Introduction
Chronic rhinosinusitis (CRS), a disease of long-standing sinonasal inflammation, is divided into two phenotypes: CRS with and without nasal polyposis (CRSwNP, CRSsNP). A unique sub-type of CRSwNP consists of a treatment-recalcitrant form termed aspirin-exacerbated respiratory disease (AERD), typified by polyposis, asthma, and sensitivity to non-steroidal anti-inflammatory drugs.1
The upper airway attempts to combat invading pathogens through mucociliary clearance (MCC) and the secretion of bactericidal compounds (nitric oxide (NO), anti-microbial peptides (AMPs)).2 Prior studies show that genetic variation in bitter taste receptors (T2Rs) contributes to the vigor of sinonasal immune response. The solitary chemosensory cell (SCC) is a heavily innervated, pro-inflammatory cell in the sinonasal epithelium which expresses T2Rs.3 Activation of SCC-based T2Rs drives AMP secretion and type-2 inflammation through IL-25 secretion and mast cell activation.4 Recent work has demonstrated that SCCs are the exclusive epithelial source of IL-25 which, together with IL-33 and thymic stromal lipoprotein, stimulate type 2 innate lymphoid cells to secrete IL-13 and drive eosinophilic chemotaxis.4,5 Studies additionally show polyps are enriched in SCCs.4
AERD patients exhibit enhanced sensitivity to the bitter agonist denatonium benzoate (DB), and degree of DB-hypersensitivity correlates with polyp recurrence.6 Eight T2Rs respond to DB, localizing to SCCs (T2R8, T2R10, T2R13, T2R46, T2R47)2,4,7 or ciliated cells (T2R4, T2R39, T2R43).8 We sought to investigate whether genetic differences in DB-sensitive T2Rs may be enriched in AERD. It was anticipated that in AERD, unique genetic polymorphisms in DB-responsive T2Rs may result in up-regulation of the receptors and resulting type-2 inflammation.
Methods
Subject Enrollment:
Following institutional review board approval, subjects were prospectively enrolled from patients treated within the University of Pennsylvania Department of Otorhinolaryngology. Adult, immunocompetent subjects were included. Exclusion criteria included history of immunodeficiency, genetic disorder affecting MCC, chemotherapy, granulomatous disease, and facial trauma. Individuals were grouped as case or control subjects based on presence or absence of CRS, respectively, with case subjects sub-divided by phenotype (CRSsNP, CRSwNP, AERD). Clinical history and 22-item Sino-Nasal Outcome Test (SNOT-22) results were collected.
Psychophysical Taste Testing:
Subjects completed taste testing as previously described.6,9
Genotyping:
Subjects provided middle turbinate tissue or saliva for genomic DNA extraction using the QuantStudio 12K Flex system (Applied Biosystems, Foster City, CA). An OpenArray single nucleotide polymorphism (SNP) gene chip (input concentration 50 ng/μl) was used to evaluate SNPs within genes for DB-responsive T2Rs and other receptors of interest (table 1).
Table 1. Taste Receptor Genotyping.
OpenArray Genotyping results of denatonium-responsive bitter taste receptors (top) and other taste receptors (bottom). Analysis with ratio and Pearson chi-squared analyses tests for between group differences. The three relevant TAS2R38 SNPs were calculated together as a haplotype given extensive prior data.

|
denotes a nominal p-value < 0.05 with the FDR-adjusted p-value in parentheses.
denotes significance after the FDR is applied.
Analysis:
SNOT-22 and taste testing results were analyzed with Stata (StataCorp, College Station, TX; version 17) using a linear regression including covariates sex, race, and age with post hoc Tukey procedure for multiple tests correction. Genotyping was analyzed with the R vcd package (RStudio, Boston MA; version 4.1.2) to calculate likelihood ratio and Pearson chi-square analyses. Association between genetic variants and disease groups was analyzed by covariates sex, race, and age with Pearson chi-square and post hoc pairwise chi-square tests with false discovery rate (FDR) for multiple tests correction (alpha=0.05), grouping variants by loci for association significance correction.
Results
108 subjects were enrolled (control n=23, CRSsNP n=24, CRSwNP n=26, AERD n=35). There were no significant between-group differences in sex, race, and age (supplementary table 1, figure 1). Control subjects had significantly reduced SNOT-22 compared with case subjects (p<0.01). AERD subjects demonstrated increased sensitivity to DB (p<0.01) and sucrose (p<0.01) compared with CRSsNP, while CRSsNP subjects demonstrated a reduced sensitivity to DB (p<0.05) and sucrose (p<0.05) compared with controls (figure 1). There were no between group differences in phenylthiocarbamide or quinine sensitivity. Genotyping showed an increased frequency of TAS2R4 A:A variant at rs2234002 in AERD subjects (p=0.022) which did not withstand the FDR correction. The remaining DB-responsive TAS2Rs showed no differences (table 1). TAS1R3 and TAS2R14 demonstrated increased frequency of C:C and C:T variants, respectively (TAS1R3 p=4.40e−4, TAS2R14 p=7.14e−5) (table 1, supplementary figure 2); both withstood the FDR correction.
