Abstract
Structured habitats play an important nursery role during the crucial early juvenile or post‐settlement stages of many fish species. Predominantly, the utility of structured habitats to juvenile fish is thought to be associated with the provisioning of food or as a refuge from predation. Although snapper (Chrysophrys auratus) in New Zealand also have a strong affinity for structured habitats during their post‐settlement phase, their predators are unknown as is the role of predation in determining habitat association. Here the authors investigated potential predators of post‐settlement snapper by remotely observing interactions of restrained post‐settlement snapper with potential predators and investigating the diet of potential predators. They also conducted tank experiments with a potential predator, both with and without the presence of structure. Restrained snapper were infrequently approached by predators, but two new nocturnal predators were identified. No snapper were observed in the diet of potential predators, although two piscivores were identified as potential candidates. No predation occurred during tank experiments, but there was a non‐significant indication that under threat of predation post‐settlement snapper may use habitat when it is present and aggregate together when it is not. The findings suggest that the pulsed nature of predation may have made it difficult to observe given the methods employed and that the threat of predation may be sufficient to drive the habitat selection of post‐settlement snapper. Investigating the significance of predation via methods that do not require direct observations may therefore be more appropriate given this context.
Keywords: biogenic nursery habitat, juvenile fish, nocturnal predators, Pagrus auratus, post‐settlement snapper, predation refuge
1. INTRODUCTION
Many fish species are associated with structured biogenic habitats during critical early life phases, before moving to separate adult habitats (Beck et al., 2001; Dahlgren et al., 2006; Heck Jr et al., 2003). These habitats are termed nurseries because they provide a disproportionately high supply of recruits to the adult population (Beck et al., 2001). For a species to have such a strong affinity to structured habitat, the associated mechanism is likely to be highly important to that species. These mechanisms are generally accepted to be protection from predation (Adams et al., 2004; Grol et al., 2011; Lindholm et al., 1999; Steele, 1999) and elevated growth rates (Harter & Heck, 2006; Nakamura et al., 2012; Tupper & Boutilier, 1997). For example, Adams et al. (2004) assessed the predation mortality of post‐settlement pinfish, Lagodon rhomboides, in aquarium experiments and when tethered in the field. In both settings, predation was reduced in the most structurally complex habitats. Alternatively, Nakamura et al. (2012) conducted field tethering and caging experiments to demonstrate that early juvenile Pacific yellowtail emperor (Lethrinus atkinsoni) had marginally higher survival, but significantly higher growth rates among structured seagrass habitats compared to coral reefs.
Snapper, Chrysophrys auratus (= Pagrus auratus), are an abundant coastal fish found in the northern half of New Zealand and are highly important at cultural, recreational, economic and ecological levels (Parsons et al., 2014b). Post‐settlement stage snapper [<60 mm fork length (FL)] spend their first few months as benthic juveniles, occupying shallow estuarine locations before dispersing to a range of habitats/locations. During this post‐settlement stage, high abundances are associated with habitat structure, whereas snapper may be almost completely absent at immediately adjacent bare sediment sites that are only tens of metres away (Parsons et al., 2013; Parsons et al., 2014a; Parsons et al., 2016).
The mechanism explaining the strong habitat association of post‐settlement snapper, however, remains unclear. It is unlikely to be associated with increased food availability, as post‐settlement snapper largely feed on free‐swimming crustaceans that are not associated with habitat structure itself (Parsons et al., 2015). There is, however, some evidence that post‐settlement snapper may be using structured habitat to shelter from water flow, which may subsequently allow snapper to access locations that have a high flux of their preferred food (Parsons et al., 2015; Parsons et al., 2018). Almost nothing is known about the predators of snapper in general, let alone whether predation is connected to habitat association. For example, a comprehensive diet study did not document snapper as a prey item (Williams, 2009), but juvenile snapper have rarely been observed in the guts of other larger snapper (Lohrer et al., 2008). Direct observations of predation attempts on juvenile snapper have only been rarely been documented (Parsons et al., 2018), and there has not been any investigation of potentially important nocturnal predators (Bassett & Montgomery, 2011). Some predation experiments have been conducted on juvenile snapper. One such experiment exposed post‐settlement snapper to predators in sea cages, but the predators used did not behave normally within the confines of the sea cage (Simon Thrush, University of Auckland, pers. comm.). Further to this, a tank experiment with post‐settlement snapper observed increased structure use in the presence of a predator (Ross et al., 2007). The lack of actual predation events, however, could have been due to the blue cod (Parapercis colias) predator used, which are unlikely to encounter post‐settlement snapper in a natural setting due to limited range and habitat overlap and are more of an opportunist than specialist piscivore (Jiang & Carbines, 2002).
Given the importance of predation as a structuring force for many other fish communities (Dahlgren & Eggleston, 2000; Forrester & Steele, 2004; Hixon & Carr, 1997; Ryer et al., 2010; Steele, 1998), the authors of this study sought to identify the predators of post‐settlement snapper and investigate predation as a potential mechanism explaining the habitat association of post‐settlement juvenile snapper. Specifically, they (a) restrained post‐settlement snapper in the field and observed interactions with potential predators during the day and night, (b) examined the diet of potential predators of post‐settlement snapper in an area where post‐settlement snapper were abundant and (c) experimentally exposed post‐settlement snapper to a potential predator with and without habitat structure. The findings do not provide strong evidence that predation is important in determining the habitat association of post‐settlement snapper, but alternative indirect observations of predation may be more appropriate.
2. MATERIALS AND METHODS
2.1. Potential predators of restrained post‐settlement snapper
Field trials assessing potential predators of post‐settlement snapper were conducted in Whangarei Harbour, northeastern Aotearoa New Zealand (Figure 1) in February 2019. Whangarei Harbour is a mesotidal estuary with a semi‐diurnal tide and has neap and spring ranges of 1.3 and 2.8 m, respectively. The harbour and the habitat features within it are an important nursery for post‐settlement snapper (Parsons et al., 2016). The authors selected a site at MacDonald Bank (174.491° E, 35.810° S) for this part of the present study for several reasons. In particular, MacDonald Bank offers an extensive area of sand flats of appropriate depth to conduct experiments, it is devoid of natural structure, artificial habitat placed on MacDonald Bank attracts high densities of post‐settlement snapper, it has reasonable water clarity for making observations and it can be accessed only by boat, reducing the potential for interference. In December 2018 [the time of peak snapper spawning (Parsons et al., 2014b)] they deployed 10 1.8 × 1.8 m artificial seagrass units (ASU) at a water depth of c. 0.4 m at low tide. ASUs were separated by 20 m. Previous investigations have demonstrated that post‐settlement snapper are attracted to ASUs in high densities, and are resident to patches separated by c. 10 m (Parsons et al., 2013), so the authors treated ASUs as independent replicates. ASUs consisted of a plastic mesh grid with bunches of ribbon (c. 30 cm long by 1 cm wide) tied to the mesh grid. All ASUs had a shoot density of 1820 blades m−2, corresponding to the upper range of subtidal seagrass densities in northeastern New Zealand (Morrison et al., 2014). ASUs were secured in place with metal pegs on the sand flats.
FIGURE 1.

