Abstract
Background
Intravenous immunoglobulin (IVIG) resistance in patients with Kawasaki disease (KD) is defined as persistent or recrudescent fever ≥36 hours after IVIG infusion. We have experienced an increase in IVIG resistance in patients with KD since the substitution of 10% IVIG for 5% IVIG. This study aimed to determine the independent association between increased IVIG resistance and 10% IVIG therapy.
Methods
Medical records of pediatric patients with KD were retrospectively reviewed. Clinical and laboratory characteristics were compared between patients receiving 5% IVIG therapy and those receiving 10% IVIG therapy. Between IVIG-responsive and IVIG-resistant patients, a multivariate analysis was performed to determine the independent factors for IVIG resistance.
Results
A total of 119 patients were included in this study: 81 (68.1%) and 38 (31.9%) patients received 5% and 10% IVIG therapy, respectively. IVIG resistance was identified in 34 (28.6%) patients: 44.7% of patients receiving 10% IVIG therapy and 21.0% of patients receiving 5% IVIG therapy (p = 0.008). The clinical manifestations and outcomes were comparable between patients who received 5% IVIG therapy and those who received 10% IVIG therapy. IVIG resistance was significantly associated with fewer fever days at IVIG administration (p = 0.032), a higher percentage of neutrophils (p = 0.013), and 10% IVIG treatment (p = 0.004) in the multivariate analysis.
Conclusion
10% IVIG therapy was significantly associated with increased reporting of IVIG resistance. However, the increase in patients with fever patterns consistent with IVIG resistance seemed to represent adverse febrile reactions resulting from using high-concentration IVIG rather than increased severity of KD.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40272-022-00537-8.
Key Points
| The substitution of 10% intravenous immunoglobulin (IVIG) for 5% IVIG was significantly associated with an increase in the rate of reported IVIG resistance in children with Kawasaki disease (KD). |
| Since the clinical severity and outcomes of KD in the periods before and after the introduction of 10% IVIG were similar, this may be due to the increase in the proportion of patients with fever patterns consistent with IVIG resistance rather than a true increase in IVIG resistance. |
| Increased adverse febrile reactions due to high-concentration IVIG use should be considered in treating patients with KD. |
Introduction
Kawasaki disease (KD) is the most common cause of acquired heart disease in children in developed countries, with an incidence of 197 per 100,000 children aged <5 years in Korea [1]. KD is characterized by acute systemic inflammation of medium-sized vessels, and coronary artery abnormalities (CAAs) are the most serious complications of KD [2]. CAAs may cause long-term sequelae, such as vascular aneurysm and stenosis, precipitating myocardial infarction, heart failure, arrhythmias, and sudden death [3, 4]. Therefore, immune-modulation therapy for systemic vasculitis is required to prevent serious complications, and high-dose intravenous immunoglobulin (IVIG) with oral aspirin is recommended as a primary treatment for KD [2]. The prevalence of CAAs was reduced from 25 to <4% with IVIG and aspirin therapy within 10 days of illness onset [5, 6]. However, 10–20% of patients with KD exhibit resistance against primary IVIG treatment, requiring additional administration of IVIG or other immune-modulating agents [2].
IVIG products have varying physical and chemical characteristics depending on their manufacturing processes and excipients [7, 8]. The final concentrations of IVIG products also vary [7]. IVIG may cause several adverse reactions, and headache, fever, nausea, and vomiting occur most frequently [7]. Considering that IVIG resistance in patients with KD is defined as persistent or recrudescent fever ≥36 hours after the end of IVIG infusion, and that fever occurs as an adverse reaction in approximately one-fifth of patients receiving IVIG [2, 8], differentiating between IVIG-resistant KD and adverse febrile IVIG reactions might be difficult in a real-life clinical setting. In Korea, 5% IVIG was used previously, and 10% IVIG was introduced in 2017. In our hospital, 10% IVIG was substituted for 5% IVIG in August 2018, and we experienced an increase in IVIG resistance in patients with KD following this substitution. Additional immune-modulation therapy for IVIG-resistant KD results in an increase in hospital days, medical costs, and potential adverse drug reactions. Therefore, whether the increase in IVIG resistance was caused by an increase in the clinical severity of KD or by the confounding effects of IVIG concentrations needs to be determined. In this study, the clinical features and IVIG resistance rates of patients treated with IVIG for KD were compared before and after the introduction of 10% IVIG, and independent factors associated with IVIG resistance were investigated.
Methods
Study Design and Subjects
Among pediatric patients aged <19 years who were admitted to the Department of Pediatrics in Daejeon St. Mary’s Hospital (Daejeon, Republic of Korea) between February 2015 and January 2022, those diagnosed with KD were included in this study. Patients who did not receive IVIG treatment during hospitalization were excluded. Electronic medical records of the included patients were retrospectively reviewed to gather demographic data, including age and gender, and clinical data, including symptoms consistent with KD, underlying diseases, and previous history of KD. The start and end times of IVIG infusion, the defervescence time, and dose and concentration of the administered IVIG were investigated. Laboratory parameters known to be associated with IVIG resistance in patients with KD were investigated, including white blood cell (WBC) count; percentage of neutrophils in WBCs (%neutrophils); hemoglobin level; platelet count; erythrocyte sedimentation rate (ESR); C-reactive protein (CRP), aspartate transaminase (AST), alanine transaminase (ALT), total bilirubin, sodium, potassium, and chloride levels. Among several scoring systems for predicting IVIG resistance, the Kobayashi score was calculated for each patient. Echocardiographic findings during the acute (<14 days after fever onset) and subacute (2–8 weeks after fever onset) phases were also reviewed.
