Abstract
The Breast Committee of the Arbeitsgemeinschaft Gynäkologische Onkologie (German Gynecological Oncology Group, AGO) presents the 2022 update of the evidence-based recommendations for the diagnosis and treatment of patients with locally advanced and metastatic breast cancer.
Keywords: Metastatic breast cancer, Arbeitsgemeinschaft Gynäkologische Onkologie, Recommendations, Diagnosis, Treatment
Introduction
For the last 20 years, the Breast Committee of the Arbeitsgemeinschaft Gynäkologische Onkologie (German Gynecological Oncology Group, AGO) has been preparing and updating evidence-based recommendations for the diagnosis and treatment of patients with early and metastatic breast cancer (MBC). The AGO Breast Committee consists of gynecological oncologists specialized in breast cancer (BC) and interdisciplinary members specialized in pathology, radiologic diagnostics, medical oncology, and radiation oncology. This update has been performed according to a documented rule-fixed algorithm by thoroughly reviewing and scoring chapter by chapter the recent publications for their scientific validity (Oxford level of evidence [LoE], www.cebm.net) and clinical relevance (AGO grades of recommendation; Table 1). Here we present the 2022 update of diagnosis and treatment of patients with locally advanced and MBC; the full version of the updated slide set is available online as a PDF file in both English and German [1]. Moreover, a special version for patients is also available at www.ago-online.de.
Table 1.
AGO grades of recommendation
| ++ | This investigation or therapeutic intervention is highly beneficial for patients, can be recommended without restrictions, and should be performed |
|
| |
| + | This investigation or therapeutic intervention is limited for patients and can be performed |
|
| |
| +/− | This investigation or therapeutic intervention has not shown benefit for patients and may be performed only in individual cases. According to current knowledge, a general recommendation cannot be given |
|
| |
| − | This investigation or therapeutic intervention can be of disadvantage for patients and might not be performed |
|
| |
| − | This investigation or therapeutic intervention is of clear disadvantage for patients and should be avoided or omitted in any case |
Prognostic and Predictive Factors
Molecular pathology for the classification of BC subtypes and prediction of targeted therapies is a key element in personalized oncology. In MBC, there are four gene mutations with therapeutical implications in routine practice. Poly(ADP-ribose)-polymerase inhibitor (PARPi) monotherapy is effective in patients with a BRCA1/2 germline mutation (gBRCA1/2 mt) (LoE 1a/A/AGO++). Recently, it was demonstrated for somatic mutations as well. Although EMA approval is based on trial results from germline mutation carriers only, in selected cases, determination of BRCA status from tumor tissue is possible to evaluate potential sensitivity of tumor cells toward poly(ADP-ribose)-polymerase inhibition.
Phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA) mutations indicate response to corresponding inhibitors such as alpelisib (LoE 1a/A/AGO++) [2]. PIK3CA is mutated in about 40% of BC, predominantly of the luminal and HER2-enriched type. Activating mutations of the estrogen receptor gene ESR1 (LoE 2b/B/AGO+/−) occur in 15–40% of hormone-treated BC patients, resulting in autocrine growth stimulation and endocrine resistance against aromatase inhibitors and tamoxifen but not fulvestrant [3].
Besides gene amplification and overexpression of the human epidermal growth factor receptor 2 (HER2), HER2 can gain transforming potential by activating gene mutation within the kinase domain. This alteration is particularly frequent in lobular cancer and results in effective growth blockade by tyrosine kinase inhibitors like tucatinib, lapatinib, or neratinib (LoE 4/C/AGO+/−) [4].
Expression of programmed cell death ligand 1 (PD-L1) by tumor-infiltrating leucocytes either in primary BC or metastatic disease is predictive for a response to checkpoint inhibitors such as atezolizumab or pembrolizumab (LoE1b/B/AGO++). Regarding the rare subpopulation of secretory BC, NTRK gene fusions detected in tumor tissue are the targets for TRK inhibitors such as larotrectinib and entrectinib (LoE 2a/B/AGO+) [5].
Circulating tumor cells represent interesting new candidates for early response evaluation (LoE 1b/A/AGO−) in the future but should not be used outside of a clinical trial [6]. Targetable mutation, like ESR1 can be determined from circulating DNA in peripheral blood, but it must be kept in mind that about 30% of metastasizing tumors are “non-shedders” without detectable DNA delivery. Consequently, DNA analysis from primary tumors (BRCA, PIK3CA) or metastases (ESR1, HER2) must be preferred.
At present, therapy-relevant mutational analysis for actionable genomic alterations in MBC represents an area of great interest. These new approaches include companion diagnostics for therapy options arising from other tumor entities (e.g., BRAF, FGR1) and large panel gene analysis to identify new treatment options in late line therapy (LoE 3a/C/AGO+/−). Use of next generation sequencing tools should be limited to situations with Tier 1 and 2 treatment options suggesting variants of strong and or potential clinical significance recommended by AMP, ACMG, and ASCO/CAP [7].
Endocrine and Targeted Therapy in MBC
Women with hormone receptor (HR)-positive, HER2-negative MBC represent the majority of BC patients. Endocrine-based therapy should be considered first choice, irrespective of menopausal status. Premenopausal women can be treated equally if they are concomitantly treated with ovarian function suppression (mainly gonadotropin-releasing hormone analogues).
Endocrine therapy should be combined with a cyclin-dependent kinase 4/6 (CDK4/6) inhibitor. The evidence concerning abemaciclib, palbociclib, and ribociclib has been completed regarding a variety of patient populations according to therapy line, menopausal status, and endocrine combination partners. Those combination therapies are rated with LoE 1a/A/AGO++ for postmenopausal patients and for premenopausal patients with LoE 1b/B/AGO++ (Ribociclib), LoE 3b/C/AGO+ (Palbociclib), and LoE 5/C/AGO+ (Abemaciclib) each in combination with aromatase inhibition and LoE 2b/B/AGO++ for all CDK 4/6 inhibitors in combination with fulvestrant. All three drugs have been thoroughly investigated in first and further therapy lines in endocrine-sensitive and -resistant MBC and demonstrated a homogeneous improvement of PFS. Thus, no subgroup could be identified neither by clinical nor by biomarkers that does not benefit from using a CDK4/6 inhibitor in addition to ET. An overall survival benefit has been reported in two first-line studies, namely the MONALEESA-7 for premenopausal patients [8] and the MONALEESA-2 trial for postmenopausal patients [9] with a median survival benefit of up to 1 year. Overall survival data from two other first-line studies are still pending (i.e., the MONARCH-3 and PALOMA-2 trials). In second-line therapy, an overall survival benefit in association with CDK4/6 inhibitor therapy was observed in the MONARCH-2 and the MONALEESA-3 study.
