ABSTRACT
Sulbactam-durlobactam is a β-lactam-β-lactamase inhibitor combination designed to treat serious Acinetobacter baumannii-calcoaceticus complex (ABC) infections, including carbapenem-non-susceptible and multidrug-resistant (MDR) isolates. The current study characterized the in vitro activity of sulbactam-durlobactam against a collection of 5,032 ABC clinical isolates collected in 33 countries across the Asia/South Pacific region, Europe, Latin America, the Middle East, and North America from 2016 to 2021. The sulbactam-durlobactam MIC50 and MIC90 were 1 and 2 μg/mL, respectively, for all ABC isolates tested. The addition of durlobactam (at a fixed concentration of 4 μg/mL) to sulbactam decreased its MIC50 by 8-fold (from 8 to 1 μg/mL) and its MIC90 by 32-fold (from 64 to 2 μg/mL) for all ABC isolates. The in vitro activity of sulbactam-durlobactam was maintained across individual ABC species, years, global regions of collection, specimen sources, and resistance phenotypes, including MDR and extensively drug-resistant (XDR) isolates. At 4 μg/mL (preliminary sulbactam-durlobactam susceptible MIC breakpoint), sulbactam-durlobactam inhibited 98.3% of all ABC isolates and >96% of sulbactam-, imipenem-, ciprofloxacin-, amikacin-, and minocycline-non-susceptible isolates; as well as colistin-resistant, MDR, and XDR isolates. Most imipenem-non-susceptible ABC isolates (96.8%, 2,488/2,570) were carbapenem-resistant A. baumannii (CRAB); 96.9% (2,410/2,488) of CRAB isolates were sulbactam-durlobactam-susceptible. More than 80% of ABC isolates had sulbactam-durlobactam MIC values that were ≥2 doubling-dilutions (4-fold) lower than sulbactam alone. Only 1.7% (84/5,032) of ABC isolates from 2016 to 2021 had sulbactam-durlobactam MIC values of >4 μg/mL. Of the 84 isolates, 94.0% were A. baumannii, 4.8% were A. pittii, and 1.2% were A. nosocomialis. In summary, sulbactam-durlobactam demonstrated potent antibacterial activity against a 2016 to 2021 collection of geographically diverse clinical isolates of ABC isolates, including carbapenem-non-susceptible and MDR isolates.
KEYWORDS: sulbactam-durlobactam, ETX2514, Acinetobacter baumannii-calcoaceticus complex, carbapenem-resistant, multidrug-resistant
INTRODUCTION
Acinetobacter baumannii-calcoaceticus complex (ABC) species (A. baumannii, A. calcoaceticus, A. dijkshoorniae, A. nosocomialis, A. pittii, A. seifertii) are the most medically important phylogroup of the genus Acinetobacter (1, 2). The majority of ABC clinical isolates are A. baumannii. ABC species are well-documented pathogens in nosocomial outbreaks and cause severe infections with high mortality rates that are often linked to aspiration and ventilator-associated pneumonia and catheter-associated bacteremia following intensive care unit admission (1–3). Globally, the susceptibility of ABC species to all first- and second-line antimicrobial agents used to treat Acinetobacter infections has declined over the last 30 years with many isolates (primarily A. baumannii) now testing as multidrug-resistant (MDR) or extensively drug-resistant (XDR) (4, 5). The identification and development of new agents to treat antimicrobial-resistant ABC infections, for which there is a high unmet medical need, is an international priority (6).
Sulbactam-durlobactam (formerly sulbactam-ETX2514) is a narrow-spectrum, parenteral β-lactam-β-lactamase inhibitor combination that recently completed a phase 3 study to evaluate its safety and efficacy for the treatment of serious infections caused by ABC, including carbapenem-resistant and MDR isolates (7, 8). Sulbactam-durlobactam has been designated a Qualified Infectious Disease Product (QIDP) by the United States Food and Drug Administration and awarded Fast Track status.
Sulbactam, a semi-synthetic penicillanic acid, is a β-lactamase inhibitor of a subset of Ambler class A enzymes (excluding TEM-1). It was initially partnered with ampicillin in the 1980s and was approved for skin and skin structure, intra-abdominal, bone and joint, and gynecological infections. Sulbactam also inhibits bacterial cell wall synthesis in ABC by binding to penicillin-binding protein (PBP) 1a/b and PBP3 (9). Sulbactam is susceptible to degradation by a variety of acquired or upregulated β-lactamases, including the serine β-lactamases of class A (TEM-1), class C (ADC-30), and class D (OXA), and class B metallo-β-lactamases (MBLs) (8). Resistance to sulbactam as well as broad-spectrum cephalosporins and carbapenems has emerged in ABC and spread widely, mainly due to the acquisition of OXA β-lactamases (OXA-23, OXA-24/40, OXA-51, OXA-58, OXA-143, OXA-235) (1, 2, 5).
Durlobactam is a rationally designed non-β-lactam diazabicyclooctane (DBO) β-lactamase inhibitor of Ambler class A, C, and D β-lactamases that can protect sulbactam from degradation by these enzymes, effectively restoring its activity against sulbactam-non-susceptible ABC isolates expressing these β-lactamases (10–16). Durlobactam does not inhibit MBLs (10). Durlobactam has a modified DBO scaffold resulting in inhibition of a broad range of class D β-lactamases, with notably more potent inhibition of class A and C β-lactamases compared to other DBO inhibitors (e.g., avibactam, relebactam) (10, 11). Ceftazidime-avibactam, imipenem-relebactam, meropenem-vaborbactam, and ceftolozane-tazobactam do not have clinically useful activity against Acinetobacter spp. (17).
The goal of the current study was to characterize the in vitro activity of sulbactam-durlobactam against a recent geographically diverse collection of clinical ABC isolates. Study isolates were chosen from −70°C frozen stocks maintained by International Health Management Associates (IHMA; Schaumburg, IL) based on geographic distribution, site of infection, and year of isolation (and therefore, this was not designed to be a prevalence-based study).
