Abstract
Traditional and phytochemical studies have confirmed the richness and diversity of medicinal plants such as Nepeta cataria (N. cataria), but more studies are needed to complete its metabolite profiling. The objective of this research was to enhance the metabolomic picture and bioactivity of N. cataria for better evaluation. Phytochemical analysis was performed by bio-guided protocols and gas chromatography-mass spectrometry (GC/MS). For this, solvents such as methanol, ethanol, water, acetone, and hexane were used to extract a wide number of chemicals. Antibacterial analysis was performed using the 96-well plate test, Kirby Bauer's disk diffusion method, and the resazurin microdilution test. Antioxidant activity was determined by the DPPH assay and radical scavenging capacity was evaluated by the oxygen radical absorbance capacity (ORAC) assay. GC/MS analysis revealed a total of 247 identified and 127 novel metabolites from all extracts of N. cataria. Water and acetone extracts had the highest identified metabolites (n = 79), whereas methanol extract was the highest in unidentified metabolites (n = 48). The most abundant phytochemicals in methanol extract were 1-isopropylcyclohex-1-ene (concentration = 27.376) and bicyclo [2.2.1] heptan-2-one (concentration = 20.437), whereas in ethanol extract, it was 9,12,15-octadecatrienoic acid (concentration = 27.308) and 1-isopropylcyclohex-1-ene (concentration = 25.854). An abundance of 2 methyl indoles, conhydrin, and coumarin was found in water extracts; a good concentration of eucalyptol was found in acetone extract; and 7,9-di-tert-butyl-1-oxaspiro is the most abundant phytochemicals in hexane extracts. The highest concentration of flavonoids and phenols were identified in hexane and methanol extracts, respectively. The highest antioxidant potential (DPPH assay) was observed in acetone extract. The ethanolic extract exhibited a two-fold higher ORAC than the methanol extract. This examination demonstrated the inhibitory effect against a set of microbes and the presence of polar and non-polar constituents of N. cataria. The results of this study provide a safe resource for the development of food, agriculture, pharmaceutical, and other industrial products upon further research validation.
Keywords: Nepeta cataria, gas chromatograph/mass spectrometry (GC/MS), antibacterial susceptibility testing (AST), antioxidants, phytochemicals
Introduction
The Nepeta genus belongs to the family Lamiaceae, which is rich in bioactive secondary metabolites. The word Cataria was derived from the Latin word for cat, “Cathus.” N. cataria is a perennial herb that grows to a height of 50–100 cm (Scott, 2003). It has been found predominately in the regions of southern and eastern Europe, the Middle East, Central Asia, and China. Bioactive compounds of N. cataria have prehistorically been used and have a wide range of biological activities, including analgesic, anti-asthmatic, anti-cancer, anti-inflammatory, and antimicrobial properties. Nepeta cataria essential oil and metabolites have important applications in the pharmaceutical, agrochemical, and food industries (Sharma et al., 2021). Researchers found them to be antifungal, antibacterial (Bandh and Kamili, 2011; Sharma et al., 2019), antioxidant (Adiguzel et al., 2009), insecticidal, anti-inflammatory, anti-nociceptive, and potentially spasmolytic (Pargaien et al., 2020; Giarratana et al., 2017). Essential oils, flavonoids, phenolic acid, steroids, terpenoids, and terpenoid hydrocarbons have all been found in this plant.
Nepeta cataria has widely been used to treat diarrhea because of spasmolytic and myorelaxant metabolites in its extracts (Gilani et al., 2009). Essential oils of N. cataria have a promising impact on raw materials of industrial food importance (Frolova et al., 2020). Studies established the presence of nepetalactones in catnip essential oil by TLC and GC–MS analysis. Using GC/MS analysis, three populations of N. crassifolia and four populations of N. nuda were studied (Sharma et al., 2021).
Essential oils and flavonoids have typically been linked to the therapeutic benefits of Nepeta species. Prior investigations on the essential oils of N. cataria identified nepetalactone as a major constituent (Mamadalieva et al., 2017; Sharma et al., 2019). In a recent study, water-based extracts of N. cataria significantly inhibited herpes virus replication in humans (Hinkov et al., 2020). Previously, N. cataria has been used to alleviate symptoms of bronchial asthma, bronchitis, and bronchial congestion. The traditional herbal medicine derived from these along with other medicinal plants may have multiple applications, including symptom relief for people with COVID-19 and the development of effective antiviral medicines. During the severe acute respiratory syndrome coronavirus (SARS-CoV-2) pandemic, also termed COVID-19, leaves of N. cataria were used to alleviate symptoms of the disease (Khan et al., 2021). Essential oils from Nepeta species that naturally produce nepetalactones can be synthesized in other regions and then be distilled to serve as a natural source of efficient Aedes aegypti repellent for effective dengue prevention (Reichert et al., 2019). Previous studies demonstrate that N. cataria essential oils effectively reduced liver damage caused by acetaminophen and enhanced mRNA expression of uridine diphosphate glucuronosyltransferases (UGTs) and sulfotransferases (SULTs) and decreased CYP2E1 activity (Tan et al., 2019). It has been shown that N. cataria and its derivatives have been used to treat gastrointestinal and respiratory disorders. They have also been reported for their effective antibacterial, antiviral, and antioxidant activities (Sharma et al., 2019). Porcine reproductive and respiratory syndrome virus (PPSRV) affects pigs and causes reproductive failure in developing pigs. According to the findings of a study, the load of PRRSV could be greatly reduced by using N. cataria hydrosol. It is strongly recommended that further research be conducted into the antiviral processes and characteristics of these plant hydrosols, both in vitro and in vivo (Kaewprom et al., 2017).
Recent research has been focused on the essential oils and antibacterial properties of plants, as they have been utilized to increase the shelf life of foods and in traditional medicine (Ergün, 2021; Özkan et al., 2021). Numerous studies demonstrate that the antibacterial and antifungal properties of N. cataria are mostly attributable to the essential oil constituents. Surprisingly, less is known about the antimicrobial activity of catnip essential oil. In these investigations, the antimicrobial activity of catnip essential oil was investigated on a limited number of bacteria or fungi (Angelini et al., 2006; Suschke et al., 2007; Bourrel et al., 2011).
In the past two decades, the antioxidant effect of the essential oils and/or extracts of medicinal and aromatic plants has received considerable attention. Therefore, these extracts can be employed as safe and effective synthetic preservative replacements. Natural antioxidants have been investigated extensively for their ability to protect organisms and cells against oxidative stress-induced damage, which is believed to be a cause of aging, degenerative illnesses, and cancer (Sharma et al., 2019). It has been known for some time that plant extracts and/or essential oils possess antioxidant properties. However, less is known about the antioxidant activity of the essential oil or extract of N. cataria.
In another study, aromatic and medicinal plants from Turkey have been characterized and reported on the antibacterial and antioxidant activities of N. cataria's essential oil, methanol extract, and its essential oil composition. They also highlighted essential oil to contain 4aβ, 7α, 7aβ-nepetalactone, 4aα, 7α, 7aβ-nepetalactone, 1,8-cineole, and elemol as major oil constituents in N. crassifolia (Dabiri and Sefidkon, 2003), while 7aβ-nepetalactone, 4aα, 7α, 7aβ-nepetalactone, pulegone, and piperitenone oxide were identified in N. nuda (Narimani et al., 2017). Research studies focused mainly on essential oil extracts of N. cataria, which indicated a need to study its metabolites in polar and nonpolar solvents. Our team was motivated to explore the constituents of N. cataria, based on polarity, via minor adjustments to already established lab protocols.
Materials and methods (experimental)
Plant collection
Nepeta cataria was collected from Swat (Himalayas), Khyber Pakhtunkhwa, Pakistan (35°22′59.99″ N, 72°10′60.00″E), locally named as catnip mint/catmint (in northern Pakistan) and Badranj boya (in central Pakistan). Species verification and identification were done at the National Herbarium, and they confirmed and identified it as N. cataria. Furthermore, it was cleaned, rinsed, dried, and preserved at the Antimicrobial Biological Laboratory (AMBL), International Islamic University Islamabad, Islamabad, Pakistan.
Plant extraction and filtration
Nepeta cataria's stem and leaves were rinsed, dried, and grounded in a fine powder by a lab grinder carefully. Fine powder was soaked separately in methanol, ethanol, water, acetone, and hexane using 1:10 ratio for 24–48 h at room temperature, to increase the maximum solubility. Filtrations and extraction were done using Whatman's # 41 and rota-evaporator at Stockbridge Medicinal and Aromatic Lab, University of Massachusetts Amherst, USA. Extracts were labeled and aliquoted in glass vials at 4°C until further use.
Phytochemical analysis
Qualitative analysis
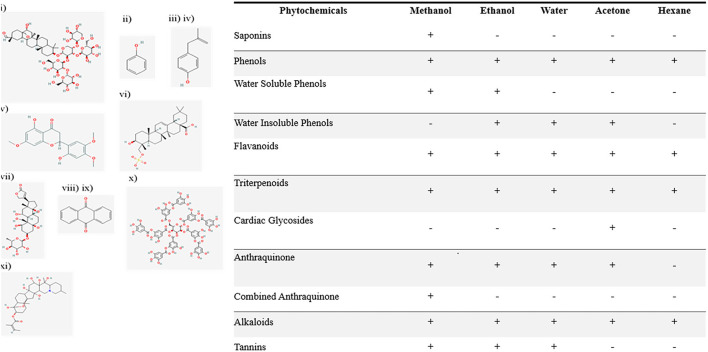
Saponins and phenolic compounds, water-soluble and insoluble phenols, alkaloid flavonoids, poly-steroids, terpenoids, cardiac glycosides, free and combined anthraquinones, tannins, and alkaloids were chemically identified in all plant extracts (Prabhavathi et al., 2016).
Quantitative analysis—Phenols and flavonoids
Concentrations of phenols and flavonoids were identified in all extracts of N. cataria via established protocols previously explained in Nadeem et al. (2021).
