Abstract
Introduction:
Antenatal ultrasound (US) is considered the gold standard tool to detect fetal anomalies during the antenatal period. However, its highly operator dependent and maybe affected with other variables. The aim of this study to compare discrepancy between antenatal and postnatal US diagnosis of congenital anomalies of the kidney and urinary tract (CAKUT) and to evaluate the incidence of parent’s consanguinity among those patients at King Abdulaziz Medical City – Western Region (KAMC-WR), as it may help changing the current practiced guidelines and applied protocols.
Methods:
This is an observational, retrospective, cross-sectional study, conducted at the Maternal Fetal Medicine Unit at KAMC-WR, reviewing antenatally detected CAKUT between the years 2009 and 2014. Utilizing the congenital anomalies database and using multiple databases collected the data. A data sheet was completed and divided into four sections, which consist of maternal data, antenatal data, delivery, and postnatal data. The analysis was performed using Statistical Package for Social Sciences program (Armonk, NY: IBM Corp).
Results:
We included 137 fetuses with renal anomalies in our study, with 17% perinatal mortality rate, and 13% loss of follow-up. Abnormal amniotic fluid was detected in 32%, and bilateral anomalies presented in 41% and it was most commonly seen in male fetuses. Added to that, 41% of the fetuses were product of consanguineous marriage and 11% had a history of other child with renal anomalies. However, the rate of discrepancy between antenatal and postnatal renal US findings was 24%. Finally, the most common anomaly found antenatally and confirmed postnatally was hydronephrosis disease spectrum (60.6%).
Conclusion:
There is a significant association between children with CAKUT and parents’ consanguinity. Furthermore, the discrepancy rate for the detection of CAKUT between antenatal and postnatal US in our study was comparable to other international studies. Further prospective studies are recommended in this field for further understanding.
Keywords: Antenatal hydronephrosis, children, congenital anomalies of the kidney and urinary tract, consanguinity, postnatal ultrasound
INTRODUCTION
Abnormality of the urinary tract determined by antenatal ultrasonography has raised several questions on years, and with the rapid improvement of medical technology, the incidence of detection of renal anomalies has changed. And with the increased use of maternal fetal ultrasound (US), the practice of prenatal urology has developed.
Congenital anomalies detected antenatal by means of US are crucially important permitting better prognosis. US antenatal screening was included in the antenatal care since early 1980s;[1] at that time, fetal screening program was considered as the gold standard method to detect different types of anomalies and related hydronephrosis during antenatal period with high specificity of 99% and variable sensitivity.[1] Currently, anteroposterior diameter (APD) of renal pelvis >5 mm with variation is demonstrating antenatal hydronephrosis and flagging the need of further radiographic documentation and classification and possible postnatal management.[2]
Antenatal hydronephrosis is identified in 1%–5% of all pregnancies[3] and its considered one of the most common birth defects detected. US is highly operator dependent and its affected by other variables. These may include: the timing of the test, quality of the machine, patient characteristics such as body habitus, gestational age, fetal position, and amniotic fluid index (AFI), knowing that the most frequently detected anomalies are congenital anomalies of the kidney and urinary tract (CAKUT) followed by head and neck and musculoskeletal system anomalies.[1]
Understanding of disease-related genes is known to be a vital step clarifying the base of a disease and its developing targeted treatment. It was studied in 1999 by Pope et al., which suggested that the genetic study of a disease occurring in the offspring of consanguineous unions is a powerful way to discover new genes, which are an excellent example because nearly 70% of cases of kidney disease in childhood are congenital with likely genetic basis, and take a specific inheritance pattern.[4] Despite that the exact risk of consanguineous unions to the health of the offspring is difficult to determine because of the presence of confounding factors.[5] Preliminary observations indicate that children in Saudi Arabia, compared with children in other parts of the world, appear to have a higher incidence of familial juvenile nephronophthisis; polycystic kidney disease; tubular diseases such as familial hypomagnesaemia, hypercalciuria, nephrocalcinosis syndrome, and renal tubular acidosis; congenital urologic anomalies; and familial nephrotic syndrome and this can be due to the evidence of consanguinity in Saudi Arabia accounting 50% of all marriages (marriages between cousins) in comparison to a global consanguinity of 10%.[5]
In Saudi Arabia, CAKUT reports of 38.6% of all fetal anomalies, detected by antenatal ultrasonography with a prevalence rate of 3:1000 births.[6] This difference could be contributed to various factors including the high rate of consanguinity previously mentioned.
