Abstract
Subclavian vein (SCV) effort thrombosis, also known as Paget-Schroetter syndrome or venous thoracic outlet syndrome, is an uncommon condition that affects individuals with an irregularly narrow thoracic outlet who engage in repetitive overhead motions of the affected arm. Venous injury arises from microtraumas that occur from the repetitive compression of the SCV between the first rib and the overlying clavicle. Additional sources of extrinsic compression can be due to the anterior scalene muscle, subclavius muscle, and costoclavicular ligament. SCV effort thrombosis is a distinct entity from other forms of deep venous thrombosis and requires unique diagnostic and treatment considerations. Early catheter-directed therapy in the form of pharmacomechanical or catheter-directed thrombolysis combined with prompt surgical thoracic outlet decompression offers patients the best chances for early and durable symptom relief.
Keywords: effort thrombosis, Paget-Schroetter, thoracic outlet syndrome, venous thrombosis, chest, thrombolysis, angioplasty, interventional radiology
Pathophysiology
Subclavian vein (SCV) effort thrombosis, also known as Paget-Schroetter Syndrome or venous thoracic outlet syndrome (TOS), is believed to result from microtrauma of the SCV endothelium and activation of the coagulation cascade after chronic repetitive stress that occurs during vigorous and sustained upper extremity (UE) movements. 1 The repetitive trauma most commonly occurs at the costoclavicular space, which is bordered by the clavicle superiorly, the first rib inferiorly, the costoclavicular ligament medially, and the anterior scalene muscle laterally ( Fig. 1 ). The repetitive stress can affect any of the neurovascular structures in the thoracic outlet and is most often a neurogenic presentation (95%) followed by venous (4%) and then arterial (1%). 1 SCV effort thrombosis accounts for 10 to 20% of all UE deep venous thrombosis (UEDVT). Other predisposing factors for UEDVT include indwelling hardware such as catheters or pacemaker wires, malignancy, and other hypercoagulable states. 2
Fig. 1.
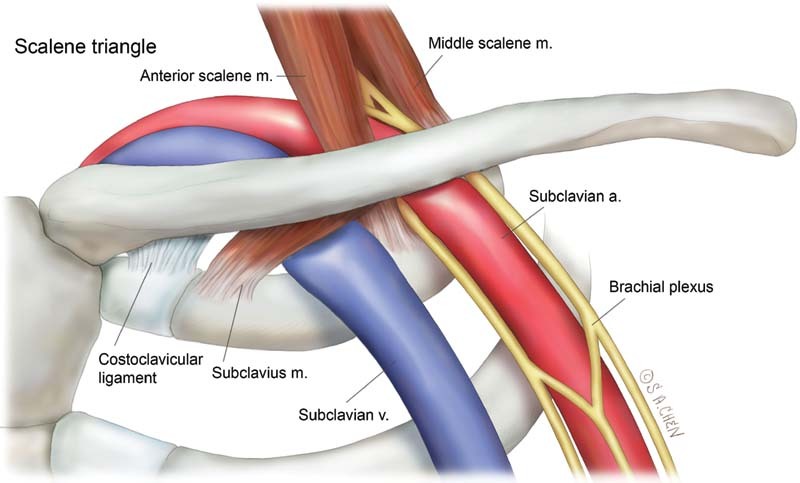
Neurovascular bundle in the scalene triangle.
Anatomic abnormalities involving the thoracic outlet are often implicated in SCV effort thrombosis. These abnormalities include congenital bands, hypertrophic scalenus anterior or subclavius muscle or tendon, abnormal clavicular or first rib anatomy or hypertrophy, and abnormal insertions of the costoclavicular ligament. The aforementioned structures exert extrinsic compression on the SCV and repetitive motion leads to endothelial trauma and intermittent thrombosis. This repetitive trauma also causes intimal hyperplasia, inflammation, and fibrosis in the form of venous webs as well as external inflammation including perivenular fibrosis and entrapment of the SCV at the level of the first rib–clavicular interval. This perivenular fibrosis worsens the costoclavicular crowding creating a negative feedback loop that culminates in thrombotic occlusion. 1 3 The thrombus then propagates upstream into the axillary vein obstructing critical collateral veins which results in the severe symptoms seen in the acute presentation of the effort thrombosis syndrome. Pulmonary embolism (PE) occurs with far less frequency than for lower extremity (LE) DVT, with wide ranging and unreliable estimates from 6 to 30%. 1 4 This may in part be related to a decreased rate of symptomatic PE due to mechanical obstruction of the SCV which might prevent embolization of any large thrombus. 1
Clinical Presentation and Diagnosis
SCV effort thrombosis occurs most frequently in adolescents and young adults between the ages of 15 and 45 years. Some studies report an equal distribution of the syndrome between males and females, although others suggest a male predominance. 3 5 6 Many of these patients will be engaged in work-related or recreational activities that involve repetitive overhead activities. The acute presentation often occurs in conjunction with recent dehydration and/or excessive activity of the affected limb. The arm at acute presentation appears congested, swollen, and sometimes cyanotic. Patients will often describe pain, heaviness, or increased fatigue in the arm with symptoms worsened by overhead positioning of the limb. Superficial veins may also be engorged and palpable, particularly around the axilla, shoulder, and upper, lateral, anterior chest wall ( Fig. 2 ). 1 3 6 Patients with the aforementioned classic presentation can be given the presumptive diagnosis of SCV effort thrombosis and started on anticoagulation without additional diagnostic delay.