GC/MS analysis of N. cataria extracts
The GC/MS is the widely adopted technique for the detection of biologically active compounds, i.e., metabolites. A set of extracts, methanol, ethanol, water, acetone, and hexane were subjected to GC/MS analysis to detect bioactive phytochemicals. Phytochemical compounds were identified and presented with their compound names, molecular formulas, molecular weight, and retention times (RT) using NIST Library 17.
Metabolic profiling of N. cataria extracts was conducted via GC/MS (Bruker Scion 456 GC, EVOQ triple quadrupole GC-MS/MS). A column of 15 m was used with a diameter and film thickness of 0.25 mm. The flow rate of helium as a carrier gas was 1.5 ml/min. For gas chromatography, temperature conditions were 45°C for 3 min, 250°C at 8°C/min for 10 min. Injection volume was 1 μl [varying split ratio (5:1/15:1/20:1), range (45–350 m/z)]. Automated Mass Spectral Deconvolution and Identification System (AMDIS) Software MSWS 8 for GC/MS and NIST library were used for compilation of all results.
Antibacterial activity
Bacterial cultures (Table 1) were grown on a tryptic soy broth (TSB) medium (Thermo Fisher Scientific, USA) (Nadeem et al., 2021). To evaluate antibacterial susceptibility testing (AST) of N. cataria extracts, three different methods were used, i.e., 96-well test, Kirby-Bauer disk diffusion, and resazurin-based well plate microdilution method.
Table 1.
Microbial profile of bacterial ingredients used in the antimicrobial analysis.
| Microorganism | Accession number | Strain |
|---|---|---|
| Escherichia coli | ATCC_25922 | Gram negative |
| Klebsiella oxytoca | ATCC_43863 | |
| Salmonella enterica | ATCC_14028 | |
| Shigella sonnei | ATCC_25931 | |
| Citrobacter ferundii | ATCC_8090 | |
| Bacillus subtilis | ATCC_6051 | Gram positive |
| Lactococcus lactis | ATCC_LMO230 | |
| Listeria monocytogenes | ATCC_LM21 | |
| Micrococcus luteus | ATCC_4698 | |
| staphylococcus aureus | ATCC_25923 |
The 96-well plate method
In each well of a 96-well microtiter plate, 100 μl of plant extract and TSB media were used. Each plant extract was checked at five bacterial concentrations (i.e., 1,000, 500, 250, 125, and 62.5 μg) for optimum antimicrobial potential. Only TSB medium was added to negative control well to ensure sterility of media. A single negative control lacked plant extract to observe normal bacterial growth. Microtiter plates were incubated for 24 h before reading at 570 nm. Chloramphenicol as standard was used to evaluate the results. Bacterial inhibition was calculated via the following formula:
Kirby-Bauer disk diffusion method
Solidified agar plates were used to analyze the antimicrobial potential of N. cataria extracts. Paper disks of 10 mm were soaked in 20 μl extracts, then placed on prepared culture plates and incubated for 24 h at a 25–35°C temperature. Paper disks (10 mm) were soaked in 20 μl of distilled water as a negative control to avoid any influence on bacterial growth (Sarin and Bafna, 2012). Aseptic conditions were maintained via working in a laminar flow. All extracts were tested in biological triplicates, and results were represented as average values of inhibition zones in mm ± standard deviation.
Resazurin-based well plate microdilution method
Resazurin solution was prepared (121.5 mg resazurin powder in 18 ml of ddH2O) and mixed for 1 h (pH = 7.4). TSB liquid medium and N. cataria extracts (100 μl each) were added to each well. Plant extract was added in serial dilution to separate wells. Each well was supplied with 106 CFU/ml of bacterial inoculum. Double negative control well was supplied with TSB media only. Single negative control well was supplied with TSB media and bacterial culture. Plates were incubated overnight and then 20 μl of resazurin was added to each well and incubated for another 4 h. Absorbance at 550–590 nm was read via spectrophotometer (SPECTRA MAX M2e plate reader) (Packialakshmi and Naziya, 2014).
DPPH antioxidant assay
The Bersuder (Edewor and Usman, 2011) method was used for antioxidant determination via DPPH radical scavenging assay. All solvent extracts were mixed with DMSO addition and DPPH-ethanol reagent was made separately. Plant-DMSO mix was saturated with DPPH-ethanol reagent for 6 h. Negative control was prepared by dissolving ascorbic acid in DMSO (50–500 μmol/L), which was used to generate calibration curve with 517 nm absorbance read via SPECTRA MAX M2e plate reader (Packialakshmi and Naziya, 2014).
Oxygen radical absorbance capacity assay protocol
Various dilutions of methanolic and extracted samples were mixed with buffered saline (10 mM, pH 7.6). Decaying of fluorescein induced by AAPH was compared to Trolox (positive control) over 120 min to evaluate the antioxidant activity via the SPECTRAMAX M2e Plate reader. Results were presented as μM Trolox Equivalent/100 μl of plant extract.
Statistical analysis
The results of all the experiments were analyzed under a complete randomized design (CRD) with three replications for each treatment. Results were statistically analyzed using GraphPad Prism and Microsoft Office Excel 2016 version. Means were calculated, and one-way analysis of variance (ANOVA) test was performed for multiple comparisons of all the mean values. Mean differences were calculated by least significant difference (LSD) at 0.05 probability.
Results
Nepeta cataria contains medicinally important phytochemicals along with many unknown metabolites that need further studies (Elshikh et al., 2016; Mir et al., 2016). High antioxidant activity was exhibited in acetone extract of N. cataria. Moreover, high flavonoid content was found in water and hexane extracts, and methanol extracts were specifically rich in phenols.
Preliminary phytochemical analysis
Qualitative phytochemical analysis of N. cataria
Saponins were found in the methanol-based extracts of N. cataria. Phenols were positive in all extracts and showed high μg/ml concentration in methanol. Water-soluble phenols were present in all the polar solvents only. Water insoluble phenols were identified in the ethanol, acetone, and hexane-based extracts. A qualitative test for flavonoids was carried out, and the development of intense yellow color indicates presence of flavonoids (Figure 1). A qualitative test for terpenoids was conducted by observing a reddish-brown coloration development, which confirms the positive test results in all extracts. Cardiac glycosides were indicated via development of green-blue color. Acetone-based extracts were positive only. Free anthraquinones were present in all extracts of N. cataria except hexane-based extract. Combined anthraquinones were only present in methanol-based extract of N. cataria. Qualitative tests for tannins were found positive only in extraction of polar solvents. Alkaloids were present in all the extracts of N. cataria.
Figure 1.
Qualitative analysis of phytochemicals in polar and non-polar extracts of Nepeta cataria. List of phytochemicals from (i) to (xi) were identified in various polar and non-polar extracts. The 2-D structure of phytochemicals are supported via PubChem.
DPPH antioxidant activity
presence of antioxidants was determined in N. cataria extracts in a set of different extractions and was measured spectrophotometrically, results were drawn as μmol of ascorbic acid equivalents/L, and the results are given in Figure 2A. The presence of antioxidants was found in the following order: acetone extracts > water extracts> ethanol extracts > methanol extracts > hexane extracts.
Figure 2.
Quantitative analysis of phytochemicals (A) DPPH mediated antioxidant activity, (B) flavonoids concentration, (C) phenols concentration, (D) oxygen radical absorbance capacity values.
Total flavonoid and phenol content
The flavonoids in polar and non-polar extracts of N. cataria were quantified in terms of μg of catechin equivalents/ml. Hexane and water-based extracts showed high levels of flavonoids as compared to acetone, methanol, and ethanol-based extracts. Flavonoid results are summarized in Figure 2B. Several other studies prove the presence of flavonoids in N. cataria extract and indicate therapeutic potential for lung cancer because of its flavonoid content (Naguib et al., 2012; Yang et al., 2020).
The methanol, ethanol, water, acetone, and hexane extracts of N. cataria were examined in terms of μg of gallic acid equivalents per ml to quantify levels of total phenols. Methanol, acetone, and ethanol-based extracts showed the maximum presence of phenols as compared to water and hexane-based extracts. The order of phenolics (Figure 2C) presence in the sample was found as follows:
ORAC assay on N. cataria extracts
Oxygen radical absorbance capacity was performed to study the antiradical activity in methanol and ethanol extract of N. cataria. Results showed two-fold higher ORAC in ethanolic extracts than methanol extract (Figure 2D), signifying our results of DPPH, free radical scavenging activity (Lucas-Abellán et al., 2008).
Determination of antibacterial activity
Percentage growth inhibition by 96-well method
Percentage growth inhibition of each tested bacteria, viz., Shigella sonnei, Bacillus subtilis, Klebsiella oxytoca, Escherichia coli, Salmonella enterica, Micrococcus luteus, and Staphylococcus aureus (S. Lactococcus lactis, Listeria monocytogenes, and Citrobacter freundii). Percentage growth inhibition of bacterial isolates is given in Figure 3.
Figure 3.
Percentage growth inhibition of bacterial strains by Nepeta cataria plant extracts in different solvents at different dose levels (96 well method).
Kirby-Bauer disk diffusion method
Kirby disk diffusion method was followed to measure the antimicrobial efficacy of plant extracts by the zone of inhibition (mm) in vitro conditions on solidifying agar media. Chloramphenicol was used as a standard and zone of inhibition was >25 mm for all strains according to CLSI guidelines (Humphries et al., 2018).
Resazurin-based well plate microdilution method
The resazurin method was used to check the antimicrobial efficacy of each prepared plant extract against tested bacterial agents. Chloramphenicol was used as a positive control at 6.25–100 μl/ml dose levels, and data on percentage bacterial growth inhibition was recorded. Plant extract of N. cataria showed a varied efficacy against all the tested bacterial isolates compared to the positive and negative control, and results are presented in Figure 4.
Figure 4.
(A,B) Percentage inhibition of bacterial growth determined in comparison with the growth inhibition by chloramphenicol (resazurin method).
