Abstract
Posttraumatic epilepsy (PTE) is a well‐known chronic complication following traumatic brain injury (TBI). Despite some evidence that age at the time of injury may influence the likelihood of PTE, the incidence of PTE in pediatric populations remains unclear. We therefore conducted a systematic review to determine the overall reported incidence of PTE, and explore potential risk factors associated with PTE after pediatric TBI. A comprehensive literature search of the PubMed, Embase, and Web of Science databases was conducted, including randomized controlled trials and cohort studies assessing the incidence of PTE in TBI pediatric patients. We excluded studies with a sample size of <10 patients and those in which a pediatric cohort was not clearly discernable. The review was conducted in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses) guidelines. We found that the overall incidence of PTE following pediatric TBI was 10% (95% confidence interval [CI] = 5.9%–15%). Subgroup analysis of a small number of studies demonstrated that the occurrence of early seizures (cumulative incidence ratio [CIR] = 7.28, 95% CI = 1.09–48.4, p = .040), severe TBI (CIR = 1.81, 95% CI = 1.23–2.67, p < .001), and intracranial hemorrhage (CIR = 1.60, 95% CI = 1.06–2.40, p = .024) increased the risk of PTE in this population. Other factors, including male sex and neurosurgical intervention, were nonsignificantly associated with a higher incidence of PTE. In conclusion, PTE is a significant chronic complication following childhood TBI, similar to in the adult population. Further standardized investigation into clinical risk factors and management guidelines is warranted. PROSPERO ID# CRD42021245802.
Keywords: childhood, epilepsy, incidence, neurotrauma, pediatric, posttraumatic epilepsy, seizure, traumatic brain injury
Key Points.
The incidence of PTE following pediatric TBI is estimated to be approximately 10%.
Early seizures, intracranial hemorrhage, and severe injury are statistically significant predictors of PTE.
Standardization of future reporting will allow for improved comparability between studies.
1. INTRODUCTION
Traumatic brain injury (TBI) affects 69 million individuals each year 1 and is associated with a number of chronic physical and cognitive complications, one of which is epilepsy. 2 , 3 Posttraumatic epilepsy (PTE) is a well‐recognized long‐term consequence of moderate and severe TBI in particular, and is defined as the occurrence of recurrent unprovoked seizures observed ≥7 days following the initial trauma. 4 , 5 The development of PTE is an added burden for affected individuals, being associated with increased neurological impairment, decreased cognitive function, and higher mortality. 6 , 7
Although epilepsy after TBI in adult populations has been increasingly studied over the past decade, clinical understanding of the chronic consequences of TBI in pediatric cohorts remains understudied. The incidence of pediatric TBI hospitalization is reported to be 70 cases per 100 000 children, 8 and the risk of PTE, as well as prognostic implications of PTE in this population, has been poorly defined to date. Known and hypothesized differences in TBI mechanisms, biomechanics, and pathophysiology in pediatric TBI compared to adult TBI require that we consider the age at which injury is sustained as a key determinant of patient outcome. For example, abusive nonaccidental acceleration–deceleration injury is a mechanism unique to pediatric populations,9 whereas developing neck muscles and a high head‐to‐body ratio results in a child's head being more vulnerable to sustaining a TBI. 10 The developmental stage of the brain at the time of injury also appears to play a role in the neuropathology of epileptogenesis, 11 including processes such as oxidative stress, neuroinflammation, and excitotoxicity. 11 , 12 , 13 Additionally, it has also been suggested that children have a higher risk of TBI following head injury. 11 , 14 , 15 As such, in the study of PTE as a clinically important outcome after TBI, it is vital to consider pediatric and adult cohorts as separate entities to aid in our understanding and guide appropriate clinical management.
A previous systematic review reported the incidence of PTE after TBI in adulthood to be approximately 15%, and a number of risk factors, including male sex, intracranial hemorrhage, and skull fracture, were identified. 16 However, in the current published literature, a consensus estimate of the incidence of PTE following TBI in pediatric patients is lacking, and the factors associated with PTE in children remain unclear. To address this knowledge gap, we conducted a systematic review to evaluate the incidence of PTE following childhood TBI. The primary aim of this study was to investigate the incidence of epilepsy diagnosis following pediatric TBI. Second, where the data were available, we aimed to identify risk factors for the development of PTE in pediatric populations.
2. MATERIALS AND METHODS
2.1. Protocol, registration, and ethics
The study was conducted as previously outlined in our registered and published protocol (PROSPERO ID# CRD42021245802) 17 and in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses) guidelines (Table S1). 18 Considering the nature of the study (derived from previously published work), ethics approval and patient consent were not required.
2.2. Search strategy
A comprehensive search of three electronic databases (PubMed, Ovid Embase, and Web of Science) was conducted in May 2021. Keywords included variations of the following: epilepsy (“epilepsy”, “seizure”, “status epilepticus”), traumatic brain injury (“traumatic brain injury” or “post‐traumatic” or “traumatic” or “TBI” or “brain injury”), and pediatric (“pediatric” or “pediatric” or “newborn” or “infan*” or “child*”). Appropriate Boolean operators were used to combine search terms. The reference lists of all included studies were also reviewed to identify any additional articles, and duplicate articles were removed.
2.3. Eligibility criteria
Our inclusion criteria allowed for randomized controlled trials and cohort studies that (1) reported TBI in patients younger than 18 years, (2) involved patients who developed PTE, and (3) included a sample size of 10 or more patients. Review publications, gray literature, conference abstracts, non‐English language publications, and animal studies were excluded. Publications in which it was not possible to ascertain a specified study population of pediatric cases were excluded. Patients with an underlying epileptogenic cause unrelated to the index TBI were also excluded.