Fig. 2.
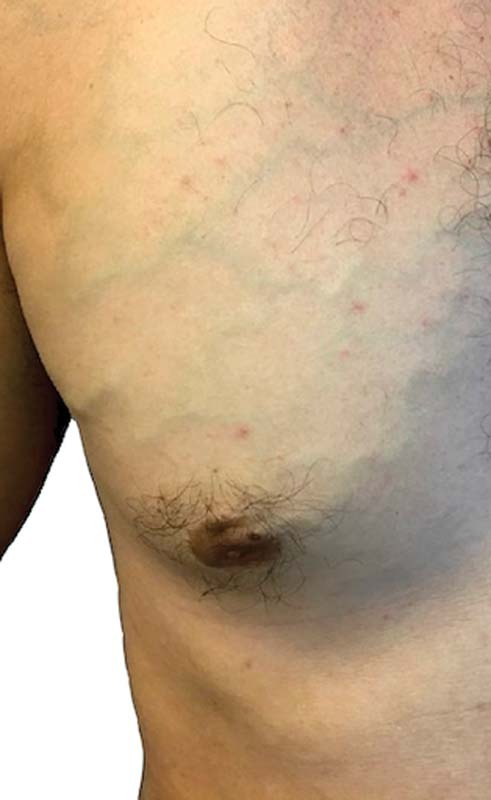
Superficial venous collaterals on physical exam suggest central venous stenosis or occlusion.
All patients with unilateral UE swelling should undergo UE duplex ultrasound evaluation which has been shown to have 80 to 100% sensitivity and specificity for axillary and/or SCV thrombosis. However, false-negative rates from ultrasound have been reported as high as 30% and duplex ultrasound is therefore insufficient to exclude the diagnosis of SCV effort thrombosis. 1 3 Patients with suggestive clinical presentations and negative ultrasounds should go on to receive contrast-enhanced computed tomography venography or magnetic resonance venography ( Fig. 3 ). These studies are also beneficial for patients with already confirmed axillary or subclavian thrombosis, as they provide anatomic confirmation that the source of the SCV stenosis or occlusion is at the level of the first rib. Additionally, they can demonstrate the presence of collateral vessels and aid in the determination of the chronicity of the clot. These studies may also be performed with the affected arm at rest or in an abducted position to elicit positional SCV obstruction ( Fig. 4 ). CT or MRI venography are usually sufficient to exclude SCV effort thrombosis if found to be negative. 1 7 The historical gold standard for diagnosing SCV effort thrombosis is catheter-directed contrast venography and/or intravascular ultrasound. This technique provides anatomic information regarding the site and extent of thrombosis and allows visualization of the collateral venous pathways. Given their invasive nature, these approaches are usually reserved for cases with high clinical suspicion and equivocal noninvasive imaging, or performed at the time of intervention.
Fig. 3.
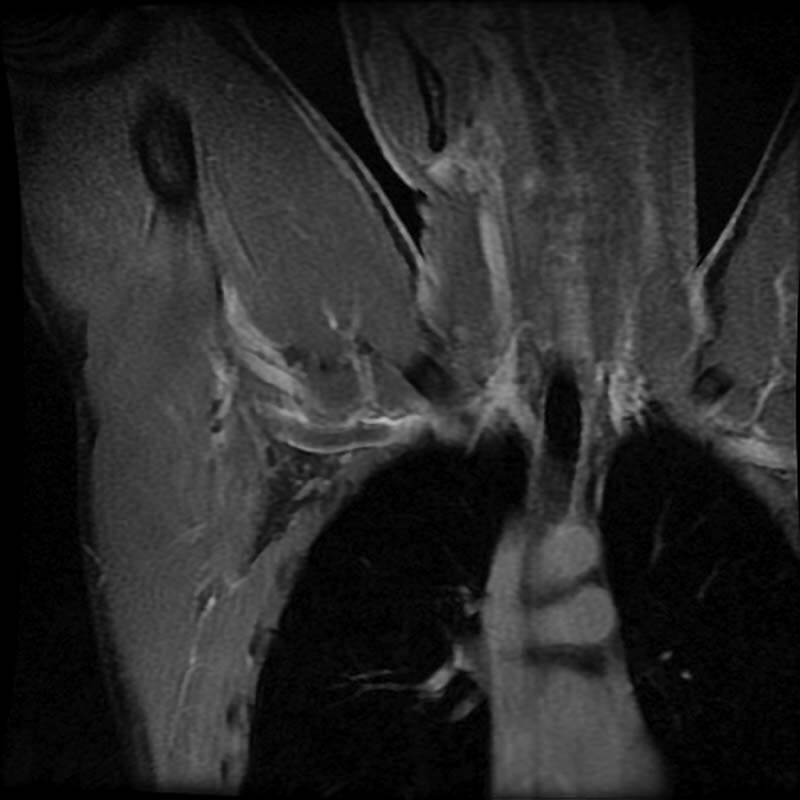
Coronal magnetic resonance venography with the patient's arms abducted. A filling defect (arrow) is visible in the left axillary and subclavian veins.
