Abstract
Background
Autism spectrum disorder (ASD; also known as autism) is a developmental disability that begins in childhood and is typically seen in around 1% to 2% of children. It is characterised by social communication difficulties and repetitive and restricted behaviours and routines that can have a negative impact on a child's quality of life, achievement at school, and social interactions with others. It has been hypothesised that memantine, which is traditionally used to treat dementia, may be effective in reducing the core symptoms of autism as well as some co‐occurring symptoms such as hyperactivity and language difficulties. If memantine is being used to treat the core symptoms of autism, it is important to review the evidence of its effectiveness.
Objectives
To assess the effects of memantine on the core symptoms of autism, including, but not limited to, social communication and stereotypical behaviours.
Search methods
We searched CENTRAL, MEDLINE, Embase, nine other databases and three trials registers up to February 2022. We also checked reference lists of key studies and checked with experts in the field for any additional papers. We searched for retractions of the included studies in MEDLINE, Embase, and the Retraction Watch Database. No retractions or corrections were found.
Selection criteria
We included randomised controlled trials (RCTs) of any dose of memantine compared with placebo in autistic people. We also included RCTs in which only one group received memantine, but both groups received the same additional therapy (e.g. a behaviour intervention).
Data collection and analysis
We used standard Cochrane methods. Our primary outcomes were core autism symptoms and adverse effects. Secondary outcomes were language, intelligence, memory, adaptive behaviour, hyperactivity, and irritability. We used GRADE to assess certainty of evidence.
Main results
We included three RCTs (two double‐blind and one single‐blind) with 204 participants that examined the short‐term effect (immediately postintervention) of memantine in autistic people. Two studies took place in the USA and the other in Iran. All three studies focused on children and adolescents, with a mean age of 9.40 (standard deviation (SD) 2.26) years. Most participants were male (range across studies 73% to 87%). The diagnosis of ASD was based on the Diagnostic and Statistical Manual of Mental Disorders (4th edition; 4th edition, text revision; or 5th edition). To confirm the diagnosis, one study used the Autism Diagnostic Observation Schedule (ADOS) and the Autism Diagnostic Interview‐Revised (ADI‐R); one used ADOS, ADI‐R or the Autism Diagnostic Interview Screener; and one used the Gilliam Autism Rating Scale. Dosage of memantine was based on the child's weight and ranged from 3 mg to 15 mg per day.
Comparisons Two studies examined memantine compared with placebo; in the other study, both groups had a behavioural intervention while only one group was given memantine.
Risk of bias All studies were rated at high risk of bias overall, as they were at high or unclear risk of bias across all but four domains in one study, and all but two domains in the other two studies. One study was funded by Forest Laboratories, LLC, (Jersey City, New Jersey), Allergan. The study sponsor was involved in the study design, data collection (via contracted clinical investigator sites), analysis and interpretation of data, and the decision to present these results. The other two studies reported no financial support or sponsorship; though in one of the two, the study medication was an in‐kind contribution from Forest Pharmaceuticals.
Primary outcomes There was no clear evidence of a difference between memantine and placebo with respect to severity of core symptoms of autism, although we are very uncertain about the evidence. The standardised mean difference in autism symptoms score in the intervention group versus the control group was –0.74 standard deviations (95% confidence interval (CI) −2.07 to 0.58; 2 studies, 181 participants; very low‐certainty evidence; medium effect size); lower scores indicate less severe autistic symptoms. Two studies (144 participants) recorded adverse effects that the authors deemed related to the study and found there may be no difference between memantine and placebo (odds ratio (OR) 0.64, 95% CI 0.17 to 2.39; low‐certainty evidence).
Secondary outcomes There may be no difference between memantine and placebo on language (2 studies, 144 participants; low‐certainty evidence); memory or adaptive behaviour (1 study, 23 participants; both low‐certainty evidence); or hyperactivity or irritability (1 study, 121 participants; both low‐certainty evidence).
Authors' conclusions
It is unclear whether memantine is an effective treatment for autistic children. None of the three included trials reported on the effectiveness of memantine in adults. Further studies using rigorous designs, larger samples, longer follow‐up and clinically meaningful outcome measures that are important to autistic people and their families will strengthen our knowledge of the effects of memantine in autism.
Keywords: Adolescent; Adult; Child; Female; Humans; Male; Autism Spectrum Disorder; Autism Spectrum Disorder/drug therapy; Memantine; Memantine/therapeutic use; Odds Ratio; Outcome Assessment, Health Care; Randomized Controlled Trials as Topic; Treatment Outcome
Plain language summary
Can a dementia medicine (memantine) help people with autism spectrum disorder?
Background Autism spectrum disorder (autism) is a condition that begins in childhood. Core symptoms include persistent difficulties with social communication (e.g. difficulties with back‐and‐forth conversations, communication without words, and in developing and maintaining relationships), and repetitive and restricted interests and behaviours (e.g. repetitive mannerisms, restricted interests and behaviours, resistance to change and sensory sensitivities). Around 1% to 2% of children have autism. Autistic people often have other conditions such as attention deficit hyperactivity disorder (ADHD), anxiety, language impairments (e.g. difficulties understanding and using grammar) and intellectual disability. Autism can have negative impacts on quality of life, school achievement and social relationships. Memantine is a medication traditionally used to treat dementia, but some studies suggest that it may decrease core autistic symptoms. If memantine is being used to change the core symptoms of autism, it is important to assess whether it works and is safe. This review combines the research evidence on the use of memantine in autism.
Review question Does memantine change the core symptoms of autism and related behaviours?
Search date The evidence is current to 14 February 2022.
Study characteristics We found three studies with 204 people that had evaluated the effectiveness of memantine in autism. All studies were randomised controlled trials, meaning participants were randomly allocated to receive either the treatment or a dummy pill (placebo). This is the best design for assessing the effectiveness of treatments. All three studies included children diagnosed with autism spectrum disorder, with an average age of 9.40 years. We found no studies in adults. The children received memantine (for 12 weeks in two studies and for 24 weeks in one study), and their behaviour was assessed before treatment and immediately after treatment.
Study funding sources One study was sponsored and funded by a laboratory that makes memantine (Forest). The study sponsor helped to design the study, collect information, analyse and interpret the information, and take the decision to publish the results. The authors of the other two smaller studies said they did not receive any funding; though in one of these studies, Forest Pharmaceuticals provided the medicine for free.
Key results It is unclear if memantine makes any difference to the core symptoms of autism. Additionally, there may be no difference between memantine and placebo in the occurrence of side effects, language ability, memory, adaptive behaviour or the autism‐related behaviours of hyperactivity and irritability.
Limitations of the evidence We are not confident about the evidence for core symptoms of autism because it comes from only three small studies; because the studies included different types of people and delivered the medicine in different ways; and because the studies did not always provide information about everything we were interested in. Additionally, we have little confidence in the evidence on side effects and language because it comes from only two small studies; and we have little confidence in the evidence on intelligence, memory, adaptive behaviour, hyperactivity and irritability, because it comes from only one small study.
Summary of findings
Summary of findings 1. Memantine versus placebo for autism.
| Memantine compared with placebo for autism | ||||||
|
Patient or population: children/adolescents diagnosed with ASD Settings: USA (2 trials) and Iran (1 trial) Intervention: memantine in the form of capsules or tablets of 3 mg or 6 mg: dosage was based on the child's weight and ranged from 3 mg to 15 mg per day (3 trials) Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Memantine | |||||
|
Core symptoms of autism Assessed with: GARS or SRS (lower scores reflect fewer autism symptoms) Follow‐up: immediately postintervention |
— | The SMD score in the intervention group was 0.74 standard deviations lower (2.07 lower to 0.58 higher) than the placebo group (i.e. they had fewer autism symptoms). | — | 181 (2 RCTs) | ⊕⊝⊝⊝ Very lowa |
There was no evidence of a difference, but results are very uncertain. The SMD was used, and a medium effect size was found (small = 0.2; medium = 0.5 and large = 0.8; Cohen 1988). |
|
Adverse effects Assessed by the study authors as those that were sufficient to discontinue treatment |
— | OR 0.64 (0.17 to 2.39) | 144 (2 RCTs) |
⊕⊕⊝⊝ Lowb |
In Aman 2017a, the authors only report adverse events that were sufficient to discontinue treatment. 1 SAE was reported: mood disorder (judged unrelated to the study medication) in a memantine‐treated participant who was one of the few participants with ASD who did not meet the criteria for the diagnosis of autistic disorder. "All treatment‐emergent AEs (TEAEs) were mild or moderate in severity, except for 3 in the memantine group (irritability, affective disorder, choking); the occurrence of affective disorder in 1 participant was also considered a serious AE (SAE)." (Aman 2017a, p 407). In Soorya 2021, 2 participants in the placebo group withdrew due to treatment‐limiting AEs including diarrhoea and emotional lability. 1 participant in the memantine group withdrew due to a treatment‐limiting AE, specifically activation (i.e. overly energetic). 75% of reported AEs were in the mild range. Gastrointestinal andmood/psychiatric symptoms were the most commonly reported AEs, and although these were experienced more in the memantine group, the difference was not "statistically significant". |
|
|
Language Assessed with: mean scores on the CCC‐2 (70 items with 10 subscales and 2 composite scores; both the composite and subscale scores were reported) and mean standard scores on the EVT‐2. Follow‐up: immediately postintervention |
Aman 2017a And Soorya 2021 collected and analysed data on language. Aman 2017a did not provide specific test statistics, and these data were not available from the authors. The study authors reported no 'statistically significant' differences between the placebo and memantine groups across all but 1 CCC‐2 subscales; the Context subscale showed a 'statistically significant' difference in favour of the placebo group i.e. the study authors reported 'significantly' greater improvement in the use of context in the placebo group compared with the memantine group (P = 0.02). In Soorya 2021, there was no evidence of a difference in standard scores for expressive vocabulary between baseline and 24 weeks (F = 0.67, P = 0.42, ES = 0.33 (CI −0.50 to 1.15)) |
— | 144 (2 RCTs) | ⊕⊕⊝⊝ Lowb |
Soorya 2021 presented data for each time point as follows. Memantine group scores: BL = 95.67 (SD 30.2); 12 wks = 98.17 (SD 31.85); 24 wks = 98.58 (SD 35.54) Placebo group scores: BL = 91 (SD 24.33); 12 wks = 87 (SD 24.23); 24 wks = 90.27 (SD 23.75) |
|
|
Memory Assessed with: mean scores on the NEPSY‐II Memory for Design and Narrative Memory subsets (4 subtests in total were completed). Follow‐up: immediately postintervention |
1 study collected and analysed data on memory (Soorya 2021). There was a treatment effect on the narrative (verbal) memory‐recognition subtest (F = 5.05, P = 0.03, ES = 0.79, 95% CI −0.06 to 1.64). There were no treatment effects for the 3 other memory scales, including Memory for Designs‐standard (F = 1.00, P = 0.33, ES = 0.39, 95% CI −0.44 to 1.22); Memory for Designs‐delayed (F = 0.06, P = 0.81, ES = −0.10, 95% CI −0.91 to 0.72) and Narrative Memory‐free and cued (F = 3.0, P = 0.1, ES = 0.69, 95% CI −0.15 to 1.53). | — | 23 (1 RCT) | ⊕⊕⊝⊝ Lowb |
— | |
|
Adaptive behaviour Assessed with: mean composite scores on the VABS (semi‐structured caregiver interview) Follow‐up: immediately postintervention |
1 study collected and analysed data on adaptive behaviour (Soorya 2021). There were no treatment effects for adaptive behaviour (F = 0.25, P = 0.62, ES = 0.19, 95% CI −0.63 to 1.01). | — | 23 (1 RCT) | ⊕⊕⊝⊝ Lowb |
— | |
|
Hyperactivity Assessed with: mean scores on the ABC‐H (caregiver‐completed questionnaire, 16 items in subscale) Follow‐up: immediately postintervention |
1 study collected and analysed data on hyperactivity (Aman 2017a). There were no specific test statistics or P values in the publication or available from the study authors. The study authors reported that there was no 'statistically significant' difference between the placebo and memantine groups on the ABC‐C. | — | 121 (1 RCT) | ⊕⊕⊝⊝ Lowb |
— | |
|
Irritability Assessed with: mean scores on the ABC‐C (caregiver‐completed questionnaire, 15 items in subscale) Follow‐up: immediately postintervention |
1 study collected and analysed data on irritability (Aman 2017a). There were no specific test statistics or P values in the publication or available from the study authors. The study authors reported that there was no 'statistically significant' difference between the placebo and memantine groups on the ABC‐C. | — | 121 (1 RCT) | ⊕⊕⊝⊝ Lowb |
— | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ABC‐C: Aberrant Behavior Checklist‐Caregiver; ABC‐H: Aberrant Behavior Checklist‐Hyperactivity; AE: adverse event; ASD: autism spectrum disorder; BL: baseline; CCC‐2: Children's Communication Checklist, 2nd edition; CI: confidence interval; ES: effect size; EVT‐2: Expressive Vocabulary Test, 2nd edition; GARS: Gilliam Autism Rating Scale; OR: odds ratio; NEPSY‐II: a developmental NEuroPSYchological assessment, 2nd edition; RCT: randomised controlled trial; SAE: serious adverse event; SD: standard deviation; SMD: standardised mean difference; SRS: Social Responsiveness Scale; VABS: Vineland Adaptive Behavior Scales; wks: weeks. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level for risk of bias, due to high or unclear risk of bias across all but one domain in one study; by one level for imprecision, due to wide CI crossing the line of no effect, small sample sizes and a small number of studies; and by one level due to inconsistency of results (I2 = 95.9%). bDowngraded one level for imprecision, because only one or two small studies contributed limited outcome data, and by one level due to high or unclear risk of bias across three or more domains in all studies.
Because studies used different measurement tools, we standardised them to a uniform scale before meta‐analysis by computing the standardised mean difference. The standardised mean difference expresses the size of the intervention effect in each study relative to the variability observed in that study.
Background
Description of the condition
Autism spectrum disorder (ASD) is a pervasive neurodevelopmental condition that is characterised by difficulties in social interaction and communication, and the presence of restricted, repetitive behaviours known as stereotypies (APA 2013; WHO 2018). The Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM‐5) uses the following five criteria for the diagnosis of ASD (APA 2013).
Persistent deficits in social communication and social interaction across multiple contexts
Restricted, repetitive patterns of behaviour, interests or activities
Behaviours are present in the early developmental period
Symptoms cause clinically significant impairment
These disturbances are not better explained by intellectual disability or global developmental delay
The International Classification of Diseases, 11th Revision (ICD‐11) is also used to diagnose ASD and mirrors the DSM‐5 criteria in most key aspects (WHO 2018). The International Classification of Diseases, 10th Revision (ICD‐10), and the previous Diagnostic and Statistical Manual of Mental Disorders, 4th edition, text revision (DSM‐IV‐TR) grouped diagnoses under 'pervasive developmental disorders', including autistic disorder, childhood autism, Asperger's disorder/syndrome, Rett syndrome, childhood disintegrative disorder, atypical autism, pervasive developmental disorder not otherwise specified (PDD‐NOS), and pervasive developmental disorder, unspecified (APA 2000; WHO 1992; Wing 1997). There was no diagnosis of ASD in the ICD‐10 and Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM‐IV), but it was generally accepted that ASD included all the diagnoses listed above, except Rett syndrome and childhood disintegrative disorder. With the publication of the DSM‐5, the term ASD replaced previous historical terms used to describe the condition, including Kanner's syndrome, infantile autism, and autistic disorder (Volkmar 2014).
There is wide variability in the severity and manifestation of behaviours in autism (Shattuck 2007; Van Wijngaarden‐Cremers 2014). However, autistic people generally have core symptoms characterised by persistent deficits in social interaction, social communication, forming and maintaining relationships, and understanding social cues from other people (APA 2013; Shattuck 2007). Other core autism symptoms include restricted, repetitive patterns and behaviours such as preoccupations or special interests, rigid adherence to routines, hypo‐ or hyper‐reactivity to or interest in sensory stimuli, and stereotypical behaviours (APA 2013). A range of patterns of early development have been reported where some children may show autism symptoms very early in development, some show a plateau of development and others seemingly develop typically but lose or regress in previously acquired social and communication skills at around two years of age (Williams 2015). Co‐occurring behaviours commonly seen in autism include anxiety, language impairments, attention deficit hyperactivity disorder (ADHD), intellectual disability, irritability and aggression; but these features do not occur in all autistic people and are not required to make a diagnosis (Lai 2014). Autistic people also commonly have sleep difficulties (ranging from half to two‐thirds of people); about one‐third have epilepsy; and up to 70% of those with low intellectual functioning have movement abnormalities (Maski 2011). Co‐occurring psychiatric conditions are prevalent and contribute significantly to functional outcomes in autistic people. The prevalence of schizophrenia is about 3.6 times higher in autistic people compared with controls (Zheng 2018), and 40% to 50% of young autistic people have at least one anxiety disorder (Vannucchi 2014; Vasa 2015).
The prevalence of autism has been estimated at around one in 44 children (2.3 %) aged eight years old in the USA (Maenner 2021), and 0.97% across 26 high‐income countries (Fombonne 2021). Some studies report increasing incidence and prevalence of autism, although this appears to be due to better case ascertainment, milder symptoms, earlier diagnosis and increased awareness and diagnostic substitution, rather than a true increase in autism (Elsabbagh 2012; Fisch 2012; Fombonne 2011; May 2020). Autism is diagnosed three to four times more often in males than in females (Loomes 2017).
There is no one cause of autism, but increasing genetic findings support what has been suspected: that rather than a true increase in autism, there are different genetic pathways to autism, with a wide range of single genes reported, known genetic problems with increased likelihood of autism, and early reports that multiple common variant genes play a role. Twin studies have found that if one identical twin has autism there is up to a 96% chance the other twin will also have autism, although the severity of their symptoms may differ (Castelbaum 2020). It is thought that genes (specifically, genes that regulate brain development) may cause disruption during early brain development. Imaging studies have identified some differences in the anatomy, functioning and connectivity of the brain in autistic people compared to those without autism. However, no consistent single pattern of brain difference has been identified for all autistic people (Ecker 2017). The possibility of gene‐environment interactions are also being explored (Bayou 2008; Hallmayer 2011; Lai 2014).
Autism is a diagnostically stable condition. The vast majority of children who are diagnosed with ASD continue to manifest autism behaviours as adults (Woolfenden 2012). However, the type and impact of behaviours may vary over the lifetime trajectories of autistic people (Vannucchi 2014). Autistic adults have variable outcomes in areas such as educational attainment, employment, relationships and functional independence. Generally, people with associated low intelligence or co‐occurring psychiatric diagnoses, such as depression or anxiety disorder, have poorer lifetime outcomes (Gotham 2015).
In recent years, there has been increased understanding of neurodiversity and greater recognition of the differences, abilities, and strengths of autistic people, and ongoing consideration of the terminology used to refer to autism. While we acknowledge that terminology for autism is varied, in this review we have used identity first language (i.e. 'autistic person'; Kenny 2016). An exception is when we refer to the diagnosis of ASD or when we present data directly from the included studies. Further to this, we acknowledge that some autistic people may not wish to reduce their autism symptoms. However, if drugs such as memantine are being prescribed by clinicians and used by autistic people, it is important to rigorously assess the quality and certainty of the evidence for their effectiveness, and to provide a clear picture of the risks, benefits and potential harms for autistic people, their families and clinicians.
Description of the intervention
Therapies for autism
There is wide variability in the manifestations and severity of core and non‐core behaviours in autistic people and different presentations are likely to need different types and amounts of interventions. Most interventions for autism target behaviour and development, and often employ a combination of behavioural and developmental, educational, medical‐related or allied‐health therapies. No pharmacological interventions have been consistently shown to change the core symptoms of autism. Most pharmacologic interventions are used as adjunctive therapy to target specific unwanted behaviours – typically non‐core behaviours of autism, such as restricted and repetitive behaviours, hyperactivity, inattention, irritability and aggression, and sleep disturbance (Farmer 2013; Henneberry 2021; Rossignol 2014) – or to assist with the management of anxiety.
More recently, there has been an interest in the potential effectiveness of pharmacological interventions that might target neuropathological pathways. These include acetylcholinesterase inhibitors (e.g. donepezil, galantamine); antidepressant selective serotonin reuptake inhibitors (SSRIs; such as fluoxetine and citalopram); antipsychotic drugs (e.g. risperidone, aripiprazole); mood stabilisers or antiepileptic agents (e.g. lamotrigine, sodium valproate); psychostimulants (e.g. methylphenidate); and glutamate receptor‐related medications such as memantine (Doyle 2012; Farmer 2013; Rossignol 2014; Siegel 2012). This study will look at the current available evidence for the use of memantine in autism.
Memantine
Memantine is a pharmacological agent that acts as a non‐competitive antagonist of glutamatergic N‐methyl‐D‐aspartate (NMDA)‐type receptors. It works by inhibiting pathological overactivation and subsequent neuroexcitation and cell death of NMDA receptor cells by glutamate (an amino acid normally found in the brain). There is evidence that autistic people have pathologically increased activity levels of glutamate and NMDA receptors (Rojas 2014), hence the aim to modulate this biochemical effect to potentially reduce the core symptoms of autism. This property of memantine has been employed in the treatment of Alzheimer's disease (McShane 2019) and is the basis for trials in the treatment of autistic people (e.g. Aman 2017a; Ghaleiha 2013; Hardan 2019; Hosenbocus 2013; Kavirajan 2009; Wei 2012). The American Psychiatric Association currently endorses the use of memantine in the treatment of moderate‐to‐severe Alzheimer's disease, and it is used off‐label in mild‐to‐moderate vascular dementia (Rabins 2007).
Research trials have also investigated the use of memantine as a pharmacological treatment for some psychiatric conditions that often co‐occur in autistic people, such as obsessive‐compulsive disorder (OCD; Haghighi 2013; Stewart 2010), ADHD (Biederman 2017; Findling 2007; Mohammadi 2015; Mohammadzadeh 2019; Surman 2013), anxiety (Feusner 2009; Rapp 2013; Schwartz 2012) and depression (Caddy 2015; McCloud 2015; Smith 2013; Strzelecki 2013; Zarate 2006). These studies have found memantine to have variable effectiveness as either single or adjuvant therapy. Hence, further research on its use is warranted.
Memantine has been used in clinical trials for the treatment of autistic people, yet the dosage used across studies has varied. One small, open‐label, retrospective study of 18 individuals with ASD aged six to 19 years used memantine up to a maximum dose of 20 mg/day. This study reported significant beneficial effects on social withdrawal and inattention (Erickson 2007). One larger prospective trial reported improvements in language and social interaction in autistic children who were treated with open‐label memantine doses of between 2.5 mg and 30 mg/day in addition to their usual medications (Chez 2007).
Trials carried out in elderly people with Alzheimer's disease have found memantine to be relatively safe and well tolerated, with an adverse effect profile between 0% and 2% higher than placebo treatment (Farlow 2008; Thomas 2009; Van Dyck 2007). Reported adverse effects associated with memantine treatment include falls, injuries, pain, arthralgia, agitation, anxiety, depression, confusion, headaches, hypertension, peripheral oedema, dizziness, fatigue, somnolence, insomnia, flu‐like symptoms, cough, dyspnoea, upper respiratory tract infections, nausea, diarrhoea, constipation, vomiting, anorexia, increase in blood urea nitrogen, urinary incontinence and urinary tract infections (Ott 2007; Thomas 2009). There are limited study data on adverse effects in children and autistic adults (Rossignol 2014).
Following oral administration, memantine is rapidly and completely absorbed through the gastrointestinal tract, with bioavailability close to 100% (Kornhuber 2007). The reported elimination half‐life of memantine is 60 to 80 hours, and the time to reach maximum plasma concentration (Tmax) is about three to eight hours (Kavirajan 2009; Kornhuber 2007). It follows a linear pharmacokinetic pattern at a single dose of up to 40 mg (or a twice daily dose of 20 mg), which implies that the half‐life will remain constant, no matter how high the concentration (Kavirajan 2009). Memantine undergoes minimal hepatic metabolism and is not strongly bound to plasma proteins, hence its minimal drug‐to‐drug interaction through these mechanisms (Kavirajan 2009; Kornhuber 2007). Over 80% of memantine undergoes renal excretion. People with significant renal impairment are recommended to have the dose of memantine limited to 5 mg twice daily (Kornhuber 2007). All data are from studies in adults, and the pharmacokinetic and pharmacodynamic properties of memantine may vary in children and adolescents.
How the intervention might work
Glutamate and the glutamatergic pathway
Glutamate, an amino acid, is the main excitatory neurotransmitter in the nervous system. It is released from vesicles in pre‐synaptic neural cells in response to transmission of a neuronal impulse, from where it goes into the extracellular space, and then into postsynaptic neuronal cells. Glutamate binds to and activates glutamate receptors in the postsynaptic cell, namely NMDA and α‐amino‐3‐hydroxy‐5‐methyl‐4‐isoxazolepropionic acid (AMPA) receptors. Glutamate is found in many parts of the brain, such as the hippocampus and parts of the cerebral cortex (Rojas 2014), and it is involved in learning and memory functions in the brain through synaptic plasticity (the modification of synaptic activity over time). Specifically, it enhances persistent synaptic activity through long‐lasting increases in neuronal transmission, known as long‐term potentiation (LTP). These actions contribute to the neuroexcitatory effects of glutamate.
Glutamate transporters are responsible for the removal of excess glutamate from the extracellular space, as increased glutamate release or reduced uptake results in accumulation of excess glutamate outside synaptic cells. The resulting accumulation of glutamate within the extracellular space causes an influx of calcium ions into the synaptic receptor cells through NMDA receptors, which leads to over activation of the NMDA receptors, neurotoxicity and eventually neuronal cell death. This process has been implicated in some conditions, including autism and Alzheimer’s disease (Rojas 2014; Uzunova 2014).
Memantine acts as a non‐competitive antagonist of glutamatergic NMDA‐type receptors, and it works by modulating NMDA receptor activity, thereby mediating the potential neurotoxic effects of glutamate. This property of memantine has been employed in the treatment of Alzheimer’s disease and has been used in trials for autistic people (Aman 2017a; Hardan 2019; Rossignol 2014; Wei 2012). Some studies have described memantine as being effective in decreasing core autism symptoms, such as social withdrawal, as well as non‐core behaviours such as irritability, hyperactivity and inattention (Chez 2007; Erickson 2007; Ghaleiha 2013; Hardan 2019; Niederhofer 2007; Owley 2006).
Memantine has other mechanisms of action besides non‐competitive NMDA receptor blockage (Sani 2012), including non‐competitive, voltage‐dependent antagonism of type 3 serotonin 5‐HT(3) receptors (Rammes 2001; Reiser 1988). 5‐HT(3) receptors are ligand‐gated ion channels that help to mediate neuronal depolarisation and excitation, including release of neurotransmitters such as glutamate. They can be found in the gut and throughout the central and peripheral nervous systems (Nichols 2008). Serotonin, the neurotransmitter that binds to 5‐HT(3) receptors, is involved in some neurocognitive functions such as memory, learning and mood. The exact mechanism of action is still unclear, but it is thought that inhibition of 5‐HT(3) receptors by memantine might improve cognition and learning and reduce anxiety, either by increasing circulating serotonin levels or by preventing receptor activation and glutamate release (Rammes 2001; Reiser 1988). Memantine also acts as a non‐competitive antagonist of nicotinic acetylcholine receptors (nACHRs) in the hippocampus and has been implicated in learning and cognitive functioning (Becker 2013). Another mechanism of action of memantine is as an agonist of dopaminergic D2 receptors, which helps to enhance neurocognitive functioning (Sani 2012; Seeman 2008). Further research is required to establish the exact mechanisms of memantine in the treatment of core and associated behaviours seen in autism.
The time needed until a therapeutic effect occurs is still uncertain for dementia, with expected improvement or stabilisation of decline over days to weeks. Memantine may be stopped if there is no reported or observed benefit after six months in some countries. Duration of treatment effectiveness in dementia is also uncertain. The time to onset of effect and the required duration of treatment have yet to be established for other conditions. One three‐stage trial of memantine in autistic children reported positive effects within nine weeks of commencing treatment (Hardan 2019). In the second stage of the study, there was a reduction in the effectiveness for 65% to 70% of children who had responded to memantine, regardless of whether they stopped or continued memantine for another 12 weeks. In stage three of the study, only 81/747 planned participants completed the 48‐week, open‐label safety trial, so little is known about the duration needed to maintain effectiveness.
Why it is important to do this review
The lifelong symptoms of autism often mean that many individuals with the condition undergo various combinations of interventions and therapies, several of which have limited evidence of effectiveness in treating the primary symptoms of autism (Aye 2021; Cheuk 2011; James 2011; James 2015; Millward 2008; Nye 2005; Sinha 2011; Williams 2012; Williams 2013; Xiong 2016). These interventions often incur a financial cost to the individual, their families and sometimes their communities. Furthermore, it is important to consider whether an individual's most significant challenges are related to their autism or the co‐occurring conditions. Most medications have side effects that must be taken into account when they are prescribed. This review will contribute to informed decision‐making for therapy and provide data for policy and guideline development for autistic people.
In this review, we described the response to memantine treatment by assessing reported core outcome measures such as: difficulties with social interaction and communication; restrictive, repetitive and rigid behaviours; and secondary co‐occurring conditions such as hyperactivity and irritability. We also assessed important functional outcomes such as improvement in language ability, adaptive behaviour, quality of life, and general health and functioning, as these outcomes are of particular importance to autistic people and their families. Finally, we assessed whether memantine treatment has any relevant side effects in autistic people.
Objectives
To assess the effects of memantine on the core symptoms of autism, including, but not limited to, social communication and stereotypical behaviours.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs). Studies were not required to use blinding (of participants, assessors or research staff). We planned to include quasi‐RCTs (where participants are allocated to study arms using a method of allocation that is not truly random) and cross‐over RCTs (where participants are randomly allocated to study arms consisting of a sequence of two or more treatments given consecutively), but did not find any that met the inclusion criteria.
Types of participants
Eligible studies enrolled children or adults of any gender who had a diagnosis of ASD and who met the diagnostic criteria for any of the following classifications of ASD: Diagnostic and Statistical Manual of Mental Disorders, 3rd edition (DSM‐III; APA 1980), Diagnostic and Statistical Manual of Mental Disorders, 3rd edition, revised (DSM‐III‐R; APA 1987), DSM‐IV (APA 1994), DSM‐IV‐TR (APA 2000), DSM‐5 (APA 2013), ICD‐10 (WHO 1992) or ICD‐11 (WHO 2018). Diagnoses may have been made with or without supporting standardised clinical instruments, including the Childhood Autism Rating Scale (CARS; Schopler 1986), Autism Diagnostic Observation Schedule (ADOS; Lord 2012), Autism Diagnostic Interview‐Revised (ADI‐R; Lord 1994), and Diagnostic Interview for Social and Communication Disorders (DISCO; Wing 2002). Children and adults with and without additional medical or developmental diagnoses were eligible, although we excluded children with a diagnosis of childhood disintegrative disorder (CDD) or Rett syndrome. This is because Rett syndrome and CDD are no longer included under DSM‐5 criteria for ASD, and the autism symptoms seen in these two conditions are only present for a narrow window of time in the life of a child.
Types of interventions
Memantine given at any dose, frequency and duration, and administered in any setting, compared with placebo or no treatment. We also included trials that gave memantine as an adjunct to behavioural interventions if the behavioural interventions were the same in both arms.
We would have included studies in which participants took memantine in addition to other pharmacological agents, provided that all participants recruited to the trial were already receiving the same or comparable medicines, and continued to receive them throughout the trial.
Types of outcome measures
Eligible studies measured outcomes using: direct, clinician‐administered tools; parent or teacher questionnaires and rating scales; and behavioural observation. We included structured and non‐structured assessments. We considered quantitative and qualitative data from all measures. Where studies presented two or more measures for one outcome, we prioritised standardised measures (e.g. published, norm‐referenced assessment tools) over non‐standardised measures (e.g. rating scales developed by the study authors). We analysed observed, parent‐reported, teacher‐reported and self‐reported data separately. If parent, teacher and self‐ratings were completed for the same outcome, we prioritised self‐ratings. If parent‐ or caregiver‐reported and teacher‐reported outcomes were available, we prioritised those of parents or caregivers, given that they have the opportunity to observe the autistic person in multiple settings and for a greater amount of time.
