Abstract
Background
Coronavirus disease 2019 (COVID‐19) vaccine–related side effects are a key concern with the emergence of various types of vaccines in the market. We aimed to assess the frequency and characteristics of headache following different types of COVID‐19 vaccines.
Methods
Fully vaccinated people were recruited by a convenience sample through an online survey from September 1 to December 1, 2021. Detailed analysis of headache following vaccination was investigated. Participants with a history of pre‐existing headaches were telephone interviewed by a neurologist to ascertain the type of headache.
Results
A total of 1372 participants participated (mean age 32.9 ± 11.1). The highest frequency of headache was reported with the adenoviral vector type (302/563, 53.6%), followed by mRNA vaccines (129/269, 48%) and then the inactivated type (188/540, 34.8%). Recipients of the adenoviral vector type had a significantly longer latency between vaccination and the headache onset (median 8 h [5:12]) than recipients of the inactivated type (median 4 h [2:8], p < 0.001). Headache intensity was significantly higher with the adenoviral vector type (median 6 [5:8]) than with the inactivated type (median 5 [4:7], p < 0.001). Adenoviral vector vaccines would increase the likelihood of headache by 2.38 times more than inactivated vaccines (odds ratio [OR] 2.38, 95% confidence interval [CI] 1.83–3.04, p < 0.001). Female sex and thyroid disease were significantly associated with headache related to COVID‐19 vaccines (OR 1.52, 95% CI 1.16–1.99; OR 3.97, 95% CI 1.55–10.2, respectively).
Conclusion
Recipients of the COVID‐19 vaccine should be counseled that they may experience headaches, especially after the adenoviral vector type. However, the intensity of such headache is mild to moderate and can resolve within a few days. Based on the current study design and the potential recall bias, these results may not be generalizable and should be preliminary.
Keywords: adenoviral vector vaccine, COVID‐19 vaccines, headache, inactivated vaccine, mRNA–based vaccine
Abbreviations
- COVID‐19
coronavirus disease 2019
- ICHD‐3
International Classification of Headache Disorders, 3rd edition
- VAS
Visual Analogue Scale
INTRODUCTION
Coronavirus disease 2019 (COVID‐19) has become a global pandemic. COVID‐19 vaccines have progressed worldwide as the most promising approach to prevent infections. 1
Regarding COVID‐19 vaccines, different types have been proposed with different mechanisms of action, such as: inactivated viral vaccine (VACSERA Sinovac [Vac Sinovac] and Sinopharm BBIBP vaccines), the newly announced mRNA vaccines (Pfizer and Moderna), and adenoviral vector–based vaccines (AstraZeneca, Johnson & Johnson, and Sputnik V vaccines). 1 , 2 , 3 The latter type presents a safe, modified version of the virus—known as “the vector,” the spike protein found on the surface of the coronavirus carrying the genetic code for the antigen. 4
Although the safety of COVID‐19 vaccines has been broadly accepted, several randomized controlled trials have reported adverse effects in the hours/days following vaccination. They are usually mild or moderate and resolve within a few days after vaccination. 5 , 6
According to the available data, the most common neurological symptom is headache in more than 50% of vaccinated individuals after the first and the second doses. 7 The International Classification of Headache Disorders, 3rd edition (ICHD‐3) does not list any diagnostic criteria for headache related to COVID‐19 vaccine. 8 However, when studying the characteristics of such headaches, the type of vaccine and its mechanism of action must be considered.
We hypothesized that headaches are the most common among the recipients of the adenoviral vector type rather than other vaccines based on the fact that the adenoviral vector vaccines can provoke more immunogenic responses than the other types. 9 , 10
We aimed in this study to compare different types of available COVID‐19 vaccines regarding frequency and in detail the clinical characteristics of headache related to COVID‐19 vaccine, and to study the predictors of such headache.