RESULTS
The current study surveyed 5,032 ABC isolates collected by clinical laboratories in 264 medical centers in 33 countries across five global regions (Europe, 42.2% of all isolates tested; North America [United States], 29.9%; Asia/South Pacific, 13.6%; Latin America, 12.6%; Middle East [Israel], 1.7%) from 2016 to 2021 and determined their in vitro susceptibility to sulbactam-durlobactam and nine comparator agents. The percentages of all isolates tested by year were 16.8% from 2016, 16.4% from 2017, 18.4% from 2018, 17.1% from 2019, 15.8% from 2020, and 15.5% from 2021. Isolates tested were limited to one isolate per patient and were primarily from five common infection sources: lower respiratory (54.3% of all isolates tested), bloodstream (20.2%), urinary tract (16.5%), skin and soft tissue (4.5%), and intraabdominal (4.3%). To be consistent with clinical experience, (1–3) 80.2% of the isolates in the survey were A. baumannii, followed by 12.7% A. pittii, 5.9% A. nosocomialis, and 1.1% A. calcoaceticus.
The sulbactam-durlobactam MIC50 and MIC90 were 1 and 2 μg/mL, respectively, for all ABC isolates tested (Table 1). The addition of durlobactam (at a fixed concentration of 4 μg/mL) to sulbactam decreased its MIC50 by 8-fold (from 8 to 1 μg/mL) and its MIC90 by 32-fold (from 64 to 2 μg/mL) for all ABC isolates. MIC50 and MIC90 values for sulbactam-durlobactam ranged from 0.5 to 1 μg/mL and from 1 to 2 μg/mL, respectively, for individual ABC species. The sulbactam-durlobactam MIC range was widest for A. baumannii (≤0.03 to >64 μg/mL), narrower for A. pittii (≤0.03 to 32 μg/mL) and A. nosocomialis (≤0.03 to 8 μg/mL), and narrowest for A. calcoaceticus (0.12 to 2 μg/mL), which correlated with the number of isolates tested for each species.
TABLE 1.
In vitro activities of sulbactam-durlobactam and comparator antimicrobial agents tested against 5,032 clinical isolates of Acinetobacter baumannii-calcoaceticus complex species collected globally from 2016 to 2021a
| Species (no. of isolates) | Antimicrobial agent | MIC (μg/mL) |
MIC interpretation (%) |
||||
|---|---|---|---|---|---|---|---|
| MIC50 | MIC90 | Range | Susceptible | Intermediate | Resistant | ||
| All isolates (5,032)b | Sulbactam-durlobactamc | 1 | 2 | ≤0.03–>64 | 98.3 | NA | 1.7 |
| Sulbactamd | 8 | 64 | 0.25–>64 | 46.9 | 8.0 | 45.1 | |
| Cefepime | 16 | >16 | ≤0.12–>16 | 44.6 | 7.9 | 47.4 | |
| Imipenem | 8 | >64 | ≤0.03–>64 | 48.9 | 0.6 | 50.5 | |
| Meropenem | 16 | >64 | ≤0.03–>64 | 47.9 | 1.1 | 51.0 | |
| Amikacin | 4 | >64 | ≤0.5–>64 | 58.6 | 3.3 | 38.1 | |
| Ciprofloxacin | >4 | >4 | ≤0.12–>4 | 44.4 | 0.7 | 54.9 | |
| Colistine | 0.5 | 1 | ≤0.25–>8 | NA | 95.9 | 4.1 | |
| Minocycline | 0.5 | 16 | ≤0.12–>16 | 78.3 | 10.1 | 11.6 | |
| Tigecyclinef | 0.5 | 2 | 0.03–32 | NA | NA | NA | |
| A. baumannii (4,038) | Sulbactam-durlobactam | 1 | 2 | ≤0.03–>64 | 98.0 | NA | 2.0 |
| Sulbactam | 16 | 64 | 0.25–>64 | 36.5 | 8.9 | 54.6 | |
| Cefepime | >16 | >16 | ≤0.12–>16 | 33.6 | 8.8 | 57.6 | |
| Imipenem | 32 | >64 | ≤0.03–>64 | 37.7 | 0.6 | 61.6 | |
| Meropenem | 64 | >64 | ≤0.03–>64 | 36.6 | 1.2 | 62.3 | |
| Amikacin | 32 | >64 | ≤0.5–>64 | 49.5 | 3.8 | 46.6 | |
| Ciprofloxacin | >4 | >4 | ≤0.12–>4 | 32.7 | 0.7 | 66.6 | |
| Colistin | 0.5 | 1 | ≤0.25–>8 | NA | 95.1 | 4.9 | |
| Minocycline | 1 | 16 | ≤0.12–>16 | 73.3 | 12.4 | 14.4 | |
| Tigecycline | 0.5 | 2 | 0.03–32 | NA | NA | NA | |
| A. calcoaceticus (55) | Sulbactam-durlobactam | 0.5 | 1 | 0.12–2 | 100 | NA | 0 |
| Sulbactam | 2 | 4 | 1–8 | 94.5 | 5.5 | 0 | |
| Cefepime | 4 | 8 | 1–>16 | 90.9 | 7.3 | 1.8 | |
| Imipenem | 0.25 | 0.25 | 0.12–1 | 100 | 0 | 0 | |
