Abstract
Aims:
This study aims to investigate the screening value of cytology, high-risk human papillomavirus (hrHPV) testing and serum CA19-9 in cervical adenocarcinomas.
Materials and Methods:
We employed HPV RNA in situ hybridization and immunohistochemistry to reclassify 209 cervical adenocarcinomas according to the International Endocervical Adenocarcinoma Criteria and Classification (IECC). We analyzed the diagnostic value of cytology, hrHPV testing and serum CA19-9 in these tumors and their detection variance among IECC histotypes.
Results:
We found that the sensitivity of cytology or hrHPV test alone was 74.1% (129/174) or 72% (131/182), respectively. Non-HPV related adenocarcinoma showed a lower detection rate of cytology (60%, 27/45 vs. 79.1%, 102/129, p=0.017) or hrHPV testing (9.8%, 4/41 vs. 90.1%, 127/141, p<0.0001), compared to HPV-related adenocarcinomas. The cytology and hrHPV co-testing significantly demonstrated a higher sensitivity (151/165, 91.5%) than single test alone (p<0.001). Nevertheless, the sensitivity of co-testing was substantially lower for gastric-type adenocarcinoma (GAC) (74.1%, 20/27) than that for non-GAC (94.9%, 131/138) (p=0.001). Serum CA19-9 (>40 U/mL) identified 44.1% (15/34) GACs including 75% (6/8) that were missed by co-testing, much higher than for non-GACs (10.7%, 19/177; p<0.001). The combination of cytology, hrHPV test and serum CA19-9 enhanced the detection rate of GACs (92.9%, 26/28).
Conclusion:
We conclude that cytology and hrHPV co-testing is not very effective for non-HPV related adenocarcinoma, particularly for GAC. As such, additional serum CA19-9 should be given in women with potential cancer risks.
Key Words: Cervical adenocarcinoma, gastric-type, cytology, high-risk human papillomavirus (hrHPV) testing, CA19-9
Introduction
Cervical carcinoma is the fourth most common cancer worldwide in women with an estimated 570,000 new cases in 2018 (Siegel et al., 2020). In China, cervical carcinoma had an estimated incidence of 98.9 and mortality of 30.5 per 1000,000 in 2015, ranking the leading cancers in the female genital tract. In the developed countries, the wide adoption of population-based screening programs not only have substantially lowered the absolute incidence of cervical carcinoma, but also have influenced the relative portions of squamous cell carcinoma and adenocarcinoma (Adams et al., 2001). In USA, cervical carcinoma is the third most common and lethal cancers in the female genital tract with estimated 13,800 new cases and 4,290 deaths in 2020 (Siegel et al., 2020). In England, the proportion of squamous cell carcinoma in cervical cancers decreased from 82.6% in 1989 to 70.4% in 2009 whereas the proportion of adenocarcinoma increased from 13.2% to 22.1% (Castanon et al., 2016).
Cytology is less effective against cervical adenocarcinoma than squamous cell carcinoma because glandular lesions in deep locations are more likely to be missed by cytology (Castanon et al., 2016). Infection with high-risk human papilloma virus (hrHPV) is well established as the causative agent in cervical cancers (Crosbie et al., 2013). However, unlike squamous cell carcinoma, cervical adenocarcinomas are a heterogeneous group of tumors with approximately 20% cases unrelated to HPV infection (Pirog et al., 2019; Stolnicu et al., 2018). An international group of gynecological pathologists have proposed the International Endocervical Adenocarcinoma Criteria and Classification (IECC), a new pathogenetic scheme based on the presence or absence of HPV infection-related morphological features (Stolnicu et al., 2018). The IECC approach appears to be valuable for prognostic assessment (Pirog et al., 2019; Stolnicu et al. , 2019). Gastric-type adenocarcinoma (GAC), the predominant subtype of non-HPV related cervical adenocarcinoma, has demonstrated a more aggressive clinical behavior than the usual HPV-related adenocarcinoma (Nishio et al., 2019; Pirog et al., 2019). hrHPV test is apparently ineffective for the detection of GAC and other non-HPV related adenocarcinomas. Accordingly, it is a permanent requirement to find additional approach for the detection of cervical adenocarcinomas.
Serum tumor biomarkers, such as cancer antigen 19-9 (CA19-9), cancer antigen 125 (CA125) and carcinoembryonic antigen (CEA), are clinically useful in the screening and diagnosis of many carcinomas including those from stomach, colorectum, pancreas and ovary (Scatena, 2015). However, the clinical significance of these biomarkers has not been well documented in cervical adenocarcinoma and its subtypes yet. In this study, we investigated the screening value of these biomarkers in combination with cytology and hrHPV co-testing for cervical adenocarcinoma, especially for GAC.