Primary outcomes
-
Core symptoms of autism.
Social communication problems, measured by diagnostic instruments such as the Childhood Autism Rating Scale (CARS; Schopler 1986), Autism Diagnostic Observation Schedule (ADOS; Lord 2012), Autism Diagnostic Interview‐Revised (ADI‐R; Lord 1994) or Diagnostic Instrument for Social Communication Disorders (DISCO; Wing 2002)
Repetitive and rigid behaviour, including stereotypy, measured by diagnostic instruments such as the CARS (Schopler 1986), ADOS (Lord 2012), ADI‐R (Lord 1994) and DISCO (Wing 2002).
-
Adverse effects, including the number of dropouts due to adverse side effects, and the risk or presence of side effects directly attributable to the use of memantine, such as:
gastrointestinal adverse effects (e.g. constipation, nausea);
neurological adverse effects (e.g. headache, irritability, somnolence); and
cardiovascular adverse effects (e.g. hypertension).
Secondary outcomes
Language in autistic people. Examples of measuring instruments include the Peabody Picture Vocabulary Test, 5th edition (PPVT‐5; Dunn 2018) or the Expressive Vocabulary Test, 3rd edition (EVT‐3; Williams 2018).
Cognition in autistic people, including specific neurocognitive skills of attention and memory, measured on a neurocognitive or intelligence quotient (IQ) scale such as the Wechsler Intelligence Scale for Children (WISC; Wechsler 2014), or NEPSY (a developmental NEuroPSYchological assessment; Korkman 2007).
Adaptive behaviour for autistic people, measured on scales such as the Vineland Adaptive Behavior Scales (VABS; Sparrow 2016).
Non‐core behaviours associated with autism, including hyperactivity, irritability and aggression. Examples of measuring instruments include: the Yale‐Brown Obsessive Compulsive Scale (Y‐BOCS; McKay 2003), which is used to measure obsessive compulsive behaviours; the Connors' Abbreviated Parent‐Teacher Questionnaire (APTQ; Conners 1997), which is used to assess hyperactivity and aggression; and the Aberrant Behaviour Checklist (ABC; Aman 2017b), which is used to assess problem behaviour such as irritability and hyperactivity.
General health and functioning at home and school, measured by tools such as the Clinical Global Impression‐Improvement subscale (CGI‐I; Guy 1976).
Quality of life for autistic people and their carers, measured by scales such as the Family Quality of Life Scale (FQOLS; Beach Center on Disabilities 2006).
Search methods for identification of studies
The Cochrane Information Specialist for Developmental, Psychosocial and Learning Problems ran searches in November 2020. We ran top‐up searches for all available years in February 2022, and removed duplicates of records retrieved in 2020.
Electronic searches
We searched the following databases and trials registers.
Cochrane Central Register of Controlled Trials (CENTRAL; 2022, Issue 2), part of the Cochrane Library, which includes the Cochrane Developmental, Psychosocial and Learning Problems Group specialised register (searched 14 February 2022)
MEDLINE Ovid (1946 to February week 1 2022)
MEDLINE In‐Process & Other Non‐indexed Citations Ovid (1946 to 11 February 2022)
MEDLINE Epub Ahead of Print Ovid (11 February 2022)
Embase Ovid (1974 to 11 February 2022)
APA PsycINFO Ovid (1806 to February week 1 2022)
CINAHL Plus EBSCOhost (Cumulative Index to Nursing and Allied Health Literature; 1937 to 14 February 2022)
ERIC EBSCOhost (Education Resources Information Center; 1966 to 14 February 2022)
Web of Science Core Collection Clarivate (Science Citation Index (SCI); Social Sciences Citation Index (SSCI); Conference Proceedings Citation Index‐Science (CPCI‐S); Conference Proceedings Citation Index‐Social Science & Humanities (CPCI‐SSH); 1970 to 15 February 2022)
SciELO Citation Index Web of Science (2002 to 5 February 2022)
LILACS (Latin American Caribbean Health Sciences Literature; search.bvsalud.org/portal/advanced/?lang=en; searched 15 February 2022)
SciELO Citation Index Web of Science (Searched 5 February 2022)
TOXLINE subset in PubMed (toxnet.nlm.nih.gov/newtoxnet/toxline.htm; searched 25 November 2020). Unavailable for later searches
PubMed using PubMed Toxicology filter (pubmed.ncbi.nlm.nih.gov/;searched 15 February 2022). Replaced TOXLINE subset
ProQuest Dissertations & Theses Global (1743 to 15 February 2022)
Cochrane Database of Systematic Reviews (CDSR; 2020 Issue 12), part of the Cochrane Library (searched 25 November 2020)
Epistemonikos (limited to systematic reviews; www.epistemonikos.org; searched 15 February 2022)
ClinicalTrials.gov (clinicaltrials.gov; searched 15 February 2022)
WHO International Clinical Trials Register Platform (WHO ICTRP ; trialsearch.who.int/; searched 15 February 2022)
EU Clinical Trials Register (www.clinicaltrialsregister.eu; searched 15 February 2022)
The search strategies for each source are reported in Appendix 1.
We did not limit the searches by publication date, language, or publication status.
Searching other resources
We contacted the first author of each included study and known experts in the field of developmental paediatrics and child psychiatry to ask if they could provide details of any additional relevant studies not already identified by the electronic searches. We also searched the reference lists of relevant studies and reviews for RCTs that met our inclusion criteria. On 4 October 2021, the Cochrane Information Specialist for Developmental Psychosocial and Learning Problems searched for retractions of the two included studies in MEDLINE, Embase and the Retraction Watch Database retractiondatabase.org/RetractionSearch.aspx?. No retractions or corrections were found.
Data collection and analysis
We report on the methods used in this review below. We were unable to use all pre‐planned methods for our review. Refer to the review protocol (Brignell 2021) and Appendix 2 for pre‐planned but unused methods.
Selection of studies
Two review authors (AB, TM) independently assessed the eligibility of the titles and abstracts identified through the searches. We then retrieved and examined full text reports for those studies deemed potentially relevant or for which more information was needed to determine relevance. We grouped multiple publications of the same study, then excluded studies that did not meet inclusion criteria. We attempted to resolve any disagreements about eligibility by discussion between the two review authors. If necessary, a third review author (KW) acted as arbiter. We calculated the Kappa statistic, which is a measure of the overall reported agreement between authors determining the eligibility of studies for inclusion, as described in Chapter 5 of the Cochrane Handbook for Systematic Reviews of Interventions (Lefebvre 2021; Li 2021). Figure 1 presents the results of our selection process in a PRISMA diagram (Moher 2009). Covidence (Covidence 2020) was used for screening the reports. We contacted authors of included, excluded, ongoing and 'awaiting classification' studies where further data were required, or further clarification was needed, around the methods or eligibility for inclusion, or both.
1.
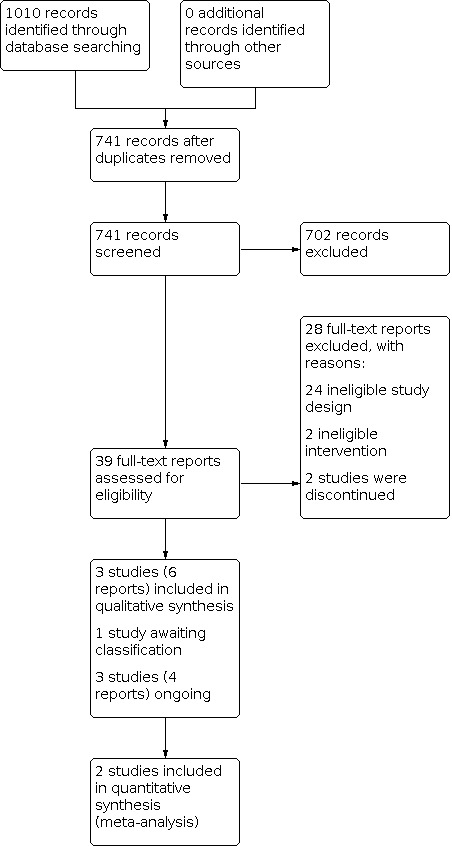
Study flow diagram.
We have listed any excluded studies that seemed relevant, together with the reasons for exclusion, in the Characteristics of excluded studies table.
Data extraction and management
We used Covidence 2020 and Excel for data organisation and management. Two review authors (AB and TM) independently extracted data from each included study. Data were extracted on the following.
Study methods and setting: study type (type of RCT), study site, country of publication, language of publication, publication type and study duration
Participant details: age, gender, diagnosis and diagnosis tool
Intervention details: intervention type, including dosage, mode of delivery, frequency and duration; placebo type, including dosage, mode of delivery, frequency and duration
Outcomes: all primary and secondary outcomes
We also extracted data for assessing risk of bias. In case of disagreement, a third review author (KW) checked the extracted data and acted as arbiter. One review author (AB) entered the extracted data into Review Manager 5 (RevMan 5; Review Manager 2020), and a second author (TM) checked the data entry. See Appendix 3 and Appendix 4 for further details on data extracted and risk of bias criteria.
Assessment of risk of bias in included studies
We used a data extraction form to collect information when assessing risk of bias. This form included the criteria described in the Cochrane risk of bias tool for randomised controlled trials (RoB 1), in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We piloted the data collection form and modified it as required. Two review authors (AB and TM) independently assessed the risk of bias of the included studies and, in case of any disagreements, a third review author (KW) acted as arbiter. The potential sources of bias that we assessed were:
sequence generation;
allocation concealment;
blinding of participants and personnel, and blinding of outcome assessment;
incomplete outcome data;
selective reporting; and
other sources of bias.
The risk of bias for each study was rated as high, low or unclear, and presented in a table, with justifications to support the decisions. We judged each study's overall validity and rated studies at high risk of bias overall if one or more domain(s) was at high risk of bias; low risk of bias overall if all five domains were at low risk of bias; and unclear risk of bias overall if the five domains were at varying risks of unclear and low risks of bias. These judgements were then used to guide overall assessments of certainty of the evidence (GRADE ratings); see Data synthesis. For example, if the body of evidence for a particular outcome came from one or more studies at high risk of bias, the overall certainty of the evidence was downgraded. If most evidence came from studies that met criteria for low risk of bias, the certainty of the evidence was not downgraded for risk of bias concerns.
Measures of treatment effect
We entered data into RevMan 5 (Review Manager 2020). We recorded data on effect size for each individual study.
Dichotomous data
We analysed dichotomous outcomes by calculating the odds ratio (OR), and corresponding 95% confidence intervals (CIs), as the OR had the most adequate properties for the outcome assessments. We calculated the OR using RevMan 5 (Review Manager 2020), as described in Chapter 10 of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2021). If the included studies presented other forms of effect measures (e.g. standardised mean difference (SMD)), we used the available information to compute the OR using the formulae given in Chapter 10 of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2021). If we were unable to enter outcome data in a 2 × 2 table, we contacted the study authors for this information.
Continuous Data
If studies used the same scales to measure continuous outcomes (for example, scores or standardised measures of improved behaviour), we estimated the effect size by computing the pooled mean difference (MD) and 95% CI using the means and standard deviations (SDs) reported in the studies. If the studies used different scales but the outcomes they measured were conceptually similar, we calculated the SMD, as recommended in Chapter 10 of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2021). We used Cohen's standards for interpreting effect sizes (small = 0.2, medium = 0.5, large = 0.8; Cohen 1988).
We focused on final values unless change scores were used in the studies. We combined studies that reported final values and studies that reported only change scores in the same meta‐analysis, provided the studies used the same rating scale. One potential problem of including change scores is that the standard deviation of the changes may not be reported in the included study (Higgins 2021a). If data were not reported or could not be extracted, we contacted the trial authors. If we were unable to contact the authors, or they did not provide the required data, we attempted to estimate the standard deviation of changes as recommended in Chapter 6.5.2.8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021b).
Multiple outcomes
If different studies provided multiple, interchangeable measures of the same construct at the same point in time, we calculated the average SMD across the outcomes and the average estimated variances, as recommended in Chapter 6 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021b). All eligible outcomes or measures were documented in the Characteristics of included studies table, where we specified which outcome measure was selected and why. If a study reported data for a particular outcome using two or more assessment instruments (e.g. a parent‐completed a questionnaire and a direct assessment of behaviour by a clinician), we chose the one that had been used most frequently by the pooled included studies for effect size calculation. This was to minimise the heterogeneity of study outcomes. We used standardised tests (as opposed to non‐standardised tests) unless non‐standardised tests were used more frequently, and data could be pooled.
Unit of analysis issues
We assessed all included trials to determine the unit of randomisation and whether this unit of randomisation was consistent with the unit of analysis. We did not use individual patient data (IPD), as we did not consider that an IPD review was warranted until a non‐IPD systematic review was completed, and more was known about whether IPD would add value to findings for this particular review. We used available published or aggregate data as our units of analysis, as described in Measures of treatment effect.
Cluster‐RCTs
Given the nature of the intervention, we did not identify any cluster‐RCTs to include in our review.
Cross‐over trials
We did not identify any cross‐over trials.
Trials with repeated measurements
Given the uncertainty about timing of onset of improvements and optimal duration of the intervention, we selected the latest available time point as most meaningful in order to enhance the applicability to clinical care settings, where people may receive medications for sustained periods of time. In studies with repeated measurements over different time points, we prioritised analysis of the most meaningful time points (e.g. outcome measures at the first point where memantine is expected to show clinical improvement or side effects). If this was not possible, then we used the most frequently reported time points (Higgins 2021a). We conducted separate analyses for data from different points of measurement (i.e. immediately post‐treatment, follow‐up data between 0 and three months, three and 12 months, and more than 12 months). If there were multiple measurements within the same interval (e.g. six to 12 months), we used the latest time point.
Trials with multiple treatment arms
We did not identify any studies with multiple treatment arms.
Dealing with missing data
We assessed missing data and dropouts for each included study and contacted study authors to obtain missing data, where relevant. We stated our assumptions regarding whether the data were 'not missing at random' (e.g. due to unfavourable outcomes or non‐adherence to treatment) or 'missing at random', and we analysed only the available data, as recommended in Chapter 10 of the Cochrane Handbook for Systematic Reviews of Intervention (Deeks 2021). We accounted for any potential bias due to missing data in the risk of bias ratings (see Risk of bias in included studies). We discussed the potential impact of the method for analysis of missing data on the interpretation of our results in the Discussion section of this review.
For missing summary data (such as the standard deviation or mean of the outcomes), we derived calculated values, as recommended in Chapter 10 of the Cochrane Handbook for Systematic Reviews of Intervention (Deeks 2021).
Assessment of heterogeneity
We assessed clinical heterogeneity by comparing the populations included in the studies (e.g. children versus adults), the settings, the treatment modalities (e.g. types of dosing regimens), and the outcomes. Additionally, we assessed methodological heterogeneity within our included studies by examining key trial characteristics (e.g. risk of bias in allocation concealment, blinding, outcome measurements, losses to follow‐up). We reported any significant sources of heterogeneity in the risk of bias tables and discussed it in the review.
We assessed statistical heterogeneity by performing a Chi2 test, with statistical significance set at P value less than 0.10, and calculating the I2 value: a quantity that describes the approximate proportion of variation in point estimates that can be attributed to heterogeneity rather than sampling error as follows.
0% to 40%: might not be important.
30% to 60%: may represent moderate heterogeneity.
50% to 90%: may represent substantial heterogeneity.
75% to 100%: considerable heterogeneity (Higgins 2021a).
To estimate the between‐study variance, we used the Tau2 in a random‐effects meta‐analysis (Deeks 2021; DerSimonian 1986), which we performed using RevMan 5 (Review Manager 2020). We incorporated the refinements to the standard approach for meta‐analysis recommended by DerSimonian 2015, using a robust variance estimator for testing the overall effect.
Assessment of reporting biases
If we had found 10 or more studies that met our inclusion criteria (Criteria for considering studies for this review), we would have created a funnel plot to explore the relationship between the intervention effect estimate and standard error of the intervention effect estimate. If there was asymmetry, we would have used Begg's test (Begg 1994), and Egger's test (Egger 1997), to explore the reasons for asymmetry, such as publication bias or low methodological quality. However, as we only identified two studies for each primary outcome, funnel plots were not indicated.
Data synthesis
See Table 1.
We extracted both change‐from‐baseline and final values, provided the data were available. We calculated the SMD in one study (Aman 2017a), by transforming the data using a random‐effects model in RevMan 5 (Review Manager 2020). The other study, Karahmadi 2018, provided baseline and follow‐up scores, so we calculated the MD based on change scores and then converted this to a SMD. Odds ratios were calculated using RevMan 5 (Review Manager 2020). We synthesised the results in meta‐analyses using a random‐effects model (Deeks 2021; DerSimonian 1986), and displayed the results in a forest plot. Where it was not possible to conduct a meta‐analysis for an outcome (e.g. data were too heterogeneous, there was high statistical heterogeneity or there were too few studies), we provided a narrative description of the results.
Our primary analysis included all eligible studies, but we did not conduct sensitivity analysis (by excluding studies at unclear or high risk of bias for lack of blinding) to test the robustness of the results to decisions made throughout the review process because of the small number of included studies (see Sensitivity analysis). As there were only two studies in each meta‐analysis, statistical heterogeneity could not be reliably estimated and investigated (see Assessment of heterogeneity).
Subgroup analysis and investigation of heterogeneity
We were unable to conduct subgroup analyses relating to the appropriateness of the measure for intellectual disability because only two studies presented mean intellectual quotient scores. Additionally, we were unable to conduct any of our other preplanned subgroup analyses due to an insufficient number of studies (see Appendix 2).
Sensitivity analysis
We were unable to conduct any of our preplanned sensitivity analyses (see Appendix 2).
Summary of findings and assessment of the certainty of the evidence
We exported data from RevMan 5 (Review Manager 2020) to GRADEpro 2020 to create our summary of findings table for the comparisons between memantine versus placebo. We included all primary outcomes (core symptoms of autism; adverse effects) and all available secondary outcomes (language, memory, adaptive behaviour, non‐core behaviours associated with autism (i.e. hyperactivity and irritability)) in the summary of findings table. Data for quality of life and general health and functioning at home and school were not available. We selected measures that were most relevant to the main construct for each outcome. We collected data for immediate, short‐term and long‐term time points, but included only the immediate time point in the table.
Using the GRADE system (Schünemann 2021), two review authors (AB, TM) independently assessed the certainty of the evidence for all primary and secondary outcomes (Types of outcome measures). The GRADE system takes into account:
directness, which assesses how well included studies address the review question;
risk of bias, which assesses the overall risk of bias of included studies that provided data for each outcome;
precision, which assesses the statistical precision of the results;
consistency, which assesses how well unexplained heterogeneity has been accounted for in the study results; and
publication bias, which assesses transparency of publication and risk of publication bias among the studies that contribute to the outcome (Schünemann 2021).
We rated the certainty of the evidence as high, moderate, low or very low. We treated evidence from RCTs as high‐certainty evidence initially, and downgraded the ratings up to a maximum of three levels depending on the presence of the aforementioned criteria (Schünemann 2021). We have indicated our reasons for downgrading the certainty of the evidence for each outcome in the footnotes to the summary of findings table (Schünemann 2021). We resolved any disagreements by discussion or in consultation with a third author (KW).
Results
Description of studies
Results of the search
The search yielded 1010 records, of which 741 remained after deduplication. Screening by title and abstract deemed 702 records to be irrelevant and these were subsequently excluded. Of the 39 remaining records, 28 were excluded in the full text review (Excluded studies). In total, three primary studies (six reports) met the inclusion criteria and were included in narrative synthesis and meta‐analysis (Aman 2017a; Karahmadi 2018; Soorya 2021). One study was awaiting classification (Martsenkovsky 2016), and three studies (four reports) were ongoing (EUCTR 2014‐003080‐38‐DE; NCT01972074; NCT03553875). See Figure 1 for the PRISMA diagram. There was 100% agreement between authors (AB, TM) for included and excluded studies, so we did not calculate a Kappa statistic.
Included studies
Three studies (six reports) met the inclusion criteria for this review and had data available for extraction (Aman 2017a; Karahmadi 2018; Soorya 2021). See the Characteristics of included studies table for further information on each study. One review author (AB) attempted to contact the corresponding study authors for relevant missing data (such as participant age, intellectual ability, memantine dose and regimen, tools used to measure change in outcomes, or reasons for dropout). We had correspondence with the authors from one study (Aman 2017a), where we requested data for missing participants; however, the authors were unable to obtain this information due to the age of the study. We clarified some details for risk of bias assessments for another study (Soorya 2021). Two studies had missing summary data (Aman 2017a; Soorya 2021), and both provided data based on intention‐to‐treat (ITT) analyses. We used the ITT data presented by these studies in the current review. Karahmadi 2018 had no missing data. Given the small number of included studies, we were unable to conduct sensitivity analyses to assess the impact of studies with high risk of bias. Regardless, all studies were rated as high risk of bias overall (Figure 2), so there was no differentiation in the ratings.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Study design
Aman 2017a was a double‐blind RCT (blind to personnel and participants), where participants received either memantine or placebo, delivered in the same manner. At the conclusion of the RCT, participants were invited to a 48‐week, open‐label extension, where all participants (50 in control group and 52 in treatment group) were given memantine (single daily dose that varied according to weight) and were followed up at 12, 24, 36, 48 and 60 weeks after RCT completion. Karahmadi 2018 was a single‐blind RCT, where participants were blinded, but personnel were not. Participants were given memantine or placebo as an adjunct to behavioural therapy (based on applied behavioural analysis; ABA). Soorya 2021 was a double‐blind placebo‐controlled RCT (blind to participants and investigators), where participants received either memantine or placebo delivered in the same manner. All included studies adopted a parallel design.
Location and setting
Two studies were conducted in the USA (Aman 2017a; Soorya 2021), and the other in Iran (Karahmadi 2018).
Aman 2017a and Soorya 2021 recruited participants across two sites, and Karahmadi 2018 recruited children via a child psychiatric clinic of Noor and Hazrat‐e‐Ali Asghar hospital in Isfahan (convenience sampling).
Participants
The review included 204 participants: 23 in Soorya 2021, 60 in Karahmadi 2018, and 121 in Aman 2017a.
Age
The mean age of participants was 9.40 years. The study by Aman 2017a included 61 participants in the control group with a mean age of 9.0 years (SD 2.2 years), and 60 participants in the treatment group with a mean age of 8.9 years (SD 2.2 years). Karahmadi 2018 included 30 participants in the control group with a mean age of 9.5 years (SD 3.86 years), and 30 participants in the treatment group with a mean age of 10.07 years (SD 3.48 years). Soorya 2021 included 12 participants in the control group with a mean age of 9.64 years (SD 2.29 years), and 11 participants in the treatment group with a mean age of 9.25 years (SD 1.76 years).
Gender
The majority of participants in the three studies were male (range 73% to 87%). In the study by Aman 2017a, 52/60 (87%) participants in the treatment group were male and 49/61 (80%) in the control group were male. In the Karahmadi 2018 study, 24/30 (73%) participants in the treatment group were male and 24/30 (80%) in the control group were male. In the third study, 20/23 (87%) participants were male (Soorya 2021).
Diagnosis
Aman 2017a included children and adolescents with a diagnosis of autistic disorder, and Soorya 2021 included children who had a primary DSM‐IV diagnosis of pervasive developmental disorder (PDD; i.e. autistic disorder, Asperger’s disorder or PDD‐NOS) or ASD as defined by DSM‐5. Karahmadi 2018 included children who were described by the authors as having ASD. The diagnosis was completed either through a clinical evaluation using the DSM‐IV‐TR and information from the ADI‐R and ADOS (Aman 2017a), use of the ADOS, ADI‐R or Autism Diagnostic Interview Screener (ADI‐S; Soorya 2021), or the Gilliam Autism Rating Scale (GARS) completed by a psychiatrist, based on DSM‐IV (Karahmadi 2018).
For ASD severity, the mean score on the Social Responsiveness Scale (SRS) was 100.75 (SD 23.3) in one study (Aman 2017a), where a score of 76 or higher suggests a clinical diagnosis of ASD. In Karahmadi 2018, participants had mean scores of 95.20 (SD 14.49) in the memantine group and 91.50 (SD 14.35) in the placebo group on the GARS at baseline. A diagnostic cutoff score of 90 or higher indicates that the child probably has autism and will be classified as ASD.
Participants in the Soorya 2021 study had mean scores on the Autism Diagnostic Observation Schedule‐Generic (ADOS‐G; social communication total score) of 12.78 (SD 3.67) in the memantine group and 15.73 (SD 4.00) in the control group. A social communication total score on the ADOS‐G of 12 or higher is indicative of autism.
Intelligence
In Aman 2017a, mean IQ was 76.8 (SD 21.3), which is in the low range (IQ of 70 or under is indicative of an intellectual disability). The average IQ score on the Kaufman‐Brief Intelligence Test (KBIT) ranges from 90 to 110. In Soorya 2021, the mean full scale IQ was 80.17 (SD 23.29) in the memantine group and 75.00 (SD 21.58) in the control group at baseline. Karahmadi 2018 did not provide data on IQ.
Language
Verbal ability was not reported in detail in Aman 2017a; however, the mean verbal score on the KBIT, 2nd edition (KBIT‐2) was 36.7 (SD 17.8), indicating most children had language difficulties. In Soorya 2021, mean standard scores on the Expressive Vocabulary Test (EVT) at baseline were 91 (SD 24.33) in the placebo group and 95.67 (SD 30.2) in the memantine group; the average score on the EVT ranges from 85 to 116. Karahmadi 2018 did not provide data on the language abilities of the participants.
Adaptive behaviour
Soorya 2021 provided adaptive behaviour scores at baseline. The mean standard score in adaptive behaviour was 74.00 (SD 8.41) in the memantine group and 74.18 (SD 19.65) in the placebo group. Aman 2017a and Karahmadi 2018 did not provide data on adaptive behaviour.
Interventions
In Aman 2017a, participants received a single daily dose of memantine (in the form of either 3 mg or 6 mg capsules), which was dependent on weight. There were four dosages based on weight: Group A (over 60 kg), maximum dose of 15 mg/day; Group B (40 kg to 59 kg), maximum of 9 mg/day; Group C (20 kg to 39 kg), maximum of 6 mg/day; and Group D (under 20 kg), maximum of 3 mg/day. The average dose for each group was: 14.4 mg/day in Group A; 8.3 mg/day in Group B; 5.9 mg/day in Group C; and 3 mg/day in Group D. Capsules were taken orally, preferably at the same time each day. Participants were not permitted to take concomitant medications. The trial duration was 12 weeks. In the 48‐week, open‐label extension (on completion of the RCT), participants were given a single daily dose, which varied according to weight.
All participants (control and treatment) in Karahmadi 2018 received an ABA intervention. In addition, the treatment group was given 2.5 mg of memantine in the morning and 2.5 mg of memantine at night (i.e. 5 mg/day in total) in oral tablet form. Participants in both groups were permitted to continue on previously used, common medications. The trial duration was three months (around 12 weeks).
In Soorya 2021, participants received 3 mg of memantine, which was titrated up by 3 mg a week to a maximum dose that was dependent on the participant's weight and tolerability. Dose adjustments were made at biweekly study visits as needed. Maximum doses were: 6 mg (20 kg to 40 kg), 9 mg (40 kg to 60 kg), and 12 mg (over 60 kg). The trial duration was 24 weeks.
Comparators
In Aman 2017a, the control group took a placebo capsule orally, once per day, in accordance with the US Food and Drug Administration.
In Karahmadi 2018, the control group received a placebo oral tablet, twice daily, which had the same appearance, odour and colour as the memantine tablet.
In Soorya 2021, the placebo mimicked the titration of memantine with increments (i.e. dose was initiated at 3 mg then titrated up by 3 mg a week to a maximum of 6 mg, 9 mg, or 12 mg). The study authors do not describe the form in which the placebo was administered (e.g. capsule), its appearance or contents, or how many times the dose was administered per day.
Outcomes
Primary outcomes
Core symptoms of autism
Two studies had core symptoms of autism as the primary outcome (Aman 2017a; Karahmadi 2018); however, each used a different tool to measure these outcomes.
For Aman 2017a, the primary outcome measure used to assess the efficacy of memantine was SRS total score. The SRS is a norm‐referenced caregiver questionnaire designed to measure autism symptoms; higher scores indicate more severe symptoms of autism. It consists of five subscales: social awareness, social cognition, social communication, social motivation, and restricted interests and repetitive behaviour. A total score can also be calculated. It was administered at week 12.
In Karahmadi 2018, the primary outcome measure was the GARS. This is a tool used to rate symptoms of autism (stereotyped behaviour, communication, social interaction); higher scores indicate more severe symptoms of autism. Both the total score and subscale scores on the GARS were reported. The GARS were administered at baseline and on completion of the trial (three months later).
Aman 2017a provided data for core autism symptoms, associated maladaptive behaviours and daily functioning on three additional scales. As all tools measure similar and overlapping domains, and we had already included data on core autism symptoms from these same participants, we did not include these data in the meta‐analysis, to avoid multiplicity of data. Instead, we prioritised the SRS tool. We describe these additional measures below and present the results in the narrative synthesis.
Core Autism Treatment Scale (CATS; severity subscale administered at baseline and the improvement subscale administered at follow‐up visits, including week 12). This is a proprietary 14‐item scale that was developed by the study authors and is not yet validated. It focuses on social interaction and communication.
Clinical Global Impression (CGI) scale (Severity subscale administered at baseline and Improvement subscale administered at follow‐up visits, including week 12). The CGI measures social interaction, communication, integrated social interaction and communication, stereotyped behaviour, restricted interests, associated maladaptive behaviours, and daily function.
Core Associated Autism Symptom Treatment Scale (CAASTS; severity subscale administered at baseline and the improvement subscale administered at follow‐up visits, including week 12). This is a 23‐item proprietary tool that was developed by the study authors and is not yet validated. It assesses stereotyped behaviour, restricted interests associated maladaptive behaviours, and daily function.
Adverse effects
Two studies collected and reported data on adverse effects (Aman 2017a; Soorya 2021). Treatment‐emergent adverse effects (TEAEs) were collected in Aman 2017a and were reported when the event had an incidence of 3% or greater in either the placebo or the memantine group. The study did not specify how adverse effects were assessed. Soorya 2021 collected data on adverse effects using the Safety Monitoring Uniform Report Form (SMURF). Routine blood haematology, blood chemistry, liver function and urinalyses were assessed at baseline, week 12 and week 24; and measures of height, weight, blood pressure, pulse, and body mass index were assessed at each in‐person visit. Karahmadi 2018 did not report on adverse effects.
Secondary outcomes
Two trials collected data on secondary outcomes (Aman 2017a; Soorya 2021). No secondary outcomes were collected in Karahmadi 2018. No study reported quality of life outcomes for children or their families (or both). All secondary outcome measures used for efficacy in Aman 2017a were collected at baseline and week 12. The secondary outcome measures in Soorya 2021 were collected at baseline, week 12 and week 24, but change was measured between baseline and 24 weeks. The following secondary outcomes were collected.
Language
Children's Communication Checklist, 2nd edition (CCC‐2). This is a 70‐item, validated, norm‐referenced caregiver questionnaire for children aged four to 16 years. The tool assesses communication skills and related areas. There are 10 subscales including speech, syntax, semantics, coherence, inappropriate initiation, scripted language, use of context, nonverbal communication, social relations, and interests. In addition to subscale standardised scores (mean 10, SD 3), the tool provides General Communication Composite and a Social Interaction Difference Index scores. This measure was used in one study (Aman 2017a).
Expressive Vocabulary Test, 2nd edition (EVT‐2). The EVT‐2 measures single word expressive vocabulary. Higher standard scores on the EVT‐2 indicate stronger abilities in naming vocabulary. This measure was used in one study (Soorya 2021).
Intelligence
The Wechsler Intelligence Scale for Children, 4th edition (WISC‐IV) and Wechsler Abbreviated Scale of Intelligence, 2nd edition (WASI‐II) are standardised assessments of intelligence. They assess multiple cognitive domains that impact performance and provide a composite standard score (full scale IQ). Higher scores indicate higher IQ. The WISC was used at baseline and the WASI at 24 weeks in one study (Soorya 2021).
