Abstract
Introduction
Clinical decision support systems (CDSSs) play an important role in summarizing the best clinical practices, thereby promoting high standards of care in specific medical fields. These systems can serve as tools for gaining knowledge and mediating between clinical guidelines and physicians thereby providing the right information to the right person at the right time.
Objective
This review aims to evaluate the effect of CDSSs on adherence to guidelines for venous thromboembolism (VTE) prophylaxis and VTE events compared to routine care without CDSSs in non-surgical patients.
Methods
In order to conduct a systematic literature review, the published studies were identified through screening EMBASE, the international clinical trials registry, OVID, Cochrane database, PubMed, ISI Web of Science, and Scopus databases, from 1982 to March 2021. The included studies were reviewed by two independent reviewers; the proportion of patients that correctly received VTE prophylaxis has been next extracted for further analysis. Additionally, patients were divided into two groups: CDSS-recommended VTE prophylaxis and routine care without using a CDSS.
Results
Twelve articles (three randomized controlled trials, seven prospective cohort trials, and two retrospective cohort trials) were in fine analyzed. The use of CDSSs is found to be associated with a significant increase in the rate of using the appropriate prophylaxis for VTE (p < 0.05) and a significant decrease in the incidence of VTE (p < 0.05).
Conclusion
Implementation of CDSSs can help improving the appropriate use of VTE prophylaxis in non-surgical patients. Further, evidence-based and interventional studies on the development of CDSSs can provide more in-depth knowledge on both this tool design and efficiency.
Keywords: Decision support systems, prophylaxis, non-surgical patients, venous thromboembolism
Introduction
Deep vein thrombosis (DVT) and pulmonary embolism (PE) known as venous thromboembolism (VTE) are adverse events among hospitalized patients and are the leading causes of mortality during and after hospitalization due to acute medical illnesses or surgery.1,2 Inappropriate VTE prophylaxis leads to adverse consequences, such as symptomatic DVT, PE, chronic post-thrombotic syndrome, and an increased risk of recurrent VTE. There are mechanical and pharmacological methods for VTE prevention in hospitalized patients. 3
Although numerous guidelines for VTE prophylaxis are developed, several recent clinical studies have shown that appropriate VTE prophylaxis is sometimes not utilized in hospitalized patients.4,5 Sometimes, physicians use risk classification tools inconsistently or incorrectly. As a result, patients do not receive proper prophylaxis for VTE, thereby increasing their VTE risk. The existence of such evidence suggests that the problem of VTE in hospitalized patients has not been given sufficient attention. In other words, evidence-based guidelines and recommendations are clearly underused.6,7
Up to now, various methods have been applied to improve prophylaxis recommendations for hospitalized patients. To this end, many hospital systems use clinical decision support systems (CDSSs), which help physicians assess VTE risk levels and provide appropriate VTE prophylaxis. 8 CDSSs use algorithms to analyze patients’ data and provide a variety of services in patient care including disease prevention, chronic disease management, healthcare screening, appropriate recommendations for diagnosis, and treatment.9,10 Borab et al. 7 show that CDSS improve VTE prophylaxis in surgical patients. 7 Additionally, Tooher et al. 11 in the systematically reviewed study show that CDSS is the most effective strategy for improve VTE prophylaxis in hospitalized patients.
CDSSs differentiate between surgical and non-surgical VTE patients, and their effects on these two groups are not the same. 12 Because, risk assessment model of VTE in two group was different, also have different recommendation. 12 Hence, the present systematic review and meta-analysis aim to determine whether the implementation of a CDSSs for VTE prophylaxis will increase adherence to VTE prophylaxis guidelines and reduce the incidence of VTE, compared to routine care without any CDSS in non-surgical patients.
Methods
This systematic review and meta-analysis were conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA). 13 Additionally, the study protocol was registered in the PROSPERO database (registration No. CRD42021225093).
Eligibility criteria
The analysis includes original peer-reviewed studies that quantitatively reported the effects of CDSSs on adherence to VTE prophylaxis guidelines among non-surgical patients. These studies provided VTE prophylaxis recommendations after CDSS implementation, regardless of the CDSS software utility, VTE risk level, and prevention of thrombosis among non-surgical patients. Language did not constitute a restriction.
We excluded studies that assessed VTE prevention in pregnant and surgical patients, did not assess prophylaxis's outcome, or did not include a non-surgical cohort.
Data sources and search strategy
We searched EMBASE, the international clinical trials registry, OVID, Cochrane database, PubMed, ISI Web of Science, and Scopus (1982-March 2021) for studies analyzing the effect of CDSSs, against no-CDSS routine care, on VTE incidence (Table 1). As the terminology for CDSS and VTE interventions has not been standardized yet, we used broad search terms. We conducted a nested search, in which we reviewed also the sources referred to in the articles we have selected for our study.
