Abstract
The role of sweat glands in hidradenitis suppurativa has been largely neglected, despite the fact that its original designation, as “hidrosadénite phlegmoneuse”, implied an inflammatory malfunction of the apocrine sweat glands as the underlying pathogenic driver. The aim of this study was to evaluate the role of apocrine sweat glands with respect to the proinflammatory environment of hidradenitis suppurativa. Therefore, gravimetric assessment and multiplex cytokine assays from sweat obtained from patients with hidradenitis suppurativa along with immunofluorescence cytokine/chemokine analysis of lesional apocrine glands-bearing hidradenitis suppurativa skin were performed. Gravimetric assessment of 17 patients with hidradenitis suppurativa revealed that the condition is not associated with hyperhidrosis. However, patients seem to be more affected by subjective sweating. The current data identified a complex proinflammatory signature in hidradenitis suppurativa sweat characterized by a significant upregulation of monocyte chemoattractant protein-1, interleukin-8 (CXCL8), and interferon-γ. In agreement with this, a strong in situ expression of these mediators could be observed in apocrine glands of lesional hidradenitis suppurativa skin. These data shed new light on the proinflammatory capacity of apocrine sweat glands in hidradenitis suppurativa, which may lead to reconsideration of the role of sweat glands in hidradenitis suppurativa pathology.
Key words: acne inversa, inflammation, interferon gamma, interleukin 8, monocyte chemoattractant protein
Hidrosadénite phlegmoneuse was the term coined by the surgeon Verneuil in 1854 (1) for a chronic, debilitating inflammatory skin disease that we now know as hidradenitis suppurativa (HS; also known as acne inversa). The reason for this naming was the idea that the main pathogenic driver was an inflammatory malfunction of the apocrine sweat glands. Verneuil related the inflammatory dermatosis to the sweat glands based solely on the coincidence between the anatomical distribution of the apocrine sweat glands (2) and the topographical occurrence of the disease (1). Current research indicate the involvement of immunological processes against the hair follicle and favours HS as a chronic T cell-mediated inflammatory skin disease (3, 4). This has resulted in reduced interest in the potential role of apocrine glands in the pathogenesis of HS. Both T-helper (Th)-17 cell activation, a relative deficiency in Treg cells, and a strong non-specific inflammation (5) correlate with disease progression. Innate proinflammatory cytokines, such as interleukin (IL)-1β and tumour necrosis factor (TNF)-α, effector mechanisms of neutrophils, macrophages and plasma cells, and cytokines of activated Th1 and Th17 cells (e.g. IL-17) are involved in disease activity (5). However, the role of sweat glands has been largely neglected over the last 100 years, although patients with HS report not only experiencing putrid discharge, malodour and pain (6), but often also (in approximately 50% of cases) a change in their sweating behaviour before an overt lesion occurs (7, 8). This raises the possibility that hyperhidrosis could be a comorbidity factor contributing to disease activity and that changes in sweat composition may be pathologically linked to HS. In order to gain better insight into the exact role of apocrine sweat glands with respect to the proinflammatory environment of HS, this study performed gravimetric assessment and multiplex cytokine assays from sweat obtained from patients with HS along with immunofluorescence cytokine analysis from lesional apocrine-bearing HS skin. The current study provides the first comprehensive data that HS is not associated with hyperhidrosis, whereas patients with HS seem to be more affected by subjective sweating. The current data refer to a complex proinflammatory signature in sweat derived from patients with HS, which is characterized by a significant upregulation of monocyte chemoattractant protein (MCP)-1, IL-8 (CXCL8), and interferon (IFN)-γ. In addition, strong in situ expression of these proinflammatory mediators in apocrine glands of lesional HS skin that probably spurs inflammation in HS was identified. These data implicate a new role for apocrine sweat glands in disease progression and chronification of HS.
SIGNIFICANCE
Hidradenitis suppurativa is a debilitating skin disease, characterized by abscesses, especially in the armpits and groins. Approximately 200 years ago the condition was associated with sweat glands, due to the localization of the painful lesions. Since then, however, our understanding of the origin of the disease has changed fundamentally. The aim of this study was to investigate whether the sweat glands and abnormal sweating may nevertheless affect hidradenitis suppurativa. It was observed that the sweat of patients with hidradenitis suppurativa is more inflammatory in nature than that of healthy individuals. Inflammatory mediators in the sweat may contribute to worsening of hidradenitis suppurativa.
