Abstract
Background and Aims:
The role of epidural analgesia in laparoscopic surgeries remains controversial. We evaluated intraoperative analgesic effects of epidural ropivacaine versus intravenous fentanyl in laparoscopic abdominal surgery and assessed postoperative analgesic requirements, hemodynamic changes, time to ambulation, and length of stay (LOS) in the ICU.
Material and Methods:
Seventy-two American Society of Anesthesiologists physical status I–III adult patients undergoing elective laparoscopic abdominal surgeries were randomized to either 0.5 mg/kg/h intravenous fentanyl (Group C) or 0.2% epidural ropivacaine at 5–8 mL/h (Group E) infusions intraoperatively and 0.25 m/kg/h fentanyl and 0.1% epidural ropivacaine infusions respectively postoperatively. Variations in mean arterial pressure (MAP) of 20% from baseline were points of intervention for propofol and analgesia with fentanyl or vasopressors. The number of interventions and total doses of fentanyl and vasopressors were noted. Postoperative analgesia was assessed at 0, 6, 12, and 24 h and when pain was reported with numerical rating scale and objective pain scores. Chi-square test and Student’s t-test were used for categorical and continuous variable analysis.
Results:
Intraoperatively, 14 patients versus 4 needed additional fentanyl and 26 versus 14 needed additional propofol in groups C and E respectively (P = 0.007, P = 0.004). MAP at 0, 6 and 18 h was lower in Group E. Pain scores were better in Group E at 6,18, and 24 h postoperatively. Time to ambulation was comparable but LOS ICU was prolonged in Group E (P = 0.05)
Conclusion:
Epidural ropivacaine produces superior intraoperative analgesia and improved postoperative pain scores without affecting ambulation but increases vasopressor need and LOS ICU in comparison with intravenous fentanyl in laparoscopic abdominal surgeries.
Keywords: Epidural analgesia, laparoscopy, ropivacaine
Introduction
Minimally invasive surgery is slowly replacing open abdominal surgery as it is associated with reduced postoperative pain and allows a faster recovery in comparison to open surgery.[1,2] Epidural analgesia has been used in laparoscopic surgery as part of the enhanced recovery after surgery (ERAS)[3,4,5] for attenuation of stress responses and enhanced recovery. While its undisputed benefits in recovery profile in open abdominal surgery is available,[5] its role in laparoscopic surgery is conflicting. The role of epidural analgesia in obese and patients at risk for pulmonary complications undergoing laparoscopic abdominal surgeries has been established in ERAS guidelines[6] but its analgesic benefit and improved bowel function have proponents for and against.[7,8]
The quality of analgesia is reportedly superior with epidural analgesia even in laparoscopic surgeries and it is associated with opioid sparing and improved ambulation benefits.[5]
At a gastro-surgical unit that had moved to laparoscopy from open surgeries which incorporated epidurals as a standard of care, we proposed to evaluate the quality of analgesia of epidural 0.2% ropivacaine versus intravenous fentanyl on the intra- and postoperative analgesic and recovery profile.
Our primary aim was the evaluation of intraoperative analgesic effects of epidural 0.2% ropivacaine versus intravenous fentanyl at 0.5 mg/kg/h in laparoscopic abdominal surgeries. The secondary objectives were postoperative analgesic requirements, hemodynamic changes, time to ambulation, and length of ICU stay (LOS ICU).
Material and Methods:
The study was commenced after the institutional ethics committee approval (IEC-AIMS-2018-ANES-055A) and prior written informed consent was taken. All procedures done in the study followed the ethical guidelines of the Declaration of Helsinki. This was a randomized control trial conducted between December 2018 and July 2019 {CTRI- 2018/12/016559}.
We included ASA I, II, and III patients undergoing elective laparoscopic abdominal surgery at our institution. Exclusion criteria were BMI >30, preoperative analgesic or opioid dependence, spinal abnormalities, and patients who needed postoperative ventilation.