The included patients were divided into two groups according to the concentration of administered IVIG: 5% IVIG and 10% IVIG groups. The collected clinical, laboratory, and echocardiographic data, proportions of the Kobayashi high-risk group for IVIG resistance, and IVIG resistance rates were compared between the two patient groups. In addition, the dose and infusion time of IVIG and the fever duration after the end of IVIG infusion were compared. The patients were further divided into IVIG-responsive and IVIG-resistant groups, and the collected data were compared between the two groups to determine independent factors associated with IVIG resistance. In the IVIG-resistant group, patients with recrudescent fever and those with persistent fever after IVIG infusion were also compared. This study was approved by the Institutional Review Board of Daejeon St. Mary’s Hospital, which waived the requirement for informed consent (approval number: DC22RASI0015).
Definition
KD was diagnosed based on the principal clinical findings in accordance with the American Heart Association (AHA) 2017 diagnostic criteria [2]. Complete KD was diagnosed based on the presence of fever for ≥5 days with at least four of the five principal clinical features of oral changes, conjunctivitis, rash, extremity changes, and cervical lymphadenopathy [2]. Complete KD could be diagnosed within 5 days of fever onset, if four or more principal clinical features were definite [2]. Incomplete KD was diagnosed when ≥5 days of fever was accompanied by fewer than four principal clinical features, in accordance with the diagnostic algorithm based on laboratory and echocardiographic findings recommended in the AHA 2017 Scientific Statement [2]. Body temperature was measured using an infrared tympanic membrane thermometer during hospitalization, and fever was defined as a body temperature ≥38 °C. Defervescence time was defined as the time when the fever was last checked, followed by a body temperature of <38 °C for >48 hours. Fever duration was defined as the time from the end of IVIG infusion to the defervescence time. Therefore, fever duration was expressed as a negative number if defervescence was achieved before the end of IVIG infusion. IVIG resistance was defined as persistent or recrudescent fever occurring ≥36 hours after the end of IVIG infusion [2]. The Kobayashi scoring system to identify patients at higher risk for IVIG resistance included sodium level ≤133 mEq/L (2 points), ≤4 days of illness at treatment (2 points), AST level ≥100 IU/L (2 points), %neutrophils ≥80% (2 points), CRP level ≥10 mg/dL (1 point), age ≤12 months (1 point), and platelet count ≤300,000/mm3 (1 point) [9]. Patients were assigned to low-risk (score <4) and high-risk (score ≥4) groups based on the sum of the points [9]. The diameters of the left main, left anterior descending, left circumflex, and right coronary arteries were measured using echocardiography, and Z-scores adjusted for body surface area were calculated. CAA was defined as a Z-score ≥2.5 in any coronary artery segment [2].
Statistical Analysis
To compare the two patient groups, the chi-square and Mann–Whitney tests were used for categorical and continuous variables, respectively. Multivariate analysis using a binary logistic regression test was performed for statistically significant factors in the comparison between the IVIG-responsive and IVIG-resistant groups to determine the independent factors associated with IVIG resistance. As a subgroup analysis, the same analyses were performed for patients who received IVIG 2 g/kg, excluding those who received IVIG 1 g/kg. The SPSS 21 program (IBM Corporation, Armonk, NY, USA) was used for statistical analyses. The threshold for statistical significance was defined as a p-value of 0.05.
Results
Characteristics of the Whole Study Population
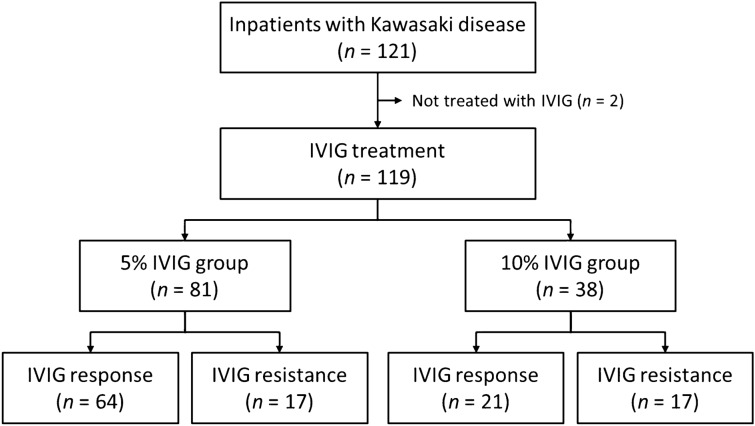
During the study period, a total of 121 inpatients were diagnosed with KD, and two (1.7%) of them who did not receive IVIG treatment were excluded (Fig. 1). The remaining 119 patients included 67 (56.3%) males and 52 (43.7%) females. Their median age was 27 months (range 2–119 months), and 17 (14.3%) patients were aged >60 months. Complete and incomplete KD were diagnosed in 105 (88.2%) and 14 (11.8%) patients, respectively. Underlying diseases were identified in three (2.5%) patients, all of whom had neurological disorders. Five (4.2%) and one (0.8%) patients experienced second and third episodes of KD, respectively. The doses of administered IVIG were 2 g/kg and 1 g/kg in 104 (87.4%) and 15 (12.6%) patients, respectively. Patients who received IVIG 1 g/kg were less likely to be assigned to the Kobayashi high-risk group and to complain of the symptoms of KD, and they had lower WBC counts, %neutrophils, and CRP levels compared with those who received IVIG 2 g/kg (Table S1 in the electronic supplementary material [ESM]): treating physicians tended to selectively administer IVIG 1 g/kg to patients who appeared to have less severe inflammation. Considering this selection bias, a subgroup analysis for patients who received IVIG 2 g/kg was performed, excluding those who received IVIG 1 g/kg. IVIG was infused for a median of 10.5 hours (range 3.9–17.9 hours). Sixty (50.4%) patients achieved defervescence before the end of IVIG infusion, and the remaining 59 (49.6%) patients became afebrile a median of 38 hours (range 0.0–172.8 hours) after the end of IVIG infusion. Echocardiography during the acute phase of the illness was performed in 103 (86.6%) patients at a median of 8 days (range 2–13 days) after fever onset, and CAAs were identified in 12 (11.7%) of them. During the subacute phase of the illness, echocardiography was performed in 106 (89.1%) patients at a median of 4 weeks (range 2–8 weeks) after fever onset, and CAAs were identified in seven (6.6%) patients.
