Abstract
One of the major challenges during glioblastoma surgery is balancing between maximizing extent of resection and preventing neurological deficits. Several surgical techniques and adjuncts have been developed to help identify eloquent areas both preoperatively (fMRI, nTMS, MEG, DTI) and intraoperatively (imaging (ultrasound, iMRI), electrostimulation (mapping), cerebral perfusion measurements (fUS)), and visualization (5-ALA, fluoresceine)). In this review, we give an update of the state-of-the-art management of both primary and recurrent glioblastomas. We will review the latest surgical advances, challenges, and approaches that define the onco-neurosurgical practice in a contemporary setting and give an overview of the current prospective scientific efforts.
Keywords: glioblastoma, intraoperative mapping, imaging, preoperative mapping, review
Glioblastoma (grade IV astrocytoma) is the most common form of primary brain malignancy in adults. Patients face a dim prognosis of approximately 16 months, which has not significantly improved over the last 15 years.1 Standard therapy includes resection followed by adjuvant chemoradiation, which can be administered in various ways dependent on the patient’s age and performance (Stupp protocol, Perry protocol).2,3
One of the most important factors in determining the patient’s prognosis is surgery (the extent of resection).4–6 First, glioblastoma patients who have undergone tumor resection experience on average a longer overall survival than those who have undergone tissue biopsy.6 Second, the extent of resection (EOR) in surgery plays a major role, since higher EOR percentages correlate with better survival outcomes.4,5
Due to their invasive nature, glioblastomas infiltrate the surrounding parenchyma and despite a gross-total resection, recurrence is inevitable. Still, neurosurgeons aim to safely resect as much tumor tissue as possible, often striving for complete resection of the contrast-enhancing (CE) part of the tumor on MR-imaging, adhering to the fact that complete resection of the contrast-enhancing tumor has shown to convey a survival benefit.7 Recent evidence suggests that it might be beneficial to expand the resection to the noncontrast-enhancing (NCE) part as well in two distinct subgroups of patients: (1) patients with IDH wildtype tumors, regardless of MGMT methylation status and (2) in younger patients, regardless of IDH status.8
Since >50% of glioblastomas are located in or near eloquent areas, aggressive resection has the potential to lead to postoperative neurological deficits, thereby severely harming the patient’s quality of life (QoL) and functioning.4–6 In order to preserve the patient’s quality of life (and protect neurological functioning), while maximizing the extent of resection, several preoperative and intraoperative methods have been developed to help the surgeon balance between these two—sometimes conflicting—goals. The postoperative functioning is of utmost importance, since suboptimal postoperative QoL or KPS negatively impact survival chances of glioblastoma patients.9
In this review, we will briefly elaborate on the standard of care for both primary and recurrent glioblastoma. We will describe the recent advances in the surgical management of glioblastoma patients and the current challenges neurosurgeons are facing. We will discuss both grade 4 astrocytoma and glioblastoma, according to the 2021 WHO classification (formerly known as IDHmt and IDHwt glioblastoma in the 2016 WHO classification). Various surgical techniques will be discussed as well as the use of intraoperative imaging and surgical adjuncts. At last, we will provide an overview of the studies that have recently been completed, are currently active, or are prospectively planned. Nonsurgical adjuncts for glioma resections such as LITT (laser interstitial thermal therapy), OCT (optical coherence tomography), mass spectrometry, and tumor treating fields (TTF) are outside the scope of this paper.
Contemporary Management of Glioblastoma
Glioblastomas can be divided in primary, secondary, and recurrent glioblastomas. Standard of care for primary glioblastoma consists of maximal safe resection followed by adjuvant chemoradiotherapy.2,3 Extent of resection (EOR), expressed as the percentage of tumor resected or postoperative residual tumor volume, has shown to be a prognostic factor.4–6 Generally, a distinction can be made between subtotal (STR) versus near-total (NTR) versus gross-total resections (GTR), but there is no consensus of standard, validated cutoff values for STR, NTR, and GTR for neither extent of resection or residual tumor volume. Other well-known prognostics include age, preoperative patient functioning (Karnofsky Performance Scale, KPS), and molecular status (MGMT and IDH).2,10–13
With very rare exceptions, these tumors regrow and no explicit standard-of-care exists at recurrence. Viable treatment options include, but are not restricted to: re-resection, re-irradiation, re-challenge TMZ, second-line chemotherapy (Lomustine), or experimental study treatments, dependent on the patient’s clinical performance.14
Previous randomized controlled trials with second-line drug regimens including i.a. anti-VEGF (Bevacizumab, Cediranib),15–17 anti-TGFβ-receptor-I (Galunisertib),18 TKI-inhibitor (Axitinib),19 anti-receptor tyrosine kinase (RTK) (Regorafenib),20 anti-protein kinase C (PKC) (Enzastaurin)21 and anti-EGFR (Depatux-M)22 failed to show significant outcome improvements.
Brain Mapping
A substantial portion of glioblastomas is located in or near eloquent areas, which can affect the patient’s neurological functioning. Eloquent brain areas include the bilateral frontal motor areas (cortical structures such as the primary motor cortex, premotor cortex, and the supplementary motor cortex, and subcortical structures such as the corticospinal tract, arcuate fasciculus, inferior fronto-occipital fasciculus, and internal capsule), the bilateral parietal somatosensory areas (postcentral gyrus), the bilateral primary visual cortex in the occipital lobes, and the speech areas of Broca and Wernicke in respectively the left frontal and temporal lobes.23
Resection of tumors in these areas proves to be challenging, since the exact location of eloquent areas differs between patients. Furthermore, delineaton of glioblastoma is often difficult due to their invasiveness. An accurate and reliable method to differentiate eloquent brain areas from both noneloquent areas and tumor tissue is therefore necessary. Since extent of resection is important for the patient’s survival, maximizing the percentage of tumor resected (minimizing the residual tumor volume) is one of the most important goals of glioblastoma surgery. For this purpose, brain mapping is one of the most commonly used methods. Brain mapping can be performed both preoperatively (nTMS, MEG, DTI, fMRI) and intraoperatively (awake mapping or asleep mapping). Motor and somatosensory mapping can be performed both awake and asleep, while speech function (Broca’s area and Wernicke’s area) can only be tested while the patient is awake.
Preoperative Brain Mapping
Four modalities are mainly used for the preoperative brain mapping in glioma and glioblastoma resections: nTMS (navigated transcranial magnetic stimulation), MEG (magnetoencephalography), fMRI (functional MRI), and DTI (diffusion tract imaging).
nTMS stimulates the brain with transcranial magnetic pulses, thereby creating a cortical electrical field that leads to neuronal stimulation or inhibition. The obtained results are then paired with the neuronavigation system, in order to combine the information regarding functional areas with the raw MRI images for intraoperative assessment. Neuronal stimulation can be achieved by a single magnetic pulse, while a repetitive pulse causes inhibition of the cortical area. nTMS is most frequently used for motor mapping,24 but retrospective evidence regarding its use for language mapping is reported as well.25,26 To reduce TMS-noise in TMS-based language mapping, automated speech algorithms have been built for which proof of concept has been established.27 A major factor of concern is the correlation between functional areas identified preoperatively by nTMS and the respective identification of these areas by direct electrostimulation intraoperatively. A recent meta-analysis by Jeltema et al. demonstrates that the average correlation between these two modalities is between 2 and 16 mm,28 but most articles found <10 mm achievable. Moreover, they found that the validity of nTMS for language mapping varied greatly when compared with DES: sensitivity differed between 10 and 100%, specificity from 13.3–98%, negative predictive value from 57 and 100%, and positive predictive value between 17 and 75%.28
The group in Munich has done extensive work on the use of nTMS in glioma surgery.26,29–31 In a retrospective 2015 paper, they found that, in comparison with the non-nTMS group, nTMS was associated with a smaller size of the craniotomy, less residual tumor tissue, shorter length-of-stay, increased proportion of patients receiving adjuvant therapy and improved survival at 3, 6, and 9 months in glioblastoma patients. No significant difference was found for surgery-induced neurological deficits.26 In contrast, Frey et al found in a prospective cohort of 250 glioma patients significant less postoperative deficits in the nTMS group than in the control group (8.5% vs. 6.1%) as well as a higher proportion of gross-total resections (59% vs. 42%).32 In 2013, Picht et al prospectively compared nTMS with DES during awake craniotomy in 20 patients with language-eloquent gliomas in a collaborative study of the Berlin and the Munich groups.33 They reported a sensitivity and negative predictive value of 100% for Broca’s area for nTMS, even though its reliability and specificity in Wernicke’s area proved to be rather limited. Moreover, they found that on a total of 10 glioblastoma patients, 6 patients maintained their preoperative speech functionality, 3 patients had an improvement and the aphasia of 1 patient was permanently worsened at 3 months postoperatively. For motor-eloquent gliomas, the Leuven group retrospectively developed a realistic electric field-based model of nTMS outperforming the point-cloud models in term of prediction of motor responses intraoperatively.34
Thus, nTMS can be used for mapping of primary motor areas during motor-eloquent glioblastoma resections. Though, due to uncertainties of nTMS and possible intraoperative confounding factors (such as brain shift), real-time intraoperative monitoring control is warranted for maximal safety. In language-eloquent gliomas, nTMS is mainly used for the preoperative surgical planning and should be mainly used as an adjunct next to conventional DES to map and resect these tumors adequately.
We searched the United States National Library of Medicine and National Institute of Health Trial Register (clinicaltrials.gov), the EU Clinical Trials Register, the Netherlands Trial Register (NTR), and the ISRCTN register for recently completed trials (between 1 January 2018 and 1 November 2020), currently active trials and planned trials evaluating the surgical management for primary or recurrent glioma. We found that the use of nTMS in motor-eloquent gliomas is currently evaluated by the Munich group in a quadruple-blinded RCT including 330 patients, comparing nTMS-guided resections with conventional resections with postoperative neurological deficits at 3 months as primary outcome (still accruing without current results, Table 1).
Table 1.