Our study focused on CAKUT due to its potential significant impact on the neonate, where the affected neonate is prone to have impaired renal function and its consequences that may include: hypertension, growth retardation, end-stage renal disease, the need for dialysis, and possible death. Therefore, this study aims at evaluating the discrepancy between antenatal and postnatal US diagnosis of CAKUT at KAMC-WR and Saudi Arabia and evaluating the incidence of consanguinity among our patients with confirmed renal anomalies.
METHODS
Study design and criteria
This study is an observational, retrospective, cross-sectional study design, where fetuses with CAKUT detected antenatal at Maternal Fetal Medicine Unit (MFMU) at KAMC-WR with US between 2009 and 2014 and then were reviewed.
Using the anomalies database, and extracting the CAKUT cases, information’s acquired represented the case files that were chosen for data collection using medical records numbers of both the mother and the newborn. In extracting the data required, four different filing systems were employed. These included the hardcopy document files, an electronic health records system, a picture archiving and communication system, and an Open Virtual Memory System.
With the data obtained from the patients’ files, a data sheet was completed to be organized in a corresponding database. This data sheet was divided into four sections, which consist of maternal data, antenatal data, delivery, and postnatal data. The vital data fields were the antenatal and postnatal US findings and diagnosis. Nonetheless, contemplations as additives were consanguinity, comorbidities of the mother, and history of other children with renal congenital anomalies.
Correspondingly, documents within the data were the AFI and other anomalies detected in utero for the fetus antenatally, and the creatinine level postnatally. Moreover, as hydronephrosis cases were of special interest in the field of both pediatric nephrology and fetal maternal medicine, all postnatal images of hydronephrosis were reassessed and reviewed by a single experienced pediatric urologist reporting the anteroposterior diameters of the renal pelvis as well as the Society of Fetal Urology (SFU) grading system.
Hydronephrosis is defined as renal pelvis dilatation of more than 4 mm with or without calyceal dilation and pyelectasis is defined fetal mild-to-moderate hydronephrosis.[7,8] The severity of hydronephrosis was determined using the grading system of the SFU and APD. The SFU developed criteria for the diagnosis and grading of antenatal hydronephrosis based on the degree of pelvic dilatation, number of calyces seen, and the presence and severity of parenchymal atrophy. This grading system ranked the severity from Grade 0 to Grade IV [Table 1].[9]
Table 1.
Society of fetal urology grading system
| Grade level | Explanation |
|---|---|
| Grade 0 | Normal examination with no dilatation of the renal pelvis |
| Grade I | Mild dilatation of the renal pelvis only |
| Grade II | Moderate dilatation of the renal pelvis including a few calyces |
| Grade III | Dilatation of the renal pelvis with visualization of all the calyces, which are uniformly dilated, and normal renal parenchyma |
| Grade IV | Similar appearance of the renal pelvis and calyces as Grade III plus thinning of the renal parenchyma |
The main measurement tool used was the US machine. The majority of antenatal US was done using the Philips UI22 US machine, which is a more advanced model. Only a few cases were done using the Philips HD11 machine, which is an older model. All sonographers were trained especially for their work in the MFMU. All have attended a yearlong course, Saudi Career Development Program, to qualify for performing antenatal USs.
Statistical data
Statistical analysis was performed using the Statistical Package for Social Sciences version 20.0 (National Gaurd hospital = KAMC WR, Jeddah, Makkah province KSA)software package for data entry. Simple descriptive statistical analysis was carried out, inform of percentages for categorical data and mean (standard deviation) for continuous data. Chi-square test, odds ratio with 95% confidence interval, and was utilized to compare between two proportions, with a P < 0.05 that was considered statistically significant.