Fig. 1.
Flow chart for the inclusion and classification of the study subjects
Comparison Between the 5% IVIG and 10% IVIG Groups
The 5% IVIG group comprised 81 (68.1%) patients admitted between February 2015 and July 2018, and the 10% IVIG group comprised 38 (31.9%) patients admitted between August 2018 and January 2022. The ESR (p < 0.001) and albumin levels (p = 0.005) in the 5% IVIG group were significantly lower than those in the 10% IVIG group (Table 1). More patients in the 5% IVIG group received IVIG 1 g/kg than those in the 10% IVIG group, although the difference was not statistically significant (p = 0.140, Table 1). The proportions of the Kobayashi high-risk group and CAAs during the acute and subacute phases were comparable between the two patient groups (Table 1). However, the IVIG resistance rate was significantly higher in the 10% IVIG group than in the 5% IVIG group (p = 0.008, Table 1). The fever duration was longer in the 10% IVIG group than in the 5% IVIG group, without statistical significance (p = 0.053, Table 1). Excluding the 60 patients who became afebrile before the end of IVIG infusion, fever duration was also longer in the 10% IVIG group than in the 5% IVIG group, without statistical significance (44.3 hours [range 1.0–167.3 hours] vs 35.5 hours [range 0.0–172.8 hours]; p = 0.077). For the 104 patients who received IVIG 2 g/kg, similar results were identified between the 5% IVIG and 10% IVIG groups (Table S2 in the ESM).
Table 1.
Comparison between the 5% IVIG and 10% IVIG groups
| Factor | 5% IVIG group (n = 81) | 10% IVIG group (n = 38) | p value |
|---|---|---|---|
| Male gender | 49 (60.5) | 18 (47.4) | 0.178 |
| Age group | 0.353 | ||
| ≤12 months | 13 (16.0) | 11 (28.9) | |
| 13–24 months | 21 (25.9) | 7 (18.4) | |
| 25–36 months | 17 (21.0) | 9 (23.7) | |
| 37–48 months | 14 (17.3) | 5 (13.2) | |
| 49–60 months | 5 (6.2) | 0 (0.0) | |
| >60 months | 11 (13.6) | 6 (15.8) | |
| Underlying diseases | 2 (2.5) | 1 (2.6) | 1.000 |
| Recurrent KD | 5 (6.2) | 1 (2.6) | 0.663 |
| KD diagnostic criteria | |||
| Fever days | 6 (2–10) | 6 (3–9) | 0.803 |
| Oral changes | 81 (100.0) | 38 (100.0) | NA |
| Conjunctivitis | 78 (96.3) | 37 (97.4) | 1.000 |
| Skin rash | 76 (93.8) | 37 (97.4) | 0.663 |
| Extremity changes | 60 (74.1) | 26 (68.4) | 0.521 |
| Cervical lymphadenopathy | 47 (58.0) | 16 (42.1) | 0.105 |
| Incomplete KD | 8 (9.9) | 6 (15.8) | 0.371 |
| Complete blood counta | |||
| White blood cell count (/mm3) | 14,000 (5000–32,000) | 13,800 (7100–26,500) | 0.878 |
| Neutrophils (%) | 65.3 (22.7–89.3) | 58.3 (37.0–94.4) | 0.231 |
| Hemoglobin (g/dL) | 11.2 (9.1–13.1) | 11.4 (10.1–12.7) | 0.392 |
| Platelet count (/mm3) | 334,000 (150,000–786,000) | 345,000 (185,000–641,000) | 0.277 |
| Inflammation marker | |||
| ESRa (mm/h) | 54 (9–120) | 34 (10–81) | <0.001 |
| C-reactive protein (mg/dL) | 6.64 (0.19–20.13) | 5.90 (0.69–19.15) | 0.341 |
| Blood chemistry | |||
| Aspartate transaminase (IU/L) | 30 (14–1110) | 30 (16–1271) | 0.661 |
| Alanine transaminase (IU/L) | 44 (4–817) | 40 (8–686) | 0.380 |
| Total bilirubinb (mg/dL) | 0.3 (0.1–4.2) | 0.3 (0.2–5.1) | 0.396 |
| Protein (g/dL) | 6.3 (4.9–7.8) | 6.5 (5.4–7.2) | 0.116 |
| Albumin (g/dL) | 3.9 (2.9–4.5) | 4.1 (3.4–4.7) | 0.005 |
| Sodium (mEq/L) | 138 (132–143) | 138 (134–142) | 0.863 |
| Potassium (mEq/L) | 4.2 (2.9–5.5) | 4.4 (3.7–5.7) | 0.212 |
| Chloride (mEq/L) | 100 (92–107) | 101 (96–105) | 0.217 |
| Kobayashi risk group | 0.859 | ||
| Low-risk | 63 (77.8) | 29 (76.3) | |
| High-risk | 18 (22.2) | 9 (23.7) | |
| IVIG treatment | |||
| Dose of 1 g/kg | 13 (16.0) | 2 (5.3) | 0.140 |
| Infusion time (hours) | 10.5 (4.5–17.9) | 10.9 (3.9–14.8) | 0.930 |
| Fever duration (hours) | −3.4 (−74.0 to 172.8) | 30.2 (−32.8 to 167.3) | 0.053 |
| IVIG resistance | 17 (21.0) | 17 (44.7) | 0.008 |
| Recrudescent fever | 10 (58.8) | 13 (76.5) | |
| Persistent fever | 7 (41.2) | 4 (23.5) | |
| CAAs on echocardiography | |||
| Acute phasec | 9 (12.9) | 3 (9.1) | 0.747 |