Patients with HR-positive BC carrying a gBRCAmt might be candidates for PARPis. Both confirmatory studies OlympiAD with olaparib (LoE 1b/A/AGO++) and EMBRACA with talazoparib (LoE 1b/A/AGO++) included about 50% HR-positive BC and showed better progression-free survival compared to standard of care mono-chemotherapies. Improved overall survival was observed with olaparib only in first-line patients. In HR+/HER2 positive patients, endocrine treatment might be an option in some cases. There are reassuring data about the use of CDK 4/6 inhibitors also in this patient group in the monarcHER trial [10]. CDK 4/6 inhibitors should only be given in HER2 positive patients in clinical trials (e.g., the DETECT V trial).
Chemotherapy with or without Targeted Drugs in MBC
In MBC, a good quality of life, as well as controlling any signs and symptoms resulting in an improved general health status, is important (A/AGO++). Mono chemotherapy is the treatment of choice in slow progressing disease or if secondary resistance to endocrine therapy arises (LoE 1b/A/AGO++). In contrast, combination chemotherapy is recommended in case of urgent remission or visceral crisis according to the ABC-5 definition. In MBC, treatment selection is based on ER and/or PR, HER2-status, PD-L1-status, as well as mutation of BRCA-genes either in the primary tumor or in the metastatic site (AGO++).
In TNBC patients with PD-L1 positive tumor disease (CPS-score ≥10) and a therapy-free interval of more than 6 months, the combination of pembrolizumab with chemotherapy is recommended (LoE 1b/B/AGO++). The addition of atezolizumab to nab-paclitaxel has resulted in a non-significant, though clinically relevant improvement in OS. Therapy should be limited to this specific combination therapy (LoE 1b/B/AGO+) [11, 12].
PARPi improved PFS in two trials (OlympiAD, EMBRACA) compared to any chemotherapy as “doctors' best choice” in HER2-negative MBC with gBRCA1/2 mt [13]. Thus, olaparib (LoE 1b/B/AGO++) or talazoparib (LoE 1b/B/AGO++) are treatment options in this setting. Furthermore, olaparib showed activity in mTNBC with either somatic BRCA (2b/B/AGO+/) or germline PALB2 (2b/B/AGO+/−) mutations [14].
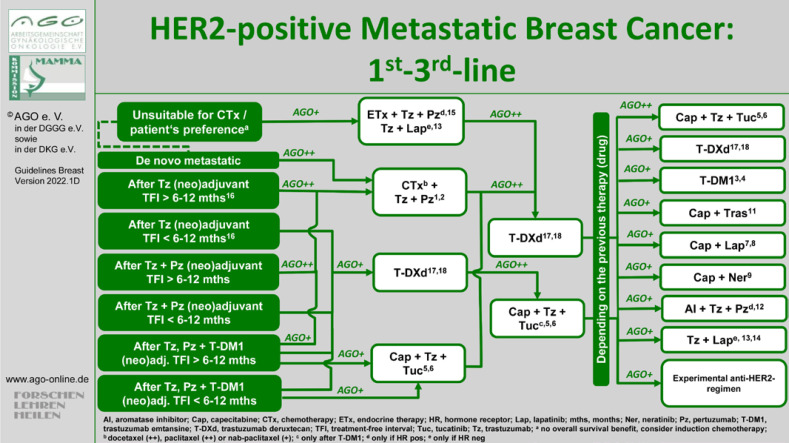
Regarding the treatment of patients with HER2-positive advanced BC, it is evident that the classic recommended sequence of taxane plus dual therapy in the first-line setting followed by T-DM1 followed by lapatinib and capecitabine can hardly be maintained for all patients, as numerous patients have already received these therapies in the (neo)-adjuvant or post-neoadjuvant situation and several new therapy options are available. Accordingly, diversified algorithms have become necessary.
In the first-line setting, dual therapy with 3-weekly docetaxel (LoE 1b/A/AGO++) or weekly paclitaxel (LoE 2b/B/AGO++) is recommended for patients with primary metastatic disease after adjuvant trastuzumab therapy prior to a treatment-free interval (TFI) of >6 months. Last year, the results of the DESTINY-Breast03 study comparing T-DM1 to trastuzumab deruxtecan (T-DXd) were presented. PFS and OS were improved significantly with HRs of 0.28 (95% CI: 0.22–0.37; p = 7.8 × 10–22) and 0.56 (95% CI: 0, 36–0.86; p = 0.007172), respectively [15]. Since T-DM1 was previously an approved option after early progression (TFI <6 months), it is now being replaced by T-DXd (LoE 5/D/AGO+). After dual HER2-targeted antibody-based therapy in the (neo)-adjuvant and a TFI of >6–12 months, reinduction of dual blockade (LoE 5/D/AGO++) and in case of a TFI of <6–12 months, T-DXd are recommended (LoE 5/D/AGO+). If patients have received both − the dual therapy and T-DM1 − in the (neo-)adjuvant setting, besides the reinduction of the dual therapy with a taxane (TFI >6–12 months) and T-DXd, tucatinib in combination with capecitabine and trastuzumab is also available according to the published data from the HER2CLIMB study (LoE 5/D/AGO+) [16].
In second-line, therapy with T-DXd (LoE 1b/B/AGO++) or tucatinib and trastuzumab with capecitabine after prior therapy with T-DM1 (LoE 1b/B/AGO++) is recommended. Several options are available for the third and later lines. The combination of tucatinib with capecitabine and trastuzumab has the highest grade of recommendation (LoE1b/B/AGO++). All treatment options are summarized in Figure 1.
