Abstract
Background
Permanent pacemaker placement (PPM) is associated with morbidity following cardiac surgery. This study identified associations between PPM placement and 5-year outcomes for patients that require PPM following valvular surgery.
Methods
All patients who underwent valvular surgery at our medical center from 2011 to 2018 were considered for analysis. Multivariable analysis identified associations between PPM placement, mortality, and readmissions. Primary outcomes were operative complications and mortality. Secondary outcomes included 5-year survival and readmission.
Results
A total of 175 (4.86%) of 3602 valvular surgery patients required postoperative PPM. The PPM cohort had significantly worse baseline comorbidities, including greater Society of Thoracic Surgeons Predicted Risk of Mortality (STS-PROM) scores (3.8 vs 2.4 P < .0001). The PPM cohort had greater rates of blood product transfusion, prolonged ventilation, and new-onset atrial fibrillation. PPM placement was significantly associated with third-degree heart block (5.26; 95% confidence interval [95% CI], 1.00-27.53; P = .0496), ventricular fibrillation/tachycardia (3.90; 95% CI, 1.59-9.59; P = .01), and atrial fibrillation/flutter (1.53; 95% CI, 1.05-2.24; P = .03). On Kaplan-Meier estimates, 5-year survival (68.8% vs 83.1%; P = 01) was significantly reduced in the PPM cohort. Five-year all-cause readmission (60.4% vs 50.04%; P = .01) and heart failure readmission (35.5% vs 20.1%; P < .000) occurred more frequently in the PPM cohort. On multivariable Cox regression analysis, PPM placement (hazard ratio, 1.12; 95% CI, 0.84-1.50; P = .444) was not an independent predictor of mortality. On competing risk analysis, PPM (hazard ratio, 1.33; 95% CI, 0.99-1.80; P = .062) was not a predictor of hospital readmission.
Conclusions
Valvular surgery patients who required postoperative PPM had elevated baseline operative risk. However, PPM implantation was not associated with mortality or readmission.
Key Words: permanent pacemaker (PPM), arrythmia, valve surgery
Abbreviations and Acronyms: CABG, coronary artery bypass grafting; CI, confidence interval; HR, hazard ratio; ICD, implantable cardioverter-defibrillator; PPM, postoperative pacemaker placement
Graphical abstract


On Kaplan–Meier estimates, 5-year survival was significantly reduced in the PPM cohort.
Central Message.
Permanent pacemaker placement was required in patients with greater comorbidities but was not independently associated with worse outcomes.
Perspective.
Permanent pacemaker placement (PPM) may be required after cardiac surgery in patients with greater baseline risk. Although PPM has been associated with worse cardiac surgery outcomes, we found no association between PPM and 5-year mortality or readmission. This suggests that PPM may be a surrogate for other clinical variables that affect outcomes following cardiac surgery.
The placement of implantable electronic devices plays an important role in the postoperative period following cardiac surgery, and the incidence is variable, ranging from <1% to nearly 10%, depending on the type of surgery.1, 2, 3, 4, 5, 6, 7, 8, 9 Patients who undergo isolated coronary artery bypass grafting10 (CABG) without associated valvular surgery tend to have the lowest need for postoperative pacemaker placement (PPM). In contrast, patients who undergo reoperative valve surgery6,11 have been shown to be at greater need for postoperative PPM compared with the general open cardiac surgery population. Known predictors of postoperative PPM include valve surgery, with a several-fold increased risk in patients with double or triple valves, reoperative surgery, and increased patient age.5,12, 13, 14
The need for permanent postoperative pacing is often due to damage to the cardiac conduction system. There is difficulty in identifying which patients will need PPM following surgery, and ubiquitous indications for device implantation may be inconsistently followed.7 Current large studies reporting the long-term impact of postoperative PPM placement on cardiac surgery outcomes are limited. The aim of this study is to provide the incidence and associations with PPM from a large single-center report of isolated valve, multiple-valve, and CABG and valve surgeries, including the association of PPM with 5-year survival and hospital readmission.
Methods
Study Population
Patients outcomes were retrospectively obtained from our medical centers prospectively maintained cardiac surgery database. Data use and analysis were approved by the institutional review board and consent waived. Elective, urgent, and emergent cases were included in the analysis. All patients who underwent isolated valve surgery and CABG with valve surgery were included (Figure 1). We excluded the patients who underwent isolated CABG surgery (due to a low likelihood of pacer requirements with isolated CABGs), had previous pacemaker placement, transplants, and ventricular assist devices.