Table 1.
Search strategies used in the different databases.
| Databases | Search items |
|---|---|
| PubMed | (“Decision Support Systems, Clinical” OR “Computerized clinical decision support systems” OR “Medical Records Systems, Computerized”) AND (“Risk Factors” OR “Risk Adjustment” OR “Risk Management” OR “Risk Assessment”) AND (“Venous Thromboembolism/prevention and control OR “Anticoagulants”) |
| Ovid | (“Decision Support Systems, Clinical” OR “computerized clinical decision support systems” OR “Medical Records Systems, Computerized”) AND (“Risk Factors” OR “Risk Adjustment “OR “Risk Management “OR “Risk Assessment”) AND (“Treatment Outcome”) AND (“Venous Thromboembolism” OR “Anticoagulants”) |
| EMBASE | Decision support systems/ and (risk factor or risk management or risk adjustment) and (treatment outcome)- remove abstracts |
| Cochrane | Venous thromboembolism and prophylaxis |
| Scopus | (“Decision Support Systems, Clinical” OR “Computerized clinical decision support systems” OR “Medical Records Systems, Computerized”) AND (Thromboembolism OR Venous thrombosis) |
| Clinicaltrials.gov | Clinical decision support and venous thromboembolism |
| ISI web of science | Title: (clinical decision support and venous thromboembolism) |
Study selection
The studies identified during the database search were downloaded from the corresponding journals. The titles and abstracts of the studies were screened, mainly by one of the co-authors (FT). Afterward, their full texts were independently screened by two experts (MK and RSH), in order to include the studies that used a CDSS to aid physicians through the recommendation of appropriate VTE prophylaxis in non-surgical patients. In case of the exclusion of reports, the reasons are reported.
Data extraction
The following data were extracted from each study, independently by two co-authors: the authors’ names, studies year, country, methods used (patient population, study design, and sample size), and an approach to VTE prevention. We also extracted primary outcomes, that is, the rate of appropriate prophylaxis for VTE before and after the CDSS implementation, and the rate of VTE events.
Statistical analysis
We analyzed studies in which patients were divided into two groups based on the type of intervention: CDSS-guided care for VTE prophylaxis, and routine care without the use of a CDSS. Data were pooled using the fixed effect model for meta-analysis when CDSS and appropriate VTE prophylaxis were sufficiently similar to compare. Between-study heterogeneity was tested using the chi-square test and I2 statistics. 14 Additionally, the potential for studies bias for subcategory meta-analysis was assessed by Cochrane collaboration risk of bias tools. 15 Sensitivity analyses were performed to use different measurements for dichotomous outcomes. 16 Hypotheses were tested using two-sided tests, at a significance level of 0.05.
Quality assessment
We assessed the quality of the studied papers using the Newcastle-Ottawa Scale (NOS) to evaluate the computerized decision support systems. The scale consists of three dimensions: the selection of study groups (four scores for cohort and five scores for cross-sectional studies), comparability of study groups (maximum two scores), and ascertainment of either the exposure (maximum three scores) or outcome of interest for non-randomized studies. Accordingly, risk assessment of VTE, age, and sex was considered for comparability items in this study.
Results
A PRISMA flow diagram, summarizing study screening (Figure 1), shows that the primary search resulted in a total of 219 potentially relevant papers. After reviewing their titles and abstracts, 191 articles were excluded. We read the full texts of the remaining 28 papers to determine if they met the inclusion criteria. The references of the eligible studies yielded no additional articles. Eventually, the screening procedure resulted in 12 papers: three randomized controlled trials, seven prospective cohort trials, and two retrospective cohort trials (Tables 2 and 3).
Figure 1.
Summary of results of search and screening of the studies.
Table 2.
Newcastle–Ottawa scale (NOS) scores and risk of bias of the studies.
