Abstract
Background
Several guidelines recommend using the Clinical Frailty Scale (CFS) for triage of critically ill coronavirus disease 2019 (COVID-19) patients. This study evaluates the impact of CFS on intensive care unit (ICU) admission rate and hospital and ICU mortality rates in hospitalized dialysis patients with COVID-19.
Methods
We analysed data of dialysis patients diagnosed with COVID-19 from the European Renal Association COVID-19 Database. The primary outcome was ICU admission rate and secondary outcomes were hospital and ICU mortality until 3 months after COVID-19 diagnosis. Cox regression analyses were performed to assess associations between CFS and outcomes.
Results
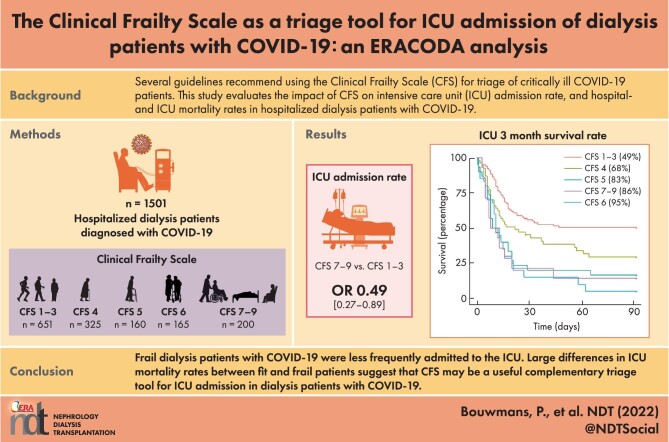
A total of 1501 dialysis patients were hospitalized due to COVID-19, of whom 219 (15%) were admitted to an ICU. The ICU admission rate was lowest (5%) in patients >75 years of age with a CFS of 7–9 and highest (27%) in patients 65–75 years of age with a CFS of 5. A CFS of 7–9 was associated with a lower ICU admission rate than a CFS of 1–3 [relative risk 0.49 (95% confidence interval 0.27–0.87)]. Overall, mortality at 3 months was 34% in hospitalized patients, 65% in ICU-admitted patients and highest in patients >75 years of age with a CFS of 7–9 (69%). Only 9% of patients with a CFS ≥6 survived after ICU admission. After adjustment for age and sex, each CFS category ≥4 was associated with higher hospital and ICU mortality compared with a CFS of 1–3.
Conclusions
Frail dialysis patients with COVID-19 were less frequently admitted to the ICU. Large differences in mortality rates between fit and frail patients suggest that the CFS may be a useful complementary triage tool for ICU admission in dialysis patients with COVID-19.
Keywords: COVID-19, dialysis, frailty, intensive care units, mortality, triage
Graphical Abstract
Graphical Abstract.
KEY LEARNING POINTS.
What is already known about this subject?
Several guidelines recommend using the Clinical Frailty Scale (CFS) for triage of critically ill COVID-19 patients.
There are no data available on the use of CFS for the decision to admit dialysis patients with COVID-19 to the intensive care unit (ICU).
We therefore assessed the relationship between CFS at presentation and ICU admission rates as well as hospital- and ICU mortality rates in dialysis patients hospitalized for COVID-19.
What this study adds?
This study demonstrates that higher clinical frailty score is associated with lower ICU admission rates and higher hospital mortality in hospitalized dialysis patients with COVID-19.
Therefore, the Clinical Frailty Scale may be a helpful tool for triage of ICU admission in hospitalized dialysis patients with COVID-19.
What impact this may have on practice or policy?
Our findings suggest that applying the CFS can help clinicians in the triage for ICU admission of dialysis patients with COVID-19.
INTRODUCTION
Patients who are admitted to the hospital with coronavirus disease 2019 (COVID-19) can develop severe COVID-19, possibly necessitating intensive care unit (ICU) admission. Whether a particular critically ill patient will benefit from ICU admission in such circumstances is a question that is difficult to answer. In addition, limited ICU capacity can necessitate a selection of patients admitted to the ICU when there is an overwhelming demand for ICU beds during a pandemic. In these situations, physicians would be greatly supported by the development of guidelines on triage of specific high-risk patients with COVID-19.
Several national guidelines have recommended the use of frailty, in addition to well-known prognostic factors such as age and comorbidity, as a tool to triage patients for ICU admission [1–3]. To assess frailty, these guidelines use the Clinical Frailty Scale (CFS), which was originally designed and validated for assessment of frailty in patients >65 years of age [4]. In patients diagnosed with COVID-19, the CFS was also found to be useful to predict adverse health outcomes and mortality in the setting of the emergency department [5] and the hospital ward [6, 7]. In patients ≥65 years of age with serious comorbidities, the proposed cut-off values of the CFS for admission of COVID-19 patients to the ICU ranges from ≥5 to ≥6 [1, 3].
Dialysis patients are particularly at risk for a severe course of COVID-19 due to their older age and high prevalence of comorbidity. Dialysis treatment was associated with higher COVID-19-related mortality rates, reported to be 16–34% in dialysis patients in the first pandemic wave [8–12]. Age and frailty strongly affect mortality risk in dialysis patients both with and without COVID-19 [8, 13–17]. Previous data from the European Renal Association COVID-19 Database (ERACODA) demonstrated a steep increase in case fatality rates with increasing age and frailty in dialysis patients and kidney transplant recipients [8]. Moreover, older age, higher frailty score and ICU admission were associated with a lower likelihood to reach pre-COVID-19 functional status in dialysis patients with COVID-19 [13]. To date, however, there are no data available on the use of CFS for the decision to admit dialysis patients with COVID-19 to the ICU. We therefore assessed the relationship between CFS at presentation and ICU admission rates as well as hospital and ICU mortality rates in dialysis patients hospitalized for COVID-19.
MATERIALS AND METHODS
Study population
The ERACODA was established in March 2020 and contains prospectively collected granular data on adult (≥18 years of age) dialysis patients or kidney transplant recipients who were diagnosed with COVID-19 [18]. The COVID-19 diagnosis was based on a positive result on a real-time polymerase chain reaction (PCR) assay or a rapid antigen test of nasal and/or pharyngeal swab specimens and/or compatible findings on a computed tomography scan or chest X-ray of the lungs. Data on patients were reported on a voluntary basis by physicians responsible for their care. For the current analysis, we included all dialysis patients admitted to the hospital with COVID-19 between 1 February 2020 and 1 May 2021 who had complete information on CFS and vital status at 3 months after first presentation.
Data collection
The ERACODA database is hosted at the University Medical Center Groningen (UMCG), Groningen, The Netherlands, and uses REDCap software (Research Electronic Data Capture, Vanderbilt University Medical Center, Nashville, TN, USA) to support data capture for research [19]. The study was assessed by the institutional review board of the UMCG. Because of the observational, non-interventional nature, the institutional review board deemed the collection and analysis of data exempt from ethics review regarding the Medical Research Involving Human Subjects Act.
Detailed information was collected on patient characteristics [demographics, frailty (referring to the clinical situation just before COVID-19), comorbidities, primary kidney disease, hospitalization, ICU admission and medication use] and COVID-19-related characteristics (presenting symptoms, vital signs and laboratory test results) at presentation. Age was categorized into three age categories: <65, 65–75 and >75 years. Frailty was assessed at presentation by using the CFS. The CFS categories range from 1 to 9, representing very fit to terminally ill patients, respectively [20]. For the current analysis, the CFS was categorized as fit to managing well (CFS 1–3), mild to moderate (CFS 4, 5 and 6) and severe (CFS 7–9). CFS categories 4–6 are presented separately because the cut off values for triage vary across countries.
Smoking status was scored as never, prior or current use. Comorbidities were recorded at presentation from the patient records and obesity was defined as a body mass index (BMI) >30 kg/m2. Primary kidney disease was recorded according to the European Renal Association (ERA) coding [21]. Contributing countries were categorized into three different regions in an arbitrary manner based on geographical location. The following regions were chosen: northwestern Europe (Austria, Belgium, France, Germany, Luxembourg, The Netherlands, Switzerland, the UK, Sweden, Norway, Latvia and Finland), southern Europe including non-European countries around the Mediterranean Sea (Spain, Portugal, Slovenia, Italy, Albania, Bosnia and Herzegovina, Croatia, Greece, Serbia, Turkey, Syria, Morocco, Egypt and Libya) and eastern Europe (Czech Republic, Poland, Romania, Russia, Slovakia and Ukraine). Information on functional and mental health outcomes was collected by treating physicians at 3 months after the COVID-19 diagnosis. Since nephrologists generally meet their haemodialysis (HD) patients every week and know their patients well, we asked them to report whether the functional and mental status of their patients had fully recovered after 3 months (yes/no). Due to the study design, we had no options to invite individual patients to report on their functional and mental outcome.