MATERIALS AND METHODS
Case selection and data assessment
Seventeen patients who were seen due to their axillary manifestation of HS were included in this study. Patients with a known history of malignancy, florid infections and/or currently under TNF-α inhibition were excluded from the study. Exclusion criteria also comprised other inflammatory skin diseases, causes of secondary hyperhidrosis, and suppurating lesions and/or Hurley stage III, where destruction of sweat glands might occur (9).The cross-sectional study protocol was in accordance with the ethics guidelines of the Declaration of Helsinki and was approved by the ethics committee (AZ-228/16) of the University of Würzburg. Signed informed consent was obtained from all patients prior to inclusion. Demographic data including sex, age, body mass index (BMI), and other pre-existing conditions to rule out causes of primary and secondary hyperhidrosis were recorded. Disease severity was staged following the modified Sartorius score (mHSS) (10). The control group consisted of 17 sex-, age- and BMI-matched healthy subjects without history of skin disease.
Quality of life and hyperhidrotic symptoms
To assess the patients’ quality of life, the Dermatology Life Quality Index (DLQI) (11) was used. The Hyperhidrosis Disease Severity Scale (HDSS) questionnaire was applied to evaluate whether patients with HS feel more affected by their sweating. A score of 1–2 indicates mild to moderate impairment due to hyperhidrosis, and a score of 3–4 indicates severe impairment due to hyperhidrosis (12). In addition to the questionnaire, all subjects were asked whether they perceived their sweating to be itchy, burning or painful.
Gravimetric measurements
Gravimetric assessment of sweat was conducted following 15 min at rest in a sitting position. All tests were performed at 25°C room temperature and 42–48% humidity at least 2 h after food intake. The axillae were thoroughly cleaned with an absorbent paper before gravimetry. A commercially available filter paper 63 cm2 was cut into 3 pieces (each 21 cm2), folded and weighed on a microbalance (Sartorius CP1245; Sartorius AG, Göttingen, Germany). Subsequently, the 3 pieces of filter paper were placed under the axilla and reweighed after 5 min at rest. The difference between the 2 weights was taken as sweat production in milligrams over 5 min. Women with a sweat production of ≥ 50 mg/5 min and men with ≥ 100 mg/5 min were considered hyperhidrotic (13). Another measurement took place after physical activity (running on the spot or squatting) for 5 min. Patients who could not participate in the exercise due to physical limitations, such as gonarthrosis or heart failure, were excluded from the study.
Sweat collection
To collect sweat for gravimetric and further proinflammatory cytokine analysis an absorbent paper (63 cm2) was cut into 3 pieces (each measuring 21 cm2), folded and placed in the centre of the left and right axillae, respectively. The topographical sweat collection area was approximately 12 cm2. Filter papers were removed after exercise and transferred into pre-weighed Eppendorf tubes, followed by centrifugation at 10,000 rpm for 2 min and freezing of sweat fluid at −20°C prior to cytokine analysis.
Multiplex assay
For cytokine quantification of IL-1β, IFN-α2, IFN-γ, TNF-α, MCP-1, IL-6, IL-8, IL-10, IL-12p70, IL-17α, IL-18, IL-23 and IL-33 bead-based multiplex LEGENDplex™ analysis (Human Inflammation Panel 1 (13-plex; Biolegend, San Diego, CA, USA) was applied according to the manufacturer’s instructions. Reactions were performed in duplicate using a Cytoflex LX flow cytometer (Beckman Coulter, Krefeld, Germany). Data were analysed by Legendplex V8.0 software (Biolegend).
Immunofluorescence
Three-µm skin sections were obtained from axillary HS skin (central region) and stained for IL-8 (1:250 dilution; #554717; BD, Germany), IFN-γ (1:200 dilution, #MAB2853, R&D Systems Inc., MN, USA), MCP-1 (1:100 dilution; #20521; BD, Germany) or IL-6 (1:400, #ab6672, Abcam, UK) as described previously (14). Healthy control skin was obtained from excess skin of the axillary region after surgery for benign tumours.