Memory
NEPSY‐II Memory for Design (standard and delayed subtests) and Narrative Memory subtests (free and cued recall and recognition). These subtests assess specific aspects of neurocognition and were used in one study (Soorya 2021).
Adaptive functioning (life skills)
Vineland Adaptive Behaviour Scales, 2nd edition (VABS‐II). This is a standardised, semi‐structured parent interview that assesses multiple domains such as communication, daily living skills, and socialisation. It also provides an overall composite score. Higher scores indicate stronger adaptive behaviour. Soorya 2021 collected data using this measure.
Non‐core behaviours associated with autism (including hyperactivity, irritability)
Aberrant Behaviour Checklist‐Community (ABC‐C; range of scores 0 to 48). This informant behaviour rating questionnaire contains 58 items and 5 subscales. The subscales consist of irritability; lethargy or social withdrawal; hyperactivity or non‐compliance; stereotypy; and inappropriate speech. Normative data in the form of T‐scores and percentiles are provided. Two studies used this measure (Aman 2017a; Soorya 2021), but Soorya 2021 used it only in the exploratory analysis (i.e. no change data provided).
Soorya 2021 also collected several exploratory measures that met our outcome inclusion criteria, including the Aberrant Behavior Checklist, Behaviour Rating Inventory of Executive Function‐Parent Questionnaire, the Sensory Profile and the SRS. The authors did not present change data for these tools, providing descriptive statistics of the data only.
The key characteristics of each study can be found in Table 2 for comparison.
1. Characteristics of included studies.
| Aman 2017a | Karahmadi 2018 | Soorya 2021 | |
| Design | RCT (single blind placebo) | RCT (single blind placebo) | RCT (double blind placebo) |
| Unit of randomisation | Individual participant | Individual participant | Individual participant |
| Location/country | USA | Iran | USA |
| Sample size (% males) | 121 (83%) | 60 (77%) | 23 (87%) |
| Tool(s) used to diagnose ASD | ADI‐R, ADOS | GARS | ADOS, ADI‐R, or ADI‐S |
| Diagnostic criteria | DSM‐IV‐TR | DSM‐IV | DSM‐IV‐TR, DSM‐5 |
| Diagnosis | Autistic disorder | ASD | ASD (DSM‐5) or PDD (DSM‐IV; autistic disorder, Asperger's disorder or PDD‐NOS) |
| Mean IQ (SD) | 76.8 (21.3) | Not reported | 80.17 (23.29) memantine; 75 (21.58) placebo |
| Mean age in years (SD) | 9 (2.2) | 9.8 | 9.25 (1.76) memantine; 9.64 (2.29) placebo |
| Inclusion criteria | Children aged 6–12 years who met DSM‐IV‐TR criteria for autistic disorder based on clinical evaluation and information from ADOS and ADI‐R; able use at least 3‐word phrases; ABC‐Irritability subscale score < 17, SRS score > 44 (for girls) and > 53 (for boys), IQ ≥ 50 | Children < 14 years; ASD diagnosed by a psychiatrist using the GARS | Verbal (ADOS module 2 or 3), outpatients; aged 6–12 years; primary DSM‐IV diagnosis of PDD (i.e. autistic disorders, Asperger's disorder, PDD‐NOS) or ASD as defined by DSM; difficulty with motor skills (caregiver report during psychiatric intake interview); stable on all nonpharmacological treatments for 3 months before randomisation; stable on up to 2 concomitant psychotropic medications 30 days before randomization; CGI‐S score ≥ 4 (i.e. moderate); ABC‐irritability subscale score < 17 |
| Exclusion criteria | Premature birth (< 35 weeks); low birth weight (< 2.3 kg); history of neurological disease; use of specific medications (antianginal, antiarrhythmic, anticoagulant, antihypertensive, antineoplastic, diuretic, hypoglycaemic or hypolipidemic agents, insulin; muscle relaxants; systemic antifungal agents or steroids; hormone suppressants; or psychotropic drugs); primary diagnosis other than autism | Major paediatric disorders (e.g. ADHD, mood disorder, ankyloglossia), any disease that may be affected by memantine (e.g. kidney disease), sensitivity to memantine | Born < 35 weeks of gestation; on d‐cycloserine or riluzole (e.g. acetazolamide, potassium citrate, and sodium bicarbonate); history of hypersensitivity reaction to dextromethorphan, amantadine, or any other NMDR antagonists; weight < 20 kg |
| Dose of memantine | 3 mg or 6 mg/day | 5 mg/day | Initiated at 3 mg, titrated up by 3 mg/week to maximum dose of 6 mg, 9 mg or 12 mg depending on weight and tolerability. Maximum dose per weight class = 6 mg (20–40 kg), 9 mg (40–60 kg) and 12 mg (> 60 kg) |
| Mean length of follow‐up in weeks | 12 weeks | 12 weeks | 24 weeks |
| Primary outcomes (measure) | Core autism symptoms (SRS total raw score) | Autism symptoms (GARS) | Apraxia (subtests from the Narrative Memory‐Recognition (NEPSY‐II) included Imitating Hand Posture, Manual Motor Sequences and Oromotor Sequences) and expressive output (subtests from the NEPSY‐II Repetition of Nonsense Words subtest; EVT‐2) |
| Secondary outcomes (measure) | Severity and improvement in social interaction and communication (CATS); communication (CCC‐2); severity and improvement in stereotyped behaviours, restricted interests, maladaptive behaviours, and daily function (CAASTS); clinical global impressions of autism symptoms and associated behaviours (CGI); aberrant behaviour (ABC); caregiver and family burden (AHEOQ) | No secondary outcomes collected | Memory (NEPSY‐II Memory for Design and Narrative Memory subtests; Stanford‐Binet‐5 Nonverbal Working Memory subtest) and adaptive behaviour (VABS‐II). Several exploratory measures were also included in the trial: ABC, Bruininks Oseretsky Test of Motor Proficiency‐2, Short Form, BRIEF, Sensory Profile, Apraxia Profile, SRS, Wechsler Intelligence Scale for Children‐IV (at baseline), Wechsler Abbreviated Scale of Intelligence (at week 24) |
ABC: Aberrant Behaviour Subscale; ADHD: attention deficit hyperactivity disorder; ADI‐R: Autism Diagnostic Interview‐Revised; ADI‐S: Autism Diagnostic Interview‐Screener; ADOS: Autism Diagnostic Observation Schedule; AHEOQ: Autism Health Economics and Outcomes Questionnaire; ASD: autism spectrum disorder; BRIEF: Behavior Rating Inventory of Executive Function‐Parent Questionnaire; CAASTS: Core and Associated Autism Symptoms Treatment Scale; CATS: Core Autism Improvement Scale; CCC‐2: Children's Communication Checklist, 2nd edition; CGI: Clinical Global Impressions scale; CGI‐S: Clinical Global Impressions‐Severity; DSM: Diagnostic and Statistical Manual of Mental Disorders; DSM‐IV: Diagnostic and Statistical Manual of Mental Disorders, 4th edition; DSM‐IV‐TR: Diagnostic and Statistical Manual of Mental Disorders, 4th edition, text revision; DSM‐5: Diagnostic and Statistical Manual of Mental Disorders, 5th edition; EVT‐2: Expressive Vocabulary Test, 2nd edition; GARS: Gilliam Autism Rating Scale; IQ: intelligence quotient; NEPSY‐II: a developmental NEuroPSYchological assessment, 2nd edition; NMDR: N‐methyl‐d‐aspartate receptor; PDD: Pervasive Developmental Disorder; PDD‐NOS: Pervasive Developmental Disorder‐not otherwise specified; RCT: randomised controlled trial; SD: standard deviation; SRS: Social Responsiveness Scale. VABS‐II: Vineland Adaptive Behavior Scales, 2nd edition.
Funding
Aman 2017a reported funding from Forest Laboratories, LLC (Jersey City, NJ), Allergan. The study sponsor was involved in the study design, data collection (via contracted clinical investigator sites), analysis and interpretation of data, and the decision to present these results. The other two studies reported that they did not receive any funding for the trial (Karahmadi 2018; Soorya 2021); however, in Soorya 2021, the medication was an in‐kind contribution from Forest Pharmaceuticals.
Excluded studies
We excluded 28 records after reading the full text paper and attempting to contact study authors for further details if there was not enough information to decide. Four studies (eight reports) appeared to meet the inclusion criteria initially but were excluded on further inspection and discussion amongst the review authors (TM, AB) or with the study authors (Ghaleiha 2013; Hardan 2019; NCT01078844; NCT02353130). Two of these studies were discontinued (NCT01078844; NCT02353130): one did not go beyond the protocol stage because the chief investigator relocated to another institution (NCT02353130); and the other was terminated because the sponsor withdrew funding (NCT01078844). One excluded study was not an RCT (Hardan 2019). Another was an RCT that compared memantine to placebo (Ghaleiha 2013), but it did not meet our inclusion criteria because both groups were also started on risperidone at the beginning of the study. See Characteristics of excluded studies table for further detail.
Ongoing studies
Three studies (four reports) were ongoing and all compared memantine to placebo (EUCTR 2014‐003080‐38‐DE; NCT01972074; NCT03553875). One registered, double‐blind, parallel, randomised controlled trial (EUCTR 2014‐003080‐38‐DE), which was conducted in the Netherlands, planned to recruit 100 participants with ASD or obsessive compulsive disorder (aged birth to 17.9 years; IQ of 70 or over). The primary outcomes were compulsivity scores and adverse effects. One 12‐week, registered, randomised controlled trial conducted in the USA recruited 84 participants with ASD aged eight to 17 years (NCT01972074). The outcomes included improvements in scores on the Social Responsiveness Scale and Clinical Global Impression Scale. A 12‐week, quadruple‐blind, randomised controlled trial (NCT03553875), which was conducted in the USA, aimed to recruit 100 participants with ASD aged eight to 18 years. The primary outcomes in that study included improvement on scores on the Clinical Global Impression Scale.
We contacted the authors from the three registered trials to obtain data, but these trials were not yet published, and the authors could not provide the required data (EUCTR 2014‐003080‐38‐DE), or did not respond to emails requesting the data (NCT01972074), or were still recruiting participants (NCT03553875).
Awaiting classification
One study, published as a conference paper, was awaiting classification because we received no reply from the authors when we requested additional data (Martsenkovsky 2016). This study was a 16‐week, double‐blind, parallel, randomised controlled trial of memantine compared with placebo. It included 76 children with ASD aged 16 to 35 months, and collected data on four primary outcomes: repetitive behaviour, eye contact, receptive language, and motivation and reciprocity. Adverse outcomes were also reported along with aberrant behaviour outcomes (e.g. irritability, stereotyped behaviours). See Characteristics of studies awaiting classification table.
Risk of bias in included studies
See the risk of bias tables (under Characteristics of included studies) for details on risk of bias of included studies, and Figure 2 for a tabular summary. We judged Aman 2017a at low risk of bias across four of the seven criteria (random sequence generation, blinding of participants and personnel, blinding of outcome assessment, selective reporting), high risk of bias in two criteria (incomplete outcome data, 'other' bias), and unclear risk of bias in one criterion (allocation concealment). We judged Karahmadi 2018 at low risk of bias in two criteria (incomplete outcome data, 'other' bias), high risk of bias across two criteria (blinding of participants and personnel, outcome assessment) and unclear risk of bias across three criteria (random sequence generation, allocation concealment, selective reporting). We judged Soorya 2021 at high risk of bias across three criteria (incomplete outcome data, selective reporting, 'other' bias), unclear risk of bias across two criteria (random sequence generation, allocation concealment) and low risk of bias across two criteria (blinding of participants and personnel, blinding of outcome assessment). Justification for each rating is provided in the risk of bias tables (under Characteristics of included studies). In assessing validity, we gave an overall rating of 'high risk of bias' to all studies (Aman 2017a; Karahmadi 2018; Soorya 2021).
Random sequence generation
We judged Aman 2017a at low risk of bias in relation to random sequence generation because it used an adequate computer‐generated random sequence. As Karahmadi 2018 and Soorya 2021 did not describe the method of randomisation, we considered them at unclear risk of bias.
Allocation concealment
No studies stated whether allocation was concealed, so we rated all studies at unclear risk of bias (Aman 2017a; Karahmadi 2018; Soorya 2021).
Participants and personnel
Double‐blinding (participants and personnel/investigators) was maintained in two studies (Aman 2017a; Soorya 2021), which we considered at low risk of bias. In Aman 2017a, caregivers completed most tools and did not know whether the child had been allocated to the intervention or the control group (i.e. caregivers were blinded). In Soorya 2021, all participants and investigators were blinded to group assignment until blinding was broken by the statistician at the end of the study. Karahmadi 2018 had a single‐blind design, so parents but not personnel were blinded to treatment allocation. We rated this study at high risk of bias.
Blinding of outcome assessment
In Aman 2017a, outcome assessors were blinded. Parents completed most tools and did not know if the child had been allocated to the treatment or placebo group. The authors state that outcome assessors were also blinded. In Soorya 2021, investigators and participants were blinded to group assignment. We contacted the study authors, who clarified that the 'investigators' were those completing the assessments. We rated both of these studies at low risk of bias. In Karahmadi 2018, personnel did not appear to have been blinded to outcome assessment, which was the GARS. It was not stated who re‐rated the GARS, but personnel were likely not blinded if the authors have described it as a single blind study. We rated this study at high risk of bias.
Incomplete outcome data
Aman 2017a had unequal loss to follow‐up across the two groups, with the placebo group retaining 50/61 participants (82%) and the memantine group 54/60 participants (90%). This study was judged as having high risk of bias due to attrition. Reasons for withdrawal were not presented by group, so it was unclear whether the data were missing at random. When we contacted the study authors, they told us they were unable to provide the missing data. Eight participants withdrew from Soorya 2021, leaving only 65% of the original sample at the follow‐up assessment. This study was rated high risk of bias in these criteria. Karahmadi 2018 had no attrition and was rated low risk of bias.
Selective reporting
In Aman 2017a, the authors had prospectively registered their protocol and the listed outcomes were consistent with those presented in the published report. We rated this study at low risk of bias. Karahmadi 2018 did not register a protocol prior to the start of the trial, so it was not clear whether the study had reported the findings selectively. We assigned this study a rating of unclear risk of bias. In both of these studies, the methods described in the publication were consistent with the results. Soorya 2021 was originally designed as a large, multisite, phase II, double‐blind, randomised placebo‐controlled trial, and planned to recruit 144 participants. However, a hold was placed on the study by the FDA to collect more toxicity data, which reduced the budget and narrowed the study to 23 participants and only two sites, and it became a signal finding study. A number of measures were reported in the publication that had not been included in the protocol, so we rated it at high risk of reporting bias.
Other potential sources of bias
The authors of Aman 2017a disclosed that the study was sponsored by the funders, which may have added potential bias. We rated this study at high risk of bias in this domain. We did not identify any additional bias in Karahmadi 2018, which we rated at low risk of bias. Soorya 2021 reported several conflicts of interest, and there were significant differences between the planned outcomes in the registered protocol and the published trial, so we rated it at high risk of other bias.
Effects of interventions
See: Table 1
See Table 1 for the main comparison. The variable measures and limited data available for the included studies precluded meta‐analysis for all but two outcomes (i.e. the primary outcomes of core symptoms of autism and adverse effects). All primary and secondary outcomes were collected immediately postintervention (12 weeks) in two studies (Aman 2017a; Karahmadi 2018) and at 24 weeks in the other study (Soorya 2021).
Comparison 1. Memantine versus placebo
Primary outcomes
Core symptoms of autism
Two studies used measures that investigated the core symptoms of autism as a single outcome (Aman 2017a; Karahmadi 2018). Core autism symptoms are measured using tools that combine scores on the domains of repetitive and restricted interests and behaviours and social communication difficulties to provide an overall score. While the two studies used different tools to assess autism symptoms (SRS and GARS), we deemed these sufficiently similar to combine in meta‐analysis. Analysis 1.1 shows a forest plot of the comparison of mean change in scores for intervention versus placebo groups. The pooled findings for autism symptoms in the intervention group resulted in a SMD of −0.74 (95% CI −2.07 to 0.58; P = 0.27, I2 = 95%; 2 studies, 181 participants; very low‐certainty evidence; Analysis 1.1). This was a medium effect size (where small = 0.2, medium = 0.5, and large = 0.8; Cohen 1988).
1.1. Analysis.

Comparison 1: Memantine versus placebo, Outcome 1: Core symptoms of autism
Aman 2017a collected additional data on ASD symptoms, maladaptive behaviours and daily functioning using several additional rating scales (CAASTS, CATS and CGI). The study authors reported no 'statistically significant' difference in mean scores on the CAASTS, CATS and CGI between the placebo and memantine groups. We were unable to combine these results with the other measures of autistic symptoms because there were no specific test statistics or P values in the publication, and were not available from study authors. Regardless, including these data in the meta‐analysis would result in multiplicity of participant data. Furthermore, in our protocol we stated that we would prioritise standardised tests unless non‐standardised tests were used more frequently, and data could be pooled (Brignell 2021). Hence, we prioritised use of the SRS for the meta‐analysis of the primary outcome.
Adverse effects
Data for adverse effects were available for two studies (Aman 2017a; Soorya 2021).
Analysis 1.2 shows a forest plot of the OR of adverse effects resulting in dropouts for intervention versus placebo groups. The pooled findings for adverse effects resulted in an OR of 0.64 (95% CI 0.17 to 2.39; P = 0.50, I2 = 0%; 2 studies, 144 participants; low‐certainty evidence; Analysis 1.2). There was no evidence of a difference in adverse effects resulting in dropouts between the memantine and placebo groups. We downgraded the certainty of the evidence by one level for imprecision because only two studies contributed limited outcome data.
1.2. Analysis.

Comparison 1: Memantine versus placebo, Outcome 2: Adverse effects
In Aman 2017a, there were four adverse effects that the authors deemed to be related to the study in the placebo group of 61 participants, and three in the 60 participants in the memantine group. The authors reported no "statistically significant" difference between the memantine and placebo groups in terms of the number of adverse effects. We calculated the OR and found that, compared to placebo, memantine did not increase the incidence of adverse effects resulting in dropouts (OR 0.75, 95% CI 0.16 to 3.50; 1 study, 121 participants; low‐certainty evidence). The study also collected data on TEAEs. During the RCT, one participant in the memantine group had a serious mood disorder (judged unrelated to the study medication). All TEAE were described as mild or moderate in severity except for three in the memantine group (irritability, affective disorder, and choking). TEAEs were experienced by 47 participants in the placebo group and 51 participants in the memantine group. Of those participants, 33 and 36, respectively, experienced TEAEs deemed related to the study medication. The most common TEAEs, which were more frequent (though not significantly more frequent) in the memantine group, were irritability (6.7% versus 3.3%) and aggression (6.7% versus 4.9%). Mean weight gain was 0.9 (SD 2.0) kg in the control group and 1.2 (SD 1.9) kg in the treatment group.
In Aman 2017a, gastrointestinal adverse effects included vomiting (6 placebo, 4 memantine), diarrhoea (3 placebo, 2 memantine), frequent bowel movements (0 placebo, 2 memantine), nausea (2 placebo, 1 memantine), abdominal pain (5 placebo, 1 memantine) in the 12‐week study period; and constipation (1 placebo, 1 memantine) in the 48‐week extension. Neurological adverse effects included irritability (3 placebo, 5 memantine), insomnia (3 placebo, 4 memantine), headache (3 placebo, 3 memantine) in the 12‐week study period; and initial insomnia (1 placebo, 1 memantine) in the 48‐week extension. The authors reported no "statistically significant" difference between the two groups in the number of TEAEs deemed related to the study. There were no cardiovascular adverse effects, with no changes in ECG findings from baseline to study completion in either group.
Soorya 2021 reported no serious adverse effects in either group. Three participants withdrew from the study due to treatment‐limiting AEs: two from the placebo group (diarrhoea and emotional lability), and one from the memantine group (activation/overly energetic). We calculated the OR and found that, compared to placebo, memantine did not increase the incidence of adverse effects resulting in dropouts (OR 0.41, 95% CI 0.03 to 5.28; 1 study, 23 participants; low‐certainty evidence). All TEAEs were mild to moderate, and 75% of reported adverse effects were in the mild range, categorised as unrelated, unlikely related, or possibly related to treatment.
In Soorya 2021, gastrointestinal and mood/psychiatric symptoms were the most commonly reported adverse effects. Gastrointestinal adverse effects affected 13 participants in the memantine group and 10 in the placebo group, while mood/psychiatric symptoms affected 11 participants in the memantine group and 6 in the placebo group. While the memantine group experienced more gastrointestinal adverse effects and mood/psychiatric symptoms, the difference was not "statistically significant". Neurological adverse effects included irritability (4 placebo, 4 memantine), sleep (4 placebo, 0 memantine), and head/nervous system (4 placebo, 3 memantine). One cardiovascular event was recorded in the placebo group and none in the memantine group.
Secondary outcomes
Two studies reported on secondary outcomes (Aman 2017a; Soorya 2021). Soorya 2021 provided change score data on language, memory, intelligence, and adaptive behaviour, but only completed exploratory analyses for the outcomes of non‐core behaviours associated with ASD (i.e. maladaptive behaviours), sensory profile, core autistic symptoms, and executive functioning. These results are not reported in this review. Aman 2017a provided a summary of results for outcomes of irritability and hyperactivity (ABC‐C) and language (CCC‐2). Aman 2017a did not provide specific test statistics or P values (except for the communication subscale of 'Use of Context') for the ABC‐C or CCC‐2, and these data were not available from the study authors. None of the three studies reported data for general health outcomes or child or family quality of life.
Language
Two studies presented data on language (Aman 2017a; Soorya 2021). We were unable to combine these results in a meta‐analysis because Aman 2017a did not contain specific test statistics or P values, and these data were not available from the study authors.
Soorya 2021 analysed change in expressive vocabulary standard scores on the EVT from baseline to 12 and 24 weeks. There were no treatment effects in expressive vocabulary (F = 0.67, P = 0.42, effect size (ES) = 0.33, 95% CI −0.50 to 1.15) at either time point. There was low‐certainty evidence for language. We downgraded the certainty of the evidence by one level for imprecision because only one very small study contributed limited outcome data.
Aman 2017a collected data on language abilities using the CCC‐2. The authors reported no "statistically significant" difference in mean scores on the CCC‐2 between placebo and memantine groups across the composite scores and all subscales of the CCC‐2 except for the 'Context' subscale. The authors reported a "statistically significant" difference in scores on the CCC‐2 Context scale, favouring the placebo group (i.e. there was "significantly" greater improvement in scores in use of context in the placebo group relative to the memantine group (P = 0.02)). There was low‐certainty evidence for communication. We downgraded the certainty of the evidence by one level for imprecision because only two small studies contributed limited outcome data, and by one level because both studies were rated at high or unclear risk of bias across three or more of the seven domains.
Cognition
Memory
Soorya 2021 investigated visual and verbal memory using four NEPSY‐II subtests at baseline and at 12 and 24 weeks. There was a treatment effect on the narrative (verbal) memory‐recognition subtest indicating improvement in verbal recognition memory (F = 5.05, P = 0.03, ES = 0.79, 95% CI −0.06 to 1.64). There were no treatment effects for the three other memory scales, including Memory for Designs‐standard (F = 1.00, P = 0.33, ES = 0.39, 95% CI −0.44 to 1.22); Memory for Designs‐delayed (F = 0.06, P = 0.81, ES = −0.10, 95% CI −0.91 to 0.72); and Narrative Memory‐free and cued (F = 3.0, P = 0.1, ES = 0.69, 95% CI −0.15 to 1.53). The authors of Soorya 2021 state that they collected data on working memory using the Stanford Binet‐5 Nonverbal Working Memory subtest; however, these results were not presented in the publication. There was low‐certainty evidence for cognition. We downgraded the certainty of the evidence by one level for imprecision because only one very small study contributed limited outcome data, and by one level due to high or unclear risk of bias across five of the seven domains for this one study.
Intelligence
Soorya 2021 investigated verbal (VIQ) and perceptual (PIQ) intelligence using the WASI and WISC at baseline and at 24 weeks. There were no treatment effects in PIQ. Results from exploratory analyses found VIQ gains of 10 points or more in five of seven participants in the memantine group at 24 weeks; no participants in the placebo group demonstrated comparable gains in VIQ at 24 weeks. There was low‐certainty evidence for IQ. We downgraded the certainty of the evidence by one level for imprecision because only one very small study contributed limited outcome data, and by one level due to high or unclear risk of bias across five of the seven domains for this one study.
Adaptive behaviour
Soorya 2021 investigated adaptive behaviour using the VABS at baseline and at 12 and 24 weeks. There were no treatment effects in adaptive behaviour (F = 0.25, P = 0.62, ES = 0.19, 95% CI −0.63 to 1.01). There was low‐certainty evidence for adaptive behaviour. We downgraded the certainty of the evidence by one level for imprecision because only one very small study contributed limited outcome data, and by one level due to high or unclear risk of bias across five of the seven domains for this one study.
Non‐core behaviours associated with autism (including hyperactivity, irritability and aggression)
Hyperactivity
Aman 2017a investigated the outcome of hyperactivity, measured using the Aberrant Behavior Checklist‐Hyperactivity. The authors reported that there was no "statistically significant" difference in mean hyperactivity scores between the placebo and memantine groups on the ABC‐C. There was low‐certainty evidence for hyperactivity. We downgraded the certainty of the evidence by one level for imprecision because only one small study contributed limited outcome data, and by one level due to high or unclear risk of bias across three of the seven domains for this one study.
Irritability
Aman 2017a investigated the outcome of irritability, measured using the Aberrant Behavior Checklist‐Irritability. The authors reported no "statistically significant" difference in mean irritability scores between the placebo and memantine groups on the ABC‐I. There was low‐certainty evidence for irritability. We downgraded the certainty of the evidence by one level for imprecision because only one small study contributed limited outcome data, and by one level due to high or unclear risk of bias across three of the seven domains for this one study.
Discussion
Summary of main results
We identified three RCTs with 204 participants that evaluated the effects of memantine compared to placebo in autistic children (mean age 9.40). In one study, both groups received a behavioural intervention in addition to memantine. We synthesised the data from the two studies that analysed the primary outcome of core autism symptoms. The meta‐analysis showed no clear evidence that memantine was more effective than placebo for the primary outcome of autism symptoms immediately postintervention. While the combined results showed a small reduction in core autism symptoms, the two studies were heterogenous, with the larger study finding no effect, although improvement from baseline was noted in both groups (Aman 2017a); and the smaller study finding an effect (Karahmadi 2018). The certainty of the evidence was very low for the outcome of core autism symptoms. The I2 statistic for the overall effect was very high, indicating that the amount of variation in autistic behaviour outcomes between the two studies was due to substantial heterogeneity (e.g. clinical diversity, methodological differences) rather than chance. We were unable to identify clear reasons for the different findings between the two studies (e.g. differences in age, IQ, dosage), although we did not complete tests to analyse these differences.
No differences were found between the memantine and placebo groups in the two studies reporting on adverse effects (Aman 2017a; Soorya 2021). The certainty of the evidence was low.
Two studies reported on secondary outcomes (Aman 2017a; Soorya 2021). Aman 2017a found no clear effect for memantine for the secondary outcomes of language, hyperactivity, or irritability (except for one subdomain of language). For the subdomain of the language construct 'use of context', the study authors reported that the placebo group had a greater increase in scores compared with the memantine group, indicating that the placebo group made greater improvement in 'use of context'. Soorya 2021 found no clear effect of memantine for the secondary outcomes of language (i.e. expressive vocabulary), intelligence, visual and verbal memory (except for the subdomain of narrative verbal memory), and adaptive behaviour. For the subdomain of narrative verbal memory, the memantine group had a greater increase in scores compared with the placebo group. The certainty of the evidence for all six secondary outcomes (language intelligence, memory, adaptive behaviour, hyperactivity, and irritability) was low.
An additional study, which appeared eligible for inclusion, is currently awaiting classification (Martsenkovsky 2016; see Characteristics of studies awaiting classification); it was published as a conference abstract, and only limited data were available. This trial of 76 children with ASD (aged 18 to 36 months) noted improvement in the frequency and duration of eye contact, motivation, reciprocity, and receptive language; and a reduction in repetitive behaviours. The authors also noted reductions in the aberrant behaviours of irritability, stereotypic behaviour, and hyperactivity or non‐compliance.
Currently, there is no clear evidence that memantine is an effective treatment for autism symptoms (and other related behaviours such as language, memory, intelligence, adaptive behaviour, hyperactivity, and irritability).
Overall completeness and applicability of evidence
No trials have evaluated the effectiveness of memantine in adults. Included studies consisted of selected clinical samples and were conducted in only two countries. Furthermore, in one of the two studies that assessed IQ (Aman 2017a), the mean IQ of participants was in the very low range (76.8; IQ of 70 or under is indicative of an intellectual disability); whereas current estimates report that around 65% of autistic people have an IQ within the average range (Maenner 2021). It is therefore unclear how representative these participants are of the general population of autistic children.
While two studies assessed a range of important outcomes (Aman 2017a; Soorya 2021), one study collected only one outcome (autism symptoms) and did not report data on adverse effects (Karahmadi 2018). The primary outcome measures used to assess change in core autism symptoms (i.e. SRS, GARS) were diagnostic tools, and the total scores from these tools were not specifically designed to measure change in symptoms over time. Rather, the authors of the SRS recommend using the individual subscale scores to assess effectiveness of interventions. By using total scores, it is not possible to detect clusters of characteristics that may change within a specific domain, especially if some domains increase over time and others decrease. Ideally, some baseline assessments to establish the stability of symptoms using the GARS or SRS should have been conducted. The choice of these two primary outcome measures may have resulted in less nuanced assessment of change over time, which may have impacted study findings and conclusions. It is worth noting that Aman 2017a did include more dimensional assessments of autism symptoms (e.g. stereotyped and repetitive behaviours, social interaction and communication) with the CAAST and the CATS tools. However, these outcomes were assessed using proprietary tools developed by the study authors that had not been validated or tested in previous randomised controlled trials (Aman 2017a). Additionally, two studies had a moderate amount of missing data that did not appear to be missing at random, and this may have impacted study findings (Aman 2017a; Soorya 2021).
At present, with only three small studies with available data on autistic children, the effectiveness of memantine compared with placebo for autism symptoms remains unclear.
Quality of the evidence
Using the GRADE approach (Schünemann 2021), we considered the certainty of the evidence very low for the primary outcome of core autistic symptoms. We downgraded the certainty of the evidence for this outcome by three levels due to imprecision, inconsistency and high risk of bias. With regard to imprecision, only two RCTs were included, and the CIs were wide, crossing the line of no effect. For inconsistency, one study showed a treatment effect, with a large effect size, while the other showed no difference in effects between the memantine and placebo groups across all measures except communication. For risk of bias, we gave all three studies an overall rating of high. Of note, two studies declared conflicts of interest (Aman 2017a; Soorya 2021) and one stated that it was sponsored (Aman 2017a), which may have introduced bias not assessed in other risk of bias domains. We rated the certainty of the evidence for adverse effects as low, downgrading by two levels, primarily due to imprecision, given only two studies reported limited outcome data on adverse effects.
The certainty of the evidence for the secondary outcomes of language, memory, intelligence, adaptive behaviour, hyperactivity and irritability was low. We downgraded these outcomes by two levels for imprecision because, in most cases, only one study contributed limited outcome data; the exception was language, which was reported by two studies.
See Table 1.
Potential biases in the review process
We declared any potential conflicts of interest as per the Cochrane guidelines (see Declarations of interest). We included studies of all languages and, where relevant, had these translated to ensure all eligible studies were included. Two studies were discontinued, in one case because the investigator moved to another institution. It is unclear how this may have impacted the results. For the other study, the sponsor withdrew funding. It is unclear why the sponsor withdrew funding, but it may have been due to lack of effect of memantine. If this is the case, the result is the exclusion from our review of a study with null findings. We contacted all authors of studies that met our inclusion criteria to obtain data, but our attempts were not always successful. We could therefore only use available data in this review, and this may have created potential bias. One study remains 'awaiting classification', as we were unable to obtain the necessary data from the author, despite repeated attempts. Because only two studies were included in each meta‐analysis, we were unable to complete funnel plots or sensitivity analysis to assess publication bias and the impact of study quality. Lastly, there was significant between‐study variation, and while we have noted this limitation, it may have biased our findings. We did not identify other biases in the review process (e.g. citation, multiple publication, outcome reporting, or location bias).