METHODS
A self‐administered, publically available online‐based questionnaire was designed using Google Forms following the Checklist for Reporting Results of Internet E‐Surveys (CHERRIES) guidelines 11 to target fully vaccinated adults aged ≥18 years. According to the Centers for Disease Control and Prevention, 12 the definition of fully vaccinated people was applied if at least 2 weeks had passed after receiving the second dose of the two‐dose vaccines or 2 weeks after receiving the single‐dose vaccine (Johnson & Johnson vaccine).
Subsequently, the questionnaire link was disseminated in Egyptian WhatsApp and Facebook groups, where vaccination is compulsory for its members, such as groups of hospital workers from medical and non‐medical staff, university students from different colleges, school teachers, in addition to groups focusing on travel facilities for those who wish to travel outside the country. We estimate the number of members of these groups to be approximately seven thousand.
The questionnaire was conducted in Arabic, which is the mother tongue of the Egyptian people. An English version is included in Supporting Information 1.
The survey was available online from September 1, 2021, and closed on December 1, 2021. The answers were collected in an online database form. The inquiry about headache, in particular, was included in the advertisement of the questionnaire and the study's objective listed at the beginning of the survey.
People who could not remember vaccine‐related events were requested not to participate in the survey. Eligible participants were recruited by a convenience sample.
The questionnaire included the following clinical information: age, gender (with provided choices of “male or female”), previous headaches, and any chronic medical illness. Anyone with a previous headache disorder was asked to complete a telephone interview with an expert neurologist to ascertain the type of headache disorder according to ICHD‐3. 8
Then, the participant was asked to choose the name of the vaccination they received from the list of vaccinations available in Egypt (Pfizer, AstraZeneca, Johnson & Johnson, Moderna, Sputnik V, Sinopharm BBIBP, and Vac Sinovac).
People who experienced a headache following COVID‐19 vaccination were asked about the clinical characteristics of this headache in terms of exact latency between vaccination and headache onset in hours, headache offset (on what day after the vaccination did the headache disappear?), pain character, site, Visual Analogue Scale (VAS) as a reliable measure of headache intensity, 13 and need for brain imaging. Anyone who underwent neuroimaging was asked to upload their imaging and to have a telephone interview with a neurologist to reveal the indications for doing the imaging. Associated symptoms were also verified, including fever, pain at the injection site, fatigue, muscle pain, and vomiting or diarrhea.
Anyone who had a headache after each of the two doses of the vaccine was requested to answer headache questions about which dose was followed by the worst headache.
The study was approved by the ethical committee of the Beni‐Seuf University, Egypt, with approval serial number REC‐HPHBSU‐21030. All responders provided an online informed consent that explained the purpose of the survey.
Sampling
The sample size was calculated using the preliminary data provided by Almufty et al. 14 with the following assumptions using PS: Power and Sample Size Calculator, for comparison of two independent proportions at type 1 error 0.05 and power of study 0.8. By comparing the proportion of 48% reported after viral vector vaccine and 33.3% after inactivated vaccines, the calculated sample size will be 173 for each group. As we collected data through Google Forms, we considered the dropout rate 40% per group according to Galesic, 15 so the minimum required number was 243 for each group.
Statistical analysis
Data cleaning was conducted before data analysis to ensure the data were free of irrelevant and incorrect information. There were no missing data. Precoded data were entered on the computer using the SPSS, version 23 (IBM). Data were summarized using mean, standard deviation, median, and interquartile range for continuous variables, and number and percent for the categorical variables. A Shapiro test was used to check data normality, and the data were nonnormally distributed. The chi‐squared test was used to compare categorical variables. Fisher's exact test was used when one expected cell or more was less than five. Mann–Whitney test was used for comparison of continuous variables between two groups. A Kruskal–Wallis test compared continuous variables between more than two groups. Pairwise comparison was conducted after the Kruskal–Wallis test. A multiple backward logistic regression model was conducted to find significant predictors of headache occurrence after the COVID‐19 vaccine. After conducting a correlation estimate in the logistic regression model in which r was <0.9, collinearity diagnostics (tolerance more than 0.1 and variance inflation factor less than 10) in all variables, and after trial of multiple models, we document the most suitable one. A p‐value equal to or less than 0.05 was considered statistically significant. All tests were two‐tailed.