Patients were randomized into one of the two groups, by a computer-generated random number sequence placed in opaque envelopes and patients grouped as per allotment. Group E was the study group with epidural and Group C was the control group with fentanyl infusion. As the drugs and routes differed, the investigator and observer were unblinded to the treatment of either strategy. Participants were enrolled by the principal and co-investigator and patients followed in the ICU by the intensive care team in accordance with the study protocols. All patients were premedicated with tablet ranitidine 150 mg and metoclopramide 10 mg on the night prior and morning of surgery except in patients where bowel obstruction was suspected. Tablet Alprazolam 0.25 mg was also added if there was no contraindication. Patients were shifted to the operating room where the preinduction monitors, non-invasive blood pressure (NIBP), electrocardiogram (ECG) and pulse oximeter were attached. Intravenous line and radial arterial line were inserted under local anesthesia as per protocol. The baseline heart rate and blood pressure were recorded.
In the intervention Group E, lower thoracic epidural between T8 and T12 was placed prior to the induction of anesthesia. An epidural test dose with 3 mL of 2% lignocaine with adrenaline was given to rule out accidental intravenous or intrathecal catheter placement and further 3 mL of saline to flush the drug. The level of sensory blockade to cold sensation was noted with ice at 5 min to confirm accurate placement of the epidural. The patients who did not manifest a specific dermatome level were inferred as having non-working epidural and excluded from the study at this point. Following confirmation, a 0.2% ropivacaine infusion at 5–8 mL/h was continued during surgery.
In the control Group C, intravenous fentanyl at 0.5 mg/kg/h as intraoperative analgesia was started following induction and continued until skin closure as per the prevailing institutional protocols for laparoscopic surgery.
All patients received general anesthesia as per a standardized protocol with intravenous midazolam 0.05 mg/kg, fentanyl 2 mg/kg, propofol titrated to loss of verbal response. Low flow anesthesia with 1.0 L air oxygen mixture (50% oxygen) and isoflurane at 0.7–1.0 minimum alveolar concentration was used and end-tidal carbon-dioxide maintained between 35 and 40 mmHg. Atracurium at 0.5 mg/kg was used for muscle relaxation. In patients with evidence of intestinal obstruction, rapid sequence induction was performed with succinylcholine 1.5 mg/kg or with rocuronium (0.9 mg/kg).
As no objective indices are available to assess the intraoperative pain and facilities for bispectral index and anti-nociceptive monitors were not available for all patients, an increase or decrease in mean arterial pressure (MAP) more than 20% of baseline was taken as the point for intervention.
For an increase in MAP >20% from baseline, the first step in management was propofol 0.5 mg/kg to a maximum of two doses at each administration. The second dose was administered after a lack of response for 5 min after the first. The second line of management was fentanyl 0.5 mg/kg for two doses over 10 min and the third labetalol incrementally in 5 mg to control blood pressure. The management for the rise in MAP was uniform among both groups.
For a fall in MAP <20% from baseline, the first line in management was incremental phenylephrine boluses not exceeding 250 mg and second-line noradrenaline infusion (0.02–0.2 mg/kg/min).
Intraoperative total number of occasions needing treatment, use of additional opioid, (mg) or need for vasopressors were noted. At the end of the surgery, the port site was infiltrated with 0.25% bupivacaine at 15 mL volume in both groups. An additional 10 mL was used for site infiltration if a stoma was present in both groups.
Postoperatively, Group E received epidural with 0.1% ropivacaine infusion at 5-8 mL/h and Group C received 0.25 mg/kg/h fentanyl infusion until they were shifted from the ICU. Quality of analgesia was assessed by numerical rating scale (NRS) and by the objective pain score (OPS)[9] [Figure 1].
Figure 1.
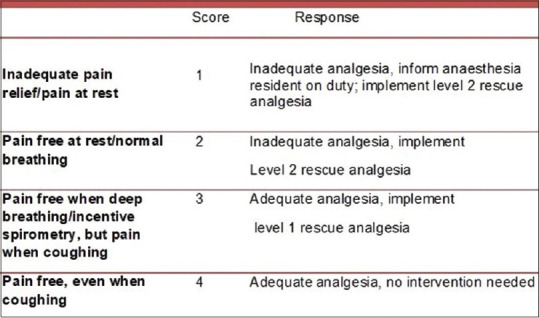
Objective pain score
For a NRS ≥3, both groups received intravenous paracetamol 1 gm as the first line of treatment as part of the protocol as they were under infusion of the study drug and were subsequently monitored for pain relief. If pain was not relieved prior to the next dose of paracetamol (8 h), epidural bolus of 10 mL 0.1% ropivacaine was given in Group E and fentanyl bolus of 0.5 mg/kg in Group C. For pain reported from site other than surgical site, 0.5 mg/kg of IV fentanyl bolus was administered. Heart rate, systolic blood pressure, and MAP were noted at the time of the shift to ICU, at 6, 12, 18, and 24 h for both groups postoperatively.
