Abstract
Background
Probiotics are live micro‐organisms that may give a beneficial physiological effect when administered in adequate amounts. Some trials show that probiotic strains can prevent respiratory infections. Even though our previously published review showed the benefits of probiotics for acute upper respiratory tract infections (URTIs), several new studies have been published. This is an update of a review first published in 2011 and updated in 2015.
Objectives
To assess the effectiveness and safety of probiotics (any specified strain or dose), compared with placebo or no treatment, in the prevention of acute URTIs in people of all ages, at risk of acute URTIs.
Search methods
We searched CENTRAL (2022, Issue 6), MEDLINE (1950 to May week 2, 2022), Embase (1974 to 10 May 2022), Web of Science (1900 to 10 May 2022), the Chinese Biomedical Literature Database, which includes the China Biological Medicine Database (from 1978 to 10 May 2022), the Chinese Medicine Popular Science Literature Database (from 2000 to 10 May 2022), and the Master's Degree Dissertation of Beijing Union Medical College Database (from 1981 to 10 May 2022). We searched the World Health Organization International Clinical Trials Registry Platform and ClinicalTrials.gov for completed and ongoing trials on 10 May 2022.
Selection criteria
We included individual randomised controlled trials (RCTs) and cluster‐RCTs comparing probiotics with placebo or no treatment to prevent acute URTIs. The participants were children, adults, or the elderly in the community, care facilities, schools, or hospitals. Our main outcomes were the number of participants diagnosed with URTIs (at least one event and at least three events), the incidence rate (number of cases/person year) of acute URTIs, and the mean duration of an episode of URTIs. Our secondary outcomes were the number of participants who were absent from childcare centre, school, or work due to acute URTIs; the number of participants who used prescribed antibiotics for acute URTIs; and the number of participants who experienced at least one adverse event from probiotics. We excluded studies if they did not specify acute respiratory infections as 'upper'; studies with more than 50% of participants vaccinated against influenza or other acute URTIs within the last 12 months; and studies with significantly different proportions of vaccinated participants between the probiotics arm and the placebo or no treatment arm.
Data collection and analysis
Two review authors independently assessed the eligibility of trials and extracted data using standard Cochrane methodological procedures. We analysed both intention‐to‐treat and per‐protocol data and used a random‐effects model. We expressed results as risk ratios (RRs) for dichotomous outcomes and mean differences (MDs) for continuous outcomes, both with 95% confidence intervals (CIs). We assessed the certainty of the evidence using the GRADE approach.
Main results
We included 23 individual RCTs and one cluster‐RCT. As one of the individual RCTs did not report outcomes in a usable way, we could only meta‐analyse data from 23 trials, involving a total of 6950 participants including children (aged from one month to 11 years old), adults (mean age 37.3), and older people (mean age 84.6 years). One trial reported 22.5% flu‐vaccine participants within the last 12 months, and 25.4% flu‐vaccine participants during the intervention. Probiotics were more likely to be given with milk‐based food in children; administered in powder form in adults; and given with milk‐based food or in capsules in the elderly. Most of the studies used one or two strains (e.g. Lactobacillus plantarum HEAL9, Lactobacillus paracasei (8700:2 or N1115)) and 109 or 1011 colony‐forming units (CFU)/day of probiotics for more than three months.
We found that probiotics may reduce the number of participants diagnosed with URTIs (at least one event) (RR 0.76, 95% CI 0.67 to 0.87; P < 0.001; 16 studies, 4798 participants; low‐certainty evidence); likely reduce the number of participants diagnosed with URTIs (at least three events) (RR 0.59, 95% CI 0.38 to 0.91; P = 0.02; 4 studies, 763 participants; moderate‐certainty evidence); may reduce the incidence rate (number of cases/person year) of URTIs (rate ratio 0.82, 95% CI 0.73 to 0.92, P = 0.001; 12 studies, 4364 participants; low‐certainty evidence); may reduce the mean duration of an episode of acute URTIs (MD −1.22 days, 95% CI −2.12 to −0.33; P = 0.007; 6 studies, 2406 participants; low‐certainty evidence); likely reduce the number of participants who used prescribed antibiotics for acute URTIs (RR 0.58, 95% CI 0.42 to 0.81; P = 0.001; 6 studies, 1548 participants; moderate‐certainty evidence); and may not increase the number of participants who experienced at least one adverse event (RR 1.02, 95% CI 0.90 to 1.15; P = 0.79; 8 studies, 2456 participants; low‐certainty evidence). Evidence showing a decrease in the number of people absent from childcare centre, school, or work due to acute URTIs with probiotics is very uncertain (RR 0.14, 95% CI 0.03 to 0.59; 1 study, 80 participants; very low‐certainty evidence). Adverse events from probiotics were minor, and most commonly gastrointestinal symptoms, such as vomiting, flatulence, diarrhoea, and bowel pain.
Authors' conclusions
Overall, we found that probiotics were better than placebo or no treatment in preventing acute URTIs.
Plain language summary
Can probiotics (live micro‐organisms) prevent upper respiratory tract infections such as the common cold?
Key messages
Probiotics may be beneficial in preventing at least one occurrence of acute upper respiratory tract infection (URTI), and are likely beneficial in preventing at least three occurrences of URTIs. More studies conducted in the elderly are needed. Larger, well‐designed studies are needed to give better estimates of the benefits and potential harms of probiotics use.
What are acute upper respiratory tract infections?
Acute URTIs include colds, influenza, and infections of the throat, nose, or sinuses. Symptoms include fever, cough, pain and headaches. Most acute URTIs are caused by viruses, and usually get better within three to seven days.
What are probiotics?
A common description of probiotics is live micro‐organisms that give a beneficial effect to the body when consumed in adequate amounts. Lactic acid bacteria and bifidobacteria are the most common types and are commonly consumed in fermented foods, such as yoghurt and soy yoghurt, or as dietary supplements.
What did we want to find out?
We wanted to find out if probiotics prevent acute URTIs in people of all ages with a healthy immune system.
What did we do?
We searched for studies that investigated probiotics for URTIs. We compared and summarised the results of the studies, and rated our confidence in the evidence, based on factors such as study methods and sizes.
What did we find?
We found 24 studies. We analysed data from 6950 people, including children (aged from 1 month to 11 years old), adults (mean age 37.3 years), and older people (mean age 84.6 years) from Italy, Japan, the United States, Croatia, England, Finland, Sweden, Chile, China, Denmark, Germany, Thailand, and Turkey. It was not clear in which countries two trials were conducted. Most of the studies were conducted in the community, care facilities, schools, and hospitals for three months during the winter/spring. Probiotics were more likely to be given with milk‐based food in children, in powder form in adults, and with milk‐based food or capsules in the elderly. One or two strains (e.g. Lactobacillus plantarum HEAL9, Lactobacillus paracasei (8700:2 or N1115)) and 109 or 1011 colony‐forming units (CFU)/day of probiotics were used in most of the studies.
Main results
Probiotics may reduce the number of people diagnosed with at least one URTI by about 24%; likely reduce the number of people diagnosed with at least three URTIs by about 41%; may reduce the incidence rate (number of new cases during a specified period of time) of acute URTIs by about 18%; may reduce the mean duration of an episode of acute URTIs by about 1.22 days; likely reduce the number of people who used antibiotics for URTIs by about 42%; and may not increase the number of people who experienced side effects (any harm). Evidence showing a decrease in the number of people absent from childcare centre, school, or work due to acute URTIs with probiotics was very uncertain.
What are the limitations of the evidence?
We are moderately confident that probiotics decrease the number of people diagnosed with at least three URTIs and the number of people who used antibiotics for URTIs, and have low confidence in the evidence that probiotics decrease the number of people diagnosed with at least one URTI, the incidence rate of acute URTIs, the mean duration of an episode of acute URTIs, and increase the number of people who experienced side effects (any harm). Evidence showing a decrease in the number of people absent from childcare centre, school, or work due to acute URTIs with probiotics was very uncertain. The main reasons for the limitations of the evidence were that people in the studies may have known which treatment they were getting, and not all of the studies provided data about everything that we were interested in.
How up‐to‐date is the evidence?
The evidence is current to 10 May 2022.
Summary of findings
Summary of findings 1. Intention‐to‐treat analysis: probiotics compared to placebo or no treatment for preventing acute upper respiratory tract infections.
| Intention‐to‐treat analysis: probiotics compared to placebo or no treatment for preventing acute upper respiratory tract infections | |||||
|
Patient or population: children, adults, and the elderly Setting: in the community, care facilities, school, or hospital Intervention: probiotics Comparison: placebo or no treatment | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with placebo or no treatment | Risk with probiotics | ||||
| Number of participants who were diagnosed with acute URTIs: at least 1 event | Study population | RR 0.76 (0.67 to 0.87) | 4798 (16 RCTs) | ⊕⊕⊝⊝ Lowa,b | |
| 447 per 1000 | 340 per 1000 (300 to 389) | ||||
| Number of participants who were diagnosed with acute URTIs: at least 3 events | Study population | RR 0.59 (0.38 to 0.91) | 763 (4 RCTs) | ⊕⊕⊕⊝ Moderatea | |
| 129 per 1000 | 76 per 1000 (49 to 117) | ||||
| Incidence rate of acute URTIs | Study population | Rate ratio 0.82 (0.73 to 0.92) | 4364 (12 RCTs) | ⊕⊕⊝⊝ Lowa,b | |
| ‐ | ‐ | ||||
| Mean duration of an episode of acute URTIs | ‐ | The mean duration of an episode of URTIs in the probiotics group was 1.22 days lower (2.12 to 0.33 lower). | ‐ | 2406 (6 RCTs) | ⊕⊕⊝⊝ Lowa,c |
| Number of participants who were absent from childcare centre, school, or work due to acute URTIs | Study population | RR 0.14 (0.03 to 0.59) | 80 (1 RCT) | ⊕⊝⊝⊝ Very lowd,e,f | |
| 35 per 1000 | 5 per 1000 (1 to 21) | ||||
| Number of participants who used prescribed antibiotics for acute URTIs | Study population | RR 0.58 (0.42 to 0.81) | 1548 (6 RCTs) | ⊕⊕⊕⊝ Moderatea | |
| 100 per 1000 | 58 per 1000 (42 to 81) | ||||
| Number of participants who experienced at least 1 side effect or adverse event | Study population | RR 1.02 (0.90 to 1.15) | 2456 (8 RCTs) | ⊕⊕⊝⊝ Lowa,g | |
| 233 per 1000 | 238 per 1000 (210 to 268) | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio; URTI: upper respiratory tract infection | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
aDowngraded once for study limitations, as most information was from studies at unclear risk of bias. bDowngraded once for publication bias, as the funnel plot was asymmetrical. cDowngraded once for inconsistency due to the higher I2 statistic (93%). dDowngraded once for study limitations due to unclear risk of bias for allocation concealment and selective reporting. eDowngraded once for imprecision due to the wide 95% CI. fDowngraded once for indirectness, as the included study only reported school absenteeism due to the common cold. gDowngraded once for imprecision, as the CI overlapped no effect.
Background
Description of the condition
Acute upper respiratory tract infections (URTIs), which include the common cold, acute sinusitis, acute pharyngitis, acute laryngotracheobronchitis (croup), acute epiglottitis (supraglottitis), acute rhinosinusitis, and acute otitis media (AOM), are a major cause of morbidity, especially in children and the elderly (Duijvestijn 2009; Kassel 2010; Liberati 2009). They are caused by a large variety of viruses and bacteria. In the United States, acute URTIs are the most common reason for people to seek medical care (Cherry 2003), and at least 1000 million colds occur there per year, with a frequency of two to six colds per person (Gwaltney 2002).
Acute URTIs are usually mild, viral infections with symptoms subsiding after a few days. They account for up to 75.8% of all antibiotic use in high‐income countries (Rún Sigurðardóttir 2015). Antibiotics are often misused in acute URTIs with viral aetiologies (Steinman 2003), despite the fact that the development of antibiotic‐resistant bacteria is inevitable. Although the causes of antibiotic resistance are multifactorial (Andersson 2020), antibiotic overuse is a major contributor (Woappi 2016).
Description of the intervention
Probiotics, a Greek word meaning 'for life', were first described by Kollath more than 50 years ago (Kollath 1953). They are now defined as "live microorganisms administered in adequate amounts which confer a beneficial physiological effect on the host" (Reid 2003). Although the underlying mechanisms of probiotics are still unclear, their application shows some promising results and trends with respect to immune modulations. Limited evidence from systematic reviews shows that probiotics are beneficial for treating infectious diarrhoea (Bernaola Aponte 2013), preventing antibiotic‐associated diarrhoea (Guo 2019), and treating vaginal infections in pregnancy (Othman 2010).
How the intervention might work
There are a number of possible means by which probiotics may improve health, one of which is the immunomodulation of local immunity (by maintaining gut wall integrity) and systemic immunity (by enhancing non‐specific and specific arms of the immune system). These include the following.
1. Probiotics and the innate immune function
Enhances phagocytic capacity of peripheral blood leucocytes (polymorphonuclear and monocytes).
Improves phagocytic activity.
Granulocytes show higher increases in phagocytic cell function compared with monocytes (Donnet‐Hughes 1999; Schiffrin 1995; Sheih 2001).
There are significant increases in the expression of receptors (CR1, CR3, FccRI, and FcaR) involved in phagocytosis (the cellular process of engulfing and ingesting solid particles, such as bacteria by the cell membrane) (Pelto 1998), the phagocytic index, oxidative burst (also known as respiratory burst, the rapid release of reactive oxygen species from some cells) (Donnet‐Hughes 1999), and microbicidal capacity in neutrophils (Arunachalam 2000). Natural killer (NK) cell (a type of cytotoxic cell that constitutes an important part of the innate immune system) activity is also markedly improved, and there are increases in the percentage of NK cells in the peripheral blood (Drakes 2004; Lee 2017).
2. Probiotics and acquired immunity
Significantly higher specific IgG, IgA, and IgM immunoglobulins (Lee 2017; Link‐Amster 1994; Majamaa 1995).
3. Probiotics and local immunity
Enhances gut barrier function and improves the local immune response (Perdigon 1995).
Increases the production of cytokines (e.g. interleukin‐1 (IL‐1), IL‐2, IL‐6, IL‐10, IL‐12, IL‐18, tumour necrosis factor alpha (TNF‐α), interferon‐α) (Gill 1998; Lee 2017; Meydani 2000; Rossol 2011).
Why it is important to do this review
More than a century ago, Nobel Prize winner Elie Metchnikoff conducted a series of trials showing that ingesting microbes that produce lactic acid by fermentation improves ailments such as digestive and respiratory tract disorders. The first evidence that probiotic strains could prevent respiratory tract infections was shown when mice were successfully protected against influenza through the administration of Bifidobacterium breve YIT4064 augmented anti‐influenza IgG (Yasui 1999). Soon after, Finnish researchers conducted trials amongst children in daycare centres who were given milk containing Lactobacillus rhamnosus GG (ATCC 53103) during winter (Hatakka 2001). However, one study showed that the probiotics did not have any effect on upper respiratory infections after the intervention (Hatakka 2007). Given the increasing consumption of probiotics, we feel there is a need to fully understand the effect of probiotics on acute URTIs and their potential adverse effects in humans.
Objectives
To assess the effectiveness and safety of probiotics (any specified strain or dose), compared with placebo or no treatment, in the prevention of acute URTIs in people of all ages, at risk of acute URTIs.
Methods
Criteria for considering studies for this review
Types of studies
Both individual and cluster‐RCTs (more than four clusters per group) of probiotics to prevent acute URTIs. We excluded cross‐over trials due to potential residual treatment effects.
Types of participants
Children and adults of all ages from the community, care facilities, schools, or hospitals. We excluded those participants whose circumstances may have affected their immunity, including those who had taken immune‐stimulating medications, undergone abnormal physical exercise (e.g. athletes (Greenham 2018)), or had known congenital or acquired immune defects (e.g. otitis‐prone children (Bardou 2020; Pichichero 2016; Pichichero 2020)) or allergies. We excluded studies that did not specify acute respiratory infections as 'upper'. We also excluded studies where more than 50% of participants had been vaccinated against influenza or other acute URTIs within the last 12 months, as vaccines will be an important effect modification factor. We also excluded studies that had significantly different proportions of vaccinated participants between the probiotics arm and the placebo or no treatment arm.
Types of interventions
Any probiotic (single or mixture of strains, any dosage regimen and any route of administration) for more than seven days, compared to placebo or no treatment.
Types of outcome measures
We only included the studies that specified acute respiratory infections as 'upper'. Cases of acute URTIs should have been confirmed by doctors, or have specific symptoms, such as nasal symptoms (e.g. runny nose, blocked nose, nose blowing, yellow secretions, bloody secretions, sneezing), pharyngeal symptoms (e.g. scratchy throat, sore throat, hoarseness), tonsillitis or pharyngitis (e.g. pain on swallowing, sore throat), laryngitis (e.g. hoarseness), and bronchial symptoms (e.g. cough, secretions), as well as headache, myalgia, red eyes (conjunctivitis), and fever (oral temperature > 37.7 °C or rectal temperature > 38 °C).
Primary outcomes
Number of participants who were diagnosed with acute URTIs.
Incidence rate (number of cases/person year) of acute URTIs.
Mean duration of an episode of acute URTIs.
Secondary outcomes
Number of participants who were absent from childcare centre, school, or work (a proxy of severity of disease).
Number of participants who used antibiotics for URTIs (a proxy of severity of disease).
Number of participants who experienced at least one side effect or adverse event.
Search methods for identification of studies
Electronic searches
For this 2022 update, we searched the Cochrane Central Register of Controlled Trials (CENTRAL; 2022, Issue 6) (accessed 10 May 2022), which includes the Cochrane Acute Respiratory Infections Group's Specialised Register; MEDLINE (July 2014 to May week 2, 2022); Embase (July 2014 to 10 May 2022); Web of Science (July 2014 to 10 May 2022); the Chinese Biomedical Literature Database, which includes the China Biological Medicine Database (from 1978 to 10 May 2022), the Chinese Medicine Popular Science Literature Database (from 2000 to 10 May 2022), and the Master's Degree Dissertation of Beijing Union Medical College Database (from 1981 to 10 May 2022). We used the search strategy described in Appendix 1 to search MEDLINE and CENTRAL. We combined the MEDLINE search strategy with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version, Ovid format (Lefebvre 2011). We adapted the search strategy to search Embase (Appendix 2), Web of Science (Appendix 3), and the Chinese Biomedical Literature Database (Figure 1). See Appendix 4 for details of previous searches.
1.
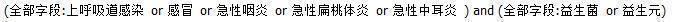
Chinese Biomedical Literature Database search strategy.
Searching other resources
We also searched the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (www.who.int/ictrp) and ClinicalTrials.gov (clinicaltrials.gov/) for completed and ongoing trials on 10 May 2022. We searched the reference sections of review articles to identify trials missed by electronic searching. We contacted the first author of the included trials and the manufacturers of probiotic agents and authors of conference literature for additional published or unpublished data. We did not impose any language or publication restrictions in the searches.
Data collection and analysis
Selection of studies
Two review authors (YZ, QH) independently performed title and abstract screening and full‐text review of studies identified by the search. We included trials using probiotic preparations containing other substances, such as vitamins and minerals, if these were also part of the placebo or no treatment arm. Any disagreements were resolved by discussion or by consulting a third review author (BD) when necessary. We discussed titles or abstracts not available in English with translators.
Data extraction and management
Two review authors (YZ, QH) independently extracted data from the included trials using the Cochrane Acute Respiratory Infection Group's data extraction form. We extracted the following data:
author;
year of publication;
language;
authors' institutions;
participants (age range, gender, inclusion and exclusion criteria);
methodological design (methods of randomisation, allocation concealment, blinding, loss to follow‐up and intention‐to‐treat analysis (ITT));
details of intervention (single or mixture of strains, dosage regimen, route of administration, duration, comparison treatment);
results (i.e. incidence of acute URTIs, reasons for withdrawal, measures of compliance and adverse effects, etc.).
Any disagreements were resolved by discussion or by consulting a third review author (BD) when necessary. We contacted trial authors and pharmaceutical companies to clarify unclear data and to request additional information on methodological certainty.
Assessment of risk of bias in included studies
Two review authors (QH, BD) independently assessed methodological certainty, as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). For trials using individual randomisation, we considered random sequence generation, allocation concealment, blinding, incomplete outcome data, selective reporting, and other bias. For trials using cluster randomisation, we assessed recruitment bias, baseline imbalance, loss of clusters, incorrect analysis and comparability with individually randomised trials.
Random sequence generation
Low risk of bias: adequate generation of allocation sequence (e.g. computer‐generated random numbers, table of random numbers, or similar).
High risk of bias: inadequate generation of allocation sequence (case record number; date of birth, day, month or year of admission (Higgins 2011); or allocation by judgement of the clinician, the participant, laboratory test or a series of tests, availability of the intervention).
Unclear risk of bias: the generation of the allocation sequence was unclear.
Allocation concealment
Low risk of bias: adequate concealment of allocation (e.g. central independent unit, non‐translucent sealed envelopes, or similar).
High risk of bias: inadequate concealment of allocation (any procedure that is transparent before allocation, e.g. alternation, the use of case record numbers, dates of birth, or open table of random numbers or similar).
Unclear risk of bias: unclear concealment of allocation (e.g. only specifying that non‐translucent sealed envelopes were used or not reporting any concealment approach) or inadequate.
Blinding of participants and personnel
Low risk of bias: we considered masking of both the participants and study personnel who implemented the study to be low risk of performance bias (e.g. identical placebo tablets or similar, and the study personnel did not know the groups).
High risk of bias: open‐label study.
Unclear risk of bias: insufficient information provided to judge the level of bias.
Blinding of outcome assessment
Low risk of bias: we considered masking of the outcome assessor to be low risk of detection bias.
High risk of bias: not used or non‐blinding of detection of outcomes (e.g. not performed or tablets versus fluids or similar).
Unclear risk of bias: insufficient information provided to judge the level of bias.
Incomplete outcome data: assessment for potential bias of exclusion and attrition
Low risk of bias: trials had no missing outcome data or few exclusions; attrition is noted; and an ITT analysis is possible.
High risk of bias: there are wide differences in exclusions between the intervention group and the control group, or the rate of exclusion and/or attrition is higher than 15%, whatever ITT analysis is used.
Unclear risk of bias: the rate of exclusions or attrition, or both, is higher than 10%, whatever ITT analysis is used.
Selective reporting
If a protocol for the included study was available, we compared the outcomes in the protocol and those in the published report.
Other bias
Any other potential biases.
Recruitment bias
Low risk of bias: the clusters were randomised after individuals recruiting.
High risk of bias: the clusters were randomised before individuals recruiting.
Unclear risk of bias: insufficient information provided to judge the level of bias.
Baseline imbalance
Low risk of bias: the trials used stratified or pair‐matched randomisation of clusters; or the trials reported the baseline comparability of clusters, or statistical adjustment for baseline characteristics.
High risk of bias: the trials did not use stratified or pair‐matched randomisation of clusters. In addition, the trials did not report the baseline comparability of clusters and statistical adjustment for baseline characteristics.
Unclear risk of bias: insufficient information provided to judge the level of bias.
Loss of clusters
Complete clusters were lost from a trial, and had to be omitted from the analysis. In addition, missing outcomes for individuals within clusters led to risk of bias.
Incorrect analysis
The trials were analysed by incorrect statistical methods and did not take the clustering into account.
Comparability with individually randomised trials
The intervention effects were different from individually randomised trials.
Measures of treatment effect
We analysed data using Review Manager Web (RevMan Web 2022). We were only able to perform limited pooled analyses. We used a random‐effects model for pooled analysis of both heterogeneous data and homogeneous data. We expressed results as risk ratios (RRs) for dichotomous outcomes and mean differences (MDs) for continuous outcomes, both with 95% confidence intervals (CIs). If the study reported the incidence rate (number of cases/person year) of URTIs using a mixed model, we would choose its effect size; otherwise, we would calculate the incidence rate of URTIs between groups and the standard error (SE) of the rate ratio according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021). We used the generic inverse‐variance weighting when pooling trials for this outcome. When the cluster‐randomised trials failed to conduct appropriate analyses and did not report their effective sample sizes, we would recalculate the design effects and the number of events. We used the intracluster correlation coefficient (ICC = 0.02) and the number of clusters to calculate the design effects and the number of events. We reported the adjusted sample sizes and numbers of events in the meta‐analyses.
Unit of analysis issues
We did not anticipate including cross‐over trials in this review. We combined similar groups to create a single pair‐wise comparison for multiple arms from one study according to the recommendations in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021). We also took care to avoid double‐counting of participants where multiple interventions were used in the same trial.
Dealing with missing data
We sought missing data from the trial authors. We analysed the outcome measures both in an ITT population (i.e. we considered participants who dropped out of a study along with those who continued) and a per‐protocol (PP) population (i.e. we excluded participants who dropped out of a study during the follow‐up period). If the study only reported data for the PP population, we imputed the missing values using single imputations and borrowed information from probiotics and placebo and no treatment group separately in ITT analysis.
Assessment of heterogeneity
We carried out tests for heterogeneity using the Chi2 test, with significance being set at P < 0.1. We used the I2 statistic to estimate the total variation across trials according to the recommendations in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021): an I2 statistic < 40% might not be important heterogeneity; 30% to 60% may represent a moderate level of heterogeneity; 50% to 90% a substantial level of heterogeneity; and 75% to 100% a considerable level of heterogeneity.
Assessment of reporting biases
We used funnel plots to assess the presence of publication bias where at least 10 studies contributed to the meta‐analysis (Egger 2007). We assessed funnel plot asymmetry visually. When there was asymmetry in the funnel plots, we would add contour‐enhanced funnel plots to differentiate the asymmetry that was due to non‐reporting biases or other factors (Higgins 2021).
Data synthesis
We used a random‐effects model to synthesise all data, irrespective of heterogeneity between the pooled trials.
Subgroup analysis and investigation of heterogeneity
We explored possible explanations for heterogeneity by conducting the following subgroup analyses:
age (children versus adults versus elderly);
treatment dose (less than 1010 colony‐forming units (CFU) per day versus more than 1010 CFU per day);
treatment duration (less than three months versus three to six months versus more than six months);
comparator (placebo versus no treatment).
Sensitivity analysis
We performed sensitivity analysis by excluding trials at high risk of bias and cluster‐RCTs.
Summary of findings and assessment of the certainty of the evidence
We only included RCTs, and downgraded the certainty of the evidence from high certainty by one level for serious (or by two for very serious) study limitations (risk of bias), indirectness of evidence, inconsistency, imprecision of effect estimates, or potential publication bias, according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021). Furthermore, we used GRADEpro GDT to create a summary of findings table, and reported primary and secondary outcomes based on an ITT population (GRADEpro GDT).
We created one summary of findings table using the following outcomes: number of participants who were diagnosed with acute URTIs (at least one event); number of participants who were diagnosed with acute URTIs (at least three events); incidence rate of acute URTIs; mean duration of an episode of acute URTIs; number of participants who were absent from childcare centre, school, or work due to acute URTIs; number of participants who used antibiotics for acute URTIs; number of participants who experienced at least one side effect or adverse event (Table 1). We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the certainty of a body of evidence as it relates to the studies which contribute data to the meta‐analyses for the prespecified outcomes (Atkins 2004). We used the methods and recommendations described in part 2 and Chapter 14 of the Cochrane Handbook for Systematic Reviews of Interventions using GRADEpro GDT (GRADEpro GDT) (Higgins 2021). We justified all decisions to down‐ or upgrade the certainty of the evidence using footnotes, and made comments to aid the reader's understanding of the review where necessary.
Results
Description of studies
Results of the search
We retrieved records from CENTRAL (517 records), MEDLINE (696 records), Embase (717 records), Web of Science (692 records), and the Chinese Biomedical Literature Database (13 records) in our electronic literature searches. After de‐duplication, 1731 records remained. We excluded 1646 records based on title and abstract, and retrieved 85 full‐text articles. We eventually included 24 trials in the review (Figure 2). We also retrieved 113 registered trials from WHO ICTRP and ClinicalTrials.gov, and identified one ongoing trial for this review after assessment (Characteristics of ongoing studies table).
2.
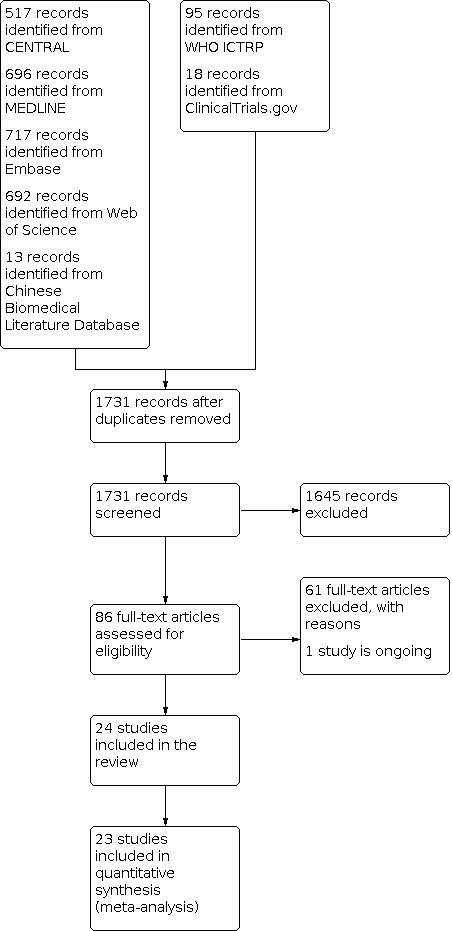
Study flow diagram.
Included studies
We identified 85 full texts of clinical trials and included 23 individual RCTs (Andaloro 2019; Berggren 2011; Butler 2020; Caceres 2010; Damholt 2022; Fujita 2013; Hojsak 2010a; Hojsak 2010b; Kara 2019; Langkamp‐Henken 2015; Laursen 2017; Lazou Ahrén 2020; Lazou Ahrén 2021; Makino 2010a; Pu 2017; Rautava 2009; Rerksuppaphol 2012; Rio 2002; Santamaria 2019; Shida 2017; Smith 2013; Taipale 2016; Vrese 2005), and one cluster‐RCT (Merenstein 2010). We also reassessed the previously included trials. We extracted and pooled data from 23 trials; we did not pool data from Makino 2010a because the outcomes were not reported in a usable way.
Design
Most of the included RCTs used a two‐arm parallel design (Andaloro 2019; Berggren 2011; Butler 2020; Caceres 2010; Damholt 2022; Fujita 2013; Hojsak 2010a; Hojsak 2010b; Kara 2019; Laursen 2017; Lazou Ahrén 2020; Lazou Ahrén 2021; Makino 2010a; Merenstein 2010; Pu 2017; Rautava 2009; Rerksuppaphol 2012; Rio 2002; Shida 2017; Smith 2013; Taipale 2016; Vrese 2005). Only two trials used a multi‐arm design (Langkamp‐Henken 2015; Santamaria 2019).
Participants
Seven trials focused on adults aged 18 to 65 years (Berggren 2011; Langkamp‐Henken 2015; Lazou Ahrén 2021; Pu 2017; Shida 2017; Smith 2013; Vrese 2005); three trials focused on older people (Butler 2020; Fujita 2013; Makino 2010a); and the remaining studies focused on children (Andaloro 2019; Damholt 2022; Hojsak 2010a; Hojsak 2010b; Kara 2019; Laursen 2017; Lazou Ahrén 2020; Merenstein 2010; Rautava 2009; Rerksuppaphol 2012; Rio 2002; Santamaria 2019; Taipale 2016). Trials were performed in Italy (Andaloro 2019; Santamaria 2019), Japan (Fujita 2013; Makino 2010a; Shida 2017), the United States (Langkamp‐Henken 2015; Merenstein 2010; Smith 2013), Croatia (Hojsak 2010a; Hojsak 2010b), England (Butler 2020; Damholt 2022), Finland (Rautava 2009; Taipale 2016), Sweden (Berggren 2011; Lazou Ahrén 2020), Chile (Caceres 2010), China (Pu 2017), Denmark (Laursen 2017), Germany (Lazou Ahrén 2021), Thailand (Rerksuppaphol 2012), and Turkey (Kara 2019). It was not clear in which countries the other two trials were conducted (Rio 2002; Vrese 2005). Baseline data were stated, and comparability was analysed in all trials except one (Rio 2002). One trial reported 22.5% flu‐vaccine participants within the last 12 months and 25.4% flu‐vaccine participants during the intervention.
Funding sources and potential conflicts of interest
Four trials did not disclose funding sources (Hojsak 2010b; Kara 2019; Rio 2002; Taipale 2016). Three trials reported that they had received no financial support (Andaloro 2019; Hojsak 2010a; Shida 2017). Fifteen studies received funding to support the research (Berggren 2011; Butler 2020; Caceres 2010; Fujita 2013; Langkamp‐Henken 2015; Laursen 2017; Lazou Ahrén 2020; Lazou Ahrén 2021; Merenstein 2010; Pu 2017; Rautava 2009; Rerksuppaphol 2012; Santamaria 2019; Smith 2013; Vrese 2005), and 10 trials received funding from the company that produced the probiotics (Berggren 2011; Caceres 2010; Damholt 2022; Fujita 2013; Laursen 2017; Lazou Ahrén 2020; Lazou Ahrén 2021; Merenstein 2010; Pu 2017; Shida 2017). Nine trials stated that they had no conflicts of interest (Andaloro 2019; Fujita 2013; Hojsak 2010a; Makino 2010a; Pu 2017; Santamaria 2019; Smith 2013; Taipale 2016; Vrese 2005), and six trials did not make declarations of interest (Caceres 2010; Hojsak 2010b; Kara 2019; Rautava 2009; Rerksuppaphol 2012; Rio 2002). Nine trials reported conflicts of interest (Berggren 2011; Butler 2020; Damholt 2022; Langkamp‐Henken 2015; Laursen 2017; Lazou Ahrén 2020; Lazou Ahrén 2021; Merenstein 2010; Shida 2017): four trials reported that the authors had received a grant from the project/company (Berggren 2011; Butler 2020; Langkamp‐Henken 2015; Laursen 2017), and seven trials reported that at least one author was an employee of the company that produced the probiotics (Berggren 2011; Damholt 2022; Langkamp‐Henken 2015; Lazou Ahrén 2020; Lazou Ahrén 2021; Merenstein 2010; Shida 2017).
Setting
Studies were conducted in various clinical settings, including the community (Berggren 2011; Laursen 2017; Lazou Ahrén 2021; Makino 2010a; Pu 2017; Shida 2017; Vrese 2005), childcare centre (Caceres 2010; Damholt 2022; Hojsak 2010a; Lazou Ahrén 2020; Merenstein 2010), hospital (Andaloro 2019; Hojsak 2010b; Kara 2019; Rio 2002; Santamaria 2019), well‐baby clinic (Rautava 2009; Taipale 2016), school (Damholt 2022; Langkamp‐Henken 2015; Rerksuppaphol 2012; Smith 2013), and care home for the elderly (Butler 2020; Fujita 2013).
Interventions
The included trials involved different types of probiotics including Lactobacillus plantarum HEAL9, Lactobacillus paracasei (8700:2 or N1115), Lactobacillus rhamnosus (GG or HN001), Lactobacillus casei strain Shirota, Lactobacillus bulgaricus OLL 073R‐1, Lactobacillus acidophilus, Lactobacillus gasseri, Lactobacillus helveticus, Streptococcus thermophilus OLS 3059, Bifidobacterium lactis BB‐12, Bifidobacterium bifidum (MF 20/5 or R0071), Bifidobacterium animalis and Bifidobacterium longum (SP 07/3 or ssp. infantis R0033), Streptococcus salivarius 24SMB, and Streptococcus oralis 89a, usually compared with placebo. Most probiotics were given along with milk‐based food (Caceres 2010; Damholt 2022; Fujita 2013; Hojsak 2010a; Hojsak 2010b; Makino 2010a; Merenstein 2010; Pu 2017; Rio 2002; Shida 2017). The probiotics were administered in powder form in seven studies (Berggren 2011; Langkamp‐Henken 2015; Laursen 2017; Lazou Ahrén 2020; Lazou Ahrén 2021; Smith 2013; Vrese 2005); in capsules in three studies (Butler 2020; Rautava 2009; Rerksuppaphol 2012); in drops in one study (Kara 2019); in tablets in one study (Taipale 2016); and in oral spray in one study (Andaloro 2019). Three strains of probiotics were used in two trials (Merenstein 2010; Vrese 2005); two strains of probiotics were used in 11 trials (Andaloro 2019; Berggren 2011; Butler 2020; Laursen 2017; Lazou Ahrén 2020; Lazou Ahrén 2021; Makino 2010a; Rautava 2009; Rerksuppaphol 2012; Rio 2002; Smith 2013); and only one strain of probiotic was used in 11 trials (Caceres 2010; Damholt 2022; Fujita 2013; Hojsak 2010a; Hojsak 2010b; Kara 2019; Langkamp‐Henken 2015; Pu 2017; Santamaria 2019; Shida 2017; Taipale 2016). Most trials were conducted for three to six months. One trial used probiotics for the duration of hospitalisation (Hojsak 2010a); one trial administered the probiotics for 8 to 12 weeks (Makino 2010a); one trial administered the probiotics for one month (Kara 2019); and six trials administered the probiotics for six months or longer (Andaloro 2019; Butler 2020; Fujita 2013; Laursen 2017; Rautava 2009; Taipale 2016). Most trials used 109 or 1011 CFU/day of probiotics, with the exception of one study which used 5 × 107 CFU/day of probiotics (Vrese 2005). Most trials used a placebo, with the exception of two trials which compared the intervention with no treatment (Kara 2019; Pu 2017). The study period in most trials included the common‐cold season (Andaloro 2019; Berggren 2011; Butler 2020; Caceres 2010; Damholt 2022; Fujita 2013; Hojsak 2010a; Hojsak 2010b; Langkamp‐Henken 2015; Laursen 2017; Lazou Ahrén 2020; Lazou Ahrén 2021; Makino 2010a; Rautava 2009; Rerksuppaphol 2012; Rio 2002; Santamaria 2019; Shida 2017; Smith 2013; Taipale 2016; Vrese 2005). Two trials took place in summer/autumn (Kara 2019; Pu 2017), and one trial did not report the season in which it was conducted (Merenstein 2010).
Outcome measures
The included trials reported different outcome measures. Most trials reported the number of participants who were diagnosed with acute URTIs or the duration of acute URTI episodes (Andaloro 2019; Berggren 2011; Damholt 2022; Fujita 2013; Hojsak 2010a; Hojsak 2010b; Kara 2019; Langkamp‐Henken 2015; Laursen 2017; Lazou Ahrén 2020; Lazou Ahrén 2021; Pu 2017; Rautava 2009; Rerksuppaphol 2012; Shida 2017; Smith 2013; Taipale 2016; Vrese 2005). The incidence rate (number of cases/person year) of acute URTIs was calculated in 12 trials (Andaloro 2019; Berggren 2011; Butler 2020; Caceres 2010; Damholt 2022; Fujita 2013; Langkamp‐Henken 2015; Lazou Ahrén 2021; Merenstein 2010; Pu 2017; Rio 2002; Santamaria 2019). The outcome measures also included symptoms of unrelated diseases and infections. Six trials reported the number of participants who used prescribed antibiotics for acute URTIs (Hojsak 2010a; Hojsak 2010b; Lazou Ahrén 2020; Pu 2017; Rautava 2009; Rerksuppaphol 2012). Eight trials reported side effects including vomiting, diarrhoea, flatulence, and increased bowel irritability (pain, loose stools, etc.) (Andaloro 2019; Berggren 2011; Lazou Ahrén 2020; Lazou Ahrén 2021; Merenstein 2010; Rautava 2009; Rerksuppaphol 2012; Smith 2013). One trial assessed the number of children who were absent from school due to the common cold (Rerksuppaphol 2012). None of the trials assessed time off from childcare centres or work due to acute URTIs. One trial reported the number of days absent from daycare centres due to "infections", but the trial did not separate URTIs from "infections" (Hojsak 2010a).
Excluded studies
We excluded 61 trials for the reasons documented in the Characteristics of excluded studies table.
Risk of bias in included studies
The overall risk of bias is presented graphically in Figure 3 and summarised in Figure 4.
3.

