Introduction
Large tumor size is an independent poor prognostic factor among patients with human epidermal growth factor receptor 2 (HER2)-positive breast cancer (BC) [1, 2]. In contrast, the risk of distant invasive recurrence of T1a was quite low, even in patients not receiving adjuvant trastuzumab and/or chemotherapy [3, 4].
In 2015, the APT trial assessed disease-free survival (DFS) in patients with HER2-positive BC, negative nodes, and tumor size ≤3 cm [5, 6]. All the patients in the trial received weekly treatment with paclitaxel and trastuzumab. The rate of serious toxic effects was low, and the long-term outcome was excellent [5, 6]. However, it remains to be seen whether similarly excellent results can be obtained in clinical practice. Most of the previous real-world data included mainly T1 tumors [4, 7, 8, 9], and very few clinical studies have been conducted to evaluate the outcome of patients with tumors of 3 cm or less in size.
The present study therefore aimed to evaluate the clinical outcomes in patients with HER2-positive, node-negative tumors ≤3 cm receiving adjuvant chemotherapy and HER2-targeted therapy.
Patients and Methods
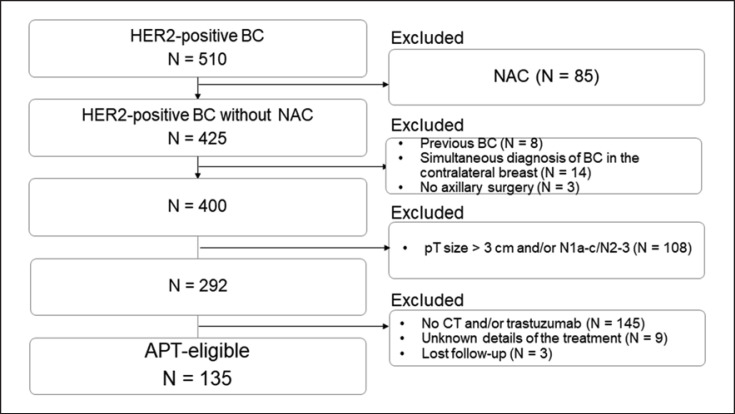
The present study enrolled consecutive patients with HER2-positive BC who underwent breast and axillary surgery between January 2007 and August 2020. Figure 1 shows the patient selection flow. Three hundred seventy-five patients were excluded for the following reasons: neoadjuvant chemotherapy (n = 85), previous diagnosis of BC (n = 8), simultaneous diagnosis of cancer in the contralateral breast (n = 14), no axillary surgery (n = 3), pathological tumor size >3 cm and/or pN1a–c to N3 (n = 108), not receiving adjuvant chemotherapy and/or HER2-targeted therapy (n = 145), unknown treatment details (n = 9), and lost to follow-up (n = 3). Finally, 135 patients who met the eligibility criteria for the APT trial were included.
Fig. 1.
Patient enrollment flow. BC, breast cancer; NAC, neoadjuvant chemotherapy; CT, chemotherapy.
Pathological Findings
Estrogen receptor (ER) status was considered positive when ≥1% of tumor cells showed positive nuclear staining. HER2 positivity status was defined as 3+ on immunohistochemistry. When HER2 IHC yielded a score of 2+, dual color in situ hybridization (DISH) was performed and the positivity status was determined according to the 2007, 2013, and 2018 ASCO/CAP guidelines at surgery [10, 11, 12].
Adjuvant Chemotherapy
Adjuvant chemotherapy was chosen according to the guidelines at the time of surgery. The anthracycline regimen included either doxorubicin (60 mg/m2) and cyclophosphamide (600 mg/m2) (AC) or fluorouracil (500 mg/m2), epirubicin (100 mg/m2), and cyclophosphamide (500 mg/m2) (FEC). FEC was repeated every 3 weeks for four cycles, and AC was repeated every 2 or 3 weeks for four cycles. The taxane regimen included either docetaxel (75 mg/m2) at 3-week intervals for four cycles, paclitaxel (80 mg/m2) at a weekly interval for 12 cycles, paclitaxel (175 mg/m2) at 2-week intervals for four cycles, or docetaxel (75 mg/m2) and carboplatin (AUC 6) at 3-week intervals for six cycles.