Statistical analysis
Continuous data are presented as mean with standard deviation (SD) or as median with interquartile range (IQR) in case of a non-normal distribution. Categorical data are presented as absolute numbers and percentages. Baseline characteristics were compared between CFS categories using Student's t-test for continuous variables (Mann–Whitney U-test for non-normally distributed data) and Pearson chi-squared statistics for categorical variables.
The primary outcome was ICU admission and secondary outcomes were hospital and ICU mortality at 3 months after the first presentation. We used Cox regression analyses with a fixed follow-up time to estimate risk ratios (RRs) of ICU admission with 95% confidence intervals (CIs). A fixed follow-up time was used because the time of ICU admission greatly depends on the disease severity at presentation in the emergency department. Cox proportional hazards regression analysis was performed to estimate hazard ratios for the association between frailty and mortality at 3 months. To account for potential confounders, multiple models were constructed. In model 1 we adjusted for age and sex and in model 2 we additionally adjusted for BMI, smoking status (current/prior/never) and region (northwestern Europe, southern Europe, eastern Europe). In model 3, we additionally adjusted for comorbidities, including obesity, hypertension, diabetes mellitus, coronary artery disease, heart failure, chronic lung disease, active malignancy and autoimmune diseases. These analyses were performed in all hospitalized patients and in patients who were admitted to the ICU. Subsequently we stratified the relationship between CFS score and 3-month in-hospital mortality by age category (<65, 65–75 and ≥75 years). In addition, we repeated the analysis stratified by region. Finally, we use descriptive statistics to compare the recovery of functional and mental health status (yes/no) at 3 months after ICU admission categorized by CFS.
Missing data in the multivariable models were handled by multiple imputation (10 imputed datasets) with the chained equations method using all variables included in the model [22]. All analyses were performed with Stata version 14 (StataCorp, College Station, TX, USA). A two-sided P-value of .05 was considered statistically significant.
RESULTS
On 1 May 2021, data on 4674 patients was collected in the ERACODA, including 3380 dialysis patients, of whom 1860 were hospitalized for COVID-19. After exclusion of patients with missing CFS data and/or lack of a 3-month follow-up, the study cohort consisted of 1501 patients (Supplementary data, Fig. S1). The treatment modality was HD in 94% of the patients and peritoneal dialysis (PD) in 6% of the patients, with an average (± SD) dialysis vintage of 5.2 ± 4.5 years (Table 1). The mean age was 68 ± 14 years, 63% were male and 81% of the patients were Caucasian. There were 651 patients with CFS 1–3 (43%), 325 patients with CFS 4 (22%), 160 patients with CFS 5 (11%), 165 patients with CFS 6 (11%) and 200 patients with CFS 7–9 (13%). Patients with CFS 1–3 were younger (61 ± 14 years); were less frequently diagnosed with diabetes (37%), coronary artery disease (24%) and heart failure (13%); and more frequently lived in eastern Europe than those with higher CFS scores. The CFS category was not associated with the duration of hospitalization.
Table 1:
Characteristics of hospitalized dialysis patients according to the CFS category.
| Characteristics | All (N = 1501) | CFS 1–3 (n = 651) | CFS 4 (n = 325) | CFS 5 (n = 160) | CFS 6 (n = 165) | CFS 7–9 (n = 200) |
|---|---|---|---|---|---|---|
| Patient characteristics | ||||||
| Age (years), mean (SD) | 68 (14) | 61 (14) | 71 (11) | 73 (11) | 74 (10) | 75 (11) |
| Sex (male), n (%) | 946 (63) | 432 (66) | 203 (62) | 89 (56) | 106 (64) | 116 (58) |
| BMI, mean (SD) | 26.5 (5.4) | 26.4 (5.2) | 26.5 (5.0) | 26.9 (5.3) | 27.6 (6.5) | 25.8 (5.6) |
| Race, n (%) | ||||||
| Asian | 48 (3) | 24 (4) | 8 (2) | 5 (3) | 2 (1) | 127 (70) |
| Black | 67 (5) | 36 (6) | 9 (3) | 7 (4) | 5 (3) | 27 (15) |
| Caucasian | 1210 (81) | 481 (74) | 276 (85) | 139 (87) | 148 (90) | 14 (8) |
| Other | 149 (10) | 100 (15) | 29 (9) | 7 (4) | 5 (3) | 14 (8) |
| Unknown | 27 (2) | 10 (2) | 3 (1) | 2 (1) | 5 (3) | 127 (70) |
| Region, n (%) | ||||||
| Northwestern Europe | 482 (32) | 134 (21) | 114 (35) | 64 (40) | 72 (44) | 38 (23) |
| Eastern Europe | 337 (22) | 186 (29) | 88 (27) | 30 (19) | 19 (12) | 162 (81) |
| Southern Europe, Middle East and Northern Africa | 677 (45) | 330 (51) | 120 (37) | 66 (41) | 73 (44) | 113 (57) |
| Unknown | 5 (0) | 1 (0) | 3 (1) | – | 1 (1) | 70 (35) |
| Smoking status, n (%) | ||||||
| Never | 662 (57.2) | 324 (61) | 142 (57) | 68 (52) | 55 (49) | 73 (54) |
| Former | 366 (31.6) | 147 (28) | 75 (30) | 47 (36) | 47 (42) | 50 (37) |
| Current | 130 (11.2) | 59 (11) | 34 (14) | 15 (12) | 11 (10) | 11 (8) |
| Reason for screening, n (%) | ||||||
| Symptoms only | 883 (64) | 358 (59) | 205 (68) | 91 (63) | 102 (67) | 127 (70) |
| Symptoms and contact | 262 (19) | 126 (21) | 59 (19) | 27 (19) | 23 (15) | 27 (15) |
| Contact only | 121 (9) | 62 (10) | 20 (7) | 13 (9) | 12 (8) | 14 (8) |
| Routine screening | 120 (9) | 58 (10) | 19 (6) | 14 (10) | 15 (10) | 14 (8) |
| Comorbidities, n (%) | ||||||
| Obesity | 293 (22) | 120 (20) | 59 (21) | 39 (28) | 37 (28) | 38 (23) |
| Hypertension | 1240 (83) | 550 (85) | 268 (82) | 126 (79) | 134 (81) | 162 (81) |
| Diabetes Mellitus | 689 (46) | 242 (37) | 163 (50) | 87 (54) | 84 (51) | 113 (57) |
| Coronary artery disease | 515 (34) | 157 (24) | 146 (45) | 73 (46) | 69 (42) | 70 (35) |
| Heart failure | 377 (25) | 86 (13) | 100 (31) | 52 (33) | 59 (36) | 80 (40) |
| Chronic lung disease | 225 (15) | 68 (10) | 58 (18) | 27 (17) | 29 (18) | 43 (22) |
| Active malignancy | 103 (7) | 29 (4) | 27 ()8 | 9 (6) | 23 (14) | 15 (8) |
| Autoimmune disease | 66 (4) | 32 (5) | 17 (5) | 5 (3) | 4 (2) | 8 (4) |
| Primary kidney disease, n (%) | ||||||
| Primary glomerulonephritis | 138 (20) | 132 (21) | 31 (10) | 23 (14) | 18 (11) | 16 (8) |
| Pyelonephritis | 26 (2) | 8 (1) | 6 (2) | 3 (2) | 5 (3) | 4 (2) |
| Interstitial nephritis | 44 (3) | 17 (3) | 10 (3) | 8 (5) | 3 (2) | 6 (3) |
| Hereditary kidney disease | 92 (6) | 57 (9) | 20 (6) | 3 (2) | 8 (5) | 4 (2) |
| Congenital diseases | 24 (2) | 14 (2) | 4 (1) | – | 4 (3) | 2 (1) |