The need for treatment for pain, and pain during ambulation and LOS ICUwas noted.
As we did not find prior literature comparing fentanyl versus epidural ropivacaine in laparoscopic surgery, we conducted a pilot study on 20 patients undergoing laparoscopic abdominal surgeries. The intraoperative need for additional fentanyl beyond the background infusion of fentanyl or epidural was compared. In our study, 5 patients (50%) in the control group (Group C) versus only 2 patients (20%) in study group (Group E) required an additional dose of fentanyl. With a 95% confidence and 80% power, the sample size was calculated as 36 patients in each group.
To compare the mean of numerical variables between groups, independent sample t-test was applied. To study the statistical significance of association between two categorical variables, the Chi-square test was applied. A P value of < 0.05 was considered statistically significant. Statistical analysis was done using IBM SPSS 20.0 (Armonk, NY, USA).
Results
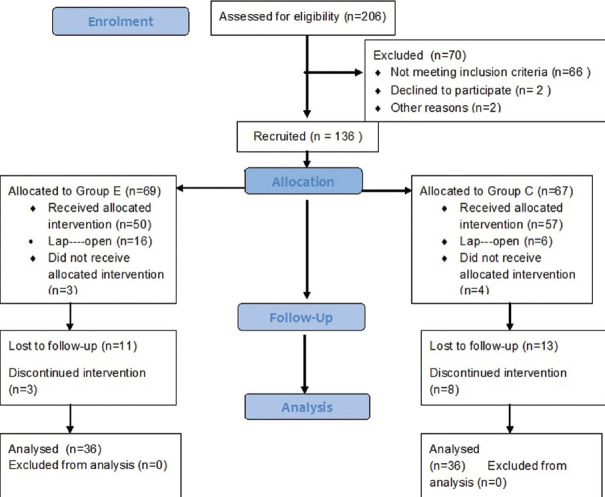
A total number of 72 patients were recruited into this study [Figure 2]. The demographic profiles between the groups were comparable. The surgeries in both groups were colorectal surgeries and distribution between the groups was comparable [Table 1].
Figure 2.
Consort diagram
Table 1.
Demographics
| Group C (n=36) Mean±SD | Group E (n=36) Mean±SD | P | |
|---|---|---|---|
| Age (years) | 57.50±13.54 | 57.44±14.23 | 0.987 |
| BMI kg/m-2 | 23.70±2.70 | 23.613±3.99 | 0.909 |
| Male/female n (%) | 19 (52.8)/17 (47.2) | 17 (47.2)/19 (52.8) | 0.637 |
| Duration of surgery (min) | 258.03±79.96 | 305.97±105.16 | 0.033 |
|
| |||
| Type of surgery | Numbers of patients | Numbers of patients | P |
|
| |||
| Sigmoid colectomy | 14 | 10 | P>0.05 |
| Anterior resection | 7 | 9 | |
| Abdominoperineal resection | 3 | 5 | |
| Hemicolectomy | 12 | 12 | |
The duration of surgery was longer in the epidural group [Table 1].
Fifteen (41.7%) patients in Group C versus only four (11.1%) patients in Group E received an additional dose of fentanyl, P = 0.007 [Table 2]. Twenty-six (72.2%) patients in Group C versus only fourteen (38.9%) patients in Group E received an additional dose of propofol, P = 0.004 [Table 2]. Two patients in Group C versus ten in Group E needed vasopressor infusions peri operatively, P = 0.024 [Table 2]. The induction doses of propofol between the groups were comparable and the doses of inhalational agents were similar between the groups [Table 2].
Table 2.
