Abstract
Background
The ongoing SARS-CoV-2 pandemic necessitates the development of accurate, rapid, and affordable diagnostics to help curb disease transmission, morbidity, and mortality. Rapid antigen tests are important tools for scaling up testing for SARS-CoV-2; however, little is known about individuals’ use of rapid antigen tests at home and how to facilitate the user experience.
Objective
This study aimed to describe the feasibility and acceptability of serial self-testing with rapid antigen tests for SARS-CoV-2, including need for assistance and the reliability of self-interpretation.
Methods
A total of 206 adults in the United States with smartphones were enrolled in this single-arm feasibility study in February and March 2021. All participants were asked to self-test for COVID-19 at home using rapid antigen tests daily for 14 days and use a smartphone app for testing assistance and to report their results. The main outcomes were adherence to the testing schedule, the acceptability of testing and smartphone app experiences, and the reliability of participants versus study team’s interpretation of test results. Descriptive statistics were used to report the acceptability, adherence, overall rating, and experience of using the at-home test and MyDataHelps app. The usability, acceptability, adherence, and quality of at-home testing were analyzed across different sociodemographic, age, and educational attainment groups.
Results
Of the 206 enrolled participants, 189 (91.7%) and 159 (77.2%) completed testing and follow-up surveys, respectively. In total, 51.3% (97/189) of study participants were women, the average age was 40.7 years, 34.4% (65/189) were non-White, and 82% (155/189) had a bachelor’s degree or higher. Most (n=133/206, 64.6%) participants showed high testing adherence, meaning they completed over 75% of the assigned tests. Participants’ interpretations of test results demonstrated high agreement (2106/2130, 98.9%) with the study verified results, with a κ score of 0.29 (P<.001). Participants reported high satisfaction with self-testing and the smartphone app, with 98.7% (157/159) reporting that they would recommend the self-test and smartphone app to others. These results were consistent across age, race/ethnicity, and gender.
Conclusions
Participants’ high adherence to the recommended testing schedule, significant reliability between participants and study staff’s test interpretation, and the acceptability of the smartphone app and self-test indicate that self-tests for SARS-CoV-2 with a smartphone app for assistance and reporting is a highly feasible testing modality among a diverse population of adults in the United States.
Keywords: COVID-19, SARS-CoV-2, rapid tests, MyDataHelps smartphone app, mHealth, mobile health, serial self-testing, digital health, pandemic, self test
Introduction
Since the emergence of the SARS-CoV-2 pandemic in late 2019, more than 590 million cases and 6.5 million deaths from COVID-19 have been reported worldwide [1]. Over 2 years into the pandemic, the United States continues to face waves of increasing SARS-CoV-2 cases. The ongoing pandemic necessitates the development of accurate, rapid, and affordable diagnostics to help curb SARS-CoV-2 disease transmission, morbidity, and mortality, as well as safely navigate social re-engagement [2].
Antigen-based rapid diagnostic tests (Ag-RDTs) detect SARS-CoV-2 viral proteins in multiple specimen types and facilitate opportunities for large-scale, cost-effective testing solutions [3,4]. Ag-RDTs are preferred by over two-thirds of SARS-CoV-2 test users, especially in comparison to molecular tests, which can take days to receive the result [5,6]. Numerous Ag-RDTs for SARS-CoV-2 have received Emergency Use Authorization by the US Food and Drug Administration for point-of-care testing in the health care setting and, more recently, for at-home use, with evidence consistently showing the validity of self-collected specimens for SARS-CoV-2 testing [7,8]. Self-testing at home offers great opportunity for scaling up and implementing regular testing of both asymptomatic and symptomatic individuals, a key step toward controlling the COVID-19 pandemic [2,9-11]. Furthermore, self-testing offers the opportunity to increase testing access across geographic, sociodemographic, and socioeconomic groups to improve health outcomes and reduce health care disparities [12-14]. However, little is known about individuals’ use of rapid antigen tests at home and how to facilitate the user experience [5,15].
