Abstract
Chronic ethanol exposure affects the glutamatergic system in several brain reward regions including the nucleus accumbens (NAc). Our laboratory has shown that chronic exposure to ethanol reduced the expression of glutamate transporter 1 (GLT-1) and cystine/glutamate exchanger (xCT) and, as a result, increased extracellular glutamate concentrations in the NAc of alcohol-preferring (P) rats. Moreover, previous studies from our laboratory reported that chronic ethanol intake altered the expression of certain metabotropic glutamate receptors in the brain. In addition to central effects, chronic ethanol consumption induced liver injury, which is associated with steatohepatitis. In the present study, we investigated the effects of chronic ethanol consumption in the brain and liver. Male P rats had access to a free choice of ethanol and water bottles for five weeks. Chronic ethanol consumption reduced GLT-1 and xCT expression in the NAc shell but not in the NAc core. Furthermore, chronic ethanol consumption increased fat droplet content as well as peroxisome proliferator-activated receptor alpha (PPAR-α) and GLT-1 expression in the liver. Importantly, treatment with the novel beta-lactam compound, MC-100093, reduced ethanol drinking behavior and normalized the levels of GLT-1 and xCT expression in the NAc shell as well as normalized GLT-1 and PPAR-α expression in the liver. In addition, MC-100093 attenuated ethanol-induced increases in fat droplet content in the liver. These findings suggest that MC-100093 may be a potential lead compound to attenuate ethanol-induced dysfunction in the glutamatergic system and liver injury.
SIGNIFICANCE STATEMENT
This study identified a novel beta-lactam, MC-100093, that has demonstrated upregulatory effects on GLT-1. MC-100093 reduced ethanol drinking behavior and normalized levels of GLT-1 and xCT expression in the NAc shell as well as normalized GLT-1 and PPAR-α expression in the liver. In addition, MC-100093 attenuated ethanol-induced increases in fat droplet content in the liver.
Introduction
Alcohol use disorders (AUDs) are complex chronic relapsing disorders that lead to long-lasting neuroadaptations of neurotransmitter systems. Changes in the glutamatergic system are implicated in the development and maintenance of ethanol dependence (Kalivas, 2009). Dysregulation of the glutamatergic system in these key brain reward regions of the mesocorticolimbic (MCL) system such as the nucleus accumbens (NAc), basal lateral amygdala, prefrontal cortex, and hippocampus are commonly observed consequences of chronic ethanol exposure, including altered expression of glutamate receptors and transporters in key reward brain regions (Goodwani et al., 2017). Among other brain regions, the NAc has been extensively studied for its crucial role in the development of substance use disorders (SUDs), including ethanol (Heinze et al., 2009; Neasta et al., 2011; Müller et al., 2016). Chronic ethanol exposure reduced the expression of glutamate transporter 1 (GLT-1) and cystine/glutamate exchanger (xCT and, consequently, increased extracellular glutamate concentration in the NAc of alcohol-preferring (P) rats (Bridges et al., 2012; Alhaddad, Kim et al., 2014; Das et al., 2015). These effects were suggested to be mediated through reduced synaptic glutamate clearance and diminished metabotropic glutamate receptor 2 (mGluR2) associated with inhibition of synaptic release. xCT is colocalized with GLT-1 in astrocytes to regulate extracellular glutamate concentrations. Reduction in xCT expression may cause a decrease in extracellular glutamate concentration in the brain, which can lead to loss of glutamatergic tone on presynaptic mGluR2/3, consequently increasing synaptic glutamate release (Moran et al., 2005; Javitt et al., 2011). We suggest that GLT-1, xCT, and mGluR2/3 are important to regulate extracellular glutamate concentrations in the brain. Moreover, treatment with GLT-1 modulators [e.g., ceftriaxone (CEF) and MS-153] (Fig. 1) upregulated GLT-1 and xCT expression in the NAc while attenuating ethanol drinking behaviors (Alhaddad, Das et al., 2014; Alhaddad, Kim et al., 2014). These studies suggest that glutamate transporters may be potential targets for the treatment of AUDs. Neuroadaptive changes involved in AUDs include downregulation of metabotropic glutamate receptor 5 (mGluR5) and its signaling pathway (Carroll, 2008; Leurquin-Sterk et al., 2018). mGluR5 has also been shown to be involved in relapse to ethanol intake as shown in a mGluR5 knockout mouse model (Parkitna et al., 2013). Additionally, 2-methyl-6-(phenylethynyl)pyridine antagonized mGluR5 action and reduced ethanol consumption in mice (Olive et al., 2005). Moreover, mGluR1 signaling within the NAc shell has a crucial role in maintaining ethanol consumption under limited access in mice (Lum et al., 2014). The expression of mGluR1 is increased in animals exposed to ethanol (Obara et al., 2009). Furthermore, mGluR1 antagonism was associated with attenuation of ethanol drinking behavior (Besheer et al., 2008a, Lum et al., 2014). These latter findings provide further support for the hypothesis that manipulation of glutamate transporters and/or receptors may serve as effective pharmacotherapeutics to treat AUDs.
Fig. 1.
Chemical structure of GLT-1 uptake modulators: MS-153, CEF, and MC-100093. MC-100093 synthesized by structural modification of the cephalosporin ring system and side chains of CEF.
In addition to its effects in the brain, ethanol exposure causes chronic liver injury, particularly steatohepatitis (De la Monte et al., 2009; Zeng et al., 2019). Peroxisome proliferator-activated receptors (PPAR-α and PPAR-γ) play critical roles in adipose expansion and in the control of its functions (You and Crabb 2004; Anghel and Wahli, 2007). Ethanol-induced fatty liver has been linked to the blocking of PPAR-α activity both in vitro (Fischer et al., 2003) and in vivo (Hong et al., 2004; Nanji et al., 2004). Moreover, PPAR agonists reduce ethanol drinking behavior (Stopponi et al., 2011), neurodegeneration (Mandrekar-Colucci et al., 2013), and ethanol-induced liver injury (Enomoto et al., 2003). It is noteworthy that ethanol, in addition to its direct action on the brain, may impair neurotransmitter function required for certain aspects of neuroplasticity, learning, and memory mediated through biochemical feedback in the liver–brain axis (De la Monte et al., 2009). Accordingly, these studies suggest a role for the liver–brain axis in ethanol dependence.