RESULTS
A total of 1372 fully vaccinated participants responded to our survey. Their ages ranged from 18 to 78 years, with a mean age of 32.9 ± 11.1. The majority were females (921, 67.1%), while 451 were males (32.9%). Only 209 participants (15.2%) had medical comorbidities (Supporting Information 2).
Five hundred sixty‐three participants (41%) were adenoviral vector vaccine recipients (448 received AstraZeneca, 77 received Johnson & Johnson, and 38 received Sputnik V vaccines), whereas 540 participants (39.4%) received inactivated COVID‐19 vaccines (310 received Vac Sinovac and 230 received Sinopharm BBIBP vaccines). Only 269 participants (19.6%) received mRNA COVID‐19 vaccines (231 had Pfizer, and 38 had Moderna vaccines).
Frequency of headache and other adverse effects following different types of COVID‐19 vaccines
The highest frequency of headache related to vaccines was reported with the adenoviral vector type (53.6%), followed by mRNA COVID‐19 vaccines (48%) and then the inactivated type (34.8%). This difference was statistically significant (p < 0.001; Figure 1).
FIGURE 1.
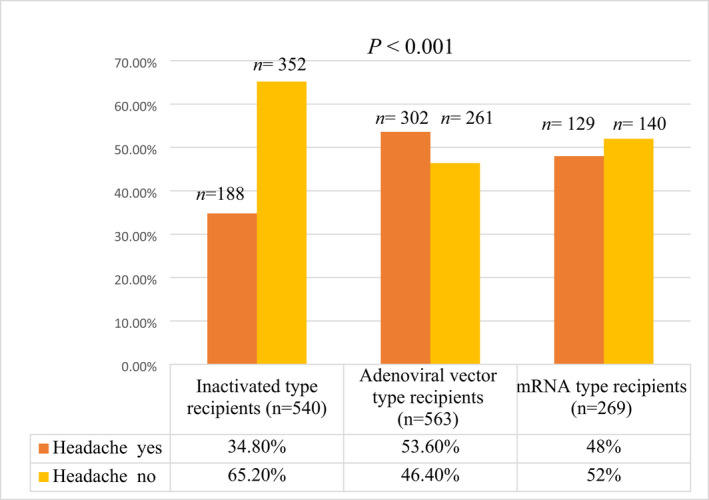
Frequency of headache related to vaccines among the recipients of different types of COVID‐19 vaccines. p‐value ≤0.05 significant. COVID‐19, coronavirus disease 2019; mRNA, messenger RNA.
Furthermore, the highest frequency of other adverse effects following vaccines (fever, pain at the injection site, fatigue, and muscle pain) was reported with the adenoviral vector type, followed by mRNA COVID‐19 vaccines and then the inactivated type (for each, p < 0.001; Figure 2).
FIGURE 2.
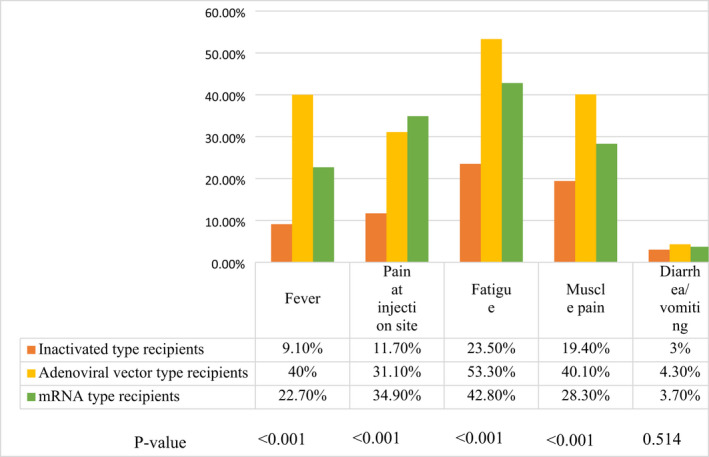
Frequency of vaccine‐related side effects among the recipients of different types of COVID‐19 vaccines. p‐value ≤0.05 significant. COVID‐19, coronavirus disease 2019; mRNA, messenger RNA.