Materials and Methods
Demographics
The Institutional Review Board of Women’s Hospital, School of Medicine, Zhejiang University approved this study (IRB: 20170139), and granted an exemption from requiring written informed consent owing to minimal risk for patient privacy exposure, healthy and other potential harms in the retrospective study. A total of 209 adenocarcinomas, accounting for 11.7% (209/1792) of all cervical carcinomas, were searched by computer (PACS system) from the archives of the Department of Surgical Pathology, Women’s Hospital, School of Medicine, Zhejiang University, China, between January 2014 and June 2019. The clinical data, including clinical presentation, preoperative hrHPV testing, and liquid-based cytology, were retrospectively obtained from the electronic medical records before de-identification. We excluded adenosquamous carcinoma, endometrioid carcinoma from the lower uterine segment and serous carcinoma with concurrent tubo-ovarian or uterine corpus carcinomas. Tumor stage was re-assessed according to the 2018 International Federation of Gynecology and Obstetrics (FIGO) cervical cancer staging system. The hrHPV test was carried out using one of the U.S. Food and Drug Administration (FDA)-approved methods, HC2 HR HPV DNA Test (Qiagen Gaithersburg, MD), Cervista HPV HR Test (Hologic, Bedford, MA) and Aptima HPV assay (Hologic, Bedford, MA). The cytological examination was performed with ThinPrep technique (Hologic, Bedford, MA). The results were interpreted according to the Bethesda System for Reporting Cervical Cytology. Both hrHPV test and cytological examination were carried out within 2 months before surgery.
Two authors (L.B. and S.H.) reviewed the archival hematoxylin-and-eosin (HandE) slides to reclassify the adenocarcinomas according to the IECC (Stolnicu et al., 2018). To reach a consensus classification, RNA in situ hybridization (ISH) and immunostaining were applied with the RNAscope HPV-HR18 Probe (Advanced Cell Diagnostics, Hayward, CA, USA) and immunostaining with a two-step Vision procedure (DAKO, Carpentaria, USA).The antibodies included p16 (G175-405; BD Bioscience, San Jose, CA, USA; 1:100), MUC6 (MRQ-20; Cell Marque, Rocklin, USA; ready-to-use), estrogen receptor (ER) (SP1; Thermo Fisher Scientific, Waltham, USA; 1:300) and progesterone receptor (PR) (SP2; Thermo Fisher Scientific, Waltham, USA; 1:500). The presence of a punctate yellow-to-brownish nuclear reaction in RNA ISH was defined as HPV RNA positive staining. Diffuse (blocking) p16 staining was considered to be positive.
Serum tumor biomarkers including CEA, CA125, and CA19-9 were measured by electrochemiluminescence assay kits (Abbott Molecular Inc., Des Plaines, IL, USA). The blood samples were collected from all patients before surgery and 91 patients 2-4 days post-operatively. The cut-off values used were those recommended by the manufacturers, i.e., 5 ng/mL for CEA, 35 U/mL for CA125, and 40 U/mL for CA19-9.
Statistical analysis
The SPSS 16.0 (SPSS, Inc., Chicago, IL, USA) software package was applied for the statistical analyses. The one-way ANOVA (LSD test), χ2 test (Fisher’s exact test) and non-parametric tests (Wilcoxon test or Mann Whitney U test) were used to detect the significance of difference in serum levels of biomarkers between different carcinoma groups. The statistical threshold was set at 0.05 (two-sided).