Title and abstract screening was conducted by two independent investigators (F.P.M. and S.S.R.). Likewise, full‐text screening was performed by two independent investigators (F.P.M. and P.S.). All conflicts were resolved by a third, senior investigator (B.D.S., T.J.O., or A.A.‐B.). The systematic review platform Covidence (www.covidence.org; Veritas Health Innovation) was used to facility the screening process. Publications found to fulfill eligibility criteria underwent data extraction.
2.4. Data extraction
Extracted variables included the number of TBI patients, number of PTE patients at follow‐up, follow‐up duration, patient demographics (age, sex, country), severity of injury, mechanism of injury (fall, motor vehicle accident, nonaccidental injury), occurrence of early seizures, skull fracture, intracranial hemorrhage, contusion, requirement of neurosurgical intervention, and use of antiseizure medications. All data extraction was performed by two independent investigators (F.P.M. and P.S.).
2.5. Evaluation of risk of bias
Critical appraisal of the risk of bias for individual studies was conducted using the Joanna Briggs Institute Critical Appraisal Checklist for Cohort Studies. 19 Each included study was scored by two independent investigators (F.P.M. and P.S.). Any discrepancies between the two reviewers were resolved by discussion and mutual agreement. Publications that scored <5 points out of 11 were considered to be of poor quality, and were excluded from further analysis.
2.6. Statistical analysis
A random‐effects meta‐analysis with DerSimonian and Laird method was used to calculate the pooled cumulative incidence of PTE after pediatric TBI. Heterogeneity was measured using the I‐squared statistic (I 2), which describes the percentage of the variability in effect estimates that is due to heterogeneity rather than chance. Meta regressions were used to explore whether publication year or country of origin was the source of heterogeneity. R‐squared (R 2) was calculated to quantify the heterogeneity that can be explained by the two variables. A funnel plot visual analysis and Egger test were applied to evaluate small study effect and publication bias for pooled PTE incidence in pediatric TBI. Freeman–Tukey double arcsine transformation was used on pooled PTE incidence in the funnel plot.
Where four or more studies were available, we performed subgroup meta‐analysis to further investigate the effects of TBI subgroups (± a priori hypothesized potential risk factors) on the incidence of PTE, and calculated estimated cumulative incidence ratios (CIRs). Statistical significance level was set at p < .05. All statistical analyses were performed using Stata version 16 (StataCorp), with the user‐written package "metaprop" for meta‐analysis of proportions. 20
3. RESULTS
3.1. Descriptive summary
Our electronic database search strategy of relevant references yielded a total of 2063 references (Figure 1). After removal of 532 duplicates, the remaining 1531 references were screened by title and abstract. A total of 114 publications were deemed to be eligible for full‐text screening, of which 95 studies were excluded with reasons. A total of 19 articles were included in our final analysis, with a median sample size of 109 (range = 41–1576) and a pooled sample size of 4374 patients who sustained a pediatric TBI. 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 Ten publications were retrospective cohort studies, seven were prospective cohort studies, and two were randomized controlled trials. In 11 publications (58%), PTE was the primary outcome variable of interest, whereas PTE was investigated as a secondary outcome in the remaining eight publications. Seventeen studies exclusively analyzed pediatric cohorts, and the other two studies described mixed adult/pediatric cohorts from which the relevant study population could be extracted. Three publications were limited to severe TBI, one only investigated mild TBI, and one publication included moderate and severe TBI. The remaining 14 articles included all TBI patients regardless of severity. All publications defined pediatric patients as <18 years of age. Geographically, two publications were from the Middle East, four were from Asia, six were from North America, and seven were from Europe. A summary of the baseline characteristics of included papers and clinical variables is provided in Table 1.
FIGURE 1.
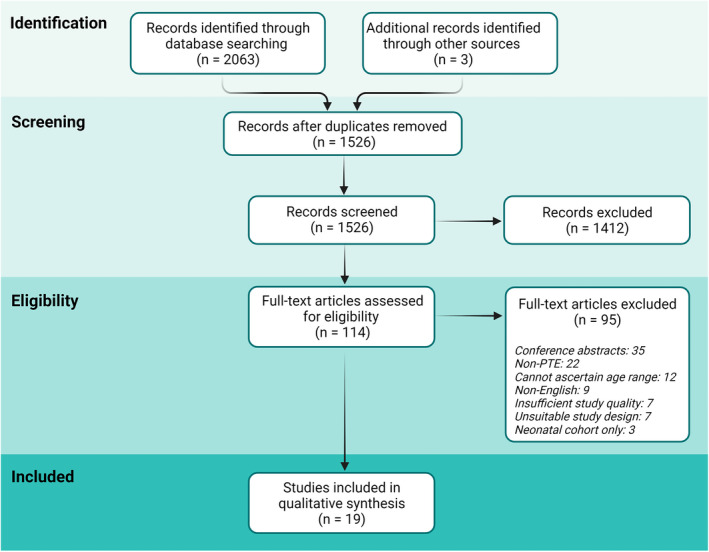
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses) flowchart of article screening. PTE, posttraumatic epilepsy.
TABLE 1.