Intraoperative interventions and duration of surgery
| Intraoperative | Number of patients needing intervention | P | |
|---|---|---|---|
|
| |||
| Group C n (%) | Group E n (%) | ||
| Add Fentanyl | 15 (41.7) | 4 (11.1) | 0.007 |
| Add Propofol | 26 (72.2) | 14 (38.9) | 0.004 |
| Vasopressors | 2 (5.6) | 10 (27.8) | 0.024 |
|
| |||
| Doses of drug | Mean±SD | Mean±SD | |
|
| |||
| Isoflurane mL/h | 8.33±2.33 | 7.18±2.92 | 0.086 |
| Induction propofol mg per patient | 112.22±37.4 | 101.94±28.06 | 0.192 |
Postoperatively, NRS was significantly lower in the epidural group at 6 h and comparable at all other points. OPS was superior (higher) with epidural at 18 and 24 h [Table 3]. MAP was significantly lower in the epidural group at 0, 6, and 18 h [Table 4]. Nineteen patients in the fentanyl group needed boluses beyond background infusion (52%) and two among them had pain outside the surgical site. Nine patients needed epidural boluses but seven patients needed fentanyl for pain beyond the surgical site (44%) and differences between the groups were not significant.
Table 3.
Postoperative pain by NRS and OPS
| Group C n=36 (mean±SD) | Group E n=36 (mean±SD) | P | |
|---|---|---|---|
| NRS# | |||
| 0 h | 3.94±2.18 | 3.5±1.7 | 0.343 |
| 6 h | 3.81±1.8 | 2.44±1.36 | 0.001 |
| 12 h | 2.94±1.5 | 2.56±1.44 | 0.271 |
| 18 h | 2.94±1.55 | 2.31±1.17 | 0.052 |
| 24 h | 2.44±1.22 | 1.94±1.09 | 0.073 |
| OPS* | |||
| 0 h | 2.06±0.893 | 2.08±0.732 | 0.886 |
| 6 h | 2.39±0.803 | 2.72±0.701 | 0.065 |
| 12 h | 2.44±0.652 | 2.69±0.624 | 0.101 |
| 18 h | 2.58±0.732 | 3.06±0.630 | 0.005 |
| 24 h | 2.83±0.697 | 3.44±0.773 | 0.001 |
# Numerical rating score. *Objective pain score [Figure 1]
Table 4.
Postoperative hemodynamics, ambulation, LOS ICU
| Time in hours | Mean Arterial Pressure (mmHg) | P | |
|---|---|---|---|
|
| |||
| Group C (n=36) Mean±SD | Group E (n=36) Mean±SD | ||
| Baseline (prior to surgery) | 92.89±14.595 | 88.39±11.200 | 0.147 |
| 0 h | 102.28±17.611 | 90.75±15.051 | 0.004 |
| 6 h | 94.33±14.448 | 85.56±19.001 | 0.031 |
| 12 h | 89.06±14.903 | 84.39±13.468 | 0.168 |
| 18 h | 92.25±16.040 | 83.97±13.259 | 0.020 |
| 24 h | 93.67±15.224 | 90.17±13.296 | 0.302 |
| Time to ambulate (h) | 20.64±3.208 | 20.03±4.55 | 0.051 |
| LOS ICU (h) | 28.64±6.787 | 37.42±25.10 | 0.050 |
LOS: Length of stay
None of the patients in the control had ileus while three patients (8.3%) in the epidural group developed postoperative ileus and one patient developed postoperative urinary retention. There were no adverse events such as nausea and pruritus in the patients studied. The mean time to ambulate was comparable between both groups but the mean LOICU stay was significantly longer in Group E [Table 4, P = 0.05].
Discussion
Our aim was to study the impact of epidural analgesia with ropivacaine on intraoperative analgesia and postoperative recovery profile at our center. The impact of postoperative epidural analgesia in laparoscopic surgery has been addressed and its analgesic benefits demonstrated with limitations on major outcomes.[10,11] However, its effect on intraoperative analgesic effects is not clear.