Fig. 4.
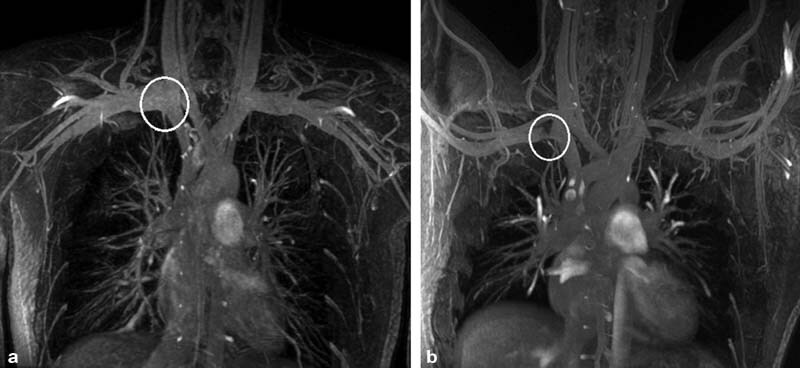
Coronal magnetic resonance venography with the patient's arms at the side ( a ) and abducted ( b ) showing physiologic compression of the subclavian vein with abduction (circles). No collateral veins are seen.
When SCV effort thrombosis is suspected, the patients should also be evaluated for arterial TOS (aTOS) and/or neurogenic TOS (nTOS). Patients with aTOS will present with digital ischemia, claudication, pallor, coldness, paresthesia, and/or pain in the hand or rarely the shoulder or neck. These symptoms are related to arterial thrombi forming in the subclavian artery and embolizing distally. aTOS is often associated with a cervical rib or an anomalous first rib which can often be detected on a neck radiograph. nTOS presents as pain, paresthesia, and weakness in the hand, arm, or shoulder, though it can sometimes also occur in the neck or occipital regions. Hand coldness and pallor (Reynaud's phenomenon) can also be seen in nTOS due to sympathetic nervous systemic overactivation from nerve compression. 6 The overlap in presentations between aTOS and nTOS can make diagnosis difficult and careful evaluation of the signs and symptoms of each syndrome must be undertaken. Ultrasound and cross-sectional imaging may assist in the differentiation of these etiologies. 8
Patients with primary UEDVT (i.e., occurring without the presence of a central venous catheter or cancer) have significantly higher rates of coagulability disorders than the general population. Some studies have calculated that patients with factor V Leiden develop a primary UEDVT over six times more frequently than patients without a known clotting disorder. 9 Furthermore, up to 67% of patients with SCV thrombosis have a coagulation disorder, and testing for thrombophilia after diagnosis is recommended. 1 10 The hypercoagulability workup should begin with a meticulous history including family history of thrombosis or hypercoagulability, medication regimen, travel and immobility, and, in women of child-bearing age, pregnancy status. Hypercoagulability testing should encompass inheritable thrombophilia panels such as activated protein C resistance, factor V Leiden, prothrombin gene mutation, proteins C and S deficiencies, and antithrombin deficiency, as well as testing for antiphospholipid syndromes. 9 10 Notably, laboratory testing should wait until 4 to 6 weeks after the acute presentation or after discontinuing anticoagulation (2 weeks for Coumadin, 2 days for a direct oral anticoagulant, or 2 days for heparin). 11 12
Patients with an unprovoked DVT or PE are more than twice as likely to have an occult malignancy as compared with patients without a DVT or PE. The occult malignancies most frequently associated with an unprovoked DVT or PE are lung, colorectal, liver, hematologic, and lymphoid cancers. 13 A workup for occult malignancy may be indicated in some patients and should include routine laboratory studies and age- or gender-specific screenings. 7 Age, comorbidities, family history, and carcinogen exposure history can be helpful in determining which patients with an UEDVT may benefit from an occult malignancy workup. 7
Treatment of Effort Vein Thrombosis
Initial Management
Once there is a high degree of suspicion for SCV effort thrombosis, anticoagulation should be initiated with intravenous or subcutaneous heparin. Despite limited evidence, some protocols will also include antiplatelet therapy with either aspirin or clopidogrel. Parenteral anticoagulation will help prevent extension of thrombus while additional workup and more definitive treatment strategies are pursued. 2 14
Catheter-Directed Thrombolysis
Anticoagulation alone has been proven to be insufficient as a monotherapy as the thrombus is only partially resolved or completely unresolved in approximately 45% of patients who were treated only with anticoagulation. 2 Additionally, rethrombosis may occur within 6 months in up to 72% of patients with SCV effort thrombosis who remain on oral anticoagulation alone. 14 Catheter-directed therapies have grown in use over time as they can alleviate symptoms by rapidly decreasing thrombus burden when compared with anticoagulation alone.
Catheter-directed thrombolysis (CDT) has largely replaced surgical thrombectomy and systemic thrombolysis given its comparative effectiveness and reduced morbidity including reduced major bleeding and mortality. 15 If the thrombus has been present for more than 2 weeks (subacute or chronic thrombus), thrombolysis is less effective than when the thrombus is in the acute phase. A recent meta-analysis found that technical success of patency restoration can be achieved with CDT in a clot that has been present for less than 2 weeks in 75 to 84% of patients versus 29% of patients with thrombosis for more than 2 weeks. 2 In cases with chronic thrombosis or restenosis after CDT, balloon angioplasty and stenting have not been shown to be effective in restoring or maintaining patency in the absence of surgical decompression ( Fig. 5 ). 16 17 18
Fig. 5.