| Meropenem | 0.25 | 1 | 0.12–4 | 98.2 | 1.8 | 0 | |
| Amikacin | 1 | 2 | ≤0.5–16 | 100 | 0 | 0 | |
| Ciprofloxacin | ≤0.12 | 0.25 | ≤0.12–0.5 | 100 | 0 | 0 | |
| Colistin | 0.5 | 1 | ≤0.25–2 | NA | 100 | 0 | |
| Minocycline | ≤0.12 | 0.25 | ≤0.12–0.25 | 100 | 0 | 0 | |
| Tigecycline | 0.12 | 0.25 | 0.03–1 | NA | NA | NA | |
| A. nosocomialis (296) | Sulbactam-durlobactam | 0.5 | 1 | ≤0.03–8 | 99.7 | NA | 0.3 |
| Sulbactam | 2 | 16 | 0.25–>64 | 81.8 | 8.1 | 10.1 | |
| Cefepime | 2 | >16 | 0.5–>16 | 85.1 | 4.4 | 10.5 | |
| Imipenem | 0.25 | 0.5 | 0.06–>64 | 92.2 | 0 | 7.8 | |
| Meropenem | 0.25 | 1 | 0.06–>64 | 92.2 | 0.3 | 7.4 | |
| Amikacin | 2 | 8 | ≤0.5–>64 | 92.6 | 2.0 | 5.4 | |
| Ciprofloxacin | 0.25 | 2 | ≤0.12–>4 | 89.9 | 1.4 | 8.8 | |
| Colistin | 0.5 | 1 | ≤0.25–>8 | NA | 98.0 | 2.0 | |
| Minocycline | ≤0.12 | 0.5 | ≤0.12–16 | 98.0 | 1.4 | 0.7 | |
| Tigecycline | 0.12 | 1 | 0.03–4 | NA | NA | NA | |
| A. pittii (638) | Sulbactam-durlobactam | 0.5 | 2 | ≤0.03–32 | 99.4 | NA | 0.6 |
| Sulbactam | 2 | 4 | 0.5–>64 | 92.9 | 2.4 | 4.7 | |
| Cefepime | 4 | 8 | ≤0.12–>16 | 91.4 | 4.2 | 4.4 | |
| Imipenem | 0.25 | 0.5 | 0.06–>64 | 95.1 | 0.3 | 4.5 | |
| Meropenem | 0.5 | 1 | ≤0.03–>64 | 95.0 | 0.8 | 4.2 | |
| Amikacin | 1 | 4 | ≤0.5–>64 | 96.6 | 1.1 | 2.4 | |
| Ciprofloxacin | ≤0.12 | 0.5 | ≤0.12–>4 | 92.8 | 0.3 | 6.9 | |
| Colistin | 0.5 | 1 | ≤0.25–4 | NA | 99.8 | 0.2 | |
| Minocycline | ≤0.12 | 0.25 | ≤0.12–16 | 98.9 | 0.6 | 0.5 | |
| Tigecycline | 0.12 | 0.5 | 0.03–4 | NA | NA | NA | |
ABC, Acinetobacter baumannii-calcoaceticus complex; NA, not available.
There were four isolates of non-identified Acinetobacter spp. and one isolate of Acinetobacter dijkshoorniae that are included in the total data set but not divided out individually in the table.
Sulbactam-durlobactam MICs were interpreted using the preliminary MIC breakpoints of ≤4 μg/mL (susceptible) and ≥8 μg/mL (resistant).
Sulbactam MICs were interpreted using the sulbactam component of CLSI M100 (2021) ampicillin-sulbactam MIC breakpoints (≤8/4 [susceptible], 16/8 [intermediate], and ≥32/16 [resistant]) given that sulbactam is well established to comprise the active component of the combination for Acinetobacter spp.
CLSI M100 (2021) lists only intermediate and resistant MIC breakpoints for colistin tested against Acinetobacter spp.
MIC interpretative criteria are not published by CLSI M100 (2021) for tigecycline tested against Acinetobacter spp.
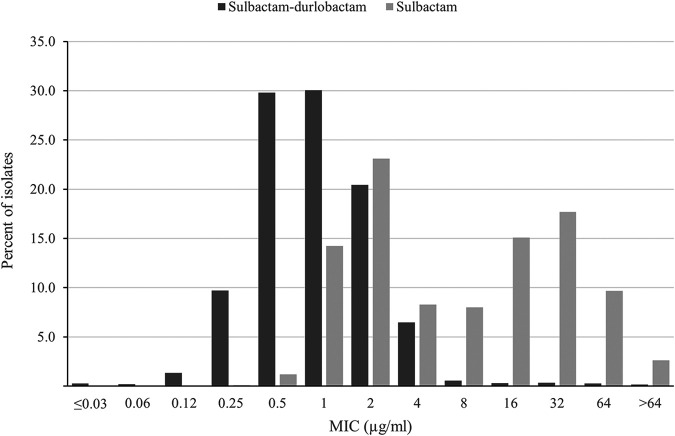
Sulbactam-durlobactam MIC values were ≤4 μg/mL (the preliminary susceptible breakpoint) (18, 19) for 98.3% of all ABC isolates: 100% of A. calcoaceticus, 99.7% of A. nosocomialis, 99.4% of A. pittii, and 98.0% of A. baumannii isolates. Sulbactam-durlobactam demonstrated a unimodal MIC distribution, with most MICs (97.9%; 4,924/5,032) measuring from 0.12 to 4 μg/mL and with modal MICs of 0.5 and 1 μg/mL (Fig. 1). In contrast to sulbactam-durlobactam, sulbactam alone showed a bimodal MIC distribution; one population with a mode of 2 μg/mL and a range of 0.5 to 4 μg/mL and a second population with a mode of 16 to 32 μg/mL and a range of 8 to >64 μg/mL (Fig. 1). Of the 2,670 isolates with sulbactam MIC values of 8 to >64 μg/mL, 96.9% (2,587) were restored to sulbactam MIC values of ≤4 μg/mL in the presence of durlobactam, suggesting that these isolates carry β-lactamases (Table 2).
FIG 1.
Sulbactam-durlobactam (black bars) and sulbactam (gray bars) MIC distributions for 5,032 isolates of Acinetobacter baumannii-calcoaceticus complex (ABC) species collected globally from 2016 to 2021.
TABLE 2.