Map of Whangarei Harbour showing the locations where restrained post‐settlement snapper were deployed on artificial seagrass units (ASUs), and the location of set nets to capture potential predators of post‐settlement snapper. New Zealand and the location of Whangarei Harbour inset.  , Artificial Seagrass Units;
, Artificial Seagrass Units;  , Set nets;
, Set nets;  , Intertidal;
, Intertidal;  , Land
, Land
In this study, the ASUs were used to attract snapper that were then observed, to document any interaction with potential predators. As predator–prey interactions can be rare events, however, increasing the frequency of these interactions was desirable. As such, the authors also placed a post‐settlement snapper restrained within a clear plastic jar in front of each camera that was recording an ASU (i.e., an ethical equivalent of tethering). The apparatus used to perform these observations consisted of a 15 cm high steel stand with a GoPro Hero 3 camera mounted on top (Figure 2). The steel stand also had a 64‐cm‐long aluminium bar extending horizontally in front of the camera with an inverted clear plastic jar (9 cm diameter by 14.2 cm high) mounted on its far end. This plastic jar had holes drilled into it, which supplied fresh sea water to the post‐settlement snapper that would be placed inside it just before the camera was deployed. Post‐settlement snapper were caught from other locations in the harbour using beach seine and retained in a small tank (c. 50 L) with an air bubbler until being deployed with the camera. Using a surface rope and float a camera was placed about 1 m from and facing an ASU, with up to five cameras deployed on different ASUs simultaneously. Each deployment would provide up to 2.5 h of footage, so once this time had elapsed the cameras were retrieved via their surface ropes, batteries and memory cards replaced, and then immediately deployed onto a different ASU. In this way camera deployments covered a range of times and tidal states.
FIGURE 2.

Vertical and horizontal diagram views of the remote camera and infrared illuminator set‐up used to observe restrained post‐settlement snapper. Note that the camera position changed depending on whether a daytime or night‐time deployment was being conducted, but was always 64 cm from the restrained post‐settlement snapper
Night‐time observations of ASUs and the restrained post‐settlement snapper were also conducted. For these night‐time deployments illumination was provided by a sealed PVC pipe containing an array of infrared illuminator light‐emitting diodes (LEDs) (model SD‐DR50WC8, MS‐Moto), other electronics and batteries, which was mounted underneath the steel stand. To reduce infrared light reflectance, the GoPro Hero 3 camera was mounted on an additional aluminium bar (54 cm long) oriented to the side, but pointing towards the jar containing the post‐settlement snapper 64 cm away (Figure 2). The infrared illuminator provided a high‐powered (35 watt) 140° wide beam of light at 850 nm [and therefore not visible to many fish (Kobayashi et al., 2002; Matsuo et al., 2021)] and was pointed at the restrained post‐settlement snapper c. 60 cm away. To enable the camera to see this infrared light source, the authors removed the infrared filter contained within each GoPro Hero 3 used for night‐time camera work. Each night‐time camera apparatus was deployed at about 18.00 hours, although it was still light during the evening. Both the infrared illuminator and the camera, however, were set up so they remotely turned on 2 h after deployment. As such, night‐time footage generally started to record from c. 20.00 hours, or c. 30 min after sunset, again providing c. 2.5 h of footage.
In terms of video analysis, each camera deployment was assigned a daytime category, being day, dusk or night, based on whether there was full light, fading light or darkness, respectively. As such, an individual camera deployment could be assigned both day and dusk or dusk and night categories (which were subsequently treated as separate replicates). All videos were watched in their entirety by the same observer, but often at double speed. When a fish, crustacean or cephalopod large enough to predate on a post‐settlement snapper was observed, the observer would note down the time when it was observed and what the species was. The relevant segment of the video would then be replayed at regular speed, noting any interaction between post‐settlement snapper (for both the individual restrained in the jar and for any other post‐settlement snapper in view of the camera at the time) and the potential predator. During some individual camera deployments, it was unclear if multiple different potential predators of the same species had approached the restrained post‐settlement snapper, or if the same potential predator had merely left the field of view and then returned. For these instances only one potential predator was denoted.
In addition to documenting predatory interactions with the restrained post‐settlement snapper, the presence of other potential predators was recorded by documenting any fish, crustacean or cephalopod species that was observed during each video deployment. In terms of age or size class categorisation, this survey was conducted during March. For snapper at this time of year, the terms 0+ and post‐settlement are synonymous because snapper spawned that year will not have yet transitioned out of their post‐settlement stage. There should also be a c. 6 cm or c. 100% difference in the FL of post‐settlement and the next oldest age class (1+ snapper) (Francis, 1994), providing adequate resolution to categorise post‐settlement snapper. Larger snapper were categorised as either sub‐adults (c. 10–25 cm FL) or adults (>25 cm FL). A similar methodology was followed for other common coastal fish species where clear size differences were present, although only 0+ and >0+ categories were determined. If no clear and distinct size differences were present, then the presence of the species was noted without size/age categorisation. The term post‐settlement was reserved for snapper as the authors have a more detailed understanding of the specific life‐history requirements of snapper during this stage that may not be applicable to 0+ individuals of other fish species.
2.2. Relative abundance and diet of potential predators of post‐settlement snapper
To provide further insight about potential predators in Whangarei Harbour, gill nets were set to capture fish large enough to eat post‐settlement snapper. This provided two pieces of information, the relative abundance of these potential predators and gut contents which provided insight about diet to better identify which species were most likely to predate on post‐settlement snapper. The authors conducted set netting in March 2021, the time of year when post‐settlement snapper should be abundant, and therefore available as a food source to potential predators. Nets were also set at locations where previous beach seining work had identified seagrass beds and the presence of post‐settlement snapper (Figure 1). Overall four separate sets were conducted: a daytime set and a night‐time set on 2 March 2021, and two night‐time sets on 12 March 2021. The nets used were made up of a combination of 90 and 115 mm nylon mesh panels, with a total net length of 200 m per set on 2 March 2021 and 400 m per set on 12 March 2021. Nets were left to soak for 2 h on 2 March 2021 and 12 h on 12 March 2021. When each net was hauled, fish that had been captured were removed and immediately placed into an ice slurry, with separate containers for each set. Fish were then immediately transported to the Northland Marine Research Centre (NMRC) where each ice slurry container was stored in a chiller until fish could be processed (1– 12 h depending on the time each net was hauled). For each individual fish, its species and length were recorded, and its gastrointestinal tract removed, opened up and immersed in 80% isopropyl alcohol (IPA) until gut contents could be examined.
Preserved gut contents were initially poured onto a 1000 μm sieve, to drain away the IPA. The sieved remains were then spread out on a sorting tray, with the fullness and digestive state of the digestive tract categorised onto a five‐point scale. Because the intention of this diet work was to identify fish species that were predating on post‐settlement snapper, gut content identification was conducted to the lowest taxonomic level that was practically able to be identified in a short (minutes) period of time per sample. This often meant that contents were identified only to the class or order level. This was sufficient to determine the general diet composition of a potential predator, but where a fish was observed in a stomach sample the authors tried to identify it to the species level so as to determine if a post‐settlement snapper had been consumed. For presentation purposes, all of the different items observed within fish guts were sorted into 10 gut content categories. The percentage volume of each of these categories was estimated and recorded, so that the total % volume of all the diet items within each gut added to 100%. The average percentage volume for each gut content category was then calculated for each fish species captured.