Figure 1.
Consolidated Standards of Reporting Trials diagram of participant inclusion and exclusion from the investigation. PPM, Permanent pacemaker placement.
PPM placement was defined as including implantable cardioverter-defibrillators (ICDs) or combined ICD/PPM implantation. The total patient population was divided into 2 cohorts: (1) PPM placement within 30 days or in hospital (postoperative) and (2) patients without PPM placement within 30 days or in hospital (postoperative).
Statistical Analysis
For continuous variables, normality was assessed via the Shapiro–Wilk test. Continuous variables that did not meet normality were analyzed via Wilcoxon rank-sum and reported as median and interquartile range (quartile 1-quartile 3). Categorical variables are reported as count and proportion. We used χ2 unless more than 20% of cells had expected frequencies <5, in which case we used the Fisher exact test.
Propensity score matching was not appropriate; after 1:1, 1:2, 1:3, and 1:4 propensity score matching, there was an imbalance between the pacemaker group and nonpacemaker group. Baseline patient characteristics were compared between PPM and non-PPM cohorts. All baseline characteristics were initially evaluated in the univariable Cox proportional hazard model (P < .2 was cutoff for inclusion in multivariable analysis) of time to death.
Our definition of 5-year mortality included all deaths starting on (and including) the date of surgery. We performed backward elimination to choose the Cox proportional hazard model for mortality and Fine and Gray model for heart failure readmission. We started with all candidate variables (Table 1), testing the deletion of each variable using the model fit criterion (significance level = .2), deleting the variable with the largest value. We repeated the process until all the variables in the model were less than or equal to .2. A limitation of backward elimination is that the deleted variables cannot go back into the model even if it is significance in a future model.
Table 1.
Baseline patient characteristics in patients who underwent isolated valve surgery and CABG with valve surgery
| Variables | No PPM (N = 3427) | PPM (N = 175) | P value |
|---|---|---|---|
| Age, y | 70 (61-77) | 74 (65-80) | <.001 |
| Women | 1302 (37.99%) | 73 (41.71%) | .32 |
| Body mass index, kg·m−2 | 28.4 (25.0-32.8) | 28.7 (25.0-33.5) | .60 |
| Diabetes mellitus | 1169 (34.1%) | 75 (42.9%) | .02 |
| Hypertension | 2780 (81.1%) | 151 (86.3%) | .09 |
| Chronic lung disease | 773 (22.2%) | 44 (25.1%) | .43 |
| Dialysis | 71 (2.1%) | 2 (1.1%) | .58 |
| Immunosuppression | 233 (6.8%) | 12 (6.9%) | .98 |
| Previous heart failure | 1040 (30.4%) | 66 (37.7%) | .04 |
| Previous myocardial infarction | 904 (26.3%) | 57 (32.6%) | .07 |
| Previous arrhythmia | 615 (18.0%) | 55 (31.4%) | <.0001 |
| Status | .001 | ||
| Elective | 2260 (66.0%) | 89 (50.9%) | |
| Urgent | 1101 (32.1%) | 83 (47.4%) | |
| Emergent | 61 (1.8%) | 3 (1.7%) | |
| Emergent salvage | 5 (0.2%) | 0 (0.0%) | |
| STS-PROM (%) | 2.4 (1.3-4.8) | 3.8 (2.0-8.0) | <.001 |
| Surgery type | .01 | ||
| Isolated MV repair | 462 (13.5%) | 12 (6.9%) | |
| Isolated MVR | 170 (5.0%) | 17 (9.7%) | |
| Isolated AVR | 1421 (41.5%) | 72 (41.1%) | |
| CABG + MVR | 87 (2.5%) | 7 (4.0%) | |
| CABG + MV repair | 303 (8.8%) | 21 (12.0%) | |
| CABG + AVR | 984 (28.7%) | 46 (26.3%) | |
| Valve type | .44 | ||
| Aortic valve | 2405 (70.2%) | 118 (67.4%) | |
| Mitral valve | 1022 (29.8%) | 57 (32.6%) | |
| Valve + CABG | .56 | ||
| Isolated valve | 2053 (59.9%) | 101 (57.7%) | |
| CABG + valve | 1374 (40.1%) | 74 (42.3%) | |
| Serum creatinine, mg per dL | 1.0 (0.8-1.2) | 1.0 (0.8-1.2) | .11 |
| Albumin, g per dL | 3.7 (3.4-4.1) | 3.6 (3.3-4.0) | .03 |
| Total bilirubin, mg per dL | 0.6 (0.5-0.9) | 0.6 (0.5-0.9) | .77 |
| Ejection fraction, % | 58.0 (50.0-63.0) | 55.0 (45.0-60.0) | .001 |
| Previous valve procedure | 84 (2.5%) | 10 (5.7%) | .02 |
| Previous CABG | 243 (7.1%) | 20 (11.4%) | .03 |
| Cardiopulmonary bypass time, min | 115 (88-151) | 116 (88-158) | .89 |
| Ischemic time, min | 89.0 (67.0-117.0) | 92.00 (68.0-123.0) | .59 |
Variables are presented as count (frequency) and median (1-3 interquartile ranges) for categorical and continuous variables, respectively. PPM, Permanent pacemaker placement; STS-PROM, Society of Thoracic Surgeons Predicted Risk of Mortality; MV, mitral valve; MVR, mitral valve replacement; AVR, aortic valve replacement; CABG, coronary artery bypass grafting.