Adjuvant HER2-Targeted Therapy
All the patients received adjuvant HER2-targeted therapy (trastuzumab and/or pertuzumab). Of the 135 patients, six (4.4%) received the combination of pertuzumab and trastuzumab. Trastuzumab was given every 3 weeks (8 mg/kg loading dose on cycle 1 then 6 mg/kg) or weekly (4 mg/kg loading dose on cycle 1 then 2 mg/kg) with chemotherapy. Pertuzumab was given at a loading dose of 840 mg followed by 420 mg every 3 weeks.
Adjuvant Endocrine Therapy and Postoperative Radiation Therapy
Patients with hormone receptor-positive tumors received adjuvant endocrine therapy at least 5 years after chemotherapy. Of 26 patients who underwent breast-conserving surgery, all the patients received the whole-breast radiation therapy after chemotherapy.
Postoperative Follow-Up
Cardiac function was assessed using echocardiography every 3 months during trastuzumab administration. A physical examination was performed every 3 months for a period of at least 3 years after surgery. After the first 3 years, a physical examination was performed every 3 to 6 months. A mammography was performed once a year, and ultrasound examination was added at the physician's discretion.
Statistical Analysis
The endpoint was the time to distant disease-free survival (D-DFS) as estimated using Kaplan-Meier curves. The secondary endpoints were DFS and overall survival (OS), which were compared among patients with pT1a or pT1b tumors (the pT1a/b group), those with pT1c tumors (the pT1c group), and those with pT2 tumors (the pT2 group). The threshold for significance was p < 0.05. The data were statistically analyzed using Bell Curve for Excel (Social Survey Research Information Co., Ltd., Tokyo, Japan). Data collection was completed by September 16, 2021.
Results
Table 1 summarizes the patient characteristics. The median age was 55 years (range: 31–78 years). In the total cohort, 28 patients (20.7%) had pT1a or pT1b tumors (the pT1a/b group), 67 (49.6%) had pT1c tumors (the pT1c group), and 40 (29.6%) had pT2 tumors. Eleven patients (8.1%) had a micrometastasis in the sentinel nodes. Taxan-based was the most common adjuvant therapy regimen and was given to 46% of the patients. Of the taxane-based regimens, paclitaxel was the most common (Table 1). During trastuzumab treatment, one patient (0.7%) had a left ventricular ejection fraction below 50% and discontinued trastuzumab before completion of the 1-year treatment.
Table 1.
Patient characteristics
| Variable | All (N = 135) | pT1a/b group (N = 28) | pT1c group (N = 67) | pT2 group (N = 40) |
|---|---|---|---|---|
| Age, median (range) cT stage | 55 (31–78) | 50 (38–73) | 57 (31–75) | 56.5 (34–78) |
| Tis | 13 (10%) | 7 (25%) | 6 (9%) | 0 |
| T1a | 0 | 0 | 0 | 0 |