Current Prospective Surgical Studies in Glioma Patients
| Study | Register | Design | Population | Intervention | Control | Primary outcome | Initiating center | Status | Timespan |
|---|---|---|---|---|---|---|---|---|---|
| General | |||||||||
| RESURGE: Randomized Controlled Comparative Phase II Trial on Surgery for Glioblastoma Recurrence | NCT02394626 | Randomized controlled trial, open label, parallel, 120 patients | Recurrent GBM | Resection followed by adjuvant second-line therapy | Adjuvant second-line therapy | Overall survival | Inselspital Bern (SUI) | Active, recruiting | 1 May 2015–1 Oct 2021 |
| Supramarginal Resection in Patients With Glioblastoma: A Randomized Controlled Trial | NCT04243005 | Randomized controlled trial, double-blinded, parallel, 90 patients | GBM | Supramarginal resection with >10mm margin on T2 MRI | Conventional resection | Overall survival | St. Olav’s University Hospital Trondheim (NOR) | Active, recruiting | 1 Jul 2020–1 Mar 2027 |
| Assessing Impact of Surgically-induced Deficits on Patient Functioning and Quality of Life (SIND Study) | NCT04007185 | Prospective cohort study, 150 patients | High-grade glioma | Maximum safe resection | Biopsy | Impact of new deficit on quality of life (EORTC QLQ-30 and BN20) | Cambridge University Hospitals NHS Foundation Trust (UK) | Not yet recruiting | 1 Feb 2020–1 Dec 2024 (Estimated) |
| Intraoperative mapping | |||||||||
| The SAFE-trial: Safe Surgery for Glioblastoma Multiforme: Awake Craniotomy versus Surgery Under General Anesthesia. A Multicenter Prospective Randomized Study | NCT03861299 | Randomized controlled trial, open label, parallel, 246 patients | Primary, eloquent GBM | Awake craniotomy | Resection under general anesthesia | Proportion of gross-total resections, postoperative neurological morbidity | Erasmus MC Rotterdam (NL) | Active, recruiting | 1 Apr 2019–1 Apr 2024 |
| Awake vs. Asleep Craniotomy for Noneloquent Gliomas | NCT03621748 | Randomized controlled trial, single-blinded, parallel, 50 patients | Primary, noneloquent glioma | Awake craniotomy | Resection under general anesthesia | Extent of resection | Mayo Clinic Jacksonville (FL, USA) | Active, recruiting | 1 Jun 2020–1 Dec 2022 |
| The PROGRAM-study: Awake mapping versus asleep mapping versus no mapping for glioblastoma resections | NCT04708171 | Prospective cohort study, open label, parallel, 453 patients | High-grade glioma | Awake or asleep mapping | Conventional resection | Extent of resection, postoperartive neurological morbidity | Erasmus MC Rotterdam (NL) | Active, recruiting | 1 April 2022-1 April 2027 (Estimated) |
| Preoperative mapping | |||||||||
| The Application of ZOOMit-fMRI to Identify Motor Functional Cortex | NCT03091270 | Prospective case-crossover study, 60 patients | Motor-eloquent gliomas | ZOOMit-fMRI-guided resection | BOLD-fMRI-guided resection | Accuracy of motor cortex localization | Beijing Neurosurgical Institute (CHN) | Active, recruiting | 1 Feb 2016–1 Jan 2025 |
| nTMS for Motor Mapping of Rolandic Lesions | NCT02879682 | Randomized controlled trial, quadruple-blinded, parallel, 330 patients | Motor-eloquent gliomas | nTMS-guided resection | Conventional resection | Postoperative neurological deficits at 3 months | Technical University Munich (GER) | Active, recruiting | 1 Aug 2016–1 Feb 2022 |
| Safety and Feasibility of Preoperative and Intraoperative Image-Guided Resection of Gliomas | NCT03542409 | Nonrandomized clinical trial, open label, parallel, 40 patients | Primary glioma | Preoperative and intraoperative 2HG spectroscopy | Preoperative and intraoperative MR perfusion | Intraoperative imaging completion, postoperative complications | University of Utah (UT, USA) | Active, recruiting | 6 Feb 2017–6 Feb 2023 |
| Predicting Sites of Tumour Progression in the Invasive Margin of Glioblastomas (PRaM-GBM Study) | NCT03294434 | Prospective cohort study, 120 patients | High-grade glioma | Resection with DTI | NA | Site of GBM true progression correctly predicted by DTI | Cambridge University Hospitals NHS Foundation Trust (UK) | Active, recruiting | 2 Mar 2017–30 Sep 2021 |
| Resting-State Functional MRI in Glioma Patients Before and After Surgery | NCT03964909 | Single-arm clinical trial, open label, 30 patients | Speech-eloquent primary glioma | fMRI, CVR MRI or rs-fMRI | NA | Detectability of language networks | M.D. Anderson Cancer Center (TX, USA) | Active, recruiting | 24 Apr 2017–12 May 2022 |
| Intraoperative fluorescence and imaging | |||||||||
| 5-Aminolevulinic Acid (5-ALA) to Enhance Visualization of Malignant Tumor | NCT02632370 | Prospective cohort study, 69 patients | Primary or recurrent glioma | 5-ALA guided resection | NA | Incidence of diagnostic tissue presence | Mount Sinai (NY, USA) | Completed | 1 May 2016–31 Dec 2018 |
| Intraoperative Ultrasound guided Glioma Surgery: a Randomized, Controlled Trial (US-GLIOMA) | NCT03531333 | Randomized controlled trial, single-blinded, parallel, 50 patients | Primary high-grade glioma | Resection with intraoperative ultrasound | Resection without intraoperative ultrasound | Proportion of patients with gross-total resection | Erasmus MC Rotterdam (NL) | Completed | 1 Nov 2016–1 Aug-2020 |
| Interest of Fluorescein in Fluorescence-guided Resection of Gliomas (FLEGME study). | NCT03291977 | Randomized controlled trial, open label, parallel, 62 patients | GBM | Resection with fluorescein | Conventional resection | Proportion of gross-total resections | Rennes University Hospital (FRA) | Active, recruiting | 5 Oct 2017–1 Oct 2021 |
| Quantification of ALA-induced PpIX Fluorescence During Brain Tumors Resection | NCT02191488 | Single-arm nonrandomized clinical trial, open label, 540 patients | Primary or recurrent glioma | 5-ALA guided resection | NA | Intraoperative PpIX measurements vs coregistered histopathology | Dartmouth-Hitchcock Medical Center (NH, USA) | Active, not recruiting | 1 Jul 2014–1 Jul 2021 (Estimated) |
| Diagnostic Performance of Fluorescein as an Intraoperative Brain Tumor Biomarker | NCT02691923 | Randomized controlled trial, open label, parallel, 30 patients | Primary glioma | Fluorescein+5-ALA guided resection | Fluorescein-guided resection | Fluorescein performance | Dartmouth-Hitchcock Medical Center (NH, USA) | Active, not recruiting | 1 Mar 2016–1 Dec 2021 (Estimated) |
| Improving Fluorescence-guided Brain Tumour Surgery With Ultra-high Sensitivity Imaging | NCT04556929 | Single-arm clinical trial, open label, 20 patients | Primary glioma | 5-ALA guided resection, biopsies from resection cavity | NA | Level of tumor fluorescence in images of resection cavity captured during surgery | Oxford University Hospitals NHS Foundation Trust (UK) | Not yet recruiting | 1 Oct 2020–1 Aug 2022 (Estimated) |
| Stereotactical Photodynamic Therapy With 5-aminolevulinic Acid (Gliolan) in Recurrent Glioblastoma | NCT04469699 | Randomized controlled trial, open label, parallel, 106 patients | Recurrent GBM | Biopsy followed by photodynamic therapy (PDT) with 5-ALA | Biopsy | Progression-free survival | University Hospital Münster (GER) | Not yet recruiting | 1 Nov 2020–1 Nov 2025 (Estimated) |
| Impact of iMRI on the Extent of Resection in Patients with Newly Diagnosed Glioblastomas | NCT02379572 | Nonrandomized clinical trial, single-blinded, parallel, 315 patients | Primary GBM | Resection with iMRI guidance | Resection with 5-ALA guidance | Proportion of gross-total resections | University Hospital Tübingen (GER) | Active, recruiting | 1 Jun 2015–1 Jun 2021 |
| FUTURE-GB study: Functional and ultrasound-guided resection of glioblastoma | ISRCTN38834571 | Randomized controlled trial, open label, parallel, 357 patients | Primary GBM | 5-ALA, DTI, and US guided resection | 5-ALA guided resection | Quality of life, overall survival, progression-free survival | Oxford University Hospitals NHS Foundation Trust (UK) | Active, recruiting | 1 Apr 2020–30 Nov 2025 |
| 3.0T High-field Intraoperative MRI Guided Extent of Resection in Cerebral Glioma Surgery: a Single Center Prospective Randomized Triple-blind Controlled Clinical Trial | NCT01479686 | Randomized controlled trial, triple-blinded, parallel, 321 patients | Primary glioma | 3.0T iMRI-guided resection | Conventional neuronavigation-guided resection | Extent of resection | Fudan University Shanghai (CHN) | Active, not recruiting | 1 Sept 2011–1 July 2021 (Estimated) |
MEG (magnetoencephalography) is a comparatively new mapping tool, which detects magnetic fields that are elicited by neuronal electrical currents in order to delineate functional from nonfunctional brain areas. MEG identifies functional areas before the operation based on task-based activity, similar to fMRI. Zimmerman et al retrospectively compared MEG with fMRI for localization of functional perirolandic areas in 13 patients with gliomas, AVMs, and hemangiomas.35 They found a solid congruency between both modalities with an average spatial distance of 10 mm. In a 2012 paper, Tarapore et al retrospectively compared MEG and nTMS with intraoperative DES in 24 glioma patients.36 They reported that the average distance between the nTMS and DES motor-eloquent sites was 2.1 mm and between nTMS and MEG 4.7 mm. nTMS was deemed reliable for negative mapping: no motor sites that were identified as negative by nTMS were found positive for motor function during intraoperative DES. Of the 7 glioblastoma patients included, only 1 patient experienced a minor postoperative deficit of the right arm (MRC grade 4 paresis).
More recently, Traut et al reported on the use of MEG for evaluating neuroplasticity and language organization after glioma surgery.37 They concluded that functional reorganization is present in most glioma patients postoperatively, more so in patients who had undergone resection of tumors in the language-dominant hemisphere.