After model selection, our model did not meet the proportional hazard assumption. For the Cox model for mortality, the supremum test found that cardiopulmonary bypass time, ischemic time, albumin, and bilirubin violated the proportional hazard assumption. To address the violation of nonproportional hazard, albumin, bilirubin, cardiopulmonary bypass time, ischemic time, and previous myocardial infarction were stratified (the cutoff of the continuous variables [albumin, bilirubin, cardiopulmonary bypass time, ischemic time] was median). After stratification, the model met the proportional hazard assumption. In the analysis, the hazard ratio (HR) refers to preset change. It indicates the change in the risk of death if the parameter (eg, age, creatinine, albumin, cardiopulmonary bypass time and ischemic time) increases by 1 unit.
We selected Firth logistic regression because of quasi-complete separation. We performed backward elimination to choose the Firth logistic model at a significance level of 0.1.
Overall mortality was calculated using Kaplan–Meier estimation and overall readmission using cumulative incidence function. The log-rank test was used for overall mortality and the Gray's test was used for overall readmission.
For readmission, cause-specific hazard was calculated using the cumulative incidence function (death as a competing risk) in both univariable and multivariable models. In the event of multiple readmissions for the same patient, time to the first readmission was used in the model. Significant covariables were adjusted in the multivariable models of time to death and readmission separately.
Five-year survival was compared for each group with the use of Kaplan–Meier curves and cumulative incidence function was used to generate a curve for 5-year readmissions.
Results
Baseline Patient Characteristics
From a total of 3602 cardiac surgery operations, 175 (4.9%) patients required PPM. Of the total, 6 (3.4%) patients had isolated ICD placement for postoperative tachyarrhythmia. Patients in the PPM cohort had significantly worse baseline comorbidities (Table 1), including, but not limited to, diabetes mellitus (42.9% vs 34.1%; P = .018), previous heart failure (37.7% vs 30.4%; P = .039), previous arrhythmia (31.4% vs 18%; P < .000), and increased Society of Thoracic Surgeons-Predicted Risk of Mortality (%) (3.8 vs 2.4; P < .000). Patients in the PPM cohort had greater previous valve surgery (5.7% vs 2.5%; P = .024) and previous CABG operations (11.4% vs 7.1%: P = .031). There were no double-valve operations. There were 25 patients with preoperative atrial fibrillation who had concomitant maze procedure.
Multivariable Regression for Association With PPM Placement
On logistic regression, age and ejection fraction were significant predictors of PPM placement. Arrhythmias significantly associated with PPM placement included third-degree heart block (5.26; 95% confidence interval [CI], 1.00-27.53; P = .0496), ventricular fibrillation/tachycardia (3.90; 95% CI, 1.59-9.59; P = .01), and atrial fibrillation/flutter (1.53; 95% CI, 1.05-2.24; P = .03) (Table 2).
Table 2.
Firth logistic model for predicting permanent pacemaker placement in patients who underwent isolated valve surgery and CABG with valve surgery
| Variable | Odds ratio (95% Confidence interval) | P value |
|---|---|---|
| Age | 1.04 (1.01-1.06) | <.001 |
| V-tach/V-fib | 3.90 (1.59-9.59) | .01 |
| Third-degree heart block | 5.26 (1.00-27.53) | .0496 |
| Atrial fibrillation/flutter | 1.53 (1.05-2.24) | .03 |
| Ejection fraction | 0.98 (0.97-1.00) | .02 |
All variables with a P value < .1 were kept in the model. V-tach, Ventricular tachycardia; V-fib, ventricular fibrillation.
