Abstract
Purpose
2021 marks the tenth anniversary of the AMNOG process and brought with it a new German administration—two good reasons to take stock of where we stand today, what has been achieved so far, and how the path of early benefit assessments in Germany should continue.
Results
From the perspective of manufacturers of cancer drugs, the AMNOG process, as a constantly evolving system, has for the most part proved itself—which does not mean that there is no longer room for improvement. Significant improvements have been achieved in the area of early consultation of medical societies regarding the selection of the appropriate comparator therapy as well as in the reimbursement of biomarker diagnostic tests in the outpatient sector. However, there is still a need for improvement, especially in the areas of patient-relevant outcomes accepted by the G-BA, the inclusion of real-world data in evidence assessments, or the transfer of evidence from certain patient groups to others.
Conclusion
The current AMNOG structures were developed for the most part at a time when no one saw immuno-oncology or gene and cell therapies coming, when there were no multi-tumor drug approvals, and when few imagined that within a few years, the established tumor entities would be broken down into dozens of sub-entities on the basis of molecular genetic markers. Society wants these and other advances, and the HTA process must, therefore, take this into account in a healthcare system based on solidarity.
Keywords: HTA, AMNOG, Drugs, Oncology, Germany
Introduction
In 2021, the COVID-19 pandemic has once again overshadowed other developments. But a round-number anniversary of a far-reaching health policy reorganization should not simply be allowed to pass unacknowledged. January 1, 2021 marked a decade since the introduction of the German Pharmaceutical Market Reorganization Act (AMNOG) (Bundesanzeiger 2010). AMNOG stands for the introduction of “early benefit assessment”, the German approach to health technology assessment (HTA). Germany was not the first country to introduce HTA, but in recognition of the AMNOG process along with its institutions, notably the Federal Joint Committee (G-BA) and the Institute for Quality and Efficiency in Health Care (IQWiG), it quickly gained a worldwide reputation as a highly regarded pioneer for patient-centered benefit assessments.
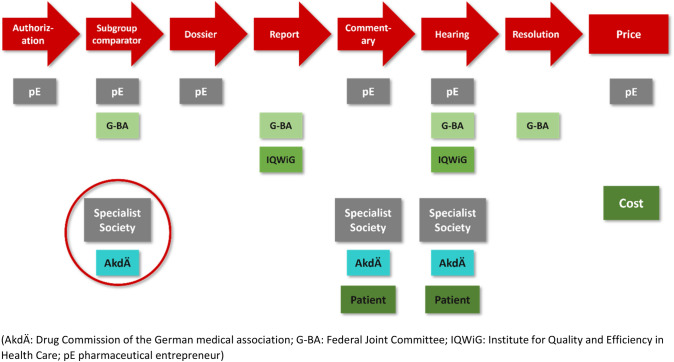
Chronologically, the AMNOG process begins after drug approval by the European Medicines Agency (EMA). The aim is to determine the additional benefit of a new drug in comparison to a standard therapy, called the appropriate comparator therapy (ACT). To this end, at the time of market entry the pharmaceutical entrepreneur (pE) must submit a dossier, which is then reviewed by the IQWiG within 3 months. “Market entry” is defined as the time at which a drug is first launched on the market or, alternatively, as one month after extension of marketing authorization for drugs that were first approved after January 1, 2011 and for which an indication with benefit assessment already exists. Based on the preliminary IQWiG recommendation and a commenting procedure with an oral hearing, the G-BA determines additional benefits in six categories (major, considerable, minor, non-quantifiable (comprising major, considerable or minor, but the data may be preliminary), no benefit, and less benefit). (Figs. 1 and 2, Table 1).
Fig. 1.
Flowchart of early benefit assessment based on AMNOG.
Modified from Trümper and Wörman (2021)
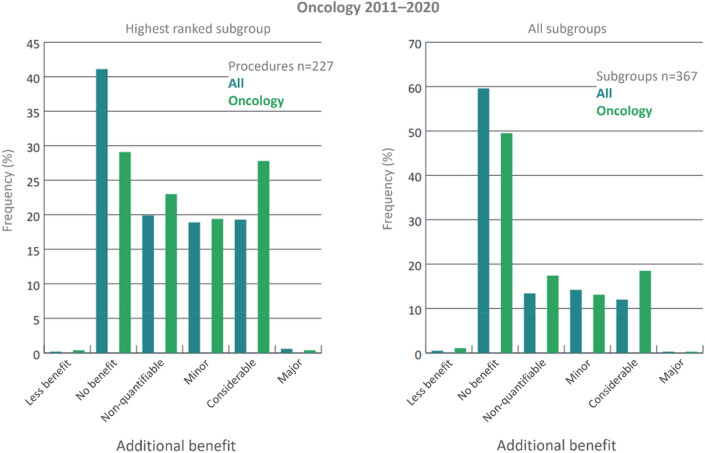
Fig. 2.