Agreements and disagreements with other studies or reviews
To our knowledge, no other systematic reviews have examined the evidence for the efficacy of memantine in autism with a focus on RCTs. Rossignol 2014 systematically reviewed the evidence on five medications (all previously approved for treating Alzheimer's disease) for autism. Memantine was one of the included medications. Nine studies using memantine for autistic people (age range 2.58 to 33.2 years) met their inclusion criteria. Seven of eight (88%) uncontrolled studies (open label or retrospective case series), with a combined total of 211 individuals (between one and 151 participants per individual study), reported improvements in some behaviours (e.g. expressive/receptive language, social interaction, irritability, hyperactivity, attention, eye contact). One of one (100%) controlled studies (40 children, aged four to 12 years) reported improvements in some behaviours (irritability, stereotypy, hyperactivity/non‐compliance). The included studies also reported side effects (e.g. worsening of irritability). Rossignol 2014 included adults and children, and was published eight years ago, so it did not include more recent evidence from RCTs. Furthermore, no studies in the Rossignol 2014 review collected outcomes on overall autism symptoms. Included studies primarily focused on co‐occurring characteristics such as language, irritability, motor planning, hyperactivity, or individual ASD domains such as repetitive behaviours or social interaction. Rossignol 2014 did not conduct a meta‐analysis to synthesise outcomes and assess the pooled effect (results were presented narratively); nor did the review authors complete a risk of bias assessment or use GRADE to rate the certainty of outcomes. It is important to note that the RCT by Ghaleiha 2013, which was included in Rossignol 2014, was excluded from our review because the participants in both groups were given risperidone at the start of the intervention, in addition to memantine or placebo. Differences between this review and those of Rossignol 2014 (memantine resulted in improvements in autism and associated behaviours in Rossignol 2014, whereas we found no evidence of an effect), are likely due to this review being limited to rigorous RCT designs.
Authors' conclusions
Implications for practice.
The evidence regarding whether memantine is an effective treatment for autistic children is unclear. We found no studies in adults. Compared to placebo, memantine may have little to no effect on the core symptoms of autism, but the evidence is very uncertain (very low‐certainty evidence). Similarly, the evidence suggests that, compared to placebo, memantine results in little to no difference in adverse effects (low‐certainty evidence). Lastly, there was no clear evidence that, compared to placebo, memantine was effective in reducing hyperactivity or irritability, or in improving communication or general health and functioning (all low‐certainty evidence). Prescribing clinicians should be clear with adults and children and their families about the lack of evidence for memantine as an effective intervention for autism and common associated difficulties, and discuss risks and other possible alternative therapeutic options.
Implications for research.
There is little research on memantine in autistic children, and no trials in autistic adults. Future studies should be blinded randomised controlled trials, ideally with larger sample sizes and participants with a broad range of characteristics (e.g. intelligence quotient (IQ), verbal ability and age, including adults). Larger sample sizes and clearly defined subgroups will enable subgroup analyses for factors that could be associated with effectiveness outcomes. Subgroups could be based on age range (in children), intelligence, language level, or presence or absence of an intellectual or language disability. Studies should collect data on adverse effects that are consistent with similar trials and use accepted adverse effect outcome measures. Information collected on the optimal dosage of memantine is also needed, as well as longer‐term follow‐up. Three ongoing studies (registered trials) identified in this review may address some gaps in the evidence for memantine and strengthen the evidence base, once complete.
Studies should consider the expected behavioural changes from use of memantine, and whether autism symptoms are the most useful outcome measure. In particular, with increased focus on acceptance of neurodivergence, studies should consider whether reducing autism symptoms is the most valid goal and one that is important to autistic people and their families. Reducing some of the more limiting co‐occurring characteristics may be a higher priority. While there is some evidence of an association between increased serum levels of the neurotransmitter glutamate in autistic people (see Rojas 2014 for a review), changes in glutamate might also alter neurological pathways that change behaviours that are not diagnostic of ASD. Current established prescribing for other interventions for autistic people target improving associated difficulties rather than autism per se. Hence, studies that only investigate autism symptoms as outcomes may miss other important improvements. We suggest that future trials provide separate data for the two diagnostic criteria domains (e.g. social communication and repetitive, restricted interests and behaviours). Future trials should also collect all outcomes that could be meaningful for children, adults and their families. These two things together will assist in our understanding of whether improving social communication or restricted and repetitive behaviours can help to improve activities, participation and quality of life, or if other changes mediate improved outcomes.
There was considerable variation between the three studies with regard to participants, sample size and methodological quality. Longer‐term observations that assess crucial outcomes relevant to individuals' participation and functioning were not assessed. In summary, the evidence is not yet complete, and applicability should be assessed for each study individually. There is an urgent need for further randomised controlled trials in this field.
History
Protocol first published: Issue 1, 2021
Acknowledgements
We wish to thank Biola Araba, Katherine Wilkins and Chidambaram Prakash for their contributions to the drafts of the protocol. We acknowledge the contribution of Kristine Egberts to the protocol for this review, who sadly passed away in March 2020.
We are also very grateful for the contributions, advice and support received from the editors, and the statisticians from the Cochrane Developmental, Psychosocial and Learning Problems (CDPLP) Review Group. We also acknowledge the valued contributions and support from the Group's Information Specialist, Margaret Anderson, in developing our search strategy and running the searches.
The CRG Editorial Team is grateful to the following peer reviewers for their time and comments: Dr Gemma Clayton, Bristol Medical School, University of Bristol (UK); Brian Duncan (USA); Christopher J McDougle, MD, Director, Lurie Center for Autism, Massachusetts General Hospital (USA); Nancy Lurie Marks, Professor of Psychiatry, Harvard Medical School (USA); and David H Skuse (Professor), University College London (UK). In addition, the CRG Editorial Team are grateful to Julia Turner for copyediting the review.
Appendices
Appendix 1. Search strategies
Cochrane Central Register of Controlled Trials (CENTRAL)
Searched 25 November 2020 (63 records) Searched 14 February 2022 (3 new records)
#1 [mh "child development disorders, pervasive"] #2 [mh ^"Developmental Disabilities"] #3 [mh ^"Neurodevelopmental Disorders"] #4 pervasive NEXT development* NEXT disorder* #5 (pervasive near/3 child) #6 (PDD or PDDs or PDD next NOS or ASD or ASDs) #7 autis* or asperger* or kanner* #8 childhood next schizophrenia #9 {or #1‐#8} #10 [mh memantine] #11 [mh Amantadine] #12 (Abixa or Adaxor or Admed or Akatinol or Alceba or Alios or Almenta or Alois or Alzant or Alzer or Alzia or Alzinex or Alzixa or Alzmenda or Alzmex or Apo‐Memantine or Axura or Biomentin or Carrier or Cogito or Cognomem or Conexine or Cordure or Dantex or Demantin or Demax or Dementa or Dementexa or Ebixa or Ebitex or Emantin or Emaxin or Esmirtal or Eutebrol or Evy or Ezemantis or Fentina or Korint or Lemix or Lindex or Lucidex or Manotin or Mantine or Mantomed or Marbodin or Mardewel or Marixino or Maruxa or Maxiram or Melanda or Memabix or Memamed or Memando or Memantin or Memantina or Memantine or Memantinol or Memantyn or Memanvitae or Memanxa or Memanzaks or Memary or Memax or Memexa or Memigmin or Memikare or Memogen or Memolan or Memorel or Memorix or Memotec or Memox or Memxa or Mentadem or Mentikline or Mentium or Mentixa or Merandex or Merital or Mexia or Mimetix or Mirvedol or Modualz or Morysa or Nemdaa or Namenda or Namzaric or Nemdatine or Neumantine or Neuro‐K or Neuroplus$ or Noojerone or PMS‐Memantine or Polmatine or Prilben or Pronervon or Ratio‐Memantine or Ravemantine or Sandoz‐Memantine or Talentum or Timantil or Tingreks or Tonibral$ or Tormoro or Valcoxia or Vilimen or Vivimex or Witgen or Xapimant or Ymana or Zalatine or Zarlyn or Zeimer or Zemertinex or Zenmem or Zenmen or Zimer) #13 {or #10‐#12} #14 #9 and #13 in Trials
Ovid MEDLINE(R)
Searched 24 November 2020 (124 records) Searched 14 February 2022 (18 new records)
1 exp child development disorders, pervasive/ 2 Developmental Disabilities/ 3 Neurodevelopmental Disorders/ 4 pervasive development$ disorder$.tw,kf. 5 (pervasive adj3 child$).tw,kf. 6 (PDD or PDDs or PDD‐NOS or ASD or ASDs).tw,kf. 7 autis$.tw,kf. 8 asperger$.tw,kf. 9 kanner$.tw,kf. 10 childhood schizophrenia.tw,kf. 11 or/1‐10 12 memantine/ 13 Amantadine/ 14 (Abixa or Adaxor or Admed or Akatinol or Alceba or Alios or Almenta or Alois or Alzant or Alzer or Alzia or Alzinex or Alzixa or Alzmenda or Alzmex or Apo‐Memantine or Axura or Biomentin or Carrier or Cogito or Cognomem or Conexine or Cordure or Dantex or Demantin or Demax or Dementa or Dementexa or Ebixa or Ebitex or Emantin or Emaxin or Esmirtal or Eutebrol or Evy or Ezemantis or Fentina or Korint or Lemix or Lindex or Lucidex or Manotin or Mantine or Mantomed or Marbodin or Mardewel or Marixino or Maruxa or Maxiram or Melanda or Memabix or Memamed or Memando or Memantin or Memantina or Memantine or Memantinol or Memantyn or Memanvitae or Memanxa or Memanzaks or Memary or Memax or Memexa or Memigmin or Memikare or Memogen or Memolan or Memorel or Memorix or Memotec or Memox or Memxa or Mentadem or Mentikline or Mentium or Mentixa or Merandex or Merital or Mexia or Mimetix or Mirvedol or Modualz or Morysa or Nemdaa or Namenda or Namzaric or Nemdatine or Neumantine or Neuro‐K or Neuroplus$ or Noojerone or PMS‐Memantine or Polmatine or Prilben or Pronervon or Ratio‐Memantine or Ravemantine or Sandoz‐Memantine or Talentum or Timantil or Tingreks or Tonibral$ or Tormoro or Valcoxia or Vilimen or Vivimex or Witgen or Xapimant or Ymana or Zalatine or Zarlyn or Zeimer or Zemertinex or Zenmem or Zenmen or Zimer).mp. 15 or/12‐14 16 11 and 15 17 randomized controlled trial.pt. 18 controlled clinical trial.pt. 19 randomi#ed.ab. 20 placebo$.ab. 21 drug therapy.fs. 22 randomly.ab. 23 trial.ab. 24 groups.ab. 25 or/17‐24 26 exp animals/ not humans.sh. 27 25 not 26 28 16 and 27
Ovid MEDLINE(R) In‐Process & Other Non‐Indexed Citations
Searched 24 November 2020 (31 records) Searched 14 February 2022 (5 new records)
1 pervasive development$ disorder$.tw,kf. 2 (pervasive adj3 child$).tw,kf. 3 (PDD or PDDs or PDD‐NOS or ASD or ASDs).tw,kf. 4 autis$.tw,kf. 5 asperger$.tw,kf. 6 kanner$.tw,kf. 7 childhood schizophrenia.tw,kf. 8 or/1‐7 9 Amantadine.mp. 10 (Abixa or Adaxor or Admed or Akatinol or Alceba or Alios or Almenta or Alois or Alzant or Alzer or Alzia or Alzinex or Alzixa or Alzmenda or Alzmex or Apo‐Memantine or Axura or Biomentin or Carrier or Cogito or Cognomem or Conexine or Cordure or Dantex or Demantin or Demax or Dementa or Dementexa or Ebixa or Ebitex or Emantin or Emaxin or Esmirtal or Eutebrol or Evy or Ezemantis or Fentina or Korint or Lemix or Lindex or Lucidex or Manotin or Mantine or Mantomed or Marbodin or Mardewel or Marixino or Maruxa or Maxiram or Melanda or Memabix or Memamed or Memando or Memantin or Memantina or Memantine or Memantinol or Memantyn or Memanvitae or Memanxa or Memanzaks or Memary or Memax or Memexa or Memigmin or Memikare or Memogen or Memolan or Memorel or Memorix or Memotec or Memox or Memxa or Mentadem or Mentikline or Mentium or Mentixa or Merandex or Merital or Mexia or Mimetix or Mirvedol or Modualz or Morysa or Nemdaa or Namenda or Namzaric or Nemdatine or Neumantine or Neuro‐K or Neuroplus$ or Noojerone or PMS‐Memantine or Polmatine or Prilben or Pronervon or Ratio‐Memantine or Ravemantine or Sandoz‐Memantine or Talentum or Timantil or Tingreks or Tonibral$ or Tormoro or Valcoxia or Vilimen or Vivimex or Witgen or Xapimant or Ymana or Zalatine or Zarlyn or Zeimer or Zemertinex or Zenmem or Zenmen or Zimer).mp. 11 9 or 10 12 8 and 11 13 (random$ or control$ or group$ or cluster$ or placebo$ or trial$ or assign$ or allocat$ or prospectiv$ or meta‐analysis or systematic review or longitudinal$).tw,kf. 14 12 and 13
Ovid MEDLINE(R) Epub Ahead of Print
Searched 24 November 2020 (4 records) Searched 14 February 2022 (3 new records)
1 pervasive development$ disorder$.tw,kf. 2 (pervasive adj3 child$).tw,kf. 3 (PDD or PDDs or PDD‐NOS or ASD or ASDs).tw,kf. 4 autis$.tw,kf. 5 asperger$.tw,kf. 6 kanner$.tw,kf. 7 childhood schizophrenia.tw,kf. 8 or/1‐7 9 Amantadine.mp. 10 (Abixa or Adaxor or Admed or Akatinol or Alceba or Alios or Almenta or Alois or Alzant or Alzer or Alzia or Alzinex or Alzixa or Alzmenda or Alzmex or Apo‐Memantine or Axura or Biomentin or Carrier or Cogito or Cognomem or Conexine or Cordure or Dantex or Demantin or Demax or Dementa or Dementexa or Ebixa or Ebitex or Emantin or Emaxin or Esmirtal or Eutebrol or Evy or Ezemantis or Fentina or Korint or Lemix or Lindex or Lucidex or Manotin or Mantine or Mantomed or Marbodin or Mardewel or Marixino or Maruxa or Maxiram or Melanda or Memabix or Memamed or Memando or Memantin or Memantina or Memantine or Memantinol or Memantyn or Memanvitae or Memanxa or Memanzaks or Memary or Memax or Memexa or Memigmin or Memikare or Memogen or Memolan or Memorel or Memorix or Memotec or Memox or Memxa or Mentadem or Mentikline or Mentium or Mentixa or Merandex or Merital or Mexia or Mimetix or Mirvedol or Modualz or Morysa or Nemdaa or Namenda or Namzaric or Nemdatine or Neumantine or Neuro‐K or Neuroplus$ or Noojerone or PMS‐Memantine or Polmatine or Prilben or Pronervon or Ratio‐Memantine or Ravemantine or Sandoz‐Memantine or Talentum or Timantil or Tingreks or Tonibral$ or Tormoro or Valcoxia or Vilimen or Vivimex or Witgen or Xapimant or Ymana or Zalatine or Zarlyn or Zeimer or Zemertinex or Zenmem or Zenmen or Zimer).mp. 11 9 or 10 12 8 and 11 13 (random$ or control$ or group$ or cluster$ or placebo$ or trial$ or assign$ or allocat$ or prospectiv$ or meta‐analysis or systematic review or longitudinal$).tw,kf. 14 12 and 13
Embase Ovid
Searched 24 November 2020 (378 records) Searched 14 February 2022 (54 new records) 1 developmental disorder/ 2 mental disease/ 3 autism/ 4 asperger syndrome/ 5 "pervasive developmental disorder not otherwise specified"/ 6 pervasive development$ disorder$.tw,kw. 7 (pervasive adj3 child$).tw,kw. 8 (PDD or PDDs or PDD‐NOS or ASD or ASDs).tw,kw. 9 autis$.tw. 10 asperger$.tw,kw. 11 kanner$.tw,kw. 12 childhood schizophreni$.tw,kw. 13 or/1‐12 14 memantine/ 15 amantadine/ 16 (Abixa or Adaxor or Admed or Akatinol or Alceba or Alios or Almenta or Alois or Alzant or Alzer or Alzia or Alzinex or Alzixa or Alzmenda or Alzmex or Apo‐Memantine or Axura or Biomentin or Carrier or Cogito or Cognomem or Conexine or Cordure or Dantex or Demantin or Demax or Dementa or Dementexa or Ebixa or Ebitex or Emantin or Emaxin or Esmirtal or Eutebrol or Evy or Ezemantis or Fentina or Korint or Lemix or Lindex or Lucidex or Manotin or Mantine or Mantomed or Marbodin or Mardewel or Marixino or Maruxa or Maxiram or Melanda or Memabix or Memamed or Memando or Memantin or Memantina or Memantine or Memantinol or Memantyn or Memanvitae or Memanxa or Memanzaks or Memary or Memax or Memexa or Memigmin or Memikare or Memogen or Memolan or Memorel or Memorix or Memotec or Memox or Memxa or Mentadem or Mentikline or Mentium or Mentixa or Merandex or Merital or Mexia or Mimetix or Mirvedol or Modualz or Morysa or Nemdaa or Namenda or Namzaric or Nemdatine or Neumantine or Neuro‐K or Neuroplus$ or Noojerone or PMS‐Memantine or Polmatine or Prilben or Pronervon or Ratio‐Memantine or Ravemantine or Sandoz‐Memantine or Talentum or Timantil or Tingreks or Tonibral$ or Tormoro or Valcoxia or Vilimen or Vivimex or Witgen or Xapimant or Ymana or Zalatine or Zarlyn or Zeimer or Zemertinex or Zenmem or Zenmen or Zimer).mp. 17 or/14‐16 18 13 and 17 19 Randomized controlled trial/ 20 Controlled clinical study/ 21 random$.ti,ab. 22 randomization/ 23 intermethod comparison/ 24 placebo.ti,ab. 25 (compare or compared or comparison).ti. 26 ((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab. 27 (open adj label).ti,ab. 28 ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab. 29 double blind procedure/ 30 parallel group$1.ti,ab. 31 (crossover or cross over).ti,ab. 32 ((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab. 33 (assigned or allocated).ti,ab. 34 (controlled adj7 (study or design or trial)).ti,ab. 35 (volunteer or volunteers).ti,ab. 36 human experiment/ 37 trial.ti. 38 or/19‐37 39 (random$ adj sampl$ adj7 ("cross section$" or questionnaire$1 or survey$ or database$1)).ti,ab. not (comparative study/ or controlled study/ or randomi?ed controlled.ti,ab. or randomly assigned.ti,ab.) 40 Cross‐sectional study/ not (randomized controlled trial/ or controlled clinical study/ or controlled study/ or randomi?ed controlled.ti,ab. or control group$1.ti,ab.) 41 (((case adj control$) and random$) not randomi?ed controlled).ti,ab. 42 (Systematic review not (trial or study)).ti. 43 (nonrandom$ not random$).ti,ab. 44 "Random field$".ti,ab. 45 (random cluster adj3 sampl$).ti,ab. 46 (review.ab. and review.pt.) not trial.ti. 47 "we searched".ab. and (review.ti. or review.pt.) 48 "update review".ab. 49 (databases adj4 searched).ab. 50 (rat or rats or mouse or mice or swine or porcine or murine or sheep or lambs or pigs or piglets or rabbit or rabbits or cat or cats or dog or dogs or cattle or bovine or monkey or monkeys or trout or marmoset$1).ti. and animal experiment/ (1094211) 51 Animal experiment/ not (human experiment/ or human/) 52 or/39‐51 53 38 not 52 54 18 and 53
APA PsycInfo Ovid
Searched 24 November 2020 (38 records) Searched 14 February 2022 (7 new records) 1 exp pervasive developmental disorders/ 2 Developmental disabilities/ 3 Neurodevelopmental Disorders/ 4 pervasive development$ disorder$.tw. 5 (pervasive adj3 child$).tw. 6 asperger$.tw. 7 autis$.tw. 8 Kanner$.tw. 9 (PDD or PDDs or PDD‐NOS or ASD or ASDs).tw. 10 childhood schizophreni$.tw. 11 or/1‐10 12 amantadine/ 13 (Abixa or Adaxor or Admed or Akatinol or Alceba or Alios or Almenta or Alois or Alzant or Alzer or Alzia or Alzinex or Alzixa or Alzmenda or Alzmex or Apo‐Memantine or Axura or Biomentin or Carrier or Cogito or Cognomem or Conexine or Cordure or Dantex or Demantin or Demax or Dementa or Dementexa or Ebixa or Ebitex or Emantin or Emaxin or Esmirtal or Eutebrol or Evy or Ezemantis or Fentina or Korint or Lemix or Lindex or Lucidex or Manotin or Mantine or Mantomed or Marbodin or Mardewel or Marixino or Maruxa or Maxiram or Melanda or Memabix or Memamed or Memando or Memantin or Memantina or Memantine or Memantinol or Memantyn or Memanvitae or Memanxa or Memanzaks or Memary or Memax or Memexa or Memigmin or Memikare or Memogen or Memolan or Memorel or Memorix or Memotec or Memox or Memxa or Mentadem or Mentikline or Mentium or Mentixa or Merandex or Merital or Mexia or Mimetix or Mirvedol or Modualz or Morysa or Nemdaa or Namenda or Namzaric or Nemdatine or Neumantine or Neuro‐K or Neuroplus$ or Noojerone or PMS‐Memantine or Polmatine or Prilben or Pronervon or Ratio‐Memantine or Ravemantine or Sandoz‐Memantine or Talentum or Timantil or Tingreks or Tonibral$ or Tormoro or Valcoxia or Vilimen or Vivimex or Witgen or Xapimant or Ymana or Zalatine or Zarlyn or Zeimer or Zemertinex or Zenmem or Zenmen or Zimer).mp. 14 12 or 13 15 11 and 14 16 clinical trials/ 17 random$.tw. 18 (allocat$ or assign$).tw. 19 ((clinic$ or control$) adj trial$).tw. 20 ((control$ or experiment$ or intervention$) adj3 group$).tw. 21 ((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$)).tw. 22 (crossover$ or "cross over$").tw. 23 random sampling/ 24 Experiment Controls/ 25 Placebo/ 26 placebo$.tw. 27 exp program evaluation/ 28 treatment effectiveness evaluation/ 29 ((effectiveness or evaluat$) adj3 (stud$ or research$)).tw. 30 or/16‐29 31 15 and 30
CINAHL Plus EBSCOhost
Searched 24 November 2020 (46 records) Searched 14 February 2022 (15 new records)
S1 MH randomized controlled trials S2 MH double‐blind studies S3 MH single‐blind studies S4 MH random assignment S5 MH pretest‐posttest design S6 MH cluster sample S7 TI (randomised OR randomized) S8 AB (random*) S9 TI (trial) S10 MH (sample size) AND AB (assigned OR allocated OR control) S11 MH (placebos) S12 PT (randomized controlled trial) S13 AB (control W5 group) S14 MH (crossover design) OR MH (comparative studies) S15 AB (cluster W3 RCT) S16 MH animals+ S17 MH (animal studies) S18 TI (animal model*) S19 S16 OR S17 OR S18 S20 MH (human) S21 S19 NOT S20 S22 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 S23 S22 NOT S21 S24 (MH "Child Development Disorders, Pervasive+") S25 (MH "Developmental Disabilities") S26 (MH "Mental Disorders Diagnosed in Childhood") S27 "pervasive development* disorder*" S28 (pervasive N3 child) S29 (PDD or PDDs or PDD‐NOS or ASD or ASDs) S30 autis* or asperger* or kanner* S31 childhood schizophrenia S32 S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 S33 (MH "Memantine") S34 (MH "Amantadine") S35 (Abixa or Adaxor or Admed or Akatinol or Alceba or Alios or Almenta or Alois or Alzant or Alzer or Alzia or Alzinex or Alzixa or Alzmenda or Alzmex or Apo‐Memantine or Axura or Biomentin or Carrier or Cogito or Cognomem or Conexine or Cordure or Dantex or Demantin or Demax or Dementa or Dementexa or Ebixa or Ebitex or Emantin or Emaxin or Esmirtal or Eutebrol or Evy or Ezemantis or Fentina or Korint or Lemix or Lindex or Lucidex or Manotin or Mantine or Mantomed or Marbodin or Mardewel or Marixino or Maruxa or Maxiram or Melanda or Memabix or Memamed or Memando or Memantin or Memantina or Memantine or Memantinol or Memantyn or Memanvitae or Memanxa or Memanzaks or Memary or Memax or Memexa or Memigmin or Memikare or Memogen or Memolan or Memorel or Memorix or Memotec or Memox or Memxa or Mentadem or Mentikline or Mentium or Mentixa or Merandex or Merital or Mexia or Mimetix or Mirvedol or Modualz or Morysa or Nemdaa or Namenda or Namzaric or Nemdatine or Neumantine or Neuro‐K or Neuroplus* or Noojerone or PMS‐Memantine or Polmatine or Prilben or Pronervon or Ratio‐Memantine or Ravemantine or Sandoz‐Memantine or Talentum or Timantil or Tingreks or Tonibral* or Tormoro or Valcoxia or Vilimen or Vivimex or Witgen or Xapimant or Ymana or Zalatine or Zarlyn or Zeimer or Zemertinex or Zenmem or Zenmen or Zimer) S36 S33 OR S34 OR S35 S37 S23 AND S32 AND S36
ERIC EBSCOhost
Searched 25 November 2020 (21 records) Searched 14 February 2022 (no new records)
S1 DE "Pervasive Developmental Disorders" OR DE "Asperger Syndrome" OR DE "Developmental Disabilities" S2 autis* or asperger* or kanner* S3 childhood schizophrenia S4 "pervasive development* disorder*" S5 (pervasive N3 child) S6 S1 OR S2 OR S3 OR S4 OR S5 S7 (Abixa or Adaxor or Admed or Akatinol or Alceba or Alios or Almenta or Alois or Alzant or Alzer or Alzia or Alzinex or Alzixa or Alzmenda or Alzmex or Apo‐Memantine or Axura or Biomentin or Carrier or Cogito or Cognomem or Conexine or Cordure or Dantex or Demantin or Demax or Dementa or Dementexa or Ebixa or Ebitex or Emantin or Emaxin or Esmirtal or Eutebrol or Evy or Ezemantis or Fentina or Korint or Lemix or Lindex or Lucidex or Manotin or Mantine or Mantomed or Marbodin or Mardewel or Marixino or Maruxa or Maxiram or Melanda or Memabix or Memamed or Memando or Memantin or Memantina or Memantine or Memantinol or Memantyn or Memanvitae or Memanxa or Memanzaks or Memary or Memax or Memexa or Memigmin or Memikare or Memogen or Memolan or Memorel or Memorix or Memotec or Memox or Memxa or Mentadem or Mentikline or Mentium or Mentixa or Merandex or Merital or Mexia or Mimetix or Mirvedol or Modualz or Morysa or Nemdaa or Namenda or Namzaric or Nemdatine or Neumantine or Neuro‐K or Neuroplus* or Noojerone or PMS‐Memantine or Polmatine or Prilben or Pronervon or Ratio‐Memantine or Ravemantine or Sandoz‐Memantine or Talentum or Timantil or Tingreks or Tonibral* or Tormoro or Valcoxia or Vilimen or Vivimex or Witgen or Xapimant or Ymana or Zalatine or Zarlyn or Zeimer or Zemertinex or Zenmem or Zenmen or Zimer) S8 S6 AND S7
Web of Science Core Collection Clarivate (Science Citation Index (SCI);Social Sciences Citation Index (SSCI); Conference Proceedings Citation Index‐Science (CPCI‐S); Conference Proceedings Citation Index‐Social Science & Humanities (CPCI‐SSH))
Searched 25 November 2020 (63 records) Searched 15 February 2022 (14 new records)
# 8 #7 AND #5 Indexes=SCI‐EXPANDED, SSCI, CPCI‐S, CPCI‐SSH Timespan=All years # 7 TS=(random* or placebo* or trial* or group* or prospective or control or TAU or "treatment as usual" ) Indexes=SCI‐EXPANDED, SSCI, CPCI‐S, CPCI‐SSH Timespan=All years # 6 #4 AND #1 Indexes=SCI‐EXPANDED, SSCI, CPCI‐S, CPCI‐SSH Timespan=All years # 5 #4 AND #3 Indexes=SCI‐EXPANDED, SSCI, CPCI‐S, CPCI‐SSH Timespan=All years # 4 TS=(Abixa or Adaxor or Admed or Akatinol or Alceba or Alios or Almenta or Alois or Alzant or Alzer or Alzia or Alzinex or Alzixa or Alzmenda or Alzmex or Apo‐Memantine or Axura or Biomentin or Cogito or Cognomem or Conexine or Cordure or Dantex or Demantin or Demax or Dementa or Dementexa or Ebixa or Ebitex or Emantin or Emaxin or Esmirtal or Eutebrol or Evy or Ezemantis or Fentina or Korint or Lemix or Lindex or Lucidex or Manotin or Mantine or Mantomed or Marbodin or Mardewel or Marixino or Maruxa or Maxiram or Melanda or Memabix or Memamed or Memando or Memantin or Memantina or Memantine or Memantinol or Memantyn or Memanvitae or Memanxa or Memanzaks or Memary or Memax or Memexa or Memigmin or Memikare or Memogen or Memolan or Memorel or Memorix or Memotec or Memox or Memxa or Mentadem or Mentikline or Mentium or Mentixa or Merandex or Merital or Mexia or Mimetix or Mirvedol or Modualz or Morysa or Nemdaa or Namenda or Namzaric or Nemdatine or Neumantine or Neuro‐K or Neuroplus* or Noojerone or PMS‐Memantine or Polmatine or Prilben or Pronervon or Ratio‐Memantine or Ravemantine or Sandoz‐Memantine or Talentum or Timantil or Tingreks or Tonibral* or Tormoro or Valcoxia or Vilimen or Vivimex or Witgen or Xapimant or Ymana or Zalatine or Zarlyn or Zeimer or Zemertinex or Zenmem or Zenmen or Zimer) Indexes=SCI‐EXPANDED, SSCI, CPCI‐S, CPCI‐SSH Timespan=All years # 3 #2 OR #1 Indexes=SCI‐EXPANDED, SSCI, CPCI‐S, CPCI‐SSH Timespan=All years # 2 TS=("Developmental Disabilit*" or "Neurodevelopmental Disorder*") Indexes=SCI‐EXPANDED, SSCI, CPCI‐S, CPCI‐SSH Timespan=All years # 1 TS=(autis* or asperger* or kanner* or "pervasive development* disorder*" or (pervasive near/3 child) or PDD or PDDs or "PDD‐NOS" or ASD or ASDs or "childhood schizophrenia") Indexes=SCI‐EXPANDED, SSCI, CPCI‐S, CPCI‐SSH Timespan=All years
SciELO (Science Electronic Library Online) Web of Science Clarivate