Statistical analysis
The results of DLQI and gravimetric sweat measurements were evaluated using the Mann–Whitney U test, HDSS by χ2 test and the association between distributed variables using the Spearman correlation. The effect sizes r were interpreted according to Cohen’s classification: r = 0.10 weak, r = 0.30 medium and r = 0.50 strong effect. The statistical significance level was set at p < 0.05. To assess the results of the current study, Microsoft Excel (Version 16.0.8431.2110, Microsoft Corporation, Redmond, CA, USA) and SPSS for Windows (Version 25.0; Statistical Package for Social Sciences; SPSS Inc., Chicago, IL, USA) were used.
RESULTS
Clinical characteristics
The study included 17 patients (mean ± standard deviation (SD) age 34 ± 9.3 years; 5 females, 12 males) diagnosed with axillary HS in Hurley stages I or II. The patients were examined clinically and their self-completed questionnaires were analysed. All patients attained a Sartorius score below 20. A mean ± SD BMI of 31 ± 5.3 kg/m2 was assessed, indicating obesity in a large proportion of patients with HS. Accordingly, BMI-, sex- and age-matched control subjects (n = 17; mean ± SD age 39 ± 15.5) years; 5 females, 12 males; mean ± SD BMI 29 ± 2.9 kg/m2) were enrolled.
Patients with hidradenitis suppurativa are more affected by subjective sweating
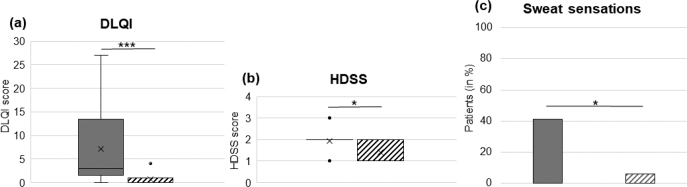
Among skin disorders, HS is one of the diseases with the most pronounced reduction in quality of life (QoL) as measured by DLQI (15). Patients with HS scored a standard error of the mean (SEM) of 7.2 ± 2.0 points compared with a mean of 0.7 ± 0.2 points in the healthy control group (p ≤ 0.001) (Fig. 1a). Impaired QoL in HS is of multifactorial origin and includes pain and pruritus. Using the HDSS to analyse subjective sweating revealed that the score was higher in HS than in healthy controls (p ≤ 0.05) (Fig. 1b). Patients with HS perceived their sweating as a burning, itching or painful sensation in 7/17 (41%) of cases, whereas only 1/17 (6%) healthy individuals reported such sensations while sweating (p ≤ 0.05) (Fig. 1c). Comparing the effects of HS on the patient’s DLQI (r = 0.644) and the subjective perception of hyperhidrosis measured by HDSS (r = 0.505) with group affiliation, a significant correlation between those items and the diseased HS group could be established. Likewise, disease severity according to mHSS significantly correlated with HDSS (r = 0.502) and DLQI (r = 0.650) (Fig. 2a, b).
Fig. 1.
Patients with hidradenistis suppurativa (HS) are more affected by subjective sweating. (a) Patients with HS scored a higher mean Dermatology Life Quality Index (DLQI) than the healthy control group (standard error of mean (SEM) 7.2 ± 2.0 vs 0.7 ± 0.2 p ≤ 0.001; box-whisker plot). (b) In addition, patients with HS were statistically significantly more affected by subjective sweating than the healthy control group (p ≤ 0.05; box-whisker-plot); and (c) report their sweating more often as a burning, itching or painful sensation (p ≤ 0.05; bar chart). Filled grey bars: HS group; striped bars: healthy control group. HDSS: Hyperhidrosis Disease Severity Scale.
Fig. 2.

Hidradenistis suppurativa (HS) disease severity correlates with subjective sweating. HS severity according to mHSS proves a significant correlation to Hyperhidrosis Disease Severity Scale (HDSS) and Dermatology Life Quality Index (DLQI).