| T1b | 6 (4%) | 6 (21%) | 0 | 0 |
| T1c | 71 (53%) | 13 (46%) | 50 (75%) | 8 (20%) |
| T2 | 45 (33%) | 2 (7%) | 11 (16%) | 32 (80%) |
| Breast surgery | ||||
| Mastectomy | 109 (81%) | 20 (71%) | 53 (79%) | 36 (90%) |
| BCS | 26 (19%) | 8 (29%) | 14 (21%) | 4 (10%) |
| Axillary surgery | ||||
| SLNB | 131 (97%) | 27 (96%) | 65 (97%) | 39 (97%) |
| SLNB and ALND | 4 (3%) | 1 (4%) | 2 (3%) | 1 (3%) |
| pT stage | ||||
| T1a | 6 (4%) | 6 (21%) | 0 | 0 |
| T1b | 22 (16%) | 22 (79%) | 0 | 0 |
| T1c | 67 (50%) | 0 | 67 (100%) | 0 |
| T2 | 40 (30%) | 0 | 0 | 40 (100%) |
| pN stage | ||||
| N0 | 126 (93%) | 26 (93%) | 62 (93%) | 38 (95%) |
| N1mic | 9 (7%) | 2 (7%) | 5 (7%) | 2 (5%) |
| Nuclear grade | ||||
| 1, 2 | 15 (11%) | 4 (14%) | 8 (12%) | 3 (7%) |
| 3 | 120 (89%) | 24 (86%) | 59 (88%) | 37 (93%) |
| Estrogen receptor status | ||||
| Positive | 68 (50%) | 11 (39%) | 35 (52%) | 22 (55%) |
| Negative | 67 (50%) | 17 (61%) | 32 (48%) | 18 (45%) |
| Chemotderapy | ||||
| Anthracycline and taxane | ||||
| AC-T | 24 (18%) | 2 (7%) | 8 (12%) | 14 (35%) |
| FEC-T | 10 (7%) | 1 (3.5%) | 5 (7%) | 4 (10%) |
| EC-T | 3 (2%) | 1 (3.5%) | 2 (3%) | 0 |
| Anthracycline alone | ||||
| FEC | 6 (4%) | 1 (3.5%) | 3 (4%) | 2 (5%) |
| EC | 30 (22%) | 10 (36%) | 12 (17%) | 8 (20%) |
| Taxane-based | ||||
| Paclitaxel | 47 (35%) | 12 (43%) | 28 (41%) | 7 (17.5%) |
| TC | 10 (7%) | 1 (3.5%) | 6 (12%) | 3 (7.5%) |
| Carboplatin + docetaxel | 5 (4%) | 0 | 3 (4%) | 2 (5%) |
| HER2-targeting therapy | ||||
| Trastuzumab | 129 (96%) | 28 (100%) | 64 (95%) | 37 (92%) |
| Trastuzumab + pertuzumab | 6 (4%) | 0 | 3 (5%) | 3 (8%) |
BCS, breast-conserving surgery; SLNB, sentinel lymph node biopsy; ALND, axillary lymph node dissection; AC-T, doxorubicin and cyclophosphamide followed by taxane; FEC-T, fluorouracil, epirubicin, and cyclophosphamide followed by taxane; EC-T, epirubicin and cyclophosphamide followed by taxane; EC, epirubicin and cyclophosphamide; FEC, fluorouracil, epirubicin, and cyclophosphamide; TC, docetaxel and cyclophosphamide.
Follow-Up Outcomes
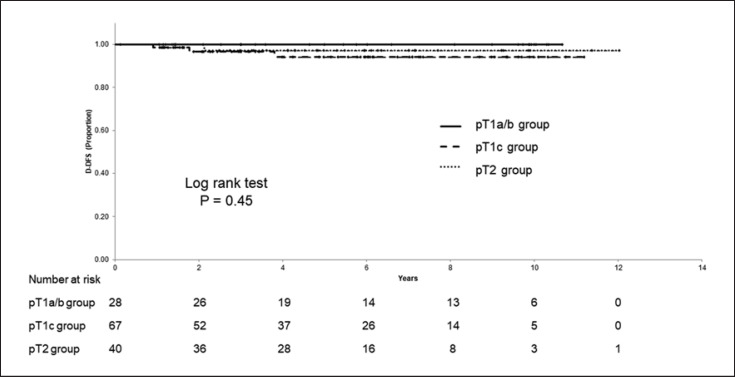
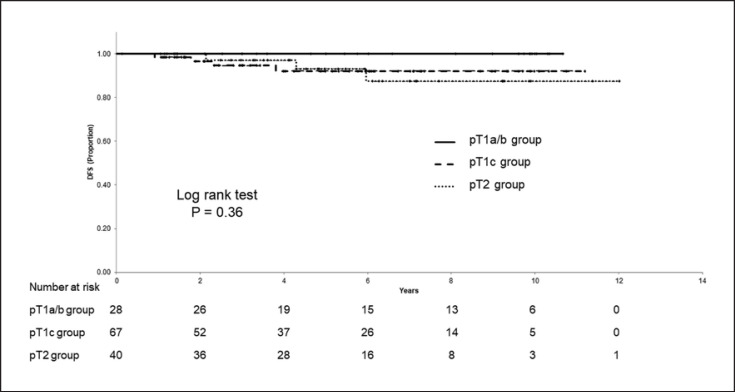
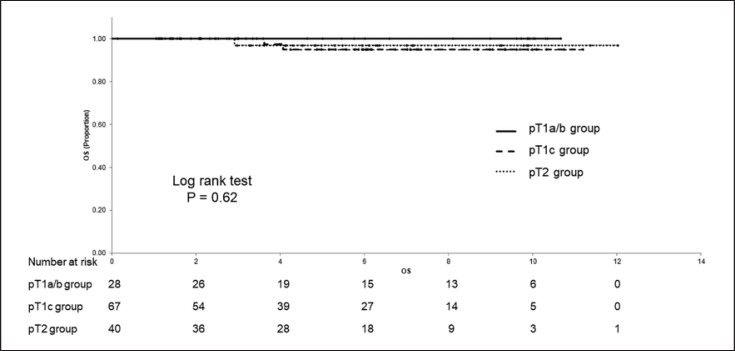
The median postoperative period was 5.4 years. The 5-year D-DFS, 5-year DFS, and 5-year OS in the total cohort was 96.3% (95% CI; 92.7–99.9%), 94.1% (95% CI; 89.5–98.8%), and 96.7% (95% CI; 92.9–100%), respectively. Distant recurrences occurred in three patients in the T1c group and one patient in the T2 group. Table 2 lists the patients with a distant recurrence. The 5-year D-DFS were 100% (95% CI; 100–100%), 94.2% (95% CI; 87.6–100%), and 97.1% (95% CI; 91.6–100%) in the T1a/b, T1c, and T2 group, respectively (Fig. 2). The 5-year DFS rate was 100% (95% CI; 100–100%), 92.1% (95% CI; 84.6–99.7%), and 93.3% (95% CI; 84.1–100%), and the 5-year OS was 100% (95% CI; 100–100%), 94.9% (95% CI; 87.9–100%), and 97.0% (95% CI; 91.1–100%), in the T1a/b, T1c, and T2 group, respectively. The T1c and T2 group had lower D-DFS, DFS, and OS rates than the T1a/b group, although not significant (Fig. 3, 4).