Results
Clinicopathological analysis
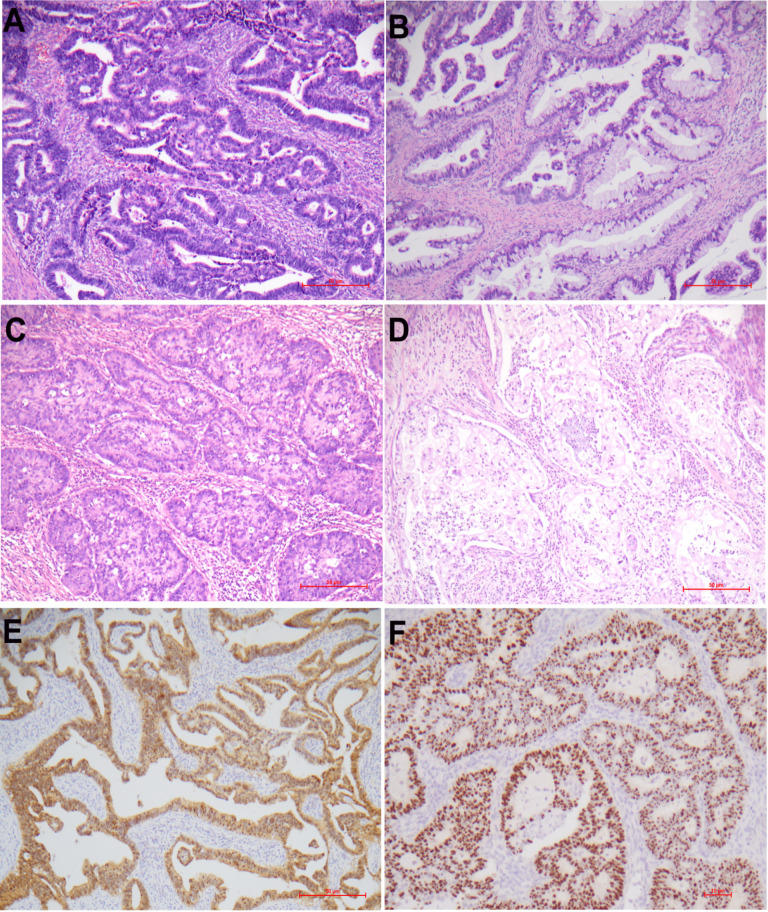
The patients with cervical adenocarcinomas ranged in age from 27 to 74 years (median: 47 years; mean±SD: 47.8±9.9 years). Most patients eventually underwent staging surgery including total abdominal hysterectomy and bilateral salpingo-oophorectom, and pelvic lymph-node dissection. There were 161 patients at stage I, 18 at stage II, 26 at stage III and 4 at stage IV. The IEEC classification included 150 (71.1%) HPV-related adenocarcinomas, 48 (22.7%) non-HPV adenocarcinomas and 13 (6.2%) adenocarcinomas-no other specified (NOS). HPV-related adenocarcinomas were composed of usual type 111 (52.6%), villoglandular type 7 (3.3%), mucinous-NOS type 20 (9.5%), stratified mucin-producing type 10 (4.7%), signet-ring cell type 1 (0.5%), and mucinous intestinal type 1 (0.5%) while non-HPV related adenocarcinomas were comprised of gastric-type 34 (16.1%), endometrioid type 5 (2.4%), serous type 4 (1.9%), clear cell type 4 (1.9%), and mesonephric type 1 (0.5%). Some histological subtypes are exemplified in Figure 1A-D. Invasive adenocarcinomas, NOS were further categorized into non-HPV (n=4) or HPV-related (n=9) adenocarcinomas for further analysis based on the results of p16 staining and HPV RNA ISH detection. Blocking p16 staining (shown in Figure 1E) or HPV signals (shown in Figure 1F) were found in 143 (89.9%) or 145 (91.2%) HPV-related adenocarcinomas, respectively. HPV signals were not detected in non-HPV related adenocarcinomas. The comparison of clinicopathological features between different adenocarcinoma groups were depicted in Table 1. GAC or non-HPV adenocarcinomas were more frequently associated with advanced FIGO stage (III/IV) than non-GAC or HPV-related adenocarcinomas (p<0.001, Table 1).
Figure 1.
Histological Subtypes of Cervical Adenocarcinomas. Depicted in A-D represent endocervical adenocarcinoma (usual-type), mucinous carcinoma (no other specified), invasive stratified mucin-producing carcinoma, and gastric-type adenocarcinoma, respectively. HPV-related adenocarcinomas showed blocking p16 staining [E] and nuclear HPV RNA signals [F]. A-D, H&E staining ×100; E, immunohistochemistry×100; F, HPV mRNA ISH ×200
Table 1.