Cumulative frequency distributions of sulbactam-durlobactam and sulbactam MICs against phenotypic subsets of antimicrobial-non-susceptible and -resistant clinical isolates of Acinetobacter baumannii-calcoaceticus complex collected globally from 2016 to 2021a
| Antimicrobial-non-susceptible/-resistant phenotype (no. of isolates) | Cumulative percentage (%) of isolates inhibited by varying MICs (no. of isolates)b |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MICs (μg/mL) | |||||||||||||
| ≤0.03 | 0.06 | 0.12 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | >64 | |
| All isolates (5,032) | |||||||||||||
| Sulbactam-durlobactam | 0.3 (14) | 0.5 (10) | 1.8 (68) | 11.5 (489) | 41.4 (1,500) | 71.4 (1,513) | 91.9 (1,029) | 98.3 (325) | 98.9 (28) | 99.2 (15) | 99.5 (18) | 99.8 (14) | 100 (9) |
| Sulbactam | 0.1 (5) | 1.3 (61) | 15.5 (716) | 38.7 (1,163) | 46.9 (417) | 54.9 (403) | 70.0 (759) | 87.7 (890) | 97.4 (486) | 100 (132) | |||
| Sulbactam-non-susceptiblec (2,670) | |||||||||||||
| Sulbactam-durlobactam | 0.1 (3) | 0.1 (1) | 0.4 (6) | 3.0 (69) | 17.0 (375) | 51.7 (926) | 85.2 (894) | 96.9 (313) | 97.9 (27) | 98.5 (15) | 99.1 (18) | 99.7 (14) | 100 (9) |
| Sulbactam | 15.1 (403) | 43.5 (759) | 76.9 (890) | 95.1 (486) | 100 (132) | ||||||||
| Imipenem-non-susceptible (2,570) | |||||||||||||
| Sulbactam-durlobactam | 0.0 (1) | 0.1 (1) | 0.4 (7) | 3.2 (73) | 17.6 (370) | 52.2 (889) | 85.1 (847) | 96.7 (298) | 97.8 (28) | 98.4 (15) | 99.1 (18) | 99.6 (14) | 100 (9) |
| Sulbactam | 0.1 (3) | 0.8 (17) | 3.7 (76) | 16.0 (314) | 43.4 (706) | 76.6 (853) | 94.9 (471) | 100 (130) | |||||
| Ciprofloxacin-non-susceptible (2,796) | |||||||||||||
| Sulbactam-durlobactam | 0.1 (2) | 0.1 (2) | 0.6 (14) | 3.6 (84) | 18.8 (425) | 53.9 (979) | 86.4 (909) | 97.5 (310) | 98.3 (24) | 98.8 (13) | 99.2 (11) | 99.7 (14) | 100 (9) |
| Sulbactam | 0.2 (5) | 1.0 (23) | 3.9 (82) | 9.0 (143) | 21.6 (350) | 47.7 (732) | 78.8 (867) | 95.7 (473) | 100 (121) | ||||
| Amikacin-non-susceptible (2,083) | |||||||||||||
| Sulbactam-durlobactam | 0.0 (1) | 0.0 (0) | 0.4 (7) | 2.9 (53) | 16.3 (279) | 48.8 (676) | 84.1 (735) | 96.9 (267) | 97.8 (20) | 98.4 (12) | 99.1 (14) | 99.6 (10) | 100 (9) |
| Sulbactam | 0.2 (5) | 0.6 (7) | 3.9 (70) | 14.2 (214) | 38.8 (512) | 74.0 (733) | 94.5 (427) | 100 (115) | |||||
| Minocycline-non-susceptible (1,092) | |||||||||||||
| Sulbactam-durlobactam | 0.6 (7) | 5.1 (49) | 28.7 (257) | 77.5 (533) | 97.7 (221) | 98.9 (13) | 99.3 (4) | 99.5 (3) | 100 (5) | ||||
| Sulbactam | 0.1 (1) | 0.3 (2) | 2.8 (28) | 10.6 (85) | 32.6 (240) | 73.3 (444) | 95.6 (244) | 100 (48) | |||||
| Colistin-resistant (204) | |||||||||||||
| Sulbactam-durlobactam | 2.9 (6) | 11.3 (17) | 44.6 (68) | 79.9 (72) | 98.0 (37) | 99.5 (3) | 99.5 (0) | 99.5 (0) | 100 (1) | ||||
| Sulbactam | 2.5 (5) | 5.4 (6) | 8.8 (7) | 20.1 (23) | 43.1 (47) | 72.5 (60) | 96.1 (48) | 100 (8) | |||||
| MDRd (2,680) | |||||||||||||
| Sulbactam-durlobactam | 0.0 (1) | 0.1 (1) | 0.3 (7) | 3.0 (71) | 17.0 (375) | 51.6 (928) | 85.3 (902) | 96.9 (311) | 97.9 (28) | 98.5 (15) | 99.1 (18) | 99.7 (14) | 100 (9) |
| Sulbactam | 0.1 (3) | 0.4 (7) | 4.0 (98) | 17.1 (350) | 44.3 (728) | 77.2 (882) | 95.1 (481) | 100 (131) | |||||
| XDRe (2,116) | |||||||||||||
| Sulbactam-durlobactam | 0.2 (4) | 2.3 (44) | 14.7 (262) | 47.1 (687) | 83.8 (776) | 97.2 (283) | 98.1 (20) | 98.6 (11) | 99.1 (10) | 99.6 (10) | 100 (9) | ||
| Sulbactam | 0.5 (11) | 11.1 (224) | 37.5 (559) | 74.0 (771) | 94.7 (438) | 100 (113) | |||||||
ABC, Acinetobacter baumannii-calcoaceticus complex; MDR, multidrug-resistant; XDR, extensively drug-resistant.
MIC90 is in boldface for each MIC distribution.
For sulbactam, a susceptibility breakpoint of ≤4 μg/mL was used, which is based on the CLSI (2021) M100 ampicillin-sulbactam (2:1) susceptible breakpoint of ≤8/4 μg/mL where sulbactam comprises the active component of the combination for Acinetobacter spp.
MDR isolates were defined as those not susceptible to agents from ≥3 different antimicrobial classes from the following list: cefepime (extended-spectrum cephalosporins), imipenem (carbapenems), amikacin (aminoglycosides), ciprofloxacin (fluoroquinolones), minocycline (tetracycline), sulbactam (penicillin plus β-lactamase inhibitor; sulbactam is the active component of ampicillin-sulbactam against Acinetobacter spp.), and colistin (polymyxins). For colistin, only colistin-resistant isolates were used in MDR and XDR determinations because colistin-non-susceptible isolates encompass all isolates of Acinetobacter spp. (CLSI [2021] M100).