| Selection | Comparability | Outcome | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Representativeness of the exposed cohort | Selection of the non-exposed cohort | Ascertainment of exposure | Demonstration that outcome was not KNOWN at the start of the study | Comparability of groups on the basis of analysis | Assessment of outcome | Follow-up being long enough for outcomes | Adequacy of follow up of cohorts | ||
| Study | Variety of nonsurgical patients (with CDSS) | Variety of nonsurgical patients (without CDSS) | CDSS | Stated in articles | Controlled for VTE risk factors between groups | Blinded or record linkage | Three months | 90%–100% complete follow up | |
| Spirk et al., 2017 19 | * | * | * | * | ** | | * | * | 8 |
| Mathers et al., 2017 20 | * | * | * | * | ** | | * | * | 8 |
| Eijgenraam et al., 2015 21 | * | * | * | * | * | | * | * | 7 |
| Amland et al., 2015 22 | * | * | * | * | ** | | * | * | 8 |
| Fuzinatto et al., 2013 23 | * | * | * | * | ** | | * | * | 8 |
| Bhalla et al., 2012 24 | * | * | * | * | * | | * | * | 7 |
| Umscheid et al., 2012 25 | * | * | * | * | * | | * | * | 7 |
| Mitchell et al., 2012 18 | * | * | * | * | * | | * | * | 7 |
| MaCauley et al., 2012 26 | * | * | * | * | ** | | * | * | 8 |
| Galanter et al.,2010 27 | * | * | * | * | ** | | * | * | 8 |
| Piazza et al., 2010 5 | * | * | * | * | ** | | * | * | 8 |
| Kucher et al., 2005 28 | * | * | * | * | ** | | * | * | 8 |
Table 3.
Characteristics of the randomized control trial.
| References | Country | Study design | Setting and participants | Time scale | Intervention | Outcomes reported |
|---|---|---|---|---|---|---|
| SPIRK et al., 2017 19 | Switzerland | Randomized controlled study | 1593 patients hospitalized in the medical, general, and internal medicine wards of the Bern University Hospital) | During hospital stay (8 months) | Electronic alert system and improving appropriate thromboprophylaxis | Appropriate VTE Prophylaxis, VTE prophylaxis underuse |
| Piazza et al., 2010 5 | USA | Randomized controlled trial | 2493 patients from 25 study sites in the intervention group (n = 1238) and the control group (n = 1255) | 90-days follow-up | Compare human alerts and electronic alert identifying the patient as high-risk for VTE and recommending | Venous thromboembolism prevention |
| Kucher et al., 2005 28 | USA | Randomized clinical trial | Medical and surgical patients (inpatient and outpatient records) 2506 patients 1255 patients to intervention group and 1251 patients in the control group | 4 years and 90-day follow-ups | Computer-alert program | Venous thromboembolism At 30 days and 90 days |
Study quality and risk of bias
Based on thresholds for converting the Newcastle-Ottawa scale (NOS) to AHRQ standards, the overall quality of the study was good. The NOS scores for the included articles are provided in Table 2. Owing to the utilized interventions, the risk of total bias was not present in the included studies. However, four studies had a moderate risk of sampling bias, because of an unbalanced patient population.
Thresholds for converting the Newcastle-Ottawa Scale to AHRQ standards (good, fair, and poor):
Good quality: 3 or 4 stars in the selection domain and 1 or 2 stars in the comparability domain and 2 or 3 stars in the outcome/exposure domain. Fair quality: 2 stars in the selection domain and 1 or 2 stars in the comparability domain and 2 or 3 stars in the outcome/exposure domain. Poor quality: 0 or 1 star in the selection domain or 0 stars in the comparability domain OR 0 or 1 star in the outcome/exposure domain.
Study characteristics
Among the studies, 12 utilized pre-post designs, but retrospective pre-post designs and randomized controlled trials were used, too. Nine studies were conducted in the United States; the remaining studies were performed in Brazil, the Netherlands, and Switzerland. The characteristics of the studies are presented in Table 2 and Table 3.
Sensitivity analysis
Initially, we conducted sensitivity analysis to evaluate how each study affected the 95% CI. The results of sensitivity analysis showed that the pooled effect size was 95% CI. In other words, removal of each study caused no significant change in the pooled studies. Sensitivity analysis was also performed in order to evaluate the impact of heterogeneous studies on the pooled estimates. For this purpose, the studies were excluded serially and pooled estimates were obtained from the remaining studies. This helped determining whether single studies with highly heterogeneous results affected the overall pooled estimates. Overall, there is no bias. Studies bias was assessed in the meta-analysis by performing Egger's rank correlation test to statistically assess the symmetries of the diagram. 17
Intervention with CDSS and control group details
Data points were pooled by the CDSSs in all the studies to risk-stratify the patients. In other words, the CDSS algorithm would risk-stratify based on the data provided. In 10 studies, control groups were determined based on the implementation of the CDSS. In the two other studies, a time series experiment was conducted and the CDSS was for the intervention periods.
VTE prophylaxis recommendation in non-surgical patients
Based on the results of this meta-analysis, risk assessment of VTE in non-surgical patients was applied in all the studies and CDSS algorithms suggested appropriate prevention based on the VTE risk scores.