Immediate Postoperative Outcomes
There was no significant difference between PPM versus non-PPM patients for postoperative mortality (1.7% vs 2.9%; P = .49) (Table 3). Patients in the PPM cohort had increased blood product transfusion (48.6% vs 39.1%; P = .013), prolonged ventilation (18.3% vs 10.2%; P = .001), and new-onset atrial fibrillation (51.4% vs 41.2%; P = .007). There was no difference between cohorts for deep sternal wound infection (0.0% vs 0.2%; P = .58), acute renal failure (3.4% vs 4.0%; P = .71), permanent stroke (3.4% vs 2.5%; P = .47), and reoperation (12.0% vs 8.6%; P = .12).
Table 3.
Postoperative outcomes in patients who underwent isolated valve surgery and CABG with valve surgery
| Variables | No PPM | PPM | P value |
|---|---|---|---|
| Operative mortality (STS definition) | 99 (2.9%) | 3 (1.7%) | .49 |
| Blood product transfusion | 1340 (39.1%) | 85 (48.6%) | .01 |
| Prolonged ventilation∗ | 351 (10.2%) | 32 (18.3%) | .00 |
| Deep sternal wound infection | 6 (0.2%) | 0 (0.0%) | .58 |
| Acute renal failure | 137 (4.0%) | 6 (3.4%) | .71 |
| Permanent stroke | 87 (2.5%) | 6 (3.4%) | .47 |
| Reoperation | 294 (8.6%) | 21 (12.0%) | .12 |
| New-onset atrial fibrillation | 1411 (41.2%) | 90 (51.4%) | .01 |
PPM, Permanent pacemaker placement; STS, Society of Thoracic Surgeons.
>24 hours.
Mortality and Readmissions
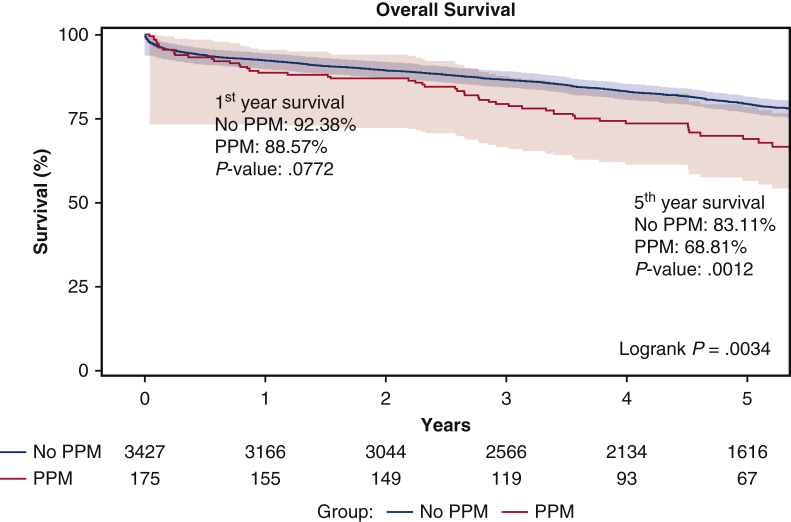
Over a median follow-up of 4.8 (3.0-6.8) years, patient in the PPM cohort had a greater mortality (31.4% vs 23.7%; P = .003), overall readmission (57.7% vs 51.0%; P = .024), cardiac readmission (53.1% vs 44.1%; P = .007), and heart failure readmission (34.3% vs 21.7%; P < .000). On Kaplan–Meier estimates, 5-year survival (68.8% vs 83.1%; P = .001) (Figure 2) was significantly reduced in the PPM cohort (see also Figure 3 for adjusted overall survival). Long-term all-cause readmission (60.4% vs 50.0%; P = .010) (Figure 4) and heart failure readmission (35.5% vs 20.1%; P < .000) (Figure 5) occurred more frequently in the PPM cohort.
Figure 2.
On Kaplan–Meier estimates, 5-year survival (68.8% vs 83.1%; P = .001) was significantly reduced in the PPM cohort among patients who underwent isolated valve surgery and coronary artery bypass grafting with valve surgery. PPM, Permanent pacemaker placement.
Figure 3.
Adjusted overall survival in the nonpacemaker and PPM cohort among patients who underwent isolated valve surgery and coronary artery bypass grafting with valve surgery. PPM, Permanent pacemaker placement.