The objectives of this study were to describe the feasibility of the at-home use of rapid antigen tests for SARS-CoV-2, as well as participants’ use of the MyDataHelps smartphone app (CareEvolution) to support at-home testing. This study aimed to describe the usability and acceptability of self-tests for SARS-CoV-2, variation in use across different sociodemographic and socioeconomic groups, and how participants interact with the MyDataHelps smartphone app to report symptoms and test results. We hypothesized that the acceptability and usability of the rapid antigen tests and smartphone app would be consistent across sociodemographic and socioeconomic groups.
Methods
Study Population
Participants were recruited from the University of Massachusetts (UMass) Chan Medical School and Northwestern University using best practices developed by the RADx Tech Community Health Equity and Engagement Team to maximize the representation of diverse age, sex, race, ethnicity, education, and socioeconomic groups [16]. Participants were enrolled in the study during February and March 2021. For inclusion in the study, individuals were required to be aged ≥18 years, be willing to use their own smartphone device and download the MyDataHelps app, have reported no symptoms attributable to COVID-19 within 48 hours prior to screening, and be proficient in English.
Ethics Approval
Details of the study procedures were explained to participants, and written informed consent was obtained from all participants. This study was approved by the Institutional Review Board (H00022342) at UMass Chan Medical School and Northwestern University, which had a reliance agreement with the UMass Chan Medical School Institutional Review Board.
Study Procedures
All participants were asked to self-test for COVID-19 at home daily over a consecutive 14-day period. Participants were mailed a QuickVue test kit (Quidel) containing testing supplies for 25 tests; written testing instructions; and a prepaid, pre-addressed return box for test strips with return instructions. All test kits used anterior nasal swabs, and instructions directed participants on how to properly swab their nasal cavity. Participants were given access to the MyDataHelps app to support testing. The MyDataHelps app allowed participants to view testing instructions, report test results, verify test results with the study team, track their testing history, respond to surveys, and access the study team’s contact information (Multimedia Appendix 1).
Participants were informed that they could report their test results to the study team either digitally through the MyDataHelps app or through manual written recording. If participants opted to use the MyDataHelps app for reporting test results, they were asked to report their interpretation of the test results—positive or negative—and upload an image of the test strip (Multimedia Appendix 1). Study coordinators validated all test results using the test strip image, with digital verification occurring within 24 hours of reporting. Written test results were mailed to study coordinators along with all test strips for verification at the end of the study. Participants were instructed to contact study coordinators with any questions during the study period. All interactions between study coordinators and participants were recorded in a contact log.
If a participant showed COVID-19 symptoms, reported close exposure to a person positive for SARS-CoV-2, or tested positive on a home test, the study team contacted the participant and scheduled confirmatory SARS-CoV-2 polymerase chain reaction testing by trained personnel using established protocols and procedures. If an individual tested positive for SARS-CoV-2 on confirmatory testing, participants were removed from the study and received an exit survey on the day of the positive results out of safety for the participants.
Study Questionnaires
Questionnaires were administered to participants through the MyDataHelps app. Eligible and consenting study participants were given a baseline survey, daily surveys, a midpoint survey, and an exit survey. The baseline survey assessed their sociodemographic characteristics, anthropometrics, health status, and social engagement. Health status included questions regarding disability, pregnancy, current alcohol and cigarette use, chronic conditions, and report of common COVID-19 symptoms. Daily surveys asked for self-interpretation of the test results (positive or negative), image upload of the test strip, and symptom reporting. The midpoint survey was given to participants on day 7, and participants were asked to self-report the total number of completed tests and their social engagement practices. Lastly, the exit survey, on day 14 of the study, asked for a self-report of the total number of tests completed, acceptability and experience of using the MyDataHelps app, acceptability of the at-home test, social engagement, insurance status (no insurance, private, or public insurance), and health status. The acceptability of the at-home test was assessed by asking participants if they would recommend the self-test to someone else using the Net Promotor Scale. The number of tests reported and number of daily image uploads over the 14-day testing period determined adherence to the testing schedule. Adherence to the testing schedule was classified into 4 categories: no (0%) adherence, low (<50%) adherence, moderate (50% to 75%) adherence, and high (>75%) adherence. Using a Likert scale, the experience and acceptability of the MyDataHelps app was assessed by asking for the participants’ overall rating of the app (1=one of the worst apps I’ve used to 5=one of the best apps I’ve used). Participants were asked how often they had difficulties using the app (1=all the time to 5=never), the usefulness of different features of the app (1=really useful to 5=really useless), whether they would recommend the app to another person to help them perform an at-home test for COVID-19 (1=definitely yes to5=definitely not), and if they would continue using the app to keep testing themselves at home for COVID-19 (1=definitely yes to 5=definitely not).