Table 2.
List of patients with a distant recurrence
| Case | cT stage | pT size, mm | ER status | Adjuvant chemotherapy | First recurrence site | Time to relapse, years |
|---|---|---|---|---|---|---|
| 1 | Tis | 20 | Negative | TC + HER | Brain | 0.9 |
| 2 | T1c | 19 | Positive | EC + HER | Bone | 1.8 |
| 3 | T2 | 21 | Negative | FEC + HER | Lung, liver | 2.9 |
| 4 | T2 | 20 | Negative | EC + HER | Lung | 3.8 |
ER, estrogen receptor; TC, docetaxel and cyclophosphamide; HER, herceptin; EC, epirubicin and cyclophosphamide; FEC, fluorouracil, epirubicin, and cyclophosphamide.
Fig. 2.
Distant disease-free survival (D-DFS) according to pT stage.
Fig. 3.
Disease-free survival (DFS) according to pT stage.
Fig. 4.
Overall survival (OS) according to pT stage.
Discussion
Currently, non-anthracycline regimens are the most common form of adjuvant chemotherapy in HER2-positive early BC [13, 14]. The APT trial demonstrated that treatment de-escalation was possible in patients with low-anatomic-risk, HER2-positive disease [15]. However, little is known about how the survival benefit of combining NAC with anthracycline and taxane compared to postoperative adjuvant chemotherapy in patients with small HER2-positive BC. Some clinical trials investigate whether anthracyclines can be safely omitted from neoadjuvant therapy for HER2-positive BC [16, 17, 18]. Although the long-term results are not yet available, the pathological complete response rate was high either with or without anthracyclines, suggesting the likelihood that anthracycline will be omitted increasingly from both NAC and adjuvant chemotherapy.
Even in clinical practice, a favorable 5-year D-DFS (96.3%) was observed in the present study. The 5-year DFS was as high as in the APT trial (94.1% vs. 96.3%). The results were better than expected, because half of the patients in the APT trial had pT1b or smaller tumors compared to 14% of the patients in the present study. Our findings underscore the fact that patients with HER2-positive, node-negative tumors ≤3 cm who received trastuzumab and chemotherapy had a favorable 5-year D-DFS.
There were few recurrence events that resulted in difficulty in identifying significant poor prognostic factors. Although not significant, larger tumor size (>1 cm) was associated with worse outcomes. None of patients with pT1a or pT1b tumors relapsed and all the patients with a distant recurrence had tumors measuring ≥19 mm.