GC/MS analysis of N. cataria
The GC/MS analysis of a methanolic extract of N. cataria showed (68 identified phytochemicals + 48 unmatched) chemicals (Table 2). Analysis of ethanol-based extracts confirmed the existence of 79 known phytochemical constituents, while 31 unmatched chemicals were detected (Table 3). Water-based extracts of N. cataria contain 28 known phytochemicals, while 11 unmatched chemicals were also detected (Table 4). Acetone-based extract confirmed the existence of 13 known compounds' extract, while 9 chemical constituents were unmatched (Table 5). Analysis of hexane-based extracts confirmed the presence of 9 known chemical constituents, while 8 unmatched chemicals were detected, as given in Table 6. GC/MS spectral chromatograms of all the solvent-based extracts are given in Figure 5 along with the most abundant metabolite in each extract. In methanol, water, and acetone extract, 1-isopropylcyclohex-1-ene was the most abundant phytochemical. The most abundant metabolite in ethanol extract is 9,12,15-octadecatrienoic acid, and the most abundant phytochemical in hexane extract is 7,9-di-tert-butyl-1-oxaspiro (Figure 5).
Table 2.
GC/MS analysis of a methanol extract of N. cataria using NIST 17 Library showed (68 identified phytochemicals + 48 unmatched) chemicals, arranged according to concentration present.
| Compound | Mol. formula | Amount/Conc.% | Mol. weight (g/mol) |
RT (Min) |
Extract |
|---|---|---|---|---|---|
| 1-Isopropylcyclohex-1-ene | C9H16 | 27.376 | 124.22 | 12.402 | Methanol |
| Bicyclo [2.2.1] heptan-2-one, | C7H10O | 20.437 | 110.15 | 7.728 | Methanol |
| gamma. -Sitosterol | C29H50O | 8.626 | 414.7 | 33.566 | Methanol |
| Eucalyptol | C10H18O | 8.505 | 154.249 | 5.112 | Methanol |
| n-Hexadecanoic acid | C16H32O2 | 7.973 | 256.4241 | 20.364 | Methanol |
| No match | – | 6.419 | – | 6.933 | Methanol |
| 9,12,15-Octadecatrienoic acid | C18H30O2 | 6.401 | 278.43 | 22.304 | Methanol |
| 1-Isopropylcyclohex-1-ene | C9H16 | 6.144 | 124.22 | 13.699 | Methanol |
| 1,6-Octadien-3-ol, 3,7-dimet | C10H18O | 5.855 | 154.25 | 9.981 | Methanol |
| Ethyl 2-5-methyl-5-vinyltet | C13H22O4 | 5.845 | 242.3114 | 6.551 | Methanol |
| Beta-Sitosterol | C29H50O | 5.461 | 414.71 | 32.541 | Methanol |
| No match | – | 4.148 | – | 13.303 | Methanol |
| No match | – | 3.893 | – | 22.205 | Methanol |
| Pentane, 1-chloro-5- methyl | C5H11Cl | 3.739 | 106.594 | 10.696 | Methanol |
| No match | – | 3.063 | – | 12.903 | Methanol |
| No match | – | 3.008 | – | 13.718 | Methanol |
| Bicyclo [3.1.0] hexane-2-undec | C6H10 | 2.974 | 82.14 | 13.804 | Methanol |
| No match | – | 2.786 | – | 26.376 | Methanol |
| Alpha-Amyrin | C30H50O | 2.691 | 426.729 | 33.062 | Methanol |
| Pregnan-18-ol, 20-methyl-20- | C22H39NO | 2.64 | 333.6 | 13.916 | Methanol |
| No match | – | 2.619 | – | 11.726 | Methanol |
| No match | – | 2.43 | – | 14.296 | Methanol |
| No match | – | 2.074 | – | 21.141 | Methanol |
| No match | – | 2.021 | – | 14.919 | Methanol |
| No match | – | 1.975 | – | 25.235 | Methanol |
| Caryophyllene oxide | C15H24O | 1.916 | 220.35 | 15.129 | Methanol |
| No match | – | 1.807 | – | 11.016 | Methanol |
| No match | – | 1.659 | – | 34.964 | Methanol |
| No match | – | 1.498 | – | 16.925 | Methanol |
| No match | – | 1.447 | – | 14.094 | Methanol |
| No match | – | 1.436 | – | 27.233 | Methanol |
| No match | – | 1.43 | – | 35.912 | Methanol |
| 2H-1-Benzopyran-2-one, 7-met | C13H15NO2 | 1.381 | 217.26 | 18.071 | Methanol |
| Uvaol | C30H50O2 | 1.365 | 442.7 | 36.319 | Methanol |
| No match | – | 1.326 | – | 35.143 | Methanol |
| Trans-Z-alpha-Bisabolene | C15H24 | 1.312 | 204.35 | 16.216 | Methanol |
| Ursolic aldehyde | C30H48O2 | 1.302 | 440.7 | 34.718 | Methanol |
| No match | – | 1.279 | – | 7.678 | Methanol |
| No match | – | 1.245 | – | 17.965 | Methanol |
| Methyl 8,11,14-heptadecatrie | C21H36O2 | 1.22 | 320.5093 | 22.864 | Methanol |
| No match | – | 1.179 | – | 12.826 | Methanol |
| Phytol | C20H40O | 1.179 | 128.1705 | 21.998 | Methanol |
| No match | – | 1.148 | – | 13.285 | Methanol |
| No match | – | 1.08 | – | 13.897 | Methanol |
| No match | – | 1.013 | – | 26.209 | Methanol |
| No match | – | 0.997 | – | 35.231 | Methanol |
| Octadecanoic acid | C18H36O2 | 0.97 | 284.48 | 22.623 | Methanol |
| Hexadecanoic acid, methyl es | C17H34O2 | 0.954 | 270.5 | 19.887 | Methanol |
| No match | – | 0.937 | – | 12.007 | Methanol |
| Methyl 8,11,14-heptadecatrie | C21H36O2 | 0.92 | 320.5093 | 21.853 | Methanol |
| Betulin | C30H50O2 | 0.91 | 442.72 | 35.472 | Methanol |
| 1,1,4a-Trimethyl-5,6-dimethyl | C15H24 | 0.891 | 204.35 | 33.896 | Methanol |
| Coumarin | C9H6O2 | 0.878 | 146.1427 | 13.867 | Methanol |
| No match | – | 0.875 | – | 12.736 | Methanol |
| 2H-1-Benzopyran-2-one, 7-met | C13H15NO2 | 0.826 | 217.26 | 17.04 | Methanol |
| 1-Chlorosulfonyl-3-methyl-1- | C9H14ClNO3S | 0.823 | 251.73 | 16.173 | Methanol |
| Beta-Amyrin | C30H50O | 0.763 | 426.729 | 33.739 | Methanol |
| Methyl 2-hydroxy-octadeca-9, | C19H32O3 | 0.754 | 308.5 | 28.775 | Methanol |
| Hexadecanoic acid, 2-hydroxy | C16H32O3 | 0.744 | 272.42 | 26.101 | Methanol |
| No match | – | 0.717 | – | 13.206 | Methanol |
| (1R,7S, E)-7-Isopropyl-4,10-d | C15H24O | 0.702 | 220.3505 | 17.243 | Methanol |
| No match | – | 0.688 | – | 35.27 | Methanol |
| Campesterol | C28H48O | 0.657 | 400.68 | 32.877 | Methanol |
| Urs-12-en-28-al | C30H48O | 0.654 | 424.7 | 35.305 | Methanol |
| 2-Butyl-5-methyl-3-2-methyl | C15H26O | 0.645 | 222.37 | 14.281 | Methanol |
| Caryophylla-4(12),8(13)-dien | C15H24O | 0.632 | 220.3505 | 16.429 | Methanol |
| endo-Borneol | C10H18O | 0.623 | 154.25 | 8.246 | Methanol |
| 1-Methyl-2-methylenecyclohex | C8H14 | 0.622 | 110.197 | 14.461 | Methanol |
| No match | – | 0.616 | – | 27.717 | Methanol |
| Caryophylla-4(12),8(13)-dien | C15H24O | 0.603 | 220.3505 | 17.45 | Methanol |
| Stigmasterol | C29H48O | 0.595 | 412.69 | 33.091 | Methanol |
| No match | – | 0.585 | – | 14.134 | Methanol |
| No match | – | 0.579 | – | 13.446 | Methanol |
| Tritetracontane | C43H88 | 0.574 | 605.2 | 27.798 | Methanol |
| No match | – | 0.566 | – | 15.531 | Methanol |
| (3S,3aS,6R,7R,9aS)-1,1,7-Tri | C15H24 | 0.562 | 204.3511 | 19.087 | Methanol |
| Megastigmatrienone | C13H18O | 0.56 | 190.28 | 16.78 | Methanol |
| No match | – | 0.553 | – | 12.88 | Methanol |
| No match | – | 0.549 | – | 11.886 | Methanol |
| Urs-12-en-28-oic acid, 3-hyd | C30H48O3 | 0.546 | 456.7 | 35.636 | Methanol |
| No match | – | 0.545 | – | 22.421 | Methanol |
| No match | – | 0.543 | – | 12.559 | Methanol |
| 3,5-Dimethylcyclohex-1-ene-4 | C8H14 | 0.542 | 110.2 | 14.226 | Methanol |
| Eicosanoic acid | C20H40O2 | 0.515 | 312.5304 | 25.775 | Methanol |
| No match | – | 0.486 | – | 13.019 | Methanol |
| Olean-12-en-3-ol, acetate, | C32H52O2 | 0.486 | 468.8 | 32.724 | Methanol |
| Alpha-Tocospiro A | C29H50O4 | 0.484 | 462.7 | 30.208 | Methanol |
| Cyclohexene,1-propyl- | C9H16 | 0.483 | 124.22 | 11.611 | Methanol |
| Alpha-Tocospiro B | C29H50O4 | 0.463 | 462.7049 | 30.023 | Methanol |
| No match | – | 0.447 | – | 11.436 | Methanol |
| No match | – | 0.447 | – | 12.434 | Methanol |
| Phenol, 2,4-bis 1-methyl-1-p | C24H26O | 0.445 | 330.5 | 26.725 | Methanol |
| 11,11-Dimethyl-4,8-dimethyl | C15H24O | 0.429 | 220.35 | 16.954 | Methanol |
| No match | – | 0.419 | – | 26.522 | Methanol |
| Tricyclo [20.8.0.07,16] tria | C30H52O2 | 0.413 | 444.7 | 18.261 | Methanol |
| No match | – | 0.394 | – | 17.818 | Methanol |
| 1,5,7-Octatrien-3-ol, 3,7-di | C10H16O | 0.39 | 152.2334 | 8.782 | Methanol |
| 2-Pentadecanone, 6,10,14-tri | C18H36O | 0.386 | 268.4778 | 18.826 | Methanol |
| 11,14-Octadecadienoic acid | C18H32O2 | 0.364 | 280.4 | 22.811 | Methanol |
| No match | – | 0.363 | – | 15.481 | Methanol |
| Caryophylla-4(12),8(13)-dien | C15H24O | 0.358 | 220.3505 | 15.937 | Methanol |
| No match | – | 0.356 | – | 34.665 | Methanol |
| 5-Cholestene-3-ol, 24-methyl | C28H48O | 0.344 | 400.7 | 31.863 | Methanol |
| No match | – | 0.325 | – | 14.381 | Methanol |
| No match | – | 0.323 | – | 11.703 | Methanol |
| No match | – | 0.322 | – | 21.365 | Methanol |
| Neophytadiene | C20H38 | 0.313 | 278.5 | 18.782 | Methanol |
| No match | – | 0.304 | – | 17.223 | Methanol |
| 2-Furanmethanol, 5-ethenylte | C10H18O2 | 0.287 | 170.2487 | 6.078 | Methanol |
| 9,12-Hexadecadienoic acid, m | C16H28O2 | 0.273 | 252.39 | 21.796 | Methanol |
| Beta-Guaiene | C15H24 | 0.271 | 204.351 | 32.882 | Methanol |
| 6-Hydroxy-4,4,7a-trimethyl-5 | C11H16O3 | 0.258 | 196.24 | 17.648 | Methanol |
| Bicyclo [2.2.1] heptane, 7,7-d | C9H16 | 0.24 | 124.22 | 9.955 | Methanol |
| 2-Cyclohexen-1-one, 3-methyl | C7H10O | 0.23 | 110.15 | 11.529 | Methanol |
| Hentriacontane | C31H64 | 0.188 | 436.85 | 28.969 | Methanol |
| Methyl octadec-6,9-dien-12-y | C18H32O2 | 0.149 | 280.4 | 15.763 | Methanol |
Table 3.