Characteristics of headache related to COVID‐19 vaccines among recipients of different types of vaccines
Most recipients of the three types of vaccines described their headaches as pressing character and often involved the entire cranium (Table 1). For the three types of vaccine, headaches were expressed predominantly with moderate intensity and ended a median of 2 days after the onset.
TABLE 1.
Characteristics of headache related to COVID‐19 vaccines among recipients of different types of vaccines
| COVID‐19 vaccines recipients who developed headache (n = 619) | p value | |||
|---|---|---|---|---|
| Inactivated type (n = 188) | Adenoviral vector type (n = 302) | mRNA type (n = 129) | ||
| Latency between vaccination and Headache onset (hours) median (IQR) | 4 (2:8) | 8 (5:12)* | 10 (4:24)* | <0.001 |
| Headache offset (days) median (IQR) | 2 (1:3) | 2 (1:3) | 2 (2:3) | 0.232 |
| VAS median (IQR) | 5 (4:7) | 6 (5:8)* | 5 (4:7) | <0.001 |
| Headache character n (%) | ||||
| Throbbing | 56 (29.8%) | 66 (21.9%) | 34 (26.4%) | 0.136 |
| Pressing | 77 (41%) | 124 (41.1%) | 58 (45%) | 0.722 |
| Dull | 42 (22.3%) | 83 (27.5%) | 31 (24%) | 0.418 |
| Bursting | 13 (6.9%) | 29 (9.6%) | 6 (4.7%) | 0.186 |
| Site of headache n (%) | ||||
| Frontal | 28 (14.9%) | 49 (16.2%) | 22 (17.1%) | 0.865 |
| Temporal | 63 (33.5%) | 74 (24.5%) | 38 (29.5%) | 0.093 |
| Occipital | 21 (11.2%) | 32 (10.6%) | 21 (16.3%) | 0.231 |
| Holecephalic | 76 (40.4%) | 147 (48.7%) | 48 (37.2%) | 0.048 |
Abbreviations: COVID‐19, coronavirus disease 2019; IQR, interquartile range; mRNA, messenger RNA; VAS, Visual Analogue Scale.
Statistically significant from inactivated vaccines (Kruskal–Wallis test), p‐value ≤0.05 significant.
Recipients of the adenoviral vector type had a significantly longer latency between vaccination and the occurrence of headache and more intense headache than those who received the inactivated type (Table 1). Furthermore, recipients of the mRNA type had a significantly longer latency between vaccination and the onset of headache than those who received the inactivated type, with no statistically significant difference regarding headache intensity (Table 1).
Only eight people reported having severe headaches that required brain imaging (three were AstraZeneca recipients, three patients were Vac Sinovac recipients, one was a Pfizer recipient, and one was a Sinopharm BBIBP recipient). There was no other indication for brain imaging other than headache severity. However, all imaging was normal.
Predictors of occurrence of headache related to COVID‐19 vaccine
Univariate analysis revealed that female gender; pre‐existing headache disorder (either migraine or tension‐type); thyroid disease; adenoviral vector vaccine; and presence of other post‐vaccination side effects such as fever, pain at the injection site, fatigue, muscle pain, and diarrhea or vomiting were significantly associated with headache related to COVID‐19 vaccine (Table 2).
TABLE 2.