| Subacute phased | 6 (7.8) | 1 (3.4) | 0.671 |
Data are presented as N (%) or median (range)
CAA coronary artery abnormality, ESR erythrocyte sedimentation rate, IVIG intravenous immunoglobulin, KD Kawasaki disease, NA not available
aComplete blood count and ESR were measured except for one patient in the 10% IVIG group
bTotal bilirubin was tested in 78 and 38 patients in the 5% IVIG and 10% IVIG groups, respectively
cEchocardiography was performed in 70 and 33 patients in the 5% IVIG and 10% IVIG groups, respectively
dEchocardiography was performed in 77 and 29 patients in the 5% IVIG and 10% IVIG groups, respectively
Comparison Between the IVIG-Responsive and IVIG-Resistant Groups
The IVIG-resistant group (n = 34, 28.6%) showed significantly fewer fever days at IVIG treatment (p = 0.015), higher %neutrophils (p = 0.007), and higher AST (p = 0.001) and ALT (p < 0.001) levels than the IVIG-responsive group (n = 85, 71.4%; Table 2). Patients in the IVIG-resistant group were more likely to be assigned to the Kobayashi high-risk group (p = 0.038) and receive 10% IVIG (p = 0.008) than those in the IVIG-responsive group (Table 2). Because fever days at IVIG treatment, %neutrophils, and ALT levels are included in the Kobayashi scoring system, a multivariate analysis to determine the independent factors for IVIG resistance was performed for two factors, the Kobayashi risk group and 10% IVIG treatment. Both factors were independently associated with IVIG resistance (Table 3A). In multivariate analysis including each factor of the Kobayashi scoring system separately, 10% IVIG treatment was still independently associated with IVIG resistance (p = 0.004, Table 3B). For the 104 patients who received 2 g/kg IVIG, the same factors, including 10% IVIG treatment, were independently associated with IVIG resistance in multivariate analysis (Table S3 in the ESM).
Table 2.
Comparison between the IVIG-responsive and IVIG-resistant groups
| Factor | IVIG-responsive group (n = 85) | IVIG-resistant group (n = 34) | p value |
|---|---|---|---|
| Male gender | 49 (57.6) | 18 (52.9) | 0.640 |
| Age group | 0.325 | ||
| ≤12 months | 19 (22.4) | 5 (14.7) | |
| 13–24 months | 21 (24.7) | 7 (20.6) | |
| 25–36 months | 17 (20.0) | 9 (26.5) | |
| 37–48 months | 10 (11.8) | 9 (26.5) | |
| 49–60 months | 4 (4.7) | 1 (2.9) | |
| >60 months | 14 (16.5) | 3 (8.8) | |
| Underlying diseases | 2 (2.4) | 1 (2.9) | 1.000 |
| Recurrent KD | 4 (4.7) | 2 (5.9) | 1.000 |
| KD diagnostic criteria | |||
| Fever days | 6 (2–10) | 5 (3–9) | 0.015 |
| Oral changes | 85 (100.0) | 34 (100.0) | NA |
| Conjunctivitis | 81 (95.3) | 34 (100.0) | 0.577 |
| Skin rash | 80 (94.1) | 33 (97.1) | 0.673 |
| Extremity changes | 62 (72.9) | 24 (70.6) | 0.796 |
| Cervical lymphadenopathy | 43 (50.6) | 20 (58.8) | 0.416 |
| Incomplete KD | 12 (14.1) | 2 (5.9) | 0.345 |
| Complete blood counta | |||
| White blood cell count (/mm3) | 14,050 (6200–28,100) | 13,850 (5000–32,000) | 0.693 |
| Neutrophils (%) | 59.7 (22.7–88.1) | 68.9 (37.0–94.4) | 0.007 |
| Hemoglobin (g/dL) | 11.2 (9.1–13.1) | 11.4 (9.5–12.9) | 0.983 |
| Platelet count (/mm3) | 337,500 (150,000–786,000) | 329,500 (185,000–519,000) | 0.274 |
| Inflammation marker | |||
| ESRa (mm/h) | 48 (9–120) | 46 (15–120) | 0.664 |
| C-reactive protein (mg/dL) | 5.71 (0.19–20.13) | 7.34 (0.85–19.26) | 0.081 |
| Blood chemistry | |||
| Aspartate transaminase (IU/L) | 28 (14–1110) | 54 (16–1271) | 0.001 |
| Alanine transaminase (IU/L) | 26 (4–817) | 134 (9–686) | <0.001 |
| Total bilirubinb (mg/dL) | 0.3 (0.1–2.8) | 0.4 (0.2–5.1) | 0.056 |
| Protein (g/dL) | 6.4 (5.2–7.8) | 6.4 (4.9–7.4) | 0.294 |
| Albumin (g/dL) | 4.0 (3.0–4.7) | 3.8 (2.9–4.5) | 0.134 |
| Sodium (mEq/L) | 138 (132–143) | 138 (133–142) | 0.939 |
| Potassium (mEq/L) | 4.3 (2.9–5.7) | 4.2 (3.3–5.4) | 0.222 |
| Chloride (mEq/L) | 100 (92–107) | 101 (96–107) | 0.926 |
| Kobayashi risk group | 0.038 | ||
| Low-risk | 70 (82.4) | 22 (64.7) | |
| High-risk | 15 (17.6) | 12 (35.3) | |
| IVIG treatment | |||