2.3. Experimental observations of post‐settlement snapper with a potential predator
Experiments to observe how physical structure influences the behaviour of post‐settlement snapper in the presence of a potential predator were conducted at the NMRC during March to June 2019 and 2020. For each of these years, post‐settlement snapper were initially captured by beach seine netting in Whangarei Harbour and held in c. 50 L tanks with oxygen bubblers while they were transported back to the NMRC and placed into two 700 L tanks receiving flow‐through sea water at 15 L min −1. Hydrogen peroxide [200 ppm (ppm)] or chloromine‐T (5 ppm) treatments were applied as needed to eliminate bacterial or fungal infections. Post‐settlement snapper were fed to satiation with Otohime EP1 (1.3 mm) extruded pellets every second day, with daily removal of mortalities and checks of water temperature and dissolved oxygen and periodic tank cleaning. Post‐settlement snapper were retained in these tanks until required for an experimental trial. Due to this period of acclimation to aquaria facilities and the introduction of a high energy diet, by the time experiments were conducted these snapper had grown to be slightly larger (mean size of 88 and 85 mm FL in 2019 and 2020, respectively) than would usually be expected for post‐settlement snapper (<60 mm FL, Parsons et al., 2014b). Although there was still a large size differential compared to kingfish (40–50 cm FL), the prey size selectivity of kingfish is unknown, but can be quite specific in some other species (Blewett et al., 2006).
Kingfish (Seriola lalandi) were chosen as the potential predator that post‐settlement snapper were exposed to as they have been observed attempting to predate on snapper (Parsons et al., 2018), and because hatchery‐reared kingfish are readily available at the NMRC. As such, prior to the commencement of experimental trials, pairs of kingfish (40–50 cm FL) were captured by hand‐netting from NMRC 25,000 L production tanks and placed into a 1700 L tank located closer to the experimental arena (see below). These kingfish then remained in this tank and were starved to encourage predation, for 5–7 days before being used in an experimental trial.
The experimental arena was an 8000 L circular black tank receiving flow‐through water at 30 L min−1. To initiate a trial, 30 individual post‐settlement snapper were captured with a dip‐net and transferred to the experimental arena, and allowed to acclimate for 24 h. At this stage, two GoPro Hero 3 cameras were suspended on poles on opposite sides of the tank and at an elevation of c. 170 mm from the tank floor. Post‐settlement snapper were then filmed for 1 h before two kingfish were captured from their holding tank with hand‐nets and transferred to the experimental arena. The authors used two kingfish because kingfish are a schooling species and the presence of a conspecific often promotes competitive predatory behaviour towards prey (D. Parsons pers. Obs.). After another hour with the presence of kingfish, the experiment and recording were stopped. The water level in the experimental arena was then lowered so that fish (post‐settlement snapper and kingfish) could be more easily captured with hand‐nets and killed in a solution of Aqui‐S at 150 mg L−1. The treatment that post‐settlement snapper were exposed to during these experimental trials was the presence (or absence for control trials) of physical structure. This structure was provided by an ASU (as per the ASUs described earlier in the section on potential predators of restrained post‐settlement snapper) weighted down in the centre of the experimental arena.
To document the interaction between post‐settlement snapper and kingfish and post‐settlement snapper and ASU structure, an observer first watched a selection of videos both with and without ASUs present. While watching these videos, the observer kept a list of behaviours, movements, locations and associations (hereafter just referred to as behaviours) that were observed. This list was then used to form an ethogram of behaviours, complete with descriptions of each behaviour and the categories that would be assigned to describe it (Table 1). While initially watching these videos, the observer also paid close attention to how behaviours changed through time. A 5 s observation period was decided on, as it provided a balance between being too short (unclear as to which behaviours were being expressed) and too long (multiple behaviours and categories expressed in the same period). The same observer then watched all of the videos, categorising behaviours according to Table 1. Data were recorded by watching a 5 s observation period every minute for the first 15 min when only snapper were present, and then every 2 min thereafter. When kingfish were released into the tank, data recording was done again every minute for the first 15 min and then every 2 min thereafter. To assist with estimating distances within the tank, tape on the tank floor denoted one‐twelfth segments. It is important to note that not all of the behaviours within Table 1 were mutually exclusive. For example, within a single observation period the avoidance, independent movement and predatory aggression behaviours could both occur if at least 25% of snapper or at least one kingfish was expressing each behaviour. For behaviours where categorical responses were recorded (such as depth and speed), the average response was recorded when there was variation within the group of fish.
TABLE 1.
Ethogram of behaviours (movements, locations or associations) expressed by post‐settlement snapper and kingfish predators during experimental trials
| Species | Behaviour | Description |
|---|---|---|
| Snapper | Aggregation | How close the 30 snapper are to each other with “1” being able to fit within an area of one‐twelfth of the tank, “2” able to fit within an area of between one‐twelfth and one‐quarter of the tank, “3” able to fit within an area of between one‐quarter and one‐half of the tank and “4” spread out across at least half of the tank. If snapper were in multiple separate clusters, then no aggregation rating was provided. |
| Avoidance | Snapper actively swimming to avoid and reduce proximity to kingfish. Assigned as either a “Yes” or “No” when at least 25% of snapper expressed this behaviour. Never assigned prior to kingfish being introduced to the arena. | |
| Independent movement | Snapper movements unaffected by the location of the kingfish. Assigned as either a “Yes” or “No” when at least 25% of snapper expressed this behaviour. | |
| Structure use | Snapper remaining in close proximity (c. two body lengths) to structure such as the ASU or the tank wall. Assigned as either a “Yes” or “No” category when at least 25% of snapper were expressing this behaviour. | |
| Speed | Relative measure of swimming speed of the snapper with “1” being slow, “2” being medium and “3” being fast. | |
| Depth | Relative measure of the depth of the snapper with “1” being near the bottom, “2” being midwater and “3” being near the surface. | |
| Kingfish | Aggregation | How close the two kingfish are to each other with “1” being less than one‐twelfth of the tank away from each other, “2”between one‐twelfth and one‐quarter of the tank away from each other, “3” between one‐quarter and one‐half of the tank away from each other and “4” at least half a tank away from each other. |
| Following | Kingfish actively following snapper by tracking their movements behind them as they swim around the tank. Assigned as either a “Yes” or “No” when either of the kingfish expressed this behaviour. | |
| Predatory aggression | Kingfish lunging for or chasing snapper at high speed. Assigned as either a “Yes” or “No” when either of the kingfish expressed this behaviour. | |
| Speed | Relative measure of swimming speed of the kingfish with “1” being slow, “2” being medium and “3” being fast. | |
| Depth | Relative measure of the depth of the kingfish with “1” being near the bottom, “2” being midwater and “3” being near the surface. | |
| Snapper and kingfish | Proximity | Distance between closest kingfish and snapper with “1” being less than one‐twelfth of the tank away from each other, “2” being within one‐twelfth and one‐quarter of the tank away from each other, “3” being within one‐quarter and one‐half of a tank away from each other, “4” being at least half a tank away from each other. |
Note: Each category was assigned a ranking or categorised as “Yes” or “No” (depending on the behaviour type) from a 5 s observation period. The “Yes” or “No” behaviours are not mutually exclusive within a species, so that within a single observation period both the avoidance and independent movement behaviours could be recorded if at least 25% of snapper were expressing each behaviour. ASU, articial seagrass units.
Behavioural data as described earlier were further refined before being analysed. Specifically, behaviours with multiple categories were first broken down into individual response variables representing each category. Only the most extreme categories were retained and used in statistical analysis as preliminary data exploration suggested these extreme categories best captured the behavioural response. For example, the aggregation of post‐settlement snapper was recorded into categories 1, 2, 3 and 4, but individual “Yes” or “No” response variables were created for categories 1 and 4. A similar process was followed for other behaviours with multiple categories. All response variables now had “Yes or “No” response categories. As such, the proportion of observation periods denoted as “Yes” was calculated for periods both before and after the introduction of kingfish into the experimental tank. The obvious exclusion was for behaviours that could not be expressed unless kingfish were present, specifically: the avoidance behaviour of post‐settlement snapper, all kingfish behaviours and the behaviour representing the proximity of post‐settlement snapper and kingfish (only response variables after the introduction of kingfish were included for these variables). This new, larger set of response variables used for data analysis is detailed in Table 2.