Comparative characteristics of headache and non‐headche groups in recepients of different types of COVID‐19 vaccines
| COVID‐19 vaccines recipients (n = 1372) | p value | ||
|---|---|---|---|
| Non headache group (n = 753) | Headache group (n = 619) | ||
| Age (mean ± SD) | 33.2 ± 11.8 | 32.5 ± 10.2 | 0.934 |
| Sex n (%) | |||
| Male | 285 (37.8%) | 166 (26.8) | <0.001 |
| Female | 468 (62.2%) | 453 (73.2%) | |
| Pre‐existing headache n (%) | |||
| None | 552 (73.3%) | 380 (61.4%) | <0.001 |
| Migraine | 71 (9.4%) | 81 (13.1%) | 0.032 |
| Tension‐type | 88 (11.7%) | 96 (15.5%) | 0.039 |
| Others | 42 a (5.6%) | 62 (10%) | 0.002 |
| Type of comorbidities n (%) | |||
| Diabetes | 27 (3.6%) | 21 (3.4%) | 0.846 |
| Hypertension | 55 (7.3%) | 45 (7.3%) | 0.981 |
| Thyroid disease | 9 (1.2%) | 27 (4.4%) | <0.001 |
| Autoimmune disease | 13 (1.7%) | 13 (2.1%) | 0.613 |
| Type of COVID‐19 vaccine | |||
| Inactivated vaccines | 352 (46.7%) | 188 (30.4%) | <0.001 |
| Adenoviral vector vaccines | 261 (34.7%) | 302 (48.8%) | <0.001 |
| mRNA vaccines | 140 (18.6%) | 129 (20.8%) | 0.279 |
| Associated symptoms n (%) | |||
| Fever | 53 (7%) | 282 (45.6%) | <0.001 |
| Pain at injection site | 93 (12.4%) | 239 (38.6%) | <0.001 |
| Fatigue | 97 (12.9%) | 445 (71.9%) | <0.001 |
| Muscle pain | 71 (9.4%) | 336 (54.3%) | <0.001 |
| Diarrhea/vomiting | 8 (1.1%) | 42 (6.8%) | <0.001 |
Note: p‐value ≤0.05 significant.
Abbreviations: COVID‐19, coronavirus disease 2019; mRNA, messenger RNA; SD, standard deviation.
Only two patients had cluster headache.
A multiple logistic regression model was conducted to find significant predictors of headache occurrence after the COVID‐19 vaccine. Gender, type of vaccine (adenoviral vector /inactivated), thyroid disease, and pre‐existing headache disorder were significant predictors for headaches.
Being female increased the odds of headache related to COVID‐19 vaccine by nearly two times (odds ratio [OR] 1.52). Patients with thyroid disease were four times more likely to develop headaches than those without (OR 3.97). Also, adenoviral vector vaccines increases the likelihood of headache by 2‐fold more than inactivated vaccines (OR 2.38; Table 3).
TABLE 3.
Multiple logistic regression model for predictors of headache occurrence after vaccine
| p value | OR | 95% CI for OR | ||
|---|---|---|---|---|
| Lower | Upper | |||
| Age in years | 0.083 | 0.99 | 0.98 | 1.00 |
| Sex (reference male) | 0.003 | 1.52 | 1.16 | 1.99 |
| Thyroid disease | 0.004 | 3.97 | 1.55 | 10.2 |
| Non‐pre‐existing headache disorder | 0.001 | 0.65 | 0.49 | 0.84 |
| Adenoviral vector /inactivated vaccines | <0.001 | 2.38 | 1.83 | 3.04 |
| Constant | 0.144 | 0.69 | ||
Note: p‐value ≤0.05 significant.
Abbreviations: CI, confidence interval; OR, odds ratio.
DISCUSSION
Headache related to vaccines is a frequently reported adverse event; it has been reported with different vaccine types at different rates. 7 Despite the advent of various COVID‐19 vaccines, the headache related to vaccines wasn't encoded yet in the ICHD‐3 and still needs further clarification. 8 This is the first study that presents a detailed comparison between the different types of COVID‐19 vaccines regarding headache related to the COVID‐19 vaccine.
This study showed that the highest frequency of headache was observed in adenoviral vector types (53.6%), followed by mRNA types (48%) that fall in the range previously reported in post‐marketing trials on the adenoviral vector type (39%–66%) 16 , 17 and mRNA type (39%–80%). 18 , 19 , 20 In contrast, the rate of headache after administration of inactivated vaccines in our study (34.8%) is much higher than previous reports regarding this vaccination type (13%). 21
Although previous studies on different COVID‐19 vaccines varied in determining the character and location of headache, 22 , 23 , 24 most recipients of the three types of vaccines in our study agreed that headache related to COVID‐19 vaccine was pressing and often involved the entire cranium.