Searched 25 November 2020 (1 record) Searched 15 February 2022 (no new records)
#5 #4 AND #3 Indexes=SCIELO Timespan=All years #4 TS=(Abixa or Adaxor or Admed or Akatinol or Alceba or Alios or Almenta or Alois or Alzant or Alzer or Alzia or Alzinex or Alzixa or Alzmenda or Alzmex or Apo‐Memantine or Axura or Biomentin or Cogito or Cognomem or Conexine or Cordure or Dantex or Demantin or Demax or Dementa or Dementexa or Ebixa or Ebitex or Emantin or Emaxin or Esmirtal or Eutebrol or Evy or Ezemantis or Fentina or Korint or Lemix or Lindex or Lucidex or Manotin or Mantine or Mantomed or Marbodin or Mardewel or Marixino or Maruxa or Maxiram or Melanda or Memabix or Memamed or Memando or Memantin or Memantina or Memantine or Memantinol or Memantyn or Memanvitae or Memanxa or Memanzaks or Memary or Memax or Memexa or Memigmin or Memikare or Memogen or Memolan or Memorel or Memorix or Memotec or Memox or Memxa or Mentadem or Mentikline or Mentium or Mentixa or Merandex or Merital or Mexia or Mimetix or Mirvedol or Modualz or Morysa or Nemdaa or Namenda or Namzaric or Nemdatine or Neumantine or Neuro‐K or Neuroplus* or Noojerone or PMS‐Memantine or Polmatine or Prilben or Pronervon or Ratio‐Memantine or Ravemantine or Sandoz‐Memantine or Talentum or Timantil or Tingreks or Tonibral* or Tormoro or Valcoxia or Vilimen or Vivimex or Witgen or Xapimant or Ymana or Zalatine or Zarlyn or Zeimer or Zemertinex or Zenmem or Zenmen or Zimer) Indexes=SCIELO Timespan=All years # 3 #2 OR #1 Indexes=SCIELO Timespan=All years # 2 TS=("Developmental Disabilit*" or "Neurodevelopmental Disorder*") Indexes=SCIELO Timespan=All years # 1 TS=(autis* or asperger* or kanner* or "pervasive development* disorder*" or (pervasive near/3 child) or PDD or PDDs or "PDD‐NOS" or ASD or ASDs or "childhood schizophrenia") Indexes=SCIELO Timespan=All years
LILACS
pesquisa.bvsalud.org/portal/?lang=en
Searched 25 November 2020 (no records) Searched 15 February 2022 (no records)
(Abixa OR AdaxOR OR Admed OR Akatinol OR Alceba OR Alios OR Almenta OR Alois OR Alzant OR Alzer OR Alzia OR Alzinex OR Alzixa OR Alzmenda OR Alzmex OR Apo‐Memantine OR Axura OR Biomentin OR Cogito OR Cognomem OR Conexine OR COR dure OR Dantex OR Demantin OR Demax OR Dementa OR Dementexa OR Ebixa OR Ebitex OR Emantin OR Emaxin OR Esmirtal OR Eutebrol OR Evy OR Ezemantis OR Fentina OR KOR int OR Lemix OR Lindex OR Lucidex OR Manotin OR Mantine OR Mantomed OR Marbodin OR Mardewel OR Marixino OR Maruxa OR Maxiram OR Melanda OR Memabix OR Memamed OR Memando OR Memantin OR Memantina OR Memantine OR Memantinol OR Memantyn OR Memanvitae OR Memanxa OR Memanzaks OR Memary OR Memax OR Memexa OR Memigmin OR Memikare OR Memogen OR Memolan OR Memorel OR Memorix OR Memotec OR Memox OR Memxa OR Mentadem OR Mentikline OR Mentium OR Mentixa OR Merandex OR Merital OR Mexia OR Mimetix OR Mirvedol OR Modualz OR MOR ysa OR Nemdaa OR Namenda OR Namzaric OR Nemdatine OR Neumantine OR Neuro‐K OR Neuroplus* OR Noojerone OR PMS‐Memantine OR Polmatine OR Prilben OR Pronervon OR Ratio‐Memantine OR Ravemantine OR Sandoz‐Memantine OR Talentum OR Timantil OR Tingreks OR Tonibral* OR Tormoro OR Valcoxia OR Vilimen OR Vivimex OR Witgen OR Xapimant OR Ymana OR Zalatine OR Zarlyn OR Zeimer OR Zemertinex OR Zenmem OR Zenmen OR Zimer) AND (autis* OR asperger* OR kanner* OR "pervasive developmental disorder" OR PDD OR PDDs OR "PDD‐NOS" OR ASD OR ASDs OR "childhood schizophrenia") AND ( db:("LILACS") AND type_of_study:("clinical_trials"))
TOXLINE
toxnet.nlm.nih.gov/newtoxnet/toxline.htm
Searched 25 November 2020 (54 records) Not available in 2022
tox [subset] AND (autis* or asperger* or kanner* or pervasive development disorder or autis* or asperger* or PDD or PDDs or PDD‐NOS or ASD or ASDs ) and (Abixa or Adaxor or Admed or Akatinol or Alceba or Alios or Almenta or Alois or Alzant or Alzer or Alzia or Alzinex or Alzixa or Alzmenda or Alzmex or Apo‐Memantine or Axura or Biomentin or Cogito or Cognomem or Conexine or Cordure or Dantex or Demantin or Demax or Dementa or Dementexa or Ebixa or Ebitex or Emantin or Emaxin or Esmirtal or Eutebrol or Evy or Ezemantis or Fentina or Korint or Lemix or Lindex or Lucidex or Manotin or Mantine or Mantomed or Marbodin or Mardewel or Marixino or Maruxa or Maxiram or Melanda or Memabix or Memamed or Memando or Memantin or Memantina or Memantine or Memantinol or Memantyn or Memanvitae or Memanxa or Memanzaks or Memary or Memax or Memexa or Memigmin or Memikare or Memogen or Memolan or Memorel or Memorix or Memotec or Memox or Memxa or Mentadem or Mentikline or Mentium or Mentixa or Merandex or Merital or Mexia or Mimetix or Mirvedol or Modualz or Morysa or Nemdaa or Namenda or Namzaric or Nemdatine or Neumantine or Neuro‐K or Neuroplus* or Noojerone or PMS‐Memantine or Polmatine or Prilben or Pronervon or Ratio‐Memantine or Ravemantine or Sandoz‐Memantine or Talentum or Timantil or Tingreks or Tonibral* or Tormoro or Valcoxia or Vilimen or Vivimex or Witgen or Xapimant or Ymana or Zalatine or Zarlyn or Zeimer or Zemertinex or Zenmem or Zenmen or Zimer)
PubMed using the PubMed Toxicology filter
Searched 15 February 2022 (14 new records) TOXLINE was replaced with a PubMed search using PubMed's toxicology filter, last reviewed in March 2020 (www.nlm.nih.gov/bsd/pubmed_subsets/tox_strategy.html)
"((autis* or asperger* or kanner* or pervasive development disorder or autis* or asperger* or PDD or PDDs or PDD‐NOS or ASD or ASDs ) and (Abixa or Adaxor or Admed or Akatinol or Alceba or Alios or Almenta or Alois or Alzant or Alzer or Alzia or Alzinex or Alzixa or Alzmenda or Alzmex or Apo‐Memantine or Axura or Biomentin or Cogito or Cognomem or Conexine or Cordure or Dantex or Demantin or Demax or Dementa or Dementexa or Ebixa or Ebitex or Emantin or Emaxin or Esmirtal or Eutebrol or Evy or Ezemantis or Fentina or Korint or Lemix or Lindex or Lucidex or Manotin or Mantine or Mantomed or Marbodin or Mardewel or Marixino or Maruxa or Maxiram or Melanda or Memabix or Memamed or Memando or Memantin or Memantina or Memantine or Memantinol or Memantyn or Memanvitae or Memanxa or Memanzaks or Memary or Memax or Memexa or Memigmin or Memikare or Memogen or Memolan or Memorel or Memorix or Memotec or Memox or Memxa or Mentadem or Mentikline or Mentium or Mentixa or Merandex or Merital or Mexia or Mimetix or Mirvedol or Modualz or Morysa or Nemdaa or Namenda or Namzaric or Nemdatine or Neumantine or Neuro‐K or Neuroplus* or Noojerone or PMS‐Memantine or Polmatine or Prilben or Pronervon or Ratio‐Memantine or Ravemantine or Sandoz‐Memantine or Talentum or Timantil or Tingreks or Tonibral* or Tormoro or Valcoxia or Vilimen or Vivimex or Witgen or Xapimant or Ymana ora Zalatine or Zarlyn or Zeimer or Zemertinex or Zenmem or Zenmen or Zimer)) AND (drug‐induced abnormalities OR occupational accidents OR adverse drug reaction reporting systems OR Drug‐Induced Akathisia OR Amino Acids, Peptides, and Proteins/ae [MH] OR Animal Diseases/CI [MH] OR poisonous animals OR Background Radiation OR biohazard release OR Biological Factors/ae [MH] OR Biomedical and Dental Materials/ae [MH] OR birth weight/de [MH] OR chemical burns OR Carbohydrates/ae [MH] OR carcinogen* OR Carcinogenesis OR cardiotox* OR Cardiotoxicity OR Cardiovascular Diseases/CI [MH] OR Chemical Actions and Uses/ae [MH] OR Chemical and Drug Induced Liver Injury OR chemical hazard release OR chemical terrorism OR Chemically‐Induced Disorders OR Climate Change OR Clin Toxicol Phila [TA] OR Colony Collapse OR Complex Mixtures/ae [MH] OR Congenital, Hereditary, and Neonatal Diseases and Abnormalities/CI [MH] OR Crit Rev Toxicol [TA] OR Digestive System Diseases/CI [MH] OR Disorders of Environmental Origin/CI [MH] OR Drug Interactions OR Drug Recalls OR drug therapy/ae [MH] OR Drug‐Induced Dyskinesia OR ecotox* OR Ecotoxicology OR Endocrine System Diseases/CI [MH] OR Environ Health Perspect [TA] OR Environ Toxicol Chem [TA] OR Environ Toxicol Pharmacol [TA] OR Environment and Public Health/ae [MH] OR Environmental Health OR environmental illness OR environmental monitoring OR environmental pollutants OR environmental pollution OR Environmental Restoration and Remediation OR Enzymes and Coenzymes/ae [MH] OR Extreme Environments OR Eye Diseases/CI [MH] OR Female Urogenital Diseases and Pregnancy Complications/CI [MH] OR Fetal Alcohol Spectrum Disorders OR food and beverages/ae [MH] OR forensic toxicology OR Genetic Phenomena/de [MH] OR Global Warming OR hazardous substances OR Hemic and Lymphatic Diseases/CI [MH] OR hepatotox* OR Heterocyclic Compounds/ae [MH] OR Hormones, Hormone Substitutes, and Hormone Antagonists/ae [MH] OR household products/ae [MH] OR Hum Exp Toxicol [TA] OR Immune System Diseases/CI [MH] OR immunotox* OR Metabolic Inactivation OR Inorganic Chemicals/ae [MH] OR Integumentary System Physiological Phenomena/de [MH] OR J Toxicol Environ Health [TA] OR J Toxicol Sci [TA] OR LC50 OR Lipids/ae [MH] OR Macromolecular Substances/ae [MH] OR Male Urogenital Diseases/CI [MH] OR manufactured materials/ae [MH] OR Material Safety Data Sheets OR mental disorders/ci [MH] OR Musculoskeletal Diseases/CI [MH] OR mutagen* OR mutagenesis OR nanostructures OR Neoplasms/CI [MH] OR nephrotox* OR Nervous System Diseases/CI [MH] OR neurotox* OR noxae OR Nuclear Power Plants OR Nucleic Acids, Nucleotides, and Nucleosides/ae [MH] OR Nutritional and Metabolic Diseases/CI [MH] OR occupational diseases OR Ocular Physiological Phenomena/de [MH] OR Organic Chemicals/ae [MH] OR Otorhinolaryngologic Diseases/CI [MH] OR Pathological Conditions, Signs and Symptoms /CI [MH] OR persian gulf syndrome OR pesticides/to [MH] OR Pharmaceutical Preparations/ae [MH] OR Phytochemicals/ae [MH] OR plants, medicinal/ae [MH] OR toxic plants OR poison* OR poisoning OR Polycyclic Compounds/ae [MH] OR substance‐induced psychoses OR radiation injuries OR Radiation Monitoring OR radiation‐induced abnormalities OR Radioactive Hazard Release OR Radioactive Pollutants OR radiotherapy/ae [MH] OR Regul Toxicol Pharmacol [TA] OR Reproductive and Urinary Physiological Phenomena/de [MH] OR Respiratory Tract Diseases/CI [MH] OR Safety‐Based Drug Withdrawals OR Skin and Connective Tissue Diseases/CI [MH] OR Stomatognathic Diseases/CI [MH] OR substance‐related disorders OR terata* OR terato* OR Teratogenesis OR Drug Therapeutic Index OR Toxic Actions OR toxic OR toxicity tests OR Toxicokinetics OR Toxicol Appl Pharmacol [TA] OR Toxicological Phenomena OR toxicology OR Toxicology [TA] OR toxif* OR toxig* OR Toxin‐Antitoxin Systems OR venoms/to [MH])" "(""autis*""[All Fields] OR ""asperger*""[All Fields] OR ""kanner*""[All Fields] OR (""child development disorders, pervasive""[MeSH Terms] OR (""child""[All Fields] AND ""development""[All Fields] AND ""Disorders""[All Fields] AND ""pervasive""[All Fields]) OR ""pervasive child development disorders""[All Fields] OR (""pervasive""[All Fields] AND ""development""[All Fields] AND ""disorder""[All Fields]) OR ""pervasive development disorder""[All Fields]) OR ""autis*""[All Fields] OR ""asperger*""[All Fields] OR ""PDD""[All Fields] OR ""PDDs""[All Fields] OR ""PDD‐NOS""[All Fields] OR (""arthropod struct dev""[Journal] OR ""agron sustain dev""[Journal] OR ""asd""[All Fields]) OR ""ASDs""[All Fields]) AND (""Abixa""[All Fields] OR ""Admed""[All Fields] OR ""Akatinol""[All Fields] OR ""Alios""[All Fields] OR ""Almenta""[All Fields] OR ""Alois""[All Fields] OR ""Alzer""[All Fields] OR (""memantine""[MeSH Terms] OR ""memantine""[All Fields] OR ""axura""[All Fields]) OR ""Cogito""[All Fields] OR ""Conexine""[All Fields] OR ""Dantex""[All Fields] OR ""Demax""[All Fields] OR ""Dementa""[All Fields] OR (""memantine""[MeSH Terms] OR ""memantine""[All Fields] OR ""ebixa""[All Fields]) OR ""Evy""[All Fields] OR ""Korint""[All Fields] OR (""lemix""[Supplementary Concept] OR ""lemix""[All Fields] OR ""lemix""[All Fields]) OR ""Lindex""[All Fields] OR ""Mantine""[All Fields] OR ""Maruxa""[All Fields] OR ""Melanda""[All Fields] OR (""memantine""[MeSH Terms] OR ""memantine""[All Fields] OR ""memantin""[All Fields] OR ""memantine s""[All Fields]) OR (""memantine""[MeSH Terms] OR ""memantine""[All Fields] OR ""memantina""[All Fields]) OR (""memantine""[MeSH Terms] OR ""memantine""[All Fields] OR ""memantin""[All Fields] OR ""memantine s""[All Fields]) OR ""Memantinol""[All Fields] OR ""Memary""[All Fields] OR ""Memax""[All Fields] OR (""nomifensine""[MeSH Terms] OR ""nomifensine""[All Fields] OR ""merital""[All Fields]) OR ""Mexia""[All Fields] OR (""memantine""[MeSH Terms] OR ""memantine""[All Fields] OR ""memantin""[All Fields] OR ""namenda""[All Fields] OR ""memantine s""[All Fields]) OR (""namzaric""[Supplementary Concept] OR ""namzaric""[All Fields] OR ""namzaric""[All Fields] OR ""donepezil""[MeSH Terms] OR ""donepezil""[All Fields] OR ""donepezil s""[All Fields] OR ""memantine""[MeSH Terms] OR ""memantine""[All Fields] OR ""memantin""[All Fields] OR ""memantine s""[All Fields]) OR ""Neuro‐K""[All Fields] OR ""neuroplus*""[All Fields] OR ""Noojerone""[All Fields] OR ""Pronervon""[All Fields] OR ""Talentum""[All Fields] OR ""tonibral*""[All Fields] OR ""Witgen""[All Fields] OR ""Ymana""[All Fields] OR ""Zeimer""[All Fields] OR ""Zenmen""[All Fields] OR ""Zimer""[All Fields]) AND (""abnormalities, drug induced""[MeSH Terms] OR (""Abnormalities""[All Fields] AND ""drug induced""[All Fields]) OR ""drug‐induced abnormalities""[All Fields] OR (""drug""[All Fields] AND ""induced""[All Fields] AND ""Abnormalities""[All Fields]) OR ""drug induced abnormalities""[All Fields] OR (""accidents, occupational""[MeSH Terms] OR (""accidents""[All Fields] AND ""occupational""[All Fields]) OR ""occupational accidents""[All Fields] OR (""occupational""[All Fields] AND ""accidents""[All Fields])) OR (""adverse drug reaction reporting systems""[MeSH Terms] OR (""adverse""[All Fields] AND ""drug""[All Fields] AND ""reaction""[All Fields] AND ""reporting""[All Fields] AND ""systems""[All Fields]) OR ""adverse drug reaction reporting systems""[All Fields]) OR (""akathisia, drug induced""[MeSH Terms] OR (""akathisia""[All Fields] AND ""drug induced""[All Fields]) OR ""drug‐induced akathisia""[All Fields] OR (""drug""[All Fields] AND ""induced""[All Fields] AND ""akathisia""[All Fields]) OR ""drug induced akathisia""[All Fields]) OR ""amino acids, peptides, and proteins/adverse effects""[MeSH Terms] OR ""animal diseases/chemically induced""[MeSH Terms] OR (""animals, poisonous""[MeSH Terms] OR (""animals""[All Fields] AND ""poisonous""[All Fields]) OR ""poisonous animals""[All Fields] OR (""poisonous""[All Fields] AND ""animals""[All Fields])) OR (""background radiation""[MeSH Terms] OR (""background""[All Fields] AND ""radiation""[All Fields]) OR ""background radiation""[All Fields]) OR (""biohazard release""[MeSH Terms] OR (""biohazard""[All Fields] AND ""release""[All Fields]) OR ""biohazard release""[All Fields]) OR ""biological factors/adverse effects""[MeSH Terms] OR ""biomedical and dental materials/adverse effects""[MeSH Terms] OR ""birth weight/drug effects""[MeSH Terms] OR (""burns, chemical""[MeSH Terms] OR (""burns""[All Fields] AND ""Chemical""[All Fields]) OR ""chemical burns""[All Fields] OR (""Chemical""[All Fields] AND ""burns""[All Fields])) OR ""carbohydrates/adverse effects""[MeSH Terms] OR ""carcinogen*""[All Fields] OR (""carcinogenesis""[MeSH Terms] OR ""carcinogenesis""[All Fields] OR ""carcinogeneses""[All Fields]) OR ""cardiotox*""[All Fields] OR (""cardiotoxic""[All Fields] OR ""cardiotoxicity""[MeSH Terms] OR ""cardiotoxicity""[All Fields] OR ""cardiotoxicities""[All Fields] OR ""cardiotoxity""[All Fields]) OR ""cardiovascular diseases/chemically induced""[MeSH Terms] OR ""chemical actions and uses/adverse effects""[MeSH Terms] OR (""chemical and drug induced liver injury""[MeSH Terms] OR (""Chemical""[All Fields] AND ""drug""[All Fields] AND ""induced""[All Fields] AND ""liver""[All Fields] AND ""injury""[All Fields]) OR ""chemical and drug induced liver injury""[All Fields]) OR (""chemical hazard release""[MeSH Terms] OR (""Chemical""[All Fields] AND ""hazard""[All Fields] AND ""release""[All Fields]) OR ""chemical hazard release""[All Fields]) OR (""chemical terrorism""[MeSH Terms] OR (""Chemical""[All Fields] AND ""terrorism""[All Fields]) OR ""chemical terrorism""[All Fields]) OR (""chemically induced disorders""[MeSH Terms] OR (""chemically induced""[All Fields] AND ""Disorders""[All Fields]) OR ""chemically induced disorders""[All Fields] OR (""chemically""[All Fields] AND ""induced""[All Fields] AND ""Disorders""[All Fields]) OR ""chemically induced disorders""[All Fields]) OR (""climate change""[MeSH Terms] OR (""climate""[All Fields] AND ""change""[All Fields]) OR ""climate change""[All Fields]) OR ""clin toxicol phila""[Journal] OR (""colony collapse""[MeSH Terms] OR (""colony""[All Fields] AND ""collapse""[All Fields]) OR ""colony collapse""[All Fields]) OR ""complex mixtures/adverse effects""[MeSH Terms] OR ""congenital, hereditary, and neonatal diseases and abnormalities/chemically induced""[MeSH Terms] OR ""crit rev toxicol""[Journal] OR ""digestive system diseases/chemically induced""[MeSH Terms] OR ""disorders of environmental origin/chemically induced""[MeSH Terms] OR (""drug interactions""[MeSH Terms] OR (""drug""[All Fields] AND ""interactions""[All Fields]) OR ""drug interactions""[All Fields]) OR (""drug recalls""[MeSH Terms] OR (""drug""[All Fields] AND ""recalls""[All Fields]) OR ""drug recalls""[All Fields]) OR ""drug therapy/adverse effects""[MeSH Terms] OR (""dyskinesia, drug induced""[MeSH Terms] OR (""dyskinesia""[All Fields] AND ""drug induced""[All Fields]) OR ""drug‐induced dyskinesia""[All Fields] OR (""drug""[All Fields] AND ""induced""[All Fields] AND ""dyskinesia""[All Fields]) OR ""drug induced dyskinesia""[All Fields]) OR ""ecotox*""[All Fields] OR (""ecotoxicology""[MeSH Terms] OR ""ecotoxicology""[All Fields]) OR ""endocrine system diseases/chemically induced""[MeSH Terms] OR ""environ health perspect""[Journal] OR ""environ toxicol chem""[Journal] OR ""environ toxicol pharmacol""[Journal] OR ""environment and public health/adverse effects""[MeSH Terms] OR (""environmental health""[MeSH Terms] OR (""Environmental""[All Fields] AND ""Health""[All Fields]) OR ""environmental health""[All Fields]) OR (""environmental illness""[MeSH Terms] OR (""Environmental""[All Fields] AND ""illness""[All Fields]) OR ""environmental illness""[All Fields]) OR (""environmental monitoring""[MeSH Terms] OR (""Environmental""[All Fields] AND ""monitoring""[All Fields]) OR ""environmental monitoring""[All Fields]) OR (""environmental pollutants""[Pharmacological Action] OR ""environmental pollutants""[MeSH Terms] OR (""Environmental""[All Fields] AND ""pollutants""[All Fields]) OR ""environmental pollutants""[All Fields]) OR (""environmental pollution""[MeSH Terms] OR (""Environmental""[All Fields] AND ""pollution""[All Fields]) OR ""environmental pollution""[All Fields]) OR (""environmental restoration and remediation""[MeSH Terms] OR (""Environmental""[All Fields] AND ""restoration""[All Fields] AND ""remediation""[All Fields]) OR ""environmental restoration and remediation""[All Fields]) OR ""enzymes and coenzymes/adverse effects""[MeSH Terms] OR (""extreme environments""[MeSH Terms] OR (""extreme""[All Fields] AND ""environments""[All Fields]) OR ""extreme environments""[All Fields]) OR ""eye diseases/chemically induced""[MeSH Terms] OR ""female urogenital diseases and pregnancy complications/chemically induced""[MeSH Terms] OR (""fetal alcohol spectrum disorders""[MeSH Terms] OR (""fetal""[All Fields] AND ""alcohol""[All Fields] AND ""spectrum""[All Fields] AND ""Disorders""[All Fields]) OR ""fetal alcohol spectrum disorders""[All Fields]) OR ""food and beverages/adverse effects""[MeSH Terms] OR (""forensic toxicology""[MeSH Terms] OR (""forensic""[All Fields] AND ""toxicology""[All Fields]) OR ""forensic toxicology""[All Fields]) OR ""genetic phenomena/drug effects""[MeSH Terms] OR (""global warming""[MeSH Terms] OR (""global""[All Fields] AND ""warming""[All Fields]) OR ""global warming""[All Fields]) OR (""hazardous substances""[Pharmacological Action] OR ""hazardous substances""[MeSH Terms] OR (""hazardous""[All Fields] AND ""Substances""[All Fields]) OR ""hazardous substances""[All Fields]) OR ""hemic and lymphatic diseases/chemically induced""[MeSH Terms] OR ""hepatotox*""[All Fields] OR ""heterocyclic compounds/adverse effects""[MeSH Terms] OR ""hormones, hormone substitutes, and hormone antagonists/adverse effects""[MeSH Terms] OR ""household products/adverse effects""[MeSH Terms] OR ""hum exp toxicol""[Journal] OR ""immune system diseases/chemically induced""[MeSH Terms] OR ""immunotox*""[All Fields] OR (""inactivation, metabolic""[MeSH Terms] OR (""inactivation""[All Fields] AND ""Metabolic""[All Fields]) OR ""metabolic inactivation""[All Fields] OR (""Metabolic""[All Fields] AND ""inactivation""[All Fields])) OR ""inorganic chemicals/adverse effects""[MeSH Terms] OR ""integumentary system physiological phenomena/drug effects""[MeSH Terms] OR ""j toxicol environ health""[Journal] OR ""j toxicol sci""[Journal] OR (""lethal dose 50""[MeSH Terms] OR ""lethal dose 50""[All Fields] OR ""lc50""[All Fields]) OR ""lipids/adverse effects""[MeSH Terms] OR ""macromolecular substances/adverse effects""[MeSH Terms] OR ""male urogenital diseases/chemically induced""[MeSH Terms] OR ""manufactured materials/adverse effects""[MeSH Terms] OR (""material safety data sheets""[MeSH Terms] OR (""material""[All Fields] AND ""safety""[All Fields] AND ""data""[All Fields] AND ""sheets""[All Fields]) OR ""material safety data sheets""[All Fields]) OR ""mental disorders/chemically induced""[MeSH Terms] OR ""musculoskeletal diseases/chemically induced""[MeSH Terms] OR ""mutagen*""[All Fields] OR (""mutagenesis""[MeSH Terms] OR ""mutagenesis""[All Fields] OR ""mutageneses""[All Fields]) OR (""nanostructural""[All Fields] OR ""nanostructuration""[All Fields] OR ""nanostructure s""[All Fields] OR ""nanostructured""[All Fields] OR ""nanostructures""[MeSH Terms] OR ""nanostructures""[All Fields] OR ""nanostructure""[All Fields] OR ""nanostructuring""[All Fields] OR ""nanostructurization""[All Fields]) OR ""neoplasms/chemically induced""[MeSH Terms] OR ""nephrotox*""[All Fields] OR ""nervous system diseases/chemically induced""[MeSH Terms] OR ""neurotox*""[All Fields] OR (""noxae""[Pharmacological Action] OR ""noxae""[MeSH Terms] OR ""noxae""[All Fields]) OR (""nuclear power plants""[MeSH Terms] OR (""nuclear""[All Fields] AND ""power""[All Fields] AND ""plants""[All Fields]) OR ""nuclear power plants""[All Fields]) OR ""nucleic acids, nucleotides, and nucleosides/adverse effects""[MeSH Terms] OR ""nutritional and metabolic diseases/chemically induced""[MeSH Terms] OR (""occupational diseases""[MeSH Terms] OR (""occupational""[All Fields] AND ""Diseases""[All Fields]) OR ""occupational diseases""[All Fields]) OR ""ocular physiological phenomena/drug effects""[MeSH Terms] OR ""organic chemicals/adverse effects""[MeSH Terms] OR ""otorhinolaryngologic diseases/chemically induced""[MeSH Terms] OR ""pathological conditions, signs and symptoms/chemically induced""[MeSH Terms] OR (""persian gulf syndrome""[MeSH Terms] OR (""persian""[All Fields] AND ""gulf""[All Fields] AND ""syndrome""[All Fields]) OR ""persian gulf syndrome""[All Fields]) OR ""pesticides/toxicity""[MeSH Terms] OR ""pharmaceutical preparations/adverse effects""[MeSH Terms] OR ""phytochemicals/adverse effects""[MeSH Terms] OR ""plants, medicinal/adverse effects""[MeSH Terms] OR (""plants, toxic""[MeSH Terms] OR (""plants""[All Fields] AND ""toxic""[All Fields]) OR ""toxic plants""[All Fields] OR (""toxic""[All Fields] AND ""plants""[All Fields])) OR ""poison*""[All Fields] OR (""poisoned""[All Fields] OR ""poisoning""[MeSH Terms] OR ""poisoning""[All Fields] OR ""poisonings""[All Fields] OR ""poisoning""[MeSH Subheading] OR ""poisonous""[All Fields] OR ""poisons""[Pharmacological Action] OR ""poisons""[MeSH Terms] OR ""poisons""[All Fields] OR ""poison""[All Fields]) OR ""polycyclic compounds/adverse effects""[MeSH Terms] OR (""psychoses, substance induced""[MeSH Terms] OR (""psychoses""[All Fields] AND ""substance induced""[All Fields]) OR ""substance‐induced psychoses""[All Fields] OR (""substance""[All Fields] AND ""induced""[All Fields] AND ""psychoses""[All Fields]) OR ""substance induced psychoses""[All Fields]) OR (""radiation injuries""[MeSH Terms] OR (""radiation""[All Fields] AND ""injuries""[All Fields]) OR ""radiation injuries""[All Fields]) OR (""radiation monitoring""[MeSH Terms] OR (""radiation""[All Fields] AND ""monitoring""[All Fields]) OR ""radiation monitoring""[All Fields]) OR (""abnormalities, radiation induced""[MeSH Terms] OR (""Abnormalities""[All Fields] AND ""radiation induced""[All Fields]) OR ""radiation‐induced abnormalities""[All Fields] OR (""radiation""[All Fields] AND ""induced""[All Fields] AND ""Abnormalities""[All Fields]) OR ""radiation induced abnormalities""[All Fields]) OR (""radioactive hazard release""[MeSH Terms] OR (""radioactive""[All Fields] AND ""hazard""[All Fields] AND ""release""[All Fields]) OR ""radioactive hazard release""[All Fields]) OR (""radioactive pollutants""[MeSH Terms] OR (""radioactive""[All Fields] AND ""pollutants""[All Fields]) OR ""radioactive pollutants""[All Fields]) OR ""radiotherapy/adverse effects""[MeSH Terms] OR ""regul toxicol pharmacol""[Journal] OR ""reproductive and urinary physiological phenomena/drug effects""[MeSH Terms] OR ""respiratory tract diseases/chemically induced""[MeSH Terms] OR (""safety based drug withdrawals""[MeSH Terms] OR (""safety based""[All Fields] AND ""drug""[All Fields] AND ""withdrawals""[All Fields]) OR ""safety based drug withdrawals""[All Fields] OR (""safety""[All Fields] AND ""based""[All Fields] AND ""drug""[All Fields] AND ""withdrawals""[All Fields]) OR ""safety based drug withdrawals""[All Fields]) OR ""skin and connective tissue diseases/chemically induced""[MeSH Terms] OR ""stomatognathic diseases/chemically induced""[MeSH Terms] OR (""substance related disorders""[MeSH Terms] OR (""substance related""[All Fields] AND ""Disorders""[All Fields]) OR ""substance related disorders""[All Fields] OR (""substance""[All Fields] AND ""related""[All Fields] AND ""Disorders""[All Fields]) OR ""substance related disorders""[All Fields]) OR ""terata*""[All Fields] OR ""terato*""[All Fields] OR (""teratogenesis""[MeSH Terms] OR ""teratogenesis""[All Fields]) OR (""therapeutic index, drug""[MeSH Terms] OR (""therapeutic""[All Fields] AND ""index""[All Fields] AND ""drug""[All Fields]) OR ""drug therapeutic index""[All Fields] OR (""drug""[All Fields] AND ""therapeutic""[All Fields] AND ""index""[All Fields])) OR (""toxic actions""[MeSH Terms] OR (""toxic""[All Fields] AND ""Actions""[All Fields]) OR ""toxic actions""[All Fields]) OR (""toxic""[All Fields] OR ""toxical""[All Fields] OR ""toxically""[All Fields] OR ""toxicant""[All Fields] OR ""toxicant s""[All Fields] OR ""toxicants""[All Fields] OR ""toxicated""[All Fields] OR ""toxication""[All Fields] OR ""toxicities""[All Fields] OR ""toxicity""[MeSH Subheading] OR ""toxicity""[All Fields] OR ""toxicity s""[All Fields] OR ""toxics""[All Fields]) OR (""toxicity tests""[MeSH Terms] OR (""toxicity""[All Fields] AND ""tests""[All Fields]) OR ""toxicity tests""[All Fields]) OR (""pharmacokinetics""[MeSH Terms] OR ""pharmacokinetics""[All Fields] OR ""toxicokinetic""[All Fields] OR ""pharmacokinetics""[MeSH Subheading] OR ""toxicokinetics""[All Fields] OR ""toxicokinetics""[MeSH Terms]) OR ""toxicol appl pharmacol""[Journal] OR (""toxicological phenomena""[MeSH Terms] OR (""toxicological""[All Fields] AND ""Phenomena""[All Fields]) OR ""toxicological phenomena""[All Fields]) OR (""toxicologies""[All Fields] OR ""toxicology""[MeSH Terms] OR ""toxicology""[All Fields]) OR (""toxicology""[Journal] OR ""toxicol open access""[Journal] OR ""open access j toxicol""[Journal] OR ""isrn toxicol""[Journal] OR ""crc crit rev toxicol""[Journal]) OR ""toxif*""[All Fields] OR ""toxig*""[All Fields] OR (""toxin antitoxin systems""[MeSH Terms] OR (""toxin antitoxin""[All Fields] AND ""systems""[All Fields]) OR ""toxin antitoxin systems""[All Fields] OR (""toxin""[All Fields] AND ""antitoxin""[All Fields] AND ""systems""[All Fields]) OR ""toxin antitoxin systems""[All Fields]) OR ""venoms/toxicity""[MeSH Terms])"