Data Analysis
Descriptive statistics were used to report the acceptability, adherence, overall rating, and experience of using the at-home test and MyDataHelps app. The usability, acceptability, adherence, and quality of at-home testing were analyzed across different sociodemographic, age, and educational attainment groups to evaluate the impact on existing socioeconomic disparities, using ANOVA to evaluate significance. The study coordinator contact log was text mined and used to generate a word cloud in R statistical software (version 4.2.1; R Foundation for Statistical Computing) to characterize participant interactions with study staff.
Results
Participant Characteristics
A total of 206 participants enrolled in the study during February and March 2021. There were 5 (5/206, 2.4%) participants who tested positive for SARS-CoV-2 during the study period, and they were removed from the study. Of the 206 participants, 189 (91.7%) and 159 (77.2%) completed testing and follow-up surveys, respectively. Among participants who completed testing, slightly more than half (97/189, 51.3%) were women, the average age of the study population was 40.7 years, 34.4% (65/189) were non-White, and 82% (155/189) had a bachelor’s degree or higher (Table 1). At the time of study enrollment (February 2021), only 2.5% of the US population were fully vaccinated for SARS-CoV-2 [17].
Table 1.
Participant characteristics stratified by testing adherence.
| Characteristic | Adherence to daily testing | |||
|
|
|
Low (n=19), n (%) | Moderate (n=37), n (%) | High (n=133), n (%) |
| Age (years) | ||||
|
|
18-39 | 11 (57.9) | 24 (64.9) | 62 (46.6) |
|
|
40-64 | 3 (15.8) | 13 (35.1) | 49 (36.8) |
|
|
≥65 | 2 (10.5) | 0 (0) | 18 (13.5) |
|
|
No answer | 3 (15.8) | 0 (0) | 4 (3) |
| Gender | ||||
|
|
Male | 8 (42.1) | 12 (32.4) | 62 (46.6) |
|
|
Female | 8 (42.1) | 24 (64.9) | 65 (48.9) |
|
|
Nonbinary or transgender | 1 (5.3) | 1 (2.7) | 3 (2.3) |
|
|
No answer | 2 (10.5) | 0 (0) | 3 (2.3) |
| Race/ethnicity | ||||
|
|
Hispanic | 2 (10.5) | 8 (21.6) | 12 (9) |
|
|
Non-Hispanic Asian | 1 (5.3) | 5 (13.5) | 13 (9.8) |
|
|
Non-Hispanic Black | 2 (10.5) | 4 (10.8) | 15 (11.3) |
|
|
Non-Hispanic Other | 1 (5.3) | 0 (0) | 2 (1.5) |
|
|
Non-Hispanic White | 11 (57.9) | 20 (54.1) | 89 (66.9) |
|
|
No answer | 2 (10.5) | 0 (0) | 2 (1.5) |
| Education level | ||||
|
|
Master’s degree or higher | 7 (36.8) | 7 (18.9) | 34 (25.6) |
|
|
Bachelor’s degree or equivalent | 6 (31.6) | 24 (64.9) | 77 (57.9) |
|
|
High school or lower | 4 (21.1) | 5 (13.5) | 19 (14.3) |
|
|
No answer | 2 (10.5) | 1 (2.7) | 3 (2.3) |
| Employment status | ||||
|
|
Working now | 10 (52.6) | 28 (75.7) | 94 (70.7) |
|
|
Student | 2 (10.5) | 5 (13.5) | 10 (7.5) |
|
|
Retired | 3 (15.8) | 1 (2.7) | 19 (14.3) |
|
|
Other | 4 (21.1) | 3 (8.1) | 10 (7.5) |
Patient-Reported Usability and Acceptability of At-Home Testing
In all, 91.7% (189/206) of the participants performed 1 or more tests during the study period (Table 2). The majority (133/206, 64.6%) of the participants showed high adherence to testing and picture upload, characterized as testing and uploading the picture of the test strip to the app on more than 75% of the indicated days (Table 1). Participants aged 18-39 years comprised the majority of the moderate (24/37, 64.9%) and low (11/19, 57.9%) adherence groups, whereas 90% (18/20) of the participants aged ≥65 years reported high adherence (P=.03; Table 1). Comparatively, only 63.9% (62/97) and 75.4% (49/65) of participants aged 18-39 years and 40-64 years demonstrated high adherence, respectively. Participants’ interpretations of test results demonstrated high agreement (2106/2130, 98.9%) with the study verified results, with a κ score of 0.29 (P<.001; Table 3). Overall, participants reported high satisfaction with at-home testing, with 98.7% (157/159) of the participants reporting that they would definitely or likely recommend the self-test to others (Figure 1).