Population characteristics
| Author and date | TBI patients, n | PTE patients, n | Female, n | Severe TBI, n | Early seizures, n | Skull fracture, n | ICH, n | Contusion, n | Required neurosurgery, n |
|---|---|---|---|---|---|---|---|---|---|
| Appleton 2002 | 102 | 9 | NR | NR | 8 | NR | NR | NR | NR |
| Arango 2012 | 130 | 22 | 44 | 130 | NR | 77 | NR | NR | NR |
| Asikainen 1999 | 241 | 77 | NR | NR | NR | NR | NR | NR | NR |
| Barlow 2000 | 42 | 8 | NR | NR | NR | NR | NR | NR | NR |
| DeSantis 1979 | 52 | 8 | NR | NR | 2 | NR | 3 | NR | NR |
| Emanuelson 2009 | 109 | 12 | 41 | 51 | 8 | NR | NR | NR | 17 |
| Hwang 2019 | 68 | 7 | 26 | NR | NR | 30 | NR | 31 | 25 |
| Kan 2006 | 51 | 10 | 18 | 51 | NR | NR | NR | NR | 51 |
| Keret 2017 | 191 | 6 | 69 | NR | 6 | 139 | 58 | 29 | 9 |
| Keret 2018 | 95 | 9 | 42 | 31 | 11 | 47 | 49 | 28 | 33 |
| Matsumoto 2013 | 294 | 32 | NR | 27 | NR | NR | NR | NR | NR |
| Mikkonen 2020 | 290 | 59 | 87 | 105 | NR | NR | 84 | NR | 57 |
| Park 2015 | 321 | 47 | NR | NR | NR | NR | NR | NR | NR |
| Pearl 2013 | 40 | 1 | NR | NR | NR | NR | NR | NR | NR |
| Petridis 2012 | 238 | 15 | 106 | 15 | 26 | 25 | NR | 50 | 29 |
| Ratan 1999 | 400 | 4 | 166 | NR | 84 | NR | NR | NR | NR |
| Shin 2019 | 1576 | 30 | 621 | 8 | NR | NR | NR | NR | NR |
| Thapa 2010 | 93 | 3 | NR | NR | NR | NR | NR | NR | NR |
| Young 1983 | 41 | 4 | NR | NR | NR | NR | NR | NR | NR |
Abbreviations: ICH, intracranial hemorrhage; NR, not reported; PTE, posttraumatic epilepsy; TBI, traumatic brain injury.
3.2. Incidence of PTE
Across the 19 included studies, the reported incidence of PTE in patients who had experienced pediatric PTE ranged from 1.9% 39 to 32%. 24 The pooled incidence of PTE, based on a random‐effects model, was 10% (95% confidence interval [CI] = 5.9%–15%; Figure 2). There was high heterogeneity among the 19 studies (I 2 = 95%). Results of the meta‐regression analysis demonstrated that country of origin could explain a substantial amount of heterogeneity (R 2 = 83%) but not year of publication (R 2 = 0%).
FIGURE 2.
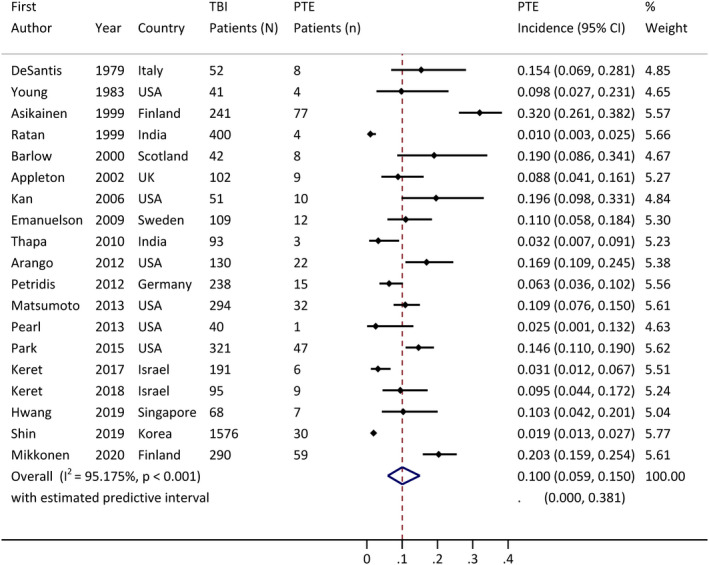
Pooled incidence of posttraumatic epilepsy (PTE) incidence, with 95% confidence interval (CI).
3.3. Subgroup analyses to explore risk factors
To explore potential risk factors of PTE in pediatric populations, subgroup analysis was performed where sufficient information was available in four or more publications. Due to limitations in reporting in individual publications, we were unable to account for potential confounding variables; thus, analysis was univariate in nature.
The correlation between early seizures and PTE was assessed in four studies (Figure 3). 28 , 31 , 32 , 37 Patients with early seizures have 7.28 times the incidence of developing PTE of those without early seizures (95% CI = 1.09–48.4, p = .040). There is substantial between‐study heterogeneity (I 2 = 73%). In addition, severe TBI (CIR = 1.81, 95% CI = 1.23–2.67, p < .001, I 2 = 0%; Figure 4) and intracranial hemorrhage (CIR = 1.60, 95% CI = 1.06–2.40, p = .024, I 2 = 0%; Figure 5) were also associated with elevated cumulative incidence of PTE, and the results of studies were relatively homogenous.
FIGURE 3.
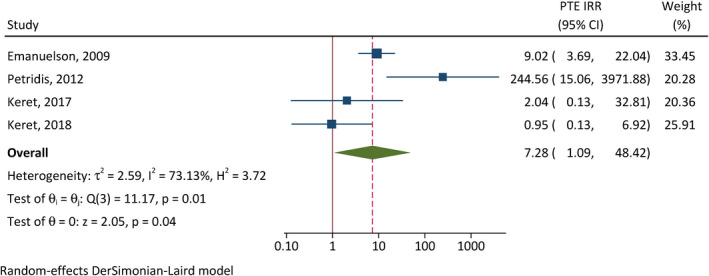
Correlation between early postinjury seizures and posttraumatic epilepsy (PTE). CI, confidence interval; IRR, incidence rate ratio.
FIGURE 4.