GC/MS analysis of ethanol extract of N. cataria using NIST 17 Library showed (79 identified phytochemicals + 31 unmatched) chemicals, arranged according to concentration present.
| Compound | Mol. formula | Amount/Conc.% |
Mol. weight (g/mol) |
RT (Min) |
Extract |
|---|---|---|---|---|---|
| No match | – | 57.084 | – | 2.058 | Ethanol |
| No match | – | 42.916 | – | 2.039 | Ethanol |
| 9,12,15-Octadecatrienoic acid | C18H30O2 | 27.308 | 278.43 | 17.266 | Ethanol |
| 1-Isopropylcyclohex-1-ene | C9H16 | 25.854 | 124.22 | 11.456 | Ethanol |
| 1-Isopropylcyclohex-1-ene | C9H16 | 14.94 | 124.22 | 9.585 | Ethanol |
| 1-Isopropylcyclohex-1-ene | C9H16 | 13.741 | 124.22 | 9.33 | Ethanol |
| Beta-Sitosterol | C29H50O | 13.312 | 414.71 | 24.939 | Ethanol |
| n-Hexadecanoic acid | C16H32O2 | 10.3 | 256.424 | 19.386 | Ethanol |
| Alpha-Amyrin | C30H50O | 6.667 | 426.729 | 25.504 | Ethanol |
| No match | – | 4.606 | – | 16.278 | Ethanol |
| Urs-12-en-28-ol | C30H50O | 4.295 | 426.7 | 23.833 | Ethanol |
| Methyl 13,14-octadecadienoate | C19H34O2 | 3.793 | 294.472 | 13.689 | Ethanol |
| Octadecanoic acid | C18H36O2 | 3.62 | 284.48 | 17.464 | Ethanol |
| Hexadecanoic acid, ethyl est | C18H36O2 | 3.361 | 284.477 | 15.865 | Ethanol |
| Ethyl 9,12,15-octadecatrieno | C20H34O2 | 3.315 | 306.5 | 21.626 | Ethanol |
| Phytol | C20H40O | 3.068 | 128.1705 | 16.907 | Ethanol |
| Coumarin | C9H6O2 | 2.94 | 146.1427 | 9.646 | Ethanol |
| 1-Chlorosulfonyl-3-methyl-1- | C9H14ClNO3S | 2.175 | 251.73 | 15.242 | Ethanol |
| Ursolic aldehyde | C30H48O2 | 2.109 | 440.7 | 33.113 | Ethanol |
| Ethyl 9.cis., 11.trans.-octad | C20H38O2 | 2.045 | 310.515 | 17.352 | Ethanol |
| No match | – | 1.95 | – | 11.165 | Ethanol |
| No match | – | 1.756 | – | 15.716 | Ethanol |
| No match | – | 1.66 | – | 9.685 | Ethanol |
| 4,4,8-Trimethyltricyclo [6.3]. | C15H26O2 | 1.458 | 238.366 | 18.101 | Ethanol |
| No match | – | 1.456 | – | 15.943 | Ethanol |
| 2H-1-Benzopyran-2-one, 7-met | C13H15NO2 | 1.381 | 217.26 | 16.049 | Ethanol |
| No match | – | 1.199 | – | 9.727 | Ethanol |
| Hentriacontane | C31H64 | 1.197 | 436.85 | 20.74 | Ethanol |
| Tetracontane, 3,5,24-trimeth | C43H88 | 1.194 | 605.2 | 20.201 | Ethanol |
| 6-Octadecynoic acid, methyl | C19H36O2 | 1.149 | 296.488 | 24.253 | Ethanol |
| Eicosanoic acid | C20H40O2 | 1.138 | 312.5304 | 19.118 | Ethanol |
| Sulfurous acid, butyl tetrad | C21H44O3S | 1.134 | 376.6 | 23.243 | Ethanol |
| Uvaol | C30H50O2 | 1.125 | 442.7 | 24.513 | Ethanol |
| Bicyclo [3.1.0] hexane-2-undec | C6H10 | 1.108 | 82.14 | 12.837 | Ethanol |
| Tetracosamethyl-cyclododecas | C16H32 | 1 | 224.425 | 27.703 | Ethanol |
| No match | – | 0.984 | – | 12.821 | Ethanol |
| Octadecanoic acid, 17-methyl | C20H40O2 | 0.982 | 312.5 | 17.68 | Ethanol |
| No match | – | 0.939 | – | 12.875 | Ethanol |
| Methyl 2-hydroxy-octadeca-9, | C19H32O3 | 0.895 | 308.5 | 21.548 | Ethanol |
| 2H-1-Benzopyran-2-one, 7-met | C13H15NO2 | 0.893 | 217.26 | 12.956 | Ethanol |
| No match | – | 0.884 | – | 11.045 | Ethanol |
| No match | – | 0.865 | – | 13.906 | Ethanol |
| No match | – | 0.836 | – | 15.628 | Ethanol |
| [1,1′-Bicyclopropyl]-2-octan | C21H38O2 | 0.823 | 322.5 | 16.857 | Ethanol |
| 11,14-Octadecadienoic acid, | C18H32O2 | 0.819 | 280.4 | 21.561 | Ethanol |
| Betulin | C30H50O2 | 0.802 | 442.72 | 33.839 | Ethanol |
| No match | – | 0.772 | – | 19.585 | Ethanol |
| 5-Hydroxymethylfurfural | C6H6O3 | 0.768 | 126.11 | 6.985 | Ethanol |
| No match | – | 0.754 | – | 12.601 | Ethanol |
| No match | – | 0.742 | – | 11.881 | Ethanol |
| No match | – | 0.727 | – | 12.675 | Ethanol |
| Urs-12-en-28-oic acid, 3-hyd | C30H48O3 | 0.722 | 456.7 | 23.776 | Ethanol |
| Sulfurous acid, butyl tetrad | C21H44O3S | 0.667 | 376.6 | 22.185 | Ethanol |
| No match | – | 0.665 | – | 11.947 | Ethanol |
| Alpha-Tocospiro A | C29H50O4 | 0.654 | 462.7 | 22.498 | Ethanol |
| Oleic Acid | C18H34O2 | 0.653 | 282.47 | 16.515 | Ethanol |
| Tricyclo [20.8.0.07,16] tria | C18H24O4 | 0.647 | 304.38 | 25.158 | Ethanol |
| No match | – | 0.643 | – | 11.217 | Ethanol |
| Stigmasterol | C29H48O | 0.63 | 412.69 | 24.507 | Ethanol |
| Methyl 10,11-tetradecadienoa | C15H26O2 | 0.573 | 238.366 | 10.069 | Ethanol |
| Sulfurous acid, butyl tridec | C17H36O3S | 0.572 | 320.5 | 22.897 | Ethanol |
| No match | – | 0.562 | – | 12.242 | Ethanol |
| 24-Noroleana-3,12-diene | C29H46 | 0.537 | 394.676 | 31.418 | Ethanol |
| No match | – | 0.534 | – | 9.47 | Ethanol |
| No match | – | 0.517 | – | 22.775 | Ethanol |
| Cholestan-3-ol, 2-methylene- | C28H48O | 0.515 | 400.7 | 15.446 | Ethanol |
| Tetracontane, 3,5,24-trimeth | C43H88 | 0.506 | 605.2 | 25.112 | Ethanol |
| No match | – | 0.501 | – | 26.914 | Ethanol |
| 2-Methylindoline | C9H11N | 0.49 | 133.19 | 6.58 | Ethanol |
| No match | – | 0.481 | – | 16.473 | Ethanol |
| No match | – | 0.457 | – | 8.924 | Ethanol |
| 3,7,11,15-Tetramethyl-2-Hexa | C20H40O | 0.444 | 296.5 | 23.191 | Ethanol |
| No match | – | 0.435 | – | 10.634 | Ethanol |
| 1-Heptatriacotanol | C37H76O | 0.432 | 537 | 13.943 | Ethanol |
| No match | – | 0.424 | – | 13.117 | Ethanol |
| 1R,4S,7S,11R-2,2,4,8-Tetrame | C15H26O | 0.419 | 222.366 | 31.553 | Ethanol |
| No match | – | 0.406 | – | 10.249 | Ethanol |
| Sulfurous acid, butyl tridec | C17H36O3S | 0.399 | 320.5 | 24.233 | Ethanol |
| No match | – | 0.395 | – | 16.248 | Ethanol |
| No match | – | 0.375 | – | 25.618 | Ethanol |
| No match | – | 0.369 | – | 23.972 | Ethanol |
| 6-Hydroxy-4,4,7a-trimethyl-5 | C11H16O3 | 0.367 | 196.24 | 16.663 | Ethanol |
| Ethyl 9.cis.,11. trans.-octad | C20H38O2 | 0.34 | 310.515 | 17.345 | Ethanol |
| Tau-Cadinol | C15H26O | 0.335 | 222.37 | 12.143 | Ethanol |
| 24(H)-Benzofuranone, 5,6,7,7 | C11H16O2 | 0.319 | 180.244 | 10.757 | Ethanol |
| Glycine, N-[3alpha, 5beta] | C30H53NO4Si | 0.313 | 519.8 | 24.109 | Ethanol |
| Tetracontane, 3,5,24-trimeth | C43H88 | 0.304 | 605.2 | 20.193 | Ethanol |
| 2-Pentadecanone, 6,10,14-tri | C18H36O | 0.298 | 268.478 | 17.839 | Ethanol |
| Neophytadiene | C20H38 | 0.294 | 278.5 | 25.337 | Ethanol |