Risk of bias graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
4.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Sixteen trials clearly described adequate sequence generation methods (Andaloro 2019; Butler 2020; Caceres 2010; Damholt 2022; Fujita 2013; Hojsak 2010a; Hojsak 2010b; Langkamp‐Henken 2015; Laursen 2017; Lazou Ahrén 2020; Lazou Ahrén 2021; Pu 2017; Rerksuppaphol 2012; Santamaria 2019; Smith 2013; Taipale 2016). Seven trials did not describe the sequence generation method. Seven trials described adequate allocation concealment (Damholt 2022; Fujita 2013; Langkamp‐Henken 2015; Laursen 2017; Lazou Ahrén 2020; Lazou Ahrén 2021; Taipale 2016), and one trial was at high risk of bias for allocation concealment (Pu 2017). We approached the authors of the remaining trials for further clarification on allocation, but did not receive any replies.
Blinding
Seventeen trials reported double‐blinding (Berggren 2011; Butler 2020; Caceres 2010; Damholt 2022; Fujita 2013; Hojsak 2010a; Hojsak 2010b; Langkamp‐Henken 2015; Laursen 2017; Lazou Ahrén 2020; Lazou Ahrén 2021; Rautava 2009; Rerksuppaphol 2012; Santamaria 2019; Smith 2013; Taipale 2016; Vrese 2005), and 11 trials described the blinding methods in detail (Butler 2020; Damholt 2022; Hojsak 2010a; Hojsak 2010b; Lazou Ahrén 2020; Lazou Ahrén 2021; Rautava 2009; Rerksuppaphol 2012; Santamaria 2019; Smith 2013; Vrese 2005). Five trials did not report the type of blinding (Andaloro 2019; Kara 2019; Makino 2010a; Rio 2002; Shida 2017), and one trial reported non‐blinding (Pu 2017).
Incomplete outcome data
All trials provided sufficient information to permit calculation of incomplete outcome data, or described the withdrawal rate. Withdrawal rates varied from 0 (Lazou Ahrén 2021; Santamaria 2019), to 42% (Rio 2002). Fifteen trials were at low risk of attrition bias (Andaloro 2019; Damholt 2022; Hojsak 2010b; Kara 2019; Langkamp‐Henken 2015; Laursen 2017; Lazou Ahrén 2020; Lazou Ahrén 2021; Makino 2010a; Pu 2017; Rerksuppaphol 2012; Santamaria 2019; Shida 2017; Smith 2013; Vrese 2005); one study was at high risk of attrition bias (Rio 2002); and the other seven trials were at unclear risk of attrition bias (Berggren 2011; Butler 2020; Caceres 2010; Fujita 2013; Hojsak 2010a; Rautava 2009; Taipale 2016).
Selective reporting
We only had access to protocols for four of the included trials, and these trials had a low risk of selective reporting bias (Damholt 2022; Hojsak 2010b; Laursen 2017; Lazou Ahrén 2021). We could not obtain the protocols for the remaining trials, which precluded an assessment of their risk of selective reporting bias.
Other potential sources of bias
We did not identify any other obvious sources of bias, therefore we judged this category as low risk for each of the individual RCTs.
Risk of bias for cluster‐RCTs
Merenstein 2010 had a low risk of recruitment bias, baseline imbalance, loss of clusters, incorrect analysis, and comparability with individually randomised trials.
Effects of interventions
See: Table 1
We meta‐analysed 23 trials with a total of 6950 participants. We analysed all outcome measures based on both an ITT population (i.e. all participants who dropped out of the study were analysed according to their original group, irrespective of whether they had completed or received that treatment) and a PP population (i.e. participants who dropped out of a study during the follow‐up period were excluded). As the inference of PP analysis was the same as ITT analysis, we only reported ITT analysis results in the main text.
Intention‐to‐treat analysis
Primary outcomes
1.1. Number of participants who were diagnosed with acute URTIs (at least one event)
Sixteen trials reported participants who experienced episodes of acute URTIs (Andaloro 2019; Berggren 2011; Damholt 2022; Fujita 2013; Hojsak 2010a; Hojsak 2010b; Kara 2019; Langkamp‐Henken 2015; Laursen 2017; Lazou Ahrén 2020; Lazou Ahrén 2021; Pu 2017; Rautava 2009; Rerksuppaphol 2012; Shida 2017; Taipale 2016). There were 2539 participants in the probiotics group and 2259 participants in the placebo or no treatment group. All trials reported the number of participants who were diagnosed with acute URTIs (at least one event).
Age
Five studies were conducted in adults, and the results showed that probiotics supplementation was negatively associated with the number of participants who were diagnosed with acute URTIs (at least one event) (RR 0.77, 95% CI 0.65 to 0.93; P = 0.006; 2132 participants; Analysis 1.1) (Berggren 2011; Langkamp‐Henken 2015; Lazou Ahrén 2021; Pu 2017; Shida 2017). Ten trials were conducted in children and showed that the probiotics intervention was better than placebo or no treatment (RR 0.72, 95% CI 0.58 to 0.89; P = 0.003; 2512 participants; Analysis 1.1) (Andaloro 2019; Damholt 2022; Hojsak 2010a; Hojsak 2010b; Kara 2019; Laursen 2017; Lazou Ahrén 2020; Rautava 2009; Rerksuppaphol 2012; Taipale 2016). Only one study was conducted on the elderly (Fujita 2013). The results showed that there was no difference between the probiotics group and the placebo group in terms of the number of older adults who were diagnosed with acute URTIs (at least one event (RR 0.99, 95% CI 0.68 to 1.45; P = 0.98; 154 participants; Analysis 1.1).
1.1. Analysis.

Comparison 1: Intention‐to‐treat analysis: probiotics versus placebo or no treatment ‐ primary outcome measures, Outcome 1: The number of participants who were diagnosed with acute URTIs: at least 1 event (age)
Treatment dose
Eleven trials used probiotics less than 1010 CFU/day (Andaloro 2019; Berggren 2011; Damholt 2022; Hojsak 2010a; Hojsak 2010b; Kara 2019; Langkamp‐Henken 2015; Laursen 2017; Lazou Ahrén 2020; Lazou Ahrén 2021; Rerksuppaphol 2012), and five trials used more than 1010 CFU/day (Fujita 2013; Pu 2017; Rautava 2009; Shida 2017; Taipale 2016). The results showed that probiotics supplementation was negatively associated with the number participants who were diagnosed with acute URTIs (at least one event) for the dose of less than 1010 CFU/day (RR 0.77, 95% CI 0.67 to 0.90; P < 0.001; 4121 participants; Analysis 1.2) and the dose of more than 1010 CFU/day (RR 0.70, 95% CI 0.50 to 0.99; P = 0.04; 677 participants; Analysis 1.2).
1.2. Analysis.

Comparison 1: Intention‐to‐treat analysis: probiotics versus placebo or no treatment ‐ primary outcome measures, Outcome 2: The number of participants who were diagnosed with acute URTIs: at least 1 event (treatment dose)
Treatment duration
Two trials were conducted for less than three months (Hojsak 2010a; Kara 2019); nine trials for three to six months (Berggren 2011; Damholt 2022; Hojsak 2010b; Langkamp‐Henken 2015; Lazou Ahrén 2020; Lazou Ahrén 2021; Pu 2017; Rerksuppaphol 2012; Shida 2017); and five trials for more than six months (Andaloro 2019; Fujita 2013; Laursen 2017; Rautava 2009; Taipale 2016). The results showed that probiotics were beneficial for preventing the occurrence of at least one episode of URTIs for both less than three months (RR 0.60, 95% CI 0.50 to 0.74; P < 0.001; 381 participants; Analysis 1.3) and three to six months (RR 0.81, 95% CI 0.71 to 0.93; P = 0.003; 3704 participants; Analysis 1.3), but not for more than six months (RR 0.78, 95% CI 0.53 to 1.13; P = 0.19; 713 participants; Analysis 1.3).
1.3. Analysis.

Comparison 1: Intention‐to‐treat analysis: probiotics versus placebo or no treatment ‐ primary outcome measures, Outcome 3: The number of participants who were diagnosed with acute URTIs: at least 1 event (treatment duration)
Comparator
Fourteen placebo‐controlled trials, Andaloro 2019; Berggren 2011; Damholt 2022; Fujita 2013; Hojsak 2010a; Hojsak 2010b; Langkamp‐Henken 2015; Laursen 2017; Lazou Ahrén 2020; Lazou Ahrén 2021; Rautava 2009; Rerksuppaphol 2012; Shida 2017; Taipale 2016, and two trials with no treatment as the control, Kara 2019; Pu 2017, showed that probiotics supplementation may reduce the number participants diagnosed with acute URTIs (at least one event) (placebo: RR 0.78, 95% CI 0.68 to 0.90; P < 0.001; 4465 participants; no treatment: RR 0.63, 95% CI 0.49 to 0.81; P < 0.001; 333 participants; Analysis 1.4).
1.4. Analysis.

Comparison 1: Intention‐to‐treat analysis: probiotics versus placebo or no treatment ‐ primary outcome measures, Outcome 4: The number of participants who were diagnosed with acute URTIs: at least 1 event (type of comparator)
Overall pooled results
Pooling of these 16 trials showed a benefit of using probiotics to prevent the occurrence of at least one episode of URTIs (RR 0.76, 95% CI 0.67 to 0.87; P < 0.001; 4798 participants; Analysis 1.1; Analysis 1.2; Analysis 1.3; Analysis 1.4). The contour‐enhanced funnel plot suggested that some studies with negative outcomes might be missing (Figure 5). The level of heterogeneity between trials was high (Chi2 = 50.94; df = 15, P < 0.001; I2 = 71%; Analysis 1.1; Analysis 1.2; Analysis 1.3; Analysis 1.4). We downgraded the certainty of evidence from high to low for this outcome due to study limitations and publication bias.
5.

Contour‐enhanced funnel plot of comparison: intention‐to‐treat analysis: probiotics versus placebo ‐ primary outcome measures, outcome: the number of participants who were diagnosed with acute URTIs: at least 1 event.
1.2. Number of participants who were diagnosed with acute URTIs (at least three events)
Four trials reported participants who were diagnosed with acute URTIs (at least three events) (Berggren 2011; Lazou Ahrén 2020; Pu 2017; Rautava 2009). There were 375 participants in the probiotics group and 388 participants in the placebo or no treatment group.
Age
Two trials were conducted in adults (Berggren 2011; Pu 2017), and two in children (Lazou Ahrén 2020; Rautava 2009). Neither group showed that probiotics made a difference in preventing the occurrence of at least three episodes of URTIs (adults: RR 0.62, 95% CI 0.20 to 1.93; P = 0.41; 551 participants; children: RR 0.64, 95% CI 0.36 to 1.14; P = 0.13; 212 participants; Analysis 1.5).
1.5. Analysis.

Comparison 1: Intention‐to‐treat analysis: probiotics versus placebo or no treatment ‐ primary outcome measures, Outcome 5: The number of participants who were diagnosed with acute URTI: at least 3 events (age)
Treatment dose
Two trials using probiotics of less than 1010 CFU/day showed that probiotics were better than placebo (RR 0.59, 95% CI 0.36 to 0.96; P = 0.03; 449 participants; Analysis 1.6) (Berggren 2011; Lazou Ahrén 2020), but not in trials using probiotics of more than 1010 CFU/day (RR 0.64, 95% CI 0.20 to 2.04; P = 0.45; 314 participants; Analysis 1.6) (Pu 2017; Rautava 2009).
1.6. Analysis.

Comparison 1: Intention‐to‐treat analysis: probiotics versus placebo or no treatment ‐ primary outcome measures, Outcome 6: The number of participants who were diagnosed with acute URTI: at least 3 events (treatment dose)
Treatment duration
Three trials used probiotics for three to six months (Berggren 2011; Lazou Ahrén 2020; Pu 2017), and one trial used probiotics for more than six months (Rautava 2009). Benefits of probiotics for preventing the occurrence of at least three episodes of URTIs were found for the treatment duration of three to six months (three to six months: RR 0.61, 95% CI 0.38 to 0.99; P = 0.05; 682 participants; more than six months: RR 0.51, 95% CI 0.20 to 1.35; P = 0.18; 81 participants; Analysis 1.7).
1.7. Analysis.

Comparison 1: Intention‐to‐treat analysis: probiotics versus placebo or no treatment ‐ primary outcome measures, Outcome 7: The number of participants who were diagnosed with acute URTI: at least 3 events (treatment duration)
Comparator
Three placebo‐controlled trials showed that probiotics reduced the number of participants who were diagnosed with acute URTIs (at least three events) (RR 0.57, 95% CI 0.37 to 0.89; P = 0.01; 530 participants; Analysis 1.8) (Berggren 2011; Lazou Ahrén 2020; Rautava 2009), whilst one trial that used no treatment as the comparator showed no difference between groups in reducing the number of participants diagnosed with acute URTIs (at least three events) (RR 3.08, 95% CI 0.13 to 74.78; P = 0.49; 233 participants; Analysis 1.8) (Pu 2017).
1.8. Analysis.

Comparison 1: Intention‐to‐treat analysis: probiotics versus placebo or no treatment ‐ primary outcome measures, Outcome 8: The number of participants who were diagnosed with acute URTI: at least 3 events (type of comparator)
Overall pooled results
We found a beneficial effect of probiotics for the outcome number of participants diagnosed with acute URTIs (at least three events) (RR 0.59, 95% CI 0.38 to 0.91; P = 0.02; 763 participants; Analysis 1.5; Analysis 1.6; Analysis 1.7; Analysis 1.8). The level of heterogeneity between these trials was high (Chi2 = 1.64; df = 3, P = 0.65; I2 = 0%; Analysis 1.5; Analysis 1.6; Analysis 1.7; Analysis 1.8). We downgraded the certainty of the evidence from high to moderate due to study limitations.
2. Incidence rate of acute URTIs
Twelve trials reported the total number of episodes of acute URTIs or the incidence of acute URTIs (Andaloro 2019; Berggren 2011; Butler 2020; Caceres 2010; Damholt 2022; Fujita 2013; Langkamp‐Henken 2015; Lazou Ahrén 2021; Merenstein 2010; Pu 2017; Rio 2002; Santamaria 2019). To perform group comparisons, we calculated the incidence ratio of episode rates (events per person/year) of acute URTIs between the probiotic and placebo or no treatment groups, and the standard error (SE) of the rate ratio according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021). There were 2320 participants in the probiotics group and 2044 participants in the placebo or no treatment group.
Age
Four studies were conducted in an adult population (Berggren 2011; Langkamp‐Henken 2015; Lazou Ahrén 2021; Pu 2017). The results showed that the probiotics intervention was better than placebo or no treatment (rate ratio 0.79, 95% CI 0.71 to 0.89; P < 0.001; 2032 participants; Analysis 1.9). Six studies were conducted in children, and showed that the probiotics intervention was better than placebo (rate ratio 0.79, 95% CI 0.65 to 0.96; P = 0.02; 1868 participants; Analysis 1.9) (Andaloro 2019; Caceres 2010; Damholt 2022; Merenstein 2010; Rio 2002; Santamaria 2019). Two studies were conducted in older people, and showed no association between probiotic intervention and incidence rate of acute URTIs (rate ratio 1.04, 95% CI 0.63 to 1.71; P = 0.88; 464 participants; Analysis 1.9) (Butler 2020; Fujita 2013).
1.9. Analysis.

Comparison 1: Intention‐to‐treat analysis: probiotics versus placebo or no treatment ‐ primary outcome measures, Outcome 9: The incidence rate of acute URTIs (age)
Treatment dose
Seven trials administered the probiotics less than 1010 CFU/day, and showed that probiotics reduced the incidence rate of acute URTIs (rate ratio 0.77, 95% CI 0.65 to 0.91; P = 0.002; 2631 participants; Analysis 1.10) (Andaloro 2019; Berggren 2011; Damholt 2022; Langkamp‐Henken 2015; Lazou Ahrén 2021; Rio 2002; Santamaria 2019). Five trials administered the probiotics more than 1010 CFU/day, and showed that probiotics did not reduce the incidence rate of acute URTIs (rate ratio 0.91, 95% CI 0.75 to 1.11; P = 0.34; 1733 participants; Analysis 1.10) (Butler 2020; Caceres 2010; Fujita 2013; Merenstein 2010; Pu 2017).
1.10. Analysis.

