Krygsman L., Yegnasubramanian S., De Marzo A.M., Shi J., et al. Redox-responsive nanoparticle-mediated systemic RNAi for effective cancer therapy. Small. 2018;14 doi: 10.1002/smll.201802565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashiro H., Siomi M.C. PIWI-interacting RNA in Drosophila: biogenesis, transposon regulation, and beyond. Chem. Rev. 2018;118:4404–4421. doi: 10.1021/acs.chemrev.7b00393. [DOI] [PubMed] [Google Scholar]
- Yan J., Lu X., Zhu X., Hu X., Wang L., Qian J., Zhang F., Liu M. Effects of miR-26a on osteogenic differentiation of bone marrow mesenchymal stem cells by a mesoporous silica nanoparticle - PEI - peptide system. Int. J. Nanomed. 2020;15:497–511. doi: 10.2147/IJN.S228797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan X., Huang X., Yu C., Deng H., Wang Y., Zhang Z., Qiao S., Lu G., Zhao D. The in-vitro bioactivity of mesoporous bioactive glasses. Biomaterials. 2006;27:3396–3403. doi: 10.1016/j.biomaterials.2006.01.043. [DOI] [PubMed] [Google Scholar]
- Yan X., Yu C., Zhou X., Tang J., Zhao D. Highly ordered mesoporous bioactive glasses with superior in vitro bone-forming bioactivities. Angew. Chem. Int. Ed. Engl. 2004;43:5980–5984. doi: 10.1002/anie.200460598. [DOI] [PubMed] [Google Scholar]
- Yang Q., Li R., Lyu Q., Hou L., Liu Z., Sun Q., Liu M., Shi H., Xu B., Yin M., et al. Single-cell CAS-seq reveals a class of short PIWI-interacting RNAs in human oocytes. Nat. Commun. 2019;10:3389. doi: 10.1038/s41467-019-11312-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Yujiao W., Fang W., Linhui Y., Ziqi G., Zhichen W., Zirui W., Shengwang W. The roles of miRNA, lncRNA and circRNA in the development of osteoporosis. Biol. Res. 2020;53:40. doi: 10.1186/s40659-020-00309-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youn H., Chung J.K. Modified mRNA as an alternative to plasmid DNA (pDNA) for transcript replacement and vaccination therapy. Expert. Opin. Biol. Ther. 2015;15:1337–1348. doi: 10.1517/14712598.2015.1057563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D., Wang J., Qian K.J., Yu J., Zhu H.Y. Effects of nanofibers on mesenchymal stem cells: environmental factors affecting cell adhesion and osteogenic differentiation and their mechanisms. J. Zhejiang Univ. - Sci. B. 2020;21:871–884. doi: 10.1631/jzus.B2000355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu T., Wang H., Zhang Y., Wang X., Han B. The delivery of RNA-interference therapies based on engineered hydrogels for bone tissue regeneration. Front. Bioeng. Biotechnol. 2020;8:445. doi: 10.3389/fbioe.2020.00445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G., Guo B., Wu H., Tang T., Zhang B.T., Zheng L., He Y., Yang Z., Pan X., Chow H., et al. A delivery system targeting bone formation surfaces to facilitate RNAi-based anabolic therapy. Nat. Med. 2012;18:307–314. doi: 10.1038/nm.2617. [DOI] [PubMed] [Google Scholar]
- Zhang P., Wu W., Chen Q., Chen M. Non-coding RNAs and their integrated networks. J. Integr. Bioinform. 2019;16 doi: 10.1515/jib-2019-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Wu Y., Shiozaki Y., Sugimoto Y., Takigawa T., Tanaka M., Matsukawa A., Ozaki T. miRNA-133a-5p inhibits the expression of osteoblast differentiation-associated markers by targeting the 3' UTR of RUNX2. DNA. Cell. Biol. 2018;37:199–209. doi: 10.1089/dna.2017.3936. [DOI] [PubMed] [Google Scholar]
- Zhang X., Li Y., Chen Y.E., Chen J., Ma P.X. Cell-free 3D scaffold with two-stage delivery of miRNA-26a to regenerate critical-sized bone defects. Nat. Commun. 2016;7 doi: 10.1038/ncomms10376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Zhu Y., Zhang C., Liu J., Sun T., Li D., Na Q., Xian C.J., Wang L., Teng Z. miR-542-3p prevents ovariectomy-induced osteoporosis in rats via targeting SFRP1. J. Cell. Physiol. 2018;233:6798–6806. doi: 10.1002/jcp.26430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Gao Y., Cai L., Li F., Lou Y., Xu N., Kang Y., Yang H. MicroRNA-221 is involved in the regulation of osteoporosis through regulates RUNX2 protein expression and osteoblast differentiation. Am. J. Transl. Res. 2017;9:126–135. [PMC free article] [PubMed] [Google Scholar]
- Zhou Z.C., Che L., Kong L., Lei D.L., Liu R., Yang X.J. CKIP-1 silencing promotes new bone formation in rat mandibular distraction osteogenesis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2017;123:e1–e9. doi: 10.1016/j.oooo.2016.07.013. [DOI] [PubMed] [Google Scholar]
- Zhu C.Y., Yao C., Zhu L.Q., She C., Zhou X.Z. Dexamethasone-induced cytotoxicity in human osteoblasts is associated with circular RNA HIPK3 downregulation. Biochem. Biophys. Res. Commun. 2019;516:645–652. doi: 10.1016/j.bbrc.2019.06.073. [DOI] [PubMed] [Google Scholar]