Figure 1: Significant difference in SNOT-22 and bitter and sweet taste rating scores.
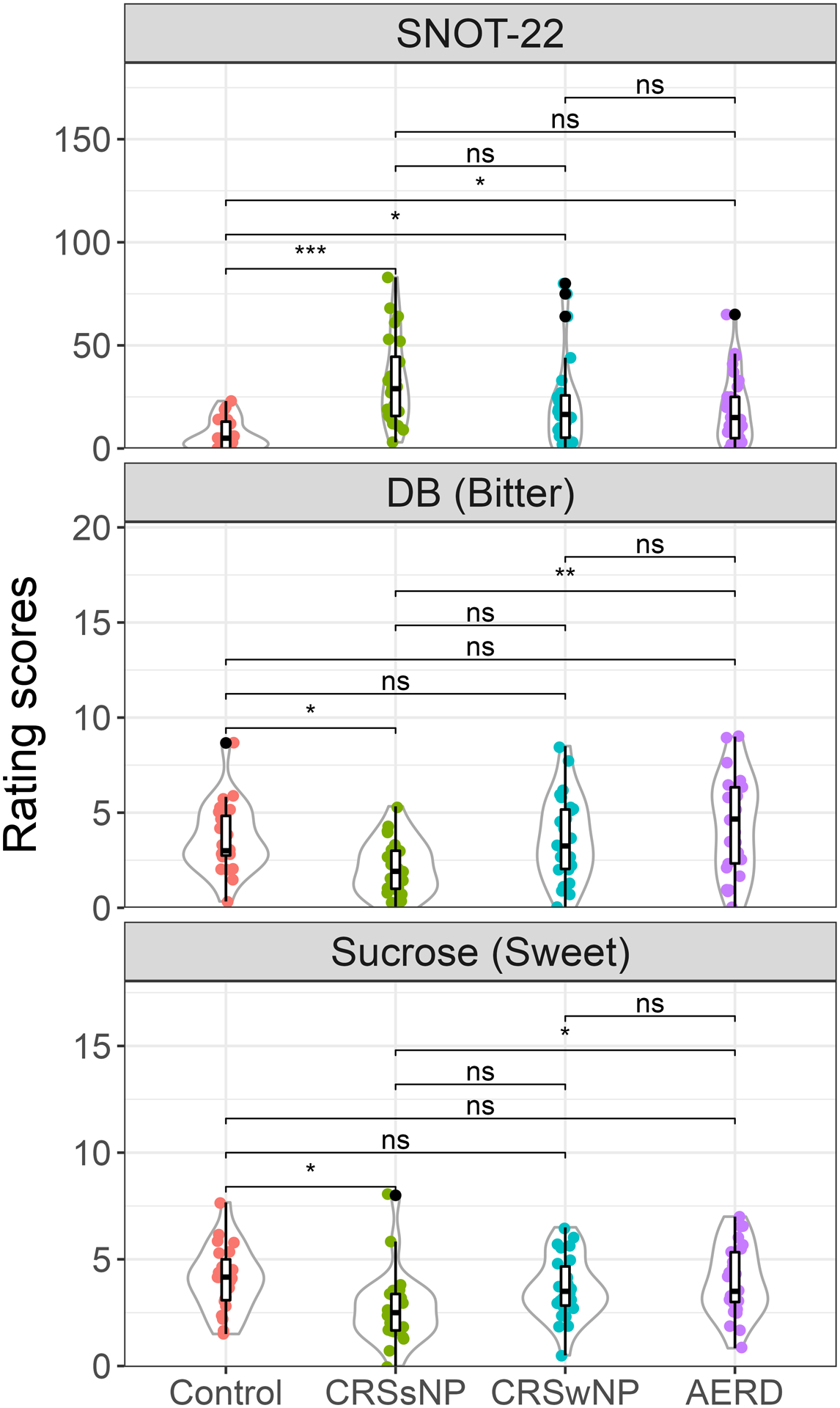
*<0.05 and **p<0.001 show significant difference. Tukey adjusted for multiple tests. ns=not significant
Discussion
Results indicate that AERD subjects exhibit an increased frequency of TAS2R4 A:A variant at rs2234002 with resulting S141N missense mutation in a coding gene region. While both serine and asparagine are polar amino acids, asparagine is found in turn motifs, making it possible the variant alters ligand binding, impacting downstream inflammatory signaling critical to AERD. The remaining DB-responsive TAS2Rs showed no differences. Interestingly, prior studies applying genome-wide association studies to AERD have been devoid of TAS2Rs.10 These results are exploratory in nature given the nominal threshold employed, however, given AERD is a rare disease, they serve as an important foundation for future work.
Between group differences in TAS1R3, which influences sweet and umami taste, are of interest given TAS1R3 localizes to upper airway SCCs. It also influences Th2 immune response by counteracting the SCC-T2R function.5,7 The importance of the TAS2R14 differences is less clear. While TAS2R14 is expressed in the upper airway and the polymorphism is present in a coding region, both amino acids (lysine and arginine) are charged, aliphatic proteins, making the mutation’s impact less straightforward.