ProQuest Dissertations & Theses Global
Searched 26 November 2020 (no records) Searched 15 February 2022 (1 new record)
ti(memantine) AND ti(autis* OR asd OR asperger*) AND ti(random* or trial* or placebo* )
Cochrane Database of Systematic Reviews (CDSR)
Searched 25 November 2020 (2 records) Searched 15 February 2022 (1 new record)
#1 [mh "child development disorders, pervasive"] #2 [mh ^"Developmental Disabilities"] #3 [mh ^"Neurodevelopmental Disorders"] #4 (pervasive NEXT development* NEXT disorder*):ti,ab,kw #5 (pervasive near/3 child):ti,ab,kw #6 (PDD or PDDs or PDD next NOS or ASD or ASDs):ti,ab,kw #7 (autis* or asperger* or kanner*):ti,ab,kw #8 (childhood next schizophrenia):ti,ab,kw #9 {or #1‐#8} #10 [mh memantine] #11 [mh Amantadine] #12 (Abixa or Adaxor or Admed or Akatinol or Alceba or Alios or Almenta or Alois or Alzant or Alzer or Alzia or Alzinex or Alzixa or Alzmenda or Alzmex or Apo‐Memantine or Axura or Biomentin or Carrier or Cogito or Cognomem or Conexine or Cordure or Dantex or Demantin or Demax or Dementa or Dementexa or Ebixa or Ebitex or Emantin or Emaxin or Esmirtal or Eutebrol or Evy or Ezemantis or Fentina or Korint or Lemix or Lindex or Lucidex or Manotin or Mantine or Mantomed or Marbodin or Mardewel or Marixino or Maruxa or Maxiram or Melanda or Memabix or Memamed or Memando or Memantin or Memantina or Memantine or Memantinol or Memantyn or Memanvitae or Memanxa or Memanzaks or Memary or Memax or Memexa or Memigmin or Memikare or Memogen or Memolan or Memorel or Memorix or Memotec or Memox or Memxa or Mentadem or Mentikline or Mentium or Mentixa or Merandex or Merital or Mexia or Mimetix or Mirvedol or Modualz or Morysa or Nemdaa or Namenda or Namzaric or Nemdatine or Neumantine or Neuro‐K or Neuroplus* or Noojerone or PMS‐Memantine or Polmatine or Prilben or Pronervon or Ratio‐Memantine or Ravemantine or Sandoz‐Memantine or Talentum or Timantil or Tingreks or Tonibral* or Tormoro or Valcoxia or Vilimen or Vivimex or Witgen or Xapimant or Ymana or Zalatine or Zarlyn or Zeimer or Zemertinex or Zenmem or Zenmen or Zimer):ti,ab,kw #13 {or #10‐#12} #14 #9 AND #13 in Cochrane Reviews, Cochrane Protocols
Epistemonikos
www.epistemonikos.org/en/ Searched 25 November 2020 (10 records) Searched 15 February 2022 (no new records)
(title:((title:((Abixa OR Adaxor OR Admed OR Akatinol OR Alceba OR Alios OR Almenta OR Alois OR Alzant OR Alzer OR Alzia OR Alzinex OR Alzixa OR Alzmenda OR Alzmex OR Apo‐Memantine OR Axura OR Biomentin OR Carrier OR Cogito OR Cognomem OR Conexine OR Cordure OR Dantex OR Demantin OR Demax OR Dementa OR Dementexa OR Ebixa OR Ebitex OR Emantin OR Emaxin OR Esmirtal OR Eutebrol OR Evy OR Ezemantis OR Fentina OR Korint OR Lemix OR Lindex OR Lucidex OR Manotin OR Mantine OR Mantomed OR Marbodin OR Mardewel OR Marixino OR Maruxa OR Maxiram OR Melanda OR Memabix OR Memamed OR Memando OR Memantin OR Memantina OR Memantine OR Memantinol OR Memantyn OR Memanvitae OR Memanxa OR Memanzaks OR Memary OR Memax OR Memexa OR Memigmin OR Memikare OR Memogen OR Memolan OR Memorel OR Memorix OR Memotec OR Memox OR Memxa OR Mentadem OR Mentikline OR Mentium OR Mentixa OR Merandex OR Merital OR Mexia OR Mimetix OR Mirvedol OR Modualz OR Morysa OR Nemdaa OR Namenda OR Namzaric OR Nemdatine OR Neumantine OR Neuro‐K OR Neuroplus* OR Noojerone OR PMS‐Memantine OR Polmatine OR Prilben OR Pronervon OR Ratio‐Memantine OR Ravemantine OR Sandoz‐Memantine OR Talentum OR Timantil OR Tingreks OR Tonibral* OR Tormoro OR Valcoxia OR Vilimen OR Vivimex OR Witgen OR Xapimant OR Ymana OR Zalatine OR Zarlyn OR Zeimer OR Zemertinex OR Zenmem OR Zenmen OR Zimer)) OR abstract:((Abixa OR Adaxor OR Admed OR Akatinol OR Alceba OR Alios OR Almenta OR Alois OR Alzant OR Alzer OR Alzia OR Alzinex OR Alzixa OR Alzmenda OR Alzmex OR Apo‐Memantine OR Axura OR Biomentin OR Carrier OR Cogito OR Cognomem OR Conexine OR Cordure OR Dantex OR Demantin OR Demax OR Dementa OR Dementexa OR Ebixa OR Ebitex OR Emantin OR Emaxin OR Esmirtal OR Eutebrol OR Evy OR Ezemantis OR Fentina OR Korint OR Lemix OR Lindex OR Lucidex OR Manotin OR Mantine OR Mantomed OR Marbodin OR Mardewel OR Marixino OR Maruxa OR Maxiram OR Melanda OR Memabix OR Memamed OR Memando OR Memantin OR Memantina OR Memantine OR Memantinol OR Memantyn OR Memanvitae OR Memanxa OR Memanzaks OR Memary OR Memax OR Memexa OR Memigmin OR Memikare OR Memogen OR Memolan OR Memorel OR Memorix OR Memotec OR Memox OR Memxa OR Mentadem OR Mentikline OR Mentium OR Mentixa OR Merandex OR Merital OR Mexia OR Mimetix OR Mirvedol OR Modualz OR Morysa OR Nemdaa OR Namenda OR Namzaric OR Nemdatine OR Neumantine OR Neuro‐K OR Neuroplus* OR Noojerone OR PMS‐Memantine OR Polmatine OR Prilben OR Pronervon OR Ratio‐Memantine OR Ravemantine OR Sandoz‐Memantine OR Talentum OR Timantil OR Tingreks OR Tonibral* OR Tormoro OR Valcoxia OR Vilimen OR Vivimex OR Witgen OR Xapimant OR Ymana OR Zalatine OR Zarlyn OR Zeimer OR Zemertinex OR Zenmem OR Zenmen OR Zimer))) AND (title:(autis* OR asperg* OR ASD OR ASDs OR PDD_NOS OR PDD OR PDDs) OR abstract:(autis* OR asperg* OR ASD OR ASDs OR PDD_NOS OR PDD OR PDDs)) NOT title:(PArkinson*)) OR abstract:((title:((Abixa OR Adaxor OR Admed OR Akatinol OR Alceba OR Alios OR Almenta OR Alois OR Alzant OR Alzer OR Alzia OR Alzinex OR Alzixa OR Alzmenda OR Alzmex OR Apo‐Memantine OR Axura OR Biomentin OR Carrier OR Cogito OR Cognomem OR Conexine OR Cordure OR Dantex OR Demantin OR Demax OR Dementa OR Dementexa OR Ebixa OR Ebitex OR Emantin OR Emaxin OR Esmirtal OR Eutebrol OR Evy OR Ezemantis OR Fentina OR Korint OR Lemix OR Lindex OR Lucidex OR Manotin OR Mantine OR Mantomed OR Marbodin OR Mardewel OR Marixino OR Maruxa OR Maxiram OR Melanda OR Memabix OR Memamed OR Memando OR Memantin OR Memantina OR Memantine OR Memantinol OR Memantyn OR Memanvitae OR Memanxa OR Memanzaks OR Memary OR Memax OR Memexa OR Memigmin OR Memikare OR Memogen OR Memolan OR Memorel OR Memorix OR Memotec OR Memox OR Memxa OR Mentadem OR Mentikline OR Mentium OR Mentixa OR Merandex OR Merital OR Mexia OR Mimetix OR Mirvedol OR Modualz OR Morysa OR Nemdaa OR Namenda OR Namzaric OR Nemdatine OR Neumantine OR Neuro‐K OR Neuroplus* OR Noojerone OR PMS‐Memantine OR Polmatine OR Prilben OR Pronervon OR Ratio‐Memantine OR Ravemantine OR Sandoz‐Memantine OR Talentum OR Timantil OR Tingreks OR Tonibral* OR Tormoro OR Valcoxia OR Vilimen OR Vivimex OR Witgen OR Xapimant OR Ymana OR Zalatine OR Zarlyn OR Zeimer OR Zemertinex OR Zenmem OR Zenmen OR Zimer)) OR abstract:((Abixa OR Adaxor OR Admed OR Akatinol OR Alceba OR Alios OR Almenta OR Alois OR Alzant OR Alzer OR Alzia OR Alzinex OR Alzixa OR Alzmenda OR Alzmex OR Apo‐Memantine OR Axura OR Biomentin OR Carrier OR Cogito OR Cognomem OR Conexine OR Cordure OR Dantex OR Demantin OR Demax OR Dementa OR Dementexa OR Ebixa OR Ebitex OR Emantin OR Emaxin OR Esmirtal OR Eutebrol OR Evy OR Ezemantis OR Fentina OR Korint OR Lemix OR Lindex OR Lucidex OR Manotin OR Mantine OR Mantomed OR Marbodin OR Mardewel OR Marixino OR Maruxa OR Maxiram OR Melanda OR Memabix OR Memamed OR Memando OR Memantin OR Memantina OR Memantine OR Memantinol OR Memantyn OR Memanvitae OR Memanxa OR Memanzaks OR Memary OR Memax OR Memexa OR Memigmin OR Memikare OR Memogen OR Memolan OR Memorel OR Memorix OR Memotec OR Memox OR Memxa OR Mentadem OR Mentikline OR Mentium OR Mentixa OR Merandex OR Merital OR Mexia OR Mimetix OR Mirvedol OR Modualz OR Morysa OR Nemdaa OR Namenda OR Namzaric OR Nemdatine OR Neumantine OR Neuro‐K OR Neuroplus* OR Noojerone OR PMS‐Memantine OR Polmatine OR Prilben OR Pronervon OR Ratio‐Memantine OR Ravemantine OR Sandoz‐Memantine OR Talentum OR Timantil OR Tingreks OR Tonibral* OR Tormoro OR Valcoxia OR Vilimen OR Vivimex OR Witgen OR Xapimant OR Ymana OR Zalatine OR Zarlyn OR Zeimer OR Zemertinex OR Zenmem OR Zenmen OR Zimer))) AND (title:(autis* OR asperg* OR ASD OR ASDs OR PDD_NOS OR PDD OR PDDs) OR abstract:(autis* OR asperg* OR ASD OR ASDs OR PDD_NOS OR PDD OR PDDs)) NOT title:(PArkinson*)))
ClinicalTrials.gov
Searched 26 November 2020 (18 records when duplicates were removed) Searched 15 February 2022 (no new records)
Abixa OR Adaxor OR Admed OR Akatinol OR Alceba OR Alios OR Almenta OR Alois OR Alzant OR Alzer OR Alzia OR Alzinex OR Alzixa OR Alzmenda OR Alzmex OR Apo‐Memantine OR Axura OR Biomentin OR Carrier OR Cogito OR Cognomem OR Conexine OR Cordure | Autism OR ASD OR Asperger OR Pervasive development OR PDD‐NOS
Dantex OR Demantin OR Demax OR Dementa OR Dementexa OR Ebixa OR Ebitex OR Emantin OR Emaxin OR Esmirtal OR Eutebrol OR Evy OR Ezemantis OR Fentina OR Korint OR Lemix OR Lindex OR Lucidex OR Manotin OR Mantine OR Mantomed | Autism OR ASD OR Asperger OR Pervasive development OR PDD‐NOS
Marbodin OR Mardewel OR Marixino OR Maruxa OR Maxiram OR Melanda OR Memabix OR Memamed OR Memando OR Memantin OR Memantina OR Memantine OR Memantinol OR Memantyn OR Memanvitae OR Memanxa OR Memanzaks OR Memary OR Memax OR Memexa OR Memigmin | Autism OR ASD OR Asperger OR Pervasive development OR PDD‐NOS
Memikare OR Memogen OR Memolan OR Memorel OR Memorix OR Memotec OR Memox OR Memxa OR Mentadem OR Mentikline OR Mentium OR Mentixa OR Merandex OR Merital OR Mexia OR Mimetix OR Mirvedol OR Modualz OR Morysa OR Nemdaa OR Namenda OR Namzaric | Autism OR ASD OR Asperger OR Pervasive development OR PDD‐NOS
Nemdatine OR Neumantine OR Neuro‐K OR Neuroplus OR Noojerone OR PMS‐Memantine OR Polmatine OR Prilben OR Pronervon OR Ratio‐Memantine OR Ravemantine OR Sandoz‐Memantine OR Talentum OR Timantil OR Tingreks OR Tonibral OR Tormoro | Autism OR ASD OR Asperger OR Pervasive development OR PDD‐NOS
Valcoxia OR Vilimen OR Vivimex OR Witgen OR Xapimant OR Ymana OR Zalatine OR Zarlyn OR Zeimer OR Zemertinex OR Zenmem OR Zenmen OR Zimer | Autism OR ASD OR Asperger OR Pervasive development OR PDD‐NOS
World Health Organisation International Clinical Trials Registry Platform
apps.who.int/trialsearch/Default.aspx
Searched 26 November 2020 (18 records)
A simplified search was used to avoid time outs due to heavy website use because of pandemic
CONDITION ( autis* OR asperger* OR kanner* OR "pervasive developmental disorder" OR PDD OR PDDs OR "PDD‐NOS" OR ASD OR ASDs OR "childhood schizophrenia") AND INTERVENTION (memantine)
Searched 15 February 2022 (no new records)
A comprehensive search was run for all years
CONDITION ( autism OR asperger OR kanner OR "pervasive developmental disorder" OR PDD OR PDDs OR "PDD‐NOS" OR ASD OR ASDs OR "childhood schizophrenia") AND Intervention (Abixa OR Adaxor OR Admed OR Akatinol OR Alceba OR Alios OR Almenta OR Alois OR Alzant OR Alzer OR Alzia OR Alzinex OR Alzixa OR Alzmenda OR Alzmex OR Apo‐Memantine OR Axura OR Biomentin OR Carrier OR Cogito OR Cognomem OR Conexine OR Cordure)
CONDITION( autism OR asperger OR kanner OR "pervasive developmental disorder" OR PDD OR PDDs OR "PDD‐NOS" OR ASD OR ASDs OR "childhood schizophrenia") AND Intervention(Dantex OR Demantin OR Demax OR Dementa OR Dementexa OR Ebixa OR Ebitex OR Emantin OR Emaxin OR Esmirtal OR Eutebrol OR Evy OR Ezemantis OR Fentina OR Korint OR Lemix OR Lindex OR Lucidex OR Manotin OR Mantine OR Mantomed)
CONDITION( autism OR asperger OR kanner OR "pervasive developmental disorder" OR PDD OR PDDs OR "PDD‐NOS" OR ASD OR ASDs OR "childhood schizophrenia") AND Intervention (Memikare OR Memogen OR Memolan OR Memorel OR Memorix OR Memotec OR Memox OR Memxa OR Mentadem OR Mentikline OR Mentium OR Mentixa OR Merandex OR Merital OR Mexia OR Mimetix OR Mirvedol OR Modualz OR Morysa OR Nemdaa OR Namenda OR Namzaric)
CONDITION ( autism OR asperger OR kanner OR "pervasive developmental disorder" OR PDD OR PDDs OR "PDD‐NOS" OR ASD OR ASDs OR "childhood schizophrenia") AND Intervention (Nemdatine OR Neumantine OR Neuro‐K OR Neuroplus OR Noojerone OR PMS‐Memantine OR Polmatine OR Prilben OR Pronervon OR Ratio‐Memantine OR Ravemantine OR Sandoz‐Memantine OR Talentum OR Timantil OR Tingreks OR Tonibral OR Tormoro)
CONDITION ( autism OR asperger OR kanner OR "pervasive developmental disorder" OR PDD OR PDDs OR "PDD‐NOS" OR ASD OR ASDs OR "childhood schizophrenia") AND Intervention (Valcoxia OR Vilimen OR Vivimex OR Witgen OR Xapimant OR Ymana OR Zalatine OR Zarlyn OR Zeimer OR Zemertinex OR Zenmem OR Zenmen OR Zimer )
EU Clinical Trials Register
www.clinicaltrialsregister.eu/
Searched 26 November 2020 (4 records when duplicates were removed) Searched 15 February 2022 (no new records)
Query did not match any clinical trials. Abixa OR Adaxor OR Admed OR Akatinol OR Alceba OR Alios OR Almenta OR Alois OR Alzant OR Alzer OR Alzia OR Alzinex OR Alzixa OR Alzmenda OR Alzmex OR Apo‐Memantine OR Axura OR Biomentin OR Carrier OR Cogito OR Cognomem OR Conexine OR Cordure | Autism OR ASD OR Asperger OR Pervasive development OR PDD‐NOS
1 result(s) found for: (Dantex OR Demantin OR Demax OR Dementa OR Dementexa OR Ebixa OR Ebitex OR Emantin OR Emaxin OR Esmirtal OR Eutebrol OR Evy OR Ezemantis OR Fentina OR Korint OR Lemix OR Lindex OR Lucidex OR Manotin OR Mantine OR Mantomed) AND (AUTISM OR ASPERGER OR ASD OR PDD‐NOS).
4 result(s) found for: (Marbodin OR Mardewel OR Marixino OR Maruxa OR Maxiram OR Melanda OR Memabix OR Memamed OR Memando OR Memantin OR Memantina OR Memantine OR Memantinol OR Memantyn OR Memanvitae OR Memanxa OR Memanzaks OR Memary OR Memax OR Memexa OR Memigmin) AND (AUTISM OR ASPERGER OR ASD OR PDD‐NOS).
Query did not match any clinical trials. Memikare OR Memogen OR Memolan OR Memorel OR Memorix OR Memotec OR Memox OR Memxa OR Mentadem OR Mentikline OR Mentium OR Mentixa OR Merandex OR Merital OR Mexia OR Mimetix OR Mirvedol OR Modualz OR Morysa OR Nemdaa OR Namenda OR Namzaric | Autism OR ASD OR Asperger OR Pervasive development OR PDD‐NOS
Query did not match any clinical trials. Nemdatine OR Neumantine OR Neuro‐K OR Neuroplus OR Noojerone OR PMS‐Memantine OR Polmatine OR Prilben OR Pronervon OR Ratio‐Memantine OR Ravemantine OR Sandoz‐Memantine OR Talentum OR Timantil OR Tingreks OR Tonibral OR Tormoro | Autism OR ASD OR Asperger OR Pervasive development OR PDD‐NOS
Query did not match any clinical trials. Valcoxia OR Vilimen OR Vivimex OR Witgen OR Xapimant OR Ymana OR Zalatine OR Zarlyn OR Zeimer OR Zemertinex OR Zenmem OR Zenmen OR Zimer | Autism OR ASD OR Asperger OR Pervasive development OR PDD‐NOS
Appendix 2. Unused methods
| Section of the protocol (Brignell 2021) | Planned approach for future updates | Why the methods were not used |
| Criteria for considering studies for this review |
Types of outcomes measures Estimates suggest that 33% to 75% of people with autism are reported to have an intellectual disability associated with their autism (Bourke 2016; Maenner 2021). Given these figures, we plan to highlight the properties of the psychometric tests used to assess outcomes in individuals treated with memantine, and discuss, in particular, how these properties may have influenced the measurement of outcomes in individuals with co‐occurring intellectual disabilities versus those without intellectual disabilities. We will indicate the evidence supporting the validity of each tool in the following categories: individuals with co‐occurring intellectual disability versus those without an intellectual disability; adults versus children; and assessment of social communication and social interaction versus repetitive and restrictive behaviours (Anagnostou 2015; McConachie 2015; Scahill 2015). For instance, in people with autism without co‐occurring intellectual disability, we will examine studies using the Social Responsiveness Scale (SRS; Constantino 2011), which measures various aspects of social cognition such as social awareness, social information processing, capacity for reciprocal social communication, social anxiety and avoidance, and preoccupations, as it has been described as a useful tool in measuring response to intervention (Payakachat 2012). On the other hand, the use of the Aberrant Behaviour Checklist (ABC; Aman 2017b) has been validated as a tool to assess treatment in individuals with an intellectual disability, and there is limited evidence at present for its use in assessing change in social function in people with autism without co‐occurring intellectual disability (Payakachat 2012). |
Only two included studies presented data on IQ, so we could not complete this analysis. |
| Unit of analysis |
Cluster randomised trials If we come across eligible studies that have used cluster‐RCT methods, we expect that the cluster effects will have been appropriately controlled for. If it is unclear whether appropriate controls for cluster effects have been carried out, we will aim to contact the study authors to obtain necessary information. If appropriate controls have not been applied, we will request the IPD and re‐analyse the data using the generic inverse variance method in order to adjust for correlation, as outlined in Chapter 10 of the Cochrane Handbookfor Systematic Reviews of Interventions (Deeks 2021). We will analyse the effect size and standard error using RevMan 5 (Review Manager 2020). Where this is not possible due to insufficient available data to control for clustering, we will use individuals as the unit of analysis, and then assess the impact of insufficient control of clustering on the effect estimate by using sensitivity analysis (Sensitivity analysis). |
We did not identify any cluster‐randomised trials in this review. |
|
Cross‐over trials We will clearly identify eligible randomised trials in which participants receive both placebo and memantine but in a different order (phase). We will analyse the data according to recommendations in the Cochrane Handbookfor Systematic Reviews of Interventions for cross‐over trials (Higgins 2021a). We will include data up until the point of the first cross‐over. We will not include data from any subsequent periods due to the likelihood of carry‐over effects from the prior intervention to the second phase of the study. We will use the effect estimate and standard deviation based on a paired t‐test. If we are able to conduct a meta‐analysis combining the results of cross‐over trials, we will use the generic inverse variance method (Deeks 2021). We will seek statistical advice for the analysis of cross‐over trials. |
We did not identify any cross‐over trials in this review. | |
|
Studies with multiple treatment arms For studies with multiple treatment arms, we will create a single pair‐wise comparison, where appropriate (for example, where memantine is given in different formulations such as immediate‐ and extended‐release formulations of memantine). If this is not feasible, we will use all treatment arms but divide the comparison arms (control arms) equally across the intervention arms (Higgins 2021a). We will determine the relevance of the treatment arms for each comparison by assessing clinical relevance (for example, if the clinical effects of memantine are comparable or not). If two formulations are used in a trial and one formulation of memantine is more readily available than another, we may choose to exclude the less commonly available formulation. If a treatment arm is not found to be relevant to our study outcomes, we will exclude the group from our analysis. We will clearly document all decisions made in the Characteristics of included studies table. |
We did not identify any trials with multiple treatment arms in this review. | |
| Dealing with missing data | If there is concern about a large amount of missing data, and we deem it inappropriate to include the data in a meta‐analysis, we will provide a qualitative summary in the text. We will perform a Sensitivity analysis to explore the validity of the imputations made by carrying out an analysis without the imputed values, and assess for any differences between the result obtained and the calculated assumed mean. We will discuss the impact the method for analysis of missing data may have on the interpretation of our results in the Discussion section of the review. If study authors provide missing data, we plan to include these data according to intention‐to‐treat (ITT) principles, and use all the data. We plan to keep participants in the treatment group to which they were originally randomised, regardless of the treatment they received, as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021a). |
It was not possible to impute missing data in the review. Authors did not provide details on missing data, so we could not complete analyses. |
| Assessment of reporting biases | If we find 10 or more studies that meet our inclusion criteria (Criteria for considering studies for this review), we will draw a funnel plot to explore the relationship between the intervention effect estimate and standard error of the intervention effect estimate. If there appears to be asymmetry, we will also use Begg's test (Begg 1994), and Egger's test (Egger 1997), to explore the reason for asymmetry, such as publication bias or low methodological quality. Begg's test ranks correlation between a standardised intervention effect and its standard error; and Egger's test analyses the linear regression of an intervention effect estimate against its standard error, weighted by the inverse of the variance of the intervention effect estimate (see Chapter 10 of the Cochrane Handbook for Systematic Reviews of Interventions; Deeks 2021). For each outcome of interest, we will explore if there is selective reporting of outcome data, which could bias the direction of results. If selective reporting of outcomes is suspected, we will contact the study authors where possible, in order to collect this missing outcome data. We will explore the impact on results of published versus any unpublished data by means of a Sensitivity analysis (Higgins 2021a). | We could not assess reporting bias (using funnel plots and testing for funnel plot asymmetry) due to the insufficient number of included studies. |
| Data synthesis |
|
We had an insufficient number of included studies to conduct all of these analyses, and some analyses were not appropriate for the type of data presented by the included studies. |
| Subgroup analysis and investigation of heterogeneity | In addition to assessing the postulated efficacy of memantine, it is also important to investigate whether memantine has an impact on, and correlates with: the severity of behaviours (for instance, individuals with verbal abilities versus those with no verbal skills; individuals with an associated low intelligence quotient (IQ)); the age of participants (children versus adults); different dosage and frequency of memantine administration (once a day versus multiple daily dosing); and duration of treatment. We will perform subgroup analyses to explore differential effects of the following, providing there is sufficient data (i.e. at least 10 observations for each characteristic being analysed).
|
We did not perform subgroup analysis due to an insufficient number of included studies. |
| Sensitivity analysis | We will conduct a sensitivity analysis to assess the impact of risk of bias on the overall result. We will do this by adding or removing studies at high or unclear risk of bias in the following assessment areas from the meta‐analysis.
We will perform a sensitivity analysis to explore the impact of missing data on the overall outcome by comparing the analyses with available outcome data with those following the ITT principle (see Dealing with missing data). |
We did not perform a sensitivity analysis due to an insufficient number of included studies. |
Appendix 3. Criteria for assigning risk of bias
Random sequence generation
Low risk of bias: the investigators described a random component in the sequence generation (e.g. referring to a random number table, using an electronic random number generator).
High risk of bias: the investigators described a component in the sequence generation process that was not strictly random or was nonrandom (e.g. by date of birth, by judgement of the clinician or by preference of the participant).
Unclear risk of bias: there was insufficient information available regarding the sequence generation process to make a judgement of high or low risk of bias.
Allocation concealment
Low risk of bias: both participants and investigators could not have foreseen assignment (e.g. due to central allocation or sealed envelopes).
High risk of bias: participants or investigators possibly could have foreseen the assignments (e.g. using an open random allocation schedule, alternation on rotation).
Unclear risk of bias: there was insufficient information available regarding allocation concealment to make a judgement of high or low risk of bias.
Blinding of participants and personnel
Low risk of bias: blinding of participants and key study personnel was ensured; or the outcomes were not likely to have been influenced by the lack of blinding.
High risk of bias: the lack of blinding was likely to have influenced the outcome; or the blinding could have been broken.
Unclear risk of bias: there was insufficient information available regarding this issue to make a judgement of high or low risk of bias.
Blinding of outcome assessment
Low risk of bias: blinding of outcome assessment was ensured; or it was judged unlikely that outcome measurement could have been influenced by the lack of blinding.
High risk of bias: a lack of blinding was likely to have influenced the outcome measurement; or the blinding could have been broken.
Unclear risk of bias: there was insufficient information available to make a judgement of high or low risk of bias.
Incomplete outcome data
Low risk of bias: there were no missing outcome data; it was unlikely that missing outcome data were related to the true outcomes; and/or missing data were imputed using appropriate methods.
High risk of bias: it was likely that missing outcome data were related to the true outcome because of, for example, imbalance in numbers, when an 'as‐treated' analysis was done with substantial differences between the intervention received and the intervention assigned in the randomisation, or when there was a potentially inappropriate application of imputation.
Unclear risk of bias: there was insufficient information reported to make a judgement of high or low risk of bias.
Selective reporting
Low risk of bias: the study protocol was available, and all of the pre‐specified outcomes were reported in the pre‐specified way; or the study protocol was not available, but the published reports included all expected or pre‐specified outcomes.
High risk of bias: there were discrepancies between reporting of outcomes that were pre‐specified and the actual reported outcomes; or outcomes were reported incompletely.
Unclear risk of bias: there was insufficient information available to make a judgement of high or low risk of bias.
Other sources of bias
Low risk of bias: no additional sources of bias was identified.
High risk of bias: a potential source of bias was identified (e.g. related to study design).
Unclear risk of bias: there was insufficient information available or there was insufficient rationale or evidence that an identified problem would introduce bias.