Fig. 1.
Treatment algorithm for metastatic HER2+ BC.
Bone Metastasis
More than 65–70% of patients with advanced BC develop skeletal metastasis (109). Bisphosphonates and denosumab (Dmab) have been successfully used to reduce hypercalcemia (LoE 1a/A/AGO++), skeletal events/complications (LoE 1a/A/AGO++), bone pain (LoE 1a/A/AGO++) and prolong bone pain-free survival (bisphosphonates: LoE 1a/A/AGO++; Dmab: LoE 1b/A/AGO++) [17]. Based on a difference regarding the evidence for a de-escalation of denosumab, pamidronate, and zoledronic acid (i.e., every 12 weeks rather than every 3–4 weeks), de-escalation is only recommended in the case of zoledronate (LoE 1a/A/AGO++) but not in the case of the other two bone-targeted agents (LoE 2b/B/AGO+/−) [18]. Severe side effects must be considered and prevention of osteonecrosis of the jaw should be performed based on the ASORS (Supportive Maßnahmen in der Onkologie, Rehabilitation und Sozialmedizin) evaluation [19]. Planned sequential therapy with multiple bone-targeted agents should be approached with caution based on higher osteonecrosis of the jaw rates (LoE 2b/B/AGO+/−) [20]. In the case of spinal cord compression, treatment should begin immediately (LoE 1c/D/AGO++) and steroids should be started at the first symptoms (LoE 2a/C/AGO+) [21]. If radiotherapy is indicated, the choice of regimen (1 × 8–10 Gy vs. multiple fractions) depends on prognosis, performance status, and patient preference.
CNS Metastases
The cumulative incidence of brain metastases in MBC increased from 10% to 40% due to the improvements in diagnostic imaging and systemic therapy in the last two decades [22]. A recent meta-analysis demonstrated a high incidence among HER2-positive and triple-negative MBC. The incidence per patient-year was 13% in each of these subtypes.
Stereotactic radiation or resection followed by radiation of the tumor bed (without whole-brain radiation) is the preferred choice of treatment of single (up to 4 cm) or oligo-brain metastases (≤4 metastases) or 5–10 metastases with limited cumulative tumor volume (<15 mL) (LoE 1b/B/AGO++) [23]. Compared to whole brain radiation (WBR), cognitive impairment is significantly reduced without any difference in overall survival. WBR in combination with supportive corticosteroid therapy is recommended for multiple brain metastases (LoE 1a/A/AGO++). WBR alone or in combination with boost irradiation should be reserved for patients in a poor general condition or with an unfavorable prognosis (LoE 2b/B/AGO+). There is increasing evidence to suggest that hippocampus sparing irradiation preserves cognitive function without impairing the brain failure rate or survival if prognosis is favorable (LoE 1b/B/AGO+).
A new chapter in this field is the differential assessment of brain metastases. The definition of a “stable” brain metastasis implies that there has been an intervention before, e.g., whole brain radiation. Different clinical trials used various time intervals after the conclusion of this intervention and allowed for different comedication like corticosteroids or anticonvulsants. Brain metastases are considered “active” if they have recently progressed or were newly diagnosed after an intervention but do not require immediate local therapy or have never been pretreated but do not need immediate treatment as well [24]. The treatment plan must be put up in an interdisciplinary team (LoE 5/D/AGO++). In the case of stable extracranial metastases and parallel new lesions in the brain, the current systemic therapy should be continued (LoE 2c/C/AGO+). Exclusive systemic treatment (chemotherapy ± targeted therapy) without local radiation is an option in selected patients (LoE 3a/D/AGO+/−). In HER2-positive MBC, tucatinib and T-DXd are recommended with an equally high level of evidence (LoE 2b/B/AGO+). Based on the efficacy of new treatment options and the old and limited data on intrathecal therapy with methotrexate in leptomeningeal disease, this option was downgraded to LoE 2b/B/AGO+/−.
Specific Sites of Metastases
Systemic therapy remains the mainstay of primary stage IV breast cancer (LoE 2a/B/AGO++). There has been an ongoing debate about whether surgical removal of the primary tumor improves survival. To date, results of four randomized phase 3 trials have been reported [25, 26, 27, 28]. Only in one of these trials, early local therapy of the primary breast tumor improved overall survival in patients with de novo metastatic disease after 10 years of follow-up in a very selected group of patients (i.e., those with HR+/HER2− BC of less than 55 years of age and solitary bone-only metastasis) [28]. Despite better local control, surgery did not improve quality of life [25, 27, 29]. In a prospective registry study (BOMET MF 14-01), 505 patients with de novo, bone-only MBC demonstrated an improvement in 3-year OS in association with locoregional therapy of the primary tumor (in combination with systemic therapy) compared to systemic therapy alone [30]. Consequently, primary tumor removal in stage IV BC is not recommended with the expectation of survival improvement even in patients with bone-only disease (LoE 1b/B/AGO+/−) [25, 26, 27, 28, 29, 30]. Only patients with limited or oligometastatic disease and a good response to systemic treatment should be considered for surgical procedures and/or stereotactic treatment of the metastatic sites (LoE 2b/C/AGO+).
AGO further acknowledges that the presence of contralateral axillary nodal metastasis (CANM) requires particular therapeutic consideration. Even though CANM (in the absence of a contralateral primary) as an initial diagnosis of recurrent disease is considered stage 4 disease, subsequent metachronous CANM after prior local therapy to the ipsilateral axilla for early BC may be considered and treated as a regional metastasis (due to altered lymphatic drainage) with a potential for long survival or cure with a multidisciplinary approach [31, 32]. However, a second cancer of unknown primary should always be excluded.