Hidradenitis suppurativa is not associated with hyperhidrosis
An association between HS and hyperhidrosis has been suggested (16, 17), but gravimetric sweat measurements have, to the best of our knowledge, never been performed. Surprisingly, this study observed a trend to higher gravimetric sweat production in the control (at rest SEM 94.9 ± 30.9 mg/5 min; under exercise SEM 190.4 ± 42.7 mg/5 min) than in the HS group (at rest SEM 77.1 ± 17.2, mg/5 min; under exercise SEM 152.5 ± 37.0 mg/5 min) (Fig. 3 a, b), identifying 35% of control and 29% of diseased individuals as hyperhidrotic. The difference was not significant (p = 0.71), indicating that HS is not associated with an increased risk of hyperhidrosis. Interestingly, the control group showed a higher sweat release upon exercise than the patient group. The gravimetric sweat measurement at rest correlated significantly with those during exercise in both groups (each r = 0.884) (Fig. 3c, d). Importanly, the BMI shows no correlation with all other parameters.
Fig. 3.
Hidradenistis suppurativa (HS) does not correlate with a higher incidence for hyperhidrosis. Both (a) at rest and (b) under excerise, the healthy control group revealed a trend to higher gravimetric sweat production than the HS group (p = 0.71). (c, d) Gravimetric sweat measurement at rest significantly correlated with that during exercise in both groups. Grey bar: HS group; striped bar: healthy control group.
Hidradenitis suppurativa sweat exhibits a proinflammatory signature
To date, there are numerous studies on HS regarding the proinflammatory milieu in blood and skin (4, 5), but not in sweat. Since hyperhidrosis is not associated with HS, but patients with HS seem subjectively to be impaired by excessive sweating or related disease progression to sweating, this study considered whether sweat from patients with HS showed an increased concentration of (proinflammatory) cytokines. Using multiplex cytokine assays significantly increased concentrations of IL-8 (p = 0.011), MCP-1 (p = 0.029), and IFN-γ (p = 0.024) were observed in the sweat of patients with HS compared with healthy controls (Table I). Other proinflammatory cytokines, such as IL-6, IL-17A, IL-23 or IL-10, displayed no significant differences between the 2 groups. Moreover, lesional axillary skin was studied for cytokine expression. Using immunofluorescence, this study confirmed increased protein levels of IL-8, MCP-1 and IFN-γ, but, for example, not of IL-6 in sweat glands (Fig. 4). These findings suggest that apocrine sweat glands in HS are a source of proinflammtory cytokines/chemokines that are released with the sweat.
Table I.
Multiplex assay. Detection of proinflammatory cytokines and chemokines in the sweat of patients with hidradenistis suppurativa (HS) and healthy control individuals by flow cytometry
| Cytokine/chemokine, pg/ml | HS Mean ± SD | Healthy Mean ± SD | p-value |
|---|---|---|---|
| IL-1β | 139.65 ± 733.8 | 3.9 ± 9.42 | 0.775 |
| IFN-α2 | 0 ± 0 | 0 ± 0 | 1.000 |
| IFN-γ | 0.14± 0.47 | 0 ± 0 | 0.024 |
| TNF-α | 0.06± 0.17 | 0.03 ± 0.10 | 0.667 |
| MCP-1 | 1.81 ± 5.69 | 12.01 ± 76.52 | 0.029 |
| IL-6 | 12.93 ± 69.69 | 0 ± 0 | 0.160 |
| IL-8 | 62.21 ± 231.55 | 1.86 ± 7.76 | 0.011 |
| IL-10 | 0.1±0.28 | 0.03 ± 0.14 | 0.238 |
| IL12-p70 | 0 ± 0 | 0 ± 0 | 1.000 |
| IL-17α | 0.28± 1.84 | 0 ± 0 | 0.323 |
| IL-18 | 5.27 ± 30.28 | 0.1 ± 0.29 | 0.158 |
| IL-23 | 0.11± 0.71 | 0 ± 0 | 0.323 |
| IL-33 | 0 ± 0 | 0 ± 0 | 1.000 |
IL: interleukin; IFN: interferon; TNF: tumour necrosis factor; MCP: monocyte chemoattractant protein; SD: standard deviation. Statistically significant values according to Mann–Whitney U test are shown in bold.