Figure 4.
Five-year all-cause readmission (60.4% vs 50.0%; P = .010) was significantly higher in the PPM cohort among patients who underwent isolated valve surgery and coronary artery bypass grafting with valve surgery. PPM, Permanent pacemaker placement.
Figure 5.
Heart failure readmission (35.5% vs 20.1%; P < .000) occurred more frequently in the PPM cohort among patients who underwent isolated valve surgery and coronary artery bypass grafting with valve surgery. PPM, Permanent pacemaker placement.
On multivariable Cox regression analysis (Table 4), PPM placement (HR, 1.1; 95% CI, 0.8-1.5; P = .444) was not an independent predictor of mortality. Numerous comorbidities were predictors of mortality including but not limited to diabetes and peripheral artery disease. The most significant predictors of readmission included immunosuppression, chronic lung disease, and elevated serum creatinine.
Table 4.
Stratified Cox model for mortality (backward elimination) in patients who underwent isolated valve surgery and CABG with valve surgery
| HR (95% CI) | P value | |
|---|---|---|
| PPM | 1.12 (0.84-1.50) | .444 |
| Ejection fraction | 0.99 (0.99-1.00) | .015 |
| Age | 1.03 (1.02-1.04) | <.0001 |
| Diabetes | 1.28 (1.09-1.50) | .003 |
| Chronic lung disease | 1.67 (1.41-1.98) | <.0001 |
| Dialysis | 2.45 (1.43-4.14) | .001 |
| Immunosuppression | 1.39 (1.07-1.81) | .014 |
| PAD | 1.54 (1.29-1.83) | <.0001 |
| Previous heart failure | 1.25 (1.06-1.48) | .010 |
| Arrhythmia | 1.43 (1.20-1.71) | <.0001 |
| Isolated MV repair | 0.74 (0.53-1.04) | .083 |
| CABG + AVR | 1.14 (0.96-1.36) | .135 |
| Serum creatinine∗ | 1.11 (1.03-1.20) | .007 |
| Redo procedure | 1.29 (1.02-1.63) | .033 |
Albumin, bilirubin, cardiopulmonary bypass time, ischemic time, previous myocardial infarction, and positive test were stratified based on median, then fit a stratified Cox model for mortality. HR, Hazard ratio; CI, confidence interval; PPM, permanent pacemaker placement; PAD, peripheral artery disease; MV, mitral valve; CABG, coronary artery bypass grafting; AVR, aortic valve replacement.
Missing data in the no PPM cohort (n = 15).
On the competing risk model for heart failure readmission, PPM placement (HR, 1.3; 95% CI, 1.0-1.8; P = .062) did not increase the likelihood or being readmitted for heart failure. Immunosuppression, previous heart failure, and isolated mitral valve replacement had the greatest HR for predictors of heart failure readmission (Table 5).
Table 5.
Fine and Gray competing risk regression for risk of heart failure readmission in patients who underwent isolated valve surgery and CABG with valve surgery (backward elimination, significance level = .2)
| HR (95% CI) | P value | |
|---|---|---|
| PPM | 1.33 (0.99-1.80) | .062 |
| Age | 1.02 (1.01-1.02) | .000 |
| Woman | 1.15 (0.96-1.37) | .121 |
| White | 0.56 (0.42-0.74) | <.001 |
| Body mass index | 1.03 (1.02-1.04) | <.001 |
| Chronic lung disease | 1.34 (1.13-1.60) | .001 |
| Immunosuppression | 1.86 (1.44-2.39) | <.001 |
| Previous heart failure | 1.64 (1.39-1.95) | <.001 |
| Isolated AVR | 0.84 (0.67-1.06) | .133 |
| Isolated MVR | 1.67 (1.20-2.32) | .003 |
| CABG + AVR | 0.70 (0.56-0.89) | .003 |
| Serum creatinine∗ | 1.13 (1.06-1.20) | .000 |
| Albumin∗ | 0.76 (0.65-0.90) | .001 |
| Bilirubin∗ | 0.85 (0.69-1.04) | .119 |
| Ejection fraction | 0.98 (0.98-0.99) | <.001 |
Continuous variables are modeled as continuous (eg, the risk of heart failure readmission decreases by 2.9% for every additional unit of BMI; the risk of heart failure readmission decreases by 1.8% for every additional percentage of ejection fraction). HR, Hazard ratio; CI, confidence interval; PPM, permanent pacemaker placement; AVR, aortic valve replacement; MVR, mitral valve replacement; CABG, coronary artery bypass grafting.