Appropriateness of prophylaxis recommendation to prevent VTE in non-surgical patients
Out of the nine studies, three revealed the effect of the VTE prophylaxis recommendation system on the appropriateness of prophylaxis for VTE in the non-surgical population (Table 5). Accordingly, the pooled meta-analysis (two of these three studies) of 2675 patients who received the CDSS intervention and 3214 controls showed that the CDSSs significantly increased the appropriate prophylaxis for VTE compared to the controls (OR = 1.69, 95% CI: 1.25–2.28, p = 0.001; I2 = 59.3%, p = 0.085). Additionally, the results of the sensitivity analysis of each study indicated that no single study contributed to a significant part of the measured I2 statistics. The largest contribution was made by the study carried out by Mitchell et al., 18 which, when excluded, comprised only 5% of the heterogeneity (Figure 2).
Table 4.
Characteristics of the observational studies.
| Outcomes reported | Intervention | Guideline | Risk assessment tools | Time scale | Setting and participants | Study design | Country | References |
|---|---|---|---|---|---|---|---|---|
| Proportion of IBD patients receiving pharmacologic VTE prophylaxis | Electronic alert | ACCP 2008 | During hospitalization, before and after electronic alert system implementation, (between January 1, 2007 and December 31, 2012) | 576 hospitalized IBD patients in PSHMC | Retrospective cohort study (pre-post implementation test) | USA | Mathers et al., 2017 20 | |
| The group in need of antithrombotic measures according to the MUMC protocol | Clinical decision support on adherence to thrombosis prophylaxis guidelines | ACCP 2008 | Padua Prediction Score | 24h after hospitalization, patients selected 3 days before and 2 days after the introduction(days were on different dates, not in a row) | Non-surgical patients, 64 medical patients before the introduction of the CDSS and 64 patients after the introduction | Pre-post implementation | The Netherlands | Eijgenraam et al., 2015 21 |
| VTE per 1000 patient days, odds ratios for differences VTE Advisor Alert and Notify Flag | CDSS to prevent venous thromboembolism | ACCP 2008 | Observation window spanned 6 months for each cohort | Adult inpatients, urban tertiary and level 1 trauma center, 45,046 hospitalizations representing 171,753 patient days | Pre-posttest, longitudinal, cohort design (retrospective for pretest-prospective for posttest) | USA | Amland et al., 2015 22 | |
| Appropriate venous thromboembolism prophylaxis | Creation of a CDSS and proportion of patients receiving appropriate VTE prophylaxis | ACCP 2008 | 3 months before and 2 months after the implementation | Clinical and surgical patients, 262 patients before and 261 patients after the implementation | Cross-sectional pre-post test | Brazil. | Fuzinatto et al., 2013 23 | |
| VTE prophylaxis ordering, pharmacological VTE prophylaxis ordering, and hospital-acquired VTE | Computerized decision support application to improve VTE prophylaxis | 6-month periods before and after the implementation | Adult inpatients on hospital medicine and non-medicine services in academic medical centers, whose discharge volume was 36,500 as the population of focus for the improvement effort | Observational cohort study(pre-posttest) | USA | Bhalla et al., 2013 24 | ||
| Estimated increase in VTE prophylaxis use | Electronic admission order set and VTE risk assessment and prophylaxis | (between April 2007 and May 2010)12 months prior to the first CDS intervention; the second period included admissions between the first and second versions of the CDS intervention; the third period included admissions in the 8 months following the implementation | Three acute care teaching hospitals, all adults admitted to an acute care inpatient service, 223,062 inpatients | Quasi-experimental study(pre-posttest) | USA | Umscheid et al., 2012 25 | ||
| Rates of prophylaxis | An electronic reminder was added to the electronic medical record admission note, prophylaxis, VTE, and bleeding rates | ACCP 2008 | 6-month period before and after the implementation system | Adult medical and surgical patients, 2888 patients before and 2350 patients after the intervention | Pre-post test | USA | Mitchell et al., 2012 18 | |
| Received prophylaxis recommendation, VTE events at 30 days | CDSS tool for VTE risk stratification and prophylaxis | During a 13-month period, a 4-month pre-implementation cohort and a 9-month post-implementation cohort | Medical and surgical patients who would have been deemed “low-risk”, 1322 patients’ pre-implementation and 3347 patients post-implementation | Pre-post test | USA | MaCauley et al., 2012 26 | ||
| Prophylactic measures | Computer alert program and prescription of prophylaxis | 90-day between the two Cohorts, 22 months | Patients at least 18 years of age who were hospitalized on medical and surgical services (880 patients), one-screen alert (n = 425), and three-screen alert (n = 455) | Control and intervention cohorts(pre-posttest) | USA | Fiumara et al., 2010 29 |
Table 5.
Appropriateness of venous thromboembolism prophylaxis in non-surgical patients.
Figure 2.