Fig. 4.
Hidradenistis suppurativa (HS) sweat glands express proinflammatory cytokines. (a) Significantly enhanced expression of interleukin (IL)-8, monocyte chemoattractant protein (MCP)-1, and interferon (IFN)-γ (but not for IL-6) in apocrine glands of lesional axillary HS skin sections. (b) Isotype control antibodies served as control. Representative immunofluorescence labelings for the indicated cytokines (red) and DAPI staining (blue) of n=13 patients. Bar: 100 µm.
DISCUSSION
Sweating has a negative effect on skin diseases such as psoriasis or atopic dermatitis (18, 19). However, the effect of sweating and the role of the apocrine glands in the pathogenesis of HS have not yet been addressed. This study provides, for the first time, evidence that HS is not associated with hyperhidrosis. On the other hand, patients with HS show an increased subjective perception that their disease would deteriorate upon sweating. The hypothesis that sweating could contribute to increased levels of disease perception was supported by significantly higher HDSS scores in the HS group compared with the control group (p ≤ 0.05; 1.94 ± 0.1 and 1.41 ± 0.12, respectively). Supporting this subjective deterioration of HS due to perspiration, several reports have described sustained improvement of HS symptoms following application of botulinum toxin A (BoNT-A) to the regions where HS was active (17, 20, 21). In contrast to previous case reports demonstrating an excellent response of HS to BoNT-A, it is notable that a similar study by Hua et al. did not confirm an improvement in any of the metrics for HS disease activity (16). Interestingly, Grimstadt et al. (17) showed that the number of active lesions and pain sensations in patients with HS were tendentially reduced after BoNT-B treatment compared with the placebo group. As BoNT-B suppresses sweating, one might speculate that this observation, at least in part, is based on the effective inhibition of pro-inflammatory and possibly pain-mediating sweat production in HS. This interesting point awaits future randomized, placebo-controlled, double-blind studies to address the potential role of proinflammatory sweat components on disease activity and pain sensations in HS. Abnormalities in the transport of sweat onto the skin’s surface, resulting in the intra-epidermal retention of sweat, can cause paraesthesia and skin inflammation, as exemplified by miliaria rubra (22). Forty-one percent of patients with HS compared with 6% of healthy controls described their sweating as a burning, itchy or painful sensation and therefore perceived sweating as impairing and disease-maintaining. This raised the question of whether patients who experience axillary HS actually have an axillary hyperhidrosis or rather have sweat retention. Gravimetric assessment showed that 29% of patients with HS are hyperhidrotic compared with 35% in the BMI-matched control group, concluding that HS is not associated with hyperhidrosis. Particularly under physical stress, the control group achieved higher values in the gravimetric sweat measurement, contradicting the clinical observation that patients with HS often report an increased tendency to perspire. One reason for the relatively lower sweat production under stress could be motor impairment due to disease-specific lesions. We previously showed that 39% of patients with HS stated a subjective motor impairment; of those, however, only a third were in Hurley stages I or II (23). Given that sweat glands and sweat gland function are integrally important for wound healing (24), it is also possible that impaired sweat gland function contribute to the pathological non-healing wound-like environment of HS (25). Microarray analysis and immunofluorescence stainings on lesional HS samples verified that expression of multiple genes (WIF1, AQP5, FOXA1, dermcidin) associated with sweat gland function are decreased in HS lesional skin (25), supporting our hypothesis of dysfunctional sweat glands and sweat retention. Another reason for a trend to lower sweat levels in HS compared with healthy controls could be a decreased overall number of eccrine sweat glands in lesional HS skin, as previously shown by Coates et al. (25).