BC − Supportive Care and Side Effect Management
In view of all new agents and indications for early and MBC, optimal side effect management and supportive care are essential for therapeutic success. Before the start of capecitabine therapy, dihydropyrimidin-dehydrogenase deficiency testing needs to be performed, preferably DPYD genotype testing (LoE 1a/A/AGO++). Even though DPYD variants (heterozygous or homozygous) are rare with about 4.1%, therapy-associated morbidity and mortality (2.3% vs. 0.1% w/o DPYD variants) is increased in patients with dihydropyrimidin-dehydrogenase deficiency under therapy with 5-fluoro-uracil and its derivates [33].
Two new antibody-drug conjugates have recently become available in Germany: main toxicities of T-DXd are interstitial lung disease (ILD), neutropenia, nausea, and alopecia [34] and those of sacituzumab govitecan are (febrile) neutropenia, leukopenia, anemia, diarrhea, nausea, alopecia [35]. Neratinib is associated with high rates of G3 diarrhea; weekly dose escalation starting with 120 mg/day, then 160 mg/day, and the full dose of 240 mg/day after 2 weeks together with loperamide prophylaxis reduces G3 diarrhea substantially [36].
Abemaciclib is associated with an invasive disease-free survival benefit in the curative setting based on the monachE results. Again, ILD is a rare side effect of CDK 4/6 inhibitor therapy; abemaciclib is associated with a 2.9% incidence (all grades) with only 0.4% > G3 events [37]. In monarchE, venous thrombotic events with abemacilib were low with 2.3% of all grades (1.2% G3/4). The incidence is about twice as high with tamoxifen than with an AI as the endocrine backbone.
ILD requires proactive management according to grade and causing agents. The diagnostic work-up should start with chest CT once symptoms arise (LoE 1a/B/AGO++). Corticosteroids (starting dose ≥0.5 mg/kg/day prednisolone-equivalent) need to be commenced early (LoE 1a/B/AGO++); recommendations for dose holds or therapy discontinuations are detailed in the respective product information.
Hepatitis B screening (HBsAG, anti-HBC, anti-HBs) should be performed before the start of adjuvant chemotherapy (LoE 2c/B/AGO+); chemotherapy does not need to be interrupted in case of positive serology or reactivation [38]. Proactive and successful side effect management requires a truly interprofessional approach by nursing staff and physicians as well as thorough patient education.
Palliative Care
It is well accepted that MBC in an early phase is incurable but treatable. However, the late “palliative” phase must be differentiated as the focus is set on end-of-life care. Early introduction of palliative care concurrent with active treatment is important to improve symptoms and quality of life. Furthermore, discussions about patient preferences at the end of life should begin early in the course of metastatic disease [39, 40, 41].
It is very important to point out that with the recent therapeutic progress with innovative and effective compounds, the patient goals are differing in each phase. Meanwhile, we are in the position to prolong progression-free survival without increasing toxicity. The very recent results of studies with CDK4/6 inhibitors, checkpoint inhibitors, antibody-drug-conjugates, and PARPis presented an overall survival benefit. With such compounds, targeted and more individual treatment strategies take center stage. Patient-reported outcome data are crucial to estimate treatment success and course.
Conflict of Interest Statement
Prof. Dr. Med. Marc Thill. Advisory boards: Agendia, Amgen, AstraZeneca, Aurikamed, Becton and Dickinson, ClearCut, Clovis, Daiichi Sankyo, Eisai, Exact Sciences, Gilead Science, Grünenthal, GSK, Lilly, MSD, Neodynamics, Novartis, Onkowissen, Organon, Pfizer, pfm Medical, Pierre-Fabre, Roche, Seagen, Sirius Pintuition, Sysmex. Manuscript support: Amgen, Clearcut, Clovis, pfm medical, Roche, Servier. Travel reimbursement: Amgen, art tempi, AstraZeneca, Clovis, Connect Medica, Daiichi Sankyo, Eisai, Exact Sciences, Lilly, MSD, Novartis, Pfizer, pfm Medical, Roche, Seagen. Congress support: Amgen, AstraZeneca, Daiichi Sanyko, Novartis, Pfizer, Roche. Lecture: Amgen, art tempi, AstraZeneca, Clovis, Connect Medica, Daiichi Sankyo, Exact Sciences, Gilead Science, Hexal, I-Med-Institute, Jörg Eickeler, Lilly, MSD, Novartis, Onkowissen, Pfizer, pfm medical, Roche, Seagen, Sysmex, Vifor, Viatris. Trial funding: Endomagnetics, Exact Sciences.
Prof. Dr. Med. Diana Lüftner. Advisory board: AstraZeneca, Eli Lilly, GSK, Novartis, Onkowissen, High5MD, Loreal, Pfizer, Roche, TEVA, Gilead Science. Lecture: AstraZeneca, Eli Lilly, GSK, Novartis, Onkowissen, High5MD, Loreal, Pfizer, Roche, TEVA, Gilead Science.
Prof. Dr. Med. Cornelia Kolberg-Liedtke. Advisory board: Aurikamed, Novartis, Pfizer, MSD, Novartis, Gilead Science, Seagen, Lilly, Agendia, Daiichi Sankyo. Lecture: Roche, Novartis, Pfizer, Exact Sciences, Amgen, AstraZeneca, Carl Zeiss Meditec, NCO Hannover. Other: Gilead Science, POMME. Stockholding: Phaon Scientific. Trial Funding: Gilead Science.
Prof. Dr. Med. Ute-Susann Albert. Lectures and/or consulting: Pfizer, Novartis, Aurikamed.
PD Dr. Malgorzata Banys-Paluchowski. Advisory board: Novartis, Roche, LIlly, Pfizer, GSK, MSD. Lecture: Novartis, Pfizer, pfm medical, Seagen, Daiichi Sankyo, Lilly, Roche, Amgen. Trial funding: Mammotome, Exact Sciences, Merit Medical, Endomag.
Dr. Med. Ingo Bauerfeind. No conflicts of interest.
Prof. Dr. Med. Jens-Uwe Blohmer. Honoraria: Astrazeneca, Eisai, Lilly, MSD, Novartis, Pfizer, Roche, Seagen.
Prof. Dr. Med. Wilfried Budach. Lecture: Merck, medpublico GmbH, BVDST.