| 10% IVIG administration | 21 (24.7) | 17 (50.0) | 0.008 |
| Dose of 1 g/kg | 14 (16.5) | 1 (2.9) | 0.064 |
| Infusion time (hours) | 10.4 (4.5–14.9) | 11.0 (3.9–17.9) | 0.210 |
| Fever duration (hours) | −8.0 (−74.0 to 35.5) | 51.4 (−36.0 to 172.8) | <0.001 |
| CAAs on echocardiography | |||
| Acute phasec | 10 (13.9) | 2 (6.5) | 0.340 |
| Subacute phased | 7 (9.2) | 0 (0.0) | 0.187 |
Data are presented as N (%) or median (range)
CAA coronary artery abnormality, ESR erythrocyte sedimentation rate, IVIG intravenous immunoglobulin, KD Kawasaki disease, NA not available
aComplete blood count and ESR were measured except for one patient in the IVIG-responsive group
bTotal bilirubin was tested in 82 and 34 patients in the IVIG-responsive and IVIG-resistant groups, respectively
cEchocardiography was performed in 72 and 31 patients in the IVIG-responsive and IVIG-resistant groups, respectively
dEchocardiography was performed in 76 and 30 patients in the IVIG-responsive and IVIG-resistant groups, respectively
Table 3.
Results of multivariate analyses to determine the independent factors for IVIG resistance while (A) including two factors of Kobayashi risk group and 10% IVIG treatment and (B) including each factor of the Kobayashi scoring system separately
| Factor | Odds ratio | 95% confidence interval | p-Value |
|---|---|---|---|
| (A) | |||
| Kobayashi high-risk group | 2.659 | 1.046–6.758 | 0.040 |
| 10% IVIG treatment | 3.143 | 1.338–7.383 | 0.009 |
| (B) | |||
| Fever days at IVIG treatment | 0.658 | 0.449–0.964 | 0.032 |
| %neutrophils | 1.049 | 1.010–1.089 | 0.013 |
| Aspartate transaminase level | 1.000 | 0.996–1.005 | 0.841 |
| Alanine transaminase level | 1.001 | 0.996–1.006 | 0.619 |
| 10% IVIG treatment | 4.140 | 1.585–10.813 | 0.004 |
IVIG intravenous immunoglobulin
In the IVIG-resistant group, recrudescent and persistent fever were identified in 23 (67.6%) and 11 (32.4%) patients, respectively. Defervescence was achieved without additional treatment in 12 (35.3%) patients, after the second IVIG treatment in three (8.8%) patients, after intravenous methyl-prednisolone treatment in 15 (44.1%) patients, and after IVIG and intravenous methyl-prednisolone combination treatment in four (11.8%) patients. Although 12 (52.2%) of the 23 patients with recrudescent fever became afebrile without additional treatment, all patients with persistent fever received additional treatment for defervescence. Patients with recrudescent fever and those with persistent fever showed comparable clinical manifestations and outcomes aside from their differing age distributions (Table S4 in the ESM).
Discussion
This study aimed to determine whether 10% IVIG treatment was associated with an increase in IVIG resistance. Although the clinical severity and outcomes of KD did not differ before and after the introduction of 10% IVIG, the IVIG resistance rate increased significantly after the introduction of 10% IVIG, and treatment with 10% IVIG was an independent factor associated with IVIG resistance in this study.
Previous studies have reported that among patients with KD, risk factors for IVIG resistance include male gender; fever days at IVIG treatment; higher %neutrophils and ESR; higher levels of CRP, AST, ALT, and total bilirubin; lower platelet counts; and lower levels of hemoglobin, albumin, and sodium [10, 11]. These findings were reaffirmed in this study. Although the Kobayashi high-risk group was significantly associated with IVIG resistance, its sensitivity and specificity in predicting IVIG resistance were 35.3% and 82.4%, respectively, in this study. Previous studies in Western countries reported the unfavorable sensitivity and specificity of various Japanese scoring systems predicting IVIG resistance and CAAs, including the Kobayashi scoring system [12–15]. In Korea and China, which are neighboring countries to Japan, Japanese scoring systems also showed unfavorable performance [16–19]. Therefore, each country should establish its own prediction system for IVIG resistance in patients with KD.