The current work showed that headaches were expressed predominantly with moderate intensity in the three vaccine categories. Also, the headache lasted for a short time as it ended with a median of 2 days after the onset in all three types of vaccine. Hence, it is worth noting that the headache related to COVID‐19 vaccine appears to have relatively milder features than that of headache associated with COVID‐19 infection, the latter being of moderate to severe intensity and longer duration. 25 The counseling letter should consider weighing this headache and constitutional manifestations following vaccine administration against the severity of these symptoms with COVID‐19 infection.
The exact pathogenesis of headache related to COVID‐19 vaccine is not yet settled. The genetic material in adenoviral vector and mRNA types may elucidate headache occurrence. It provokes intracellular synthesis of S glycoprotein that, in turn, causes headache directly or indirectly via triggering an immune response. 26 , 27 Furthermore, Zhu et al. 28 hypothesized that the viral shell component in the adenoviral vector type can trigger an additional immune response, which might explain the most severe headache intensity with this vaccine type in our study.
On the other hand, vaccine adjuvants such as aluminium salt in inactivated COVID‐19 vaccines can trigger a headache‐inducing immune response. 29 This may also explain the rapidity of the immune response elicited by the ready‐made antigen components causing headaches to appear with shorter latency in this type of inactivated vaccine, as our results showed.
The occurrence of headache in the context of other adverse effects related to the vaccine, such as fever, fatigue, myalgia, vomiting, or diarrhea, may add another proposal for how the COVID‐19 vaccine may induce a headache. The frequency of these vaccine‐related adverse effects concurred with the same order of headache frequency among the three types of vaccine recipients in the present study, supporting this assumption. These symptoms are also associated with headaches during COVID‐19 infection mediated by pro‐inflammatory cytokines. 30 , 31
Identifying people at risk of developing headache related to a COVID‐19 vaccine is a crucial cornerstone of counseling. In agreement with preceding reports, 23 , 24 this study showed that females were more predisposed to headache related to a COVID‐19 vaccine. These two studies also showed that younger age was associated with headaches, which our study could not prove.
Patients with thyroid disorders are another vulnerable category declared in all studies on the different types of COVID‐19 vaccination. 22 , 23 , 24 This finding may be explained by the deleterious hormonal effects on the pain modulating system, particularly in hypothyroid patients. 32
It is broadly accepted that patients with a pre‐existing primary headache disorder, particularly migraine, can lead to the hyperexcitability of trigeminovascular neurons resulting in a lowered pain threshold, making patients with primary headache disorders more susceptible to secondary headaches than others. 33 However, migraine could not predict developing headache related to COVID‐19 vaccine in the present study.
Our study had some limitations. Although using an online questionnaire is an accepted method in the pandemic, it increases bias in survey studies. Because the usage rate of the various social media platforms is affected by age, gender, and education level, 34 the willingness to participate in our online surveys was high among the female and the young age groups. Also, as a convenience sample is well known to be biased and non‐representative, the results of the current study cannot be generalizable. Furthermore, few participants received mRNA vaccines, as they were available in Egypt several months after other types of vaccines became available. Last, case–control studies are very informative in the absence of clear diagnostic criteria for headache related to vaccines, which we hope will soon be released by ICHD‐3.
CONCLUSION
The current study provided the following preliminary data; however, they cannot be generalizable due to the risk of biased recall in the online survey. Recipients of the COVID‐19 vaccine should be counseled that they may experience headache related to COVID‐19 vaccine, especially following the adenoviral vector type. Females or patients with thyroid disorders are also more likely to develop this type of headache. However, they must be reassured that such headache is generally mild to moderate in intensity and remits within a few days for most people.