The Comparison of Clinicopathological Features between Gastric-Type and Non-Gastric-Type, or Non-HPV and HPV-Related Adenocarcinomas
| Clinicopathological features | Histotype | Subcategory of adenocarcinoma † | ||||
|---|---|---|---|---|---|---|
| GAC | non-GAC | p | non-HPV | HPV-related | p | |
| No. (n=211, 100%) | 34 (16.3%) | 175 (83.7%) | 50(24.9%) | 159(75.1%) | ||
| Age, mean ±SD (yr) | 48.8±10.3 | 48±9.9 | 0.696 | 52.4±10.5 | 46.7±9.4 | <0.001 |
| FIGO Stage (I,II/III,IV) | 21/13 | 158/17 | <0.001 | 33/17 | 146/13 | <0.001 |
| ‡Cytological abnormalities (+/-, sensitivity§) | 20/10 (66.7%) | 109/35 (75.7%) | 0.65 | 27/18 (60%) | 102/27 (79.1%) | 0.017 |
| hrHPV test (+/-; sensitivity) | 3/26 (10.3%) | 128/25 (83.7%) | <0.001 | 4/37 (9.8%) | 127/14 (90.1%) | <0.001 |
| CEA, mean ±SD, ng/mL(+/-, sensitivity) | 4.3±9.2 (5/29, 14.7%) | 4.3±18.5 (13/164, 7.3%) | 0.978 | 3.7±7.9 (6/46, 11.5%) | 4.5±19.5 (12/147, 7.5%) | 0.091 |
| CA19-9, mean ±SD, U/mL (+/-, sensitivity) | 422.9±833.6 (15/19, 44.1%) | 41.3±144.3 (19/158, 10.7%) | <0.001 | 277.2±292.7 (16/36, 30.7%) | 44.2±152.3 (18/141, 11.3%) | <0.001 |
| CA125, mean ±SD, U/mL (+/-, sensitivity) | 29.0±29.7 (8/26, 23.5%) | 20.8±18.0 (21/156, 11.9%) | 0.033 | 26.1±26.3 (11/41, 21.2%) | 20.7±18.0 (9/150, 5.7%) | 0.1 |
Annotation: †Invasive adenocarcinomas, NOS were re-categorized into non-HPV (n=4) or HPV-related (n=9) adenocarcinomas based on p16 staining and HPV RNA ISH detection. ‡Cytological abnormalities are defined as any cytological findings above negative for malignancy or intraepithelial lesions. §The sensitivity was calculated as positive/total cases (positive plus negative cases)*100% while the histopathological diagnosis as the gold standard; Abbreviations: FIGO, International Federation of Gynecology & Obstetrics; GAC, gastric-type adenocarcinoma.
Results of cytology and hrHPV tests
Most patients (n=174) received preoperative liquid-based cytology. The cytological abnormalities (atypical squamous cells of undetermined significance (ASCUS)/ atypical glandular cells, NOS (AGC-NOS) or worse) were identified in 129 patients (74.1%) composing of ASCUS 12 (9.3%) cases, atypical squamous cells, cannot exclude HSIL (ASCH) 20 (15.5%) cases, low-grade squamous intraepithelial lesion (LSIL) 2 (1.6%) cases, high-grade squamous intraepithelial lesion (HSIL) 18 (14.0%) cases, squamous cell carcinoma (SCC) 2 (1.6%) cases, AGC-NOS 24 (18.6%) cases, atypical glandular cells, favor neoplastic (AGC-N) 35 (27.1%) cases, adenocarcinoma in situ (AIS) 6 (4.7%) cases, and adenocarcinoma 10 (7.8%) cases. Cytological abnormalities were more frequently present in HPV-related adenocarcinomas than in non-HPV-related adenocarcinomas (p=0.017, Table 1).
Prior hrHPV tests were performed in 182 patients, of which, 131 (72%) were hrHPV positive. Eight patients with prior negative hrHPV testing showed HPV RNA positive signals while 10 patients with positive hrHPV testing were negative by RNA ISH. However, the positive results between prior hrHPV tests and RNA ISH in paraffin slides were highly concordant (p<0.0001, R=0.761). hrHPV genotyping was available in 101 patients composing of HPV16 alone 31 (30.7%) cases, HPV18 alone 20 (19.8%) cases, HPV16 and 18 12 (11.9%) cases, multiple infection with HPV16 7 (6.9%) cases, multiple infection with HPV18 18 (17.8%) cases, and multiple infection without HPV16/18 13 (12.9%) cases. HPV16 and 18 were the predominant genotypes in cervical adenocarcinomas (88/101, 87.1%). The frequency of hrHPV test was much lower in GAC and non-HPV related adenocarcinomas than non-GAC and HPV-related adenocarcinomas (p<0.001, Table 1), respectively.
In 165 patients with cytological and hrHPV co-testing, 89 (53.9%) harbored abnormal findings on both cytology and hrHPV testing. Cytological abnormalities were found in 124 (75.2%), and positive hrHPV in 116 (70.3%) patients. Cytological and hrHPV test alone showed no significant difference in the detection of cervical adenocarcinomas (p=0.437). The cytology and hrHPV co-testing exhibited a significantly higher frequency of abnormal findings (151/165, 91.5%) than cytological or hrHPV test alone (p<0.001). Nevertheless, the sensitivity of co-testing for GACs (74.1%, 20/27) was substantially lower than that for non-GACs (94.9%, 131/138) (p=0.001).