| 2-Pentadecanone, 6,10,14-tri | C18H36O | 0.286 | 268.478 | 14.346 | Ethanol |
| 2,4-Dihydroxy-2,5-dimethyl-3 | C6H8O4 | 0.284 | 144.12 | 3.404 | Ethanol |
| n-Propyl 9,12-hexadecadienoa | C19H34O2 | 0.262 | 294.5 | 11.116 | Ethanol |
| Tetradecanoic acid | C14H28O2 | 0.25 | 228.3709 | 13.552 | Ethanol |
| 10,10-Dimethyl-2,6-dimethyle | C15H24 | 0.199 | 204.351 | 12.067 | Ethanol |
| Ergost-5-en-3-ol (3beta)- | C28H48O | 0.18 | 400.7 | 24.141 | Ethanol |
| Fumaric acid, ethyl 2-methyl | C10H14O4 | 0.179 | 198.22 | 11.356 | Ethanol |
| Tritetracontane | C43H88 | 0.177 | 605.2 | 22.18 | Ethanol |
| Azulene, 1,2,3,3a,4,5,6,7-oc | C15H24 | 0.17 | 204.351 | 15.056 | Ethanol |
| (4aS,7S,7aR)-4,7-Dimethyl-2 | C10H14O2 | 0.169 | 166.217 | 10.885 | Ethanol |
| cis-5,8,11,14,17-Eicosapenta | C20H30O2 | 0.148 | 302.5 | 13.276 | Ethanol |
| Carbamic acid, N-[1,1-bis tr] | C12H24N2O4 | 0.124 | 260.33 | 13.319 | Ethanol |
| Bicyclo [4.4.0] dec-1-ene, 2-i | C15H24 | 0.116 | 204.35 | 11.54 | Ethanol |
| 2-Cyclohexen-1-one, 4,5-dime | C8H12O | 0.113 | 124.18 | 10.585 | Ethanol |
| 12-Methyl-E, E-2,13-octadecad | C19H36O | 0.113 | 280.489 | 11.164 | Ethanol |
| Stigmasterol | C29H48O | 0.102 | 412.69 | 24.241 | Ethanol |
| 2-Cyclohexen-1-one, 3-methyl | C7H10O | 0.083 | 110.15 | 8.592 | Ethanol |
| Megastigmatrienone | C13H18O | 0.081 | 190.28 | 11.924 | Ethanol |
| 2,4-Dihydroxy-2,5-dimethyl-3 | C6H8O4 | 0.032 | 144.12 | 3.23 | Ethanol |
| Cyclopentanecarboxylic acid | C6H10O2 | 0.008 | 114.14 | 9.434 | Ethanol |
| 2-Methylindoline | C9H11N | 133.19 | 8.12 | Ethanol |
Table 4.
GC/MS analysis of water extract of N. cataria using NIST 17 Library showed (79 identified phytochemicals + 31 unmatched) chemicals, arranged according to concentration present.
| Compound | Mol. formula | Amount/Conc.% | Mol. weight (g/mol) | RT (Min) | Extract |
|---|---|---|---|---|---|
| 1-Isopropylcyclohex-1-ene | C9H16 | 22.387 | 124.22 | 10.657 | Water |
| 7-Methylhexahydrocyclopenta | C9H14O2 | 5.399 | 154.21 | 11.265 | Water |
| 2H-1-Benzopyran-2-one, 7-met | C13H15NO2 | 5.336 | 217.26 | 14.95 | Water |
| (R) -(-)-14-Methyl-8-hexadecy | C17H34O | 5.106 | 254.4513 | 10.79 | Water |
| No match | – | 4.917 | – | 15.825 | Water |
| Benzofuran, 2,3-dihydro- | C8H8O | 4.002 | 120.15 | 8.48 | Water |
| Hydro coumarin | C9H8O2 | 3.699 | 148.1586 | 10.843 | Water |
| Bicyclo [3.1.0] hexane-2-undec | C6H10 | 3.1 | 82.14 | 12.831 | Water |
| No match | – | 2.942 | – | 13.861 | Water |
| Coumarin | C9H6O2 | 2.265 | 146.1427 | 11.545 | Water |
| Cyclopentane carboxylic acid, | C6H10O2 | 2.165 | 114.14 | 10.486 | Water |
| 13-Tetradece-11-yn-1-ol | C14H24O | 2.146 | 208.34 | 11.581 | Water |
| No match | – | 1.738 | – | 11.098 | Water |
| No match | – | 1.63 | – | 14.825 | Water |
| No match | – | 1.472 | – | 15.575 | Water |
| (S-2-1R,4R)-4-Methyl-2-oxo | C4H6O3 | 1.274 | 102.0886 | 12.723 | Water |
| (4R,4aR,7S,7aR)-4,7-Dimethyl | C10H18O | 1.17 | 154.25 | 11.326 | Water |
| Homovanillyl alcohol | C9H12O3 | 1.118 | 168.19 | 12.621 | Water |
| 2-Cyclohexene-1-one, 4-3-hyd | C13H20O2 | 0.997 | 208.2967 | 13.984 | Water |
| No match | – | 0.932 | – | 13.036 | Water |
| 2-Methylindoline | C9H11N | 0.825 | 133.19 | 8.353 | Water |
| (E)-2,6-Dimethylocta-3,7-die | C10H18O2 | 0.67 | 170.25 | 8.078 | Water |
| No match | – | 0.562 | – | 11.045 | Water |
| Ethanone, 1-2-hydroxyphenyl | C8H8O2 | 0.559 | 136.15 | 11.463 | Water |
| 2-Methoxy-4-vinyl phenol | 0.53 | 9.845 | Water | ||
| 6-Hydroxy-4,4,7a-trimethyl-5 | C11H16O3 | 0.496 | 196.24 | 15.394 | Water |
| No match | – | 0.469 | – | 3.652 | Water |
| No match | – | 0.437 | – | 5.652 | Water |
| No match | – | 0.404 | – | 16.262 | Water |
| 3-Acetylthymine | C5H6N2O2 | 0.402 | 126.1133 | 13.283 | Water |
| 3-Oxo-4-phenylbutyronitrile | C10H9NO | 0.371 | 159.18 | 8.825 | Water |
| No match | – | 0.337 | – | 4.368 | Water |
| 7-Oxabicyclo [4.1.0] heptan-3- | C6H10O2 | 0.295 | 114.14 | 16.821 | Water |
| n-Hexadecanoic acid | C16H32O2 | 0.263 | 256.4241 | 17.288 | Water |
| 1H-Pyrrole-2,5-dione, 3-ethy | – | 0.25 | – | 8.69 | Water |
| 1,7-Octadiene-3,6-diol, 2,6- | C10H18O2 | 0.238 | 170.25 | 9.271 | Water |
| Conhydrin | C8H17NO | 0.212 | 143.23 | 7.847 | Water |
| Methyl 7,8-octadecadienoate | C19H34O2 | 0.206 | 294.4721 | 12.898 | Water |
| 1H-Indene, 1-ethylideneoctah | C11H10 | 0.07 | 142.2 | 14.737 | Water |
Table 5.
GC/MS analysis of an acetone-based extract of N. cataria using NIST 17 Library showed (12 identified phytochemicals + 9 unmatched) chemicals, arranged according to concentration present.
| Compound | Mol. formula | Amount/conc. % | Mol. weight (g/mol) | RT (Min) | Extract |
|---|---|---|---|---|---|
| Oxime-, methoxy-phenyl- | C8H9NO2 | 2.849 | 151.16 | 3.685 | Acetone |
| 1-Isopropylcyclohex-1-ene | C9H16 | 29.552 | 124.22 | 8.206 | Acetone |
| Caryophyllene oxide | C15H24O | 6.868 | 220.35 | 11.452 | Acetone |
| (+)-2-Bornanone | C10H16O | 6.365 | 152.233 | 5.984 | Acetone |
| n-Hexadecanoic acid | C16H32O2 | 5.337 | 256.424 | 17.237 | Acetone |
| No match | – | 3.443 | - | 10.391 | Acetone |
| Endo-Borneol | C10H18O | 3.083 | 154.25 | 6.191 | Acetone |
| Hotrienol | C10H16O | 2.947 | 152.23 | 7.692 | Acetone |
| No match | – | 2.573 | - | 13.475 | Acetone |
| (E)-2,6-Dimethylocta-3,7-die | C10H18O2 | 2.572 | 170.25 | 6.217 | Acetone |
| No match | – | 2.57 | - | 8.885 | Acetone |
| Cyclopentanecarboxylic acid | C6H10O2 | 2.496 | 114.14 | 8.04 | Acetone |
| No match | – | 2.289 | - | 9.631 | Acetone |
| No match | – | 2.038 | - | 8.424 | Acetone |
| No match | – | 2.007 | - | 8.53 | Acetone |
| No match | – | 1.947 | - | 8.127 | Acetone |
| No match | – | 1.844 | - | 9.297 | Acetone |
| Eucalyptol | C10H18O | 1.513 | 154.249 | 5.004 | Acetone |
| No match | – | 1.196 | - | 7.749 | Acetone |
| Cyclohexane, 1-propyl- | C9H16 | 1.093 | 124.22 | 7.507 | Acetone |
| alpha-methyl- alpha-[4-methyl] | C6H11NO2 | 1.026 | 129.16 | 5.292 | Acetone |
| 1,7-Octadiene-3,6-diol, 2,6-dimethyl | C10H18O2 | 0.819 | 170.25 | 7.049 | Acetone |
Table 6.