Missing data in the no PPM cohort (creatinine [n = 13], albumin [n = 477], bilirubin [n = 481]).
Discussion
The current study is composed of outcomes from a large single-center analysis of isolated valve operations and CABG + valve operations, with analysis focusing on patients who required permanent pacemakers in the immediate postoperative period. Of the total cohort, nearly 5% of patients required PPM placement, which included ICD or combined ICD/PPM placement. The PPM cohort represents a patient group with significantly increased baseline comorbidities, including but not limited to increased patient age, cerebrovascular disease, peripheral artery disease, diabetes mellitus, previous heart failure, and increased Society of Thoracic Surgeons-Predicted Risk of Mortality. There was no significant difference in postoperative mortality between PPM and non-PPM cohorts; however, patients requiring PPM had increased postoperative complications. In addition, patients in the PPM cohort had reduced 5-year survival and increased cumulative incidence of hospital readmission and heart failure readmissions over the study follow-up period. However, on multivariable regression analysis, PPM placement was not an independent predictor of mortality or readmission, indicating that worse 5-year outcomes in this patient population are multifactorial and likely due to heightened baseline preoperative risk.
Existing literature reports variable postoperative PPM requirements for cardiac surgery patients and incidence ranges from approximately 1% to 10%.6,7,9,10,15 Importantly, surgery type plays an significant role in determining the associated risk of pacemaker placement.6,10 Recent large studies1,16,17 report the incidence of postoperative PPM implantation ranging from 1.2% to 3.2%, although patient cohorts were largely composed of isolated aortic valve replacement16,17 and isolated CABG procedures were included.1 In the current study, we excluded all isolated CABG procedures and included all patients who underwent isolated-valve and valves in combination with CABG procedures. Furthermore, we included all patients that underwent reoperative cardiac surgery, which has been identified as a risk factor for worse cardiac surgery outcomes including both short- and long-term mortality,11 and heightened risk for postoperative PPM need.6 On multivariable analysis, previous arrhythmia was an independent predictor of PPM placement and ventricular fibrillation/ventricular tachycardia, third-degree heart block, and atrial fibrillation/flutter were significantly associated with PPM. Reoperative cardiac surgery was not independently associated with PPM placement; however, it was predictive of increased mortality risk on 5-year follow-up. Likewise, numerous baseline patient comorbidities (eg, chronic obstructive pulmonary disease, peripheral artery disease, dialysis) including previous heart failure, were significantly associated with mortality. Moreover, we found that patients in the PPM cohort had a significantly greater cumulative incidence of heart failure hospital readmissions on 5-year follow-up. There is an important known association with reduced left ventricular ejection fraction and poor outcomes in cardiac surgery18 and our findings are similar to previous literature that identified an association between low left ventricular ejection fraction and heart failure hospitalization in patients with permanent pacemakers.19
Multiple studies have reported associated baseline patient comorbidities20, 21, 22 and the impact of comorbid disease on cardiac permanent pacemaker requirements and survival following pacemaker implantation. In a large population-based study,21 including nearly 9000 patients with initial PPM placement, 5-year mortality was associated with a greater Charlson Comorbidity Index and a history of heart failure, among other comorbidities. Importantly, life expectancy in patients with a PPM without significant comorbid disease was similar to that of the general population. According to trends in national data,22 PPM placement has increased a significant degree within the past 2 decades, which was likely due to increasing patient age and comorbid disease. Furthermore, a recent report20 shows that, compared with the general population, patients who require PPM implantation have an age-independent increase in medical comorbidities. Although PPM literature is not limited to postcardiotomy patients, the importance of recognizing that patients who required PPMs often have heightened baseline risk, due to comorbidities, has significant implications and is consistent with our findings. The current study shows that the need for postoperative pacemaker is representative of a complicated interplay between numerous factors including patient comorbidities, surgery type, and previous arrhythmia. This underscores the importance of careful preoperative workup by a multidisciplinary team to determine appropriate preoperative risk assessment.
With a rapid increase in using transcatheter valve therapies across the board, the data regarding postoperative PPM have become highly relevant23 after valvular surgery. Complications associated with a PPM include infection, cardiomyopathy, and potential heart failure symptoms. These complications are not trivial, and patients continue to take this into consideration when deciding between transcatheter aortic valve replacement or surgical aortic valve replacement. With a clear decreasing rate of PPM after transcatheter aortic valve replacement with current generation devices and improvement in valve implant technique, surgeons should similarly continue to pay attention to fine-tuning surgical technique in minimizing PPM.