Prof. Dr. Med. Peter Dall. Advisory Boards: Gilead Science, Roche. Lecture: Novartis, AstraZeneca, Pfizer.
PD Dr. Med. Eva Maria Fallenberg. No conflicts of interest.
Prof. Dr. Med. Peter A. Fasching. Advisory board: Pfizer, Novartis, Roche, Daiichi Sankyo, Eisai, AstraZeneca, Lilly, MSD, Seagen, Agendia, Pierre Fabre, Sanofi Aventis, Gilead Science. Lecture: Pfizer, Novartis, Roche, Daiichi Sankyo, Eisai, AstraZeneca, Lilly, MSD, Seagen, Gilead Science. Other: Onkowissen, art tempi.
Prof. Dr. Med. Tanja N. Fehm. Onkowissen.
Prof. Dr. Med. Michael Friedrich. Advisory Board: Gilead Science. Other honoraria: Roche, MSD. Stockholding: Biontech, Curevac.
Prof. Dr. Med. Bernd Gerber. Lecture honoraria: Roche, AstraZeneca, Seagen, Novartis, Pfizer, MedConcept. Others: Pfizer.
PD Dr. Med. Oleg Gluz. No conflicts of interest.
Prof. Dr. Med. Nadia Harbeck. Honoraria for lectures and/or consulting: Amgen, AstraZeneca, Daiichi Sanyko, Gilead Science, Lilly, MSD, Novartis, Pierre-Fabre, Pfizer, Roche, Sandoz, Seagen, Exact Sciences. Minority shareholder: Westdeutsche Studiengruppe (WSG).
Prof. Dr. Med. Jörg Heil. Advisory board: Polytech. Trial funding: pfm medical.
Prof. Dr. Med. Jens Huober. Lecture: Gilead Science, Seagen, Lilly, Novartis, Daiichi Sankyo, GSK, Pfizer.
Prof. Dr. Med. Christian Jackisch. Advisory board: Exact Sciences, Pfizer, Roche, GSK, Pierre-Fabre. Lecture: AstraZeneca, Lilly, Novartis.
Prof. Dr. Med. Hans-Heinrich Kreipe. Advisory board: Lilly. Lecture: AstraZeneca, Roche, Daiichi Sankyo, Pfizer.
Dr. David Krug. Lecture: MSD, ESO, ESMO, medupdate GmbH. Trial funding: Merck.
Prof. Dr.med. Thorsten Kühn. Advisory Board: Sysmex, Neodynamics. Trial funding: Merit Medical, Endomag, Mammotome. Lecture: Pfizer.
Prof. Dr. Med. Sherko Kümmel. Lecture: Roche, Lilly, Exact Sciences, Novartis, Amgen, Daiichi Sankyo, AstraZeneca, Somatex, MSD, Pfizer, pfm medical, Seagen, Gilead Science, Agendia. Other honoraria: Roche, Daiichi Sankyo, Sonoscape Advisory board: Lilly, MSD, Roche.
Prof. Dr. Med. Sibylle Loibl. Trial funding: Abbvie, AstraZeneca, Celgene, Daiichi Sankyo, Gilead Science, Novartis, Pfizer, Roche. Lecture: Abbvie, Amgen, AstraZeneca, Bayer, BMS, Celgene, Daiichi Sankyo, Eirgenix, GSK, Gilead Science, Lilly, Merck, Novartis, Pfizer, Pierre Fabre, Prime/Medscape, Puma, Roche, Samsung.
Prof. Dr. Med. Michael Patrick Lux. Lecture: Lilly, Roche, MSD, Onkowissen, Novartis, Pfizer, Exact Sciences, Daiichi Sankyo, Gilead, Grünenthal, Eisai, AstraZeneca. Advisory board: pfm medical, Saman Tree, Sysmex, Lilly, AstraZeneca, MSD, Novartis, Pfizer, Eisai, Gilead, Exact Sciences, Daiichi Sankyo, Grünenthal, Pierre Fabre, PharmaMar, Roche. Other: Medac, Pfizer.
Prof. Dr. Med. Nicolai Maass. Advisory board: Amgen, Grünenthal. Lecture: AstraZeneca, Pierre Fabre, Clovis, Seagen, Lilly, MSD, Novartis, Pfizer.
Prof. Dr. Med. Christoph Mundhenke. Not specified.
Prof. Dr. Med. Ulrike Nitz. Not specified.
Prof. Dr. Tjoung-Won Park-Simon. Advisory board: Roche, AstraZeneca, GSK, Pfizer, Lilly, MSD, Exact Sciences, Daiichi Sankyo, Seagen, Novartis, Gilead Science. Lecture: Roche, AstraZeneca, GSK, Pfizer, Lilly, MSD, NCO, Exact Sciences, Daiichi Sankyo, Seagen, Novartis, Gilead Science.
Prof. Dr. Med. Toralf Reimer. Trial funding: Novartis, German Cancer Aid and Else Kroener-Fresenius-Stiftung. Advisory board: MSD, Novartis, Myriad. Lecture: Pfizer, Novartis, Roche, AstraZeneca.
PD. Dr. Med. Kerstin Rhiem. Lecture: AstraZeneca, Amgen, MDS, Medupdate, Medconcept.
Prof. Dr. Med. Achim Rody. Advisory board: AstraZeneca, Novartis, Roche, Exact Sciences, Pierre Fabre, Lilly, Seagen, Amgen, MSD. Lecture: Pfizer, Celgene, Eisai. Trial funding: Eisai.
Prof. Dr. Med. Marcus Schmidt. Advisory board: AstraZeneca, Novartis, Eisai, Lilly, MSD, Pierre Fabre, Pfizer, Roche, Seagen.
Prof. Dr. Med. Andreas Schneeweiss. Lecture: Amgen, AstraZeneca, Aurikamed, Clinsol, ConnectMedica, Gilead Science, GSK, I-Med, Lilly, MSD, Nanostring, Novartis, Onkowissen, Promedicis, Pfizer, Pierre Fabre, Roche, Seagen, StreamedUp. Other: Thieme.