GC/MS analysis of a hexane-based extract of N. cataria using NIST 17 Library showed (9 identified phytochemicals + 8 unmatched) chemicals, arranged according to concentration present.
| Compound | Mol. formula | Amount/Conc. % |
Mol. weight (g/mol) |
RT (Min) |
Extract |
|---|---|---|---|---|---|
| (+)-2-Bornanone | C10H16O | 6.809 | 152.233 | 9.187 | Hexane |
| Methyl 6,9,12,15,18-heneicos | – | 11.008 | – | 16.663 | Hexane |
| 1,2-Benzenedicarboxylic acid | C8H6O4 | 8.551 | 166.14 | 10.181 | Hexane |
| Dibutyl phthalate | C16H22O4 | 5.877 | 278.34 | 21.611 | Hexane |
| No match | – | 5.199 | – | 12.947 | Hexane |
| No match | – | 4.075 | – | 12.537 | Hexane |
| No match | – | 3.969 | – | 19.713 | Hexane |
| endo-Borneol | C10H18O | 3.719 | 154.25 | 9.774 | Hexane |
| Benzophenone | C13H10O | 3.591 | 182.217 | 17.321 | Hexane |
| No match | – | 3.472 | – | 18.437 | Hexane |
| No match | – | 3.172 | – | 12.675 | Hexane |
| Tetracontane, 3,5,24-trimeth | C43H88 | 2.939 | 605.2 | 8.975 | Hexane |
| No match | – | 2.756 | – | 12.391 | Hexane |
| Benzoic acid, 4-ethoxy-, eth | C11H14O3 | 2.535 | 194.23 | 15.905 | Hexane |
| 7,9-Di-tert-butyl-1-oxaspiro | C17H24O3 | 1.956 | 276.4 | 20.957 | Hexane |
| No match | – | 1.421 | – | 12.21 | Hexane |
| No match | – | 1.176 | – | 17.73 | Hexane |
Figure 5.
(A) GC/MS chromatogram of set of extracts of Nepeta cataria showing peaks of metabolites in each extract. (B) The 2-D structures of important phytochemicals are retrieved via PubChem. i. Betulin was most abundant phytochemical in methanol and ethanol (ME), ii. Uvol in ethanol, iii. 2-methyl Indole in water, iv. Eucalyptol in acetone and methanol (AM) and v. 7,9-Di-ter-butyl-1-oxaspiro is most abundant phytochemical in hexane extract.
Discussion
One of the most well-known species in the genus Nepeta is N. cataria. Several studies have performed qualitative identification of phytochemical constituents from leaves and flowers of N. cataria extract as well as oils from the plant (Edewor and Usman, 2011; Reichert et al., 2018; Azizian et al., 2021). The antibacterial of N. cataria from previous research likewise demonstrated sufficient antibacterial activity against S. aureus, K. pneumoniae and S. typhi (Mukhtar and Singh, 2019). The results from our studies corroborate the results exhibited in previous studies. In addition to N. cataria, other species of the Nepeta genus have also been studied extensively for their phytochemical analysis, and among all species, N. cataria is the most promising of all species (Azizian et al., 2021).
Several studies corroborate our findings and indicate high DPPH activity in acetone extracts while others exhibit versatile results (Dienaite et al., 2018). Some studies presented more efficient DPPH activity in methanol, 70% ethanol and others in aqueous extract of N. cataria (Kraujalis et al., 2011; Mihaylova et al., 2013; Dienaite et al., 2018). Modernized extraction protocols, i.e., ultrasound-based microextraction, are being used to maximize output of phenolic compounds from methanol extract of N. cataria, which corroborates with our study (Hajmohammadi et al., 2021). Several other studies also indicate rosmarinic acid as a prominent phenolic compound in N. cataria extracts (Hadi et al., 2017).
Water extracts of N. cataria exhibit reasonable ORAC activity as per different studies (Dienaite et al., 2018; Baranauskiene et al., 2019). Another study showed excellent radical scavenging properties of N. cataria via FRAP assay, which improves the confidence in this plant (Duda et al., 2015).
Among all the treatments, ethanol-based extracts of N. cataria showed maximum percentage inhibition of all the tested bacteria at 1,000–250 μg/ml concentration, followed by methanolic extracts at 1,000 and 500 μg/ml dose levels and water-based extracts at 1,000 and 500 μg/ml dose levels. In contrast, acetone and hexane-based extracts of N. cataria did not significantly inhibit all the tested bacterial isolates compared to control treatments. Many studies provide insights for the use of N. cataria extract in inhibition of S. aureus and B. subtilis and its oil as a topical treatment of respiratory tract infections (Suschke et al., 2007; Bandh and Kamili, 2011). MIC values indicated that the ethanol-based extract of all N. cataria extracts showed maximum inhibition of B. subtilis, followed by C. freundii and M. luteus. At the same time, methanol-based extracts also showed maximum efficacy against S. sonnei, E. coli, M. luteus, and C. freundii. Water, acetone, and hexane-based extracts were almost equally effective against tested bacterial isolates, as given in Table 7. Studies indicate promising effect of N. cataria extract as antibacterial agent against S. aureus, K. pneumoniae, and Salmonella typhi (Edewor and Usman, 2011). Considering resazurin methodology, by using combined extractions of all solvents in DMSO, N. cataria plant extract at the dose level of 12.5 μl/ml showed maximum inhibition of all the bacterial strains, followed by 6.25 μl/ml. The antibacterial screening of the N. cataria from other studies also exhibited sufficient evidence of antibacterial activity against S. aureus, K. pneumoniae, and S. typhi (Morombaye et al., 2018).
Table 7.
Antimicrobial efficacy of N. cataria extracts against a set of gram-negative and gram-positive bacterial strains.
| Bacterial pathogens | Zone of inhibition (mm) | ||||||
|---|---|---|---|---|---|---|---|
| Methanol | Ethanol | Water | Acetone | Hexane | Chloramphenicol | ||
| Gram negative | E. coli | 15 ± 0.1 | 14 ± 0.1 | 12 ± 0.1 | 0 | 14 ± 0.1 | 25 ± 0.2 |
| K. oxytoca | 14 ± 0.2 | 14 ± 0.1 | 16 ± 0.1 | 14 ± 0.2 | 13 ± 0.3 | 26 ± 0.1 | |
| S. enterica | 13 ± 0.1 | 14 ± 0.1 | 0 | 0 | 0 | 25 ± 0.1 | |
| S. sonnei | 15 ± 0.2 | 15 ± 0.1 | 0 | 16 ± 0.2 | 14 ± 0.1 | 26 ± 0.2 | |
| C. ferundii | 15 ± 0.2 | 22 ± 0.4 | 12 ± 0.1 | 12 ± 0.2 | 11 ± 0.1 | 25 ± 0.2 | |
| Gram positive | B. subtilis | 14 ± 0.1 | 21 ± 0.5 | 0 | 0 | 14 ± 0.2 | 31 ± 0.1 |
| L. lactis | 0 | 0 | 0 | 13 ± 0.1 | 0 | 25 ± 0.2 | |
| L. monocytogenes | 13 ± 0.1 | 14 ± 0.2 | 13 ± 0.1 | 13 ± 0.1 | 0 | 25 ± 0.2 | |
| M. luteus | 15 ± 0.2 | 16 ± 0.2 | 16 ± 0.1 | 16 ± 0.2 | 0 | 26 ± 0.1 | |
| S. aureus | 13 ± 0.1 | 13 ± 0.1 | 0 | 0 | 0 | 20 ± 0.1 | |
GC/MS analysis of methanol and ethanol revealed the presence of betulin extracts, which is a promising antitumorigenic candidate and escalates the importance of N. cataria in cancer treatment (Liu et al., 2009). Arachidic acid (eicosanoic acid) is used to produce detergents, photographic materials, and lubricants. Caryophyllene oxide is a potential preservative used in food, drugs, and cosmetics. It also displays anti-inflammatory and anti-carcinogenic properties (Salaria et al., 2020). Uvaol also displays anti-inflammatory properties and antioxidant effects (Botelho et al., 2019). Campesterols found in methanol extracts is phytosterol, used in growth induction in animals, commonly abused anabolic steroid in sports can also reduce the absorption of cholesterol in intestine by targeting transporter protein, minimizing the effect of cardiovascular disease (Choudhary and Tran, 2011). Phytol in ethanol has been investigated for its potential anxiolytic, metabolism-modulating, cytotoxic, antioxidant, autophagy- and apoptosis-inducing, antinociceptive, anti-inflammatory, immune-modulating, and antimicrobial effects (Islam et al., 2018). Phytol is likely the most abundant acyclic isoprenoid compound present in the biosphere and its degradation products have been used as biogeochemical tracers in aquatic environments (Rontani and Volkman, 2003). Phytol is used in the fragrance industry and is used in cosmetics, shampoos, toilet soaps, household cleaners, and detergents (McGinty et al., 2010). Coumarin (2H-1-benzopyran-2-one) in methanol and ethanol is famous for pharmacological properties such as anti-inflammatory, anticoagulant, antibacterial, antifungal, antiviral, anticancer, antihypertensive, antitubercular, anticonvulsant, antiadipogenic, antihyperglycemic, antioxidant, and neuroprotective properties (Venugopala et al., 2013). Similarly in water extracts, 2-methylindole is used as an intermediate to synthesize dyes, pigments, and pharmaceuticals. Conhydrin is a poisonous alkaloid, when ingested interruption with the central nervous system, paralyzing respiratory muscles and causing failure (Hotti and Rischer, 2017). Likewise, extracts of hexane contain eucalyptol, an active ingredient as a cough suppressant as it controls mucus secretion from airway and asthma via anti-inflammatory cytokines (Juergens, 2014). Hexane soluble constituents conformed to identification of 7, 9-di-tert-butyl-1-oxaspiro which is used against skin diseases, gonorrhea, migraine, intestinal parasites, and warts (Sharif et al., 2015), and dibutyl phthalate is used in making flexible plastics. In addition to this, several other studies indicate presence of nepetalactone and other terpenoids as essential components of oil extracts of N. cataria (Handjieva et al., 2011; Sharma et al., 2019).