| Vascular diseases | 269 (18) | 100 (16) | 73 (23) | 25 (16) | 31 (19) | 40 (21) |
| Secondary glomerular disease | 91 (6) | 32 (5) | 25 (8) | 9 (6) | 8 (5) | 17 (9) |
| Diabetic kidney disease | 481 (32) | 190 (30) | 110 (34) | 57 (36) | 55 (34) | 69 (35) |
| Other | 79 (5) | 28 (4) | 15 (5) | 13 (8) | 12 (8) | 11 (6) |
| Unknown | 155 (10) | 66 (10) | 29 (9) | 18 (11) | 16 (10) | 26 (13) |
| Dialysis modality, n (%) | ||||||
| HD | 1404 (94) | 601 (93) | 306 (95) | 154 (96) | 153 (93) | 190 (95) |
| PD | 91 (6) | 47 (7) | 17 (5) | 6 (4) | 12 (7) | 9 (5) |
| Dialysis vintage (years), mean (SD) | 5.2 (4.5) | 5.3 (4.7) | 5.0 (3.9) | 5.0 (4.0) | 4.9 (4.0) | 5.4 (5.1) |
| COVID-19-related characteristics | ||||||
| Presenting symptoms, n (%) | ||||||
| Sore throat (yes), n (%) | 196 (14) | 93 (15) | 46 (15) | 19 (13) | 19 (13) | 19 (10) |
| Cough | 772 (52) | 324 (50) | 189 (59) | 81 (51) | 91 (56) | 87 (45) |
| Shortness of breath | 630 (42) | 217 (33) | 168 (52) | 78 (49) | 69 (42) | 98 (50) |
| Fever | 914 (61) | 405 (62) | 212 (65) | 88 (56) | 92 (57) | 117 (59) |
| Headache | 162 (11) | 87 (14) | 34 (11) | 16 (11) | 11 (7) | 14 (8) |
| Nausea or vomiting | 180 (12) | 72 (11) | 53 (17) | 20 (13) | 17 (11) | 18 (9) |
| Diarrhoea | 209 (14) | 89 (14) | 51 (16) | 23 (15) | 18 (11) | 28 (15) |
| Myalgia or arthralgia | 364 (26) | 169 (27) | 81 (26) | 43 (28) | 33 (21) | 38 (21) |
| Vital signs | ||||||
| Temperature (°C), mean (SD) | 37.5 (1.0) | 37.5 (1.0) | 37.5 (1.0) | 37.5 (1.0) | 37.4 (1.0) | 37.4 (1.0) |
| Respiration rate (/min), mean (SD) | 19 (5) | 18 (4) | 19 (5) | 20 (5) | 19 (5) | 21 (6) |
| O2 saturation room air (%), mean (SD) | 93 (6) | 94 (6) | 93 (5) | 92 (6) | 93 (6) | 92 (5) |
| Systolic BP (mmHg), mean (SD) | 135 (26) | 137 (23) | 136 (26) | 136 (28) | 132 (29) | 130 (28) |
| Diastolic BP (mmHg), mean (SD) | 74 (15) | 77 (14) | 73 (15) | 73 (15) | 70 (17) | 68 (16) |
| Pulse rate (bpm), mean (SD) | 83 (16) | 83 (15) | 84 (17) | 84 (18) | 82 (16) | 84 (15) |
| Laboratory test results | ||||||
| Lymphocytes (×1000/µl), median (IQR) | 0.9 (0.6–1.3) | 0.9 (0.6–1.4) | 0.8 (0.5–1.2) | 0.9 (0.6–1.3) | 0.8 (0.6–1.2) | 0.9 (0.6–1.3) |
| CRP (mg/l), median (IQR) | 92 (42–212) | 120 (33–430) | 119 (40–429) | 107 (46–359) | 90 (36–215) | 113 (44–333) |
Numbers may not add up to the total because of missing values.
bpm, beats per minute; CRP, C-reactive protein.
As shown in Supplementary data, Table S1, hospitalized patients with missing CFS values more often had obesity (32% versus 22%), coronary artery disease (55% versus 34%) and heart failure (36% versus 25%) than patients with available CFS data.
ICU admission
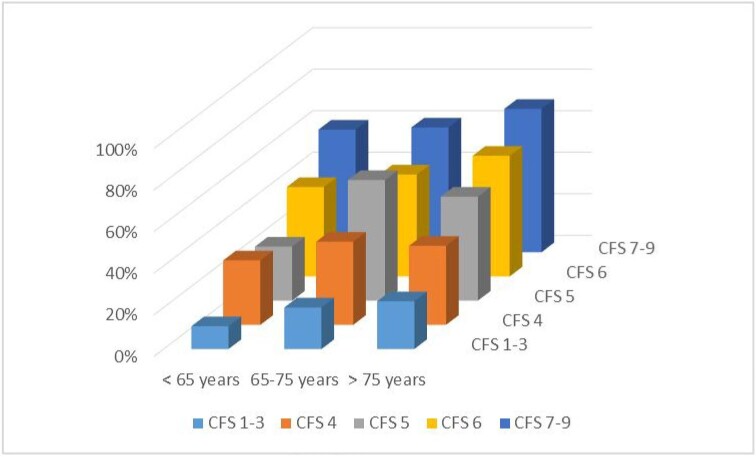
Of the 1501 hospitalized dialysis patients, 219 (15%) were admitted to the ICU. The median time between hospital admission and ICU admission was 3 days (IQR 1–7) and did not differ between CFS categories (P = .4). The ICU admission rates ranged from 5% to 27% across different CFS and age categories (Table 2). The highest ICU admission rate was 27% in patients 65–75 years of age with CFS 5 and the lowest was 5% in patients >75 years of age with CFS 7–9. Multivariable analysis revealed that CFS 7–9 was associated with a lower rate of ICU admission compared with CFS 1–3 after adjustment for age and sex [7% versus 16%; RR 0.49 (95% CI 0.27–0.87), P = .02; Table 3]. Additional adjustment for BMI, smoking status, region and comorbidity did not change this result. Supplementary data, Table S2 shows the chance of ICU admission for the three European regions separately. In northwestern and southern Europe, the chance of being admitted to an ICU was lower in frail patients compared with patients with CFS 1–3. The opposite was true in eastern Europe, where a higher frailty score increased the chance of being admitted to an ICU.
Table 2:
ICU admission rates in hospitalized dialysis patients with COVID-19.
| All | <65 years | 65–75 years | >75 years | |||||
|---|---|---|---|---|---|---|---|---|
| CFS | Hospital, n | ICU, n (%) | Hospital, n | ICU, n (%) | Hospital, n | ICU, n (%) | Hospital, n | ICU, n (%) |
| 1–3 | 651 | 102 (15) | 380 | 65 (17) | 154 | 25 (16) | 117 | 12 (10) |
| 4 | 325 | 53 (16) | 85 | 14 (16) | 110 | 28 (26) | 130 | 11 (9) |
| 5 | 160 | 30 (19) | 34 | 6 (18) | 48 | 13 (27) | 78 | 11 (14) |
| 6 | 165 | 20 (12) | 28 | 3 (11) | 49 | 5 (10) | 88 | 12 (14) |
| 7–9 | 200 | 14 (7) | 32 | 5 (16) | 55 | 3 (6) | 113 | 6 (5) |
| Total | 1501 | 219 (15) | 559 | 93 (17) | 416 | 74 (18) | 526 | 52 (10) |
Table 3:
Multivariable analysis of ICU admission in hospitalized dialysis patients with COVID-19.
| Model 1a | Model 2b | Model 3c | ||||||
|---|---|---|---|---|---|---|---|---|
| CFS | n | ICU, n (%) | RR (95% CI) | P-value | RR (95% CI) | P-value | RR (95% CI) | P-value |
| 1–3 | 651 | 102 (16) | Ref. | Ref. | Ref. | – | ||
| 4 | 325 | 53 (16) | 1.11 (0.78–1.58) | .6 | 1.09 (0.76–1.56) | .6 | Ref. | .9 |
| 5 | 160 | 30 (19) | 1.29 (0.84–1.99) | .2 | 1.30 (0.84–2.01) | .2 | 1.03 (0.72–1.49) | .4 |
| 6 | 165 | 20 (12) | 0.84 (0.51–1.40) | .5 | 0.85 (0.51–1.42) | .5 | 1.21 (0.78–1.88) | .4 |
| 7–9 | 200 | 14 (7) | 0.49 (0.27–0.87) | .02 | 0.52 (0.29–0.94) | .03 | 0.82 (0.49–1.37) | .02 |
Adjusted for age and sex.
Adjusted for age, sex, BMI, smoking status and region.
Adjusted for age, sex, BMI, smoking status, region and comorbidity (obesity, hypertension, diabetes mellitus, coronary artery disease, heart failure, chronic lung disease, active malignancy and autoimmune diseases).
Hospital mortality
After 3 months of follow-up, the mortality rate was 34% in the 1501 hospitalized patients and this rate rose with increasing age and CFS category, as shown in Fig. 1 and Supplementary data, Fig. S2. The highest mortality rate was 69% in patients >75 years of age with CFS 7–9, whereas the lowest mortality rate was 11% in patients <65 years of age with CFS 1–3 (Table 4). Multivariable analysis revealed that each of the CFS categories 4, 5, 6 and 7–9 were significantly associated with a higher hospital mortality rate compared with CFS 1–3 [CFS 4: RR 2.4 (95% CI 1.8–3.1), CFS 5: 3.3 (2.4–4.4), CFS 6: 3.6 (2.7–4.9), CFS 7–9: 4.9 (3.7–6.5); see Supplementary data, Table S3]. Additional adjustment for BMI, smoking status, region and comorbidity did not change the results. The association between CFS and 3-month mortality remained statistically significant across all age categories (Supplementary data, Table S4).