A few studies have reported on the association between IVIG concentration and IVIG resistance in patients with KD [20–22]. Oda et al. reported that the frequencies of adverse reactions and IVIG resistance were comparable between patients with KD who received 5% IVIG and those who received 10% IVIG and that fever duration after IVIG infusion was significantly shorter in patients who received 10% IVIG than in those who received 5% IVIG [21]. However, a recent nationwide Japanese study including a larger number of patients with KD reported a significantly higher IVIG resistance rate without an increase in CAAs in patients who received 10% IVIG than in those who received 5% IVIG [22]. In Canadian children, a higher IVIG resistance rate was reported in patients who received 10% IVIG compared with those who received 5% IVIG [20]. Our study also showed a significant association between 10% IVIG treatment and increased IVIG resistance. We previously reported that clinical characteristics and IVIG resistance rates in patients with KD in Korea did not change significantly from the 1990s to the 2010s, before the introduction of 10% IVIG [23, 24]. In this study, comparing patients with KD treated with 5% IVIG with those treated with 10% IVIG, we found comparable proportions of patients belonging to the Kobayashi high-risk group and those developing CAAs. IVIG resistance is based on the duration of fever after IVIG treatment, and fever is one of the most frequent adverse reactions of IVIG. Therefore, we hypothesized that the substitution of 10% IVIG for 5% IVIG increased febrile reactions after IVIG infusion and that this was clinically recognized as IVIG resistance; that is, an increase in patients with fever patterns consistent with the definition of IVIG resistance rather than an increase in patients with true IVIG resistance after the use of 10% IVIG.
IVIG dose, IVIG infusion rate, patient age, and underlying conditions were considered to influence the development of adverse reactions linked to IVIG treatment [25]. To address these confounding factors, we only included pediatric patients with KD and conducted a subgroup analysis for patients receiving IVIG 2 g/kg, which is the standard therapy for KD. The infusion rates in the 5% IVIG and 10% IVIG groups were similar to each other; therefore, in this study, the infused dose of IVIG in grams per hour was lower in the 10% IVIG group than in the 5% IVIG group. Administration of 10% IVIG results in earlier determination of IVIG resistance due to the reduced infusion time compared with 5% IVIG. This can shorten the timeframe required to determine IVIG resistance after IVIG infusion. Therefore, the 2020 Japanese KD guideline changed the time for defining IVIG resistance from 24 hours to 24–36 hours after the end of IVIG infusion based on the results of the above-mentioned nationwide study [22, 26]. However, the AHA 2017 guideline had already recommended defining IVIG resistance ≥36 hours after the end of IVIG infusion [2], and this study adopted the AHA guideline. Although the exact mechanisms responsible for the adverse febrile reactions of IVIG are unknown, they might be related to the complement activation mediated by the IgG aggregates included in IVIG [25]. The presence of serum proteins, such as IgA, IgM, CD4, CD8, and cytokines; viruses; and excipients added during manufacturing may cause adverse reactions because these components cannot be completely eliminated despite the use of several manufacturing processes [25]. However, we used 5% IVIG and 10% IVIG from the same manufacturer, and they only differed in concentration. Considering that the components of 5% IVIG and 10% IVIG were identical in this study, individual patient factors might have influenced the development of adverse febrile reactions after infusion of a higher concentration of IVIG. Genetic variations in Fc receptors and proteins in the complement pathway that participate in IVIG actions need to be evaluated to define the independent association between the use of 10% IVIG and an increase in the occurrence of adverse febrile reactions.
Efforts should be made to differentiate between adverse febrile reactions due to a higher concentration of IVIG and true IVIG resistance, which is accompanied by an increased risk for CAAs. Downie et al. reported that patients with persistent fever after IVIG treatment were more likely to develop CAAs than those with recrudescent fever after IVIG treatment [20]. Among the laboratory test results, the WBC count, %neutrophils, and CRP and N-terminal pro-brain natriuretic peptide levels significantly decreased after IVIG treatment in IVIG-responsive patients but not in IVIG-resistant patients [27]. Therefore, the fever pattern and laboratory test results after IVIG treatment can be considered adjunctively to determine true IVIG resistance. In this study, the frequencies of CAAs in patients with recrudescent fever and those in patients with persistent fever were comparable. Unfortunately, laboratory data after IVIG infusion were not systemically collected in our patients; therefore, the association between IVIG resistance and changes in laboratory test results after IVIG treatment could not be determined. Genetic variations associated with IVIG resistance, as well as with the incidence of KD, have been reported. Various genes involved in T-cell stimulation, interleukin-1β and -2 and transforming growth factor-β signaling pathways, cell apoptosis, and angiogenesis have been identified [28, 29]. Although using a combination of genetic and clinical factors improved the predictability of IVIG resistance [28, 29], routine genetic testing might not be currently applicable in real-life clinical settings. Reductions in the infusion rate and dose of IVIG can help decrease adverse IVIG reactions [7]. However, in this study, the infusion times of 5% IVIG (median of 10.5 hours) and 10% IVIG (median of 10.9 hours) were comparable; further extension of the infusion time might not be practical. Recent studies have reported comparable therapeutic effects of IVIG 1 g/kg and IVIG 2 g/kg [30–32]. In patients at low risk for IVIG resistance, IVIG 1 g/kg was expected to be as effective as IVIG 2 g/kg, with decreases in adverse IVIG reactions [32]. We could not determine the association between 10% IVIG therapy and IVIG resistance in patients receiving IVIG 1 g/kg due to the small numbers of patients receiving IVIG 1 g/kg, with IVIG-resistant patients among them.