XDR isolates were defined as those not susceptible to at least 5 of the following 7 agents or agent classes from the following list: cefepime (extended-spectrum cephalosporins), imipenem (carbapenems), amikacin (aminoglycosides), ciprofloxacin (fluoroquinolones), minocycline (tetracycline), sulbactam (penicillin plus β-lactamase inhibitor; sulbactam is the active component of ampicillin-sulbactam against Acinetobacter spp.), and colistin (polymyxins). For colistin, only colistin-resistant isolates were used in XDR determinations because colistin-non-susceptible isolates encompass all isolates of Acinetobacter spp. (CLSI [2021] M100).
Susceptibility to comparator agents varied by ABC species (Table 1). Less than 50% of A. baumannii isolates were susceptible to sulbactam, cefepime, imipenem, meropenem, amikacin, and colistin; 73.3% of isolates were minocycline-susceptible. Percentages of susceptible values were >90% for all agents tested against A. calcoaceticus and A. pittii, and for all agents except sulbactam, cefepime, and ciprofloxacin against A. nosocomialis. Colistin (MIC90, 1 μg/mL) and tigecycline (MIC90, 2 μg/mL) were the only two comparator agents tested which demonstrated in vitro potency equivalent to sulbactam-durlobactam against all ABC isolates tested; however, these in vitro potencies often do not translate into efficacy due to toxicities and suboptimal pharmacokinetics (20).
MIC50 and MIC90 values for sulbactam-durlobactam did not show appreciable differences when ABC isolates were analyzed by global region (MIC50 range, 1 μg/mL; MIC90 range, 2 to 4 μg/mL) (Table S1 in Supplemental File 1) and specimen source (MIC50 range, 1 μg/mL; MIC90 range, 2 μg/mL) (Table S2). From 2016 to 2021, MIC90 values for sulbactam-durlobactam for all ABC isolates fluctuated by one doubling-dilution (between 2 and 4 μg/mL) without any discernible trend (Table S3). The percentages of isolates with sulbactam-durlobactam MICs ≤4 μg/mL did not differ significantly (by <3%; P = 0.572) across the 6 years and ranged from a low of 97.0% in 2017 to a high of 99.3% in 2018. Individual ABC species also showed random fluctuations in MIC50 and MIC90 values across years without identifiable trends.
A total of 84 ABC isolates from 2016 to 2021 had sulbactam-durlobactam MICs of >4 μg/mL (Table 2). Of these 84 isolates, 79 (94.0%) were A. baumannii, 4 (4.8%) were A. pittii, and 1 (1.2%) was A. nosocomialis. By year, the percentages of isolates with sulbactam-durlobactam MICs of >4 μg/mL were: 1.2% (10/843) in 2016, 3.0% (25/826) in 2017, 0.8% (7/928) in 2018, 2.2% (19/860) in 2019, 1.8% (14/795) in 2020, and 1.2% (9/780) in 2021. The specimen sources associated with the 84 isolates were 1.6% (16/1,015) bloodstream, 2.3% (5/217) intraabdominal, 1.9% (52/2,731) lower respiratory, 0.9% (2/227) skin and soft tissue, and 1.1% (9/832) urinary tract. The 84 isolates were spread across the five regions: 4.7% (30/632) Latin America, 2.3% (2/88) Middle East (Israel), 1.6% (11/685) Asia/South Pacific, 1.4% (29/2,121) Europe, and 0.8% (12/1,506) North America (United States).
The MIC90 for sulbactam-durlobactam was 4 μg/mL for all antimicrobial-non-susceptible phenotypes of ABC studied, including sulbactam-non-susceptible (defined as MIC ≥ 8 μg/mL), carbapenem-non-susceptible, colistin-resistant, MDR, and XDR isolates (Table 2). At 4 μg/mL (the preliminary susceptibility breakpoint), sulbactam-durlobactam inhibited >96% of sulbactam-, imipenem-, ciprofloxacin-, amikacin-, and minocycline-non-susceptible, and colistin-resistant, MDR, and XDR isolates. Most imipenem-non-susceptible ABC isolates (96.8%, 2,488/2,570) were carbapenem-resistant A. baumannii (CRAB); 96.9% (2,410/2,488) of CRAB isolates were sulbactam-durlobactam-susceptible. At a concentration of ≤4 μg/mL, sulbactam alone inhibited <10% of isolates in all antimicrobial-non-susceptible phenotype subsets. The MIC90 value for sulbactam alone was 64 μg/mL for all ABC isolates tested and for all antimicrobial-non-susceptible phenotypes studied. Taken together, these results suggest there is little to no pre-existing cross-resistance between sulbactam-durlobactam and other classes of antimicrobial agents.
DISCUSSION
The treatment of ABC infections is clinically challenging. Current first-line therapies include ampicillin-sulbactam, carbapenems (imipenem, meropenem), and broad-spectrum cephalosporins (ceftazidime, cefepime) when isolates demonstrate in vitro susceptibility (3, 21). In the collection of 5,032 ABC surveillance isolates tested in the current study, <50% were susceptible to sulbactam, cefepime, imipenem, meropenem, ciprofloxacin, and colistin (Table 1). Additionally, 53.3% (2,680/5,032) and 42.1% (2,116/5,032) of isolates, respectively, demonstrated MDR or XDR phenotypes (Table 2). Our results showing high rates of in vitro resistance to first-line therapies confirm those reported in earlier studies (4, 22) and reinforce the importance of identifying new therapies to treat ABC infections. Sulbactam-durlobactam has the potential to significantly lower the high incidence of difficult-to-treat resistance identified in ABC isolates (DTR; defined as intermediate/resistant in vitro to all β-lactam categories, including carbapenems, and fluoroquinolones) (23) by providing an active β-lactam-based therapy that would reduce reliance on less effective and more toxic reserve agents (aminoglycosides, colistin, tigecycline).