Figure 1:
Three-month mortality of hospitalized patients per age and CFS categories.
Table 4:
Hospital mortality (upper panel) and ICU mortality (lower panel) rates at the 3-month follow-up in dialysis patients with COVID-19.
| All | <65 years | 65–75 years | >75 years | |||||
|---|---|---|---|---|---|---|---|---|
| CFS | n | Mortality, n (%) | n | Mortality, n (%) | n | Mortality, n (%) | n | Mortality, n (%) |
| Hospital mortality | ||||||||
| 1–3 | 651 | 99 (15) | 380 | 41 (11) | 154 | 31 (20) | 117 | 27 (23) |
| 4 | 325 | 119 (37) | 85 | 26 (31) | 110 | 44 (40) | 130 | 49 (37) |
| 5 | 160 | 76 (48) | 34 | 9 (27) | 48 | 28 (58) | 78 | 39 (50) |
| 6 | 165 | 87 (53) | 28 | 12 (43) | 49 | 24 (49) | 88 | 51 (58) |
| 7–9 | 200 | 130 (65) | 32 | 19 (59) | 55 | 33 (60) | 113 | 78 (69) |
| ICU mortality | ||||||||
| 1–3 | 102 | 50 (49) | 65 | 29 (45) | 25 | 15 (60) | 12 | 6 (50) |
| 4 | 53 | 36 (68) | 14 | 7 (50) | 28 | 21 (75) | 11 | 8 (73) |
| 5 | 30 | 25 (83) | 6 | 5 (83) | 13 | 10 (77) | 11 | 10 (91) |
| 6 | 20 | 19 (95) | 3 | 2 (67) | 5 | 5 (100) | 12 | 12 (100) |
ICU mortality
The 3-month mortality rate in ICU-admitted patients was 65% (Supplementary data, Fig. S2). Only 3 of 34 patients (9%) with a CFS of 6–9 survived ICU admission, irrespective of age (Table 4). The lowest mortality rate was ∼50% in patients with CFS 1–3, with no clear difference between age groups. Multivariable analysis revealed that each of the CFS categories 4, 5, 6 and 7–9 were significantly associated with a higher ICU mortality rate compared with CFS 1–3 [CFS 4: RR 1.6 (95% CI 1.0–2.5), CFS 5: 2.4 (1.4–3.9), CFS 6: 2.8 (1.6–4.9), CFS 7–9: 2.7 (1.4–5.1); see Supplementary data, Table S3]. Adjustment for age and sex only led to a non-significant difference in ICU mortality for CFS 4 [RR 1.5 (95% CI 0.9–2.3)]. Additional adjustment for other confounders did not change the results.
Functional and mental health outcomes after ICU admission
Of the 219 ICU-admitted patients, 77 were still alive 3 months after ICU admission and data were available on functional and mental recovery for 52 of them (Supplementary data, Table S5). In 37 patients with CFS 1–3, recovery of functional status to a pre-existing level was observed in 70% of the patients, whereas mental status had recovered in 84% of the patients (Supplementary data, Table S5). Only six patients had a CFS ≥5, but all showed an almost complete recovery of functional and mental status.
DISCUSSION
This study demonstrates that during the first year of the COVID-19 pandemic, ICU admission rates were lower among the frailest hospitalized dialysis patients with COVID-19. In addition, hospital and ICU mortality rates were highest in these frailest dialysis patients when admitted for COVID-19. Of the ICU-admitted dialysis patients with CFS ≥5, more than 80% died within 3 months after ICU admission, irrespective of age. In contrast, fit to mildly frail dialysis patients with COVID-19 had a 40–55% survival rate when admitted to the ICU, with physician-reported functional and mental recovery at 3 months of follow-up. These findings suggest that the use of CFS can help clinicians to triage dialysis patients for ICU admission in cases of COVID-19.
The use of CFS for estimating a patient's prognosis in case of ICU admission has been studied mainly in patients >80 years of age. Both Flaatten et al. [15] and Guidet et al. [16] found a positive association between CFS and 30-day ICU mortality in patients >80 years of age without kidney disease. In addition, frailty has recently been associated with worse outcome during long-stay ICU admission [23]. The surge of COVID-19 during the first year of the pandemic caused a great need for available ICU capacity in many countries. The international consensus is that optimal patient care and allocation of ICU resources should be based on an estimation of the prognosis to survive [24, 25]. This led to the implementation of guidelines for ICU triage of COVID-19 patients in which CFS was introduced as an instrument for triage. The proposed CFS cut-off values ranged from ≥5 to ≥6 in different guidelines [1, 3]. In response to the National Institute for Health and Care Excellence guideline recommendations, Darvall et al. [26] investigated the utility of CFS for triage in critically ill adults with non-COVID-19 pneumonia in Australia and New Zealand. Using a multicentre retrospective cohort design, they found that compared with lower frailty scores, severe frailty (CFS ≥7) was associated with increased ICU mortality. Although this patient group accounted for only 7% of the total ICU population, they recommended the use of CFS ≥7 as a threshold for excluding patients from ICU admission if triage was based on expected mortality. If the goal was to reduce the ICU occupancy, then a stricter CFS cut-off value of ≥4 was advised.
The use of CFS to predict hospital outcomes in patients with COVID-19 has been studied mainly in patients without kidney disease. In patients with suspected COVID-19, Simon et al. [5] found a higher risk of being admitted to the ICU when the CFS was ≥5 at presentation in the emergency department. In a large multicentre retrospective cohort study, Sablerolles et al. [7] found a higher risk of ICU admission in patients with CFS ≥6 in all age categories when compared with fit patients with CFS 1–3. In dialysis patients with COVID-19, highly varying ICU admission rates were reported. In their systematic review, Alfano et al. [27] report on ICU admission rates ranging from 2.6% to 70.5%. In our study, we observed a lower ICU admission rate in dialysis patients with CFS 7–9 compared with dialysis patients with CFS 1–3. In dialysis patients with CFS 4, 5 or 6, ICU admission rates were similar to those in patients with CFS 1–3. Since the use of CFS in triaging critically ill COVID-19 patients was already recommended early in the first pandemic waves by national guidelines in different countries across Europe, we presume that CFS was already applied in ICU triage of dialysis patients in our cohort, explaining the lower ICU admission rates in patients with CFS 7–9. Interestingly, we observed differences between European regions. While higher frailty was associated with a lower chance of being admitted to the ICU in northwestern and southern Europe, ICU admission rates were the lowest in patients with CFS 1–3 in eastern Europe. Differences in ICU admission rates might be explained by variations in COVID-19 healthcare policies, limited ICU capacity or cultural and religious interregional variations. Reasons for ICU admission were often not registered and therefore we cannot further explore our hypotheses for these interregional differences in ICU admission rates.
Dialysis patients were found to be at high risk for a severe course of COVID-19. Hospital mortality rates at 28 days ranged from 16% to 26% during the first year of the pandemic [8–12, 28, 29]. Nevertheless, associations between CFS and mortality have rarely been studied in dialysis patients, whereas in COVID-19 patients without kidney disease, this association has previously been observed [17]. A previous analysis of ERACODA data demonstrated a steep increase in case fatality rates with increasing age and frailty in dialysis patients and kidney transplant recipients [8]. In addition, higher hospital and ICU mortality rates were observed in dialysis patients with higher frailty scores after adjustment for age and comorbidity. We showed that the survival chances of dialysis patients strongly decline in all age categories if frailty increased. This suggests that frailty assessed by CFS may also be useful in dialysis patients <65 years of age. However, the significance of frailty in younger patients differs from that of frailty in the elderly. The CFS has not been widely validated in patients <65 years of age [20]. In dialysis patients <65 years of age, it is therefore important to use the CFS with caution.
Besides mortality as an objective endpoint to determine a patient's prognosis, the functional or mental outcome after ICU admission due to COVID-19 is also of importance. It has been observed that older age, higher frailty score and ICU admission were associated with a lower likelihood to reach pre-COVID-19 functional status in dialysis patients with COVID-19 [13]. Although our present data represent a limited number of dialysis patients who survived ICU admission, 70–80% of those with low to mild frailty scores at hospital admission did show recovery of functional and mental status. This indicates that it was probably the right decision to admit these patients to the ICU.