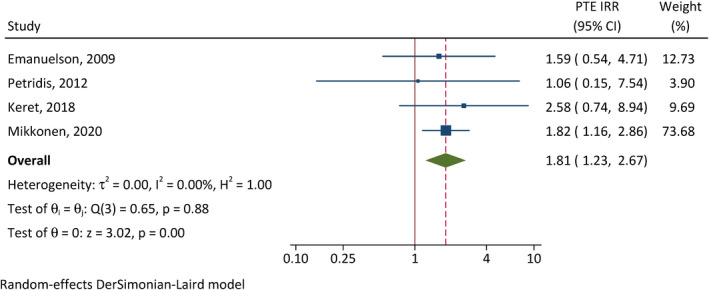
Correlation between traumatic brain injury severity and posttraumatic epilepsy (PTE). CI, confidence interval; IRR, incidence rate ratio.
FIGURE 5.
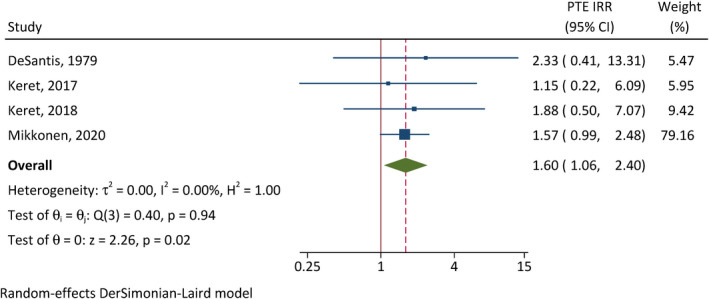
Correlation between intracranial hemorrhage and posttraumatic epilepsy (PTE). CI, confidence interval; IRR, incidence rate ratio.
On the other hand, the cumulative incidences of PTE were not significantly different between females and males (CIR = .89, 95% CI = .46–1.71, p = .72, I 2 = 33%; Figure 6), and between patients requiring neurosurgical intervention and those not (CIR = 1.43, 95% CI = .91–2.26, p = .12, I 2 = 0; Figure 7).
FIGURE 6.
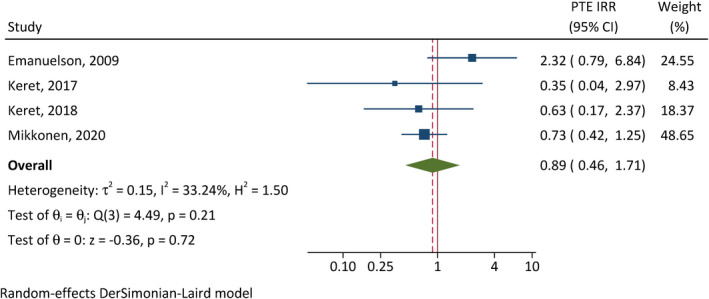
Correlation between female sex and posttraumatic epilepsy (PTE). CI, confidence interval; IRR, incidence rate ratio.
FIGURE 7.
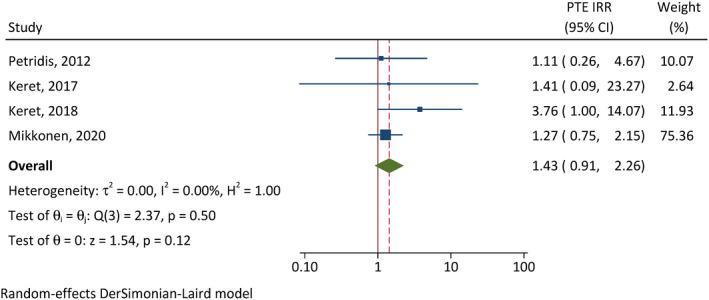
Correlation between neurosurgical intervention and posttraumatic epilepsy (PTE). CI, confidence interval; IRR, incidence rate ratio.
Unfortunately, due to a lack of individual patient data, and between‐study variation in the reporting of age ranges, it was not possible to perform subgroup analysis on the impact of age on PTE incidence.
3.4. Study quality
Risk of bias was evaluated using the Joanna Briggs Institute Critical Appraisal Checklist for Cohort Studies (Table 2). 19 A total of 26 publications were eligible for quality assessment, of which seven publications were of low quality (score < 5/11) and excluded, and 19 publications were of sufficient quality to be included for further analysis (detailed above). The median score was 8 points out of a total of 11 (range = 5–11). The funnel plot examination did not demonstrate any apparent asymmetry (Figure 8), and Egger test (bias = 1.43, 95% CI = −2.13–4.99, p = .43) did not suggest presence of small study effect or publication bias. Of the included studies, the most common limitation in reporting was related to confounding variables and strategies of addressing incomplete follow‐up. Only 11 publications (57.9%) identified and addressed confounding factors such as sex and other demographics in their analysis, and only seven publications (37%) specified strategies to address incomplete follow‐up.
TABLE 2.