Limitations
The current study is limited by retrospective design and may be prone to selection bias. Although we have a large hospital network with more than 40 divisions, a small percentage of patients may be readmitted to other centers and lost to follow-up. Our database was unable to separate paroxysmal, persistent, and permanent atrial fibrillation, which is an ideal area for future work. Model selection was done in a stepwise manner, although it may be limited by small P values, narrow confidence intervals, and coefficients too far from the null. Finally, complications to PPM tend to accumulate over time, but this investigation only examined 5-year outcomes. This length of time may not have revealed issues with device failure that occur over time.
Conclusions
Cardiac surgery patients who required postoperative PPM placement have heightened postoperative complications and increased 5-year mortality and heart failure readmissions. However, PPM placement is not an independent predictor of worse outcomes (Figure 6). Patients requiring PPM represent a group with increased comorbid conditions, and careful preoperative risk assessment should guide surgical decision making in this patient population.
Figure 6.
Study design and outcomes.
Conflict of Interest Statement
The authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
References
- 1.Bis J., Gościńska-Bis K., Gołba K.S., Gocoł R., Zębalski M., Deja M.A. Permanent pacemaker implantation after cardiac surgery: optimization of the decision making process. J Thorac Cardiovasc Surg. February 19, 2020 doi: 10.1016/j.jtcvs.2020.01.082. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 2.Kho J., Ioannou A., O'Sullivan K.E., Jones M. Permanent pacemaker implantation rates following cardiac surgery in the modern era. Ir J Med Sci. 2020;189:1289–1294. doi: 10.1007/s11845-020-02254-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mehaffey J.H., Haywood N.S., Hawkins R.B., Kern J.A., Teman N.R., Kron I.L., et al. Need for permanent pacemaker after surgical aortic valve replacement reduces long-term survival. Ann Thorac Surg. 2018;106:460–465. doi: 10.1016/j.athoracsur.2018.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Del Rizzo D.F., Nishimura S., Lau C., Sever J., Goldman B.S. Cardiac pacing following surgery for acquired heart disease. J Card Surg. 1996;11:332–340. doi: 10.1111/j.1540-8191.1996.tb00059.x. [DOI] [PubMed] [Google Scholar]
- 5.Gordon R.S., Ivanov J., Cohen G., Ralph-Edwards A.L. Permanent cardiac pacing after a cardiac operation: predicting the use of permanent pacemakers. Ann Thorac Surg. 1998;66:1698–1704. doi: 10.1016/s0003-4975(98)00889-3. [DOI] [PubMed] [Google Scholar]
- 6.Lewis J.W., Jr., Webb C.R., Pickard S.D., Lehman J., Jacobsen G. The increased need for a permanent pacemaker after reoperative cardiac surgery. J Thorac Cardiovasc Surg. 1998;116:74–81. doi: 10.1016/S0022-5223(98)70245-4. [DOI] [PubMed] [Google Scholar]
- 7.Wiggins N.B., Chong D.T., Houghtaling P.L., Hussein A.A., Saliba W., Sabik J.F., et al. Incidence, indications, risk factors, and survival of patients undergoing cardiac implantable electronic device implantation after open heart surgery. Europace. 2017;19:1335–1342. doi: 10.1093/europace/euw234. [DOI] [PubMed] [Google Scholar]
- 8.Elahi M.M., Lee D., Dhannapuneni R.R. Predictors of permanent pacemaker implantation during the early postoperative period after valve surgery. Tex Heart Inst J. 2006;33:455–457. [PMC free article] [PubMed] [Google Scholar]
- 9.Schurr U.P., Berli J., Berdajs D., Häusler A., Dzemali O., Emmert M., et al. Incidence and risk factors for pacemaker implantation following aortic valve replacement. Interact Cardiovasc Thorac Surg. 2010;11:556–560. doi: 10.1510/icvts.2010.249904. [DOI] [PubMed] [Google Scholar]