β-lactam compounds have shown promise as pharmacotherapeutics to treat SUDs, including AUDs. CEF is a prototypical β-lactam antibiotic that has been shown to reduce ethanol intake, while upregulating GLT-1 and xCT levels in the MCL (Rao and Sari, 2012). However, because CEF is an antibiotic, efforts have been made to construct β-lactam products that lack antibiotic activity. For instance, structural modification of the cephalosporin ring system and side chains of CEF resulted in the creation of a novel beta-lactam compound, MC-100093 (Fig. 1), which has enhanced GLT-1 upregulatory properties compared with CEF (Childers et al., 2020; Abou-Gharbia et al., 2017). In vitro pharmacokinetics and pharmacology and in vivo pharmacokinetic data for MC-100093 have been previously reported (Knackstedt et al., 2021). MC-100093 enhanced glutamate uptake in an astrocyte–neuron coculture model by 23.5% over that of control values with an ED50 value of 0.1 μM. CEF displayed similar efficacy but was significantly less potent (ED50 approximately 3 μM). Unlike CEF, MC-100093 showed no antimicrobial effects against select gram-positive and gram-negative bacteria at concentrations up to 256 μg/mL. MC-100093 displayed no affinity for 43 G-protein coupled receptors and transporters at a concentration of 10 μM as assessed through the National Institute of Mental Health Psychoactive Drug Screening Program (Besnard et al., 2012). In vitro, MC-100093 demonstrated high aqueous solubility (>10 mM in 2% DMSO/PBS), high stability in rodent and human liver microsomes, low binding to plasma protein (35% bound), and low partitioning into lipid membranes (83% free, unbound). In vivo pharmacokinetic studies found that MC-100093 displayed reasonable bioavailability following oral administration (F% = 28%). The lack of significant oxidative metabolism suggests that bioavailability following intraperitoneal administration should be similar to that found with oral dosing. The brain-to-plasma ratio for MC-100093 was 0.28 (28%). In vivo half-life was found to be moderate (4.2 hours i.p., 5.26 hours p.o.) and was likely the result of renal elimination, given the lack of oxidative metabolism found in liver microsomes and the long half-life found with MC-100093 in plasma (t1/2 ≫ 240 minutes). These results contrast with those revealed with CEF, which displays essentially no significant oral bioavailability and very low brain penetration (brain-to-plasma ratio = 1%) (Granero et al., 1995).
Given the above, we hypothesized that treatment with MC-100093 would upregulate GLT-1 in key MCL regions while attenuating ethanol intake. Using P rats as an established animal model of chronic ethanol drinking and AUD (Bell et al., 2017), we investigated the effects of chronic ethanol exposure and MC-100093 treatment on glutamatergic receptor and transporter levels as well as that of PPARs in the liver and brain.
Materials and Methods
Animals
Male P rats were received from Indiana University School of Medicine (Indianapolis, IN, USA), and were housed in a 21°C vivarium on a standard 12/12-hour light/dark cycle. Rats had a free access to water and food throughout the experiment. Institutional Animal Care and Use Committee of the University of Toledo approved all experimental procedures, in accordance with the guidelines governing the use of animals in research of National Institutes of Health as described in the Guide for the Care and Use of Laboratory Animals.
Ethanol Drinking Protocol
We used an ethanol drinking procedure as described previously (Alhaddad, Kim, et al., 2014). Briefly, animals started the experimental drinking procedure at an age of 90 days. Rats had five weeks of free-choice access to three-bottle choice drinking, 0%, 15% and 30%, v/v ethanol. Daily measurement of ethanol intake was performed (grams of ethanol intake/kg of body weight/day). We measured the baseline ethanol drinking by averaging the intake during the last three weeks. Three groups of P rats (n = 8–9/group) were exposed to ethanol for five weeks. At week 6, rats received either MC-100093 (50 mg/kg, i.p.; ethanol–MC-100093 group), CEF (200 mg/kg, i.p.; ethanol–CEF group, which served as a positive control), or equivolume of saline vehicle (ethanol group) for five days. Following criteria for the development of ethanol dependence, rats whose average ethanol intake ≤ 4 g/kg/day were excluded from the study (Li et al., 1987; Bell et al., 2012; Sari and Sreemantula, 2012). Beside the ethanol exposed groups, another group of P rats (n = 8), was exposed to only water and food throughout the exposure procedure (control group). This procedure results in pharmacologically relevant blood ethanol concentration (50–200 mg%) (Bell et al., 2006).
Brain and Liver Tissue Dissection
At the completion of pharmacological challenges while the rats had free-choice access to ethanol, P rats were removed from their home cage and rapidly euthanized by CO2 inhalation followed by decapitation with the guillotine the day following the fifth and final injection. Brains were isolated and immediately frozen on dry ice and stored at −80°C. The stereotaxic coordinates provided by Paxinos et al. (2007) rat brain stereotactic atlas were used to isolate NAc core and NAc shell using a cryostat apparatus (−20°C), and samples were returned to −80°C for subsequent Western blot analyses. We used a micropunch procedure under a laboratory microscope (10×) to dissect the NAc core and NAc shell. Liver samples were extracted after decapitation, with the anterior-ventral lobe removed and stored at −20°C for subsequent analyses.
Western Blot Analyses
Western blot assays were performed to measure the expression of GLT-1, xCT, mGluR1, mGluR5, PPAR-α, PPAR-γ, glyceraldehyde-3-phosphate dehydrogenase, and β-tubulin in the NAc core and NAc shell as has been done in previous studies from our laboratory (Alhaddad, Das et al., 2014, Alhaddad, Kim et al., 2014). Liver, NAc core, and NAc shell tissues were lysed using a lysis buffer containing protease and phosphatase inhibitors. A detergent compatible protein assay (Bio-Rad, Hercules, CA, USA) was used to quantify the amount of protein for each sample. Samples were mixed with Laemmli dye and loaded with equal amounts on polyacrylamide gels (10%) for protein separation using an electrophoresis apparatus. Polyvinylidene difluoride membranes were further used to transfer proteins electrophoretically from the gels. Membranes were incubated in 5% fat-free milk in Tris-buffered saline with Tween-20 for 30 to 60 minutes at room temperature. Membranes were then incubated with appropriate primary antibodies overnight at 4°C: rabbit anti-GLT-1 (1:5000, Abcam, ab41621), rabbit anti-xCT (1:2000, Abcam, ab175186), rabbit anti-mGluR1 (1:1000, Abcam, ab82211), rabbit anti-mGluR5 (1:1000, Abcam, ab76316), rabbit anti-PPAR-α (1:1000, Abcam, ab24509), and rabbit anti-PPAR-γ (1:1000, Abcam, ab209350). Mouse anti-β-tubulin (1:1000; BioLegend) was used as a loading control antibody for the brain tissue and rabbit anti-glyceraldehyde-3-phosphate dehydrogenase (1:5000; cell signaling) was used as a loading control antibody for the liver tissues. Membranes were washed five times on the next day with Tris-buffered saline with Tween-20 followed by incubation with appropriate secondary antibody (1:5000) for 90 minutes at room temperature. Chemiluminescent reagents (Super Signal West Pico, Pierce Inc.) were used to detect proteins using a GeneSys imaging system, which digitized the blot images. Water-control group data were represented as 100%, and all other values were expressed relative to this control group for detection of changes in the expression of all targeted proteins in the brain and liver samples as previously described (Alhaddad, Alasmari et al., 2020).