Coronal digital subtraction venogram demonstrating left subclavian vein occlusion with collateral veins ( a , arrow) which was subsequently treated with angioplasty with persistent waist of the balloon ( b , arrow). After angioplasty, the left subclavian vein remains small in caliber with filling defects consistent with vascular webs and persistent venous collaterals ( c ). The patient went on to surgical decompression.
CDT has traditionally been performed by continuous infusion of a thrombolytic agent, such as alteplase, through a multihole catheter placed directly within the region of thrombosis. This infusion is typically continued for 24 to 48 hours with interval venograms performed to assess venous patency. Some studies have shown that CDT beyond 48 hours may be associated with significantly higher rates of major bleeding. 19 20 The goal of thrombolysis is to clear acute thrombus from the affected veins as well as any occluded collateral vessels. 5 Recombinant tissue plasminogen activator (alteplase) is the most commonly used lytic medication in the United States. Alteplase is often cheaper and more widely accessible than urokinase. It is also cleared more rapidly and may have a reduced bleeding risk profile as it will be eliminated more quickly in scenarios where systemic thrombolysis is occurring. However, this theoretical benefit has yet to be demonstrated. 21 The PURPOSE trial demonstrated that lower infusion rates of thrombolytic clear thrombus more slowly but have lower rates of major bleeding. 22 As such, the current recommendation is to limit alteplase infusion rates to 1 mg/hour or less. 23
Concurrent anticoagulation and CDT has been shown to have similar risks of major bleeding as compared with CDT alone in multiple studies, but no study has demonstrated any benefit of concurrent administration. When compared with traditional CDT, ultrasound-assisted CDT has not been shown to have any significant differences including thrombus load reduction, primary patency, severity of postthrombotic syndrome (PTS), or treatment-related complication rates in PE or iliofemoral venous thrombolysis and may not have added benefit in SCV thrombolysis. 24 25
Protocols for monitoring during CDT vary between institutions and practitioners. Patients are typically roomed in the intensive care unit (ICU) due to the increased nursing tasks. Physical exam to include neurologic monitoring and assessment of bleeding is performed at regular intervals. During the period of thrombolysis, some or all of the following laboratory data are tested every 6 to 24 hours: hemoglobin, fibrinogen, fibrin D-dimer, antithrombin, and platelets. 21 26 Fibrinogen is often elevated in the acute phase and decreases during CDT. A rapid and pronounced decrease in fibrinogen may indicate systemic fibrinolysis and an increased risk of bleeding. Many protocols will reduce the infusion rate of the thrombolytic or may stop the infusion entirely if the fibrinogen level reaches 150 mg/dL, as some studies have shown higher rates of major bleeding events below this level. 19 Multiple studies have shown no increased risk of bleeding at this level or even below 100 mg/dL and the utility of fibrinogen testing is in question. 20 22 27 Consumption of antithrombin requiring antithrombin substitution may indicate ongoing bleeding and should prompt an evaluation of the patient for signs of bleeding. D-dimer measurements may help guide the duration of CDT, as it is elevated when thrombus degradation is ongoing. If D-dimer is still significantly elevated in a patient with a normal venogram, they may benefit from additional lysis. However, if D-dimer is not elevated or only minimally elevated, there may be no benefit from additional thrombolytic therapy. 21
Percutaneous Thrombectomy
As CDT requires overnight admission to an ICU to monitor for bleeding complications, there has been increased interest in pharmacomechanical thrombectomy (PMT) or entirely mechanical thrombectomy. These approaches can restore venous patency in a single session and do not require admission. 5 28 Additionally, mechanical thrombectomy may offer an endovascular treatment in patients with contraindications to pharmacologic thrombolysis.
Available devices utilize various mechanisms for thrombus removal. The ClotTriever (Inari Medical, Irvine, CA) utilizes a mechanical coring mechanism and collection bag to remove thrombus. Rheolytic thrombectomy devices, such as the AngioJet (Boston Scientific, Marlborough, MA), use high-velocity saline jets and the Venturi-Bernoulli effect to break up and aspirate clot. Rotational thrombectomy devices such as the Cleaner (Argon, Frisco, TX) and Arrow-Trerotola (TeleFlex, Wayne, PA) macerate clot with a rotating sinusoidal wire or cage but do not remove thrombus. Circuit thrombectomy systems like the AngioVac (AngioDynamics, Latham, NY) use a large caliber suction cannula and filter as part of an extracorporeal veno-venous bypass circuit to remove clot and redeposit aspirated blood back into the venous system. Finally, aspiration thrombectomy may be performed using a large bore sheath and suction or as part of an endovascular device such as the Indigo (Penumbra, Alameda, CA), FlowTriever (Inari Medical, Irvine, CA), 28 or through a large bore sheath. Many operators will pursue PMT by infusing 5 to 25 mg of adjunctive alteplase via one of the systems or directly into the thrombosed segment via a separate catheter. PMT has been shown to be safe and effective for restoring patency after venous thrombosis with reduced ICU utilization and decreased lengths of hospital stay. 28 A recent meta-analysis comparing PMT to CDT concluded PMT may reduce rates of PTS when compared with CDT alone. 29 Table 1 outlines mechanical thrombectomy devices commonly used during the treatment of SCV effort thrombosis.