The current study reports in vitro susceptibility testing results for sulbactam-durlobactam against >5,000 clinical isolates of ABC collected in five global regions. Previously published reports have described the in vitro activity of sulbactam-durlobactam against far smaller, often regional isolate collections (<100 to 1,722 isolates) (11–16). The current study determined that a sulbactam-durlobactam concentration of 2 μg/mL (MIC90) inhibited 91.9% of 5,032 ABC isolates and that 98.3% of isolates tested with a sulbactam-durlobactam MIC value of ≤4 μg/mL, the preliminary susceptible MIC breakpoint for sulbactam-durlobactam (18, 19). The in vitro activity of sulbactam-durlobactam was shown to be consistent for A. baumannii and three additional ABC species, as well as for isolates across five geographical regions, isolates from five common infection sources, and isolates with multiple clinically relevant resistance phenotypes. Five of the six previously published studies also reported a sulbactam-durlobactam MIC90 of 1 to 2 μg/mL, irrespective of international clonal lineage, and the presence of various OXA-type β-lactamases in isolates, each study showing an MIC distribution similar to that shown in Fig. 1; a single study from Greece describing 190 carbapenem-resistant A. baumannii isolates reported an MIC90 of 8 μg/mL for sulbactam-durlobactam (15). The observation that durlobactam lowered the MIC of sulbactam for almost all (96.9%, 2,587/2,670) ABC isolates with sulbactam MICs of >4 μg/mL (sulbactam-non-susceptible) suggests that durlobactam inhibited class A, C, and D (OXA) β-lactamases in those isolates that would have otherwise hydrolyzed sulbactam (as well as imipenem, meropenem, and cefepime). In addition, these results collectively indicate that PBP mutations or MBLs in global isolates of ABC that can confer sulbactam-durlobactam resistance are currently rare, as discussed below.
In the current study, a small percentage (1.7%, 84/5,032) of isolates had sulbactam-durlobactam MIC values above the preliminary susceptible MIC breakpoint of 4 μg/mL (18, 19), an observation also reported in most earlier publications (11–14, 16). Clinical isolates with sulbactam-durlobactam MICs of >4 μg/mL have been shown to be comprised of two main types, and some may be clonal (12). The first group are isolates with mutations in PBP3 near its active site serine (S336) (24), the sulbactam-binding site. Common PBP3 mutations include A515V and T526S and confer sulbactam-durlobactam MICs of 8 to 32 μg/mL (12, 15). The second group are isolates that carry an MBL (NDM) and test with higher sulbactam-durlobactam MICs (32 to >64 μg/mL) (12). Currently, the prevalence of MBLs in ABC isolates is very low in most regions of the world (12, 25, 26).
In summary, the current study demonstrated the consistent and potent in vitro activity of sulbactam-durlobactam against recent, global clinical isolates of ABC. There are currently no reliably effective antimicrobial agents for the treatment of carbapenem-resistant A. baumannii infections. Our results suggest that sulbactam-durlobactam, if approved, may be useful for the treatment of infections caused by ABC, for which there is currently a high unmet medical need.
MATERIALS AND METHODS
Bacterial isolates.
From 2016 to 2021, 5,032 ABC isolates were collected by clinical laboratories in 264 medical centers in 33 countries (Table S4) and shipped to IHMA; 4,038 were A. baumannii, 638 were A. pittii, 296 were A. nosocomialis, and 55 were A. calcoaceticus. Four isolates of non-identified Acinetobacter species and one isolate of A. dijkshoorniae were also included in the collection. All isolates were cultured from patients receiving care in hospital and were limited to one isolate per infected patient. The identities of all isolates were confirmed by IHMA using matrix-assisted laser desorption ionization–time of flight mass spectrometry (Bruker Daltonics, Billerica, MA). Isolate collection employed annual, specimen source (bloodstream, intra-abdominal, lower respiratory, skin and soft tissue, and urinary tract), and geographic (country or region) quotas. Therefore, this study was not designed to evaluate the prevalence of individual species of Acinetobacter (or to estimate antimicrobial resistance) in the countries or regions from which participating laboratories supplied isolates to the study.
Antimicrobial susceptibility testing.
The CLSI standard broth microdilution antimicrobial susceptibility testing method was used to determine MICs for sulbactam-durlobactam and nine comparator agents (27, 28). All testing was performed using cation-adjusted Mueller-Hinton broth in IHMA in-house-prepared custom broth microdilution panels (27, 28). Sulbactam-durlobactam was tested using 2-fold dilutions of sulbactam in combination with a fixed concentration of 4 μg/mL of durlobactam (27). MIC values for each agent were read and interpreted using CLSI standardized methods (27, 28). MIC breakpoint criteria are not currently published by CLSI for sulbactam-durlobactam, sulbactam alone, or tigecycline (27). Sulbactam-durlobactam MICs were interpreted using a preliminary susceptible MIC breakpoint of ≤4 μg/mL and a resistant MIC breakpoint of ≥8 μg/mL (18, 19). For comparative purposes, susceptible (≤4 μg/mL), intermediate (8 μg/mL), and resistant (≥16 μg/mL) MIC breakpoints were used for sulbactam (alone), based on the ampicillin-sulbactam (in vitro testing ratio 2:1)-susceptible, —intermediate, and -resistant breakpoints of 8/4, 16/8, and 32/16 μg/mL, respectively, where sulbactam is well established to comprise the active component of the combination against Acinetobacter spp. (27).
MDR isolates were defined as those not susceptible to agents from ≥3 different antimicrobial classes from the following list: cefepime (extended-spectrum cephalosporins), imipenem (carbapenems), amikacin (aminoglycosides), ciprofloxacin (fluoroquinolones), minocycline (tetracycline), sulbactam (penicillin plus β-lactamase inhibitor [sulbactam was used in lieu of ampicillin-sulbactam because it is the active component of the combination against Acinetobacter spp.]), and colistin (polymyxins) (29). XDR isolates were defined as those not susceptible to at least 5 of the 7 agents or agent classes listed above for MDR determination (i.e., isolates that were non-susceptible to ≥1 agent in all but ≤2 categories) (29). For colistin, only colistin-resistant isolates were used in MDR and XDR determinations because all isolates of Acinetobacter spp. are now classified as colistin-non-susceptible by CLSI (27).