Table 2.
Total number of tests performed in the 14-day study period.
| Total number tests performed in the 14-day study period | Participants (N=206), n (%) |
| 0 | 17 (8.3) |
| 1 | 2 (1) |
| 2 | 3 (1.5) |
| 3 | 2 (1) |
| 4 | 2 (1) |
| 5 | 2 (1) |
| 6 | 2 (1) |
| 7 | 6 (2.9) |
| 8 | 7 (3.4) |
| 9 | 9 (4.4) |
| 10 | 22 (10.7) |
| 11 | 24 (11.7) |
| 12 | 32 (15.5) |
| 13 | 35 (17) |
| 14 | 41 (19.9) |
Table 3.
Reliability of self-interpretation versus study verification of at-home antigen-based rapid diagnostic tests.
| Self-interpretation | Study verification | |||
|
|
Negative | Positive | Invalid | Total |
| Negative | 2102 | 5 | 11 | 2118 |
| Positive | 4 | 4 | 2 | 10 |
| Invalid | 2 | 0 | 0 | 2 |
| Total | 2108 | 9 | 13 | 2130 |
Figure 1.
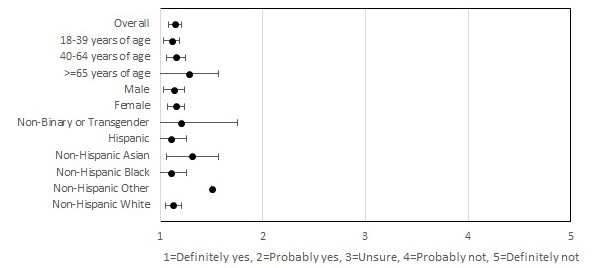
Usability and acceptability of self-tests.
MyDataHelps Participant Usability
Participants also reported high satisfaction with the MyDataHelps app. In all, 98.7% (157/159) of the participants indicated that they would definitely or probably recommend the app to others, with 91.8% (146/159) indicating that they would continue using the app for at-home testing if possible (Figures 2 and 3). These results were consistent across all age, race/ethnicity, and gender groups. In all, 77.4% (123/159) of the participants reported never having difficulties using the app, and 3.8% (6/159) reported having difficulties with the app most or all of the time. Among participants who reported difficulties, internet connection issues (5/6, 83%) were the most common reason. Participants on average found the COVID-19 testing instructions to be the most useful feature of the app, with 88.1% (140/159) of the participants finding this feature “very useful.” The overall rating of the app was 4.4 out of 5, and the overall rating did not differ by age, gender, or race/ethnicity (Figure 4).
Figure 2.

Participants’ willingness to recommend smartphone app to others.
Figure 3.

Participants’ interest in continuing to use the smartphone app after the study period.
Figure 4.

Participants’ overall rating of the smartphone app.
Participant and Study Coordinator Interactions
Over the study period, there were a total of 117 phone and email interactions between study staff and participants. The most discussed topics were test kit return (43/117, 36.8%), test results (28/117, 23.9%), image upload (21/117, 17.9%), and scheduling confirmatory polymerase chain reaction testing (25/117, 21.4%; Figure 5). These topics were not mutually exclusive.
Figure 5.

Word cloud of participant interactions with study coordinators.