AUTHOR CONTRIBUTIONS
Study concept and design: Rehab Magdy. Acquisition of data: Rehab Magdy, Diana Khedr, Osama Yacoub, Doaa Mekkawy, and Mona A. Abdelrahman. Analysis and interpretation of data: Abeer Attia. Drafting of the manuscript: Rehab Magdy, Doaa Mekkawy, Diana Khedr, Osama Yacoub, and Abeer Attia. Revising it for intellectual content: Rehab Magdy, Doaa Mekkawy, Diana Khedr, Osama Yacoub, Abeer Attia, and Mona A. Abdelrahman. Final approval of the completed manuscript: Rehab Magdy, Doaa Mekkawy, Diana Khedr, Osama Yacoub, Abeer Attia, and Mona A. Abdelrahman.
CONFLICTS OF INTEREST
All authors had no disclosures.
Supporting information
Appendix S1
Appendix S2
Magdy R, Khedr D, Yacoub O, Attia A, Abdelrahman MA, Mekkawy D. Epidemiological aspects of headache after different types of COVID‐19 vaccines: An online survey. Headache. 2022;62:1046‐1052. doi: 10.1111/head.14374
REFERENCES
- 1. Krammer F. SARS‐CoV‐2 vaccines in development. Nature. 2020;586:516‐527. [DOI] [PubMed] [Google Scholar]
- 2. Kyriakidis NC, López‐Cortés A, González EV, Grimaldos AB, Prado EO. SARS‐CoV‐2 vaccines strategies: a comprehensive review of phase 3 candidates. NPJ Vaccines. 2021;6:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nagy A, Alhatlani B. An overview of current COVID‐19 vaccine platforms. Comput Struct Biotechnol J. 2021;19:2508‐2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mendonça SA, Lorincz R, Boucher P, Curiel DT. Adenoviral vector vaccine platforms in the SARS‐CoV‐2 pandemic. NPJ Vaccines. 2021;6:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid‐19 vaccine. N Engl J Med. 2020;383:2603‐2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Haas JW, Bender FL, Ballou S, et al. Frequency of adverse events in the placebo arms of COVID‐19 vaccine trials: a systematic review and meta‐analysis. JAMA Netw Open. 2022;5:e2143955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Menni C, Klaser K, May A, et al. Vaccine side‐effects and SARS‐CoV‐2 infection after vaccination in users of the COVID symptom study app in the UK: a prospective observational study. Lancet Infect Dis. 2021;21:939‐949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia. 2013;33:629‐808. [DOI] [PubMed] [Google Scholar]
- 9. Coughlan L. Factors which contribute to the immunogenicity of non‐replicating adenoviral vectored vaccines. Front Immunol. 2020;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Coughlan L, Kremer EJ, Shayakhmetov DM. Adenovirus‐based vaccines—a platform for pandemic preparedness against emerging viral pathogens. Mol Ther. 2022;30:1822‐1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Eysenbach G. Improving the quality of web surveys: the checklist for reporting results of internet E‐surveys (CHERRIES). J Med Internet Res. 2004;6:e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Center of Disease and Control Prevention . Stay Up to Date with Your Vaccines. COVID‐19. 2022. [Google Scholar]
- 13. Bijur PE, Silver W, Gallagher EJ. Reliability of the visual analog scale for measurement of acute pain. Acad Emerg Med. 2001;8:1153‐1157. [DOI] [PubMed] [Google Scholar]
- 14. Almufty HB, Mohammed SA, Abdullah AM, Merza MA. Potential adverse effects of COVID19 vaccines among Iraqi population; a comparison between the three available vaccines in Iraq; a retrospective cross‐sectional study. Diabetes Metab Syndr. 2021;15:102207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Galesic M. Dropouts on the web: effects of interest and burden experienced during an online survey. Qual Eng. 2007;52:617‐618. [Google Scholar]