This study gave a thorough brief of antibacterial and antioxidant activity and its constituents. Present methodology can be beneficial in devising and exploring different bioactive compounds that can be exploited for the constructing novel antimicrobial agents for alternative therapeutic intervention against several bacterial and viral infections after processing. It may also help to treat different antibiotic-resistant pathogens. Its chemicals if used in pharmacology industries can serve as indigenous, cheaper, and readily available source.
Conclusion
Many aspects of plants were studied, but complete metabolomic profiling and identification of unmatched chemicals remain a question mark. MS-MS analysis of plant metabolites should be considered for knowing the medicinal potential of unknown and novel plant metabolites. Data compilation and individual chemical studies need a larger scale with a set of skills to combat emerging diseases. Yet, to the best of our knowledge, the concluded information, reported results, and this research is comprehensive to the best of our scale, our team tried to achieve.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
Practical performance and data compilation were performed solely by AN. Experimental assistance for GC/MS, and antibacterial analysis was given by BA. Data analysis was performed by HS. Manuscript drafting and proofreading were conducted by HS, in assistance with MW and AT. All authors contributed to the study design and implementation. All authors contributed to the article and approved the submitted version.
Funding
The authors acknowledge the Higher Education Commission, Government of Pakistan, for funding part of the research under the International Research Support Initiative Program (IRSIP) at the University of Massachusetts, USA.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors acknowledge the Higher Education Commission, Government of Pakistan and University of Massachusetts, USA, for providing us with the research facilities and support to publish this article.
References
- Adiguzel A., Ozer H., Sokmen M., Gulluce M., Sokmen A., Kilic H., et al. (2009). Antimicrobial and antioxidant activity of the essential oil and methanol extract of Nepeta cataria. Polish J. Microbiol. 58, 69-76. Available at: www.microbiology.pl/pjm [PubMed] [Google Scholar]
- Angelini P., Pagiotti R., Menghini A., Vianello B. (2006). Antimicrobial activities of various essential oils against foodborne pathogenic or spoilage moulds. Ann. Microbiol. 56, 65–69. 10.1007/BF03174972 [DOI] [Google Scholar]
- Azizian T., Alirezalu A., Hassani A., Bahadori S., Sonboli A. (2021). Phytochemical analysis of selected Nepeta species by HPLC-ESI-MS/MS and GC-MS methods and exploring their antioxidant and antifungal potentials. J. Food Meas. Charact. 15, 2417–2429. 10.1007/s11694-021-00819-8 [DOI] [Google Scholar]
- Bandh S., Kamili A. (2011). Evaluation of antimicrobial activity of aqueous extracts of Nepeta cataria Cellular and Humoral Immune Responses following Immunization with Surface/excretory secretory Antigens of Abomasal Nematodes of Sheep View project BOOK PROJECTS View project. Artic. J. Pharm. Res. Available online at: https://www.researchgate.net/publication/235769154 (accessed July 14, 2022).
- Baranauskiene R., BendŽiuviene V., RagaŽinskiene O., Venskutonis P. R. (2019). Essential oil composition of five Nepeta species cultivated in Lithuania and evaluation of their bioactivities, toxicity and antioxidant potential of hydrodistillation residues. Food Chem. Toxicol. 129, 269–280. 10.1016/j.fct.2019.04.039 [DOI] [PubMed] [Google Scholar]
- Botelho R. M., Tenorio L. P. G., Silva A. L. M., Tanabe E. L. L., Pires K. S. N., Gonçalves C. M., et al. (2019). Biomechanical and functional properties of trophoblast cells exposed to Group B Streptococcus in vitro and the beneficial effects of uvaol treatment. Biochim. Biophys. Acta Gen. Subj. 1863, 1417–1428. 10.1016/j.bbagen.2019.06.012 [DOI] [PubMed] [Google Scholar]
- Bourrel C., Perineau F., Michel G., Bessiere J. M. (2011). Catnip (Nepeta cataria L.) essential oil: analysis of chemical constituents, bacteriostatic and fungistatic properties. J. Essent. Oil Res. 5, 159–167. 10.1080/10412905.1993.9698195 [DOI] [Google Scholar]
- Choudhary S., Tran L. (2011). Phytosterols: perspectives in human nutrition and clinical therapy. Curr. Med. Chem. 18, 4557–4567. 10.2174/092986711797287593 [DOI] [PubMed] [Google Scholar]
- Dabiri M., Sefidkon F. (2003). Chemical composition of Nepeta crassifolia Boiss. and Buhse oil from Iran. Flavour Fragr. J. 18, 225–227. 10.1002/ffj.1199 [DOI] [Google Scholar]
- Dienaite L., Pukalskiene M., Matias A. A., Pereira C. V., Pukalskas A., Venskutonis P. R. (2018). Valorization of six Nepeta species by assessing the antioxidant potential, phytochemical composition and bioactivity of their extracts in cell cultures. J. Funct. Foods 45, 512–522. 10.1016/j.jff.2018.04.004 [DOI] [Google Scholar]
- Duda S. C., Mărghita,ş L. A., Dezmirean D., Duda M., Mărgăoan R., Bobi,ş O. (2015). Changes in major bioactive compounds with antioxidant activity of Agastache foeniculum, Lavandula angustifolia, Melissa officinalis and Nepeta cataria: effect of harvest time and plant species. Ind. Crops Prod. 77, 499–507. 10.1016/j.indcrop.2015.09.045 [DOI] [Google Scholar]
- Edewor I. T., Usman A. L. (2011). Phytochemical and antibacterial activities of leaf extracts of Nepeta cataria. African J. Pure Appl. Chem. 5, 503–506. 10.5897/AJPAC11.074 [DOI] [Google Scholar]
- Elshikh M., Ahmed S., Funston S., Dunlop P., McGaw M., Marchant R., et al. (2016). Resazurin-based 96-well plate microdilution method for the determination of minimum inhibitory concentration of biosurfactants. Biotechnol. Lett. 38, 1015–1019. 10.1007/s10529-016-2079-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ergün Z. (2021). Seed oil content and fatty acid profiles of endemic Phoenix theophrasti greuter, Phoenix roebelenii o'brien, Phoenix caneriensis hort. Ex chabaud, and Phoenix dactylifera l. grown in the same locality in Turkey. Turkish J. Agric. For. 45, 557–564. 10.3906/tar-2105-34 [DOI] [Google Scholar]
- Frolova N., Uktainets A., Korablova O., Voitsekhivskyi V. (2020). Plants of Nepeta cataria var. citriodora Beck. and essential oils from them for food industry. Potravin. J. Food Sci. 13, 449–455. 10.5219/1109 [DOI] [Google Scholar]
- Giarratana F., Muscolino D., Ziino G., Lo Presti V., Rao R., Chiofalo V., et al. (2017). Activity of Catmint (Nepeta cataria) essential oil against Anisakis larvae. Trop. Biomed. 34, 22–31. [PubMed] [Google Scholar]
- Gilani A. H., Shah A. J., Zubair A., Khalid S., Kiani J., Ahmed A., et al. (2009). Chemical composition and mechanisms underlying the spasmolytic and bronchodilatory properties of the essential oil of Nepeta cataria L. J. Ethnopharmacol. 121, 405–411. 10.1016/j.jep.2008.11.004 [DOI] [PubMed] [Google Scholar]
- Hadi N., Sefidkon F., Shojaeiyan A., Šiler B., Jafari A. A., Aničić N., et al. (2017). Phenolics' composition in four endemic Nepeta species from Iran cultivated under experimental field conditions: the possibility of the exploitation of Nepeta germplasm. Ind. Crops Prod. 95, 475–484. 10.1016/j.indcrop.2016.10.059 [DOI] [Google Scholar]
- Hajmohammadi M. R., Najafi AsliPashaki S., Rajab Dizavandi Z., Amiri A. (2021). Ultrasound-assisted vesicle-based microextraction as a novel method for determination of phenolic acid compounds in Nepeta cataria L. samples. J. Iran. Chem. Soc. 18, 1559–1566. 10.1007/s13738-020-02131-6 [DOI] [Google Scholar]
- Handjieva N. V., Popov S. S., Evstatieva L. N. (2011). Constituents of essential oils from Nepeta cataria L., N. grandiflora M.B. and N. nuda L. 8, 639–643. 10.1080/10412905.1996.9701032 [DOI] [Google Scholar]
- Hinkov A., Angelova P., Marchev A., Hodzhev Y., Tsvetkov V., Dragolova D., et al. (2020). Nepeta nuda ssp. nuda L. water extract: inhibition of replication of some strains of human alpha herpes virus (genus simplex virus) in vitro, mode of action and NMR-based metabolomics. J. Herb. Med. 21, 100334. 10.1016/j.hermed.2020.100334 [DOI] [Google Scholar]