A major strength of our study is the prospective data collection on frailty and clinical outcomes in a large cohort of dialysis patients with COVID-19 from the start of the pandemic. This resulted in a large dataset of patients with a wide representation of CFS scores and age categories. Because of the international collaboration, we were able to provide data from many predominantly European countries. This enabled correcting for region, taking into account the diverse cultural background and ethical considerations across the European continent. The observational design of our study also has its limitations. First, the study design is not suitable for making a recommendation whether a specific CFS cut-off value could be applied for ICU triage in critically ill dialysis patients with COVID-19, due to confounding by indication. This is a result of the guidelines in force at that time advocating the use of frailty in ICU triage, contributing to the observed association between frailty and lower ICU admission rates. Further studies are needed to validate the use of a certain CFS cut-off value in this population. Second, the majority of patients were included before the start of vaccination campaigns in the different countries. This limits the generalizability of our findings to the actual situation in which a large proportion of dialysis patients have been vaccinated. However, the immune response to vaccination is weaker in dialysis patients than in healthy controls [30, 31]. Dialysis patients are therefore considered to remain at risk for a serious course of COVID-19 and in case of new sever acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants. The course of future COVID-19 in dialysis patients may vary depending on the SARS-CoV-2 variant responsible for infection, the vaccination status and the vaccination response. Third, data registration in the ERACODA took place on a voluntary base, resulting in differences in contributions by countries across Europe. For this reason, results might not be generalizable to each individual European country. Fourth, the number of PD patients in our cohort was too low to perform a valid comparison between outcomes in PD and HD patients. Ultimately we observed a selection bias caused by the exclusion of patients without data on CFS who had a higher prevalence of obesity, coronary artery disease and heart failure and who are prone to a more severe disease course. We expect this to have led to bias away from the null, which means a stronger association between frailty and health outcomes than we have observed.
In conclusion, the frailest dialysis patients hospitalized for COVID-19 were less often admitted to an ICU. Dialysis patients with moderate to serious frailty who were admitted to the ICU had a very high risk for mortality, irrespective of age. Outcomes were better for mildly frail dialysis patients who were admitted to the ICU. These findings suggest that use of the CFS can help clinicians to triage dialysis patients for ICU admission in case of COVID-19. Further research is required to establish whether use of the CFS can also be recommended for dialysis patients in other healthcare settings. It remains crucial to take into account the personal values of patients and relatives as well as the considerations for individual decision making for ICU triage in dialysis patients with COVID-19.
Supplementary Material
ACKNOWLEDGEMENTS
We thank all the people that entered information in the ERACODA database for their participation and especially all healthcare workers who have taken care of the included COVID-19 patients. The ERACODA collaboration is an initiative endorsed by the ERA to study the prognosis and risk factors for mortality due to COVID-19 in patients with a kidney transplant or on dialysis. The organizational structure contains a Working Group [C. F. M. Franssen, R. T. Gansevoort (coordinator), M. H. Hemmelder, L. B. Hilbrands and K. J. Jager] assisted by a Management Team (R. Duivenvoorden, M. Noordzij, P. Vart) and an Advisory Board (D. Abramowicz, C. Basile, A. Covic, M. Crespo, Z. A. Massy, S. Mitra, E. Petridou, J. E. Sanchez and C. White).
APPENDIX
Names and affiliations of ERACODA collaborators: Jeroen B. van der Net (Albert Schweitzer Hospital, Dordrecht, The Netherlands); Marie Essig (Ambroise Pare Hospital, APHP Paris-Saclay University, Boulogne Billancourt, France); Peggy W. G. du Buf-Vereijken, Betty van Ginneken and Nanda Maas (Amphia Hospital, Breda, The Netherlands); Brigit C. van Jaarsveld, Frederike J. Bemelman, Farah Klingenberg-Salahova, Frederiek Heenan-Vos, Marc G. Vervloet, Azam Nurmohamed and Liffert Vogt (Amsterdam UMC, Amsterdam, The Netherlands); Daniel Abramowicz and Sabine Verhofstede (Antwerp University Hospital, Antwerp, Belgium); Omar Maoujoud (Avicennes Military Hospital, Faculty of Medicine, Cadi Ayyad University, Marrakech Morocco); Thomas Malfait (AZ Delta, Roeselare, Belgium); Jana Fialova (B. Braun Avitum, Litomerice, Czech Republic); Edoardo Melilli, Alexandre Favà, Josep M. Cruzado and Nuria Montero Perez (Bellvitge University Hospital, Hospitalet de Llobregat, Barcelona, Spain); Joy Lips (Bernhoven Hospital, Uden, The Netherlands); Harmen Krepel (Bravis Hospital, Roosendaal/Bergen op Zoom, The Netherlands); Harun Adilovic (Cantonal Hospital Zenica, Bosnia and Herzegovina); Daniela Radulescu (‘Carol Davila’ University of Medicine and Pharmacy, Bucharest, Romania / Emergency Clinical Hospital ‘Sf. Ioan’); Maaike Hengst and Constantijn Konings (Catharina Hospital, Eindhoven, The Netherlands); Andrzej Rydzewski (Central Clinical Hospital of the Ministry of Interior, Warsaw, Poland); Philippe Braconnier and Daniel Weis (Centre Hospitalier du Nord, Luxembourg); Ryszard Gellert (Centre of Postgraduate Medical Education, Warsaw, Poland); João Oliveira (Centrodial, São João da Madeira, Portugal); Daniela G. Alferes (Centro Hospitalar Vila Nova de Gaia/Espinho, Vila Nova de Gaia, Portugal); Elena V. Zakharova (City Hospital n.a. S.P. Botkin, Moscow, Russia); Patrice Max Ambühl, Rebecca Guidotti and Andrea Walker (City Hospital Zürich, Zürich, Switzerland); Fanny Lepeytre and Guy Rostoker (Claude Galien Hospital Ramsay santé, Quincy-sous-Sénart, France); Sofia Marques (Clínica de Hemodiálise de Felgueiras, Felgueiras, Portugal); Tijana Azasevac and Gordana Strazmester Majstorovic (Clinical Centre of Vojvodina, Novi Sad, Serbia); Dajana Katicic (Croatian Society of Nephrology, Dialysis and Transplantation, Croatia); Marc ten Dam (CWZ Nijmegen, Nijmegen, The Netherlands); Thilo Krüger (DaVita Geilenkirchen, Geilenkirchen, Germany); Szymon Brzosko (DaVita, Wrocław, Poland); Vassilios Liakopoulos (1st Department of Internal Medicine, Medical School, Aristotle University of Thessaloniki, Thessaloniki, Greece); Adriaan L. Zanen (Deventer Ziekenhuis, Deventer, The Netherlands); Susan J. J. Logtenberg (Dianet Dialysis Center, Utrecht, The Netherlands); Lutz Fricke (Dialysis Center Bochum, Bochum, Germany); Olexandr Kuryata (Dnipro State Medical University, Dnipro, Ukraine); Jeroen J. P. Slebe (Elyse Klinieken voor Nierzorg, Kerkrade, The Netherlands); Samar Abd ElHafeez (Epidemiology Department, High Institute of Public Health, Alexandria University, Alexandria, Egypt); Delphine Kemlin (Erasme Hospital, Brussels, Belgium); Jacqueline van de Wetering, Marlies E. J. Reinders, Dennis A. Hesselink and Judith Kal-van Gestel (Erasmus MC Transplant Institute, Department of Internal Medicine, University Medical Center Rotterdam, Rotterdam, The Netherlands); Jaromir Eiselt and Lukas Kielberger (Faculty of Medicine in Pilsen, Charles University, Pilsen, Czech Republic); Hala S. El-Wakil (Faculty of Medicine, Alexandria University, Alexandria, Egypt); Martine Verhoeven (Franciscus Gasthuis & Vlietland, Schiedam, The Netherlands); Ian Logan (Freeman Hospital, Newcastle upon Tyne, UK); Cristina Canal and Carme Facundo (Fundació Puigvert, Barcelona, Spain); Ana M. Ramos (Fundación Jiménez Díaz, Madrid, Spain); Alicja Debska-Slizien (Gdansk Medical University, Gdansk, Poland); Nicoline M. H. Veldhuizen (Gelre Hospital, Apeldoorn, The Netherlands); Eirini Tigka (General Hospital of Athens “G. Gennimatas”, Athens, Greece); Maria Anna Polyzou Konsta (General Hospital of Serres, Serres, Greece); Stylianos Panagoutsos (General University