TABLE 2.
Behavioural response variables expressed by post‐settlement snapper and kingfish predators during experimental trials and used in statistical analysis
| Species | Period (before or after kingfish introduction) | Behaviour (from Table 1) | Response category represented | Response variable abbreviation |
|---|---|---|---|---|
| Snapper | Before | Aggregation | “1” | SNA_agg_1 |
| Aggregation | “4” | SNA_agg_4 | ||
| Independent movement | NA | SNA_ind | ||
| Structure use | NA | SNA_structure | ||
| Speed | “1” | SNA_speed_1 | ||
| Speed | “3” | SNA_speed_3 | ||
| Depth | “1” | SNA_depth_1 | ||
| Depth | “3” | SNA_depth_3 | ||
| After | Aggregation | “1” | SNA_agg_1_A | |
| Aggregation | “4” | SNA_agg_4_A | ||
| Independent movement | NA | SNA_ind_A | ||
| Structure use | NA | SNA_structure_A | ||
| Speed | “1” | SNA_speed_1_A | ||
| Speed | “3” | SNA_speed_3_A | ||
| Depth | “1” | SNA_depth_1_A | ||
| Depth | “3” | SNA_depth_3_A | ||
| Avoidance | NA | SNA_avoidance | ||
| Kingfish | Aggregation | “1” | KIN_agg_1 | |
| Aggregation | “4” | KIN_agg_4 | ||
| Following | NA | KIN_follow | ||
| Predatory aggression | NA | KIN_pred | ||
| Speed | “1” | KIN_speed_1 | ||
| Speed | “3” | KIN_speed_3 | ||
| Depth | “1” | KIN_depth_1 | ||
| Depth | “3” | KIN_depth_3 | ||
| Snapper and kingfish | Proximity | “1” | Proximity_1 |
Note: Behaviours listed relate to those described on Table 1. Where a behaviour was originally recorded on a categorical scale (i.e., from 1 to 4), only the extreme categories were chosen for use in statistical analysis. The “response category represented” column refers to which of these categories each response variable relates to. NA, not applicable because that behaviour was already a “Yes” or “No” variable. Response variable abbreviations are those as displayed in statistical analysis.
3. STATISTICAL ANALYSIS
3.1. Potential predators of restrained post‐settlement snapper
The community of potential predators observed during restrained post‐settlement snapper camera deployments was initially explored using non‐parametric multi‐dimensional scaling (MDS) ordination. This analysis was performed on a Jaccard resemblance matrix of presence‐absence data. A dummy variable was included because some samples were exactly identical (i.e., samples which did not observe any potential predators). MDS ordination was followed up with hypothesis testing via a one‐way analysis of similarity (ANOSIM) which assessed the influence of daytime category on the observed potential predator community.
3.2. Experimental observations of post‐settlement snapper with a potential predator
The behaviours observed during experimental trials and documented in Table 2 were initially explored using non‐parametric MDS ordination. This analysis was performed on a Jaccard resemblance matrix of presence‐absence data. MDS ordination was followed up with hypothesis testing via a two‐way ANOSIM which assessed the influence of treatment (ASU vs. Control) and Year (2019 vs. 2020) on the behaviours expressed by post‐settlement snapper and kingfish predators. All multivariate analyses were conducted with Primer Version 7 (Devon, United Kingdom), using non‐parametric methods that do not have assumptions of balanced replication and the equivalent of ANOVA variance homogeneity (Clarke & Gorman, 2015).
4. RESULTS
4.1. Potential predators of restrained post‐settlement snapper
A total of 46 individual camera deployments were conducted totalling >110 h of video footage of restrained post‐settlement snapper (Table 3). On six occasions (all at night) separate predators closely approached the restrained post‐settlement snapper, causing it to swim vigorously from one side of the jar to the other (Figure 3). On each of these six occasions the predator remained close to the restrained post‐settlement snapper for an extended period, often lasting many minutes, with the restrained post‐settlement snapper reacting to the presence of the predator often multiple times. Broad squid (Sepioteuthis australis) and conger eel (Conger sp.) were each responsible for three of these six nocturnal predatory interactions (Table 3; Figure 3). In addition to these positive predation encounters, it is important to note occasions when post‐settlement snapper (restrained or not restrained) did not react to a nearby potential predator. This occurred on multiple occasions with large eagle ray (Myliobatis tenuicaudatus).
TABLE 3.
Number of restrained post‐settlement snapper camera deployments, their duration and observed potential predation events by daytime category
| Daytime category | No. of deployments | Hours of video footage | Squid predation attempts | Conger eel predation attempts |
|---|---|---|---|---|
| Day | 36 | 75.97 | 0 | 0 |
| Dusk | 9 | 3.47 | 0 | 0 |
| Night | 9 | 31.11 | 3 | 3 |
Note: On eight occasions two different time categories occurred within an individual camera deployment (e.g., day and dusk or dusk and night categories both occurred within the same individual deployment).
FIGURE 3.

Screenshot images of predators taken during night‐time deployments of restrained post‐settlement snapper (in plastic jar at centre of image). (a) Conger eel and (b) broad squid
The fish and invertebrate community that were filmed around the ASU in general were dominated by snapper (mostly post‐settlement snapper), piper (Hyporhamphus ihi), 0+ trevally (Pseudocaranx georgianus), eagle ray (Myliobatis tenuicaudatus), spotty (Notolabrus celidotus), conger eel and broad squid (Figure 4). MDS ordination indicated that separate fish and invertebrate communities were present at night compared to during the day and at dusk (Figure 5). This was confirmed by a one‐way ANOSIM assessing the influence of daytime category (R = 0.421, significance level = 0.001). Correlations of individual species with the MDS axes were dominated by piper (−0.78), post‐settlement snapper (−0.53), 0+ trevally (−0.46), eagle ray (−0.45) and 0+ spotty (−0.42) (all abundant during the day), as well as adult snapper (0.46) and conger eel (0.44) (abundant at night).
FIGURE 4.

Fish and invertebrate species and age categories (at different times of the day) observed by camera deployments conducted with restrained post‐settlement snapper. Species names not mentioned elsewhere: goatfish (Upeneichthys lineatus).  , Day;
, Day;  , Dusk;
, Dusk;  , Night
, Night
FIGURE 5.