Prof. Dr. Med. Florian Schütz. Lecture: Amgen, AstraZeneca, Eisai, Pfizer, Novartis, Onkowissen. Advisory board: Amgen, Lilly, MSD, Eisai.
Prof. Dr. Med. Hans-Peter Sinn. Advisory board: Diaceutics.
Prof. Dr. Med. Christine Solbach. Lecture: DiaLog Service GmbH, Jörg Eickeler, Pfizer, MedConcept, Medicultus, GBG, Dt. Röntgengesellschaft, BVF Akademie, LÄK Hessen Akademie, Meet the Expert Academy. Advisory board: MSD, Roche.
Prof. Dr. Med. Erich-Franz Solomayer. Lecture: Universitätsklinikum Freiburg, MedConcept, Saarländische Krebsgesellschaft, Bsh medical Communications GmbH, Pfizer, AstraZeneca. Other: Roche, Amgen, Clovis, AstraZeneca, Novartis, GSK, MSD, Eisai, Pfizer, Medac, Pierre Fabre, PharmaMar, Daiichi Sankyo, Samsung, Primus, matramed, GE Healthcare. Trial funding: Medac, GBG, AMS, AstraZeneca, Pfizer, Novartis, AGO Research GmbH, WSG, Universitätsklinikum Tübingen, Roche.
Prof. Dr. Med. Elmar Stickeler. Advisory boards: Gilead, Iomedico, Lilly, MSD, Seagen. Lecture: Pfizer, Bsh Düsseldorf, Gilead, Iomedico, PharmaMar, Onkowissen, Roche.
Prof. Dr. Med. Christoph Thomssen. Advisory board: Amgen, AstraZeneca, Hexal, Lilly, MSD, Pfizer, Roche, Seagen. Lecture: Klnikum Wolfsburg, Medicultus, Medupdate, Novartis, Pfizer, Roche, AstraZeneca, Daiichi Sankyo, Jörg Eickeler, Evang. KH Wittenberg, Gilead Science. Other: Aurikamed, ForumSanitas/Merit Corporate, Onkowissen, Pfizer, Daiichi Sankyo.
Prof. Dr. Med. Michael Untch. Advisory board: Lilly, AstraZeneca, Pfizer, Roche, Pierre Fabre, Sanofi Aventis, Gilead Science. Lecture: Daiichi Sankyo, Lilly, Seagen, Novartis, AstraZeneca, Roche, Eisai, MSD, I-Med_Insitute, Onkowissen, art tempi, High5Med.
Prof. Dr. Isabell Witzel. Lecture: Daiichi Sankyo, Pfizer, MSD, Lilly, Seagen, AstraZeneca. Other: Onkowissen
Prof. Dr. Med. Achim Wöckel. Advisory board: Amgen, AstraZeneca, Aurikamed, Celgene, Eisai, Lilly, Novartis, Pfizer, Roche, Tesaro, Sirtex, MSD, Genomic Health, Pierre Fabre, Clovis, Organon.
Prof. Dr. Med. Volkmar Müller. Lecture: Amgen, AstraZeneca, Daiichi Sankyo, eisai, GSK, Pfizer, MSD, Medac, Novartis, Roche, Teva, Seagen, Onkowissen, High5Oncology, Medscape, Gilead. Advisory board: Hexal, Roche, Pierre Fabre, Amgen, Clinsol, Novartis, MSD, Daiichi Sankyo, Eisai, Lilly, Sanofi Aventis, Seagen, Gilead Science. Trial funding: Novartis, Roche, Seagen, Genetech. Other: Daiichi Sankyo.
Prof. Dr. Med. Wolfgang Janni. Lecture: Amgen, AstraZeneca, Daiichi Sankyo, Lilly, MSD, Novartis, Pfizer, Roche, Seagen, Gilead Science. Trial Funding: Amgen, AstraZeneca, Lilly, Novartis, Roche.
Prof. Dr. Med. Nina Ditsch. Not specified.
Funding Sources
No funding.
Author Contributions
Paper is written by all the authors.
References
- 1.Empfehlungen Gynäkologische Onkologie Kommission Mamma 2022. Available from: www.ago-online.
- 2.André F, Ciruelos E, Rubovszky G, Campone M, Loibl S, Rugo HS, et al. Alpelisib for PIK3CA-mutated, hormone receptor-positive advanced breast cancer. N Engl J Med. 2019 May;380:1929–40. doi: 10.1056/NEJMoa1813904. [DOI] [PubMed] [Google Scholar]
- 3.Fribbens C, O'Leary B, Kilburn L, Hrebien S, Garcia-Murillas I, Beaney M, et al. Plasma ESR1 mutations and the treatment of estrogen receptor-positive advanced breast cancer. J Clin Oncol. 2016 Sep;34:2961–8. doi: 10.1200/JCO.2016.67.3061. [DOI] [PubMed] [Google Scholar]
- 4.Hymann DM, Piha-Paul SA, Won H, Rodon J, Saura C, Shapiro GI, et al. HER kinase inhibition in patients with HER2- and HER3-mutant cancers. Nature. 2018 Feb;554:189–94. doi: 10.1038/nature25475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cocco E, Scaltriti M, Drilon A. NTRK fusion-positive cancers and TRK inhibitor therapy. Nat Rev Clin Oncol. 2018 Dec;15:731–47. doi: 10.1038/s41571-018-0113-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bidard FC, Peeters DJ, Fehm T, Nolè F, Gisbert-Criado R, Mavroudis D, et al. Clinical validity of circulating tumor cells in patients with metastatic breast cancer. A pooled analysis of individual patient data. Lancet Oncol. 2014 Apr;15((4)):406–14. doi: 10.1016/S1470-2045(14)70069-5. [DOI] [PubMed] [Google Scholar]