Hospital of Alexandroupolis, Alexandroupolis, Greece); Francesca Mallamaci, Adele Postorino and Francesco Cambareri (Grande Ospedale Metropolitano and CNR, Reggio Calabria, Italy); Irina Matceac and Ionut Nistor (Grigore T. Popa University of Medicine and Pharmacy, Iasi, Romania/Dr Ci Parhon Hospital, Iasi, Romania); J. H. M. Groeneveld and Jolanda Jousma (Haaglanden Medisch Centrum, The Hague, The Netherlands); Marjolijn van Buren (Haga Hospital, The Hague, The Netherlands); Fritz Diekmann, Federico Oppenheimer and Miquel Blasco (Hospital Clínic de Barcelona, Barcelona, Spain); Tiago Assis Pereira (Hospital Curry Cabral – Central Lisbon University Hospital Center, Lisbon, Portugal); Augusto Cesar S. Santos Jr. (Hospital das Clinicas, Universidade Federal de Minas Gerais, Brazil); Carlos Arias-Cabrales, Marta Crespo, Laura Llinàs-Mallol, Anna Buxeda, Carla Burballa Tàrrega, Dolores Redondo-Pachon, Maria Dolores Arenas Jimenez and Alberto Mendoza-Valderrey (Hospital del Mar, Barcelona, Spain); Ana Cristina Martins, Catarina Mateus, Goncalo Alvila and Ivo Laranjinha (Hospital de Santa Cruz, Centro Hospitalar de Lisboa Ocidental, Lisbon, Portugal); Julia M. Hofstra and Machiel A. Siezenga (Hospital Gelderse Vallei, Ede, The Netherlands); Antonio Franco (Hospital General of Alicante, Alicante, Spain); David Arroyo, Sandra Castellano and Maria Luisa Rodríguez-Ferrero (Hospital General Universitario Gregorio Marañón, Madrid, Spain); Sagrario Balda Manzanos (Hospital Obispo Polanco, Salud Aragón, Spain); R. Haridian Sosa Barrios (Hospital Universitario Ramón y Cajal, Madrid, Spain); Wim Lemahieu (Imelda Hospital, Bonheiden, Belgium); Karlijn Bartelet (Isala, Zwolle, The Netherlands); Ahmet Burak Dirim, Erol Demir, Mehmet Sukru Sever, Aydin Turkmen and Seda Şafak (Istanbul Faculty of Medicine, Istanbul University, Istanbul, Turkey); Daan A. M. J. Hollander (Jeroen Bosch Ziekenhuis, Den Bosch, The Netherlands); Stefan Büttner (Klinikum Aschaffenburg-Alzenau, Aschaffenburg, Germany); Aiko P.J. de Vries, Soufian Meziyerh, Danny van der Helm, Marko Mallat and Hanneke Bouwsma (Leiden University Medical Center, Leiden, The Netherlands); Sivakumar Sridharan (Lister Hospital, Stevenage, UK); Kristina Petruliene (Lithuanian University of Health Sciences, Kaunas, Lithuania); Sharon-Rose Maloney (Luzerner Kantonsspital, Luzern, Switzerland); Iris Verberk (Maasstad Ziekenhuis, Rotterdam, The Netherlands); Frank M. van der Sande and Maarten H. L. Christiaans (Maastricht University Medical Center, Maastricht, The Netherlands); MohanKumar N (Manipal Hospital, Manipal, India); Marina Di Luca (Marche Nord Hospital, Pesaro, Italy); Serhan Z. Tuğlular (Marmara University School of Medicine, Istanbul, Turkey); Andrea B. Kramer (Martini Ziekenhuis, Groningen, The Netherlands); Charles Beerenhout (Maxima Medisch Centrum, Veldhoven, The Netherlands); Peter T. Luik (Meander Medisch Centrum, Amersfoort, The Netherlands); Julia Kerschbaum (Austrian Dialysis and Transplant Registry) and Martin Tiefenthaler (Medical University Innsbruck, Innsbruck, Austria); Bruno Watschinger (Medical University of Vienna, Vienna, Austria); Aaltje Y. Adema (Medisch Centrum Leeuwarden, Leeuwarden, The Netherlands); Vadim A. Stepanov and Alexey B. Zulkarnaev (Moscow Regional Research and Clinical Institute, Moscow, Russia); Kultigin Turkmen (Necmettin Erbakan University Meram School of Medicine, Konya, Turkey); Ilaria Gandolfini and Umberto Maggiore (Nephrology Unit, Department of Medicine and Surgery, University of Parma, Parma, Italy); Anselm Fliedner (Nierenzentrum Reutlingen-Tübingen, Reutlingen, Germany); Anders Åsberg and Geir Mjoen (Norwegian Renal Registry, Oslo University Hospital–Rikshospitalet, Olso, Norway); Hitoshi Miyasato (Okinawa Chubu Hospital, Uruma, Japan); Carola W. H. de Fijter (Onze Lieve Vrouwe Gasthuis, Amsterdam, The Netherlands); Nicola Mongera (Ospedale S. Maurizio Bolzano, Bolzano, Italy); Stefano Pini (Padua University Hospital, Padua, Italy); Consuelo de Biase, Angele Kerckhoffs, Anne Els van de Logt and Rutger Maas (Radboud University Medical Center, Nijmegen, The Netherlands); Olga Lebedeva (Regional Clinical Hospital, Yaroslavl, Russia); Veronica Lopez (Regional Hospital of Malaga, Malaga, Spain); Louis J. M. Reichert and Jacobien Verhave (Rijnstate Hospital, Arnhem, The Netherlands); Denis Titov (People's Friendship University of Russia, Moscow, Russia); Ekaterina V. Parshina (Saint Petersburg State University Hospital, Saint Petersburg, Russia); Luca Zanoli and Carmelita Marcantoni (San Marco Hospital, University of Catania, Catania, Italy); Gijs van Kempen (Saxenburgh Medisch Centrum, Hardenberg, The Netherlands); Liesbeth E. A. van Gils-Verrij (Sint Antonius Ziekenhuis, Nieuwegein, The Netherlands); John C. Harty (Southern Health and Social Care Trust, Newry, Northern Ireland); Marleen Meurs (Spaarne Gasthuis, Haarlem, The Netherlands); Marek Myslak (Samodzielny Publiczny Wojewódzki Szpital Zespolony Hospital, Szczecinie, Poland); Yuri Battaglia (St. Anna University Hospital, Ferrara, Italy); Paolo Lentini (St. Bassiano Hospital, Bassano del Grappo, Italy); Edwin den Deurwaarder (Streekziekenhuis Koningin Beatrix, Winterswijk, The Netherlands); Maria Stendahl (Swedish Renal Registry, Jönköping, Sweden); Hormat Rahimzadeh (Tehran University of Medical Sciences, Tehran, Iran); Marcel Schouten (Tergooi Medical Center, Hilversum, The Netherlands); Ivan Rychlik (Third Faculty of Medicine, Charles University and Faculty Hospital Kralovske Vinohrady, Prague, Czech Republic); Carlos J. Cabezas-Reina and Ana Maria Roca (Toledo University Hospital, Toledo, Spain); Ferdau Nauta (Treant/Scheper Ziekenhuis, Emmen, The Netherlands); İdris Sahin (Turgut Ozal Medical Center, Malatya, Turkey); Eric Goffin, Nada Kanaan, Laura Labriola and Arnaud Devresse (Université Catholique de Louvain, Cliniques Universitaires St Luc, Brussels, Belgium); Anabel Diaz-Mareque (University Clinical Hospital of Santiago de Compostela, Santiago de Compostela, Spain); Armando Coca (University Clinical Hospital of Valladolid, Valladolid, Spain); Gabriel de Arriba (University Hospital of Guadalajara, Guadalajara, Spain); Björn K. I. Meijers, Maarten Naesens, Dirk Kuypers and Bruno Desschans (University Hospital Leuven, Leuven, Belgium); Annelies Tonnerlier and Karl M. Wissing (University Hospital Brussels, Brussels, Belgium); Ivana Dedinska (University Hospital Martin and Jessenius Faculty of Medicine Comenius University, Martin, Slovakia); Giuseppina Pessolano (University Hospital Medical Center Verona, Verona, Italy); Shafi Malik (University Hospitals of Coventry and Warwickshire NHS Trust, Coventry, UK); Evangelia Dounousi (University Hospital of Ioannina, Ioannina, Greece); Evangelos Papachristou (University Hospital of Patras, Patras, Greece); Stefan P. Berger, Esther Meijer, Jan Stephan F. Sanders (University Medical Center Groningen, Groningen, The Netherlands) and Akin Özyilmaz (Dialysis Center Groningen); Jadranka Buturović Ponikvar, Andreja Marn Pernat, Damjan Kovac and Miha Arnol (University Medical Center Ljubljana, Ljubljana, Slovenia); Robert Ekart (University Medical Centre Maribor, Maribor, Slovenia); Alferso C. Abrahams, Femke M. Molenaar, Arjan D. van Zuilen, Sabine C. A. Meijvis and Helma Dolmans (University Medical Center Utrecht, Utrecht, The Netherlands); Ekamol Tantisattamo (University of California Irvine School of Medicine, Orange, CA, USA); Pasquale Esposito (University of Genoa, Genoa, Italy); Jean-Marie Krzesinski and Jean Damacène Barahira (University of Liège, Liège, Belgium); Maurizio Gallieni (University of Milan, Milan, Italy); Paloma Leticia Martin-Moreno (University of Navarra Clinic, Pamplona, Spain); Gabriele Guglielmetti (University of Piemonte Orientale, Novara, Italy); Gabriella Guzzo (Valais Hospital, Sion and Lausanne University Hospital, Lausanne, Switzerland); Nestor Toapanta and Maria Jose Soler (Vall d'Hebron University Hospital, Barcelona, Spain); Antinus J. Luik, Willi H. M. van Kuijk, Lonneke W. H. Stikkelbroeck and Marc M. H. Hermans (VieCuri Medical Centre, Venlo, The Netherlands); Laurynas Rimsevicius (Vilnius University, Vilnius, Lithuania); Marco Righetti (Vimercate Hospital, Vimercate, Italy); Nicole Heitink-ter Braak (Zuyderland Medical Center, Geleen and Heerlen, The Netherlands).