Among patients with HER2-positive BC, hormone receptor (HR)-negative disease often recurred earlier than HR-positive disease [19]. In the present study, three of the four recurrent cases had HR-negative tumors. Whereas patients with HR-negative tumors had a higher risk of recurrence within postoperative 3 years, those with HR-positive tumors had a higher incidence of recurrence in postoperative 6 to 8 years; thus, the survival outcomes at 8 years were similar between the patients with HR-positive and those with HR-negative/HER2-positive early BC [20]. The median postoperative period in the present study was 5.4 years; longer follow-up is required to understand the long-term outcomes.
Based on the findings of the present study, patients with HER2-positive BC with a tumor 20 mm or more in size should be candidates for NAC. These patients may have had a better outcome if they had received NAC and anti-HER2 therapy based on their treatment response.
The present study has several limitations. First, the sample size was small, and the study design a retrospective design. Second, it included patients who received various regimens, making a simple comparison with the APT trial difficult. Third, the follow-up period for the patients who received taxane-based therapy was short, and further follow-up is needed.
Conclusions
In the present study, patients with HER2-positive, node-negative tumors ≤3 cm who received trastuzumab and chemotherapy had a favorable 5-year D-DFS (96.3%). Among them, those with a tumor 1 cm or less in size had better outcomes.
Statement of Ethics
This study was approved by the institutional ethics committee of Tokyo Metropolitan Cancer and Infectious Diseases Center, Komagome Hospital and written informed consent was waived because of the study's retrospective design. All the experimental protocols were approved on December 2, 2020 (No. 2641).
Conflict of Interest Statement
T. Aruga has received honoraria from Chugai Pharmaceutical Co. Ltd., Eisai Co. Ltd., and Pfizer Japan Inc. N. Iwamoto has no conflict of interest to declare.
Funding Sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author Contributions
All the authors contributed to the conception and design of the study. Naoko Iwamoto collected the data and prepared the first draft of the manuscript. Tomoyuki Aruga commented on previous versions of the manuscript and approved the final version.
Data Availability Statement
The datasets generated during and/or analyzed during the present study are not publicly available due to privacy but are available from the corresponding author on reasonable request.
References
- 1.Perez EA, Romond EH, Suman VJ, et al. Trastuzumab plus adjuvant chemotherapy for human epidermal growth factor receptor 2-positive breast cancer: planned joint analysis of overall survival from NSABP B-31 and NCCTG N9831. J Clin Oncol. 2018;20:3744–52. doi: 10.1200/JCO.2014.55.5730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gingras I, Holmes E, Azambuja ED, et al. Regional nodal irradiation after breast conserving surgery for early HER2-positive breast cancer: results of a subanalysis from the ALTTO trial. J Natl Cancer Inst. 2017;109:djw331. doi: 10.1093/jnci/djw331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fehrenbacher L, Capra AM, Quesenberry CP, Jr, et al. Distant invasive breast cancer recurrence risk in human epidermal growth factor receptor 2-positive T1a and T1b node-negative localized breast cancer diagnosed from 2000 to 2006: a cohort from an integrated health care delivery system. J Clin Oncol. 2014;32:2151–8. doi: 10.1200/JCO.2013.52.0858. [DOI] [PubMed] [Google Scholar]
- 4.Horio A, Fujita T, Hayashi H, et al. High recurrence risk and use of adjuvant trastuzumab in patients with small, HER2-positive, node-negative breast cancers. Int J Clin Oncol. 2012;17:131–6. doi: 10.1007/s10147-011-0269-4. [DOI] [PubMed] [Google Scholar]