This study had some limitations, including potential selection bias due to its retrospective nature and the restricted hospital admission of febrile patients due to the recent coronavirus disease 2019 pandemic. Although we performed multivariate analysis, we might have missed some factors associated with IVIG resistance that work concurrently with the introduction of 10% IVIG. In addition, various IVIG products may have different adverse reaction profiles depending on their composition. After the pandemic ends, prolonged prospective studies including a sufficient number of patients should be conducted, while controlling for disease-, treatment-, and patient-related confounding factors.
Conclusion
Infusion with 10% IVIG was independently associated with treatment resistance. However, this resistance seemed to represent an adverse febrile reaction linked to the use of a higher concentration of IVIG rather than a true treatment resistance, as no associations with other significant changes in clinical manifestations were observed.
Supplementary Information
Below is the link to the electronic supplementary material.
Declarations
Funding
The authors received no financial support for this study.
Conflict of interest
All authors had no conflict of interest.
Ethics approval
This study was conducted in compliance with ethical principles originating in the Declaration of Helsinki, and was approved by the Institutional Review Board of Daejeon St. Mary’s Hospital, which waived the requirement for informed consent (approval number: DC22RASI0015).
Consent to participate
The Institutional Review Board of Daejeon St. Mary’s Hospital waived the requirement for informed consent.
Consent to publish
Not applicable.
Statement of data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Author contributions
Cenceptualization: SBH and J-WR; methodology: SBH; formal analysis and investigation: SBH, WS and J-WR; writing—original draft preparation: SBH and WS; writing—review and editing: J-WR; supervision: J-WR.
References
- 1.Kim GB, Eun LY, Han JW, Kim SH, Yoon KL, Han MY, et al. Epidemiology of Kawasaki disease in South Korea: a nationwide survey 2015–2017. Pediatr Infect Dis J. 2020;39:1012–1016. doi: 10.1097/INF.0000000000002793. [DOI] [PubMed] [Google Scholar]
- 2.McCrindle BW, Rowley AH, Newburger JW, Burns JC, Bolger AF, Gewitz M, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a scientific statement for health professionals from the American Heart Association. Circulation. 2017;135:e927–e999. doi: 10.1161/CIR.0000000000000484. [DOI] [PubMed] [Google Scholar]
- 3.Kato H, Inoue O, Kawasaki T, Fujiwara H, Watanabe T, Toshima H. Adult coronary artery disease probably due to childhood Kawasaki disease. Lancet. 1992;340:1127–1129. doi: 10.1016/0140-6736(92)93152-D. [DOI] [PubMed] [Google Scholar]
- 4.Burns JC, Shike H, Gordon JB, Malhotra A, Schoenwetter M, Kawasaki T. Sequelae of Kawasaki disease in adolescents and young adults. J Am Coll Cardiol. 1996;28:253–257. doi: 10.1016/0735-1097(96)00099-X. [DOI] [PubMed] [Google Scholar]
- 5.Furusho K, Kamiya T, Nakano H, Kiyosawa N, Shinomiya K, Hayashidera T, et al. High-dose intravenous gammaglobulin for Kawasaki disease. Lancet. 1984;2:1055–1058. doi: 10.1016/S0140-6736(84)91504-6. [DOI] [PubMed] [Google Scholar]
- 6.Newburger JW, Takahashi M, Burns JC, Beiser AS, Chung KJ, Duffy CE, et al. The treatment of Kawasaki syndrome with intravenous gamma globulin. N Engl J Med. 1986;315:341–347. doi: 10.1056/NEJM198608073150601. [DOI] [PubMed] [Google Scholar]
- 7.Gelfand EW. Differences between IGIV products: impact on clinical outcome. Int Immunopharmacol. 2006;6:592–599. doi: 10.1016/j.intimp.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 8.Bonilla FA. Intravenous immunoglobulin: adverse reactions and management. J Allergy Clin Immunol. 2008;122:1238–1239. doi: 10.1016/j.jaci.2008.08.033. [DOI] [PubMed] [Google Scholar]
- 9.Kobayashi T, Inoue Y, Takeuchi K, Okada Y, Tamura K, Tomomasa T, et al. Prediction of intravenous immunoglobulin unresponsiveness in patients with Kawasaki disease. Circulation. 2006;113:2606–2612. doi: 10.1161/CIRCULATIONAHA.105.592865. [DOI] [PubMed] [Google Scholar]
- 10.Li X, Chen Y, Tang Y, Ding Y, Xu Q, Sun L, et al. Predictors of intravenous immunoglobulin-resistant Kawasaki disease in children: a meta-analysis of 4442 cases. Eur J Pediatr. 2018;177:1279–1292. doi: 10.1007/s00431-018-3182-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu G, Wang S, Du Z. Risk Factors of intravenous immunoglobulin resistance in children with Kawasaki disease: a meta-analysis of case-control studies. Front Pediatr. 2020;8:187. doi: 10.3389/fped.2020.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tremoulet AH, Best BM, Song S, Wang S, Corinaldesi E, Eichenfield JR, et al. Resistance to intravenous immunoglobulin in children with Kawasaki disease. J Pediatr. 2008;153:117–121. doi: 10.1016/j.jpeds.2007.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davies S, Sutton N, Blackstock S, Gormley S, Hoggart CJ, Levin M, et al. Predicting IVIG resistance in UK Kawasaki disease. Arch Dis Child. 2015;100:366–368. doi: 10.1136/archdischild-2014-307397. [DOI] [PubMed] [Google Scholar]
