Abstract
Studies on the relationship between vitamin D (VitD) and glucose homeostasis usually consider either total VitD or 25OHD3 but not 25OHD2 and epimers. We aimed to evaluate the cross-sectional association of VitD compounds with glucose homeostasis measurements in pregnant women with overweight/obesity participating in the Vitamin D And Lifestyle Intervention for Gestational Diabetes Mellitus Prevention study. Methods: The analysis included 912 women. Inclusion criteria: <20 weeks gestation, body mass index ≥29 kg/m2 and information on exposure and outcome variables at baseline. Measurements: A 75 g OGTT at <20, 24–28 and 35–37 weeks gestation (except if previous diabetes diagnosis). Exposure variables: 25OHD2, 25OHD3 and C3-epimer. Outcome variables: fasting and post-challenge insulin sensitivity and secretion indices, corresponding disposition indices (DI), plasma glucose at fasting and 1 and 2 h, hyperglycemia in pregnancy (HiP). Statistics: Multivariate regression analyses with adjustment. Results: Baseline VitD sufficiency was 66.3%. Overall, VitD compounds did not show strong associations with any glucose homeostasis measures. 25OHD3 showed direct significant associations with: FPG at <20 and 24–28 weeks (standardized β coefficient (β) 0.124, p = 0.030 and 0.111, p = 0.026 respectively), 2 h plasma glucose at 24–28 weeks (β 0.120, p = 0.018), and insulin sensitivity (1/HOMA-IR, β 0.127, p = 0.027) at 35–37 weeks; it showed an inverse association with fasting DI (QUCKI*HOMA-β) at <20 and 24–28 weeks (β −0.124, p = 0.045 and β −0.148, p = 0.004 respectively). 25OHD2 showed direct associations with post-challenge insulin sensitivity (Matsuda, β 0.149, p = 0.048) at 24–28 weeks) and post-challenge DI (Matsuda*Stumvoll phase 1) at 24–28 and 35–37 weeks (β 0.168, p = 0.030, β 0.239, p = 0.006). No significant association with C3-epimer was observed at any time period. Conclusions: In these women with average baseline VitD in sufficiency range, VitD compounds did not show clear beneficial associations with glucose homeostasis measures.
Keywords: vitamin D compounds, 25OHD2, 25OHD3, C3-epimer, glucose homeostasis, pregnancy, obesity
1. Introduction
Vitamin D (VitD), a cholesterol-derived hormone mainly involved in calcium homeostasis, has been shown to have pleotropic effects including glucose metabolism [1].
VitD is available in two forms, ergocalciferol (VitD2), mainly derived from mushrooms and fortified foods, and cholecalciferol (VitD3), obtained from different dietary sources, mainly oil-rich fish or by skin synthesis from 7-dehydrocholesterol under the influence of UV light. While both are commonly used in nutritional supplements, certain differences according to VitD compound have been described. VitD enters the circulation bound to the vitamin D binding protein and is then hydroxylated to 25-hydroxycholecalciferol (25OHD), the main circulating form, in the liver. 25OHD3 binds with greater affinity to VitD-binding protein and is more efficiently hydroxylated and converted to 1,25-dihydroxycholecalciferol (1,25OH2D) than 25OHD2 [2,3]. Thus, 25OHD3 seems to display greater biological activity compared with 25OHD2 and has been shown to be more efficient in raising total 25OHD concentrations [4]. Therefore, supplementation studies commonly use 25OHD3.
In recent years, there has been growing interest in exploring the role of VitD in glucose metabolism. In vitro, 1,25OH2D stimulates the expression of insulin receptors [5,6] and modulates cytokine expression and activity [7,8], hence improving insulin sensitivity. On the other hand, in animal models, VitD deficiency has been shown to impair glucose-mediated insulin secretion [9,10,11], which can be restored after 1,25OH2D3 supplementation [12]. In in vitro models, 1,25OH2D3 enhances glucose-stimulated insulin secretion (GSIS) via calcium channel up-regulation [13]. Changes in β-cell gene expression affecting viability and apoptosis that also enhance GSIS have also been reported in cell lines treated with 1,25OH2D2 [14].
3-epi-25OHD3 (C3-epimer) results from the epimerization of 25OHD3 and represents 3.5 to 7% of the total 25OHD3 concentration in the general adult population, reaching up to 26% in a pediatric population [15]. In pregnancy, a sub-analysis from the Hyperglycemia And Pregnancy Outcomes study reported that C3-epimer can account for ~20% of 25OHD3 concentrations [16]. Recent publications support a possible biological role for C3-epimer. It has been reported that C3-epimer can suppress parathyroid hormone secretion and modulate cell differentiation and apoptosis [17,18]. Inverse associations have been described between C3-epimer and body mass index (BMI) and low density lipoprotein cholesterol [19]. Regarding glucose homeostasis, in 2019, Zheng et al. observed in the EPIC-InterAct case cohort an inverse association between non-epimeric 25OHD3 and the incidence of type 2 diabetes mellitus, while the C3-epimer showed a direct association. No association was observed between 25OHD2 and incidence of type 2 diabetes mellitus [20]. In pregnancy, an abstract published in 2019 observed no association between either 23OHD3 or C3-epimer and the risk of gestational diabetes mellitus (GDM) [21].
Circulating total 25OHD concentrations have been inversely associated with fasting plasma glucose (FPG) and essentially with improvements in insulin resistance [22,23,24,25]. However, randomized controlled trials of VitD3 supplementation aiming at type 2 diabetes mellitus prevention have had inconsistent results [26,27,28,29]. Studies investigating the effect of VitD3 on glucose homeostasis usually address FPG and insulin sensitivity and less often include measures of β-cell function. Improvements in FPG [26], insulin resistance, and 2 h plasma glucose [26,27] have been described in some trials, while others have not observed such benefits [30,31]. Insulin sensitivity and secretion can be separately measured, but it is the paired secretion-sensitivity relationship that is relevant for glucose homeostasis. The disposition index reflects insulin response at a given insulin sensitivity, which makes it a useful integrated β-cell function measure. Regarding β-cell function, a study by Mitri in 2011 described an improvement in the disposition index that was dependent on improved insulin secretion but not insulin sensitivity [32]. However, more recent studies have observed no effect in these parameters [33,34].
In pregnancy, VitD supplementation trials for GDM prevention have been conducted using VitD3 or a non-specified VitD [35,36,37,38] showing potential benefits according to the latest Cochrane review [39]. A beneficial effect on FPG and fasting insulin sensitivity has consistently been reported [40,41]. There are scarcer data on insulin secretion, but a recent metanalysis found an improvement in HOMA-β and 2 h plasma glucose [41].
To assess glucose homeostasis, several oral glucose tolerance test (OGTT)-derived indices to measure insulin sensitivity and secretion have been used in pregnancy studies (HOMA-IR, QUICKI, Matsuda, OGIS, HOMA-β, IGI, AUCins/glu, Stumvoll), but only some of them have been validated during pregnancy. Kirwan et al. described a correlation between clamp and OGTT-derived HOMA-IR, QUICKI, and Matsuda indices, with the Matsuda index showing the strongest correlation [42]. As for insulin secretion, a recent report by Powe et al. evaluated different indices and concluded that Stumvoll phase 1 and AUCins/glu were valid OGTT-based (vs. clamp) insulin secretory response measures for pregnancy studies [43]. HOMA-β, the only fasting insulin secretion index evaluated, showed a weak positive correlation with first-phase insulin response in early pregnancy but not in late pregnancy [43].