Comparison 1: Intention‐to‐treat analysis: probiotics versus placebo or no treatment ‐ primary outcome measures, Outcome 10: The incidence rate of acute URTIs (treatment dose)
Treatment duration
Ten trials were conducted for three to six months, and showed that antibiotics reduced the incidence rate of acute URTIs (rate ratio 0.79, 95% CI 0.70 to 0.90; P < 0.001; 3900 participants; Analysis 1.11) (Andaloro 2019; Berggren 2011; Caceres 2010; Damholt 2022; Langkamp‐Henken 2015; Lazou Ahrén 2021; Merenstein 2010; Pu 2017; Rio 2002; Santamaria 2019). Two trials were conducted for more than six months, and showed that probiotics were not associated with incidence rate of acute URTIs (rate ratio 1.04, 95% CI 0.63 to 1.71; P = 0.88; 464 participants; Analysis 1.11) (Butler 2020; Fujita 2013).
1.11. Analysis.

Comparison 1: Intention‐to‐treat analysis: probiotics versus placebo or no treatment ‐ primary outcome measures, Outcome 11: The incidence rate of acute URTIs (treatment duration)
Comparator
Eleven placebo‐controlled trials showed that probiotics were better than placebo (rate ratio 0.83, 95% CI 0.73 to 0.94; P = 0.003; 4131 participants; Analysis 1.12) (Andaloro 2019; Berggren 2011; Butler 2020; Caceres 2010; Damholt 2022; Fujita 2013; Langkamp‐Henken 2015; Lazou Ahrén 2021; Merenstein 2010; Rio 2002; Santamaria 2019), and one trial using no treatment as the comparator did not find an association between probiotics and incidence rate of acute URTIs (rate ratio 0.70, 95% CI 0.47 to 1.04; P = 0.08; 233 participants; Analysis 1.12) (Pu 2017).
1.12. Analysis.

Comparison 1: Intention‐to‐treat analysis: probiotics versus placebo or no treatment ‐ primary outcome measures, Outcome 12: The incidence rate of acute URTIs (type of comparator)
Overall pooled results
Pooled analyses showed that across these trials, probiotics decreased the incidence rate of acute URTIs (rate ratio 0.82, 95% CI 0.73 to 0.92; P = 0.001; 4364 participants; Analysis 1.9; Analysis 1.10; Analysis 1.11; Analysis 1.12). The contour‐enhanced funnel plot for the pooled analysis suggested that some studies with a lower incidence rate of URTIs in the intervention group might be missing (Figure 6). The level of heterogeneity between these trials was high (Chi2 = 36.40; df = 11, P = 0.001; I2 = 70%; Analysis 1.9; Analysis 1.10; Analysis 1.11; Analysis 1.12). We downgraded the certainty of the evidence for this outcome from high to low due to study limitations and publication bias.
6.

Contour‐enhanced funnel plot of comparison: intention‐to‐treat analysis: probiotics versus placebo ‐ primary outcome measures, outcome: the incidence rate of acute URTIs.
3. Mean duration of an episode of acute URTIs
Six trials reported the mean duration of an episode of acute URTIs (Fujita 2013; Langkamp‐Henken 2015; Lazou Ahrén 2021; Shida 2017; Smith 2013; Vrese 2005). There were 1343 participants in the probiotics group and 1063 participants in the placebo or no treatment group.
Age
In five trials conducted in adults, the results showed that the probiotics intervention was better than placebo (MD −1.14, 95% CI −2.15 to −0.13; P = 0.03; 2252 participants; Analysis 1.13) (Langkamp‐Henken 2015; Lazou Ahrén 2021; Shida 2017; Smith 2013; Vrese 2005). One study included older people (the mean age was 83 years old), and showed that probiotics were better than placebo (MD −1.69, 95% CI −2.75 to −0.63; P = 0.002; 154 participants; Analysis 1.13) (Fujita 2013).
1.13. Analysis.

Comparison 1: Intention‐to‐treat analysis: probiotics versus placebo or no treatment ‐ primary outcome measures, Outcome 13: The mean duration of an episode of acute URTIs (age)
Treatment dose
Four trials used a dose of less than 1010 CFU/day, and showed that probiotics were not associated with the mean duration of an episode of acute URTIs (MD −0.89, 95% CI −2.09 to 0.31; P = 0.14; 2152 participants; Analysis 1.14) (Langkamp‐Henken 2015; Lazou Ahrén 2021; Smith 2013; Vrese 2005). Two trials used a dose of more than 1010 CFU/day, and showed that probiotics were better in reducing the mean duration of an episode of acute URTIs (MD −2.01, 95% CI −2.66 to −1.36; P < 0.001; 254 participants; Analysis 1.14) (Fujita 2013; Shida 2017).
1.14. Analysis.

Comparison 1: Intention‐to‐treat analysis: probiotics versus placebo or no treatment ‐ primary outcome measures, Outcome 14: The mean duration of an episode of acute URTIs (treatment dose)
Treatment duration
Four trials were conducted for three to six months, and showed that antibiotics did not affect the mean duration of an episode of acute URTIs (MD −0.90, 95% CI −1.86 to 0.05; P = 0.06; 1773 participants; Analysis 1.15) (Langkamp‐Henken 2015; Lazou Ahrén 2021; Shida 2017; Smith 2013). Two trials were conducted for more than six months, and showed that antibiotics reduce the mean duration of an episode of acute URTIs (MD −1.90, 95% CI −2.04 to −1.76; P < 0.001; 633 participants; Analysis 1.15) (Fujita 2013; Vrese 2005).
1.15. Analysis.

Comparison 1: Intention‐to‐treat analysis: probiotics versus placebo or no treatment ‐ primary outcome measures, Outcome 15: The mean duration of an episode of acute URTIs (treatment duration)
Overall pooled results
Pooled analyses showed that the MD of an episode of acute URTIs after probiotic treatment decreased (MD −1.22, 95% CI −2.12 to −0.33; P = 0.007; 6 trials, 2406 participants; Analysis 1.13, Analysis 1.14; Analysis 1.15). The level of heterogeneity in terms of the MD of an episode of acute URTIs was high (Chi2 = 75.60; df = 5, P < 0.001; I2 = 94%). We downgraded the certainty of the evidence for this outcome from high to low due to study limitations and high heterogeneity.
See Table 1 for an overall assessment of the primary outcomes.
Secondary outcomes
1. Number of participants who were absent from childcare centre, school, or work
One trial reported the number of participants who experienced cold‐related school absence during the follow‐up period (Rerksuppaphol 2012). This study involved 40 participants in the probiotics group and 40 participants in the placebo group. There were 14 participants in the placebo group who experienced cold‐related absence, whereas there were only two in the probiotics group. The results showed that probiotics decreased the number of participants who were absent from childcare centre, school, or work (RR 0.14, 95% CI 0.03 to 0.59; 1 study, 80 participants; Analysis 2.1). None of the included trials reported time off from childcare centres or work for acute URTIs. We downgraded the certainty of the evidence for this outcome from high to very low due to study limitations, imprecision, and indirectness.
2.1. Analysis.

Comparison 2: Intention‐to‐treat analysis: probiotics versus placebo or no treatment ‐ time off from childcare centre, school, or work, Outcome 1: The number of participants who were absent from childcare centre, school, or work due to acute URTIs
2. Number of participants who used antibiotics for acute URTIs
Six trials reported the number of participants who used prescription antibiotics for acute URTIs (Hojsak 2010a; Hojsak 2010b; Lazou Ahrén 2020; Pu 2017; Rautava 2009; Rerksuppaphol 2012). One trial was a two‐stage study reporting the number of participants using antibiotics (Rautava 2009). There were 771 participants in the probiotics group and 777 participants in the placebo or no treatment group.
Age
In five trials conducted in children, the results showed that the probiotics intervention was better than placebo or no treatment (RR 0.59, 95% CI 0.43 to 0.83, P = 0.002; 1315 participants; Analysis 3.1) (Hojsak 2010a; Hojsak 2010b; Lazou Ahrén 2020; Rautava 2009; Rerksuppaphol 2012). One study included an adult population, and showed that probiotics did not decrease the number of participants who used prescribed antibiotics for acute URTIs (RR 0.09, 95% CI 0.01 to 1.67; P = 0.11; 233 participants; Analysis 3.1) (Pu 2017).
3.1. Analysis.

Comparison 3: Intention‐to‐treat analysis: probiotics versus placebo or no treatment ‐ prescribed antibiotics for acute URTIs, Outcome 1: The number of participants who used antibiotics for acute URTIs (age)
Treatment dose
Four trials used a dose of less than 1010 CFU/day, and the results showed that the probiotics reduced the number of participants who used prescribed antibiotics for acute URTIs (RR 0.65, 95% CI 0.42 to 0.99; P = 0.05; 1234 participants; Analysis 3.2) (Hojsak 2010a; Hojsak 2010b; Lazou Ahrén 2020; Rerksuppaphol 2012). Two trials used a dose of less than 1010 CFU/day, and showed no difference between probiotics and placebo or no treatment in reducing the number of participants who used prescribed antibiotics for acute URTIs (RR 0.38, 95% CI 0.09 to 1.52; P = 0.17; 314 participants; Analysis 3.2) (Pu 2017; Rautava 2009).
3.2. Analysis.

Comparison 3: Intention‐to‐treat analysis: probiotics versus placebo or no treatment ‐ prescribed antibiotics for acute URTIs, Outcome 2: The number of participants who used antibiotics for acute URTIs (treatment dose)
Treatment duration
One trial was conducted for less than three months (Hojsak 2010a), four trials for three to six months (Hojsak 2010b; Lazou Ahrén 2020; Pu 2017; Rerksuppaphol 2012), and one trial for more than six months (Rautava 2009). Benefits of probiotics in decreasing in the number of participants who used prescribed antibiotics for acute URTIs were found for the treatment duration more than six months (less than three months: RR 0.68, 95% CI 0.42 to 1.11; P = 0.12; 281 participants; three to six months: RR 0.46, 95% CI 0.20 to 1.10; P = 0.08; 1186 participants; more than six months: RR 0.52, 95% CI 0.31 to 0.88; P = 0.02; 81 participants; Analysis 3.3).
3.3. Analysis.

Comparison 3: Intention‐to‐treat analysis: probiotics versus placebo or no treatment ‐ prescribed antibiotics for acute URTIs, Outcome 3: The number of participants who used antibiotics for acute URTIs (treatment duration)
Comparator
Five placebo‐controlled trials showed that probiotic interventions reduced the number of participants who used prescribed antibiotics for acute URTIs (RR 0.59, 95% CI 0.43 to 0.83; P = 0.02; 1315 participants; Analysis 3.4). One trial using no treatment as the comparator did not find an association between the probiotic intervention and the number of participants who used prescribed antibiotics for acute URTIs (Pu 2017) (RR 0.09, 95% CI 0.01 to 1.67; P = 0.11; 233 participants; Analysis 3.4).
3.4. Analysis.

Comparison 3: Intention‐to‐treat analysis: probiotics versus placebo or no treatment ‐ prescribed antibiotics for acute URTIs, Outcome 4: The number of participants who used antibiotics for acute URTIs (type of comparator)
Overall pooled results
Pooled analyses showed that probiotic treatment reduced the number of participants who used prescribed antibiotics for acute URTIs (RR 0.58, 95% CI 0.42 to 0.81; P = 0.001; 1548 participants; Analysis 3.1; Analysis 3.2; Analysis 3.3; Analysis 3.4). We found no heterogeneity (Chi2 = 3.36; df = 5, P = 0.64; I2 = 0%; Analysis 3.1; Analysis 3.2; Analysis 3.3; Analysis 3.4). This indicates that the numbers of participants using antibiotics and infections requiring antibiotic prescriptions were lower in the probiotics group than in the placebo or no treatment group. We downgraded the certainty of the evidence for this outcome from high to moderate due to study limitations.
3. Number of participants who experienced at least one side effect or adverse event of probiotics
Most of the included trials reported that side effects or adverse events from the intervention were minor. One study described the main adverse effects as gastrointestinal symptoms such as vomiting, flatulence, and increased irritability (Rautava 2009). The probiotics used in the study were Lactobacillus rhamnosus and Bifidobacterium lactis Bb‐12. Eight trials reported side effects including diarrhoea, vomiting, bowel pain, loose stools, flatulence, nausea, etc. (Andaloro 2019; Berggren 2011; Lazou Ahrén 2020; Lazou Ahrén 2021; Merenstein 2010; Rerksuppaphol 2012; Smith 2013; Taipale 2016). There were 1222 participants in the probiotics group and 1234 participants in the placebo or no treatment group.
Age
Three studies included adults (Berggren 2011; Lazou Ahrén 2021; Smith 2013), and five trials included children (Andaloro 2019; Lazou Ahrén 2020; Merenstein 2010; Rerksuppaphol 2012; Taipale 2016). We found no associations between probiotics and the number of participants who experienced at least one side effect or adverse event in either adults (RR 1.03, 95% CI 0.89 to 1.19; P = 0.71; 1414 participants; Analysis 4.1) or children (RR 0.99, 95% CI 0.71 to 1.37; P = 0.93; 1042 participants; Analysis 4.1).
4.1. Analysis.

Comparison 4: Intention‐to‐treat analysis: probiotics versus placebo or no treatment ‐ adverse events, Outcome 1: The number of participants who experienced at least 1 adverse event (age)
Treatment dose
Six trials used a dose of probiotics of less than 1010 CFU/day (Andaloro 2019; Berggren 2011; Lazou Ahrén 2020; Lazou Ahrén 2021; Rerksuppaphol 2012; Smith 2013), and two trials used a dose of more than 1010 CFU/day (Merenstein 2010; Taipale 2016). We found no associations between probiotics and the number of participants who experienced at least one side effect or adverse event for either the lower dose (RR 1.02, 95% CI 0.90 to 1.16; P = 0.74; 1709 participants; Analysis 4.2) or the higher dose of probiotics (RR 1.46, 95% CI 0.23 to 9.09; P = 0.69; 747 participants; Analysis 4.2).
4.2. Analysis.

Comparison 4: Intention‐to‐treat analysis: probiotics versus placebo or no treatment ‐ adverse events, Outcome 2: The number of participants who experienced at least 1 adverse event (treatment dose)
Treatment duration
Six trials were conducted for three to six months (Berggren 2011; Lazou Ahrén 2020; Lazou Ahrén 2021; Merenstein 2010; Rerksuppaphol 2012; Smith 2013), and two trials were conducted for more than six months (Andaloro 2019; Taipale 2016). Neither result differed between the probiotics group and the placebo or no treatment group in number of participants who experienced at least one side effect or adverse event of probiotics (three to six months: RR 1.01, 95% CI 0.89 to 1.15; P = 0.88; 2263 participants; more than six months: RR 4.06, 95% CI 0.69 to 23.88; P = 0.12; 193 participants; Analysis 4.3).
4.3. Analysis.

Comparison 4: Intention‐to‐treat analysis: probiotics versus placebo or no treatment ‐ adverse events, Outcome 3: The number of participants who experienced at least 1 adverse event (treatment duration)
Overall pooled results
Pooled analyses showed that there was no difference in side effects between the probiotics group and the placebo or no treatment group (RR 1.02, 95% CI 0.90 to 1.15; P = 0.79; 2456 participants; Analysis 4.1; Analysis 4.2; Analysis 4.3). We found no heterogeneity (Chi2 = 5.93; df = 7, P = 0.55; I2 = 0%; Analysis 4.1; Analysis 4.2; Analysis 4.3). We downgraded the certainty of the evidence for this outcome from high to low due to study limitations and imprecision.
Sensitivity analysis
We conducted sensitivity analyses by excluding trials at high risk of bias and cluster‐RCTs. The results did not change.
Per‐protocol analysis
We also conducted per‐protocol analyses, and these did not change the inference of the original analyses. See Analysis 5.1; Analysis 5.2; Analysis 5.3; Analysis 5.4; Analysis 5.5; Analysis 5.6; Analysis 5.7; Analysis 5.8; Analysis 5.9; Analysis 5.10; Analysis 5.11; Analysis 5.12; Analysis 5.13; Analysis 5.14; Analysis 5.15; Analysis 6.1; Analysis 7.1; Analysis 7.2; Analysis 7.3; Analysis 7.4; Analysis 8.1; Analysis 8.2; Analysis 8.3.
5.1. Analysis.

Comparison 5: Per‐protocol analysis: probiotics versus placebo or no treatment ‐ primary outcome measures, Outcome 1: The number of participants who were diagnosed with acute URTIs: at least 1 event (age)
5.2. Analysis.

Comparison 5: Per‐protocol analysis: probiotics versus placebo or no treatment ‐ primary outcome measures, Outcome 2: The number of participants who were diagnosed with acute URTIs: at least 1 event (treatment dose)
5.3. Analysis.

Comparison 5: Per‐protocol analysis: probiotics versus placebo or no treatment ‐ primary outcome measures, Outcome 3: The number of participants who were diagnosed with acute URTIs: at least 1 event (treatment duration)
5.4. Analysis.

Comparison 5: Per‐protocol analysis: probiotics versus placebo or no treatment ‐ primary outcome measures, Outcome 4: The number of participants who were diagnosed with acute URTIs: at least 1 event (type of comparator)
5.5. Analysis.

Comparison 5: Per‐protocol analysis: probiotics versus placebo or no treatment ‐ primary outcome measures, Outcome 5: The number of participants who were diagnosed with acute URTIs: at least 3 events (age)
5.6. Analysis.

Comparison 5: Per‐protocol analysis: probiotics versus placebo or no treatment ‐ primary outcome measures, Outcome 6: The number of participants who were diagnosed with acute URTIs: at least 3 events (treatment dose)
5.7. Analysis.

Comparison 5: Per‐protocol analysis: probiotics versus placebo or no treatment ‐ primary outcome measures, Outcome 7: The number of participants who were diagnosed with acute URTIs: at least 3 events (treatment duration)
5.8. Analysis.

Comparison 5: Per‐protocol analysis: probiotics versus placebo or no treatment ‐ primary outcome measures, Outcome 8: The number of participants who were diagnosed with acute URTIs: at least 3 events (type of comparator)
5.9. Analysis.

Comparison 5: Per‐protocol analysis: probiotics versus placebo or no treatment ‐ primary outcome measures, Outcome 9: The incidence rate of acute URTIs (age)
5.10. Analysis.

Comparison 5: Per‐protocol analysis: probiotics versus placebo or no treatment ‐ primary outcome measures, Outcome 10: The incidence rate of acute URTIs (treatment dose)
5.11. Analysis.

Comparison 5: Per‐protocol analysis: probiotics versus placebo or no treatment ‐ primary outcome measures, Outcome 11: The incidence rate of acute URTIs (treatment duration)
5.12. Analysis.

Comparison 5: Per‐protocol analysis: probiotics versus placebo or no treatment ‐ primary outcome measures, Outcome 12: The incidence rate of acute URTIs (type of comparator)
5.13. Analysis.

Comparison 5: Per‐protocol analysis: probiotics versus placebo or no treatment ‐ primary outcome measures, Outcome 13: The mean duration of an episode of acute URTIs (age)
5.14. Analysis.

Comparison 5: Per‐protocol analysis: probiotics versus placebo or no treatment ‐ primary outcome measures, Outcome 14: The mean duration of an episode of acute URTIs (treatment dose)
5.15. Analysis.

Comparison 5: Per‐protocol analysis: probiotics versus placebo or no treatment ‐ primary outcome measures, Outcome 15: The mean duration of an episode of acute URTIs (treatment duration)
6.1. Analysis.

Comparison 6: Per‐protocol analysis: probiotics versus placebo or no treatment ‐ time off from childcare centre, school, or work, Outcome 1: The number of participants who experienced time off from childcare centre, school, or work due to acute URTIs
7.1. Analysis.

Comparison 7: Per‐protocol analysis: probiotics versus placebo or no treatment ‐ prescribed antibiotics for acute URTIs, Outcome 1: The number of participants who used antibiotics for acute URTIs (age)
7.2. Analysis.

Comparison 7: Per‐protocol analysis: probiotics versus placebo or no treatment ‐ prescribed antibiotics for acute URTIs, Outcome 2: The number of participants who used antibiotics for acute URTIs (treatment dose)
7.3. Analysis.

Comparison 7: Per‐protocol analysis: probiotics versus placebo or no treatment ‐ prescribed antibiotics for acute URTIs, Outcome 3: The number of participants who used antibiotics for acute URTIs (treatment duration)
7.4. Analysis.

Comparison 7: Per‐protocol analysis: probiotics versus placebo or no treatment ‐ prescribed antibiotics for acute URTIs, Outcome 4: The number of participants who used antibiotics for acute URTIs (type of comparator)
8.1. Analysis.

Comparison 8: Per‐protocol analysis: probiotics versus placebo or no treatment ‐ adverse events, Outcome 1: The number of participants who experienced at least 1 adverse event (age)
8.2. Analysis.

Comparison 8: Per‐protocol analysis: probiotics versus placebo or no treatment ‐ adverse events, Outcome 2: The number of participants who experienced at least 1 adverse event (treatment dose)
8.3. Analysis.