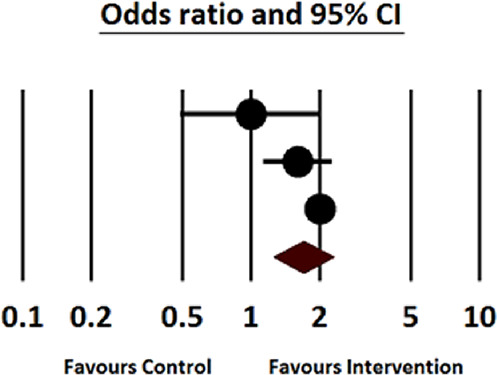
Appropriateness of venous thromboembolism prophylaxis in non-surgical patients.
Receive VTE prophylaxis in non-surgical patients
Seven out of the nine studies reported the effect of the prophylaxis recommendation system on receiving VTE prophylaxis in the non-surgical population (Table 6). These studies were conducted on 55,258 patients receiving the CDSS intervention and 60,664 control patients; the results demonstrated the effect of CDSSs on the reception of VTE prophylaxis in non-surgical patients. Accordingly, the use of a CDSS was associated with significantly increased rates of receiving VTE prophylaxis (six out of these seven studies) among the non-surgical patients compared to the controls (OR = 2.02, 95% CI: 1.66–2.45, p < 0.001; I2 = 97.1%, p < 0.001) (Figure 3).
Table 6.
Receive venous thromboembolism prophylaxis in non-surgical patients.
| References | Statistics for each study | |||
|---|---|---|---|---|
| Odds ratio | Lower limit | Upper limit | P value | |
| Mathers et al., 2017 20 | 4.827 | 2.768 | 8.429 | 0.000 |
| Eijgenraam et al., 2015 21 | 1.207 | 0.603 | 2.417 | 0.596 |
| Bhalla et al., 2012 24 | 2.813 | 2.667 | 2.966 | 0.000 |
| Umscheid et al., 2012 25 | 1.969 | 1.900 | 2.041 | 0.000 |
| Mitchell et al., 2012 18 | 1.514 | 1.341 | 1.710 | 0.000 |
| MaCauley et al., 2012 25 | 1.381 | 1.200 | 1.591 | 0.000 |
| Galanter et al., 2010 27 | 2.306 | 2.161 | 2.461 | 0.000 |
| Total | 2.023 | 1.666 | 2.457 | 0.000 |
Figure 3.

Receive venous thromboembolism prophylaxis in non-surgical patients.
Incidence of VTE in non-surgical patients
Three out of the nine studies referred to the effect of the VTE prophylaxis recommendation system on the VTE incidence in the non-surgical population (Table 7). These studies were conducted on 243,530 patients receiving the CDSS intervention and 242,973 control patients; the results showed the effect of CDSSs on the number of VTE events experienced. Accordingly, the use of a CDSS was associated with significantly decreased rates of VTE events among the patients compared to the controls (OR = 0.68, 95% CI: 0.54–0.85, p = 0.001; I2 = 31.5%, p = 0.211) (Figure 4).
Table 7.
The incidence of venous thromboembolism in non-surgical patients.
| References | Statistics for each study | |||
|---|---|---|---|---|
| Odds ratio | Lower limit | Upper limit | P value | |
| Amland et al., 2015 22 | 0.468 | 0.244 | 0.894 | 0.022 |
| Umscheid et al., 2012 25 | 0.790 | 0.757 | 0.824 | 0.000 |
| Mitchell et al., 2012 18 | 0.748 | 0.344 | 1.624 | 0.463 |
| MaCauley et al., 2012 25 | 0.459 | 0.212 | 0.994 | 0.048 |
| Galanter et al., 2010 27 | 0.598 | 0.383 | 0.934 | 0.024 |
| Total | 0.682 | 0.545 | 0.855 | 0.001 |
Figure 4.

The incidence of venous thromboembolism in non-surgical patients.
Discussion
This systematic review and meta-analysis included 12 studies to determine the impact of the computerized CDSSs on appropriate VTE prophylaxis orders in non-surgical patients. According to this systematic review and meta-analysis results, the use of CDSSs among non-surgical patients is associated with a considerable increase in the proportion of patients who received appropriate VTE prophylaxis recommendation, especially if the CDSS could auto-populate patient’s prescriptions. Additionally, using CDSSs is associated with a considerable reduction in the risk of developing VTE.