Table 1. Examples of FDA-approved venous thrombectomy devices.
| Device name | Manufacturer | Type of thrombectomy | Sheath size (Fr) |
|---|---|---|---|
| AngioVac | AngioDynamics (New York, NY) | Aspiration | 26 |
| Cleaner | Argon Medical (Frisco, TX) | Rotational | 6, 7 |
| AngioJet | Boston Scientific (Marlborough, MA) | Rheolytic | 6, 8 |
| ClotTriever | Inari Medical (Irvine, CA) | Mechanical | 13, 16 |
| FlowTriever | Inari Medical | Aspiration | 20–26 |
| Indigo | Penumbra (Alameda, CA) | Aspiration | 5–12 |
| QuickClear | Philips Healthcare (Andover, MA) | Aspiration | 10 |
| Trerotola | TeleFlex Medical (Gurnee, IL) | Rotational | 5, 7 |
| Jeti | Walk Vascular (Irvine, CA) | Aspiration | 6, 8 |
Successful thrombolysis and/or thrombectomy results in a prompt reduction in the venous obstructive symptoms in the affected limb. After thrombus debulking, there is often a high-grade focal stenosis in the proximal SCV at the level of the first rib that is visible on venography. 5 Balloon angioplasty may be helpful in restoring some temporary improvement in this stenosis, but any improvement is short-lived as elastic recoil after angioplasty is expected. Stenting improves elastic recoil in other vascular territories but has been plagued by failures when attempted at the thoracic outlet. The first rib, costoclavicular ligament, anterior scalene muscle, and the clavicle exert significant external compressive stress on the stent leading to stent fracture and recurrent thrombosis. 16 17 18
Surgical Decompression
The evolution of Paget-Schroetter Syndrome management over the past few decades has highlighted the role of surgical decompression as an important tool in the treatment and prevention of recurrent symptomatology. While controversy may exist regarding the timing and approach to surgery, most institutions advocate pursuing early surgical decompression, in conjunction with thrombolysis and anticoagulation, to ensure optimal long-term outcomes—especially in younger, more active patients. In a large systematic review by Lugo et al, patients treated conservatively with anticoagulation and thrombolysis had a 55% rate of venous patency and a 63% resolution of symptomatology. 30 In comparison, the addition of first rib resection (FRR) conferred an 88 to 98% venous patency and a 95% rate of symptomatic relief, respectively. Urschel and Razzuk's landmark 30-year experience portends the importance of early surgical decompression with FRR in preventing recurrent symptomatology. 31 In their series, there was a 60% recurrence in patients treated with an anticoagulation-only strategy and a 56% recurrence in patients treated with thrombolysis in the first 6 weeks post-insult. Other reports have underscored a similarly high rate of 30-day recurrence, ranging from 20 to 33% in patients who are treated with an anticoagulation and thrombolysis-only strategy. 2 5 32 Long-term reviews cite similar rates of high recurrence. Lee et al reported a 23% post-lytic, rethrombosis rate at 13 months in patients who underwent nonoperative management. 17
Current indications for surgical intervention include (1) moderate-to-severe symptoms despite thrombolysis due to axillary–subclavian venous thrombosis; (2) positive venogram demonstrating extrinsic compression postthrombolysis; (3) symptomatic subacute (>14 days) thrombosis; and (4) symptomatic chronic (>90 days) thrombosis. 33
While the timing of surgical decompression remains a matter of debate, most institutions favor surgery 2 to 4 weeks postthrombolysis. In a large systematic review, de Kleijn et al revealed a higher rate of pre- and postsurgical rethrombosis in those who had surgery delayed beyond 14 days. 34 While early surgical decompression has been shown in multiple series as providing the best results, clinical acumen should ultimately dictate the optimal window for intervention on a case-by-case basis. Having said that, younger, more active patients, who will likely engage in strenuous exercises ad lib are one group that should be offered early surgical decompression.
The most common surgical approaches for venous thoracic outlet syndrome include trans-axillary, infraclavicular, supraclavicular, or through a claviculectomy ( Fig. 6 ). Regardless of the approach, surgery should be performed to meet two principal goals: (1) decompression of the SCV through removal of the first rib with its associated scalene and subclavius muscles and (2) restoration of venous patency through its liberation from any bands, ligaments, or constricting scar tissue—coupled with balloon venoplasty or venous reconstruction if necessary. If venotomy, thrombectomy, or venous reconstruction is anticipated, then an infraclavicular approach provides optimal visualization and access. Trans-axillary approach elevates the vein, artery, and lower trunk of brachial plexus off the rib, providing for a more complete rib resection. In addition, it confers a more satisfactory aesthetic outcome. It is important to note that trans-axillary and infraclavicular approaches have comparable success and complication rates. 35 While the supraclavicular approach has been described in the past, it is not the common contemporary approach to FRR, as it often requires an additional infraclavicular incision for complete first rib excision. In the larger patient with difficult visualization, a claviculectomy can be performed by removing the medial two-thirds of the clavicle to provide excellent exposure of pertinent anatomy. Unless necessary, this is generally not favored, as it carries the risk of developing shoulder instability. Regardless of the approach, intraoperative venography should be used to assess residual stenosis and need for additional surgical intervention, venoplasty, or stent placement. Complication rates after FRR, with or without venoplasty, are relatively low. There is a 3% risk of an ipsilateral pneumothorax, 2.5% risk of bleeding complication, and <0.05% risk of nerve injury—most commonly to the lower brachial plexus, phrenic, or C8, T1 nerve roots, which are adjacent to the neck of the first rib. 35 36 As the benefits of FRR after early thrombolysis outweigh its relatively low complication rates, it should be offered to most, but the highest surgical risk patients.