Comparison 8: Per‐protocol analysis: probiotics versus placebo or no treatment ‐ adverse events, Outcome 3: The number of participants who experienced at least 1 adverse event (treatment duration)
Discussion
Summary of main results
In this review, we found that probiotics may reduce the number of participants who were diagnosed with URTIs (at least one event); likely reduce the number of participants diagnosed with URTIs (at least three events); may reduce the incidence rate of acute URTIs; may reduce the mean duration of an episode of acute URTIs; likely reduce the number of participants who used antibiotics for URTIs; and may not increase the number of participants who experienced at least one adverse event. Adverse events were minor. Evidence showing a decrease in the number of people absent from childcare centre, school, or work due to acute URTIs with probiotics is very uncertain. However, these results must be interpreted with caution because the included outcomes were unsatisfactory and susceptible to bias given that some of them were extracted from only one or two trials, and in some subgroups the level of heterogeneity between pooled trials was substantial. In addition, some trials had small sample sizes and limitations in their methods. Furthermore, the outcomes prespecified as most important in this review were not the main outcomes in some of the original trials.
Overall completeness and applicability of evidence
Probiotics for acute URTIs in children
The majority of the trials included in this review were conducted in children (Andaloro 2019; Caceres 2010; Damholt 2022; Hojsak 2010a; Hojsak 2010b; Kara 2019; Laursen 2017; Lazou Ahrén 2020; Merenstein 2010; Rautava 2009; Rerksuppaphol 2012; Rio 2002; Santamaria 2019; Taipale 2016). We analysed subgroups according to different ages of participants and found that probiotics showed a benefit in reducing the number of children who experienced URTIs episodes (at least one event), the incidence rate of acute URTIs, and the number of participants who used antibiotics for acute URTIs. In most trials the probiotics were given in milk‐based food, such as yoghurt, for three months or more.
A double‐blind, placebo‐controlled RCT, conducted in 18 municipal daycare centres in similar socioeconomic areas in north, west, and northeast Helsinki, found that Lactobacillusrhamnosus GG milk may reduce the rate and severity of respiratory infections and antibiotic treatment amongst children in daycare centres (Hatakka 2001). One study included 309 otitis‐prone children (with at least four episodes of acute otitis media) (Hatakka 2007). We included Hatakka 2007 in the previous version of the review, but after reassessing it in this update, we excluded it because otitis‐prone children may have immunodeficiency. In Hatakka 2007, the trial authors also found that probiotics did not prevent the occurrence of acute otitis media or the nasopharyngeal carriage of otitis pathogens in otitis‐prone children.
Probiotics for acute URTIs in adults
Six studies were conducted in adults (Berggren 2011; Langkamp‐Henken 2015; Pu 2017; Shida 2017; Smith 2013; Vrese 2005). Subgroup analysis showed that probiotics were associated with a reduction in the number of adults who experienced URTIs episodes (only at least one event), the incidence rate of acute URTIs, and the mean duration of an episode of URTIs. However, we did not find that probiotics were beneficial in reducing the number of participants who needed a prescription for antibiotics. In most trials two or three strains of probiotics were given through powder‐like food.
Probiotics for acute URTIs in the elderly
Infections often occur in the elderly as the immune system weakens with age (Valente 2009). As such, it is very important to compare the treatment effect in older people. To date, we have only found five trials that compared probiotics to placebo in older people (Butler 2020; Fujita 2013; Guillemard 2010; Makino 2010a; Turchet 2003). One study was a unicentric, randomised, stratified, open pilot study, in which 360 community residents over 60 years of age were randomised to receive (a) one 100‐millilitre bottle of Actimel (milk fermented with yoghurt cultures and Lactobacillus casei DN‐114 001, containing 108 CFU/mL L casei DN‐114 001) twice daily for three weeks, or (b) control (Turchet 2003). The study found no difference in the incidence of infections during the winter between groups. However, the study authors found that the duration of all pathologies and maximum temperature were lower in the treatment group than in the control group. The other study was also a multicentre, double‐blind controlled trial, involving 1072 participants (median age 76 years) randomised to either probiotic strain L casei DN‐114 001 or placebo for three months (Guillemard 2010). The probiotic group was associated with a decreased duration of common infectious diseases in comparison to the placebo, especially URTIs.
In our Criteria for considering studies for this review, we included studies with participants who had been vaccinated against influenza or other acute URTIs within the last 12 months, if the percentage of participants was less than 50%, and there was no difference between groups. Eighty‐two per cent of participants in one study had been vaccinated against influenza three months before the study (Turchet 2003). In addition, the study did not separate acute URTIs from other infections commonly experienced in winter. Another study included participants who were vaccinated against influenza prior to receiving the intervention (Guillemard 2010). We therefore decided to exclude these two studies.
One included study contained reports from two trials: the Funagata study and the Arita study (Makino 2010a). The Arita study was not an RCT, so we excluded Makino 2010b. However, the Funagata study had no available data that could be extracted to conduct a meta‐analysis. The study reported that the risk of catching a common cold or influenza was about 3.4 times lower in the probiotic group than in the placebo group.
In this 2022 review, we only included two studies considering the effect of probiotics amongst older people (Butler 2020; Fujita 2013). The probiotics were given with milk‐based food or in capsules, for more than six months. The results showed that probiotics did not reduce the incidence rate of acute URTIs, but did reduce the duration of acute URTIs in older people. More trials are needed in elderly populations.
Intervention dose and duration
In this review, we conducted subgroup analyses according to the intervention dose and duration. For the intervention dose, we found that a lower dose of probiotics (less than 1010 CFU/day) showed a benefit in reducing the number of participants who experienced URTIs episodes (at least one event and at least three events), the incidence rate of acute URTIs, and the number of participants who used antibiotics for acute URTIs, whilst a higher dose of probiotics (more than 1010 CFU/day) showed a benefit in reducing the number of participants who experienced URTIs episodes (at least one event) and the mean duration of an episode of URTIs. No associations between probiotics and the number of participants who experienced at least one side effect or adverse event were found with either a lower dose (less than 1010 CFU/day) or a higher dose of probiotics (more than 1010 CFU/day). Regarding intervention duration, we found that a shorter intervention duration (less than three months) of probiotics was associated with a reduction in the number of participants diagnosed with acute URTIs (at least one event); a moderate intervention duration (three to six months) was associated with a reduction in the number of participants who experienced URTIs episodes (at least one event and at least three events) and the incidence rate of acute URTIs; and a longer intervention duration (more than six months) was associated with a reduction in the mean duration of an episode of URTIs and the number of participants who used antibiotics for acute URTIs. Neither moderate nor longer intervention duration of probiotics was associated with the number of participants who experienced at least one side effect or adverse event.
Type of comparator
Two trials used no treatment as the comparison group (Kara 2019; Pu 2017). Subgroup analysis according to type of comparator found that probiotics reduced the number of participants who experienced URTIs episodes (both at least one event and at least three events), the incidence rate of acute URTIs, and the number of participants who used antibiotics for acute URTIs in the placebo‐controlled trials, and the number of participants who experienced URTIs episodes (at least one event) in the trials using no treatment group as the control. The results of the placebo‐controlled studies were consistent with the pooled analysis.
Clinical interpretation of the data
The analyses showed that probiotics may reduce the number of participants who were diagnosed with URTIs (at least one event); likely reduce the number of participants diagnosed with URTIs (at least three events); may reduce the incidence rate of acute URTIs; may reduce the mean duration of an episode of acute URTIs; likely reduce the number of participants who used antibiotics for URTIs; and may not increase the number of participants who experienced at least one adverse event. Evidence showing a decrease in the number of people absent from childcare centre, school, or work due to acute URTIs with probiotics is very uncertain. The primary outcome of mean duration of an episode of acute URTIs was based on only one or two trials in the elderly and children subgroups. We found only one study that reported school absenteeism due to the common cold; more trials are needed to measure this outcome (Rerksuppaphol 2012). There were insufficient data for adults and older people in our review. In addition, different ages and settings of participants, types of probiotics, and follow‐up periods were used in the trials, so that heterogeneity in some outcomes could not be avoided. We thus could not conclude a clear intervention dose or duration of probiotics to prevent acute URTIs. Most of trials included in this review used 109 or 1011 CFU/day of probiotics for more than three months, which may indicate that people should take at least 109 CFU/day of probiotics for three months to experience these benefits. According to the included trials, probiotics are safe and adverse effects are minor. The major adverse events of probiotics were gastrointestinal symptoms such as diarrhoea, vomiting, flatulence, and increased irritability. The limited results showed that probiotic therapy probably provides more benefits than placebo or no treatment in terms of decreasing the number of participants who were diagnosed with acute URTIs (at least three events) and the number of participants who were prescribed antibiotics for acute URTIs; and may provide more benefits in terms of decreasing the number of participants who were diagnosed with acute URTIs (at least one event), the episodes of infection, and the duration of an episode of acute URTIs.
Certainty of the evidence
Limitations of the included trials
Allocation concealment was only described in seven individual RCTs (Damholt 2022; Fujita 2013; Langkamp‐Henken 2015; Laursen 2017; Lazou Ahrén 2020; Lazou Ahrén 2021; Taipale 2016). Double‐blinding was reported in 17 trials, and details of the blinding methods were reported in 11 trials. However, four trials did not report the type of blinding, and one trial was unblinded to participants and outcome assessors. All of this could potentially have biased the results in favour of treatment (Figure 3).
After assessment of the overall certainty of the evidence, we downgraded the certainty of the evidence for our primary outcomes from high to moderate or low, usually due to study limitations, inconsistency, imprecision, indirectness, or publication bias. The results of trials were more likely to be positive if the trials were funded by industry or if the authors had a financial conflict of interest.
Potential biases in the review process
We included 24 studies in this 2022 review, 23 of which were used to extract data for meta‐analysis. The limited number of included studies may introduce potential bias. Meanwhile, we excluded some studies that simply reported respiratory tract infection without specifying whether it was lower or upper respiratory tract infection. The effects of probiotics may be underestimated or overestimated, as we may have missed some related studies. However, we attempted to identify all relevant studies, and performed analyses based on both intention‐to‐treat and per‐protocol populations. These would be helpful in reducing potential bias in the review process.
Agreements and disagreements with other studies or reviews
One systematic review was conducted to investigate probiotics supplementation effects on respiratory tract infections in children attending day care (Laursen 2018). That review suggested low‐certainty evidence for probiotics preventing the number of children with acute URTIs based on five trials. However, Laursen 2018 included another three trials with insufficient information to assess the number of patients with acute URTIs.
Another systematic review focused on probiotics supplementation for preventing URTIs amongst adults (Li 2020). The authors included six RCTs and found that probiotics reduce the incidence of URTIs episodes and the mean duration of URTIs compared with placebo. However, the results were based on per‐protocol analysis, which may overestimate the effect of probiotics.
We excluded trials performed on athletes, as the excessive training may have influenced the effect of the probiotics or immune system (Greenham 2018). A systematic review was conducted to evaluate probiotics supplementation's effectiveness on athletes' URTIs (Łagowska 2021). The authors found that probiotics supplementation had no effect on the duration of URTIs episodes, but had an effect on total symptom severity score. We also identified one study conducted amongst older people that found that probiotics only reduced the duration of acute URTIs, rather than the number of participants who experienced URTIs episodes (Fujita 2013). Another study found that probiotics did not reduce the rate ratio of episodes of URTIs in older adults (Butler 2020). We have not found any other systematic reviews that conflict with this review to date. However, there are systematic reviews that focus on critically ill patients. Based on the current evidence, probiotics could not only reduce hospital mortality, but also reduce the incidence of intensive care unit (ICU)‐acquired pneumonia, and were associated with a shorter ICU stay (Batra 2020).
Authors' conclusions
Implications for practice.
When compared to placebo or no treatment, probiotics may reduce the number of participants diagnosed with acute upper respiratory tract infections (URTIs) (at least one event) (low‐certainty evidence); likely reduce the number of participants diagnosed with URTIs (at least three events) (moderate‐certainty evidence); may reduce the incidence rate of acute URTIs (low‐certainty evidence); may reduce the mean duration of an episode of acute URTIs (low‐certainty evidence); likely reduce the number of participants who used antibiotics for URTIs (moderate‐certainty evidence); and may not increase the number of participants who experienced at least one adverse event (low‐certainty evidence). The evidence regarding the number of participants who were absent from childcare centre, school, or work due to acute URTIs is very uncertain.
Implications for research.
Future randomised controlled trials should consider:
a study design that incorporates adequate blinding and concealment of the allocation sequence;
assessment of common outcomes (e.g. the number of episodes of acute URTIs and the mean duration of an episode of acute URTIs should be primary outcome measures);
focusing on older people or performing a subgroup analysis of older people;
adverse event outcomes: time off from childcare centre, school, or work; and
cost‐effectiveness and quality of life.
Additionally, studies should not be influenced by funds from manufacturers of the tested probiotics.
What's new
| Date | Event | Description |
|---|---|---|
| 10 May 2022 | New citation required but conclusions have not changed | Our conclusions remain unchanged. |
| 10 May 2022 | New search has been performed | We included 16 new trials in this update (Andaloro 2019; Butler 2020; Damholt 2022; Kara 2019; Langkamp‐Henken 2015; Laursen 2017; Lazou Ahrén 2020; Lazou Ahrén 2021; Pu 2017; Santamaria 2019; Shida 2017; Taipale 2016; Little 2017; Maya‐Barrios 2021; Murata 2018; Nocerino 2017), and excluded 27 new trials (Altadill 2021; Anaya‐Loyola 2019; Aryayev 2018; Campanella 2018; Corsello 2017; Di Pierro 2021; Dziechciarz 2020; Gerasimov 2016; Hishiki 2020; Hojsak 2015; Hojsak 2016; Hor 2018; Kosek 2019; Lau 2018; Lefevre 2015; Li 2014; Mai 2021; Maldonado‐Lobón 2015; Manti 2020; Meng 2016; Michael 2020; Mullish 2021; Ringel‐Kulka 2015; Sanz 2006; Stojković 2016; Turner 2017; Zhang 2018). |
History
Protocol first published: Issue 1, 2008 Review first published: Issue 9, 2011
| Date | Event | Description |
|---|---|---|
| 10 May 2009 | Amended | Contact details updated. |
| 17 May 2008 | Amended | Converted to new review format |
Notes
In the next update of this review, we will include a subgroup to assess the effects of different probiotics on acute URTIs.
Acknowledgements
The following people conducted the editorial process for this review.
Sign‐off Editors (final editorial decision): Mark Jones (Bond University, Australia); Mieke van Driel (The University of Queensland, Australia).
Managing Editors (provided editorial guidance to authors, edited the review, selected peer reviewers, collated peer reviewer comments): Liz Dooley (Bond University, Australia); Fiona Russell (Bond University, Australia).
Contact Editor (assessed peer review comments and recommended an editorial decision): Roger Damoiseaux (Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht University, Utrecht, Netherlands).
Statistical Editor (provided comments): Teresa Neeman, PhD statistics, AStat (Biology Data Science Institute, Australian National University).
Copy Editor (copy‐editing and production): Lisa Winer, Cochrane Copy Edit Support.
Peer reviewers (provided comments and recommended an editorial decision) were as follows.
Clinical/content review: Iva Hojsak (Children's Hospital Zagreb, Croatia).
Consumer review: Ann E Fonfa (Annie Appleseed Project).
Methods review: has requested to remain anonymous.
Search review: Justin Clark (Institute for Evidence‐Based Healthcare, Bond University, Australia).
The authors wish to thank Janet Wale, Shilpa Amin, Simone Guglielmetti, Michael de Vrese, Karin Stockert, Nelcy Rodriguez, and the Chinese Cochrane Center for commenting on previous versions of this review. The authors also want to thank Dr Zhenchan Lu and Dr Changquan Huang for their co‐authoring contributions to earlier versions of this review.
Appendices
Appendix 1. MEDLINE (Ovid) search strategy
1 Common Cold/ 2 common cold*.tw. 3 exp Sinusitis/ 4 sinusit*.tw. 5 Pharyngitis/ 6 pharyngit*.tw. 7 exp Laryngitis/ 8 laryngit*.tw. 9 laryngotracheobronchit*.tw. 10 Rhinitis/ 11 rhinit*.tw. 12 Tonsillitis/ 13 tonsillit*.tw. 14 peritonsillar abscess*.tw. 15 Croup/ 16 croup*.tw. 17 Epiglottitis/ 18 epiglottit*.tw. 19 supraglottit*.tw. 20 rhinosinusit*.tw. 21 exp Otitis Media/ 22 (otitis media or aom or ome).tw. 23 (inner ear* adj2 (inflamm* or infection*)).tw. 24 Respiratory Tract Infections/ 25 respiratory tract infection*.tw. 26 upper respiratory infection*.tw. 27 urti.tw. 28 (acute infection* adj5 respirat*).tw. 29 or/1‐28 30 Probiotics/ 31 probiotic*.tw. 32 exp Lactobacillus/ 33 lactobacill*.tw. 34 Bifidobacterium/ 35 (bifido* or bifidu*).tw. 36 exp Lactococcus/ 37 lactococc*.tw. 38 exp Saccharomyces/ 39 saccharomyc*.tw. 40 Streptococcus thermophilus/ 41 streptococcus thermophilus.tw. 42 Bacillus subtilis/ 43 bacillus subtilis.tw. 44 exp Enterococcus/ 45 enterococcus faec*.tw. 46 bulgarian bacillus.tw. 47 or/30‐46 48 29 and 47
Appendix 2. Embase.com search strategy
#52 #44 AND #51 #51 #47 NOT #50 #50 #49 NOT #48 #49 [animals]/lim #48 'human'/exp #47 #45 OR #46 #46 random*:ab,ti OR placebo*:ab,ti OR crossover*:ab,ti OR 'cross over':ab,ti OR allocat*:ab,ti OR ((singl* OR doubl*) NEXT/1 blind*):ab,ti OR trial:ti #45 'randomized controlled trial'/exp OR 'single blind procedure'/exp OR 'double blind procedure'/exp OR 'crossover procedure'/exp #44 #27 AND #43 #43 #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 #42 'bulgarian bacillus':ab,ti #41 (enterococcus NEXT/1 faec*):ab,ti #40 'enterococcus'/exp #39 'bacillus subtilis':ab,ti #38 'bacillus subtilis'/de #37 'streptococcus thermophilus':ab,ti #36 'streptococcus thermophilus'/exp #35 saccharomyc*:ab,ti #34 'saccharomyces'/exp #33 'lactococcus'/exp #32 bifido*:ab,ti OR bifidu*:ab,ti #31 'bifidobacterium'/exp #30 lactobacill*:ab,ti #29 'lactobacillus'/exp #28 'probiotic agent'/de #27 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 #26 urti:ab,ti #25 (upper NEAR/2 'respiratory infection'):ab,ti OR ('acute infection' NEAR/3 respiratory):ab,ti #24 'upper respiratory tract infection'/de OR 'viral upper respiratory tract infection'/de #23 'respiratory tract infection'/de #22 ('middle ear' NEAR/2 (infect* OR inflam*)):ab,ti #21 'otitis media':ab,ti OR aom:ab,ti OR ome:ab,ti #20 'otitis media'/exp #19 nasopharyngit*:ab,ti OR rhinopharyngit*:ab,ti #18 nasosinusit*:ab,ti OR rhinosinusit*:ab,ti #17 epiglottit*:ab,ti OR supraglottit*:ab,ti #16 'epiglottitis'/exp #15 croup:ab,ti #14 'croup'/de #13 tonsillit*:ab,ti OR 'peritonsillar abscess':ab,ti #12 'tonsillitis'/exp #11 rhinit*:ab,ti #10 'rhinitis'/exp #9 laryngotracheobronchit*:ab,ti #8 laryngit*:ab,ti #7 'laryngitis'/exp #6 pharyngit*:ab,ti #5 'pharyngitis'/exp #4 sinusit*:ab,ti #3 'sinusitis'/exp #2 'common cold':ab,ti OR 'common colds':ab,ti #1 'common cold'/de OR 'common cold symptom'/de
Appendix 3. Web of Science search strategy
Topic=(probiotic* or lactobacill* or bifido* or bifidu* or lactococc* or saccharomyc* or streptococcus thermophilus or bacillus subtilis or enterococcus faec* or bulgarian bacillus) AND
Topic=(common cold* or sinusit* or pharyngit* or laryngit* or laryngotracheobronchit* or rhinit* or tonsillit* or peritonsillar abscess* or croup or epiglottit* or supraglottit* or rhinosinusit* or otitis media or aom or ome or respiratory tract infection* or upper respiratory infection* or acute respiratory infection*)
Refined by: Topic=(placebo* or random* or clinical trial* or double blind* or single blind* or rct)
Timespan=All Years. Databases=SCI‐EXPANDED, CPCI‐S.
Appendix 4. Details of previous search strategy
Previously we searched the Cochrane Central Register of Controlled Trials (CENTRAL 2014, Issue 6), part of The Cochrane Library,www.thecochranelibrary.com (accessed 25 July 2014), which includes the Cochrane Acute Respiratory Infections Group's Specialised Register, MEDLINE (Ovid) (1950 to July week 3, 2014), EMBASE (1974 to July 2014), Web of Science, which includes Science Citation Index (from 1900 to July 2014) and Conference Proceedings Citation Index (from 1991 to July 2014), the Chinese Biomedical Literature Database, which includes the China Biological Medicine Database (from 1978 to July 2014), the Chinese Medicine Popular Science Literature Database (from 2000 to July 2014) and the Masters Degree Dissertation of Beijing Union Medical College Database (from 1981 to July 2014).
We used the following search strategy to search MEDLINE and CENTRAL. We combined the MEDLINE search strategy with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version (2008 revision); Ovid format (Lefebvre 2011). We adapted the search strategy to search EMBASE; Web of Science and the Chinese Biomedical Literature Database (see Figure 1).
MEDLINE (Ovid)
1 Common Cold/ 2 common cold*.tw. 3 exp Sinusitis/ 4 sinusit*.tw. 5 Pharyngitis/ 6 pharyngit*.tw. 7 exp Laryngitis/ 8 laryngit*.tw. 9 laryngotracheobronchit*.tw. 10 Rhinitis/ 11 rhinit*.tw. 12 Tonsillitis/ 13 tonsillit*.tw. 14 peritonsillar abscess*.tw. 15 Croup/ 16 croup*.tw. 17 Epiglottitis/ 18 epiglottit*.tw. 19 supraglottit*.tw. 20 rhinosinusit*.tw. 21 exp Otitis Media/ 22 (otitis media or aom or ome).tw. 23 (inner ear* adj2 (inflamm* or infection*)).tw. 24 Respiratory Tract Infections/ 25 respiratory tract infection*.tw. 26 upper respiratory infection*.tw. 27 urti.tw. 28 (acute infection* adj5 respirat*).tw. 29 or/1‐28 30 Probiotics/ 31 probiotic*.tw. 32 exp Lactobacillus/ 33 lactobacill*.tw. 34 Bifidobacterium/ 35 (bifido* or bifidu*).tw. 36 exp Lactococcus/ 37 lactococc*.tw. 38 exp Saccharomyces/ 39 saccharomyc*.tw. 40 Streptococcus thermophilus/ 41 streptococcus thermophilus.tw. 42 Bacillus subtilis/ 43 bacillus subtilis.tw. 44 exp Enterococcus/ 45 enterococcus faec*.tw. 46 bulgarian bacillus.tw. 47 or/30‐46 48 29 and 47
Data and analyses
Comparison 1. Intention‐to‐treat analysis: probiotics versus placebo or no treatment ‐ primary outcome measures.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 The number of participants who were diagnosed with acute URTIs: at least 1 event (age) | 16 | 4798 | Risk Ratio (IV, Random, 95% CI) | 0.76 [0.67, 0.87] |
| 1.1.1 Adults | 5 | 2132 | Risk Ratio (IV, Random, 95% CI) | 0.77 [0.65, 0.93] |
| 1.1.2 Children | 10 | 2512 | Risk Ratio (IV, Random, 95% CI) | 0.72 [0.58, 0.89] |
| 1.1.3 Elderly | 1 | 154 | Risk Ratio (IV, Random, 95% CI) | 0.99 [0.68, 1.45] |
| 1.2 The number of participants who were diagnosed with acute URTIs: at least 1 event (treatment dose) | 16 | 4798 | Risk Ratio (IV, Random, 95% CI) | 0.76 [0.67, 0.87] |
| 1.2.1 Less than 1010 CFU per day | 11 | 4121 | Risk Ratio (IV, Random, 95% CI) | 0.77 [0.67, 0.90] |
| 1.2.2 More than 1010 CFU per day | 5 | 677 | Risk Ratio (IV, Random, 95% CI) | 0.70 [0.50, 0.99] |
| 1.3 The number of participants who were diagnosed with acute URTIs: at least 1 event (treatment duration) | 16 | 4798 | Risk Ratio (IV, Random, 95% CI) | 0.76 [0.67, 0.87] |
| 1.3.1 Less than 3 months | 2 | 381 | Risk Ratio (IV, Random, 95% CI) | 0.60 [0.50, 0.74] |
| 1.3.2 3 to 6 months | 9 | 3704 | Risk Ratio (IV, Random, 95% CI) | 0.81 [0.71, 0.93] |
| 1.3.3 More than 6 months | 5 | 713 | Risk Ratio (IV, Random, 95% CI) | 0.78 [0.53, 1.13] |
| 1.4 The number of participants who were diagnosed with acute URTIs: at least 1 event (type of comparator) | 16 | 4798 | Risk Ratio (IV, Random, 95% CI) | 0.76 [0.67, 0.87] |
| 1.4.1 Placebo | 14 | 4465 | Risk Ratio (IV, Random, 95% CI) | 0.78 [0.68, 0.90] |
| 1.4.2 No treatment | 2 | 333 | Risk Ratio (IV, Random, 95% CI) | 0.63 [0.49, 0.81] |
| 1.5 The number of participants who were diagnosed with acute URTI: at least 3 events (age) | 4 | 763 | Risk Ratio (IV, Random, 95% CI) | 0.59 [0.38, 0.91] |
| 1.5.1 Adults | 2 | 551 | Risk Ratio (IV, Random, 95% CI) | 0.62 [0.20, 1.93] |
| 1.5.2 Children | 2 | 212 | Risk Ratio (IV, Random, 95% CI) | 0.64 [0.36, 1.14] |
| 1.6 The number of participants who were diagnosed with acute URTI: at least 3 events (treatment dose) | 4 | 763 | Risk Ratio (IV, Random, 95% CI) | 0.59 [0.38, 0.91] |
| 1.6.1 Less than 1010 CFU per day | 2 | 449 | Risk Ratio (IV, Random, 95% CI) | 0.59 [0.36, 0.96] |
| 1.6.2 More than 1010 CFU per day | 2 | 314 | Risk Ratio (IV, Random, 95% CI) | 0.64 [0.20, 2.04] |
| 1.7 The number of participants who were diagnosed with acute URTI: at least 3 events (treatment duration) | 4 | 763 | Risk Ratio (IV, Random, 95% CI) | 0.59 [0.38, 0.91] |
| 1.7.1 3 to 6 months | 3 | 682 | Risk Ratio (IV, Random, 95% CI) | 0.61 [0.38, 0.99] |
| 1.7.2 More than 6 months | 1 | 81 | Risk Ratio (IV, Random, 95% CI) | 0.51 [0.20, 1.35] |
| 1.8 The number of participants who were diagnosed with acute URTI: at least 3 events (type of comparator) | 4 | 763 | Risk Ratio (IV, Random, 95% CI) | 0.59 [0.38, 0.91] |
| 1.8.1 Placebo | 3 | 530 | Risk Ratio (IV, Random, 95% CI) | 0.57 [0.37, 0.89] |
| 1.8.2 No treatment | 1 | 233 | Risk Ratio (IV, Random, 95% CI) | 3.08 [0.13, 74.78] |
| 1.9 The incidence rate of acute URTIs (age) | 12 | 4364 | Rate Ratio (IV, Random, 95% CI) | 0.82 [0.73, 0.92] |
| 1.9.1 Adults | 4 | 2032 | Rate Ratio (IV, Random, 95% CI) | 0.79 [0.71, 0.89] |
| 1.9.2 Children | 6 | 1868 | Rate Ratio (IV, Random, 95% CI) | 0.79 [0.65, 0.96] |
| 1.9.3 Elderly | 2 | 464 | Rate Ratio (IV, Random, 95% CI) | 1.04 [0.63, 1.71] |
| 1.10 The incidence rate of acute URTIs (treatment dose) | 12 | 4364 | Rate Ratio (IV, Random, 95% CI) | 0.82 [0.73, 0.92] |
| 1.10.1 Less than 1010 CFU per day | 7 | 2631 | Rate Ratio (IV, Random, 95% CI) | 0.77 [0.65, 0.91] |
| 1.10.2 More than 1010 CFU per day | 5 | 1733 | Rate Ratio (IV, Random, 95% CI) | 0.91 [0.75, 1.11] |
| 1.11 The incidence rate of acute URTIs (treatment duration) | 12 | 4364 | Rate Ratio (IV, Random, 95% CI) | 0.82 [0.73, 0.92] |
| 1.11.1 3 to 6 months | 10 | 3900 | Rate Ratio (IV, Random, 95% CI) | 0.79 [0.70, 0.90] |
| 1.11.2 More than 6 months | 2 | 464 | Rate Ratio (IV, Random, 95% CI) | 1.04 [0.63, 1.71] |
| 1.12 The incidence rate of acute URTIs (type of comparator) | 12 | 4364 | Rate Ratio (IV, Random, 95% CI) | 0.82 [0.73, 0.92] |
| 1.12.1 Placebo | 11 | 4131 | Rate Ratio (IV, Random, 95% CI) | 0.83 [0.73, 0.94] |
| 1.12.2 No treatment | 1 | 233 | Rate Ratio (IV, Random, 95% CI) | 0.70 [0.47, 1.04] |
| 1.13 The mean duration of an episode of acute URTIs (age) | 6 | 2406 | Mean Difference (IV, Random, 95% CI) | ‐1.22 [‐2.12, ‐0.33] |
| 1.13.1 Adults | 5 | 2252 | Mean Difference (IV, Random, 95% CI) | ‐1.14 [‐2.15, ‐0.13] |
| 1.13.2 Elderly | 1 | 154 | Mean Difference (IV, Random, 95% CI) | ‐1.69 [‐2.75, ‐0.63] |
| 1.14 The mean duration of an episode of acute URTIs (treatment dose) | 6 | 2406 | Mean Difference (IV, Random, 95% CI) | ‐1.22 [‐2.12, ‐0.33] |
| 1.14.1 Less than 1010 CFU per day | 4 | 2152 | Mean Difference (IV, Random, 95% CI) | ‐0.89 [‐2.09, 0.31] |
| 1.14.2 More than 1010 CFU per day | 2 | 254 | Mean Difference (IV, Random, 95% CI) | ‐2.01 [‐2.66, ‐1.36] |
| 1.15 The mean duration of an episode of acute URTIs (treatment duration) | 6 | 2406 | Mean Difference (IV, Random, 95% CI) | ‐1.22 [‐2.12, ‐0.33] |
| 1.15.1 3 to 6 months | 4 | 1773 | Mean Difference (IV, Random, 95% CI) | ‐0.90 [‐1.86, 0.05] |
| 1.15.2 More than 6 months | 2 | 633 | Mean Difference (IV, Random, 95% CI) | ‐1.90 [‐2.04, ‐1.76] |
Comparison 2. Intention‐to‐treat analysis: probiotics versus placebo or no treatment ‐ time off from childcare centre, school, or work.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 The number of participants who were absent from childcare centre, school, or work due to acute URTIs | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 3. Intention‐to‐treat analysis: probiotics versus placebo or no treatment ‐ prescribed antibiotics for acute URTIs.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 The number of participants who used antibiotics for acute URTIs (age) | 6 | 1548 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.42, 0.81] |