Most of the literature available on epidural analgesia has incorporated bupivacaine[6,12] and limited reviews are available on the use of ropivacaine.[13] The limitations in literature on intraoperative analgesic requirements perhaps exist because of a lack of definitive measurement of intraoperative analgesia. Erol and colleagues[14] had made comparisons between intravenous fentanyl and epidural bupivacaine with improved results on early postoperative analgesia. We chose to compare the intraoperative analgesic dose of ropivacaine 0.2% to the analgesic dose of fentanyl at 0.5 mg/kg/h that is our standard opioid regime intraoperatively for major laparoscopic abdominal surgery.
Our analysis showed that the analgesic benefits were less in Group C that received fentanyl despite a context-sensitive half-life of more than 300 min documenting analgesic efficacy of the epidural in the early postoperative period. The epidural group had a longer duration of surgery and better analgesia despite a beneficial effect of fentanyl with an extended half-life.
We proposed that increases in blood pressure could be an indirect measurement of pain and analgesic requirements during surgery. We used an increase in MAP above 20% from the baseline value as an endpoint for treatment. As the heart rate could vary due to different causes during surgery including hypovolemia, vagal stretch responses, we believed that a rise in blood pressure could be a more accurate reflection of pain. The bispectral index monitors or the anti-nociceptive monitors were not consistently available for our patients and we looked at the utility of hemodynamic changes in predicting intraoperative pain.
Patients who were hypertensives were controlled at the time of surgery as these were elective procedures and were comparable between the groups. The point for intervention was an increase from the basal MAP measured when the patient was calm and resting. The administration of fentanyl was after checking the response to propofol in both groups wherein transient responses were eliminated. The need for fentanyl was a sustained blood pressure rise suggestive of pain and was higher in Group C (41.1% vs. 11.7%, P = 0.007) documenting opioid-sparing effects of epidural analgesia.
There was a higher need for vasopressors in the epidural group 27.8% vs. 5.6% as per our protocol with intervention after initial resuscitation with phenylephrine. As our intervention was targeted at a fall in MAP less than 20% from baseline greater numbers of patients received norepinephrine as a rescue strategy even when the MAP was greater than 65 mmHg and this eliminated problems due to reduction in MAP. Norepinephrine was used in doses between 0.02 and 0.08 mg/kg/min, which is unlikely to cause adverse effects. A meta-analysis comparing epidural versus opioids in largely open colorectal surgery had shown significant hypotension in the epidural group,[10] however epidurals exclusively in laparoscopic surgeries have not reported hypotension during surgery.[11,13,14] This could be an advantage of epidural in laparoscopic surgery wherein the hypotension of sympathetic blockade is partly offset by sympathetic responses to peritoneal stretch and handling.
The MAP was also lower in the epidural group postoperatively but none of the patients needed norepinephrine as the intervention was based on an actual MAP and not from its baseline value [Table 4].
We had also looked at the consumption of volatile anesthetic and induction doses of propofol between the groups to avoid the confounding effects of these agents on hemodynamics. The usage between both groups was similar [Table 2].
Postoperative analgesia was compared by the standard numerical rating score and an objective index, OPS.[6] A numerical rating score may not pick up the pain when the patient is at rest in bed and we evaluated by the OPS patient’s comfort or pain at specific efforts such as deep breathing or coughing during incentive spirometry. This was also used to evaluate pain when the patient was ambulated the day after surgery but we did not compare pain scores at the time of ambulation. In the first 6 h patients are usually asleep and the movements for care, spirometry, and ambulation start after 12 h. We believed that the pain scores were assessed at those times when standard rehabilitation measures were employed and would be reflective of the overall pain perception. It is evident that the epidural group was more pain-free 18 h after surgery as assessed by the OPS even when the NRS did not pick up the pain difference. Significantly, better scores were seen on patient movement and coughing at 18 and 24 h and when patient ambulation was instituted as seen with other workers.[11,12,13,14,15]
Paracetamol was used as part of the postoperative pain management strategy at our institute. Prolonged surgery and positioning can cause myalgia and it can be managed with paracetamol. Pain persisting beyond this dosing was considered for bolus treatment or fentanyl bolus for pain outside the surgical site.