Liver Oil Red O Staining
Fat content was measured in formalin-fixed liver sections (frozen liver sections placed in 4% formalin, 10 µm thick) using Oil Red O (Sigma, CAS no. 1320-06-5) staining. Liver sections were placed on slides and stained with freshly prepared Oil Red O solution for 15 minutes and then rinsed with 60% isopropanol. Slides were then rinsed with distilled water and mounted in aqueous mounting media and prepared for imaging. The magnitude of Oil Red O staining was determined using a color video camera attached to an Olympus VS120 slide scanning microscope at 20× magnification. Olympus OlyVIA software was used to analyze images. Lipid droplets were quantified using Image J pro (National Institutes of Health). Data are presented as the mean ± S.E.M. of the Oil Red O staining for each group.
Statistical Analyses
All statistical analyses were conducted using GraphPad Prism software, with a P value ≤ 0.05 considered statistically significant. We performed a two-way (mixed model) ANOVA followed by Bonferroni multiple comparison post hoc test to analyze daily ethanol and water consumption, as well as body weight changes. For the Western blot and Oil Red O staining analysis, we performed one-way ANOVA with Newman–Keuls post hoc tests to measure differences between groups as a percentage (relative to control values).
Results
Effect of MC-100093 and Ceftriaxone Treatment on Ethanol Consumption
Treatment with MC-100093 or CEF significantly reduced ethanol consumption compared with saline treatment. Statistical analysis of ethanol drinking data revealed a significant drug by day interaction (F10,138 = 17.49, P < 0.0001). Tukey’s multiple comparison tests showed a significant decrease in ethanol consumption from treatment day 2 through treatment day 5 in the MC-100093 and CEF groups compared with the ethanol–saline group (Fig. 2A). However, ethanol consumption was significantly reduced in CEF group compared with MC-100093 group in day 2 through day 5.
Fig. 2.
Effects of five consecutive days of MC-100093 (50 mg/kg, i.p.) or CEF (200 mg/kg, i.p.) treatment on (A) ethanol consumption (g/kg of average body weight/24 h), (B) water intake (mL/day), and (C) body weight (g). Statistical analyses revealed that treatment with MC-100093 or CEF significantly reduced ethanol consumption from day 2 through day 5, with a concomitant significant increase in water consumption from day 2 through day 5 by the ethanol–CEF group and on day 5 by the ethanol–MC-100093 group as compared with the ethanol–saline group. However, ethanol consumption was significantly lower, and water consumption was significantly higher in the ethanol–CEF group as compared with the ethanol–MC-100093 group on day 2 through day 5. There were no significant effects of MC-100093 or CEF treatment on body weight. The values are expressed as mean ± S.E.M. (n = 8/group for the ethanol–saline group and n = 9/group for ethanol–MC-100093 and ethanol–CEF groups). *P < 0.05; **P < 0.01; #P < 0.0001.
Effects of MC-100093 and Ceftriaxone Treatment on Water Intake
Water consumption was significantly increased in MC-100093- and CEF-treated groups compared with the saline treated group. Statistical analysis of water consumption data revealed a significant drug by day interaction (F10,138 = 6.987, P < 0.0001). Tukey’s multiple comparison tests showed a significant increase in water consumption from treatment day 2 through treatment day 5 in the CEF group and at treatment day 5 in the MC-100093 group compared with the ethanol–saline group (Fig. 2B). Noteworthy, there was no significant difference in the total fluid intake across all groups (data not shown).
Effects of MC-100093 and Ceftriaxone Treatment on Body Weight
Statistical analysis of body weight data revealed a nonsignificant drug by day interaction (F10,138 = 0.0045, P > 0.999). Neither MC-100093 nor CEF treatment had a significant effect on animal body weights (Fig. 2C).
Effect of MC-100093 and Ceftriaxone on the Expression of GLT-1 in the NAc-Core and NAc-Shell
We investigated the effects of MC-100093 or CEF on GLT-1 expression in P rats exposed to a chronic ethanol drinking protocol. One-way ANOVA revealed no significant difference in GLT-1 expression in the NAc core, among all tested groups (F3,28 = 0.734, P > 0.05, n = 8/group) (Fig. 3A). However, there was a significant difference in GLT-1 expression among the four groups in the NAc shell (F3,28 = 4.85, P < 0.01, n = 8/group). Newman–Keuls post hoc analyses revealed a significant decrease in GLT-1 expression in the NAc shell of the ethanol group compared with the water control group (P < 0.05), while its expression was significantly increased in groups treated with MC-100093 (P < 0.05) or CEF (P < 0.01) in the NAc shell as compared with the ethanol–saline group. No significant differences were detected between the water control, ethanol–MC-100093, and ethanol–CEF groups as shown in Fig. 3B.
Fig. 3.
Effects of five consecutive days of MC-100093 or CEF treatment on (A) GLT-1 expression in the NAc core. Upper panel: Representative immunoblot of GLT-1 and β-tubulin in the NAc core. Lower panel: Quantitative analysis revealed a nonsignificant difference in GLT-1 expression between water control, ethanol, ethanol–MC-100093, and ethanol–CEF groups in the NAc core. (B) GLT-1 expression in the NAc shell. Upper panel: Representative immunoblot of GLT-1 and β-tubulin in the NAc shell. Lower panel: Quantitative analysis revealed a significant downregulation of GLT-1 expression in the ethanol group compared to the water control group. However, the ethanol–MC-100093 and ethanol–CEF groups showed significantly higher levels of GLT-1 expression compared with the ethanol group, with no significant difference between the water control, ethanol–MC-100093, and ethanol–CEF groups in the NAc shell. (C) xCT expression in NAc core. Upper panel: Representative immunoblot of xCT and β-tubulin in the NAc core. Lower panel: Quantitative analysis revealed a nonsignificant difference in xCT expression between the water control, ethanol, ethanol–MC-100093, and ethanol–CEF groups in the NAc core. (D) xCT expression in the NAc shell. Upper panel: Representative immunoblot of xCT and β-tubulin in the NAc shell. Lower panel: Quantitative analysis revealed a significant downregulation of xCT expression in the ethanol group compared to the water control group. However, the ethanol–MC-100093 and ethanol–CEF groups showed significantly higher level of xCT compared to the ethanol group, with no significant difference between the water control, ethanol–MC-100093, and ethanol–CEF groups in the NAc shell. Control group data were represented as 100% (i.e., relative to water control). The values are expressed as mean ± S.E.M (n = 8/group). *P < 0.05; **P < 0.01.