Oncology is the field with most new drugs and indication extensions within the German benefit assessment system. The category “significant additional benefit” is granted in oncological benefit assessments at an above-average rate.
Modified from Trümper and Wörman (2021), Dabisch et al. (2014) and Federal Joint Committee (2021a)
Table 1.
Benefit assessment procedure in Germany from 2011 to 2020. The G-BA recognizes additional benefits in six categories (Federal Joint Committee 2021a)
| Additional benefit category | Definition |
|---|---|
| Major additional benefit | Persistent and previously unattained substantial improvement of the treatment-relevant benefit |
| Considerable additional benefit | Previously unattained marked improvement of the treatment-relevant benefit |
| Minor additional benefit | Previously unattained moderate—not just small—improvement of the treatment-relevant benefit |
| Non-quantifiable additional benefit | Scientific basis does not allow quantification (can potentially summarize all benefit categories—major/considerable/minor) |
| No additional benefit | No additional benefit demonstrated |
| Less benefit | Benefit is inferior in comparison to the ACT |
The categorization of the additional benefit provides the basis for negotiating the reimbursement price between the National Association of Statutory Health Insurance Funds (GKV-SV) and the pE, which must be completed within 6 months after the G-BA decision or, if an arbitration board is consulted, up to 15 months after the G-BA decision. Free pricing applies for 12 months from the time of first market entry. From month 13 after market entry or—in the case of an extension of the market authorization—approval of a new indication, the agreed or arbitrated reimbursement amount applies. It is important to note that the AMNOG process—in contrast to EMA approval—says nothing about the underlying efficacy and tolerability of a treatment. It is NOT a second approval procedure and does not involve a risk–benefit evaluation. It merely serves as a price-determining basis predicated upon a patient-relevant additional benefit. Hence, the G-BA decision does not affect the approval status: the drug in question can still be prescribed without restriction—as well as reimbursed—irrespective of the additional benefit category to which it is assigned.
Cancer drugs in the AMNOG process: trial successfully completed
The AMNOG process affects all medical fields, but it presents each discipline with different challenges. The aim of this article is to review the first AMNOG decade from the perspective of cancer care. From the outset it was a political aspiration in Germany—especially with regard to cancer patients—to ensure that benefit evaluations would not lead to the availability of drugs being delayed for months or even years after approval.
That has been successfully averted, which is very good news for all cancer patients, who often simply do not have the time to wait for a delayed market launch. Speedy availability of new drugs in Germany is not a matter of course, as the recent annual WAIT survey by the European Federation of Pharmaceutical Industries and Associations (EFPIA) has once again shown. In 2020 Germany led Europe with a median of 50 days between approval and the granting of prescription status. By comparison, that figure was 87 days in Switzerland, 357 days in Italy and 474 days in France (Association of Research-Based Pharmaceutical Companies 2021).
There have been ample oncological examples in recent years that illustrate this clear strength of the German AMNOG process—for which the Federal Republic is envied internationally. Mention should be made of the consistent introduction of immuno-cancer drugs such as ipilimumab in 2011 and nivolumab and pembrolizumab in 2015—an entirely novel pillar of cancer therapy previously unknown within the system. Tyrosine kinase inhibitors as well as the Advanced Therapy Medicinal Products (ATMP) are also notable, with the latter still in the “introductory phase” in the AMNOG system and certainly still needing some “tweaking”. Nevertheless, the fact that the first oncological CAR-T cell therapies, such as tisagenlecleucel and axicabtagene ciloleucel, have swiftly found their way into routine care and statutory health insurance reimbursement schemes and the fact that the system has successfully established quality assurance measures (Federal Joint Committee 2021e) underscore the efficiency and adaptability of the AMNOG process.
AMNOG as an evolving system
In retrospect, the adaptability of the AMNOG process may have been the key to the fact that the German HTA approach can look back on a relatively successful decade. Early on, oncology associations within the Association of Scientific Medical Societies in Germany (AWMF) as well as representatives from politics, medicine, science and business promoted the AMNOG process as a system that must evolve over the years.