| 3.1.1 Adults | 1 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 0.09 [0.01, 1.67] |
| 3.1.2 Children | 5 | 1315 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.43, 0.83] |
| 3.2 The number of participants who used antibiotics for acute URTIs (treatment dose) | 6 | 1548 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.42, 0.81] |
| 3.2.1 Less than 1010 CFU per day | 4 | 1234 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.42, 0.99] |
| 3.2.2 More than 1010 CFU per day | 2 | 314 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.09, 1.52] |
| 3.3 The number of participants who used antibiotics for acute URTIs (treatment duration) | 6 | 1548 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.42, 0.81] |
| 3.3.1 Less than 3 months | 1 | 281 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.42, 1.11] |
| 3.3.2 3 to 6 months | 4 | 1186 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.20, 1.10] |
| 3.3.3 More than 6 months | 1 | 81 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.31, 0.88] |
| 3.4 The number of participants who used antibiotics for acute URTIs (type of comparator) | 6 | 1548 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.42, 0.81] |
| 3.4.1 Placebo | 5 | 1315 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.43, 0.83] |
| 3.4.2 No treatment | 1 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 0.09 [0.01, 1.67] |
Comparison 4. Intention‐to‐treat analysis: probiotics versus placebo or no treatment ‐ adverse events.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 The number of participants who experienced at least 1 adverse event (age) | 8 | 2456 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.90, 1.15] |
| 4.1.1 Adults | 3 | 1414 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.89, 1.19] |
| 4.1.2 Children | 5 | 1042 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.71, 1.37] |
| 4.2 The number of participants who experienced at least 1 adverse event (treatment dose) | 8 | 2456 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.90, 1.15] |
| 4.2.1 Less than 1010 CFU per day | 6 | 1709 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.90, 1.16] |
| 4.2.2 More than 1010 CFU per day | 2 | 747 | Risk Ratio (M‐H, Random, 95% CI) | 1.46 [0.23, 9.09] |
| 4.3 The number of participants who experienced at least 1 adverse event (treatment duration) | 8 | 2456 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.90, 1.15] |
| 4.3.1 3 to 6 months | 6 | 2263 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.89, 1.15] |
| 4.3.2 More than 6 months | 2 | 193 | Risk Ratio (M‐H, Random, 95% CI) | 4.06 [0.69, 23.88] |
Comparison 5. Per‐protocol analysis: probiotics versus placebo or no treatment ‐ primary outcome measures.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 The number of participants who were diagnosed with acute URTIs: at least 1 event (age) | 16 | 4446 | Risk Ratio (IV, Random, 95% CI) | 0.77 [0.67, 0.88] |
| 5.1.1 Adults | 5 | 2023 | Risk Ratio (IV, Random, 95% CI) | 0.78 [0.65, 0.93] |
| 5.1.2 Children | 10 | 2289 | Risk Ratio (IV, Random, 95% CI) | 0.74 [0.60, 0.91] |
| 5.1.3 Elderly | 1 | 134 | Risk Ratio (IV, Random, 95% CI) | 0.99 [0.66, 1.49] |
| 5.2 The number of participants who were diagnosed with acute URTIs: at least 1 event (treatment dose) | 16 | 4446 | Risk Ratio (IV, Random, 95% CI) | 0.77 [0.67, 0.88] |
| 5.2.1 Less than 1010 CFU per day | 11 | 3872 | Risk Ratio (IV, Random, 95% CI) | 0.79 [0.68, 0.91] |
| 5.2.2 Less than 1010 CFU per day | 5 | 574 | Risk Ratio (IV, Random, 95% CI) | 0.71 [0.51, 0.98] |
| 5.3 The number of participants who were diagnosed with acute URTIs: at least 1 event (treatment duration) | 16 | 4446 | Risk Ratio (IV, Random, 95% CI) | 0.77 [0.67, 0.88] |
| 5.3.1 Less than 3 months | 2 | 325 | Risk Ratio (IV, Random, 95% CI) | 0.60 [0.49, 0.75] |
| 5.3.2 3 to 6 months | 9 | 3506 | Risk Ratio (IV, Random, 95% CI) | 0.83 [0.72, 0.94] |
| 5.3.3 More than 6 months | 5 | 615 | Risk Ratio (IV, Random, 95% CI) | 0.78 [0.54, 1.14] |
| 5.4 The number of participants who were diagnosed with acute URTIs: at least 1 event (type of comparator) | 16 | 4446 | Risk Ratio (IV, Random, 95% CI) | 0.77 [0.67, 0.88] |
| 5.4.1 Placebo | 14 | 4170 | Risk Ratio (IV, Random, 95% CI) | 0.79 [0.69, 0.91] |
| 5.4.2 No treatment | 2 | 276 | Risk Ratio (IV, Random, 95% CI) | 0.62 [0.47, 0.83] |
| 5.5 The number of participants who were diagnosed with acute URTIs: at least 3 events (age) | 4 | 648 | Risk Ratio (IV, Random, 95% CI) | 0.57 [0.36, 0.91] |
| 5.5.1 Adults | 2 | 477 | Risk Ratio (IV, Random, 95% CI) | 0.60 [0.20, 1.82] |
| 5.5.2 Children | 2 | 171 | Risk Ratio (IV, Random, 95% CI) | 0.60 [0.33, 1.12] |
| 5.6 The number of participants who were diagnosed with acute URTIs: at least 3 events (treatment dose) | 4 | 648 | Risk Ratio (IV, Random, 95% CI) | 0.57 [0.36, 0.91] |
| 5.6.1 Less than 1010 CFU per day | 2 | 371 | Risk Ratio (IV, Random, 95% CI) | 0.57 [0.34, 0.96] |
| 5.6.2 More than 1010 CFU per day | 2 | 277 | Risk Ratio (IV, Random, 95% CI) | 0.63 [0.19, 2.03] |
| 5.7 The number of participants who were diagnosed with acute URTIs: at least 3 events (treatment duration) | 4 | 648 | Risk Ratio (IV, Random, 95% CI) | 0.57 [0.36, 0.91] |
| 5.7.1 3 to 6 months | 3 | 576 | Risk Ratio (IV, Random, 95% CI) | 0.59 [0.35, 1.00] |
| 5.7.2 More than 6 months | 1 | 72 | Risk Ratio (IV, Random, 95% CI) | 0.50 [0.17, 1.45] |
| 5.8 The number of participants who were diagnosed with acute URTIs: at least 3 events (type of comparator) | 4 | 648 | Risk Ratio (IV, Random, 95% CI) | 0.57 [0.36, 0.91] |
| 5.8.1 Placebo | 3 | 443 | Risk Ratio (IV, Random, 95% CI) | 0.55 [0.35, 0.89] |
| 5.8.2 No treatment | 1 | 205 | Risk Ratio (IV, Random, 95% CI) | 2.97 [0.12, 72.09] |
| 5.9 The incidence rate of acute URTIs (age) | 12 | 3932 | Rate Ratio (IV, Random, 95% CI) | 0.82 [0.73, 0.91] |
| 5.9.1 Adults | 4 | 1927 | Rate Ratio (IV, Random, 95% CI) | 0.79 [0.70, 0.89] |
| 5.9.2 Children | 6 | 1676 | Rate Ratio (IV, Random, 95% CI) | 0.79 [0.66, 0.95] |
| 5.9.3 Elderly | 2 | 329 | Rate Ratio (IV, Random, 95% CI) | 1.04 [0.63, 1.70] |
| 5.10 The incidence rate of acute URTIs (treatment dose) | 12 | 3932 | Rate Ratio (IV, Random, 95% CI) | 0.82 [0.73, 0.91] |
| 5.10.1 Less than 1010 CFU per day | 7 | 2485 | Rate Ratio (IV, Random, 95% CI) | 0.76 [0.64, 0.90] |
| 5.10.2 More than 1010 CFU per day | 5 | 1447 | Rate Ratio (IV, Random, 95% CI) | 0.90 [0.75, 1.08] |
| 5.11 The incidence rate of acute URTIs (treatment duration) | 12 | 3932 | Rate Ratio (IV, Random, 95% CI) | 0.82 [0.73, 0.91] |
| 5.11.1 3 to 6 months | 10 | 3603 | Rate Ratio (IV, Random, 95% CI) | 0.79 [0.71, 0.89] |
| 5.11.2 More than 6 months | 2 | 329 | Rate Ratio (IV, Random, 95% CI) | 1.04 [0.63, 1.70] |
| 5.12 The incidence rate of acute URTIs (type of comparator) | 12 | 3932 | Rate Ratio (IV, Random, 95% CI) | 0.82 [0.73, 0.91] |
| 5.12.1 Placebo | 11 | 3727 | Rate Ratio (IV, Random, 95% CI) | 0.82 [0.73, 0.93] |
| 5.12.2 No treatment | 1 | 205 | Rate Ratio (IV, Random, 95% CI) | 0.70 [0.46, 1.07] |
| 5.13 The mean duration of an episode of acute URTIs (age) | 6 | 2308 | Mean Difference (IV, Random, 95% CI) | ‐1.22 [‐2.12, ‐0.32] |
| 5.13.1 Adults | 5 | 2174 | Mean Difference (IV, Random, 95% CI) | ‐1.14 [‐2.16, ‐0.13] |
| 5.13.2 Elderly | 1 | 134 | Mean Difference (IV, Random, 95% CI) | ‐1.69 [‐2.83, ‐0.55] |
| 5.14 The mean duration of an episode of acute URTIs (treatment dose) | 6 | 2308 | Mean Difference (IV, Random, 95% CI) | ‐1.22 [‐2.12, ‐0.32] |
| 5.14.1 Less than 1010 CFU per day | 4 | 2078 | Mean Difference (IV, Random, 95% CI) | ‐0.89 [‐2.09, 0.31] |
| 5.14.2 More than 1010 CFU per day | 2 | 230 | Mean Difference (IV, Random, 95% CI) | ‐2.02 [‐2.70, ‐1.34] |
| 5.15 The mean duration of an episode of acute URTIs (treatment duration) | 6 | 2308 | Mean Difference (IV, Random, 95% CI) | ‐1.22 [‐2.12, ‐0.32] |
| 5.15.1 3 to 6 months | 4 | 1720 | Mean Difference (IV, Random, 95% CI) | ‐0.89 [‐1.85, 0.06] |
| 5.15.2 More than 6 months | 2 | 588 | Mean Difference (IV, Random, 95% CI) | ‐1.90 [‐2.04, ‐1.75] |
Comparison 6. Per‐protocol analysis: probiotics versus placebo or no treatment ‐ time off from childcare centre, school, or work.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 The number of participants who experienced time off from childcare centre, school, or work due to acute URTIs | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 7. Per‐protocol analysis: probiotics versus placebo or no treatment ‐ prescribed antibiotics for acute URTIs.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 The number of participants who used antibiotics for acute URTIs (age) | 6 | 1420 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.40, 0.82] |
| 7.1.1 Adults | 1 | 205 | Risk Ratio (M‐H, Random, 95% CI) | 0.11 [0.01, 2.02] |
| 7.1.2 Children | 5 | 1215 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.41, 0.84] |
| 7.2 The number of participants who used antibiotics for acute URTIs (treatment dose) | 6 | 1420 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.40, 0.82] |
| 7.2.1 Less than 1010 CFU per day | 4 | 1143 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.40, 0.99] |
| 7.2.2 More than 1010 CFU per day | 2 | 277 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.17, 1.16] |
| 7.3 The number of participants who used antibiotics for acute URTIs (treatment duration) | 6 | 1420 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.40, 0.82] |
| 7.3.1 Less than 3 months | 1 | 254 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.40, 1.11] |
| 7.3.2 3 to 6 months | 4 | 1094 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.18, 1.13] |
| 7.3.3 More than 6 months | 1 | 72 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.29, 0.92] |
| 7.4 The number of participants who used antibiotics for acute URTIs (type of comparator) | 6 | 1420 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.40, 0.82] |
| 7.4.1 Placebo | 5 | 1215 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.41, 0.84] |
| 7.4.2 No treatment | 1 | 205 | Risk Ratio (M‐H, Random, 95% CI) | 0.11 [0.01, 2.02] |
Comparison 8. Per‐protocol analysis: probiotics versus placebo or no treatment ‐ adverse events.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 8.1 The number of participants who experienced at least 1 adverse event (age) | 8 | 2209 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.89, 1.16] |
| 8.1.1 Adults | 3 | 1321 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.89, 1.20] |
| 8.1.2 Children | 5 | 888 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.71, 1.30] |
| 8.2 The number of participants who experienced at least 1 adverse event (treatment dose) | 8 | 2209 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.89, 1.16] |
| 8.2.1 Less than 1010 CFU per day | 6 | 1578 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.89, 1.16] |
| 8.2.2 More than 1010 CFU per day | 2 | 631 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.31, 4.61] |
| 8.3 The number of participants who experienced at least 1 adverse event (treatment duration) | 8 | 2209 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.89, 1.16] |
| 8.3.1 3 to 6 months | 6 | 2060 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.88, 1.15] |
| 8.3.2 More than 6 months | 2 | 149 | Risk Ratio (M‐H, Random, 95% CI) | 3.71 [0.62, 22.10] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Andaloro 2019.
| Study characteristics | ||
| Methods | Study design: prospective, randomised, single‐blind, placebo‐controlled pilot trial Method of randomisation: randomisation was carried out using a statistical computing web programming Blinding: single‐blind: participants were blinded to their treatment group throughout the study; however, investigators and study site staff were unblinded Duration: 9 months; 90‐day treatment and 6‐month follow‐up, start from November 2017 and ends in July 2018 Exclusions postrandomisation: 0 Losses to follow‐up: 2: 1 in the probiotic bacteria group, 1 in the placebo group |
|
| Participants | Country: Italy Setting: outpatient section No. of participants: 84; 42 in the probiotic bacteria group, 42 in the placebo group Age: 6 to 11 years Inclusion criteria: patients were eligible to participate if they were 6 to 11 years old and who had at least 3 episodes of microbiologically documented GABHS infections with clinical symptoms suggesting GABHS pharyngitis in the period from November 2016 to July 2017. Exclusion criteria: non‐completion of the entire study protocol; the presence of symptoms of another infective disease at the time of enrolment; severe respiratory or systemic pathologies, or both; current antibiotics, corticoids, or montelukast treatment; asthma; known immunological deficiency; had undergone tonsillectomy, adenoidectomy, or a previous reduction of tonsils; healthy carriage of Streptococcus pyogenes; and hypersensitivity to penicillin. |
|
| Interventions | Treatment group: Streptococcus salivarius 24SMB and Streptococcus oralis 89a (4 × 109 CFU/day) for 3 months Control group: placebo: an identical‐looking and ‐tasting control group |
|
| Outcomes | Primary outcome: the number of new episodes of GABHS pharyngotonsillar infections Secondary endpoints:
|
|
| Funding and conflicts of interest statements | Funding source: none Conflicts of interest: none |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was carried out using a statistical computing web programming. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Assessor was unblinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 2 participants were lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | No information provided. |
| Other bias | Low risk | No other potential source of bias was found. |
Berggren 2011.
| Study characteristics | ||
| Methods | Study design: a double‐blind, placebo‐controlled, randomised clinical study with 2 parallel arms Method of randomisation: not clearly stated Blinding: double‐blind. Not clearly stated. The children may have been blinded. Duration: between January 2007 and May 2007 Exclusions postrandomisation: 0 Losses to follow‐up: 43; 20 in the probiotic bacteria group, 23 in the placebo group |
|
| Participants | Country: Sweden Setting: community residents No. of participants: 318; 159 in the probiotic bacteria group, 159 in the placebo group Age: aged 18 to 65 Inclusion criteria: healthy volunteers Exclusion criteria: known intolerance or allergy to any ingredient included in the formulations, medically treated allergy, current treatment for severe gastrointestinal disorders, pregnancy or lactation, vaccination against influenza within the last 12 months, or smoking |
|
| Interventions | Treatment group: Lactobacillus plantarum HEAL 9 and Lactobacillus paracasei 8700:2 (1 × 109 CFU/day) for 12 weeks Control: placebo: an identical‐looking and ‐tasting control product |
|
| Outcomes |
|
|
| Funding and conflicts of interest statements | Funding source: Probi AB Conflicts of interest: the authors are employees at Probi AB, and the study was funded by Probi AB |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Double‐blind, but details not provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 40 participants lost to follow‐up, and the analysis of the study was not based on the intention‐to‐treat population. |
| Selective reporting (reporting bias) | Unclear risk | No information provided. |
| Other bias | Low risk | No other potential source of bias was found. |
Butler 2020.
| Study characteristics | ||
| Methods | Study design: multicentre, parallel, individually randomised, placebo‐controlled, double‐blind clinical trial Method of randomisation: randomisation was carried out using an online computerised randomisation system created by the University of Oxford Primary Care Clinical trials Unit Blinding: double‐blind: the participants, care home staff, treating clinicians, and trial team were blinded Duration: 12 months; between December 2016 and May 2018 Exclusions postrandomisation: 30: 14 in the probiotic bacteria group, 16 in the placebo group Losses to follow‐up: 32: 16 in the probiotic bacteria group, 16 in the placebo group |
|
| Participants | Country: United Kingdom Setting: care home No. of participants: 310; 155 in the probiotic bacteria group, 155 in the placebo group Age: ≥ 65 years old Inclusion criteria: care home residents aged 65 years or older Exclusion criteria: being immunocompromised (ongoing immune‐suppressants; long‐term, high‐dose, oral, intramuscular, or intravenous steroids) or taking ongoing regular probiotics |
|
| Interventions | Treatment group: probiotic combination of LGG and Bifidobacterium animalis subsp lactis BB‐12 (total cell count per capsule, 1.3 × 1010 to 1.6 × 1010) once a day for 12 months Control group: an identical‐looking and ‐tasting control product |
|
| Outcomes | Primary outcome: cumulative systemic antibiotic administration days for all‐cause infections Secondary endpoints:
|
|
| Funding and conflicts of interest statements | Funding source: the Efficacy and Mechanism Evaluation Programme, the Medical Research Council, and the National Institute for Health Research Conflicts of interest: many authors of this study were funded by the Efficacy and Mechanism Evaluation Programme, the National Institute for Health Research, and the Medical Research Council. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was carried out using an online computerised randomisation system created by the University of Oxford Primary Care Clinical Trials Unit. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind: participant and provider were blinded |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessor was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 10% participants lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No information provided. |
| Other bias | Low risk | No other potential source of bias was found. |
Caceres 2010.
| Study characteristics | ||
| Methods | Study design: prospective, multicentre, randomised, controlled, double‐blind trial Method of randomisation: using a computer‐generated random numbers table Blinding: double‐blinding not clearly stated. The children may have been blinded. Duration: 3 months of the cold season: June to September 2006 Exclusions postrandomisation: 0 Losses to follow‐up: 49 (33 in the probiotic bacteria group, 16 in the placebo group) |
|
| Participants | Country: Chile Setting: Santiago: 4 daycare centres No. of participants: 398 (203 in the probiotic bacteria group, 195 in the placebo group) Age: 1 to 5 Inclusion criteria: asymptomatic children of both sexes and attending day centres regularly Exclusion criteria: antibiotic treatment at the time of enrolment; unwillingness on the part of the parents to interrupt the intake of other probiotic‐containing products, signs of current respiratory insufficiency, immune deficiency, congenital malformations including heart disease, inborn errors of metabolism, cystic fibrosis, chronic enteropathies or malabsorption, diabetes mellitus, treatment with prokinetic drugs or with systemic or inhaled corticosteroids, children whose parents would not comply with the requirements of the study protocol or who had been participating in another clinical trial during the 4 weeks prior the beginning of this study |
|
| Interventions | Treatment group: milk‐based product containing approximately 1010 CFU/day of the probiotic strain (Lactobacillus rhamnosus HN001) for 3 months Control group: placebo (an identical‐looking control product that did not contain the probiotic) |
|
| Outcomes | Primary outcome: the number of episodes of ARI per child Secondary endpoints:
|
|
| Funding and conflicts of interest statements | Funding source: Danisco, Copenhagen, Denmark Conflicts of interest: not reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was carried out using a computer‐generated random numbers table. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Double‐blind, but no details provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 49 participants were lost to follow‐up, and the analysis of the study was based on the intention‐to‐treat population. |
| Selective reporting (reporting bias) | Unclear risk | No information provided. |
| Other bias | Low risk | No other potential source of bias was found. |
Damholt 2022.
| Study characteristics | ||
| Methods | Study design: randomised, double‐blind, placebo‐controlled, 2‐armed, parallel‐group trial Method of randomisation: the randomisation list was generated by a statistician not involved in the study using the PROC PLAN procedure in SAS version 9.3 (SAS Institute, Cary, NC, USA). The enrolled children were randomised to active product or placebo in a ratio of 1:1 in blocks of 6. Neither the investigator site nor the sponsor received any information about randomisation blocks. Randomisation was stratified in 2 strata, ‘attending day care’ or ‘attending school’, with the aim of enrolling at least 40% in the ‘attending day care’ group. Blinding: all the children, parents/guardians, investigators, trial team, and sponsor staff involved in the trial were blinded until the final database was locked. Duration: 16 weeks; 20 September 2018 (screening of the first child), and 15 April 2019 (the last trial visit) Exclusions postrandomisation: 2 in the placebo group Losses to follow‐up: 14: 5 in the probiotics group, 9 in the placebo group |
|
| Participants | Country: United Kingdom Setting: day care or primary school Age: 2 to 6 years (mean 4 years) Inclusion criteria: children were aged 2 to 6 years, inclusive, at the time of informed consent, with no URTI (as assessed by a general practitioner), were able to consume the trial product, attended day care or primary school with at least 10 children, and were generally healthy (as determined by the investigator). Exclusion criteria: any known concomitant chronic infections, chronic systemic diseases, autoimmune diseases (e.g. asthma), immunodeficiency, metabolic diseases, chronic respiratory tract diseases including respiratory allergies and cystic fibrosis, or congenital cardiac defects; suspected or challenge‐proven food allergy; use of any prescribed immune suppressive medications at enrolment; use of oral antibiotics in the 1 month before randomisation; use of pre‐/pro‐/synbiotics during the trial; intake of Echinacea, high‐dose vitamins, or zinc during both the run‐in period and the intervention period; language limitations regarding interviews or questionnaires; participation in other clinical trials in the previous 2 months; and planned extensive travel (for > 1 month) over the duration of the trial |
|
| Interventions | Treatment group: 109 CFU of Lactobacillus rhamnosus GG DSM 33156 Control group: an identical‐looking and ‐tasting control product |
|
| Outcomes | Primary outcome: incidence of URTIs Secondary endpoints:
|
|
| Funding and conflicts of interest statements | Funding source: Chr. Hansen A/S Conflicts of interest: many authors of this study were funded by Chr. Hansen A/S. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation list was generated by a statistician not involved in the study using the PROC PLAN procedure in SAS version 9.3 (SAS Institute, Cary, NC, USA). |
| Allocation concealment (selection bias) | Low risk | Test and placebo products were similar in smell, taste, colour, and overall appearance. All trial products were packaged in identical packs with identical labelling, except for the randomisation number. In case siblings were enrolled in the trial, the carton boxes were colour coded as an extra precaution. The boxes were blinded with respect to the parent/guardian, child, general practitioners, and trial team. Labelling was performed by a third party not otherwise involved in the conduct of the trial. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind: parent/guardian, child, general practitioners, and trial team |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind: parent/guardian, child, general practitioners, and trial team |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessor was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2.3% participants lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported. |
| Other bias | Low risk | No other potential source of bias was found. |
Fujita 2013.
| Study characteristics | ||
| Methods | Study design: a multicentre, double‐blind, placebo‐controlled, randomised clinical study with 2 parallel arms Method of randomisation: central enrolment system (block size 4). Allocation was performed independently from researchers by the data centre allocation co‐ordinator, who also retained the allocation list until observations were complete. Blinding: double‐blind, but no details provided. The participants and study personnel may have been blinded. Duration: 7 months: 1 December 2009 to 30 June 2010 Exclusions postrandomisation: 14 Losses to follow‐up: 20 |
|
| Participants | Country: Japan Setting: 4 daycare facilities for elderly people located around Tokyo No. of participants: 154; 76 in the LcS group, 78 in the placebo group Age: 83.2 ± 9.1 years Inclusion criteria: older volunteers in daycare facilities Exclusion criteria: people with a history of allergy to dairy products or people consuming lactic acid bacteria‐containing food or drink on a regular basis (at least 4 days per week) |
|
| Interventions | Treatment group: Lactobacillus casei strain Shirota (4.0 × 1010 CFU/day) with high‐fructose corn syrup, sugar, and skimmed milk powder for 5 months Control group: placebo: an identical‐looking and ‐tasting control product with the same energy (62 kcal) as the intervention group |
|
| Outcomes |
|
|
| Funding and conflicts of interest statements | Funding source: Yakult Honsha Co Ltd Conflicts of interest: none |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central enrolment system |
| Allocation concealment (selection bias) | Low risk | Allocation was performed independently from the researchers by the data centre allocation co‐ordinator. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Double‐blind, but no details provided; the participants and study personnel may have been blinded |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details provided. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | About 13% of participants were lost to follow‐up, and the analysis of the study was based on the intention‐to‐treat population. |
| Selective reporting (reporting bias) | Unclear risk | No information provided. |
| Other bias | Low risk | No other potential source of bias was found. |
Hojsak 2010a.
| Study characteristics | ||
| Methods | Study design: a double‐blind, placebo‐controlled, randomised clinical study Method of randomisation: randomisation procedure performed with computer‐generated numbers Blinding: double‐blind. Participant, provider, and assessor were blinded. Duration: during the 4‐month intervention period (from 19 November 2007 to 20 February 2008) Exclusions postrandomisation: 0 Losses to follow‐up: 27; 12 in the probiotic bacteria group, 15 in the placebo group |
|
| Participants | Country: Croatia (Zagreb area) Setting: daycare centres No. of participants: 281; 139 in the probiotic bacteria group, 142 in the placebo group Age: 13 to 86 months Inclusion criteria: those attending a daycare centre and whose parents or legal guardians provided written informed consent Exclusion criteria: children with cow's milk allergy (probiotics were given in a fermented cow's milk product); those who were receiving probiotic or prebiotic products (or both) prior to or at the time of enrolment; those who had a neoplasm, other chronic severe illness, or immunodeficency; and children who disliked fermented milk products |
|
| Interventions | Treatment group: Lactobacillus rhamnosus strain GG (LGG strain from Valio) was administered in 100 mL of a fermented milk product at a dose of 109 CFU/day. Control group: the same postpasteurised fermented milk product (100 mL) without LGG Length of follow‐up: 3‐month period |
|
| Outcomes | Primary outcomes:
Secondary endpoints:
|
|
| Funding and conflicts of interest statements | Funding source: none Conflicts of interest: none |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation procedure performed with computer‐generated numbers. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind: participant, provider, and assessor were blinded |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 27 participants were lost to follow‐up, and the analysis of the study was based on the intention‐to‐treat population. |
| Selective reporting (reporting bias) | Unclear risk | No information provided. |
| Other bias | Low risk | No other potential source of bias was found. |
Hojsak 2010b.
| Study characteristics | ||
| Methods | Study design: a double‐blind, placebo‐controlled, randomised clinical study Method of randomisation: randomisation procedure performed with computer‐generated numbers Blinding: double‐blind. Participant, provider, and assessor were blinded. Duration: November 2007 to May 2008 Exclusions postrandomisation: 0 Losses to follow‐up: 28; 16 in the probiotic bacteria group, 12 in the placebo group |
|
| Participants | Country: Zagreb, Croatia Setting: hospitalised at the paediatric department No. of participants: 742; 376 in the probiotic bacteria group, 366 in the placebo group Age: older than 12 months Inclusion criteria: all patients who were older than 12 months and hospitalised at the paediatric department Exclusion criteria: children with gastrointestinal or respiratory tract infections (or both) on admission; children with immunodeficency, cow milk allergy, neoplasm, chronic severe illnesses, or an anticipated hospital stay of 3 days; children who had received probiotic or prebiotic products (or both) before enrolment (7 days before hospitalisation); and children who disliked fermented milk products |
|
| Interventions | Treatment group: Lactobacillus rhamnosus strain GG (LGG strain (Valio Ltd, Helsinki, Finland)) was administered in 100 mL of a fermented milk product at a dose of 109 CFU/day. Control group: the same postpasteurised fermented milk product (100 mL) without LGG Length of follow‐up: duration of the hospitalisation |
|
| Outcomes | Primary outcomes:
Secondary endpoints:
|
|
| Funding and conflicts of interest statements | Funding source: not reported Conflicts of interest: not reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation procedure performed with computer‐generated numbers. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind: participant, provider, and assessor were blinded |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The assessor was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 28 participants were lost to follow‐up, and the analysis of the study was based on the intention‐to‐treat population. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported. |
| Other bias | Low risk | No other potential source of bias was found. |
Kara 2019.
| Study characteristics | ||
| Methods | Study design: randomised, prospective trial Method of randomisation: not clearly stated Blinding: not clearly stated Duration: 3 months, between 1 June and 31 December 2016 Exclusions postrandomisation: 29; 12 in the probiotic bacteria group, 17 in the placebo group Losses to follow‐up: 0 |