Effect of MC-100093 and Ceftriaxone on the Expression of xCT in the NAc-Core and NAc-Shell
One-way ANOVA revealed no significant difference in xCT expression in the NAc core, among all tested groups (F3,28 = 0.265, P > 0.05, n = 8/group) (Fig. 3C). However, there was a significant difference in protein expression of xCT between the four groups in the NAc shell (F3,28 = 4.48, P < 0.05, n = 8-9/group). Newman–Keuls post hoc analyses showed a significant decrease in xCT expression in NAc shell of the ethanol group compared with the water control group (P < 0.05). The analysis also showed that MC-100093 (P < 0.01) and CEF (P < 0.05) significantly increased xCT expression in the NAc shell as compared with the ethanol–saline group. No significant differences were detected between the water control, ethanol–MC-100093, and ethanol–CEF groups in the NAc shell as shown in Fig. 3D.
Effect of MC-100093 and Ceftriaxone on the Expression of Metabotropic Glutamate Receptors in the NAc-Core and NAc-Shell
We next investigated the effects of MC-100093 or CEF on protein expression of mGluRs in the NAc. One-way ANOVA revealed no significant difference in mGluR1 expression between all tested groups in the NAc core (F3,27 = 0.802, P > 0.05, n = 7–8/group), and NAc shell (F3,28 = 0.890, P > 0.05, n = 8/group) (Fig. 4, A and B, respectively). There was no significant difference in mGluR5 expression between all groups in the NAc core (F3,27 = 1.084, P > 0.05, n = 7–8/group) (Fig. 4C). However, mGluR5 expression was significantly downregulated in the ethanol group compared with the water control, ethanol–MC-100093, and ethanol–CEF groups in the NAc shell (F3,28 = 2.707, P < 0.05, n = 8/group). No significant changes in mGluR5 expression were detected between the water control, ethanol–MC-100093, and ethanol–CEF groups (Fig. 4D).
Fig. 4.
Effects of five consecutive days of MC-100093 or CEF treatment on (A) protein expression of mGluR1 in the NAc core. Upper panel: Representative immunoblot of mGluR1 and β-tubulin in the NAc core. Lower panel: Quantitative analysis revealed a nonsignificant difference in mGluR1 expression between the water control, ethanol, ethanol–MC-100093, and ethanol–CEF groups in the NAc core. (B) mGluR1 expression in the NAc shell. Upper panel: Representative immunoblot of mGluR1 and β-tubulin in the NAc shell. Lower panel: Quantitative analysis revealed non-significant differences in mGluR1 expression between the water control, ethanol, ethanol–MC-100093, and ethanol–CEF groups in the NAc shell. (C) mGluR5 expression in the NAc core. Upper panel: Representative immunoblot of mGluR5 and β-tubulin in the NAc core. Lower panel: Quantitative analysis revealed a nonsignificant difference in mGluR5 expression between the water control, ethanol, ethanol–MC-100093, and ethanol–CEF groups in the NAc core. (D) mGluR5 expression in NAc shell. Upper panel: Representative immunoblot of mGluR5 and β-tubulin in the NAc shell. Lower panel: Quantitative analysis revealed a significant downregulation in mGluR5 expression in the ethanol group compared to the water control group in the NAc shell. However, there was no significant difference in mGluR5 expression in the ethanol–MC-100093 and ethanol–CEF groups compared to the water control group. (E) PPAR-α expression in the NAc core. Upper panel: Representative immunoblot of PPAR-α and β-tubulin in the NAc core. Lower panel: Quantitative analysis revealed nonsignificant differences in PPAR-α expression between the water control, ethanol, ethanol–MC-100093, and ethanol–CEF groups in the NAc core. (F) PPAR-α expression in the NAc shell. Upper panel: Representative immunoblot of PPAR-α and β-tubulin in the NAc shell. Lower panel: Quantitative analysis revealed nonsignificant differences in PPAR-α expression between the water control, ethanol, ethanol–MC-100093, and ethanol–CEF groups in the NAc shell. Control group data were represented as 100% (i.e., relative to water control). The values are expressed as mean ± S.E.M. (n = 7–8/group). *P < 0.05; **P < 0.01.
Effect of MC-100093 and Ceftriaxone on the Expression of PPAR-α in the NAc-Core and NAc-Shell
There were no significant difference in PPAR-α expression between all tested groups in the NAc core (F3,28 = 0.922, P > 0.05, n = 8/group) and NAc shell (F3,20 = 1.609, P > 0.05, n = 6/group) as shown in Fig. 4, E and F, respectively.
Effect of Chronic Ethanol Exposure, MC-100093, and Ceftriaxone on Fat Deposition in the Liver
Oil Red O staining was used to detect the liver fat content for each group. One-way ANOVA revealed a significant difference in fat content between groups (F3,15 = 5.543, P < 0.01, n = 4–5/group. Newman–Keuls post hoc analyses revealed higher fat droplet content in the ethanol group (P < 0.05) compared with the water control, ethanol–MC-100093, or ethanol–CEF groups. However, no significant differences were observed between the water control, ethanol–MC-100093, or ethanol–CEF group (Fig. 5A).
Fig. 5.
Fat droplets in Oil Red O-stained liver sections. (A) Quantitative analysis revealed a significant increase in liver fat content (high fat deposition) in the ethanol group compared with the water control group. There was no significant difference in fat content between the water control, ethanol–MC-100093, and ethanol–CEF groups. (B) Representative Oil Red O-stained liver sections of the water control, ethanol, ethanol–MC, and ethanol–CEF groups. Higher fat content was observed in liver sections of the ethanol group as compared to the water control, ethanol–MC-100093, or ethanol–CEF groups. The values are expressed as mean ± S.E.M. (n = 4-5/group). *P < 0.05.