In this exploratory sub-analysis of the Vitamin D And Lifestyle Intervention for Gestational Diabetes Mellitus Prevention (DALI) study, we aimed to evaluate the association of the serum concentrations of different VitD compounds with glucose homeostasis measures (FPG and post-challenge plasma glucose and insulin sensitivity, insulin secretion and disposition indices).
2. Materials and Methods
2.1. Study Population
A total of 984 women were recruited to participate in the DALI trials [38,44,45]. The full study protocol has been previously published [46]. In brief, the DALI project was a multicenter study conducted in nine European countries testing different strategies for GDM prevention. The study was approved by the ethics committees of all participating sites (NRES Committee East of England-Norfolk: 11/EE/0221; Medical University Poznan: 1165/12; UZ KU Leuven: ML7625; Hospital De La Santa Creu i Sant Pau Barcelona 13/006 (OBS); Medical University Vienna: 2022/2012-1369/2013; Province of Padua and Pisa: 4201 Å~11; Galway University Hospitals: 7/12; Ethical comittee VU medisch centrum Amsterdam: nr. 2012/400; Copenhague and Odense: Scientific Ethics Committee for the Capital Region, Hillerod, Denmark, Protokol nr.: H-4-2013-005). Women with <20 weeks’ gestation and BMI ≥29 Kg/m2 who signed a written consent were eligible for the study. Exclusion criteria were being unable to walk at least 100 m safely or to speak the language of the recruitment site, having complex diet requirements and chronic medical or psychiatric conditions, and, for the VitD trial, having current or past abnormal calcium metabolism or having hypercalciuria or hypercalcemia detected at baseline measurement.
All participants underwent a 75-g, 2 h OGTT at <20 weeks using IADPSG/WHO2013 criteria for GDM diagnosis. Women with normal glucose tolerance were randomized to three lifestyle arms [44]: lifestyle vs usual care [45] or lifestyle and/or VitD vs. usual care [38]. The lifestyle intervention consisted of healthy eating (promoting a high-fiber diet, lower in simple and complex carbohydrate, lower in fat, and limited intake of total calories), physical activity (promoting both aerobic and resistance activity according to American College of Obstetricians and Gynecologists (ACOG) guidelines), and combined healthy eating and physical activity interventions. Participants who signed informed consent and were excluded from the original trial allowed their data to be used in secondary analyses.
In this exploratory observational sub-analysis, all women (n = 912) with available OGTT and VitD data at baseline were included.
2.2. Data Collection and Assessments
Data from participating women were collected at 3 time points: <20 weeks (baseline), at 24–28, and at 35–37 weeks’ gestation.
Sociodemographic data and medical history were recorded at baseline. Blood samples and anthropometric measures were obtained at each time point. A standard 75-g, 2 h OGTT, after 10 h fasting, was performed at each time point (unless women had been previously diagnosed with overt diabetes/GDM). GDM was defined after IADPS/WHO 2013 diagnostic criteria (FPG ≥ 5.1 mmol/L and/or 1 h plasma glucose ≥ 10 mmol/L and/or 2 h plasma glucose ≥ 8.5 mmol/L). Overt diabetes was diagnosed if FPG ≥ 7.0 mmol/L or 2 h plasma glucose ≥ 11.1 mmol/L. Hyperglycemia in pregnancy (HiP) was defined as either GDM or overt diabetes. Blood samples were analyzed at local and central laboratories, with local results being used for clinical management. For the current analysis, central laboratory values were used (local data when central values were unavailable).
2.3. Measurements
During the OGTT, blood samples were drawn for the measurement of glucose at fasting and at 1 and 2 h (and additionally at 30 and 90 min in some study sites).
Glucose was measured using the hexokinase method (DiaSys Diagnostic Systems, Holzheim, Germany) with a lower limit of sensitivity of 0.1 mmol/L. Insulin was measured by a sandwich immunoassay (ADVIA Centaur; Siemens Health Care Diagnostics Inc., Vienna, Austria) with an analytical sensitivity of 0.5 mU/L, intra-assay coefficient of variation of 3.3% to 4.6%, and inter-assay coefficient of variation of 2.6% to 5.9%.
The following pregnancy-validated glucose homeostasis indices were calculated:
Insulin sensitivity at fasting using Homeostasis model assessment (HOMA-IR) [47] and Quantitative insulin check index (QUICKI) [48] and post-challenge using Matsuda and De Fronzo’s (ISI (comp)) [49].
Insulin secretion at fasting using Homeostasis model assessment β (HOMA-β) [47] and post-challenge using Stumvoll phase 1 [50] and area under the curve insulin/glucose (AUCins/glu) [43].
The corresponding disposition indices: at fasting, QUICKI* HOMA-β and 1/HOMA-IR* HOMA-β and post-challenge, Matsuda*AUCins/glu, and Matsuda*Stumvoll phase 1.
Additional commonly used glucose homeostasis indices were also calculated as complementary information.
Post-challenge insulin sensitivity using oral glucose insulin sensitivity (OGIS) [51].
Postchallenge insulin secretion using early insulinogenic index (IGI) [52] and Stumvoll phase 2 [50].
The corresponding disposition index (OGIS*IGI) plus those for combining OGIS and IGI with pregnancy-validated indices (Matsuda*IGI, OGIS*AUCins/glu, OGIS*Stumvoll phase 1).
Serum 25(OH)D concentrations were measured using a ClinMass® liquid chromatography–mass spectrometry/mass spectrometry (LCeMS/MS) complete kit (RECIPE Chemicals. Instruments GmbH, Munich, Germany). Vitamin D compounds 25OHD2, 25OHD3 were quantified, and C3-epimerwas qualitatively measured. All measurements were performed in the central laboratory.
2.4. Statistical Analysis
Categorical variables are presented as counts and percentages and continuous variables as median and percentile 25–75. At visual inspection, distributions did not substantially deviate from normal.
Multivariate regression analyses (forward method) were used to assess the relationships between individual VitD compounds (25OHD2, 25OHD3 and C3-epimer) and glucose homeostasis variables: FPG, 1 and 2 h plasma glucose, pregnancy-validated indices (HOMA-IR, QUICKI, Matsuda, HOMA-β, Stumvoll phase 1, AUCIns/Glu), additional glucose homeostasis indices (OGIS, IGI, Stumvoll phase 2), and fasting and post-challenge disposition indices at each pregnancy period evaluated. At <20 weeks, the regression model included VitD compounds (25OHD2, 25OHD3 and C3-epimer), age, BMI (at the time of evaluation), ethnicity, family history of diabetes, prior GDM, and recruitment site. At 24–28 and 35–37 weeks, the regression model included the abovementioned variables and DALI lifestyle intervention.
Statistical assumptions for regression analyses were checked including lack of influential case detection (Cook’s distance < 1), collinearity diagnosis (variable inflation scores < 10), and residual independence (Durbin–Watson statistic between 1 and 3).