|
| Participants | Country: Turkey Setting: tertiary hospital No. of participants: 100; 50 in the probiotic bacteria group, 50 in the placebo group Age: 6 months to 5 years Inclusion criteria: aged between 6 months and 5 years, with body weight and height below −2 SD Exclusion criteria: a history of prematurity, or any chronic disorders such as chronic liver, renal, or cardiac diseases, malignancy, malabsorption disorders, primary immunodeficiencies, HIV positivity, or malnutrition with any metabolic deficiencies requiring replacement therapy (anaemia (haemoglobin < 11 g/dL), zinc, vitamin D, or any other supplement), using any antibiotic or probiotics during the previous 3 months |
|
| Interventions | Treatment group: 5 drops (approximately 109 micro‐organisms) of Lactobacillus rhamnosus GG together with diet (containing age‐appropriate calorie and protein levels) once a day for 3 months Control group: the same diet (containing age‐appropriate calorie and protein levels) without L rhamnosus GG |
|
| Outcomes | Primary outcome: the prevalence of infections (respiratory, gastrointestinal, and urinary infections), and hospitalisation Secondary endpoints:
|
|
| Funding and conflicts of interest statements | Funding source: not reported Conflicts of interest: not reported |
|
| Notes | The trial reported the incidence of URTIs per month, therefore we only extracted the number of participants who experienced episodes of acute URTIs in the first month. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No participants lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No information provided. |
| Other bias | Low risk | No other potential source of bias was found. |
Langkamp‐Henken 2015.
| Study characteristics | ||
| Methods | Study design: a prospective, randomised, double‐blind, placebo‐controlled trial Method of randomisation: randomisation was carried out using sealed envelopes. The study statistician (MCC) generated the stratification and randomisation scheme using a random number generator available in JMP Pro 10.0 (SAS Institute Inc), and had no direct contact with the study participants. Blinding: double‐blind: participants, provider, and assessor were blinded Duration: 6 weeks; participants were randomly assigned to receive probiotics or placebo during the 3rd week of November 2012. Participants completed daily questionnaires, consumed the study supplements, and were followed for the next 6 weeks. Exclusions postrandomisation: 0 Losses to follow‐up: 2; 2 in the probiotic bacteria group, 0 in the placebo group |
|
| Participants | Country: United States Setting: university No. of participants: 583; 436 in the probiotic bacteria group, 147 in the placebo group Age: mean 19.8 years Inclusion criteria: participants were healthy, full‐time undergraduate students aged ≥ 18 years who reported at least 1 cold in the past year. Exclusion criteria: current smokers; individuals with chronic allergies (defined as taking daily allergy medicine); those who did not have at least 1 final exam scheduled during the week of final exams; those who did not have daily access to the internet during the study period; those who were unwilling to discontinue the consumption of probiotic‐ and prebiotic‐containing foods or potentially immune‐enhancing dietary supplements (i.e. prebiotics, probiotics, Echinacea, fish oil, and vitamin E (> 400% of the RDA or > 60 mg/d)); those who received an immune‐suppressing intervention or had an immunosuppressive illness within the last year; those who received antibiotic therapy within 2 months of their study start date; those who had a cold on the 1st day of the study |
|
| Interventions | Treatment group: each supplement capsule contained 3 × 109 CFU of 1 of the following: Lactobacillus helveticus, Bifidobacterium bifidum, Bifidobacterium infantis. Control group: placebo (an identical‐looking control product that did not contain the probiotic) |
|
| Outcomes | Primary outcome: proportion of healthy days Secondary endpoints:
|
|
| Funding and conflicts of interest statements | Funding source: Lallemand Health Solutions and the University of Florida Agriculture Experiment Station Conflicts of interest: 3 authors received research support or contract funding from Lallemand Health Solutions for other projects. 1 author was employed by Lallemand Health Solutions. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The study statistician (MCC) generated the stratification and randomisation scheme using a random number generator available in JMPÒ Pro 10.0 (SAS Institute Inc), and had no direct contact with the study participants. |
| Allocation concealment (selection bias) | Low risk | Randomisation was carried out using sealed envelopes. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind: participant and provider were blinded. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessor was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 participants were lost to follow‐up, and the analysis of the study was based on intention‐to‐treat population. |
| Selective reporting (reporting bias) | Unclear risk | No information provided. |
| Other bias | Low risk | No other potential source of bias was found. |
Laursen 2017.
| Study characteristics | ||
| Methods | Study design: a randomised, double‐blind, placebo‐controlled trial Method of randomisation: randomisation was carried out using block randomisation (randomisation.com) Blinding: double‐blind: participant and provider were blinded Duration: 6 months: between September and February Exclusions postrandomisation: 5; 1 in the probiotic bacteria group, 4 in the placebo group Losses to follow‐up: 25; 13 in the probiotic bacteria group, 12 in the placebo group |
|
| Participants | Country: Denmark Setting: living in the capital region of Denmark No. of participants: 290; 144 in the probiotic bacteria group, 146 in the placebo group Age: 8 to 14 months Inclusion criteria: single born and expected to start in child care at age 8 to 14 months between September and February Exclusion criteria: birth weight < 2500 g; gestational age < 36 weeks; severe chronic illnesses; regular medication; antibiotic treatment within 4 weeks before baseline examination; non‐Danish‐speaking parents; intake of supplements or fermented milk products with probiotics for 2 weeks and during the whole intervention period; intake of other yoghurt products more than 1 to 2 meals per week |
|
| Interventions | Treatment group: sachets with 2 probiotics BB‐12 and LGG at a dose of 109 CFU/day Control group: same sachets without probiotics |
|
| Outcomes | Primary outcome: number of days absent from child care because of respiratory or gastrointestinal infections Secondary endpoints:
|
|
| Funding and conflicts of interest statements | Funding source: Innovation Fund Denmark, the University of Copenhagen, and Chr. Hansen A/S Conflicts of interest: 2 authors received a grant from Chr. Hansen for the current study and for another study. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was carried out using block randomisation (randomisation.com). |
| Allocation concealment (selection bias) | Low risk | Allocation was performed independently by a university employee with no involvement in the study. The sachets were provided to the families for the intervention period at baseline and were packed in boxes labelled with unique participant identification numbers to ensure allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind: participant and provider were blinded |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessor was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5 participants were lost to follow‐up, and the analysis of the study was based on intention‐to‐treat population. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported. |
| Other bias | Low risk | No other potential source of bias was found. |
Lazou Ahrén 2020.
| Study characteristics | ||
| Methods | Study design: randomised, double‐blind, placebo‐controlled trial Method of randomisation: randomisation was carried out using computer‐generated randomisation list. Blinding: double‐blinded: participant and provider were blinded Duration: 3 months; recruitment was initiated in September 2013 and by the end of the common cold season 2013 to 2014 Exclusions postrandomisation: 30; 17 in the probiotic bacteria group, 13 in the placebo group Losses to follow‐up: 2; 2 in the probiotic bacteria group, 0 in the placebo group |
|
| Participants | Country: Sweden Setting: day care No. of participants: 131; 63 in the probiotic bacteria group, 68 in the placebo group Age: 1 to 6 years old Inclusion criteria: healthy children attending day care and whose caregivers had given a signed informed consent were eligible for participation in the study. Exclusion criteria: significant illness (including common cold) within the 2 weeks prior to intervention, or any active systemic infection or medical condition that might require treatment or therapeutic intervention during the study; history of severe allergic reactions or anaphylaxis or any allergy to compounds of the investigational product to an extent that would jeopardise the participant or the study purpose as judged by the investigator; treatment with immune‐modulatory or ‐stimulating medication or botanicals/herbal supplements (e.g. Echinacea) within 4 weeks before randomisation in the study; antibiotic treatment 30 days before randomisation; a history or current signs of perennial allergic rhinitis or asthma; influenza vaccination within 3 months before the start of the intervention; caregiver/caregivers smoking at home; regular consumption of probiotics as food supplements in the past 3 months before randomisation; regular consumption of probiotics or probiotic fermented milk in 4 weeks prior to randomisation; participation in another clinical trial during the last 4 weeks prior to the beginning of this study; incapability to comply with the study procedures; any other reason which in the opinion of the investigator might either put the person at risk because of participation in the study, or influence the results or the person’s ability to participate in the study |
|
| Interventions | Treatment group: 2 probiotic bacterial strains (Lactobacillus plantarum HEAL9 (DSM 15312) and Lactobacillus paracasei 8700:2 (DSM 13434), 1 × 109 CFU/sachet), once daily for 3 months Control group: an identical‐looking and ‐tasting control product |
|
| Outcomes | Primary outcome: the incidence of upper respiratory infections Secondary endpoints:
|
|
| Funding and conflicts of interest statements | Funding source: Probi AB Conflicts of interest: Probi AB is the sponsor of this clinical study, and the authors of the manuscript are employed by Probi AB or were employed at the time the study was initiated. |
|
| Notes | The study reports the mean duration of URTIs for the first, second, and third episodes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was carried out using a computer‐generated randomisation list. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes were prepared for the allocation concealment and were safely stored by the principal investigator throughout the study. The labelling of the study product and the preparation of the sealed code envelopes were done by employees at Probi not otherwise involved in any study‐related activities. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind: participant and provider were blinded |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessor was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 2 participants were lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | No information provided. |
| Other bias | Low risk | No other potential source of bias was found. |
Lazou Ahrén 2021.
| Study characteristics | ||
| Methods | Study design: randomised, double‐blind, and placebo‐controlled trial Method of randomisation: randomisation was carried out using the randomisation scheme BiAS V 9.2 (2009). Blinding: double‐blind: participants and investigators were blinded Duration: 3 months: recruitment was initiated in October 2013 and completed in February 2016 during the 3 common‐cold seasons of 2013 to 2014, 2014 to 2015, and 2015 to 2016. Exclusions postrandomisation: 29; 15 in the probiotic bacteria group, 14 in the placebo group Losses to follow‐up: 0 |
|
| Participants | Country: Germany Setting: community residents No. of participants: 898; 448 in the probiotic bacteria group, 450 in the placebo group Age: 18 to 70 years old Inclusion criteria: healthy men and women (18 to 70 years of age) with susceptibility to the common cold (a minimum of 4 colds in the last 12 months) were eligible for inclusion in the study provided they agreed to refrain from major changes in their diet and physical activity and committed to not using any products that might influence the study outcome (pharmaceuticals or botanicals). Exclusion criteria: acute or chronic disease in the airways or gut; a history of nasal reconstructive surgery; the presence of nasal ulcers/nasal polyps or other conditions that could cause nasal obstruction; congenital or acquired immunodeficiency disease; Bechterew’s disease; a body temperature above 37.5 °C; suspected swine flu or influenza; vaccination with an adjuvanted vaccine within 3 months or a non‐adjuvanted vaccine within 6 weeks prior to the study start; serious organ or systemic diseases; sleep disorder; psychiatric disorders; known sensitivity to the ingredients of the investigational product; any allergic reaction or regular intake of products that might influence the study outcome (e.g. immune suppressants/immune stimulants, including paramedication, such as Echinacea, analgesics/antirheumatics, antiphlogistics, antitussives/expectorants, influenza remedies, mouth or throat therapeutics, decongestants, antibiotics, antihistaminergic drugs, probiotics) within the last 4 weeks prior to the study start; habitual usage of nasal drops/spray; pregnancy or nursing; alcohol or drug abuse; simultaneous participation in another clinical trial, or participation in a clinical trial within the last 30 days; use of other products with functional food or dietary supplements containing live bacteria cultures |
|
| Interventions | Treatment group: Probi Defendum (Lactiplantibacillus plantarum HEAL9 (DSM 15312) and Lacticaseibacillus paracasei 8700:2) (DSM 13434) 1 × 109 CFU/day for 12 weeks Control group: an identical‐looking and ‐tasting control product |
|
| Outcomes | Primary outcome: the severity of cold episodes Secondary endpoints:
|
|
| Funding and conflicts of interest statements | Funding source: Probi AB Conflicts of interest: Probi AB provided the probiotic product in this clinical study, and the authors of the manuscript are employed by Probi AB or were employed at the time the study was initiated. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was carried out using the randomisation scheme BiAS V 92 (2009). |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes were prepared for the allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind: participant and provider were blinded |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind: participant and provider were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessor was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No participants were lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported. |
| Other bias | Low risk | No other potential source of bias was found. |
Makino 2010a.
| Study characteristics | ||
| Methods | Study design: randomised, placebo‐controlled, parallel‐group intervention study Method of randomisation: not clearly stated Blinding: not clearly stated Duration: 8 weeks: 13 March 2006 to 5 February 2007 Exclusions postrandomisation: 0 Losses to follow‐up: 3; 1 in the probiotic group, 2 in the placebo group |
|
| Participants | Country: Japan Setting: Yamagata Prefecture No. of participants: 60; 30 in the probiotic bacteria group, 30 in the placebo group Age: 69 to 80 years Inclusion criteria: residents of Funagata who were in good health with no previous history of relevant physical or psychiatric illness Exclusion criteria: any recent history of virus infection, cancer, or immunological disorders and abnormalities in haematological or biochemical serum parameters |
|
| Interventions | Treatment group: the cell counts of Lactobacillus delbrueckii ssp. bulgaricus OLL1073R‐1 and Streptococcus thermophilus OLS3059 in the yoghurts were 1.8 to 3.2 × 1010 CFU/day and 5.7 to 7.9 × 1010 CFU/day, respectively. Control group: milk was used as a reference food. | |
| Outcomes |
|
|
| Funding and conflicts of interest statements | Funding source: not reported Conflicts of interest: none |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 participants were lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | No information provided. |
| Other bias | Low risk | No other potential source of bias was found. |
Merenstein 2010.
| Study characteristics | ||
| Methods | Study design: double‐blind, placebo‐controlled, randomised, patient‐oriented trial Method of randomisation: randomisation scheme was generated using SAS software by data managers; study identification was generated and a number from 0 to 9 was assigned. Blinding: double‐blind. Participant, provider, and assessor were blinded. Duration: 90 consecutive days Exclusions postrandomisation: 0 Losses to follow‐up: 74; 22 in the probiotic bacteria group, 52 in the placebo group |
|
| Participants | Country: Washington, DC, USA Setting: attending daycare centre/school 5 days a week No. of participants: 638; 314 in the probiotics group, 324 in the placebo group Age: between the ages of 3 and 6 years Gender: 309 female, 329 male: probiotics group (157 female, 157 male); placebo group (152 female, 172 male) Inclusion criteria: healthy children between the ages of 3 and 6 years attending daycare centre/school 5 days a week in Washington, DC area Exclusion criteria: taking any regular medicines at initiation of study, lactose intolerance, allergy to strawberry, inability of a parent to speak English or Spanish, active respiratory or gastrointestinal infection, or chronic disease or consuming other probiotic foods or supplements |
|
| Interventions | Treatment group: 'Actimel' contains the probiotic strain Lacticaseibacillus casei DN‐114 001/CNCM I‐1518 (also named Lactobacillus paracasei subsp. paracasei after the current nomenclature) combined with 2 cultures commonly used in yoghurt, Streptococcus thermophilus and Lactobacillus bulgaricus. 1 bottle per day, at the end of shelf life met targets of 2 × 1010 CFU/day of L casei DN‐114001; symbiotic cultures S thermophilus and L bulgaricus were also present in the final product at levels 109 CFU/day. Control group: a sweetened, flavoured, non‐fermented, acidified dairy drink without the active components of the tested product: 1 bottle per day Length of follow‐up: 90 consecutive days | |
| Outcomes | Primary outcomes:
Secondary endpoints:
|
|
| Funding and conflicts of interest statements | Funding source: The Dannon Company Conflicts of interest: 3 authors were employees of The Dannon Company during this trial; however, they did not develop the initial protocol, gather, supervise double data entry, or analyse the data. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Recruitment bias (cluster‐RCTs only) | Low risk | The clusters were randomised after individuals recruiting. |
| Baseline imbalance (cluster‐RCTs only) | Low risk | The trial reported the baseline comparability of clusters. |
| Loss of clusters (cluster‐RCTs only) | Low risk | Complete clusters were saved, and the missing outcomes for individuals within clusters did not lead to risk of bias in the trial. |
| Incorrect analysis (cluster‐RCTs only) | Low risk | The trial took the clustering into account. |
| Comparability with individually randomised trials (cluster‐RCTs only) | Low risk | The intervention effects were the same as with individually randomised trials. |
Pu 2017.
| Study characteristics | ||
| Methods | Study design: a randomised, open‐label trial Method of randomisation: randomisation was carried out using a computer‐generated random sequence Blinding: open‐label Duration: 12 weeks: 31 March and 30 June 2013 Exclusions postrandomisation: 18; 7 in the probiotic bacteria group, 11 in the placebo group Losses to follow‐up: 10; 5 in the probiotic bacteria group, 5 in the placebo group |
|
| Participants | Country: China Setting: community‐dwelling adults No. of participants: 233; 115 in the probiotic bacteria group, 118 in the placebo group Age: ≥ 45 years Inclusion criteria: healthy adults aged ≥ 45 years; lived around the West China School of Public Health, Sichuan University, Chengdu, China; be able to physically adapt to the long‐term daily consumption of 300 mL of yoghurt Exclusion criteria: were unable to communicate; had been hospitalised in the previous 3 months; were suffering from any severe acute and chronic diseases; were using long‐term antibiotics before study entry; had intolerance or an allergy to milk; had markedly abnormal results in any of immunity‐ and nutrition‐related parameters blood tests |
|
| Interventions | Treatment group: 100‐millilitre bottles of test yoghurt with living N1115 3.6 × 109 CFU Control group: none |
|
| Outcomes | Primary outcome: the incidence of all acute URTIs Secondary endpoints: changes in a series of blood indicators |
|
| Funding and conflicts of interest statements | Funding source: Shijiazhuang Junlebao Dairy Co Ltd Conflicts of interest: none |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was carried out using a computer‐generated random sequence. |
| Allocation concealment (selection bias) | High risk | Open‐label trial |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label trial |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 10 participants were lost to follow‐up, and the analysis of the study was based on intention‐to‐treat population. |
| Selective reporting (reporting bias) | Unclear risk | No information provided. |
| Other bias | Low risk | No other potential source of bias was found. |
Rautava 2009.
| Study characteristics | ||
| Methods | Study design: randomised, double‐blind, placebo‐controlled trial Method of randomisation: random allocation was generated independently from the investigators by the manufacturer of the capsules. Blinding: double‐blind: participant, provider, and assessor were blinded Duration: between September 2000 and May 2002 Losses to follow‐up: 9; 6 in the probiotic bacteria group, 3 in the placebo group |
|
| Participants | Country: Finland Setting: well‐baby clinics No. of participants: 81; 38 in the probiotic bacteria group, 43 in the placebo group Age: 0‐ to 2‐month‐old infants Gender: male 35: 16 in the probiotic bacteria group, 19 in the placebo group Inclusion criteria: need for infant formula before the age of 2 months Exclusion criteria: infants with chronic disease were excluded. |
|
| Interventions | Treatment group: 1 × 1010 CFU/day of both Lactobacillus rhamnosus and Bifidobacterium lactis BB‐12 Control group: placebo Length of follow‐up: 12 months after birth |
|
| Outcomes |
|
|
| Funding and conflicts of interest statements | Funding source: the Microbes and Man research programme, the Academy of Finland, and the Bristol‐Myer Squibb Mead Johnson Foundation Unrestricted Research Grant Conflicts of interest: not reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind: participant, provider, and assessor were blinded |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The assessor was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 11.1% participants lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No information provided. |
| Other bias | Low risk | No other potential source of bias was found. |
Rerksuppaphol 2012.
| Study characteristics | ||
| Methods | Study design: a double‐blind, placebo‐controlled, randomised clinical study with 2 parallel arms Method of randomisation: a computerised program using blocks of 2 by a person not involved in the study Blinding: double‐blind: the investigators, teachers, children, and parents were blinded Duration: November 2010 to January 2011 Exclusions postrandomisation and losses to follow‐up: 4; 2 in the probiotic group, 2 in the placebo group |
|
| Participants | Country: Thailand Setting: a public school in a rural area No. of participants: 80; 40 in the probiotic group, 40 in the placebo group Age: aged 8 to 13 years Inclusion criteria: healthy children Exclusion criteria: history of chronic illnesses such as chronic cough or chronic respiratory disease, asthma, chronic gastrointestinal conditions, behavioural or psychiatric problems or other neurological conditions, immune deficiency, diabetes mellitus, malignancy, chronic renal diseases, congenital heart diseases, or chronic liver disease were excluded. Children who were taking vitamin or mineral supplements or who had a history of any drug allergy were also excluded. |
|
| Interventions | Treatment group: Lactobacillus acidophilus (minimum of 109/capsule) and Bifidobacterium bifidum (minimum of 109/capsule) twice a day for 3 months Control group: placebo: an identical‐looking control |
|
| Outcomes |
|
|
| Funding and conflicts of interest statements | Funding source: Faculty of Medicine, Srinakharinwirot University, Thailand Conflicts of interest: not reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computerised program was used. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind: the investigators, teachers, children, and parents were blinded |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The investigators, teachers, children, and parents were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The investigators, teachers, children, and parents were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5% of participants were lost to follow‐up, and the analysis of the study was based on intention‐to‐treat population. |
| Selective reporting (reporting bias) | Unclear risk | No information provided. |
| Other bias | Low risk | No other potential source of bias was found. |
Rio 2002.
| Study characteristics | ||
| Methods | Study design: randomised, placebo‐controlled trial Method of randomisation: not clearly stated Blinding: not clearly stated Duration: during autumn and winter, April to September, at least 90 days Exclusions postrandomisation: 0 Losses to follow‐up: 42; 28 in the probiotic bacteria group, 14 in the placebo group |
|
| Participants | Country: not clearly stated Setting: study was performed on an outpatient basis, except when there were cases of pneumonia that necessitated hospitalisation No. of participants: 100; 50 in the probiotic bacteria group, 50 in the placebo group Age: between 6 and 24 months of age Gender: not clearly stated Inclusion criteria: study was conducted in 100 children between 6 and 24 months of age, selected according to the following schedule: anthropometrical children, clinically normal and healthy or malnourished Grade I or II depending on the parameter weight/height % according to the classification of Ariza Macias, without another medical condition diagnosed at baseline. Exclusion criteria: none |
|
| Interventions | Treatment group: dietary supplement of Lactobacillus acidophilus and Lactobacillus casei 250 to 300 mL of fermented milk to a concentration of 107 to 108/mL (109/1010 CFU/day) Control group: an equivalent amount of fluid milk Length of follow‐up: at least 90 days |
|
| Outcomes |
|
|
| Funding and conflicts of interest statements | Funding source: not reported Conflicts of interest: not reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 42% of participants lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No information provided. |
| Other bias | Low risk | No other potential source of bias was found. |
Santamaria 2019.
| Study characteristics | ||
| Methods | Study design: prospective, randomised, double‐blinded, placebo‐controlled clinical trial Method of randomisation: not clearly stated Blinding: double‐blinded: participants and investigators were blinded Duration: 6 months: started on October 2015, with follow‐up of the last child completed in May 2017. Participants received oral active medication or placebo for the first 10 days of each month for 4 months, and were subsequently followed up for an additional period of 2 months. Exclusions postrandomisation: 0 Losses to follow‐up: 0 |
|
| Participants | Country: Italy Setting: paediatric pulmonology unit No. of participants: 29; 13 in the probiotic bacteria group, 16 in the placebo group Age: 3 to 6 years Inclusion criteria: age 3 to 6 years; attendance at nursery school/kindergarten; diagnosis of RRI Exclusion criteria: not meeting inclusion criteria; presence of chronic medical conditions, including cardiovascular or any systemic disease, neurological disorders, primary or secondary immunodeficiency, cystic fibrosis, or primary ciliary dyskinesia; Down's syndrome; airways malformation; recurrent wheezing; administration of immunomodulators or systemic steroids in the previous 4 weeks; current acute respiratory and/or any other infection requiring hospital admission |
|
| Interventions | Treatment group: bifidobacteria mixture (Bifidobacterium longum BB536, 3 × 109 CFU; Bifidobacterium infantis M‐63, 1 x 109 105 CFU; Bifidobacterium breve M‐16 V, 1 x 109 106 CFU) as powder in 3‐gram sachet (1 sachet/day) for the first 10 days of each month for 4 months Control group: placebo: an identical‐looking and ‐tasting control |
|
| Outcomes | Primary outcome: the morbidity of RRI Secondary endpoints: modify the urine metabolomic profile of preschool children with RRI |
|
| Funding and conflicts of interest statements | Funding source: an unrestricted grant (Medical Department, Valeas Spa) Conflicts of interest: none |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was carried out using a randomisation list. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind: participant and provider were blinded |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessor was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No participants lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No information provided. |
| Other bias | Low risk | No other potential source of bias was found. |
Shida 2017.
| Study characteristics | ||
| Methods | Study design: randomised controlled trial Method of randomisation: not clearly stated Blinding: not clearly stated Duration: 12 weeks: from 8 December 2012 through 5 March 2013 Exclusions postrandomisation: 3; 1 in the probiotic bacteria group, 2 in the placebo group Losses to follow‐up: 0 |
|