In this context, the question arose as to whether HS could even be associated with lesional sweat retention leading to compensatory sweating in non-lesional skin. This, in turn, would be in line with the clinical observation that patients with HS report pronounced perspiration more often than the control group. Follicular hyperkeratosis and plugging, key steps during the pathogenesis of HS (4), might cause sweat retention and accumulation of proinflammatory chemokines and cytokines, and therefore trigger disease progression. To investigate this hypothesis, the current study examined HS sweat for the presence of proinflammatory mediators. A complex proinflammatory signature was identified in sweat derived from patients with HS, which is characterized by a significant upregulation of MCP-1, IL-8, and IFN-γ compared with healthy controls. In addition, a strong expression of these proinflammatory mediators, which probably drive inflammation in HS, was identified in apocrine glands of lesional HS skin by immunofluorescence. Dai et al. (26) demonstrated that sweat activates NF-κB, ERK and JNK signalling pathways and induces IL-8, IL-1b, NOD2, and RIG-I in epidermal keratinocytes. Moreover, Emelianov et al. (27) revealed significantly increased concentrations of the antimicriobial peptide (AMP) cathelicidin (LL-37) in the epithelium of eccrine and apocrine sweat glands in patients with HS. The pro-inflammatory functions of LL-37 could trigger local disease exacerbation and thus promote HS development (28). In addition, IL-8 produced by sweat and lesional apocrine glands may trigger LL-37 production in neutrophils. Moreover, IL-8 can be rapidly induced in response to several proinflammatory cytokines, such as TNF-α and IL-1β, bacterial and viral products and cellular stress (29), as well as by IL-17 (30), all of which are key proinflammatory mediators in HS (5). Therefore, it is possible that upregulation of IL-8 expression in HS apocrine glands and sweat could propagate a proinflammatory circle that promotes keratinocytic stress signalling as well as neutrophil, mast- and T-cell chemotaxis, and thus the characteristic inflammatory infiltrate and milieu seen in HS (5).
Among T cell-typical mediators, the Th1 cytokine IFN-γ is highly expressed in HS lesions, with levels comparable to those in psoriasis (5). IFN-γ pushes proinflammatory cytokine production by macrophages and regulates B cell functions. Moreover, it induces the surface expression of the major histocompatibility complex and costimulatory molecules on both resident tissue cells as well as antigen-presenting immune cells, thus supporting local T-cell activation in HS (31). In accordance with prior work showing that IFN-γ-producing effector T cells activate the JAK-STAT cascade and other downstream pathways for inflammatory responses and immunoregulation (32), we recently showed that IFN-γ stimulation of epidermal HS skin isolates triggers a sustained STAT1 phosphorylation (33). It is feasible, that this keratinocytic activation partly results from sweatderived IFN-γ. Likewise, MCP-1 (also known as CCL2) has recently been shown as elevated in lesional and nonlesional HS epidermis (34) and could also contribute to the proinflamatory milieu.
The current study suggests an altered sweat gland function in HS disease pathology. Sweat glands may contribute to pathological cutaneous immunity in HS beyond their role in wound repair through production of inflammatory cytokines. It is possible that sweat glands trigger multiple host factors, including AMPs, proinflammatory cytokines and chemokines, thereby spurring disease progression and chronification. These results suggest that the role of apocrine sweat glands in the pathology of HS should be revisited.
ACKNOWLEDGEMENTS
We thank Daniela Schillinger, Claudia Rüth, and Nadine Vornberger for excellent technical assistance. D.P. is member of the European Hidradenitis Suppurativa Foundation (EHSF) e.V. The Department of Dermatology, Venereology and Allergology, University Hospital Würzburg is – with active members D. P. and M. G. – a health provider centre of the European Network for Rare and Low Prevalence Complex Skin diseases (ERN Skin).
This work was supported by the Interdisziplinäres Zentrum für Klinische Forschung (IZKF, AdvCSP-2 to AK; ZZ/25 to VGF), Medical Faculty of the University of Würzburg, Würzburg, Germany.
Footnotes
The authors have no conflicts of interest to declare.