Initially, this was by no means the consensus, but it has turned out to be the right approach and an important one. The continuous process of evolution is evident not least from the long list of legislation relating to early benefit assessment that followed the introduction of AMNOG. Examples include the 2012 Second Law Amending Pharmaceutical and Other Regulations, which merged several assessment procedures for one active substance and implemented benefit assessment decisions in physician´s practice management software, the 2017 Act to Strengthen the Supply of Medicines in Statutory Health Insurance and the 2019 Act for More Safety in the Supply of Pharmaceuticals, which introduced indication-related data collection and greater participation by medical societies. Outside the field of oncology, the 2020 Act for Fair Competition Among Health Insurance clarified that an additional benefit is considered a given for exempted reserve antibiotics.
Even if we focus solely on the field of oncology, it would be beyond the scope of this article to acknowledge the multitude of G-BA decisions made over the past decade. A glance at the G-BA’s 2020 annual report is enough to illustrate how hopeless such a task would be. In this one (COVID) year alone, pharmaceutical companies submitted a record number of 116 benefit dossiers to the G-BA. Forty-two of them were in the field of oncology (Federal Joint Committee 2021b). Also in the nine preceding years, oncology was far and away the front runner in benefit assessments (Table 2). Against this backdrop, the aim of this article is to emphasize not so much individual decisions but rather some of the structural innovations that have distinguished AMNOG as an evolving system over the past decade.
Table 2.
Number of completed early benefit assessments by medical field (modified from Trümper and Wörmann 2021)
| Medical field | 2011–2018 | 2019/2020 |
|---|---|---|
| Dermatology | 35 | 15 |
| Diabetology | 36 | 9 |
| Endocrinology | 46 | 10 |
| Gynecology | 12 | 17 |
| Hematology | 59 | 38 |
| Hemostaseology | 15 | 7 |
| Otolaryngology | 2 | 5 |
| Hepatogastroenterology | 34 | 10 |
| Infectious diseases | 36 | 9 |
| Cardiology | 12 | 4 |
| Nephrology | 13 | 5 |
| Neurology | 30 | 14 |
| Oncology | 139 | 89 |
| Ophthalmology | 10 | 3 |
| Pediatrics | 30 | 38 |
| Pulmonology | 47 | 33 |
| Psychiatry | 5 | 1 |
| Rheumatology | 6 | 4 |
| Metabolism | 27 | 26 |
| Urology | 18 | 12 |
Constructive cooperation in the run-up to benefit assessment
The opportunities for targeted coordination between the pEs and the HTA bodies in accordance with Art. 35a (7) of the German Social Code Book V (SGB V) have developed positively over the years. This relates on the one hand to “late” consultation during compilation of the dossier. In particular, however, we will emphasize “early” advice on studies and ACTs here. The successively expanded participation of the regulatory authorities in these consultations, as introduced in 2012 (Bundesanzeiger 2012), provides assistance with the planning of studies and study endpoints in such a way that the needs of both the EMA and the Federal Institute for Drugs and Medical Devices (BfArM) or the Paul Ehrlich Institute (PEI) as well as the G-BA are taken into account. And in 2019 (Bundesanzeiger 2019), the introduction of the mandatorily requested input of medical societies and/or the Drug Commission of the German Medical Association (AkdÄ), improved the acceptance of the AMNOG process further. Ideally, this facilitates a shared understanding of an ACT in the relevant indication.
This shared understanding with regard to the ACT should not be called into question again. In situations where the G-BA has stipulated multiple therapeutic alternatives, they should be treated as equally appropriate comparators for demonstrating an additional benefit in the context of a benefit assessment. It is unacceptable if later, in the context of the actual benefit assessment, criticism is suddenly voiced of the pharmaceutical company’s choice of an ACT or if the options communicated by the G-BA during the planning of the study are being limited during or after the process [See, for example, the G-BA hearing of August 20, 2020 on apalutamide (Federal Joint Committee 2021d)]. Also, the real-world value of a drug may not be reflected in the individual benefit category assigned by the G-BA and thus it is up to the negotiating parties to find an adequate negotiation price. To provide planning security for the pharmaceutical companies and especially in recognition of the very complex, international coordination of study designs required for drug development, it is essential to maintain a consistent ACT as well as flexibility in refining ACT definitions throughout the AMNOG process.