RESULTS
Our sample size included 137 case files found within the MFMU with complete antenatal data. About 96 fetuses (70%) had complete data with both antenatal and postnatal US data. Twenty-three fetuses (17%) were perinatal deaths, including two medical terminations and one miscarriage, with the remaining being neonatal deaths. The rest of the case files were loss of follow-up, rounding up to 16 case files and the remaining two cases had missing data (13%) [Figure 1]. Therefore, the sum of case files used within this study that had both antenatal and postnatal US recorded were a total of 96 cases and we found the discrepancy rate between antenatal and postnatal US to be 24%.
Figure 1.
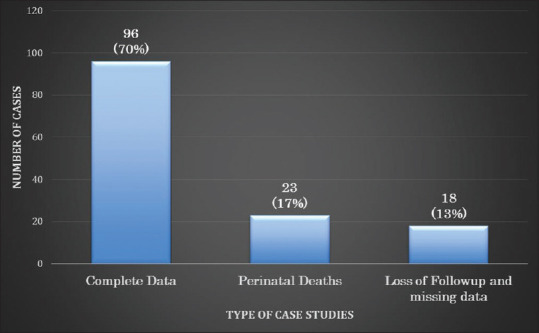
Types of case files of the study population from the years 2009 to 2014
The most commonly found antenatal US diagnosis was hydronephrosis, followed by multicystic dysplastic kidney disease [Figure 2]. On the other hand, the most commonly affected site found was bilateral except in pelvic kidney in which the right side was the most frequently identified [Table 2].
Figure 2.
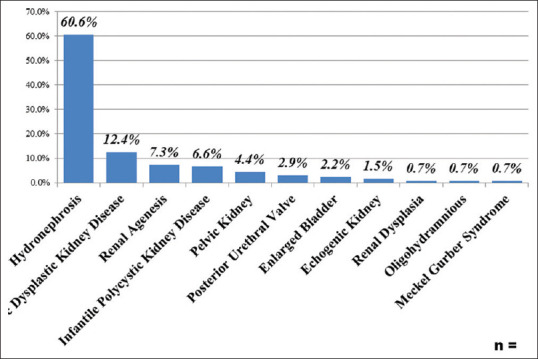
The most common congenital anomalies of the kidney and urinary tract diagnosis during antenatal follow-up
Table 2.
The laterality of antenatal diagnosis on ultrasound
| Antenatal diagnosis (n=137) | Site | ||
|---|---|---|---|
|
| |||
| Bilateral (%) | Right (%) | Left (%) | |
| Hydronephrosis (60.9%) | 39.8 (24.1) | 32.5 (19.7) | 27.7 (16.8) |
| Multicystic dysplastic kidney disease (12.4%) | 41.2 (5.1) | 35.5 (4.4) | 23.5 (2.9) |
| Renal agenesis (7.3%) | 50 (3.6) | 40 (2.9) | 10 (0.7) |
| Infantile polycystic kidney disease (6.6%) | 100 (6.6) | ||
| Pelvic kidney (4.4%) | 16.7 (0.7) | 66.7 (2.9) | 16.7 (0.7) |
| Echogenic kidney (1.5%) | 0 | 0 | 100 (1.5) |
| Renal dysplasia (0.7%) | 100 (0.7) | ||
The gestational age at diagnosis of CAKUT was observed most commonly before 25 weeks of gestation in 35%, 14% between 25 and 29 weeks of gestation, 22% in 30–34 weeks of gestation, and in 29% it was 35 weeks or above [Figure 3].
Figure 3.
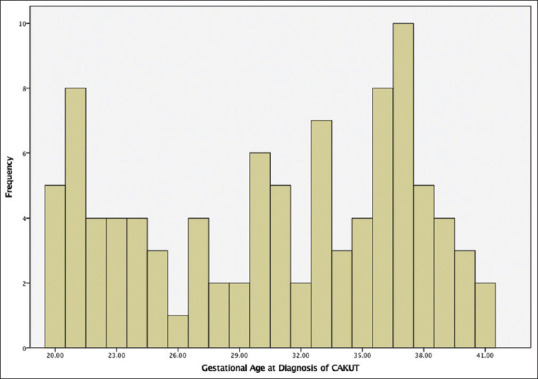
Gestational age at diagnosis of congenital anomalies of the kidney and urinary tract
The most frequently occurred gender was male with 69.8% of the population.