Taken from Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Appendix 4. Data extracted from included studies
Study methods and setting: study ID, study type, study site, country of publication, language of publication, publication type and study duration
Participant details: sample size, age, gender, diagnosis (including subtypes), diagnostic tool, intelligence and/or adaptive behaviour level
Intervention details: number of participants in each group; missing participants; intervention type, including dosage, mode of delivery, frequency and duration; placebo type, including dosage, mode of delivery, frequency and duration
Outcomes: all primary and secondary outcomes (see Primary outcomes; Secondary outcomes) and time points were collected (2 × 2 table for dichotomous data; means and standard deviations for continuous data)
Risk of bias and the rating for each criterion: randomisation process, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, other sources of bias
Data and analyses
Comparison 1. Memantine versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Core symptoms of autism | 2 | 181 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.74 [‐2.07, 0.58] |
| 1.2 Adverse effects | 2 | 144 | Odds Ratio (M‐H, Random, 95% CI) | 0.64 [0.17, 2.39] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aman 2017a.
| Study characteristics | ||
| Methods | Design: parallel‐group, double‐blind RCT of memantine versus placebo (with follow‐up period) Unit of randomisation: individual participant; does not specifically state the process for participants being recruited to the study Randomisation: participants were randomised 1:1, in accordance with the US Food and Drug Administration Guidance for Industry, to receive placebo or memantine. Randomisation codes generated and securely retained. Statistical methods used: mixed‐effects model with repeated measures, including treatment group, study centre, visit, and treatment‐by‐visit interaction as fixed effects, and the baseline score and baseline‐score‐by visit interaction as covariates based on the observed cases. Used an unstructured covariance matrix to model covariance of within‐patient scores. Secondary/additional efficacy variables analysed using a mixed model, with repeated measures or ANCOVA model, except for CGI‐I (analysed using a Cochran–Mantel–Haenszel test) | |
| Participants |
Location/country: multiple sites in the USA
Setting: not described
Sample size: 121; 60 in treatment group; 61 in control group
Gender: 101 male participants; 52 in treatment group (87%); 49 in control group (80.3%)
Mean age: not reported for entire group; in treatment group, 9.0 years (SD 2.2); in control group, 8.9 years (SD 2.2)
Diagnosis: participants met criteria based on a clinical evaluation and information derived from ADOS and ADI‐R (diagnostic tools); autism severity score on SRS was 100.75 (SD 23.3)
Verbal language: mean verbal ability on the KBIT‐2 was 36.7 (SD 17.8)
Mean IQ: 76.8 (SD 21.3)
Inclusion criteria
Exclusion criteria: participants not permitted to use the following medications concurrently: antianginal, antiarrhythmic, anticoagulant, antihypertensive, antineoplastic, diuretic, hypoglycaemic or hypolipidemic agents; insulin; muscle relaxants; systemic antifungal agents or steroids; hormone suppressants; or psychotropic drugs. Authors did not state if concurrent behavioural or psychosocial interventions were allowed or received by participants. |
|
| Interventions | Treatment (N = 60): single dose of memantine (3 mg or 6 mg) via capsule (up to 3 capsules) Control (N = 61): placebo, taken orally at the same time each day, for 12 weeks | |
| Outcomes | Outcomes collected at multiple times points; however, we only report on the points of assessment used in this review (12 weeks). Primary outcomes
Secondary outcomes
|
|
| Notes | Study start date: April 2009 Study end date: February 2013 Funding source: Forest Laboratories, LLC (Jersey City, New Jersey), Allergan. The study sponsor was involved in the study design, data collection (via contracted clinical investigator sites), analysis and interpretation of data, and the decision to present these results. Declaration of interest: declared (p 411) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: randomisation codes were generated and securely retained by the Statistical Programming Institute, Inc. (Aman 2017a, p 404). |
| Allocation concealment (selection bias) | Unclear risk | Comment: authors state randomisation codes were securely retained, but authors do not state allocation concealment, so this remains unclear (Aman 2017a, p 404). |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk |
Comment: authors state study was double‐blind to participants and personnel. Quote: "double‐blind dose titration period followed by 42 week open label maintenance period. Participants retained their randomisation codes from the lead in study; the protocol required maintaining the double‐blind for the first 6 weeks of the extension trial." (Aman 2017a, p 404) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk |
Comment: depended on the outcome, but caregivers completed most tools and did not know whether child had been allocated to the intervention or the control group (i.e. caregivers were blinded). Outcome assessors were also blinded. Quote: "The study consisted of a 6 week, double‐blind, dose titration period" (Aman 2017a, p 404) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: 50/61 participants (82%) and 54/60 participants (90%) retained until study completion. Loss to follow‐up was therefore moderate. Loss to follow‐up was not equal across the 2 groups. |
| Selective reporting (reporting bias) | Low risk | Comment: followed registered trial protocol |
| Other bias | High risk |
Comment: study was sponsored, which may introduce risk of bias Quote: "This study was supported by funding from Forest Laboratories, LLC, (Jersey City, New Jersey), Allergan. The study sponsor was involved in the study design, data collection (via contracted clinical investigator sites), analysis and interpretation of data, and the decision to present these results." (Aman 2017a, p 403) |
Karahmadi 2018.
| Study characteristics | ||
| Methods | Design: parallel group, single blind RCT of memantine versus placebo (12 weeks) Unit of randomisation: individual child; children recruited through convenience sampling Randomisation: children allocated randomly into 2 groups (ABA and memantine or ABA and placebo), via randomised block design (15 blocks with size of 4). Statistical analysis: parametric tests (including independent samples t‐test, paired samples t‐test, Chi2 test, and Fisher's exact test) | |
| Participants |
Location/country: single site study in Iran
Setting: children who presented to the psychiatric clinic of Noor and Hazrat‐e‐Ali Asghar hospital in Isfahan
Sample size: 60 in total; 30 in treatment group, 30 in control group
Gender: 46 male participants; 22 in treatment group (73%), 24 in control group (80%)
Mean age: not reported for all participants; 10.07 (SD 3.48) years in treatment group, 9.5 (SD 3.86) years in control group
Diagnosis: mean GARS at baseline: treatment group 95.20 (SD 14.49), control group 91.50 (SD 14.35)
Verbal language: not reported
IQ: not reported
Inclusion criteria
Exclusion criteria
|
|
| Interventions | Treatment (N = 30): memantine tablet 5 mg/day, given orally, twice daily (morning and evening) Control (N = 30): placebo; contents of placebo not described but states it had same appearance, odour and colour as memantine Both groups also received behavioural training based on ABA + previous common medications. | |
| Outcomes |
Primary outcomes
|
|
| Notes | Study start date: March 2016 Study end date: March 2017 Funding source: authors' report that the study was not funded Declaration of interest: authors state there were no conflicts of interest to declare | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The children were allocated randomly divided into two groups of control group […] and intervention group" (Karahmadi 2018, p2) |
| Allocation concealment (selection bias) | Unclear risk |
Quote: "Through randomized block design (15 blocks with size of 4 to compose two groups of 30)." (Karahmadi 2018, p2) Comment: does not specifically state there was allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: appears to be single blind, so only parents were blinded, not personnel. Both drugs (memantine and placebo) were the same in "appearance, shape, color, and odor" (Karahmadi 2018, p3), and encoded with A and B; thus, the participants were blinded to the intervention. Personnel: high risk of bias; participants: low risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: study did not report who re‐rated the GARS and whether they were blinded to treatment (likely not if single blind study) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: all data collected, no loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol obtained. Unclear what was intended regarding study aims and reporting |
| Other bias | Low risk | Comment: we did not identify any other sources of potential bias such as sponsorship or conflicts of interest. |
Soorya 2021.
| Study characteristics | ||
| Methods | Design: RCT of memantine versus placebo (24 weeks) Intervention model: parallel assignment; participants randomised 1:1 to groups Blinding: double (participant, investigator) | |
| Participants |
Location/country: USA; two sites
Recruitment status: completed
Sample size: 23 (actual enrolment; 12 memantine; 11 placebo)
Inclusion criteria
Exclusion criteria
*These items were listed in the study protocol but not in the publication. |
|
| Interventions | Treatment: memantine, initiated at 3 mg. Dose increased by 3 mg every week to a maximum of 12 mg for participants weighing ≥ 60 kg, 9 mg for participants weighing ≥ 40 kg but < 60 kg, and 6 mg for participants weighing ≥ 20 kg but < 40 kg Control: placebo | |
| Outcomes |
Primary outcomes Apraxia and expressive speech/language.
Secondary outcomes Memory, adaptive behaviour and adverse effects.
Exploratory outcomes
Authors did not present change data for these tools and provide descriptive statistics only. Timing of outcome assessment: all outcomes assessed at baseline, week 12 and week 24, except for adverse effects, which were assessed at screening, baseline, week 2, week 4, week 6, week 8, week 10, week 12, week 16, week 20, and week 24. Effect size was calculated using baseline and 24 week assessment. *These items were listed in the publication but not in the protocol |
|
| Notes | Trial registry: clinicaltrials.gov Trial registration number:NCT01372449 Declaration of interest: EA received consultation fees from Roche and Quadrant, research funding from Roche, in‐kind supports from AMO Pharma, royalties from APPI and Springer, and editorial honorarium from Wiley. LVS received consultation fees from Roche, royalties from Hogrefe Publications, and holds equity/ownership interest in Argus Cognitive, Inc. AK receives research support from AMO Pharma and consults to Ovid Therapeutics, Acadia, and sema4. All other authors declared that they had no conflicts of interest. Funding: study medication was provided through an in‐kind contribution from Forest Pharmaceuticals Comment(s): none | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "Participants (n = 23), ages 6–12, were randomized at a 1:1 ratio to treatment with memantine or placebo by the study pharmacist at the Icahn School of Medicine at Mount Sinai (original coordinating site)." Comment: does not state type of method used to generate the sequence (e.g. coin toss, computer) |
| Allocation concealment (selection bias) | Unclear risk | Quote: "...randomized at a 1:1 ratio to treatment with memantine or placebo by the study pharmacist at the Icahn School of Medicine at Mount Sinai (original coordinating site)." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "All participants and investigators were blind to group assignment until the blind was broken by the study statistician at the end of the study." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk |
Quote: "Investigators were blind to group assignment" Comment: we contacted the study authors, who confirmed the 'investigators' were the assessors and the assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: 15 participants completed week 24; 8 participants withdrew (Figure 1). High loss to follow‐up and missing data (only 65% retained in the study) |
| Selective reporting (reporting bias) | High risk | Comment: only some measures were reported. The ABC and BRIEF are reported here to provide metrics of impact on behavioural and EF domains, and IQ measures are included to provide preliminary data on the overall neurocognitive effects. Did not report on SRS despite it being completed (as per methods). However, did report all other negative results, and SRS was only an exploratory outcome. |
| Other bias | High risk | Comment: there were some COI reported and funding was provided by pharmaceutical company. |
ABA: Applied Behaviour Analysis; ABC: Aberrant Behavior Checklist; ABC‐C: Aberrant Behavior Checklist‐Community; ADHD: attention deficit hyperactivity disorder; ADI‐R: Autism Diagnostic Interview‐Revised; ADOS: Autism Diagnostic Observation Schedule; AHEOQ: Autism Health Economics and Outcomes Questionnaire; ANCOVA: analysis of covariance; ASD: autism spectrum disorder; BRIEF: Behavior Rating Inventory of Executive Function; CAASTS: Core Associated Autism Symptom Treatment Scale; CATS: Core Autism Treatment Scale; CCC‐2: Children's Communication Checklist, 2nd edition; CGI: Clinical Global Impression; CGI‐I: Clinical Global Impression‐Improvement subscale; CGI‐S: Clinical Global Impression‐Severity subscale; COI: conflicts of interest; DSM‐IV: Diagnostic Statistical Manual for Mental Disorders, 4th edition; DSM‐IV‐TR: Diagnostic Statistical Manual for Mental Disorders, 4th edition, text revision; DSM‐5: Diagnostic Statistical Manual for Mental Disorders, 5th edition; EF: executive function; EVT: Expressive Vocabulary Test; EVT‐2: Expressive Vocabulary Test, 2nd edition; GARS: Gilliam Autism Rating Scale; Inc.: incorporated; IQ: intelligence quotient; KBIT‐2: Kaufman Brief Intelligence Test, 2nd edition; LLC: limited liability company; MRI: magnetic resonance imaging; NEPSY‐II: a developmental NEuroPSYchological assessment, 2nd edition; NMDA: N‐methyl‐D‐aspartate; PDD: pervasive developmental disorder; PDD‐NOS: pervasive developmental disorder not otherwise specified; RCT: randomised controlled trial; SD: standard deviation; SRS: Social Responsiveness Scale; VABS‐II: Vineland Adaptive Behavior Scales, 2nd edition; WASI: Wechsler Abbreviated Scale of Intelligence; WISC: Wechsler Intelligence Scale for Children.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ghaleiha 2013 | RCT of risperidone + memantine and risperidone + placebo. Risperidone was started within the study and not before. For our inclusion criteria, we required participants to have already started taking the other medication (i.e. risperidone) before entry to the study. |
| Hardan 2019 | Study design (e.g. open label or withdrawal trial); includes several related studies, with some overlapping participants; related to Aman 2017a |
| NCT01078844 | Study was terminated in 2017, as sponsor withdrew funds |
| NCT02353130 | Study was withdrawn and protocol did not continue once investigator had relocated to another institution |
RCT: randomised controlled trial.
Characteristics of studies awaiting classification [ordered by study ID]
Martsenkovsky 2016.
| Methods | Design: double‐blind, parallel, placebo‐controlled study of memantine versus placebo (16 weeks) Unit of randomisation: individual participants, randomly assigned to 2 groups of equal size of either memantine or placebo Statistical analysis: ANOVA used to compare baseline data; differences in frequency of side effects were assessed using Fisher’s exact test |
| Participants | Location/country: Ukraine Sample size: 76 children with ASD (numbers per group not reported) Gender: not reported Age: 18–36 months Inclusion criteria: diagnosis met DSM‐IV criteria and was confirmed by the ADI‐R and ADOS‐G |
| Interventions | Treatment (N = not reported): memantine; initial daily dose of 3 mg/day, which was increased to 3 mg/week to 15 mg/day or occurrence of side effects. The study authors do not provide further detail here, but we presume they increased the dose until side effects were noticed, or they increased to 15 mg/day maximum. The mean daily dosage of memantine for participants who completed the study was 7.5 mg (for those aged 18–25 months), and 10.3 mg (for those aged 25–36 months). Control (N = not reported): placebo; further details not provided |
| Outcomes |
Primary outcomes: not specifically described as primary outcomes and measures were not clearly linked to tools (we have made some assumptions based on the areas assessed by each tool). Timing of outcome assessment not stated by the study authors, but treatment was for 16 weeks.
The study used the following tools to evaluate efficacy of memantine.
Secondary outcome (s): not explicitly stated but appear to be aberrant behaviours, including irritability, lethargy/withdrawal, stereotypic behaviour, hyperactivity/non‐compliance, inappropriate speech, assessed using the ABC. Results Primary outcomes Memantine had a large effect on basic subdomains of autistic behaviour.
Secondary outcomes On the ABC‐C, the difference between the memantine and placebo groups were:
Memantine has influenced the level of functioning of children with ASDs. The highest increase was observed in communication. There were no significant side effects in comparison groups (Martsenkovsky 2016, p S729). |
| Notes | Funding source(s): not reported Declarations of interest: not reported Comment(s): study was only available as an abstract from a conference, so limited data were available. We contacted the study authors twice but were unable to obtain the required data to include the study in the data synthesis. |
ABC: Aberrant Behavior Checklist; ADI‐R: Autism Diagnostic Interview‐Revised; ADOS‐G: Autism Diagnostic Observation Schedule‐Generic; ANOVA: analysis of variance; ASD: autism spectrum disorder; CCC‐2: Children's Communication Checklist, 2nd edition; CGAS: Children's Global Assessment Scale; CGI‐S/I: Clinical Global Impression‐Severity/Improvement subscales; DSM‐IV: Diagnostic Statistical Manual for Mental Disorders, 4th edition; PAERS: Pediatric Adverse Event Rating Scale; PEP‐R: Psycho‐educational Profile‐Revised; RCT: randomised controlled trial; SD: standard deviation; SRS: Social Responsiveness Scale.
Characteristics of ongoing studies [ordered by study ID]
EUCTR 2014‐003080‐38‐DE.
| Study name | Public title: Glutamatergic medication in the treatment of obsessive compulsive disorder (OCD) and autism spectrum disorder (ASD) Scientific title: Glutamatergic medication in the treatment of obsessive compulsive disorder (OCD) and autism spectrum disorder (ASD) |
| Methods | Design: RCT of memantine hydrochloride versus placebo (2 years) Intervention model: parallel assignment Blinding: double |
| Participants |
Location/country: Netherlands
Recruitment status: completed
Sample size: 100 (actual enrolment)
Inclusion criteria for the ASD cohort
Exclusion criteria
|
| Interventions | Treatment: memantine hydrochloride; 5 mg film‐coated tablets, given orally Control: placebo; coated tablet, given orally |
| Outcomes |
Primary outcomes
Secondary outcomes
Timing of outcome assessment: 12 weeks |
| Starting date | 2017 End date: 27 September 2018 (actual) |
| Contact information | Primary investigator: not reported Contact name: CIMH, CAP, Medical Faculty Mannheim, University of Heidelberg Telephone number: + 4962117034532 Email address: alexander.haege@zi‐mannheim.de; ZI_AGKPPKJ@zi‐mannheim.de |
| Notes | Trial registry: EU Clinical Trials Register Trial registration number:EUCTR 2014-003080-38-DE Funding source: Radboud University, Nijmegen Medical Centre, Donders Institute for Brain, Cognition and Behaviour (non‐commercial sponsor) Declaration of interest: not reported Comment(s): none |
NCT01972074.
| Study name | Public title: Behavioral and neural response to memantine in adolescents with autism spectrum disorder Scientific title: Behavioral and neural response to memantine in adolescents with autism spectrum disorder |
| Methods | Design: RCT of memantine hydrochloride versus placebo (12 weeks). Investigators also conducted pre‐ and post‐treatment neuroimaging (functional MRI and hydrogen MRS to assess neural functional deficits in adolescents with autism spectrum disorder compared with typical adolescents Intervention model: parallel assignment Blinding: quadruple (participant, care provider, investigator, outcomes assessor) |
| Participants |
Location/country: USA
Recruitment status: completed
Sample size: 84 participants (actual enrolment)
Inclusion criteria
Exclusion criteria
|
| Interventions | Treatment: memantine in capsule form, given twice daily for 12 weeks (including a 4‐week titration phase to a maximum dose of 20 mg/day) Control: placebo; capsule with no active ingredients, given twice daily for 12 weeks |
| Outcomes |
Primary outcome: treatment responder, defined as having:
Adverse effect outcomes were not measured. Secondary outcomes: none reported Timing of outcome assessment: 12 weeks, from baseline (week 0) to endpoint (week 12) |
| Starting date | 17 February 2015 End date: 7 May 2018 (actual) |
| Contact information | Principal investigator(s): Gagan Joshi, Massachusetts General Hospital Contact name: not reported Telephone number: not reported Email address: not reported |
| Notes | Trial registry: clinicaltrials.gov Trial registration number:NCT01972074 Declaration of interest: not reported Comment(s): none |
NCT03553875.
| Study name | Public title: Memantine for the treatment of social deficits in youth with disorders of impaired social interactions Scientific title: Memantine for the treatment of social deficits in youth with disorders of impaired social interactions: a randomized‐controlled trial |
| Methods | Design: randomised controlled trial of memantine hydrochloride (Namenda) versus placebo (12 weeks) Intervention model: parallel assignment Blinding: quadruple (participant, care provider, investigator, outcomes assessor) |
| Participants |
Location/country: USA
Recruitment status: recruiting
Sample size: 100 participants (target)
Inclusion criteria
Exclusion criteria
|
| Interventions | Treatment: memantine hydrochloride, administered in tablet form twice daily, titrated to a maximum dose of 20 mg for 12 weeks Control: matched placebo pill, with no active ingredients, administered twice daily for 12 weeks |
| Outcomes |
Primary outcome: not mentioned specifically but likely treatment responders, as measured by CGI‐I. The CGI‐I is a clinician‐rated measure of the improvement of ASD symptoms. The subscale is rated on a 7‐point Likert scale; higher scores indicate less symptom improvement. The CGI measures social interaction, communication, integrated social interaction and communication, stereotyped behaviour, restricted interests, associated maladaptive behaviours, and daily function. Adverse effect outcomes were not measured. Secondary outcomes: none reported Timing of outcome assessment: baseline to 12 weeks |
| Starting date | 13 November 2018 End date: June 2022 (estimated) |
| Contact information | Principal investigator(s): Gagan Joshi, Massachusetts General Hospital Contact name: Chloe Hutt Vater Telephone number: 617‐724‐7301 Email address: chuttvater@mgh.harvard.edu |
| Notes | Trial registry: clinicaltrials.gov Registration number:NCT03553875 Declaration of Interest: not reported Comment(s): Adverse effects were not named as an outcome. |
ABC: Aberrant Behavior Checklist; ADI‐R: Autism Diagnostic Interview‐Revised; ADHD: attention deficit hyperactivity disorder; ADOS: Autism Diagnostic Observation Schedule; ASD: autism spectrum disorder; CCC‐2: Children's Communication Checklist, 2nd edition; CGAS: Children's Global Assessment Scale; CGI‐I: Clinical Global Impression‐Improvement subscale; CGI‐S: Clinical Global Impression‐Severity subscale; CY‐BOCS: the Children's Yale‐Brown Obsessive Compulsive Scale; DNA: Developmental Neuropsychological Assessment; DSM‐5: Diagnostic Statistical Manual for Mental Disorders, 5th edition; EVT: Expressive Vocabulary Test; IQ: intelligence quotient; K‐SADS‐E: Kiddie Schedule for Affective Disorders and Schizophrenia‐Epidemiological Version; MRI: magnetic resonance imaging; MRS: magnetic resonance spectroscopy; OCD: obsessive compulsive disorder; RCT: randomised controlled trial; SRS‐2: Social Responsiveness Scale, 2nd edition; VABS: Vineland Adaptive Behavior Scale; WASI‐II: Wechsler Abbreviated Scale of Intelligence, 2nd edition.
Differences between protocol and review
Review authors
Dr Chadambaram Prakash was an author of the protocol (Brignell 2021), but stepped down as an author of this review. We gratefully acknowledge his contribution in the Acknowledgements section.
Methods
We added 'language' as a separate secondary outcome. This had been accidentally omitted. It is an important outcome to measure because language difficulties commonly co‐occur in autism and constitute a key outcome. Difficulties with language significantly impact quality of social relationships, activity, participation (e.g. in education and employment), and quality of life.
We added 'adaptive behaviour' as an additional secondary outcome as this is an important outcome for managing daily life activities and is commonly used as an outcome in intervention studies.
We prioritised presentation of adaptive behaviour and language scores over quality of life and general health functioning outcomes in the summary of findings table, as these were standardised and objective measures of outcomes. Language and adaptive behaviour are also two essential ingredients of learning and participation.
Although we had included 'other sources of bias' in our appendices, we accidentally omitted it from the methods section of the protocol. We have now added 'other sources of bias' to the methods section of the current review and assessed it along with the other risk of bias domains.
We added "the number of dropouts due to adverse effects" as an important outcome to measure tolerability.
ProQuest Dissertations & Theses A&I was replaced by ProQuest Dissertations & Theses Global. The TOXLINE subset was no longer available in PubMed in 2022, so we appended the PubMed Toxicology filter to our PubMed search.
We were unable to use all our pre‐planned analyses (Brignell 2021). See Appendix 2.
Contributions of authors
AB designed the review; co‐ordinated the review; searched other resources (such as reference lists from included studies); selected studies for inclusion in the review; collected data for the review and entered them into RevMan 5; assessed risk of bias in the included studies; assessed the certainty of the body of evidence; interpreted the data; and wrote the review. AB is the guarantor for the review.
CM provided feedback on drafts of the review.
KW conceived the review; designed the review; arbitrated, if necessary, in the case of disagreements regarding study selection, data extraction, assessment of risk of bias, and grading the certainty of the evidence; interpreted the data; and contributed to writing the review.
TM selected studies for inclusion in the review; collected data for the review and checked the data entry into RevMan 5; completed data analysis; assessed risk of bias in the included studies; assessed the certainty in the body of evidence; interpreted the data; and contributed to writing the review.
All authors revised the final manuscript version, provided expert comments, and approved the final version.
Sources of support
Internal sources
-
The Royal Children's Hospital, Melbourne, Australia
Salary support for Catherine Marraffa
-
Monash University, Melbourne, Australia, Australia
Salary support for Amanda Brignell, Tamara May and Katrina Williams
External sources
-
None, Other
N/A
Declarations of interest
AB is a Speech Pathologist in developmental paediatrics at Monash Children's Hospital; her role is assessment and not ongoing management. AB is also an Associate Editor with Cochrane Developmental, Psychosocial and Learning Problems (DPLP).
CM is a Consultant Paediatrician at the Royal Children's Hospital Melbourne, where she is involved in both clinical care and research with pharmacological and non‐pharmacological interventions for individuals with ASD. CM has recently received commercial funding paid to her research institute (MCRI) to establish safety and efficacy for a drug called AB‐2004 for children with ASD (Axial Therapeutics). She has received no commercial funding for clinical care.
KW is a Paediatrician in developmental paediatrics at Monash Children's Hospital; her role is assessment and not ongoing management. KW reports a grant from the National Health And Medical Research Council (NHMRC) for a phase III trial of cannabidiol for severe behaviour disorder in children with an intellectual disability, with or without autism, on which she is Chief Investigator (1 January 2020 to 31 May 2023); this grant was paid to the institution, but KW benefited from the payment or had access to the funds. KW also reports contracts with Epsilon Healthcare (formerly THC Global Group Ltd; ongoing since 1 January 2020) to develop an interventional product and placebo for children, which was provided by the Victorian Government, for a Medical Research Future Fund funded phase III multisite trial; and with Tilray (from 28 November 2018 to 27 November 2019) for the investigation drug and placebo for a pilot trial of cannabidiol for severe behaviour problems in children with intellectual disability, with or without autism; both paid to institution. KW reports a grant from the NHMRC for an ongoing prognosis study about predictors of autism outcome that will also publish diagnostic stability outcomes and that could be included in an update of this systematic review; paid to institution. Lastly, KW reports that she is an Editor with DPLP.
TM is a Senior Research Fellow at Monash University and a Psychologist in private practice, involved in both clinical care and research with non‐pharmacological interventions for individuals with autism.
New
References
References to studies included in this review
Aman 2017a {published data only}
- Aman MG, Findling RL, Hardan AY, Hendren RL, Melmed RD, Kehinde-Nelson O, et al. Safety and efficacy of memantine in children with autism: randomized, placebo-controlled study and open-label extension. Journal of Child and Adolescent Psychopharmacology 2017;27(5):403-12. [DOI: 10.1089/cap.2015.0146] [PMCID: PMC5510039] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00872898. Study of pharmacokinetics, safety, efficacy, and tolerability of memantine in children with autism [An open-label (part one) and a randomized, double-blind, placebo-controlled (part two) study of the pharmacokinetics, safety, efficacy, and tolerability of memantine in pediatric patients with autism]. clinicaltrials.gov/ct2/show/NCT00872898 (first received 31 March 2009).
Karahmadi 2018 {published data only}
- Karahmadi M, Tarrahi MJ, Vatankhah Ardestani SS, Omranifard V, Farzaneh B. Efficacy of memantine as adjunct therapy for autism spectrum disorder in children aged <14 years. Advanced Biomedical Research 2018;7:131. [DOI: 10.4103/abr.abr_100_18] [PMCID: PMC6176686] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Soorya 2021 {published data only}
- Bader YK. A double-blind placebo-controlled trial of memantine vs placebo in children with autism spectrum disorder (ASD) targeting memory and motor planning [MS thesis]. Ann Arbor (MI): Rush University, 2016. [Google Scholar]
- NCT01372449. A multi-site double-blind placebo-controlled trial of memantine versus placebo in children with autism (MEM) [A multi-site double-blind placebo-controlled trial of memantine versus placebo in children with autism targeting memory and motor planning]. clinicaltrials.gov/ct2/show/NCT01372449 (first received 14 June 2011).
- Soorya LV, Fogg L, Ocampo E, Printen M, Youngkin S, Halpern D, et al. Neurocognitive outcomes from memantine: a pilot, double-blind, placebo-controlled trial in children with autism spectrum disorder. Journal of Child and Adolescent Psychopharmacology 2021;31(7):475-84. [DOI: 10.1089/cap.2021.0010] [PMID: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Ghaleiha 2013 {published data only}IRCT1138901151556N10
- Ghaleiha A, Asadabadi M, Mohammadi M-R, Shahei M, Tabrizi M, Hajiaghaee R, et al. Memantine as adjunctive treatment to risperidone in children with autistic disorder: a randomized, double-blind, placebo-controlled trial. International Journal of Neuropsychopharmacology 2013;16(4):783-9. [DOI: 10.1017/S1461145712000880] [DOI] [PubMed] [Google Scholar]
- IRCT1138901151556N10. Memantine in the treatment of autism [A double- blind placebo controlled trial of memantine added to risperidone in patients with autistic disorder]. www.irct.ir/trial/860 (first received 1 May 2010).
Hardan 2019 {published data only}
- EUCTR 2012-001568-31-BE. A double-blind, placebo-controlled, randomized withdrawal study of the safety and efficacy of memantine in pediatric patients with autism, Asperger's disorder, or pervasive developmental disorder not otherwise specified (PDD-NOS) previously treated with memantine. www.clinicaltrialsregister.eu/ctr-search/trial/2012-001568-31/BE (first received 19 July 2012).
- Hardan AY, Hendren RL, Aman MG, Robb A, Melmed RD, Andersen KA, et al. Efficacy and safety of memantine in children with autism spectrum disorder: results from three phase 2 multicenter studies. Autism 2019;23(8):2096-111. [DOI: 10.1177/1362361318824103] [PMCID: PMC6779018] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01592747. Withdrawal study of memantine in pediatric patients with autism, Asperger's disorder, or pervasive developmental disorder not otherwise specified previously treated with memantine [A double-blind, placebo-controlled, randomised withdrawal study of the safety and efficacy of memantine in pediatric patients with autism, Asperger's disorder, or pervasive developmental disorder not otherwise specified previously treated with memantine]. clinicaltrials.gov/ct2/show/NCT01592747 (first received 7 May 2012).
- NCT01592773. Safety study of memantine in pediatric patients with autism, Asperger's disorder or pervasive developmental disorder not otherwise specified (PDD-NOS) [An open-label extension study of the safety and tolerability of memantine in pediatric patients with autism, Asperger's disorder, or pervasive developmental disorder not otherwise specified (PDD-NOS)]. clinicaltrials.gov/ct2/show/NCT01592773 (first received 7 May 2012).
NCT01078844 {published data only}
- NCT01078844. Memantine in adult autism spectrum disorder. clinicaltrials.gov/ct2/show/NCT01078844 (first received 2 March 2010).
NCT02353130 {published data only}
- NCT02353130. Cortical metrics assessment outcome measure development in autism with memantine treatment [Development of cortical metrics assessment outcome measures in response to memantine treatment in autism spectrum disorders]. clinicaltrials.gov/ct2/show/NCT02353130 (first received 2 February 2015).
References to studies awaiting assessment
Martsenkovsky 2016 {published data only}
- Martsenkovsky I, Martsenkovska I. The safety and efficacy of memantine hydrochloride versus placebo for children under 3 years old with autism spectrum disorders. European Neuropsychopharmacology 2016;26(Suppl 2):S729. [DOI: 10.1016/S0924-977X(16)31879-X] [DOI] [Google Scholar]
References to ongoing studies
EUCTR 2014‐003080‐38‐DE {published data only}
- EUCTR 2014-003080-38-DE. Glutamatergic medication in the treatment of obsessive compulsive disorder (OCD) and autism spectrum disorder (ASD). www.clinicaltrialsregister.eu/ctr-search/trial/2014-003080-38/DE (first received 22 December 2014).
- Häge A, Banaschewski T, Buitelaar JK, Dijkhuizen RM, Franke B, Lythgoe DJ, et al. Glutamatergic medication in the treatment of obsessive compulsive disorder (OCD) and autism spectrum disorder (ASD) – study protocol for a randomised controlled trial. Trials 2016;17(1 ):141. [DOI: 10.1186/s13063-016-1266-8] [PMCID: PMC4794817] [PMID: ] [DOI] [PMC free article] [PubMed]
NCT01972074 {published data only}
- NCT01972074. Behavioral and neural response to memantine in adolescents with autism spectrum disorder. clinicaltrials.gov/ct2/show/NCT01972074 (first received 30 October 2013).
NCT03553875 {published data only}
- NCT03553875. Memantine for the treatment of social deficits in youth with disorders of impaired social interactions [Memantine for the treatment of social deficits in youth with disorders of impaired social interactions: a randomized-controlled trial]. clinicaltrials.gov/ct2/show/NCT03553875 (first received 12 June 2018).