Logistic regression analysis with the same exposure variables was used to assess the relationships between VitD compounds and HiP. Significance was defined as a two-sided p < 0.05. As this was an exploratory analysis, no sample size calculation was performed, and corrections for multiple comparisons were not applied.
To explore if vitamin D compounds had different impacts on glucose homeostasis variables in participants with different vitamin D concentrations, we performed a subgroup analysis using vitamin D cut-offs <30 mmol/L for deficiency, 30–50 mmol/L for insufficiency, and ≥50 mmol/L for sufficiency.
All analyses were performed with IBM SPSS Statistics package (IBM Corp., version 26, Armonk, NY, USA).
3. Results
A total of 912 participants were included in the present analysis. Maternal characteristics are described in Table 1. In summary, average maternal age was 32 years, and average pre-pregnancy BMI was 32.9 kg/m2. Of the women, 86% were Caucasian, 25% had a family history of diabetes, and 62% had had previous pregnancies. Two thirds of the participants (66.3%) had total 25OHD concentrations ≥ 50 nmol/L at baseline. VitD concentrations and glucose homeostasis variables according to pregnancy period are summarized in Table 2.
Table 1.
Maternal characteristics and sociodemographic data.
| Variables | Median (P25–75) or n (%) | n |
|---|---|---|
| Maternal age (years) | 32.1 (28.4–36.0) | 912 |
| Height (cm) | 166 (161–170) | 912 |
| Gestational age at baseline (weeks) | 15.0 (13.2–16.7) | 912 |
| Ethnicity (Caucasian) | 784 (86.2) | 910 |
| Family history of DM | 228 (25.0) | 912 |
| Previous pregnancies | 567 (62.3) | 910 |
| GDM | 58 (10.4) | 558 |
| Stillbirth | 64 (11.5) | 558 |
| Congenital anomalies | 24 (4.3) | 560 |
| Macrosomia | 120 (21.6) | 556 |
| BMI according to pregnancy period | ||
| Pre-pregnancy BMI (kg/m2) | 32.9 (30.6–36.2) | 912 |
| <20 weeks | 33.7 (31.6–36.8) | 911 |
| 24–28 weeks | 34.9 (33.1–37.9) | 655 |
| 35–37 weeks | 36.6 (34.2–39.2) | 500 |
BMI: body mass index, DM: diabetes mellitus, GDM: gestational diabetes mellitus.
Table 2.
Vitamin D compounds and pregnancy-validated glucose homeostasis variables according to pregnancy period.
| Variable | <20 Weeks | 24–28 Weeks | 35–37 Weeks | |||
|---|---|---|---|---|---|---|
| Median (P25-75) or n (%) | n | Median (P25-75) or n (%) | n | Median (P25-75) or n (%) | n | |
| Vitamin D compounds | ||||||
| 25OHDtotal (nmol/L) | 61.8 (43.5–79.3) | 912 | 70.4 (47.9–98.7) | 660 | 71.1 (45.7–101.4) | 502 |
| 25OHD2 (nmol/L) | 0.025 (0.025–8.310) ‡ | 912 | 0.025 (0.025–8.299) | 660 | 0.025 (0.025–6.027) | 502 |
| 25OHD3 (nmol/L) | 60.1 (42.0–77.2) | 912 | 68.3 (46.8–97.0) | 660 | 67.2 (42.5–99.0) | 502 |
| C3-epimer (+) | 323 (36%) | 896 | 296 (46%) | 643 | 233 (47.6%) | 490 |
| Vitamin D sufficiency (≥50 nmol/L) | 605 (66.3%) | 912 | 476 (72.1%) | 660 | 348 (69.3%) | 502 |
| Glucose | ||||||
| FPG (mmol/L) | 4.7 (4.4–5.0) | 912 | 4.6 (4.3–4.8) | 660 | 4.5 (4.2–4.8) | 502 |
| 1 h PG (mmol/L) | 7.0 (5.8–8.3) | 902 | 7.6 (6.6–8.8) | 652 | 8.2 (7.1–9.2) | 485 |
| 2 h PG (mmol/L) | 6.0 (5.2–6.9) | 904 | 6.2 (5.4–7.0) | 652 | 6.4 (5.6–7.2) | 485 |
| Fasting glucose homeostasis indexes | ||||||
| HOMA-IR (mUI/L*mmol/L) | 2.8 (2.1–3.9) | 892 | 3.0 (2.2–4.1) | 645 | 3.3 (2.4–4.4) | 491 |
| QUICKI (1/(log μUI/mL + log mg/dL) | 0.33 (0.31–0.34) | 892 | 0.32 (0.31–0.34) | 645 | 0.32 (0.31–0.33) | 491 |
| HOMA-β (mUI/mmol) | 237 (177–340) | 890 | 287 (206–405) | 642 | 348 (252–528) | 488 |
| Fasting DI | ||||||
|
76.7 (58.6–108.9) | 890 | 92.9 (67.3–127) | 642 | 111 (81.9–164.5) | 488 |
|
79.8 (60–114) | 890 | 88.9 (72.1–130.8) | 642 | 100 (72.1–153) | 488 |
| Post-challenge glucose homeostasis indexes | ||||||
| Matsuda (μUI/mg) | 3.1 (2.1–4.3) | 408 | 2.6 (1.9–3.5) | 311 | 2.2 (1.6–2.9) | 220 |
| Stumvoll phase 1 (pmol) | 1623 (1257–2117) | 845 | 1843 (1368–2347) | 622 | 2200 (1732–2775) | 470 |
| AUCins/glu (μUI/mmol | 12.7 (9.0–19.0) | 414 | 14.8 (10.3–19.5) | 311 | 17.6 (13.0–23.4) | 224 |
| Post-challenge DI | ||||||
|
38.4 (29.8–47.6) | 408 | 34.9 (30.0–43.2) | 310 | 38.1 (29.9–46.1) | 220 |
|
4892 (4029–5979) | 408 | 4595 (3836–5469) | 311 | 4576 (3940–5599) | 220 |
| HiP | 245 (27.1%) | 904 | 140 (21.5%) | 652 | 97 (20%) | 486 |
‡ Expressed as P10–90 due to low concentration, FPG: fasting plasma glucose, PG: plasma glucose, HOMA-IR: homeostasis model assessment insulin resistance, HOMA- β: homeostasis model assessment beta, QUICKI: quantitative insulin sensitivity check index, AUCins/glu: area under the curve insulin/glucose, DI: disposition index, HiP: hyperglycemia in pregnancy, C3-epimer (+): C3-epimer positivity.
Overall, statistical assumptions for regression analyses were met, with the exception of 3 indices displaying a Durbin–Watson statistic and/or Cook’s distance close to 1 at 24–28 weeks and a single index that showed a Durbin–Watson statistic close to 1 at 35–37 weeks.