With regards to AFI, 93 (67.9%) had normal AFI, 24 (17.5%) had oligohydramnios, 17 (12.4) had anhydramnios, and 3 (2.2%) were observed to have polyhydramnios.
The data revealed that 93 (67.9%) of the patients had follow-up US s done antenatally, ranging from 2 to 8 visits with a progression rate of 41%; while 44 (32.1%) had no follow-up US done.
About 56 (40.9%) cases files of the chosen population were formed of consanguineous marriages with the remaining 81 (59.1%) cases being nonconsanguineous [Table 3]. In spite of the markedly high consanguinity rate, only 1 (0.7%) patient had agreed to have a chromosomal testing procedure done at the advisement of her physician even though 28 (20.4%) patients were shown to have associated congenital anomalies antenatally.
Table 3.
Gender distribution and prevalence of consanguinity among study population
| Sociodemographic data | Percentage |
|---|---|
| Consanguinity | |
| Yes | 41 |
| No | 59 |
| Gender | |
| Male | 70 |
| Female | 29 |
| Ambiguous | 1 |
It is noteworthy that of the 96 fetuses that had a postnatal US done, 56 (60.9%) had it done in the first 24 h and the remaining 40 (39.1%) had it performed after the first 24-h. Abnormal examination on their postnatal ultrasound was identified in 21% of patients.
Furthermore, demographically, the case files contained a mean age of mothers to be 31.40-year-old, the mean gestational age at US was 28.96 weeks, and the mean gestational age at delivery was 37.87 weeks and the mean neonatal weight found to be 2.90 kg [Table 4]. The progression rate on follow-up US was 41%, and 21% of those cases had abnormal examination on their postnatal evaluation. Finally, the most common anomaly found antenatally and confirmed postnatally was hydronephrosis disease spectrum (60%) followed by multicystic dysplastic kidney disease (12%), renal agenesis (8%), infantile polycystic kidney disease (7%), and posterior urethral valve (3%).
Table 4.
Demographic features of the study population
| Maternal and neonatal data | Mean (SD) | Range |
|---|---|---|
| Age of mother (years) | 31.4 (5.83) | 20-53 |
| Gestational age at ultrasound (weeks) | 29 (7.05) | 12-41 |
| Gestational age at birth (weeks) | 38 (3.54) | 22-42 |
| Birth weight (kg) | 2.9 (0.68) | 0.88-4.11 |
SD: Standard deviation
DISCUSSION
CAKUT is a term used broadly to describe developmental defects in the urinary system that can occur in isolation, in combination or as a part of other syndromes. It comprises a wide spectrum of congenital defects in the urinary system, ranging from renal agenesis and hypoplasia, structural duplication, and malpositioning to defects in the ureter and bladder. It originates from the disruption of regulatory circuitry and the progenitor cells involved in the development process.[10]
Genetic, epigenetic, and environmental factors can all cause these abnormalities. Research in animal models, especially transgenic mouse models, has contributed to a better understanding of the genetic factors and mechanisms of the pathogenesis of CAKUT.