Risk of bias evaluation
| Author and date | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | Q11 | Score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Appleton 2002 | Y | Y | Y | Y | U | Y | Y | Y | U | U | Y | 8 |
| Arango 2012 | Y | Y | Y | Y | Y | Y | Y | Y | U | U | Y | 8 |
| Asikainen 1999 | Y | Y | U | Y | Y | Y | Y | Y | Y | Y | Y | 9 |
| Barlow 2000 | Y | Y | Y | Y | Y | U | Y | Y | Y | Y | Y | 10 |
| DeSantis 1979 | Y | Y | Y | U | U | U | Y | Y | U | U | Y | 5 |
| Emanuelson 2009 | Y | Y | Y | Y | Y | U | Y | Y | Y | Y | Y | 10 |
| Hwang 2019 | Y | Y | Y | Y | Y | Y | Y | N | U | U | Y | 8 |
| Kan 2006 | Y | Y | Y | Y | Y | U | U | N | Y | Y | Y | 8 |
| Keret 2017 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 11 |
| Keret 2018 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 11 |
| Matsumoto 2013 | Y | Y | Y | U | U | Y | Y | U | U | U | Y | 6 |
| Mikkonen 2020 | Y | Y | Y | Y | Y | Y | U | Y | Y | U | Y | 9 |
| Park 2015 | Y | Y | Y | Y | U | Y | Y | U | U | U | U | 6 |
| Pearl 2013 | Y | Y | Y | Y | U | Y | Y | Y | Y | U | U | 8 |
| Petridis 2012 | Y | Y | Y | U | U | Y | Y | U | U | U | Y | 6 |
| Ratan 1999 | Y | Y | Y | U | U | Y | Y | U | U | U | Y | 6 |
| Shin 2019 | Y | Y | U | Y | U | Y | Y | U | U | U | Y | 6 |
| Thapa 2010 | Y | Y | Y | Y | Y | U | Y | N | U | U | Y | 7 |
| Young 1983 | Y | Y | Y | Y | Y | U | Y | N | Y | Y | Y | 9 |
Note: The Joanna Briggs Institute Critical Appraisal Checklist for Cohort Studies, 19 as used, incorporated the following series of questions. Q1: Were subjects recruited from the same population? Q2: Was TBI measured comparatively? Q3: Was TBI measured in a valid and reliable way? Q4: Were confounding variables identified? Q5: Were strategies to deal with confounding factors stated? Q6: Were subjects free from epilepsy at the start of the study / at exposure to TBI? Q7: Was epilepsy measured in a valid and reliable way? Q8: Was the follow‐up time reported and sufficient to be long enough for epilepsy to occur? Q9: Was follow‐up complete, and if not, were the reasons described/explored? Q10: Were strategies to address incomplete follow‐up utilized? Q11: Was appropriate statistical analysis used?
Abbreviations: N, no; TBI, traumatic brain injury; U, unknown; Y, yes.
FIGURE 8.
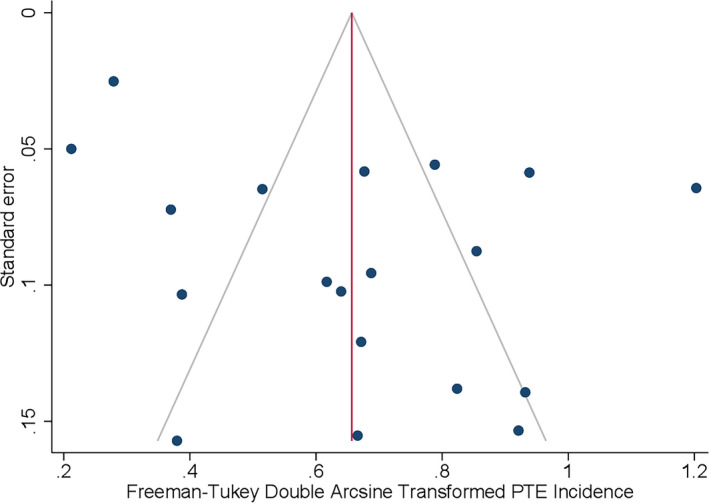
Funnel plot analysis. The apparent asymmetry suggests a lack of publication bias. PTE, posttraumatic epilepsy.
4. DISCUSSION
Our systematic review of the current literature found that the pooled incidence of PTE reported following childhood TBI is 10% (95% CI = 5.9%–15%), with quite a high level of heterogeneity in the 19 included publications (I 2 = 95%). Subgroup analyses revealed that early seizures (i.e., seizures within 7 days of sustaining a TBI), intracranial hemorrhage, and severe injury increase the risk of PTE. Other clinical variables, including male sex and requirement of neurosurgical intervention, tended to also be associated with a greater incidence of PTE, but did not reach statistical significance, likely due to the low sample sizes.
Establishing the incidence of PTE following TBI in childhood is vital in understanding and highlighting the magnitude of TBI and its long‐term complications. Sustaining a TBI during early life, particularly when the injury is severe, results in a substantial life‐long burden to the individual, their families/carers, society, and the economy, not just in terms of health care resources but also in loss of earning potential across a lifespan. 42 PTE increases this burden, as the development of epilepsy post‐TBI is associated with increased morbidity and mortality. 6 , 7 In adults, the risk factors and prevalence of PTE have been well described. 16 In pediatric cohorts, however, the clinical predictors of PTE are less clear, and a widely variable incidence has been reported by only a few studies. As secondary pathophysiological processes after TBI are increasingly understood to be dependent on the development age at the time of injury, 11 , 13 we now have a greater appreciation that findings from adult populations may not be directly applicable to a pediatric population.
This systematic review demonstrates that PTE is a significant outcome after TBI in childhood, with one in 10 children going on to develop epilepsy following TBI. A recent systematic review including adults found that PTE had a prevalence of 15%. 16 There may be several reasons for the increased incidence reported when compared to our review of pediatric cohorts alone. First, a very wide range has been reported for PTE incidence in adult studies, from 1% to 53%. Additionally, adult studies often include PTE in military veterans, which may add a layer of complexity due to different injury mechanisms and comorbidities in this population. In our review, the vast majority of studies included mild TBI cases, which may have impacted the overall incidence. For instance, Shin et al. 39 reported an incidence of 3.4% in 1576 patients, with 99% of patients only experiencing mild TBI. Finally, our study investigated all patients younger than 18 years, which encompasses a wide range of distinct neurodevelopmental periods. It has been suggested that developmental windows during infancy and early childhood may be particularly susceptible to seizures and epilepsy. 43 , 44 Due to limitations in reporting by the included studies, we were unable to stratify according to age, which may have provided a more accurate representation of epilepsy vulnerability in children of different developmental stages. We recommend that future studies pay close attention to age at the time of injury as a potential variable that may influence the incidence of PTE.
In this study, we determined that early seizures increased the risk of PTE, and analysis also demonstrated that severe TBI was strongly associated with PTE. Ultimately, inconsistencies in the definitions and classification of TBI severity prevented the inclusion of more studies in this subgroup analysis. Future studies would be well served by a standardization of definitions (e.g., Glasgow Coma Scale) and reporting to ensure comparability.