Fig. 6.
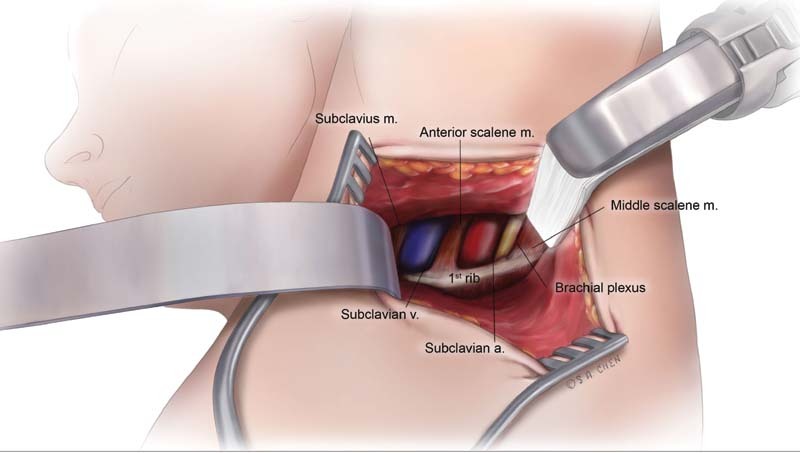
Transaxillary surgical exposure.
Postoperative Management
Therapeutic anticoagulation should be initiated within a few days after surgery and continued for 12 weeks. The ideal choice of oral anticoagulant has yet to be determined due to a lack of randomized controlled trials evaluating the topic. The 2021 American College of Chest Physicians Evidence-Based Clinical Practice Guidelines recommend apixaban, dabigatran, edoxaban, or rivaroxaban over vitamin K antagonists (VKAs) with moderate-certainty evidence. This is due to the comparable efficacy and improved safety profile of direct oral anticoagulants over VKA. VKA should be considered in patients with severe renal impairment or antiphospholipid syndrome. 37
If a stent was placed, adjunctive antiplatelet therapy may be considered. Expected postoperative hospital stay is 2 to 4 days following FFR. 36 Inpatient physical therapy should be started the day following surgery to maintain range of motion and continued as an outpatient. No restrictions need to be placed on the affected extremity beyond 12 weeks of surgery. Full recovery is expected within 3 months and patients who recover fully should expect to return to prior levels of functional activity. 3
No studies have yet compared open venous reconstruction to angioplasty and stenting for residual venous disease after FRR. Some centers will attempt to resolve residual venous disease after FRR with balloon angioplasty and/or stenting to avoid the added complexity and morbidity of open venous reconstruction in all but the most severely stenotic lesions. These patients may benefit from planned venography and possible balloon angioplasty in the weeks to months following decompression. 14 The use of dedicated venous stents after surgical decompression for recurrent short segment stenoses has been shown to have primary, primary-assisted, and secondary patency rates at 24 months of 55, 95, and 100%, respectively. 38
Conclusion
Subclavian effort thrombosis is an uncommon sequela of TOS, which manifests as debilitating axillosubclavian thrombosis due to chronic repetitive microtrauma to the vein causing endothelial damage, fibrosis, and webs. Catheter-directed thrombolysis and/or percutaneous thrombectomy is used to debulk the thrombus and restore venous outflow. As stenting in the setting of extrinsic compression of the vein leads to poor outcomes, surgical costoclavicular decompression must be performed first. However, recurrent venous thrombosis following surgical decompression may necessitate stenting. Thus far, preliminary data for modern-generation venous stents in the thoracic outlet appear promising.
Acknowledgments
The authors thank Sarah A. Chen, MD, MS, for her generous, beautiful, and educational artwork.
Footnotes
Conflict of Interest None declared.