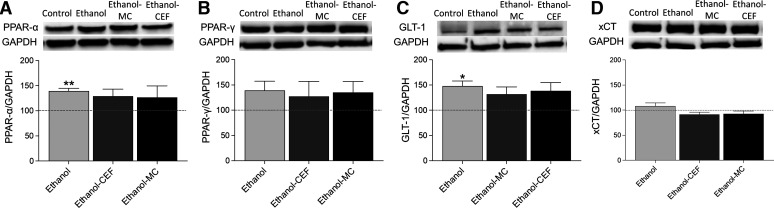
Effects of MC-100093 and Ceftriaxone on the Protein Expression of PPARs in the Liver
We also investigated the effects of MC-100093 or CEF on protein expression of PPAR-α in the liver of P rats exposed to a chronic ethanol drinking protocol. One-way ANOVA revealed a significant change in PPAR-α expression (F3,28 = 4.223, P < 0.05, n = 8/group) (Fig. 6A). Newman–Keuls post hoc analyses showed that PPAR-α expression was significantly upregulated in the ethanol group compared with the water control group (P < 0.01) and that treatment with MC-100093 or CEF normalized PPAR-α expression. However, PPAR-γ expression was not significantly different between all treatment groups (F3,28 = 0.747, P > 0.05, n = 8/group) (Fig. 6B).
Fig. 6.
Effects of five consecutive days of MC-100093 or CEF injections on (A) PPAR-α expression in the liver. Upper panel: Representative immunoblot of PPAR-α and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) in the liver. Lower panel: Quantitative analysis revealed a significant increase in PPAR-α expression in the ethanol group compared with control the water control group in the liver. However, there was no significant change in PPAR-α expression in the ethanol–MC-100093 and ethanol–CEF groups compared to the water control group. (B) PPAR-γ expression in the liver. Upper panel: Representative immunoblot of PPAR-γ and GAPDH in the liver. Lower panel: Quantitative analysis revealed no significant difference in PPAR-γ expression between all groups. (C) GLT-1 expression in the liver. Upper panel: Representative immunoblot of GLT-1 and GAPDH in the liver. Lower panel: Quantitative analysis revealed a significant increase in GLT-1 expression in the ethanol group compared with the water control group in the liver. However, there was no significant change in GLT-1 expression in the ethanol–MC-100093 and ethanol–CEF groups compared to the water control group. (D) xCT expression in the liver. Upper panel: Representative immunoblot of xCT and GAPDH in the liver. Lower panel: Quantitative analysis revealed no significant difference in xCT expression between all groups. Control group data were represented as 100% (i.e., relative to water control). The values are expressed as mean ± S.E.M. (n = 8/group). *P < 0.05; **P < 0.01.
Effects of MC-100093 and Ceftriaxone on GLT-1 and xCT Expression in the Liver
There was a significant increase in GLT-1 protein expression in the liver of the ethanol group (F3,28 = 3.042, P < 0.05, n = 8/group) (Fig. 6C). Newman–Keuls post hoc analyses showed that GLT-1 expression was significantly upregulated in the ethanol group compared with the water control group (P < 0.05), and that treatment with MC-100093 or CEF normalized GLT-1 expression. However, one-way ANOVA revealed no significant difference in xCT expression between all treatment groups (F3,28 = 2.613, P > 0.05, n = 8/group) (Fig. 6D).
Discussion
Chronic ethanol consumption was associated with dysregulation of the glutamatergic system in both the brain and liver of P rats. More specifically, the expression of GLT-1, xCT, and mGluR5 were downregulated in the NAc shell, while the liver exhibited upregulation in GLT-1 expression. Moreover, the steatotic liver in rats exposed to ethanol showed higher protein expression of PPAR-α and GLT-1. More important, the novel beta lactam compound, MC-100093, attenuated ethanol drinking behavior, and this effect was associated with normalized levels in several glutamatergic and PPAR protein products associated with chronic ethanol drinking. It is noteworthy that MC-100093 is orally bioavailable with essentially no antibiotic activity and is found to be a potent GLT-1 upregulator, which has the potential to attenuate cocaine reinstatement and reverses a neurodegenerative phenotype in an animal model of cerebral palsy (Childers et al., 2020). Our results demonstrate that MC-100093 may be a potential pharmacotherapeutic to treat AUDs with preferable characteristics (i.e., high oral bioavailability and no antibiotic actions) compared to CEF. Nevertheless, CEF was able to attenuate ethanol drinking behavior to a greater extent than MC-100093. This is probably due to the use of one low dose of MC-100093 (50 mg/kg), which represents one of the limitations in this study. Further studies are warranted to explore the effect of higher doses. MC-100093 (50 mg/kg, i.p.) attenuated ethanol intake, and this effect was associated with normalized GLT-1 and xCT levels in the NAc shell. This is in line with our previous finding that demonstrated chronic ethanol drinking induces downregulation of glutamate transporters in the NAc shell and treatment with GLT-1 up-regulators attenuated ethanol drinking behaviors (Alasmari et al., 2020; Alhaddad, Alasmari et al., 2020). Furthermore, we previously found that chronic ethanol consumption induced glucocorticoid resistance and a neuroinflammatory response in the NAc shell but not in the NAc core (Alasmari et al., 2020; Alhaddad, Gordon et al., 2020), which supports the involvement of neuroimmune signaling in the NAc shell in the pathology of AUDs, as well as, putatively, SUDs. Likewise, chronic ethanol consumption reduced the expression of mGluR5 in the NAc shell, and its normalization was associated with attenuation of drinking behavior and neuroimmune signaling (Alasmari et al., 2020). It is noteworthy that mGluR5 signaling is strongly implicated in the development and maintenance of ethanol dependence in animal models. For instance, blockade of mGluR5’s action resulted in reduction in ethanol consumption by mice (Hodge et al., 2006), as well as reductions in both consumption and ethanol self-administration under a progressive ratio schedule of reinforcement by P rats (Schroeder et al., 2005; Besheer et al., 2008b). Moreover, activation of mGluR5 resulted in anti-inflammatory effects by inhibiting microglial activation and the induction of neuroimmune signaling (Byrnes et al., 2009; Loane et al., 2009). In this study, we further confirmed the role of mGluR5 in ethanol drinking behavior by demonstrating that chronic ethanol consumption induced downregulation of mGluR5 expression in the NAc shell. Additionally, MC-100093 or CEF treatment was associated with normalizing levels of mGluR5 expression in alcohol dependent animals, which may contribute to previously observed attenuation of ethanol drinking behavior by type I mGluR antagonists. Together, the previous studies along with the current findings provide strong support that the NAc shell mediates continuous ethanol consumption and/or self-administration. In this regard, chronic ethanol exposure induced dysregulation of glutamate receptors and/or transporters in the NAc shell provides a potential pharmacotherapeutic target to treat AUDs and, possibly, SUDs.