REFERENCES
- 1.Verneuil A. Études sur les tumeurs de la peau; des quelques maladies des glandes sudoripares. Arch Gén Méd 1854; 5: 447–468. [Google Scholar]
- 2.Robin C. Note sur une espèce particulière de glandes de la peau de l’homme. 3° série, Zoologie et Biologie Animale. Ann Sci Nat Paris 1845; 4: 380–381. [Google Scholar]
- 3.Hotz C, Boniotto M, Guguin A, Surenaud M, Jean-Louis F, Tisserand P, et al. Intrinsic defect in keratinocyte function leads to inflammation in hidradenitis suppurativa. J Invest Dermatol 2016; 136: 1768–1780. [DOI] [PubMed] [Google Scholar]
- 4.Zouboulis CC, Nogueira da Costa A, Makrantonaki E, Hou XX, Almansouri D, Dudley JT, et al. Alterations in innate immunity and epithelial cell differentiation are the molecular pillars of hidradenitis suppurativa. J Eur Acad Dermatol Venereol 2020; 34: 846–861. [DOI] [PubMed] [Google Scholar]
- 5.Wolk K, Join-Lambert O, Sabat R. Aetiology and pathogenesis of hidradenitis suppurativa. Br J Dermatol 2020; 183: 999–1010. [DOI] [PubMed] [Google Scholar]
- 6.Matusiak L, Szczech J, Kaaz K, Lelonek E, Szepietowski JC. Clinical characteristics of pruritus and pain in patients with hidradenitis suppurativa. Acta Derm Venereol 2018; 98: 191–194. [DOI] [PubMed] [Google Scholar]
- 7.Ring HC, Theut Riis P, Zarchi K, Miller IM, Saunte DM, Jemec GB. Prodromal symptoms in hidradenitis suppurativa. Clin Exp Dermatol 2017; 42: 261–265. [DOI] [PubMed] [Google Scholar]
- 8.Hoffman LK, Ghias MH, Lowes MA. Pathophysiology of hidradenitis suppurativa. Semin Cutan Med Surg 2017; 36: 47–54. [DOI] [PubMed] [Google Scholar]
- 9.Hurley HJ. Axillary hyperhidrosis, apocrine bromhidrosis, hidradenitis suppurativa, and familial benign pemphigus: surgical approach. In: Roenigk RK, Roenigk HH, editors. Dermatologic surgery. Marcel Dekker, New York, 1989, pp 729–739. [Google Scholar]
- 10.Sartorius K, Killasli H, Heilborn J, Jemec GB, Lapins J, Emtestam L. Interobserver variability of clinical scores in hidradenitis suppurativa is low. Br J Dermatol 2010; 162: 1261–1268. [DOI] [PubMed] [Google Scholar]
- 11.Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI) – a simple practical measure for routine clinical use. Clin Exp Dermatol 1994; 19: 210–216. [DOI] [PubMed] [Google Scholar]
- 12.Solish N, Bertucci V, Dansereau A, Hong HC, Lynde C, Lupin M, et al. A comprehensive approach to the recognition, diagnosis, and severity-based treatment of focal hyperhidrosis: recommendations of the Canadian Hyperhidrosis Advisory Committee. Dermatol Surg 2007; 33: 908–923. [DOI] [PubMed] [Google Scholar]
- 13.Hund M, Kinkelin I, Naumann M, Hamm H. Definition of axillary hyperhidrosis by gravimetric assessment. Arch Dermatol 2002; 138: 539–541. [DOI] [PubMed] [Google Scholar]
- 14.Rauschenberger T, Schmitt V, Azeem M, Klein-Hessling S, Murti K, Grän F, et al. T cells control chemokine secretion by keratinocytes. Front Immunol 2019; 10: 1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matusiak L, Bieniek A, Szepietowski JC. Hidradenitis suppurativa markedly decreases quality of life and professional activity. J Am Acad Dermatol 2010; 62: 706–708, 708e1. [DOI] [PubMed] [Google Scholar]
- 16.Hua VJ, Kuo KY, Cho HG, Sarin KY. Hyperhidrosis affects quality of life in hidradenitis suppurativa: a prospective analysis. J Am Acad Dermatol 2020; 82: 753–754. [DOI] [PubMed] [Google Scholar]
- 17.Grimstad O, Kvammen BO, Swartling C. Botulinum toxin type B for hidradenitis suppurativa: a randomised, double-blind, placebo-controlled pilot study. Am J Clin Dermatol 2020; 21: 741–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teodossieva E. [Activity of the eccrine sweat glands in patients with psoriasis]. Dermatol Monatsschr 1986; 172: 585–588. [PubMed] [Google Scholar]