Non‐metric multiple dimensional scaling (MDS) ordination of the presence of fish and invertebrate species and age categories (at different times of the day) observed by camera deployments conducted with restrained post‐settlement snapper. A biplot of Pearson correlations of contributing species/age groups with canonical axes is overlaid on the ordination (correlations >0.4 represented). Abbreviations as follows: 0 + _TRE = 0+ trevally, 0+ STY = 0+ spotty, GAR = piper, EGR = eagle ray, PS_SNA = post‐settlement snapper, A_SNA = adult snapper, CON = conger eel.  , Day time;
, Day time;  , Dusk time;
, Dusk time;  , Night time
, Night time
4.2. Relative abundance and diet of potential predators of post‐settlement snapper
A total of 237 individual fish from 10 different species were captured from the four nets that were set in Whangarei Harbour (Table 4). The most abundant fish species caught were snapper (C. auratus, 83 individuals), trevally (67 individuals), parore (Girella tricuspidata, 29 individuals), grey mullet (Mugil cephalus, 24 individuals) and rig (Mustelus lenticulatus, 21 individuals). The average size of all but two of the species caught [leatherjacket (Parika scaber) and jack mackerel (Trachurus sp.)] was in excess of 300 mm. Of the 237 fish caught, 187 had at least some contents within their digestive tract. Diet varied by species, although some species could be grouped together with relatively similar diets (Figure 6). For example, trevally, rig, snapper and eagle ray had diets dominated by benthic invertebrates such as benthic crustacea, polychaetes, bivalves and shell/sand/gravel; parore and leatherjacket had herbivorous or omnivorous diets dominated by algae/seagrass; kahawai (Arripis trutta) and hammerhead shark (Sphyrna zygaena) had piscivorous diets dominated by teleosts; jack mackerel and grey mullet diets did not fit any categories, with jack mackerel diet dominated by gastropods and grey mullet diet dominated by shell/sand/gravel (Figure 6). Across all of the fish guts examined, 10 individuals contained teleosts as part of their diet. Although the majority of these teleost diet samples were not able to be identified to species level (except for one spotty and one piper), the unidentified teleost samples had sufficient basic morphological features present for the authors to be confident that they were not snapper (e.g., the samples had the wrong body shape or skin colour where it was present).
TABLE 4.
Fish species caught by set net within Whangarei Harbour and associated length distributions
| Fish species | Number caught (number with gut contents present in brackets) | Minimum length | Average length | Maximum length |
|---|---|---|---|---|
| Kahawai (Arripis trutta) | 1(1) | 369 | 369 | 369 |
| Eagle ray (Myliobatis tenuicaudatus) | 3(3) | 258 | 469 | 680 |
| Grey mullet (Mugil cephalus) | 24(6) | 370 | 408 | 510 |
| Hammerhead shark (Sphyrna zygaena) | 5(5) | 600 | 650 | 706 |
| Jack mackerel (Trachurus sp.) | 2(1) | 229 | 236 | 242 |
| Leatherjacket (Meuschenia scaber) | 2(2) | 264 | 276 | 287 |
| Parore (Girella tricuspidata) | 29(29) | 317 | 388 | 447 |
| Snapper (Chrysophrys auratus) | 83(72) | 210 | 301 | 410 |
| Rig (Mustelus lenticulatus) | 21(20) | 410 | 661 | 1005 |
| Trevally (Pseudocaranx georgianus) | 67(48) | 210 | 334 | 525 |
Note: Lengths listed are in millimetre and are either fork length or total length, depending on the species.
FIGURE 6.
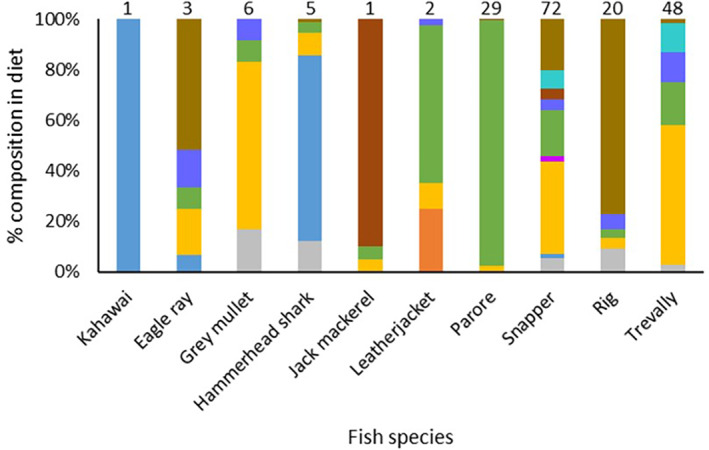
Diet composition (% volume) of fish species captured by set net from four sites in Whangarei Harbour. Numbers above each bar refer to the number of stomachs that contained at least some gut contents for each species.  , Benthic Crustacea;
, Benthic Crustacea;  , Bivalvia;
, Bivalvia;  , Gastropoda;
, Gastropoda;  , Polychaeta;
, Polychaeta;  , Algae/Seagrass;
, Algae/Seagrass;  , Porifera;
, Porifera;  , Shell/sand/gravel;
, Shell/sand/gravel;  , Teleostei;
, Teleostei;  , Tunicata;
, Tunicata;  , Unidentified invertebrate
, Unidentified invertebrate
4.3. Experimental observations of post‐settlement snapper with a potential predator
A total of 12 experimental trials observing the interaction of post‐settlement snapper with a potential kingfish predator were conducted across 2 years. Due to a communication error within the research team, however, no control trials were conducted in the second year.
Overall, there were no successful predation events on post‐settlement snapper across all of the trials, regardless of treatment or the year a trial was conducted in. In general a range of different behaviours were expressed by both post‐settlement snapper and kingfish. The most interaction between kingfish and post‐settlement snapper appeared to occur immediately after kingfish were released into the experimental tank, when kingfish would follow post‐settlement snapper around the tank. MDS ordination illustrated some visual separation of the suite of behaviours expressed by post‐settlement snapper and kingfish depending on whether ASU habitat was present (Figure 7). In particular, a biplot overlay suggested that when ASUs were present, post‐settlement snapper exhibited a strong correlation between structure use (SNA_structure and SNA_structure_A) and MDS axis 1 (−0.74 before and − 0.83 after kingfish were introduced). Conversely, for control trials (i.e., no ASU present) the response variables denoting high levels of aggregation (SNA_agg_4 and SNA_agg_4_A) had strong correlations with MDS axis 1 (0.77 before and 0.71 after kingfish were introduced). Overall, these observations qualitatively suggest that post‐settlement snapper was associated with habitat when it was present, and aggregated together when it was not. Furthermore, the presence or absence of kingfish did not have a large influence on either structure use or aggregation. Two‐way ANOSIM analysis, however, produced a significant year effect (R = 0.852, significance level = 0.012), but not a significant effect of the presence of ASU structure (R = 0.593, significance level = 0.1).
FIGURE 7.

Non‐metric multiple dimensional scaling (MDS) ordination of behaviours expressed by post‐settlement snapper and kingfish predators [with and without the presence of physical structure provided by artificial seagrass units (ASUs)] as observed in tank experiment trials conducted at the the Northland Marine Research Centre (NMRC). Each data point is labelled with the year in which that particular experimental trial was conducted. A biplot of Pearson correlations of contributing behaviours (labels as per Table 2) with canonical axes is overlaid on the ordination (correlations >0.65 represented).  , Control treatment;
, Control treatment;  , ASU
, ASU
5. DISCUSSION
This study investigated potential predators of post‐settlement snapper and whether predation might be linked to habitat association. Perhaps the main result was the seemingly low levels of interaction with predators. This occurred despite three separate lines of investigation that included >100 h of video observation of post‐settlement snapper, some of the first night‐time observations of potential snapper predators enabled by infrared illumination, diet assessment for a large number of individual potential predators and experimental manipulations of predators and post‐settlement snapper. In other systems the interaction between juvenile fish and predators is often conspicuous (Adams et al., 2004; Hixon & Carr, 1997; Holbrook & Schmitt, 2002; Steele & Forrester, 2002), but it seems in this system predation may influence post‐settlement snapper and their strong habitat association in more subtle ways (Preisser et al., 2005).