Analysis of serum CEA, CA19-9, and CA125
We observed that postoperative serum CA19-9 level was significantly lower than that prior to surgery (p=0.012). Across the IECC subtypes, GAC harbored a much higher serum CA19-9 level than other types or non-GAC (Table 1, p<0.001). Serum CA19-9 significantly elevated in non-HPV adenocarcinomas than in HPV-related adenocarcinomas (p<0.001, Table 1).
According to the recommended cut-off values, the sensitivity of serum CEA, CA19-9 and CA125 for cervical adenocarcinomas was 8.5% (18/211), 16.1% (34/211), and 13.7% (29/211), respectively. CA19-9 showed a relatively high sensitivity for GAC (44.1%, Table 1). The detection rate of the biomarker combination was 64.1% (22/34) for GAC and 28.8% (51/177) for non-GAC. Particularly, 87.5% (7/8) GACs with negative cytology and hrHPV co-testing were detected by these markers, mostly by CA19-9 alone (75%, 6/8 GACs). The combination of cytology, hrHPV test and serum CA19-9 could detect 92.9% (26/28) GACs and 97.1% (133/137) non-GACs.
Discussion
In this retrospective study from a tertiary center in China, we investigated prior hrHPV tests and cytology results in patients with invasive cervical adenocarcinoma, and comprehensively analyzed their diagnostic efficiency among histotypes according to a recent pathogenetic classification. We found that the rate of cytological abnormalities was 74.1% (129/174) and that of hrHPV test was 72% (131/182). Our findings indicate that even in the setting of invasive cervical adenocarcinomas, cytology or hrHPV test alone has a high negative rate. However, cytology and hrHPV co-testing can significantly improve the sensitivity for cervical adenocarinomas (91.5%); therefore, they may represent the most efficient approach for the screening of invasive cervical adenocarcinoma to date.
The percentage of patients with invasive cervical adenocarcinoma showing positive Pap tests (ASCUS or worse) varies widely. The rate of positive Pap testing was 64.0% (87/136) in a retrospective study with large case series from KinMed Diagnostics (Xie et al., 2019). Other small studies also indicated a similar frequency of positive cytological findings in cervical adenocarcinomas (Krane et al., 2001; Zhang et al., 2017; Tao et al., 2015). However, data from Kaiser Permanente Northern California (KPNC) showed that only 4/27 (14.8%) cervical adenocarcinomas had abnormal cytological findings (Katki et al., 2011). The early tumor stage of the disease in the screening population may be associated with the low rate of positive cytology in the KPNC program. There are at least a certain percentage of false negative cytological tests for cervical adenocarcinoma. The possible explanations include the deep location of tumors in the endocervix, inadequate specimen and interpretation challenges limited by benign-looking morphology in minimally deviated adenocarcinomas (the well differentiated form of GAC) and diagnostic unfamiliarity with adenocarcinomas by cytopathologists. Recently, a large case-control study from regular screening project has indicated that cytological test alone is less efficient for cervical adenocarcinomas than for squamous cell carcinomas, but it can lead to a down-staging of disease by detecting adenocarcinoma earlier than diagnosis in the absence of screening (Castanon et al., 2016).