Discussion
Principal Findings
We described the feasibility of at-home rapid antigen testing for COVID-19 using a mobile app for testing support. Most participants displayed high adherence to the recommended testing schedule and were very satisfied with both the app and testing experience. Adherence to testing significantly differed by age; however, the usability and acceptability of at-home testing and the MyDataHelps app did not differ by age, race/ethnicity, or gender. The majority of patients aged ≥65 years belonged to the high adherence group, whereas the proportion of participants with high adherence was lower among those aged 18-39 years and 40-64 years. The COVID-19 pandemic has hit those aged ≥65 years the hardest, with mortality rates over 60 times higher among those aged ≥65 years than those aged ≤54 years [18]. The difference in adherence by age group may reflect differences in risk perception influencing testing behaviors. Additionally, participants’ interpretation of the test results showed significant reliability with the study team’s interpretations, further demonstrating the feasibility of using self-tests outside the clinical environment. Participants were very capable of administering, reading, and reporting test results at home without clinical assistance.
Comparison With Prior Work
Although many previous studies have analyzed the performance of rapid antigen tests for the detection of SARS-CoV-2, few studies have looked at users’ testing behavior. It is important to understand who uses rapid antigen tests, when people use rapid antigen tests, and how people test to facilitate the development of effective testing interventions. Nguyen et al [5] found that, among 31 employees of a large company, a daily serial rapid antigen testing intervention with an associated mobile app was highly acceptable, with mean adherence of 88% over a 21-day period. This finding is similar to our own findings of adherence, with over 60% of participants displaying high adherence (>75%) during the 14-day study period. Although the study by Nguyen et al [5] was nested in an employer testing program, with weekly COVID-19 testing required as a condition for employment, our study was based among households residing in 2 large metropolitan cities. The consistency of daily testing adherence across these 2 populations is notable and adds to the external validity of these results. Nguyen et al [5] also found that the acceptability of daily testing was related to the perceived threat of COVID-19, and participants were more likely to find daily testing acceptable in times of high SARS-CoV-2 prevalence. Our study was conducted prior to the widespread distribution of vaccines for SARS-CoV-2; therefore, it is possible that the perceived threat of COVID-19 was generally high throughout the population, contributing to high acceptability and adherence. It is important to reassess COVID-19 testing behaviors as the pandemic continues to evolve to understand the motivations and challenges with testing.
Additionally, the COVID-19 pandemic has escalated prepandemic health care disparities within the United States, and geographic inequities in COVID-19 incidence and testing availability persist [19-21]. Non-English speakers, persons of color, and those of lower socioeconomic status are less likely to have access to testing for SARS-CoV-2 than their counterparts, despite simultaneously having an increased proportion of positive cases and mortality [22,23]. Bringing health care services outside the traditional clinical environment offers solutions to accessibility, as well as bridging the gap to populations who have been systematically exploited by the health care system. In this study, we found that the acceptability and usability of at-home testing was consistent across all race/ethnicity categorizations, indicating that at-home testing could be a promising tool in addressing COVID-19 disparities.
As individuals navigate the return to work and school in the age of COVID-19, it is important that individuals have access to frequent and rapid testing to guide social engagement [24]. However, more information is needed on the diagnostic capabilities and limitations of these tools to ensure that individuals interpret the implications of their test results properly [25]. Additionally, although participants were asked to adhere to a 14-day continuous testing schedule for the purpose of this study, it is important to investigate further optimal testing schedules for SARS-CoV-2 detection [11]. We must also continue to evaluate the accessibility of at-home testing and the MyDataHelps app among diverse communities, including non-English speakers [26].
Strengths and Limitations
This is the first study to look at the feasibility of at-home Ag-RDTs, filling an important gap in the literature. The strengths of this study include the longitudinal design, which allowed us to analyze adherence over time, and the use of a digital app for testing assistance and survey administration. The use of a digital app allowed participants to engage in the study from their homes, decreasing the burden of participation. Additionally, the wide inclusion criteria allowed the enrollment of a diverse cohort. However, this study is not without limitations. Study participants were required to speak English and have access to a smartphone, which limits the generalizability of our findings. Over 85% of adults in the United States own a smartphone; however, smartphone users vary from nonsmartphone users in terms of education, income, and age [27]. Additionally, only rapid antigen tests using nasal specimen collection were analyzed in this study; therefore, additional work may be necessary to evaluate the feasibility and acceptability of alternative SARS-CoV-2 testing modalities.