As mentioned earlier, the investigation of potential predators through the use of restrained post‐settlement snapper provided a comprehensive volume of remotely recorded observations. Daytime recordings did not observe any predation attempts, and the fish communities observed were lacking in potential predators (dominated by post‐settlement snapper, 0+ trevally, piper and eagle ray). This lack of potential predators could have potentially been influenced by the site selected, as habitats lacking in structure and disconnected from reefs are known to contain fewer predators (Dorenbosch et al., 2009; Grol et al., 2011) and the predators that are present are more likely to be vagile or transient piscivores (Ault & Johnson, 1998; Hixon & Carr, 1997). Although eagle ray are large enough to predate on post‐settlement snapper, there was a lack of reaction from post‐settlement snapper towards passing eagle ray. Given the strong reaction of the restrained snapper to night‐time predation attempts (see below), this may rule eagle ray out as an important predator.
Despite the reduced field of view illuminated at night, three species or life stages that were rare or absent during the day (adult snapper, conger eel and broad squid) dominated nocturnal fish communities. Nocturnal fish communities are often overlooked (Bassett & Montgomery, 2011) but, in some cases, have been found to exert more predation impact than even more abundant diurnal fish communities (Danilowicz & Sale, 1999; Helfman, 1978). In the present study two nocturnal potential predators (conger eel and broad squid) were revealed for the first time. On multiple occasions conger eel and broad squid attempted to predate on restrained snapper. Although these simple observations are not able to be extrapolated to properly understand the scale of the predation effect that these two predators represent, further investigation focusing on these two species (potentially targeted diet work) might be informative. Alternatively, it is possible that the importance of diurnal predators was underestimated due to an artefact of the sampling method (Peterson & Black, 1994). Specifically, active diurnal piscivores, such as kingfish, may have been less likely to attack post‐settlement snapper that were unable to express a full range of behaviours and therefore visual cues when restrained within a jar. Alternatively, visual cues are less likely to be important to the nocturnal broad squid and conger eel. Although traditional tethering may have proved more appealing to diurnal predators, this was not possible due to animal ethics considerations.
Diet analysis of fish caught by set net within Whangarei Harbour did not identify any predation on snapper. Although this result is similar to that of Williams (2009), it is worth noting a difference of the present study in that nets were specifically set at a time of year and at locations where post‐settlement snapper were abundant. Despite the lack of post‐settlement snapper, set netting did confirm the presence of some piscivorous fish species, specifically kahawai and hammerhead shark. Further investigation of the diet of these two species may be warranted, although obtaining appropriate sample sizes may prove challenging considering that catch rates are likely to be low and that the consumption of small fishes by these predators can be extremely sporadic (Baker & Sheaves, 2009b). As such, a much more extensive and temporally replicated set netting effort would likely be required to identify important predators. Another relevant observation was the lack of or low proportion of fish in the diet of a number of common estuarine fish species that were large enough to predate on post‐settlement snapper, such as trevally, rig, eagle ray and larger snapper. This may suggest these species are not ecologically relevant predators of post‐settlement snapper, although in other systems “minor” piscivores have been observed to inflict greater mortality than more conspicuous piscivores (Baker & Sheaves, 2009a), especially on immediately post‐settlement stages which are known to be more vulnerable (Almany, 2004). To this end, the sheer abundance of snapper in general [over 90% of fish biomass in a recent trawl survey was snapper (Parsons et al., 2021)], however, could potentially suggest that even low individual rates of piscivory and cannibalism could add up to being important to post‐settlement snapper as a whole. Further to this, cannibalism has been observed in snapper previously (Lohrer et al., 2008). Overall, the observations of the diet study conducted here largely agree with that of a comprehensive study of fish diet within two northeastern New Zealand estuaries (Williams, 2009). Another piscivorous species that Williams (2009) identified that the authors of this study did not, however, is the spotted stargazer (Genyagnus monopterygius). As such, spotted stargazer are another species where more focused diet assessment may be justified.
Although the lack of replication of control trials in the second year of tank experiments was ultimately limiting, there was some indication that post‐settlement snapper used habitat when it was present, and aggregated together when it was not. Although not statistically significant, this result is consistent with the use of structure by post‐settlement snapper in field studies (Parsons et al., 2013; Parsons et al., 2014a), with elevated habitat use observed under predation threat by Ross et al. (2007), and an adaptive antipredator defence strategy (i.e., schooling) based on environmental context (e.g., Creel et al., 2014). The lack of observations of actual predation, however, is a limitation as it prevented comparison of survival rates with and without the presence of habitat structure. Longer trials may have encouraged predation by allowing predators to become accustomed to the experimental tank or for low probability events such as predation to play out (previous predation trials have been conducted over periods of 24 h or more: Lindholm et al., 1999; Manderson et al., 2000; Adams et al., 2004; Scharf et al., 2006). Longer trials, however, were not a possibility here due to animal ethics considerations, but the use of a divider within the tank to allow predator acclimation before the trial started may have been prudent. In addition, the use of kingfish that were not experienced with live prey may have influenced the level of predatory aggression expressed by kingfish and the perception of threat expressed by post‐settlement snapper. Although some have indeed observed a reduction in predation for fish that are not experienced with live prey (Ellis et al., 2002), others have observed some level of predation for inexperienced fish and there is sometimes little difference in predation rates compared to wild fish (Donadelli et al., 2015; Gillen et al., 1981; Paszkowski & Olla, 1985). For hatchery‐reared juvenile kingfish, the authors know that these fish display aggression and even cannibalise other kingfish that are slightly smaller than them (S. Pether, NIWA, pers. comm., Moran, 2007). Therefore, although some level of predatory aggression maybe innate, training the kingfish used in the experiment that juvenile snapper were a potential food source would likely have improved the experiment. For example, acclimation of hatchery‐reared fish to wild environments prior to release can improve behavioural competence leading to higher survival (Brennan et al., 2006), suggesting that an improvement in predation competence might also have been achieved if kingfish were trained. Although it is likely the kingfish did not fully perceive the juvenile snapper as food, the snapper themselves were wild caught, so likely perceived the kingfish as a predator. Furthermore, that the presence of kingfish predators did not change the response of the snapper (they used structure when it was present and schooled when it was not) is consistent with how an antipredation strategy should be effectively applied; a defensive strategy is likely to work best if it is being applied before a predator is present. As such, although this predation trial could undoubtedly have been improved, it still provided some useful insights.
The nocturnal observations of predation attempts on post‐settlement snapper presented here are a new observation and could represent a worthy future avenue of more focused investigation. It is possible, however, that other mechanisms are involved in this habitat association (e.g., shelter from water flow Parsons et al., 2018), or that predation is occurring in a temporally pulsed or spatially patchy nature making it difficult to observe. As such, predation can appear to be rare, given the methods of sampling employed, but is likely to be ecologically significant because it either occurred at different times or places to where and when the authors were observing (Baker & Sheaves, 2009b), or because the mere threat of predation had already caused post‐settlement snapper to modify their behaviour (such as the selection of habitat to occupy) to avoid predation in the first place (Abrams, 1993; Baker & Sheaves, 2007; Preisser et al., 2005). A potentially more fruitful line of investigation to understanding the mechanisms associated with this crucial life stage would be to indirectly observe the effect of predation on the abundance of post‐settlement snapper using predator exclosure cages and potentially individual marking. Such an approach has the advantage of accumulating the influence of predation within census numbers of the animal of interest without the need to directly observe infrequent events, and has been successfully applied in other systems (Heinlein et al., 2010; Hixon & Carr, 1997; Steele & Forrester, 2002).
AUTHOR CONTRIBUTIONS
Conceptualisation (PD), experimental design (PD, TR, MC, GG, LD), data collection (PD, TR, HR, MC, GY, LD), animal husbandry (GY, LD, HR), data analysis (PD, TR), writing – original draft preparation (PD), writing – review and editing (PD, TR, HR, MC, GY, LD).
CONFLICTS OF INTEREST
The authors have no conflicts of interest to declare.