Reimbursement of biomarker diagnostic tests no longer an issue
An especially welcome achievement from the point of view of cancer care in particular is the fact that changes to the German Social Code Book V] succeeded in establishing an efficient billing method (Bundesanzeiger 2017) for the increasingly important field of biomarker diagnostics, which in Germany is typically carried out on in the outpatient setting. The trend towards more biomarker diagnostic tests is driven by the increasing availability of targeted anti-tumor therapies. Biomarker tests identify, among other things, those cancer patients who are highly likely to benefit from targeted anti-tumor therapy. Testing of EGFR inhibitors set a negative precedent, as it had been initially partly paid for by the pharmaceutical company and therefore a code had not been provided for in the pre-AMNOG reimbursement system.
That has now changed. Specifically, since 2017, a process is automatically triggered upon submission of the dossier for all oncological products for which a diagnostic marker is explicitly mentioned in the approval, leading to an adjustment of the outpatient statutory health insurance Uniform Assessment Standard Tariff (EBM) at the same time as the benefit assessment decision is finalized, which is decisive for outpatient billing. This pragmatic approach to companion biomarker reimbursement is expedient. And it also makes good economic and medical sense, because it effectively establishes the primacy of therapy for the reimbursement of testing. Tests are not pushed onto the market that “seek” a therapy; rather, reimbursable companion tests are made available if they actually make sense or are required by virtue of the marketing authorization of the respective drug. By contrast, there is unfortunately still no formalized reimbursement scheme for biomarker diagnostics in the inpatient sector.
Endpoints in evidence evaluation
As far as the evidence recognized by the G-BA is concerned, the entire first AMNOG decade in oncology has been accompanied by a debate on progression-free survival (PFS), which is still not recognized by the IQWiG as a patient-relevant endpoint and is still a matter of dispute within the G-BA with regard to its patient relevance. On this point there is contention between the majority of the pEs, the medical societies and many patient representatives on the one hand and the German HTA bodies on the other. Overall, we believe it is important to emphasize that a benefit assessment should not oversimplify. Rather, patient-relevant endpoints should be selected on the basis of the tumor entity and stage so as to take into account the complexity of the cancer therapy (Dabisch et al. 2014).
A learning AMNOG process along these lines with regard to the evaluation of evidence can be clearly seen from the overall picture—despite all the dissent regarding PFS. In this context, the recognition of intermediate imaging endpoints in some treatment situations should be mentioned. This is particularly important in adjuvant cancer therapy, where the G-BA recognizes tumor recurrence as being patient-relevant in tumor-free patients even if it is only diagnosed using biomarkers or imaging and is not yet clinically manifest. Thus, radiologically manifest recurrences have recently been recognized for both tyrosine kinase inhibitors (BRAF/MEK) and immunotherapeutic agents (PD-1) (Federal Joint Committee 2019a, b). However, the G-BA also recognized such an endpoint in hematological oncological indications in the form of event-free survival (EFS), for the first time specifically in the benefit assessment procedure for brentuximab vedotin in anaplastic large-cell lymphoma (Federal Joint Committee 2019c).
Process innovations in evidence evaluation
In addition, some—only ostensibly secondary—process innovations should be mentioned that have improved the routine AMNOG process from the pharmaceutical companies’ point of view with regard to evidence and evidence evaluation. Since 2017, it is possible to apply to the G-BA for a benefit reassessment based on new scientific findings within one year (Bundesanzeiger 2017). This expedites the process in the case of dynamically shifting evidence situations, although the reassessment process does not start until the one-year period has elapsed.
The possibility of bundling several benefit assessment procedures into one drug with a new active ingredient is likewise helpful (Bundesanzeiger 2017). The bundling option enables pharmaceutical companies to “merge” submission of dossiers if approval of further indications is expected within six months of market entry. In this way, pharmaceutical companies can deviate from the statutory deadlines in individual cases and submit the required evidence for a benefit assessment in “bundled” form. This also ultimately leads to enhanced procedural efficiency on the part of the G-BA, the GKV-SV and the PEs.
It should also be emphasized here that the G-BA unfortunately so far only accepts indirect comparisons in the context of a benefit assessment in exceptional cases. Such indirect comparisons allow an additional benefit compared to an ACT to be assessed beyond study boundaries. Indirect comparisons are always useful if randomized clinical trials are not possible or are ethically unacceptable. In the case of rare diseases with small numbers of patients, a high disease burden and a paucity of treatment options, it makes good sense to generate evidence using indirect comparisons. This was the case, for example, with the benefit assessment of pembrolizumab in combination with pemetrexed and platinum in non-small cell lung cancer, where superiority to monotherapy was demonstrated via an indirect route using chemotherapy as a “bridging comparator” (Federal Joint Committee (2019d).