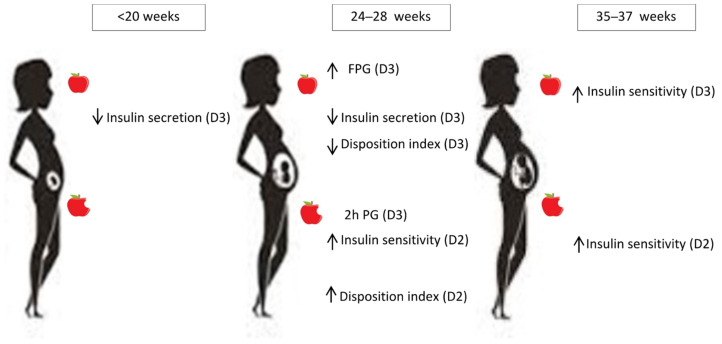
Only the significant associations observed in multivariate analysis between the VitD compounds and the pregnancy-validated glucose homeostasis variables are displayed in Table 3. The associations are schematically summarized in Figure 1.
Table 3.
Associations between vitamin D compounds and pregnancy-validated glucose homeostasis variables in a multivariate model adjusted for age, body mass index, ethnicity, family history of diabetes, prior GDM, recruitment site, and, at 24–28 and 35–37 weeks, DALI lifestyle intervention.
| Outcome Variables | β Values/OR for Significant Associations | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| <20 Weeks | 24–28 Weeks | 35–37 Weeks | |||||||
| 25OHD2 | 25OHD3 | C3-Epimer | 25OHD2 | 25OHD3 | C3-Epimer | 25OHD2 | 25OHD3 | C3-Epimer | |
| Fasting | |||||||||
| FPG | 0.124 * | 0.111 * | |||||||
| 1/HOMA-IR (sens) | 0.127 * | ||||||||
| QUICKI (sens) | |||||||||
| HOMA-β (sec) | −0.117 * | −0.145 ** | |||||||
| Fasting DI | |||||||||
|
−0.124 * | −0.148 ** | |||||||
|
−0.093 * | ||||||||
| Post-challenge | |||||||||
| 1 h PG | |||||||||
| 2 h PG | 0.120* | ||||||||
| Matsuda (sens) | 0.149 * | ||||||||
| Stumvoll phase 1 (sec) | |||||||||
| AUCins/glu (sec) | |||||||||
| Post-challenge DI | |||||||||
|
|||||||||
|
−0.103 * | 0.168 * | 0.239 ** | ||||||
|
|||||||||
* = p < 0.05, ** = p < 0.01. FPG: fasting plasma glucose, HOMA-IR: homeostasis model assessment insulin resistance, sens: insulin sensitivity index, QUICKI: quantitative insulin sensitivity check index, HOMA- β: homeostasis model assessment beta, sec: insulin secretion index; DI: disposition index, PG: plasma glucose, AUCins/glu: area under the curve insulin/glucose, HiP: Hyperglycemia in pregnancy.
Figure 1.
Associations between vitamin D compounds and pregnancy-validated glucose homeostasis variables according to pregnancy period.
At first assessment (<20 weeks’ gestation), 25OHD3 was associated with higher FPG, decreased fasting insulin secretion (HOMA-β) and decreased fasting DI (1/HOMA-IR* HOMA-β and QUICKI* HOMA-β).
At 24–28 weeks, 25OHD3 was associated with higher FPG and 2 h plasma glucose, lower fasting insulin secretion (HOMA-β), and lower fasting DI (QUCKI* HOMA-β). 25OHD2 was associated with higher post-challenge insulin sensitivity (Matsuda) and higher post-challenge DI (Matsuda*Stumvoll phase 1).
At 35–37 weeks, 25OHD3 was associated with higher fasting insulin sensitivity (1/HOMA-IR), and 25OHD2 was associated with higher post-challenge DI (Matsuda*Stumvoll phase 1).
No significant associations between glucose homeostasis variables and C3-epimer were observed at any pregnancy period.
Furthermore, no significant associations between any vitamin D compounds and HiP were observed at any pregnancy period assessed.
Coefficients for the associations were weak or very weak, with global R2 changes ranging from 0.010 to 0.144 for the different glucose homeostasis indices.
No significant associations between the additional glucose homeostasis indices (OGIS, IGI, Stumvoll phase 2, or their calculated disposition indices) were observed.
In the subgroup analysis, both favorable and unfavorable associations were present in different sufficiency groups, pregnancy periods, and VitD compounds with the exceptions of only favorable associations being observed with 25OHD2 or at 35–37 weeks (Supplementary Table S1). The associations were not strong.
4. Discussion
In this exploratory sub-analysis of the DALI study, we observed direct associations between VitD compounds and insulin sensitivity and inverse associations with fasting insulin secretion throughout pregnancy. The association between VitD and β-cell function (measured as DI) was inverse at fasting and direct post-challenge, while that for glucose concentrations was direct. It is important to note that all coefficients were weak or very weak, suggesting a small contribution from VitD in glucose homeostasis. Most associations were observed with 25OHD3.
Our observations indicate a very weak but significant association between VitD and fasting (35–37 weeks) and post-challenge (24–28 weeks) insulin sensitivity indices validated in pregnancy. Several observational studies have reported positive associations between VitD concentrations and insulin sensitivity, generally assessed by HOMA-IR and QUICKI [25,53,54]. This association has been confirmed in VitD supplementation trials, both in and outside pregnancy [23,30,47], with insulin sensitivity usually measured in fasted state [25,27].
As to insulin secretion, we observed an association between VitD compounds and lower fasting insulin secretion (<20 and 24–28 weeks). While in animal models, the association between VitD deficiency and insulin secretion impairment is well-characterized, human observational and intervention trials tend to focus on insulin sensitivity rather than secretion. Reports involving VitD and insulin secretion have been inconsistent. Small intervention studies have shown increased insulin secretion with supplementation with VitD3 in four subjects with insulin deficiency [55] or with 1-alfa-OH-D3 in seven subjects with diabetes [56]. On the other hand, in line with our observations, significant inverse associations between total 25OHD and OGTT-induced insulin secretion [22] and hyperglycemic clamp-induced insulin response have been reported [24].
As to more relevant β-cell function indices, we observed a consistent inverse association between VitD compounds and fasting DI (QUICKI*HOMA-β in early and mid-pregnancy, 1/HOMA-IR*HOMA-β in early pregnancy) and a direct association with post-challenge DI (Matsuda*Stumvoll phase1) in mid- and late pregnancy.
A direct association between 25OHD3 and glucose (fasting at <20 and 24–28 weeks, and post-challenge at 24–28 weeks) was observed.
Though weak, the associations with 25OHD3 can be viewed as unfavorable (mainly reduced fasting insulin, decreased fasting DI, and higher glucose concentrations), while those for 25OHD2 were favorable (increased post-challenge sensitivity (Matsuda) and increased post-challenge β-cell function, estimated by the Matsuda*Stumvoll phase 1 index).
Reports addressing VitD and glucose homeostasis usually study the associations between 25OHD3 or total 25OHD and different glucose-related variables, but less is known about the influence of individual VitD compounds. An analysis from the European Prospective Investigation into Cancer and Nutrition (EPIC) case–cohort study for type 2 diabetes mellitus involving 9671 incident cases described an inverse association between 25OHD3 and incident type 2 diabetes melitus, suggesting a favorable effect, while C3-epimer concentrations were directly associated with incident type 2 diabetes mellitus. This pointed to a potential negative role of C3-epimer in glucose-homeostasis regulation. There was no statistically significant association with type 2 diabetes mellitus for 25OHD2 in this report [20]. We did not find any significant association between C3-epimer and any glucose homeostasis variable.