Conclusions
As society establishes a new normal amid an ongoing pandemic, the development of accurate and rapid diagnostics is necessary to help curb SARS-CoV-2 disease transmission and safely navigate social re-engagement. The use of self-tests for COVID-19 with the MyDataHelps app for testing assistance was shown to be a feasible and accessible testing modality across gender, age, and racial groups, and more investigations into the efficacy of these testing modalities is indicated.
Acknowledgments
This work was supported by the National Heart, Lung, and Blood Institute at the National Institutes of Health (3U54HL143541-02S1 and 3U54HL143541-02S2) through the RADx-Tech program.
Abbreviations
- Ag-RDTs
antigen-based rapid diagnostic tests
- UMass
University of Massachusetts
Screenshots of the MyDataHelps app.
Footnotes
Conflicts of Interest: CN and VK are employees of CareEvolution. DM has received personal fees and grants from Bristol Myers Squibb, Pfizer, Flexcon, and Fitbit; personal fees for editorial work from Heart Rhythm Society and from Avania for work on Data Safety Monitoring Boards; financial research support from Boehringer Ingelheim; and non-financial research support from Apple, Samsung, and Fitbit.
References
- 1.Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020 May;20(5):533–534. doi: 10.1016/S1473-3099(20)30120-1. https://europepmc.org/abstract/MED/32087114 .S1473-3099(20)30120-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tromberg BJ, Schwetz TA, Pérez-Stable Eliseo J, Hodes RJ, Woychik RP, Bright RA, Fleurence RL, Collins FS. Rapid scaling up of COVID-19 diagnostic testing in the United States - the NIH RADx Initiative. N Engl J Med. 2020 Sep 10;383(11):1071–1077. doi: 10.1056/NEJMsr2022263. https://europepmc.org/abstract/MED/32706958 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ricks S, Kendall EA, Dowdy DW, Sacks JA, Schumacher SG, Arinaminpathy N. Quantifying the potential value of antigen-detection rapid diagnostic tests for COVID-19: a modelling analysis. BMC Med. 2021 Mar 09;19(1):75. doi: 10.1186/s12916-021-01948-z. https://bmcmedicine.biomedcentral.com/articles/10.1186/s12916-021-01948-z .10.1186/s12916-021-01948-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dinnes J, Deeks JJ, Berhane S, Taylor M, Adriano A, Davenport C, Dittrich S, Emperador D, Takwoingi Y, Cunningham J, Beese S, Domen J, Dretzke J, Ferrante di Ruffano Lavinia, Harris I, Price M, Taylor-Phillips S, Hooft L, Leeflang M, McInnes M, Spijker R, Van den Bruel Ann, Cochrane COVID-19 Diagnostic Test Accuracy Group Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection. Cochrane Database Syst Rev. 2021 Mar 24;3:CD013705. doi: 10.1002/14651858.CD013705.pub2. https://europepmc.org/abstract/MED/33760236 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nguyen N, Lane B, Lee IS, Gorman S, Wu Yumeng, Li A, Lu H, Elhadad N, Yin M, Meyers IK. A mixed methods study evaluating acceptability of a daily COVID-19 testing regimen with a mobile-app connected, at-home, rapid antigen test: implications for current and future pandemics. PLoS One. 2022 Aug 08;17(8):e0267766. doi: 10.1371/journal.pone.0267766. https://dx.plos.org/10.1371/journal.pone.0267766 .PONE-D-22-10858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Møller IJB, Utke AR, Rysgaard UK, Østergaard LJ, Jespersen S. Diagnostic performance, user acceptability, and safety of unsupervised SARS-CoV-2 rapid antigen-detecting tests performed at home. Int J Infect Dis. 2022 Mar;116:358–364. doi: 10.1016/j.ijid.2022.01.019. https://linkinghub.elsevier.com/retrieve/pii/S1201-9712(22)00020-0 .S1201-9712(22)00020-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tu YP, Jennings R, Hart B, Cangelosi GA, Wood RC, Wehber K, Verma P, Vojta D, Berke EM. Swabs collected by patients or health care workers for SARS-CoV-2 testing. N Engl J Med. 2020 Jul 30;383(5):494–496. doi: 10.1056/NEJMc2016321. https://europepmc.org/abstract/MED/32492294 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hanson KE, Barker AP, Hillyard DR, Gilmore N, Barrett JW, Orlandi RR, Shakir SM. Self-collected anterior nasal and saliva specimens versus health care worker-collected nasopharyngeal swabs for the molecular detection of SARS-CoV-2. J Clin Microbiol. 