- Hotti H., Rischer H. (2017). The killer of Socrates: coniine and related alkaloids in the plant kingdom. Molecule 22, 1962. 10.3390/molecules22111962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries R. M., Ambler J., Mitchell S. L., Castanheira M., Dingle T., Hindler J. A., et al. (2018). CLSI methods development and standardization working group best practices for evaluation of antimicrobial susceptibility tests. J. Clin. Microbiol. 56, e01934-17. 10.1128/JCM.01934-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam M. T., Ali E. S., Uddin S. J., Shaw S., Islam M. A., Ahmed M. I., et al. (2018). Phytol: a review of biomedical activities. Food Chem. Toxicol. 121, 82–94. 10.1016/j.fct.2018.08.032 [DOI] [PubMed] [Google Scholar]
- Juergens U. R. (2014). Anti-inflammatory properties of the monoterpene 18-cineole: current evidence for co-medication in inflammatory airway diseases. Drug Res. (Stuttg). 64, 638–646. 10.1055/s-0034-1372609 [DOI] [PubMed] [Google Scholar]
- Kaewprom K., Chen Y. H., Lin C. F., Chiou M. T., Lin C. N. (2017). Antiviral activity of Thymus vulgaris and Nepeta cataria hydrosols against porcine reproductive and respiratory syndrome virus. Thai J. Vet. Med. 47, 25–33. [Google Scholar]
- Khan T., Khan M. A., Mashwani Z., ur R., Ullah N., Nadhman A. (2021). Therapeutic potential of medicinal plants against COVID-19: the role of antiviral medicinal metabolites. Biocatal. Agric. Biotechnol. 31, 101890. 10.1016/j.bcab.2020.101890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraujalis P., Rimantas Venskutonis P., Ragazinskiene O. (2011). “Antioxidant activities and phenolic composition of extracts from nepeta plant species,” in Proceedings of the 6th Baltic Conference on Food Science and Technology.36015417 [Google Scholar]
- Liu H., Wang S., Cai B., Yao X. (2009). Anticancer activity of compounds isolated from Engelhardtia serrata Stem Bark. Pharm. Biol. 42, 475–477. 10.3109/13880200490889028 [DOI] [Google Scholar]
- Lucas-Abellán C., Mercader-Ros M. T., Zafrilla M. P., Fortea M. I., Gabaldón J. A., Núñez-Delicado E. (2008). ORAC-fluorescein assay to determine the oxygen radical absorbance capacity of resveratrol complexed in cyclodextrins. J. Agric. Food Chem. 56, 2254–2259. 10.1021/jf0731088 [DOI] [PubMed] [Google Scholar]
- Mamadalieva N. Z., Akramov D. K., Ovidi E., Tiezzi A., Nahar L., Azimova S. S., et al. (2017). Aromatic medicinal plants of the Lamiaceae family from Uzbekistan: ethnopharmacology, essential oils composition, and biological activities. Medicine 4, 8. 10.3390/medicines4010008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinty D., Letizia C. S., Api A. M. (2010). Fragrance material review on phytol. Food Chem. Toxicol. 48, S59–S63. 10.1016/j.fct.2009.11.012 [DOI] [PubMed] [Google Scholar]
- Mihaylova D., Popova A., Deseva I. N. (2013). In vitro antioxidant activity and phenolic composition of Nepeta cataria L. extracts. Int. J. Agric. Sci. Technol. 1, 74–79. [Google Scholar]
- Mir M. A., Parihar K., Tabasum U., Kumari E., Mir A., Amin Mir M. (2016). Estimation of alkaloid, saponin and flavonoid, content in various extracts of Crocus sativa. J. Med. Plants Stud. 4, 171–174. [Google Scholar]
- Morombaye S. M., Kangogo M., Revathi G., Nyerere A., Ochora J., Morombaye S. M., et al. (2018). Evaluation of the antimicrobial effect of Nepeta cataria and Basella alba against clinically resistant Acinetobacter baumannii in Nairobi, Kenya. Adv. Microbiol. 8, 790–803. 10.4236/aim.2018.810052 [DOI] [Google Scholar]
- Mukhtar H. M., Singh G. P. (2019). Pharmacognostic and phytochemical investigations of aerial parts of Nepeta cataria Linn. Asian J. Pharm. Pharmacol. 5, 810–815. 10.31024/ajpp.2019.5.4.23 [DOI] [Google Scholar]
- Nadeem A., Ahmed B., Shahzad H., Craker L. E., Muntean T. (2021). Verbascum Thapsus (Mullein) versatile polarity extracts: GC-MS analysis, phytochemical profiling, anti-bacterial potential and anti-oxidant activity. Res. Artic. Pharmacogn. J. 13, 1488–1497. 10.5530/pj.2021.13.189 [DOI] [Google Scholar]
- Naguib A. M. M., Ebrahim M. E., Aly H. F., Metawaa H. M., Mahmoud A. H., Mahmoud E. A., et al. (2012). Phytochemical screening of Nepeta cataria extracts and their in vitro inhibitory effects on free radicals and carbohydrate-metabolising enzymes. Nat. Product Res. 26, 2196–2198. 10.1080/14786419.2011.635342 [DOI] [PubMed] [Google Scholar]
- Narimani R., Moghaddam M., Ghasemi Pirbalouti A., Mojarab S. (2017). Essential oil composition of seven populations belonging to two Nepeta species from Northwestern Iran. Int. J. Food Proper. 20, 2272–2279. 10.1080/10942912.2017.1369104 [DOI] [Google Scholar]
- Özkan K., Karadag A., Sagdiç O. (2021). Determination of the in vitro bioaccessibility of phenolic compounds and antioxidant capacity of Juniper berry (Juniperus drupacea Labill.) pekmez. Turk. J. Agric. For. 45, 290–300. 10.3906/tar-2009-2 [DOI] [Google Scholar]
- Packialakshmi N., Naziya S. (2014). Phytochemical and antimicrobial screening of the polar and non-polar solvent stem extract of Caralluma fimbriyata. Int. J. Pure Appl. Biosci. 2, 32–37. 10.3126/ijasbt.v2i3.10796 [DOI] [Google Scholar]
- Pargaien A. V., Bisht N., Joshi H., Pargaien S., Adhikari M. (2020). Analysis of ethanomedicinally potential extract of Nepeta cataria. Eco. Env. Cons. 26, 90–95. Available online at: http://www.envirobiotechjournals.com/EEC/Vol26OctSuppl20/EEC-15.pdf21843624 [Google Scholar]
- Prabhavathi R. M., Prasad M. P., Jayaramu M. (2016). Studies on qualitative and quantitative phytochemical analysis of Cissus quadrangularis. Adv. Appl. Sci. Res. 7, 11–17. [Google Scholar]
- Reichert W., Ejercito J., Guda T., Dong X., Wu Q., Ray A., et al. (2019). Repellency assessment of Nepeta cataria essential oils and isolated nepetalactones on Aedes aegypti. Sci. Rep. 9, 1–9. 10.1038/s41598-018-36814-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichert W., Villani T., Pan M. H., Ho C. T., Simon J. E., Wu Q. (2018). Phytochemical analysis and anti-inflammatory activity of Nepeta cataria accessions. J. Med. Active Plants 7, 19–27. 10.7275/1mca-ez51 [DOI] [Google Scholar]
- Rontani J. F., Volkman J. K. (2003). Phytol degradation products as biogeochemical tracers in aquatic environments. Org. Geochem. 34, 1–35. 10.1016/S0146-6380(02)00185-7 [DOI] [Google Scholar]
- Salaria D., Rolta R., Sharma N., Dev K., Sourirajan A., Kumar V. (2020). In silico and in vitro evaluation of the anti-inflammatory and antioxidant potential of Cymbopogon citratus from North-western Himalayas. bioRxiv 2020.05.31.124982. 10.1101/2020.05.31.124982 [DOI] [Google Scholar]
- Sarin R. V., Bafna P. A. (2012). Herbal antidiarrhoeals: a review. Int. J. Res Pharmacol. Biomed. Sci. 3, 637–649. [Google Scholar]
- Scott H. (2003). Catnip, Nepeta cataria, a morphological comparison of mutant and wild type specimens to gain an ethnobotanical perspective. Econ. Bot. 57, 135–142. 10.1663/0013-0001(2003)057[0135:CNCAMC]2.0.CO;2 [DOI] [Google Scholar]
- Sharif H. B., Mukhtar M. D., Mustapha Y., Lawal A. O. (2015). Preliminary investigation of bioactive compounds and bioautographic studies of whole plant extract of Euphorbia pulcherrima on Escherichia coli, Staphylococcus aureus, Salmonella typhi, and Pseudomonas aeruginosa. Adv. Pharm. 2015, 14. 10.1155/2015/485469 [DOI] [Google Scholar]
- Sharma A., Cooper R., Bhardwaj G., Cannoo D. S. (2021). The genus Nepeta: traditional uses, phytochemicals and pharmacological properties. J. Ethnopharmacol. 268, 113679. 10.1016/j.jep.2020.113679 [DOI] [PubMed] [Google Scholar]
- Sharma A., Nayik G. A., Cannoo D. S. (2019). Pharmacology and Toxicology of Nepeta cataria (Catmint) Species of Genus Nepeta: A Review. Cham: Springer. [Google Scholar]
- Suschke U., Sporer F., Schneele J., Geiss H. K., Reichling J. (2007). Antibacterial and cytotoxic activity of Nepeta cataria L., N. cataria Var. Citriodora (Beck.) Balb. and Melissa officinalis L. essential oils. Nat. Prod.Commun. 2, 1277–1286. 10.1177/1934578X0700201218 [DOI] [Google Scholar]
- Tan J., Li J., Ma J., Qiao F. (2019). Hepatoprotective effect of essential oils of Nepeta cataria L. On acetaminophen-induced liver dysfunction. Biosci. Rep. 39, BSR20190697. 10.1042/BSR20190697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venugopala K. N., Rashmi V., Odhav B. (2013). Review on natural coumarin lead compounds for their pharmacological activity. Biomed Res. Int. 2013, 963248. 10.1155/2013/963248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F., Chen Y., Xue Z., Lv Y., Shen L., Li K., et al. (2020). High-throughput sequencing and exploration of the lncRNA-circRNA-miRNA-mRNA network in type 2 diabetes Mellitus. Biomed. Res. Int. 2020, 13. 10.1155/2020/8162524 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.