- 10.Emlein G., Huang S.K., Pires L.A., Rofino K., Okike O.N., Vander Salm T.J. Prolonged bradyarrhythmias after isolated coronary artery bypass graft surgery. Am Heart J. 1993;126:1084–1090. doi: 10.1016/0002-8703(93)90658-v. [DOI] [PubMed] [Google Scholar]
- 11.Bianco V., Kilic A., Gleason T.G., Aranda-Michel E., Habertheuer A., Wang Y., et al. Reoperative cardiac surgery is a risk factor for long-term mortality. Ann Thorac Surg. 2020;110:1235–1242. doi: 10.1016/j.athoracsur.2020.02.028. [DOI] [PubMed] [Google Scholar]
- 12.Sultan I., Bianco V., Kilic A., Chu D., Navid F., Gleason T.G. Aortic root replacement with cryopreserved homograft for infective endocarditis in the modern North American opioid epidemic. J Thorac Cardiovasc Surg. 2019;157:45–50. doi: 10.1016/j.jtcvs.2018.05.050. [DOI] [PubMed] [Google Scholar]
- 13.Sultan I., Dufendach K.A., Kilic A., Bianco V., Navid F., Gleason T.G. A running suture line for aortic valve replacement does not increase the rate of postoperative complete heart block. Gen Thorac Cardiovasc Surg. 2019;67:283–288. doi: 10.1007/s11748-018-1011-1. [DOI] [PubMed] [Google Scholar]
- 14.Wallen T.J., Szeto W., Williams M., Atluri P., Arnaoutakis G., Fults M., et al. Tricuspid valve endocarditis in the era of the opioid epidemic. J Card Surg. 2018;33:260–264. doi: 10.1111/jocs.13600. [DOI] [PubMed] [Google Scholar]
- 15.Song J., Liang Z., Wang Y., Han Z., Ren X. Incidence of permanent pacemaker implantation after valve replacement surgery: cardiac structure and function at 1-year follow-up. Herz. 2021;46(Suppl 1):109–114. doi: 10.1007/s00059-020-04895-2. [DOI] [PubMed] [Google Scholar]
- 16.Bagur R., Manazzoni J.M., Dumont É., Doyle D., Perron J., Dagenais F., et al. Permanent pacemaker implantation following isolated aortic valve replacement in a large cohort of elderly patients with severe aortic stenosis. Heart. 2011;97:1687–1694. doi: 10.1136/heartjnl-2011-300308. [DOI] [PubMed] [Google Scholar]
- 17.Greason K.L., Lahr B.D., Stulak J.M., Cha Y.M., Rea R.F., Schaff H.V., et al. Long-term mortality effect of early pacemaker implantation after surgical aortic valve replacement. Ann Thorac Surg. 2017;104:1259–1264. doi: 10.1016/j.athoracsur.2017.01.083. [DOI] [PubMed] [Google Scholar]
- 18.Pieri M., Belletti A., Monaco F., Pisano A., Musu M., Dalessandro V., et al. Outcome of cardiac surgery in patients with low preoperative ejection fraction. BMC Anesthesiol. 2016;16:97. doi: 10.1186/s12871-016-0271-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mazza A., Bendini M.G., Leggio M., Riva U., Ciardiello C., Valsecchi S., et al. Incidence and predictors of heart failure hospitalization and death in permanent pacemaker patients: a single-centre experience over medium-term follow-up. Europace. 2013;15:1267–1272. doi: 10.1093/europace/eut041. [DOI] [PubMed] [Google Scholar]
- 20.Uslan D.Z., Tleyjeh I.M., Baddour L.M., Friedman P.A., Jenkins S.M., St Sauver J.L., et al. Temporal trends in permanent pacemaker implantation: a population-based study. Am Heart J. 2008;155:896–903. doi: 10.1016/j.ahj.2007.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bradshaw P.J., Stobie P., Knuiman M.W., Briffa T.G., Hobbs M.S. Life expectancy after implantation of a first cardiac permanent pacemaker (1995-2008): a population-based study. Int J Cardiol. 2015;190:42–46. doi: 10.1016/j.ijcard.2015.04.099. [DOI] [PubMed] [Google Scholar]
- 22.Greenspon A.J., Patel J.D., Lau E., Ochoa J.A., Frisch D.R., Ho R.T., et al. Trends in permanent pacemaker implantation in the United States from 1993 to 2009: increasing complexity of patients and procedures. J Am Coll Cardiol. 2012;60:1540–1545. doi: 10.1016/j.jacc.2012.07.017. [DOI] [PubMed] [Google Scholar]
- 23.Kim K.M., Shannon F., Paone G., Lall S., Batra S., Boeve T., et al. Evolving trends in aortic valve replacement: a statewide experience. J Card Surg. 2018;33:424–430. doi: 10.1111/jocs.13740. [DOI] [PubMed] [Google Scholar]