- 16. Sadoff J, Gray G, Vandebosch A, et al. Safety and efficacy of single‐dose Ad26.COV2.S vaccine against Covid‐19. N Engl J Med. 2021;384:2187‐2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhu FC, Li YH, Guan XH, et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type‐5 vectored COVID‐19 vaccine: a dose‐escalation, open‐label, non‐randomised, first‐in‐human trial. Lancet. 2020;395:1845‐1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Anderson EJ, Rouphael NG, Widge AT, et al. Safety and immunogenicity of SARS‐CoV‐2 mRNA‐1273 vaccine in older adults. N Engl J Med. 2020;383:2427‐2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Walsh EE, Frenck RW Jr, Falsey AR, et al. Safety and immunogenicity of two RNA‐based Covid‐19 vaccine candidates. N Engl J Med. 2020;383:2439‐2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Thomas SJ, Moreira ED, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid‐19 vaccine through 6 months. N Engl J Med. 2021;385:1761‐1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Al Kaabi N, Zhang Y, Xia S, et al. Effect of 2 inactivated SARS‐CoV‐2 vaccines on symptomatic COVID‐19 infection in adults: a randomized clinical trial. JAMA. 2021;326:35‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Göbel CH, Heinze A, Karstedt S, et al. Clinical characteristics of headache after vaccination against COVID‐19 (coronavirus SARS‐CoV‐2) with the BNT162b2 mRNA vaccine: a multicentre observational cohort study. Brain Commun. 2021;3:fcab169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ekizoglu E, Gezegen H, Yalınay Dikmen P, Orhan EK, Ertaş M, Baykan B. The characteristics of COVID‐19 vaccine‐related headache: clues gathered from the healthcare personnel in the pandemic. Cephalalgia. 2021;42:3331024211042390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Göbel C, Heinze A, Karstedt S, et al. Headache attributed to vaccination against COVID‐19 (coronavirus SARS‐CoV‐2) with the ChAdOx1 nCoV‐19 (AZD1222) vaccine: a multicenter observational cohort study. Pain Ther. 2021;10:1309‐1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Magdy R, Hussein M, Ragaie C. Characteristics of headache attributed to COVID‐19 infection and predictors of its frequency and intensity: a cross sectional study. Cephalalgia. 2020;40:1422‐1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. De León‐Rodríguez SG, Hernández‐Rico B, Olmo‐Vázquez GD, Cruz‐Dávalos I, Bonifaz LC. SARS‐CoV‐2: previous coronaviruses, immune response, and development of vaccines. Bol Med Hosp Infant Mex. 2020;77:252‐261. [DOI] [PubMed] [Google Scholar]
- 27. Alnefaie A, Albogami S. Current approaches used in treating COVID‐19 from a molecular mechanisms and immune response perspective. Saudi Pharm J. 2020;28:1333‐1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhu J, Huang X, Yang Y. Innate immune response to adenoviral vectors is mediated by both toll‐like receptor‐dependent and ‐independent pathways. J Virol. 2007;81:3170‐3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pulendran B, Ahmed R. Immunological mechanisms of vaccination. Nat Immunol. 2011;12:509‐517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mekkawy DA, Hamdy S, Abdel‐Naseer M, et al. Neurological manifestations in a cohort of Egyptian patients with COVID‐19: a prospective, multicenter, observational study. Brain Sci. 2022;12:1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hussein M, Fathy W. Relative frequency and risk factors of COVID‐19 related headache in a sample of Egyptian population: a hospital‐based study. Pain Med. 2021;22:2092‐2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Spanou I, Bougea A, Liakakis G, et al. Relationship of migraine and tension‐type headache with hypothyroidism: a literature review. Headache. 2019;59:1174‐1186. [DOI] [PubMed] [Google Scholar]
- 33. Goadsby PJ, Holland PR, Martins‐Oliveira M, Hoffmann J, Schankin C, Akerman S. Pathophysiology of migraine: a disorder of sensory processing. Physiol Rev. 2017;97:553‐622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Elhadidi MAF. Beyond access to social media: a comparison of gratifications, interactivity, and content usage among Egyptian adults. Glob Media J. 2018;16:1. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Appendix S2