- 19.Murota H, Yamaga K, Ono E, Katayama I. Sweat in the pathogenesis of atopic dermatitis. Allergol Int 2018; 67: 455–459. [DOI] [PubMed] [Google Scholar]
- 20.Shi W, Schultz S, Strouse A, Gater DR. Successful treatment of stage III hidradenitis suppurativa with botulinum toxin A. BMJ Case Rep 2019; 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Campanati A, Martina E, Giuliodori K, Bobyr I, Consales V, Offidani A. Two cases of hidradenitis suppurativa and botulinum toxin type a therapy: a novel approach for a pathology that is still difficult to manage. Dermatol Ther 2019; 32: e12841. [DOI] [PubMed] [Google Scholar]
- 22.Wenzel FG, Horn TD. Nonneoplastic disorders of the eccrine glands. J Am Acad Dermatol 1998; 38: 1–17; quiz 18–20. [DOI] [PubMed] [Google Scholar]
- 23.Frings VG, Bauer B, Glöditzsch M, Goebeler M, Presser D. Assessing the psychological burden of patients with hidradenitis suppurativa. Eur J Dermatol 2019; 29: 294–301. [DOI] [PubMed] [Google Scholar]
- 24.Rittie L, Sachs DL, Orringer JS, Voorhees JJ, Fisher GJ. Eccrine sweat glands are major contributors to reepithelialization of human wounds. Am J Pathol 2013; 182: 163–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coates M, Mariottoni P, Corcoran DL, Kirshner HF, Jaleel T, Brown DA, et al. The skin transcriptome in hidradenitis suppurativa uncovers an antimicrobial and sweat gland gene signature which has distinct overlap with wounded skin. PLoS One 2019; 14: e0216249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dai X, Okazaki H, Hanakawa Y, Murakami M, Tohyama M, Shirakata Y, et al. Eccrine sweat contains IL-1alpha, IL-1beta and IL-31 and activates epidermal keratinocytes as a danger signal. PLoS One 2013; 8: e67666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Emelianov VU, Bechara FG, Glaser R, Langan EA, Taungjaruwinai WM, Schroder JM, et al. Immunohistological pointers to a possible role for excessive cathelicidin (LL-37) expression by apocrine sweat glands in the pathogenesis of hidradenitis suppurativa/acne inversa. Br J Dermatol 2012; 166: 1023–1034. [DOI] [PubMed] [Google Scholar]
- 28.Thomi R, Schlapbach C, Yawalkar N, Simon D, Yerly D, Hunger RE. Elevated levels of the antimicrobial peptide LL-37 in hidradenitis suppurativa are associated with a Th1/Th17 immune response. Exp Dermatol 2018; 27: 172–177. [DOI] [PubMed] [Google Scholar]
- 29.Hoffmann E, Dittrich-Breiholz O, Holtmann H, Kracht M. Multiple control of interleukin-8 gene expression. J Leukoc Biol 2002; 72: 847–855. [PubMed] [Google Scholar]
- 30.Mirandola P, Gobbi G, Micheloni C, Vaccarezza M, Di Marcantonio D, Ruscitti F, et al. Hydrogen sulfide inhibits IL-8 expression in human keratinocytes via MAP kinase signaling. Lab Invest 2011; 91: 1188–1194. [DOI] [PubMed] [Google Scholar]
- 31.Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol 2004; 75: 163–189. [DOI] [PubMed] [Google Scholar]
- 32.Qing Y, Stark GR. Alternative activation of STAT1 and STAT3 in response to interferon-gamma. J Biol Chem 2004; 279: 41679–41685. [DOI] [PubMed] [Google Scholar]
- 33.Frings VG, Jopp L, Srivastava M, Presser D, Goebeler M, Schmidt M. Stress signaling and STAT1 activation characterize the keratinocytic gene expression pattern in Hidradenitis suppurativa. J Eur Acad Dermatol Venereol 2022. Jul 26 [Online ahead of print]. [DOI] [PubMed] [Google Scholar]
- 34.Dajnoki Z, Somogyi O, Medgyesi B, Jenei A, Szabo L, Gaspar K, et al. Primary alterations during the development of hidradenitis suppurativa. J Eur Acad Dermatol Venereol 2022; 36: 462–471. [DOI] [PMC free article] [PubMed] [Google Scholar]