Benefit from the patient’s point of view taken into account
The AMNOG process has undergone significant further development in recent years in the area of patient-reported outcomes (PROs). PROs are recorded by patients themselves by means of standardized, validated questionnaires. Based on patient-reported symptoms, an additional benefit can be achieved in the morbidity category and patient-reported psychosocial and behavioral aspects in the health-related quality of life category. Although patient-reported symptoms (as morbidity endpoints) and health-related quality of life—in addition to mortality and safety—were defined as benefit categories within the AMNOG process from the outset, their importance clearly straggled behind an emphasis on reduction in mortality in the first few years. This has repeatedly caused difficulties, especially in the metastatic therapy situation.
An important breakthrough in taking patient-reported endpoints into account in benefit assessments was the initial benefit assessment procedure for crizotinib in ALK-positive, advanced non-small-cell lung cancer (Federal Joint Committee 2013). The AMNOG process for carfilzomib in combination with dexamethasone or lenalidomide/dexamethasone in previously treated multiple myeloma is a prime example of the inclusion of PROs in the benefit assessment for hematological neoplasms (Federal Joint Committee 2018). Here, the established quality of life questionnaire EORTC QLQ-C30, developed with cancer patients, as well as the myeloma-specific EORTC-QLQ-MY20 questionnaire were used as a PRO measure for recording symptoms and health-related quality of life. Overall, the collection of PROs for many anti-tumor therapies is essential for understanding the patient’s perspective. Their growing consideration in benefit assessments is all the more welcome.
Open issues for the new legislature
Recognizing that the AMNOG process is an evolving system also means that the learning process is continuous and does not end in the second decade of existence or when a legislature expires. At this point, some currently open issues should be mentioned which, from the point of view of pharmaceutical companies involved in oncology, should be addressed by the G-BA and/or a new federal administration:
Evidence transfer: The transfer of evidence from certain patient groups to others is provided for in the AMNOG process and regulated by law. In the case of drugs approved for pediatric use (“PUMA drugs”), the G-BA is currently working on a code of procedure to improve the transfer of evidence during benefit assessments to patient groups which, although covered by the approval, have not been sufficiently included in relevant studies. Such a transfer of evidence can also be very helpful in adult oncology and PIP-based approval extensions. A clearly defined, methodological set of rules for the implementation of such a transfer is important in order to achieve the required broad acceptance.
Responder thresholds for recording PROs: This is an important topic and the subject of many ongoing discussions between the HTA bodies, medical societies and PEs. Specifically, it is a question of what percentage change in PROs should be deemed relevant for benefit assessments. In its methodology paper (Institute for Quality and Efficiency in Health Care 2020), the IQWiG advocates a general and indication-independent threshold of 15% of the respective scale range based on several reviews from various indications. Critics point out that such a blanket procedure does not do justice to the recording of PROs and is simply an empirical determination. In the view of the PEs, scientifically established thresholds should continue to be accepted as part of benefit assessments, and the scientific evaluation of further thresholds should continue to be carried out independently of AMNOG.
Documentation bureaucracy: There is a need for action with regard to the bureaucracy needed for an HTA process. This primarily relates to the new dossier templates that the G-BA introduced in its resolution of June 20, 2019 and which have been mandatory since April 2020. Their analytical scope has increased sharply compared to the previous dossier templates, exemplified by the requirements for non-predefined subgroup analyses without significant interaction. This has significantly increased the workload for pharmaceutical companies without as yet having any apparent relevance to benefit assessments.
Inclusion of real-world evidence: The inclusion of real-world data in evidence evaluations is still a recent AMNOG innovation that was introduced into benefit assessments in 2019 (Bundesanzeiger 2019). The pharmaceutical industry generally welcomes this innovation but believes that it should be reserved for clearly defined exceptional situations. As an example, an expedient procedure for establishing knowledge-generating registers was set out in a methodology guideline of the German Network Health Services Research (DNVF) (Apfelbacher et al. 2020). Unlike procedural regulations defined by the G-BA resolution of July 16, 2020 (Federal Joint Committee 2020b), it sets out a transparent consensus-finding process. By contrast, all parties agree that registers for a benefit assessment must be prospective and must be set up outside the AMNOG process. In principle, clinical cancer registers are good candidates but are not yet comprehensive enough for the purpose of benefit assessments, so that additional prospective registers will remain necessary for the foreseeable future.