AVAILABILITY OF DATA AND MATERIAL
The data sets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
ETHICS APPROVAL
The use of animals for the observations and experiments described in this article were approved by the NIWA Animal Ethics Committee (AEC215).
ACKNOWLEDGEMENTS
Funding for this project was provided by NIWA's Coasts and Oceans research programme 3 (2017/18 Statement of Corporate Intent). We thank Chris Ray for constructing the infrared illuminators and camera set‐up as well as Steve Pether, Glenn Irvine and the staff at NIWA's Northland Marine Research Centre for supporting the tank experiments. Thanks to Ricky Jones and Greg Hayes for set netting work and Ian Tuck (Fisheries New Zealand) for statistical advice. Open access publishing facilitated by National Institute of Water and Atmospheric Research, as part of the Wiley ‐ National Institute of Water and Atmospheric Research agreement via the Council of Australian University Librarians.
Parsons, D. , Taylor, R. , Hughes, R. , Middleton, C. , Gublin, Y. , & Levell, D. (2022). Predators and habitat association of post‐settlement snapper (Chrysophrys auratus). Journal of Fish Biology, 101(6), 1509–1521. 10.1111/jfb.15222
Funding information NIWA's Coasts and Oceans research programme 3 (2017/18 Statement of Corporate Intent)
REFERENCES
- Abrams, P. A. (1993). Why predation rate should not be proportional to predator density. Ecology, 74, 726–733. [Google Scholar]
- Adams, A. J. , Locascio, J. V. , & Robbins, B. D. (2004). Microhabitat use by a post‐settlement stage estuarine fish: Evidence from relative abundance and predation among habitats. Journal of Experimental Marine Biology and Ecology, 299, 17–33. [Google Scholar]
- Almany, G. R. (2004). Priority effects in coral reef fish communities on the Great Barrier Reef. Ecology, 85, 2872–2880. [Google Scholar]
- Ault, T. R. , & Johnson, C. R. (1998). Spatially and temporally predictable fish communities on coral reefs. Ecological Monographs, 68, 25–50. [Google Scholar]
- Baker, R. , & Sheaves, M. (2007). Shallow‐water refuge paradigm: Conflicting evidence from tethering experiments in a tropical estuary. Marine Ecology Progress Series, 349, 13–22. [Google Scholar]
- Baker, R. , & Sheaves, M. (2009a). Overlooked small and juvenile piscivores dominate shallow‐water estuarine “refuges” in tropical Australia. Estuarine, Coastal and Shelf Science, 85, 618–626. [Google Scholar]
- Baker, R. , & Sheaves, M. (2009b). Refugees or ravenous predators: Detecting predation on new recruits to tropical estuarine nurseries. Wetlands Ecology and Management, 17, 317–330. [Google Scholar]
- Bassett, D. K. , & Montgomery, J. C. (2011). Investigating nocturnal fish populations in situ using baited underwater video: With special reference to their olfactory capabilities. Journal of Experimental Marine Biology and Ecology, 409, 194–199. [Google Scholar]
- Beck, M. W. , Heck, K. L. , Able, K. W. , Childers, D. L. , Eggleston, D. B. , Gillanders, B. M. , … Weinstein, M. P. (2001). The identification, conservation, and management of estuarine and marine nurseries for fish and invertebrates. Bioscience, 51, 633–641. [Google Scholar]
- Blewett, D. A. , Hensley, R. A. , & Stevens, P. W. (2006). Feeding habits of common Snook, Centropomus undecimalis, in Charlotte Harbor, Florida. Gulf and Caribbean Research, 18, 1–14. [Google Scholar]
- Brennan, N. P. , Darcy, M. C. , & Leber, K. M. (2006). Predator‐free enclosures improve post‐release survival of stocked common Snook. Journal of Experimental Marine Biology and Ecology, 335, 302–311. [Google Scholar]
- Clarke, K. R. & Gorman, R. N. (2015). PRIMER c7: User manual/tutorial. Available from: http://updates.primer-e.com/primer7/manuals/User_manual_v7a.pdf [Accessed 16/8/2022]. 300 p.
- Creel, S. , Schuette, P. , & Christianson, D. (2014). Effects of predation risk on group size, vigilance, and foraging behavior in an African ungulate community. Behavioral Ecology, 25, 773–784. [Google Scholar]
- Dahlgren, C. P. , & Eggleston, D. B. (2000). Ecological processes underlying ontogenetic habitat shifts in a coral reef fish. Ecology, 81, 2227–2240. [Google Scholar]
- Dahlgren, C. P. , Todd, G. T. , Adams, A. J. , Gillanders, B. M. , Kendall, M. S. , Layman, C. A. , … Serafy, J. E. (2006). Marine nurseries and effective juvenile habitats: Concepts and applications. Marine Ecology Progress Series, 312, 291–295. [Google Scholar]
- Danilowicz, B. S. , & Sale, P. F. (1999). Relative intensity of predation on the French grunt, Haemulon flavolineatum, during diurnal, dusk, and nocturnal periods on a coral reef. Marine Biology, 133, 337–343. [Google Scholar]
- Donadelli, V. , Longobardi, A. , Finoia, M. G. , & Marino, G. (2015). Feeding hatchery‐reared dusky grouper Epinephelus marginatus juveniles on live prey: Implications for restocking. Environmental Biology of Fishes, 98, 1757–1766. [Google Scholar]
- Dorenbosch, M. , Grol, M. G. G. , de Groene, A. , van der Velde, G. , & Nagelkerken, I. (2009). Piscivore assemblages and predation pressure affect relative safety of some back‐reef habitats for juvenile fish in a Caribbean bay. Marine Ecology Progress Series, 379, 181–196. [Google Scholar]
- Ellis, T. , Hughes, R. N. , & Howell, B. R. (2002). Artificial dietary regime may impair subsequent foraging behaviour of hatchery‐reared turbot released into the natural environment. Journal of Fish Biology, 61, 252–264. [Google Scholar]
- Forrester, G. E. , & Steele, M. A. (2004). Predators, prey refuges, and the spatial scaling of density‐dependent prey mortality. Ecology, 85, 1332–1342. [Google Scholar]
- Gillen, A. L. , Stein, R. A. , & Carline, R. F. (1981). Predation by pellet‐reared tiger muskellunge on minnows and bluegills in experimental systems. Transactions of the American Fisheries Society, 110, 197–209. [Google Scholar]
- Grol, M. G. G. , Nagelkerken, I. , Rypel, A. L. , & Layman, C. A. (2011). Simple ecological trade‐offs give rise to emergent cross‐ecosystem distributions of a coral reef fish. Oecologia, 165, 79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harter, S. L. , & Heck, K. L. (2006). Growth rates of juvenile pinfish (Lagodon rhomboides): Effects of habitat and predation risk. Estuaries and Coasts, 29, 318–327. [Google Scholar]
- Heck, K. L., Jr. , Hays, G. , & Orth, R. J. (2003). Critical evaluation of the nursery role hypothesis for seagrass meadows. Marine Ecology Progress Series, 253, 123–136. [Google Scholar]
- Heinlein, J. M. , Stier, A. C. , & Steele, M. A. (2010). Predators reduce abundance and species richness of coral reef fish recruits via non‐selective predation. Coral Reefs, 29, 527–532. [Google Scholar]
- Helfman, G. S. (1978). Patterns of community structure in fishes: Summary and overview. Environmental Biology of Fishes, 3, 129–148. [Google Scholar]
- Hixon, M. A. , & Carr, M. H. (1997). Synergistic predation, density dependence, and population regulation in marine fish. Science, 277, 946–949. [Google Scholar]
- Holbrook, S. J. , & Schmitt, R. J. (2002). Competition for shelter space causes density‐dependent predation mortality in damselfishes. Ecology, 83, 2855–2868. [Google Scholar]
- Jiang, W. M. , & Carbines, G. (2002). Diet of blue cod, Parapercis colias, living on undisturbed biogenic reefs and on seabed modified by oyster dredging in Foveaux Strait, New Zealand. Aquatic Conservation: Marine and Freshwater Ecosystems, 12, 257–272. [Google Scholar]
- Kobayashi, R. , Endo, M. , Yoshizaki, G. , & Takeuchi, T. (2002). Sensitivity of tilapia to infrared light measured using a rotating striped drum differs between two strains. Nippon Suisan Gakkaishi, 68, 646–651. [Google Scholar]
- Lindholm, J. B. , Auster, P. J. , & Kaufman, L. S. (1999). Habitat‐mediated survivorship of juvenile (0‐year) Atlantic cod Gadus morhua. Marine Ecology Progress Series, 180, 247–255. [Google Scholar]
- Lohrer, D. , Townsend, M. , Morrison, M. & Hewitt, J. (2008). Change in the benthic assemblages of the Waitemata Harbour: Invasion risk as a function of community structure. Biosecurity New Zealand Technical Paper No.: 2008/17. Available from https://www.marinebiosecurity.org.nz/changes-in-marine-systems-over-time/ [accessed November 6, 2021].