The role of PPARs in ethanol dependence is well documented. Several studies have shown that PPAR agonists reduced ethanol drinking behavior in animal models. For instance, gemfibrozil, a PPAR-α agonist, reduced ethanol drinking behavior in outbred rats (Barson et al., 2009); pioglitazone and rosiglitazone reduced ethanol drinking and stress-induced relapse in selected Sardinian alcohol-preferring (sP) rats (Stopponi et al., 2011, 2013). The mechanism underlying PPAR-associated reduction in ethanol consumption is possibly mediated through modulation of the neuroimmune system centrally (Blednov et al., 2017). However, PPAR-α activation in the liver also stimulates hepatic catalase and hydrogen peroxide that leads to ethanol aversion via conversion of ethanol into acetaldehyde, which represents another possible mechanism for its reduction of drinking behavior (Karahanian et al., 2015). To assess this hypothesis, we used our chronic ethanol drinking model and measured the expression of PPARs in the liver and brain. The expression of PPAR-α in the NAc core and NAc shell were not significantly changed. The present experimental conditions prevented the detection of PPAR-γ in the brain, probably due to lower expression levels compared with other organs (Cullingford et al., 1998; Moreno et al., 2004). In the liver, although the PPAR-γ expression was not changed, we found that chronic ethanol consumption significantly increased PPAR-α level. In line with findings from a previous study (Fischer et al., 2003), we found that our ethanol drinking paradigm was associated with the development of fatty liver. Previously, exposure to ethanol for four weeks did not alter the expression of PPAR-α but was associated with reduced PPAR/RXR binding to its consensus sequence in mice (Fischer et al., 2003). Our results suggest that increased PPAR-α expression in P rats exposed to ethanol for six weeks increased liver fat contents. It is important to note that PPAR-α agonists reduced ethanol consumption, and this effect might be mediated through activation of PPAR-α in the liver (Karahanian et al., 2015). Interestingly, our results showed that the expression of GLT-1 was upregulated in the liver, with no change in xCT levels. Previous work indicated that PPAR-γ activation increased the expression of GLT-1 at the transcriptional level (Romera et al., 2007). However, little is known about the involvement of PPAR-α in GLT-1 function. Nevertheless, it has been shown that PPAR-α signaling promotes GLT-1 endocytosis in astrocytes (Huang et al., 2017). The present findings provide further support for a connection between PPAR-α and GLT-1 activity. Further, we showed that treatment with MC-100093 or CEF alleviated liver steatosis and normalized PPAR-α as well as GLT-1 levels in the liver. However, whether these effects are directly related to MC-100093 and CEF action on the liver is not investigated in the present study. Further studies are warranted to explore the effect of MC-100093 treatment on PPAR-α and GLT-1 levels in the liver as well as other glutamate receptors (e.g., N-methyl-D-aspartate receptors) in the brain. Thus, this study revealed the efficacy of both MC-100093 and CEF in mitigating chronic ethanol intake and its consequences in both the brain and liver.
In summary, we demonstrated here that chronic ethanol drinking induced dysregulation of the glutamatergic system in both the brain and liver. In addition to liver steatosis, chronic ethanol consumption was associated with increased PPAR-α expression in the liver. Treatment with MC-100093 or CEF attenuated ethanol drinking behavior, which was associated with normalization of glutamatergic-associated proteins in both the brain and liver as well as attenuating fatty deposition in the liver. This study presents a new potential candidate, MC-100093, as pharmacotherapeutic for the treatment of AUDs and liver injury caused by chronic ethanol consumption.
Acknowledgments
The authors would like to thank the National Institutes of Health and The University of Toledo for their continuous support.
Abbreviations
- AUD
Alcohol use disorder
- CEF
ceftriaxone
- GLT-1
glutamate transporter 1
- MCL
mesocorticolimbic
- mGluR
metabotropic glutamate receptor
- NAc
nucleus accumbens
- P
alcohol-preferring
- PPAR
peroxisome proliferator-activated receptor
- SUDs
substance use disorders
- xCT
cystine/glutamate exchanger
Authorship Contributions
Participated in research design: Alhaddad, Sari.
Conducted experiments: Alhaddad, Wong.
Contributed new reagents or analytic tools: Abou-Gharbia, Childers, Melenski, Bell, Sari.
Performed data analysis: Alhaddad, Wong.
Contributed to the writing of the manuscript: Alhaddad, Wong, Abou-Gharbia, Childers, Melenski, Bell, Sari.
Footnotes
This study was support in part by National Institutes of Health National Institute on Alcohol Abuse and Alcoholism [Grant U24-AA013522] (R.L.B.) to provide the animals. Funding from the University of Toledo through the deArce-Koch award fund [RSP N-127483-01] (Y.S.) supported the experiments for animal treatment and performance of Western blot for protein detection in brain and liver and histological analysis of liver.
The authors declare no conflict of interest.