| Participants | Country: Japan Setting: working within office buildings No. of participants: 100; 50 in the probiotic bacteria group, 50 in the placebo group Age: 30 to 49 years Inclusion criteria: healthy male workers living in Tokyo or its suburbs Exclusion criteria: working outside the office building twice or more a week; difficulty providing saliva and blood samples; pollinosis, chronic rhinitis, asthma, or milk allergy; periodontitis or gingivitis; history of serious liver, kidney, heart, lung, or gut disease; receiving current medical treatment; regularly consuming probiotics or fermented milk; taking drugs or supplements that might affect the outcome of the study; history of influenza vaccination or infection within the last 6 months; and being deemed ineligible for this study by a physician, based on blood chemistry, blood pressure, pulse rate, or other reasons |
|
| Interventions | Treatment group: Lactobacillus casei strain Shirota fermented milk Control group: control milk |
|
| Outcomes | Primary outcome: incidence of URTIs Secondary endpoints:
|
|
| Funding and conflicts of interest statements | Funding source: none Conflicts of interest: all of the authors are employed by the Yakult Honsha, which produces fermented dairy products using the probiotic strain LcS. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 participants were lost to follow‐up, and the analysis of the study was based on intention‐to‐treat population. |
| Selective reporting (reporting bias) | Unclear risk | No information provided. |
| Other bias | Low risk | No other potential source of bias was found. |
Smith 2013.
| Study characteristics | ||
| Methods | Study design: a double‐blind, placebo‐controlled, randomised clinical study with 2 parallel arms Method of randomisation: using an internet‐based random number generator (GraphPad Random Number Generator, 2005) Blinding: double‐blind: the participants, investigators, and outcome assessors were blinded Duration: February to May 2011 Exclusions postrandomisation: 23; 13 in the probiotic group, 20 in the placebo group Losses to follow‐up: 18; 6 in the probiotic group, 12 in the placebo group |
|
| Participants | Country: United States Setting: Framingham State University No. of participants: 198; 97 in the probiotic group, 101 in the placebo group Age: aged 18 to 24 years Inclusion criteria: all students living on campus in residence halls Exclusion criteria: under 18 years of age or over 25 years of age; experienced chronic perennial allergies; pregnant; with medical conditions affecting immune function; acute pancreatitis, undergoing treatment for cancer, or taking immunosuppressive drugs for an autoimmune disease or post‐transplant |
|
| Interventions | Treatment group: 109 CFU Lactobacillus rhamnosus LGG and Bifidobacterium animalis ssp. lactis BB‐12 in powder form (Chr. Hansen A/S)/stick/day for 12 weeks Control group: placebo: an identical‐looking and ‐tasting control |
|
| Outcomes |
|
|
| Funding and conflicts of interest statements | Funding source: Chr. Hansen A/S (Hoersholm, Denmark) Conflicts of interest: none |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Internet‐based random number generator (GraphPad Random Number Generator, 2005) |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind: the investigators and participants were blinded |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Investigators and participants were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 9% of participants were lost to follow‐up, and the analysis of the study was based on intention‐to‐treat population. |
| Selective reporting (reporting bias) | Unclear risk | No information provided. |
| Other bias | Low risk | No other potential source of bias was found. |
Taipale 2016.
| Study characteristics | ||
| Methods | Study design: double‐blind, placebo‐controlled trial Method of randomisation: randomisation was carried out using a computer‐generated randomisation list. Blinding: double‐blind: participants, provider, and assessor were blinded Duration: 2 years; participants were recruited from Muurame and Korpilahti, Finland, between September 2004 and February 2007 Exclusions postrandomisation: 34; 17 in the probiotic bacteria group, 17 in the placebo group Losses to follow‐up: 11; 7 in the probiotic bacteria group, 4 in the placebo group |
|
| Participants | Country: Finland Setting: well‐baby clinics No. of participants: 109; 55 in the probiotic bacteria group, 54 in the placebo group Age: 1 month old Inclusion criteria: the child was healthy; the parents agreed to use the novel slow‐release pacifier; the child started to use the pacifier before the age of 2 months. Exclusion criteria: moving out of the area; miscarriage; and lack of interest in the trial |
|
| Interventions | Treatment group: Bifidobacterium animalis subsp. lactis BB‐12 (5.0 × 1010 CFU) with xylitol (the smaller tablet contained 100 mg xylitol, the larger tablet 300 mg xylitol) twice a day Control group: xylitol in respective amounts |
|
| Outcomes | Primary outcome: the prevalence of overall acute infections occurring before the age of 2 years Secondary endpoints: successful intestinal passage of BB‐12 |
|
| Funding and conflicts of interest statements | Funding source: not reported Conflicts of interest: none |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was carried out using a computer‐generated randomisation list. |
| Allocation concealment (selection bias) | Low risk | All tablets were manufactured by Oy Karl Fazer Ab (Vantaa, Finland) and packed in white plastic bottles with colour codes. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind: participant and provider were blinded |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessor was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 10% participants lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No information provided. |
| Other bias | Low risk | No other potential source of bias was found. |
Vrese 2005.
| Study characteristics | ||
| Methods | Study design: randomised, double‐blind, placebo‐controlled, parallel‐group intervention study Method of randomisation: not clearly stated Blinding: double‐blind: participant and assessor were blinded Duration: 242 participants during a 3‐month period (between January and May 2001); 237 participants during a 5.5‐month period (between December 2001 and June 2002) Exclusions postrandomisation: 0 Losses to follow‐up: 25; 13 in the probiotic bacteria group, 12 in the placebo group |
|
| Participants | Country and setting: community‐dwelling adults No. of participants: 479; 238 in the probiotic bacteria group, 241 in the placebo group Age: (average age 38 ± 13): probiotic bacteria group (average age 37 ± 12); placebo group (average age 38 ± 14) Gender: male: 185; 86 in the probiotic bacteria group, 99 in the placebo group Inclusion criteria: 479 healthy women and men were included after physical examination. Exclusion criteria: those with laboratory parameters outside the normal range, known congenital or acquired immune defects, allergies and other chronic or acute diseases requiring treatment, alcohol or drug misuse or both, pregnancy or lactation, interfering dietary habits, or vaccination against influenza within the last 12 months were excluded. |
|
| Interventions | Treatment group: 5 × 107 CFU of the spray‐dried probiotic bacteria with vitamins and minerals. (The probiotic strains used in this study were Lactobacillus gasseri PA 16/8, Bifidobacterium longum SP 07/3, and Bifidobacterium bifidum MF 20/5.) Control group: just the vitamin‐mineral preparation Length of follow‐up: 8.5 months |
|
| Outcomes |
|
|
| Funding and conflicts of interest statements | Funding source: Merck Consumer Health Care Conflicts of interest: none |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participant and assessor were blinded. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5.2% of participants lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No information provided. |
| Other bias | Low risk | No other potential source of bias was found. |
AOM: acute otitis media ARI: acute respiratory infection BMI: body mass index CFU: colony‐forming units CIDs: common infectious diseases FOS: fructo‐oligosaccharides GABHS: Group A beta‐haemolytic Streptococcus GI: gastrointestinal GOS: galacto‐oligosaccharides HRQL: health‐related quality of life IcFOS: long‐chain fructo‐oligosaccharides kcal: kilocalorie LcS: Lactobacillus casei strain Shirota LGG: Lactobacillus rhamnosus GG LRTIs: lower respiratory tract infections NK: natural killer RDA: Recommended Dietary Allowance RRI: recurrent respiratory infections scGOS: short‐chain galacto‐oligosaccharides SD: standard deviation sIgA: secretory immunoglobulin A URI: upper respiratory infection URTIs: upper respiratory tract infections
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Agustina 2012 | The study did not separate URTIs from other respiratory infections. |
| Altadill 2021 | The study only reported the total days of acute URTIs and not the mean duration of an episode of acute URTIs. |
| Anaya‐Loyola 2019 | The study only reported cold or influenza‐like symptoms and did not diagnose URTIs. |
| Arslanoglu 2008 | The study used prebiotics (GOS and FOS). |
| Aryayev 2018 | Not an RCT |
| Campanella 2018 | The study did not separate URTIs from other respiratory infections. |
| Corsello 2017 | The interventions had "no living organisms". |
| Di Pierro 2014 | The study is a non‐randomised trial. |
| Di Pierro 2021 | The study only reported the signs and symptoms of URTIs and did not diagnose URTIs. |
| Dziechciarz 2020 | The study did not separate URTIs from other respiratory infections. |
| Gerasimov 2016 | The study did not separate URTIs from other respiratory infections. |
| Gil‐Campos 2012 | The study did not separate URTIs from other respiratory infections. |
| Gleeson 2010 | The study focused on endurance‐training athletes. |
| Gleeson 2012 | The study focused on endurance‐training athletes. |
| Guillemard 2010 | The study included participants vaccinated against the influenza virus. |
| Gutierrez‐Castrellon 2014 | The study did not separate URTIs from other respiratory infections. |
| Hatakka 2001 | The study did not separate URTIs from other respiratory infections. |
| Hatakka 2007 | The participants in this study were otitis‐prone children, which may be associated with immunodeficency. |
| Haywood 2014 | This is a cross‐over study. |
| Hishiki 2020 | The study did not separate URTIs from other influenza virus infections. |
| Hojsak 2015 | Supplementation of probiotic was less than 7 days. |
| Hojsak 2016 | The study separately reported the number of participants with different URTIs during the intervention period. Neither the total number of participants who experienced episodes of acute URTIs nor the overall events of URTIs was clear. |
| Hor 2018 | The study only reported cold or influenza‐like symptoms and did not diagnose URTIs. |
| Kekkonen 2007 | The study focused on marathon‐running athletes. |
| Kloster Smerud 2008 | The study did not separate URTIs from other respiratory infections. |
| Kosek 2019 | The study only reported cold or influenza‐like symptoms and did not diagnose URTIs. |
| Kukkonen 2008 | The study did not separate URTIs from other respiratory infections and did not separate AOM from middle ear infections. |
| Kumpu 2012 | The study did not separate URTIs from other respiratory infections. |
| Kumpu 2013 | The study did not separate URTIs from other respiratory infections. |
| Lau 2018 | The study only reported cold or influenza‐like symptoms and did not diagnose URTIs. |
| Lefevre 2015 | The study did not separate URTIs from other respiratory infections. |
| Lehtoranta 2012 | The study only analysed the bocavirus in the nasopharynx and included children who had had at least 3 episodes during the preceding 12 months. |
| Leyer 2009 | The study only reported cold or influenza‐like symptoms and did not diagnose URTIs. |
| Li 2014 | The study did not separate URTIs from other respiratory infections. |
| Lin 2009 | The study compared 2 different probiotics. |
| Little 2017 | This is a treatment study. |
| Luoto 2013 | The study only reported symptoms of respiratory tract infection and did not diagnose URTIs. |
| Mai 2021 | The study did not separate URTIs from other respiratory infections. |
| Makino 2010b | The Arita study was reported in this trial, but was not an RCT. |
| Maldonado 2012 | Roughly 70% of included participants were vaccinated against rotavirus. |
| Maldonado‐Lobón 2015 | The study did not separate URTIs from other respiratory infections. |
| Manti 2020 | The study only reported cold or influenza‐like symptoms and did not diagnose URTIs in the children with recurrent respiratory infections. |
| Maya‐Barrios 2021 | This is a treatment study. |
| Meng 2016 | Not an RCT |
| Michael 2020 | The study only reported the symptoms of URTI and no diagnosis of rhinitis. |
| Moyad 2010 | The study did not use probiotics as the intervention. |
| Mullish 2021 | The study only reported the symptoms of URTIs and no diagnosis of rhinitis. |
| Murata 2018 | The intervention groups of the study are “heat‐killed L. paracasei MCC1849 cells”, and there are no living organisms. |
| Nocerino 2017 | There are no living organisms in the intervention groups. |
| Pitkaranta 2003 | The study was published as an abstract. We cannot find the unpublished data, and data for extraction from the study were inadequate. |
| Pregliasco 2008 | The study used symbiotic formulas: probiotics plus prebiotics (FOS/GOS). |
| Ringel‐Kulka 2015 | The study used symbiotic formulas: probiotics plus prebiotics. |
| Sanz 2006 | Not a proper RCT, as study had only 2 clusters. |
| Stojković 2016 | The study used symbiotic formulas: probiotics plus prebiotics. |
| Tajima 1995 | Not an RCT |
| Tiollier 2007 | The study did not separate URTIs from other respiratory infections. |
| Turchet 2003 | 82% of participants had been vaccinated against influenza 3 months before the study, and the study did not separate URTIs from other respiratory infections. |
| Turner 2017 | The study only reported rhinorrhoea or nasal obstruction symptom scores and no diagnosis of rhinitis. |
| West 2011 | The study focused on cyclists and triathletes. |
| West 2014 | The participants in this study included competitive athletes at a regional level. |
| Zhang 2018 | The study used placebo yoghurt drink that contained probiotics as the control. |
AOM: acute otitis media FOS: fructo‐oligosaccharides GOS: galacto‐oligosaccharides RCT: randomised controlled trial URTIs: upper respiratory tract infections
Characteristics of studies awaiting classification [ordered by study ID]
Kaplan 1968.
| Methods | Details of this study were not available. |
| Participants | Unknown |
| Interventions | Unknown |
| Outcomes | Unknown |
| Notes | Unknown |
Marushko 2000.
| Methods | Details of this study were not available. |
| Participants | Unknown |
| Interventions | Unknown |
| Outcomes | Unknown |
| Notes | Unknown |
Characteristics of ongoing studies [ordered by study ID]
NCT01782118.
| Study name | LGG for prevention of infectious complications during PPI treatment in children |
| Methods | Randomised, double‐blind, placebo‐controlled trial |
| Participants | Children aged 1 month to 5 years |
| Interventions | Lactobacillus rhamnosus GG |
| Outcomes | Number of upper and lower respiratory tract infections during intervention plus 3 months after termination of the intervention |
| Starting date | February 2013 |
| Contact information | katarzynakrenke@gmail.com |
| Notes |
LGG: Lactobacillus rhamnosus GG PPI: proton pump inhibitors
Differences between protocol and review
Changes from the 2015 update
There is increasing awareness that vaccination is the most effective means of preventing influenza infection. In the 2019 to 2020 influenza season, seasonal flu vaccination coverage was 63.8% amongst children and 48.4% amongst adults ≥ 18 years in the United States (Flu Vaccination Coverage, United States) and 42% amongst adults ≥ 18 years in Canada (Vaccine uptake in Canadian adults). Given the high influenza vaccination rate, it would be hard to include new trials in which no one had received the seasonal influenza vaccination within the previous 12 months. As a result, in this version of the review we replaced the exclusion criterion 'those who had been vaccinated against influenza or other acute URTIs within the last 12 months' with 'studies where more than 50% of participants had been vaccinated against influenza or other acute URTIs within the last 12 months' and 'studies that had significantly different proportions of vaccinated participants between the probiotics arm and the placebo or no treatment arm'. We also replaced the methods of analysis according to the new version of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021). It is very unlikely to achieve the goal of randomisation when there are few clusters per group in cluster‐randomised controlled trials (cluster‐RCTs). In the current version of the review, we added an exclusion criterion of cluster‐RCTs with fewer than four clusters per group, and excluded Sanz 2006, which only had two clusters. We evaluated the risk of bias and calculated the design effects and the numbers of events of cluster‐RCTs according to Higgins 2011. We assessed the heterogeneity of each outcome according to Higgins 2021. We added contour‐enhanced funnel plots to differentiate the asymmetry that was due to non‐reporting biases or other factors. We reported risk ratio and rate ratios rather than odds ratio and the number needed to treat, as the included studies were all RCTs, and outcomes are not rare. As the mixed model adjusts more effect factors, it may be better at reflecting the effect size of probiotics for upper respiratory tract infection (URTI). We chose the effect size of the incidence rate from the study if the study used the mixed model to calculate the incidence rate. We used the GRADE approach to assess the overall certainty of the evidence following the instructions in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021). We included all outcomes in a single summary of findings table. We added subgroup analyses of treatment dose, treatment duration, and type of comparator.
Previous changes from protocol
We excluded studies conducted amongst otitis‐prone children, as these children may have immunodeficiency (Bardou 2020; Pichichero 2016; Pichichero 2020). To clarify our outcomes, we specified that cases of acute URTIs should have been confirmed by doctors, or have specific symptoms. We added 'number of participants who were diagnosed with acute URTIs (at least three events)' and 'incidence rate of acute URTIs' to our primary outcomes. Due to the complex composition of herbal medicines, we removed the outcome using herbal medicines for acute URTIs. Since our aim was to verify whether probiotics are beneficial for preventing acute URTIs, we removed the outcome 'number of participants who experienced acute lower respiratory tract infections'. We did not include all respiratory tract infections because many studies reported only respiratory tract infection, rather than specifying lower or upper respiratory tract infection, which may increase the levels of clinical heterogeneity. We used a random‐effects model to conduct the analysis, as the random‐effects model meta‐analysis is a more conservative estimate than the fixed‐effect model. We could not conduct subgroup analysis according to the type of probiotics and chronic diseases in this version due to the limited number of included studies.
Contributions of authors
Yunli Zhao (YZ) searched for trials, assessed the certainty of the evidence, extracted data, analysed data, and drafted the review. Bi Rong Dong (BD) advised and assisted in writing the protocol and the review, searched for trials, and developed the review. Qiukui Hao (QH) contributed to the development of the methods of the review and assisted with data extraction and analysis.
Sources of support
Internal sources
Chinese Cochrane Center, West China Hospital of Sichuan University, China
External sources
Editorial base and team of the Cochrane Acute Respiratory Infections Group, Australia
Declarations of interest
Yunli Zhao: has declared that they have no conflict of interest. Bi Rong Dong: has declared that they have no conflict of interest. Qiukui Hao: has declared that they have no conflict of interest.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Andaloro 2019 {published data only}
- Andaloro C, Santagati M, Stefani S, La Mantia I. Bacteriotherapy with Streptococcus salivarius 24SMB and Streptococcus oralis 89a oral spray for children with recurrent streptococcal pharyngotonsillitis: a randomized placebo-controlled clinical study. European Archives of Oto-Rhino-Laryngology 2019;276(3):879-87. [DOI: 10.1007/s00405-019-05346-3] [DOI] [PubMed] [Google Scholar]
Berggren 2011 {published data only}
Butler 2020 {published data only}
- Butler CC, Lau M, Gillespie D, Owen-Jones E, Lown M, Wootton M, et al. Effect of probiotic use on antibiotic administration among care home residents: a randomized clinical trial. JAMA 2020;324(1):47-56. [DOI: 10.1001/jama.2020.8556] [DOI] [PMC free article] [PubMed] [Google Scholar]
Caceres 2010 {published data only}
- Caceres P, Montes S, Vega N, Cruchet S, Brunser O, Gotteland M. Effects of Lactobacillus rhamnosus HN001 on acute respiratory infections and intestinal secretory IgA in children. Journal of Pediatric Infectious Diseases 2010;5(4):353-62. [Google Scholar]
Damholt 2022 {published data only}
- Damholt A, Keller MK, Baranowski K, Brown B, Wichmann A, Melsaether C, et al. Lacticaseibacillus rhamnosus GG DSM 33156 effects on pathogen defence in the upper respiratory tract: a randomised, double-blind, placebo-controlled paediatric trial. Beneficial Microbes 2022;13(1):13-23. [DOI] [PubMed] [Google Scholar]
Fujita 2013 {published data only}
- Fujita R, Iimuro S, Shinozaki T, Sakamaki K, Uemura Y, Takeuchi A, et al. Decreased duration of acute upper respiratory tract infections with daily intake of fermented milk: a multicenter, double-blinded, randomized comparative study in users of day care facilities for the elderly population. American Journal of Infection Control 2013;41(12):1231-5. [DOI] [PubMed] [Google Scholar]
Hojsak 2010a {published data only}
- Hojsak I, Snovak N, Abdovic S, Szajewska H, Misak Z, Kolacek S. Lactobacillus GG in the prevention of gastrointestinal and respiratory tract infections in children who attend day care centers: a randomized, double-blind, placebo-controlled trial. Clinical Nutrition 2010;29(3):312-6. [DOI] [PubMed] [Google Scholar]
Hojsak 2010b {published data only}
- Hojsak I, Abdovic S, Szajewska H, Milosevic M, Krznaric Z, Kolacek S. Lactobacillus GG in the prevention of nosocomial gastrointestinal and respiratory tract infections. Pediatrics 2010;125(5):e1171-7. [DOI] [PubMed] [Google Scholar]
Kara 2019 {published data only}
- Kara SS, Volkan B, Erten I. Lactobacillus rhamnosus GG can protect malnourished children. Beneficial Microbes 2019;10(3):237-44. [DOI: 10.3920/BM2018.0071] [DOI] [PubMed] [Google Scholar]
Langkamp‐Henken 2015 {published data only}
- Langkamp-Henken B, Rowe CC, Ford AL, Christman MC, Nieves C Jr, Khouri L, et al. Bifidobacterium bifidum R0071 results in a greater proportion of healthy days and a lower percentage of academically stressed students reporting a day of cold/flu: a randomised, double-blind, placebo-controlled study. British Journal of Nutrition 2015;113(3):426-34. [DOI] [PubMed] [Google Scholar]
Laursen 2017 {published data only}
- Laursen RP, Larnkjaer A, Ritz C, Hauger H, Michaelsen KF, Mølgaard C. Probiotics and child care absence due to infections: a randomized controlled trial. Pediatrics 2017;140(2):e20170735. [DOI: 10.1542/peds.2017-0735] [DOI] [PubMed] [Google Scholar]
Lazou Ahrén 2020 {published data only}
- Lazou Ahrén I, Berggren A, Teixeira C, Martinsson Niskanen T, Larsson N. Evaluation of the efficacy of Lactobacillus plantarum HEAL9 and Lactobacillus paracasei 8700:2 on aspects of common cold infections in children attending day care: a randomised, double-blind, placebo-controlled clinical study. European Journal of Nutrition 2020;59(1):409-17. [DOI: 10.1007/s00394-019-02137-8] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lazou Ahrén 2021 {published data only}
- Lazou Ahrén I, Hillman M, Nordström EA, Larsson N, Niskanen TM. Fewer community-acquired colds with daily consumption of Lactiplantibacillus plantarum HEAL9 and Lacticaseibacillus paracasei 8700:2. A randomised, placebo-controlled clinical trial. Journal of Nutrition 2021;151(1):214-22. [DOI: 10.1093/jn/nxaa353] [DOI] [PMC free article] [PubMed] [Google Scholar]
Makino 2010a {published data only (unpublished sought but not used)}
- Makino S, Ikegami S, Kume A, Horiuchi H, Sasaki H, Orii N. Reducing the risk of infection in the elderly by dietary intake of yoghurt fermented with Lactobacillus delbrueckii ssp. bulgaricus OLL1073R-1. British Journal of Nutrition 2010;104(7):998-1006. [DOI] [PubMed] [Google Scholar]
Merenstein 2010 {published data only}
- Merenstein D, Murphy M, Fokar A, Hernandez RK, Park H, Nsouli H, et al. Use of a fermented dairy probiotic drink containing Lactobacillus casei (DN-114 001) to decrease the rate of illness in kids: the DRINK study. A patient-oriented, double-blind, cluster-randomized, placebo-controlled, clinical trial. European Journal of Clinical Nutrition 2010;64(7):669-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pu 2017 {published data only}
- Pu F, Guo Y, Li M, Zhu H, Wang S, Shen X, et al. Yogurt supplemented with probiotics can protect the healthy elderly from respiratory infections: a randomized controlled open-label trial. Clinical Interventions in Aging 2017;12:1223-31. [DOI: 10.2147/CIA.S141518] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rautava 2009 {published data only}
- Rautava S, Salminen S, Isolauri E. Specific probiotics in reducing the risk of acute infections in infancy - a randomised, double-blind, placebo-controlled study. British Journal of Nutrition 2009;101(11):1722-6. [DOI] [PubMed] [Google Scholar]
Rerksuppaphol 2012 {published data only}
- Rerksuppaphol S, Rerksuppaphol L. Randomized controlled trial of probiotics to reduce common cold in schoolchildren. Pediatrics International 2012;54(5):682-7. [DOI] [PubMed] [Google Scholar]
Rio 2002 {published data only}
- Rio ME, Zago Beatriz L, Garcia H, Winter L. The nutritional status change the effectiveness of a dietary supplement of lactic bacteria on the emerging of respiratory tract diseases in children [El estado nutricional modifica la efectividad de un suplemento dietario de bacterias lácticas sobre la aparición de patologías de vías respiratorias en niños]. Archivos Latinoamericanos de Nutricion 2002;52(1):29-34. [PubMed] [Google Scholar]
Santamaria 2019 {published data only}
- Santamaria F, Montella S, Stocchero M, Pirillo P, Bozzetto S, Giordano G, et al. Effects of pidotimod and bifidobacteria mixture on clinical symptoms and urinary metabolomic profile of children with recurrent respiratory infections: a randomized placebo-controlled trial. Pulmonary Pharmacology and Therapeutics 2019;58:101818. [DOI: 10.1016/j.pupt.2019.101818] [DOI] [PubMed] [Google Scholar]
Shida 2017 {published data only}
- Shida K, Sato T, Iizuka R, Hoshi R, Watanabe O, Igarashi T, et al. Daily intake of fermented milk with Lactobacillus casei strain Shirota reduces the incidence and duration of upper respiratory tract infections in healthy middle-aged office workers. European Journal of Nutrition 2017;56(1):45-53. [DOI: 10.1007/s00394-015-1056-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
Smith 2013 {published data only}
- Smith TJ, Rigassio-Radler D, Denmark R, Haley T, Touger-Decker R. Effect of Lactobacillus rhamnosus LGGw and Bifidobacterium animalis ssp. lactis BB-12w on health-related quality of life in college students affected by upper respiratory infections. British Journal of Nutrition 2013;109(11):1999-2007. [DOI] [PubMed] [Google Scholar]
Taipale 2016 {published data only}
- Taipale TJ, Pienihäkkinen K, Isolauri E, Jokela JT, Söderling EM. Bifidobacterium animalis subsp. lactis BB-12 in reducing the risk of infections in early childhood. Pediatric Research 2016;79(1-1):65-9. [DOI: 10.1038/pr.2015.174.] [DOI] [PubMed] [Google Scholar]
Vrese 2005 {published data only}
- Vrese DM, Winkler P, Rautenberg P, Harder T, Noah C, Laue C, et al. Effect of Lactobacillus gasseri PA 16/8, Bifidobacterium longum SP 07/3, B. bifidum MF 20/5 on common cold episodes: a double blind, randomized, controlled trial. Clinical Nutrition 2005;24(4):481-91. [DOI] [PubMed] [Google Scholar]
- Vrese DM, Winkler P, Rautenberg P, Harder T, Noah C, Laue C, et al. Probiotic bacteria reduced duration and severity but not the incidence of common cold episodes in a double blind, randomized, controlled trial. Vaccine 2006;24(44-6):6670-4. [DOI] [PubMed] [Google Scholar]
- Winkler P, Vrese DM, Laue CH, Schrezenmeir J. Effect of a dietary supplement containing probiotic bacteria plus vitamins and minerals on common cold infections and cellular immune parameters. International Journal of Clinical Pharmacology and Therapeutics 2005;43(7):318-26. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Agustina 2012 {published data only}
- Agustina R, Kok FJ, de Rest O, Fahmida U, Firmansyah A, Lukito W, et al. Randomized trial of probiotics and calcium on diarrhea and respiratory tract infections in Indonesian children. Pediatrics 2012;129(5):e1155-64. [DOI] [PubMed] [Google Scholar]
Altadill 2021 {published data only}
- Altadill T, Espadaler-Mazo J, Liong MT. Effects of a Lactobacilli probiotic on reducing duration of URTI and fever, and use of URTI-associated medicine: a re-analysis of a randomized, placebo-controlled study. Microorganisms 2021;9(3):528. [DOI: 10.3390/microorganisms9030528] [DOI] [PMC free article] [PubMed] [Google Scholar]
Anaya‐Loyola 2019 {published data only}
- Anaya-Loyola MA, Enciso-Moreno JA, López-Ramos JE, García-Marín G, Orozco Álvarez MY, Vega-García AM, et al. Bacillus coagulans GBI-30, 6068 decreases upper respiratory and gastrointestinal tract symptoms in healthy Mexican scholar-aged children by modulating immune-related proteins. Food Research International 2019;125:108567. [DOI: 10.1016/j.foodres.2019.108567] [DOI] [PubMed] [Google Scholar]
Arslanoglu 2008 {published data only}
- Arslanoglu S, Moro GE, Schmitt J, Tandoi L, Rizzardi S, Boehm G. Early dietary intervention with a mixture of prebiotic oligosaccharides reduces the incidence of allergic manifestations and infections during the first two years of life. Journal of Nutrition 2008;128(6):1091-5. [DOI] [PubMed] [Google Scholar]
Aryayev 2018 {published data only}
- Aryayev ML, Senkivska LI, Bredeleva NK, Talashova IV. Prophylaxis of acute respiratory infections via improving the immune system in late preterm newborns with E. coli strain Nissle 1917: a controlled pilot trial. Pilot Feasibility Study 2018;4:79. [DOI: 10.1186/s40814-018-0271-y] [DOI] [PMC free article] [PubMed] [Google Scholar]
Campanella 2018 {published data only}
- Campanella V, Syed J, Santacroce L, Saini R, Ballini A, Inchingolo F. Oral probiotics influence oral and respiratory tract infections in pediatric population: a randomized double-blinded placebo-controlled pilot study. European Review for Medical and Pharmacological Sciences 2018;22(22):8034-41. [DOI: 10.26355/eurrev_201811_16433] [DOI] [PubMed] [Google Scholar]
Corsello 2017 {published data only}