- 5.Tolaney SM, Barry WT, Dang CT, et al. Adjuvant paclitaxel and trastuzumab for node-negative, HER2-positive breast cancer. N Engl J Med. 2015;372:134–41. doi: 10.1056/NEJMoa1406281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tolaney SM, Guo H, Pernas S, et al. Seven-year follow-up analysis of adjuvant paclitaxel and trastuzumab trial for node-negative, human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol. 2019;37:1868–75. doi: 10.1200/JCO.19.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin H, Zheng H, Ge C, et al. The effect of adjuvant treatment in small node-negative HER2-positive breast cancer: which subgroup will benefit? Clin Breast Cancer. 2020;20:503–10. doi: 10.1016/j.clbc.2020.05.012. [DOI] [PubMed] [Google Scholar]
- 8.de Nonneville Gonçalves A, Zemmour C, et al. Benefit of adjuvant chemotherapy with our without trastuzumab in pT1ab node-negative human epidermal growth factor receptor 2-positive breast carcinomas: results of a national multi-institutional study. Breast Cancer Res Treat. 2017;162:307–16. doi: 10.1007/s10549-017-4136-5. [DOI] [PubMed] [Google Scholar]
- 9.He X, Ji J, Tian M, et al. Long-term survival analysis of adjuvant chemotherapy with or without trastuzumab in patients with T1, node-negative HER2-positive breast cancer. Clin Cancer Res. 2019;25:7388–95. doi: 10.1158/1078-0432.CCR-19-0463. [DOI] [PubMed] [Google Scholar]
- 10.Wolff AC, Hammond ME, Schwartz JN, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25:118–45. doi: 10.1200/JCO.2006.09.2775. [DOI] [PubMed] [Google Scholar]
- 11.Wolff AC, Hammond ME, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31:3997–4013. doi: 10.1200/JCO.2013.50.9984. [DOI] [PubMed] [Google Scholar]
- 12.Wolff AC, Hammond MEH, Allison KH, et al. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline focused update. J Clin Oncol. 2018;36:2105–22. doi: 10.1200/JCO.2018.77.8738. [DOI] [PubMed] [Google Scholar]
- 13.Byfield SD, Buck PO, Blauer-Peterson C, et al. Treatment patterns and cost of care associated with initial therapy among patients diagnosed with operable early-stage human epidermal growth factor receptor 2-overexpressed breast cancer in the United States: A real-world retrospective study. J Oncol Pract. 2016;12:159–67. doi: 10.1200/JOP.2015.004747. [DOI] [PubMed] [Google Scholar]
- 14.Tang M, Schaffer A, Kiely BE, et al. Treatment patterns and survival in HER2-positive early breast cancer: a whole-of-population Australian cohort study (2007-2016) Br J Cancer. 2019;121:904–11. doi: 10.1038/s41416-019-0612-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choong GM, Cullen GD, O'Sullivan CC. Evolving standards of care and new challenges in the management of HER2-positive breast cancer. CA Cancer J Clin. 2020;70:355–74. doi: 10.3322/caac.21634. [DOI] [PubMed] [Google Scholar]
- 16.van Ramshorst MS, van der Voort A, van Werkhoven ED, et al. Neoadjuvant chemotherapy with or without anthracyclines in the presence of dual HER2 blockade for HER2-positive breast cancer (TRAIN-2): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2018;19:1630–40. doi: 10.1016/S1470-2045(18)30570-9. [DOI] [PubMed] [Google Scholar]
- 17.Tanaka S, Matsunami N, Morishima H, et al. De-escalated neoadjuvant therapy with nanoparticle albumin-bound paclitaxel and trastuzumab for low-risk pure HER2 breast cancer. Cancer Chemother Pharmacol. 2019;83:1099–104. doi: 10.1007/s00280-019-03836-z. [DOI] [PubMed] [Google Scholar]
- 18.Gao HF, Wu Z, Lin Y, et al. Anthracycline-containing versus carboplatin-containing neoadjuvant chemotherapy in combination with trastuzumab for HER2-positive breast cancer: the neoCARH phase ll randomized clinical trial. Ther Adv Med Oncol. 2021;13:17588359211009003. doi: 10.1177/17588359211009003. eCollection 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamashiro H, Iwata H, Masuda N, et al. Outcomes of trastuzumab therapy in HER2-positive early breast cancer patients: extended follow-up of JBCRG-cohort study 01. Breast Cancer. 2020;27:631–41. doi: 10.1007/s12282-020-01057-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lambertini M, Campbell C, Gelber RD, et al. Dissecting the effect of hormone receptor status in patients with HER2-positive early breast cancer: exploratory analysis from the ALTTO (BIG 2-06) randomized clinical trial. Breast Cancer Res Treat. 2019;177:103–14. doi: 10.1007/s10549-019-05284-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the present study are not publicly available due to privacy but are available from the corresponding author on reasonable request.