Statistical analysis.
The Cochran-Armitage test was used to assess linear trends in annual proportions of isolates with sulbactam-durlobactam MICs of ≤4 μg/mL from 2016 to 2021 (XLSTAT version 2020.2.1). A P value of <0.05 was considered statistically significant.
ACKNOWLEDGMENTS
This study was sponsored by Entasis Therapeutics. J.A.K. is a consultant to IHMA, and M.A.H. is an employee of IHMA. J.A.K. and M.A.H. have no personal financial interest in the sponsor of this paper (Entasis Therapeutics). S.M.M. and A.A.M. are employees and shareholders of Entasis Therapeutics.
All authors provided analysis input and have read and approved the final manuscript.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Moubareck CA, Halat DH. 2020. Insights into Acinetobacter baumannii: a review of microbiological, virulence, and resistance traits in a threatening nosocomial pathogen. Antibiotics (Basel) 9:119. doi: 10.3390/antibiotics9030119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wong D, Nielsen TB, Bonomo RA, Pantapalangkoor P, Luna B, Spellberg B. 2017. Clinical and pathophysiological overview of Acinetobacter infections: a century of challenges. Clin Microbiol Rev 30:409–447. doi: 10.1128/CMR.00058-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kanafani ZA, Kanj SS. 2022. Acinetobacter infection: treatment and prevention. Wolters Kluwer, Alphen aan de Rijn, The Netherlands.https://www.uptodate.com/contents/acinetobacter-infection-treatment-and-prevention. Accessed 1 March 2022. [Google Scholar]
- 4.Gales AC, Seifert H, Gur D, Castanheira M, Jones RN, Sader HS. 2019. Antimicrobial susceptibility of Acinetobacter calcoaceticus-Acinetobacter baumannii complex and Stenotrophomonas maltophilia clinical isolates: results from the SENTRY antimicrobial surveillance program (1997–2016). Open Forum Infect Dis 6:S34–S46. doi: 10.1093/ofid/ofy293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Potron A, Poirel L, Nordmann P. 2015. Emerging broad-spectrum resistance in Pseudomonas aeruginosa and Acinetobacter baumannii: mechanisms and epidemiology. Int J Antimicrob Agents 45:568–585. doi: 10.1016/j.ijantimicag.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 6.Tacconelli E, Carrara E, Savoldi A, Harbarth S, Mendelson M, Monnet DL, Pulcini C, Kahlmeter G, Kluytmans J, Carmeli Y, Ouellette M, Outterson K, Patel J, Cavaleri M, Cox EM, Houchens CR, Grayson ML, Hansen P, Singh N, Theuretzbacher U, Magrini N, WHO Pathogens Priority List Working Group . 2018. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis 18:318–327. doi: 10.1016/S1473-3099(17)30753-3. [DOI] [PubMed] [Google Scholar]
- 7.ClinicalTrials.gov. 2019. Study to evaluate the efficacy and safety of intravenous sulbactam-ETX2514 in the treatment of patients with infections caused by Acinetobacter baumannii-calcoaceticus complex (ATTACK). U.S. National Library of Medicine, Bethesda, MD. https://clinicaltrials.gov/ct2/show/NCT03894046. Accessed 14 February 2022. [Google Scholar]
- 8.Shapiro AB, Moussa SH, McLeod SM, Durand-Réville T, Miller AA. 2021. Durlobactam, a new diazabicyclooctane β-lactamase inhibitor for the treatment of Acinetobacter infections in combination with sulbactam. Front Microbiol 12:709974. doi: 10.3389/fmicb.2021.709974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Penwell WF, Shapiro AB, Giacobbe RA, Gu R-F, Gao N, Thresher J, McLaughlin RE, Huband MD, de Jonge BLM, Ehmann DE, Miller AA. 2015. Molecular mechanisms of sulbactam antibacterial activity and resistance determinants in Acinetobacter baumannii. Antimicrob Agents Chemother 59:1680–1689. doi: 10.1128/AAC.04808-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Durand-Réville TF, Guler S, Comita-Prevoir J, Chen B, Bifulco N, Huynh H, Lahiri S, Shapiro AB, McLeod SM, Carter NM, Moussa SH, Velez-Vega C, Olivier NB, McLaughlin R, Gao N, Thresher J, Palmer T, Andrews B, Giacobbe RA, Newman JV, Ehmann DE, de Jonge B, O'Donnell J, Mueller JP, Tommasi RA, Miller AA. 2017. ETX2514 is a broad-spectrum β-lactamase inhibitor for the treatment of drug-resistant Gram-negative bacteria including Acinetobacter baumannii. Nat Microbiol 2:e17104. doi: 10.1038/nmicrobiol.2017.104. [DOI] [PubMed] [Google Scholar]
- 11.Barnes MD, Kumar V, Bethel CR, Moussa SH, O’Donnell J, Rutter JD, Good CE, Hujer KM, Hujer AM, Marshall SH, Kreiswirth BN, Richter SS, Rather PN, Jacobs MR, Papp-Wallace KM, van den Akker F, Bonomo RA. 2019. Targeting multidrug-resistant Acinetobacter spp.: sulbactam and the diazabicyclooctenone β-lactamase inhibitor ETX2514 as a novel therapeutic agent. mBio 10:e00159-19. doi: 10.1128/mBio.00159-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McLeod SM, Moussa SH, Hackel MA, Miller AA. 2020. In vitro activity of sulbactam-durlobactam against Acinetobacter baumannii-calcoaceticus complex isolates collected globally in 2016 and 2017. Antimicrob Agents Chemother 64:e02534-19. doi: 10.1128/AAC.02534-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seifert H, Müller C, Stefanik D, Higgins PG, Miller A, Kresken M. 2020. In vitro activity of sulbactam/durlobactam against global isolates of carbapenem-resistant Acinetobacter baumannii. J Antimicrob Chemother 75:2616–2621. doi: 10.1093/jac/dkaa208. [DOI] [PubMed] [Google Scholar]