References
- Abou-Gharbia M, Blass B, Childers W, Ramanjulu M, Melenski E (2017) Glutamate transporter-1 (GLT-1): a potential therapeutic target for the treatment of central nervous system diseases and disorders. Drugs Fut 42:489–509. [Google Scholar]
- Alasmari F, Alhaddad H, Wong W, Bell RL, Sari Y (2020) Ampicillin/sulbactam treatment modulates NMDA receptor NR2B subunit and attenuates neuroinflammation and alcohol intake in male high alcohol drinking rats. Biomolecules 10:1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhaddad H, Alasmari F, Alhamadani B, Wong W, Bell RL, Sari Y (2020) Effects of chronic ethanol consumption on the expression of GLT-1 and neuroplasticity-related proteins in the nucleus accumbens of alcohol-preferring rats. Brain Res Bull 165:272–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhaddad H, Das SC, Sari YJP. (2014) Effects of ceftriaxone on ethanol intake: a possible role for xCT and GLT-1 isoforms modulation of glutamate levels in P rats. Psychopharmacology (Berl) 231:4049–4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhaddad H, Gordon DM, Bell RL, Jarvis EE, Kipp ZA, Hinds TD Jr, Sari Y (2020) Chronic ethanol consumption alters glucocorticoid receptor isoform expression in stress neurocircuits and mesocorticolimbic brain regions of alcohol-preferring rats. Neuroscience 437:107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhaddad H, Kim NT, Aal-Aaboda M, Althobaiti YS, Leighton J, Boddu SHS, Wei Y, Sari Y (2014) Effects of MS-153 on chronic ethanol consumption and GLT1 modulation of glutamate levels in male alcohol-preferring rats. Front Behav Neurosci 8:366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anghel SI, Wahli W (2007) Fat poetry: a kingdom for PPAR γ. Cell Res 17:486–511. [DOI] [PubMed] [Google Scholar]
- Barson JR, Karatayev O, Chang G-Q, Johnson DF, Bocarsly ME, Hoebel BG, Leibowitz SF (2009) Positive relationship between dietary fat, ethanol intake, triglycerides, and hypothalamic peptides: counteraction by lipid-lowering drugs. Alcohol 43:433–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RL, Hauser SR, Liang T, Sari Y, Maldonado-Devincci A, Rodd ZA (2017) Rat animal models for screening medications to treat alcohol use disorders. Neuropharmacology 122:201–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RL, Rodd ZA, Lumeng L, Murphy JM, McBride WJ (2006) The alcohol-preferring P rat and animal models of excessive alcohol drinking. Addict Biol 11:270–288. [DOI] [PubMed] [Google Scholar]
- Bell RL, Sable HJ, Colombo G, Hyytia P, Rodd ZA, Lumeng L (2012) Animal models for medications development targeting alcohol abuse using selectively bred rat lines: neurobiological and pharmacological validity. Pharmacol Biochem Behav 103:119–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besheer J, Faccidomo S, Grondin JJ, Hodge CW (2008a) Effects of mGlu1-receptor blockade on ethanol self-administration in inbred alcohol-preferring rats. Alcohol 42:13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besheer J, Faccidomo S, Grondin JJ, Hodge CW (2008b) Regulation of motivation to self-administer ethanol by mGluR5 in alcohol-preferring (P) rats. Alcohol Clin Exp Res 32:209–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besnard JRuda GFSetola VAbecassis KRodriguiz RMHuang XPNorval SSassano MFShin AIWebster LA, et al. (2012) Automated design of ligands to polypharmacological profiles. Nature 492:215–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Black M, Chernis J, Da Costa A, Mayfield J, Harris RA (2017) Ethanol consumption in mice lacking CD14, TLR2, TLR4, or MyD88. Alcohol Clin Exp Res 41:516–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges R, Lutgen V, Lobner D, Baker DA (2012) Thinking outside the cleft to understand synaptic activity: contribution of the cystine-glutamate antiporter (System xc-) to normal and pathological glutamatergic signaling. Pharmacol Rev 64:780–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrnes KR, Stoica B, Loane DJ, Riccio A, Davis MI, Faden AI (2009) Metabotropic glutamate receptor 5 activation inhibits microglial associated inflammation and neurotoxicity. Glia 57:550–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll FI (2008) Antagonists at metabotropic glutamate receptor subtype 5: structure activity relationships and therapeutic potential for addiction. Ann N Y Acad Sci 1141:221–232. [DOI] [PubMed] [Google Scholar]
- Childers WE, Elokely KM, Abou-Gharbia M (2020) The resurrection of phenotypic drug discovery. ACS Med Chem Lett 11:1820–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullingford TE, Bhakoo K, Peuchen S, Dolphin CT, Patel R, Clark JB (1998) Distribution of mRNAs encoding the peroxisome proliferator-activated receptor α, β, and γ and the retinoid X receptor α, β, and γ in rat central nervous system. J Neurochem 70:1366–1375. [DOI] [PubMed] [Google Scholar]
- Das SC, Yamamoto BK, Hristov AM, Sari Y. (2015) Ceftriaxone attenuates ethanol drinking and restores extracellular glutamate concentration through normalization of GLT-1 in nucleus accumbens of male alcohol-preferring rats. Neuropharmacology 97:67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Monte SM, Longato L, Tong M, DeNucci S, Wands JR (2009) The liver-brain axis of alcohol-mediated neurodegeneration: role of toxic lipids. Int J Environ Res Public Health 6:2055–2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enomoto N, Takei Y, Hirose M, Konno A, Shibuya T, Matsuyama S, Suzuki S, Kitamura KI, Sato N (2003) Prevention of ethanol-induced liver injury in rats by an agonist of peroxisome proliferator-activated receptor-γ, pioglitazone. J Pharmacol Exp Ther 306:846–854. [DOI] [PubMed] [Google Scholar]
- Fischer M, You M, Matsumoto M, Crabb DW (2003) Peroxisome proliferator-activated receptor α (PPARalpha) agonist treatment reverses PPARalpha dysfunction and abnormalities in hepatic lipid metabolism in ethanol-fed mice. J Biol Chem 278:27997–28004. [DOI] [PubMed] [Google Scholar]
- Goodwani S, Saternos H, Alasmari F, Sari YJN, Reviews B. (2017) Metabotropic and ionotropic glutamate receptors as potential targets for the treatment of alcohol use disorder. Neurosci Biobehav Rev 77:14–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granero L, Santiago M, Cano J, Machado A, Peris J-E (1995) Analysis of ceftriaxone and ceftazidime distribution in cerebrospinal fluid of and cerebral extracellular space in awake rats by in vivo microdialysis. Antimicrob Agents Chemother 39:2728–2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinze H-JHeldmann MVoges JHinrichs HMarco-Pallares JHopf J-MMüller UJGalazky ISturm VBogerts B, et al. (2009) Counteracting incentive sensitization in severe alcohol dependence using deep brain stimulation of the nucleus accumbens: clinical and basic science aspects. Front Hum Neurosci 3:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge CW, Miles MF, Sharko AC, Stevenson RA, Hillmann JR, Lepoutre V, Besheer J, Schroeder JP (2006) The mGluR5 antagonist MPEP selectively inhibits the onset and maintenance of ethanol self-administration in C57BL/6J mice. Psychopharmacology (Berl) 183:429–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong F, Radaeva S, Pan HN, Tian Z, Veech R, Gao B (2004) Interleukin 6 alleviates hepatic steatosis and ischemia/reperfusion injury in mice with fatty liver disease. Hepatology 40:933–941. [DOI] [PubMed] [Google Scholar]