Further development of evidence requirements: In the past decade, the initially very rigid evidence requirements in the AMNOG process have become more flexible in some respects. In order to continue along this path, one point of discussion should be the expansion of patient-relevant endpoints accepted by the G-BA so as to include clinically established, morbidity-related endpoints. For example, metastasis-free survival (MFS) is an endpoint recognized by regulatory authorities in advanced prostate cancer. And endpoints such as the time to subsequent therapy (TTST) and the time to first chemotherapy are gaining increased attention by patients and their caregivers because they are a measure of how long burdensome follow-up interventions can be delayed. In this context, it should be noted that the now accepted endpoint of event/recurrence-free survival (EFS/RFS) in adjuvant therapy still requires clarification, since the type of treatment regarded as curative, thus warranting use of this endpoint, is sometimes called into question.
Benefit assessments for multi-tumor approvals: Clinical research faces an ethical dilemma with regard to tumors triggered by rare mutations. There are often no specific drug treatment options available; at the same time large-scale phase III studies are not possible. However, it is possible to generate evidence from single-arm, multi-tumor phase II safety and efficacy studies in such cases. In the past, the EMA has already granted approval if robust results are available from such “basket studies”. However, in previous benefit assessments of entrectinib (Federal Joint Committee 2021c) and larotrectinib (Federal Joint Committee 2020a), both cancer drugs with multi-tumor (‘tumor-agnostic’) approval, the benefit of those substances—as demonstrated in the regulatory process—did not translate into an additional benefit. In addition to the presentation of multi-tumor analyses of the general population, one approach is to consider the data situation for individual tumor entities, known as lead entities. An indirect comparative assessment is then carried out on the basis of data collected on the lead entities. However, the challenge posed by small populations also applies to this approach, since lead entities represent subpopulations of the often already very small, tumor-agnostic target population.
Benefit assessment of advanced therapy medicinal products (ATMPs): ATMPs are drugs for use in humans that are based on genes, tissues or cells. In principle, the AMNOG process is also a suitable regulatory framework for ATMPs, though it will still need fine-tuning in the coming years. The problems outlined above in connection with the harmonization of the evidence requirements of the EMA and G-BA are particularly challenging here. Registers and indirect comparisons are very important in the case of diseases that are often rare in the ATMP context. Apart from questions concerning the evaluation of evidence, it must also be clarified how single-use therapies should be handled within the AMNOG process and subsequent price negotiations. Appropriate regulations are important to ensure planning security for all parties.
Governance: In conclusion, it should be pointed out that since its implementation the AMNOG process has been beset by a governance problem which stems from the “double role” of the GKV-SV and has not been satisfactorily resolved from the pharmaceutical companies’ point of view. The GKV-SV is a key element in determining additional benefits through the G-BA. At the same time, it acts as the company’s negotiating partner in downstream price negotiations.
Conclusion
Overall, it can be concluded that a constructive, inter-stakeholder communication climate has developed with the AMNOG process that endeavors to take into account the requirement of a certain level of planning security on the part of pharmaceutical companies and has also had a confidence-building effect. From the perspective of cancer care, the introduction of an HTA system in Germany was NOT accompanied by a decline in access to cancer drugs. We should definitely try to maintain this, even if the impending surge of innovations in cancer care and the financial consequences of the pandemic pose parallel challenges.
The current AMNOG structures were developed for the most part at a time when no one saw immuno-oncology or gene and cell therapies coming, when there were no multi-tumor drug approvals and when few imagined that within a few years established tumor entities would be broken down into dozens of sub entities on the basis of molecular genetic markers. Society wants these and other advances, and the HTA process must therefore take this into account in a healthcare system based on solidarity.
However, it would not be fair to pretend that only the HTA bodies need to do their homework. Indeed, medicine and science as a whole are evolving. The real message that should gleaned from this paper is therefore a broader one: a healthcare system that claims to be one of the best in the world and that hopes to remain so must keep a close eye on medical-scientific progress and, if necessary, continuously adapt its structures and evaluation systems accordingly.
Acknowledgements
The authors wish to thank Philipp Grätzel von Grätz, Berlin, for conceiving and editing the manuscript, and Michael Capone for translation into the English language, which was financially sponsored by Section C of the German Cancer Society.