- 14.Jakob A, von Kries R, Horstmann J, Hufnagel M, Stiller B, Berner R, et al. Failure to predict high-risk Kawasaki disease patients in a population-based study cohort in Germany. Pediatr Infect Dis J. 2018;37:850–855. doi: 10.1097/INF.0000000000001923. [DOI] [PubMed] [Google Scholar]
- 15.Piram M, Darce Bello M, Tellier S, Di Filippo S, Boralevi F, Madhi F, et al. Defining the risk of first intravenous immunoglobulin unresponsiveness in non-Asian patients with Kawasaki disease. Sci Rep. 2020;10:3125. doi: 10.1038/s41598-020-59972-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park HM, Lee DW, Hyun MC, Lee SB. Predictors of nonresponse to intravenous immunoglobulin therapy in Kawasaki disease. Korean J Pediatr. 2013;56:75–79. doi: 10.3345/kjp.2013.56.2.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim BY, Kim D, Kim YH, Ryoo E, Sun YH, Jeon IS, et al. Non-responders to intravenous immunoglobulin and coronary artery dilatation in Kawasaki disease: predictive parameters in Korean children. Korean Circ J. 2016;46:542–549. doi: 10.4070/kcj.2016.46.4.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shin J, Lee H, Eun L. Verification of current risk scores for Kawasaki disease in Korean children. J Korean Med Sci. 2017;32:1991–1996. doi: 10.3346/jkms.2017.32.12.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qian W, Tang Y, Yan W, Sun L, Lv H. A comparison of efficacy of six prediction models for intravenous immunoglobulin resistance in Kawasaki disease. Ital J Pediatr. 2018;44:33. doi: 10.1186/s13052-018-0475-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Downie ML, Manlhiot C, Latino GA, Collins TH, Chahal N, Yeung RS, et al. Variability in response to intravenous immunoglobulin in the treatment of Kawasaki disease. J Pediatr. 2016;179:124 e1–130. doi: 10.1016/j.jpeds.2016.08.060. [DOI] [PubMed] [Google Scholar]
- 21.Oda T, Nagata H, Nakashima Y, Nanishi E, Takada Y, Nishimura M, et al. Clinical utility of highly purified 10% liquid intravenous immunoglobulin in Kawasaki disease. J Pediatr. 2019;214:227–230. doi: 10.1016/j.jpeds.2019.06.018. [DOI] [PubMed] [Google Scholar]
- 22.Suzuki T, Michihata N, Yoshikawa T, Saito K, Matsui H, Fushimi K, et al. Low- versus high-concentration intravenous immunoglobulin for children with Kawasaki disease in the acute phase. Int J Rheum Dis. 2022;25:576–583. doi: 10.1111/1756-185X.14309. [DOI] [PubMed] [Google Scholar]
- 23.Rhim JW, Youn YS, Han JW, Lee SJ, Oh JH, Lee KY. Changes in Kawasaki disease during 2 decades at a single institution in Daejeon, Korea. Pediatr Infect Dis J. 2014;33:372–375. doi: 10.1097/INF.0000000000000123. [DOI] [PubMed] [Google Scholar]
- 24.Kil HR, Yu JW, Lee SC, Rhim JW, Lee KY. Changes in clinical and laboratory features of Kawasaki disease noted over time in Daejeon, Korea. Pediatr Rheumatol Online J. 2017;15:60. doi: 10.1186/s12969-017-0192-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nydegger UE, Sturzenegger M. Adverse effects of intravenous immunoglobulin therapy. Drug Saf. 1999;21:171–185. doi: 10.2165/00002018-199921030-00003. [DOI] [PubMed] [Google Scholar]
- 26.Research Committee of the Japanese Society of Pediatric Cardiology and Cardiac Surgery Guidelines for medical treatment of acute Kawasaki disease (2020 revised version) J Pediatr Cardiol Cardiac Surg. 2021;5:41–73. [Google Scholar]
- 27.Kim HK, Oh J, Hong YM, Sohn S. Parameters to guide retreatment after initial intravenous immunoglobulin therapy in kawasaki disease. Korean Circ J. 2011;41:379–384. doi: 10.4070/kcj.2011.41.7.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuo HC, Wong HS, Chang WP, Chen BK, Wu MS, Yang KD, et al. Prediction for intravenous immunoglobulin resistance by using weighted genetic risk score identified from genome-wide association study in Kawasaki disease. Circ Cardiovasc Genet. 2017;10:e001625. doi: 10.1161/CIRCGENETICS.116.001625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen L, Song S, Ning Q, Zhu D, Jia J, Zhang H, et al. Prediction for intravenous immunoglobulin resistance combining genetic risk loci identified from next generation sequencing and laboratory data in Kawasaki disease. Front Pediatr. 2020;8:462367. doi: 10.3389/fped.2020.462367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suzuki T, Michihata N, Yoshikawa T, Hata T, Matsui H, Fushimi K, et al. High-dose versus low-dose intravenous immunoglobulin for treatment of children with Kawasaki disease weighing 25 kg or more. Eur J Pediatr. 2020;179:1901–1907. doi: 10.1007/s00431-020-03794-2. [DOI] [PubMed] [Google Scholar]
- 31.He L, Liu F, Yan W, Huang M, Huang M, Xie L, et al. Randomized trial of different initial intravenous immunoglobulin regimens in Kawasaki disease. Pediatr Int. 2021;63:757–763. doi: 10.1111/ped.14656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matsuura M, Sugawara D, Makita E, Hirakubo Y, Nonaka K, Yamashita S, et al. Stratified therapy for Kawasaki disease using a new scoring system to predict the response to a lower dose of intravenous immunoglobulin therapy. Cardiol Young. 2022;32:405–409. doi: 10.1017/S1047951121002237. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.