Our findings add to the growing body of evidence emphasizing the long‐term ramifications of childhood TBI and highlighting the vital need for public education regarding TBI in children. In particular, the incidence of abusive head trauma may be increasing, according to recent reports, 45 , 46 and the implementation of preventive measures may improve awareness and increase the identification of red flags and warning signs. 47 , 48 Additionally, motor vehicle accidents are a significant source of head injury in children, and parental education concerning motor vehicle safety in children may play a key role in reducing the risk of head injury secondary to road accidents. 49
This study has a number of strengths. It is relatively representative of the worldwide incidence of PTE following pediatric TBI, with studies sourced from more than 12 countries across Asia, Europe, North America, and the Middle East. Our systematic review also incorporated a relatively large total sample size of almost 5000 patients from 19 studies. Our eligibility criteria were stringently enforced, ensuring that publications were of sound quality, with a particular emphasis on including pediatric patients only, and excluding cohorts where age was unclear.
However, we also note several limitations to the analysis. There was significant heterogeneity in the clinical variables reported by different publications, with very limited reporting of relevant variables, including clinical, radiological, and treatment characteristics. Consequently, despite a relatively large sample size, very few studies reported sufficient detail to be included for further subgroup analyses. As such, we were unable to thoroughly investigate predisposing factors and clinical variables that may increase the risk of PTE. Furthermore, many survivors of TBI report new onset neuropsychiatric conditions and neurocognitive deficits—comorbidities that are also common in individuals with epilepsy. 50 Nonetheless, the potential for such comorbidities to influence PTE incidence, in either adult or pediatric populations, remains poorly explored. Another limitation to note was that our search strategy and article selection excluded non‐English publications, which may have provided more data. Finally, most studies included in this review were retrospective in nature, which introduces several forms of bias into the literature, limiting the strength of conclusions drawn.
Regardless, our findings highlight that future investigation into childhood TBI and its ramifications is clearly warranted, and provides direction for improving future research in the field. Despite increasing awareness and interest in pediatric TBI and epilepsy, this review identifies a strong need for further and more rigorous examination. Large, prospective studies with a focus on identifying population subgroups at increased risk of developing PTE would provide significant insight. Additional variables such as patient demographics, clinical presentation, severity of injury, and radiological findings may also provide prognostic value. Additionally, investigation into injury mechanisms would have widespread implications, with specific consideration of nonaccidental injury and its long‐term impacts on children. Furthermore, study into the clinical management of TBI and its impact on developing PTE may propel investigation into treatments and strategies to reduce the risk of PTE. Finally, future studies should adopt more standardized reporting of results and utilization of consensus‐agreed clinical definitions to allow for better comparability between studies, allowing for a more uniform body of evidence from which robust conclusions can be drawn.
5. CONCLUSIONS
In the reported literature, the overall incidence of PTE following pediatric TBI is 10.0%. Seizures within 1 week of initial presentation, intracranial hemorrhage, and severe injury were significantly associated with an increased risk of PTE, and other factors such as male sex and neurosurgical intervention showed a greater risk that was not statistically significant. This review ultimately highlights the vital need for further investigation into childhood TBI and its complications, with a focus on standardized reporting of clinical findings.
AUTHOR CONTRIBUTIONS
Frederick P. Mariajoseph, Terence J. O'Brien, Ana Antonic‐Baker, and Bridgette D. Semple conceived and designed the project. Frederick P. Mariajoseph, Praba Sekhar, and Sarah S. Rewell conducted the systematic review with guidance from Bridgette D. Semple and Ana Antonic‐Baker. Zhibin Chen performed the statistical analyses, and Terence J. O'Brien provided critical input in planning the study protocol. Frederick P. Mariajoseph, Ana Antonic‐Baker, and Bridgette D. Semple drafted the manuscript. All authors read, edited, and approved the final manuscript prior to submission.
CONFLICT OF INTEREST
None of the authors has any conflict of interest to disclose.
Supporting information
Appendix S1
ACKNOWLEDGMENTS
Although this project received no specific funding, the authors are currently supported by funding from the National Health and Medical Research Council of Australia, the US Department of Defense, and Monash University Central Clinical School. Open access publishing facilitated by Monash University, as part of the Wiley ‐ Monash University agreement via the Council of Australian University Librarians. [Correction added on 30 November 2022, after first online publication: funding statement has been added.]
Mariajoseph FP, Chen Z, Sekhar P, Rewell SS, O’Brien TJ, Antonic‐Baker A, et al. Incidence and risk factors of posttraumatic epilepsy following pediatric traumatic brain injury: A systematic review and meta‐analysis. Epilepsia. 2022;63:2802–2812. 10.1111/epi.17398
Contributor Information
Ana Antonic‐Baker, Email: Ana.Antonic-Baker@monash.edu.
Bridgette D. Semple, Email: bridgette.semple@monash.edu.