Author contributions
This paper was produced by the author team of the Health Policy and Market Access Working Group of Section C of the German Cancer Society. All the authors contributed equally to the concept, development and realization of the manuscript.
Funding
Amgen GmbH, Bayer Vital GmbH, Janssen-Cilag GmbH, MSD SHARP & DOHME GmbH, Novartis Pharma GmbH and Takeda Pharma Vertrieb GmbH & Co. KG through salaries of the authors and financial support of the Section C of the German Cancer Society, Berlin, Germany.
Data availability
Not applicable—the data presented are from referenced original work.
Declarations
Conflict of interest
AB is an employee of Janssen-Cilag GmbH, Neuss, Germany; KD is an employee of Bayer Vital GmbH, Berlin, Germany; at the time of the concept development and realization, PK was an employee of MSD Sharp & Dohme GmbH (a German subsidiary of Merck & Co., Inc., Rahway, NJ, USA), and is a shareholder in Merck & Co., Inc., Rahway, NJ, USA); CL is an employee of Novartis Pharma GmbH; CL is an employee of Amgen GmbH; MP is a full-time employee of Takeda Pharma Vertrieb GmbH & Co. KG.
Ethical approval
Not applicable—no clinical study was conducted.
Consent to participate
Not applicable—no clinical study was conducted.
Consent to publish
Not applicable—no clinical study was conducted.
Footnotes
This article has primarily been published in German under the title "Zehn Jahre AMNOG-Prozess aus Sicht der Onkologie" in Forum 37, 84–90 (2022). 10.1007/s12312-021-01031-x.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Apfelbacher C et al (2020) Manual for methods and use of routine practice data for knowledge generation. Das Gesundheitswesen. 10.1055/a-1237-4011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Association of Research-Based Pharmaceutical Companies (2021) Press release—Germany is quick. Association of Research-Based Pharmaceutical Companies. https://www.vfa.de/de/presse/pressemitteilungen/pm-009-2021-deutschland-ist-schnell.html. Accessed 10 Aug 2021
- Bundesanzeiger (2010) Act to reorganize the pharmaceuticals’ market in the statutory health insurance. Bundesanzeiger Verlag. http://www.bgbl.de/xaver/bgbl/start.xav?startbk=Bundesanzeiger_BGBl&jumpTo=bgbl110s2262.pdf. Accessed 10 Aug 2021
- Bundesanzeiger (2012) Second act amending pharmaceutical and other regulations. Bundesanzeiger Verlag. http://www.bgbl.de/xaver/bgbl/start.xav?startbk=Bundesanzeiger_BGBl&jumpTo=bgbl112s2192.pdf. Accessed 16 Aug 2021
- Bundesanzeiger (2017) Act to strengthen the supply of medicines in the statutory health insurance. Bundesanzeiger Verlag. http://www.bgbl.de/xaver/bgbl/start.xav?startbk=Bundesanzeiger_BGBl&jumpTo=bgbl117s1050.pdf. Accessed 10 Aug 2021
- Bundesanzeiger (2019) Act for more safety in the supply of pharmaceuticals. Bundesanzeiger Verlag. http://www.bgbl.de/xaver/bgbl/start.xav?startbk=Bundesanzeiger_BGBl&jumpTo=bgbl119s1202.pdf. Accessed 16 Aug 2021
- Dabisch I et al (2014) Patient relevant endpoints in oncology: current issues in the context of early benefit assessment in Germany. Health Econ Rev. 10.1186/2191-1991-4-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federal Joint Committee (2013) Supporting evidence for the decision of the Federal Joint Committee on an amendment to the Pharmaceuticals Guideline (AM-RL): Annex XII—Decisions on the benefit assessment of medicinal products with new active substances according to § 35a SGB V Crizotinib. Federal Joint Committee. https://www.g-ba.de/downloads/40-268-2301/2013-05-02_AM-RL-XII_Crizotinib_TrG.pdf. Accessed 10 Aug 2021
- Federal Joint Committee (2018) Benefit assessment according to § 35a SGB V-Benefit assessment procedure for the active substance Carfilzomib (exceedance of € 50 million threshold: multiple myeloma, at least 1 prior therapy, combination with Dexamethasone or Lenalidomide and Dexamethasone). Federal Joint Committee. https://www.g-ba.de/bewertungsverfahren/nutzenbewertung/308/. Accessed 10 Aug 2021