- 7.Mosele F, Remon J, Mateo J, Westphalen CB, Barlesi F, Lolkema MP, et al. Recommendations for the use of next-generation sequencing (NGS) for patients with metastatic cancers: a report from the ESMO precision medicine working group. Ann Oncol. 2020 Nov;31((3)):1491–505. doi: 10.1016/j.annonc.2020.07.014. [DOI] [PubMed] [Google Scholar]
- 8.Im SA, Lu YS, Bardia A, Harbeck N, Colleoni M, Franke F, et al. Overall survival with Ribociclib plus endocrine therapy in breast cancer. N Engl J Med. 2019 Jul;381((4)):307–16. doi: 10.1056/NEJMoa1903765. [DOI] [PubMed] [Google Scholar]
- 9.Hortobagyi GN, Stemmer SM, Burris HA, Yap YS, Sonke GS, Hart L, et al. Overall survival with Ribociclib plus letrozole in advanced breast cancer. N Engl J Med. 2022 Mar;386((10)):942–50. doi: 10.1056/NEJMoa2114663. [DOI] [PubMed] [Google Scholar]
- 10.Tolaney SM, Wardley AM, Zambelli S, Hilton JF, Troso-Sandoval TA, Ricci F, et al. Abemaciclib plus trastuzumab with or without fulvestrant versus trastuzumab plus standard-of-care chemotherapy in women with hormone receptor-positive, HER2-positive advanced breast cancer (monarcHER): a randomised, open-label, phase 2 trial. Lancet Oncol. 2020 Jun;21((6)):763–75. doi: 10.1016/S1470-2045(20)30112-1. [DOI] [PubMed] [Google Scholar]
- 11.Schmid P, Rugo HS, Adams S, Schneeweiss A, Barrios CH, Iwata H, et al. Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2020 Dec;21:44–59. doi: 10.1016/S1470-2045(19)30689-8. [DOI] [PubMed] [Google Scholar]
- 12.Miles D, Gligorov J, Andre F, Cameron D, Schneeweiss A, Barrios CH, et al. Primary results from impassion131, a double-blind placebo-controlled randmised phase III trial of first-line paclitaxel +/− atezolizumab for unresectable locally advanced/metastatic triple-negative breast cancer. Ann Oncol. 2020 Aug;31((Suppl 4)):S1142–215. doi: 10.1016/j.annonc.2021.05.801. [DOI] [PubMed] [Google Scholar]
- 13.Taylor AM, Chan DLH, Tio M, Patil SM, Traina TA, Robson ME, et al. PARP (Poly ADP-Ribose Polymerase) inhibitors for locally advanced or metastatic breast cancer. Cochrane Database Syst Rev. 2021 Apr;4((4)):CD011395. doi: 10.1002/14651858.CD011395.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tung NM, Robson ME, Ventz S, Santa-Maria CA, Nanda R, Marcom PK, et al. TBCRC 048: phase II study of olaparib for metastatic breast cancer and mutations in homologous recombination-related genes. J Clin Oncol. 2020 Dec;38:4274–82. doi: 10.1200/JCO.20.02151. [DOI] [PubMed] [Google Scholar]
- 15.Cortés J, Kim SB, Chung WP, Im SA, Park YH, Hegg R, et al. Trastuzumab deruxtecan versus trastuzumab emtansine for breast cancer. N Engl J Med. 2022 Mar;386((12)):1143–54. doi: 10.1056/NEJMoa2115022. [DOI] [PubMed] [Google Scholar]
- 16.Murthy RK, Loi S, Okines A, Paplomata E, Hamilton E, Hurvitz SA, et al. Tucatinib, trastuzumab, and capecitabine for HER2-positive metastatic breast cancer. N Engl J Med. 2020 Feb;382((7)):597–609. doi: 10.1056/NEJMoa1914609. [DOI] [PubMed] [Google Scholar]
- 17.Tesfamariam Y, Jakob T, Wöckel A, Adams A, Weigl A, Monsef I, et al. Adjuvant bisphosphonates or RANK-ligand inhibitors for patients with breast cancer and bone metastases: a systematic review and network meta-analysis. Crit Rev Oncol Hematol. 2019 May;137:1–8. doi: 10.1016/j.critrevonc.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 18.Clemons M, Ong M, Stober C, Ernst S, Booth C, Canil C, et al. A randomised trial of 4- versus 12-weekly administration of bone-targeted agents in patients with bone metastases from breast or castration-resistant prostate cancer. Eur J Cancer. 2021 Jan;142:132–40. doi: 10.1016/j.ejca.2020.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. https://www.onkosupport.de/asors/content/e4126/e1743/e1861/e1862/e4628/LaufzettelAGSMOFarbefinal.pdf .