According to the findings of this review, for any intervention to significantly improve the prevention of VTE, it is necessary to have at least two basic factors: the intervention should assist clinicians in assessing patients’ risk status for VTE, and it should assist clinicians in prescribing the appropriate prophylaxis for risk classification.30,31
Several studies have evaluated computer alerts to improve thromboprophylaxis in surgical and non-surgical patients. For instance, Kucher et al. conducted a randomized clinical trial using a computer program including risk factors and special diagnostic codes and identified both surgical and non-surgical patients who were at risk of VTE. Accordingly, the hospital's VTE-prevention guidelines were linked to this computer-alert system. The authors also found that using CDSSs reduced the incidence of DVT and PE. 28 The use of computerized alerts for appropriate VTE prophylaxis was also studied in large-scale research in Switzerland (1593 non-surgical patients divided into an alert group (804) and a control group (789)). 19 Therefore, the alert was considered a support system for clinical decision-making, which played an important role in helping physicians comply with the current international consensus guidelines. 19
Computerized CDSSs have been used successfully to provide preventive care services to non-surgical patients. 26 In this context, CDSSs have been shown to increase adherence to guidelines based on the overall VTE prevention medical services. 7 Bhalla et al. disclosed that the VTE preventive medical services increased from 61.9% to 82.1% (p < 0.001), and pharmacological VTE prophylaxis increased from 59.0% to 74.5% (p < 0.001). 24 Moreover, Fuzinatto et al. conducted a research on a specific population of cancer patients and revealed a significant increase in the use of appropriate VTE prophylaxis (from 18.1% to 44.1%; p = 0.002). 23 In contrast, the results of the study by Eijgenraam et al. showed no improvements in the adherence to antithrombotic prophylaxis guidelines in medical patients. 21 These positive results indicated CDSSs to be a powerful tool for implementing current evidence-based medicine as a standard of care.
From the present meta-analysis, it follows that an increase in the appropriate prophylaxis rate for VTE with the implementation of CDSSs was associated with a reduction in the incidence of VTE events. Previous studies demonstrated that using CDSSs resulted in an increase in the rate of VTE prevention. Nonetheless, there is still insufficient evidence to link the increased rate of prophylaxis with the clinical endpoint of decline in the VTE rate. 6
Among the 12 studies analyzed here, five used the American College of Chest Physicians (ACCP) guideline to prevent thrombosis. In the other studies, the guidelines were developed by local experts. 32 Overall, more efficient and powerful CDSSs are associated with electronic medical records. This strategy significantly improves the prevention of VTE, which may lead to a significant reduction in VTE incidents, especially in non-surgical patients. 11 Since many hospitals under investigation in the analyzed studies did not have the necessary IT resources, most studies were limited to individual centers, whence their results could not be generalized to other institutions. 33
To address the fatigue symptoms, the staff had direct relationships with together and made decisions in the presence of the physician. 34 In a large randomized multicenter study (n = 2493), in the alert group, the physician was informed by another staff member that the patient was at a high risk for VTE but was not receiving VTE prophylaxis. Then, prophylaxis was recommended for the patient. Based on the previous study findings, the patients who were warned by their physicians were more than twice as likely to be prevented from VTE compared to the control patients who were not warned (p < 0.0001). Additionally, a decrease was found in the VTE rate in the intervention group at 90 days. However, no significant difference was observed between the human alert trial and the computer alert trial. Yet, the slightly less prominent reduction in VTE in the human alert trial suggested the higher effectiveness of the computer alerting system. 5
Although our analysis indicates that the CDSS-guided prevention was associated with a reduction in VTE incidents in non-surgical patients, no sufficient data were provided regarding the side effects of prophylaxis. These risks include hematoma formation, bleeding events, and thrombocytopenia. Therefore, it is vitally important to further evaluate the advantages and disadvantages of prescribing additional anticoagulants for VTE patients.
Strengths of the review
This review provides readers with a comprehensive overview of the range of approaches taken to date to evaluate the effectiveness of computerized CDSSs in the improvement of using appropriate VTE prophylaxis in non-surgical patients. The information presented in Table 2 and Table 3 provides a useful basis for other researchers to evaluate their intervention elements in relation to previous approaches to designing interventions. Our overall goal was to help future researchers to improve these efforts by highlighting the strengths and weaknesses of the previous studies and to consider appropriate measures and methods, if possible.
Limitations of the review
One of the limitations of this study was that only the evaluation of quantitative studies was focused and qualitative studies were excluded. Moreover, our review only includes the studies that assessed the CDSS implementation process associated with the prescription of VTE prophylaxis such as those describing successful implementation strategies. Since most studies reported demographic information without separating surgical and non-surgical patients, this information as well as patients’ risk levels could not be extracted. Besides, each individual study did not have sufficient power to detect changes in the VTE incidence; however, this limitation was softened by pooling the data. Furthermore, considering the 90-day follow-up, the incidence of VTE could be potentially underestimated. Finally, the results might be context-dependent since most studies were conducted in the United States. This could affect the validity of the CDSS system and its impact on appropriate VTE prophylaxis orders in non-surgical patients.