- Federal Joint Committee (2019a) Benefit assessment according to § 35a SGB V—benefit assessment procedure for the active substance Pembrolizumab (new indication: melanoma, adjuvant therapy). Federal Joint Committee. https://www.g-ba.de/bewertungsverfahren/nutzenbewertung/451/. Accessed 10 Aug 2021
- Federal Joint Committee (2019b) Benefit assessment according to § 35a SGB V—Benefit assessment procedure for the active substance Trametinib (new indication: melanoma, in combination with Dabrafenib, BRAF V600 mutation, adjuvant therapy). Federal Joint Committee. https://www.gba.de/bewertungsverfahren/nutzenbewertung/387/. Accessed 10 Aug 2021
- Federal Joint Committee (2019c) Benefit assessment according to § 35a SGB V - Benefit assessment procedure for the active substance Brentuximab Vedotin (new indication: systemic anaplastic large cell lymphoma; first-line; combination with Cyclophosphamide, Doxorubicin and Prednisone). Federal Joint Committee. https://www.g-ba.de/bewertungsverfahren/nutzenbewertung/557/. Accessed 16 Sep 2021
- Federal Joint Committee (2019d) Supporting evidence for the decision of the Federal Joint Committee on an amendment to the German Medicines Directive (AM-RL): Annex XII—Benefit assessment of medicinal products with new active substances according to Section 35a SGB V Pembrolizumab (new indication: non-small cell lung cancer, non-plate epithelial, first-line, combination with pemetrexed and platinum chemotherapy). Federal Joint Committee. https://www.g-ba.de/downloads/40-268-6021/201.9-09-19_AM-RL-XII_Pembrolizumab_D-447_TrG.pdf. Accessed 10 Aug 2021
- Federal Joint Committee (2020a) Benefit assessment according to § 35a SGB V—Benefit assessment procedure for the active substance Larotrectinib (solid tumors, neurotrophic tyrosine receptor kinase (NTRK) gene fusion, histology-independent). Federal Joint Committee. https://www.g-ba.de/bewertungsverfahren/nutzenbewertung/502/. Accessed 17 Aug 2021
- Federal Joint Committee (2020b) Resolution—rules of procedure: procedure for requesting a register study. Federal Joint Committee. https://www.g-ba.de/beschluesse/4402/. Accessed 10 Aug 2021
- Federal Joint Committee (2021a) Additional benefit of new drugs – Categories. Federal Joint Committee. https://www.g-ba.de/themen/arzneimittel/arzneimittel-richtlinie-anlagen/nutzenbewertung-35a/zusatznutzen/. Accessed 16 Aug 2021a
- Federal Joint Committee (2021b) Annual Report 2020. Federal Joint Committee. https://www.g-ba.de/downloads/17-98-5148/202.1-07-01_G-BA_Geschaeftsbericht_2020_bf.pdf. Accessed 10 Aug 2021b
- Federal Joint Committee (2021c) Benefit assessment according to Section 35a SGB V - Benefit assessment procedure for the active substance Entrectinib (solid tumors, neurotrophic tyrosine receptor kinase (NTRK) gene fusion, histology-independent). Federal Joint Committee. https://www.g-ba.de/bewertungsverfahren/nutzenbewertung/588/#beschluesse-mobile. Accessed 17 Aug 2021c
- Federal Joint Committee (2021d) Decision of benefit assessment of August 20 2020—from minute 47.20. Federal Joint Committee. https://www.g-ba.de/service/livestream-mediathek/. Accessed 16 Sep 2021d
- Federal Joint Committee (2021e) Resolution—Measures regarding quality assurance according to § 136a paragraph 5 SGB V: CAR-T cells in B cell neoplasms and on the amendment of Annex XII. Federal Joint Committee. https://www.g-ba.de/beschluesse/4477/. Accessed 16 Sep 2021e
- Institute for Quality and Efficiency in Health Care (2020) General methods—version 6.0 of 5 November 2020. Institute for Quality and Efficiency in Health Care. https://www.iqwig.de/methoden/general-methods_version-6-0.pdf. Accessed 10 Aug 2021
- Trümper L, Wörmann B (2021) Early benefit assessment of new drugs in Germany 2011–2020—pricing and more. German Society for Haematology and Medical Oncology (DGHO) and Association of the Scientific Medical Societies in Germany (AWMF). https://www.dgho.de/publikationen/schriftenreihen/fruehe-nutzenbewertung/awmf_amnog_2021_210x297_ok_ansicht_es.pdf. Accessed 16 Aug 2021
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable—the data presented are from referenced original work.