References
- 1.Hangge P, Rotellini-Coltvet L, Deipolyi A R, Albadawi H, Oklu R. Paget-Schroetter syndrome: treatment of venous thrombosis and outcomes. Cardiovasc Diagn Ther. 2017;7 03:S285–S290. doi: 10.21037/cdt.2017.08.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karaolanis G, Antonopoulos C N, Koutsias S G. A systematic review and meta-analysis for the management of Paget-Schroetter syndrome. J Vasc Surg Venous Lymphat Disord. 2021;9(03):801–8.1E7. doi: 10.1016/j.jvsv.2021.01.011. [DOI] [PubMed] [Google Scholar]
- 3.Thompson R W. Comprehensive management of subclavian vein effort thrombosis. Semin Intervent Radiol. 2012;29(01):44–51. doi: 10.1055/s-0032-1302451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kucher N. Clinical practice. Deep-vein thrombosis of the upper extremities. N Engl J Med. 2011;364(09):861–869. doi: 10.1056/NEJMcp1008740. [DOI] [PubMed] [Google Scholar]
- 5.Illig K A, Doyle A J. A comprehensive review of Paget-Schroetter syndrome. J Vasc Surg. 2010;51(06):1538–1547. doi: 10.1016/j.jvs.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 6.Sanders R J, Hammond S L, Rao N M. Diagnosis of thoracic outlet syndrome. J Vasc Surg. 2007;46(03):601–604. doi: 10.1016/j.jvs.2007.04.050. [DOI] [PubMed] [Google Scholar]
- 7.Kraaijpoel N, van Es N, Porreca E, Büller H R, Di Nisio M. The diagnostic management of upper extremity deep vein thrombosis: a review of the literature. Thromb Res. 2017;156:54–59. doi: 10.1016/j.thromres.2017.05.035. [DOI] [PubMed] [Google Scholar]
- 8.Raptis C A, Sridhar S, Thompson R W, Fowler K J, Bhalla S. Imaging of the patient with thoracic outlet syndrome. Radiographics. 2016;36(04):984–1000. doi: 10.1148/rg.2016150221. [DOI] [PubMed] [Google Scholar]
- 9.Martinelli I, Battaglioli T, Bucciarelli P, Passamonti S M, Mannucci P M. Risk factors and recurrence rate of primary deep vein thrombosis of the upper extremities. Circulation. 2004;110(05):566–570. doi: 10.1161/01.CIR.0000137123.55051.9B. [DOI] [PubMed] [Google Scholar]
- 10.Cassada D C, Lipscomb A L, Stevens S L, Freeman M B, Grandas O H, Goldman M H. The importance of thrombophilia in the treatment of Paget-Schroetter syndrome. Ann Vasc Surg. 2006;20(05):596–601. doi: 10.1007/s10016-006-9106-z. [DOI] [PubMed] [Google Scholar]
- 11.Nakashima M O, Rogers H J. Hypercoagulable states: an algorithmic approach to laboratory testing and update on monitoring of direct oral anticoagulants. Blood Res. 2014;49(02):85–94. doi: 10.5045/br.2014.49.2.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Connors J M. Thrombophilia testing and venous thrombosis. N Engl J Med. 2017;377(23):2298. doi: 10.1056/NEJMc1713797. [DOI] [PubMed] [Google Scholar]
- 13.Sun L-M, Chung W-S, Lin C-L, Liang J-A, Kao C-H. Unprovoked venous thromboembolism and subsequent cancer risk: a population-based cohort study. J Thromb Haemost. 2016;14(03):495–503. doi: 10.1111/jth.13251. [DOI] [PubMed] [Google Scholar]
- 14.Cai T Y, Rajendran S, Saha P, Dubenec S. Paget-Schroetter syndrome: a contemporary review of the controversies in management. Phlebology. 2020;35(07):461–471. doi: 10.1177/0268355519898920. [DOI] [PubMed] [Google Scholar]
- 15.Furfaro D, Stephens R S, Streiff M B, Brower R. Catheter-directed thrombolysis for intermediate-risk pulmonary embolism. Ann Am Thorac Soc. 2018;15(02):134–144. doi: 10.1513/AnnalsATS.201706-467FR. [DOI] [PubMed] [Google Scholar]
- 16.Urschel H C, Jr, Patel A N.Paget-Schroetter syndrome therapy: failure of intravenous stents Ann Thorac Surg 200375061693–1696., discussion 1696 [DOI] [PubMed] [Google Scholar]
- 17.Lee J T, Karwowski J K, Harris E J, Haukoos J S, Olcott C., IV Long-term thrombotic recurrence after nonoperative management of Paget-Schroetter syndrome. J Vasc Surg. 2006;43(06):1236–1243. doi: 10.1016/j.jvs.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 18.Meier G H, Pollak J S, Rosenblatt M, Dickey K W, Gusberg R J.Initial experience with venous stents in exertional axillary-subclavian vein thrombosis J Vasc Surg 19962406974–981., discussion 981–983 [DOI] [PubMed] [Google Scholar]
- 19.Skeik N, Gits C C, Ehrenwald E, Cragg A H. Fibrinogen level as a surrogate for the outcome of thrombolytic therapy using tissue plasminogen activator for acute lower extremity intravascular thrombosis. Vasc Endovascular Surg. 2013;47(07):519–523. doi: 10.1177/1538574413497107. [DOI] [PubMed] [Google Scholar]
- 20.Lee K, Istl A, Dubois L. Fibrinogen level and bleeding risk during catheter-directed thrombolysis using tissue plasminogen activator. Vasc Endovascular Surg. 2015;49(07):175–179. doi: 10.1177/1538574415611234. [DOI] [PubMed] [Google Scholar]