Notes
The ERACODA collaborators are listed in the appendix.
Contributor Information
Pim Bouwmans, Department of Internal Medicine, Division of Nephrology, Maastricht University Medical Center, Maastricht, The Netherlands; CARIM School for Cardiovascular Disease, University of Maastricht, Maastricht, The Netherlands.
Lloyd Brandts, Department of Clinical Epidemiology and Medical Technology Assessment, Maastricht University Medical Center, Maastricht, The Netherlands.
Luuk B Hilbrands, Department of Nephrology, Radboud University Medical Center, Nijmegen, The Netherlands.
Raphaël Duivenvoorden, Department of Nephrology, Radboud University Medical Center, Nijmegen, The Netherlands.
Priya Vart, Department of Internal Medicine, University Medical Center Groningen, Groningen, The Netherlands; Department of Clinical Pharmacy and Pharmacology, University Medical Center Groningen, Groningen, The Netherlands.
Casper F M Franssen, Department of Clinical Pharmacy and Pharmacology, University Medical Center Groningen, Groningen, The Netherlands.
Adrian Covic, Grigore T. Popa University of Medicine and Pharmacy, Iasi, Romania; Dr Ci Parhon Hospital, Iasi, Romania.
Mahmud Islam, Zonguldak Ataturk State Hospital, Zonguldak, Turkey; Claude Galien Hospital Ramsay Santé, Quincy-sous-Sénart, France.
Clémentine Rabaté, Claude Galien Hospital Ramsay Santé, Quincy-sous-Sénart, France.
Kitty J Jager, ERA Registry, Amsterdam UMC location University of Amsterdam, Medical Informatics, Amsterdam, The Netherlands; Amsterdam Public Health Research Institute, Quality of Care, Amsterdam, The Netherlands.
Marlies Noordzij, Department of Clinical Pharmacy and Pharmacology, University Medical Center Groningen, Groningen, The Netherlands.
Ron T Gansevoort, Department of Clinical Pharmacy and Pharmacology, University Medical Center Groningen, Groningen, The Netherlands.
Marc H Hemmelder, Department of Internal Medicine, Division of Nephrology, Maastricht University Medical Center, Maastricht, The Netherlands; CARIM School for Cardiovascular Disease, University of Maastricht, Maastricht, The Netherlands.
for the ERACODA collaborators:
Jeroen B van der Net, Marie Essig, Peggy W G du Buf-Vereijken, Betty van Ginneken, Nanda Maas, Brigit C van Jaarsveld, Frederike J Bemelman, Farah Klingenberg-Salahova, Frederiek Heenan-Vos, Marc G Vervloet, Azam Nurmohamed, Liffert Vogt, Daniel Abramowicz, Sabine Verhofstede, Omar Maoujoud, Thomas Malfait, Jana Fialova, Edoardo Melilli, Alexandre Favà, Josep M Cruzado, Nuria Montero Perez, Joy Lips, Harmen Krepel, Harun Adilovic, Daniela Radulescu, Maaike Hengst, Constantijn Konings, Andrzej Rydzewski, Philippe Braconnier, Daniel Weis, Ryszard Gellert, João Oliveira, Daniela G Alferes, Elena V Zakharova, Patrice Max Ambühl, Rebecca Guidotti, Andrea Walker, Fanny Lepeytre, Guy Rostoker, Sofia Marques, Tijana Azasevac, Gordana Strazmester Majstorovic, Dajana Katicic, Marc ten Dam, Thilo Krüger, Szymon Brzosko, Vassilios Liakopoulos, Adriaan L Zanen, Susan J J Logtenberg, Lutz Fricke, Olexandr Kuryata, Jeroen J P Slebe, Samar Abd ElHafeez, Delphine Kemlin, Jacqueline van de Wetering, Marlies E J Reinders, Dennis A Hesselink, Judith Kal-van Gestel, Jaromir Eiselt, Lukas Kielberger, Hala S El-Wakil, Martine Verhoeven, Ian Logan, Cristina Canal, Carme Facundo, Ana M Ramos, Alicja Debska-Slizien, Nicoline M H Veldhuizen, Eirini Tigka, Maria Anna Polyzou Konsta, Stylianos Panagoutsos, Francesca Mallamaci, Adele Postorino, Francesco Cambareri, Irina Matceac, Ionut Nistor, J H M Groeneveld, Jolanda Jousma, Marjolijn van Buren, Fritz Diekmann, Federico Oppenheimer, Miquel Blasco, Tiago Assis Pereira, Augusto Cesar S Santos, Jr., Carlos Arias-Cabrales, Marta Crespo, Laura Llinàs-Mallol, Anna Buxeda, Carla Burballa Tàrrega, Dolores Redondo-Pachon, Maria Dolores Arenas Jimenez, Alberto Mendoza-Valderrey, Ana Cristina Martins, Catarina Mateus, Goncalo Alvila, Ivo Laranjinha, Julia M Hofstra, Machiel A Siezenga, Antonio Franco, David Arroyo, Sandra Castellano, Maria Luisa Rodríguez-Ferrero, Sagrario Balda Manzanos, R Haridian Sosa Barrios, Wim Lemahieu, Karlijn Bartelet, Ahmet Burak Dirim, Erol Demir, Mehmet Sukru Sever, Aydin Turkmen, Seda Şafak, Daan A M J Hollander, Stefan Büttner, Aiko P J de Vries, Soufian Meziyerh, Danny van der Helm, Marko Mallat, Hanneke Bouwsma, Sivakumar Sridharan, Kristina Petruliene, Sharon-Rose Maloney, Iris Verberk, Frank M van der Sande, Maarten H L Christiaans, MohanKumar N, Marina Di Luca, Serhan Z Tuğlular, Andrea B Kramer, Charles Beerenhout, Peter T Luik, Julia Kerschbaum, Martin Tiefenthaler, Bruno Watschinger, Aaltje Y Adema, Vadim A Stepanov, Alexey B Zulkarnaev, Kultigin Turkmen, Ilaria Gandolfini, Umberto Maggiore, Anselm Fliedner, Anders Åsberg, Geir Mjoen, Hitoshi Miyasato, Carola W H de Fijter, Nicola Mongera, Stefano Pini, Consuelo de Biase, Angele Kerckhoffs, AnneEls van de Logt, Rutger Maas, Olga Lebedeva, Veronica Lopez, Louis J M Reichert, Jacobien Verhave, Denis Titov, Ekaterina V Parshina, Luca Zanoli, Carmelita Marcantoni, Gijs van Kempen, Liesbeth E A van Gils-Verrij, John C Harty, Marleen Meurs, Marek Myslak, Yuri Battaglia, Paolo Lentini, Edwin den Deurwaarder, Maria Stendahl, Hormat Rahimzadeh, Marcel Schouten, Ivan Rychlik, Carlos J Cabezas-Reina, Ana Maria Roca, Ferdau Nauta, İdris Sahin, Eric Goffin, Nada Kanaan, Laura Labriola, Arnaud Devresse, Anabel Diaz-Mareque, Armando Coca, Gabriel de Arriba, Björn K I Meijers, Maarten Naesens, Dirk Kuypers, Bruno Desschans, Annelies Tonnerlier, Karl M Wissing, Ivana Dedinska, Giuseppina Pessolano, Shafi Malik, Evangelia Dounousi, Evangelos Papachristou, Stefan P Berger, Esther Meijer, Jan Stephan F Sanders, Akin Özyilmaz, Jadranka Buturović Ponikvar, Andreja Marn Pernat, Damjan Kovac, Miha Arnol, Robert Ekart, Alferso C Abrahams, Femke M Molenaar, Arjan D van Zuilen, Sabine C A Meijvis, Helma Dolmans, Ekamol Tantisattamo, Pasquale Esposito, Jean-Marie Krzesinski, Jean Damacène Barahira, Maurizio Gallieni, Paloma Leticia Martin-Moreno, Gabriele Guglielmetti, Gabriella Guzzo, Nestor Toapanta, Maria Jose Soler, Antinus J Luik, Willi H M van Kuijk, Lonneke W H Stikkelbroeck, Marc M H Hermans, Laurynas Rimsevicius, Marco Righetti, and Nicole Heitink-ter Braak
FUNDING
The ERACODA received unrestricted research grants from the ERA, the Dutch Kidney Foundation, Baxter and Sandoz. None of these organizations had any role in the design of the study, interpretation of results or in writing of the manuscript.