One of the major drawbacks of MEG is the cost of the necessary equipment and the need for a dedicated setting with adequate expertise. Consequently, this modality is still scarcely used despite its potential in clinical practice.
DTI (diffusion tract imaging) is used for white-matter fiber tracking based on diffusion-weighted imaging (DWI) MRI sequences. Four tracts are commonly visualized by DTI: the corticospinal tract (CST), arcuate fasciculus (AF), optic radiation (OR), and inferior fronto-occipital fasciculus (IFOF). DTI is based on the anisotropy (diffusion varies with direction) of water molecules, thereby deriving the precise direction of the axons within every voxel. The white matter tracts can be derived from the magnetic gradients of all voxels combined, indicating the orientation of single fibers. FA (fractional anisotropy) is the most frequently used method to measure these gradients. When these measurements are combined with anatomical ROIs (regions-of-interest), a 3D map of the four tracts mentioned above can be incorporated in weighted MR-images to visualize the specific, individual trajectory in which the color represents the orientation of the most dominant eigenvector of that particular voxel. It, therefore, supplies information regarding displacement, disruption and infiltration of the white matter with the concurrent presence or absence of edema. Therefore, DTI is often used in glioblastoma patients as a tool for preoperative surgical planning,38 outcome prediction,39,40 and intraoperative decision making.41,42
Sensitivity and specificity of DTI in comparison with DES are >90% but it suffers from important limitations.43 Since there is no standard protocol for DTI (e.g., selecting ROIs and fiber tracking), external generalizability, precision, and accuracy can be adversely affected. Furthermore, it is susceptible to challenges that are common to preoperatively conducted imaging such as unreliable spatial congruency due to brain shift. Last, an important inherent limitation of DTI is commonly described as the “crossing fiber problem”, for which DTI has a very limited visualization accuracy. Advanced DTI techniques such as HARDI q-ball imaging have been tested. Although they are effective in identifying language tracts preoperatively and in predicting functional outcome postoperatively, they generally suffer from the same limitations as standard DTI.44 New techniques such as CSD (constrained spherical deconvolution), DKI (diffusional kurtosis imaging), and DSI (diffusion spectrum imaging) show promising results and are potentially more adept at improving reproducibility and intraoperative accuracy.45–47
Two British studies are currently investigating the use of DTI in glioma patients in the PRaM-GBM study (Cambridge) and the FUTURE-GBM study (Oxford) (Table 1).
fMRI (functional MRI) identifies eloquent areas based on task paradigms and consequently increased levels of blood oxygen in the respective functional areas as a surrogate for increased neuronal activity. BOLD (blood oxygen level-dependent) MRI sequences are used as contrast images. The correlation between fMRI-identified eloquent areas is high with Wada testing but not always with direct electrostimulation, with considerable variances being found in different retrospective and review studies.48,49 Moreover, fMRI has been shown to suffer from suboptimal specificity caused by neurovascular uncoupling. This can occur due to disruption of regular white matter perfusion as caused by intraparenchymal tumors.50–52 fMRI-based detection of eloquent areas can therefore only be used as a surgical adjunct and remains heavily reliable on confirmation by intraoperative methods. As of now, the Beijing Neurosurgical Institute and the M.D. Anderson Cancer Center are prospectively evaluating the use of fMRI in glioma patients (Table 1).
Intraoperative Brain Mapping: Awake and Asleep
Motor mapping can be performed when the patient is awake (awake craniotomy under local anesthesia) or asleep (general anesthesia). Cortical stimulation of the motor areas can be performed with two methods: direct electrostimulation (DES) with a handheld probe or the usage of a subdural grid with strip (grid) electrodes (adjacent to the central sulcus).53,54 DES in its turn can be performed with the low-frequency technique, in which a stimulator with a 50-Hz (Europe) or 60-Hz (USA, Canada) frequency is used for functional localization, or with the high-frequency technique (train-of-five stimulation).53,55 Both the low-frequency technique and the high-frequency technique can be carried out safely with a monopolar or bipolar stimulation device. The stimulation intensity of the device ranges between 1 and 20 mA with increasing steps of 0.5–1.0 mA. Subcortical motor mapping can be achieved by DES with a handheld probe with similar or slightly adjusted stimulation settings. Gogos et al recently reported on their prospective study evaluating “triple motor mapping” (transcranial, bipolar, and monopolar), in which they found that monopolar high-frequency stimulation was more effective at identification of subcortical motor pathways (86.4% of cases) than bipolar stimulation (10.2% of cases).56
The identification of motor-eloquent areas under awake circumstances differs from mapping when the patient is asleep. During awake mapping, motor function is assessed by the involuntary movement (positive response) or impaired motor function (negative response) of muscles in the face, arm, or leg. In contrast, during asleep mapping, MEPs (motor-evoked potentials) are used to assess the integrity of cortical motor structures and its descending subcortical tracts.57 Evoked potentials are recorded with the use of EMG needle electrodes in the contralateral extremity. Generally, reduction of the amplitude of the evoked potentials of more than 50% or the necessity to increase the stimulation current significantly represent clinically significant changes. Amplitude reductions can be reversible, which generally are a sign of temporary motor deficits, and irreversible, rather suggesting new motor deficits.58,59
Speech mapping can be performed only when the patient is awake. Cortical stimulation near speech areas is performed most commonly with the use of a bipolar stimulator with the electrodes 0.5 cm apart. The surgeon usually starts with a low stimulus between 1.0 and 2.0 mA and maps the cortex for 2 seconds every 0.5–1.0 cm. Positive or negative stimulation sites are noted and eloquent areas are avoided. Frequently used tests for language function include the Boston naming test, Token test, semantic associations, counting, verb generation, and word fluency.59 The surgeon maps the surface various times with increasing currents. Subcortical stimulation of language-associated fibers can be performed similarly (Figure 1).53,59
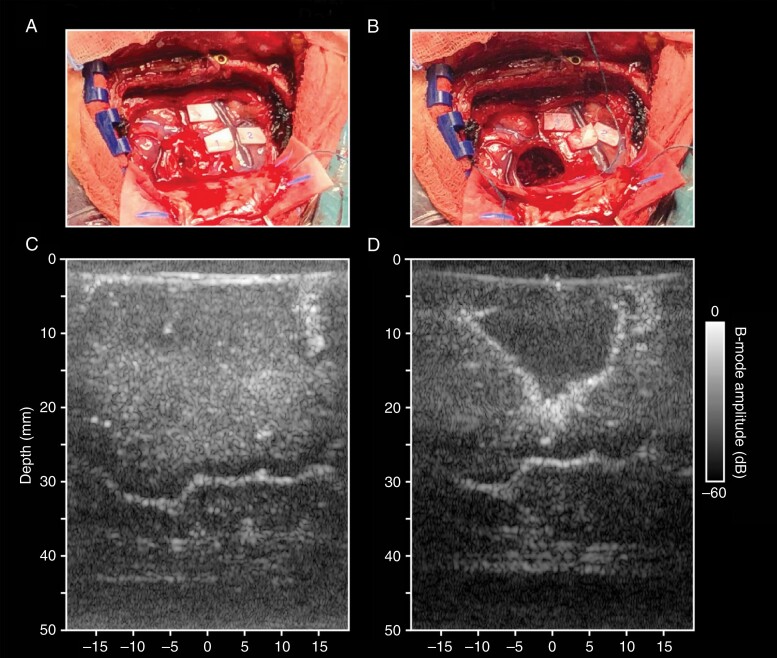
Figure 1.
Intraoperative ultrasound. A: Intraoperative image of a glioma in the right parietal lobe. B: Intraoperative image of the cavity after tumor resection. C: Pre-resection B-mode image of the tumor and surrounding tissue. D: Post-resection B-mode ultrasound image of the resection cavity.
One of the most promising new awake mapping techniques includes functional ultrasound (fUS). fUS uses Doppler ultrasound images to detect changes in brain tissue perfusion while the patient carries out certain motor or linguistic tasks, allowing the surgeon to identify eloquent areas based on a vascular, rather than a mechanical basis. Advantages of fUS include its high spatiotemporal resolution, wide field of view, high depth penetration, and its low-cost of implementation. Imbault et al described this technique in 2017 as a proof-of-principle, using fUS to successfully identify eloquent areas in all 28 low-grade glioma patients.60 In 2020, the Rotterdam group published their experience with using fUS during awake surgery in 10 low-grade and high-grade glioma patients. They demonstrated with this prospective study that fUS can be used to map both motor and language function accurately.61
New developments in asleep mapping techniques led to the progression towards continuous monitoring of the motor structures’ integrity with a technique called continuous dynamic mapping (CDM). This technique utilizes a monopolar probe at the tip of the suction device and has been pioneered by the team from Bern. Thanks to the known current-distance relationship of monopolar stimulation, the surgeon can resect tumor tissue close to motor pathways with stepwise decreasing stimulation intensity while continuously being guided by the different sounds of the device (indicating the distance to the motor fibers).62 Subcortical mapping is performed using a monopolar with the train-of-five technique with a 0.5 ms pulse duration, an interval of 4 ms, and an intensity ranging from 1 to 20 mA. Recently, they published their update on the CDM technique in 182 patients with intra-axial tumors within 1 cm of the CST.63 Six of those patients (3%) had a permanent motor decrease of 0.5 points or more on the MRC scale: half of them were due to ischemic injury, half of them were due to mechanic injury (1.7%).63 CDM can therefore be deemed as a very safe, feasible, and intuitive alternative for conventional asleep mapping methods in order to prevent neurological deficits after motor-eloquent glioma surgery.