2020 Oct 21;58(11):1824–1844. doi: 10.1128/JCM.01824-20. https://europepmc.org/abstract/MED/32817233 .JCM.01824-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Subramanian R, He Q, Pascual M. Quantifying asymptomatic infection and transmission of COVID-19 in New York City using observed cases, serology, and testing capacity. Proc Natl Acad Sci U S A. 2021 Mar 02;118(9):e2019716118. doi: 10.1073/pnas.2019716118. https://www.pnas.org/doi/abs/10.1073/pnas.2019716118?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%3dpubmed .2019716118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vermund SH, Pitzer VE. Asymptomatic transmission and the infection fatality risk for COVID-19: implications for school reopening. Clin Infect Dis. 2021 May 04;72(9):1493–1496. doi: 10.1093/cid/ciaa855. https://europepmc.org/abstract/MED/32584967 .5862668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soni A, Herbert C, Lin H, Pretz C, Stamegna P, Orwig T, Wright C, Tarrant S, Behar S, Suvarna T, Schrader S, Harman E, Nowak C, Kheterpal V, Rao LV, Cashman L, Orvek E, Ayturk D, Lazar P, Wang Z, Barton B, Achenbach C, Murphy R, Robinson Matthew, Manabe Yuka, Wang B, Pandey S, Colubri A, Oâ Connor Laurel, Lemon S, Fahey N, Luzuriaga K, Hafer N, Heetderks B, Broach J, McManus D. Performance of screening for SARS-CoV-2 using rapid antigen tests to detect incidence of symptomatic and asymptomatic SARS-CoV-2 infection: findings from the Test Us at Home prospective cohort study. medRxiv. doi: 10.1101/2022.08.05.22278466. doi: 10.1101/2022.08.05.22278466. Preprint posted online on August 06, 2022 . [DOI] [Google Scholar]
- 12.Hu T, Yue H, Wang C, She B, Ye X, Liu R, Zhu X, Guan WW, Bao S. Racial segregation, testing site access, and COVID-19 incidence rate in Massachusetts, USA. Int J Environ Res Public Health. 2020 Dec 19;17(24):9528. doi: 10.3390/ijerph17249528. https://www.mdpi.com/resolver?pii=ijerph17249528 .ijerph17249528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hernandez JH, Karletsos D, Avegno J, Reed CH. Is COVID-19 community level testing effective in reaching at-risk populations? evidence from spatial analysis of New Orleans patient data at walk-up sites. BMC Public Health. 2021 Apr 01;21(1):632. doi: 10.1186/s12889-021-10717-9. https://bmcpublichealth.biomedcentral.com/articles/10.1186/s12889-021-10717-9 .10.1186/s12889-021-10717-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dalva-Baird NP, Alobuia WM, Bendavid E, Bhattacharya J. Racial and ethnic inequities in the early distribution of U.S. COVID-19 testing sites and mortality. Eur J Clin Invest. 2021 Nov 27;51(11):e13669. doi: 10.1111/eci.13669. https://europepmc.org/abstract/MED/34390487 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herbert C, Kheterpal V, Suvarna T, Broach J, Marquez JL, Gerber B, Schrader S, Nowak C, Harman E, Heetderks W, Fahey N, Orvek E, Lazar P, Ferranto J, Noorishirazi K, Valpady S, Shi Q, Lin H, Marvel K, Gibson L, Barton B, Lemon S, Hafer N, McManus D, Soni A. Design and preliminary findings of adherence to the self-testing for our protection from COVID-19 (STOP COVID-19) risk-based testing protocol: prospective digital study. JMIR Form Res. 2022 Jun 16;6(6):e38113. doi: 10.2196/38113. https://formative.jmir.org/2022/6/e38113/ v6i6e38113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gibson LL, Fahey NM, Hafer N, Buchholz B, Dunlap DR, Murphy RL, Achenbach C, Stone C, Cleeton R, O'Neal J, Frediani J, Vos M, Brand O, Nayee R, Wells L, Lam W, Martin G, Manabe Y, Robinson M, Broach J, Olgin J, Barton B, Lemon S, Blodgett A, McManus D. The RADx Tech Clinical Studies Core: a model for academic based clinical studies. IEEE Open J Eng Med Biol. 2021 Apr 28;2:152–157. doi: 10.1109/OJEMB.2021.3070830. https://europepmc.org/abstract/MED/34192287 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.COVID data tracker. Centers for Disease Control and Prevention. [2021-05-12]. https://covid.cdc.gov/covid-data-tracker/#variant-proportions .