AUTHORS’ CONTRIBUTIONS
P.B., L.B., P.V., M.N., R.T.G. and M.H.H. designed the study, drafted the article and performed data analysis. P.B., L.B., L.B.H., R.D., P.V., C.F.M.F., A.C., M.I., C.R., K.J.J., M.N., R.T.G. and M.H.H. contributed to the data collection, contributed important intellectual content during the interpretation of the results and manuscript drafting and agree to be personally accountable for the individual's own contributions.
DATA AVAILABILITY STATEMENT
Collaborators that entered data in the ERACODA remain owner of these data. Therefore the database cannot be disclosed to any third party without the prior written consent of all data providers, but the database will be made available to the editorial offices of medical journals when requested.
CONFLICT OF INTEREST STATEMENT
On behalf of all authors, the corresponding author states that there are no conflicts of interest.
The results presented in this article have not been published previously in whole or part, except in abstract format.
References
- 1. National Institute for Health and Care Excellence . COVID-19 rapid guideline: managing COVID-19. https://www.nice.org.uk/guidance/ng191 (30 December 2021, date last accessed). [PubMed] [Google Scholar]
- 2. Riccioni L, Ingravallo F, Grasselli Get al. The Italian document: decisions for intensive care when there is an imbalance between care needs and resources during the COVID-19 pandemic. Ann Intensive Care 2021;11:100. 10.1186/s13613-021-00888-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. COVID-19 pandemic: triage for intensive-care treatment under resource scarcity (3rd, updated version). Swiss Med Wkly 2020;150:w20401. 10.4414/smw.2020.20401 [DOI] [PubMed] [Google Scholar]
- 4. Rockwood K, Song X, MacKnight Cet al. A global clinical measure of fitness and frailty in elderly people. Can Med Assoc J 2005;173:489–95. 10.1503/cmaj.050051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Simon NR, Jauslin AS, Rueegg Met al. Association of frailty with adverse outcomes in patients with suspected COVID-19 infection. J Clin Med 2021;10:2472. 10.3390/jcm10112472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hewitt J, Carter B, Vilches-Moraga Aet al. The effect of frailty on survival in patients with COVID-19 (COPE): a multicentre, European, observational cohort study. Lancet Public Health 2020;5:e444–51. 10.1016/S2468-2667(20)30146-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sablerolles RSG, Lafeber M, van Kempen JALet al. Association between Clinical Frailty Scale score and hospital mortality in adult patients with COVID-19 (COMET): an international, multicentre, retrospective, observational cohort study. Lancet Healthy Longev 2021;2:e163–70. 10.1016/S2666-7568(21)00006-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hilbrands LB, Duivenvoorden R, Vart Pet al. COVID-19-related mortality in kidney transplant and dialysis patients: results of the ERACODA collaboration. Nephrol Dial Transplant 2020;35:1973–83. 10.1093/ndt/gfaa261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Williamson EJ, Walker AJ, Bhaskaran Ket al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020;584:430–6. 10.1038/s41586-020-2521-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jager KJ, Kramer A, Chesnaye NCet al. Results from the ERA-EDTA Registry indicate a high mortality due to COVID-19 in dialysis patients and kidney transplant recipients across Europe. Kidney Int 2020;98:1540–8. 10.1016/j.kint.2020.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Salerno S, Messana JM, Gremel GWet al. COVID-19 risk factors and mortality outcomes among Medicare patients receiving long-term dialysis. JAMA Netw Open 2021;4:e2135379. 10.1001/jamanetworkopen.2021.35379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ozturk S, Turgutalp K, Arici Met al. Mortality analysis of COVID-19 infection in chronic kidney disease, haemodialysis and renal transplant patients compared with patients without kidney disease: a nationwide analysis from Turkey. Nephrol Dial Transplant 2020;35:2083–95. 10.1093/ndt/gfaa271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hemmelder MH, Noordzij M, Vart Pet al. Recovery of dialysis patients with COVID-19: health outcomes 3 months after diagnosis in ERACODA. Nephrol Dial Transplant 2022;37:1140–51. 10.1093/ndt/gfac008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Flythe JE, Assimon MM, Tugman MJet al. Characteristics and outcomes of individuals with pre-existing kidney disease and COVID-19 admitted to intensive care units in the United States. Am J Kidney Dis 2021;77:190–203.e1. 10.1053/j.ajkd.2020.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Flaatten H, De Lange DW, Morandi Aet al. The impact of frailty on ICU and 30-day mortality and the level of care in very elderly patients (≥80 years). Intensive Care Med 2017;43:1820–8. 10.1007/s00134-017-4940-8 [DOI] [PubMed] [Google Scholar]
- 16. Guidet B, de Lange DW, Boumendil Aet al. The contribution of frailty, cognition, activity of daily life and comorbidities on outcome in acutely admitted patients over 80 years in European ICUs: the VIP2 study. Intensive Care Med 2020;46:57–69. 10.1007/s00134-019-05853-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cosco TD, Best J, Davis Det al. What is the relationship between validated frailty scores and mortality for adults with COVID-19 in acute hospital care? A systematic review. Age Ageing 2021;50:608–16. 10.1093/ageing/afab008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Noordzij M, Duivenvoorden R, Pena MJet al. ERACODA: the European database collecting clinical information of patients on kidney replacement therapy with COVID-19. Nephrol Dial Transplant 2020;35:2023–5. 10.1093/ndt/gfaa179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Harris PA, Taylor R, Minor BLet al. The REDCap consortium: building an international community of software partners. J Biomed Inform 2019;95:103208. 10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rockwood K, Theou O.. Using the Clinical Frailty Scale in allocating scarce health care resources. Can Geriatr J 2020;23:254–9. 10.5770/cgj.23.463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. ERA-EDTA Registry . ERA-EDTA Registry Annual Report2019. Amsterdam:Amsterdam UMC, location AMC, Department of Medical Informatics, 2021. [Google Scholar]
- 22. Liu Y, De A. Multiple imputation by fully conditional specification for dealing with missing data in a large epidemiologic study. Int J Stat Med Res 2015;4:287–95. 10.6000/1929-6029.2015.04.03.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Darvall JN, Bellomo R, Bailey M.. Impact of frailty on persistent critical illness: a population-based cohort study. Intensive Care Med 2022;48:343–51. 10.1007/s00134-022-06617-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Emanuel EJ, Persad G, Upshur Ret al. Fair allocation of scarce medical resources in the time of Covid-19. N Engl J Med 2020;382:2049–55. 10.1056/NEJMsb2005114 [DOI] [PubMed] [Google Scholar]
- 25. Vergano M, Bertolini G, Giannini Aet al. Clinical ethics recommendations for the allocation of intensive care treatments in exceptional, resource-limited circumstances: the Italian perspective during the COVID-19 epidemic. Crit Care 2020;24:165. 10.1186/s13054-020-02891-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Darvall JN, Bellomo R, Bailey Met al. Frailty and outcomes from pneumonia in critical illness: a population-based cohort study. Br J Anaesth 2020;125:730–8. 10.1016/j.bja.2020.07.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Alfano G, Ferrari A, Magistroni Ret al. The frail world of haemodialysis patients in the COVID-19 pandemic era: a systematic scoping review. J Nephrol 2021;34:1387–403. 10.1007/s40620-021-01136-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Couchoud C, Bayer F, Ayav Cet al. Low incidence of SARS-CoV-2, risk factors of mortality and the course of illness in the French national cohort of dialysis patients. Kidney Int 2020;98:1519–29. 10.1016/j.kint.2020.07.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. De Meester J, De Bacquer D, Naesens Met al. Incidence, characteristics, and outcome of COVID-19 in adults on kidney replacement therapy: a regionwide registry study. J Am Soc Nephrol 2021;32:385–96. 10.1681/ASN.2020060875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sanders JF, Bemelman FJ, Messchendorp ALet al. The RECOVAC immune-response study: the immunogenicity, tolerability, and safety of COVID-19 vaccination in patients with chronic kidney disease, on dialysis, or living with a kidney transplant. Transplantation 2022;106:821–34. 10.1097/TP.0000000000003983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Stumpf J, Siepmann T, Lindner Tet al. Humoral and cellular immunity to SARS-CoV-2 vaccination in renal transplant versus dialysis patients: a prospective, multicenter observational study using mRNA-1273 or BNT162b2 mRNA vaccine. Lancet Reg Health Eur 2021;9:100178. 10.1016/j.lanepe.2021.100178 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Collaborators that entered data in the ERACODA remain owner of these data. Therefore the database cannot be disclosed to any third party without the prior written consent of all data providers, but the database will be made available to the editorial offices of medical journals when requested.