The benefit of brain mapping in glioma surgery has been demonstrated by various groups. Sanai et al published in 2008 a large well-known study investigating 245 patients undergoing awake craniotomy (AC) for speech-eloquent gliomas.53 They found that the use of AC permits the surgeon to maximize extent of resection while minimizing language deficits: the incidence of permanent language deficits after 6 months was 1.6% with a mean extent of resection of 69.0% among glioblastoma patients. In 2011, Sacko et al prospectively compared awake craniotomy with surgery under general anesthesia for resections of supratentorial lesions in a prospective setting.64 They included 575 patients with gliomas, metastases, cavernous malformations, and meningiomas, and found that patients who had undergone awake craniotomy had better postoperative neurological outcomes and increased extent of resection rates. They observed permanent postoperative neurological deficits in 4.6% of patients operated with awake craniotomy and in 16% of patients operated under general anesthesia. De Witt Hamer et al published their landmark paper in 2012, evaluating the impact of intraoperative stimulation mapping (ISM) in a meta-analysis including 90 papers covering a total of 8,091 patients.65 They found that resections with mapping led to fewer late severe neurologic deficits (3.4% vs. 8.2%) and were simultaneously more extensive (GTR in 75% vs 58%). These results were in line with the meta-analysis of Gerritsen et al published in 2018 which evaluated the impact of mapping techniques in high-grade glioma specifically.66 They found that ISM-led resections were associated with improved overall survival (16.9 months in the ISM group vs. 12.0 months in the GA group), less postoperative complications (13% vs. 21%), and a higher incidence of GTR (79% vs 48%).
Awake mapping has several limitations. First, reliable mapping information often can be obtained only when patients have near-intact or intact function of language or motor-based tasks. Function impairments can hamper the reliability of the procedure which can harm the accuracy and precision of the mapping. Second, awake craniotomies are known to have the potential to cause after-discharges (ADs—stimulation-induced epileptic discharges) and stimulation-evoked seizures.67 ADs can be recorded with EEG or ECoG and are electro-encephalographic alterations after electrostimulation that are similar to seizures or can progress into them.68 Intraoperative seizures can be managed by applying ice-cold saline to the exposed brain surface, administration of anti-epileptic drugs (AEDs), benzodiazepines, propofol, or even by terminating the mapping procedure and continuing the resection under general anesthesia.69,70 However, intraoperative stimulation-evoked seizures tend to not occur if the current is low (i.e., 2–2.5 mA). Third, extreme obesity could interfere with a safe airway surveillance and is, therefore, an important anesthesiological contraindication for awake craniotomies. Last, false positive findings during intraoperative stimulation can occur due to mental fatigue of patients during long procedures which may challenge the interpretation of the patient’s performance and the identification of eloquent areas, consequently.
There is no general consensus regarding mapping techniques and procedures. A 2014 survey evaluating stimulation mapping techniques in epilepsy surgery found a wide range of local paradigms.71 Though, the inconsistencies between centers and countries in glioma mapping are virtually unknown at this moment. For example, the choice between awake mapping and asleep mapping is largely based on the surgeon’s expertise, as is the preference for DES versus subdural grid electrodes, bipolar versus monopolar probe, the current’s range and increasing steps, the assessment of motor and speech function during awake craniotomy (neurophysiologist/neuro-linguist vs. trained assessor vs. patient himself/herself), the use of ECoG or intraoperative EEG to detect epileptic activity intraoperatively, the use of additional surgical adjuncts during mapping procedures such as 5-ALA, DTI, ioMRI, and ultrasound; and the anesthesia technique during awake craniotomy (awake-awake-awake versus asleep-awake-asleep or asleep-awake-awake) for example. Moreover, one of the most challenging parts of mapping techniques during glioma surgery is the decision-making process, i.e., on which information the decision to alter the surgical strategy or to end the resection is based. For many surgeons, this decision frequently is based on the combination of multiple concurrent information sources: the patient’s task performance (during awake craniotomy), the evoked potentials’ amplitude (during asleep mapping), the imaging (neuronavigation with or without DTI), and the macroscopy (expertise and fluorescence). To gain understanding in the local techniques and procedures that are used for glioma resections in different centers and countries, the ENCRAM Consortium has carried out two international surveys evaluating this inter-center variability in mapping procedures and decision making.72,73 Together with large, well-designed prospective studies, the results from this survey may be the first step towards reaching a general consensus regarding the use of these techniques in glioblastoma patients.
Currently, three prospective clinical studies are currently evaluating the use of intraoperative mapping techniques in glioma patients: two randomized controlled trials (RCT): a large one in the Netherlands and Belgium (SAFE trial, 246 patients) and a smaller one at the Mayo clinic (50 patients); and one prospective cohort study from the transatlantic ENCRAM Consortium (PROGRAM study) (Table 1).74
Intraoperative Fluorescence and Imaging
Three main tools are used during surgery to increase the extent of resection and minimize residual tumor volume: fluorescence (including 5- aminolevulinic acid (5-ALA) and fluorescein), ultrasound, and intraoperative MRI (ioMRI).
The use of 5-ALA (Gliolan®), a precursor of hemoglobin, results in the accumulation of fluorescent porphyrin IX in cells lacking ferrochelatase (e.g. glioblastoma cells) and is therefore used to visualize tumor cells in vivo with the use of an adjusted neurosurgical microscope. Another fluorescence agent, (sodium) fluoresceine, designed to be an intravascular fluorophore, passes the (dysfunctional) blood-brain barrier in glioma patients, as opposed to the intratumoral synthesis of 5-ALA.
Fluorescence is mainly used to increase extent of resection in glioma surgery. However, the ultimate goal is maximizing EOR while minimizing postoperative deficits. Stummer et al, found that GTR was confirmed in 65% of the patients in the 5-ALA group which was a significantly higher proportion than in the white light group (36%).75 Moreover, the 5-ALA group had a higher progression-free survival at 6 months postoperatively (41% vs. 21%). Although their study was not powered for overall survival, they found that the 5-ALA group had a nonsignificant shorter OS than the white-light group (13.5 months vs. 15.2 months, P = .1). Notably, in 2011 a supplemental analysis was published which showed that patients in the 5-ALA group had more early postoperative neurological deficits.76 Forty-eight hours after surgery, the proportion of patients with NIHSS (National Institute of Health Stroke Scale) deterioration of 1 point of more in the 5-ALA group was 26.2% versus 14.5% of patients in the white light group. After 6 weeks, this was decreased to 17.1% in the 5-ALA group and 11.3% in the white light group (P = .29) and 3 months postoperatively, the difference was negligible between groups (19.6% in the 5-ALA group and 18.6% in the white light group, P = .77). KPS deterioration did not differ significantly between groups during follow-up. They concluded that a postoperative transient deficit weighs up against the long-term benefits of using 5-ALA (longer PFS, higher chance of GTR).75,76 Since then, various studies have demonstrated the benefit of 5-ALA among different subgroups of brain tumor patients.77–79 However, the differentiation between tumorous and healthy tissue in the marginal area of the tumor remains a common challenge during 5-ALA guided resections.80 Since the levels of fluorescence are much lower in this area, the delineation between different tissues is obscured which makes 5-ALA guided resections somewhat subjective to the surgeon’s expertise. Objective quantification remains therefore moderately limited. Another major limitation of 5-ALA is the lack of guidance in the resection of the noncontrast-enhancing part of the tumor, which has recently been shown to be of utmost importance in glioma surgery. Molinaro et al from the UCSF group demonstrated in a large retrospective cohort of 761 patients that maximum resection of the noncontrast-enhancing part of the tumor leads to increased overall survival, regardless of their IDH status.8
A recent study by Hansen et al retrospectively compared the use of 5-ALA with fluorescein during high-grade glioma resections,81 which showed no difference regarding mean extent of resection (96.9% in the 5-ALA group, 97.4% in the fluorescein group), the proportion of patients with GTR (defined as residual tumor volume of <0.175m3; 29.5% in the 5-ALA group and 36.2% in the fluorescein group), median overall survival (14.8 months in the 5-ALA group and 19.7 months in the fluorescein group) or median progression-free survival (8.7 months in the 5-ALA group and 9.2 months in the fluorescein group).
Two prospective studies have investigated the use of yellow fluorescein in high-grade glioma patients. Falco et al reported on their preliminary results of the FLUOCERTUM study, in which they found a 74.2% rate of GTR in their high-grade glioma subgroup of 128 patients.82 Acerbi et al found in their FLUOGLIO study that GTR was achieved in 82.6% of their HGG patients (n = 57).83 Moreover, 6-month PFS was 56.6%, 12 month PFS was 15.2% and median overall survival was 12 months.
Recently, Schipmann et al reported on the combined use of 5-ALA and photodynamic therapy (PDT) in a prospective cohort study in recurrent high-grade glioma patients.84 The accumulated porphyrins caused by 5-ALA are both fluorescence agents and photosensitizers, which in combination with PDT leads to cellular damage by reactive oxygen species (ROS). They included 20 patients in their series in which they achieved GTR in 45% of patients, median PFS of 6 months (95% CI 4.8–7.2), and no adverse events, deeming this novel application of 5-ALA a safe and promising tool for recurrent glioma surgery. Therefore, the team from Münster (Germany) has planned a randomized controlled trial including 106 patients in which biopsy will be compared with biopsy + PDT with 5-ALA for recurrent glioblastoma patients with PFS as primary outcome (Table 1).
Intraoperative ultrasound (ioUS) is the use of sonography to locate tumor tissue during surgery and to delineate it from healthy brain tissue (Figure 2). Similar to 5-ALA, ioUS is one of the tools to potentially increase the extent of resection. However, ioUS is able to identify both low-grade and high-grade glioma (as opposed to 5-ALA, which can only identify high-grade glioma). Theoretically, 5-ALA and ioUS can be considered complementary techniques since the former visualizes tumor tissue macroscopically and the latter is able to detect nodular remnants that might get hidden behind collapsing cavity walls after large tumor resections. One of the main advantages of ioUS over preoperative imaging modalities is the possibility to visualize the tumor in real-time (with taking into account brain shift), which is especially useful for subcortical lesions. Moreover, its corresponding costs (and duration to acquire images) are much lower than other intraoperative imaging methods, such as intraoperative MRI (ioMRI; the cost of which is a well-known limitation), with a significantly lower spatial resolution than ioMRI as a consequence.