The role of hrHPV test in cervical carcinoma screening is increasing because of the causative link of persistent hrHPV infection to most cervical carcinomas. Primary screening with hrHPV test has been considered as an independent approach (Rijkaart et al., 2012). Recent population-based studies confirmed that hrHPV testing had a higher sensitivity, but lower specificity for the detection of cervical carcinomas and precursor lesions, compared with cytology (Katki et al., 2011; Ronco et al., 2014; Zhao et al., 2010). The overall hrHPV prevalence in cervical adenocarcinomas varied from 62% to 94% (Chen et al., 2018; de Sanjose et al., 2010; Holl et al. 2015; Tao et al. 2015; Xie et al. 2019; Zhang et al. 2017), lower than in squamous cell carcinoma and precursor lesions (~97.5% in CIN3 or worse lesions) (Zhao et al., 2010). It becomes evident that there is a substantially high rate of negative hrHPV testing in cervical adenocarcinomas. The question on negative hrHPV tests in adenocarcinomas is whether they are true or false negativity. In this study, 8 of 14 patients with prior negative hrHPV testing showed HPV RNA positive staining in tissue slides by RNA ISH. This finding suggests the presence of false negativity to a certain degrees. The potential reasons may include some technical issues, such as the limited detection sensitivity and coverage for specific HPV types, low HPV copies in cancers, and inadequate cellularity. A possible explanation for true HPV negativity may be due to the misclassification of cases including endometrial carcinoma with endocervical involvement and metastasis to the cervix from the ovary and fallopian tube. Such cases were excluded in our study since primary endometrioid carcinoma, serous carcinoma and other rare types of the cervix, were only diagnosed when no concurrent carcinomas were found in the ovary, fallopian tube, uterus and lower uterine segment by staging surgery. In fact, cervical adenocarcinoma represents a heterogenous group with many different subtypes. The rate of hrHPV positivity was reported to vary among cervical adenocarcinoma subtypes, with a high prevalence in the usual type (~90.4%), and much lower prevalence in other subtypes (0%-34%), such as serous carcinoma, endometrial carcinoma, clear cell carcinoma and gastric-type adenocarcinoma (An et al., 2005; Holl et al., 2015; Pirog et al., 2014). These subtypes were regarded as non-HPV related adenocarcinomas, accounting for 15.2% (55/361), in the new IECC classification as supported by negative RNAscope signals for HPV mRNA and non-blocking P16 staining (Stolnicu et al., 2018). Likewise, we here also find that the non-HPV related subtypes (23.7%, 50/211) showed no evidence of HPV infection in histological slides. Therefore, we believe that a substantial number of cervical adenocarcinomas are truly negative for hrHPV tests indeed.
We here showed the high efficiency of cytological and hrHPV co-testing in the detection of cervical adenocarcinoma. However, very limited data were available on the co-testing for cervical adenocarcinoma screening due to the relatively low incidence. A recent study from China showed consistent findings with ours (Xie et al., 2019) whereas the KPNC co-testing program suggested that cytology added little to the efficiency of HPV testing for cervical adenocarcinoma (Katki et al., 2011). Besides the relatively earlier stages in screening patients, another major factor that affects the screening efficiency of HPV testing is the wide geographic distribution of adenocarcinoma subtypes. For example, GAC, the predominant histotype of non-HPV related adenocarcioma, has a prevalence of up to 20% of cervical adenocarcinomas in Japanese, and only 10% in the IECC cohort (Stolnicu et al., 2018). Our study contained a substantial number of non-HPV related adenocarcinomas (23.9%) or GAC (16.3%) that were confirmed by histopathology, HPV E6/E7 mRNA ISH, and immunostaining with a panel of antibodies (ER, PR, P16 and P53). We did find a substantially lower detection rate of co-testing for GACs (74.1 %) than for non-GACs (94.9%). To the best of knowledge, there was no clinical analysis specifically on the diagnostic value of cytological and hrHPV co-testing in GAC to date.
We observed that serum CEA, CA19-9 and CA125 were not good markers for detecting cervical adenocarcinomas because of their low sensitivity. Nevertheless, we found that serum CA19-9 was relatively sensitive for GAC (44.1%) including 75% (6/8) cases that were missed by cytology and hrHPV co-testing. The combination of cytology, hrHPV test and serum CA19-9 generated a high sensitivity for GACs (92.9%) and non-GACs (97.1%). The investigation on serum CA19-9 in cervical adenocarcinoma is very limited at present. Two early studies with a small sample size showed that CA19-9 had a detection rate of 43.7% (7/16) in cervical adenocarcinomas (Borras et al., 1995) and 62% (13/21) in recurrent adenocarcinomas (Tabata et al., 2000). Recently, Nakamura et al. (2019) reported that 53.8% (7/13) of GACs harbored an increased CA19-9 level (>40U/mL), much higher than non-GACs (21.4%, 12/56). Our study on serum CA19-9 was comparable to previous studies. However, several critical aspects limited the clinical utility of CA19-9. First, CA19-9 is not sensitive for the detection of cervical adenocarcinoma. Second, CA19-9 is a broad tumor marker primarily for pancreatic cancer and to a lesser degree for malignancies from other sites, such as hepatobilliary tract, esophagus, stomach, colorectum, liver, breast and ovary (Bagde, 2020; Das, 2020; Scatena, 2015). Third, transient elevation of CA19-9 (false positivity) is occasionally found in a variety of benign entities including pancreatitis, liver cirrhosis, and ovarian cysts. However, considering the aggressive clinical behavior in GAC and lack of powerful biomarkers currently (Nishio et al., 2019; Pirog et al., 2019), we believe that serum CA19-9 remain an alternative for the detection of GACs particularly in women with clinical manifestations (such as vaginal discharge) and negative cytology, and monitoring the clinical course in patients with elevated CA19-9 levels.