It is generally accepted that 25OHD3 has greater biological activity compared with 25OHD2, as it has proven to be more efficient at raising total serum 25OHD concentrations [4]. However, studies comparing health outcomes after different regimes of vitamin supplementation are scarce. Curiously, a study involving mice exclusively fed either VitD2 or VitD3 showed that D2-mice had lower serum 1,25(OH)2D relative to D3 mice, but in contrast, free 25OHD was significantly higher. Furthermore, D2-mice had significantly higher bone volume/total volume and trabecular number compared with D3-mice [57]. Thus, the ideal supplementation strategy might not be completely clarified.
Our observations showed a mixed relationship between VitD compounds and glucose homeostasis variables, hinting to a more favorable association with 25OHD2 compared with 25OHD3. However, the magnitudes of the associations observed suggest that VitD does not have a pivotal role in glucose homeostasis regulation in this study group. Nonetheless, results might not be extrapolated to other populations of pregnant women (i.e., non-obese)
Evidence from recent reports addressing the prevention of type 2 diabetes mellitus have shown a beneficial effect from VitD supplementation [58,59] that seems to be limited to non-obese subjects [59]. More recently, a secondary analysis from the D2d trial (comparing VitD with placebo for the prevention of type 2 diabetes mellitus) investigated the effects of VitD3 supplementation on insulin sensitivity and β-cell function. The authors of the analysis observed that supplementation with VitD3 did not improve insulin sensitivity or β-cell function in people with prediabetes, but there was benefit among those with very low baseline total 25OHD status [60]. In light of these observations, the authors speculated that the potential effect of VitD on the risk of type 2 diabetes mellitus might be mediated through other pathways independent of insulin sensitivity and β-cell function [60].
In pregnancy, a Cochrane review examining the effect of VitD supplementation reported a GDM risk reduction (RR 0.51, 95CI 0.27–0.97) [39]. Interestingly, average baseline total 25OHD concentration in the women included in this meta-analysis was <50 nmol/L. We reported similar observations commenting on a metanalysis where VitD supplementation showed a GDM risk reduction. Studies included comprised women with VitD deficiency at baseline and high supplementation doses (~3500 Ui/day) [61]. In this report, we did not observe differences between sufficiency groups that suggest more favorable or unfavorable associations according to total 25OHD concentrations. As abovementioned, this could be due to different relationships between VitD metabolites and glucose homeostasis indices in obese subjects.
We reported different associations between VitD compounds and glucose homeostasis variables. Though our observations hint at a more favorable role for 25OHD2, the magnitudes of the associations observed suggest that vitamin D may not play a key role in glucose homeostasis regulation in pregnancy. Little is known of the associations between individual VitD compounds and glucose metabolism in pregnancy, and further studies are needed in order to define their roles in glucose homeostasis.
Strengths/Limitations
The main novelty presented in this report is the relationships between different VitD compounds (instead of total VitD) and glucose homeostasis. Another strength is the use of pregnancy-validated sensitivity and secretion indices and the use of the disposition index as a measure of β-cell function, which can be more informative than isolated insulin sensitivity and secretion values.
As for the limitations, this was an exploratory analysis, so the associations observed must be interpreted with care. C3-epimer was not quantitatively measured. Additionally, in our study, only overweight and obese women were included, and the majority of the participants were VitD sufficient at baseline and throughout pregnancy. Considering recent evidence from type 2 diabetes mellitus prevention trials, the characteristics of our population may not have allowed us to fully explore the associations between VitD compounds and glucose homeostasis in pregnancy.
5. Conclusions
In women participating in the DALI study, most of them with baseline VitD in sufficiency range, VitD compounds were not unequivocally associated with more favorable glucose homeostasis measures. This suggests a limited potential of VitD compounds for HiP prevention in populations with similar characteristics. Whether different VitD compounds have different impacts on glucose homeostasis is not clear, but further exploring their role might be of interest for the design of supplementation studies.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu14163256/s1, Table S1: Associations between vitamin D compounds and pregnancy-validated glucose homeostasis variables according to 25OHD sufficiency, in a multivariate model adjusted for age, body mass index, ethnicity, family history of diabetes, prior GDM, recruitment site and (at 24–28 and 35–37 weeks) DALI lifestyle intervention.
Author Contributions
R.C. and L.C.M. designed and executed the study, provided input for the interpretation of the results and wrote the manuscript. J.H., G.D., D.S., J.M.A., A.K.-W., A.Z., E.W.-O., A.L., M.G.D., A.B., R.D., F.D., E.R.M., P.D., L.L.A., D.M.J., D.H. and M.N.M.v.P. provided input for the interpretation of the results and reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Ethical approval for the study was granted by local ethics committees: NRES Committee East of England-Norfolk: 11/EE/0221; Medical University Poznan: 1165/12; UZ KU Leuven: ML7625; Hospital De La Santa Creu i Sant Pau Barcelona 13/006 (OBS); Medical University Vienna: 2022/2012-1369/2013; Province of Padua and Pisa: 4201 Å~ 11; Galway University Hospitals: 7/12; Ethical comittee VU medisch centrum Amsterdam: nr. 2012/400; Copenhague and Odense: Scientific Ethics Committee for the Capital Region, Hillerod, Denmark, Protokol nr.: H-4-2013-005. The study was performed according to the Declaration of Helsinki II. Trial registration was ISRCTN70595832.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, RC, upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