Conclusion
This study showed that CDSS interventions could be effective in improving the use of VTE prophylaxis in non-surgical patients. Nonetheless, higher-quality studies are required to confirm the results. Currently, little evidence is available to assist physicians in consciously selecting CDSSs for VTE prescription. This will inevitably lead to many choices, each of which can make CDSSs easier to use. This suggests the need for higher-quality systematic comparative studies.
Acknowledgments
The authors would like to thank all the professors and staff of Shiraz Medical Sciences University for their contributions to this study. Also, the authors would like to acknowledge Marcel Ausloos from the University of Leicester in the UK for proofreading and improving the language.
Footnotes
Conflict of interest: The authors have no conflicts of interest to declare.
Contributorship: M.K. was responsible for the study's conception and design. F.T. searched the relevant databases and included the appropriate articles according to the study objective. At the same time, R.S. supervised the whole thesis. H.R.S. preparing the first draft of the manuscript and revise the manuscript. H.R.S. and S.H. did the analysis of the results, made critical revisions to the paper for important intellectual content, and supervised the study. All authors have read and approved the final manuscript.
Ethical approval: CRD42021225093 is the study protocol number registered in PROSPERO.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Guarantor: MK.
ORCID iDs: Mehrdad Karajizadeh https://orcid.org/0000-0002-9297-3488
Hamid Reza Saeidnia https://orcid.org/0000-0001-5430-2416
References
- 1.Schünemann HJ, Cushman M, Burnett AE, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: prophylaxis for hospitalized and nonhospitalized medical patients. Blood Adv 2018; 2: 3198–3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gould MK, Garcia DA, Wren SM, et al. Prevention of VTE in nonorthopedic surgical patients: antithrombotic therapy and prevention of thrombosis: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2012; 141: e227S–ee77S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumar P, Dhillon MS, Kansal R. Routine chemoprophylaxis for venous thromboembolism in orthopedic patients: is it justified?. J Postgrad Med Educ Res 2019; 53: 152–157. [Google Scholar]
- 4.Amin A, Spyropoulos A, Dobesh P, et al. Are hospitals delivering appropriate VTE prevention? The venous thromboembolism study to assess the rate of thromboprophylaxis (VTE start). J Thromb Thrombolysis 2010; 29: 326–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Piazza. Physician alerts to prevent symptomatic venous thromboembolism in hospitalized patients (vol 119, pg 2196, 2009). Circulation. 2010; 122: E4–EE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Veeramootoo D, Harrower L, Saunders Ret al. et al. Prophylaxis of venous thromboembolism in general surgery: guidelines differ and we still need local policies. Ann R Coll Surg Engl 2011; 93: 370–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borab ZM, Lanni MA, Tecce MGet al. et al. Use of computerized clinical decision support systems to prevent venous thromboembolism in surgical patients: a systematic review and meta-analysis. JAMA Surg 2017; 152: 638–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qatawneh Z, Alshraideh M, Almasri Net al. et al. Clinical decision support system for venous thromboembolism risk classification. Appl Comput Inform 2019; 15: 12–18. [Google Scholar]
- 9.Delvaux N, Van Thienen K, Heselmans Aet al. et al. The effects of computerized clinical decision support systems on laboratory test ordering: a systematic review. Arch Pathol Lab Med 2017; 141: 585–595. [DOI] [PubMed] [Google Scholar]
- 10.Bennett P, Hardiker NR. The use of computerized clinical decision support systems in emergency care: a substantive review of the literature. J Am Med Inform Assoc 2017; 24: 655–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tooher R, Middleton P, Pham C, et al. A systematic review of strategies to improve prophylaxis for venous thromboembolism in hospitals. Ann Surg 2005; 241: 397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jaspers T, Duisenberg-van Essenberg M, Maat Bet al. et al. A multifaceted clinical decision support intervention to improve adherence to thromboprophylaxis guidelines. Int J Clin Pharm 2021; 43: 1327–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moher D, Liberati A, Tetzlaff Jet al. et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Higgins JP, Thompson SG, Deeks JJet al. et al. Measuring inconsistency in meta-analyses. Br Med J 2003; 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Armijo-Olivo S, Stiles CR, Hagen NAet al. et al. Assessment of study quality for systematic reviews: a comparison of the Cochrane Collaboration Risk of Bias Tool and the Effective Public Health Practice Project Quality Assessment Tool: methodological research. J Eval Clin Pract 2012; 18: 12–18. [DOI] [PubMed] [Google Scholar]