A prominent feature of the development of the urinary system is the dynamic temporal and spatial integration of progenitor tissues of distinct embryonic origins.[10]
With the widespread use of US in pregnancies the recognition of prenatal hydronephrosis has increased. Moreover, the most involved infants are asymptomatic, that support the importance of prenatal diagnosis by at least one prenatal ultrasonography.[3]
On a different study was made addressing the postnatal outcome on prenatal hydronephrosis, showed the most common cause of hydronephrosis was transient ureteropelvic junction obstruction (UPJO) in 28%, the second cause was UPJO with unilateral or bilateral in 18%. The third cause was vesicoureteral reflux in (15%). The other causes consisted of dilated ureter 8.5%, neurogenic bladder 2.5%, duplicated ureter 2%, multicystic dysplastic kidney 1.5%, and ureterocele 1%.[3]
In this study, 82.8% of cases corresponded to the antenatal diagnosis of urinary tract anomalies, while the other 17.2% of cases had different diagnosis, which was interestingly not concordant with the recent international study by Policiano et al., stating that antenatal detection of urinary tract anomalies is 11.8%.[11]
The literature up to our knowledge did not reveal other recent studies addressing the discrepancy rate of CAKUT diagnosis by US. However, there was another study conducted in late 80s showed a difference between the antenatal and postnatal CAKUT diagnosis in 35.2% of cases,[12] Added to another study emphasizing on whether normal postnatal US, as a part of a strict screening protocol for the detection and follow-up of antenatal hydronephrosis, is effectively exclude the majority of babies with congenital urinary tract abnormalities that would otherwise present with a urinary tract infection, and that study found to have a supporting results. Which means that we need to point out that these pre- and post-natal screening protocols is underestimated and it could have been of great assistance to both local and international Unmanned Aerial Systems programs.
Most of the antenatal diagnoses of CAKUT were made on the third trimester rather than early screening, which is considered an advantage as it improves accuracy.[13] The most common diagnosis that was expected is isolated hydronephrosis accounting for 60.6% of cases and a second trimester anomaly scan is important in routine antenatal care to increase the prenatal detection of fetal defects.[14]
The figures we had in our study which showed a significant increase in number of cases correspond to the antenatal diagnoses in comparison to the previous 80s Policiano et al.’s study can be explained by the difference in the study design and sample size but it implement the importance of the patient education and awareness to adherence of both antenatal and postnatal follow-up along with understanding of possible morbidity if not done.
It is crucial to mention the importance of performing the postnatal US at least after the first 24 h, due to the fact that during the early hours after birth the US images is underestimated and often unclear. The only exception is neonate with bilateral involvement, a solitary kidney, and/or a history of oligohydroamnious because they are at increased risk for a serious renal anomaly that maybe amenable to intervention.[15] Knowing that the best cutoff point of APD renal pelvis diameter that led to surgery was 15 mm, with sensitivity 88% and specificity 74%.[3]
Correspondingly, we investigated consanguinity, which accounted for 41% of study population, and which was consistent with other studies in Jeddah, Saudi Arabia,[6] especially when compared to other international publication of only being 10% of the cases reviewed.[5] Nevertheless, in order to maintain an updated methods and procedures we first thought to identify and research retrospective cases to validate changes needed. Further studies are recommended investigating the possible link between CAKUT with genetic background and the role of consanguinity, especially since only one patient in our study agreed to do antenatal chromosomal testing with understanding the possible consequent of intrauterine intervention.
CONCLUSION
The rate of discrepancy for detection of CAKUT between antenatal and postnatal US in our study was different than the recent published data. However, our results were significant in the era of antenatal diagnosis of CAKUT, despite that further prospective studies are recommended in this field for further understanding. In addition, the causes, genetics, and pathogenesis must be included and studied in depth to adequately diagnose, prepare, and evaluate the management of CAKUT, added to the fact of having a significant higher local consanguinity rate in comparison with the global population.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
This acknowledgment goes to medical records team at King Abdualziz Medical City, due to their help in extracting the files for data collection. And, our special thanks to fetal maternal unite for their assistance in the execution of the project. Furthermore, we would like to thank Rawan Algarni and Ghassan Sukkar for their help in preparing the proposal and in data collection.