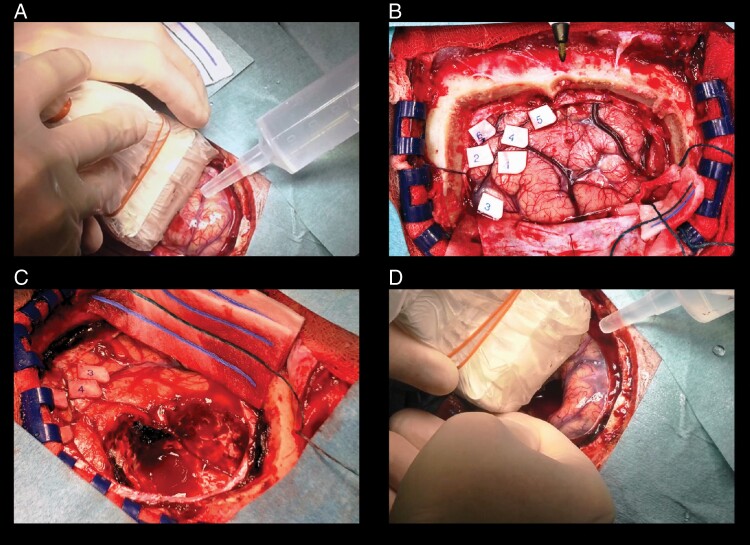
Figure 2.
Electrocortical stimulation with intraoperative ultrasound. A: Intraoperative ultrasound before starting tumor removal. B: Electrocortical stimulation mapping using awake craniotomy to determine eloquent brain areas. C: Tumor resection based on mapping procedure, aided by the neuro-linguist. D: Intraoperative ultrasound after tumor resection to identify potential residual tumor.
There is an increasing amount of research interest in using ioUS in glioma surgery, in particular retrospective evidence in low-grade glioma patients. In 2015, Petridis et al evaluated the use of ioUS in low-grade glioma surgery.85 They found that it was well-suited for identification of tumor tissue and major blood vessels. Gerganov et al compared ioUS with ioMRI for resections of low-grade gliomas and concluded that both modalities are well-suited to locate the tumor and its borders before resection starts.86 However, based on their results the quality of ioMRI proves to be superior to ioUS during the resection, and is better suited to detect residual tumor, particularly because the difference in spatial resolution and the subsequent interpretation of the images. ioUS proved to be prone to problems in differentiating artifacts such as blood clots and fluids from true residual tumor tissue, which has been reported before.87 Though, other studies found ioUS to be accurate in identifying tumor tissue after glioma resection and assessing extent of resection.88,89 Coburger et al suggested a comparable sensitivity and specificity of ioMRI to ioUS, deeming ioUS ideal for centers lacking a ioMRI.90 Trevisi et al recently published a large meta-analysis regarding the use of ioUS in glioma patients including 13 studies.91 They demonstrated that the pooled sensitivity of ioUS in detecting residual tumor tissue was 72.2% and the specificity was 93.5%. Detection was complicated by artifacts, small volume of residual tumor (<5 ml), and previous radiotherapy.89
Scientific evidence for the use of ioUS in high-grade glioma is rarer. Incekara et al published the results of their single-center randomized controlled trial in 2021.92 They included 50 glioblastoma patients and randomized them with a 1:1 ratio between resection with or without the use of ioUS. They found that gross-total resection was achieved more often in the ioUS group (8 of 23 vs. 2 of 24, P = .036) without increased rates of postoperative neurological deficits. Furthermore, there is evidence that ioUS can be used to detect residual tumor and therefore could increase extent of resection in high-grade glioma, equal to ioMRI.93 This is supported by the study of Solheim et al, in which they used ioUS in a series of 156 high-grade glioma patients. They found that medium or good ultrasound image quality was independently associated with a higher incidence of gross-total resection.94
Wang et al prospectively compared 137 patients undergoing glioma resection with the help of ioUS with a control group of 60 patients.95 They found that the 1-year and 2-year survival in for both low-grade and high-grade glioma patients was longer in the ioUS group than in the control group. Recently, Liang et al and Prada et al have reported on their use of contrast-enhanced ultrasound (CEUS) in high-grade glioma patients with improved differentiation between artifacts and residual tumor tissue.96–98 Colleagues from Norway are working on improving the spatial resolution of ioUS by developing a new fluid (as compared to the conventional Ringer’s lactate) to decrease image noise.99 Another development is the integration of ioUS with neuronavigation (navigated intraoperative ultrasound; nUS) with subsequent 3D image acquisition (n3DUS).100 nUS has been shown to be able to detect residual tumor volume more reliably than conventional ultrasound.101
The use of ioUS in glioma surgery is promising but is currently subject to contradictory results, since studies are mostly retrospective, small and heterogenous in study population. Currently, two prospective studies are evaluating its use for this patient group: the US-GLIOMA trial (results are expected soon) and the FUTURE-GBM study (recently started) (Table 1).
ioMRI is used to assess tumor extent of resection intraoperatively with the highest spatial resolution currently possible. Senft et al published their RCT evaluating the use of ioMRI in glioma surgery in 2011, including 58 patients.102 They found that tumor resections in the ioMRI arm proved more often GTR than in the control group (96% versus 68%) with no difference in postoperative neurological complications. Furthermore, no patients in the ioMRI with GTR experienced postoperative neurological deterioration. Whiting et al reported on their retrospective series regarding the combined use of minimal access craniotomy with ioMRI and awake mapping in grade I–IV gliomas.103 They found a median EOR of 98.5%, with GTR being achieved in 60.7% of LGG cases and in 30.3% of HGG cases. More than twenty-seven percent of the total group achieved an increase in EOR of more than 15% due to the use of ioMRI. A recent paper by Pichierri et al retrospectively compared the combined use of ioMRI and awake mapping with ioMRI in asleep patients and a (third) control group.104 They found that the addition of ioMRI led to increased GTR rates among resections of all glioma grades, but there were no significant differences in EOR, tumor recurrences, or overall survival between the awake ioMRI and asleep ioMRI group, although the three groups were biased for patient selection.
Recent evidence suggests that ioMRI might play a major role in enabling supratotal resection (i.e. resection of the tumor beyond the contrast-enhancing (CE) part into the surrounding noncontrast-enhancing (NCE) part, but with radiological abnormalities on T2/FLAIR images). Two retrospective studies evaluated the association between ioMRI and supratotal resection. Li et al demonstrated that resection 53% of the NCE part led to additional survival benefit,105 whereas Pessina et al found that 45% would already lead to a significant improvement in survival outcomes.106 Furthermore, Eyüpoglu et al showed in a prospective cohort series that the addition of ioMRI to resections with 5-ALA increased the NCE extent of resection, which was directly correlated to overall survival.107
Major limitations of ioMRI are its high costs of installation and maintenance and the increased duration of the operation. Moreover, the use of ioMRI during eloquent gliomas is ideally combined with intraoperative mapping such as awake craniotomy or asleep mapping to test for tissue functionality and preserve speech and motor tracts.
Prospective evidence is needed to provide Level I evidence for the use of ioMRI. Currently, two prospective studies are conducted at the University Hospital Tübingen (Germany) and University Hospital Fudan (China) (Still accruing without current results, Table 1).
Intraoperative Tissue Sampling
Currently there are a few emerging techniques for intraoperative tissue sampling as an alternative to fluorescence. Vibrational spectroscopy is one of the most notable new techniques, with Raman spectroscopy (RS, based on inelastic scattering of photons) and Fourier-Transform Infrared spectroscopy (FTIS, based on the interaction of infrared radiation with tissue) as the two main modalities. RS and FTIS provide in a noninvasive manner real-time information about the molecular buildup of specific tissues. Consequently, they can potentially be used intraoperatively to assist the surgeon in distinguishing healthy brain parenchyma from tumor tissue. Recent evidence indeed suggests that spectroscopy can be used (1) to delineate the tumor margin, (2) to discern between specific histological tumor areas (e.g. tumor core, necrosis, infiltrative zone), (3) to evaluate the molecular tumor buildup (e.g. IDH status), and (4) to identify molecular tumor heterogeneity on both fresh tissue, frozen tissue, and formalin-fixed paraffin-embedded (FFPE) brain tissue samples.108–111 However, the use of these techniques is still in its experimental phase: studies focusing on in vivo validation, the interplay with intraoperative fluorescence and imaging, and the added benefit when employed simultaneously with intraoperative mapping techniques are awaited.
Supratotal Resection
Recently there has been growing interest in evaluating the benefit of “supratotal resection” (also called “supramarginal” or “supramaximal” resection, abbreviated: SpTR). The term “supratotal” applies to the extent of resection of the tumor outside the borders of the contrast-enhancing part of the tumor (as evaluated on T1 + Gd images), i.e., the noncontrast-enhancing part (as evaluated on T2/FLAIR images). It can therefore be defined as GTR plus resection of some noncontrast-enhancement, as concluded by a recent crowdsourced consensus.112 2019, colleagues De Leeuw and Vogelbaum evaluated the use of supratotal resection in glioma in a systematic review.113 They concluded that the available evidence was insufficient for “carte blanche” application and stressed the importance of validation in prospective cohort studies. In 2020, Molinaro et al published their well-known multicenter, retrospective cohort study, including 716 patients from UCSF, the Mayo Clinic, and the Cleveland Clinic.8 They found a significant association between supratotal resection and longer overall survival in younger patients, regardless of IDH status, as well as in patients with IDHwt tumors regardless of MGMT status. Therefore, they proposed that in younger patients (<65 years old), maximal resection of the contrast-enhancing part should be pursued; and when safely feasible, the noncontrast-enhancing part as well (regardless of molecular status). Based on their dataset, maximal resection of the noncontrast-enhancing part was not recommended for patients aged >65 years. A smaller retrospective study by Hirono et al, which included 30 glioblastoma patients, also found that supratotal resection led to improved survival outcomes and was not associated with increased postoperative neurological deficits.114 The results of these retrospective studies will be validated in the ENCRAM Consortium’s prospective PROGRAM study.74
Conclusions and Future Directions
Glioma surgery means balancing between maximizing extent of resection and preventing postoperative neurological complications. Various surgical techniques and adjuncts can be used, either to detect (residual) tumor tissue and to increase EOR (decrease residual volume), or to identify eloquent brain areas to preserve functionality. In recent years, a sizable amount of progress has been made for both goals by numerous scientific efforts. Neurosurgeons can choose from a wide array of possibilities their preoperative and intraoperative modality of choice. Different modalities can be used for the same goal, often with comparable outcomes or without strong, prospective evidence for one modality in particular. For some of these modalities and patient subgroups, the clinical impact is not always based on high-level evidence. Therefore, sizable prospective studies such as RCTs or multicenter cohort studies are needed to compare various modalities in a multimodal setting to determine which modality is best suited for which patient (grade, location, etc.). We gave an overview of current evidence for different surgical modalities and adjuncts for glioma surgery. Furthermore, we elaborated on the current prospective scientific efforts which will define the neurosurgical practice and decision making in the near future.