- 20.Srivastava A, Nogueras Gonzales GM, Geng Y, Won AM, Cabanillas ME, Naing A, et al. Prevalence of medication related osteonecrosis of the jaw in patients treated with sequential antiresorptive drugs: systematic review and meta-analysis. Support Care Cancer. 2021 May;29((5)):2305–17. doi: 10.1007/s00520-020-05882-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumar A, Weber MH, Gokaslan Z, Wolinsky JP, Schmidt M, Rhines L, et al. Metastatic spinal cord compression and steroid treatment a systematic review. Clin Spine Surg. 2017;30((4)):156–63. doi: 10.1097/BSD.0000000000000528. [DOI] [PubMed] [Google Scholar]
- 22.Kuksis M, Gao Y, Tran W, Hoey C, Kiss A, Komorowski AS, et al. The incidence of brain metastases among patients with metastatic breast cancer: a systematic review and meta-analysis. Neuro Oncol. 2021 May;23((6)):894–904. doi: 10.1093/neuonc/noaa285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Le Rhun E, Guckenberger M, Smits M, Dummer R, Bachelot T, Sahm F, et al. EANO-ESMO clinical practice guidelines for diagnosis, treatment and follow-up of patients with brain metastasis from solid tumours. Ann Oncol. 2021 Mar;32((11)):1332–47. doi: 10.1016/j.annonc.2021.07.016. [DOI] [PubMed] [Google Scholar]
- 24.Lin N, Borges V, Anders C, Murthy R, Paplomata E, Hamilton E, et al. Intracranial efficacy and survival with tucatinib plus trastuzumab and capecitabine for previously treated HER2-positive breast cancer with brain metastases in the HER2CLIMB trial. J Clin Oncol. 2020 Aug;38((23)):610–2619. doi: 10.1200/JCO.20.00775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fitzal F, Bjelic-Radisic V, Knauer M, Steger G, Hubalek M, Balic M, et al. Impact of breast surgery in primary metastasized breast cancer: outcomes of the prospective randomized phase III ABCSG-28 POSYTIVE trial. Ann Surg. 2019 Jun;269((6)):1163–9. doi: 10.1097/SLA.0000000000002771. [DOI] [PubMed] [Google Scholar]
- 26.Khan SA, Zhao F, Solin LJ, Goldstein LJ, Cella D, Basik M, et al. A randomized phase III trial of systemic therapy plus early local therapy versus systemic therapy alone in women with de novo stage IV breast cancer: a trial of the ECOG-ACRIN research group (E2108) J Clin Oncol. 2020;38((18 Suppl) [Google Scholar]
- 27.Badwe R, Hawaldar R, Nair N, Kaushik R, Parmar V, Siddique S, et al. Locoregional treatment versus no treatment of the primary tumour in metastatic breast cancer: an open-label randomised controlled trial. Lancet. 2015 Oct;16((13)):1380–8. doi: 10.1016/S1470-2045(15)00135-7. [DOI] [PubMed] [Google Scholar]
- 28.Soran A, Ozmen V, Ozbas S, Karanlik H, Muslumanoglu M, Igci A, et al. Primary surgery with systemic therapy in patients with de Novo stage IV breast cancer: 10-year follow-up; protocol MF07-01 randomized clinical trial. J Am Coll Surg. 2021 Dec;233((6)):742–51. doi: 10.1016/j.jamcollsurg.2021.08.686. [DOI] [PubMed] [Google Scholar]
- 29.Bjelic-Radisic V, Fitzal F, Knauer M, Steger G, Egle D, Greil R, et al. Primary surgery versus no surgery in synchronous metastatic breast cancer: patient-reported quality-of-life outcomes of the prospective randomized multicenter ABCSG-28 Posytive trial. BMC Cancer. 2020 May;20((1)):392. doi: 10.1186/s12885-020-06894-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soran A, Dogan L, Isik A, Ozbas S, Trabulus DC, Demirci U, et al. The effect of primary surgery in patients with De Novo stage IV breast cancer with bone metastasis only (Protocol BOMET MF 14-01): a multi-center, prospective registry study. Ann Surg Oncol. 2021 Sep;28((9)):5048–57. doi: 10.1245/s10434-021-09621-8. [DOI] [PubMed] [Google Scholar]
- 31.Magnoni F, Colleoni M, Mattar D, Corso G, Bagnardi V, Frassoni S, et al. Contralateral axillary lymph node metastases from breast carcinoma: is it time to review TNM cancer staging? Ann Surg Oncol. 2020 Oct;27((11)):4488–99. doi: 10.1245/s10434-020-08605-4. [DOI] [PubMed] [Google Scholar]
- 32.Nash AL, Thomas SM, Plichta JK, Fayanju OM, Hwang ES, Greenup RA, et al. Contralateral axillary nodal metastases: stage IV disease or a manifestation of progressive locally advanced breast cancer? Ann Surg Oncol. 2021 Oct;28((10)):5544–52. doi: 10.1245/s10434-021-10461-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sharma BB, Rai K, Blunt H, Zhao W, Tosteson TD, Brooks GA. Pathogenic DPYD variants and treatment-related mortality in patients receiving fluoropyrimidine chemotherapy: a systematic review and meta-analysis. Oncologist. 2021 Dec;26((12)):1008–16. doi: 10.1002/onco.13967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Modi S, Saura C, Yamashita T, Park YH, Kim SB, Tamura K, et al. Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N Engl J Med. 2020 Feb;382((7)):610–21. doi: 10.1056/NEJMoa1914510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bardia A, Hurvitz SA, Tolaney SM, Loirat D, Punie K, Oliveira M, et al. Sacituzumab govitecan in metastatic triple-negative breast cancer. N Engl J Med. 2021;384((16)):1529–41. doi: 10.1056/NEJMoa2028485. [DOI] [PubMed] [Google Scholar]
- 36.Barcenas CH, Hurvitz SA, Di Palma JA, Bose R, Chien AJ, Iannotti N, et al. Improved tolerability of neratinib in patients with HER2-positive early-stage breast cancer: the CONTROL trial. Ann Oncol. 2020 Sep;31:1223–30. doi: 10.1016/j.annonc.2020.05.012. [DOI] [PubMed] [Google Scholar]
- 37.Raschi E, Fusaroli M, Ardizzoni A, Poluzzi E, De Ponti F. Cyclin-dependent kinase 4/6 inhibitors and interstitial lung disease in the FDA adverse event reporting system: a pharmacovigilance assessment. Breast Cancer Res Treat. 2021 Feb;186((1)):219–27. doi: 10.1007/s10549-020-06001-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hwang JP, Feld JJ, Hammond SP, Wang SH, Alston-Johnson DE, Cryer DR, et al. Hepatitis B virus screening and management for patients with cancer prior to therapy: ASCO provisional clinical opinion update. J Clin Oncol. 2020 Nov;38:3698–715. doi: 10.1200/JCO.20.01757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cardoso F, Paluch-Shimon S, Senkus E, Curigliano G, Aapro MS, André F, et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5) Ann Oncol. 2020 Dec;31((12)):1623–49. doi: 10.1016/j.annonc.2020.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin NU, Thomssen C, Cardoso F, Cameron D, Cufer T, Fallowfield L, et al. International guidelines for management of metastatic breast cancer (MBC) from the European School of Oncology (ESO)-MBC task force: surveillance, staging, and evaluation of patients with early-stage and metastatic breast cancer. Breast. 2013 Jun;22((3)):203–10. doi: 10.1016/j.breast.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 41.Ferrell BR, Temel JS, Temin S, Smith TJ. Integration of palliative care into standard oncology care: ASCO clinical practice guideline update summary. J Oncol Pract. 2017 Jan;13((2)):119–21. doi: 10.1200/JOP.2016.017897. [DOI] [PubMed] [Google Scholar]