- Corsello G, Carta M, Marinello R, Picca M, De Marco G, Micillo M, et al. Preventive effect of cow's milk fermented with Lactobacillus paracasei CBA L74 on common infectious diseases in children: a multicenter randomized controlled trial. Nutrients 2017;9(7):669. [DOI: 10.3390/nu9070669] [DOI] [PMC free article] [PubMed] [Google Scholar]
Di Pierro 2014 {published data only}
- Di Pierro F, Colombo M, Zanvit A, Risso P, Rottoli AS. Use of Streptococcus salivarius K12 in the prevention of streptococcal and viral pharyngotonsillitis in children. Drug, Healthcare and Patient Safety 2014;13(6):15-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Di Pierro 2021 {published data only}
- Di Pierro F, Lo Russo P, Danza ML, Basile I, Soardo S, Capocasale G, et al. Use of a probiotic mixture containing Bifidobacterium animalis subsp. lactis BB-12 and Enterococcus faecium L3 as prophylaxis to reduce the incidence of acute gastroenteritis and upper respiratory tract infections in children. Minerva Pediatrica (Torino) 2021;73(3):222-9. [DOI: 10.23736/S0026-4946.20.05925-3] [DOI] [PubMed] [Google Scholar]
Dziechciarz 2020 {published data only}
- Dziechciarz P, Krenke K, Szajewska H, Horvath A. Lactobacillus rhamnosus GG usage in the prevention of gastrointestinal and respiratory tract infections in children with gastroesophageal reflux disease treated with proton pump inhibitors: a randomized double-blinded placebo-controlled trial. European Review for Medical and Pharmacological Sciences 2020;23(3):251-8. [DOI: 10.5223/pghn.2020.23.3.251] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gerasimov 2016 {published data only}
- Gerasimov SV, Ivantsiv VA, Bobryk LM, Tsitsura OO, Dedyshin LP, Guta NV, et al. Role of short-term use of L. acidophilus DDS-1 and B. lactis UABLA-12 in acute respiratory infections in children: a randomized controlled trial. European Journal if Clinical Nutrition 2016;70(4):463-9. [DOI: 10.1038/ejcn.2015.171] [DOI] [PubMed] [Google Scholar]
Gil‐Campos 2012 {published data only}
- Gil-Campos M, López MÁ, Rodriguez-Benítez MV, Romero J, Roncero I, Linares MD, et al. Lactobacillus fermentum CECT 5716 is safe and well tolerated in infants of 1-6 months of age: a randomized controlled trial. Pharmacological Research 2012;65(2):231-8. [DOI] [PubMed] [Google Scholar]
Gleeson 2010 {published data only}
- Gleeson M, Bishop NC, Oliveira M, Tauler P. Daily probiotic's (Lactobacillus casei Shirota) reduction of infection incidence in athletes. International Journal of Sport Nutrition & Exercise Metabolism 2011;21(1):55-64. [DOI] [PubMed] [Google Scholar]
Gleeson 2012 {published data only}
- Gleeson M, Bishop NC, Oliveira M, McCauley T, Tauler P, Lawrence C. Effects of a Lactobacillus salivarius probiotic intervention on infection, cold symptom duration and severity, and mucosal immunity in endurance athletes. International Journal of Sport Nutrition and Exercise Metabolism 2012;22(4):235-42. [DOI] [PubMed] [Google Scholar]
Guillemard 2010 {published data only}
- Guillemard E, Tondu F, Lacoin F, Schrezenmeir J. Consumption of a fermented dairy product containing the probiotic Lactobacillus casei DN-114001 reduces the duration of respiratory infections in the elderly in a randomised controlled trial. British Journal of Nutrition 2010;103(1):58-68. [DOI] [PubMed] [Google Scholar]
Gutierrez‐Castrellon 2014 {published data only}
- Gutierrez-Castrellon P, Lopez-Velazquez G, Diaz-Garcia L, Jimenez-Gutierrez C, Mancilla-Ramirez J, Estevez-Jimenez J, et al. Diarrhea in preschool children and Lactobacillus reuteri: a randomized controlled trial. Pediatrics 2014;133(4):e904-9. [DOI] [PubMed] [Google Scholar]
Hatakka 2001 {published data only}
- Hatakka K, Savilahti E, Ponka A, Meurman JH, Poussa T, Nase L, et al. Effect of long term consumption of probiotic milk on infections in children attending day care centres: double blind, randomised trial. BMJ 2001;322(1298):1327-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hatakka 2007 {published data only}
- Hatakka K, Blomgren K, Pohjavuori S, Kaijalainen T, Poussa T, Leinonen M, et al. Treatment of acute otitis media with probiotics in otitis-prone children - a double-blind, placebo-controlled randomised study. Clinical Nutrition 2007;26(3):314-21. [DOI] [PubMed] [Google Scholar]
Haywood 2014 {published data only}
- Haywood BA, Black KE, Baker D, McGarvey J, Healey P, Brown RC. Probiotic supplementation reduces the duration and incidence of infections but not severity in elite rugby union players. Journal of Science and Medicine in Sport 2014;17(4):356-60. [DOI] [PubMed] [Google Scholar]
Hishiki 2020 {published data only}
- Hishiki H, Kawashima T, Tsuji NM, Ikari N, Takemura R, Kido H, et al. A double-blind, randomized, placebo-controlled trial of heat-killed Pediococcus acidilactici K15 for prevention of respiratory tract infections among preschool children. Nutrients 2020;12(7):1989. [DOI: 10.3390/nu12071989] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hojsak 2015 {published data only}
- Hojsak I, Tokić Pivac V, Močić Pavić A, Pasini AM, Kolaček S. Bifidobacterium animalis subsp. lactis fails to prevent common infections in hospitalized children: a randomized, double-blind, placebo-controlled study. American Journal of Clinical Nutrition 2015;101(3):680-4. [DOI: 10.3945/ajcn.114.102004] [DOI] [PubMed] [Google Scholar]
Hojsak 2016 {published data only}
- Hojsak I, Močić Pavić A, Kos T, Dumančić J, Kolaček S. Bifidobacterium animalis subsp. lactis in prevention of common infections in healthy children attending day care centers - randomized, double blind, placebo-controlled study. Clinical Nutrition 2016;35(3):587-91. [DOI: 10.1016/j.clnu.2015.05.004] [DOI] [PubMed] [Google Scholar]
Hor 2018 {published data only}
- Hor YY, Lew LC, Lau ASY, Ong JS, Chuah LO, Lee YY, et al. Probiotic Lactobacillus casei Zhang (LCZ) alleviates respiratory, gastrointestinal & RBC abnormality via immuno-modulatory, anti-inflammatory & anti-oxidative actions. Journal of Functional Foods 2018;44:235-45. [DOI: 10.1016/j.jff.2018.03.017] [DOI] [Google Scholar]
Kekkonen 2007 {published data only}
- Kekkonen RA, Vasankari TJ, Vuorimaa T, Haahtela T, Julkunen I, Korpela R. The effect of probiotics on respiratory infections and gastrointestinal symptoms during training in marathon runners. International Journal of Sport Nutrition & Exercise Metabolism 2007;17(4):352-63. [DOI] [PubMed] [Google Scholar]
Kloster Smerud 2008 {published data only}
- Kloster Smerud H, Ramstad Kleiveland C, Roll Mosland A, Grave G, Birkeland SE. Effect of a probiotic milk product on gastrointestinal and respiratory infections in children attending day-care. Microbial Ecology in Health and Disease 2008;20(2):80-5. [Google Scholar]
Kosek 2019 {published data only}
- Kosek MN, Peñataro-Yori P, Paredes-Olortegui M, Lefante J, Ramal-Asayag C, Zamora-Babilonia M, et al. Safety of Lactobacillus Reuteri DSM 17938 in healthy children 2-5 years of age. Pediatric Infectious Disease Journal 2019;38(8):e178-80. [DOI: 10.1097/INF.0000000000002267] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kukkonen 2008 {published data only}
- Kukkonen K, Savilahti E, Haahtela T, Juntunen-Backman K, Korpela R, Poussa T, et al. Long-term safety and impact on infection rates of postnatal probiotic and prebiotic (synbiotic) treatment: randomized, double-blind, placebo-controlled trial. Pediatrics 2008;122(1):8-12. [DOI] [PubMed] [Google Scholar]
Kumpu 2012 {published data only}
- Kumpu M, Kekkonen RA, Kautiainen H, Järvenpää S, Kristo A, Huovinen P, et al. Milk containing probiotic Lactobacillus rhamnosus GG and respiratory illness in children: a randomized, double-blind, placebo-controlled trial. European Journal of Clinical Nutrition 2012;66(9):1020-3. [DOI] [PubMed] [Google Scholar]
Kumpu 2013 {published data only}
- Kumpu M, Lehtoranta L, Roivainen M, Rönkkö E, Ziegler T, Söderlund-Venermo M. The use of the probiotic Lactobacillus rhamnosus GG and viral findings in the nasopharynx of children attending day care. Journal of Medical Virology 2013;85(9):1632-8. [DOI] [PubMed] [Google Scholar]
Lau 2018 {published data only}
- Lau AS, Yanagisawa N, Hor YY, Lew LC, Ong JS, Chuah LO, et al. Bifidobacterium longum BB536 alleviated upper respiratory illnesses and modulated gut microbiota profiles in Malaysian pre-school children. Beneficial Microbes 2018;9(1):61-70. [DOI: 10.3920/BM2017.0063] [DOI] [PubMed] [Google Scholar]
Lefevre 2015 {published data only}
- Lefevre M, Racedo SM, Ripert G, Housez B, Cazaubiel M, Maudet C, et al. Probiotic strain Bacillus subtilis CU1 stimulates immune system of elderly during common infectious disease period: a randomized, double-blind placebo-controlled study. Immunity & Ageing 2015;12:24. [DOI: 10.1186/s12979-015-0051-y] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lehtoranta 2012 {published data only}
- Lehtoranta L, Söderlund-Venermo M, Nokso-Koivisto J, Toivola H, Blomgren K, Hatakka K, et al. Human bocavirus in the nasopharynx of otitis-prone children. International Journal of Pediatric Otorhinolaryngology 2012;76(2):206-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Leyer 2009 {published data only}
- Leyer GJ, Li S, Mubasher ME, Reifer C, Ouwehand AC. Probiotic effects on cold and influenza-like symptom incidence and duration in children. Pediatrics 2009;124(2):e172-9. [DOI] [PubMed] [Google Scholar]
Li 2014 {published data only}
- Li F, Jin X, Liu B, Zhuang W, Scalabrin D. Follow-up formula consumption in 3- to 4-year-olds and respiratory infections: an RCT. Pediatrics 2014;133(6):e1533-40. [DOI: 10.1542/peds.2013-3598] [DOI] [PubMed] [Google Scholar]
Lin 2009 {published data only}
- Lin JS, Chiu YH, Lin NT, Chu CH, Huang KC, Liao KW, et al. Different effects of probiotic species/strains on infections in preschool children: a double-blind, randomized, controlled study. Vaccine 2009;27(7):1073-9. [DOI] [PubMed] [Google Scholar]
Little 2017 {published data only}
- Little P, Stuart B, Wingrove Z, Mullee M, Thomas T, Johnson S, et al. Probiotic capsules and xylitol chewing gum to manage symptoms of pharyngitis: a randomized controlled factorial trial. Canadian Medical Association Journal 2017;189(50):E1543-50. [DOI: 10.1503/cmaj.170599] [DOI] [PMC free article] [PubMed] [Google Scholar]
Luoto 2013 {published data only}
- Luoto R, Ruuskanen O, Waris M, Kalliomäki M, Salminen S, Isolauri E. Prebiotic and probiotic supplementation prevents rhinovirus infections in preterm infants: a randomized, placebo-controlled trial. Journal of Allergy and Clinical Immunology 2014;133(2):405-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mai 2021 {published data only}
- Mai TT, Thi Thu P, Thi Hang H, Trang TT, Yui S, Shigehisa A, et al. Efficacy of probiotics on digestive disorders and acute respiratory infections: a controlled clinical trial in young Vietnamese children. European Journal of Clinical Nutrition 2021;75(3):513-20. [DOI: 10.1038/s41430-020-00754-9] [DOI] [PMC free article] [PubMed] [Google Scholar]
Makino 2010b {published data only (unpublished sought but not used)}
- Makino S, Ikegami S, Kume A, Horiuchi H, Sasaki H, Orii N. Reducing the risk of infection in the elderly by dietary intake of yoghurt fermented with Lactobacillus delbrueckii ssp. bulgaricus OLL1073R-1. British Journal of Nutrition 2010;104(7):998-1006. [DOI] [PubMed] [Google Scholar]
Maldonado 2012 {published data only}
- Maldonado J, Cañabate F, Sempere L, Vela F, Sánchez AR, Narbona E, et al. Human milk probiotic Lactobacillus fermentum CECT5716 reduces the incidence of gastrointestinal and upper respiratory tract infections in infants. Journal of Pediatric Gastroenterology and Nutrition 2012;54(1):55-61. [DOI] [PubMed] [Google Scholar]
Maldonado‐Lobón 2015 {published data only}
- Maldonado-Lobón JA, Gil-Campos M, Maldonado J, López-Huertas E, Flores-Rojas K, Valero AD, et al. Long-term safety of early consumption of Lactobacillus fermentum CECT5716: a 3-year follow-up of a randomized controlled trial. Pharmacological Research 2015;95-6:12-9. [DOI: 10.1016/j.phrs.2015.01.006] [DOI] [PubMed] [Google Scholar]
Manti 2020 {published data only}
- Manti S, Parisi GF, Papale M, Licari A, Salpietro C, Miraglia Del Giudice M, et al. Bacteriotherapy with Streptococcus salivarius 24SMB and Streptococcus oralis 89a nasal spray for treatment of upper respiratory tract infections in children: a pilot study on short-term efficacy. Italian Journal of Pediatrics 2020;46(1):42. [DOI: 10.1186/s13052-020-0798-4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Maya‐Barrios 2021 {published data only}
- Maya-Barrios A, Lira-Hernandez K, Jiménez-Escobar I, Hernández L, Ortiz-Hernandez A, Jiménez-Gutiérrez C, et al. Limosilactobacillus reuteri ATCC PTA 5289 and DSM 17938 as adjuvants to improve evolution of pharyngitis/tonsillitis in children: randomised controlled trial. Beneficial Microbes 2021;12(2):137-45. [DOI: 10.3920/BM2020.0171] [DOI] [PubMed] [Google Scholar]
Meng 2016 {published data only}
- Meng H, Lee Y, Ba Z, Peng J, Lin J, Boyer AS, et al. Consumption of Bifidobacterium animalis subsp. lactis BB-12 impacts upper respiratory tract infection and the function of NK and T cells in healthy adults. Molecular Nutrition & Food Research 2016;60(5):1161-71. [DOI: 10.1002/mnfr.201500665] [DOI] [PubMed] [Google Scholar]
Michael 2020 {published data only}
- Michael DR, Jack AA, Masetti G, Davies TS, Loxley KE, Kerry-Smith J, et al. A randomised controlled study shows supplementation of overweight and obese adults with lactobacilli and bifidobacteria reduces bodyweight and improves well-being. Scientific Reports 2020;10(1):4183. [DOI: 10.1038/s41598-020-60991-7] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moyad 2010 {published data only}
- Moyad MA, Robinson LE, Zawada ET, Kittelsrud J, Chen DG, Reeves SG. Immunogenic yeast-based fermentate for cold/flu-like symptoms in nonvaccinated individuals. Journal of Alternative & Complementary Medicine 2010;16(2):213-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mullish 2021 {published data only}
- Mullish BH, Marchesi JR, McDonald JA, Pass DA, Masetti G, Michael DR, et al. Probiotics reduce self-reported symptoms of upper respiratory tract infection in overweight and obese adults: should we be considering probiotics during viral pandemics? Gut Microbes 2021;13(1):1-9. [DOI: 10.1080/19490976.2021.1900997] [DOI] [PMC free article] [PubMed] [Google Scholar]
Murata 2018 {published data only}
- Murata M, Kondo J, Iwabuchi N, Takahashi S, Yamauchi K, Abe F, et al. Effects of paraprobiotic Lactobacillus paracasei MCC1849 supplementation on symptoms of the common cold and mood states in healthy adults. Beneficial Microbes 2018;9(6):855-64. [DOI: 10.3920/BM2017.0197] [DOI] [PubMed] [Google Scholar]
Nocerino 2017 {published data only}
- Nocerino R, Paparo L, Terrin G, Pezzella V, Amoroso A, Cosenza L, et al. Cow's milk and rice fermented with Lactobacillus paracasei CBA L74 prevent infectious diseases in children: a randomized controlled trial. Clinical Nutrition 2017;36(1):118-25. [DOI: 10.1016/j.clnu.2015.12.004] [DOI] [PubMed] [Google Scholar]
Pitkaranta 2003 {published data only (unpublished sought but not used)}
- Pitkaranta A, Hatakka K, Blomgren K, Pohjavuori S, Korpela R. Probiotics in prevention of acute otitis media in otitis prone children. In: 8th International Symposium on Recent Advances in Otitis Media. 2003. [ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=cctr&NEWS=N&AN=CN-00449364]
Pregliasco 2008 {published data only}
- Pregliasco F, Anselmi G, Fonte L, Giussani F, Schieppati S, Soletti L. A new chance of preventing winter diseases by the administration of symbiotic formulations. Journal of Clinical Gastroenterology 2008;42(Suppl 3 Pt 2):224-33. [DOI] [PubMed] [Google Scholar]
Ringel‐Kulka 2015 {published data only}
- Ringel-Kulka T, Kotch JB, Jensen ET, Savage E, Weber DJ. Randomized, double-blind, placebo-controlled study of synbiotic yogurt effect on the health of children. Journal of Pediatrics 2015;166(6):1475-81. [DOI: 10.1016/j.jpeds.2015.02.038] [DOI] [PubMed] [Google Scholar]
Sanz 2006 {published data only}
- Sanz JM, Mateos JA, Conejo AM. Effect of Lactobacillus casei on the incidence of infectious conditions in children [Efecto de Lactobacillus casei sobre la incidencia de procesos infecciosos enniños/as]. Nutricion Hospitalaria 2006;21(4):547-51. [PubMed] [Google Scholar]
Stojković 2016 {published data only}
- Stojković A, Simović A, Bogdanović Z, Banković D, Poskurica M. Clinical trial/experimental study (consort compliant): optimal time period to achieve the effects on synbiotic-controlled wheezing and respiratory infections in young children. Srpski Arhiv za Celokupno Lekarstvo 2016;144(1-2):38-45. [DOI: 10.2298/sarh1602038s] [DOI] [PubMed] [Google Scholar]
Tajima 1995 {published data only}
- Tajima T, Kobayashi M, Hata M, Negishi S, Kubota K, Iitsuka T, et al. Pharmacokinetic, bacteriological, and clinical studies on SY5555 in children. Japanese Journal of Antibiotics 1995;48(1):31-40. [PubMed] [Google Scholar]
Tiollier 2007 {published data only}
- Tiollier E, Chennaoui M, Gomez-Merino D, Drogou C, Filaire E, Guezennec CY, et al. Effect of a probiotics supplementation on respiratory infections and immune and hormonal parameters during intense military training. Military Medicine 2007;172(9):1006-11. [DOI] [PubMed] [Google Scholar]
Turchet 2003 {published data only}
- Turchet P, Laurenzano M, Auboiron S, Antoine JM. Effect of fermented milk containing the probiotic Lactobacillus casei DN-114001 on winter infections in free-living elderly subjects: a randomised, controlled pilot study. Journal of Nutrition, Health and Aging 2003;7(2):75-7. [PubMed] [Google Scholar]
Turner 2017 {published data only}
- Turner RB, Woodfolk JA, Borish L, Steinke JW, Patrie JT, Muehling LM, et al. Effect of probiotic on innate inflammatory response and viral shedding in experimental rhinovirus infection - a randomised controlled trial. Beneficial Microbes 2017;8(2):207-15. [DOI: 10.3920/BM2016.0160] [DOI] [PMC free article] [PubMed] [Google Scholar]
West 2011 {published data only}
- West NP, Pyne DB, Cripps AW, Hopkins WG, Eskesen DC, Jairath A, et al. Lactobacillus fermentum (PCC) supplementation and gastrointestinal and respiratory-tract illness symptoms: a randomised control trial in athletes. Nutrition Journal 2011;10(1):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
West 2014 {published data only}
- West NP, Horn PL, Pyne DB, Gebski VJ, Lahtinen SJ, Fricker PA, et al. Probiotic supplementation for respiratory and gastrointestinal illness symptoms in healthy physically active individuals. Clinical Nutrition 2014;33(4):581-7. [DOI] [PubMed] [Google Scholar]
Zhang 2018 {published data only}
- Zhang H, Yeh C, Jin Z, Ding L, Liu BY, Zhang L, et al. Prospective study of probiotic supplementation results in immune stimulation and improvement of upper respiratory infection rate. Synthetic and Systems Biotechnology 2018;3(2):113-20. [DOI: 10.1016/j.synbio.2018.03.001] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Kaplan 1968 {published data only}
- Kaplan M, Fischgrund A, Dobrowolski B. Therapeutic effectiveness and clinical tolerance in children of a medication combining in the same preparation of a tetracycline base with lyophilized lactobacillus. Semaine des Hopitaux 1968;44(28):1889-93. [PubMed] [Google Scholar]
Marushko 2000 {published data only}
- Marushko IV. The development of a treatment method for streptococcal tonsillitis in children. Likarska Sprava 2000;1:79-82. [PubMed] [Google Scholar]
References to ongoing studies
NCT01782118 {published data only}
- NCT01782118. LGG for prevention of infectious complications during PPI treatment in children [Effectiveness of Lactobacillus GG in the prevention of gastrointestinal and respiratory tract infections in children with gastroesophageal reflux disease treated with proton pump inhibitors: randomized double-blind placebo, controlled trial]. clinicaltrials.gov/show/NCT01782118 (first received 1 February 2013).
Additional references
Andersson 2020
- Andersson DI, Balaban NQ, Baquero F, Courvalin P, Glaser P, Gophna U, et al. Antibiotic resistance: turning evolutionary principles into clinical reality. FEMS Microbiolgy Reviews 2020;44(2):171-88. [DOI: 10.1093/femsre/fuaa001] [DOI] [PubMed] [Google Scholar]
Arunachalam 2000
- Arunachalam K, Gill HS, Chandro RK. Enhancement of natural immune function by dietary consumption of Bifidobacterium lactis (HN019). European Journal of Clinical Nutrition 2000;54(3):263-7. [DOI] [PubMed] [Google Scholar]
Atkins 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, et al, GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bardou 2020
- Bardou ML, Pontarolli D, Grumach AS. Otitis media and inborn errors of immunity. Current Allergy and Asthma Reports 2020;20(10):59. [DOI: 10.1007/s11882-020-00957-x] [DOI] [PubMed] [Google Scholar]
Batra 2020
- Batra P, Soni KD, Mathur P. Efficacy of probiotics in the prevention of VAP in critically ill ICU patients: an updated systematic review and meta-analysis of randomized control trials. Journal of Intensive Care 2020;15(8):81. [DOI: 10.1186/s40560-020-00487-8] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bernaola Aponte 2013
- Bernaola Aponte G, Bada Mancilla CA, Carreazo NY, Rojas Galarza RA. Probiotics for treating persistent diarrhoea in children. Cochrane Database of Systematic Reviews 2013, Issue 8. Art. No: CD007401. [DOI: 10.1002/14651858.CD007401.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cherry 2003
- Cherry DK, Burt CW, Woodwell DA. National ambulatory medical care survey: 2001 summary. Advance Data 2003;337:1-44. [PubMed] [Google Scholar]
Donnet‐Hughes 1999
- Donnet-Hughes A, Rochat F, Serrant P, Aeschlimann JM, Schifferin EJ. Modulation of nonspecific mechanisms of defense by lactic acid bacteria: effective dose. Journal of Dairy Science 1999;82(5):863-9. [DOI] [PubMed] [Google Scholar]
Drakes 2004
- Drakes M, Blanchard T, Czinn S. Bacterial probiotic modulation of dendritic cell. Infection and Immunity 2004;72(6):3299-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
Duijvestijn 2009
- Duijvestijn YC, Mourdi N, Smucny J, Pons G, Chalumeau M. Acetylcysteine and carbocysteine for acute upper and lower respiratory tract infections in paediatric patients without chronic broncho-pulmonary disease. Cochrane Database of Systematic Reviews 2009, Issue 1. Art. No: CD003124. [DOI: 10.1002/14651858.CD003124.pub3] [DOI] [PubMed] [Google Scholar]
Egger 2007
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315(7109):629-34. [DOI: 10.1136/bmj.315.7109.629] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gill 1998
- Gill HS. Stimulation of the immune system by lactic cultures. International Dairy Journal 1998;8:535-44. [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT. Version accessed 20 May 2022. Hamilton (ON): McMaster University (developed by Evidence Prime). Available at gradepro.org.
Greenham 2018
- Greenham G, Buckley JD, Garrett J, Eston R, Norton K. Biomarkers of physiological responses to periods of intensified, non-resistance-based exercise training in well-trained male athletes: a systematic review and meta-analysis. Sports Medicine 2018;48(11):2517-48. [DOI: 10.1007/s40279-018-0969-2] [DOI] [PubMed] [Google Scholar]
Guo 2019
- Guo Q, Goldenberg JZ, Humphrey C, El Dib R, Johnston BC. Probiotics for the prevention of pediatric antibiotic-associated diarrhea. Cochrane Database of Systematic Reviews 2019, Issue 4. Art. No: CD004827. [DOI: 10.1002/14651858.CD004827.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gwaltney 2002
- Gwaltney JM Jr. Clinical significance and pathogenesis of viral respiratory infections. American Journal of Medicine 2002;112(Suppl):13-8. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Higgins 2021
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch V, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook/archive/v6.2.
Kassel 2010
- Kassel JC, King D, Spurling GK. Saline nasal irrigation for acute upper respiratory tract infections. Cochrane Database of Systematic Reviews 2010, Issue 3. Art. No: CD006821. [DOI: 10.1002/14651858.CD006821.pub2] [DOI] [PubMed] [Google Scholar]
Kollath 1953
- Kollath W. Nutrition and the tooth system [Ernahrung und Zahnsystem]. Deutsche Zahnarztliche Zeitschrift 1953;8:7-16. [PubMed] [Google Scholar]
Laursen 2018
- Laursen RP, Hojsak I. Probiotics for respiratory tract infections in children attending day care centers - a systematic review. European Journal of Pediatrics 2018;177(7):979-94. [DOI: 10.1007/s00431-018-3167-1] [DOI] [PubMed] [Google Scholar]
Lee 2017
- Lee A, Lee YJ, Yoo HJ, Kim M, Chang Y, Lee DS, et al. Consumption of dairy yogurt containing Lactobacillus paracasei ssp. paracasei, Bifidobacterium animalis ssp. lactis and heat-treated Lactobacillus plantarum improves immune function including natural killer cell activity. Nutrients 2017;9(6):558. [DOI: 10.3390/nu9060558] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Li 2020
- Li L, Hong K, Sun Q, Xiao H, Lai L, Ming M, et al. Probiotics for preventing upper respiratory tract infections in adults: a systematic review and meta-analysis of randomized controlled trials. Evidence-Based Complementary and Alternative Medicine 2020;2020:8734140. [DOI: 10.1155/2020/8734140] [DOI] [PMC free article] [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, D'Amico R, Pifferi S, Torri V, Brazzi L, Parmelli E. Antibiotic prophylaxis to reduce respiratory tract infections and mortality in adults receiving intensive care. Cochrane Database of Systematic Reviews 2009, Issue 4. Art. No: CD000022. [DOI: 10.1002/14651858.CD000022.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Link‐Amster 1994
- Link-Amster H, Rochat F, Saudan KY, Mignot O, Aeschlimann JM. Modulation of a specific humoral immune response and changes in intestinal flora mediated through fermented milk intake. Immunology and Medical Microbiology 1994;10(1):55-63. [DOI] [PubMed] [Google Scholar]
Majamaa 1995
- Majamaa H, Isolauri E, Saxelin M, Vesikari T. Lactic acid bacteria in the treatment of acute rotavirus gastroenteritis. Journal of Pediatric Gastroenterology and Nutrition 1995;20(3):333-8. [DOI] [PubMed] [Google Scholar]
Meydani 2000
- Meydani SN, Ha WK. Immunologic effects of yogurt. American Journal of Clinical Nutrition 2000;71(4):861-72. [DOI] [PubMed] [Google Scholar]
Othman 2010
- Othman M, Meilson JP, Alfirevic Z. Probiotics for preventing preterm labour. Cochrane Database of Systematic Reviews 2010, Issue 6. Art. No: CD005941. [DOI: 10.1002/14651858.CD005941.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pelto 1998
- Pelto L, Isolauri E, Lilius EM, Nuutila J, Salminen S. Probiotic bacteria down-regulate the milk-induced inflammatory response in milk-hypersensitive subjects but have an immunostimulatory effect in healthy subjects. Clinical and Experimental Allergy 1998;28(12):1474-9. [DOI] [PubMed] [Google Scholar]
Perdigon 1995
- Perdigon G, Alvarez S, Rachid M, Aguero G, Gobbato N. Immune system stimulation by probiotics. Journal of Dairy Science 1995;78(7):1597-606. [DOI] [PubMed] [Google Scholar]
Pichichero 2016
- Pichichero ME. Ten-year study of the stringently defined otitis-prone child in Rochester, NY. Pediatric Infectious Disease Journal 2016;35(9):1033-9. [DOI: 10.1097/INF.0000000000001217] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pichichero 2020
- Pichichero ME. Immunologic dysfunction contributes to the otitis prone condition. Journal of Infection 2020;80(6):614-22. [DOI: 10.1016/j.jinf.2020.03.017] [DOI] [PMC free article] [PubMed] [Google Scholar]
Reid 2003
- Reid G, Sanders ME, Gaskins HR, Gibson GR, Mercenier A, Rastall R, et al. New scientific paradigms for probiotics and prebiotics. Journal of Clinical Gastroenterology 2003;37(2):105-18. [DOI] [PubMed] [Google Scholar]
RevMan Web 2022 [Computer program]
- Review Manager Web (RevMan Web). Version 4.7.1. The Cochrane Collaboration, 2022. Available at revman.cochrane.org.
Rossol 2011
- Rossol M, Heine H, Meusch U, Quandt D, Klein C, Sweet MJ, et al. LPS-induced cytokine production in human monocytes and macrophages. Critical Reviews in Immunology 2011;31(5):379-446. [DOI: 10.1615/critrevimmunol.v31.i5.20] [DOI] [PubMed] [Google Scholar]
Rún Sigurðardóttir 2015
- Rún Sigurðardóttir N, Nielsen AB, Munck A, Bjerrum L. Appropriateness of antibiotic prescribing for upper respiratory tract infections in general practice: comparison between Denmark and Iceland. Scandinavian Journal of Primary Health Care 2015;33(4):269-74. [DOI: 10.3109/02813432.2015.1114349] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schiffrin 1995
- Schiffrin EJ, Rochar F, Link-Amster H, Aeschlimann JM, Donnet-Hughes A. Immunomodulation of human blood cells following the ingestion of lactic acid bacteria. Journal of Dairy Science 1995;78:491-7. [DOI] [PubMed] [Google Scholar]
Sheih 2001
- Sheih YH, Chiary BL, Wang LH, Liao CK, Gill HS. Systemic immunity-enhancing effects in healthy subjects following dietary consumption of the lactic acid bacterium lactobacillus rhamnosus HN001. Journal of the American College of Nutrition 2001;20(Suppl 2):149-56. [DOI] [PubMed] [Google Scholar]
Steinman 2003
- Steinman MA, Gonzales R, Linder JA, Landefeld CS. Changing use of antibiotics in community-based outpatient practice, 1991-1999. Annals of Internal Medicine 2003;738:525-33. [DOI] [PubMed] [Google Scholar]
Valente 2009
- Valente SA, Fallon WF Jr, Alexander TS, Tomas ER, Evancho-Chapman MM, Schmidt SP, et al. Immunologic function in the elderly after injury - the neutrophil and innate immunity. Journal of Trauma 2009;67:968-74. [DOI] [PubMed] [Google Scholar]
Woappi 2016
- Woappi Y, Gabani P, Singh A, Singh OV. Antibiotrophs: the complexity of antibiotic-subsisting and antibiotic-resistant microorganisms. Critical Reviews in Microbiology 2016;42(1):17-30. [DOI: 10.3109/1040841X.2013.875982] [DOI] [PubMed] [Google Scholar]
Yasui 1999
- Yasui H, Kiyosima J, Hori T, Shida K. Protection against influenza infection of mice fed Bifido bacterium breve YIT 4064. Clinical and Diagnostic Laboratory Immunology 1999;6:186-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Łagowska 2021
- Łagowska K, Bajerska J. Effects of probiotic supplementation on respiratory infection and immune function in athletes: systematic review and meta-analysis of randomized controlled trials. Journal of Athletic Training 2021;56(11):1213-23. [DOI: 10.4085/592-20] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Hao 2011
- Hao Q, Lu Z, Dong BR, Huang CU, Wu T. Probiotics for preventing acute upper respiratory tract infections. Cochrane Database of Systematic Reviews 2011, Issue 9. Art. No: CD006895. [DOI: 10.1002/14651858.CD006895.pub2] [DOI] [PubMed] [Google Scholar]
Hao 2015
- Hao Q, Dong BR, Wu T. Probiotics for preventing acute upper respiratory tract infections. Cochrane Database of Systematic Reviews 2015, Issue 2. Art. No: CD006895. [DOI: 10.1002/14651858.CD006895.pub3] [DOI] [PubMed] [Google Scholar]
Lu 2008
- Lu Z, Dong B, Huang C, Wu T. Probiotics for preventing acute upper respiratory tract infections. Cochrane Database of Systematic Reviews 2008, Issue 1. Art. No: CD006895. [DOI: 10.1002/14651858.CD006895] [DOI] [PubMed] [Google Scholar]