- 18.Yanez ND, Weiss NS, Romand JA, Treggiari MM. COVID-19 mortality risk for older men and women. BMC Public Health. 2020 Nov 19;20(1):1742. doi: 10.1186/s12889-020-09826-8. https://bmcpublichealth.biomedcentral.com/articles/10.1186/s12889-020-09826-8 .10.1186/s12889-020-09826-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zalla LC, Martin CL, Edwards JK, Gartner DR, Noppert GA. A geography of risk: structural racism and coronavirus disease 2019 mortality in the United States. Am J Epidemiol. 2021 Aug 01;190(8):1439–1446. doi: 10.1093/aje/kwab059. https://europepmc.org/abstract/MED/33710272 .6168675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsiao CJ, Patel AGM, Fasanya HO, Stoffel MR, Beal SG, Winston-McPherson GN, Campbell S, Cotten S, Crews B, Kuan K, Lapedis C, Mathias P, Peck Palmer Octavia M, Greene D. The lines that held us: assessing racial and socioeconomic disparities in SARS-CoV-2 testing. J Appl Lab Med. 2021 Sep 01;6(5):1143–1154. doi: 10.1093/jalm/jfab059. https://europepmc.org/abstract/MED/34240171 .6316654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bilal U, Tabb LP, Barber S, Diez Roux AV. Spatial inequities in COVID-19 testing, positivity, confirmed cases, and mortality in 3 U.S. cities : an ecological study. Ann Intern Med. 2021 Jul;174(7):936–944. doi: 10.7326/M20-3936. https://www.acpjournals.org/doi/abs/10.7326/M20-3936?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%3dpubmed . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grigsby-Toussaint DS, Shin JC, Jones A. Disparities in the distribution of COVID-19 testing sites in black and Latino areas in new York City. Prev Med. 2021 Jun;147:106463. doi: 10.1016/j.ypmed.2021.106463.S0091-7435(21)00047-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johansson MA, Quandelacy TM, Kada S, Prasad PV, Steele M, Brooks JT, Slayton RB, Biggerstaff M, Butler JC. SARS-CoV-2 transmission from people without COVID-19 symptoms. JAMA Netw Open. 2021 Jan 04;4(1):e2035057. doi: 10.1001/jamanetworkopen.2020.35057. https://jamanetwork.com/journals/jamanetworkopen/fullarticle/10.1001/jamanetworkopen.2020.35057 .2774707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mina MJ, Andersen KG. COVID-19 testing: one size does not fit all. Science. 2021 Jan 08;371(6525):126–127. doi: 10.1126/science.abe9187.science.abe9187 [DOI] [PubMed] [Google Scholar]
- 25.Woloshin S, Dewitt B, Krishnamurti T, Fischhoff B. Assessing how consumers interpret and act on results from at-home COVID-19 self-test kits: a randomized clinical trial. JAMA Intern Med. 2022 Mar 01;182(3):332–341. doi: 10.1001/jamainternmed.2021.8075.2788656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fulmer AA, Abboud GA, Wallace LS. Health literacy characteristics of over-the-counter rapid antigen COVID-19 test materials. Res Social Adm Pharm. 2022 Aug 15;S1551-7411(22):00269–8. doi: 10.1016/j.sapharm.2022.08.003. https://europepmc.org/abstract/MED/35987673 .S1551-7411(22)00269-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Demographics of mobile device ownership and adoption in the United States. Pew Research Center. 2021. Apr 07, [2022-08-14]. https://www.pewresearch.org/internet/fact-sheet/mobile/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Screenshots of the MyDataHelps app.