REFERENCES
- 1. Dewan MC, Rattani A, Gupta S, Baticulon RE, Hung YC, Punchak M, et al. Estimating the global incidence of traumatic brain injury. J Neurosurg. 2018;130(4):1080–97. [DOI] [PubMed] [Google Scholar]
- 2. Ahmed S, Venigalla H, Mekala HM, Dar S, Hassan M, Ayub S. Traumatic brain injury and neuropsychiatric complications. Indian J Psychol Med. 2017;39(2):114–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bramlett HM, Dietrich WD. Long‐term consequences of traumatic brain injury: current status of potential mechanisms of injury and neurological outcomes. J Neurotrauma. 2015;32(23):1834–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fisher RS. An overview of the 2017 ILAE operational classification of seizure types. Epilepsy Behav. 2017;70:271–3. [DOI] [PubMed] [Google Scholar]
- 5. Fisher RS, van Emde Boas W, Blume W, Elger C, Genton P, Lee P, et al. Epileptic seizures and epilepsy: definitions proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE). Epilepsia. 2005;46(4):470–2. [DOI] [PubMed] [Google Scholar]
- 6. Englander J, Bushnik T, Duong TT, Cifu DX, Zafonte R, Wright J, et al. Analyzing risk factors for late posttraumatic seizures: a prospective, multicenter investigation. Arch Phys Med Rehabil. 2003;84(3):365–73. [DOI] [PubMed] [Google Scholar]
- 7. Mazzini L, Cossa FM, Angelino E, Campini R, Pastore I, Monaco F. Posttraumatic epilepsy: neuroradiologic and neuropsychological assessment of long‐term outcome. Epilepsia. 2003;44(4):569–74. [DOI] [PubMed] [Google Scholar]
- 8. Schneier AJ, Shields BJ, Hostetler SG, Xiang H, Smith GA. Incidence of pediatric traumatic brain injury and associated hospital resource utilization in the United States. Pediatrics. 2006;118(2):483–92. [DOI] [PubMed] [Google Scholar]
- 9. Mok JY. Non‐accidental injury in children—an update. Injury. 2008;39(9):978–85. [DOI] [PubMed] [Google Scholar]
- 10. Giza CC, Mink RB, Madikians A. Pediatric traumatic brain injury: not just little adults. Curr Opin Crit Care. 2007;13(2):143–52. [DOI] [PubMed] [Google Scholar]
- 11. Potts MB, Koh S‐E, Whetstone WD, Walker BA, Yoneyama T, Claus CP, et al. Traumatic injury to the immature brain: inflammation, oxidative injury, and iron‐mediated damage as potential therapeutic targets. NeuroRx. 2006;3(2):143–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sharma S, Tiarks G, Haight J, Bassuk AG. Neuropathophysiological mechanisms and treatment strategies for post‐traumatic epilepsy. Front Mol Neurosci. 2021;14:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Semple BD, O'Brien TJ, Gimlin K, Wright DK, Kim SE, Casillas‐Espinosa PM, et al. Interleukin‐1 receptor in seizure susceptibility after traumatic injury to the pediatric brain. J Neurosci. 2017;37(33):7864–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mazzola CA, Adelson PD. Critical care management of head trauma in children. Crit Care Med. 2002;30(11):S393–401. [DOI] [PubMed] [Google Scholar]
- 15. Adelson D, Clyde B, Kochanek PM, Wisniewski SR, Marion DW, Yonas H. Cerebrovascular response in infants and young children following severe traumatic brain injury: a preliminary report. Pediatr Neurosurg. 1997;26(4):200–7. [DOI] [PubMed] [Google Scholar]
- 16. Xu T, Yu X, Ou S, Liu X, Yuan J, Huang H, et al. Risk factors for posttraumatic epilepsy: a systematic review and meta‐analysis. Epilepsy Behav. 2017;67:1–6. [DOI] [PubMed] [Google Scholar]
- 17. Mariajoseph FP, Rewell SS, O'Brien TJ, Semple BD, Antonic‐Baker A. Incidence of post‐traumatic epilepsy following paediatric traumatic brain injury: protocol for systematic review and meta‐analysis. BMJ Open. 2021;11(11):e054034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta‐analysis protocols (PRISMA‐P) 2015 statement. Syst Rev. 2015;4(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Moola S, Munn Z, Tufanaru C, et al. Chapter 7: Systematic reviews of etiology and risk. Joanna briggs institute reviewer's manual The Joanna Briggs Institute. 2017; 5.
- 20. Nyaga VN, Arbyn M, Aerts M. Metaprop: a Stata command to perform meta‐analysis of binomial data. Arch Public Health. 2014;72(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Annegers JF, Coan SP. The risks of epilepsy after traumatic brain injury. Seizure. 2000;9(7):453–7. [DOI] [PubMed] [Google Scholar]
- 22. Appleton R, Demellweek C. Post‐traumatic epilepsy in children requiring inpatient rehabilitation following head injury. J Neurol Neurosurg Psychiatry. 2002;72(5):669–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Arango JI, Deibert CP, Brown D, Bell M, Dvorchik I, Adelson PD. Posttraumatic seizures in children with severe traumatic brain injury. Childs Nerv Syst. 2012;28(11):1925–9. [DOI] [PubMed] [Google Scholar]
- 24. Asikainen I, Kaste M, Sarna S. Early and late posttraumatic seizures in traumatic brain injury rehabilitation patients: brain injury factors causing late seizures and influence of seizures on long‐term outcome. Epilepsia. 1999;40(5):584–9. [DOI] [PubMed] [Google Scholar]
- 25. Barlow KM, Spowart JJ, Minns RA. Early posttraumatic seizures in non‐accidental head injury: relation to outcome. Dev Med Child Neurol. 2000;42(9):591–4. [DOI] [PubMed] [Google Scholar]
- 26. Christensen J, Pedersen MG, Pedersen CB, Sidenius P, Olsen J, Vestergaard M. Long‐term risk of epilepsy after traumatic brain injury in children and young adults: a population‐based cohort study. Lancet. 2009;373(9669):1105–10. [DOI] [PubMed] [Google Scholar]