- 16.Schober P, Bossers SM, Schwarte LA. Statistical significance versus clinical importance of observed effect sizes: what do P values and confidence intervals really represent? Anesth Analg 2018; 126: 1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sterne JA, Egger M. Regression methods to detect publication and other bias in meta-analysis. Publ Bias Meta Anal 2005: 99–110. [Google Scholar]
- 18.Mitchell JD, Collen JF, Petteys Set al. et al. A simple reminder system improves venous thromboembolism prophylaxis rates and reduces thrombotic events for hospitalized patients1. J Thromb Haemost 2012; 10: 236–243. [DOI] [PubMed] [Google Scholar]
- 19.Spirk D, Stuck A, Hager Aet al. et al. Electronic alert system for improving appropriate thromboprophylaxis in hospitalized medical patients: a randomized controlled trial. J Thromb Haemostasis 2017; 15: 2138–2146. [DOI] [PubMed] [Google Scholar]
- 20.Mathers B, Williams E, Bedi Get al. et al. An electronic alert system is associated with a significant increase in pharmacologic venous thromboembolism prophylaxis rates among hospitalized inflammatory bowel disease patients. J Healthc Qual 2017; 39: 307–314. [DOI] [PubMed] [Google Scholar]
- 21.Eijgenraam P, Meertens N, van den Ham Ret al. et al. The effect of clinical decision support on adherence to thrombosis prophylaxis guidelines in medical patients; A single center experience. Thromb Res 2015; 135: 464–471. [DOI] [PubMed] [Google Scholar]
- 22.Amland RC, Dean BB, Yu H, et al. Computerized clinical decision support to prevent venous thromboembolism among hospitalized patients: proximal outcomes from a multiyear quality improvement project. J Healthc Qual 2015; 37: 221–231. [DOI] [PubMed] [Google Scholar]
- 23.Fuzinatto F, Waldemar F, Wajner A, et al. A clinical decision support system for venous thromboembolism prophylaxis at a general hospital in a middle-income country. J Bras Pneumol 2013; 39: 138–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bhalla R, Berger MA, Reissman SH, et al. Improving hospital venous thromboembolism prophylaxis with electronic decision support. J Hosp Med 2013; 8: 115–120. [DOI] [PubMed] [Google Scholar]
- 25.Umscheid CA, Hanish A, Chittams Jet al. et al. Effectiveness of a novel and scalable clinical decision support intervention to improve venous thromboembolism prophylaxis: a quasi-experimental study. BMC Med Inform Decis Mak 2012; 12: 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.MaCauley MJ, Showalter JW, Beck MJet al. et al. The effect of a provider-enhanced clinical decision support tool for guiding venous thromboembolism pharmacoprophylaxis in low-risk patients. Hosp Pract 2012; 40: 7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Galanter WL, Thambi M, Rosencranz H, et al. Effects of clinical decision support on venous thromboembolism risk assessment, prophylaxis, and prevention at a university teaching hospital. Am J Health Syst Pharm 2010; 67: 1265–1273. [DOI] [PubMed] [Google Scholar]
- 28.Kucher N, Koo S, Quiroz R, et al. Electronic alerts to prevent venous thromboembolism among hospitalized patients. N Engl J Med 2005; 352: 969–977. [DOI] [PubMed] [Google Scholar]
- 29.Fiumara K, Piovella C, Hurwitz S, et al. Multi-screen electronic alerts to augment venous thromboembolism prophylaxis. Thromb Haemostasis 2010; 103: 312–317. [DOI] [PubMed] [Google Scholar]
- 30.Adams P, Riggio JM, Thomson Let al. et al. Clinical decision support systems to improve utilization of thromboprophylaxis: a review of the literature and experience with implementation of a computerized physician order entry program. Hosp Pract 2012; 40: 27–39. [DOI] [PubMed] [Google Scholar]
- 31.Michota FA. Bridging the gap between evidence and practice in venous thromboembolism prophylaxis: the quality improvement process. J Gen Intern Med 2007; 22: 1762–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eikelboom JW, Karthikeyan G, Fagel Net al. et al. American Association of Orthopedic Surgeons and American College of Chest Physicians guidelines for venous thromboembolism prevention in hip and knee arthroplasty differ: what are the implications for clinicians and patients? Chest 2009; 135: 513–520. [DOI] [PubMed] [Google Scholar]
- 33.Al-Jaghbeer M, Dealmeida D, Bilderback Aet al. et al. Clinical decision support for in-hospital AKI. J Am Soc Nephrol 2018; 29: 654–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Dort BA, Zheng WY, Baysari MT. Prescriber perceptions of medication-related computerized decision support systems in hospitals: a synthesis of qualitative research. Int J Med Inf 2019; 129: 285–295. [DOI] [PubMed] [Google Scholar]