Additional references
Aman 2017b
- Aman MG, Singh NN. Aberrant Behavior Checklist Manual. 2nd edition. East Aurora, NY: Slosson Educational Publications, 2017. [Google Scholar]
Anagnostou 2015
- Anagnostou E, Jones N, Huerta M, Halladay AK, Wang P, Scahill L, et al. Measuring social communication behaviors as a treatment endpoint in individuals with autism spectrum disorder. Autism 2015;19(5):622-36. [DOI: 10.1177/1362361314542955] [PMID: ] [DOI] [PubMed] [Google Scholar]
APA 1980
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-III). 3rd edition. Washington (DC): American Psychiatric Association, 1980. [Google Scholar]
APA 1987
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-III-R). 3rd edition, revised. American Psychiatric Association, 1987. [Google Scholar]
APA 1994
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV). 4th edition. Washington (DC): American Psychiatric Association, 1994. [Google Scholar]
APA 2000
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR). 4th edition, text revision. Washington (DC): American Psychiatric Association, 2000. [Google Scholar]
APA 2013
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5). 5th edition. Arlington (VA): American Psychiatric Association, 2013. [Google Scholar]
Aye 2021
- Aye SZ, Ni H, Sein HH, Mon ST, Zheng Q, Wong YK. The effectiveness and adverse effects of D‐cycloserine compared with placebo on social and communication skills in individuals with autism spectrum disorder. Cochrane Database of Systematic Reviews 2021, Issue 2. Art. No: CD013457. [DOI: 10.1002/14651858.CD013457.pub2] [PMCID: PMC8094878 (available on 14 February 2022)] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bayou 2008
- Bayou N, M'Rad R, Ahlem B, Béchir Helayem M, Chaabouni H. Autism: an overview of genetic aetiology. La Tunisie Médicale 2008;86(6):573-8. [PMID: ] [PubMed] [Google Scholar]
Beach Center on Disabilities 2006
- Beach Center on Disabilities. Family Quality of Life Scale. Lawrence (KS): Beach Center on Disabilities, 2006. [Google Scholar]
Becker 2013
- Becker B, Klein EM, Striepens N, Mihov Y, Schlaepfer TE, Reul J, et al. Nicotinic acetylcholine receptors contribute to learning-induced metaplasticity in the hippocampus. Journal of Cognitive Neuroscience 2013;25(7):986-97. [DOI: 10.1162/jocn_a_00383] [PMID: ] [DOI] [PubMed] [Google Scholar]
Begg 1994
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50(4):1088-101. [DOI: 10.2307/2533446] [PMID: ] [DOI] [PubMed] [Google Scholar]
Biederman 2017
- Biederman J, Fried R, Tarko L, Surman C, Spencer T, Pope A, et al. Memantine in the treatment of executive function deficits in adults with ADHD: a pilot-randomized double-blind controlled clinical trial. Journal of Attention Disorders 2017;21(4):343-52. [DOI: 10.1177/1087054714538656] [PMID: ] [DOI] [PubMed] [Google Scholar]
Bourke 2016
- Bourke J, De Klerk N, Smith T, Leonard H. Population-based prevalence of intellectual disability and autism spectrum disorders in western Australia: a comparison with previous estimates. Medicine 2016;95(21):e3737. [DOI: 10.1097/MD.0000000000003737] [PMCID: PMC4902360 ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Caddy 2015
- Caddy C, Amit BH, McCloud TL, Rendell JM, Furukawa TA, McShane R, et al. Ketamine and other glutamate receptor modulators for depression in adults. Cochrane Database of Systematic Reviews 2015, Issue 9. Art. No: CD011612. [DOI: 10.1002/14651858.CD011612.pub2] [PMID: ] [DOI] [PubMed] [Google Scholar]
Castelbaum 2020
- Castelbaum L, Sylvester CM, Zhang Y, Yu Q, Constantino JN. On the nature of monozygotic twin concordance and discordance for autistic trait severity: a quantitative analysis. Behavior Genetics 2020;50(4):263-72. [DOI: 10.1007/s10519-019-09987-2] [PMCID: PMC7355281] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cheuk 2011
- Cheuk DK, Wong V, Chen WX. Acupuncture for autism spectrum disorders (ASD). Cochrane Database of Systematic Reviews 2011, Issue 9. Art. No: CD007849. [DOI: 10.1002/14651858.CD007849.pub2] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chez 2007
- Chez MG, Burton Q, Dowling T, Chang M, Khanna P, Kramer C. Memantine as adjunctive therapy in children diagnosed with autistic spectrum disorders: an observation of initial clinical response and maintenance tolerability. Journal of Child Neurology 2007;22(5):574-9. [DOI: 10.1177/0883073807302611] [PMID: ] [DOI] [PubMed] [Google Scholar]
Cohen 1988
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd edition. Hillsdale (NJ): Erlbaum, 1988. [ISBN: 0-8058-0283-5] [Google Scholar]
Conners 1997
- Conners CK. Conners' Rating Scales – Revised. North Tonawanda (NY): Multi-Health Systems, Inc, 1997. [Google Scholar]
Constantino 2011
- Constantino J. Social Responsiveness Scale. 2nd edition. Los Angeles (CA): Western Psychological Services, 2011. [Google Scholar]
Covidence 2020 [Computer program]
- Covidence 2020. Version accessed 20 December 2020. Melbourne, Australia: Veritas Health Innovation, 2020. Available at covidence.org.
Deeks 2021
- Deeks JJ, Higgins JP, Altman DG, editor(s). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
DerSimonian 1986
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177-88. [DOI: 10.1016/0197-2456(86)90046-2] [PMID: ] [DOI] [PubMed] [Google Scholar]
DerSimonian 2015
- DerSimonian R, Laird N. Meta-analysis in clinical trials revisited. Contemporary Clinical Trials 2015;45(Pt A):139-45. [DOI: 10.1016/j.cct.2015.09.002] [PMCID: PMC4639420] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Doyle 2012
- Doyle CA, McDougle CJ. Pharmacotherapy to control behavioral symptoms in children with autism. Expert Opinion on Pharmacotherapy 2012;13(11):1615-29. [DOI: 10.1517/14656566.2012.674110] [PMID: ] [DOI] [PubMed] [Google Scholar]
Dunn 2018
- Dunn D. Peabody Picture Vocabulary Test. 5th edition. Minneapolis (MN): Pearson, 2018. [Google Scholar]
Ecker 2017
- Ecker C. The neuroanatomy of autism spectrum disorder: an overview of structural neuroimaging findings and their translatability to the clinical setting. Autism 2017;21(1):18-28. [DOI: 10.1177/1362361315627136] [PMID: ] [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315(7109):629-34. [DOI: 10.1136/bmj.315.7109.629] [PMCID: PMC2127453] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Elsabbagh 2012
- Elsabbagh M, Divan G, Koh YJ, Kim YS, Kauchali S, Marcín C, et al. Global prevalence of autism and other pervasive developmental disorders. Autism Research 2012;5(3):160-79. [DOI: 10.1002/aur.239] [PMCID: PMC3763210] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Erickson 2007
- Erickson CA, Posey DJ, Stigler KA, Mullett J, Katschke AR, McDougle CJ. A retrospective study of memantine in children and adolescents with pervasive developmental disorders. Psychopharmacology 2007;191(1):141-7. [DOI: 10.1007/s00213-006-0518-9] [PMID: ] [DOI] [PubMed] [Google Scholar]
Farlow 2008
- Farlow MR, Graham SM, Alva G. Memantine for the treatment of Alzheimer's disease: tolerability and safety data from clinical trials. Drug Safety 2008;31(7):577-85. [DOI: 10.2165/00002018-200831070-00003] [PMID: ] [DOI] [PubMed] [Google Scholar]
Farmer 2013
- Farmer C, Thurm A, Grant P. Pharmacotherapy for the core symptoms in autistic disorder: current status of the research. Drugs 2013;73(4):303-14. [DOI: 10.1007/s40265-013-0021-7] [PMCID: PMC3632301] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Feusner 2009
- Feusner JD, Kerwin L, Saxena S, Bystritsky A. Differential efficacy of memantine for obsessive-compulsive disorder vs generalized anxiety disorder: an open-label trial. Psychopharmacology Bulletin 2009;42(1):81-93. [PMID: ] [PubMed] [Google Scholar]
Findling 2007
- Findling RL, McNamara NK, Stansbrey RJ, Maxhimer R, Periclou A, Mann A, et al. A pilot evaluation of the safety, tolerability, pharmacokinetics, and effectiveness of memantine in pediatric patients with attention-deficit/hyperactivity disorder combined type. Journal of Child Adolescent Psychopharmacology 2007;17(1):19-33. [DOI: 10.1089/cap.2006.0044] [PMID: ] [DOI] [PubMed] [Google Scholar]
Fisch 2012
- Fisch GS. Nosology and epidemiology in autism: classification counts. American Journal of Medical Genetics. Part C, Seminars in Medical Genetics 2012;160C(2):91-103. [DOI: 10.1002/ajmg.c.31325] [PMID: ] [DOI] [PubMed] [Google Scholar]
Fombonne 2011
- Fombonne E, Quirke S, Hagen A. Epidemiology of pervasive developmental disorders. In: Amaral DG, Dawson G, Geschwind DH, editors(s). Autism Spectrum Disorders. Oxford (UK): Oxford University Press, 2011:90-111. [Google Scholar]
Fombonne 2021
Gotham 2015
- Gotham K, Marvin AR, Taylor JL, Warren Z, Anderson CM, Law PA, et al. Characterizing the daily life, needs, and priorities of adults with autism spectrum disorder from Interactive Autism Network data. Autism 2015;19(7):794-804. [DOI: 10.1177/1362361315583818] [PMCID: PMC4581903] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro 2020 [Computer program]
- GRADEpro GDT. Version accessed 20 October 2020. Hamilton (ON): McMaster University (developed by Evidence Prime), 2020. Available at gradepro.org.
Guy 1976
- Guy W. ECDEU Assessment Manual for Psychopharmacology. Rockville (MD): US Department of Health, Education, and Welfare, Public Health Service, Alcohol, Drug Abuse, and Mental Health Administration; National Institute of Mental Health, Psychopharmacology Research Branch, Division of Extramural Research Programs, 1976. [Google Scholar]
Haghighi 2013
- Haghighi M, Jahangard L, Mohammad-Beigi H, Bajoghli H, Hafezian H, Rahimi A, et al. In a double-blind, randomized and placebo-controlled trial, adjuvant memantine improved symptoms in inpatients suffering from refractory obsessive-compulsive disorders (OCD). Psychopharmacology 2013;228(4):633-40. [DOI: 10.1007/s00213-013-3067-z] [PMID: ] [DOI] [PubMed] [Google Scholar]
Hallmayer 2011
- Hallmayer J, Cleveland S, Torres A, Phillips J, Cohen B, Torigoe T, et al. Genetic heritability and shared environmental factors among twin pairs with autism. Archives of General Psychiatry 2011;68(11):1095-102. [DOI: 10.1001/archgenpsychiatry.2011.76] [PMCID: PMC4440679] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Henneberry 2021
- Henneberry E, Lamy M, Dominick KC, Erickson CA. Decades of progress in the psychopharmacology of autism spectrum disorder. Journal of Autism and Developmental Disorders 2021;51(12):4370-94. [DOI: 10.1007/s10803-021-05237-9] [PMID: ] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Altman DG, Sterne JA, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1.
Higgins 2021a
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
Higgins 2021b
- Higgins JP, Li T, Deeks JJ, editor(s). Chapter 6: Choosing effect measures and computing estimates of effect. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
Hosenbocus 2013
- Hosenbocus S, Chahal R. Memantine: a review of possible uses in child and adolescent psychiatry. Journal of the Canadian Academy of Child and Adolescent Psychiatry 2013;22(2):166-71. [PMCID: PMC3647634] [PMID: ] [PMC free article] [PubMed] [Google Scholar]
James 2011
- James S, Montgomery P, Williams K. Omega-3 fatty acids supplementation for autism spectrum disorders (ASD). Cochrane Database of Systematic Reviews 2011, Issue 11. Art. No: CD007992. [DOI: 10.1002/14651858.CD007992.pub2] [PMID: ] [DOI] [PubMed] [Google Scholar]
James 2015
- James S, Stevenson SW, Silove N, Williams K. Chelation for autism spectrum disorder (ASD). Cochrane Database of Systematic Reviews 2015, Issue 5. Art. No: CD010766. [DOI: 10.1002/14651858.CD010766.pub2] [PMCID: PMC6457964] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kavirajan 2009
- Kavirajan H. Memantine: a comprehensive review of safety and efficacy. Expert Opinion on Drug Safety 2009;8(1):89-109. [DOI: 10.1517/14740330802528420] [PMID: ] [DOI] [PubMed] [Google Scholar]
Kenny 2016
- Kenny L, Hattersley C, Molins B, Buckley C, Povey C, Pellicano E. Which terms should be used to describe autism? Perspectives from the UK autism community. Autism 2016;20(4):442-462. [DOI: 10.1177/1362361315588200] [DOI] [PubMed] [Google Scholar]
Korkman 2007
- Korkman M, Kirk U, Kemp S. NEPSY-II. 2nd edition. San Antonio (TX): Psychological Assessment, 2007. [Google Scholar]
Kornhuber 2007
- Kornhuber J, Kennepohl EM, Bleich S, Wiltfang J, Kraus T, Reulbach U, et al. Memantine pharmacotherapy: a naturalistic study using a population pharmacokinetic approach. Clinical Pharmacokinetics 2007;46(7):599-612. [DOI: 10.2165/00003088-200746070-00005] [PMID: ] [DOI] [PubMed] [Google Scholar]
Lai 2014
- Lai MC, Lombardo MV, Baron-Cohen S. Autism. Lancet 2014;383(9920):896-910. [DOI: 10.1016/S0140-6736(13)61539-1] [PMID: ] [DOI] [PubMed] [Google Scholar]
Lefebvre 2021
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M-I, et al. Chapter 4: Searching for and selecting studies. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
Li 2021
- Li T, Higgins JP, Deeks JJ, editor(s). Chapter 5: Collecting data. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
Loomes 2017
- Loomes R, Hull L, Mandy WP. What is the male-to-female ratio in autism spectrum disorder? A systematic review and meta-analysis. Journal of the American Academy of Child & Adolescent Psychiatry 2017;56(6):466-74. [DOI: 10.1016/j.jaac.2017.03.013] [PMID: ] [DOI] [PubMed] [Google Scholar]
Lord 1994
- Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview – Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders 1994;24(5):659-85. [DOI: 10.1007/BF02172145] [PMID: ] [DOI] [PubMed] [Google Scholar]
Lord 2012
- Lord C, Rutter M, DiLavore PC, Risi S, Gotham K, Bishop SL. ADOS-2: Autism Diagnostic Observation Schedule, Manual (Part 1): Modules 1–4. 2nd edition. Torrance (CA): Western Psychological Services, 2012. [Google Scholar]
Maenner 2021
- Maenner MJ, Shaw KA, Bakian AV, Bilder DA, Durkin MA, Esler A, et al. Prevalence and characteristics of autism spectrum disorder among children aged 8 years — Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2018. MMWR Surveillance Summaries 2021;70(11):1–16. [DOI: 10.15585/mmwr.ss7011a1] [PMCID: PMC8639024] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Maski 2011
- Maski KP, Jeste SS, Spence SJ. Common neurological co-morbidities in autism spectrum disorders. Current Opinions in Pediatrics 2011;23(6):609-15. [DOI: 10.1097/MOP.0b013e32834c9282] [PMCID: PMC4229811] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
May 2020
- May T, Brignell A, Williams K. Autism spectrum disorder prevalence in children aged 12–13 years from the Longitudinal Study of Australian Children. Autism Research 2020;13(5):821-7. [DOI: 10.1002/aur.2286] [PMID: ] [DOI] [PubMed] [Google Scholar]
McCloud 2015
- McCloud TL, Caddy C, Jochim J, Rendell JM, Diamond PR, Shuttleworth C, et al. Ketamine and other glutamate receptor modulators for depression in bipolar disorder in adults. Cochrane Database of Systematic Reviews 2015, Issue 9. Art. No: CD011611. [DOI: 10.1002/14651858.CD011611.pub2] [PMID: ] [DOI] [PubMed] [Google Scholar]
McConachie 2015
- McConachie H, Parr JR, Glod M, Hanratty J, Livingstone N, Oono IP, et al. Systematic review of tools to measure outcomes for young children with autism spectrum disorder. Health Technology Assessments 2015;19(41):1-506. [DOI: 10.3310/hta19410] [PMCID: PMC4781156] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
McKay 2003
- McKay D, Piacentini J, Greisberg S, Graae F, Jaffer M, Miller J, et al. The Children's Yale-Brown Obsessive-Compulsive Scale: item structure in an outpatient setting. Psychological Assessment 2003;15(4):578-81. [DOI: 10.1037/1040-3590.15.4.578] [PMID: ] [DOI] [PubMed] [Google Scholar]
McShane 2019
- McShane R, Westby MJ, Roberts E, Minakaran N, Schneider L, Farrimond LE, et al. Memantine for dementia. Cochrane Database of Systematic Reviews 2019, Issue 3. Art. No: CD003154. [DOI: 10.1002/14651858.CD003154.pub6] [PMCID: PMC6425228] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Millward 2008
- Millward C, Ferriter M, Calver SJ, Connell-Jones GG. Gluten and casein-free diets for autistic spectrum disorder. Cochrane Database of Systematic Reviews 2008, Issue 2. Art. No: CD003498. [DOI: 10.1002/14651858.CD003498.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mohammadi 2015
- Mohammadi MR, Mohammadzadeh S, Akhondzadeh S. Memantine versus methylphenidate in children and adolescents with attention deficit hyperactivity disorder: a double-blind, randomized clinical trial. Iran Journal of Psychiatry 2015;10(2):106-14. [PMCID: PMC4752523] [PMID: ] [PMC free article] [PubMed] [Google Scholar]
Mohammadzadeh 2019
- Mohammadzadeh S, Ahangari TK, Yousefi F. The effect of memantine in adult patients with attention deficit hyperactivity disorder. Human Psychopharmacology 2019;34(1):e2687. [DOI: 10.1002/hup.2687] [PMID: ] [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Medicine 2009;6(7):e1000097. [DOI: 10.1371/journal.pmed.1000097] [PMCID: PMC2707599] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nichols 2008
- Nichols DE, Nichols CD. Serotonin receptors. Chemical Reviews 2008;108(5):1614-41. [DOI: 10.1021/cr078224o] [PMID: ] [DOI] [PubMed] [Google Scholar]
Niederhofer 2007
- Niederhofer H. Glutamate antagonists seem to be slightly effective in psychopharmacologic treatment of autism. Journal of Clinical Psychopharmacology 2007;27(3):317-8. [DOI: 10.1097/01.jcp.0000270082.30500.69] [PMID: ] [DOI] [PubMed] [Google Scholar]
Nye 2005
- Nye C, Brice A. Combined vitamin B6-magnesium treatment in autism spectrum disorder. Cochrane Database of Systematic Reviews 2005, Issue 4. Art. No: CD003497. [DOI: 10.1002/14651858.CD003497.pub2] [PMCID: PMC7003675] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ott 2007
- Ott BR, Blake LM, Kagan E, Resnick M, Memantine MEM-MD-11AB Study Group. Open label, multicenter, 28-week extension study of the safety and tolerability of memantine in patients with mild to moderate Alzheimer's disease. Journal of Neurology 2007;254(3):351-8. [DOI: 10.1007/s00415-006-0374-x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Owley 2006
- Owley T, Salt J, Guter S, Grieve A, Walton L, Ayuyao N, et al. A prospective, open-label trial of memantine in the treatment of cognitive, behavioral, and memory dysfunction in pervasive developmental disorders. Journal of Child and Adolescent Psychopharmacology 2006;16(5):517-24. [DOI: 10.1089/cap.2006.16.517] [PMID: ] [DOI] [PubMed] [Google Scholar]
Payakachat 2012
- Payakachat N, Tilford JM, Kovacs E, Kuhlthau K. Autism spectrum disorders: a review of measures for clinical, health services and cost-effectiveness applications. Expert Review of Pharmacoeconomics & Outcomes Research 2012;12(4):485-503. [DOI: 10.1586/erp.12.29] [PMCID: PMC3502071] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rabins 2007
- Rabins PV, Blacker D, Rovner BW, Rummans T, et al, APA Work Group on Alzheimer's Disease and other Dementias. American Psychiatric Association practice guideline for the treatment of patients with Alzheimer's disease and other dementias. 2nd edition. American Journal of Psychiatry 2007;164(12 Suppl):5-56. [PMID: ] [PubMed] [Google Scholar]
Rammes 2001
- Rammes G, Rupprecht R, Ferrari U, Zieglgänsberger W, Parsons CG. The N-methyl-D-aspartate receptor channel blockers memantine, MRZ 2/579 and other amino-alkyl-cyclohexanes antagonise 5-HT(3) receptor currents in cultured HEK-293 and N1E-115 cell systems in a non-competitive manner. Neuroscience Letters 2001;306(1-2):81-4. [DOI: 10.1016/s0304-3940(01)01872-9] [PMID: ] [DOI] [PubMed] [Google Scholar]
Rapp 2013
- Rapp A, Dodds A, Walkup JT, Rynn M. Treatment of pediatric anxiety disorders. Annals of the New York Academy of Sciences 2013;1304(1):52-61. [DOI: 10.1111/nyas.12318] [PMID: ] [DOI] [PubMed] [Google Scholar]
Reiser 1988
- Reiser G, Binmöller FJ, Koch R. Memantine (1-amino-3,5-dimethyladamantane) blocks the serotonin-induced depolarization response in a neuronal cell line. Brain Research 1988;443(1-2):338-44. [DOI: 10.1016/0006-8993(88)91630-7] [PMID: ] [DOI] [PubMed] [Google Scholar]
Review Manager 2020 [Computer program]
- Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2020.
Rojas 2014
- Rojas DC. The role of glutamate and its receptors in autism and the use of glutamate receptor antagonists in treatment. Journal of Neural Transmission 2014;121(8):891-905. [DOI: 10.1007/s00702-014-1216-0] [PMCID: PMC4134390] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rossignol 2014
- Rossignol DA, Frye RE. The use of medications approved for Alzheimer's disease in autism spectrum disorder: a systematic review. Frontiers in Pediatrics 2014;2:87. [DOI: 10.3389/fped.2014.00087] [PMCID: PMC4141213] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sani 2012
- Sani G, Serra G, Kotzalidis GD, Romano S, Tamorri SM, Manfredi G, et al. The role of memantine in the treatment of psychiatric disorders other than the dementias: a review of current preclinical and clinical evidence. CNS Drugs 2012;26(8):663-90. [DOI: 10.2165/11634390-000000000-00000] [PMID: ] [DOI] [PubMed] [Google Scholar]
Scahill 2015
- Scahill L, Aman MG, Lecavalier L, Halladay AK, Bishop SL, Bodfish JW, et al. Measuring repetitive behaviors as a treatment endpoint in youth with autism spectrum disorder. Autism 2015;19(1):38-52. [DOI: 10.1177/1362361313510069] [PMID: ] [DOI] [PubMed] [Google Scholar]
Schopler 1986
- Schopler E, Reichler RJ, Renner BR. The Childhood Autism Rating Scale (CARS): For Diagnostic Screening and Classification of Autism. New York (NY): Irvington, 1986. [Google Scholar]
Schünemann 2021
- Schünemann HJ, Vist GE, Higgins JP, Santesso N, Deeks JJ, Glasziou P, et al. Chapter 15: Interpreting results and drawing conclusions. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
Schwartz 2012
- Schwartz TL, Siddiqui UA, Raza S. Memantine as an augmentation therapy for anxiety disorders. Case Reports in Psychiatry 2012;2012:749796. [DOI: 10.1155/2012/749796] [PMCID: PMC3420624] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Seeman 2008
- Seeman P, Caruso C, Lasaga M. Memantine agonist action at dopamine D2High receptors. Synapse 2008;62(2):149-53. [DOI: 10.1002/syn.20472] [PMID: ] [DOI] [PubMed] [Google Scholar]
Shattuck 2007
- Shattuck PT, Seltzer MM, Greenberg JS, Orsmond GI, Bolt D, Kring S, et al. Change in autism symptoms and maladaptive behaviors in adolescents and adults with an autism spectrum disorder. Journal of Autism and Developmental Disorders 2007;37(9):1735-47. [DOI: 10.1007/s10803-006-0307-7] [PMCID: PMC3265360] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Siegel 2012
- Siegel M, Beaulieu AA. Psychotropic medications in children with autism spectrum disorders: a systematic review and synthesis for evidence-based practice. Journal of Autism and Developmental Disorders 2012;42(8):1592-605. [DOI: 10.1007/s10803-011-1399-2] [PMID: ] [DOI] [PubMed] [Google Scholar]
Sinha 2011
- Sinha Y, Silove N, Hayen A, Williams K. Auditory integration training and other sound therapies for autism spectrum disorders (ASD). Cochrane Database of Systematic Reviews 2011, Issue 12. Art. No: CD003681. [DOI: 10.1002/14651858.CD003681.pub3] [PMCID: PMC7173755] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Smith 2013
- Smith EG, Deligiannidis KM, Ulbricht CM, Landolin CS, Patel JK, Rothschild AJ. Antidepressant augmentation using the N-methyl-D-aspartate antagonist memantine: a randomized, double-blind, placebo-controlled trial. Journal of Clinical Psychiatry 2013;74(10):966-73. [DOI: 10.4088/JCP.12m08252] [PMCID: PMC4000742] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sparrow 2016
- Sparrow SS, Cicchetti DV, Saulnier CA. Vineland-3: Vineland Adaptive Behavior Scales. 3rd edition. San Antonio (TX): Pearson, 2016. [Google Scholar]
Stewart 2010
- Stewart SE, Jenike EA, Hezel DM, Stack DE, Dodman NH, Shuster L, et al. A single-blinded case-control study of memantine in severe obsessive-compulsive disorder. Journal of Clinical Psychopharmacology 2010;30(1):34-9. [DOI: 10.1097/JCP.0b013e3181c856de] [PMID: 20075645] [DOI] [PubMed] [Google Scholar]
Strzelecki 2013
- Strzelecki D, Tabaszewska A, Barszcz Z, Józefowicz O, Kropiwnicki P, Rabe-Jabłońska J. A 10-week memantine treatment in bipolar depression: a case report. Focus on depressive symptomatology, cognitive parameters and quality of life. Psychiatry Investigation 2013;10(4):421-4. [DOI: 10.4306/pi.2013.10.4.421] [PMCID: PMC3902162] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Surman 2013
- Surman CB, Hammerness PG, Petty C, Spencer T, Doyle R, Napolean S, et al. A pilot open label prospective study of memantine monotherapy in adults with ADHD. World Journal of Biological Psychiatry 2013;14(4):291-8. [DOI: 10.3109/15622975.2011.623716] [PMID: ] [DOI] [PubMed] [Google Scholar]
Thomas 2009
- Thomas SJ, Grossberg GT. Memantine: a review of studies into its safety and efficacy in treating Alzheimer's disease and other dementias. Clinical Interventions in Aging 2009;4:367-77. [DOI: 10.2147/cia.s6666] [PMCID: PMC2762361] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Uzunova 2014
- Uzunova G, Hollander E, Shepherd J. The role of ionotropic glutamate receptors in childhood neurodevelopmental disorders: autism spectrum disorders and fragile x syndrome. Current Neuropharmacology 2014;12(1):71-98. [DOI: 10.2174/1570159X113116660046] [PMCID: PMC3915351] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Dyck 2007
- Van Dyck CH, Tariot PN, Meyers B, Malca Resnick E, Memantine MEM-MD-01 Study Group. A 24-week randomized, controlled trial of memantine in patients with moderate-to-severe Alzheimer disease. Alzheimer Disease & Associated Disorders 2007;21(2):136-43. [DOI: 10.1097/WAD.0b013e318065c495] [PMID: ] [DOI] [PubMed] [Google Scholar]
Vannucchi 2014
- Vannucchi G, Masi G, Toni C, Dell'Osso L, Marazziti D, Perugi G. Clinical features, developmental course, and psychiatric comorbidity of adult autism spectrum disorders. CNS Spectrums 2014;19(2):157-64. [DOI: 10.1017/S1092852913000941] [PMID: ] [DOI] [PubMed] [Google Scholar]
Van Wijngaarden‐Cremers 2014
- Van Wijngaarden-Cremers PJ, Van Eeten E, Groen WB, Van Deurzen PA, Oosterling IJ, Van der Gaag RJ. Gender and age differences in the core triad of impairments in autism spectrum disorders: a systematic review and meta-analysis. Journal of Autism and Developmental Disorders 2014;44(3):627-35. [DOI: 10.1007/s10803-013-1913-9] [PMID: ] [DOI] [PubMed] [Google Scholar]
Vasa 2015
- Vasa RA, Mazurek MO. An update on anxiety in youth with autism spectrum disorders. Current Opinion in Psychiatry 2015;28(2):83-90. [DOI: 10.1097/YCO.0000000000000133] [PMCID: PMC5764108] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Volkmar 2014
- Volkmar FR, McPartland JC. From Kanner to DSM-5: autism as an evolving diagnostic concept. Annual Review of Clinical Psychology 2014;10:193-212. [DOI: 10.1146/annurev-clinpsy-032813-153710] [PMID: ] [DOI] [PubMed] [Google Scholar]
Wechsler 2014
- Wechsler D. Wechsler Intelligence Scale for Children. 5th edition. Bloomington (MN): Pearson, 2014. [Google Scholar]
Wei 2012
- Wei H, Dobkin C, Sheikh AM, Malik M, Brown WT, Li X. The therapeutic effect of memantine through the stimulation of synapse formation and dendritic spine maturation in autism and fragile X syndrome. PLoS One 2012;7(5):e36981. [DOI: 10.1371/journal.pone.0036981] [PMCID: PMC3352866] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 1992
- World Health Organization (WHO). The ICD-10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines. Geneva (CH): WHO, 1992. [Google Scholar]
WHO 2018
- World Health Organization. International classification of diseases for mortality and morbidity statistics (11th Revision). www.icd.who.int/browse11/l-m/en (accessed 1 December 2020).
Williams 2012
- Williams K, Wray JA, Wheeler DM. Intravenous secretin for autism spectrum disorders (ASD). Cochrane Database of Systematic Reviews 2012, Issue 4. Art. No: CD003495. [DOI: 10.1002/14651858.CD003495.pub3] [PMCID: PMC7154585] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Williams 2013
- Williams K, Brignell A, Randall M, Silove N, Hazell P. Selective serotonin reuptake inhibitors (SSRIs) for autism spectrum disorders (ASD). Cochrane Database of Systematic Reviews 2013, Issue 8. Art. No: CD004677. [DOI: 10.1002/14651858.CD004677.pub3] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Williams 2015
- Williams K, Brignell A, Prior M, Bartak L, Roberts J. Regression in autism spectrum disorders. Journal of Paediatrics and Child Health 2015;51(1):61-4. [DOI: 10.1111/jpc.12805] [DOI] [PubMed] [Google Scholar]
Williams 2018
- Williams KT. EVT-3: Expressive Vocabulary Test. 3rd edition. San Antonio (TX): Pearson, 2018. [Google Scholar]
Wing 1997
- Wing L. The autistic spectrum. Lancet 1997;350(9093):1761-6. [DOI: 10.1016/S0140-6736(97)09218-0] [PMID: ] [DOI] [PubMed] [Google Scholar]
Wing 2002
- Wing L, Leekam SR, Libby SJ, Gould J, Larcombe M. The Diagnostic Interview for Social and Communication Disorders: background, inter-rater reliability and clinical use. Journal of Child Psychology and Psychiatry 2002;43(3):307-25. [DOI: 10.1111/1469-7610.00023] [PMID: ] [DOI] [PubMed] [Google Scholar]
Woolfenden 2012
- Woolfenden S, Sarkozy V, Ridley G, Williams K. A systematic review of the diagnostic stability of autism spectrum disorder. Research in Autism Spectrum Disorders 2012;6(1):345-54. [DOI: 10.1016/j.rasd.2011.06.008] [DOI] [PubMed] [Google Scholar]
Xiong 2016
- Xiong T, Chen H, Luo R, Mu D. Hyperbaric oxygen therapy for people with autism spectrum disorder (ASD). Cochrane Database of Systematic Reviews 2016, Issue 10. Art. No: CD010922. [DOI: 10.1002/14651858.CD010922.pub2] [PMCID: PMC6464144] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zarate 2006
- Zarate CA Jr, Singh JB, Quiroz JA, De Jesus G, Denicoff KK, Luckenbaugh DA, et al. A double-blind, placebo-controlled study of memantine in the treatment of major depression. American Journal of Psychiatry 2006;163(1):153-5. [DOI: 10.1176/appi.ajp.163.1.153] [PMID: ] [DOI] [PubMed] [Google Scholar]
Zheng 2018
- Zheng Z, Zheng P, Zou X. Association between schizophrenia and autism spectrum disorder: a systematic review and meta-analysis. Autism Research 2018;11(8):1110-9. [DOI: 10.1002/aur.1977] [PMID: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Brignell 2021
- Brignell A, Prakash C, Marraffa C, Williams K, May T. Memantine for autism spectrum disorder. Cochrane Database of Systematic Reviews 2021, Issue 1. Art. No: CD013845. [DOI: 10.1002/14651858.CD013845] [DOI] [PMC free article] [PubMed] [Google Scholar]