Contributor Information
Jasper Kees Wim Gerritsen, Department of Neurosurgery, Erasmus Medical Center, Rotterdam, The Netherlands.
Marike Lianne Daphne Broekman, Department of Neurosurgery, Haaglanden Medical Center, The Hague, The Netherlands.
Steven De Vleeschouwer, Department of Neurosurgery, University Hospital–Leuven, Leuven, Belgium.
Philippe Schucht, Department of Neurosurgery, University Hospital–Bern, Bern, Switzerland.
Brian Vala Nahed, Department of Neurosurgery, Massachusetts General Hospital/Harvard Medical School, Boston, Massachusetts, USA.
Mitchel Stuart Berger, Department of Neurosurgery, University of California, San Francisco, California, USA.
Arnaud Jean Pierre Edouard Vincent, Department of Neurosurgery, Erasmus Medical Center, Rotterdam, The Netherlands.
Funding
None.
Conflict of interest statement. None.
References
- 1. Ho VK, Reijneveld JC, Enting RH, et al. Changing incidence and improved survival of gliomas. Eur J Cancer. 2014;50(13):2309–2318. [DOI] [PubMed] [Google Scholar]
- 2. Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 3. Perry JR, Laperriere N, O’Callaghan CJ. Short-course radiation plus temozolomide in elderly patients with glioblastoma. N Engl J Med. 2017;376(11):1027–1037. [DOI] [PubMed] [Google Scholar]
- 4. Stummer W, Meinel T, Pichlmeier U, et al. Extent of resection and survival in glioblastoma multiforme: identification of and adjustment for bias. Neurosurgery 2008;62(3):564–576. [DOI] [PubMed] [Google Scholar]
- 5. Brown TJ, Brandmeir NJ, Church EW, et al. Association of the extent of resection with survival in glioblastoma: a systematic review and meta-analysis. JAMA Oncol 2016;2(11):1460–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Almenawer SA, Badhiwala JH, Alhazzani W, et al. Biopsy versus partial versus gross total resection in older patients with high-grade glioma: a systematic review and meta-analysis. Neuro-Oncology 2015; 17(6):868–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stummer W, Reulen HJ, Meinel T, et al. Extent of resection and survival in glioblastoma multiforme: identification of and adjustment for bias. Neurosurgery 2008;62(3):564–576; discussion 564. [DOI] [PubMed] [Google Scholar]
- 8. Molinaro AM, Hervey-Jumper S, Morshed RA, et al. Association of maximal extent of resection of contrast-enhanced and non-contrast-enhanced tumor with survival within molecular subgroups of patients with newly diagnosed glioblastoma. JAMA Oncol 2020;6(4):495–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McGirt MJ, Mukherjee D, Chaichana KL, et al. Association of surgically acquired motor and language deficits on overall survival after resection of glioblastoma multiforme. Neurosurgery 2009;65(3):463–469; discussion 469. [DOI] [PubMed] [Google Scholar]
- 10. Lian J, Lv X, Lu C, et al. Prognostic factors of patients with gliomas—an analysis on 335 patients with glioblastoma and other forms of gliomas. BMC Cancer 2020;20(1):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lamborn KR, Chang SM, Prados MD, et al. Prognostic factors for survival of patients with gliobastoma: recursive partitioning analysis. Neuro-Oncol 2004;6(3):227–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kitange GJ, Carlson BL, Schroeder MA, et al. Induction of MGMT expression is associated with temozolomide resistance in glioblastoma xenografts. Neuro Oncol 2009;11(3):281–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hartmann C, Meyer J, Balss J, et al. Type and frequency of IDH1 and IDH2 mutations are related to oligodendroglial differentiation and age: a study of 1,010 diffuse gliomas. Acta Neuropathol. 2009;118(4):469–474. [DOI] [PubMed] [Google Scholar]
- 14. Weller M, van den Bent M, Tonn JC, et al. European Association for Neuro-Oncology (EANO) guideline on the diagnosis and treatment of adult astrocytic and oligodendroglial gliomas. Lancet Oncol. 2017;18(6):e315–e329. [DOI] [PubMed] [Google Scholar]
- 15. Taal W, Oosterkamp H, Walenkamp AME, et al. Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): a randomised controlled phase 2 trial. Lancet Oncol. 2014; 15(9):943–953. [DOI] [PubMed] [Google Scholar]
- 16. Wick W, Gorlia T, Bendszus M, et al. Lomustine and bevacizumab in progressive glioblastoma. N Engl J Med. 2017;377(20):1954–1963. [DOI] [PubMed] [Google Scholar]
- 17. Batchelor TT, Mulholland P, Neyns B, et al. Phase III randomized trial comparing the efficacy of cediranib as monotherapy, and in combination with lomustine, versus lomustine alone in patients with recurrent glioblastoma. J Clin Oncol. 2013;31(26):3212–3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brandes AA, Carpentier AF, Kesari S, et al. A phase II randomized study of galunisertib monotheraepy or galunisertib plus lomustine compared with lomustine monotherapy in patients with recurrent glioblastoma. Neuro-Oncology 2016;18(8):1146–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Duerinck J, Du Four S, Bouttens F, et al. Randomized phase II trial comparing axitinib with the combination of axitinib and lomustine in patients with recurrent glioblastoma. J Neurooncol. 2018;136(1): 115–125. [DOI] [PubMed] [Google Scholar]
- 20. Lombardi G, De Salvo GL, Brandes AA, et al. Regorafenib compared with lomustine in patients with relapsed glioblastoma (REGOMA): a multicentre, open-label, randomised, controlled, phase 2 trial. Lancet Oncol. 2019;20(1):110–119. [DOI] [PubMed] [Google Scholar]
- 21. Wick W, Puduvalli VK, Chamberlain MC, et al. Phase III study of enzastaurin compared with lomustine in the treatment of recurrent intracranial glioblastoma. J Clin Oncol. 1168;28(7):74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Van den Bent M, Eoli M, Sepulveda JM, et al. INTELLANCE 2/EORTC 1410 randomized phase II study of Depatux-M alone and with temozolomide vs temozolomide or lomustine in recurrent EGFRamplified glioblastoma. Neuro-Oncology 2019;22(8):684–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Spetzler RF, Martin NA. A proposed grading system for arteriovenous malformations. J Neurosurg. 1986. 65(4):476–483. [DOI] [PubMed] [Google Scholar]
- 24. Takakura T, Muragaki Y, Tamura M, et al. Navigated transcranial magnetic stimulation for glioma removal: prognostic value in motor function recovery from postsurgical neurological deficits. J Neurosurg. 2017;127(4):877–891. [DOI] [PubMed] [Google Scholar]
- 25. Sollmann N, Picht T, Mäkelä JP, et al. Navigated transcranial magnetic stimulation for preoperative language mapping in a patient with a left frontooperatcular glioblastoma. J Neurosurg. 2013;118(1):175–179. [DOI] [PubMed] [Google Scholar]
- 26. Ille S, Sollmann N, Hauck T, et al. Combined noninvasive language mapping by navigated transcranial magnetic stimulation and functional MRI and its comparison with direct cortical stimulation. J Neurosurg. 2015;123(1):212–225. [DOI] [PubMed] [Google Scholar]
- 27. Seynaeve L, Baby D, Van Hamme H, et al. Automated speech analysis to improve TMS-based language mapping: algorithm and proof-of-concept. Brain Stimul 2020;13(1):2367–2369. [DOI] [PubMed] [Google Scholar]
- 28. Jeltema HR, Ohlerth AK, de Wit A, et al. Comparing navigated transcranial magnetic stimulation mapping and “gold standard” direct cortical stimulation mapping in neurosurgery: a systematic review. Neurosurg Rev. 2021;44(4):1903–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Krieg SM, Sollmann N, Obermueller T, et al. Changing the clinical course of glioma patients by preoperative motor mapping with navigated transcranial magnetic brain stimulation. BMC Cancer 2015;15:231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Krieg SM, Sollmann N, Hauck T, et al. Repeated mapping of cortical language sites by preoperative navigated transcranial magnetic stimulation compared to repeated intraoperative DCS mapping in awake craniotomy. BMC Neurosci. 2014;15:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ille S, Sollmann N, Butenschoen VM, et al. Resection of highly language-eloquent brain lesions based purely on rTMS language mapping without awake surgery. Acta Neurochir (Wien) 2016;158(12):2265–2275. [DOI] [PubMed] [Google Scholar]
- 32. Frey D, Schilt S, Strack V, et al. Navigated transcranial magnetic stimulation improves the treatment outcome in patients with brain tumors in motor eloquent locations. Neuro Oncol 2014;16(10):1365–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Picht T, Krieg SM, Sollmann N, et al. A comparison of language mapping by preoperative navigated transcranial magnetic stimulation and direct cortical stimulation during awake surgery. Neurosurgery 2013;72(5):808–819. [DOI] [PubMed] [Google Scholar]
- 34. Seynaeve L, Haeck T, Gramer M, et al. Optimized preoperative motor cortex mapping in brain tumors using advanced processing of transcranial magnetic stimulation data. Neurimage Clin. 2019;21:101657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zimmerman M, Rössler K, Kaltenhäuser M, et al. Comparative fMRI and MEG localization of cortical sensiomotor function: bimodal mapping supports motor area reorganization in glioma patients. PLoS One. 3371;14(3):e021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tarapore PE, Tate MC, Honma SM, et al. Preoperative multimodal motor mapping: a comparison of magnetoencephalography imaging, navigated transcranial magnetic stimulation, and direct cortical stimulation: clinical article. J Neurosurg. 2012;117(2):354–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Traut T, Sardesh N, Bulubas L, et al. MEG imaging of recurrent gliomas reveals functional plasticity of hemispheric language specialization. Hum Brain Mapp. 2019;40(4):1082–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dimou S, Battisti RA, Hermens DF, Lagopoulos JA. systematic review of functional magnetic resonance imaging and diffusion tensor imaging modalities used in presurgical planning of brain tumour resection. Neurosurg Rev. 2013;36(2):205–214. [DOI] [PubMed] [Google Scholar]