- 27. de Santis A, Marossero F, Pagni C, Rampini P. Long‐term prognosis of early epilepsy in juvenile head‐injured patients (preliminary results). J Neurosurg Sci. 1979;23(2):105–8. [PubMed] [Google Scholar]
- 28. Emanuelson I, Uvebrant P. Occurrence of epilepsy during the first 10 years after traumatic brain injury acquired in childhood up to the age of 18 years in the south western Swedish population‐based series. Brain Inj. 2009;23(7–8):612–6. [DOI] [PubMed] [Google Scholar]
- 29. Hwang SY, Ong JW, Ng ZM, Foo CY, Chua SZ, Sri D, et al. Long‐term outcomes in children with moderate to severe traumatic brain injury: a single‐centre retrospective study. Brain Inj. 2019;33(11):1420–4. [DOI] [PubMed] [Google Scholar]
- 30. Kan P, Amini A, Hansen K, White GL Jr, Brockmeyer DL, Walker ML, et al. Outcomes after decompressive craniectomy for severe traumatic brain injury in children. J Neurosurg Pediatr. 2006;105(5):337–42. [DOI] [PubMed] [Google Scholar]
- 31. Keret A, Bennett‐Back O, Rosenthal G, Gilboa T, Shweiki M, Shoshan Y, et al. Posttraumatic epilepsy: long‐term follow‐up of children with mild traumatic brain injury. J Neurosurg Pediatr. 2017;20(1):64–70. [DOI] [PubMed] [Google Scholar]
- 32. Keret A, Shweiki M, Bennett‐Back O, Abed‐Fteiha F, Matoth I, Shoshan Y, et al. The clinical characteristics of posttraumatic epilepsy following moderate‐to‐severe traumatic brain injury in children. Seizure. 2018;58:29–34. [DOI] [PubMed] [Google Scholar]
- 33. Matsumoto JH, Caplan R, McArthur DL, Forgey MJ, Yudovin S, Giza CC. Prevalence of epileptic and nonepileptic events after pediatric traumatic brain injury. Epilepsy Behav. 2013;27(1):233–7. [DOI] [PubMed] [Google Scholar]
- 34. Mikkonen ED, Skrifvars MB, Reinikainen M, Bendel S, Laitio R, Hoppu S, et al. Posttraumatic epilepsy in intensive care unit–treated pediatric traumatic brain injury patients. Epilepsia. 2020;61(4):693–701. [DOI] [PubMed] [Google Scholar]
- 35. Park JT, Chugani HT. Post‐traumatic epilepsy in children—experience from a tertiary referral center. Pediatr Neurol. 2015;52(2):174–81. [DOI] [PubMed] [Google Scholar]
- 36. Pearl PL, McCarter R, McGavin CL, Yu Y, Sandoval F, Trzcinski S, et al. Results of phase II levetiracetam trial following acute head injury in children at risk for posttraumatic epilepsy. Epilepsia. 2013;54(9):e135–e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Petridis AK, Doukas A, Maslehaty H, Mehdorn HM. Predictors and incidence of posttraumatic seizures in children and adolescents after brain injury. Clin Prac. 2012;2(3):164–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ratan SK, Kulshreshtha R, Pandey RM. Predictors of posttraumatic convulsions in head‐injured children. Pediatr Neurosurg. 1999;30(3):127–31. [DOI] [PubMed] [Google Scholar]
- 39. Shin T, Oh K, Cha BH. The risk factors and clinical features of posttraumatic seizure in preschool‐aged children. Ann Child Neurol. 2019;27(1):22–8. [Google Scholar]
- 40. Thapa A, Chandra SP, Sinha S, Sreenivas V, Sharma BS, Tripathi M. Post‐traumatic seizures—a prospective study from a tertiary level trauma center in a developing country. Seizure. 2010;19(4):211–6. [DOI] [PubMed] [Google Scholar]
- 41. Young B, Rapp RP, Norton J, Haack D, Tibbs PA, Bean JR. Failure of prophylactically administered phenytoin to prevent early posttraumatic seizures. J Neurosurg. 1983;58(2):231–5. [DOI] [PubMed] [Google Scholar]
- 42. Fu TS, Jing R, McFaull SR, Cusimano MD. Health & economic burden of traumatic brain injury in the emergency department. Can J Neurol Sci. 2016;43(2):238–47. [DOI] [PubMed] [Google Scholar]
- 43. Semple BD, Dill LK, O'Brien TJ. Immune challenges and seizures: how do early life insults influence epileptogenesis? Front Pharmacol. 2020;11(2). 10.3389/fphar.2020.00002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kieslich M, Marquardt G, Galow G, Lorenz R, Jacobit G. Neurological and mental outcome after severe head injury in childhood: a long‐term follow‐up of 318 children. Disabil Rehabil. 2001;23(15):665–9. [DOI] [PubMed] [Google Scholar]
- 45. Rebbe R, Mienko JA, Martinson ML. Incidence and risk factors for abusive head trauma: a population‐based study. Child Abuse Rev. 2020;29(3):195–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sidpra J, Abomeli D, Hameed B, Baker J, Mankad K. Rise in the incidence of abusive head trauma during the COVID‐19 pandemic. Arch Dis Child. 2021;106(3):e14. [DOI] [PubMed] [Google Scholar]
- 47. Ashraf IJ, Pekarsky AR, Race JE, Botash AS. Making the most of clinical encounters: prevention of child abuse and maltreatment. Pediatr Clin. 2020;67(3):481–98. [DOI] [PubMed] [Google Scholar]
- 48. Dias MS, Smith K, DeGuehery K, Mazur P, Li V, Shaffer ML. Preventing abusive head trauma among infants and young children: a hospital‐based, parent education program. Pediatrics. 2005;115(4):e470–e7. [DOI] [PubMed] [Google Scholar]
- 49. Stewart CL, Moscariello MA, Hansen KW, Moulton SL. Infant car safety seats and risk of head injury. J Pediatr Surg. 2014;49(1):193–7. [DOI] [PubMed] [Google Scholar]
- 50. Semple BD, Zamani A, Rayner G, Shultz SR, Jones NC. Affective, neurocognitive and psychosocial disorders associated with traumatic brain injury and post‐traumatic epilepsy. Neurobiol Dis. 2019;123:27–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1