The advantage of our study is the presentation of a large cohort of cervical adenocarcinomas with detailed clinicopathological features. Therefore, it may provide a relatively robust analysis of the efficacy of cytology, hrHPV test and serum CA19-9 in the detection of adenocarcinoma and its subtypes. However, this study is limited by missing values in some patients, sampling bias owing to retrospective study, and the rarity of GAC. Future multiple-institutional studies and large population-based screen programs are critically required to consolidate these findings.
In conclusion, we find that cytology and hrHPV co-testing is much more efficient than cytology or hrHPV test alone in the detection of cervical adenocarcinoma. However, the co-testing remains less effective for non-HPV related adenocarcinoma, particularly GAC. As such, serum CA19-9 should be given for women with suspected clinical manifestations on account of its aggressive clinical behavior.
Author Contribution Statement
All authors contributed equally in this study.
Acknowledgments
We thank Dr Brian Eyden (Manchester, United Kingdom) for English language editing of the manuscript. This manuscript is supported by National Natural Scientific Foundations of China (81872112).
References
- Adams J, Sasieni P. Changing rates of adenocarcinoma and adenosquamous carcinoma of the cervix in England. Lancet. 2001;357:1490–3. doi: 10.1016/S0140-6736(00)04646-8. [DOI] [PubMed] [Google Scholar]
- An HJ, Kim KR, Kim IS, et al. Prevalence of human papillomavirus DNA in various histological subtypes of cervical adenocarcinoma: a population-based study. Mod Pathol. 2005;18:528–34. doi: 10.1038/modpathol.3800316. [DOI] [PubMed] [Google Scholar]
- Bagde ND, Bagde MN, Lone ZA. Relationship between serum tumor markers, CA-125, CEA, CA19-9, LDH, and βHCG with histopathology and age in women with ovarian tumors. Asian Pac J Cancer Biol. 2020;5:167–71. [Google Scholar]
- Borras G, Molina R, Xercavins J, Ballesta A, Iglesias J. Tumor Antigens CA 19 9, CA 125, and CEA in carcinoma of the uterine cervix. Gynecol Oncol. 1995;57:205–11. doi: 10.1006/gyno.1995.1126. [DOI] [PubMed] [Google Scholar]
- Castanon A, Landy R, Sasieni PD. Is cervical screening preventing adenocarcinoma and adenosquamous carcinoma of the cervix? Int J Cancer. 2016;139:1040–5. doi: 10.1002/ijc.30152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Sun H, Molijn A, et al. The variable characteristics of human papillomavirus in squamous cell carcinoma and adenocarcinoma of cervix in China. J Low Genit Tract Dis. 2018;22:355–61. doi: 10.1097/LGT.0000000000000408. [DOI] [PubMed] [Google Scholar]
- Crosbie EJ, Einstein MH, Franceschi S, Kitchener HC. Human papillomavirus and cervical cancer. Lancet. 2013;382:889–99. doi: 10.1016/S0140-6736(13)60022-7. [DOI] [PubMed] [Google Scholar]
- Das S, Kakoti L, Ahmed S, et al. Study of serum CEA and Ca 19 9 in esophageal squamous carcinoma and ROC curve analysis. Asian Pac J Cancer Biol. 2020;5:141–5. [Google Scholar]
- de Sanjose S, Quint WG, Alemany L, et al. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11:1048–56. doi: 10.1016/S1470-2045(10)70230-8. [DOI] [PubMed] [Google Scholar]
- Holl K, Nowakowski AM, Powell N, et al. Human papillomavirus prevalence and type-distribution in cervical glandular neoplasias: Results from a European multinational epidemiological study. Int J Cancer. 2015;137:2858–68. doi: 10.1002/ijc.29651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katki HA, Kinney WK, Fetterman B, et al. Cervical cancer risk for women undergoing concurrent testing for human papillomavirus and cervical cytology: a population-based study in routine clinical practice. Lancet Oncol. 2011;12:663–72. doi: 10.1016/S1470-2045(11)70145-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krane JF, Granter SR, Trask CE, Hogan CL, Lee KR. Papanicolaou smear sensitivity for the detection of adenocarcinoma of the cervix: a study of 49 cases. Cancer. 2001;93:8–15. [PubMed] [Google Scholar]