- 21.Bækgaard N, Klitfod L, Jørgensen M.Should catheter-directed thrombolysis be monitored? Phlebology 201631(1, Suppl):5–10. [DOI] [PubMed] [Google Scholar]
- 22.Ouriel K, Kandarpa K, Schuerr D M, Hultquist M, Hodkinson G, Wallin B. Prourokinase versus urokinase for recanalization of peripheral occlusions, safety and efficacy: the PURPOSE trial. J Vasc Interv Radiol. 1999;10(08):1083–1091. doi: 10.1016/s1051-0443(99)70196-x. [DOI] [PubMed] [Google Scholar]
- 23.Fleck D, Albadawi H, Shamoun F, Knuttinen G, Naidu S, Oklu R. Catheter-directed thrombolysis of deep vein thrombosis: literature review and practice considerations. Cardiovasc Diagn Ther. 2017;7 03:S228–S237. doi: 10.21037/cdt.2017.09.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Engelberger R P, Spirk D, Willenberg T. Ultrasound-assisted versus conventional catheter-directed thrombolysis for acute iliofemoral deep vein thrombosis. Circ Cardiovasc Interv. 2015;8(01):e002027. doi: 10.1161/CIRCINTERVENTIONS.114.002027. [DOI] [PubMed] [Google Scholar]
- 25.Liang N L, Avgerinos E D, Marone L K, Singh M J, Makaroun M S, Chaer R A. Comparative outcomes of ultrasound-assisted thrombolysis and standard catheter-directed thrombolysis in the treatment of acute pulmonary embolism. Vasc Endovascular Surg. 2016;50(06):405–410. doi: 10.1177/1538574416666228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaufman C, Kinney T, Quencer K. Practice trends of fibrinogen monitoring in thrombolysis. J Clin Med. 2018;7(05):E111. doi: 10.3390/jcm7050111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Poorthuis M HF, Brand E C, Hazenberg C EVB. Plasma fibrinogen level as a potential predictor of hemorrhagic complications after catheter-directed thrombolysis for peripheral arterial occlusions. J Vasc Surg. 2017;65(05):1519–1.527E29. doi: 10.1016/j.jvs.2016.11.025. [DOI] [PubMed] [Google Scholar]
- 28.Kohi M P, Kohlbrenner R, Kolli K P, Lehrman E, Taylor A G, Fidelman N. Catheter directed interventions for acute deep vein thrombosis. Cardiovasc Diagn Ther. 2016;6(06):599–611. doi: 10.21037/cdt.2016.11.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang T, Chen L, Chen J, Mei T, Lu Y. Pharmacomechanical thrombectomy versus catheter-directed thrombolysis for iliofemoral deep vein thrombosis: a meta-analysis of clinical trials. Clin Appl Thromb Hemost. 2019;25:1.07602961882119E15. doi: 10.1177/1076029618821190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lugo J, Tanious A, Armstrong P. Acute Paget–Schroetter Syndrome: does the first rib routinely need to be removed after thrombolysis? Ann Vasc Surg. 2015;29(06):1073–1077. doi: 10.1016/j.avsg.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 31.Urschel HC, Razzuk MA. Paget-Schroetter syndrome: what is the best management? Ann Thorac Surg. 2000;69(06):1663–1668. doi: 10.1016/s0003-4975(00)01151-6. [DOI] [PubMed] [Google Scholar]
- 32.Kärkkäinen J M, Nuutinen H, Riekkinen T. Pharmacomechanical thrombectomy in Paget-Schroetter syndrome. Cardiovasc Intervent Radiol. 2016;39(09):1272–1279. doi: 10.1007/s00270-016-1376-4. [DOI] [PubMed] [Google Scholar]
- 33.Kumar R, Harsh K, Saini S. Treatment-related outcomes in Paget-Schroetter syndrome - a cross-sectional investigation. J Pediatr. 2019;207:226–2320. doi: 10.1016/j.jpeds.2018.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Kleijn R JCMF, Schropp L, Westerink J, de Borst G J, Petri B-J. Timing of thoracic outlet decompression after thrombolysis for primary upper extremity deep venous thrombosis: a systematic review. Ann Vasc Surg. 2020;66:654–661. doi: 10.1016/j.avsg.2020.01.083. [DOI] [PubMed] [Google Scholar]
- 35.Aboul Hosn M, Goffredo P, Man J. Supraclavicular versus transaxillary first rib resection for thoracic outlet syndrome. J Laparoendosc Adv Surg Tech A. 2020;30(07):737–741. doi: 10.1089/lap.2019.0722. [DOI] [PubMed] [Google Scholar]
- 36.Rinehardt E K, Scarborough J E, Bennett K M. Current practice of thoracic outlet decompression surgery in the United States. J Vasc Surg. 2017;66(03):858–865. doi: 10.1016/j.jvs.2017.03.436. [DOI] [PubMed] [Google Scholar]
- 37.Stevens S M, Woller S C, Kreuziger L B. Antithrombotic therapy for VTE disease: second update of the CHEST guideline and expert panel report. Chest. 2021;160(06):e545–e608. doi: 10.1016/j.chest.2021.07.055. [DOI] [PubMed] [Google Scholar]
- 38.Rajendran S, Cai T Y, Loa J, Saha P, Dubenec S. Early outcomes using dedicated venous stents in the upper limb of patients with venous thoracic outlet syndrome: a single centre experience. CVIR Endovasc. 2019;2(01):22. doi: 10.1186/s42155-019-0066-0. [DOI] [PMC free article] [PubMed] [Google Scholar]