- Huang H-T, Liao C-K, Chiu W-T, Tzeng S-F (2017) Ligands of peroxisome proliferator-activated receptor-alpha promote glutamate transporter-1 endocytosis in astrocytes. Int J Biochem Cell Biol 86:42–53. [DOI] [PubMed] [Google Scholar]
- Javitt DCSchoepp DKalivas PWVolkow NDZarate CMerchant KBear MFUmbricht DHajos MPotter WZ, et al. (2011) Translating glutamate: from pathophysiology to treatment. Sci Transl Med 3:102mr2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW (2009) The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci 10:561–572. [DOI] [PubMed] [Google Scholar]
- Karahanian E, Rivera-Meza M, Quintanilla ME, Muñoz D, Fernández K, Israel Y (2015) PPARα agonists reduce alcohol drinking: do they act in the brain or in the liver? Alcohol Alcohol 50:717–718. [DOI] [PubMed] [Google Scholar]
- Knackstedt LA, Wu L, Rothstein J, Vidensky S, Gordon J, Ramanjulu M, Dunman P, Blass B, Childers W, Abou-Gharbia M (2021) MC-100093, a novel β-lactam glutamate transporter-1 enhancer devoid of antimicrobial properties, attenuates cocaine relapse in rats. J Pharmacol Exp Ther 378:51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leurquin-Sterk G, Ceccarini J, Crunelle CL, de Laat B, Verbeek J, Deman S, Neels H, Bormans G, Peuskens H, Van Laere K (2018) Lower limbic metabotropic glutamate receptor 5 availability in alcohol dependence. J Nucl Med 59:682–690. [DOI] [PubMed] [Google Scholar]
- Li TK, Lumeng L, McBride WJ, Murphy JM (1987) Rodent lines selected for factors affecting alcohol consumption. Alcohol Alcohol Suppl 1:91–96. [PubMed] [Google Scholar]
- Loane DJ, Stoica BA, Pajoohesh-Ganji A, Byrnes KR, Faden AI (2009) Activation of metabotropic glutamate receptor 5 modulates microglial reactivity and neurotoxicity by inhibiting NADPH oxidase. J Biol Chem 284:15629–15639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lum EN, Campbell RR, Rostock C, Szumlinski KK (2014) mGluR1 within the nucleus accumbens regulates alcohol intake in mice under limited-access conditions. Neuropharmacology 79:679–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandrekar-Colucci S, Sauerbeck A, Popovich PG, McTigue DM (2013) PPAR agonists as therapeutics for CNS trauma and neurological diseases. ASN Neuro 5:e00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran MM, McFarland K, Melendez RI, Kalivas PW, Seamans JK (2005) Cystine/glutamate exchange regulates metabotropic glutamate receptor presynaptic inhibition of excitatory transmission and vulnerability to cocaine seeking. J Neurosci 25:6389–6393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno S, Farioli-Vecchioli S, Cerù MP (2004) Immunolocalization of peroxisome proliferator-activated receptors and retinoid X receptors in the adult rat CNS. Neuroscience 123:131–145. [DOI] [PubMed] [Google Scholar]
- Müller U, Sturm V, Voges J, Heinze H-J, Galazky I, Büntjen L, Heldmann M, Frodl T, Steiner J, Bogerts B (2016) Nucleus accumbens deep brain stimulation for alcohol addiction–safety and clinical long-term results of a pilot trial. Pharmacopsychiatry 49:170–173. [DOI] [PubMed] [Google Scholar]
- Nanji AA, Dannenberg AJ, Jokelainen K, Bass NM (2004) Alcoholic liver injury in the rat is associated with reduced expression of peroxisome proliferator-α (PPARalpha)-regulated genes and is ameliorated by PPARalpha activation. J Pharmacol Exp Ther 310:417–424. [DOI] [PubMed] [Google Scholar]
- Neasta J, Hamida SB, Yowell QV, Carnicella S, Ron D. (2011) AKT signaling pathway in the nucleus accumbens mediates excessive alcohol drinking behaviors. Biol Psychiatry 70:575–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obara I, Bell RL, Goulding SP, Reyes CM, Larson LA, Ary AW, Truitt WA, Szumlinski KK (2009) Differential effects of chronic ethanol consumption and withdrawal on homer/glutamate receptor expression in subregions of the accumbens and amygdala of P rats. Alcohol Clin Exp Res 33:1924–1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive MF, McGeehan AJ, Kinder JR, McMahon T, Hodge CW, Janak PH, Messing RO (2005) The mGluR5 antagonist 6-methyl-2-(phenylethynyl)pyridine decreases ethanol consumption via a protein kinase C ϵ-dependent mechanism. Mol Pharmacol 67:349–355. [DOI] [PubMed] [Google Scholar]
- Parkitna JR, Sikora M, Gołda S, Gołembiowska K, Bystrowska B, Engblom D, Bilbao A, Przewlocki R (2013) Novelty-seeking behaviors and the escalation of alcohol drinking after abstinence in mice are controlled by metabotropic glutamate receptor 5 on neurons expressing dopamine d1 receptors. Biol Psychiatry 73:263–270. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Koutcherov Y, Halliday GM, Watson C, Wang H (2007) Atlas of the Developing Mouse Brain: At E17.5, P0, and P6, Academic Press, Amsterdam. [Google Scholar]
- Rao PS, Sari Y (2012) Glutamate transporter 1: target for the treatment of alcohol dependence. Curr Med Chem 19:5148–5156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romera CHurtado OMallolas JPereira MPMorales JRRomera ASerena JVivancos JNombela FLorenzo P, et al. (2007) Ischemic preconditioning reveals that GLT1/EAAT2 glutamate transporter is a novel PPARgamma target gene involved in neuroprotection. J Cereb Blood Flow Metab 27:1327–1338. [DOI] [PubMed] [Google Scholar]
- Sari Y, Sreemantula SN (2012) Neuroimmunophilin GPI-1046 reduces ethanol consumption in part through activation of GLT1 in alcohol-preferring rats. Neuroscience 227:327–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder JP, Overstreet DH, Hodge CW (2005) The mGluR5 antagonist MPEP decreases operant ethanol self-administration during maintenance and after repeated alcohol deprivations in alcohol-preferring (P) rats. Psychopharmacology (Berl) 179:262–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stopponi Sde Guglielmo GSomaini LCippitelli ACannella NKallupi MUbaldi MHeilig MDemopulos GGaitanaris G, et al. (2013) Activation of PPARγ by pioglitazone potentiates the effects of naltrexone on alcohol drinking and relapse in msP rats. Alcohol Clin Exp Res 37:1351–1360. [DOI] [PubMed] [Google Scholar]
- Stopponi SSomaini LCippitelli ACannella NBraconi SKallupi MRuggeri BHeilig MDemopulos GGaitanaris G, et al. (2011) Activation of nuclear PPARγ receptors by the antidiabetic agent pioglitazone suppresses alcohol drinking and relapse to alcohol seeking. Biol Psychiatry 69:642–649. [DOI] [PubMed] [Google Scholar]
- You M, Crabb DW (2004) Recent advances in alcoholic liver disease II. Minireview: molecular mechanisms of alcoholic fatty liver. Am J Physiol Gastrointest Liver Physiol 287:G1–G6. [DOI] [PubMed] [Google Scholar]
- Zeng H, Guo X, Zhou F, Xiao L, Liu J, Jiang C, Xing M, Yao P (2019) Quercetin alleviates ethanol-induced liver steatosis associated with improvement of lipophagy. Food Chem Toxicol 125:21–28. [DOI] [PubMed] [Google Scholar]