Limitations of the study include its’ retrospective design. From a population perspective, it is also notable that initial ligand screening was done on a genetically homogenous cohort (e.g., European ancestry), making it possible that other T2Rs are involved but the study is not best-suited to capture these complex interactions.
In summary, while the genetic differences may be small and the study may be underpowered to detect this, taste receptors may act as gene modulators, exerting effects in an indirect fashion. The present data thus demonstrate that individuals with AERD exhibit an increased frequency of TAS2R4 S141N variant compared with control and non-AERD CRS subjects. This substitution may alter ligand binding and contribute to the heightened DB-sensitivity and increased Th2 inflammatory response characteristic of AERD.
Supplementary Material
Supplementary Figure 1. There is no significant association between patient cohort and patient age, sex and race. Age grouped with equal window size as young (18–39, n=27), middle (40–61, n=52) and old (62–82; n=28) and the race grouped as white (European; n=87) and non-white (others, n=21)
Supplementary Table 1. Subject Characteristics
Supplementary Figure 2. Genetic variants of taste receptors norminally associated with disease cohort. (A) AERD has more heterozygous genotype C:T of rs111614880 (TAS2R14) compared to other patient groups; (B and C) Control subjects have more heterozygous genotype C:T of rs307365 (TAS1R3) and homozygous G:G of rs2234002 (TAS2R4) relative to other patient groups.
Funding:
This research was supported by NIH R01 DC013588 (NAC, DRR), VA Merit CX001617 (NAC, DRR), NIH P30 DC011735 (DRR), NIH R01 AI167971 (NDA, JNP), and a University of Pennsylvania Department of Otorhinolaryngology - Head & Neck Surgery Pilot Research Grant.
Footnotes
Conflicts of interest: The authors declare they have no relevant conflicts of interest.
References
- 1.White AA, Stevenson DD. Aspirin-Exacerbated Respiratory Disease. N Engl J Med. Sep 2018;379(11):1060–1070. doi: 10.1056/NEJMra1712125 [DOI] [PubMed] [Google Scholar]
- 2.Lee RJ, Kofonow JM, Rosen PL, et al. Bitter and sweet taste receptors regulate human upper respiratory innate immunity. J Clin Invest. Mar 2014;124(3):1393–405. doi: 10.1172/jci72094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barham HP, Cooper SE, Anderson CB, et al. Solitary chemosensory cells and bitter taste receptor signaling in human sinonasal mucosa. Int Forum Allergy Rhinol. Jun 2013;3(6):450–7. doi: 10.1002/alr.21149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kohanski MA, Workman AD, Patel NN, et al. Solitary chemosensory cells are a primary epithelial source of IL-25 in patients with chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 08 2018;142(2):460–469.e7. doi: 10.1016/j.jaci.2018.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Howitt MR, Lavoie S, Michaud M, et al. Tuft cells, taste-chemosensory cells, orchestrate parasite type 2 immunity in the gut. Science. Mar 2016;351(6279):1329–33. doi: 10.1126/science.aaf1648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Civantos AM, Maina IW, Arnold M, et al. Denatonium benzoate bitter taste perception in chronic rhinosinusitis subgroups. Int Forum Allergy Rhinol. Sep 2020;doi: 10.1002/alr.22687 [DOI] [PubMed] [Google Scholar]
- 7.Tizzano M, Cristofoletti M, Sbarbati A, Finger TE. Expression of taste receptors in solitary chemosensory cells of rodent airways. BMC Pulm Med. 2011;11:3. doi: 10.1186/1471-2466-11-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yan CH, Hahn S, McMahon D, et al. Nitric oxide production is stimulated by bitter taste receptors ubiquitously expressed in the sinonasal cavity. Am J Rhinol Allergy. Mar 2017;31(2):85–92. doi: 10.2500/ajra.2017.31.4424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Douglas JE, Mansfield CJ, Arayata CJ, et al. Taste Exam: A Brief and Validated Test. J Vis Exp. 08 2018;(138)doi: 10.3791/56705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang S, Jeong HH, Kim D, et al. Integrative information theoretic network analysis for genome-wide association study of aspirin exacerbated respiratory disease in Korean population. BMC Med Genomics. 05 24 2017;10(Suppl 1):31. doi: 10.1186/s12920-017-0266-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. There is no significant association between patient cohort and patient age, sex and race. Age grouped with equal window size as young (18–39, n=27), middle (40–61, n=52) and old (62–82; n=28) and the race grouped as white (European; n=87) and non-white (others, n=21)
Supplementary Table 1. Subject Characteristics
Supplementary Figure 2. Genetic variants of taste receptors norminally associated with disease cohort. (A) AERD has more heterozygous genotype C:T of rs111614880 (TAS2R14) compared to other patient groups; (B and C) Control subjects have more heterozygous genotype C:T of rs307365 (TAS1R3) and homozygous G:G of rs2234002 (TAS2R4) relative to other patient groups.