- Nakamura A, Yamaguchi K, Minamiguchi S, et al. Mucinous adenocarcinoma, gastric type of the uterine cervix: clinical features and HER2 amplification. Med Mol Morphol. 2019;52:52–9. doi: 10.1007/s00795-018-0202-2. [DOI] [PubMed] [Google Scholar]
- Nishio S, Mikami Y, Tokunaga H, et al. Analysis of gastric-type mucinous carcinoma of the uterine cervix - An aggressive tumor with a poor prognosis: A multi-institutional study. Gynecol Oncol. 2019;153:13–9. doi: 10.1016/j.ygyno.2019.01.022. [DOI] [PubMed] [Google Scholar]
- Pirog EC, Lloveras B, Molijn A, et al. RIS HPV TT study group HPV prevalence and genotypes in different histological subtypes of cervical adenocarcinoma, a worldwide analysis of 760 cases. Mod Pathol. 2014;27:1559–67. doi: 10.1038/modpathol.2014.55. [DOI] [PubMed] [Google Scholar]
- Pirog EC, Park KJ, Kiyokawa T, et al. Gastric-type adenocarcinoma of the cervix: tumor with wide range of histologic appearances. Adv Anat Pathol. 2019;26:1–12. doi: 10.1097/PAP.0000000000000216. [DOI] [PubMed] [Google Scholar]
- Rijkaart DC, Berkhof J, Rozendaal L, et al. Human papillomavirus testing for the detection of high-grade cervical intraepithelial neoplasia and cancer: final results of the POBASCAM randomised controlled trial. Lancet Oncol. 2012;13:78–88. doi: 10.1016/S1470-2045(11)70296-0. [DOI] [PubMed] [Google Scholar]
- Ronco G, Dillner J, Elfström KM, et al. International HPV screening working group Efficacy of HPV-based screening for prevention of invasive cervical cancer: follow-up of four European randomised controlled trials. Lancet. 2014;383:524–32. doi: 10.1016/S0140-6736(13)62218-7. [DOI] [PubMed] [Google Scholar]
- Scatena R. Advances in cancer biomarkers: from biochemistry to clinic for a critical revision. Springer. 2015;New York:pp 27–262. [Google Scholar]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- Stolnicu S, Barsan I, Hoang L, et al. International Endocervical Adenocarcinoma Criteria and Classification (IECC): a new pathogenetic classification for invasive adenocarcinomas of the endocervix. Am J Surg Pathol. 2018;42:214–26. doi: 10.1097/PAS.0000000000000986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolnicu S, Hoang L, Chiu D, et al. Clinical outcomes of HPV-associated and unassociated endocervical adenocarcinomas categorized by the International Endocervical Adenocarcinoma Criteria and Classification (IECC) Am J Surg Pathol. 2019;43:466–74. doi: 10.1097/PAS.0000000000001224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao X, Griffith CC, Zhou X, et al. History of high-risk HPV and Pap test results in a large cohort of patients with invasive cervical carcinoma: experience from the largest women’s hospital in China. Cancer Cytopathol. 2015;123:421–7. doi: 10.1002/cncy.21545. [DOI] [PubMed] [Google Scholar]
- Tabata T, Takeshima N, Tanaka N, Hirai Y, Hasumi K. Clinical value of tumor markers for early detection of recurrence in patients with cervical adenocarcinoma and adenosquamous carcinoma. Tumour Biol. 2000;21:375–80. doi: 10.1159/000030143. [DOI] [PubMed] [Google Scholar]
- Xie F, Zhang L, Zhao D, et al. Prior cervical cytology and high-risk HPV testing results for 311 patients with invasive cervical adenocarcinoma: a multicenter retrospective study from China’s largest independent operator of pathology laboratories. BMC Infect Dis. 2019;19:962. doi: 10.1186/s12879-019-4614-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Xie F, Wang X, et al. Previous cervical cytology and high-risk human papillomavirus testing in a cohort of patients with invasive cervical carcinoma in Shandong Province, China. PLoS One. 2017;12:e0180618. doi: 10.1371/journal.pone.0180618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao FH, Lin MJ, Chen F, et al. Performance of high-risk human papillomavirus DNA testing as a primary screen for cervical cancer: a pooled analysis of individual patient data from 17 population-based studies from China. Lancet Oncol. 2010;11:1160–71. doi: 10.1016/S1470-2045(10)70256-4. [DOI] [PMC free article] [PubMed] [Google Scholar]