DALI has received funding from the European Community’s 7th Framework Programme (FP7/2007–2013) under grant agreement no. 242187. In the Netherlands, additional funding was provided by the Netherlands Organization for Health Research and Development (ZonMw) (grant no. 200310013). In Poland, additional funding was obtained from Polish Ministry of Science (grant no. 2203/7. PR/2011/2). In Denmark, additional funding was provided by the Odense University Free Research Fund. In the United Kingdom, the local DALI team was supported by NIHR Clinical Research Network: Eastern. In Spain, additional funding was provided by CAIBER 1527-B-226. The funders had no role in any aspect of the study beyond funding. This sub-study also received funding from the Spanish Diabetes Society (SED) XI Grant for clinical research projects in diabetes lead by young investigators to LM in 2020.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Pittas A.G., Lau J., Hu F.B., Dawson-Hughes B. Review: The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 2007;92:2017–2029. doi: 10.1210/jc.2007-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haddad J.G., Matsuoka L.Y., Hollis B.W., Hu Y.Z., Wortsman J. Human plasma transport of vitamin D after its endogenous synthesis. J. Clin. Investig. 1993;91:2552–2555. doi: 10.1172/JCI116492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zarei A., Hulley P.A., Sabokbar A., Javaid M.K., Morovat A. 25-Hydroxy- and 1A,25-Dihydroxycholecalciferol Have Greater Potencies Than 25-Hydroxy- and 1A,25-Dihydroxyergocalciferol in Modulating Cultured Human and Mouse Osteoblast Activities. PLoS ONE. 2016;11:e0165462. doi: 10.1371/journal.pone.0165462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tripkovic L., Lambert H., Hart K., Smith C., Giselda B., Penson S., Chope G., Hyppönen E., Berry J., Vieth J. Comparison of vitamin D2 and vitamin D3 supplementation in raising serum 25-hydroxyvitamin D status: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2012;95:1357–1364. doi: 10.3945/ajcn.111.031070. Available online: http://www.academia.edu/download/31819832/Birkenmaier__Revista_Iberoamericana_2008.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maestro B., Campion J., Dávila N., Calle C. Stimulation by 1,25-Dihydroxyvitamin D3 of Insulin Receptor Expression and Insulin Responsiveness for Glucose Transport in U-937 Human Promonocytic Cells. Endocr. J. 2000;47:383–391. doi: 10.1507/endocrj.47.383. [DOI] [PubMed] [Google Scholar]
- 6.Maestro B., Molero S., Bajo S., Dávila N., Calle C. Transcriptional activation of the human insulin receptor gene by 1,25-dihydroxyvitamin D3. Cell Biochem. Funct. 2002;20:227–232. doi: 10.1002/cbf.951. [DOI] [PubMed] [Google Scholar]
- 7.Pittas A.G., Joseph N.A., Greenberg A.S. Adipocytokines and Insulin Resistance. J. Clin. Endocrinol. Metab. 2004;89:447–452. doi: 10.1210/jc.2003-031005. [DOI] [PubMed] [Google Scholar]
- 8.Gysemans C., Cardozo A.K., Callewaert H., Giulietti A., Hulshagen L., Bouillon R., Eizirik D.L., Mathieu C. 1,25-Dihydroxyvitamin D3 Modulates Expression of Chemokines and Cytokines in Pancreatic Islets: Implications for Prevention of Diabetes in Nonobese Diabetic Mice. Endocrinology. 2005;146:1956–1964. doi: 10.1210/en.2004-1322. [DOI] [PubMed] [Google Scholar]
- 9.Norman A.W., Frankel B.J., Heldt A.M., Grodsky G.M. Vitamin D Deficiency Inhibits Pancreatic Secretion of Insulin. Science. 1980;209:823–825. doi: 10.1126/science.6250216. [DOI] [PubMed] [Google Scholar]
- 10.Zeitz U., Weber K., Soegiarto D.W., Wolf E., Balling R., Erben R.G. Impaired insulin secretory capacity in mice lacking a functional vitamin D receptor. FASEB J. 2003;17:509–511. doi: 10.1096/fj.02-0424fje. [DOI] [PubMed] [Google Scholar]
- 11.Bland R., Markovic D., Hills C.E., Hughes S.V., Chan S.L., Squires P.E., Hewison M. Expression of 25-hydroxyvitamin D3-1α-hydroxylase in pancreatic islets. J. Steroid Biochem. Mol. Biol. 2004;89-90:121–125. doi: 10.1016/j.jsbmb.2004.03.115. [DOI] [PubMed] [Google Scholar]
- 12.Clark S.A., Stumpf W.E., Sar M. Effect of 1,25 Dihydroxy vitamin D3 on Insulin Secretion. Diabetes. 1981;30:382–386. doi: 10.2337/diab.30.5.382. [DOI] [PubMed] [Google Scholar]
- 13.Kjalarsdottir L., Tersey S., Vishwanath M., Chuang J., Posner B., Mirmira R., Repa J.J. 1,25-Dihydroxyvitamin D3 enhances glu-cose-stimulated insulin secretion in mouse and human islets: A role for transcriptional regulation of voltage-gated calcium channels by the vitamin D receptor. J. Steroid Biochem. Mol. Biol. 2019;185:17–26. doi: 10.1016/j.jsbmb.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 14.Bornstedt M.E., Gjerlaugsen N., Olstad O.K., Berg J.P., Bredahl M.K., Thorsby P.M. Vitamin D metabolites influence expression of genes concerning cellular viability and function in insulin producing β-cells (INS1E) Gene. 2020;746:144649. doi: 10.1016/j.gene.2020.144649. [DOI] [PubMed] [Google Scholar]
- 15.Bailey D., Veljkovic K., Yazdanpanah M., Adeli K. Analytical measurement and clinical relevance of vitamin D3 C3-epimer. Clin. Biochem. 2013;46:190–196. doi: 10.1016/j.clinbiochem.2012.10.037. [DOI] [PubMed] [Google Scholar]
- 16.Mao D., Yuen L.-Y., Ho C.-S., Wang C.-C., Tam C.H.-T., Chan M.H.-M., Lowe W.L., Ma R.C.-W., Tam W.-H. Maternal and Neonatal 3-epi-25-hydroxyvitamin D Concentration and Factors Influencing Their Concentrations. J. Endocr. Soc. 2021;6 doi: 10.1210/jendso/bvab170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown A.J., Ritter C.S., Weiskopf A.S., Vouros P., Sasso G.J., Uskokovic M.R., Wang G., Satyanarayana G.R. Isolation and identification of 1α-hydroxy-3-epi-vitamin D3, a potent suppressor of parathyroid hormone secretion. J. Cell Biochem. 2005;96:569–578. doi: 10.1002/jcb.20553. [DOI] [PubMed] [Google Scholar]
- 18.Nakagawa K., Sowa Y., Kurobe M., Ozono K., Siu–Caldera M.-L., Reddy G., Uskokovic M.R., Okano T. Differential activities of 1α,25-dihydroxy-16-ene-vitamin D3 analogs and their 3-epimers on human promyelocytic leukemia (HL-60) cell differentiation and apoptosis. Steroids. 2001;66:327–337. doi: 10.1016/S0039-128X(00)00142-2. [DOI] [PubMed] [Google Scholar]