The amount of ropivacaine used in the epidural group was between 10 and 16 mg/h which was well below the 3 mg/kg dose associated with toxicity. An additional 15 mL of 0.25% was used and 10 mL additionally for the stoma in both groups. A study on infusions of 0.2% ropivacaine for 120 h post knee surgery with boluses of higher concentrations has shown that even at an absolute duration of 1,786 h of infusion, the plasma-free ropivacaine that is linked to toxicity was only 0.16 mg/mL and that levels plateaued after an initial rise emphasizing safety in long-term epidural infusions in patients.[16] We did not encounter any clinically apparent toxicity among our patients but did not measure serum levels of ropivacaine for clarification.
A meta-analysis by Joshi and colleagues[11] critically evaluated the optimal analgesic options in laparoscopic colorectal surgery and recommended the use of cyclooxygenase inhibitors and steroids in conjunction with paracetamol as part of multimodal analgesia. Among our patients, 40% had diabetes mellitus which precluded routine use of perioperative steroids. Cyclooxygenase inhibitors are not used at our center because of concerns of major gastrointestinal bleed and renal dysfunction after surgery. Some studies have shown a higher incidence of anastomotic leakage after their use.[17,18] The incorporation of an epidural analgesic may help in the practice of opioid-free anesthesia when the safe use of non-steroidal anti-inflammatory agents is not possible.[19]
While it is true that the quality of analgesia with opioids does not differ from an epidural in laparoscopic colorectal surgery,[20] opioids can increase isedation, cause respiratory depression, pruritus, and other side effects including dependence that are not seen with epidural analgesia.
The proposed advantage of the decrease in ileus[21,22] with use of epidural was not observed in our patients. We had looked at the time to appearance of bowel sounds or passage of flatus or functioning of the stoma as the time to bowel function. The overall incidence of ileus was low among our patients, but three patients in the epidural group had ileus by our definition.
As per our protocol, both sets of patients had indwelling urinary catheters at the time of shift from the ICU. The removal of the catheters was in the surgical wards and any retention may have been overlooked in our study.
The intent to fast track was standard management but the shifting of patients may have been influenced by the need for ICU beds or surgical reasons in some patients. However, LOS ICU was longer in the epidural group in our study, 37 h against 28 h in the opioid group and this is similar to the reports of Halabi[23] and Borzellino.[24] We feel that protocols relating to indwelling epidural catheters and deep vein thrombosis prophylaxis besides the ileus may have contributed to an increase in their ICU stay.
Our study had its limitations. We relied on blood pressure changes to indicate intraoperative pain and did not use depth of anesthesia monitors to support our assumption. As we believed that heart rate changes could occur independent of pain, we relied on the sustained elevation of blood pressure as the trigger for intervention. We also felt that it would have been difficult to fix a target for intervention for both heart rate and blood pressure simultaneously. We did not specifically look for hemodynamic responses at port insertion, pneumoperitoneum or at closure. This may have added inputs on analgesic efficacy. Obese patients were excluded to avoid errors on dosing of fentanyl according to body weight and technical difficulties in epidural catheter placements but they may have added to our understanding.
The heart rate changes were monitored postoperatively and hemodynamic comparisons were made. The intraoperative heart rate changes were not compared between the two groups in our study. We did not follow-up patients for pain after 24 h for uniformity as most Group C patients were shifted after 24 h in the ICU. Patient controlled analgesia pumps were not available at our center; the use of these pumps may have regulated the volume of fentanyl given postoperatively.
Although perioperative pain evaluation has progressed and more objective dimensions have been introduced, the quest for an ideal intraoperative nociceptive monitor continues. Newer monitors that are based upon skin conductance, pupillometric responses, nociceptive flexion responses, and the surgical pleth index are perhaps tools to consider for the future. The q-NOX monitor that incorporates pain assessment in addition to consciousness level correlates well with the standard bispectral index and could be the tool for the future.[25]
We believe with the current evidence and using blood pressure measurements, the use of an epidural can reduce pain and intra- and postoperative opioid requirements and allow more comfortable ambulation postoperatively. Although ERAS guidelines are moving away from central neuraxial blockade, limited use in high volume centers in high-risk patients may reduce opioid dependence and gastrointestinal side effects.