REFERENCES
- 1.Carrera JM, Torrents M, Mortera C, Cusí V, Muñoz A. Routine prenatal ultrasound screening for fetal abnormalities:22 years'experience. Ultrasound Obstet Gynecol. 1995;5:174–9. doi: 10.1046/j.1469-0705.1995.05030174.x. [DOI] [PubMed] [Google Scholar]
- 2.Naglaa E, Yasser A. Neonatal Hydronephrosis With Review of Initial Ultrasound Imaging and Follow-Up Protocols. Australian Journal of Basic and Applied Sciences. 2014;8:476–83. [Google Scholar]
- 3.Sadeghi-Bojd S, Kajbafzadeh AM, Ansari-Moghadam A, Rashidi S. Postnatal evaluation and outcome of prenatal hydronephrosis. Iran J Pediatr. 2016;26:e3667. doi: 10.5812/ijp.3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pope JC, 4th, Brock JW, 3rd, Adams MC, Stephens FD, Ichikawa I. How they begin and how they end:Classic and new theories for the development and deterioration of congenital anomalies of the kidney and urinary tract, CAKUT. J Am Soc Nephrol. 1999;10:2018–28. doi: 10.1681/ASN.V1092018. [DOI] [PubMed] [Google Scholar]
- 5.Kari JA, Bockenhauer D, Stanescu H, Gari M, Kleta R, Singh AK. Consanguinity in Saudi Arabia:A unique opportunity for pediatric kidney research. Am J Kidney Dis. 2014;63:304–10. doi: 10.1053/j.ajkd.2013.08.033. [DOI] [PubMed] [Google Scholar]
- 6.Bondagji NS. Antenatal diagnosis, prevalence and outcome of congenital anomalies of the kidney and urinary tract in Saudi Arabia. Urol Ann. 2014;6:36–40. doi: 10.4103/0974-7796.127021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ouzounian JG, Castro MA, Fresquez M, Al-Sulyman OM, Kovacs BW. Prognostic significance of antenatally detected fetal pyelectasis. Ultrasound Obstet Gynecol. 1996;7:424–8. doi: 10.1046/j.1469-0705.1996.07060424.x. [DOI] [PubMed] [Google Scholar]
- 8.Ahmad G, Green P. Outcome of fetal pyelectasis diagnosed antenatally. J Obstet Gynaecol. 2005;25:119–22. doi: 10.1080/01443610500041446. [DOI] [PubMed] [Google Scholar]
- 9.Kim SY, Kim MJ, Yoon CS, Lee MS, Han KH, Lee MJ. Comparison of the reliability of two hydronephrosis grading systems:The society for foetal urology grading system vs. the onen grading system. Clin Radiol. 2013;68:e484–90. doi: 10.1016/j.crad.2013.03.023. [DOI] [PubMed] [Google Scholar]
- 10.Jain S, Chen F. Developmental pathology of congenital kidney and urinary tract anomalies. Clin Kidney J. 2019;12:382–99. doi: 10.1093/ckj/sfy112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Policiano C, Djokovic D, Carvalho R, Monteiro C, Melo MA, Graça LM. Ultrasound antenatal detection of urinary tract anomalies in the last decade:Outcome and prognosis. J Matern Fetal Neonatal Med. 2015;28:959–63. doi: 10.3109/14767058.2014.939065. [DOI] [PubMed] [Google Scholar]
- 12.Scott JE, Renwick M. Antenatal diagnosis of congenital abnormalities in the urinary tract. Results from the Northern Region Fetal Abnormality Survey. Br J Urol. 1988;62:295–300. doi: 10.1111/j.1464-410x.1988.tb04351.x. [DOI] [PubMed] [Google Scholar]
- 13.Tabel Y, Haskologlu ZS, Karakas HM, Yakinci C. Ultrasonographic screening of newborns for congenital anomalies of the kidney and the urinary tracts. Urol J. 2010;7:161–7. [PubMed] [Google Scholar]
- 14.Carvalho MH, Brizot ML, Lopes LM, Chiba CH, Miyadahira S, Zugaib M. Detection of fetal structural abnormalities at the 11-14 week ultrasound scan. Prenat Diagn. 2002;22:1–4. doi: 10.1002/pd.200. [DOI] [PubMed] [Google Scholar]
- 15.Laing FC, Burke VD, Wing VW, Jeffrey RB, Jr, Hashimoto B, et al. Postpartum evaluation of fetal hydronephrosis:Optimal timing for follow-up sonography. Radiol. 1984;152:423–4. doi: 10.1148/radiology.152.2.6539930. [DOI] [PubMed] [Google Scholar]