- 39. Berntsen EM, Gulati S, Solheim O, et al. Functional magnetic resonance imaging and diffusion tensor tractography incorporated into an intraoperative 3-dimensional ultrasound-based neuronavigation system: impact on therapeutic strategies, extent of resection, and clinical outcome. Neurosurgery 2010;67(2):251–264. [DOI] [PubMed] [Google Scholar]
- 40. Castellano A, Bello L, Michelozzi C, et al. Role of diffusion tensor magnetic resonance tractography in predicting the extent of resection in glioma surgery. Neuro Oncol 2012;14(2):192–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Frati A, Pesce A, D’Andrea G, et al. A purely functional Imaging based approach for transcortical resection of lesion involving the dominant atrium: toward safer, imaging-guided, tailored cortico-leucotomies. J Clin Neurosci. 2018;50:252–261. [DOI] [PubMed] [Google Scholar]
- 42. Sollmann N, Wildschuetz N, Kelm A, et al. Associations between clinical outcome and navigated transcranial magnetic stimulation characteristics in patients with motor-eloquent brain lesions: a combined navigated transcranial magnetic stimulation–diffusion tensor imaging fiber tracking approach. J Neurosurg. 2017;128(3):800–810. [DOI] [PubMed] [Google Scholar]
- 43. Zhu FP, Wu JS, Song YY, et al. Clinical application of motor pathway mapping using diffusion tensor imaging tractography and intraoperative direct subcortical stimulation in cerebral glioma surgery: a prospective cohort study. Neurosurgery 2012;71(6):1170–1183; discussion 1183. [DOI] [PubMed] [Google Scholar]
- 44. Caverzasi E, Hervey-Jumper SL, Jordan KM, et al. Identifying preoperative language tracts and predicting postoperative functional recovery using HARDI q-ball fiber tractography in patients with gliomas. J Neurosurg. 2016;125(1):33–45. [DOI] [PubMed] [Google Scholar]
- 45. Mormina E, Longo M, Arrigo A, et al. MRI tractography of corticospinal tract and arcuate fasciculus in high-grade gliomas performed by constrained spherical deconvolution: qualitative and quantitative analysis. ANJR AM J Neuroradiol 2015;36(10):1853–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sotiropoulos SN, Jbabdi S, Xu J, et al. Advances in diffusion MRI acquisition and processing in the Human Connectome Project. Neuroimage 2013;80:125–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Glenn GR, Kuo LW, Chao YP, et al. Mapping the orientation of white matter fiber bundles: a comparative study of diffusion tensor imaging, diffusional kurtosis imaging, and diffusion spectrum imaging. AJNR Am J Neuroradiol. 2016;37(7):1216–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Roux FE, Boulanouar K, Ranjeva JP, et al. Usefulness of motor functional MRI correlated to cortical mapping in Rolandic low-grade astrocytomas. Acta Neurochir (Wien) 1999;141(1):71–79. [DOI] [PubMed] [Google Scholar]
- 49. Giussani C, Roux FE, Ojemann J, et al. Is preoperative functional magnetic resonance imaging reliable for language areas mapping in brain tumor surgery? Review of language functional magnetic resonance imaging and direct cortical stimulation correlation studies. Neurosurgery 2010;66(1):113–120. [DOI] [PubMed] [Google Scholar]
- 50. Austermuehler A, Cojin J, Reynolds R, et al. Language functional MRI and direct cortical stimulation in epilepsy preoperative planning. Ann Neurol. 2017;81(4):526–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ulmer JL, Hacein-Bey L, Mathews VP, et al. Lesion-induced pseudo- dominance at functional magnetic resonance imaging: implications for preoperative assessments. Neurosurgery 2004;55(3):569–579; discussion 580. [DOI] [PubMed] [Google Scholar]
- 52. Van Niftrik CHB, Piccirelli M, Muscas G, et al. The voxel-wise analysis of false negative fMRI activation in regions of provoked impared cerebrovascular reactivity. PLoS One. 2019;14:e0215294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sanai N, Mirzadeh Z, Berger MS. Functional outcome after language mapping for glioma resection. N Engl J Med. 2008;358(1):18–27. [DOI] [PubMed] [Google Scholar]
- 54. Kral T, Kurthen M, Schramm J, et al. Stimulation mapping via implanted grid electrodes prior to surgery for gliomas in highly eloquent cortex. Neurosurgery 2006;58(1 Suppl):36–43. [DOI] [PubMed] [Google Scholar]
- 55. Bander ED, Shelkov E, Modik O, et al. Use of the train-of-five bipolar technique to provide reliable, spatially accurate motor cortex identification in asleep patients. Neurosurg Focus. 2020;48(2):E4. [DOI] [PubMed] [Google Scholar]
- 56. Gogos AJ, Young JS, Morshed RA, et al. Triple motor mapping: transcranial, bipolar, and monopolar mapping for supratentorial glioma resection adjacent to motor pathways. J Neurosurg. 2020;134(6):1728–1737. [DOI] [PubMed] [Google Scholar]
- 57. Krieg SM, Shiban E, Droese D, et al. Predictive value and safety of intraoperative neurophysiological monitoring with motor evoked potentials in glioma surgery. Neurosurgery 2012;70(5):1060–1070; discussion 1070. [DOI] [PubMed] [Google Scholar]
- 58. Neuloh G, Pechstein U, Cedzich C, et al. Motor evoked potential monitoring with supratentorial surgery. Neurosurgery 2007;61(1 Suppl):337–46; discussion 346. [DOI] [PubMed] [Google Scholar]
- 59. De Witte E, Satoer DD, Robert E, et al. The Dutch linguistic intraoperative protocol: a valid linguistic appraoch to awake brain surgery. Brain Lang. 2015;140:35–48. [DOI] [PubMed] [Google Scholar]
- 60. Imbault M, Chauvet D, Gennisson JL, et al. Intraoperative functional ultrasound imaging of human brain activity. Sci Rep. 2017;7(1):7304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Soloukey S, Vincent AJPE, Satoer DD, et al. Functional ultrasound (fUS) during awake brain surgery: the clinical potential of intra-operative functional and vascular brain mapping. Front Neurosci. 2020;13:1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Raabe A, Beck J, Schucht P, et al. Continuous dynamic mapping of the corticospinal tract during surgery of motor eloquent brain tumors: evaluation of a new method. J Neurosurg. 2014;120(5):1015–1024. [DOI] [PubMed] [Google Scholar]
- 63. Seidel K, Schucht P, Beck J, et al. Continuous dynamic mapping to identify the corticospinal tract in motor eloquent brain tumors: an update. J Neurol Surg A Cent Eur Neurosurg 2020;81(2):105–110. [DOI] [PubMed] [Google Scholar]
- 64. Sacko O, Lauwers-Cances V, Brauge D, et al. Awake craniotomy vs surgery under general anesthesia for resection of supratentorial lesions. Neurosurgery 2011;68(5):11921992–11921199. [DOI] [PubMed] [Google Scholar]
- 65. De Witt Hamer PC, Robles SG, Zwinderman AH, Duffau H, Berger MS. Impact of intraoperative stimulation brain mapping on glioma surgery outcome: a meta-analysis. J Clin Oncol. 2012;30(20):2559–2565. [DOI] [PubMed] [Google Scholar]
- 66. Gerritsen JKW, Arends LR, Dirven CMF, et al. Impact of intraoperative stimulation mapping and awake craniotomy on high-grade glioma surgery outcome: a meta-analysis. Acta Neurochirurgica (Wien) 2017;161(1):99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Szelényi A, Bello L, Duffau H, et al. Intraoperative electrical stimulation in awake craniotomy: methodological aspects of current practice. Neurosurg Focus. 2010;28(2):E7. [DOI] [PubMed] [Google Scholar]
- 68. Yao PS, Zheng SF, Wang F, et al. Surgery guided with intraoperative electrocorticography in patients with low-grade glioma and refractory seizures. J Neurosurg. 2018;128(3):840–845. [DOI] [PubMed] [Google Scholar]
- 69. Sartorius CJ, Berger MS. Rapid termination of intraoperative stimulation-evoked seizures with application of cold Ringer’s lactate to the cortex. Technical note. J Neurosurg. 1998;88(2):349–351. [DOI] [PubMed] [Google Scholar]
- 70. Roca E, Pallud J, Guerrini F, et al. Stimulation-related intraoperative seizures during awake surgery: a review of available evidences. Neurosurg Rev. 2020;43(1):87–93. [DOI] [PubMed] [Google Scholar]
- 71. Hamberger MJ, Williams AC, Schevon CA. Extraoperative neurostimulation mapping: results from an international survey of epilepsy surgery programs. Epilepsia 2014;55(6):933–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Gerritsen JKW, Broekman MLD, De Vleeschouwer S, et al. Decision making and surgical modality selection in glioblastoma patients: an international multicenter survey. J Neurooncol. 2022;156(3):465–482. doi: 10.1007/s11060-021-03894-5. [DOI] [PubMed] [Google Scholar]
- 73. Gerritsen JKW, Broekman MLD, De Vleeschouwer S, et al. Global comparison of awake and asleep mapping procedures in glioma surgery: an international multicenter survey. Neuro Oncology Practice. 2022;9(2):123–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Gerritsen JKW, Dirven CMF, De Vleeschouwer S, et al. The PROGRAM study: awake mapping versus asleep mapping versus no mapping for high-grade glioma resections: study protocol for an international multicenter prospective three-arm cohort study. BMJ Open 7306;11(7):e04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Stummer W, Pichlmeier U, Meinel T, et al. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol. 2006;7(5):392–401. [DOI] [PubMed] [Google Scholar]
- 76. Stummer W, Tonn, JC, Mehdorn HM, et al. Counterbalancing risks and gains from extended resections in malignant glioma surgery: a supplemental analysis from the randomized 5-aminolevulinic acid glioma resection study. J Neurosurg. 2011;114(3):613–623. [DOI] [PubMed] [Google Scholar]