Conclusion
We conclude that epidural ropivacaine produces superior intraoperative analgesia and improved postoperative pain scores but increased intraoperative need for vasopressors and ICU stay in comparison with intravenous fentanyl in laparoscopic abdominal surgeries.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Ekstein P, Szold A, Sagie B, Werbin N, Klausner JM, Weinbroum AA. Laparoscopic surgery may be associated with severe pain and high analgesia requirements in the immediate postoperative period. Ann Surg. 2006;243:41–6. doi: 10.1097/01.sla.0000193806.81428.6f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bruce J, Krukowski ZH. Quality of life and chronic pain four years after gastrointestinal surgery. Dis Colon Rectum. 2006;49:1362–70. doi: 10.1007/s10350-006-0575-5. [DOI] [PubMed] [Google Scholar]
- 3.Sarin A, Litonius ES, Naidu R, Yost CS, Varma MG, Chen LL. Successful implementation of an Enhanced Recovery After Surgery program shortens length of stay and improves postoperative pain, and bowel and bladder function after colorectal surgery. BMC Anesthesiol. 2016;16:55. doi: 10.1186/s12871-016-0223-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wind J, Polle SW, Fung Kon Jin PH, Dejong CH, von Meyenfeldt MF, Ubbink DT, et al. Laparoscopy and/or Fast Track Multimodal Management Versus Standard Care (LAFA) Study Group;Enhanced Recovery after Surgery (ERAS) Group. Systematic review of enhanced recovery programmes in colonic surgery. Br J Surg. 2006;93:800–9. doi: 10.1002/bjs.5384. [DOI] [PubMed] [Google Scholar]
- 5.Gustafsson UO, Scott MJ, Hubner M, Nygren J, Demartines N, Francis N, et al. Guidelines for perioperative care in elective colorectal surgery. Enhanced Recovery After Surgery (ERAS) Recommendations:|y2018. World J Surg. 2019;43:659–95. doi: 10.1007/s00268-018-4844-y. [DOI] [PubMed] [Google Scholar]
- 6.Pöpping DM, Elia N, Van Aken HK, Marret E, Schug SA, Kranke P, et al. Impact of epidural analgesia on mortality and morbidity after surgery:Systematic review and meta-analysis of randomized controlled trials. Ann Surg. 2014;259:1056–67. doi: 10.1097/SLA.0000000000000237. [DOI] [PubMed] [Google Scholar]
- 7.Guay J, Nishimori M, Kopp S. Epidural local anaesthetics versus opioid based analgesic regimens for postoperative gastrointestinal paralysis, vomiting, and pain after abdominal surgery. Cochrane Database Syst Rev. 2016;7:CD001893. doi: 10.1002/14651858.CD001893.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yanagimoto Y, Takiguchi S, Miyazaki Y, Mikami J, Makino T, Takahashi T, et al. Comparison of pain management after laparoscopic distal gastrectomy with and without epidural analgesia. Surg Today. 2016;46:229–34. doi: 10.1007/s00595-015-1162-y. [DOI] [PubMed] [Google Scholar]
- 9.Tandon M, Singh A, Saluja V, Dhankhar M, Pandey CK, Jain P. Validation of a new “OPS” vs. “Numeric rating scale” for the evaluation of acute pain:A comparative study. Anesth Pain Med. 2016;6:e32101. doi: 10.5812/aapm.32101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marret E, Remy C, Bonnet F. Postoperative Pain Forum Group. |iMeta-analysis of epidural analgesia versus parenteral opioid analgesia after colorectal surgery. Br J Surg. 2007;94:665–73. doi: 10.1002/bjs.5825. [DOI] [PubMed] [Google Scholar]
- 11.Joshi GP, Bonnet F, Kehlet H. Evidence-based postoperative pain management after laparoscopic colorectal surgery. Colorectal Dis. 2013;15:146–55. doi: 10.1111/j.1463-1318.2012.03062.x. [DOI] [PubMed] [Google Scholar]