- 19.Chailurkit L.-O., Aekplakorn W., Srijaruskul K., Ongphiphadhanakul B. Discrepant association of serum C-3 epimer of 25-hydroxyvitamin D versus non-epimeric 25-hydroxyvitamin D with serum lipid levels. Lipids Health Dis. 2016;15:157. doi: 10.1186/s12944-016-0333-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zheng J.S., Imamura F., Sharp S.J., Van Der Schouw Y.T., Sluijs I., Gundersen T.E., Ardanaz E., Boeing H., Bonet C., Humberto Gómez J. Association of plasma Vitamin D metabolites with incident type 2 diabetes: EPIC-InterAct case-cohort study. J. Clin. Endocrinol. Metab. 2019;104:1293–1303. doi: 10.1210/jc.2018-01522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xia J., Song Y., Wu J., Hinkle S., Li M., Tsai M.Y., Zhang C. Abstract P266: Biomarkers of Vitamin D3 C-3 Epimers During Pregnancy and the Risk of Gestational Diabetes Mellitus: A Longitudinal Study in a Multiracial Cohort. Circulation. 2019;139 doi: 10.1161/circ.139.suppl_1.P266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baynes K.C.R., Boucher B.J., Feskens E.J.M., Kromhout D. Vitamin D, glucose tolerance and insulinaemia in elderly men. Diabetologia. 1997;40:344–347. doi: 10.1007/s001250050685. [DOI] [PubMed] [Google Scholar]
- 23.Kamycheva E., Jorde R., Figenschau Y., Haug E. Insulin sensitivity in subjects with secondary hyperparathyroidism and the effect of a low serum 25-hydroxyvitamin D level on insulin sensitivity. J. Endocrinol. Investig. 2007;30:126–132. doi: 10.1007/BF03347410. [DOI] [PubMed] [Google Scholar]
- 24.Chiu K.C., Chu A., Go V.L.W., Saad M.F. Hypovitaminosis D is associated with insulin resistance and β cell dysfunction. Am. J. Clin. Nutr. 2004;79:820–825. doi: 10.1093/ajcn/79.5.820. [DOI] [PubMed] [Google Scholar]
- 25.Kayaniyil S., Vieth R., Retnakaran R., Knight J.A., Qi Y., Gerstein H.C., Perkins B.A., Harris S.B., Zinman B., Hanley A.J. Association of vitamin D with insulin resistance and β-cell dysfunction in subjects at risk for type 2 diabetes. Diabetes Care. 2010;33:1379–1381. doi: 10.2337/dc09-2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dutta D., Mondal S.A., Choudhuri S., Maisnam I., Reza A.H.H., Bhattacharya B., Chowdhury S., Mukhopadhyay S. Vitamin-D supplementation in prediabetes reduced progression to type 2 diabetes and was associated with decreased insulin resistance and systemic inflammation: An open label randomized prospective study from Eastern India. Diabetes Res. Clin. Pract. 2014;103:e18–e23. doi: 10.1016/j.diabres.2013.12.044. [DOI] [PubMed] [Google Scholar]
- 27.Niroomand M., Fotouhi A., Irannejad N., Hosseinpanah F. Does high-dose vitamin D supplementation impact insulin resistance and risk of development of diabetes in patients with pre-diabetes? A double-blind randomized clinical trial. Diabetes Res. Clin. Pract. 2018;148:1–9. doi: 10.1016/j.diabres.2018.12.008. [DOI] [PubMed] [Google Scholar]
- 28.Jorde R., Sollid S.T., Svartberg J., Schirmer H., Joakimsen R.M., Njølstad I., Fuskevåg O.M., Figenschau Y., Hutchinson M.Y. Vitamin D 20 000 IU per Week for Five Years Does Not Prevent Progression from Prediabetes to Diabetes. J. Clin. Endocrinol. Metab. 2016;101:1647–1655. doi: 10.1210/jc.2015-4013. [DOI] [PubMed] [Google Scholar]
- 29.Pittas A.G., Dawson-Hughes B., Sheehan P., Ware J.H., Knowler W.C., Aroda V.R., Brodsky I., Ceglia L., Chadha C., Chatterjee R., et al. Vitamin D Supplementation and Prevention of Type 2 Diabetes. N. Engl. J. Med. 2019;381:520–530. doi: 10.1056/NEJMoa1900906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wagner C.L., McNeil R., Hamilton S.A., Winkler J., Cook C.R., Warner G., Bivens B., Davis D.J., Smith P.G., Murphy M., et al. A randomized trial of vitamin D supplementation in 2 community health center networks in South Carolina. Am. J. Obstet. Gynecol. 2012;208:137.e1–137.e13. doi: 10.1016/j.ajog.2012.10.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davidson M.B., Duran P., Lee M.L., Friedman T.C. High-dose vitamin D supplementation in people with prediabetes and hypo-vitaminosis D. Diabetes Care. 2013;36:260–266. doi: 10.2337/dc12-1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mitri J., Dawson-Hughes B., Hu F.B., Pittas A.G. Effects of vitamin D and calcium supplementation on pancreatic β cell function, insulin sensitivity, and glycemia in adults at high risk of diabetes: The Calcium and Vitamin D for Diabetes Mellitus (CaDDM) randomized controlled trial. Am. J. Clin. Nutr. 2011;94:486–494. doi: 10.3945/ajcn.111.011684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gagnon C., Daly R.M., Carpentier A., Lu Z.X., Shore-Lorenti C., Sikaris K., Jean S., Ebeling P.R. Effects of combined calcium and vitamin D sup-plementation on insulin secretion, insulin sensitivity and β-cell function in multi-ethnic vitamin D-deficient adults at risk for type 2 diabetes: A pilot randomized, placebo-controlled trial. PLoS ONE. 2014;9:e109607. doi: 10.1371/journal.pone.0109607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lemieux P., Weisnagel S.J., Caron A.Z., Julien A.S., Morisset A.S., Carreau A.M., Poirier J., Tchernof A., Robitaille J., Bergeron J. Effects of 6-month Vitamin D supple-mentation on insulin sensitivity and secretion: A randomised, placebo-controlled trial. Eur. J. Endocrinol. 2019;181:287–299. doi: 10.1530/EJE-19-0156. [DOI] [PubMed] [Google Scholar]
- 35.Asemi Z., Samimi M., Tabassi Z., Shakeri H., Esmaillzadeh A. Retracted: Vitamin D Supplementation Affects Serum High-Sensitivity C-Reactive Protein, Insulin Resistance, and Biomarkers of Oxidative Stress in Pregnant Women. J. Nutr. 2013;143:1432–1438. doi: 10.3945/jn.113.177550. [DOI] [PubMed] [Google Scholar]
- 36.Tehrani H.G., Mostajeran F., Banihashemi B. Effect of Vitamin D Supplementation on the Incidence of Gestational Diabetes. Adv. Biomed. Res. 2017;6 doi: 10.4103/2277-9175.210658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shahgheibi S., Farhadifar F., Pouya B. The effect of vitamin D supplementation on gestational diabetes in high-risk women: Results from a randomized placebo-controlled trial. J. Res. Med. Sci. 2018;21:2. doi: 10.4103/1735-1995.175148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Corcoy R., Mendoza L.C., Simmons D., Desoye G., Adelantado J., Chico A., Devlieger R., van Assche A., Galjaard S., Timmerman D., et al. The DALI vitamin D randomized controlled trial for gestational diabetes mellitus prevention: No major benefit shown besides vitamin D sufficiency. Clin. Nutr. 2019;39:976–984. doi: 10.1016/j.clnu.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 39.Palacios C., Kostiuk L., Peña-Rosas J. Vitamin D supplementation for women during pregnancy: Summary of a Cochrane review. Cochrane Database Syst. Rev. Database Syst. Rev. 2019;7:CD008873. doi: 10.1002/14651858.CD008873.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Y., Gong Y., Xue H., Xiong J., Cheng G. Vitamin D and gestational diabetes mellitus: A systematic review based on data free of Hawthorne effect. BJOG Int. J. Obstet. Gynaecol. 2018;125:784–793. doi: 10.1111/1471-0528.15060. [DOI] [PubMed] [Google Scholar]