- 77. Yamada S, Muragaki Y, Maruyama T, et al. Role of neurochemical navigation with 5-aminolevulinic acid during intraoperative MRI-guided resection of intracranial malignant gliomas. Clin Neurol Neurosurg. 2015;130:134–139. [DOI] [PubMed] [Google Scholar]
- 78. Schucht P, Beck J, Abu-Isa J, et al. Gross total resection rates in contemporary glioblastoma surgery: results of an institutional protocol combining 5-aminolevulinic acid intraoperative fluorescence imaging and brain mapping. Neurosurgery 2012;71(5):927–935; discussion 935. [DOI] [PubMed] [Google Scholar]
- 79. Stummer W, Tonn JC, Goetz C, et al. 5-aminolevulinic acid-derived tumor fluorescence: the diagnostic accuracy of visible fluorescence qualities as corroborated by spectrometry and histology and postoperative imaging. Neurosurgery 2014;74(3):310–319; discussion 319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Utsuki S, Oka H, Sato S, et al. Possibility of using laser spectroscopy for the intraoperative detection of nonfluorescing brain tumors and the boundaries of brain tumor infiltraties. Technical note. J Neurosurg. 2006;104(4):618–620. [DOI] [PubMed] [Google Scholar]
- 81. Hansen RW, Pedersen CB, Halle B, et al. Comparison of 5-aminolevulinic acid and sodium fluorescein for intraoperative tumor visualization in patients with high-grade gliomas: a single-center retrospective study. J Neurosurg. 2019;1:8. [DOI] [PubMed] [Google Scholar]
- 82. Falco J, Cavallo C, Vetrano IG, et al. Fluorescein application in cranial and spinal tumors enhancing at preoperative MRI and operated with a dedicated filter on the surgical microscope: preliminary results in 279 patients enrolled in the FLUOCERTUM prospective study. Front Surg 2019;6:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Acerbi F, Broggi M, Schebesch KM, et al. Fluorescein-guided surgery for resection of high-grade gliomas: a multicentric prospective phase II study (FLUOGLIO). Clin Cancer Res. 2018;24(1):52–61. [DOI] [PubMed] [Google Scholar]
- 84. Schipmann S, Müther M, Stögbauer L, et al. Combination of ALA-induced fluorescence-guided resection and intraoperative open photodynamic therapy for recurrent glioblastoma: case series on a promising dual strategy for local tumor control. J Neurosurg. 2020;1:11. [DOI] [PubMed] [Google Scholar]
- 85. Petridis AK, Anokhin M, Vavruska J, et al. The value of intraoperative sonography in low grade glioma surgery. Clin Neurol Neurosurg. 2015;131:64–68. [DOI] [PubMed] [Google Scholar]
- 86. Gerganov VM, Samii A, Giordano M, Samii M, Fahlbusch R. Two-dimensional high-end ultrasound imaging compared to intraoperative MRI during resection of low-grade gliomas. J Clin Neurosci. 2011;18(5):669–673. [DOI] [PubMed] [Google Scholar]
- 87. Hammoud MA, Ligon BL, el Souki R, et al. Use of intraoperative ultrasound for localizing tumors and determining the amount of resection: a comparative study with magnetic resonance imaging. J Neurosurg. 1996;84(5):737–741. [DOI] [PubMed] [Google Scholar]
- 88. Le Roux PD, Winter TC, Berger MS, et al. A comparison between preoperative magnetic resonance and intraoperative ultrasound tumor volumes and margins. J Clin Ultrasound. 1994;22(1):29–36. [DOI] [PubMed] [Google Scholar]
- 89. Erdogan N, Tucer B, Mavili E, et al. Ultrasound guidance in intracranial tumor resection: correlation with postoperative magnetic resonance findings. Acta Radiol 2005;46(7):743–749. [DOI] [PubMed] [Google Scholar]
- 90. Coburger J, Scheuerle A, Rudolf Thal D, et al. Linear array ultrasound in low-grade glioma surgery: histology-based assessment of accuracy in comparison to conventional intraoperative ultrasound and intraoperative MRI. Acta Neurochir (Wien) 2015;157(2):195–206. [DOI] [PubMed] [Google Scholar]
- 91. Trevisi G, Barbone P, Treglia G, Mattoli MV, Mangiola A. Reliability of intraoperative ultrasound in detecting tumor residual after brain diffuse glioma surgery: a systematic review and meta-analysis. Neurosurg Rev. 2019;43(5):1221–1233. [DOI] [PubMed] [Google Scholar]
- 92. Incekara F, Smits M, Dirven L, et al. Intraoperative B-mode ultrasound guided surgery and the extent of glioblastoma resection: a randomized controlled trial. Front Oncol. 2021;11:649797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Moiraghi S, Prada F, Delaidelli A, et al. Navigated intraoperative 2-dimensional ultrasound in high-grade glioma surgery: impact on extent of resection and patient outcome. Oper Neurosurg (Hagerstown) 2020;18(4):363–373. [DOI] [PubMed] [Google Scholar]
- 94. Solheim O, Selbekk T, Jakola AS, et al. Ultrasound-guided operations in unselected high-grade gliomas—overall results, impact of image quality and patient selection. Acta Neurochir (Wien) 2010;152(11):1873–1886. [DOI] [PubMed] [Google Scholar]
- 95. Wang J, Liu X, Mei Ba Y, et al. Effect of sonographically guided cerebral glioma surgery on survival time. J Ultrasound Med. 2012;31(5):757–762. [DOI] [PubMed] [Google Scholar]
- 96. Liang C, Li M, Gong J, et al. A new application of ultrasound-magnetic resonance multimodal fusion virtual navigation in glioma surgery. Ann Transl Med 2019;7(23):736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Prada F, Perin A, Martegani A, et al. Intraoperative contrast-enhanced ultrasound for brain tumor surgery. Neurosurgery 2014;74(5):542–552; discussion 552. [DOI] [PubMed] [Google Scholar]
- 98. Prada F, Vitale V, Del Bene M, et al. Contrast-enhanced MR imaging versus contrast-enhanced US: a comparison in glioblastoma surgery by using intraoperative fusion imaging. Radiology 2017;285(1):242–249. [DOI] [PubMed] [Google Scholar]
- 99. Unsgård G, Sagberg LM, Müller S, et al. A new acoustic coupling fluid with ability to reduce ultrasound imaging artefacts in brain tumour surgery-a phase I study. Acta Neurochir (Wien) 2019;161(7):1475–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Yeole U, Singh V, Mishra A, et al. Navigated intraoperative ultrasonography for brain tumors: a pictorial essay on the technique, its utility, and its benefits in neuro-oncology. Ultrasonography 2020;39(4):394–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Renovanz M, Hickmann AK, Henkel C, Nadji-Ohl M, Hopf NJ. Navigated versus non-navigated intraoperative ultrasound: is there any impact on the extent of resection of high-grade gliomas? A retrospective clinical analysis. J Neurol Surg A Cent Eur Neurosurg 2014;75(3):224–230. [DOI] [PubMed] [Google Scholar]
- 102. Senft C, Bink A, Franz K, et al. Intraoperative MRI guidance and extent of resection in glioma surgery: a randomized, controlled trial. Lancet Oncol. 2011;12(11):997–1003. [DOI] [PubMed] [Google Scholar]
- 103. Whiting B, Lee BS, Mahadev V, et al. Combined use of minimal access craniotomy, intraoperative magnetic resonance imaging, and awake functional mapping for the resection of gliomas in 61 patients. J Neurosurg. 2019;1:9. [DOI] [PubMed] [Google Scholar]
- 104. Pichierri A, Bradley M, Iyer V. Intraoperative magnetic resonance imaging-guided glioma resections in awake and asleep settings and feasibility in the context of a public health system. World Neurosurg X 2019;3: 100022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Li YM, Suki D, Hess K, et al. The influence of maximum safe resection of glioblastoma on survival in 1229 patients: can we do better than gross-total resection? J Neurosurg. 2016;124(4):977–988. [DOI] [PubMed] [Google Scholar]
- 106. Pessina F, Navarria P, Cozzi L, et al. Maximize surgical resection beyond contrast-enhancing boundaries in newly diagnosed glioblastoma multiforme: is it useful and safe? A single institution retrospective experience. J Neurooncol. 2017;135(1):129–139. [DOI] [PubMed] [Google Scholar]
- 107. Eyüpoglu IY, Hore N, Merkel A, et al. Supra-complete surgery via dual intraoperative visualization approach (DiVA) prolongs patient survival in glioblastoma. Oncotarget 2016;7(18):25755–25768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Uckermann O, Juratli TA, Galli R, et al. Optical analysis of glioma: Fourier-Transform Infrared Spectroscopy reveals the IDH1 mutation status. Clin Cancer Res. 2018;24:2530–2538. [DOI] [PubMed] [Google Scholar]
- 109. Anna I, Bartosz P, Lech P, et al. Novel strategies of Raman imaging for brain tumor research. Oncotarget 2017;8(11):852902530–852985310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Zhang J, Fan Y, He M, et al. Accuracy of Raman spectroscopy in differentiating brain tumor from normal brain tissue. Oncotarget 2017;8(22):36824–36831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Klamminger GG, Gérardy JJ, Jelke F, et al. Application of Raman spectroscopy for detection of histologically distinct areas in formalin-fixed paraffin-embedded glioblastoma. Neuro-Oncology Advances 2021;3(1): vdab077. doi: 10.1093/noajnl/vdab077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Khalafallah AM, Rakovec M, Bettegowda C, et al. A crowdsources consensus on supratotal resection versus gross total resection for anatomically distinct primary glioblastoma. Neurosurgery 2021;89(4):712–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. De Leeuw CN, Vogelbaum MA. Supratotal resection in glioma: a systematic review. Neuro Oncol 2019;21(2):179–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Hirono S, Ozaki K, Kobayashi M, et al. Oncological and functional outcomes of supratotal resection of IDH1 wild-type glioblastoma based on 11C-methionine PET: a retrospective, single-center study. Sci Rep. 2021;11(1):14554. [DOI] [PMC free article] [PubMed] [Google Scholar]