- 14.Nodari C, Santos F, Kurihara M, Valiatti T, Cayo R, Gales A. 2021. In vitro activity of sulbactam-durlobactam against extensively-drug-resistant Acinetobacter baumannii isolates belonging to South America major clones. J Glob Antimicrob Resist 25:363–366. doi: 10.1016/j.jgar.2021.05.001. [DOI] [PubMed] [Google Scholar]
- 15.Petropoulou D, Siopi M, Vourli S, Pournaras S. 2021. Activity of sulbactam-durlobactam and comparators against a national collection of carbapenem-resistant Acinetobacter baumannii isolates from Greece. Front Cell Infect Microbiol 11:814530. doi: 10.3389/fcimb.2021.814530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang Q, Xu Y, Jia P, Zhu Y, Zhang J, Zhang G, Deng J, Hackel M, Bradford PA, Reinhart H. 2020. In vitro activity of sulbactam/durlobactam against clinical isolates of Acinetobacter baumannii collected in China. J Antimicrob Chemother 75:1833–1839. doi: 10.1093/jac/dkaa119. [DOI] [PubMed] [Google Scholar]
- 17.Karlowsky JA, Hackel MA, Takemura M, Yamano Y, Echols R, Sahm DF. 2022. In vitro susceptibility of Gram-negative pathogens to cefiderocol in five consecutive annual multinational SIDERO-WT surveillance studies, 2014 to 2019. Antimicrob Agents Chemother 66:e0199021. doi: 10.1128/AAC.01990-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O’Donnell J, Rubino C, Onufrak NJ, Bhaunani SM, Ambrose PG, Tommasi R, Stone E, Rodvold K, Mueller J, Isaacs R, Miller A, Srinivasan S. 2019. Pharmacokinetics/pharmacodynamics and phase 3 dose projection for the novel β-lactamase inhibitor ETX2514 in combination with sulbactam against Acinetobacter baumannii-calcoaceticus complex (ABC), poster AAR-LB-14. Microbe 2019, San Francisco, CA. [Google Scholar]
- 19.Rodvold KA, Gotfried MH, Isaacs RD, O'Donnell JP, Stone E. 2018. Plasma and intrapulmonary concentrations of ETX2514 and sulbactam following intravenous administration of ETX2514SUL to healthy adult subjects. Antimicrob Agents Chemother 62:e01089-18. doi: 10.1128/AAC.01089-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Isler B, Doi Y, Bonomo RA, Paterson DL. 2019. New treatment options against carbapenem-resistant Acinetobacter baumannii infections. Antimicrob Agents Chemother 63:e01110-18. doi: 10.1128/AAC.01110-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kalil AC, Metersky ML, Klompas M, Muscedere J, Sweeney DA, Palmer LB, Napolitano LM, O'Grady NP, Bartlett JG, Carratalà J, El Solh AA, Ewig S, Fey PD, File TM, Jr., Restrepo MI, Roberts JA, Waterer GW, Cruse P, Knight SL, Brozek JL. 2016. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis 63:e61–e111. doi: 10.1093/cid/ciw353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morrissey I, Olesky M, Hawser S, Lob SH, Karlowsky JA, Corey GR, Bassetti M, Fyfe C. 2020. In vitro activity of eravacycline against Gram-negative bacilli isolated in clinical laboratories worldwide from 2013 to 2017. Antimicrob Agents Chemother 64:e01699-19. doi: 10.1128/AAC.01699-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kadri SS, Adjemian J, Lai YL, Spaulding AB, Ricotta E, Prevots DR, Palmore TN, Rhee C, Klompas M, Dekker JP, Powers JH, Suffredini AF, Hooper DC, Fridkin S, Danner RL, National Institutes of Health Antimicrobial Resistance Outcomes Research Initiative (NIH–ARORI) . 2018. Difficult-to-treat resistance in Gram-negative bacteremia at 173 US hospitals: retrospective cohort analysis of prevalence, predictors, and outcome of resistance to all first-line agents. Clin Infect Dis 67:1803–1814. doi: 10.1093/cid/ciy378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Han S, Caspers N, Zaniewski RP, Lacey BM, Tomaras AP, Feng X, Geoghegan KF, Shanmugasundaram V. 2011. Distinctive attributes of β-lactam target proteins in Acinetobacter baumannii relevant to development of new antibiotics. J Am Chem Soc 133:20536–20545. doi: 10.1021/ja208835z. [DOI] [PubMed] [Google Scholar]
- 25.Biedenbach D, Bouchillon S, Hackel M, Hoban D, Kazmierczak K, Hawser S, Badal R. 2015. Dissemination of NDM metallo-β-lactamase genes among clinical isolates of Enterobacteriaceae collected during the SMART global surveillance study from 2008 to 2012. Antimicrob Agents Chemother 59:826–830. doi: 10.1128/AAC.03938-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kostyanev T, Xavier BB, García-Castillo M, Lammens C, Bravo-Ferrer Acosta J, Rodríguez-Baño J, Cantón R, Glupczynski Y, Goossens H, EURECA/WP1B Group . 2021. Phenotypic and molecular characterizations of carbapenem-resistant Acinetobacter baumannii isolates collected within the EURECA study. Int J Antimicrob Agents 57:106345. doi: 10.1016/j.ijantimicag.2021.106345. [DOI] [PubMed] [Google Scholar]
- 27.Clinical and Laboratory Standards Institute. 2021. Performance standards for antimicrobial susceptibility testing. M100, 31st ed. CLSI, Wayne, PA. [Google Scholar]
- 28.Clinical and Laboratory Standards Institute. 2018. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved Standard M07-A11, 11th ed. CLSI, Wayne, PA. [Google Scholar]
- 29.Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler JF, Kahlmeter G, Olsson-Liljequist B, Paterson DL, Rice LB, Stelling J, Struelens MJ, Vatopoulos A, Weber JT, Monnet DL. 2012. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material. Download aac.00781-22-s0001.pdf, PDF file, 0.3 MB (278KB, pdf)