- 41.Chan K.Y., Wong M.M.H., Pang S.S.H., Lo K.K.H. Dietary supplementation for gestational diabetes prevention and management: A meta-analysis of randomized controlled trials. Arch. Gynecol. Obstet. 2021;303:1381–1391. doi: 10.1007/s00404-021-06023-9. [DOI] [PubMed] [Google Scholar]
- 42.Kirwan J.P., Huston-Presley L., Kalhan S.C., Catalano P.M. Clinically Useful Estimates of Insulin Sensitivity During Pregnancy. Diabetes Care. 2001;24:1602–1607. doi: 10.2337/diacare.24.9.1602. [DOI] [PubMed] [Google Scholar]
- 43.Powe C.E., Locascio J.J., Gordesky L.H., Florez J.C., Catalano P.M. Oral Glucose Tolerance Test-Based Measures of Insulin Secretory Response in Pregnancy. J. Clin. Endocrinol. Metab. 2022 doi: 10.1210/clinem/dgac041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Simmons D., Jelsma J.G., Galjaard S., Devlieger R., van Assche A., Jans G., Corcoy R., Adelantado J.M., Dunne F., Desoye G., et al. Results From a European Multicenter Randomized Trial of Physical Activity and/or Healthy Eating to Reduce the Risk of Gestational Diabetes Mellitus: The DALI Lifestyle Pilot. Diabetes Care. 2015;38:1650–1656. doi: 10.2337/dc15-0360. [DOI] [PubMed] [Google Scholar]
- 45.Simmons D., Devlieger R., Van Assche A., Jans G., Galjaard S., Corcoy R., Adelantado J.M., Dunne F., Desoye G., Harreiter J., et al. Effect of Physical Activity and/or Healthy Eating on GDM Risk: The DALI Lifestyle Study. J. Clin. Endocrinol. Metab. 2016;102:903–913. doi: 10.1210/jc.2016-3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jelsma J.M., van Poppel M.N., Galjaard S., Desoye G., Corcoy R., Devlieger R., van Assche A., Timmerman D., Jans G., Harreiter J., et al. DALI: Vitamin D and lifestyle intervention for gestational diabetes mellitus (GDM) prevention: An European multicentre, randomised trial—Study protocol. BMC Pregnancy Childbirth. 2013;13:16. doi: 10.1186/1471-2393-13-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Matthews D.R., Hosker J.P., Rudenski A.S., Naylor B.A., Treacher D.F., Turner R.C. Homeostasis model assessment: Insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 48.Katz A., Nambi S.S., Mather K., Baron A.D., Follmann D.A., Sullivan G., Quon M.J. Quantitative Insulin Sensitivity Check Index: A Simple, Accurate Method for Assessing Insulin Sensitivity in Humans. J. Clin. Endocrinol. Metab. 2000;85:2402–2410. doi: 10.1210/jcem.85.7.6661. [DOI] [PubMed] [Google Scholar]
- 49.Matsuda M., DeFronzo R.A. Insulin sensitivity indices obtained from oral glucose tolerance testing: Comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22:1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 50.Stumvoll M., Mitrakou A., Pimenta W., Jenssen T., Yki-Järvinen H., Van Haeften T., Renn W., Gerich J. Use of the oral glucose tolerance test to assess insulin release and insulin sensitivity. Diabetes Care. 2000;23:295–301. doi: 10.2337/diacare.23.3.295. [DOI] [PubMed] [Google Scholar]
- 51.Mari A., Pacini G., Murphy E., Ludvik B., Nolan J.J. A Model-Based Method for Assessing Insulin Sensitivity from the Oral Glucose Tolerance Test. Diabetes Care. 2001;24:539–548. doi: 10.2337/diacare.24.3.539. [DOI] [PubMed] [Google Scholar]
- 52.Pjillips D., Clark P., Hales C., Osmond C. Understanding Oral Glucose Tolerance: Comparison of Glucose or Insulin Measurements During the Oral Glucose Tolerance Test with Specific Measurements of Insulin Resistance and Insulin Secretion. Diabet Med. 1994;11:286–292. doi: 10.1111/j.1464-5491.1994.tb00273.x. [DOI] [PubMed] [Google Scholar]
- 53.Maghbooli Z., Hossein-Nezahd A., Karimi F., Shafaei A., Larijani B. Correlation between vitamin D3 deficiency and insulin resistance in pregnancy. Diabetes Metab. Res. Rev. 2008;24:27–32. doi: 10.1002/dmrr.737. [DOI] [PubMed] [Google Scholar]
- 54.Karamali M., Beihaghi E., Mohammadi A., Asemi Z. Effects of High-Dose Vitamin D Supplementation on Metabolic Status and Pregnancy Outcomes in Pregnant Women at Risk for Pre-Eclampsia. Horm. Metab. Res. 2015;47:867–872. doi: 10.1055/s-0035-1548835. [DOI] [PubMed] [Google Scholar]
- 55.Gedik O., Akahn S. Effects of vitamin D deficiency and repletion on insulin and glucagon secretion in man. Diabetologia. 1986;29:142–145. doi: 10.1007/BF02427083. [DOI] [PubMed] [Google Scholar]
- 56.Inomata S., Kadowaki S., Yamatani T., Fukase M., Fujita T. Effect of 1 alpha (OH)-vitamin D3 on. Bone Miner. 1983;1:187–192. [PubMed] [Google Scholar]
- 57.Chun R.F., Hernandez I., Pereira R., Swinkles L., Huijs T., Zhou R., Liu N.Q., Shieh A., Guemes M., Mallya S.M., et al. Differential Responses to Vitamin D2 and Vitamin D3 Are Associated with Variations in Free 25-Hydroxyvitamin D. Endocrinology. 2016;157:3420–3430. doi: 10.1210/en.2016-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Barbarawi M., Zayed Y., Barbarawi O., Bala A., Alabdouh A., Gakhal I., Rizk F., Alkasasbeh M., Bachuwa G., Manson J.E. Effect of Vitamin D Supplementation on the Incidence of Diabetes Mellitus. J. Clin. Endocrinol. Metab. 2020;105:2857–2868. doi: 10.1210/clinem/dgaa335. [DOI] [PubMed] [Google Scholar]
- 59.Zhang Y., Tan H., Tang J., Li J., Chong W., Hai Y., Feng Y., Lunsford L.D., Xu P., Jia D., et al. Effects of Vitamin D Supplementation on Prevention of Type 2 Diabetes in Patients with Prediabetes: A Systematic Review and Meta-analysis. Diabetes Care. 2020;43:1650–1658. doi: 10.2337/dc19-1708. [DOI] [PubMed] [Google Scholar]
- 60.Rasouli N., Brodsky I.G., Chatterjee R., Kim S.H., Pratley R.E., Staten M.A., Pittas A.G., Ceglia L., Chadha C., Dawson-Hughes B., et al. Effects of Vitamin D Supplementation on Insulin Sensitivity and Secretion in Prediabetes. J. Clin. Endocrinol. Metab. 2021;107:230–240. doi: 10.1210/clinem/dgab649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Corcoy R., Mendoza L.C., Simmons D., Desoye G., Mathiesen E.R., Kautzky-Willer A., Damm P., Dunne F.P., Wender-Ozegowska E., Lapolla A., et al. Re: Vitamin D and gestational diabetes mellitus: A systematic review based on data free of Hawthorne effect. BJOG Int. J. Obstet. Gynaecol. 2018;125:784–793. doi: 10.1111/1471-0528.15278. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, RC, upon reasonable request.