- Manderson, J. P. , Phelan, B. A. , Stoner, A. W. , & Hilbert, J. (2000). Predator‐prey relations between age‐1 + summer flounder (Paralichthys dentatus, Linnaeus) and age‐0 winter flounder (Pseudopleuronectes americanus, Walbaum): Predator diets, prey selection, and effects of sediments and macrophytes. Journal of Experimental Marine Biology and Ecology, 251, 17–39. [DOI] [PubMed] [Google Scholar]
- Matsuo, M. , Kamei, Y. , & Fukamachi, S. (2021). Behavioural red‐light sensitivity in fish according to the optomotor response. Royal Society Open Science, 8, 210415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran, D. (2007). Size heterogeneity, growth potential and aggression in juvenile yellowtail kingfish (Seriola lalandi Valenciennes). Aquaculture Research, 38, 1254–1264. [Google Scholar]
- Morrison, M. A. , Lowe, M. L. , Grant, C. , Smith, P. J. , Carbines, G. , Reed, J. , … Brown, J. (2014). Seagrass meadows as biodiversity and productivity hotspots. New Zealand Aquatic Environment and Biodiversity Report (Vol. 2014; No. 137. Available from:). Ministry for Primary Industries. https://www.mpi.govt.nz/document-vault/4409. [Google Scholar]
- Nakamura, Y. , Hirota, K. , Shibuno, T. , & Watanabe, Y. (2012). Variability in nursery function of tropical seagrass beds during fish ontogeny: Timing of ontogenetic habitat shift. Marine Biology, 159, 1305–1315. [Google Scholar]
- Parsons, D. , Morrison, M. , Thrush, S. F. , Middleton, C. , Smith, M. , Spong, K. , & Buckthought, D. (2013). The influence of habitat structure on juvenile fish in a New Zealand estuary. Marine Ecology, 34, 492–500. [Google Scholar]
- Parsons, D. M. , Bian, R. , Parkinson, D. & MacGibbon, D. J. (2021). Trawl surveys of the Hauraki Gulf and Bay of Plenty in 2019 abd 2020 to estimate the abundance of juvenile snapper. New Zealand Fisheries Assessment Report 2021/08.
- Parsons, D. M. , Buckthought, D. , Middleton, C. , & MacKay, G. (2016). Relative abundance of snapper (Chrysophrys auratus) across habitats within an estuarine system. New Zealand Journal of Marine and Freshwater Research, 50, 358–370. [Google Scholar]
- Parsons, D. M. , MacDonald, I. , Buckthought, D. , & Middleton, C. (2018). Do nursery habitats provide shelter from flow for juvenile fish? PLoS One, 13, e0186889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons, D. M. , Middleton, C. , Smith, M. D. , & Cole, R. G. (2014a). The influence of habitat availability on juvenile fish abundance in a northeastern New Zealand estuary. New Zealand Journal of Marine and Freshwater Research, 48, 216–228. [Google Scholar]
- Parsons, D. M. , Middleton, C. , Spong, K. T. , Mackay, G. , Smith, M. D. , & Buckthought, D. (2015). Mechanisms explaining nursery habitat association: How do juvenile snapper (Chrysophrys auratus) benefit from their nursery habitat? PLoS One, 10, e0122137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons, D. M. , Sim‐Smith, C. J. , Cryer, M. , Francis, M. P. , Hartill, B. , Jones, E. G. , … Zeldis, J. (2014b). Snapper (Chrysophrys auratus): A review of life history and key vulnerabilities in New Zealand. New Zealand Journal of Marine and Freshwater Research, 48, 256–283. [Google Scholar]
- Paszkowski, C. A. , & Olla, B. L. (1985). Foraging behavior of hacthery‐produced coho salmon (Oncorhynchus‐kisutch) smolts on live prey. Canadian Journal of Fisheries and Aquatic Sciences, 42, 1915–1921. [Google Scholar]
- Peterson, C. H. , & Black, R. (1994). An experimenatalists challenge—when artifacts of intervention interact with treatments. Marine Ecology Progress Series, 111, 289–297. [Google Scholar]
- Preisser, E. L. , Bolnick, D. I. , & Benard, M. F. (2005). Scared to death? The effects of intimidation and consumption in predator‐prey interactions. Ecology, 86, 501–509. [Google Scholar]
- Ross, P. M. , Thrush, S. F. , Montgomery, J. C. , Walker, J. W. , & Parsons, D. M. (2007). Habitat complexity and predation risk determine juvenile snapper (Pagrus auratus) and goatfish (Upeneichthys lineatus) behaviour and distribution. Marine and Freshwater Research, 58, 1144–1151. [Google Scholar]
- Ryer, C. H. , Laurel, B. J. , & Stoner, A. W. (2010). Testing the shallow water refuge hypothesis in flatfish nurseries. Marine Ecology Progress Series, 415, 275–282. [Google Scholar]
- Scharf, F. S. , Manderson, J. P. , & Fabrizio, M. C. (2006). The effects of seafloor habitat complexity on survival of juvenile fishes: Species‐specific interactions with structural refuge. Journal of Experimental Marine Biology and Ecology, 335, 167–176. [Google Scholar]
- Steele, M. A. (1998). The relative importance of predation and competition in two reef fishes. Oecologia, 115, 222–232. [DOI] [PubMed] [Google Scholar]
- Steele, M. A. (1999). Effects of shelter and predators on reef fishes. Journal of Experimental Marine Biology and Ecology, 233, 65–79. [Google Scholar]
- Steele, M. A. , & Forrester, G. E. (2002). Early postsettlement predation on three reef fishes: Effects on spatial patterns of recruitment. Ecology, 83, 1076–1091. [Google Scholar]
- Tupper, M. , & Boutilier, R. G. (1997). Effects of habitat on settlement, growth, predation risk and survival of a temperate reef fish. Marine Ecology Progress Series, 151, 225–236. [Google Scholar]
- Williams, P. J. (2009). Diets of larger (>10cm) fish in two North‐Eastern New Zealand estuaries (p. 63). Unpublished MSc thesis, University of Auckland. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.