- 12.Levy BF, Scott MJ, Fawcett W, Fry C, Rockall TA. Randomized clinical trial of epidural, spinal or patient-controlled analgesia for patients undergoing laparoscopic colorectal surgery. Br J Surg. 2011;98:1068–78. doi: 10.1002/bjs.7545. [DOI] [PubMed] [Google Scholar]
- 13.Turunen P, Carpelan-Holmstorm M, Kairaluoma P, Wikstrom H, Kruuna O, Pere P, et al. Epidural analgesia diminished pain but did not otherwise improve enhanced recovery after laparoscopic sigmoidectomy:A prospective randomized study. Surg Endosc. 2009;23:31–7. doi: 10.1007/s00464-008-0100-0. [DOI] [PubMed] [Google Scholar]
- 14.Erol DD, Yilmaz S, Polat C, Arikan Y. Efficacy of thoracic epidural analgesia for laparoscopic cholecystectomy. Adv Ther. 2008;25:45–52. doi: 10.1007/s12325-008-0005-2. [DOI] [PubMed] [Google Scholar]
- 15.Zingg U, Miskovic D, Hamel CT, Erni L, Oertli D, Metzger U. Influence of thoracic epidural analgesia on postoperative pain relief and ileus after laparoscopic colorectal resection:Benefit with epidural analgesia. Surg Endosc. 2009;23:276–82. doi: 10.1007/s00464-008-9888-x. [DOI] [PubMed] [Google Scholar]
- 16.Wiedemann D, Mühlnickel B, Staroske E, Neumann W, Röse W. Ropivacaine plasma concentrations during 120-hour epidural infusion. Br J Anaesth. 2000;85:830–5. doi: 10.1093/bja/85.6.830. [DOI] [PubMed] [Google Scholar]
- 17.Holte K, Andersen J, Jakobsen DH, Kehlet H. Cyclo- oxygenase 2 inhibitors and the risk of anastomotic leakage after fast-track colonic surgery. Br J Surg. 2009;96:650–4. doi: 10.1002/bjs.6598. [DOI] [PubMed] [Google Scholar]
- 18.Klein M, Krarup PM, Burcharth J, Agren MS, Gogenur I, Jorgensen LN, et al. Effect of diclofenac on cyclooxygenase-2 levels and early breaking strength of experimental colonic anastomoses and skin incisions. Eur Surg Res. 2011;46:26–31. doi: 10.1159/000321706. [DOI] [PubMed] [Google Scholar]
- 19.Veyckemans F. Opioid free anesthesia-still a debate? Eur J Anaesthesiol. 2019;36:245–6. doi: 10.1097/EJA.0000000000000964. [DOI] [PubMed] [Google Scholar]
- 20.Turi S, Gemma M, Braga M, Monzani R, Radrizzani D, Beretta L. Peri operative Italian Society-ERAS Italian chapter. Int J Colorectal Dis. 2019;34:915–21. doi: 10.1007/s00384-019-03284-4. [DOI] [PubMed] [Google Scholar]
- 21.Taqi A, Hong X, Mistraletti G, Stein B, Charlebois P, Carli F. Thoracic epidural analgesia facilitates the restoration of bowel function and dietary intake in patients undergoing laparoscopic colon resection using a traditional, non-accelerated, perioperative care program. Surg Endosc. 2007;21:247–52. doi: 10.1007/s00464-006-0069-5. [DOI] [PubMed] [Google Scholar]
- 22.Bardram L, Funch-Jensen P, Gensen P, Crawford ME, Kehlet H. Recovery after laparoscopic colonic surgery with epidural analgesia, and early oral nutrition and mobilization. Lancet. 1995;345:763–4. doi: 10.1016/s0140-6736(95)90643-6. [DOI] [PubMed] [Google Scholar]
- 23.Halabi WJ, Kang CY, Nguyen VQ, Carmichael JC, Mills S, Stamos MJ, et al. Epidural analgesia in laparoscopic colorectal surgery. A nationwide analysis of use and outcomes. JAMA Surg. 2014;149:130–6. doi: 10.1001/jamasurg.2013.3186. [DOI] [PubMed] [Google Scholar]
- 24.Borzellino G, Francis NK, Chapuis O, Krastinova E, Dyevre V, Genna M. Role of epidural analgesia within an ERAS Program after Laparoscopic Colorectal Surgery:A review and meta-analysis of randomised controlled studies. Surg Res Pract. 2016;2016:7543684. doi: 10.1155/2016/7543684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ledowski T. Objective monitoring of nociception:A review of current commercial solutions. Br J Anaesth. 2019;123:e312–21. doi: 10.1016/j.bja.2019.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]